Abstract
Background
Repetitive transcranial magnetic stimulation (rTMS) is a potential treatment option for Parkinson’s disease patients with depression (DPD), but conflicting results in previous studies have questioned its efficacy.
Method
To investigate the safety and efficacy of neuronavigated high-frequency rTMS at the left DLPFC in DPD patients, we conducted a randomized, double-blind, sham-controlled study (NCT04707378). Sixty patients were randomly assigned to either a sham or active stimulation group and received rTMS for ten consecutive days. The primary outcome was HAMD, while secondary outcomes included HAMA, MMSE, MoCA and MDS-UPDRS-III. Assessments were performed at baseline, immediately after treatment, 2 weeks, and 4 weeks post-treatment.
Results
The GEE analysis showed that the active stimulation group had significant improvements in depression, anxiety, and motor symptoms at various time points. Specifically, there were significant time-by-group interaction effects in depression immediately after treatment (β, −4.34 [95% CI, −6.90 to −1.74; P = 0.001]), at 2 weeks post-treatment (β, −3.66 [95% CI, −6.43 to −0.90; P = 0.010]), and at 4 weeks post-treatment (β, −4.94 [95% CI, −7.60 to −2.29; P < 0.001]). Similarly, there were significant time-by-group interaction effects in anxiety at 4 weeks post-treatment (β, −2.65 [95% CI, −4.96 to −0.34; P = 0.024]) and in motor symptoms immediately after treatment (β, −5.72 [95% CI, −9.10 to −2.34; P = 0.001] and at 4 weeks post-treatment (β, −5.43 [95% CI, −10.24 to −0.61; P = 0.027]).
Conclusion
The study suggested that neuronavigated high-frequency rTMS at left DLPFC is effective for depression, anxiety, and motor symptoms in PD patients.
Keywords: Parkinson’s disease, Depression, Transcranial magnetic stimulation, Dorsolateral prefrontal cortex, Neuronavigation
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease that poses a substantial burden on patients, families, and society [1,2]. Among the various non-motor symptoms associated with PD, depression (DPD) is particularly common, affecting up to 22.9% of PD patients [3,4]. The onset of depression in PD is insidious and often precedes the onset of typical motor symptoms [5]. Moreover, research suggests that depression is a clinical prodromal sign of PD and an independent risk factor for its development, with depressed individuals being 3.24 times more likely to develop PD than the general population [6,7].
Compared to primary depression, patients with DPD often experience additional symptoms such as anxiety, irritability, sadness, pessimism about the future, and a higher prevalence of death and suicidal ideation. Studies indicated that 28% of PD patients have experienced death ideation, 11% have had suicidal ideation, and 4% have even attempted suicide [8]. These symptoms have a substantial negative impact on the quality of life for patients and their families [9,10]. While typical antidepressant medications like selective serotonin-norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SNRIs) and tricyclic antidepressants (TAs) are commonly feasible for DPD [11], SSRIs and SNRIs may worsen the symptoms of Parkinsonism, such as dystonia and tremor, and may induce 5-hydroxytryptamine syndrome; TAs, on the other hand, have anticholinergic effects, cognitive impairment, postural hypotension, and increase risk of falls in patients. Given that PD patients are typically older and often have multiple comorbidities and medications [12], treating depression becomes more complicated. Therefore, exploring non-pharmacological alternatives with fewer side effects and risks is crucial for alleviating depressive symptoms in PD.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulation technique based on the electromagnetic induction principle. In recent years, clinicians have shown increasing interest in its potential role in the treatment of neuropsychiatric disorders [13,14]. Several studies have investigated the effects of rTMS on depression in PD, but the results have been inconsistent [15]. A recent randomized, sham-stimulation-controlled study reported no significant improvement in depression symptoms [16]. Variations in stimulation targets, parameters, and localization methods may contribute to these discrepancies. The selection of stimulation targets in rTMS studies for DPD has primarily focused on the primary motor cortex (M1) and the left dorsolateral prefrontal cortex (DLPFC). While M1 stimulation has demonstrated efficacy in improving motor symptoms, DLPFC stimulation has shown potential in addressing depressive symptoms. Abnormal functional connections within the prefrontal-limbic network have been observed in patients with DPD [17,18]. The dorsolateral prefrontal cortex (DLPFC), with its extensive connectivity to the limbic system, other prefrontal and parietal regions, and subcortical structures, has emerged as a promising target for rTMS in DPD. Animal studies have demonstrated the beneficial effects of rTMS stimulation of the left DLPFC on dopamine synthesis and release in PD models, which has been shown to increase dopamine levels in regions such as the hippocampus, striatum, and nucleus accumbens, reduce inflammation-related factors, and protect against the loss of dopaminergic neurons in the nigrostriatal pathway. Moreover, these findings have been further supported by evidence of improved mood and motor function recovery in PD animal models [[19], [20], [21], [22]]. Furthermore, human studies have corroborated the beneficial effects of rTMS stimulation of the left DLPFC, which have shown increased dopamine release [23] and cortical excitation [24] in specific regions such as the left subgenual anterior cingulate cortex and medial orbitofrontal cortex following high-frequency rTMS stimulation of the left DLPFC. In addition, the localization methods used to target the DLPFC can impact the effectiveness of rTMS. Previous studies have mainly relied on the “standard” localization method, which positions the target approximately 5 cm in front of M1 without considering individual differences in skull and brain morphology. However, the use of neuronavigation, a novel method that incorporates individual brain information, allows for more precise targeting of specific regions. Studies have found that using neuronavigation resulted in the DLPFC being positioned approximately 2 cm more anterolateral than conventional methods, which may have implications for treatment outcomes [25,26]. Lastly, the after-effect of rTMS treatment may depend on stimulation frequency, stimulation time, and the duration of stimulation, with longer stimulation time leading to longer after-effects [4,27]. Previous studies have mainly focused on a stimulation frequency of 5 Hz and a daily total of 600 pulses [28,29], which may can be explored for a higher stimulation frequency and larger number of stimulation pulses.
Therefore, in this randomized, double-blind, sham-controlled clinical trial, we aimed to evaluate the safety and efficacy of high-frequency (10 Hz) and a daily total of 1200 pulses rTMS in patients with DPD by precisely localizing the left DLPFC using a neuronavigation system with high-resolution structural magnetic resonance imaging (MRI). We hypothesized that the active stimulation group guided by neuronavigation will show greater efficacy in alleviating depression in PD compared to the sham-stimulation controlled group.
2. Methods
2.1. Study design
This was a randomized, double-blind, sham-controlled study, which recruited 60 patients with DPD from the Department of Neurology, Guangdong Provincial People’s Hospital, Guangdong Province. The study was approved by the Institutional Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2020181H(R1)) and registered at ClinicalTrials.gov (registration code: NCT04707378). Before the trial, all participants provided informed consent.
2.2. Participants recruitment
Inclusion criteria: (a) aged between 18 and 85 years and diagnosed with idiopathic Parkinson’s Disease by two neurologists based on the 2015 MDS diagnostic criteria for Parkinson’s Disease, with either “clinically established PD” or “clinically probable PD” [30]; (b) met the DSM-IV diagnostic criteria for depression; (c) had a stable drug regimen for at least 28 days if taking a combination of antiparkinson agents, which was maintained during treatment and follow-ups; (d) had not taken any antidepressants for at least 2 months; (e) subjects and their families were able to comply with the study protocol; (f) subjects and their legal representatives agreed to participate in the study and signed the informed consent form.
Exclusion criteria: (a) Secondary Parkinsonism or Parkinsonism Plus Syndrome caused by drugs, vascular lesions, toxic, tumors, trauma, and other insults; (b) unable to cooperate with MRI scanning or rTMS treatment due to persistent head tremor; (c) dementia; (d) severe psychiatric symptoms and suicidal tendencies; (e) history of impaired consciousness, stroke, or severe neuropsychiatric disease within 1 year before screening; (f) contraindications to rTMS, including cochlear implants, deep brain stimulation, pacemakers, medical pumps, and other metal-containing devices close to stimulation sites; history of epilepsy, traumatic brain injury, brain tumor, encephalitis, cerebrovascular disease, or cerebral metabolic disease; sleep deprivation, unrecovered jet lag, intoxication, overexertion; pregnancy and heart disease; (g) contraindications to MRI scanning, including metal implants, retractors, stents, and claustrophobia.
2.3. Neuronavigated rTMS
All treatments were performed using the Visor 2 neuronavigation system (ANT Neuro, Berlin, Germany), which enables the modeling of individual brains based on high-resolution cranial structural MRI. This system allowed for precise targeting of the left DLPFC, of which the Talairach coordinates is (−42.17, 33.73, 34.74) [31]. The rTMS stimulator used was the YRD CCY-1 transcranial magnetic stimulator with an 8-character coil (Yiruide, Wuhan, China). In the active stimulation group, the coil was kept in a horizontal position, while in the sham stimulation group, it was kept vertical. Stimulation frequency was set at 10 Hz, and intensity was set at 100% resting motor threshold [32]. Each session consisted of 2s stimulation followed by an 18s interval, for a total of 60 sessions and 1200 pulses of stimulation per day over 10 consecutive days.
2.4. Clinical symptoms assessment
Subjects underwent evaluations at baseline, immediately after treatment, as well as 2 and 4 weeks post-treatment, respectively. The primary outcome was the change in Hamilton Rating Scale for Depression (HAMD) scores. HAMD, known for its satisfactory reliability and high validity, is recommended for assessing depression in PD [33,34]. Response was defined as a decrease of more than 50% in HAMD score from baseline, while remission was defined as a HAMD score of less than 8 [35]. Secondary outcomes included the Hamilton Rating Scale for Anxiety (HAMA) to assess anxiety status; the Mini-mental State Examination (MMSE) and the Montreal Cognitive Assessment Scale (MoCA) to evaluate cognitive function, and the Part III of MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS-III) to assess motor symptoms. We have implemented few measures to manage inter-rater reliability in the administration of the HAMD and all the other rating scales, including training sessions, regular meeting and discussions.
2.5. Statistical methods
2.5.1. Sample size calculation
Sample size calculation was conducted using R 3.6.2. Based on the pre-experiments’ results and previous study [36], we assumed that the response rate of high frequency rTMS stimulation in patients with Parkinson’s disease and depression was 45% in the active stimulation group and 10% in the sham stimulation group. To achieve a power of 80% and a significance level of 0.05 for the difference testing of two sample rates, a required sample size of 56 cases was calculated, assuming a dropout rate of 20%. We increased the sample size to 60, with 30 subjects randomly assigned to each group.
2.5.2. Statistical analysis
Statistical analysis was performed using a modified intention-to-treat analysis in R 3.6.2. Normality tests were conducted on continuous numerical data at baseline, in which age, years of education, LED, HAMD, HAMA, and MDS-UPDRS-III were normally distributed, thus reported as “mean (standard deviation)” and compared using a student t-test between groups. On the other hand, for non-normally distributed data such as disease duration, MMSE and MoCA, the median and interquartile range (25th–75th percentile) were reported, and the Wilcoxon rank sum test was used for group comparisons. Categorical data such as gender, H–Y stage, response, and remission were reported as frequencies (percentages) and compared using the chi-square test. The generalized estimating equation (GEE) analysis was employed to analyze the repeated primary and secondary outcome measures over time, with treatment group (active vs. sham) as the between-subject factor and time (baseline, post-treatment, 2-week post-treatment, and 4-week post-treatment) as the within-subject factor. Furthermore, age, gender, education, disease duration, LED and baseline MDS-UPDRS-III were included in the operational correlation matrix by considering the QIC (Quasi-likelihood under the independence model criterion) and clinical practice, and the correlation between any two follow-up visits was assumed to be equal. All statistical analyses were two-tailed, and P values less than 0.05 were considered statistically significant.
3. Results
3.1. Baseline period characteristics
As shown in Fig. 1, a total of 188 potential participants were initially screened for eligibility, of whom 60 were randomized to receive the intervention. Two patients were unable to complete the treatment due to COVID-19 epidemic prevention and control measures in Guangzhou, and one patient in the active stimulation group was excluded for self-adjustment of antiparkinson drugs without informing the researchers in advance. The demographic and clinical characteristics of the two groups were similar at baseline, as summarized in Table 1. Notably, there was a statistically significant difference in years of education between the two groups (P = 0.009), with the active stimulation group having a longer education than the sham stimulation group. However, there were no statistically significant differences in HAMD and MDS-UPDRS-III scores between groups at baseline (P = 0.367 and P = 0.362, respectively).
Fig. 1.
Flow diagram of neuronavigated rTMS to treat depression in Parkinson’s disease.
Table 1.
Participants' demographic and clinical features at the baseline.
| Sham stimulation (n = 29) | Active stimulation (n = 28) | P | |
|---|---|---|---|
| Age | 64.3 (8.9) | 62.7 (12.9) | 0.580 |
| Gender | 0.514 | ||
| Female | 17 (58.6%) | 14 (50%) | |
| Male | 12 (41.4%) | 14 (50%) | |
| Education year | 8.4 (4.2) | 11.3 (3.9) | 0.009 |
| Disease onset | 60 (54,65) | 57.5 (46,62.2) | 0.354 |
| Disease duration | 3 (2,7) | 7 (3,10.2) | 0.071 |
| LED | 396.1 (340.9) | 391.5 (235.4) | 0.953 |
| HAMD | 21.1 (6.2) | 19.5 (7.1) | 0.367 |
| HAMA | 14.2 (4.7) | 13.7 (6) | 0.712 |
| MDS-UPDRS-III | 38 (13) | 41.7 (17) | 0.362 |
| H–Y stage | 0.140 | ||
| 1 | 0 (0%) | 1 (3.6%) | |
| 1.5 | 0 (0%) | 1 (3.6%) | |
| 2 | 13 (44.8%) | 16 (57.1%) | |
| 2.5 | 14 (48.3%) | 5 (17.9%) | |
| 3 | 2 (6.9%) | 4 (14.3%) | |
| 4 | 0 (0%) | 1 (3.6%) | |
| MMSE | 28 (26,29) | 28 (26,29) | 0.871 |
| MoCA | 25 (22,27) | 25.5 (23,28) | 0.466 |
LED: Levodopa Equivalent Dose, HAMD: Hamilton Rating Scale for Depression, HAMA: Hamilton Rating Scale for Anxiety, MDS-UPDRS-III: Part III of Movement Disorders Society-Unified Parkinson’s Disease Rating Scale, H–Y Scale: Modified Hoehn-Yahr Stage, MMSE: Mini-mental State Examination and MoCA: Montreal Cognitive Assessment Scale.
3.2. Safety analysis
The patients in both groups tolerated the treatment well. Only two subjects in the active stimulation group reported mild transient headaches at the stimulation target during the initial treatment, which subsided soon after treatment. No adverse effect was reported in the sham stimulation group.
3.3. Effect of rTMS treatment
3.3.1. Primary outcome: depression
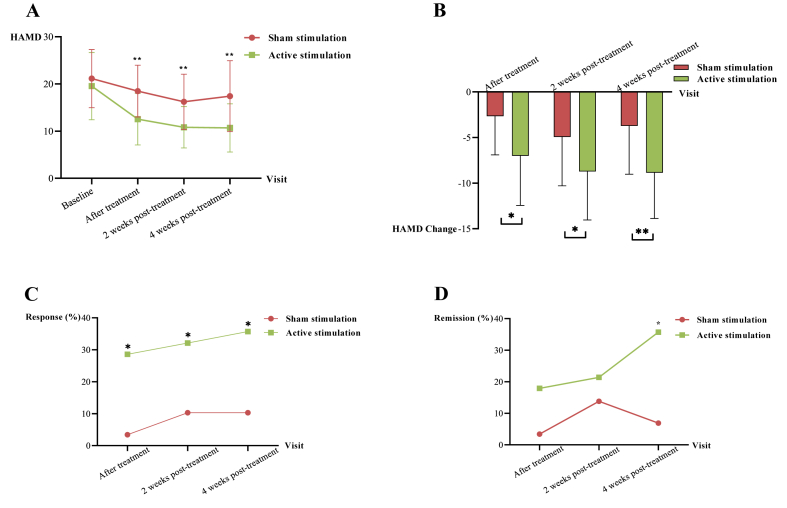
Table 2, Fig. 2A and B illustrated the results of the primary outcome, HAMD. In the active stimulation group, the HAMD score reduced by 7, 8.7, and 8.9 points (P = 0.001, P = 0.010 and P < 0.001) from baseline immediately after treatment, at 2 weeks post-treatment, and at 4 weeks post-treatment, respectively. The GEE analysis of HAMD in Table 3 showed significant time effects, revealing significant improvements of 2.66, 4.93, and 3.72 points (P = 0.001, P < 0.001, P < 0.001) in the sham stimulation group. Additionally, there were significant time-by-group interaction effects in depression immediately after treatment (β, −4.34 [95% CI, −6.90 to −1.74; P = 0.001]), at 2 weeks post-treatment (β, −3.66 [95% CI, −6.43 to −0.90; P = 0.010]), and at 4 weeks post-treatment (β, −4.94 [95% CI, −7.60 to −2.29; P < 0.001]), revealing that the active stimulation group had significant improvement than the sham stimulation group in depression at all follow-up visits.
Table 2.
Measurements of primary depression and secondary outcomes.
| Scales |
Visit |
Scale scores |
Changes in scale scores |
||||
|---|---|---|---|---|---|---|---|
| Sham stimulation |
Active stimulation |
P | Sham stimulation |
Active stimulation |
P | ||
| (n = 29) | (n = 28) | (n = 29) | (n = 28) | ||||
| HAMD | Baseline | 21.1 (6.2) | 19.5 (7.1) | 0.367 | – | – | – |
| After treatment | 18.5 (5.5) | 12.5 (5.5) | <0.001 | −2.7 (4.3) | −7 (5.4) | 0.001 | |
| 2 weeks post-treatment | 16.2 (5.9) | 10.8 (4.4) | <0.001 | −4.9 (5.4) | −8.7 (5.3) | 0.010 | |
| 4 weeks post-treatment | 17.4 (7.5) | 10.7 (5.1) | <0.001 | −3.7 (5.3) | −8.9 (5) | <0.001 | |
| MDS-UPDRS-III | Baseline | 38 (13) | 41.7 (17) | 0.362 | – | – | – |
| After treatment | 39 (33,46) | 37.5 (23,48) | 0.507 | 0.8 (7.7) | −4.8 (5.4) | 0.003 | |
| 2 weeks post-treatment | 35 (31,42) | 35.5 (19.8,49.8) | 0.981 | −3 (−10,2) | −5 (−9.2,-2) | 0.174 | |
| 4 weeks post-treatment | 36.2 (10.8) | 34.5 (17.7) | 0.654 | −1.8 (9.9) | −7.2 (8.6) | 0.032 | |
| HAMA | Baseline | 14.2 (4.7) | 13.7 (6) | 0.712 | – | – | – |
| After treatment | 12.1 (4.1) | 9.6 (5.4) | 0.057 | −2 (−4,-1) | −3.5 (−7.2,-0.8) | 0.100 | |
| 2 weeks post-treatment | 11.6 (4.2) | 8.6 (4.2) | 0.011 | −2.7 (4.1) | −5.1 (5) | 0.052 | |
| 4 weeks post-treatment | 11.9 (5.5) | 8.7 (3.6) | 0.011 | −2.3 (3.8) | −5 (4.9) | 0.023 | |
| MMSE | Baseline | 28 (26,29) | 28 (26,29) | 0.871 | – | – | – |
| 4 weeks post-treatment | 28 (28,30) | 28 (27,30) | 0.654 | 0.7 (1.5) | 0.6 (1.8) | 0.786 | |
| MoCA | Baseline | 25 (22,27) | 25.5 (23,28) | 0.466 | – | – | – |
| 4 weeks post-treatment | 26 (25,28) | 26.5 (24.8,28.2) | 0.724 | 1.4 (2.3) | 0.6 (2.5) | 0.228 | |
LED: Levodopa Equivalent Dose, HAMD: Hamilton Rating Scale for Depression, HAMA: Hamilton Rating Scale for Anxiety, MDS-UPDRS-III: Part III of Movement Disorders Society-Unified Parkinson’s Disease Rating Scale, MMSE: Mini-mental State Examination, and MoCA: Montreal Cognitive Assessment Scale. “--” indicates no data at this visit.
Fig. 2.
A. HAMD at each follow-up visit. B. Changes of HAMD at each visit. The red line represents sham stimulation group, and green line represents active stimulation group. C. Response at each visit, and response was defined as a more than 50% reduction in HAMD score from baseline. D. Remission at each visit, and remission was defined as HAMD score <8. *P < 0.05, **P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Estimates of group, time, and interaction effects in HAMD.
| Parameter | Estimate (β) | Standard Error | 95% Confidence Limits | Z | P | |
|---|---|---|---|---|---|---|
| Intercept | 19.27 | 3.80 | 11.82 | 26.72 | 5.07 | <.001 |
| Group | −0.80 | 1.87 | −4.47 | 2.87 | −0.43 | 0.670 |
| Time 2 vs 1 | −2.66 | 0.78 | −4.18 | −1.13 | −3.42 | 0.001 |
| Time 3 vs 1 | −4.93 | 0.98 | −6.85 | −3.01 | −5.04 | <.001 |
| Time 4 vs 1 | −3.72 | 0.97 | −5.62 | −1.83 | −3.85 | <.001 |
| Group*Time (1 vs 2) | −4.34 | 1.30 | −6.90 | −1.79 | −3.34 | 0.001 |
| Group*Time (1 vs 3) | −3.66 | 1.41 | −6.43 | −0.90 | −2.60 | 0.010 |
| Group*Time (1 vs 4) | −4.94 | 1.35 | −7.60 | −2.29 | −3.65 | <.001 |
| Age | 0.10 | 0.06 | −0.01 | 0.21 | 1.71 | 0.087 |
| Sex | −2.20 | 1.63 | −5.39 | 0.99 | −1.35 | 0.177 |
| Education | −0.30 | 0.17 | −0.64 | 0.04 | −1.71 | 0.088 |
| Disease Duration | −0.05 | 0.16 | −0.37 | 0.26 | −0.34 | 0.735 |
| LED | 0.00 | 0.00 | −0.01 | 0.01 | −0.21 | 0.834 |
Generalized estimating equation was used for group main effect, time main effect, and group*time interaction effect analysis. Time 2 vs 1, time 3 vs 1 and time 4 vs 1 represent HAMD changes in sham stimulation group between immediately, 2 weeks post-treatment, 4 weeks post-treatment and baseline, respectively. Group*Time 1 vs 2, 1 vs 3, 1 vs 4 represent changes of HAMD-change between active stimulation and sham stimulation group after treatment, 2 weeks post-treatment, and 4 weeks post-treatment, respectively. LED: Levodopa Equivalent Dose, HAMD: Hamilton Rating Scale for Depression.
Response and remission are also typical indicators for evaluating antidepressant effects. As demonstrated in Fig. 2C and Supplementary Table 1, the response rates in the active stimulation group were 28.6%, 32.1%, and 35.7%, respectively, which were higher than those in the sham stimulation group, and the differences were statistically significant (P = 0.011, P = 0.044, P = 0.022). Meanwhile, in Fig. 2D and Supplementary Table 1, the remission rates in the active stimulation group were 17.9%, 21.4%, and 35.7%, respectively, which was higher than in the sham stimulation group at 4 weeks post-treatment, and the difference was statistically significant (P = 0.008).
3.4. Secondary outcomes
3.4.1. Motor symptoms
Table 2, Fig. 3A and B presented the results of the motor symptom, MDS-UPDRS-III. In the active stimulation group, the MDS-UPDRS-III score reduced by 4.8 and 7.2 points (P = 0.003 and P = 0.032) from baseline immediately after treatment and at 4 weeks post-treatment. The GEE analysis of MDS-UPDRS-III in Supplementary Table 2 showed no significant time effects in the sham stimulation group. However, there were significant time-by-group interaction effects in motor symptoms immediately after treatment (β, −5.72 [95% CI, −9.10 to −2.34; P = 0.001] and at 4 weeks post-treatment (β, −5.43 [95% CI, −10.24 to −0.61; P = 0.027]), revealing that the active stimulation group had significant improvement than the sham stimulation group in motor symptoms immediately after treatment and at 4 weeks post-treatment.
Fig. 3.
A. MDS-UPDRS-III at each follow-up. B. Changes of MDS-UPDRS-III at each visit. The red line represents sham stimulation group, and the green line represents active stimulation group. *P < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4.2. Anxiety
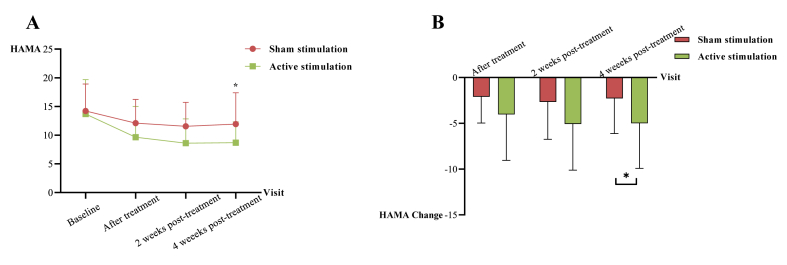
Table 2Fig. 4A and B illustrated the results of the anxiety, HAMA. In the active stimulation group, the HAMA score decreased by 5 points (P = 0.023) from baseline at 4 weeks post-treatment. The GEE analysis of HAMA in Supplementary Table 3 showed significant time effects in the sham stimulation group, with improvements of 2, 2.7, and 2.3 points (P = 0.001, P < 0.001, P < 0.001), indicating a placebo effect on anxiety symptoms. Similarly, there were significant time-by-group interaction effects in anxiety at 4 weeks post-treatment (β, −2.65 [95% CI, −4.96 to −0.34; P = 0.024]), revealing that the active stimulation group had significant improvement than the sham stimulation group in anxiety at 4 weeks post-treatment.
Fig. 4.
A. HAMA at each follow-up visits. B. Changes of HAMA at each visit. The red line represents sham stimulation group, and green line represents active stimulation group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4.3. Cognitive function
The study also examined the impact of the treatment on cognitive function, as measured by MMSE and MoCA. The results, shown in Table 2, Supplementary Tables 4 and 5, indicate that there was a slight improvement of 0.69 and 1.41 points in MMSE and MoCA in the sham stimulation group at 4 weeks post-treatment, and these improvements were statistically significant (P = 0.011 and P = 0.001). However, GEE analysis showed that there was no statistically significant time-by-group interaction effect in MMSE and MoCA between groups, revealing that there was no significant difference between the two groups in cognition function changes.
4. Discussion
In light of the current dearth of effective therapies and the inconsistent findings regarding the impact of rTMS on depression in Parkinson’s disease (PD), the present study sought to examine the safety and efficacy of high frequency (10 Hz) neuronavigated rTMS on the left DLPFC in DPD patients. Our findings demonstrated that DPD patients tolerated the neuronavigated rTMS procedure well, with only minor and transient head pain localized to the stimulation target noted in the active stimulation group and no reports of serious adverse reactions. Notably, the treatment resulted in a reduction in depression symptoms that persisted for four weeks and was also associated with improvements in anxiety and motor symptoms in Parkinson’s disease patients.
The results of this study demonstrated that high-frequency neuronavigated rTMS stimulation on the left DLPFC can effectively improve depressive symptoms in DPD patients over a 10-day treatment period. The improvement was found to be significant compared to the sham stimulation group. Although depressive symptoms also improved in the sham stimulation group, they worsened 4 weeks after treatment, possibly due to a reduction in the placebo effect. In contrast, the improvement in the active stimulation group remained stable up to 4 weeks after treatment. The study observed a significant decrease in HAMD scores by 7, 8.7, and 8.9 points in active stimulation patients, with response rates of 28.6%, 32.1%, and 35.7% after treatment, 2 weeks and 4 weeks post-treatment, respectively. The results of this study were consistent with previous small sample size research that indicates high-frequency rTMS stimulation of the left DLPFC as an effective method for alleviating depressive symptoms [15,24,28,29,37]. However, the Brys study did not show significant improvement [38], possibly due to the small sample size of each group, which was not statistically valid enough to show statistical differences. Moreover, Brys conducted interim analyses on various subgroups, which were prematurely discontinued due to the absence of demonstrated improvement. This premature termination potentially augmented the likelihood of committing a type II error. Additionally, the study included a heterogeneous patient population encompassing both early and late stage disease, with participants who were not currently using antidepressants. This heterogeneity might have obscured the effects observed within specific subgroups, such as the sham-stimulation group consisting of younger Parkinson’s disease patients with shorter disease duration, who exhibited a noteworthy placebo effect. Furthermore, it is worth noting that participants were informed that this study aimed to enhance both motor and nonmotor symptoms, a factor that could have heightened the placebo effect and consequently influenced the overall outcomes. A recent meta-analysis that included five eligible studies with 202 patients supports the potential effectiveness of DLPFC stimulation in alleviating depressive symptoms [39], which further strengthens our conclusion. Compared to previous studies, this study showed a more significant remission of depressive symptoms. In 2010, Pal conducted a study including 22 patients with DPD who underwent 10-day rTMS treatment with a frequency of 5 Hz and 600 pulses per day. The study found that BDI scores decreased from 9 to 5 points and MADRS scores decreased from 11.5 to 10 points after treatment. One month after treatment, BDI scores remained at 5 points and MADRS scores decreased to 8.5 points, resulting in treatment responsiveness of 75%. It is worth noting that Pal defined “treatment responsiveness” as a minimally important clinical difference, i.e., a decrease of at least 2 points in MADRS score, which is much lower than the “response” criteria used in our study (HAMD score decreased by 50% or more) [40]. Similarly, Shin included 21 patients and used similar treatment parameters to the Pal study. Although the results showed improvement in patients' depressive symptoms after treatment, and the effect lasted for six weeks, this study did not calculate the difference in changes between the active and sham stimulation groups [29]. Compared to these two studies, our study demonstrated a more substantial improvement in depressive symptoms among individuals with DPD, which can be attributed to the higher stimulation frequency, more pulsed stimulation, and the use of neuronavigation to localize the stimulation target accurately [41].
Besides relieving depressive symptoms, high-frequency neuronavigated rTMS treatment also demonstrated a positive effect on motor symptoms that persisted for up to 4 weeks. The MDS-UPDRS-III scores decreased by 4.8, 5, and 7.2 points from baseline at post-treatment, 2 weeks post-treatment, and 4 weeks post-treatment, respectively. Previous studies focusing on rTMS treatment for motor symptoms in Parkinson’s disease (PD) have predominantly concentrated on high-frequency stimulation on M1 or supplementary motor area (SMA) [[42], [43], [44]]. Conversely, no noteworthy effects have been observed when targeting the left DLPFC [45]. The Pal study, employing 5 Hz stimulation, demonstrated a reduction of 2.5 and 7.5 points on the UPDRS-III scale at the post-treatment and 1-month post-treatment, respectively. However, the improvement in motor symptoms observed after treatment was comparatively weaker than the current study, which showcased a similar sustained effect on motor symptoms at the 1-month follow-up period [28]. In the Brys study, where the HAMD and UPDRS-III were employed as primary outcome measures [16], the results indicated a 3.5-point decrease in UPDRS-III one month after high frequency stimulation of the left DLPFC, combined with sham stimulation of bilateral M1 in PD patients. However, this difference did not reach statistical significance, and no amelioration in depressive symptoms or motor symptoms was observed with combined stimulation of the left DLPFC and bilateral M1. Nonetheless, a study targeting bilateral M1 sites in PD with depressive symptoms as the primary outcome found improvements in both depressive symptoms and motor symptoms [46]. This suggests that rTMS treatment may be more likely to benefit both symptoms in PD patients with depression, and the precise targeting of neuronavigation can improve the outcomes. Additionally, it is acknowledged that the impact of the left DLPFC stimulation on motor symptoms in PD patients may be attributed to either a direct effect or an indirect effect resulting from the antidepressant effect. However, due to the limitations of a comparatively small sample size trial with 60 subjects, further investigation with larger sample sizes, such as path analysis, would be needed to address this question.
In addition to improvements in depressive symptoms, our study also demonstrated significant improvements in anxiety symptoms immediately after treatment and at 4 weeks post-treatment, with possible reasons including the combination of the elimination of the placebo effect in the sham stimulation group and the post-treatment effect of rTMS. The prevalence of anxiety in PD patients is estimated to be around 31% [47]. While there has been no randomized controlled trial investigating the use of rTMS for PD with anxiety, reductions in anxiety symptoms have been observed in some other interventions such as antidepressants [48,49], cognitive behavioral therapy [50] and atomoxetine [51]. A non-randomized controlled trial conducted in 2007 on seven patients with DPD found that 10 sessions of high-frequency (20 Hz) stimulation on left DLPFC led to improvements in both trait and state anxiety improved [52]. As depression and anxiety are highly correlated, with significant symptom overlap, improvements in depressive symptoms may also contribute to the improvements seen in anxiety symptoms among patients with DPD following rTMS treatment [53]. Furthermore, the findings of this study, which indicate higher levels of anxiety symptoms in older individuals with DPD, are inconsistent with previous research. Previous studies have demonstrated that early-onset PD is more susceptible to anxiety compared to late-onset PD, irrespective of the severity of motor symptoms [54]. Moreover, it has been observed that anxiety symptoms tend to gradually decline after the age of 45 in middle-aged and older populations, whereas depressive symptoms gradually decrease after the age of 65 [55]. However, it is important to note that the patient population in this study was primarily concentrated between the ages of 60 and 65, at which point the severity of depressive symptoms may be peaking. Furthermore, as age increases, motor symptoms tend to become more severe, leading to heightened anxiety among patients. Anxiety symptoms often receive less attention than depressive symptoms in individuals with Parkinson’s disease, but the results of this study suggest that rTMS treatment for depression in PD patients may also have a positive impact on alleviating anxiety symptoms.
Finally, the study found no improvement in cognitive symptoms after rTMS treatment, which is consistent with previous studies [56]. Current evidence does not yet support the use of rTMS to improve cognitive function in PD patients. However, the one-month follow-up in this study may not have been sufficient to evaluate changes in cognitive function.
Moreover, it is also worth noting that the improvements in this study present a tendency towards after-effects. Specifically, the improvements didn’t occur or change that much immediately after the treatment, but rather at 2 weeks or 4 weeks post-treatment. For example, depression improved more at the last two visits and anxiety showed improvement only at the last visit. This observation is consistent with previous studies, which have also reported slight after-effects of rTMS treatment [57,58]. However, the improvements of both depressive symptoms and motor symptoms in our study showed a more pronounced after-effect and were overall stronger. The after-effect of TMS treatment may depend on stimulation frequency, stimulation time, and the duration of stimulation, with longer stimulation times leading to longer after-effects [4,27]. The physiological mechanisms underlying after-effects are not yet fully understood, but some studies suggest that they may be related to long-term potentiation and long-term depression, which modulate neural circuitry over time by modulating neuroplasticity [40].
The study had several limitations that should be acknowledged. Firstly, the generalizability of the findings may be limited due to certain factors. The characteristics of the participants, such as their specific demographic group and particular clinical profiles, may restrict the applicability of the results to broader populations of PD patients. For instance, most of the patients included in the study were at an age where the severity of depressive symptoms may be reaching its peak. Therefore, caution should be exercised when extrapolating these findings to PD patineds. Additionally, the intervention parameters, such as stimulation frequency, stimulation pulses, and stimulation mode, may not be directly applicable to other interventions or variations in clinical practice. These intervention-specific factors should be carefully considered when extrapolating the findings to different subgroups of PD patients. Furthermore, it is important to recognize the inherent limitations of the study design, including sample size, study duration, and potential biases. For example, the follow-up duration was short, which may not have been sufficient to detect the long-term benefits of the intervention. These design limitations can impact the reliability and generalizability of the results. Secondly, while high-frequency rTMS stimulation of the left DLPFC is currently the most established modality for treating depressive symptoms in PD, low-frequency stimulation of the right DLPFC has also been shown to be potentially effective, and combined bilateral target treatment may produce a superimposed therapeutic effect. Therefore, more research is needed to explore the use of various combined stimulation modalities. Furthermore, recent studies have suggested that utilizing functional MRI to identify abnormal functional connectivity for treatment target selection may be more effective in alleviating depression than high-resolution structural MRI-guided rTMS [59,60]. Although neuronavigation was employed for precise target localization in the current study, real-time coil adjustment during treatment was not achieved. Future studies may benefit from the use of TMS robots that integrate neuronavigation and allow for real-time coil adjustment.
In summary, our findings indicate that high-frequency rTMS stimulation of the left DLPFC with neuronavigation is a safe and non-invasive treatment technique that significantly alleviates depressive, anxiety and motor symptoms in PD patients. The treatment can be repeated as needed, making it a valuable tool for treating PD patients with depression. Further studies are required to optimize treatment parameters and explore the mechanism of effects.
Declarations
The study was approved by the Institutional Ethics Committee of Guangdong Provincial People’s Hospital [No. GDREC2020181H(R1)] and registered at ClinicalTrials.gov (registration code: NCT04707378). Before the trial, all participants provided informed consent.
Author contribution statement
Shuolin Jiang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Cuijing Zhan: Shujun Feng: Yuyuan Gao: Performed the experiments.
Peikun He: Performed the experiments; Analyzed and interpreted the data.
Jiehao Zhao: Limin Wang: Yuhu Zhang: Contributed reagents, materials, analysis tools or data.
Kun Nie: Yihui Qiu: Lijuan Wang: Conceived and designed the experiments.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
This work was supported by the National Key R&D Program of China under Grant (2017YFC1310200); and Science and Technology Planning Project of Guangzhou under Grant (202201000005).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18364.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rocca W.A. The burden of Parkinson’s disease: a worldwide perspective. Lancet Neurol. 2018;17:928–929. doi: 10.1016/S1474-4422(18)30355-7. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators G.B.D.N. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langston J.W. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann. Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 4.Simonetta-Moreau M. Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann. Phys. Rehabil. Med. 2014;57:530–542. doi: 10.1016/j.rehab.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 5.van der Hoek T.C., Bus B.A., Matui P., van der Marck M.A., Esselink R.A., Tendolkar I. Prevalence of depression in Parkinson’s disease: effects of disease stage, motor subtype and gender. J. Neurol. Sci. 2011;310:220–224. doi: 10.1016/j.jns.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Shen C.C., Tsai S.J., Perng C.L., Kuo B.I., Yang A.C. Risk of Parkinson disease after depression: a nationwide population-based study. Neurology. 2013;81:1538–1544. doi: 10.1212/WNL.0b013e3182a956ad. [DOI] [PubMed] [Google Scholar]
- 7.Berg D., Postuma R.B., Adler C.H., Bloem B.R., Chan P., Dubois B., Gasser T., Goetz C.G., Halliday G., Joseph L., Lang A.E., Liepelt-Scarfone I., Litvan I., Marek K., Obeso J., Oertel W., Olanow C.W., Poewe W., Stern M., Deuschl G. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015;30:1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 8.Nazem S., Siderowf A.D., Duda J.E., Brown G.K., Ten Have T., Stern M.B., Weintraub D. Suicidal and death ideation in Parkinson’s disease. Mov. Disord. 2008;23:1573–1579. doi: 10.1002/mds.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravina B., Camicioli R., Como P.G., Marsh L., Jankovic J., Weintraub D., Elm J. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69:342–347. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones J.D., Marsiske M., Okun M.S., Bowers D. Latent growth-curve analysis reveals that worsening Parkinson’s disease quality of life is driven by depression. Neuropsychology. 2015;29:603–609. doi: 10.1037/neu0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seppi K., Ray Chaudhuri K., Coelho M., Fox S.H., Katzenschlager R., Perez Lloret S., Weintraub D., Sampaio C., the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine C Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov. Disord. 2019;34:180–198. doi: 10.1002/mds.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyasaki J.M., Shannon K., Voon V., Ravina B., Kleiner-Fisman G., Anderson K., Shulman L.M., Gronseth G., Weiner W.J., Quality Standards Subcommittee of the American Academy of N Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:996–1002. doi: 10.1212/01.wnl.0000215428.46057.3d. [DOI] [PubMed] [Google Scholar]
- 13.Avery D.H., Isenberg K.E., Sampson S.M., Janicak P.G., Lisanby S.H., Maixner D.F., Loo C., Thase M.E., Demitrack M.A., George M.S. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J. Clin. Psychiatry. 2008;69:441–451. doi: 10.4088/jcp.v69n0315. [DOI] [PubMed] [Google Scholar]
- 14.George M.S., Lisanby S.H., Avery D., McDonald W.M., Durkalski V., Pavlicova M., Anderson B., Nahas Z., Bulow P., Zarkowski P., Holtzheimer P.E., 3rd, Schwartz T., Sackeim H.A. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch. Gen. Psychiatr. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 15.Fregni F., Santos C.M., Myczkowski M.L., Rigolino R., Gallucci-Neto J., Barbosa E.R., Valente K.D., Pascual-Leone A., Marcolin M.A. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2004;75:1171–1174. doi: 10.1136/jnnp.2003.027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brys M., Fox M.D., Agarwal S., Biagioni M., Dacpano G., Kumar P., Pirraglia E., Chen R., Wu A., Fernandez H., Wagle Shukla A., Lou J.S., Gray Z., Simon D.K., Di Rocco A., Pascual-Leone A. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial. Neurology. 2016;87:1907–1915. doi: 10.1212/WNL.0000000000003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng K., Fang W., Su M., Li R., Zou D., Han Y., Wang X., Cheng O. Altered spontaneous brain activity in patients with Parkinson’s disease accompanied by depressive symptoms, as revealed by regional homogeneity and functional connectivity in the prefrontal-limbic system. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X., Song X., Yuan Y., Li E., Liu J., Liu W., Liu Y. Abnormal functional connectivity of the amygdala is associated with depression in Parkinson’s disease. Mov. Disord. 2015;30:238–244. doi: 10.1002/mds.26087. [DOI] [PubMed] [Google Scholar]
- 19.Keck M.E., Welt T., Muller M.B., Erhardt A., Ohl F., Toschi N., Holsboer F., Sillaber I. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology. 2002;43:101–109. doi: 10.1016/s0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 20.Kanno M., Matsumoto M., Togashi H., Yoshioka M., Mano Y. Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. J. Neurol. Sci. 2004;217:73–81. doi: 10.1016/j.jns.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Yang X., Song L., Liu Z. The effect of repetitive transcranial magnetic stimulation on a model rat of Parkinson’s disease. Neuroreport. 2010;21:268–272. doi: 10.1097/WNR.0b013e328335b411. [DOI] [PubMed] [Google Scholar]
- 22.Ba M., Ma G., Ren C., Sun X., Kong M. Repetitive transcranial magnetic stimulation for treatment of lactacystin-induced Parkinsonian rat model. Oncotarget. 2017;8:50921–50929. doi: 10.18632/oncotarget.17285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho S.S., Strafella A.P. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso E.F., Fregni F., Martins Maia F., Boggio P.S., Luis Myczkowski M., Coracini K., Lopes Vieira A., Melo L.M., Sato J.R., Antonio Marcolin M., Rigonatti S.P., Cruz A.C., Jr., Reis Barbosa E., Amaro E., Jr. rTMS treatment for depression in Parkinson’s disease increases BOLD responses in the left prefrontal cortex. Int. J. Neuropsychopharmacol. 2008;11:173–183. doi: 10.1017/S1461145707007961. [DOI] [PubMed] [Google Scholar]
- 25.Peleman K., Van Schuerbeek P., Luypaert R., Stadnik T., De Raedt R., De Mey J., Bossuyt A., Baeken C. Using 3D-MRI to localize the dorsolateral prefrontal cortex in TMS research. World J. Biol. Psychiatr. 2010;11:425–430. doi: 10.1080/15622970802669564. [DOI] [PubMed] [Google Scholar]
- 26.Ahdab R., Ayache S.S., Brugieres P., Goujon C., Lefaucheur J.P. Comparison of "standard" and "navigated" procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol. Clin. 2010;40:27–36. doi: 10.1016/j.neucli.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Klomjai W., Katz R., Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS) Ann. Phys. Rehabil. Med. 2015;58:208–213. doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Pal E., Nagy F., Aschermann Z., Balazs E., Kovacs N. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: a randomized, double-blind, placebo-controlled study. Mov. Disord. 2010;25:2311–2317. doi: 10.1002/mds.23270. [DOI] [PubMed] [Google Scholar]
- 29.Shin H.W., Youn Y.C., Chung S.J., Sohn Y.H. Effect of high-frequency repetitive transcranial magnetic stimulation on major depressive disorder in patients with Parkinson’s disease. J. Neurol. 2016;263:1442–1448. doi: 10.1007/s00415-016-8160-x. [DOI] [PubMed] [Google Scholar]
- 30.Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., Halliday G., Goetz C.G., Gasser T., Dubois B., Chan P., Bloem B.R., Adler C.H., Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 31.Mylius V., Ayache S.S., Ahdab R., Farhat W.H., Zouari H.G., Belke M., Brugieres P., Wehrmann E., Krakow K., Timmesfeld N., Schmidt S., Oertel W.H., Knake S., Lefaucheur J.P. Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: inter-rater reliability, accuracy, and influence of gender and age. Neuroimage. 2013;78:224–232. doi: 10.1016/j.neuroimage.2013.03.061. [DOI] [PubMed] [Google Scholar]
- 32.Borckardt J.J., Nahas Z., Koola J., George M.S. Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods. J. ECT. 2006;22:169–175. doi: 10.1097/01.yct.0000235923.52741.72. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naarding P., Leentjens A.F., van Kooten F., Verhey F.R. Disease-specific properties of the Rating Scale for Depression in patients with stroke, Alzheimer’s dementia, and Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 2002;14:329–334. doi: 10.1176/jnp.14.3.329. [DOI] [PubMed] [Google Scholar]
- 35.Keller M.B. Remission versus response: the new gold standard of antidepressant care. J. Clin. Psychiatry. 2004;65(Suppl 4):53–59. [PubMed] [Google Scholar]
- 36.Filipcic I., Simunovic Filipcic I., Milovac Z., Sucic S., Gajsak T., Ivezic E., Basic S., Bajic Z., Heilig M. Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-Coil in treatment of major depressive disorder; A randomized clinical trial. J. Psychiatr. Res. 2019;114:113–119. doi: 10.1016/j.jpsychires.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 37.Boggio P.S., Fregni F., Bermpohl F., Mansur C.G., Rosa M., Rumi D.O., Barbosa E.R., Odebrecht Rosa M., Pascual-Leone A., Rigonatti S.P., Marcolin M.A., Araujo Silva M.T. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov. Disord. 2005;20:1178–1184. doi: 10.1002/mds.20508. [DOI] [PubMed] [Google Scholar]
- 38.Brys M., Fox M.D., Agarwal S., Biagioni M., Dacpano G., Kumar P., Pirraglia E., Chen R., Wu A., Fernandez H., Shukla A.W., Lou J.-S., Gray Z., Simon D.K., Rocco A.D., Pascual-Leone A. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial. Neurology. 2016;87:1907–1915. doi: 10.1212/WNL.0000000000003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W., Deng B., Xie F., Zhou H., Guo J.F., Jiang H., Sim A., Tang B., Wang Q. Efficacy of repetitive transcranial magnetic stimulation in Parkinson’s disease: a systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine. 2022;52 doi: 10.1016/j.eclinm.2022.101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoogendam J.M., Ramakers G.M., Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Herbsman T., Avery D., Ramsey D., Holtzheimer P., Wadjik C., Hardaway F., Haynor D., George M.S., Nahas Z. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol. Psychiatr. 2009;66:509–515. doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 42.Shirota Y., Ohtsu H., Hamada M., Enomoto H., Ugawa Y., Research Committee on r TMSToPsD Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology. 2013;80:1400–1405. doi: 10.1212/WNL.0b013e31828c2f66. [DOI] [PubMed] [Google Scholar]
- 43.Kim M.S., Chang W.H., Cho J.W., Youn J., Kim Y.K., Kim S.W., Kim Y.H. Efficacy of cumulative high-frequency rTMS on freezing of gait in Parkinson’s disease. Restor. Neurol. Neurosci. 2015;33:521–530. doi: 10.3233/RNN-140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang W.H., Kim M.S., Park E., Cho J.W., Youn J., Kim Y.K., Kim Y.H. Effect of dual-mode and dual-site noninvasive brain stimulation on freezing of gait in patients with Parkinson disease. Arch. Phys. Med. Rehabil. 2017;98:1283–1290. doi: 10.1016/j.apmr.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Yokoe M., Mano T., Maruo T., Hosomi K., Shimokawa T., Kishima H., Oshino S., Morris S., Kageyama Y., Goto Y., Shimizu T., Mochizuki H., Yoshimine T., Saitoh Y. The optimal stimulation site for high-frequency repetitive transcranial magnetic stimulation in Parkinson’s disease: a double-blind crossover pilot study. J. Clin. Neurosci. 2018;47:72–78. doi: 10.1016/j.jocn.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Makkos A., Pal E., Aschermann Z., Janszky J., Balazs E., Takacs K., Karadi K., Komoly S., Kovacs N. High-frequency repetitive transcranial magnetic stimulation can improve depression in Parkinson’s disease: a randomized, double-blind, placebo-controlled study. Neuropsychobiology. 2016;73:169–177. doi: 10.1159/000445296. [DOI] [PubMed] [Google Scholar]
- 47.Broen M.P., Narayen N.E., Kuijf M.L., Dissanayaka N.N., Leentjens A.F. Prevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 2016;31:1125–1133. doi: 10.1002/mds.26643. [DOI] [PubMed] [Google Scholar]
- 48.Devos D., Dujardin K., Poirot I., Moreau C., Cottencin O., Thomas P., Destee A., Bordet R., Defebvre L. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov. Disord. 2008;23:850–857. doi: 10.1002/mds.21966. [DOI] [PubMed] [Google Scholar]
- 49.Menza M., Dobkin R.D., Marin H., Mark M.H., Gara M., Buyske S., Bienfait K., Dicke A. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology. 2009;72:886–892. doi: 10.1212/01.wnl.0000336340.89821.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moonen A.J.H., Mulders A.E.P., Defebvre L., Duits A., Flinois B., Köhler S., Kuijf M.L., Leterme A.C., Servant D., de Vugt M., Dujardin K., Leentjens A.F.G. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov. Disord. 2021;36:2539–2548. doi: 10.1002/mds.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weintraub D., Mavandadi S., Mamikonyan E., Siderowf A.D., Duda J.E., Hurtig H.I., Colcher A., Horn S.S., Nazem S., Ten Have T.R., Stern M.B. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology. 2010;75:448–455. doi: 10.1212/WNL.0b013e3181ebdd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kormos T.C. Efficacy of rTMS in the treatment of co-morbid anxiety in depressed patients with Parkinson’s disease. Mov. Disord. 2007;22:1836. doi: 10.1002/mds.21613. [DOI] [PubMed] [Google Scholar]
- 53.Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dissanayaka N.N., Sellbach A., Matheson S., O’Sullivan J.D., Silburn P.A., Byrne G.J., Marsh R., Mellick G.D. Anxiety disorders in Parkinson’s disease: prevalence and risk factors. Mov. Disord. 2010;25:838–845. doi: 10.1002/mds.22833. [DOI] [PubMed] [Google Scholar]
- 55.O’Connor D.W. Do older Australians truly have low rates of anxiety and depression? A critique of the 1997 National Survey of Mental Health and Wellbeing. Aust. N. Z. J. Psychiatr. 2006;40:623–631. doi: 10.1080/j.1440-1614.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 56.He P.K., Wang L.M., Chen J.N., Zhang Y.H., Gao Y.Y., Xu Q.H., Qiu Y.H., Cai H.M., Li Y., Huang Z.H., Feng S.J., Zhao J.H., Ma G.X., Nie K., Wang L.J. Repetitive transcranial magnetic stimulation (rTMS) fails to improve cognition in patients with Parkinson’s disease: a Meta-analysis of randomized controlled trials. Int. J. Neurosci. 2022;132:269–282. doi: 10.1080/00207454.2020.1809394. [DOI] [PubMed] [Google Scholar]
- 57.Filipovic S.R., Rothwell J.C., Bhatia K. Slow (1 Hz) repetitive transcranial magnetic stimulation (rTMS) induces a sustained change in cortical excitability in patients with Parkinson’s disease. Clin. Neurophysiol. 2010;121:1129–1137. doi: 10.1016/j.clinph.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang Y., Chen Q., Cao M., Zhao Q., Qiu Y. A study on high-and low-frequency repetitive transcranial magnetic stimulation in the treatment of Parkinson’s disease complicated with depressive disorder. China Modern Doctor. 2015;53:7–10+15. [Google Scholar]
- 59.Cole E.J., Stimpson K.H., Bentzley B.S., Gulser M., Cherian K., Tischler C., Nejad R., Pankow H., Choi E., Aaron H., Espil F.M., Pannu J., Xiao X., Duvio D., Solvason H.B., Hawkins J., Guerra A., Jo B., Raj K.S., Phillips A.L., Barmak F., Bishop J.H., Coetzee J.P., DeBattista C., Keller J., Schatzberg A.F., Sudheimer K.D., Williams N.R. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am. J. Psychiatr. 2020;177:716–726. doi: 10.1176/appi.ajp.2019.19070720. [DOI] [PubMed] [Google Scholar]
- 60.Cole E.J., Phillips A.L., Bentzley B.S., Stimpson K.H., Nejad R., Barmak F., Veerapal C., Khan N., Cherian K., Felber E., Brown R., Choi E., King S., Pankow H., Bishop J.H., Azeez A., Coetzee J., Rapier R., Odenwald N., Carreon D., Hawkins J., Chang M., Keller J., Raj K., DeBattista C., Jo B., Espil F.M., Schatzberg A.F., Sudheimer K.D., Williams N.R. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am. J. Psychiatr. 2022;179:132–141. doi: 10.1176/appi.ajp.2021.20101429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.