Immune dysregulation is suggested to underlie the mechanism of itch in the pruritic inflammatory skin condition prurigo nodularis (PN), with type 2 immune mediators found to be upregulated in PN lesional skin (1). Recent proteomic studies utilizing Olink, the most comprehensive inflammatory panel to date, with the ability to simultaneously analyse 92 protein biomarkers associated with inflammatory diseases and related biological processes (2), have found inflammatory serum biomarkers in atopic dermatitis that correlate with clinical severity and/or therapeutic responses (3). To our knowledge, no such similar study characterizing the systemic inflammatory signature of PN has been conducted. With increasing evidence of systemic involvement in PN pathogenesis (4), a comprehensive proteomic profile of PN was conducted to evaluate the immune axis via the blood to understand the correlation of the proteomic signature to patient itch severity scores.
MATERIALS AND METHODS
Serum samples were obtained from 33 patients with PN from the Miami Itch Center and from 20 age- and sex-matched healthy controls (HC). PN patients with concomitant atopy (defined as reported or documented medical history of allergic rhinitis, atopic dermatitis, or asthma) were excluded. Demographics and clinical data, including duration of itch and average 24 h-numerical rating scale (NRS, 0–10) for self-reported itch intensity (Table I), were collected from patients.
Table I.
Patient demographics
| Category | PN patients | Healthy controls |
|---|---|---|
| Age, years, mean ± SEM [range] | 64 ± 1.9 [42–85] | 63.1 ± 2.18 [44–78] |
| Sex (F/M) | 23 [70%] / 10 [30%] | 14 [70%] / 6 [30%] |
| Race | ||
| Caucasian | 19 (58%) | 13 (65%) |
| African American | 8 (24%) | 4 (20%) |
| Hispanic | 3 (9%) | 2 (10%) |
| Asian | 3 (9%) | 1 (5%) |
| Average itch intensity, mean ± SEM [range] | 7.67 ± 0.4 [0–10] | N/A |
| Duration of itch, mean ± SEM [range], years | 10.3 ± 1.5 [1–30] | N/A |
F: female; M: male; SEM: standard error of the mean; PN: prurigo nodularis.
All samples were analysed by immunoassay. Interleukin (IL)-13 and IL-17A were measured using the Simoa® human IL-13 Advantage HD-1/HD-X kit and Simoa human IL-17A HD-1/HD-X 2.0 kit (Quanterix, Billerica, MA, USA). IL-4 and IL-5 were measured with S-PLEX Human IL-4 and IL-5 kits (MSD, Rockville, MD, USA). Periostin was assayed with the Invitrogen human periostin enzyme-linked immunoassay (ELISA) kit and IgE was measured with Invitrogen human IgE ELISA kit (Thermo-Fisher Scientific, Frederick, MD, USA). IL-22 was measured using MSD immunoassay (Lilly in-house assay) (5). In addition, samples were tested with the Olink Target 96 Inflammation panel (Uppsala, Sweden).
For statistical analysis, one-way analysis of variance (ANOVA) was utilized to compare between patients with PN and healthy controls HC using log-transformed Olink proteomic data and single-plex data. For between-markers multiplicity adjustment, q-value was calculated with a Benjamini-Hochberg procedure with the significance threshold at 0.05. Spearman’s correlation was utilized to find relationships between assays and clinical data.
RESULTS
In the Olink panel, 13 proteins with missing data frequency greater than 80% were removed from analysis (IL-20RA, IL-2RB, IL-1 alpha, IL2, IL-22RA1, Beta-NGF, IL13, ARTN, IL20, IL33, LIF, NRTN, IL5). Of the remaining proteins and using 1.3-fold changes (FC) as the minimum threshold to ensure biological relevance, caspase 8 (CASP8) (FC = 1.56, p = 9.35E–5) was significantly elevated, while IL-17C (FC = – 2.61, p = 1.38E–9) was significantly decreased (Fig. 1). Single-plex cytokine analysis revealed a significant elevation in IL-13 (FC = 4.39, p = 0.0003). Other biomarkers IL-4, IL-5, IL-17A, IL-22, periostin, and IgE did not yield significant results (not shown). Average NRS itch scores did not correlate with IL-13 or CASP8.
Fig. 1.
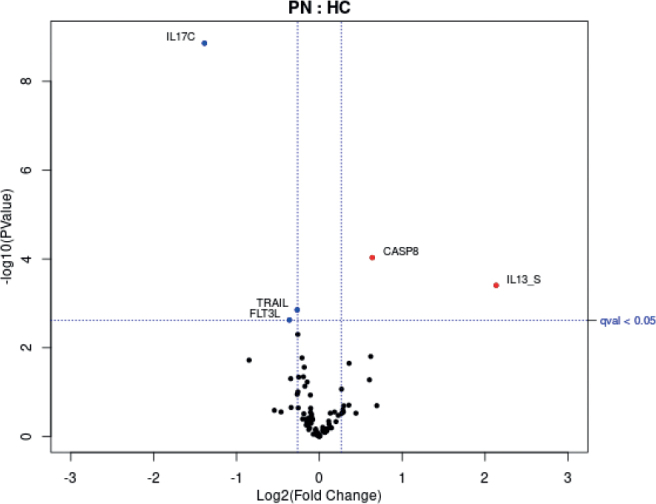
Volcano plot of proteomic assays showing analytes with > 1.3-fold changes that were significant (qval < 0.05). Blue circles represent analytes significantly reduced in prurigo nodularis (PN) vs healthy controls (HC). Red circles represent analytes significantly elevated in PN vs HC. “S” indicates single-plex assay. Protein markers with missing data frequency > 80% were removed from Olink data analysis (IL20RA, IL2RB, IL1 alpha, IL2, IL22RA1, Beta-NGF, IL13, ARTN, IL20, IL33, LIF, NRTN, IL5). FLT3L: fms-related tyrosine kinase 3 ligand. IL13_S: interleukin 13 from single-plex analysis. TRAIL: tumour necrosis factor-related apoptosis-inducing ligand. IL17C: interleukin-17 C.
DISCUSSION
Interleukins have been difficult to accurately and reproducibly quantify within human serum due to low abundance. Many available immunoassays have not been able to specifically detect these circulating makers to the sub-picogram/ml levels, leading to inconsistencies in reported values. This study employed ultrasensitive assays for the detection of IL-13, IL-4, IL-5 and IL-17 to better understand their serum concentrations in healthy control and PN populations. IL-13 was found elevated in the blood of patients with PN, although no correlation between IL-13 and itch severity was demonstrated, perhaps due to the similar high itch intensity levels reported by the patients. IL-13 is a proinflammatory Th2 cytokine that is frequently implicated in pruritic skin conditions, including PN, and binds to IL-4R on keratinocytes, sensory neurones, and dermal immune cells (6). Dupilumab, an IL-4 and IL-13 inhibitor, has been shown to be efficacious in the treatment of PN (1). Therefore, the current study, in combination with the literature, suggests that IL-13 is an important immunotarget for future PN therapies.
Levels of apoptotic markers were found to be irregular in the serum of patients with PN, with CASP8 significantly upregulated. The role of CASP8 in the setting of inflammation is complex. Although CASP8 has generally been regarded to have anti-inflammatory properties (inhibiting inflammasome activation, necroptosis, and inflammatory signalling complexes), there are findings that suggest CASP8 may also be proinflammatory by inducing cytokine and chemokine expression depending on cell type and context (6). To date, no literature points to a relationship between CASP8 and the pathogenesis of PN. As CASP8 was upregulated in this study, it may play a more proinflammatory role in the pathogenesis of PN. Interestingly, no other signals of fibrosis or pro-fibrosis were detected in the serum of these patients, as we expected initially, as the skin of PN has abundant profibrotic cascade (5).
Serum analysis did not demonstrate significant elevation in many key players of itch found in PN tissue, such as IL-17 and IL-22, which were recently reported in PN and work downstream of IL-31. A study on transcriptomic characterization of PN and nemolizumab, an IL-31 receptor antagonist, found that IL-17A and IL-22 levels were normalized after treatment in PN skin (5, 8). Interestingly, IL-17C, which is upregulated in lesional PN, was downregulated in the serum. However, IL-17C is mainly expressed by epithelial cells rather than immune cells, which may explain this phenomenon (9). Other autoimmune inflammatory skin diseases have also shown no significant difference or decreased levels of serum IL-17 (10, 11).
In conclusion, this is the first study of serum proteomic signature of PN that further provides support that a key Th2 cytokine has a significant role in PN and may serve as a serum biomarker for the disease. This study was limited by small sample sizes and by the sensitivity of the immunoassays and Olink panel when evaluating serum. IL-31, a common pruritogen found in the lesional skin of patients with PN, was not evaluated, due to limitations in the sensitivity and reliability of current available immunoassays. In addition, no validated tool beyond investigator global assessment was used to assess PN disease severity, although the majority of patients had extensive disease of IGA ≥ 3. Many of the inflammatory markers identified are not widely recognized and their role in PN pathogenesis is still unknown. As the majority of the PN cases had similar itch intensities and PN severity, further studies are needed to elucidate the inflammatory and itch pathways related to PN and to evaluate the roles of the identified serum cytokines in PN disease severity.
ACKNOWLEDGEMENTS
IRB approval status
This study was reviewed and approved by the IRB at the University of Miami.
Conflict of interest disclosures
GY has served as an advisory board member, investigator and/or received consulting fees from Galderma, Pfizer, Sanofi Regeneron, Novartis, Eli Lilly, Kiniksa, Vellus and Cell Dex. SE, AA, SS, JTS, ZS and AO are all employees and potentially stockholders of Eli Lilly. All other authors have no additional conflicts of interest to disclose.
REFERENCES
- 1.Ingrasci G, Lipman ZM, Hawash AA, Girolomoni G, Yosipovitch G. The pruritogenic role of the type 2 immune response in diseases associated with chronic itch. Exp Dermatol 2021; 30: 1208–1217. [DOI] [PubMed] [Google Scholar]
- 2.Mikhaylov D, Del Duca E, Guttman-Yassky E. Proteomic signatures of inflammatory skin diseases: a focus on atopic dermatitis. Expert Rev Proteomics 2021; 18: 345–361. [DOI] [PubMed] [Google Scholar]
- 3.Engle SM, Chang CY, Ulrich BJ, Satterwhite A, Hayes T, Robling K, et al. Predictive biomarker modeling of pediatric atopic dermatitis severity based on longitudinal serum collection. Clin Exp Immunol 2021; 207: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belzberg M, Alphonse MP, Brown I, Williams KA, Khanna R, Ho B, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol 2021; 141: 2208–2218.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsoi LC, Hacini-Rachinel F, Fogel P, Rousseau F, Xing X, Patrick MT, et al., Transcriptomic characterization of prurigo nodularis and the therapeutic response to nemolizumab. J Allergy Clin Immunol 2022; 149: 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miron Y, Miller PE, Hughes C, Indersmitten T, Lerner EA, Cevikbas F. Mechanistic insights into the antipruritic effects of lebrikizumab, an anti-IL-13 mAb. J Allergy Clin Immunol 2022; 150: 690–700. [DOI] [PubMed] [Google Scholar]
- 7.Han JH, Park J, Kang TB, Lee KH. Regulation of caspase-8 activity at the crossroads of pro-inflammation and anti-inflammation. Int J Mol Sci 2021; 22: 3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol 2020; 83: 1567–1575. [DOI] [PubMed] [Google Scholar]
- 9.Brembilla NC, Senra L, Boehncke WH. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front Immunol 2018; 9: 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gholibeigian Z, Izad M, Daneshpazhooh M, Mortazavi H, Salehi Z, Behruzifar S, Akhdar, et al. Decreased serum levels of interleukin-17, interleukin-23, TGF-β in pemphigus vulgaris patients, and their association with disease phase. Dermatol Ther 2020; 33: e14071. [DOI] [PubMed] [Google Scholar]
- 11.Aşkın Ö, Yücesoy SN, Coşkun E, Engin B, Serdaroğlu S. Evaluation of the level of serum interleukins (IL-2, IL-4, IL-15 andIL-17) and its relationship with disease severity in patients with alopecia areata. An Bras Dermatol 2021; 96: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]


