Abstract
Purpose
To investigate whether the use of absorble AZ31B magnesium alloys over distraction gaps improves the quality and quantity of regenerated bone better than the use of Collagen membranes.
Methods
Fifteen mixed-breed dogs were randomly divided into the experimental (n = 10) and control (n = 5) groups. In the experimental group, two devices were implanted along the mandible; one side with absorble AZ31B and the other side with Collagen. The control animals did not undergo osteotomy or distraction. After a consolidation time of two months, 30 specimens were harvested, and newly created bone was identified using CBCT and micro-CT.
Results
The Collagen membranes were absorbed completely, and the AZ31B membranes became irregular and rough. Mandible length was successfully extended approximately 1 cm. More bone formation was found after using AZ31B than Collagen, and there was a significant difference in width reduction between experimental sites treated with AZ31B (0.11 ± 0.04 cm) and Collagen (0.42 ± 0.06 cm) (p < 0.05). Trabecular thickness was also significantly higher in AZ31B (0.338 ± 0.08 cm) and control (0.417 ± 0.05 cm) than Collagen (0.178 ± 0.04 cm) (p < 0.05).
Conclusion
An AZ31B membrane barrier is biocompatible and absorbable which can maintain the distraction gap and provide support to the attached osteoprogenitors by providing space for them to proliferate.
Keywords: Distraction osteogenesis, Absorble, AZ31B magnesium alloy
1. Introduction
Distraction osteogenesis (DO) is a powerful tool in craniomaxillofacial surgery [[1], [2], [3]] that is based on the “law of tension-stress” introduced by Ilizarov [4]. In 1992, McCarthy performed this technique on the human mandible, and ever since, DO has become a widely accepted approach for the treatment of craniofacial deformities.
The main disadvantage of DO is the time the fixator has to stay in place, which can lead to multiple complications. However, the distraction phase cannot be accelerated as this will lead to poor bone quality. Another problem associated with DO is the possibility of having less new bone regeneration than is needed in the bone segment, causing a bottleneck bone or open gap at the distraction gap [[5], [6], [7]]. This may be caused by pressure from soft tissue or the invasion of surrounding fibroblasts into the distraction gap prior to osteoblasts, both of which can lead to incomplete bone in the distraction gap, which may cause inadequacies in the implants or even fracture [8]. Currently, there are several techniques to address these problems, such as onlay bone grafts, guided bone regeneration and using bone substitutes [9]. Additionally, several materials, including collagen, fibrin, synthetic polymers (PLA) and hydroxyapatite (HA) have been used to maintain the osteogenic space and provide support to the attached osteoprogenitor cells, by providing space for them to proliferate [10], in addition to preventing fibroblasts from migrating into the distraction gap. These materials have limitations when used on their own, including brittleness and softness, which is especially an issue for PLA and collagen [[11], [12], [13]] In this study, we investigated a membrane made of magnesium alloy AZ31B, which is a new material that has many advantages compared with other metal membranes, including biocompatibility, biodegradability, and decreasing the stress shielding effect [14,15]. Previous studies have also found AZ31B can promote new bone regeneration and decrease healing times of bone fractures, while also not requiring a second surgical procedure for its removal, due to its biodegradability [16,17].
2. Materials and methods
2.1. Materials
AZ31B (Mg–3%Al–1%Zn) magnesium alloys were provided by the Institute of Metal Research, Chinese Academy of Sciences. The chemical composition of AZ31B included aluminum (2.5%–3.5%), zinc (0.6%–1.4%), manganese (0.2%–1.0%), silicon (0.1%), iron (0.005%), copper (0.005%) and nickel (0.005%, all percentages represent weights). The magnesium alloys were fabricated as 0.5 mm thick “U” shapes, 15 mm in height and 10 mm in width. These materials degrade after 2 weeks in saline and 4–20 weeks in vivo, according to our previous study [18]. To delay degradation time, we introduced a surface barrier technique. The AZ31B magnesium alloy barrier membranes were ultrasonically cleaned in alcohol for 5 min, air dried, and then coated with fluoride (patent number: 200710159202).
Collagen membranes were obtained from (B. Braun Melsungen AG, Carl-Braun-Straße 1, 34212 Melsungen, Germany).
2.2. Animals
Fifteen 8-month-old mixed-breed dogs, weighing between 20 and 22 Kg were provided by the Experimental Animal Centre of Jinzhou Medical University (license number: SYXK(Liao)2019-0007, Liaoning, China) and were randomly divided into the experimental (n = 10) and control (n = 5) groups. In the experimental group, one side of the mandible was randomly selected as the AZ31B group and the contralateral side was the Collagen group. Control animals did not undergo osteotomy and distraction. The housing, care and experimental protocol were in accordance with the guidelines established by the Ethics Committee of Jinzhou Medical University and registered as number 15/0058. The experimental procedures were performed according to the Author's Guide Consensus on Animal Ethics and Welfare enacted by the International Association of Veterinary Editors and local and national regulations.
2.3. Surgical procedures
Before surgery, the experimental dogs were injected with 0.01 mg/kg atropine 15 min prior to the intravenous administration of 3% pentobarbital (0.1 mg/kg) by a veterinarian. Then, the dogs were given 40,000 units penicillin G Benzedrine, which was also repeated for 7 days after the operation. Subcutaneous infiltration was conducted under aseptic conditions with 4 ml of 0.5% lidocaine and epinephrine at a 1:200,000 ratio. Then a longitudinal skin incision approximately 5 cm was made along the inferior border of the mandible, and the subcutaneous, platysma, and periosteum were dissected to expose the mandible. To cover the bone gap with a magnesium membrane, we “opened” the device to 10 mm at the beginning. The distraction devices (CBX0101, Xi'an Zhongbang Titanium Biological Materials Co., Ltd., Xi'an, China) with a total action length of 20 mm were temporarily “opened” to 10 mm and attached to the bone via screws. After the device was removed, the mandible osteotomy was performed with a standard surgical reciprocating saw blade under irrigation with sterile saline solution. Then, the distraction device was reattached, and the AZ31B magnesium alloy membrane was shaped along the mandible to cover the site of the osteotomy (Fig. 1). To rotate the screw smoothly, a small incision (approximately 0.4 cm) was made near the top of the distractor rod to expose it from the mouth. The surgical incisions were then closed with 3-0 sutures. The same procedures were performed on the contralateral side of the mandible except covered with Collagen membranes.
Fig. 1.
After osteotomy, the distraction device was fixed and an AZ31B magnesium alloy barrier membrane was shaped along the mandible to cover the site of the osteotomy gap.
After 7 days, the mandible distraction was carried out at the rate of 0.9 mm/day, three times per day, for 11 consecutive days. Buprenorphine was given at 0.004 mg/kg every 12 h for pain. Food and water consumption was monitored to make sure the animals were eating and drinking properly. The progress of the distraction and consolidation was assessed through clinical observations and X-ray scans. All animals were sacrificed by intravenous pentobarbital injections (10 mg/kg) 2 months after the consolidation period, in accordance with the recommendations of the Veterinary Medical Association Panel.
Following sacrifice, the entire mandibular segments with the distractor was dissected and immersed in neutral 10% formalin for 10 days. A sliding caliper (Syntek, Sy01-150mm, Zhejiang, China) was used to measure the length, thickness and altitude of the mandibles.
2.4. Cone beam computed tomography (CBCT) measurements
To measure the width of the regenerated mandibles, dissected mandibles were scanned using a Rayscan CBCT (Samsung, Seoul, South Korea) under 80 kvp, 5 mA and 0.4 mm voxel size. The exposure time for each specimen was 30 s. The CBCT scans were saved as single DICOM files and imported into ANALYZE 3D Imaging software (Samsung, Seoul, South Korea). To obtain a precise mean value for the new mandible width, five parallel lines were made. Two of the five lines were located on the distal and mesial side of the distraction gap. After calculating the distance between them, the other equally divided three lines were made to analyze the mean mandible width value.
2.5. Micro-computed tomography (Micro-CT) measurements
After CBCT, mandibles were then sectioned for further analysis. Each side of the mandible was initially sectioned into two segments, the interest region containing new bone (approximately 0.6 cm) and native bone adjacent to the regenerated bone (approximately 0.4 cm). Regenerated and native bone blocks from both sides of the mandible were initially scanned using a micro-CT (Skyscan 1076; Skyscan Co., Antwerp, Belgium). Each specimen was placed in a midsize sample holder between the x-ray source and the camera. The micro-CT parameters were 9.5 mm pixel size, 100 kV peak, 100 mA, 0.5 mm aluminum filter, angular rotation step 0.8°, 360° scanning, 450 projections and an exposure time of 1.5 s with total scan duration of 1360 s. The total area of the distraction gap and native bone were outlined on each scanned image as the region of interest. The serial scanned images of each specimen were analyzed using version 1.14.4.1 Bruker micro-CT software (Kontich, Belgium). The final obtained parameters included: 1) trabecular thickness (Tb.Th), determined as the diameter of spheres filling the bone structures and providing detailed microstructure parameters, 2) trabecular number (Tb.N), determined as the inverse of the mean distances of the skeletonized bone structure, 3) trabecular spacing (Tb.Sp), determined as the diameter of spheres filling the marrow spaces, 4) bone mineral density (BMD), defined as the total mineral content of the newly remodeled bone, which is an important indicator of bone strength and 5) bone volume fraction (BV/TV), which indicates the degree of bone tissue volume in cancellous bone (described in detail by Hildebrand & Ruegsegger) [19].
2.6. Statistical analysis
Statistical evaluations of the clinical and CBCT examinations were done with independent Student's t-tests. The Tb.Th, Tb.N, Tb.Sp, BMD and BV/TV results were analyzed with two-way analysis of variance (ANOVA) parametric tests. All calculations were carried out using SPSS version 17.0 (IBM, Armonk, NY, USA). Results were accepted as statistically significant at a value of P < 0.05.
3. Results
3.1. Clinical evaluation
The progress of distractor activation, stability, signs of infection and eating habits were followed up clinically and radiographically. One dog died due to digestive infection and was replaced. The surgical/distraction procedure went smoothly in the remaining animals, although, three animals showed a little discomfort. Mild wound swelling around the incisions were found on the AZ31B sides and lasted for 10 d; on the Collagen sides these only lasted 4 d. As expected, at the time of sacrifice the dogs in the distracted groups developed an overgrowth of their lower jaws, with average lengths of 16.02 ± 0.34, 15.97 ± 0.54 and 15.05 ± 0.39 cm in the AZ31B, Collagen and control groups, respectively. The mean mandible prolonged length in the experimental groups was 0.95 ± 0.11 cm longer than controls. After dissection, all distracter devices maintained adequate stability to mandibles. As the integrity of the magnesium alloy disappeared, the surface became irregular and rough due to biodegradation. New bone formation was found on and under the devices in the AZ31B group. The Collagen membranes were absorbed completely, and the bone surface was smooth but shaped like a bottleneck (Fig. 2). The difference in width of the new bone was statistically significant between the experimental and control groups (P < 0.05). No significant difference was found between the groups in alveolar height (Table 1).
Fig. 2.
The mandibular with the distractor was dissected after the consolidation period. Co: Group Collagen, Obvious depressions can be seen on both sides and the surface is smooth. AZ: Group AZ31B, There are no obvious depressions on both sides, and the surface is uneven.
Table 1.
Group comparison (mean ± SD) for mandible length, height, width (cm).
| length | height | width | |
|---|---|---|---|
| AZ31B | 16.02 ± 0.34 | 2.89 ± 0.13 | 1.47 ± 0.1 |
| Collagen | 15.97 ± 0.54 | 2.87 ± 0.22 | 1.16 ± 0.05 |
| control | 15.05 ± 0.39 | 2.93 ± 0.36 | 1.58 ± 0.12 |
| Difference A | 0.97 ± 0.06 | 0.04 ± 0.00 | 0.11 ± 0.04 |
| Difference C | 0.92 ± 0.04 | 0.05 ± 0.01 | 0.42 ± 0.06∗ |
Difference A, C:The difference between Group AZ31B and control; Collagen and control. ∗ The width difference between AZ31B and Collagen (P<0.05).
3.2. CBCT evaluation
CBCT analysis showed that the width of new bone (distance between the buccal and lingual mandible) induced by distraction was 1.48 ± 0.25 cm for the AZ31B group, 1.14 ± 0.13 cm for the Collagen group and 1.62 ± 0.12 cm for controls (Fig. 3). The width was reduced by 0.14 ± 0.05 cm and 0.48 ± 0.05 cm in the AZ31B group and the Collagen group, respectively, compared to controls. The increased bone width can be explained by the higher mechanical strength provided by the AZ31B membrane barrier, which allowed more space for osteoblasts to proliferate and prevented fibroblast invasion better than low strength Collagen.
Fig. 3.
The width between the buccal and lingual of the mandible was measured by CBCT, and the results showed AZ31B: 1.48 ± 0.25 cm, Collagen: 1.14 ± 0.13 cm and control: 1.62 ± 0.12 cm. The wider bone width suggests that the higher mechanical strength of AZ31B provides more space for osteoblasts to proliferate than low strength Collagen.
3.3. Micro-CT evaluation
All experimental mandibles, including from the AZ31B and Collagen groups, showed the presence of an almost integrated cortical bone and a well-defined alveolar canal. A dense trabecular network was visible in all segments through high resolution micro-CT images in all groups. However, much thinner bone and less newly formed trabecular bone were found in the distracted callus from the Collagen group (Fig. 4) compared with the AZ31B group (Fig. 5). The structures treated with AZ31B magnesium alloy were similar to the adjacent bone, displaying a dense cortical bone-like structure peripherally and a large region of newly formed trabecular bone in between (Table 2). Tb.Th was significantly higher in the AZ31B (0.338 ± 0.08) and control groups (0.417 ± 0.05) than the Collagen (0.178 ± 0.04) group (P < 0.05). Tb.Sp was significantly lower in the Collagen group than the controls (P < 0.05). Higher Tb.N, BMD and Bv/Tv results were found in the AZ31B group than the Collagen group; however, these results were not significantly different.
Fig. 4.

Micro-CT images from the Collagen group showed a thinner mandible with a bottleneck and less newly formed bone trabecular. Smooth bone surfaces and a clearly alveolar canal were found.
Fig. 5.
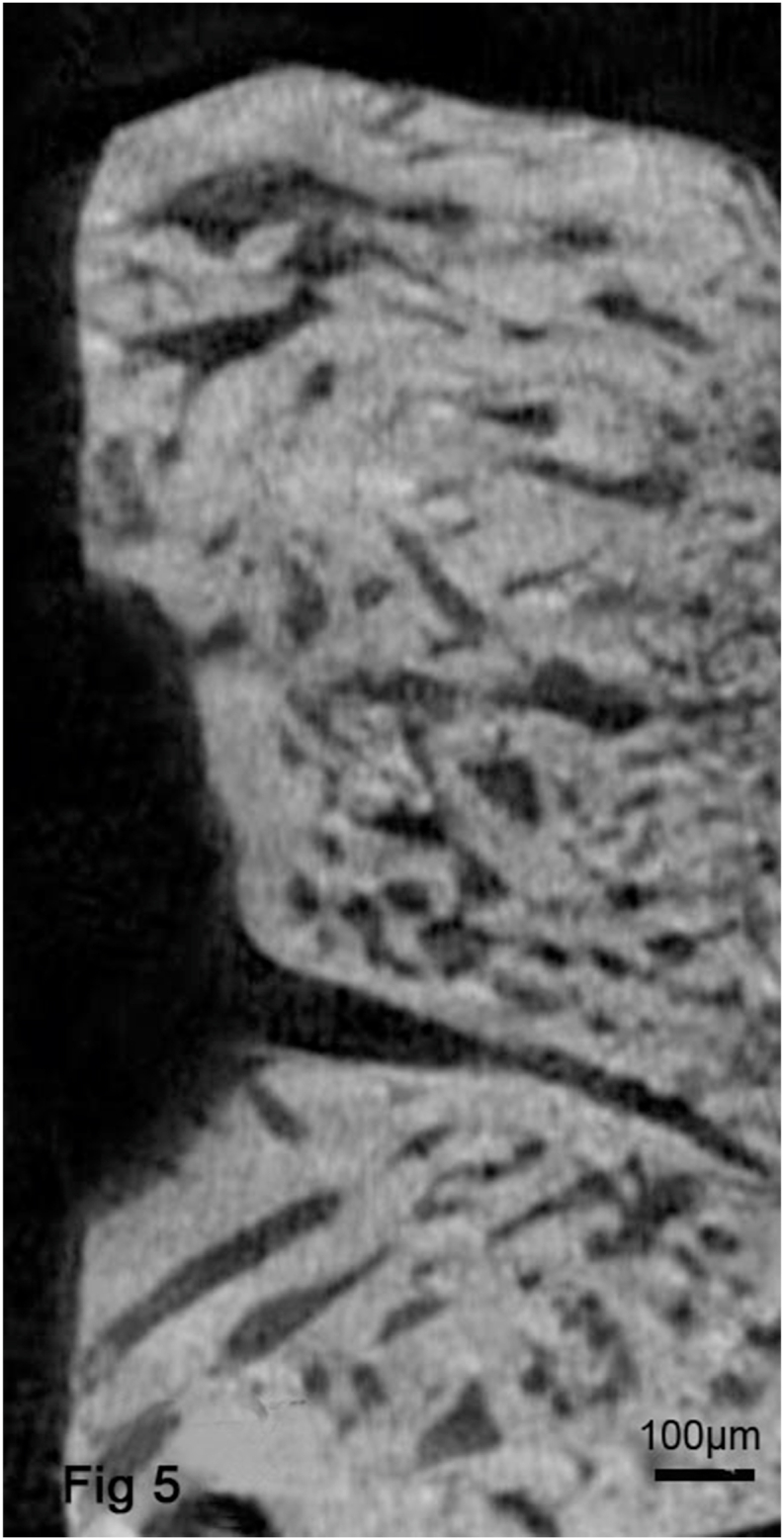
Micro-CT images from the AZ31B group showed a rough and uneven bone surface and the thick trabecular bone similar to that of adjacent bone. From the analysis of these images, significantly higher Tb.Th, and lower Tb.Sp were obtained.
Table 2.
The quantitative analysis of all groups through micro-CT examination. (mean ± SD).
| Tb.Th (цm) | Tb.N (1/цm) | Tb.Sp (цm) | BMD (mg/цm2) | Bv/Tv (%) | |
|---|---|---|---|---|---|
| AZ31B | 0.338 ± 0.08∗ | 5.066 ± 0.31 | 0.145 ± 0.47 | 6.79 ± 0.13 | 0.68 ± 0.02 |
| Collagen | 0.178 ± 0.04# | 4.824 ± 0.54 | 0.197 ± 0.37# | 5.04 ± 0.08 | 0.57 ± 0.05 |
| control | 0.417 ± 0.05 | 7.352 ± 0.29 | 0.094 ± 0.35 | 8.37 ± 0.09 | 0.83 ± 0,06 |
∗p<0.05: AZ31B vs Collagen.
#p<0.05: Collagen vs control.
4. Discussion
Distraction with membrane addition is a promising method to promote new bone formation. Theoretically, using a mechanical hindrance such as a membrane can prevent fibroblasts and other soft connective tissue cells from entering the bone defect, allowing osteogenic cells to fill the distraction gap and repopulate the bone. This not only increases the volume of new bone, but also prevents fibrotic growth in the distraction gap. Although concave bone defects are frequently seen in the clinic, only a few studies about them have been reported. Klug et al. [20] performed vertical distraction in 10 patients (13 sites) and 3 patients (4 sites) resulting in concave defects. For this reason, Klug applied titanium membranes in the distraction gap to prevent the invasion of the soft tissues into the distraction gap. At the end of treatment, the membranes and distractors were removed and alveolar height was sufficient to make dental implantation. In 2004, Elshahat et al. [21] reported that use of a collagen membrane could prevent rapidly migrating fibroblasts from entering the site of an osteotomy with a high distraction ratio in 14 rabbits. After 4 weeks of consolidation, they obtained better quantity bone in the membrane group than in the controls. Together, these studies demonstrated that barrier membranes can increase the quantity of bone during distraction osteogenesis.
Numerous barrier membranes are grouped as non-resorbable and require a second surgery for removal [22,23]. In contrast, resorbable membranes are often made of natural or synthetic polymers and undergo an unpredicted degree of resorption, which can alter the mechanical strength of the membrane or even cause it to collapse into the space, leading to a failed reconstruction. Non-resorbable membranes include polytetrafluoroethylene (PTFE) and titanium mesh. Compared with other resorbable membranes, these can maintain the space beneath the membrane for a sufficient period to treat bone defect.
In recent years, new innovative magnesium alloys have been intensively investigated as potential resorbable materials 13 with appropriate mechanical and corrosion properties for these procedures. Particularly, the mechanical properties of magnesium alloys are close to those of natural bone, preventing both stress shielding and the not need to remove the implant after surgery [[24], [25], [26]]. Magnesium alloys are also very active alloys that can dissolve and turn into magnesium and zinc ions [27], releasing hydrogen when immersed in a phosphate bath. Zhang et al. also tested the production of hydraxide ions, demonstrating that the pH increases only for the first few days, then reaches equilibrium after two weeks. This stable neutral pH promotes cell proliferation and maintains a safe environment for cells [28]. Furthermore, Zhang [18] showed that the regulation of magnesium occurs in the kidney, where excess is excreted in the urine, suggesting magnesium and other metal elements have no toxic effects on the liver, kidneys and blood composition. To take advantage of absorbable membranes, and at same time, avoid the drawbacks of non-absorbable membranes, a membrane made of AZ31B magnesium alloy coated with fluoride was used to maintain the osteogenic space, and to allow the attached osteoprogenitor cells to proliferate. The amount of hydrogen gas production is dependent on the corrosion rate of the magnesium alloy, although it did not become clinically visible. To depress its corrosion rate, a binary fluoride-coated magnesium alloy was created to improve degradation kinetics. The decreased electrical conductivity of the fluoride insulates the magnesium alloy membrane from the surrounding tissue fluid (patent application number 200710159202.0). Through this method, we hope to take advantage of magnesium alloys to get high calcium and magnesium ion concentrations to accelerate the deposition of biological calcium phosphate on the surface of the membrane and stimulate cell proliferation.
According to Berglund et al. [29] magnesium implants are almost completely degraded after 6 weeks, and as the alloy degrades it is consistently replaced by bone. At this time point, hydrogen gas formed during degradation process was not observed, suggesting degradation is slower and gas was systemically absorbed. To further test the influence of direct exposure to magnesium alloys, Charyeva et al. evaluated the reactions of humans to cellular debris. From these experiments, they found that the number of viable cells in the presence of Mg2Ag was high over the entire observation period [30]. Thus, they concluded that magnesium-based materials are beneficial for orthopedic and dental applications. Like those previous studies, we also found mild wound swelling in mandibles of the AZ31B group. However, this mild swelling only lasted for 10 days, then, slowly dissipated. Additionally, hydrogen gas was not observed; thus, we hypothesize that, fluoride coating technology is effective to decrease the degradation rate of magnesium and reduce infections and swelling.
Significant differences in new bone width reduction (AZ31B: 0.14 ± 0.05 cm and Collagen: 0.48 ± 0.05 cm) (Table 1) indicate that magnesium alloy barrier membranes can maintain the distraction gap better than low strength Collagen and provide more space for attached osteoprogenitor cells to proliferate. Another possibility is that during the degradation process the AZ31B alloy was consistently replaced by bone matrix along the surface of the membrane. Thus, bone width in AZ31B (1.48 ± 0.25 cm) was close to that of controls (1.62 ± 0.12 cm).
The increase in trabecular volume and mineral content translated to an increase in strength [31]. From micro-CT evaluation, during distraction osteogenesis, increased results were found in Tb.N, BMD and Bv/Tv; however, there was no significant difference between AZ31B and Collagen. Whereas, dramatically increased Tb.Th was found in AZ31B. This finding indicates that AZ31B magnesium alloy helped to increase Tb.N, BMD and Bv/Tv, especially to recover trabecular thickness. Moreover, previous studies have demonstrated that the release of free Mg2+ ions during the biodegradation process can contribute to enhanced activity of osteoblasts and a consequent increase in bone quantity [25,32]. Both the reduced Tb.Sp (0.145 ± 0.47) as well as the tendency to a higher Tb.Th around the magnesium membrane indicates a more mature bone and a better mechanical support in the process of osseointegration. Another hypothesis is that magnesium, as a key component of the ribosome that translates the genetic information encoded by mRNA into polypeptide structures [33], is an indispensable osteogenic stimulus for the initiation of fracture healing.
Micro-CT is a validated method for 3D quantitative structural analysis of bone, but does not give insight into the cell population, mineral apposition rate as bone histomorphometry does. In future studies, we will further evaluate the histology and structure, including osteoblasts and osteoclast reactions after AZ31B magnesium implantation.
5. Conclusions
No inflammatory responses or side effects were found during this study, showing that AZ31B magnesium alloy is a biocompatible and absorbable material that combines the advantages of traditional metallic implants and biodegradable polymers. An AZ31B membrane barrier can maintain the distraction gap and provide support for attached osteoprogenitor cells and space for them to proliferate. Finally, the use of magnesium barrier membranes to promote bone regeneration and exclude other cells such as epithelium and connective tissue cells is feasible.
Author contribution statement
Zhao Guiran: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Wang Shu: Performed the experiments; Analyzed and interpreted the data.
Wang Guijun: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zhang Bin: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Huang Han: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Yao yusheng: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Funding statement
This study was supported by the Science and Technology Research of Liaoning Education Department (No. JYTJCZR2020081).
Ethics approval
Our study was approved by the Ethics Committee of Jinzhou Medical University and registered as number 15/0058.
Statement of clinical relevance
Distraction osteogenesis with guided bone regeneration is a promising treatment for deformity and bone defects in the oral and maxillofacial area. Our findings will provides a good theoretical reference for clinicians in planning therapeutic approaches for such patients.
Declaration of competing interest
We declare that we have no any financial interests and any such interests of immediate family members, including financial holdings, professional affiliations, advisory positions, board memberships, receipt of consulting fees etc.
Acknowledgments
We are grateful to Yang Ke and Tan Lili(Institute of Metal Research, Chinese Academy of Sciences, Shenyang City, Liaoning Province, China.) for technical assistance.
References
- 1.Discolo C. Paediatric mandibular distraction: optimizing outcomes. Curr. Opin. Otolaryngol. Head Neck Surg. 2022;30:426–430. doi: 10.1097/MOO.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 2.R Zhao G., Wang Y., J Wang G., Wang C.Y., Yao Y.S. A new way to accelerate the distraction of the transpalatal suture in growing dogs using recombinant human bone morphogenetic protein-2. Cleft Palate Craniofac. J. 2017;54:193–201. doi: 10.1597/15-044. [DOI] [PubMed] [Google Scholar]
- 3.Holzinger D., D Carvalho P.H., C dos Santos J., Wagner F., C Gabrielli M.A., R Gabrielli M.F., Pereira V.A. Bone formation after surgically assisted rapid maxillary expansion: comparison of 2 distraction osteogenesis protocols. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022;133:271–276. doi: 10.1016/j.oooo.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 4.A Ilizarov G. The tension-stress effect on the genesis and growth of tissues. Part I: the influence of stability of fixation and soft tissue preservation. Clin. Orthop. Relat. Res. 1989;238:249–281. [PubMed] [Google Scholar]
- 5.Datarkar A., Valvi B., Parmar S., Patil J. Qualitative assessment of newly formed bone after distraction osteogenesis of mandible in patients with facial asymmetry using 3 dimensional computed tomography. J. Oral Biol. Craniofac. Res. 2021;11:410–414. doi: 10.1016/j.jobcr.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.E Elsalanty M., Malavia V., Zakhary I., Mulone T., D Kontogiorgos E., C Dechow P., Opperman L.A. Dentate transport discs can be used to reconstruct large segmental mandibular defects. J. Oral Maxillofac. Surg. 2015;73:745–758. doi: 10.1016/j.joms.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.J Kim S., C Balce G., V Agashe M., H Song S., R Song H. Is bilateral lower limb lengthening appropriate for achondroplasia?: midterm analysis of the complications and quality of life. Clin. Orthop. Relat. Res. 2012;470:616–621. doi: 10.1007/s11999-011-1983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Grandjean A., Reininger D., Lopez-Quiles J. Complications in the treatment with alveolar extraosseous distractors. Literature review. Med. Oral Patol. Oral Cir. Bucal. 2015;20:e518–e524. doi: 10.4317/medoral.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatelet M., Afota F., Savoldelli C. Review of bone graft and implant survival rate: a comparison between autogenous bone block versus guided bone regeneration. J. Stom. Oral Maxi. 2022;123:222–227. doi: 10.1016/j.jormas.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Nakahara K., Haga-Tsujimura M., Sawada K., Mottini M., Schaller B., Saulacic N. Effects of collagen membrane application and cortical bone perforation on de novo bone formation in periosteal distraction: an experimental study in a rabbit calvaria. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017;123:173–182. doi: 10.1016/j.oooo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Wang B., M Feng C., M Liu Y., Mi F.L., Dong J. Recent advances in biofunctional guided bone regeneration materials for repairing defective alveolar and maxillofacial bone: a review. Jpn Dent. Sci. Rev. 2022;58:233–248. doi: 10.1016/j.jdsr.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang J.W., Kim S., W Kim S., H Lee J. Effect of extracellular matrix membrane on bone formation in a rabbit tibial defect model. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/6715295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panseri S., Montesi M., M Dozio S., Savini E., Tampieri A., Sandri M. Biomimetic scaffold with aligned microporosity designed for dentin regeneration. Front Bioeng. Biothch. 2016;4:48. doi: 10.3389/fbioe.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adetunla A., Fide-Akwuobi A., Benjamin H., Adeyinka A., Kolawole A. A study of degradable orthopedic implant: an insight in magnesium metal matrix composites. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.P Kacarevic Z., Rider P., Elad A., Tadic D., Rothamel D., Sauer G., Bornert F., Windisch P., B Hangyasi D., Molnar B., Kammerer T., Hesse B., Bortel E., Bartosch M., Witte F. Biodegradable magnesium fixation screw for barrier membranes used in guided bone regeneration. Bioact. Mater. 2022;14:15–30. doi: 10.1016/j.bioactmat.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D., Zhang D.C., Yuan Q., H Liu L., Li H., Xiong L., Guo X.N., Y Yan K Yu, L Dai Y., Xiao T., Li Y.C., Wen C.E. In vitro and in vivo assessment of the effect of biodegradable magnesium alloys on osteogenesis. Acta Biomater. 2022;141:454–465. doi: 10.1016/j.actbio.2021.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Moravej M., Mantovani D. Biodegradable metals for cardiovascular stent application: interests and new opportunities. Int. J. Mol. Sci. 2011;12:4250–4270. doi: 10.3390/ijms12074250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang E., Xu L., Yu G., Pan F., Yang K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. 2009;90:882–893. doi: 10.1002/jbm.a.32132. [DOI] [PubMed] [Google Scholar]
- 19.Hildebrand T., Laib A., Muller R., Dequeker J., Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J. Bone Miner. Res. 1999;14(7):1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 20.N Klug C., Millesi-Schobel G.A., Millesi W., Watzinger F., Ewers R. Preprosthetic vertical distraction osteogenesis of the mandible using an L-shaped osteotomy and titanium membranes for guided bone regeneration. J. Oral Maxillofac. Surg. 2001;59:1302–1308. doi: 10.1053/joms.2001.27520. discussion 1309-10. [DOI] [PubMed] [Google Scholar]
- 21.Elshahat A., Inoue N., Marti G., Safe I., Manson P., Vanderkolk C. Role of guided bone regeneration principle in preventing fibrous healing in distraction osteogenesis at high speed: experimental study in rabbit mandibles. J. Craniofac. Surg. 2004;15:916–921. doi: 10.1097/00001665-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 22.E Zakhary I., El-Mekkawi H.A., E Elsalanty M. Alveolar ridge augmentation for implant fixation: status review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;114:s179–s189. doi: 10.1016/j.oooo.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Gentile P., Chiono V., Tonda-Turo C., M Ferreira A., Ciardelli G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011;6:1187–1197. doi: 10.1002/biot.201100294. [DOI] [PubMed] [Google Scholar]
- 24.Zhao N., Watson N., Xu Z., Chen Y., Waterman J., Sankar J., Zhu D. In vitro biocompatibility and endothelialization of novel magnesium-rare earth alloys for improved stent applications. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mushahary D., Sravanthi R., Li Y., J Kumar M., Harishankar N., D Hodgson P., Wen C., Pande G. Zirconium, calcium, and strontium contents in magnesium based biodegradable alloys modulate the efficiency of implant-induced osseointegration. Int. J. Nanomed. 2013;8:2887–2902. doi: 10.2147/IJN.S47378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waizy H., Weizbauer A., Modrejewski C., Witte F., Denkena B., Behrens P., Windhagen H., Lucas A., Kieke M., et al. In vitro corrosion of ZEK100 plates in Hank's balanced salt solution. Biomed. Eng. Online. 2012;11:12. doi: 10.1186/1475-925X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uppal G., Thakur A., Chauhan A., Bala S. Magnesium based implants for functional bone tissue regeneration–A review. J. Magnesium Alloys. 2022;10:356–386. [Google Scholar]
- 28.Zhang X., Zhang C., Xu W., Zhong B., Lin F., Zhang J., Wang Q., Ji J., Wei J., Zhang Y. Biodegradable mesoporous calcium-magnesium silicate-polybutylene succinate scaffolds for osseous tissue engineering. Int. J. Nanomed. 2015;10:6699–6708. doi: 10.2147/IJN.S92598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.S Berglund I., Jacobs B.Y., D Allen K., E Kim S., Pozzi A., Allen J.B., V Manuel M. Peri-implant tissue response and biodegradation performance of a Mg-1.0Ca-0.5Sr alloy in rat tibia. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;62:79–85. doi: 10.1016/j.msec.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Charyeva O., Dakischew O., Sommer U., Heiss C., R Schnettler K.S. Lips, Biocompatibility of magnesium implants in primary human reaming debris-derived cells stem cells in vitro. J. Orthop. Traumatol. 2016;17:63–73. doi: 10.1007/s10195-015-0364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little D.G., C Smith N., R Williams P., N Briody J., E Bilston L., J Smith E., Gardiner E.M., T Cowell C. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J. Bone Miner. Res. 2003;18:1300–1307. doi: 10.1359/jbmr.2003.18.7.1300. [DOI] [PubMed] [Google Scholar]
- 32.Z Zhang X., Zu H.Y., Zhao D.W., Yang K., M Tian S., Yu X.M., Lu F.Q., Liu B.Y., Yu X.B., J Wang B. Ion channel functional protein kinase TRPM7 regulates Mg ions to promote the osteoinduction of human osteoblast via PI3K pathway: in vitro simulation of the bone-repairing effect of Mg-based alloy implant. Acta Biomater. 2017;63:369–382. doi: 10.1016/j.actbio.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T., Shimizu Y., Ueda T., Shiro Y. Mg2+ dependence of 70 S ribosomal protein flexibility revealed by hydrogen/deuterium exchange and mass spectrometry. J. Biol. Chem. 2010;285:5646–5652. doi: 10.1074/jbc.M109.081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.





