Abstract
The neurotoxicity of heavy metals received increasingly attention in recent years. Sleeping is regulated and coordinated by nervous system, however, the health hazard of heavy metal like cadmium (Cd) exposure on sleep health remained unknown. Rescue strategies like physical exercise (PE) has emerged to mitigate such influence. An epidemiological design with cross-sectional data from National Health and Nutrition Examination Survey 2007–2010 was applied. The relationship between three blood heavy metals [cadmium (Cd), lead (Pb), mercury (Hg)] and sleep disturbance was analyzed. A total of 8,751 participants were finally included in and the weighted participants were 330,239,463. Weighted quantile sum (WQS) regression indicated that mixed blood metals were positively related to risk of sleep disturbance and the mixture effect of exposure to heavy metals was mainly attributable to Cd (89.1%). Weighted logistic regression showed a significant positive association between the highest quartile of blood Cd and sleep disturbance [(OR (95% CI)): 1.191 (1.014,1.400), p = 0.036] in the fully adjusted model, while no association was found under Pb and Hg exposure. In the association between Q3 and Q4 level of blood Cd and sleep disturbance, moderate-to-vigorous physical exercise group had lower risks than none and low exercise group. In the restricted cubic spline model, it was also verified that higher PE participation was associated with the lowest incidence of sleep disturbance with the increment in Cd concentration. Our study suggested that both policy makers and the public should minimize heavy metal exposure. Moreover, conducting moderate to vigorous physical exercise is a protecting factor to mitigate Cd’s influence on sleep health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-023-16358-4.
Keywords: Heavy metal, Cadmium, Physical exercise, NHANES, Sleep disturbance, Cross-sectional study
Introduction
Environmental toxic metals, including cadmium (Cd), lead (Pb) and mercury (Hg), have recently received intense attention in consideration of their widespread exposure and adverse health effects [1–3]. Heavy metals, which are widely used in electronics, optics, as well as medical industries, can affect the human system through ingestion of contaminated water and food, inhalation of ambient air, and skin contact. Compared with the broad consensus of metals on cardiovascular or metabolism system, metal toxicity on the function and progression of multiple neurological diseases (such as sleep disturbance, stroke, Alzheimer’s disease) is emerging topics [4] and has not been fully examined.
On the basis of the close relationship between neurological and brain health with sleep, the influence of metal pollutants on sleep health is another research topic worth exploring. As a rising public health issue, poor sleep and sleep disturbance is more common in modern society and one third of the US general population self-reported troubling sleeping experiences [5]. Relative to other toxic metals, more research has been done on lead and cadmium, perhaps attributed to their significant morbidity and mortality. One previous case–control study found that higher blood lead and cadmium concentrations were significantly associated with obstructive sleep apnea (OSA, one of a sleep breathing disorder) [6]. There are mainly two pathways that environmental pollutants can affect the brain as well as sleep: one is through food intake and the metabolic system, and another is through the circulation system and the blood-brain barrier (BBB) [7, 8].
Although there were hypotheses and concerns about sleep troubles due to metal exposure, the existing research was hard to fully explain the etiology of sleep related disorders, and epidemiological study on the nationally population remained scarce. Over recent years, one cross-sectional research using nationwide samples from the National Health and Nutrition Examination Surveys (NHANES) showed that higher urinary arsenic level was related to frequent night awakenings [9], and higher urinary antimony concentration was associated with sleep health concerns including OSA [10]. Nevertheless, to the best of our knowledge, limited evidence has explored the relationship between heavy metal (including Cd and Pb) exposure and sleep disturbance.
As a rescue strategy, physical exercise (PE) has been reported to improve sleep quality and efficiency. In recent years, plentiful trials focused on the benefits of exercise on sleep health and a number of systematic reviews and meta-analyses were conducted to verify the efficiency of exercise on sleep related disorders [11–13]. Abundant evidence suggested that there are biological and psychosocial mechanisms induced by exercise [14–16], which may improve sleep symptoms and quality of life. Given that PE can induce beneficial effects on sleep health, while exposure to heavy metal was harmful on sleep disturbance, these facts, led us to propose whether PE could mitigate the negative effects of metal exposure. The major weaknesses of previous studies included shortage of specific metal exposure (such as Cd) assessments, as well as the lack of data on confounders (e.g., body mass index, chronic diseases). Additionally, the joint effect of environmental exposure and lifestyle factor including PE on sleep disturbance was unknown.
Using nationwide–represented data from the NHANES, we aimed to explore the association between the exposure of blood metal with sleep disturbance as well as the mitigation effects of exercise. We hypothesized that toxic metal exposure was positively associated with sleep disturbance and PE can alleviate this association. Collectively, this cross-sectional study aimed to: (i) investigate the associations between metal exposure and sleep disturbance; (ii) identify the most relevant metal on sleep disturbance and further explore the influence of different subgroups; (iii) explore the mitigation effect of PE on relationship to the associations mentioned above.
Methods
Study population
All data used in this current research were obtained from the National Health and Nutrition Examination Survey, which was implemented by the National Center for Health Statistics. In order to reflect the noninstitutionalized civilian population residing in the United States, NHANES was designed as a stratified, multistage probability samples through a complex statistical process to reflect all resident population information. The interview of NHANES covered demographic, socioeconomic, physiological and biochemical indexes as well as other health-related issues. Unique sampling weight was assigned to each participant and multidimensional index such as interviewed weights (interviews in the home), mobile examination center (MEC) weights, or blood metals should be carefully applied in different situations. Sample information and processing methods from NHANES for epidemiological and health related research can be publicly achieved from the online website (https://www.cdc.gov/nchs/nhanes/index.htm). Written informed consent was obtained from each participant in NHANES and the survey protocol was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board.
We aggregated two year cycles’ (four years) data from NHANES (2007–2010). Among the 20,015 participants in the total sample, adult’s samples (n = 11,766) were used for further research. A total of 2,238 samples were excluded due to lack of information of blood metal, sleep status, and information of physical exercise behavior. Participants without covariates’ data (n = 777) were further excluded. Finally, 8,751 adults were enrolled in for analysis (Fig. 1).
Fig. 1.
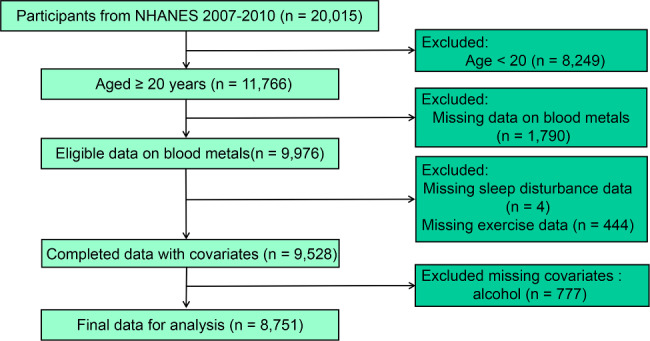
The flow chart of participant selection using the National Health and Nutrition Examination Survey data 2007–2010
Blood metals exposure assessment
Blood metals’ sampling weights were applied to analyze these data properly. In NHANES 2007–2008 and 2009–2010, three indexes of heavy metal including Cadmium (Cd), Lead (Pb), and Mercury (Hg) were evaluated by the inductively coupled plasma-mass spectrometer (ICP-MS). Whole blood specimens were processed and stored to the Division of Laboratory Sciences and Vials were stored under frozen (–30 °C) conditions until they were shipped to National Center for Environmental Health for testing. Details of laboratory processing and quality assurance were available in the survey website of the two years’ cycle [17, 18]. Lower limits of detection (LLOD) for each metal, as well as the number and percentage of samples below the LLOD were presented in detection rate. In cases where the result was below the limit of detection, the value below the LLOD was imputed as the detection limit divided by the square root of 2.
Ascertainment of sleep disturbance outcomes
In the present analysis, the identification of sleep disturbance cases was conducted through interview and self-reported sleeping status in the NHANES 2007–2010 survey. Referring to the interview guideline, cases of sleep disturbance were defined by utilizing a computer-assisted personal interviewing system by trained interviewers in the home as the following question “Have you ever told a doctor or other health professional that you have trouble sleeping?”, and this classification method was used by prior published studies [19, 20].
Ascertainment of physical exercise exposure
Physical exercise (PE) information was obtained during the household interviews utilizing the Physical Activity Questionnaire. PE was defined as leisure time or recreational physical engagement (including sports, fitness and other recreational activities) in the NHANES. Before NHANES 2007, it was difficult to calculate volume of exercising in detail, while the NHANES physical exercise questionnaire changed after 2007, and low, moderate and vigorous recreational activity can be assessed by calculating the metabolic equivalent of task (MET). Days and minutes of exercise in a typical week was extracted to calculate the total time (counted in minutes). Subsequently, the MET value for PE was obtained by the following formula: PE (MET-minutes per week) = MET (vigorous as 8 and moderate as 4) × weekly frequency ×duration of each recreational activity, the detailed calculation method was described elsewhere [21]. According to the previous literature, PE status was categorized into three level (none, low, and moderate-to-vigorous) [22].
.
Covariates determination
Referring to previous literature, relevant covariates were considered as potential confounding factors in our analysis [21, 23], including socio-demographic characteristics (age, gender, race/ethnicity, marital status, education, poverty income ratio), lifestyle factors [body mass index (BMI), smoking status, alcohol use] and chronic disease conditions [diabetes mellitus (DM) and cardiovascular disease (CVD)]. Age was grouped into < 40, [40, 60), and ≥ 60. Gender was dichotomized into male and female. Education was categorized into three groups (below high school, high school, and college or above). Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American and others. Marital statuses were grouped into married/living with partner, never married, widowed/ divorced. Family poverty-to-income ratio was divided into three groups (< 1, [1,3), and ≥ 3). BMI was calculated as body weight in kilograms divided by meters squared and categorized as < 25, [25, 30), and ≥ 30. According to previous literature [24, 25], smoking status was categorized into never, former, and current; and alcohol use status was categorized into never, moderate drinkers, heavy drinkers.
Statistical analysis
Due to the probability sampling design, sample weights, strata, and primary sampling units of NHANES program, all analysis strategy in this study accounted for the complex survey design, survey non-response, and post-stratification adjustment. Weighted percentages of variables were reported and weighted chi-square test was used to show the differences in baseline characteristics. Further, survey-weighted logistic regression model were performed to estimate odds ratios and 95% CI for associations between heavy metal exposure and the sleep disturbance. There were three modeling strategies applied in this study: [1] single-metal analysis, which used separate model for each blood metal; [2] weighted quantile sum (WQS) analysis, in order to detect the importance of each metal and evaluating the mixture effect; [3] sensitive analysis using two-metal or multiple-metal model detecting blood metals simultaneously. For the weighted logistic regression, the crude model with adjusted for no covariates. Age, gender, race/ethnicity were adjusted in Model 1. Fully adjusted covariates, including BMI, marital status, education, poverty income ratio, smoking status, alcohol use and chronic diseases were adjusted in Model 2.
Referring to several previous studies [26, 27], we assumed that the direction of the association between metal exposures (Cd, Pb, and Hg) and sleep disturbances was positive. In order to verify the assumption, a regression analysis was conducted between individual blood metals and sleep disturbances. If the assumption held, the WQS regression would be applied to explore multi-metal exposures and sleep disturbance risks. For the WQS model, in detail, all blood metals were combined into a WQS index which was generated through bootstrap sampling and then scored into quartiles. The weights of each metal were constrained between 0 and 1 and can identify important (highly weighted) metals. Blood metals were randomly split into training and validation sets in WQS model (rate was 40:60), where the training set was used to estimate variable weights via 1000 bootstrap samplings and the validation set was used to test the significance of the mixture. In the WQS regression, covariates were adjusted using the fully adjusted model (Model 2).
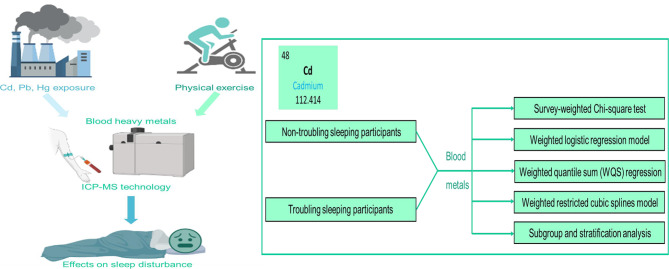
Based on the results of weighted logistic regression model, significant relationship between specific metal and sleep disturbance was further examined. Dose-response relationship between specific metal exposure and sleep disturbance was estimated using weighted restricted cubic spline (RCS), and 3 knots were established in our models [28]. Additionally, subgroup analyses were conducted to explore the influence of different PE levels on the relationship between heavy metal and sleep disturbance. An illustration of the research design and analytical procedures can be seen in Fig. 2. All analyses were performed using R software (version 4.2.0) and P value less than 0.05 was regarded as statistically significant.
Fig. 2.
Brief introduction of the research design and analytical procedures
Results
Basic characteristics of the study participants
We included 8,751 adults aged 20 years or older in the present analyses and the weighted participants were 330,239,463. The weighted prevalence of sleep disturbance was 25.2%. The blood metal concentration was categorized into four quartiles [Cd: Q1 (< 0.22 µg/l), Q2 (0.25–0.35 µg/l), Q3 (0.35–0.62 µg/l), Q4 (> 0.62 µg/l); Pb: Q1 (< 0.89 µg/dl), Q2 (0.89–1.37 µg/dl), Q3 (1.37–2.13 µg/dl), Q4 (> 2.13 µg/dl); Hg: Q1 (< 0.49 µg/l), Q2 (0.49–0.88 µg/l), Q3 (0.88–1.68 µg/l), Q4 (> 1.68 µg/l)]. The median age was 50 years, and 47.73% of them were men. Over half of the total participants were more likely to be non-Hispanic white and married, with a higher education level (College or above) and higher income, and less likely to be diabetes mellitus or cardiovascular patients. Detailed characteristics of participants in this study is shown in Table 1.
Table 1.
Weighted characteristics of participants in the NHANES (2007–2010) by sleep disturbance
| Variable | All participants | Non-troubling sleeping | Troubling sleeping | P-value |
|---|---|---|---|---|
| Age (year) | 50.45 ± 0.35 | 46.38 ± 0.40 | 50.58 ± 0.36 | < 0.001 |
| BMI(kg/m2) | 28.89 ± 0.10 | 28.52 ± 0.13 | 29.96 ± 0.17 | < 0.001 |
| Blood Cd (µg/l) | 0.52 ± 0.01 | 0.50 ± 0.01 | 0.58 ± 0.02 | < 0.001 |
| Blood Pb (µg/dl) | 1.61 ± 0.03 | 1.61 ± 0.03 | 1.59 ± 0.05 | 0.629 |
| Blood Hg (µg/l) | 1.63 ± 0.07 | 1.70 ± 0.08 | 1.41 ± 0.05 | < 0.001 |
| Age | < 0.001 | |||
| < 40 | 35.40 | 39.00 | 24.75 | |
| [40, 60) | 39.83 | 37.35 | 47.15 | |
| ≥ 60 | 24.77 | 23.64 | 28.10 | |
| BMI(kg/m2) | < 0.001 | |||
| < 25 | 30.05 | 31.41 | 26.05 | |
| [25, 30) | 34.03 | 34.54 | 32.53 | |
| ≥ 30 | 35.91 | 34.05 | 41.42 | |
| Gender | < 0.001 | |||
| Male | 47.73 | 50.48 | 39.60 | |
| Female | 52.27 | 49.52 | 60.40 | |
| Race/ethnicity | < 0.001 | |||
| non-Hispanic White | 71.55 | 69.14 | 78.68 | |
| non-Hispanic Black | 10.18 | 10.28 | 9.89 | |
| Mexican American | 8.23 | 9.46 | 4.58 | |
| Other Race/ethnicity | 10.04 | 11.12 | 6.85 | |
| Marital status | < 0.001 | |||
| Never married | 15.93 | 16.71 | 13.61 | |
| Married/living with partner | 65.30 | 66.68 | 61.21 | |
| Widowed/ divorced | 18.77 | 16.61 | 25.18 | |
| Poverty income ratio. | 0.293 | |||
| < 1 | 13.57 | 13.14 | 14.84 | |
| [1,3) | 35.68 | 36.05 | 34.57 | |
| ≥ 3 | 50.75 | 50.81 | 50.59 | |
| Education | 0.344 | |||
| Below high school | 5.95 | 6.07 | 5.60 | |
| High school | 37.30 | 36.76 | 38.90 | |
| College or above | 56.75 | 57.17 | 55.50 | |
| Smoking status | < 0.001 | |||
| None | 53.34 | 55.82 | 46.00 | |
| Former smoking | 24.98 | 23.68 | 28.81 | |
| Current smoking | 21.68 | 20.50 | 25.19 | |
| Alcohol use | 0.036 | |||
| None | 27.36 | 26.68 | 29.36 | |
| Moderate alcohol use | 51.18 | 51.01 | 51.68 | |
| High alcohol use | 21.46 | 22.30 | 18.96 | |
| Physical exercise | < 0.001 | |||
| None | 49.86 | 48.07 | 55.17 | |
| Low | 17.51 | 17.62 | 17.19 | |
| Moderate to vigorous | 32.63 | 34.31 | 27.64 | |
| DM | < 0.001 | |||
| No | 84.45 | 85.98 | 79.96 | |
| Yes | 15.55 | 14.02 | 20.04 | |
| CVD | < 0.001 | |||
| No | 91.19 | 93.00 | 85.83 | |
| Yes | 8.81 | 7.00 | 14.17 | |
| Blood Cd (µg/l) | < 0.001 | |||
| Q1 (< 0.22) | 26.09 | 27.09 | 23.12 | |
| Q2 (0.25–0.35) | 27.54 | 28.24 | 25.47 | |
| Q3 (0.35–0.62) | 23.02 | 22.71 | 23.93 | |
| Q4 (> 0.62) | 23.35 | 21.96 | 27.47 | |
| Blood Pb (µg/dl) | 0.766 | |||
| Q1 (< 0.89) | 27.84 | 28.23 | 26.70 | |
| Q2 (0.89–1.37) | 27.06 | 26.73 | 28.03 | |
| Q3 (1.37–2.13) | 24.21 | 24.15 | 24.38 | |
| Q4 (> 2.13) | 20.89 | 20.89 | 20.90 | |
| Blood Hg (µg/l) | 0.033 | |||
| Q1 (< 0.49) | 23.66 | 22.84 | 26.09 | |
| Q2 (0.49–0.88) | 24.14 | 24.18 | 24.03 | |
| Q3 (0.88–1.68) | 24.85 | 24.57 | 25.69 | |
| Q4 (> 1.68) | 27.34 | 28.41 | 24.19 | |
Notes: % for categorical variables. Abbreviations: NHANES, National Health and Nutrition Examination Survey; BMI, body mass index; PIR, poverty income ratio; Cd, cadmium; Pb, lead; Hg, mercury; DM, diabetes mellitus; CVD, cardiovascular diseases. For categorical variables, data was presented as survey-weighted percentage, P-value was calculated by survey-weighted Chi-square test; For continuous variables, data was presented as survey-weighted mean ± SE (standard error), P-value was calculated by survey-weighted linear regression
Associations between heavy metal exposure and sleep disturbance
The associations between blood Cd, Pb, Hg level and sleep disturbance are shown in Table 2 and Supplementary Table 1. Participants were categorized according to interquartile range of Cd concentration with Q1 level as reference. A positive association between Cd and sleep disturbance was identified in Q4 level for all three models [Crude Model: OR (95% CI) as 1.466 (1.270,1.692), p < 0.001; Model 1: OR (95% CI) as 1.233 (1.058,1.436), p = 0.009; and Model 2: OR (95% CI) as 1.191 (1.014,1.400), p = 0.036]. Although a positive trend was identified, no statistically significant relationship was found between Pb, Hg and risk of sleep disturbance after adjusting for covariates in the fully adjusted model (Model 2).
Table 2.
Weighted logistic regression results for the relationship between blood Cd and sleep disturbance
| Crude model | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||||
| Cd (µg/l) | |||||||||
| Q1 (< 0.22) | Ref. | Ref. | Ref. | ||||||
| Q2 (0.25–0.35) | 1.057(0.903,1.238) | 0.479 | 0.927(0.796,1.080) | 0.317 | 0.961(0.821,1.123) | 0.582 | |||
| Q3 (0.35–0.62) | 1.235(1.024,1.489) | 0.028 | 1.011(0.824,1.240) | 0.913 | 1.012(0.808,1.267) | 0.909 | |||
| Q4 (> 0.62) | 1.466(1.270,1.692) | < 0.001 | 1.233(1.058,1.436) | 0.009 | 1.191(1.014,1.400) | 0.036 | |||
| Trend test | < 0.001 | 0.012 | 0.019 | ||||||
Notes: Crude model, no covariates were adjusted. Model 1, age, gender, race/ethnicity were adjusted. Model 2, age, gender, race/ethnicity, body mass index, marital status, education, poverty income ratio, smoking status, alcohol use, and chronic diseases were adjusted
Pearson correlations among blood metals were displayed as a correlation matrix in Supplementary Fig. 1(A). Considering that a unidirectional (positive) assumption was already verified, a multivariable WQS model was applied. Results showed that mixed blood metals were positively related to risk of sleep disturbance. The mixture effect of exposure to heavy metals was mainly attributable to Cd (89.1%), followed by Hg (10.8%) and Pb (0.1%) (Supplementary Fig. 1(B)), which showed that Cd exposure was the most important influencing factor of sleep disturbance in this study. Moreover, the smooth curve fitting method was used to describe the relationship between WQS index and risks of sleep disturbance (Supplementary Fig. 1(C)).
To test the robustness of the correlation between Cd level and sleep disturbance, we also performed two-metal and multi-metal regression models (incorporating multiple metals simultaneously). After adjustment for all covariates, we estimated the association between Cd exposure and sleep disturbance. The association persisted in multi-metal models and Cd in the Q4 level was positively correlated with the risk of sleep disturbance (Table 3).
Table 3.
Weighted logistic regression results for the two-metal model and multi-metal model on the relationship between blood Cd and sleep disturbance
| Heavy metal | Cd (µg/L) | ||||
|---|---|---|---|---|---|
| Q1 (< 0.25) | Q2 (0.25–0.35) | Q3 (0.35–0.62) | Q4 (> 0.62) | ||
| Two-metal model | |||||
| Cd | + Pb | Ref. | 0.971(0.821,1.149) | 1.035(0.816,1.311) | 1.240(1.029,1.494) |
| + Hg | Ref. | 0.962(0.813,1.138) | 1.012(0.795,1.288) | 1.180(0.991,1.406) | |
| Multi-metal model | |||||
| Cd | + Pb + Hg | Ref. | 0.971(0.801,1.176) | 1.029(0.790,1.340) | 1.218(0.984,1.509) |
Notes: Fully adjusted model were used. Age, gender, race/ethnicity, body mass index, marital status, education, poverty income ratio, alcohol use, and chronic diseases were adjusted
Stratified analysis
Based on above results, Supplementary Table 2 demonstrates the association between blood Cd and sleep disturbance by stratified analysis. It was found that females [OR (95% CI): 1.509(1.185,1.923), p = 0.002] had a greater risk of sleep disturbance than males. Additionally, participants who were non-Hispanic White [OR (95% CI): 1.419(1.189,1.694), p < 0.001], non-Hispanic Black [OR (95% CI): 1.501(1.001,2.251), p = 0.049], or Mexican American [OR (95% CI): 2.383(1.490,3.810), p < 0.001] were more vulnerable to cadmium exposure with sleep disturbance. Moreover, population younger than 60 years old, BMI < 30 kg/m2, education level below high school or above college, unmarried, worse family income, smokers, alcohol consumption, diagnosed with cardiovascular diseases were more likely to suffer from sleep disturbance with the increment in blood Cd concentrations.
Potential mitigation role of exercise in the relationship between blood Cd and sleep disturbance
The weighted logistic regression analyses showed significant negative associations between moderate to vigorous physical exercise (MVPE) and sleep disturbance (Table 4). In Crude Model, (MVPE) vs. (None), OR (95% CI): 0.702 (0.598,0.824), p < 0.001; In Model 1, (MVPE) vs. (None), OR (95% CI): 0.736 (0.626,0.865), p < 0.001; In Model 2, (MVPE) vs. (None), OR (95% CI): 0.804 (0.660,0.981), p = 0.034. However, no significant association was found between low level exercise and sleep disturbance.
Table 4.
Weighted logistic regression results for physical exercise with sleep disturbance
| Crude model | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||||
| Physical exercise | |||||||||
| None | Reference | Reference | Reference | ||||||
| Low | 0.850(0.704,1.027) | 0.089 | 0.848(0.710,1.013) | 0.068 | 0.910(0.726,1.140) | 0.378 | |||
| Moderate to vigorous | 0.702(0.598,0.824) | < 0.001 | 0.736(0.626,0.865) | < 0.001 | 0.804(0.660,0.981) | 0.034 | |||
Notes: Crude model, no covariates were adjusted. Model 1, age, gender, race/ethnicity were adjusted. Model 2, age, gender, race/ethnicity, body mass index, marital status, education, poverty income ratio, smoking status, alcohol use, and chronic diseases were adjusted
Moreover, subgroup analysis was conducted on the association between Cd exposure and sleep disturbance under different levels of PE (Fig. 3). In the association between Q4 of Cd exposure and sleep disturbance, the MVPE group (OR = 1.333, 95%CI: 0.954 ~ 1.863) had lower risks than the none exercise group (OR = 1.520, 95%CI: 1.286 ~ 1.797). Additionally, this trend was also identified in the relationship between Q3 level of blood Cd and sleep disturbance, that MVPE group (OR = 1.106) was associated with lower OR than the none exercise (OR = 1.361) and low exercise group (OR = 1.251). This indicated that in higher Cd exposure, maintaining moderate-to-vigorous exercise was associated with lower sleep disturbance rate. We also conducted analysis between PE volume and sleep disturbance under different levels of blood cadmium (Supplementary Fig. 2). In Cd = Q3, a significant association was observed between MVPE and sleep disturbance [OR (95% CI): 0.699 (0.533,0.916), p = 0.014].
Fig. 3.
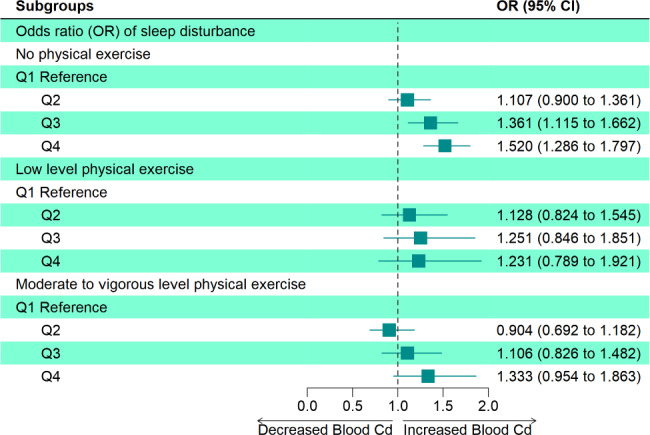
Association between blood Cd and sleep disturbance under different physical exercise levels
Based on the fully adjusted model, weighted restricted cubic spline was used to examine the dose-response relations between Cd exposure and sleep disturbance under different levels of PE (Fig. 4). It was found that blood Cd was positively associated with the incidence of sleep disturbance. In lower Cd exposure, there was not a significant trend between Cd level and sleep disturbance with different PE volume. However, with the increment in Cd concentration before it reached 1 µg/l, this relationship weakened with the increase in exercise volume, with the highest risk in the no exercise activity group, but the weakest risk in the MVPE group.
Fig. 4.
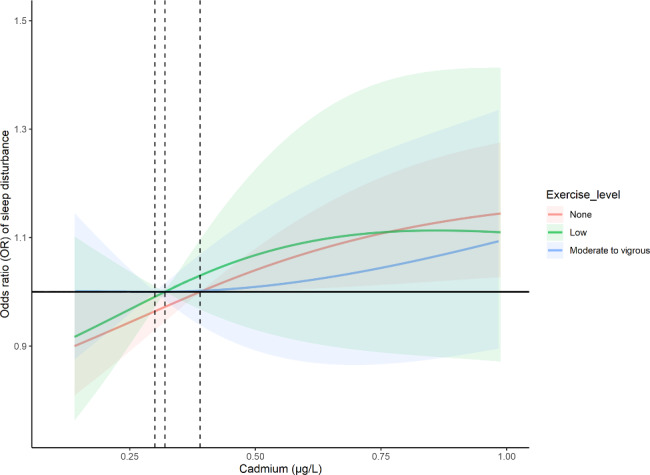
Dose-response relationship between blood Cd and sleep disturbance under different physical exercise levels
Discussions
Using a representative cross-sectional survey, this study first identified that higher blood Cd exposure was significantly associated with sleep disturbance after adjustment for confounding factors. WQS model detected that the mixture effect of exposure to heavy metals was mainly attributable to Cd. Moreover, two-metal and multi-metal verified this association. However, no significant associations were found between Pb, Hg and risk of sleep disturbance. In addition, we not only elucidated negative effects of Cd exposure on sleep health, but also found countermeasures by doing more exercise to mitigate this association. Despite the fact that low level PE could not produce significant mitigation effects, MVPE could alleviate such associations in higher levels of Cd exposure. Further RCS model analysis verified these findings in a dose-response manager.
To date, our findings offered insights for the relationship between Cd exposure and the risk of sleep disturbance in the largest nationwide population. The impacts of Cd exposure on health outcomes such as cognition and mortality have been widely reported in previous studies [29–31], however the influence of Cd exposure on sleep health remained unknown. One animal study explored the effect of Cd exposure on brain structure and function in rats, results showed that one single injections of cadmium chloride lead to significant changes of wakefulness-sleep cycle [32]. Neurodegeneration due to the generation of reactive oxygen species (ROS) by other heavy metals, whether directly or not has been previously reported [33, 34]. From the mechanism, an increase and accumulation of ROS after Cd exposure might help to explain this phenomenon. Another rats study verified this hypothesis that Cd-induced sleep disturbance as a consequence of oxidative stress [35]. Considering that oxidized glutathione is one of endogenous sleep substances, Cd could induce abnormal sleep wake cycle by occupying intrinsic sleep-inducing neurotransmitters. In detail, the dysregulated activity of neurotransmitters involved in sleep regulation such as dopamine and serotonin [36] could be affected by high doses of metal exposure in the brain. Accounting for the facts that neurotransmitters regulating circadian rhythms also played important roles in neurodegenerative disorders [37], knowing the mechanism of Cd exposure on sleep might also help to prevent metal induced sleep problems and its related diseases.
Benefits of physical exercise on sleep status has received increasingly attention in recent years. Several epidemiological research reported the positive effects of exercise on sleep efficiency and quality [38–40], while the evidence on its rescue effects on heavy metal exposure was limited. According to our results, with the increment in Cd level, people performing regular MVPE were less likely to suffer from sleep disturbance. When it comes to the mechanism, inflammation and ROS and its related neurotransmitters may produce a marked effect on this process. Exercise-induced cytokine response, including predominant anti-inflammatory effect, may explain part of the improvement of sleep condition [41]. Early literature reported that long term regular PE can have an anti-inflammatory effect and reduce C- reaction protein levels [42]. Notably, the brain-derived neurotrophic factor (BDNF) as well as the glial cell-derived neurotrophic factor (GDNF) were other key factors induced by exercise. Studies have shown that exercise can strikingly upregulate the BDNF’s expression [43, 44]. As one of the most versatile neurotrophic factors in the brain, BDNF and GDNF played critical roles in the brain function, which mediated the protection of neurons, which in turn, could protect oxidative stress caused by ROS. Additionally, intensity mattered in the mitigation effects of metal exposure. In consideration of the cumulative effect of PE, previous publications suggested that chronic vigorous exercise was positively related to sleep quality enhancement. This was consistent with our findings that none or low level exercise was not enough to induce the rescue influence. Performing moderate to high level exercise could trigger secretions of multiple hormones in the neuro system, such as dopamine and serotonin [45], which were closely related to the ROS elimination.
This study reinforced the findings of adverse effects of heavy metals, especially Cd exposure on the sleep health. The strengths included the general-population study design, appreciable sample size, survey-weighted based analytical methods based on the complex multistage sampling design. Comprehensive baseline characteristics from the representative US adults’ sample enabled us to use multiple adjusted model for potential confounding factors. Moreover, compared with previous studies that only focused on the hazard effects of metals on human health, we not only verified the negative effects of Cd exposure, but also put forward a valid strategy, performing moderate to vigorous exercise, to mitigate sleep disturbances associated with Cd exposure.
Potential limitations should be acknowledged. First, although NHANES database only included three blood heavy metals in the 2007–2010 cycle, it was possible that there were still other metals with not available data may influence the results. Thus the findings of this study should be interpreted with caution due to the mixed-metal model only included three metals for analysis. Secondly, given the cross-sectional study design, a causal relationship between metal exposure and sleep disturbance cannot be confirmed. The assessment of sleep disturbance and physical exercise was based on the self-reported interview. This might cause report bias, although most previous studies tended to use such questionnaires rather than wearable fitness tracker devices [46]. Third, special groups such as industrial workers who were routinely at risk of heavy metal exposure, including those working in chemical plants, were worthy of further attention. Additionally, although several potential covariates including demographic and lifestyle factors in the analyses were adjusted, future studies should consider incorporating a wider array of chronic diseases and relevant comorbidity measures to capture a more comprehensive picture of sleep disturbance and its associations with metal exposures. Last but not least, other specific target population, mostly the child, teenager and pregnant woman did not receive strong evidence for reference. Intergenerational effect (e.g., influence of maternal exposure on offspring) of metal exposure and other rescue methods requested further validation.
Conclusion
In summary, this study was the first to examine the relationship between blood Cd exposure and sleep disturbance in a nationwide population. Our results revealed the hazard impact of Cd exposure, highlighted the positive effects of exercise in mitigating such influence and expressed the underlying biology of pathways among these associations. This study indicated that lower Cd exposure and more PE were associated with a lower prevalence of sleep disturbance. Although our preliminary analysis found the association between blood Cd and sleep related disorder, such findings warranted biological investigations. Moreover, further studies employing longitudinal designs and objective measurements of sleep disturbance and physical exercise would provide more robust and specific insights into these relationships.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Yanwei You: Conceptualization, Methodology, Software, Data curation, Writing - original draft. Yuquan Chen: Conceptualization, Software, Data curation, Writing - original draft. Yangchang Zhang: Methodology, Data curation, Writing - review & editing. Qi Zhang: Methodology. Yaohui Yu: Writing - review & editing. Qiang Cao: Conceptualization, Supervision. All authors reviewed the manuscript.
Funding
Not applicable.
Data Availability
(ADM)
The datasets generated and analyzed for the current study are available in the NHANES repository. These data can be accessed using the following link: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All information from the NHANES program is available and free for public, so the agreement of the medical ethics committee board was not necessary.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanwei You, Yuquan Chen and Yangchang Zhang contributed equally to this work.
References
- 1.Health Risks from Toxic Pollution Lancet. 2012;380(9853):1532. doi: 10.1016/S0140-6736(12)61862-5. [DOI] [PubMed] [Google Scholar]
- 2.White AR, Kanninen KM, Crouch PJ, Editorial Metals and Neurodegeneration: restoring the balance. Front Aging Neurosci. 2015;7:127. doi: 10.3389/fnagi.2015.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom AJ. Metal Regulation of Metabolism. Curr Opin Chem Biol (2019) 49:33 – 8. Epub 2018/10/09. doi: 10.1016/j.cbpa.2018.09.017. [DOI] [PubMed]
- 4.Jomova K, Vondrakova D, Lawson M, Valko M, Metals Oxidative stress and neurodegenerative Disorders. Mol Cell Biochem. 2010;345(1–2):91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 5.Cdc/Sleep and Sleep Disorders. [cited 2022 12 Oct]. Available from: https://www.cdc.gov/sleep/index.html.
- 6.Asker S, Asker M, Yeltekin AC, Aslan M, Demir H. Serum levels of Trace Minerals and Heavy Metals in severe obstructive sleep apnea patients: correlates and clinical implications. Sleep Breath. 2015;19(2):547–52. doi: 10.1007/s11325-014-1045-2. [DOI] [PubMed] [Google Scholar]
- 7.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled Ultrafine particles to the brain. Inhal Toxicol. 2004;16(6–7):437–45. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 8.Lampron A, Elali A, Rivest S. Innate Immunity in the Cns: Redefining the Relationship between the Cns and Its Environment. Neuron (2013) 78(2):214 – 32. Epub 2013/04/30. doi: 10.1016/j.neuron.2013.04.005. [DOI] [PubMed]
- 9.Shiue I, Urinary Arsenic P, Metals H. Phthalates, polyaromatic hydrocarbons, and Polyfluoroalkyl Compounds are Associated with sleep troubles in adults: USA Nhanes, 2005–2006. Environ Sci Pollut Res Int. 2017;24(3):3108–16. doi: 10.1007/s11356-016-8054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scinicariello F, Buser MC, Feroe AG, Attanasio R, Antimony, Disorders S-R. Nhanes 2005–2008. Environ Res (2017) 156:247 – 52. Epub 2017/04/01. doi: 10.1016/j.envres.2017.03.036. [DOI] [PMC free article] [PubMed]
- 11.Yang PY, Ho KH, Chen HC, Chien MY. Exercise Training improves Sleep Quality in Middle-Aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58(3):157–63. doi: 10.1016/S1836-9553(12)70106-6. [DOI] [PubMed] [Google Scholar]
- 12.Aiello KD, Caughey WG, Nelluri B, Sharma A, Mookadam F, Mookadam M. Effect of Exercise Training on Sleep Apnea: a systematic review and Meta-analysis. Respir Med. 2016;116:85–92. doi: 10.1016/j.rmed.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Xie Y, Liu S, Chen XJ, Yu HH, Yang Y, Wang W. Effects of Exercise on Sleep Quality and Insomnia in adults: a systematic review and Meta-analysis of Randomized controlled trials. Front Psychiatry. 2021;12:664499. doi: 10.3389/fpsyt.2021.664499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.You Y, Chen Y, Zhang Q, Yan N, Ning Y, Cao Q. Muscle Quality Index is Associated with trouble sleeping: a cross-sectional Population based study. BMC Public Health. 2023;23(1):489. doi: 10.1186/s12889-023-15411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley GA, Kelley KS. Exercise and Sleep: a systematic review of previous Meta-analyses. J Evid Based Med. 2017;10(1):26–36. doi: 10.1111/jebm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You Y, Liu J, Wang D, Fu Y, Liu R, Ma X. Cognitive Performance in Short Sleep Young Adults with Different Physical Activity Levels: A Cross-Sectional Fnirs Study. Brain Sci (2023) 13(2). Epub 2023/02/26. doi: 10.3390/brainsci13020171. [DOI] [PMC free article] [PubMed]
- 17.Cdc/National Center for Health Statistics. National Health and Nutrition Examination Survey 2007–2008. Description of Laboratory Methodology [cited 2022 16 Sep]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/PBCD_E.htm.
- 18.Cdc/National Center for Health Statistics. National Health and Nutrition Examination Survey 2009–2010. Description of Laboratory Methodology [cited 2022 16 Sep]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/PBCD_F.htm.
- 19.Beydoun HA, Beydoun MA, Jeng HA, Zonderman AB, Eid SM. Bisphenol-a and sleep adequacy among adults in the National Health and Nutrition examination surveys. Sleep. 2016;39(2):467–76. doi: 10.5665/sleep.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel P, Shiff B, Kohn TP, Ramasamy R. Impaired sleep is Associated with low testosterone in us adult males: results from the National Health and Nutrition Examination Survey. World J Urol. 2019;37(7):1449–53. doi: 10.1007/s00345-018-2485-2. [DOI] [PubMed] [Google Scholar]
- 21.You Y, Chen Y, Yin J, Zhang Z, Zhang K, Zhou J, et al. Relationship between leisure-time physical activity and depressive symptoms under different levels of Dietary Inflammatory Index. Front Nutr. 2022;9:983511. doi: 10.3389/fnut.2022.983511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Xu P, Fu X, Ren Z, Cheng J, Lin Z, et al. The Effect of Triglycerides in the Associations between physical activity, sedentary behavior and depression: an Interaction and Mediation Analysis. J Affect Disord. 2021;295:1377–85. doi: 10.1016/j.jad.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 23.You Y, Chen Y, Fang W, Li X, Wang R, Liu J, et al. The Association between Sedentary Behavior, Exercise, and Sleep Disturbance: a mediation analysis of inflammatory biomarkers. Front Immunol. 2022;13:1080782. doi: 10.3389/fimmu.2022.1080782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun WJ, Xu L, Chan WM, Lam TH, Schooling CM. Are depressive symptoms Associated with Cardiovascular Mortality among older Chinese: a cohort study of 64,000 people in Hong Kong? Am J Geriatr Psychiatry. 2013;21(11):1107–15. doi: 10.1016/j.jagp.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 25.Taylor AL, Denniston MM, Klevens RM, McKnight-Eily LR, Jiles RB. Association of Hepatitis C virus with Alcohol Use among U.S. adults: Nhanes 2003–2010. Am J Prev Med. 2016;51(2):206–15. doi: 10.1016/j.amepre.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Duan W, Xu C, Liu Q, Xu J, Weng Z, Zhang X et al. Levels of a Mixture of Heavy Metals in Blood and Urine and All-Cause, Cardiovascular Disease and Cancer Mortality: A Population-Based Cohort Study. Environ Pollut (2020) 263(Pt A):114630. Epub 2021/02/24. doi: 10.1016/j.envpol.2020.114630. [DOI] [PubMed]
- 27.Tian X, Xue B, Wang B, Lei R, Shan X, Niu J, et al. Physical activity reduces the role of blood cadmium on Depression: a cross-sectional analysis with Nhanes Data. Environ Pollut. 2022;304:119211. doi: 10.1016/j.envpol.2022.119211. [DOI] [PubMed] [Google Scholar]
- 28.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 29.Nogawa K, Suwazono Y, Nishijo M, Sakurai M, Ishizaki M, Morikawa Y et al. Increase of Lifetime Cadmium Intake Dose-Dependently Increased All Cause of Mortality in Female Inhabitants of the Cadmium-Polluted Jinzu River Basin, Toyama, Japan. Environ Res (2018) 164:379 – 84. Epub 2018/03/24. doi: 10.1016/j.envres.2018.03.019. [DOI] [PubMed]
- 30.Nishijo M, Nogawa K, Suwazono Y, Kido T, Sakurai M, Nakagawa H. Lifetime Cadmium Exposure and Mortality for Renal Diseases in Residents of the Cadmium-Polluted Kakehashi River Basin in Japan. Toxics (2020) 8(4). Epub 2020/10/07. doi: 10.3390/toxics8040081. [DOI] [PMC free article] [PubMed]
- 31.You Y, Chen Y, Li J, Zhang Q, Zhang Y, Yang P, et al. Physical activity mitigates the influence of blood cadmium on memory function: a cross-sectional analysis in us Elderly Population. Environ Sci Pollut Res Int. 2023;30(26):68809–20. doi: 10.1007/s11356-023-27053-7. [DOI] [PubMed] [Google Scholar]
- 32.Vataev SI, Mal’gina NA, Oganesian GA. [the Effect of Cadmium on the structure of the circadian cycle of Waking-Sleep and on the Eeg in Wistar rats] Zh Evol Biokhim Fiziol. 1994;30(3):408–19. [PubMed] [Google Scholar]
- 33.Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and Mixtures of Heavy Metals in the Environment. Environ Sci Pollut Res Int. 2016;23(9):8244–59. doi: 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- 34.Nava-Ruiz C, Mendez-Armenta M, Rios C. Lead Neurotoxicity: Effects on Brain Nitric Oxide Synthase. J Mol Histol (2012) 43(5):553 – 63. Epub 2012/04/25. doi: 10.1007/s10735-012-9414-2. [DOI] [PubMed]
- 35.Unno K, Yamoto K, Takeuchi K, Kataoka A, Ozaki T, Mochizuki T, et al. Acute Enhancement of Non-Rapid Eye Movement Sleep in rats after drinking Water contaminated with Cadmium Chloride. J Appl Toxicol. 2014;34(2):205–13. doi: 10.1002/jat.2853. [DOI] [PubMed] [Google Scholar]
- 36.Lidsky TI, Schneider JS. Lead neurotoxicity in children: Basic Mechanisms and clinical correlates. Brain. 2003;126(Pt 1):5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 37.Videnovic A, Lazar AS, Barker RA, Overeem S. The Clocks that time us’--Circadian rhythms in neurodegenerative Disorders. Nat Rev Neurol. 2014;10(12):683–93. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Q, Zhang QL, Du Y, Ye YL, He QQ. Associations of physical activity, screen time with Depression, anxiety and Sleep Quality among Chinese College Freshmen. PLoS ONE. 2014;9(6):e100914. doi: 10.1371/journal.pone.0100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaingankar JA, Muller-Riemenschneider F, Chu AHY, Subramaniam M, Tan LWL, Chong SA, et al. Sleep duration, Sleep Quality and Physical Activity, but not sedentary Behaviour, are Associated with positive Mental Health in a multi-ethnic Asian Population: a cross-sectional evaluation. Int J Environ Res Public Health. 2020;17(22). 10.3390/ijerph17228489. Epub 2020/11/20. [DOI] [PMC free article] [PubMed]
- 40.St Laurent CW, Burkart S, Rodheim K, Marcotte R, Spencer RMC. Cross-Sectional Associations of 24-Hour Sedentary Time, Physical Activity, and Sleep Duration Compositions with Sleep Quality and Habits in Preschoolers. Int J Environ Res Public Health (2020) 17(19). Epub 2020/10/03. doi: 10.3390/ijerph17197148. [DOI] [PMC free article] [PubMed]
- 41.Abd El-Kader SM, Al-Jiffri OH. Aerobic Exercise modulates Cytokine Profile and Sleep Quality in Elderly. Afr Health Sci. 2019;19(2):2198–207. doi: 10.4314/ahs.v19i2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dufaux B, Order U, Muller R, Hollmann W. Delayed Effects of prolonged Exercise on serum lipoproteins. Metabolism. 1986;35(2):105–9. doi: 10.1016/0026-0495(86)90108-3. [DOI] [PubMed] [Google Scholar]
- 43.Aguiar AS, Jr, Stragier E, da Luz Scheffer D, Remor AP, Oliveira PA, Prediger RD, et al. Effects of Exercise on mitochondrial function, neuroplasticity and anxio-depressive behavior of mice. Neuroscience. 2014;271:56–63. doi: 10.1016/j.neuroscience.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Soke F, Kocer B, Fidan I, Keskinoglu P, Guclu-Gunduz A. Effects of Task-Oriented training combined with aerobic training on serum bdnf, Gdnf, Igf-1, Vegf, Tnf-Alpha, and Il-1beta levels in people with Parkinson’s Disease: a randomized controlled study. Exp Gerontol. 2021;150:111384. doi: 10.1016/j.exger.2021.111384. [DOI] [PubMed] [Google Scholar]
- 45.You Y, Li W, Liu J, Li X, Fu Y, Ma X. Bibliometric Review to explore emerging high-intensity interval training in Health Promotion: a New Century Picture. Front Public Health. 2021;9:697633. doi: 10.3389/fpubh.2021.697633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chuang HC, Su TY, Chuang KJ, Hsiao TC, Lin HL, Hsu YT et al. Pulmonary Exposure to Metal Fume Particulate Matter Cause Sleep Disturbances in Shipyard Welders. Environ Pollut (2018) 232:523 – 32. Epub 2017/10/11. doi: 10.1016/j.envpol.2017.09.082. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
(ADM)
The datasets generated and analyzed for the current study are available in the NHANES repository. These data can be accessed using the following link: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.