Abstract
Although the etiology of multiple sclerosis (MS) is not known, several factors play a role in this disease: genetic contributions, immunologic elements, and environmental factors. Viruses and virus infections have been associated with the initiation and/or enhancement of exacerbations in MS. Theiler’s murine encephalomyelitis virus (TMEV) infection of mice is one of the animal models used to mimic MS. In other animal model systems, DNA vaccination has been used to protect animals against a variety of virus infections. To explore the utility of DNA vaccination, we have constructed eukaryotic expression vectors encoding the TMEV capsid proteins VP1, VP2, and VP3. SJL/J mice were vaccinated intramuscularly once, twice, or three times with the different capsid protein cDNAs. This was followed by intracerebral TMEV infection to determine the effects of DNA vaccination on the course of TMEV-induced central nervous system (CNS) demyelinating disease. We found that vaccination of mice three times with cDNA encoding VP2 led to partial protection of mice from CNS demyelinating disease as determined by a decrease in clinical symptoms and histopathology. Vaccination of mice with cDNA encoding VP3 also led to a decrease in clinical symptoms. In contrast, mice vaccinated with cDNA encoding VP1 experienced a more severe disease with an earlier onset of clinical signs and enhanced histopathology compared with control mice. There was no correlation between anti-TMEV antibody titers and disease course. These results indicate that DNA immunization can modify chronic virus-induced demyelinating disease and may eventually lead to potential treatments for illnesses such as MS.
Multiple sclerosis (MS) is the most common human demyelinating disease, affecting thousands of individuals a year, with an estimated 2 million cases worldwide (12, 26). Several etiologies have been proposed for the disease, but none has clearly been established. However, several factors, including genetic (3, 13, 17, 36, 37), immunologic (14, 15, 24, 31, 39), and environmental factors such as viral infections (4, 21, 29, 38, 40, 47), appear to play a role. Typical clinical symptoms and signs of MS include ataxia, optic neuritis, incontinence, and spastic paralysis. Histologically, areas of demyelination associated with inflammation in the brain and spinal cord are observed (2).
Myelin breakdown appears to be mediated by infiltrating cells of the immune system. These activated immune cells are found in active lesions of MS. For this reason, it is believed that MS is an immune system-mediated disease. Infiltrating cells include CD4+ and CD8+ T cells, B cells, and macrophages, with the presence of activated astrocytes in the lesions. These cells are involved in direct or indirect damage to the myelin sheath (24, 31). A similar picture in the central nervous system (CNS) can occur by viral infection of the CNS, leading to immune system-mediated killing of virus-infected cells, virus-induced autoimmunity through molecular mimicry, or direct viral lysis of infected oligodendrocytes (4, 6).
A viral model for MS is Theiler’s murine encephalomyelitis virus (TMEV) infection of SJL/J mice (23, 42). TMEV, a member of the family Picornaviridae, contains a single, positive genomic strand of RNA. Like other picornaviruses, TMEV undergoes proteolytic processing of a large polyprotein encoded by the viral RNA. This allows for the production of the viral capsid proteins VP4, VP2, VP1, and VP3 (27, 42). Recognition of viral capsid proteins is crucial in immune clearance of TMEV. Major T- and B-cell epitopes have been found in VP1 and VP2 (10, 11, 18, 51). Specific immune responses to VP1 and VP2 have been shown to play detrimental roles in TMEV-induced disease (8, 10, 11, 49). In addition, VP3 contains a dominant T-cell epitope recognized during viral infection or immunization with an epitope-containing peptide (50). As in MS, areas of demyelination due to TMEV infection contain B and T cells, macrophages, and reactive astrocytes (44). Demyelination in TMEV infection may be due to cell death from direct viral lysis of oligodendrocytes or T-cell-mediated killing of virus-infected oligodendrocytes. Another possible source of myelin damage is nonspecific damage of oligodendrocytes or myelin by a delayed-type hypersensitivity response of CD4+ Th1 T cells recognizing other antigens (11). Autoimmunity to myelin may also develop through molecular mimicry leading to demyelination (7).
DNA vaccination has been considered a viable option to traditional immunization methods for a number of reasons (16, 45). First, there is no possibility for viral reversion to an infectious form or infection in immunocompromised individuals, since the DNA encodes only portions of the virus. Second, no laborious protein purification procedures are required. Third, because the protein(s) is being produced in the host cell itself, it is mimicking viral protein production and/or processing following infection, which leads to the induction of cellular and humoral immune responses. In conjunction with this last advantage, long-lasting immunity is more likely to occur. An added benefit is the option of integrating multiple vaccines to particular virus subtypes or even different viruses in the same plasmid (48).
Immunodominant epitopes of TMEV have been reported to be located in capsid proteins VP1, VP2, and VP3 (10, 11, 18, 50, 51). We created plasmids capable of expressing TMEV capsid proteins VP1, VP2, and VP3 to determine whether vaccination of mice with these plasmids led to demonstrable alterations in TMEV-induced demyelinating disease. We found that injection with cDNA encoding VP1 resulted in enhanced disease in SJL/J mice, whereas SJL/J mice vaccinated with cDNA encoding VP2 and VP3 exhibited attenuation of disease.
MATERIALS AND METHODS
Virus.
A working stock of the DA strain of TMEV was prepared in BHK-21 cells and used for all experiments (20). Virus titer was determined by a plaque assay in BHK-21 cells.
Plasmids.
VP1, VP2, and VP3 of the DA strain of TMEV (35) were cloned into the NotI site in plasmid pCMV (52), resulting in pCMV/VP1, pCMV/VP2, and pCMV/VP3. The pCMV vector, derived by excision of the β-galactosidase gene from pCMVβ (Clontech, Palo Alto, Calif.), contains the strong immediate-early gene promoter/enhancer from human cytomegalovirus, the polyadenylation signal and splice donor/acceptor from simian virus 40, and the Escherichia coli β-galactosidase gene. Each construct was confirmed by restriction enzyme digestion and was sequenced at the Huntsman Cancer Center DNA Sequencing Facility (Salt Lake City, Utah). The sequence for VP2 was identical to that of the original TMEV template. A single base in VP1 at amino acid position 2 changed a serine to a threonine at this position, and a single base in VP3 at amino acid position 202 changed alanine to threonine. For all experiments, plasmids were extracted by using an Endo-Free Plasmid Maxi kit (Qiagen, Inc., Chatworth, Calif.).
Mice.
Four- to six-week-old female SJL/J mice (National Cancer Institute, Bethesda, Md.) were injected with plasmid pCMV/VP1, pCMV/VP2, pCMV/VP3, or vector pCMV alone as a control. Each injection contained 100 μg of endotoxin-free plasmid DNA in 100 μl of saline introduced equally into each tibialis anterior muscle. Two weeks following the final plasmid injection, each mouse was challenged intracerebrally with 2 × 105 PFU of DA virus. To confirm that plasmid expression had occurred in the muscle, pCMVβ encoding β-galactosidase was injected into the leg muscle of mouse. Three days after injection, the muscle was removed and frozen.
Clinical signs.
Throughout the course of disease, mice were weighed to help gauge the severity of disease. Weighing was performed daily during the acute stage of disease and biweekly during the chronic stage. A modified righting reflex was also measured at the time of weighing as described by Rauch et al. (32). A healthy mouse is able to resist being turned over and is scored 0. If the mouse is flipped onto its back but immediately rights itself, it is given a score of 1; if it rights itself in 1 to 20 s, the score is 2; if righting takes >20 s, the score is 3. The modification from the scheme of Rauch et al. (32) is that if the mouse is not completely flipped but slips on one or both hind limbs, it is given a score of 0.25 or 0.5, respectively. In addition, mice were viewed for obvious clinical signs of disease, consisting mainly of a waddling gait, ataxia, and/or paralysis.
Histology.
Sixty days after viral challenge, mice were anesthetized, exsanguinated, and perfused with phosphate-buffered saline. The tibialis anterior muscle was collected and frozen in O.C.T. compound (VWR, Salt Lake City, Utah). Mice were then perfused with 4% paraformaldehyde in phosphate-buffered saline. Once fixed, the brains were cut coronally into five sections, and the spinal cord was cut into 6 to 12 cross sections. The sections were embedded in paraffin. Sections of 3 μm were cut and stained with luxol fast blue. Scoring for brain and spinal cord was performed as described by Barnett et al. (1) and Rodriguez et al. (33), respectively (45). Each brain slide contained five coronal sections, representing areas dorsal to caudal along the length of the brain. Each section was scored for perivascular cuffing, demyelination, and meningitis as follows: for perivascular cuffing, no lesion = 0, 1 to 20 lesions = 1, 21 to 50 lesions = 2, and >50 lesions = 3; for demyelination, not present = 0 and present = 1; for meningitis, none = 0, slight = 1, and severe = 2. The final score was determined by adding the individual scores in each group. Each spinal cord slide contained 6 to 12 cross sections representing areas along the length of the cord. Each cross section was divided into quadrants: dorsal column, ventral column, and two lateral columns. Any quadrant containing inflammation, demyelination, or meningitis was given a score of 1 in that pathologic class. The total number of positive quadrants for each pathologic class was determined, then divided by the total number of quadrants present on the slide, and multiplied by 100 to give percent involvement for each pathologic class. An overall pathologic score was also determined by assigning a positive score if any pathology was present in the quadrant. This, too, was presented as percent involvement.
Frozen muscle was stored at −75°C until sectioned. Sections of 6 μm were cut and then air dried. Expression of β-galactosidase was tested with Bluo-Gal (Gibco/BRL, Rockville, Md.).
Antibodies.
Three to five mice were injected one, two, or three times at 1-week intervals. One day before each DNA injection, 1 week after the final DNA injection, 1 day before viral challenge, 8 days after challenge, and when mice were sacrificed, serum was collected and stored at −20°C. Concentrations of TMEV-specific antibodies were determined by enzyme-linked immunosorbent assay (ELISA) using purified TMEV as the antigen (20). To demonstrate specificity of TMEV antibodies to capsid proteins, Western blot analyses were performed as described previously (8). Briefly, purified DA virus proteins were resolved on a polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane. The membrane was blocked with milk and cut into strips containing a protein lane and molecular weight marker. Each protein/marker strip was incubated with serum collected from mice from each treatment. Binding specificity was demonstrated by using horseradish peroxidase-conjugated secondary antibody with 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, Mo.).
RESULTS
Clinical signs.
A decrease in weight gain is associated with TMEV-induced disease (46), as is a loss in righting reflex (32), indicated by an increase in righting reflex grade. These characteristics were analyzed throughout the course of this study. We also observed obvious abnormal physical signs (waddling gait, spastic paralysis, and other movement problems) in the mice during the course of disease. Mice demonstrating any of these symptoms were given a positive score, and data are presented as the percentage of mice in each group showing positive signs. The results correlate well with those demonstrated by other methods, that is, mice having greater weight gain and a smaller righting reflex grade than controls exhibiting fewer movement abnormalities.
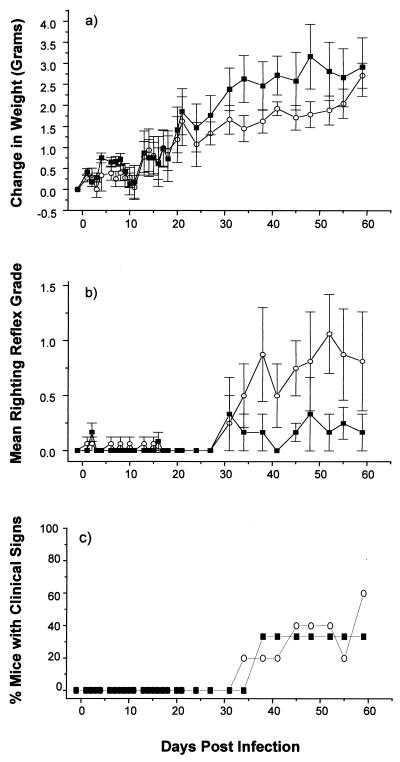
Among the pCMV/VP1-immunized groups, we found that the singly immunized mice showed both less weight gain and a greater righting reflex score than control mice, while there was no difference in obvious clinical signs (Fig. 1). Interestingly, however, there was no exacerbation of clinical disease in mice injected with pCMV/VP1 two and three times (data not shown). These results suggest that pCMV/VP1 vaccination could exacerbate TMEV-induced demyelinating disease, the effect appearing in a non-dose-dependent fashion.
FIG. 1.
Effect of plasmid pCMV/VP1 vaccination on the clinical course of TMEV infection. pCMV/VP1 encodes TMEV capsid protein VP1. Two weeks after a single vaccination with vector pCMV (■) or pCMV/VP1 (○), SJL/J mice were injected intracerebrally with TMEV (day 0). Mice were monitored for weight change (a), righting reflex disturbance (b), and obvious clinical signs (c). Means ± standard errors are shown. Each experimental group consisted of three to five mice. pCMV/VP1-vaccinated mice showed less weight gain and more impaired righting reflex than control mice.
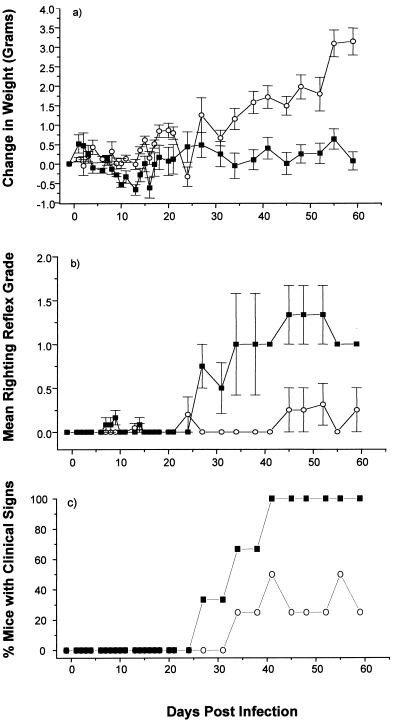
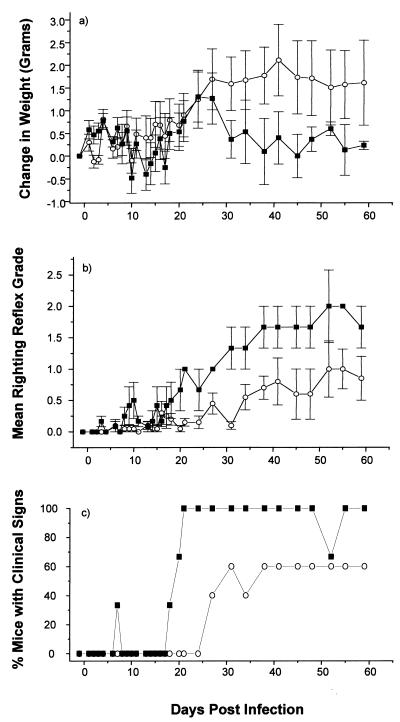
In contrast, both pCMV/VP2 and pCMV/VP3-immunized mice gained more weight and showed a much less severe righting reflex grade than did control mice (Fig. 2 and 3). These mice also showed late onset and less severe obvious clinical signs of TMEV-induced demyelinating disease. The mice injected twice with either pCMV/VP2 or pCMV/VP3 also showed less severe clinical signs than controls, but none of these differences were significant (data not shown). Neither the weight differences nor the righting reflex grades were different between mice injected once with pCMV/VP2 or pCMV/VP3 and the corresponding controls (data not shown). These results suggest a dose-dependent attenuation of disease in pCMV/VP2- and pCMV/VP3-vaccinated mice.
FIG. 2.
Effect of plasmid pCMV/VP2 vaccination on the clinical course of TMEV infection. pCMV/VP2 encodes TMEV capsid protein VP2. Two weeks after three vaccinations with vector pCMV (■) or pCMV/VP2 (○), SJL/J mice were injected intracerebrally with TMEV (day 0). Mice were monitored for weight change (a), righting reflex disturbance (b), and obvious clinical signs (c). Means ± standard errors are shown. Each experimental group consisted of three to five mice. pCMV/VP2-vaccinated mice gained more weight and showed less severe righting reflex grade and later onset of the disease than control mice.
FIG. 3.
Effect of plasmid pCMV/VP3 vaccination on the clinical course of TMEV infection. pCMV/VP3 encodes TMEV capsid protein VP3. Two weeks after three vaccinations with vector pCMV (■) or pCMV/VP3 (○), SJL/J mice were injected intracerebrally with TMEV (day 0). Mice were monitored for weight change (a), righting reflex disturbance (b), and obvious clinical signs (c). Means ± standard errors are shown. Each experimental group consisted of three to five mice. pCMV/VP3-vaccinated mice gained more weight and showed less severe righting reflex grade and later onset of the disease than control mice.
Histology. (i) Spinal cords.
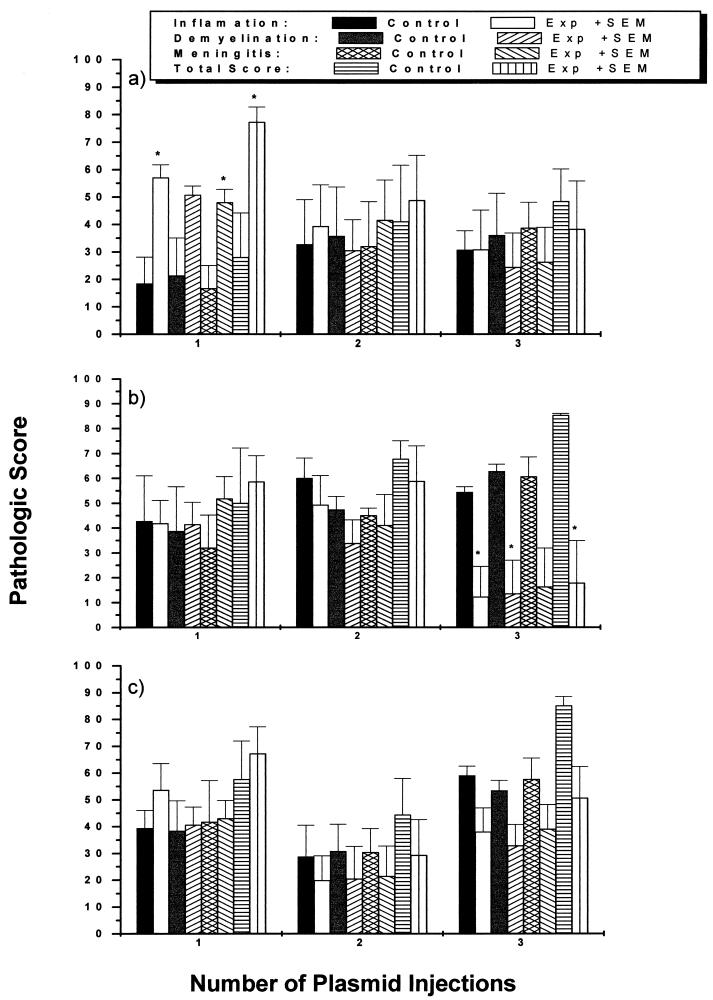
The spinal cord is consistently involved during the chronic stage of TMEV infection in SJL/J mice. Areas of demyelination associated with perivascular cuffing can be found throughout the spinal cord white matter, particularly in the ventral root entry zone. In addition, meningitis is frequently seen. Spinal cords were analyzed and graded for the percentage of area involved in inflammation, demyelination, and meningitis separately and for the percentage of area involved in any of the three, combined. Mice injected once with pCMV/VP1 showed more inflammation, meningitis, and combined pathology than did parallel controls (P < 0.05, analysis of variance [ANOVA]) (Fig. 4a, 5a, and 5b). There were no differences among the groups injected two and three times with pCMV/VP1 and control vector pCMV. In contrast, mice injected three times with pCMV/VP2 or pCMV/VP3 were found to have less inflammation and demyelination, and overall much less pathology of the spinal cord, than mice injected with vector alone (Fig. 4b, 4c, 5c, and 5d). The mice injected twice with either pCMV/VP2 or pCMV/VP3 also showed less pathology than controls, while none of these differences were significant. No difference was found between mice injected once with pCMV/VP2 or pCMV/VP3 and the corresponding controls. Therefore, the pathological scores for the spinal cord correlate well with clinical scores.
FIG. 4.
Pathologic scores of the spinal cord of TMEV-infected mice following DNA vaccination with pCMV/VP1 (a), pCMV/VP2 (b), or pCMV/VP3 (c) one, two, or three times. Control mice were injected with vector pCMV. Mice were infected with TMEV intracerebrally 2 weeks after the final DNA vaccination and sacrificed 60 days after TMEV injection. Three injections with cDNA encoding VP2 and VP3 led to a decrease in all pathologic scores, while mice injected once with pCMV/VP1 showed enhanced histopathology. ∗, P < 0.05 compared with mice immunized with pCMV, determined by ANOVA.
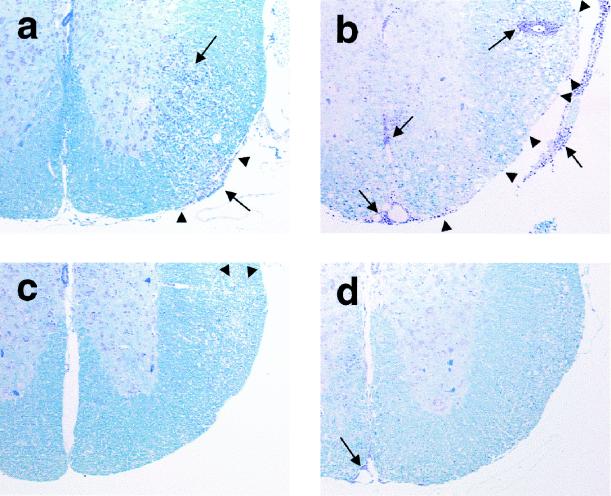
FIG. 5.
Histopathology of the ventral root entry zone of the spinal cord of TMEV-infected mice following DNA vaccination with pCMV (a), pCMV/VP1 (b), pCMV/VP2 (c), or pCMV/VP3 (d). Mice injected once with pCMV/VP1 showed more inflammation (➞) and demyelination (▸) than control mice, while those injected three times with pCMV/VP2 and pCMV/VP3 had less inflammation (➞) and demyelination (▸). Luxol fast blue stain; magnification, ×50.
(ii) Brains.
Since pathologic evidence in chronic TMEV infection is generally localized to the spinal cord, little pathology was seen in the brains of these mice at day 60 after viral challenge. When pathology was evident in the brain, it was localized to the brainstem area. Five sections of each brain, representing five different areas, rostral to caudal, were scored for inflammation, demyelination, and meningitis. Mice vaccinated three times with pCMV/VP2 had significantly less pathology (pathology score = 1.0 ± 1.0) than did the control mice (pathology score = 5.0 ± 0.6; P < 0.05, ANOVA). No differences were found in any other groups (data not shown).
(iii) Muscle.
To confirm that intramuscular injection of plasmid DNA results in expression of the plasmid-encoded gene within the muscle, we injected into the tibialis anterior muscle either plasmid pCMVβ or plasmid pCMV. Three days after injection, the muscles were removed, and the section was histochemically stained for β-galactosidase activity. With the pCMVβ injection, positive β-galactosidase staining was found within muscle cells (data not shown). No staining was seen in control pCMV-injected mice. We also examined the expression of TMEV capsid proteins in muscle injected with pCMV/VP1-VP3, using immunohistochemistry against TMEV antigen (46). Capsid protein expression was not detected at day 3 or 60; most likely the antigen level was below the limit of our system. However, antibody to capsid protein was detected before TMEV infection, indicating that in vivo expression by plasmid DNA did occur (see below).
Antibodies. (i) ELISA.
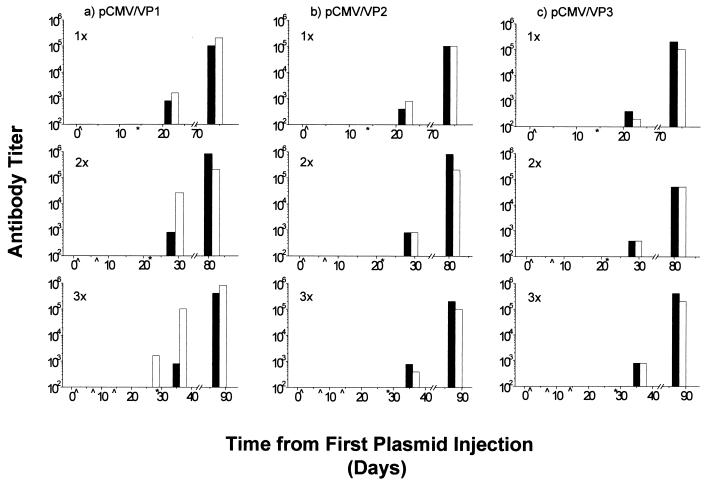
Blood was taken from mice before treatment and throughout the experiment before each injection of plasmid, before and after challenge with virus, and at the time of sacrifice. Sera for antibody titers to TMEV were analyzed by ELISA. Of interest are the apparent high antibody titers for TMEV found in mice injected three times with pCMV/VP1 prior to challenge with the virus itself. The antibody titers in all groups, control and experimental, increased over the course of the experiment (Fig. 6). There was no correlation between anti-TMEV antibody titer and disease course.
FIG. 6.
Serum anti-TMEV antibody titers of mice following DNA vaccination with pCMV/VP1 (a), pCMV/VP2 (b), or pCMV/VP3 (c) one, two, or three times (empty columns). Control mice were injected with vector pCMV (solid columns). After the final DNA vaccination, mice were injected intracerebrally with TMEV. ELISA was used to measure the level of serum anti-TMEV antibody. Λ, day of vaccination; ∗, day of viral inoculation. A significant anti-TMEV antibody titer was detected in mice injected three times with pCMV/VP1 prior to TMEV inoculation.
Western blot analysis.
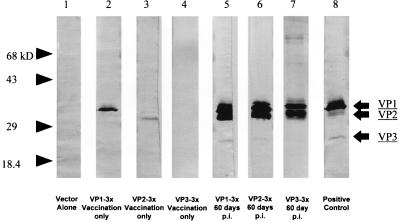
Western blot analyses were performed with pooled mouse sera for each group from various time points throughout the experiment. Sera from mice after challenge with virus, in all cases, contained antibodies to capsid proteins VP1, VP2, and at times VP3. No responses to VP4 were detectable. We also detected antibodies to VP1 and VP2 in mice treated with pCMV/VP1 and pCMV/VP2, respectively, prior to challenge with TMEV but not in mice treated with pCMV/VP3 (Fig. 7). This finding indicates that viral protein was being produced in vivo by using these plasmids and that an immune response was made to the protein before viral challenge.
FIG. 7.
Sera from various time points were tested for TMEV-specific antibodies by Western blot analysis. Two weeks after three DNA injections, mice were injected with TMEV intracerebrally. Prior to TMEV inoculation, mice vaccinated three times with pCMV/VP1 (lane 2) and pCMV/VP2 (lane 3), but not with pCMV (lane 1) or pCMV/VP3 (lane 3), demonstrated antibodies to TMEV capsid proteins VP1 and VP2, respectively. Sixty days postinfection (p.i.), antibodies to VP1, VP2, and at times VP3 were detectable in all vaccinated mice (lanes 5 to 7). Hyperimmune serum to TMEV was used as a positive control (lane 8). Molecular weights: VP1, 37,000; VP2, 34,000; VP3, 27,000.
DISCUSSION
We demonstrate that vaccination of SJL/J mice with cDNA encoding the capsid proteins of TMEV can alter the course of TMEV-induced demyelinating disease. A single vaccination with cDNA encoding VP1 led to increased pathology in the CNS and increased clinical demonstration of disease. In contrast, vaccinating mice three times with plasmids encoding the capsid proteins VP2 and VP3 reduced TMEV-induced disease, as demonstrated by a decrease in clinical expression of disease and reduced CNS pathology.
Because vaccination with cDNA encoding the capsid proteins of TMEV led to alteration in disease expression, it can be inferred that host cells incorporated the cDNA and proteins were expressed. In support of this possibility is the detection of β-galactosidase expression in muscle by using the same vector system and also detection of antibodies to VP1 and VP2 by Western blot analyses after vaccination with plasmid DNA but before challenge with TMEV.
Recently, bone marrow-derived macrophages or other professional antigen-presenting cells have been suggested to process plasmid DNA encoding proteins for expression in major histocompatibility complex (MHC) class I molecules (5, 19, 41). Thus, one explanation for the modulation found in the disease course is that cDNA encoding viral capsid protein was synthesized intracellularly, followed by normal cellular processing for expression in association with MHC class I molecules by professional antigen-presenting cells. This could lead to induction of MHC class I-restricted TMEV-specific T-cell responses. MHC class I-restricted T-cell responses, including cytotoxic T-lymphocyte (CTL) response, are suggested to play a role as either suppressor or effector in TMEV-induced demyelinating disease (22, 34).
Another possible explanation for our observations is that these proteins were released into the extracellular milieu and recognized by specific B cells, leading to antibody production and/or uptake by macrophages or other professional antigen-presenting cells, processing, and presentation in MHC class II molecules. There is evidence that influenza virus proteins are secreted extracellularly by some cell types (30).
Although antibody expression detected by ELISA was not found in our work to be correlated with disease modulation, antibodies to both VP1 and VP2 were detectable with Western blot analysis prior to infection with TMEV in pCMV/VP1- and pCMV/VP2-treated mice, respectively. Inoue et al. (18) demonstrated several linear antibody epitopes to TMEV. These epitopes were located in VP1, VP2, and VP3. Interestingly, these investigators found that the major antibody epitope in disease-susceptible SJL mice infected with TMEV was to VP1; however, in mice resistant to TMEV-induced disease, the major epitopes were located in VP2. These investigators suggest that antibody to VP1, which is high in SJL mice, may be involved in disease, whereas antibody to VP2, which is high in BALB/c and C57BL/6 mice, could be a reason for protection. They also found that even with high neutralizing antibody titers to VP1 in SJL mice, protection was not effective. In addition, we have found a monoclonal antibody, H8, which reacts both with TMEV VP1 and with galactocerebroside, a major lipid component of myelin (8). When injected into mice with experimental allergic encephalomyelitis, H8 increased the size of demyelinating lesions 10-fold, while no demyelination was observed in mice treated with H8 alone (49). pCMV/VP1 injection could induce the production of a similar antibody, leading to the enhancement of TMEV demyelinating disease. Induction of antibody production to VP1 detected by Western blot analysis, before challenge with live virus, had no beneficial effect and may even have been detrimental in some mice. However, upon introduction of VP2 prior to TMEV infection, mice were able to establish an antibody response to epitopes in this protein. This may have allowed animals to produce virus-neutralizing antibodies.
In other DNA vaccination studies, both CTL and antibody responses have been shown to increase after boosting with plasmid injections (52). This is in accord with our observation that pCMV/VP2- and pCMV/VP3-immunized mice showed highest protection against TMEV-induced demyelinating disease after three plasmid injections, an effect that appeared to be dose dependent.
Interestingly, however, the mice immunized with pCMV/VP1 showed exacerbation of demyelinating disease only after a single plasmid injection, not after two or three injections. Using a plasmid encoding influenza virus nucleoprotein, Pertmer et al. (28) demonstrated that nucleoprotein-specific CTL activities were highest in mice that received a single immunization and significantly lower in groups that received additional immunizations. They also showed that the decline in CTL activity following the administration of booster plasmid immunizations appeared to be correlated to decreasing gamma interferon (IFN-γ) production after boosting. A decreased CTL response following additional plasmid immunizations has also been reported for mice receiving human immunodeficiency virus type 1 gp120 DNA vaccine; the decline in the CTL response is paralleled by a decrease in IFN-γ production and increase in interleukin-4 production (9). On the other hand, Mor et al. (25) reported a similar but reversed cytokine pattern following DNA immunization with a Plasmodium yoelii circumsporozoite protein plasmid DNA. In this case, initial interleukin-4 production was replaced by IFN-γ production upon boosting. Pertmer et al. (28) suggest that the identity of the encoded antigen may be one of the most important factors in determining the ultimate responses generated. Cytokine milieu is known to affect both humoral and cellular immune responses as well as TMEV-induced demyelinating disease course (40a, 43). Therefore, in our studies, divergent immune responses, including alteration in cytokine microenvironment, might be induced with pCMV/VP constructs, leading to different modulation in TMEV-induced demyelinating disease.
This work demonstrates that DNA immunization can be a viable method to modulate CNS viral infections that may contribute to MS. Although further work will be needed to determine specific T-cell responses, T-cell types, and B-cell involvement in our model, this work may lead to vaccination against virus-induced demyelinating disease and MS.
ACKNOWLEDGMENTS
We thank Kornelia Edes, Amy Perou, and Li-Qing Kuang for excellent technical assistance, and we thank J. Lindsay Whitton and Daniel E. Hasset for many helpful discussions. We are grateful to Kathleen Borick for preparation of the manuscript.
This study was supported by NIH grant NS34497.
REFERENCES
- 1.Barnett L A, Whitton J L, Wada Y, Fujinami R S. Enhancement of autoimmune disease using recombinant vaccinia virus encoding myelin proteolipid protein. J Neuroimmunol. 1993;44:15–25. doi: 10.1016/0165-5728(93)90263-x. . (Erratum, 48:120.) [DOI] [PubMed] [Google Scholar]
- 2.Bradley W G, Daroff R B, Fenichel G M, Marsden C D. Neurology in clinical practice. Newton, Mass: Butterworth-Heinemann; 1996. pp. 1307–1343. [Google Scholar]
- 3.Compston D A, Kellar W H, Robertson N, Sawcer S, Wood N W. Genes and susceptibility to multiple sclerosis. Acta Neurol Scand Suppl. 1995;161:43–51. doi: 10.1111/j.1600-0404.1995.tb05855.x. [DOI] [PubMed] [Google Scholar]
- 4.Cook S D, Rohowsky K C, Bansil S, Dowling P C. Evidence for multiple sclerosis as an infectious disease. Acta Neurol Scand Suppl. 1995;161:34–42. doi: 10.1111/j.1600-0404.1995.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corr M, Lee D J, Carson D A, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujinami R S. Viruses and autoimmunity. In: van Regenmortel M H V, Neurath A R, editors. Immunochemistry of viruses. II. The basis for serodiagnosis and vaccines. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1990. pp. 77–82. [Google Scholar]
- 7.Fujinami R S, Zurbriggen A. Is Theiler’s murine encephalomyelitis virus infection of mice an autoimmune disease? APMIS. 1989;97:1–8. doi: 10.1111/j.1699-0463.1989.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujinami R S, Zurbriggen A, Powell H C. Monoclonal antibody defines determinant between Theiler’s virus and lipid-like structures. J Neuroimmunol. 1988;20:25–32. doi: 10.1016/0165-5728(88)90110-5. [DOI] [PubMed] [Google Scholar]
- 9.Fuller D H, Haynes J R. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein 120 vaccine. AIDS Res Hum Retroviruses. 1994;10:1433–1441. doi: 10.1089/aid.1994.10.1433. [DOI] [PubMed] [Google Scholar]
- 10.Gerety S J, Karpus W J, Cubbon A R, Goswami R G, Rundell M K, Peterson J D, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J Immunol. 1994;152:908–918. [PubMed] [Google Scholar]
- 11.Gerety S J, Rundell M K, Dal Canto M C, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- 12.Goodkin D E. Role of steroids and immunosuppression and effects of interferon beta-1b in multiple sclerosis. West J Med. 1994;161:292–298. [PMC free article] [PubMed] [Google Scholar]
- 13.Haegert, D. G., and M. G. Marrosu. 1994. Genetic susceptibility to multiple sclerosis. Ann. Neurol. 36(Suppl. 2):S204–S210. [DOI] [PubMed]
- 14.Hafler D A. Recognition of self antigens in multiple sclerosis. In: Abramsky O, Ovadia H, editors. Frontiers in multiple sclerosis: clinical research and therapy. St. Louis, Mo: Mosby-Year Book; 1997. pp. 71–79. [Google Scholar]
- 15.Hartung H P. Pathogenesis of multiple sclerosis. In: Abramsky O, Ovadia H, editors. Frontiers in multiple sclerosis: clinical research and therapy. St. Louis, Mo: Mosby-Year Book; 1997. pp. 45–59. [Google Scholar]
- 16.Hassett D E, Whitton J L. DNA immunization. Trends Microbiol. 1996;4:307–312. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 17.Hillert, J. 1994. Human leukocyte antigen studies in multiple sclerosis. Ann. Neurol. 36(Suppl.):S15–S17. [DOI] [PubMed]
- 18.Inoue A, Choe Y K, Kim B S. Analysis of antibody responses to predominant linear epitopes of Theiler’s murine encephalomyelitis virus. J Virol. 1994;68:3324–3333. doi: 10.1128/jvi.68.5.3324-3333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki A, Torres C A, Ohashi P S, Robinson H L, Barber B H. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–14. [PubMed] [Google Scholar]
- 20.Kurtz C I B, Sun X, Fujinami R S. Protection of SJL/J mice from demyelinating disease mediated by Theiler’s murine encephalomyelitis virus. Microb Pathog. 1995;18:11–27. doi: 10.1016/s0882-4010(05)80009-9. [DOI] [PubMed] [Google Scholar]
- 21.Kurtzke J F, Beebe G W, Norman J E. Epidemiology of multiple sclerosis in U.S. veterans. 1. Race, sex, and geographic distribution. Neurology. 1979;29:1228–1235. doi: 10.1212/wnl.29.9_part_1.1228. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Thiemann N R, Pease L R, Rodriguez M. VP1 and VP2 capsid proteins of Theiler’s virus are targets of H-2D-restricted cytotoxic lymphocytes in the central nervous system of B10 mice. Virology. 1995;214:91–99. doi: 10.1006/viro.1995.9951. [DOI] [PubMed] [Google Scholar]
- 23.Lipton H L. Theiler’s viruses. In: Webster R G, Granoff A, editors. Encyclopedia of virology plus CD-ROM product. London, England: Academic Press; 1996. [Google Scholar]
- 24.Lisak R P. Multiple sclerosis: evidence for immunopathogenesis. Neurology. 1980;30:99–105. doi: 10.1212/wnl.30.7_part_2.99. [DOI] [PubMed] [Google Scholar]
- 25.Mor G, Klinman D M, Shapiro S, Hagiwara E, Sedegah M, Norman J A, Hoffman S L, Steinberg A D. Complexity of the cytokine and antibody response elicited by immunizing mice with Plasmodium yoelii circumsporozoite protein plasmid DNA. J Immunol. 1995;155:2039–2046. [PubMed] [Google Scholar]
- 26.Nelson D A. Dorsal root ganglia may be reservoirs of viral infection in multiple sclerosis. Med Hypotheses. 1993;40:278–283. doi: 10.1016/0306-9877(93)90006-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohara Y, Stein S, Fu J, Stillman L, Klaman L, Roos R P. Molecular cloning and sequence determination of DA strain of Theiler’s murine encephalomyelitis viruses. Virology. 1988;164:245–255. doi: 10.1016/0042-6822(88)90642-3. [DOI] [PubMed] [Google Scholar]
- 28.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poser, C. M. 1994. The epidemiology of multiple sclerosis: a general overview. Ann. Neurol. 36(Suppl. 2):S180–S193. [DOI] [PubMed]
- 30.Prokudina E N, Semenova N P. Localization of the influenza virus nucleoprotein: cell-associated and extracellular non-virion forms. J Gen Virol. 1991;72:1699–1702. doi: 10.1099/0022-1317-72-7-1699. [DOI] [PubMed] [Google Scholar]
- 31.Raine, C. S. 1994. The Dale E. McFarlin Memorial Lecture: the immunology of the multiple sclerosis lesion. Ann. Neurol. 36(Suppl.):S61–S72. [DOI] [PubMed]
- 32.Rauch H C, Montgomery I N, Hinman C L, Harb W, Benjamins J A. Chronic Theiler’s virus infection in mice: appearance of myelin basic protein in the cerebrospinal fluid and serum antibody directed against MBP. J Neuroimmunol. 1987;14:35–48. doi: 10.1016/0165-5728(87)90099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez M, Pease L R, David C S. Immune-mediated injury of virus-infected oligodendrocytes: a model of multiple sclerosis. Immunol Today. 1986;7:359–362. doi: 10.1016/0167-5699(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez, M., C. Rivera-Quiñones, P. D. Murray, N. M. Kariuki, P. J. Wettstein, and T. Mak. 1997. The role of CD4+ and CD8+ T cells in demyelinating disease following Theiler’s virus infection: a model for multiple sclerosis. J. Neurovirol. 3(Suppl. 1):S43–S45. [PubMed]
- 35.Roos R P, Stein S, Ohara Y, Fu J, Semler B L. Infectious cDNA clones of the DA strain of Theiler’s murine encephalomyelitis virus. J Virol. 1989;63:5492–5496. doi: 10.1128/jvi.63.12.5492-5496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose J, Gerken S, Lynch S, Pisani P, Varvil T, Otterud B, Leppert M. Genetic susceptibility in familial multiple sclerosis not linked to the myelin basic protein gene. Lancet. 1993;341:1179–1181. doi: 10.1016/0140-6736(93)91003-5. [DOI] [PubMed] [Google Scholar]
- 37.Sadovnick A D, Ebers G C. Basic, clinical and genetic epidemiology of multiple sclerosis. In: Abramsky O, Ovadia H, editors. Frontiers in multiple sclerosis: clinical research and therapy. St. Louis, Mo: Mosby-Year Book; 1997. pp. 14–21. [Google Scholar]
- 38.Schattner A, Rager-Zisman B. Virus-induced autoimmunity. Rev Infect Dis. 1990;12:204–222. doi: 10.1093/clinids/12.2.204. [DOI] [PubMed] [Google Scholar]
- 39.Seboun E, Oksenberg J R, Hauser S L. Molecular and genetic aspects of multiple sclerosis. In: Rosenberg R N, Prusiner S B, DiMauro S, Barchi R L, editors. The molecular and genetic basis of neurological diseases. Newton, Mass: Butterworth-Heinemann; 1997. pp. 631–660. [Google Scholar]
- 40.Sibley W A, Bamford C R, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;i:1313–1315. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a.Theil, D., and R. S. Fujinami. Unpublished data.
- 41.Torres C A, Iwasaki A, Barber B H, Robinson H L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 42.Tsunoda I, Fujinami R S. Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J Neuropathol Exp Neurol. 1996;55:673–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Tsunoda I, Fujinami R S. Theiler’s murine encephalomyelitis virus (TMEV) In: Ahmed R, Chen I, editors. Persistent viral infections. Chichester, England: John Wiley & Sons; 1998. pp. 517–536. [Google Scholar]
- 44.Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler’s murine encephalomyelitis virus. Acta Neuropathol (Berlin) 1996;91:595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- 45.Tsunoda I, Kuang L-Q, Tolley N D, Whitton J L, Fujinami R S. Enhancement of experimental allergic encephalomyelitis (EAE) by DNA immunization with myelin proteolipid protein (PLP) plasmid DNA. J Neuropathol Exp Neurol. 1998;57:758–767. doi: 10.1097/00005072-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Tsunoda I, McCright I J, Kuang L-Q, Zurbriggen A, Fujinami R S. Hydrocephalus in mice infected with a Theiler’s murine encephalomyelitis virus variant. J Neuropathol Exp Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Welsh C T, Fujinami R S. Neuropathic viruses and autoimmunity. In: Friedman H, Rose N R, Bendinelli M, editors. Microorganisms and autoimmune diseases. New York, N.Y: Plenum Press; 1996. pp. 159–180. [Google Scholar]
- 48.Whitton J L, Sheng N, Oldstone M B, McKee T A. A “string-of-beads” vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993;67:348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada M, Zurbriggen A, Fujinami R S. Monoclonal antibody to Theiler’s murine encephalomyelitis virus defines a determinant on myelin and oligodendrocytes, and augments demyelination in experimental allergic encephalomyelitis. J Exp Med. 1990;171:1893–1907. doi: 10.1084/jem.171.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yauch R L, Kerekes K, Saujani K, Kim B S. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler’s virus in demyelination-susceptible SJL/J mice. J Virol. 1995;69:7315–7318. doi: 10.1128/jvi.69.11.7315-7318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yauch R L, Kim B S. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler’s virus is located within VP1(233-244) J Immunol. 1994;153:4508–4519. [PubMed] [Google Scholar]
- 52.Yokoyama M, Zhang J, Whitton J L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]