Summary
Background
In a parallel-group, international, phase 3 study (ClinicalTrials.govNCT04762680), we evaluated prototype (D614) and Beta (B.1.351) variant recombinant spike protein booster vaccines with AS03-adjuvant (CoV2 preS dTM-AS03).
Methods
Adults, previously primed with mRNA (BNT162b2, mRNA-1273), adenovirus-vectored (Ad26.CoV2.S, ChAdOx1nCoV-19) or protein (CoV2 preS dTM-AS03 [monovalent D614; MV(D614)]) vaccines were enrolled between 29 July 2021 and 22 February 2022. Participants were stratified by age (18–55 and ≥ 56 years) and received one of the following CoV2 preS dTM-AS03 booster formulations: MV(D614) (n = 1285), MV(B.1.351) (n = 707) or bivalent D614 + B.1.351 (BiV; n = 625). Unvaccinated adults who tested negative on a SARS-CoV-2 rapid diagnostic test (control group, n = 479) received two primary doses, 21 days apart, of MV(D614). Anti-D614G and anti-B.1.351 antibodies were evaluated using validated pseudovirus (lentivirus) neutralization (PsVN) assay 14 days post-booster (day [D]15) in 18–55-year-old BNT162b2-primed participants and compared with those pre-booster (D1) and on D36 in 18–55-year-old controls (primary immunogenicity endpoints). PsVN titers to Omicron BA.1, BA.2 and BA.4/5 subvariants were also evaluated. Safety was evaluated over a 12-month follow-up period. Planned interim analyses are presented up to 14 days post-last vaccination for immunogenicity and over a median duration of 5 months for safety.
Findings
All three boosters elicited robust anti-D614G or -B.1.351 PsVN responses for mRNA, adenovirus-vectored and protein vaccine-primed groups. Among BNT162b2-primed adults (18–55 years), geometric means of the individual post-booster versus pre-booster titer ratio (95% confidence interval [CI]) were: for MV (D614), 23.37 (18.58–29.38) (anti-D614G); for MV(B.1.351), 35.41 (26.71–46.95) (anti-B.1.351); and for BiV, 14.39 (11.39–18.28) (anti-D614G) and 34.18 (25.84–45.22 (anti-B.1.351). GMT ratios (98.3% CI) versus post-primary vaccination GMTs in controls, were: for MV(D614) booster, 2.16 (1.69; 2.75) [anti-D614G]; for MV(B.1.351), 1.96 (1.54; 2.50) [anti-B.1.351]; and for BiV, 2.34 (1.84; 2.96) [anti-D614G] and 1.39 (1.09; 1.77) [anti-B.1.351]. All booster formulations elicited cross-neutralizing antibodies against Omicron BA.2 (across priming vaccine subgroups), Omicron BA.1 (BNT162b2-primed participants) and Omicron BA.4/5 (BNT162b2-primed participants and MV D614-primed participants). Similar patterns in antibody responses were observed for participants aged ≥56 years. Reactogenicity tended to be transient and mild-to-moderate severity in all booster groups. No safety concerns were identified.
Interpretation
CoV2 preS dTM-AS03 boosters demonstrated acceptable safety and elicited robust neutralizing antibodies against multiple variants, regardless of priming vaccine.
Funding
Sanofi and Biomedical Advanced Research and Development Authority (BARDA).
Keywords: AS03 adjuvant, Beta, Booster, B.1.351, COVID-19, CoV2 preS dTM-AS03, Immunogenicity, Omicron, Recombinant protein vaccine, Safety, SARS-CoV-2
Research in context.
Evidence before this study
Given the constant threat of newly emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants or subvariants, COVID-19 vaccines that include strains that provide broad cross-protection against emergent variants may offer improved protection. We searched PubMed from database inception up to 05 January 2022, with no language restrictions, for studies reporting the safety and immunogenicity of protein-based vaccine candidates used as boosters against SARS-CoV-2 using the search terms ‘COVID-19 OR SARS-CoV-2’, ‘vaccine AND protein’, ‘adjuvant’ and ‘booster’, limiting the results to clinical trial data. Among the published trials retrieved, booster doses of recombinant S protein vaccines NVXCoV2373 (Novavax; phase 2 study), COVAX-19 (Cinnagen; phase 3 study) and MVC-COV1901 (phase 1 study), all of which are based on the ancestral SARS-CoV-2 S protein, were shown to elicit antibody responses against ancestral vaccine strain and SARS-CoV-2 variants, including Alpha, Beta, Delta, and Omicron (BA.1 and/or BA.2).
The current phase 3 study (ClinicalTrials.gov NCT04762680) evaluated neutralizing antibody responses elicited by three booster formulations of a SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant (CoV2 preS dTM-AS03) containing the ancestral D614 or variant B.1.351 S protein (monovalent [MV] D614 or MV B.1.351) or both (bivalent [BiV] D614 + B.1.351) in adults who were previously primed with mRNA (BNT162b2, mRNA-1273), adenovirus-vectored (Ad26.CoV2.S, ChAdOx1nCoV-19) or protein (MV D614) vaccines.
Added value of this study
In our study, all three CoV2 preS dTM-AS03 booster formulations demonstrated acceptable safety and elicited robust neutralizing antibodies against multiple variants, in young and older adults previously primed with mRNA and adenovirus-vectored vaccines. The cross-neutralization of Omicron subvariants with a Beta-variant containing vaccine, with greater neutralizing antibody responses against both BA.2 and BA.4/5 after boosting with MV B.1.351 than after the BiV or MV D614 formulations, is consistent with initial findings from the independently conducted COVIBOOST phase 3 trial, in which the MV B.1.351 booster achieved higher neutralizing antibody titers against evaluated variants than either MV D614 or a third dose of BNT162b2, when administered to adults previously vaccinated with two doses of BNT162b2.
Implications of all the available evidence
These data support the use of Beta-containing CoV2 preS dTM-AS03 boosters in individuals primed with globally deployed vaccines to provide broad immunogenicity against SARS-CoV-2 variants, including Omicron BA.4/5 subvariant, which was circulating at time of study conduct.
Introduction
The continued emergence of new variants of concern (VoC) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), together with waning antibody-mediated immunity and protection against mild and moderately symptomatic infection, underscore the need for new and updated booster vaccines to enhance and broaden protection.1, 2, 3, 4, 5, 6 Bivalent boosters, targeting the prototype virus and Omicron BA.1 or BA.4 and BA.5 subvariants have recently been authorized for use in the EU, USA and the UK.7, 8, 9, 10 However, while these were designed to include the predominant circulating strain at the time, new subvariants have continued to emerge and to replace the predominant strain.11 Thus an alternative approach is to include strains that provide broad cross-protection against emergent variants.12
Sanofi and GSK have developed a SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant (CoV2 preS dTM-AS03) using a baculovirus vector system to express stabilized SARS-CoV-2 pre-fusion S protein.13 In parallel with development of a D614 strain containing booster and primary series vaccine,14, 15, 16 we developed a CoV2 preS dTM-AS03 booster formulation containing 5 μg of B.1.351 variant S protein (monovalent [MV] B.1.351) and a formulation containing 2.5 μg of D614 plus 2.5 μg of B.1.351 variants (bivalent [BiV] D614 + B.1.351). Here, we present interim safety and immunogenicity data for MV D614, MV B.1.351 and BiV D614 + B.1.351 in participants who were previously primed with authorized COVID-19 mRNA or adenovirus-vectored vaccines, or with MV D614 (5, 10 or 15 μg of D614 antigen).
Based on data from the current study and an independently conducted phase 3 trial showing higher neutralizing antibody titers induced by MV B.1.351 booster than either MV D614 or a third dose of BNT162b2 in adults previously vaccinated with two doses of BNT162b2,17 the MV B.1.351 formulation has been authorized for booster vaccination by the European Medicines Agency (EMA) and the UK's Medicines and Healthcare products Regulatory Agency (MHRA).18,19
Methods
Study design and participants
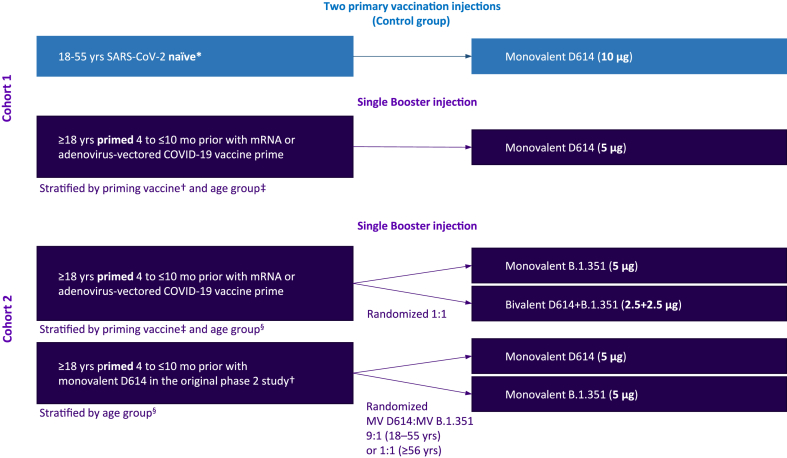
In an amendment to a phase 2 randomized, dose-finding study (NCT04762680),14 we added two supplemental cohorts (cohorts 1 and 2), to be enrolled independently and sequentially, for phase 3 evaluation of the safety and immunogenicity of MV D614, MV B.1.351 and BiV D614 + B.1.351 booster formulations (Fig. 1). We firstly enrolled adults (≥18 years) who were previously vaccinated with approved mRNA or adenovirus-vectored vaccines into cohort 1 (between 29 July 2021 and 22 October 2021) to evaluate the MV D614 booster candidate, which was available at that time. The variant-containing vaccine candidates became available later and were evaluated in cohort 2, with enrolment between 15 November 2021 and 22 February 2022. In cohort 2, we evaluated: (i) MV B.1.351 or BiV D614 + B.1.351 as a booster following primary vaccination with an approved mRNA or adenovirus-vectored vaccine and (ii) MV D614 or MV B.1.351 as boosters following primary vaccination 4–10 months earlier with two doses of MV D614 in the original phase 2 study (Fig. 1). For both booster cohorts, adults from France, the USA, the UK, Australia and Spain, who had completed a primary series of an authorized or approved D614 mRNA vaccine (2 injections of BNT162b2 [30 μg; Pfizer/BioNTech] or mRNA-1273 [100 μg; Moderna]) or adenovirus-vectored vaccine (2 injections of ChAdOx1 nCoV-19 [5 × 1010 vp; Oxford University/AstraZeneca] or 1 injection of Ad26.CoV2.S [5 × 1010 vp; J&J/Janssen]) at least 4 months and no longer than 10 months prior to enrolment, were included. Cohort 2 additionally included a subgroup of adults from the USA and Honduras who were primed 4–10 months earlier with two injections of MV D614 in the original phase 2 study. Unvaccinated adults, with a target age range of 18–55 years, who tested negative for antibodies to SARS-CoV-2 with a rapid diagnostic test (COVID-19 IgG and IgM Rapid Test Cassette; Healgen Scientific, Houston, TX, USA), were enrolled from the USA and Australia into a parallel, non-randomized control group. Enrolment into the control group was contemporaneous with cohort 1 (29 July–22 October 2021). Participants were stratified by priming vaccine (booster groups only) and by age category (18–55 years; ≥56 years). Individuals with pre-existing medical conditions, those who were immunocompromised (except those with organ transplant in the past 180 days, chemotherapy in the past 90 days or with HIV and CD4 counts <200/mm3) and those with a potentially increased risk for severe COVID-1920 were eligible for inclusion. Exclusion criteria for both cohorts and controls are described in full in the Appendix. Vaccine administration was open-label in cohort 1 booster and control groups and was modified double-blind (observer-blinded) in cohort 2.
Fig. 1.
Study design. Footnote: ∗Naïve or non-naïve status (control group) was determined based on serological detection of anti-S antibodies on study day 1 and detection of SARS-CoV-2 nucleic acid in nasopharyngeal swabs on days 1 and 22. Participants were categorized as naïve if both tests showed negative results on day 1 and day 22. †Participants were primed with two injections of a monovalent CoV2 preS dTM-AS03 D614 formulation containing 5, 10 or 15 μg antigen as part of the original phase 2 study. ‡mRNA COVID19 priming vaccines: BNT162b2 or mRNA-1273; adenovirus-vectored COVID19-priming vaccine: ChAdOx1nCoV-19 or Ad26.CoV2.S. §Age groups: 18–55 years and ≥56 years mo, months; MV, monovalent.
Here, we report interim immunogenicity data up to 14 days after last vaccination in all participants and safety data up to cut-off dates 18 February 2022 (cohort 1) and 13 May 2022 (cohort 2 and control group).
Ethics
The study was undertaken in compliance with the International Conference on Harmonization guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The protocol and amendments were approved by applicable Independent Ethics Committees/Institutional Review Boards (listed in the Appendix) and regulatory agencies as per local regulations; ethical approval was received for each site involved in the study. Written informed consent was obtained from all participants before any study procedures were performed.
Procedures
In cohort 1, all participants primed with an mRNA or adenovirus-vectored vaccine were offered a dose of MV D614. In cohort 2, participants primed with an mRNA or adenovirus-vectored vaccine were randomized 1:1, using interactive response technology, to receive MV B.1.351 or BiV D614 + B.1.351. Those in cohort 2 who were previously primed with MV D614 were randomized 9:1 (18–55-year-olds) or 1:1 (≥56-year-olds) to receive MV D614 or MV B.1.351, respectively. Participants in the control group received two injections, 21 days apart (D1 and D22), of MV D614 containing 10 μg of D614 antigen (Fig. 1).
MV D614 was described previously.13,14 Preparation of the booster formulations and intramuscular administration are described in the Appendix.
Immunogenicity
SARS-CoV-2 neutralizing antibody responses were measured using a lentivirus-based pseudovirus neutralization (PsVN) assay expressing the full-length S protein of the SARS-CoV-2 D614G, Beta (B.1.351) or Omicron BA.1, BA.2 or BA.4/5 variants (Monogram Biosciences LabCorp, South San Francisco, CA, USA; Appendix).21 The assay lower limit of quantification (LLOQ) was a PSVN titer of 40. For values less than the LLOQ, the computed value LLOQ/2 (20) was used. Primary immunogenicity endpoints were PsVN titers pre- (D1) and post-booster (D15) in BNT162b2-primed adults (18–55 years) in each booster group: anti-D614G titers for MV D614 booster, anti-B.1.351 titers for MV B.1.351 and both anti-D614G and anti-B.1.351 titers for the bivalent booster were measured. PsVN titers against D614G were measured at D36 for the control group. Secondary immunogenicity endpoints included the measurement of D614G and/or B.1.351 titers pre- and post-booster for participants who previously received primary vaccination with mRNA or adenovirus-vectored vaccines, or MV D614, in all booster groups. Other secondary endpoints included seroresponse rates at D15 (a four-fold or greater rise in PsVN titers relative to D1) and individual PsVN titer fold-rise at D15 (relative to D1) in all study groups. The study endpoints are detailed in full in the study protocol.
Safety
Safety was assessed for each treatment group and by age (18–55 or ≥56 years). Safety endpoints were described in the original phase 2 study,14 with the list of adverse events (AEs) of special interest (AESIs) revised per the Safety Platform for Emergency Vaccines updates.22,23 AEs were graded from grade 1 (mild) to 3 (severe; prevents normal daily activities) and assessed by the site principal investigator as related or unrelated to the study vaccine. Safety is being monitored for up to 12 months following last vaccination.
Statistics
Results are presented by booster vaccine candidate, overall and by priming vaccine group. Co-primary immunogenicity objectives were to demonstrate, for BNT162b2-primed participants 18–55 years of age: i) the non-inferiority of post-booster D614G PsVN responses following MVD614, post-booster B.1.351 PsVN responses following MV B.1.351 or both responses following BiV, compared to the D614G PsVN response elicited by primary vaccination in the control group; and ii) the superiority of the post-booster relative to pre-booster PsVN response. Statistical inference was based on the use of two-sided 98.3% CIs. Non-inferiority in terms of neutralizing antibody titers was concluded if the lower bound of the 98.3% CI for the between-group (booster versus control) GMT ratio was >0.67. Superiority in terms of neutralizing antibody titers was concluded if the lower limit of the 2-sided 98.3% CI of the geometric mean of the individual titer ratio (GMTR; post-booster versus pre-booster) was >2. Exploratory endpoints included individual serum PsVN titers against Omicron subvariants BA.1, BA.2 for each booster formulation in available samples in BNT162b2-primed participants (BA.1) or across all priming vaccine subgroups (BA.2). Individual serum PsVN titers against BA.4/5 were assessed for MV B.1.351 and BiV D614 + B.1.351 in available samples from BNT162b2-primed participants and for MV D614 and MV B.1351 in MV D614-primed participants. The study objectives assessed here are described in more detail in the Appendix, together with the sequential testing strategy and statistical analyses performed. A full list of all objectives for this study is available in the study protocol (link).
Immunogenicity was assessed in the per-protocol analysis set (PPAS) and safety in the safety analysis set (SafAS). In the control group, immunogenicity analyses were performed on SARS-CoV-2 naïve participants from the PPAS. Naïve or non-naïve status was determined based on serological detection of anti-S antibodies on D1 and detection of SARS-CoV-2 nucleic acid in nasopharyngeal swabs on D1 and D22 (Appendix).
For full details on statistical considerations, including planned sample sizes, see the Appendix.
Role of the funding source
Funding was provided by Sanofi and by federal funds from the Biomedical Advanced Research and Development Authority, part of the office of the Administration for Strategic Preparedness and Response at the U.S. Department of Health and Human Services under Contract # HHSO100201600005I, and in collaboration with the U.S. Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense under Contract #W15QKN-16-9-1002. The funders were involved in the study design, data collection, data analysis, data interpretation, writing of the report, and the decision to submit the paper for publication. GSK provided access to, and use of, the AS03 Adjuvant System.
Results
Participants and participant disposition
Booster doses were administered to 803 of 806 participants in the MV D614 group, 705 of 707 participants in the MV B.1.351 group and 621 of 625 in the BiV group. In the control group, 473 of 479 enrolled SARS-CoV-2-naïve participants received at least one primary dose of MV D614. A total of 18 MV D614 booster recipients, 34 MV B.1.351 booster recipients, 26 BiV recipients and 43 control group participants discontinued the study before the analysis cut-off (Figure S1). The median interval between last primary and booster doses was slightly shorter for MV D614 recipients (5.75 months) than for MV B.1.351 or BiV recipients (6.96 and 6.50 months, respectively) (Table S1a). Across the three booster groups (SafAS), the mean age (standard deviation [SD]) ranged from 43.7 (14.3) to 50.4 (15.0) years and the proportion of those aged ≥56 years old ranged from 22.2% to 37.5%. Control group participants had a mean age of 37.5 (11.2) years. The majority of participants were white and just over half had at least one high-risk medical condition (Table S1a), of which obesity was the most common (Table S2). The subgroup of MV D614-primed participants had a longer median primary to booster vaccination interval (8.2 months, in both the MV D614 and MV B.1.351 booster groups) than the other priming vaccine groups (Table S1b and S1c). MV D614-primed participants also tended to be older (mean age [SD]: 54.9 [14.8] years for MV D614-primed, MV D614-boosted participants; and 65.9 [11.5] years for MV D614-primed, MV B.1.351-boosted participants). Among booster groups in the PPAS, prior SARS-CoV-2 infection based on anti-nucleoprotein seropositivity at baseline was detected for 68/734 (9.3%) of MV D614 recipients, 138/615 (22.4%) of MV B.1.351 recipients and 124/561 (22.1%) of BiV recipients.
Immunogenicity
Monovalent D614 booster
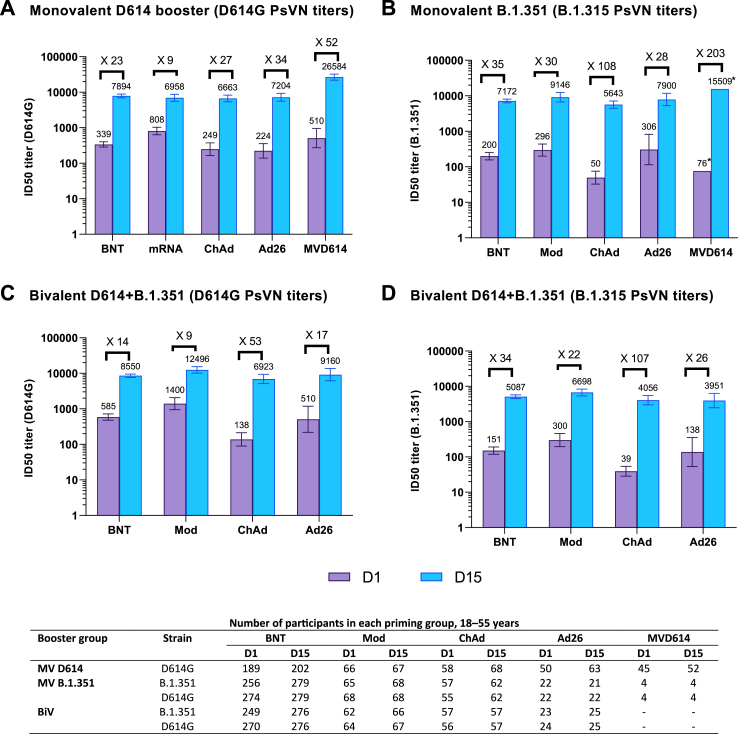
Among participants aged 18–55 years, the D614G PsVN GMTs (95% CI) for BNT162b2-primed participants increased from 339 (285; 402) at D1 to 7894 (6993; 8911) at D15 following MV D614, demonstrating superiority of the PsVN response post-booster compared to pre-booster (post/pre-booster ratio, 23.37 [98.3% CI: 18.58; 29.38]) and non-inferiority compared to primary vaccination with MV D614 in the control group (GMT ratio [post-booster/control]: 2.16 [98.3% CI: 1.69; 2.75]) to the control group. Thus, the co-primary objectives for the MV D614 group were met (Table 1A; see Table S3 for PsVN titers in the control group).
Table 1.
Non-inferiority and superiority of PsVN titers against D614G and B.1.351 following monovalent and bivalent boosters compared with MV D614 primary vaccination or compared with pre-booster PsVN titers–PPAS.
| A. Monovalent D614 booster group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Priming vaccine or vaccine platform subgroup | Booster D15 |
Control D36 |
Post-booster vs control |
Post-booster vs pre-booster |
|||||||
| Strain | M | Strain | M | GMT ratio | 98.3% CI | NIa | M | GMTR | 98.3% CI | Superiorityb | |
| BNT162b2 | D614G | 202 | D614G | 302 | 2.16 | 1.69; 2.75 | Yes | 189 | 23.37 | 18.58; 29.38 | Yes |
| mRNA vaccine platform | D614G | 269 | D614G | 302 | 2.09 | 1.66; 2.64 | Yes | 255 | 18.08 | 14.75; 22.16 | Yes |
| Pooled Ad virus vectored platform | D614G | 131 | D614G | 302 | 1.89 | 1.43; 2.49 | Yes | 108 | 30.18 | 20.56; 44.31 | Yes |
| MV D614 | D614G | 52 | D614G | 302 | 7.27 | 5.34; 9.89 | Yes | 45 | 51.63 | 22.29; 119.57 | Yes |
| B. Monovalent B.1.351 booster group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Priming vaccine or vaccine platform subgroup | Booster D15 |
Control D36 |
Post-booster vs control |
Post-booster vs pre-booster |
|||||||
| Strain | M | Strain | M | GMT ratio | 98.3% CI | NIa | M | GMTR | 98.3% CI | Superiorityb | |
| BNT162b2 | B.1.351 | 279 | D614G | 302 | 1.96 | 1.54; 2.50 | Yes | 256 | 35.41 | 26.71; 46.95 | Yes |
| B.1.351 | 279 | B.1.351 | 291 | 17.36 | 13.39; 22.50 | Yesc | – | – | – | – | |
| mRNA vaccine platform | B.1.351 | 347 | D614G | 302 | 2.06 | 1.62; 2.61 | Yes | 321 | 34.19 | 26.58; 43.98 | Yes |
| C. Bivalent D614+B.1.351 booster group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Priming vaccine or vaccine platform subgroup | Booster D15 |
Control D36 |
Post-booster vs control |
Post-booster vs pre-booster |
|||||||
| Strain | M | Strain | M | GMT ratio | 98.3% CI | NIa | M | GMTR | 98.3% CI | Superiorityb | |
| BNT162b2 | D614G | 276 | D614G | 302 | 2.34 | 1.84; 2.96 | Yes | 269 | 14.39 | 11.34; 18.28 | Yes |
| B.1.351 | 276 | D614G | 302 | 1.39 | 1.09; 1.77 | Yes | 248 | 34.18 | 25.84; 45.22 | Yes | |
| B.1.351 | 276 | B.1.351 | 291 | 12.31 | 9.50; 15.97 | Yesc | – | – | – | – | |
| mRNA vaccine platform | D614G | 343 | D614G | 302 | 2.52 | 2.00; 3.16 | Yes | 332 | 13.04 | 10.56; 16.11 | Yes |
| B.1.351 | 342 | D614G | 302 | 1.47 | 1.16; 1.85 | Yes | 309 | 31.19 | 24.33; 39.97 | Yes | |
D, study day; M, number of participants with available data for the analysis; NI, non-inferiority.
The two-sided 98.3% confidence intervals presented were adjusted for multiple testing using Bonferroni method to adjust the Type 1 error at 0.025 (one-sided) over the 3 comparisons (each booster candidate versus the control group).
Non-inferiority of a booster response against D614G or B.1.351 compared to a response against D614G in the control group was concluded if the lower bound of the 98.3% CI for the between-group (booster versus control) GMT ratio was >0.67.
Superiority of a booster response compared to a pre-booster response was concluded if the lower limit of the 2-sided 98.3% CI of the fold-rise (post-booster versus pre-booster) was >2.
Superiority, rather than non-inferiority, was demonstrated for this comparison (conditional secondary objective). The superiority of a booster dose response against B.1.351 of monovalent B.1.351 or bivalent D614+B.1.351 in BNT162b2 primed participants aged 18-55 years compared to a response against B.1.351 in the control group was concluded if the lower limit of the 2-sided 98.3% CI of the ratio of GMTs between groups was > 1.5.
Non-inferiority versus the control group was demonstrated for the pooled mRNA vaccine-primed group, the pooled adenovirus vectored vaccine-primed group and the MV D614-primed group (lower bound of the 98.3% CI for the between-group GMT ratio was >0.67; Table 1A). Superiority versus the pre-booster response was also demonstrated for all three vaccine priming groups (lower limit of the 2-sided 98.3% CI of post-booster versus pre-booster GMTR was >2; Table 1A). The magnitude of D614G PsVN titers at D15 following MV D614 booster in 18–55-year-olds was similar in the mRNA- and adenoviral-vectored priming groups but higher in the group primed with MV D614 (Fig. 2A; Table S4). Seroresponse rates (95% CI) to D614G ranged from 71.2% (58.7; 81.7) to 93.1% (88.5; 96.3) across priming vaccine subgroups following MV D614 booster and was 99.0% (on D36) in the control group among 18–55-year-olds (Tables S3 and S4).
Fig. 2.
Booster pseudovirus neutralizing antibody responses against D614G or B.1.351 strains for (A) monovalent D614 (B) monovalent B.1.351 and (C and D) bivalent D614 + B.1.351 among 18–55-year-olds, by prior priming vaccine – PPAS. Antibody levels were measured using a lentivirus-based pseudovirus neutralization assay. Graphs are annotated with geometric mean titers (above each bar) and geometric means of individual titer ratios (above grouped bars), calculated based on paired post-vaccination/pre-vaccination results. Error bars denote 95% CIs for the GMTs. ∗95% confidence not calculated due to small number of participants analyzed (n = 4). Table shows number of participants with available data for at each timepoint. D1, study day 1 (pre-booster); D15, study day 15 (14 days post-booster); BNT, BNT162b2-primed; Mod, mRNA-1273-primed; ChAd, ChAdOx1nCoV-19-primed; Ad26, Ad26.CoV2.S-primed; MVD614, CoV2 preS dTM-AS03 monovalent (D614)-primed.
A similar pattern in D614G PsVN titers was observed in older adults (≥56 years) (Figure S2). While post-booster GMTs in older adults tended to be of lower magnitude than those in younger adults, they were still numerically higher than D36 GMTs post-primary vaccination in the control group, for all vaccine-primed subgroups (Table S4).
Monovalent B.1.351 booster
In BNT162b2-primed participants aged 18–55 years, B.1.351 PsVN GMTs (95% CI) increased from 200 (157; 254) at D1 to 7172 (6363; 8083) at D15 after MV B.1.351 (Fig. 2B), demonstrating superiority to pre-booster titers (Table 1B). B.1.351 PsVN titers post-booster were non-inferior to D614G PsVN titers post-primary vaccination and superior to B.1.351 PsVN titers post-primary vaccination (Table 1B).
.Among participants aged 18–55 years, B.1.351 PsVN GMTs (95% CI) increased post-booster with MV B.1.351, with D15/D1 GMTRs (95% CIs) ranging from 28 to 108 across the four mRNA and adenovirus-vectored priming vaccine subgroups (Table S5). Post-booster D15 GMTs were 1.5- to 2.5-fold higher than D614G PsVN GMTs following primary vaccination in the control group. PsVN GMTs (95% CI) against D614G also increased in the MV B.1.351 booster group (18–55 years) across mRNA or adenovirus-vectored priming vaccine subgroups from between 165 (105; 258) and 1331 (917; 1931) at D1 to between 6817 (5453; 8521) and 13,189 (9836; 17,684) at D15. Seroresponse rates (95% CI) to B.1.351 ranged from 71.4% (47.8; 88.7) to 96.5% (87.9; 99.6) across priming vaccine subgroups (Table S5).
PsVN GMTs against B.1.351 and D614G following MV B.1.351 booster also increased among older adults (≥56 years), with D15 GMTs in all vaccine priming subgroups exceeding those following primary vaccination in the control group (Figure S2; Table S5). D15 GMTs in older adults tended to be lower than those in younger participants in all but the mRNA-1273-primed group. Notably, the increase in post-booster PsVN titers following MV B.1.351 booster was particularly marked for MV D614-primed participants (≥56 years; N = 68), with PsVN GMTs (95% CI) rising to 24,407 (17,705; 33,647) (anti-D614G) and 13,180 (9571; 18,151) (anti-B.1.351). This marked increase in titers was also seen in the 18–55 years age category for a small number (n = 4) of participants (Table S5).
Bivalent D614 + B.1.351 booster
Among BNT162b2-primed participants aged 18–55 years, PsVN GMTs (95% CI) to D614G increased from 585 (477; 718) at D1 to 8550 (7638; 9571) at D15 and PsVN GMTs to B.1.351 increased from 151 (119; 191) at D1 to 5087 (4511; 5737) at D15 following BiV booster, demonstrating superiority of D614G and B.1.351 booster responses at D15 compared to pre-booster (Fig. 2C and D; Table 1C). The non-inferiority of PsVN titers against D614G and B.1.351 following BiV compared to post-primary D614G PsVN titers in the control group was also demonstrated, as was the superiority of B.1.351 PsVN titers induced by BiV compared with B.1.351 PsVN titers in the control group (Table 1C).
Among 18–55-year-olds, D614G and B.1.351 PsVN GMTs following BiV increased substantially across all vaccine priming subgroups, with GMTRs (D15/D1) ranging 9–53 for anti-D614G and 21–107 for anti-B.1.351 (Fig. 2C and D). Seroresponse rates (95% CI) to D614G ranged from 61.9% (48.8; 73.9) to 89.1% (77.8; 95.9) and those to B.1.351 ranged from 78.3% (56.3; 92.5) to 96.4% (87.7; 99.6) across subgroups (Table S6).
Increases in neutralizing antibody titers (against B.1.351 and against D614G) following BiV were also seen among older adults (≥56 years) (D15 GMTs: 2025–10035 [B.1.351]; 4492–22531 [D614G] across priming vaccine subgroups), with similar or lower PsVN titers compared to younger adults in all but the Ad26.CoV2.S-primed subgroup, in which GMTs were higher in older adults (Table S6).
Neutralizing antibody responses to Omicron subvariant strains (exploratory analysis)
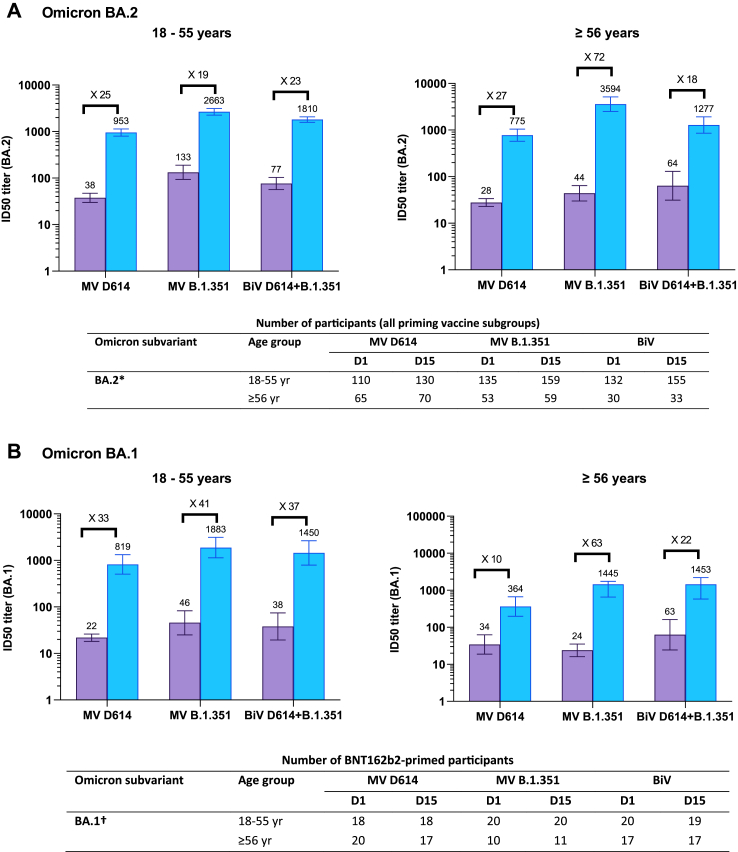
In a subset of participants aged 18–55 years from the PPAS (MV D614, N = 132; MV B.1.351, N = 160; BiV, N = 156), baseline BA.2 PsVN titers were higher in the MV B.1.351 and BiV groups than in the MV D614 group, with the highest titers induced by MV B.1.351 (2663 [95% CI: 2260; 3137]) (Fig. 3A). BA.2 PsVN GMTRs (D15/D1) ranged from 19 (for MV B.1.351) to 25 (for MV D614) across the three booster groups. Similar results were obtained for booster responses against the Omicron BA.1 subvariant in BNT162b2-primed participants (Fig. 3B).
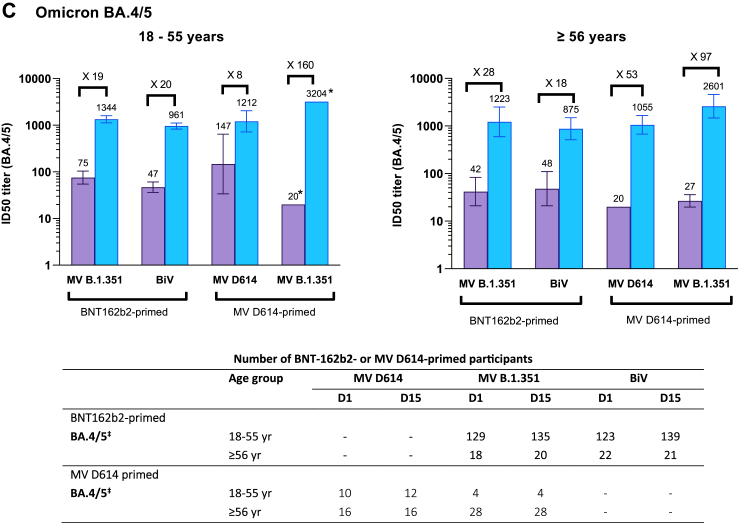
Fig. 3.
Cross-neutralizing titers elicited by monovalent and bivalent CoV2 preS dTM-AS03 formulations against Omicron subvariants (A) BA.2 (B) BA.1 and (C) BA.4/5, by age category. BiV, bivalent; MV, monovalent. Antibody levels were measured using a lentivirus-based pseudovirus neutralization assay. Graphs are annotated with geometric mean titers (above each bar) and geometric means of individual titer ratios (above grouped bars), calculated based on paired post-vaccination/pre-vaccination results. Error bars denote 95% CIs for the GMTs, calculated using normal approximation of log-transformed titers. Tables show number of participants with available data at each timepoint. ∗Assessed on available samples across all vaccine priming vaccine subgroups; †Assessed on available samples in BNT162b2-primed subgroups; ‡Assessed on available samples in BNT162b2-primed subgroups and in CoV2 preS dTM-AS03 primed subgroups.
In BNT162b2-primed participants aged 18–55 years, PSVN titers against BA.4/5 increased from a GMTs (95% CI) of 75.2 (54.7; 104) at D1 to 1344 (1125; 1604) at D15 after a MV B.1.351 booster and from 46.9 (36.2; 60.8) to 961 (828; 1115) after BiV D614 + B.1351 (Fig. 3C). Similar magnitudes of titers were observed for older adults (aged ≥56 years) following MV B.1.351. Among 18–55-year-old participants primed and boosted with MV D614, anti-BA.4/5 GMTs were slightly higher at baseline (147 [33.8; 643]) than in the other groups and increased to 1212 (720; 2041) at D15 following booster. MV B.1.351 induced a large increase in anti-BA.4/5 titers, reaching a GMT of 3204 (95% CI not calculated) at D15, albeit for only four available participant samples in the 18–55 years age group. Large increases in BA.4/5 GMTs, with GMTRs of 53 and 97, were also achieved after MV D614 and MV B.1.351 booster, respectively, among older adults (Fig. 3C).
Safety
In each booster group, ≥98.0% of participants completed at least 2 months of safety follow-up by the analysis cut-off dates (median duration of safety follow-up, 144–150 days across booster groups). In the control group, 70.9% completed ≥2 months safety follow-up (median duration, 148 days).
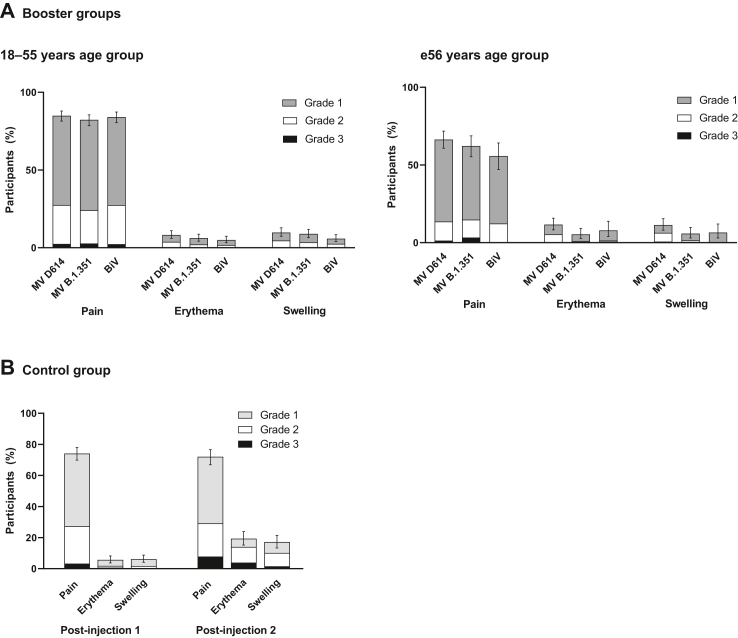
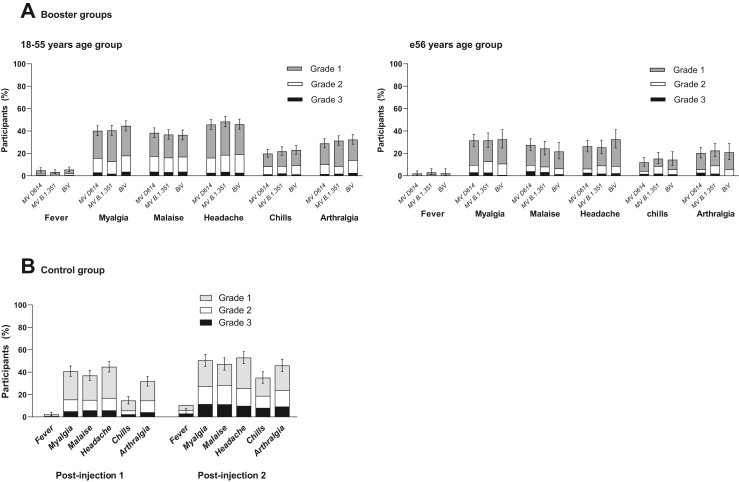
AEs for each booster formulation are summarized in Table S7. There were no immediate unsolicited AEs reported in any booster group. Within 7 days post-booster, grade 3 solicited injection reactions were reported by 2.5% participants in the MV D614 group, 3.3% in the MV B.1.351 group and 1.9% in the BiV group (Fig. 4); grade 3 solicited systemic reactions were reported by 6.6%, 6.9% and 6.3%, respectively (Fig. 5). Most solicited reactions were transient (lasting 1–3 days) and self-limited; all participants fully recovered. Within 21 days post-booster, 7.1–8.8% participants reported at least one unsolicited adverse reaction. Across priming vaccine subgroups, solicited systemic reactions tended to be more frequently reported for MV D614-primed participants than for other priming vaccines (Figure S3). Solicited and unsolicited events were mostly reactogenicity-type events and generally more common in the 18–55 years than the ≥56 years group, in both booster cohorts (Table S7).
Fig. 4.
Solicited injection site reactions up to 7 days following vaccination in CoV2 preS dTM-AS03 booster groups and the control group, by age category – SafAS. The percentages of participants experiencing at least one of the specified reactions are shown. Error bars denote 95% CIs for the percentages after any injection, calculated using the Clopper-Pearson method. MV, monovalent; BiV, bivalent.
Fig. 5.
Solicited systemic reactions up to 7 days following vaccination in CoV2 preS dTM-AS03 booster groups and the control group, by age category – SafAS. The percentages of participants experiencing at least one of the specified reactions are shown. Error bars denote 95% CIs for the percentages after any injection, calculated using the Clopper-Pearson method. MV, monovalent; BiV, bivalent.
One of 28 SAEs reported up to the analysis cut-off date was assessed as related to the study vaccine (serum sickness-like reaction in a participant primed with mRNA-1273 and boosted with bivalent D614 + B.1.351), which resolved 11 days after onset following treatment (antihistamines and steroid cream). One participant aged ≥56 years, primed with MV D614 and boosted with MV B.1.351, reported an AESI (trigeminal neuralgia), assessed as unrelated to vaccination. Of 356 MAAEs reported by 264 booster recipients, eight (2 in 2 participants in the MV D614 booster group, 5 in 3 participants in the MV B.1.351 group and 1 in 1 participant in the BiV group) were assessed as related. All MAAEs resolved. No deaths and no AEs leading to study discontinuation were reported. No cases of unintentional exposure during pregnancy were reported in any booster group.
The safety profile of the control group is described in the Appendix and summarized in Table S8. Solicited injection site and systemic reactions are shown in Fig. 4, Fig. 5, respectively.
Discussion
In this study, boosters containing the prototype D614 and/or Beta (B.1.351) variants MV D614, MV B.1.351 and BiV were well tolerated and elicited robust neutralizing antibody responses against variants including Omicron in younger and older adults, regardless of the vaccine platform used for primary vaccination.
The cross-neutralization of Omicron subvariants with the monovalent B.1.351- containing vaccine, whereby neutralizing antibody levels against BA.2 and BA.4/5 tended to be higher than after either of the D614-containing formulations, is consistent with initial findings from an independently conducted phase 3 trial in adults previously vaccinated with two doses of BNT162b2, in which the monovalent B.1.351-containing booster achieved higher neutralizing antibody titers against Beta, Delta and Omicron BA.1 variants than the monovalent D614 booster or a third dose of BNT162b2 in.17 Notably, data from that previous study, together with those from the current study, led to authorization of the monovalent B.1.351 formulation for use as a booster in Europe and the UK.
Although rare, higher than expected frequencies of myocarditis and pericarditis have been reported following receipt of COVID-19 mRNA vaccines24 or the protein-based vaccine NVX-CoV2373,25,26 and an increased risk of thrombosis with thrombocytopenia following receipt of adenovirus-vectored vaccines.27 While we did not identify any safety concerns in the current study, this will continue to be monitored in larger sample sizes. Reactogenicity in all booster groups tended to be transient and mild-to-moderate in severity. The frequency of reactogenicity events (any grade or grade 3) in all booster groups appeared to be similar to or lower than after licensed COVID-19 boosters (BNT162b2, mRNA-1273, NVX-CoV2373).28, 29, 30
This study benefits from a large global study population, inclusive of high-risk groups, closely representing the potential target populations. The inclusion of participants previously primed with different vaccine platforms (mRNA, adenovirus-vectored and adjuvant recombinant protein vaccines), including globally deployed vaccines, allowed a comprehensive analysis of prime-boosting options and make our findings applicable to a broad population. While our immunogenicity results are promising, further evaluation on the durability of the responses observed in this study will be needed, along with effectiveness against disease outcomes. Some limitations should also be noted. The later enrolment of cohort 2 will have increased the likelihood of the exposure of participants to prior infection in this cohort compared to cohort 1. Given the separation of cohort enrolment in time, comparisons between the cohorts need to be done with caution. Additionally, due to the limited number of participants anticipated to be available from the CoV2 preS dTM-AS03-primed arms and the need to support the then-current regulatory strategy, it was decided not to randomise the CoV2 preS dTM-AS03 D614-primed group to the bivalent booster to focus on the evaluation of the MV D614 and MV B.1.351 vaccines. The inclusion of participants who were immunocompromised in this study may have affected the observed responses. The variability in the interval between last primary vaccine dose and booster may also have affected the antibody responses to booster vaccination. Indeed, recent BNT162b2 booster vaccination data suggest that a longer interval (35 weeks or more) may increase vaccine effectiveness,3 and previous data have suggested that extended intervals between doses in a primary series of COVID-19 vaccination result in higher neutralizing antibody titers.31 Additionally, while the control group was age-matched for the 18–55 years age category, they were not age-matched for older participants. We also acknowledge that our data could be extended to assess the more recent circulating BQ.1.1, BA.2.75 and XBB Omicron subvariants.
In summary, B.1.351-containing CoV2 preS dTM-AS03 boosters in individuals primed with globally deployed vaccines elicited strong cross-neutralizing antibody responses against SARS-CoV-2 variants, including Omicron subvariants BA.2 and BA.4/5, and had acceptable safety profiles. Further humoral and cellular analysis may support the deployment of MV B.1.351 as a universal booster against COVID-19.
Contributors
GdB, MAC, FTDS, BF, MHG, OH, MK, NLM, RMC, NR, LS, SG, SSa and SSr contributed to the concept or design of the study and data analysis and interpretation; MIB and RM contributed to the conception or design of the study and data acquisition; MSR, HA, SA, DB, AB, RC, SD, DD, AF, RF, JG, PG, DH, OL, JAH, FMT, JP, DMRM, CR, LDS and AW contributed to data acquisition; AP and JW were involved in the analysis and interpretation of the data. All authors were involved in drafting or critically revising the manuscript, and all authors approved the final version and are accountable for the accuracy and integrity of the manuscript. All authors had full access to all the data and accept responsibility to submit for publication. JW, AP and JG have accessed and verified the underlying data reported in this manuscript.
Data sharing statement
Qualified researchers can request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
Declaration of interests
GdB, JW, AP, MSR, HA, MIB, RMCa, RF, BF, JG, M-HG, SG, OH, RM, JP, NR, RMCh and SSr are Sanofi employees. GdB, AP, MIB, BF, OH, NR, RMCh and SSr hold stock or stock options in Sanofi. SSa was a Sanofi employee at the time of study conduct and held shares and/or stock options in the company at the time of study conduct. GdB, SSr, RMCh, and SSa are inventors on a pending patent application filed by Sanofi and GSK for the development of the CoV-2 dTM vaccine. MAC, LS and MK are employed by GSK and hold restricted shares in the GSK group of companies. FTDS was employed by GSK and held restricted shares in the GSK group of companies, at the time of the study. FMT declares trial fees paid to their institution by Sanofi; honoraria received from GSK group of companies, Pfizer Inc., Sanofi, MSD, Moderna, Biofabri, AstraZeneca, Novavax, Janssen; meeting and/or travel fees received from Pfizer Inc, MSD, GSK and Sanofi; data safety monitoring board or advisory board participation for Pfizer and Biofabri; being a member of ETAGE–WHO Europe, coordinator of the Spanish Pediatric Critical Trials Network and coordinator of the WHO Collaborating Centre for Vaccines Safety of Santiago de Compostela; and payments made to their institution for their role as principal investigator in randomized controlled trials for Ablynx, Abbot, Seqirus, Sanofi, MSD, Merck, Pfizer, Roche, Regeneron, Janssen, Medimmune, Novavax, Novartis and GSK. DMRM declares that her institution received funding from Sanofi. AF receives research funding, paid to his employers, from Sanofi both for work related to this study and other unrelated vaccine trials and from GSK for other unrelated studies. He receives research funding from other vaccine manufacturers relating to trials and studies and undertakes paid consultancy related to a number of developmental antimicrobial drugs and vaccines. OL declares that their institution received funding for conducting the trial. NLM received travel support from Sanofi and chaired a Scientific Advisory Board for them, unrelated to current study. LDS received a research grant from Sanofi. SD, SA, DB, AB, DD, PG, JAH, CR and AW declare no conflicts of interest.
Acknowledgements
The authors thank all participants, investigators, and study site personnel who took part in this study. The authors acknowledge Juliette Gray of inScience Communications, Springer Healthcare Ltd, London, UK, for providing editorial assistance with the preparation of this manuscript, funded by Sanofi. The authors also thank Hanson Geevarghese for providing manuscript coordination on behalf of Sanofi. This work was done in collaboration with GSK, who provided access to, and use of, the AS03 Adjuvant System.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102109.
Contributor Information
Guy de Bruyn, Email: guy.debruyn@sanofi.com.
VAT00002 booster cohorts study team:
Guy de Bruyn, Joyce Wang, Annie Purvis, Martin Sanchez Ruiz, Haritha Adhikarla, Saad Alvi, Matthew I. Bonaparte, Daniel Brune, Agustin Bueso, Richard M. Canter, Maria Angeles Ceregido, Sachin Deshmukh, David Diemert, Adam Finn, Remi Forrat, Bo Fu, Julie Gallais, Paul Griffin, Marie-Helene Grillet, Owen Haney, Jeffrey A. Henderson, Marguerite Koutsoukos, Odile Launay, Federico Martinon Torres, Roger Masotti, Nelson L. Michael, Juliana Park, Doris Maribel Rivera-Medina, Natalya Romanyak, Chris Rook, Lode Schuerman, Lawrence D. Sher, Fernanda Tavares-Da-Silva, Ashley Whittington, Roman M. Chicz, Sanjay Gurunathan, Stephen Savarino, Saranya Sridhar, Allaw Mohammed, Babin Valérie, Babyak Jennifer, Ines Ben-Ghezala, Thomas Breuer, Corinne Breymeier, Anne Conrad, Ciarrah Holmqvist, Cristiana Costa-Araujo, Florence Coux, Christine Dellanno, Bertrand Dussol, Brandon Essink, Jesús Garrido, Pierre-Olivier Girodet, Claudia Gonzalez, Marie-Ange Grosbois, Justin Hammond, Chelsea He, Ciarrah Homlqvist, Kathy Hudzina, Mark Hutchens, Peta-Gay Jackson Booth, Arnel Joaquin, Rama Kandasamy, Jennifer Kasztejna, Michael Keefer, Murray Kimmel, Matthew Kresge, Fabrice Laine, Maeva Lefebvre, Denise Lopez, Malaborbor Perpetua Lourdes, Zoha Maakaroun-Vermesse, Caitlin Malishchak, Lisa Menard, Sandra Mendoza, Patrick Moore, Mounika Mulamalla, Patrick Mulholland, Jean-Francois Nicolas, Onyema Ogbuagu, Juan Ortiz, Ana Paula Perroud, Gina Peyton, Ya-Fen Purvis, Vanessa Raabe, Enrique Rivas, Nadine Rouphael, Beatrice Roy, Lola Sagot, Nessryne Sater, Howard Schwartz, Randall Severance, Jiayuan Shi, Magdalena Sobieszczyk, Charlene Stevens, Tran Phuong Thuy, Ramy Toma, Tina Tong, Sophie Tourneux, John Treanor, Núria Turet, Rachel Froget, Stephen Walsh, Judith White, Victor del Campo Perez, Lina Perez Breva, Pablo Rojo Conejo, Maria Belen Ruiz Antoraz, Toong Chin, Charlotte Fribbens, Adrian Phillipson, Rachel Kaminski, Stevan Emmett, Corey Hebert, Thomas Birch, Russell Roberson, Jeffrey Zacher, Sophie Gelu-Maury, Loron Loryne, and Yvonne Davis
Appendix A. Supplementary data
References
- 1.World Health Organization WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ Available at:
- 2.World Health Organization Interim statement on decision-making considerations for the use of variant updated COVID-19 vaccines. 2022. https://www.who.int/news/item/17-06-2022-interim-statement-on-decision-making-considerations-for-the-use-of-variant-updated-covid-19-vaccines Available at:
- 3.Andrews N., Stowe J., Kirsebom F., et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28:831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386(18):1712–1720. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferdinands J.M., Rao S., Dixon B.E., et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION network, 10 states, august 2021-january 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menni C., May A., Polidori L., et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022;22(7):1002–1010. doi: 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency First adapted COVID-19 booster vaccines recommended for approval in the EU. https://www.ema.europa.eu/en/news/first-adapted-covid-19-booster-vaccines-recommended-approval-eu Available at:
- 8.U.S. Food & Drug Administration Coronavirus (COVID-19) update: FDA authorizes Moderna, pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use Available at:
- 9.UK Medicines and Healthcare Products Regulatory Agency Press release: first bivalent COVID-19 booster vaccine approved by UK medicines regulator. https://www.gov.uk/government/news/first-bivalent-covid-19-booster-vaccine-approved-by-uk-medicines-regulator Available at:
- 10.UK Medicines and Healthcare Products Regulatory Agency. Press release: Pfizer/BioNTech bivalent COVID-19 booster approved by UK medicines regulator. https://www.gov.uk/government/news/pfizerbiontech-bivalent-covid-19-booster-approved-by-uk-medicines-regulator Available at:
- 11.Wang Q., Iketani S., Li Z., et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186(2):279–286.e8. doi: 10.1016/j.cell.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sridhar S., Chicz R.M., Warren W., et al. The potential of Beta variant containing COVID booster vaccines for chasing Omicron in 2022. Nat Commun. 2022;13(1):5794. doi: 10.1038/s41467-022-33549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goepfert P.A., Fu B., Chabanon A.L., et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. Lancet Infect Dis. 2021;21(9):1257–1270. doi: 10.1016/S1473-3099(21)00147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sridhar S., Joaquin A., Bonaparte M.I., et al. Safety and immunogenicity of an AS03-adjuvanted SARS-CoV-2 recombinant protein vaccine (CoV2 preS dTM) in healthy adults: interim findings from a phase 2, randomised, dose-finding, multicentre study. Lancet Infect Dis. 2022;22(5):636–648. doi: 10.1016/S1473-3099(21)00764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbett K.S., Gagne M., Wagner D.A., et al. Protection against SARS-CoV-2 Beta variant in mRNA-1273 vaccine-boosted nonhuman primates. Science. 2021;374(6573):1343–1353. doi: 10.1126/science.abl8912. [DOI] [PubMed] [Google Scholar]
- 16.Pavot V., Berry C., Kishko M., et al. Protein-based SARS-CoV-2 spike vaccine booster increases cross-neutralization against SARS-CoV-2 variants of concern in non-human primates. Nat Commun. 2022;13(1):1699. doi: 10.1038/s41467-022-29219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launay O., Cachanado M., Luong Nguyen L.B., et al. Immunogenicity and safety of beta-adjuvanted recombinant booster vaccine. N Engl J Med. 2022;387(4):374–376. doi: 10.1056/NEJMc2206711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Medicines Agency EMA recommends approval of VidPrevtyn Beta as a COVID 19 booster vaccine. New 10/11/2022. 2022. https://www.ema.europa.eu/en/news/ema-recommends-approval-vidprevtyn-beta-covid-19-booster-vaccine Available at:
- 19.Medicines and Healthcare products regulatory agency. Sanofi pasteur COVID-19 vaccine authorised by MHRA. 2022. https://www.gov.uk/government/news/sanofi-pasteur-covid-19-vaccine-authorised-by-mhra Available at:
- 20.Centers for disease control and prevention Underlying medical conditions associated with high risk for severe COVID-19: information for Healthcare providers. 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html Available at: [PubMed]
- 21.Petropoulos C.J., Parkin N.T., Limoli K.L., et al. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2000;44(4):920–928. doi: 10.1128/aac.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavares Da Silva F., De Keyser F., Lambert P.-H., Robinson W.H., Westhovens R., Sindic C. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine. 2013;31(14):1870–1876. doi: 10.1016/j.vaccine.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Law B. Safety platform for emergency vaccines. SO2-D2.1.2 priority list of COVID-19 adverse events of special interest: quarterly update december 2020. The coalition for epidemic preparedness innovations (CEPI) and the brighton collaboration; december 23, 2020. 2020. https://brightoncollaboration.us/wp-content/uploads/2021/01/SO2_D2.1.2_V1.2_COVID-19_AESI-update-23Dec2020-review_final.pdf Available at:
- 24.Gargano J.W., Wallace M., Hadler S.C., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States, june 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Health and aged care AG. Nuvaxovid (Novavax) https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/approved-vaccines/novavax Available at:
- 26.Twentyman E., Wallace M., Roper L.E., et al. Interim recommendation of the advisory committee on immunization practices for use of the Novavax COVID-19 vaccine in persons aged ≥18 years - United States, july 2022. MMWR Morb Mortal Wkly Rep. 2022;71(31):988–992. doi: 10.15585/mmwr.mm7131a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Burn E., Duarte-Salles T., et al. Comparative risk of thrombosis with thrombocytopenia syndrome or thromboembolic events associated with different covid-19 vaccines: international network cohort study from five European countries and the US. BMJ. 2022;379 doi: 10.1136/bmj-2022-071594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi A., Koch M., Wu K., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallory R.M., Formica N., Pfeiffer S., et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2022;22(11):1565–1576. doi: 10.1016/S1473-3099(22)00420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration Application for licensure of a booster dose for COMINARTY (COVID-19 vaccine, mRNA). BNT162b2: evaluation of a booster dose (third dose) 2021. https://www.fda.gov/media/152176/download
- 31.Martinez D.R., Ooi E.E. A potential silver lining of delaying the second dose. Nat Immunol. 2022;23(3):349–351. doi: 10.1038/s41590-022-01143-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.