Abstract
Background
People with diabetes mellitus are at increased risk of postoperative complications. Data from randomised clinical trials and meta‐analyses point to a potential benefit of intensive glycaemic control, targeting near‐normal blood glucose, in people with hyperglycaemia (with and without diabetes mellitus) being submitted for surgical procedures. However, there is limited evidence concerning this question in people with diabetes mellitus undergoing surgery.
Objectives
To assess the effects of perioperative glycaemic control for people with diabetes undergoing surgery.
Search methods
For this update, we searched the databases CENTRAL, MEDLINE, LILACS, WHO ICTRP and ClinicalTrials.gov. The date of last search for all databases was 25 July 2022. We applied no language restrictions.
Selection criteria
We included randomised controlled clinical trials (RCTs) that prespecified different targets of perioperative glycaemic control for participants with diabetes (intensive versus conventional or standard care).
Data collection and analysis
Two authors independently extracted data and assessed the risk of bias. Our primary outcomes were all‐cause mortality, hypoglycaemic events and infectious complications. Secondary outcomes were cardiovascular events, renal failure, length of hospital and intensive care unit (ICU) stay, health‐related quality of life, socioeconomic effects, weight gain and mean blood glucose during the intervention. We summarised studies using meta‐analysis with a random‐effects model and calculated the risk ratio (RR) for dichotomous outcomes and the mean difference (MD) for continuous outcomes, using a 95% confidence interval (CI), or summarised outcomes with descriptive methods. We used the GRADE approach to evaluate the certainty of the evidence (CoE).
Main results
A total of eight additional studies were added to the 12 included studies in the previous review leading to 20 RCTs included in this update. A total of 2670 participants were randomised, of which 1320 were allocated to the intensive treatment group and 1350 to the comparison group. The duration of the intervention varied from during surgery to five days postoperative. No included trial had an overall low risk of bias.
Intensive glycaemic control resulted in little or no difference in all‐cause mortality compared to conventional glycaemic control (130/1263 (10.3%) and 117/1288 (9.1%) events, RR 1.08, 95% CI 0.88 to 1.33; I2 = 0%; 2551 participants, 18 studies; high CoE).
Hypoglycaemic events, both severe and non‐severe, were mainly experienced in the intensive glycaemic control group. Intensive glycaemic control may slightly increase hypoglycaemic events compared to conventional glycaemic control (141/1184 (11.9%) and 41/1226 (3.3%) events, RR 3.36, 95% CI 1.69 to 6.67; I2 = 64%; 2410 participants, 17 studies; low CoE), as well as those considered severe events (37/927 (4.0%) and 6/969 (0.6%), RR 4.73, 95% CI 2.12 to 10.55; I2 = 0%; 1896 participants, 11 studies; low CoE).
Intensive glycaemic control, compared to conventional glycaemic control, may result in little to no difference in the rate of infectious complications (160/1228 (13.0%) versus 224/1225 (18.2%) events, RR 0.75, 95% CI 0.55 to 1.04; P = 0.09; I2 = 55%; 2453 participants, 18 studies; low CoE).
Analysis of the predefined secondary outcomes revealed that intensive glycaemic control may result in a decrease in cardiovascular events compared to conventional glycaemic control (107/955 (11.2%) versus 125/978 (12.7%) events, RR 0.73, 95% CI 0.55 to 0.97; P = 0.03; I2 = 44%; 1454 participants, 12 studies; low CoE). Further, intensive glycaemic control resulted in little or no difference in renal failure events compared to conventional glycaemic control (137/1029 (13.3%) and 158/1057 (14.9%), RR 0.92, 95% CI 0.69 to 1.22; P = 0.56; I2 = 38%; 2086 participants, 14 studies; low CoE).
We found little to no difference between intensive glycaemic control and conventional glycaemic control in length of ICU stay (MD ‐0.10 days, 95% CI ‐0.57 to 0.38; P = 0.69; I2 = 69%; 1687 participants, 11 studies; low CoE), and length of hospital stay (MD ‐0.79 days, 95% CI ‐1.79 to 0.21; P = 0.12; I2 = 77%; 1520 participants, 12 studies; very low CoE). Due to the differences within included studies, we did not pool data for the reduction of mean blood glucose. Intensive glycaemic control resulted in a mean lowering of blood glucose, ranging from 13.42 mg/dL to 91.30 mg/dL. One trial assessed health‐related quality of life in 12/37 participants in the intensive glycaemic control group, and 13/44 participants in the conventional glycaemic control group; no important difference was shown in the measured physical health composite score of the short‐form 12‐item health survey (SF‐12). One substudy reported a cost analysis of the population of an included study showing a higher total hospital cost in the conventional glycaemic control group, USD 42,052 (32,858 to 56,421) compared to the intensive glycaemic control group, USD 40,884 (31.216 to 49,992). It is important to point out that there is relevant heterogeneity between studies for several outcomes.
We identified two ongoing trials. The results of these studies could add new information in future updates on this topic.
Authors' conclusions
High‐certainty evidence indicates that perioperative intensive glycaemic control in people with diabetes undergoing surgery does not reduce all‐cause mortality compared to conventional glycaemic control. There is low‐certainty evidence that intensive glycaemic control may reduce the risk of cardiovascular events, but cause little to no difference to the risk of infectious complications after the intervention, while it may increase the risk of hypoglycaemia. There are no clear differences between the groups for the other outcomes. There are uncertainties among the intensive and conventional groups regarding the optimal glycaemic algorithm and target blood glucose concentrations. In addition, we found poor data on health‐related quality of life, socio‐economic effects and weight gain. It is also relevant to underline the heterogeneity among studies regarding clinical outcomes and methodological approaches. More studies are needed that consider these factors and provide a higher quality of evidence, especially for outcomes such as hypoglycaemia and infectious complications.
Plain language summary
What are the effects of intensive control of blood sugar before, during and after surgery in people with diabetes?
Key messages
‐ Intensive blood sugar control leads to lower levels, which may increase the risk of 'hypoglycaemia' (low blood sugar levels below what is healthy).
‐ Intensive control does not reduce mortality. Moreover, it may not reduce the risk of infections or kidney problems, or time in the hospital or intensive care unit. However, intensive control may reduce the risk of cardiovascular problems.
‐ More studies are needed to understand the effect of this intervention across different types of surgeries.
What is already known?
The perioperative period is the time surrounding an individual's surgical procedure, involving ward admission, anaesthesia and recovery after surgery, covering the preoperative (before operation), intraoperative (during operation) and postoperative (after operation) phases of surgery. People with diabetes mellitus are at more risk of complications after surgery than the general population. Diabetes is a well‐known risk factor for complications after surgery, causing more extended hospital stays, higher healthcare resource utilisation and even more deaths. One of the most important medical complications is the increased risk of infections in the period around a surgical procedure. However, it is still unclear whether targeting more intensive blood glucose control (glycaemic control) during the perioperative period is better than targeting conventional blood glucose to reduce surgical risk in people with diabetes mellitus.
What did we want to find out?
The results of the previous review were not clear on how to handle blood glucose control during surgery in people with diabetes. Therefore, we have performed an update to obtain the most recent scientific evidence available on glucose management in people undergoing surgery.
What did we find?
We identified eight new studies that add to the previous 12 included in the last review, so a total of 20 trials are now included in this review. All the trials evaluated intensive control of blood sugar. We included 1320 participants with diabetes randomised to perioperative intensive glucose control and 1350 participants with diabetes randomised to conventional or regular glucose control in our analyses. The trials were conducted on all continents. The mean duration of the intervention period varied from during surgery to five days. The mean age of the participants was 63 years.
What were the main results of our review?
Despite lower blood sugar concentrations during the perioperative period, intensive glucose control may lead to little or no reduction in relevant postoperative outcomes such as risk of infection, kidney problems, and hospital and intensive care unit stay. Likewise, intensive glycaemic control results in little or no difference in all‐cause mortality.
Compared with conventional glucose control, intensive glucose control may reduce the risk of cardiovascular problems.
Intensive glucose control may slightly increase the risk of hypoglycaemia events, including serious ones.
What are the limitations of the evidence?
We have high confidence in the results for mortality, but our confidence is low or very low for the other results. This is because of limitations in the studies, and imprecise and inconsistent results.
How up‐to‐date is the evidence?
This evidence is current to 25 July 2022
Summary of findings
Summary of findings 1. Summary of findings: perioperative control for people with diabetes undergoing surgery.
| Perioperative glycaemic control for people with diabetes undergoing surgery | ||||||
|
Patients: people with diabetes undergoing surgery Settings: hospital Intervention: intensive blood glucose control Comparison: conventional blood glucose control | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comment | |
| Risk with conventional glucose control a | Risk with intensive glucose control | |||||
|
All‐cause mortality (death from any cause) Follow‐up: from 28 days to 1 year |
91 per 1000 | 98 per 1000 (80 to 121) | RR 1.08 (0.88 to 1.33) | 2551 (18) | ⊕⊕⊕⊕ highb,c,d | The pooled relative effect was based on 15 studies; 3 studies could not be included as they had zero events in both the intervention and control groups. |
|
Severe hypoglycaemic episodes (number of severe hypoglycaemic episodes) Follow‐up: from 28 days to 1 year |
6 per 1000 | 29 per 1000 (13 to 65) | RR 4.73 (2.12 to 10.55) | 1896 (11) | ⊕⊕⊝⊝ lowc,e,f | The pooled relative effect was based on 8 studies; 3 studies could not be included as they had zero events in both the intervention and control groups. |
|
Infectious complications (infectious complication after surgery (e.g. pneumonia, urinary tract infection)) Follow‐up: from 28 days to 1 year |
183 per 1000 | 137 per 1000 (101 to 190) | RR 0.75 (0.55 to 1.04) | 2453 (18) | ⊕⊕⊕⊝ lowc,g,h | The pooled relative effect was based on 16 studies; 2 studies could not be included as they had zero events in both the intervention and control groups. |
|
Cardiovascular events (incidents that may cause damage to the cardiovascular system) Follow‐up: from 28 days to 1 year |
244 per 1000 | 178 per 1000 (134 to 237) | RR 0.73 (0.55 to 0.97) | 1454 (12) | ⊕⊕⊕⊝ lowc,h,i | The pooled relative effect was based on 11 studies; one study could not be included as it had zero events in both the intervention and control groups. |
|
Renal failure (number of individuals with an elevation of serum creatinine greater than 2 mg/dL or requiring dialysis) Follow‐up: from 28 days to 1 year |
149 per 1000 | 138 per 1000 (103 to 182) | RR 0.92 (0.69 to 1.22) | 2086 (14) | ⊕⊕⊕⊝ lowc,g,j | — |
|
Length of ICU stay (days admitted to the ICU unit) Follow‐up: from 28 days to 1 year |
The mean length of ICU stay ranged across control groups from 1.2 to 7.4 days | The mean length of ICU stay in the intervention groups was 0.1 days shorter (0.57 days shorter to 0.38 days longer) | — | 1687 (11) | ⊕⊕⊕⊝ lowh,k |
— |
|
Length of hospital stay (days admitted to the hospital) Follow‐up: from 28 days to 1 year |
The mean length of hospital stay ranged across control groups from 5.0 to 19.6 days | The mean length of hospital stay in the intervention groups was 0.79 days shorter (1.79 days shorter to 0.21 days longer) | — | 1520 (12) | ⊕⊕⊝⊝ very lowh,k,l |
— |
| CI: confidence interval; ICU: intensive care unit; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group quality of evidence grades High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aMean baseline risk from the included studies.
bNot downgraded for risk of bias: studies with an unclear risk of selection bias did not have major impact on certainty. A sensitivity analysis omitting these studies showed a similar effect estimate (RR 1.09, 95% CI 0.88 to 1.36).
cOptimal information sizes are estimated to assess the precision of the effect estimates according to a threshold of an anticipated 25% relative risk reduction.
dNot downgraded for imprecision: according to the control group event rate (0.1) and an anticipated 25% relative risk reduction, the optimal information size has been reached.
eDowngraded by one level due to the impact of risk of bias: the trial informing the outcome was open for participants and personnel, and many did not provide details on blinding of outcome assessment.
fDowngraded by one level for imprecision: optimal information size probably not met; with a 0.05 control group event rate and an anticipated 25% relative risk reduction, about 3500 participants would be required.
gDowngraded by one level for imprecision: optimal information size probably not met; with a 0.10 control group event rate and an anticipated 25% relative risk reduction, about 3000 participants would be required.
hDowngraded by one level due to risk of bias: all included studies were at an overall high risk of bias.
iDowngraded by one level for imprecision: optimal information size probably not met; with a 0.10 control group event rate and an anticipated 25% relative risk reduction, about 2000 participants would be required.
jDowngraded by one level for indirectness: heterogeneous definition of the outcome measure.
kDowngraded by one level for inconsistency: unexplained statistical heterogeneity within effect estimates from trials informing this outcome (I2 = 69% for length of ICU stay, I2 = 77% for length of hospital stay) with large differences in point estimates.
lDowngraded by one level for imprecision: lower 95% CI boundary includes the possibility of an important benefit.
Background
Description of the condition
Diabetes mellitus is a chronic disease that occurs when the pancreas does not secrete enough insulin or the body does not use insulin effectively, leading to hyperglycaemia. Different types of diabetes mellitus have been identified according to aetiological criteria that focus on the impact that various factors, such as genetics, insulin resistance, environmental markers and immune system inflammation, have on the progressive loss of the β‐cell mass and/or function. The most prevalent types of diabetes are type 1 and type 2, which are heterogeneous in their presentation and progression, and challenging to diagnose and treat. Treatment depends on the typology and must be individualised. Currently, non‐pharmacological actions (diet and exercise) are combined with oral hypoglycaemic agents (in type 2 diabetes) and/or insulin (ADA 2021).
The World Health Organization (WHO) considers diabetes mellitus to be a silent epidemic and estimates that more than 400 million people worldwide suffer from this condition, with a higher prevalence in low‐ and middle‐income countries. In 2019, diabetes was the ninth leading cause of death with an estimated 1.5 million deaths directly caused by diabetes; 48% of deaths are premature (under the age of 70 years). Deaths due to diabetes are associated with complications arising from the impact of chronic hyperglycaemia at the micro‐ and macrovascular levels. Diabetes is a significant cause of blindness, kidney failure, heart attacks, stroke and lower limb amputation (WHO 2021).
People with diabetes mellitus are particularly vulnerable to surgical procedures due to the complex idiosyncrasy of the perioperative period. This period ranges from ward admission (preoperative), to anaesthesia and surgery (intraoperative), to recovery (postoperative). During the perioperative process, it is important to be aware of the interference of the disease in multiple organ systems and the well‐known potential complications, such as increased length of hospital stay, higher health care resource utilisation, and greater perioperative morbidity (particularly due to infection) and mortality (Drayton 2022).
The increased risk of infection in the perioperative period is thought to be due to a combination of the long‐term effects of hyperglycaemia on blood vessels (occlusion) and neutrophil dysfunction due to immune‐mediated impairment. Hyperglycaemia also compromises phagocytosis, thus lowering the barriers to infection. Although the perioperative period is relatively short and many of the implications of hyperglycaemia are difficult to reverse, there is literature suggesting that improving glycaemic control enhances immune function and consequently reduces the risk of infection (AHRQ 2001; Smiley 2006).
Description of the intervention
In the perioperative glycaemic management of people with diabetes, it is common practice to suspend or administer a minimal dose of hypoglycaemic drugs and start an intravenous infusion of glucose at a low rate while the individual is in fasting status. This infusion is prolonged until the person is able to start eating, at which point the usual drug regimen is restored. Often, a sliding‐scale insulin regimen or a schedule of regular, subcutaneous insulin dosages after capillary blood glucose measurements are also continued through the perioperative period. However, the use of a sliding scale may result in wide variations in serum glucose, questioning the rationale for this method (AHRQ 2001).
The importance of measuring the relationship between glycaemic control and the risk of complications before, during and after surgery for people with diabetes lies in the possibility of getting a better prediction of risk for individual people and of improving clinical surveillance during surgery. Pomposelli 1998 studied the relationship between glycaemic perioperative control and postoperative nosocomial infection in 100 participants with diabetes undergoing elective surgery and found that a single blood glucose level greater than 220 mg/dL on the first postoperative day was a sensitive (87.5%) predictor of postoperative infection, with 2.7 times higher infection rates and, if minor infections were excluded, 5.7 times higher (serious) infection rates. Furnary 1999 studied the relationship in 1499 individuals undergoing coronary artery bypass grafting and reported that a continuous insulin infusion protocol aimed at maintaining blood glucose within 150 mg/dL to 200 mg/dL was associated with a significant reduction in perioperative blood glucose levels, which led to a significant reduction in the incidence of deep sternal wounds. Golden 1999 studied a cohort of 411 adults undergoing coronary artery surgery and found that, compared with participants with postoperative glucose levels within 121 mg/dL to 206 mg/dL, the risk of infection (defined as at least one of the following events 36 hours after surgery: pneumonia, urinary tract infection, wound infection or other infections with either positive culture or associated fever) was increased by 17% for those with blood glucose levels between 207 mg/dL and 229 mg/dL, by 78% in individuals with blood glucose levels between 253 mg/dL and 352 mg/dL, and by 86% for blood glucose levels between 230 mg/dL and 252 mg/dL. Van den Berghe 2001studied whether the normalisation of blood glucose levels with insulin therapy improved the prognosis in a sample of 1548 critically ill participants receiving mechanical ventilation in a surgical intensive care unit (ICU) (783 and 765 assigned to conventional and intensive treatment, respectively). They reported that, at 12 months, intensive insulin therapy showed a significant mortality reduction from 8.0% with conventional treatment to 4.6%, even greater when analysing deaths due to multiple organ failure with a proven septic focus. Intensive insulin therapy also reduced overall in‐hospital mortality by 34%, bloodstream infections by 46%, acute renal failure requiring dialysis or haemofiltration by 41%, the median number of red cell transfusions by 50% and critical illness polyneuropathy by 44%.
Controlled clinical trials studying the benefits of intensive glycaemic control versus conventional glycaemic control reported inconsistent findings. Thus, for example, Van den Berghe 2001demonstrated significant benefits in mortality reduction associated with intensive insulin therapy. However, Gandhi 2007 did not find significant benefits when analysing 371 adults undergoing on‐pump cardiac surgery.
Adverse effects of the intervention
The main adverse event that may be expected when introducing an intensive insulin regimen aimed at reaching normoglycaemia is hypoglycaemia. There is increasing evidence pointing to a potential link between hypoglycaemia and the risk of cardio/cerebrovascular events in people with diabetes (Gandhi 2007; Seaquist 2013). Actually, hypoglycaemia is clearly associated with an increased risk of cardiovascular events in people treated with glucose‐lowering medications that increase the risk of hypoglycaemia (mainly insulin and sulphonylureas); this risk has also been shown to be present in inpatients during hospitalisation periods (International Hypoglycaemia Study Group 2019). Although more research insight is needed to clarify the pathogenetic pathway through which hypoglycaemia may lead to cardiovascular disease events, several mechanisms may be involved, i.e. blood coagulation abnormalities, sympathoadrenal response (cardiac arrhythmia and haemodynamic changes), endothelial dysfunction and inflammatory response (International Hypoglycaemia Study Group 2019; Seaquist 2013).
How the intervention might work
Hyperglycaemia has been identified as an independent risk factor for perioperative surgical complications, including death (Gandhi 2005). Hyperglycaemia is known to impact immune status, wound healing and vascular function. It is therefore conceivable that normalising an individual's blood glucose levels could reduce the morbidity and mortality associated with surgical interventions.
Why it is important to do this review
Currently, the available data point to a positive effect of improved glycaemic control on infections and other medical complications after surgical procedures. Although our previous systematic review demonstrated no clear differences for most outcomes when intensive perioperative glycaemic control was compared to conventional glycaemic control in participants with diabetes mellitus (Buchleitner 2012), a post hoc analysis indicated that intensive glycaemic control was associated with a higher number of participants experiencing episodes of hypoglycaemia. Since the publication of the previous version of our review in 2012, several new eligible trials have been published. These events have thus triggered an update of this review. We have attempted to identify, appraise and synthesise all newly published research evidence relevant to assessing the effect of intensive glycaemic control on surgical adverse events and other outcomes, to update our previous findings in order to inform decision‐making and the development of guidelines for the perioperative management of people with diabetes undergoing major surgery.
Objectives
To assess the effects of perioperative glycaemic control for patients with diabetes undergoing surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCTs).
Types of participants
Participants of any age, sex or ethnicity with previously diagnosed type 1 or 2 diabetes mellitus and submitted to perioperative glycaemic control. We contacted the authors of trials reporting on people with and without diabetes and asked them for separate data on people with diabetes to include in this review.
Diagnostic criteria for diabetes mellitus
In order to be consistent with changes in the classification of, and diagnostic criteria for, diabetes mellitus over the years, the diagnosis should have been established using the standard criteria valid at the time of the trial commencing (for example, ADA 2003; ADA 2010; Alberti 1998; WHO 1999). Ideally, the diagnostic criteria should have been described. We used the trial authors' definition of diabetes mellitus if necessary. We planned to subject diagnostic criteria to a sensitivity analysis.
Definition of perioperative period
We considered the perioperative period as the time elapsed between admission, anaesthesia, surgery and recovery.
Changes in diagnostic criteria may have produced significant variability in the clinical characteristics of the participants included as well as in the results obtained (which have been investigated through sensitivity analysis).
Types of interventions
We planned to investigate the following comparison of intervention versus control/comparator.
Intervention
Perioperative glycaemic control protocol proposed by the trial authors that involves a more intensive control than conventional care.
Comparison
Perioperative glycaemic control protocol defined as standard or conventional care by the trial authors.
Concomitant interventions had to be identical in both the intervention and comparator groups to establish fair comparisons. If a trial included multiple arms, we included any arm that met the inclusion criteria for this review.
Minimum duration of intervention
The minimal clinically meaningful duration of the intervention ranged from just the duration of the surgical procedure up to 90 days of follow‐up.
Minimum duration of follow‐up
The minimal duration of follow‐up was one day (24 hours) after the perioperative glycaemic intervention.
We defined any follow‐up period going beyond the original time frame for the primary outcome measure as specified in the power calculation of the trials' protocol as an extended follow‐up period (also called an open‐label extension study) (Buch 2011; Megan 2012).
Summary of specific exclusion criteria
We excluded trials in the following categories:
Participants with different morbidities that could influence the results or some kind of co‐medication.
No separate data available in studies involving people with and without diabetes.
Paediatric population.
Emergency surgeries.
People who had off‐pump cardiopulmonary bypass procedures.
Study designs other than RCTs.
Types of outcome measures
We did not exclude a trial if it failed to report one or several of our primary or secondary outcome measures. If none of our primary or secondary outcomes were reported in the trial, we did not include the trial but provided some basic information in an additional table.
We extracted the following outcomes, using the methods and time points specified below (Lefebvre 2022).
Primary outcomes
All‐cause mortality
Severe hypoglycaemic episodes
Hypoglycaemic episodes
Infectious complications
Secondary outcomes
Cardiovascular events
Renal failure
Length of ICU stay
Length of hospital stay
Health‐related quality of life
Socioeconomic effects
Weight gain
Mean blood glucose during the intervention
Method of outcome measurement
All‐cause mortality: defined as death from any cause
Hypoglycaemic episodes: number of overall, severe and non‐severe hypoglycaemic episodes (subdivided by time of day of occurrence)
Infectious complications: any kind of infectious complication (e.g. pneumonia, urinary tract infection)
Cardiovascular events: defined as any incidents that may cause damage to the cardiovascular system
Renal failure: defined as an elevation of the serum creatinine greater than 2 mg/dL or need for dialysis
Length of ICU stay: defined as days admitted to the ICU
Length of hospital stay: defined as days admitted to the hospital unit
Health‐related quality of life: evaluated by a validated instrument such as QOLS (Quality of Life Scale) or WHOQOL (World Health Organization Quality of Life Questionnaire), SF36 (36‐Item Short Form Survey), etc.
Socioeconomic effects: such as direct costs defined as admission or readmission rates; the average length of stay; visits to general practitioner; accident or emergency visits; medication consumption; indirect costs defined as resources lost due to illness by the participant or their family member
Weight gain: defined as the difference between the weight (kg) before and after the intervention
Mean blood glucose during the intervention: defined as the mean of total glucose values (mg/dL) obtained from the start to the end of the intervention
Timing of outcome measurement
All‐cause mortality, hypoglycaemic episodes, infectious complications, cardiovascular events, renal failure, health‐related quality of life, socioeconomic effects, weight gain and mean blood glucose: any time after participants were randomised to the intervention/comparator groups
Length of ICU and hospital stay: at ICU and hospital discharge
Search methods for identification of studies
Electronic searches
For this update, we searched the following sources from 1 January 2012 to 25 July 2022 and placed no restrictions on the language of publication:
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO) (last searched on 25 July 2022);
MEDLINE OvidSP (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE; from 1946 to present) (last searched on 25 July 2022);
LILACS (last searched on 25 July 2022);
ClinicalTrials.gov (www.clinicaltrials.gov) (last searched on 25 July 2022);
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch/) (last searched on 25 July 2022).
We did not include Embase in our search, as RCTs indexed in Embase are now prospectively added to CENTRAL via a highly sensitive screening process (Cochrane 2022). For detailed search strategies, see Appendix 1.
In addition to the Boolean searches described above, our Information Specialist carried out a PubMed 'Similar articles' search. This search was based on 12 records of included studies from the previous review (see Appendix 1). It was conducted on 12 September 2018 and subsequently imported into CRS Web (Cochrane Register of Studies), where the Cochrane RCT classifier was applied and records removed (Marshall 2018), when they were categorised by the classifier as having less than 74% probability of being an RCT. We screened the records with a probability ≥ 75% together with the search results from the Boolean searches.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials, systematic reviews and meta‐analyses. In addition, we contacted authors of included trials to identify any additional information on the retrieved trials and establish whether we may have missed further trials.
We did not use abstracts or conference proceedings for data extraction unless full data were available from trial authors because this information source does not fulfil the CONSORT requirements, which consist of "an evidence‐based, minimum set of recommendations for reporting randomized trials" (CONSORT 2016; Scherer 2007). We presented information on abstracts or conference proceedings in the Characteristics of studies awaiting classification table.
Data collection and analysis
Selection of studies
Two review authors (MH, GG) independently screened the abstract, title, or both, of every record retrieved by the literature searches to determine which trials we should assess further. We obtained the full texts of all potentially relevant records. We resolved any disagreements through consensus or by recourse to a third review author (DM). If we could not resolve a disagreement, we categorised the trial as a 'study awaiting classification' and contacted the trial authors for clarification. We presented an adapted PRISMA flow diagram to show the process of trial selection (Page 2020). We listed all articles excluded after full‐text assessment in a Characteristics of excluded studies table and provided the reasons for exclusion.
Data extraction and management
For studies that fulfilled our inclusion criteria, two review authors (ER, FB) independently extracted key information on participants, interventions and comparators. We described interventions according to the 'template for intervention description and replication' (TIDieR) checklist (Hoffmann 2014; Hoffmann 2017).
We reported data on efficacy outcomes and adverse events using standardised data extraction sheets from the CMED Group. We resolved any disagreements by discussion or, if required, by consultation with a third review author (DM) (for details see Characteristics of included studies; Table 2; Table 3; Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15; Appendix 16).
1. Overview of study population (participants with diabetes undergoing surgery).
| Study (design) | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible (N) | Randomised (N) | Analysed (primary outcome) (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up |
|
Duncan 2018 Parallel‐group RCT |
I: hyperinsulinaemic normoglycaemia | — | — | 226* | 226* | 226* | 100* | Follow ‐up 30 months 475 * Follow‐up 1 year: — |
| C: standard therapy | — | — | 249* | 249* | 249* | 100* | ||
| Total: | 475* | 475* | 475* | 100* | ||||
|
Wallia 2017 Parallel‐group RCT |
I: intensive (140) | — | — | 23* | 23* | 23* | 100* | 1 year follow‐up: 49* |
| C: moderate (180) | — | — | 26* | 26* | 26* | 100* | ||
| Total: | 49* | 49* | 49* | 100* | ||||
|
Wahby 2016 Parallel‐group RCT |
I: tight glycaemic control | — | — | 67 | 67 | 67 | 100 | 30 days follow up: 135 |
| C: conventional moderate glycaemic control | 68 | 68 | 68 | 100 | ||||
| Total: | 135 | 135 | 135 | 100 | ||||
|
Parekh 2016 Parallel‐group RCT Study terminated early |
I: moderately intense control | With power of 80% and a P value of 0.05 the sample size is 40 recipients per study group | — | 30 | 30 | 30 | 100 | Follow up 30 days: 58 (97%) Follow up 6 months: 51 (83.3%) Follow up 1 year: 42 (70%) |
| C: standard glucose control | 30 | 30 | 30 | 100 | ||||
| Total: | 60 | 60 | 60 | 100 | ||||
|
Yuan 2015 Parallel‐group RCT |
I: intensive glycaemic (IG) management | — | 248 | 106 | 106 | 105 | 99 | — |
| C: conventional glycaemic (CG) management | 106 | 106 | 105 | 99 | ||||
| Total: | 212 | 212 | 210 | 99 | ||||
|
Umpierrez 2015 Parallel‐group RCT |
I: intensive group | 152* | 77* | 77* | 77* | 100* | — | |
| C: conservative group | 75* | 75* | 75* | 100* | ||||
| Total: | 152* | 152* | 152* | 100* | ||||
|
Abdelmalak 2013 Parallel‐group RCT Study terminated early |
I: intensive glucose management | — | — | 54 | 54* | 54* | 100* | Follow up 30 days: 103* Follow up 1 year: 103* |
| C: conventional glucose management | 49 | 49* | 49* | 100* | ||||
| Total: | 103* | 103* | 100 | |||||
|
Hermayer 2012 Parallel‐group RCT |
I: intensive glycaemic control | "The statistical error rates were established a priori at 0.15 and 0.20 for the type I and type II error rates, respectively. The sample size for the clinical trial was based on feasibility of enrollment along with power to detect a clinically relevant effect size in secondary endpoints (e.g. BG levels and biomarkers) at the P 0.15 level of significance. The upper bound for the recruitment potential of this single clinical site was based on transplant volume. The transplant center estimated approximately 150 renal transplants per year; of these, it was conservatively estimated that approximately 30% of these patients would be have diabetes, eligible, and willing to participate in this study. Thus, approximately 45 patients per year would be the expected accrual annual rate. This rate for a projected accrual period of 27 months would be expected to yield approximately 90 participants in the trial. Using the feasible sample size of 90 participants, there was 80% power to detect an effect size of 0.48 for the secondary outcomes (i.e. a 0.48‐SD difference in continuous outcomes)" | 104 | 52 | 44 | 44 | 85 | Follow‐up for at least 2 weeks (interim analysis): 51 |
| C: standard glycaemic control | 52 | 49 | 49 | 94 | ||||
| Total: | 104 | 93 | 93 | 89 | ||||
|
Desai 2012 Parallel‐group RCT |
I: strict blood glucose control | — | 223* | 44* | 44* | 44* | 100* | Follow up 30 days: 81* |
| C: liberal blood glucose control | 37* | 37* | 37* | 100* | ||||
| Total: | 81* | 81* | 81* | 100 | ||||
|
Lazar 2011 Parallel‐group RCT |
I: aggressive glucose control | — | — | 40 | 40 | 40 | 100 | Follow up 30 days: 82 |
| C: moderate glucose control | 42 | 42 | 42 | 100 | ||||
| Total: | 82 | 82 | 82 | 100 | ||||
|
Cao 2010 Parallel‐group RCT |
I: intensive insulin therapy | "The minimum required sample size was determined by using an appropriate formula that would provide 80% power to detect a 16% difference in the postoperative complication rate at a 0.05 level (two sided test)" | 220 | 92 | 92 | 92 | 100 | Follow up 28 days: 179 |
| C: conventional insulin therapy | 87 | 87 | 87 | 100 | ||||
| Total: | 179 | 179 | 179 | 100 | ||||
|
Glucontrol 2009 Parallel‐group RCT Study terminated early |
I: intensive insulin therapy | — | — | 55 | 55* | 55* | 100* | Follow up 28 days: 124* |
| C: intermediate glucose control | 69 | 69* | 69* | 100* | ||||
| Total: | 124* | 124* | ||||||
|
NICE SUGAR 2009 Parallel‐group RCT |
I: intensive glucose control | — | — | 213* | 213* | 203* | 95* | Follow up 90 days: 402* |
| C: conventional glucose control | 208* | 208* | 199* | 96* | ||||
| Total: | 421* | 421* | 402* | 95 | ||||
|
Subramaniam 2009 Parallel‐group RCT Study terminated early |
I: continuous insulin infusion | — | — | 62 | 62 | 62 | 100 | Follow up 30 days: 126 |
| C: standard intermittent insulin bolus | 64 | 64 | 64 | 100 | ||||
| Total: | 126 | 126 | 100 | |||||
|
Chan 2009a Parallel‐group RCT |
I: intensive Insulin treatment | — | — | 10* | 10* | 10* | 100* | Follow up 30 days: 32* |
| C: conventional insulin treatment | 22* | 22* | 22* | 100* | ||||
| Total: | 32* | 32* | 32* | 100 | ||||
|
De La Rosa 2008 Parallel‐group RCT |
I: intensive insulin therapy | — | — | 11* | 11* | 11* | 100* | Follow up 28 days: 13* |
| C: standard insulin therapy | 2* | 2* | 2* | 100* | ||||
| Total: | 13* | 13* | 13* | 100* | ||||
|
Gandhi 2007 Parallel‐group RCT |
I: intensive treatment | — | — | 38 | 37 | 37 | 97 | Follow up 30 days: 73 |
| C: conventional treatment | 40 | 36 | 36 | 90 | ||||
| Total: | 78 | 73 | 73 | 94 | ||||
|
Li 2006b Parallel‐group RCT |
I: continuous insulin infusion (CII) | — | — | 51 | 51 | 51 | 100 | — |
| C: glucometer‐guided insulin (GGI) | 49 | 42 | 42 | 86 | ||||
| Total: | 100 | 93 | 93 | 93 | ||||
|
Lazar 2004 Parallel‐group RCT |
I: tight glycaemic control with GIK | — | — | 72 | 72 | 72 | 100 | Follow up 30 days: 141 Follow up 5 years: 120 |
| C: standard therapy | 69 | 69 | 69 | 100 | ||||
| Total: | 141 | 141 | 141 | 100 | ||||
|
Rassias 1999 Parallel‐group RCT |
I: aggressive insulin therapy | "Using published data, we determined that group sizes of 13 patients per study group would give us 84% power to detect a significant (20%) increase in Polymorphonuclear neutrophils function in the treatment groups after surgery at a 5% level of significance" | — | 13 | 13 | 13 | 100 | — |
| C: standard insulin therapy | 13 | 13 | 13 | 100 | ||||
| Total: | 26 | 26 | 26 | 100 | ||||
| Grand total | All interventions | 1320 | 1316 | |||||
| All comparators | 1350 | 1333 | ||||||
| All interventions and comparators | 2670 | 2649 | ||||||
aFrom the total population 11 participants withdrew from both groups after randomisation. bSeven participants in the control group dropped out of the study after surgery and were switched to the intervention regimen because their personal surgeon considered their degree of blood glucose control to be unacceptable.
Number of randomised participants in Glucontrol 2009 and Subramaniam 2009 not available; ITT population used instead.
*Data provided by study authors.
—: not reported
C: control group; GIK: glucose‐insulin‐potassium; I: intervention group; ITT: intention‐to‐treat: RCT: randomised controlled trial.
2. Overview of study population (total participants of studies).
| Study (design) | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible (N) | Randomised (N) | Analysed (primary outcome) (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up |
|
Duncan 2018 Parallel‐group RCT |
I: hyperinsulinaemic normoglycaemia | "A maximum of 2,790 patients was required to detect a 30% relative reduction in the composite of any major complications (i.e., any vs. none) from an expected 15% incidence of complications in the standard group at the overall 0.05 significance level with 90% power" | — | 709 | 709 | 709 | 100 | Follow up 30 days: 1439 Follow up 1 year: 1335 |
| C: standard therapy | 730 | 730 | 730 | 100 | ||||
| Total: | 1439 | 1439 | 1439 | 100 | ||||
|
Wallia 2017 Parallel‐group RCT |
I: intensive (140 group) | "Based on our cross‐sectional analyses, the 1‐year rejection rate in the 140‐mg/dL group was assumed to be 20% and in the 180‐mg/dL group was assumed to be 44%. Using these rates, we estimated that a total sample size of 136 patients, 68 in each group, would give us 80% power (a = 0.05, 2‐sided) to detect a statistically significant difference between these groups. We estimated a potential withdrawal rate of 20%, giving a total of 82 patients in each group, for a total of 164 patients to be randomized" | 733 | 82 | 82 | 82 | 100 | 1 year follow‐up: 164 |
| C: moderate (180 group) | 82 | 82 | 82 | 100 | ||||
| Total: | 164 | 164 | 164 | 100 | ||||
|
Wahby 2016 Parallel‐group RCT |
I: tight glycaemic control | — | — | 67 | 67 | 67 | 100 | 30 days follow‐up: 67 |
| C: conventional moderate glycaemic control | 68 | 68 | 68 | 100 | 30 days follow‐up: 68 | |||
| Total: | 135 | 135 | 135 | 100 | ||||
|
Parekh 2016 Parallel‐group RCT Study terminated early |
I: moderately intense control | "With a power of 80% and P‐value of.05, we estimated a needed sample size of 40 recipients per study group" | 327 (69) | 30 | 30 | 30 | 100 | Follow up 30 days: 58 Follow up 6 months: 51 Follow up 1 year: 42 |
| C: standard glucose control | 30 | 30 | 30 | 100 | ||||
| Total: | 60 | 60 | 60 | 100 | ||||
|
Yuan 2015 Parallel‐group RCT |
I: intensive glycaemic (IG) management | — | 248 | 106 | 106 | 105 | 99 | — |
| C: conventional glycaemic (CG) management | 106 | 106 | 105 | 99 | ||||
| Total: | 212 | 212 | 210 | 99 | ||||
|
Umpierrez 2015 Parallel‐group RCT |
I: intensive group | "Sample size was based on previous studies by van den Berghe et al. and Umpierrez et al. We estimated an incidence rate of the primary end point in the control group of 20% and odds ratio for the intensive versus conservative glucose control group of 0.35. We expected a low attrition rate of, 10% in the ICU; using two sided Fisher exact test, with a = 0.05, we estimated that the sample size required for 80% power to be 148 patients per group (a total of 296 patients) for the primary end point. For all analyses, reported P values are two‐sided, and P values 0.05 were considered significant. All analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC)" | 855 | 152 | 151 | 151 | 99 | Follow‐up 90 days after discharge 151 |
| C: conservative group | 153 | 151 | 151 | 99 | ||||
| Total: | 305 | 302 | 302 | 99 | ||||
|
Abdelmalak 2013 Parallel‐group RCT Study terminated early |
I: intensive glucose management | "A maximum of 970 total patients were required to have 90% power at the 0.05 significance level to detect a 40% relative reduction on the primary outcome for the most effective intervention (whichever of the three), assuming effects of 20% and 10% for the other two interventions. If only one of the three factors had any effect, we had 90% power to detect a slightly narrower 37% relative reduction" | 2222 | 196 | 196 | 196 | 100 | Follow up 30 days: 381 Follow up 1 year: 381 |
| C: conventional glucose management | 185 | 185 | 185 | 100 | ||||
| Total: | 381 | 381 | 381 | 100 | ||||
|
Hermayer 2012 Parallel‐group RCT |
I: intensive glycaemic control | "The statistical error rates were established a priori at 0.15 and 0.20 for the type I and type II error rates, respectively. The sample size for the clinical trial was based on feasibility of enrollment along with power to detect a clinically relevant effect size in secondary endpoints (e.g. BG levels and biomarkers) at the P 0.15 level of significance. The upper bound for the recruitment potential of this single clinical site was based on transplant volume. The transplant center estimated approximately 150 renal transplants per year; of these, it was conservatively estimated that approximately 30% of these patients would have diabetes, eligible, and willing to participate in this study. Thus, approximately 45 patients per year would be the expected accrual annual rate. This rate for a projected accrual period of 27 months would be expected to yield approximately 90 participants in the trial. Using the feasible sample size of 90 participants, there was 80% power to detect an effect size of 0.48 for the secondary outcomes (i.e. a 0.48‐SD difference in continuous outcomes)" | 104 | 52 | 44 | 44 | 85 | Follow‐up for at least 2 weeks (interim analysis): 51 |
| C: standard glycaemic control | 52 | 49 | 49 | 94 | ||||
| Total: | 104 | 93 | 93 | 89 | ||||
|
Desai 2012 Parallel‐group RCT |
I: strict blood glucose control | "The a priori sample size of this study (N=200) was determined to be sufficient using a medium effect size, an alpha level of 0.05, and 80% power.20 Observed power was found to be robust for significant results, but not robust for nonsignificant results as expected, given the much smaller observed effect sizes for those comparisons" | 223 | 91 | 91 | 91 | 118 | Follow up 30 days: 189 |
| C: liberal blood glucose control | 98 | 98 | 98 | 84 | ||||
| Total: | 189 | 189 | 189 | 100 | ||||
|
Lazar 2011 Parallel‐group RCT |
I: aggressive glucose control | — | — | 40 | 40 | 40 | 40 | Follow up 30 days: 82 |
| C: moderate glucose control | 42 | 42 | 42 | 42 | ||||
| Total: | 82 | 82 | 82 | 82 | ||||
|
Cao 2010 Parallel‐group RCT |
I: intensive insulin therapy | "The minimum required sample size was determined by using an appropriate formula that would provide 80% power to detect a 16% difference in the postoperative complication rate at a 0.05 level (two‐sided test)" | 220 | 92 | 92 | 92 | 100 | Follow up 28 days: 179 |
| C: conventional insulin therapy | 87 | 87 | 87 | 100 | ||||
| Total: | 179 | 179 | 179 | 100 | ||||
|
Glucontrol 2009 Parallel‐group RCT Study terminated early |
I: intensive insulin therapy | "The power of the sample size of 1,078 patients to detect a 4% difference in ICU mortality with an alpha error rate of 5% and beta error rate of 20% was 32%. The expected mortality in the ‘‘control’’ group (group 1) was based on the data recorded in the preliminary survey and was used to calculate the sample size needed to detect a 4% decrease in mortality with an alpha error rate of 5% and beta error rate of 20% (n = 1,496 patients in each group). A total of 1,750 patients per group were deemed necessary to account for drop‐outs" |
7747 | 550 | 536 | 536 | 97 | Follow up 28 days: 1078 |
| C: intermediate glucose control | 551 | 542 | 542 | 98 | ||||
| Total: | 1101 | 1078 | 1078 | 98 | ||||
|
NICE SUGAR 2009 Parallel‐group RCT |
I: intensive glucose control | "The study was originally designed to enroll 4000 patients. On the basis of data reported by Van den Berghe et al. in 2006, 13 the sample size was increased to 6100, thereby providing a statistical power of 90% to detect an absolute difference in mortality between the two groups of 3.8 percentage points, assuming a baseline mortality of 30% at a two‐sided alpha level of less than 0.05" | 40,171 | 3054 | 3054 | 3010 | 99 | 90 days follow‐up 6022 |
| C: conventional glucose control | 3050 | 3050 | 3012 | 99 | ||||
| Total: | 6104 | 6104 | 6022 | 99 | ||||
|
Subramaniam 2009 Parallel‐group RCT Study terminated early |
I: continuous insulin infusion | "A conservative estimate of 5% rate of MACEs in patients undergoing vascular surgery was assumed for the current study. Assuming a 10% dropout rate, this study needed 993 patients in each group to show a 50% reduction in MACEs for 80% (1‐Beta) power and a statistical significance of P 0.05 (alfa) in patients receiving continuous intravenous insulin infusion compared with conventional therapy. An interim analysis was planned at 452 patients" | 252 | 117 | 114 | 114 | 97 | Follow up 30 days: 236 |
| C: standard intermittent insulin bolus | 125 | 122 | 122 | 98 | ||||
| Total: | 242 | 236 | 236 | 98 | ||||
|
Chan 2009 Parallel‐group RCT |
I: intensive Insulin treatment | — | 300 | 54 | 54 | 47 | 87 | Follow‐up 30 days: 98 |
| C: conventional insulin treatment | 55 | 55 | 51 | 93 | ||||
| Total: | 109 | 109 | 98 | 90 | ||||
|
De La Rosa 2008 Parallel‐group RCT |
I: intensive insulin therapy | "We estimated that the enrollment of 504 patients would provide a power of 80% to detect an absolute reduction of 10% in the 28‐day mortality rate with an alpha error (two‐sided test) of 0.05. We assumed a 25% mortality rate in the control group" | 1643 | 254 | 254 | 252 | 99 | Follow up 28 days: 502 |
| C: standard insulin therapy | 250 | 250 | 250 | 100 | ||||
| Total: | 504 | 504 | 502 | 99 | ||||
|
Gandhi 2007 Parallel‐group RCT |
I: intensive treatment | "On the basis of a composite outcome rate of 40% in the conventional treatment group. We needed to enroll 177 patients per treatment group to have 90% power (2‐sided " level of 0.05) of finding a 40% decrease in the composite outcome with intensive insulin therapy (decrease from 40% to 24%). Because we expected that approximately 10% of patients would not experience hyperglycemia during surgery, we randomly assigned 200 patients per treatment group to ensure a sufficient number with outcome information" | 502 | 199 | 188 | 185 | 93 | Follow up 30 days: 371 |
| C: conventional treatment | 201 | 191 | 186 | 93 | ||||
| Total: | 400 | 379 | 371 | 93 | ||||
|
Li 2006 Parallel‐group RCT |
I: continuous insulin infusion (CII) | — | — | 51 | 51 | 51 | 100 | — |
| C: glucometer‐guided insulin (GGI) | 49 | 42 | 42 | 86 | ||||
| Total: | 100 | 93 | 93 | 93 | ||||
|
Lazar 2004 Parallel‐group RCT |
I: tight glycaemic control with GIK | — | — | 72 | 72 | 72 | 100 | Follow up 30 days: 141 Follow up 5 years: 120 |
| C: standard therapy | 69 | 69 | 69 | 100 | ||||
| Total: | 141 | 141 | 141 | 100 | ||||
|
Rassias 1999 Parallel‐group RCT |
I: intensive insulin therapy | "Using published data, we determined that group sizes of 13 patients per study group would give us 84% power to detect a significant (20%) increase in Polymorphonuclear neutrophils function in the treatment groups after surgery at a 5% level of significance" | — | 13 | 13 | 13 | 100 | — |
| C: standard insulin therapy | 13 | 13 | 13 | 100 | ||||
| Total: | 26 | 26 | 26 | 100 | ||||
| Grand total | All interventions | 5981 | 5887 | |||||
| All comparators | 5996 | 5914 | ||||||
| All interventions and comparators | 11,977 | 11,801 | ||||||
Number of randomised participants in Glucontrol 2009 and Subramaniam 2009 not available; ITT population used instead.
*Data provided by study authors.
—: not reported
C: control group; GIK: glucose‐insulin‐potassium; I: intervention group; ITT: intention‐to‐treat; RCT: randomised controlled trial.
We provided information, including the study identifier for potentially relevant ongoing trials in the Characteristics of ongoing studies table and in Appendix 9 'Matrix of trial endpoint (publications and trial documents)'. We attempted to find the protocol for each included study, and we reported in Appendix 9 the primary, secondary and other outcomes from these protocols, alongside the date from the study publications.
We emailed all authors of included trials to enquire whether they would be willing to answer questions regarding their trials. We presented the results of this survey in Appendix 17. We thereafter sought relevant missing information on the trial from the primary trial author(s), if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary trial, we maximised the information yield by collating all available data, and we used the most complete data set aggregated across all known publications. We listed duplicate publications, companion documents, multiple reports of a primary trial and trial documents of included trials (such as trial registry information) as secondary references under the study ID of the included trial. Furthermore, we also listed duplicate publications, companion documents, multiple reports of a trial and trial documents of excluded trials (such as trial registry information) as secondary references under the study ID of the excluded trial.
Data from clinical trials registers
If data from included studies were available as study results in clinical trials registers, such as ClinicalTrials.gov or similar sources, we made full use of this information and extracted the data. If there was also a full publication of the study, we collated and critically appraised all available data. If an included study was marked as a completed study in a clinical trial register but no additional information was available (study results, publication, or both), we added this study to the Characteristics of studies awaiting classification table.
Assessment of risk of bias in included studies
Two review authors (IS, FB) independently assessed the risk of bias for each included trial. We resolved disagreements by consensus or by consulting a third review author (DM). In the case of disagreement, we consulted the remainder of the review author team and made a judgement based on consensus. If adequate information was unavailable from the trial publications, trial protocols or other sources, we contacted the trial authors for more detail to request missing data on risk of bias items.
We used the Cochrane risk of bias assessment tool (Higgins 2019b), assigning assessments of low, high or unclear risk of bias (for details see Appendix 2; Appendix 3). We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions according to the criteria and associated categorisations contained therein (Higgins 2019b).
Summary assessment of risk of bias
We presented a risk of bias graph and a risk of bias summary figure.
We distinguished between self‐reported and investigator‐assessed and adjudicated outcome measures.
We considered the following self‐reported outcomes.
Health‐related quality of life
We considered the following outcomes to be investigator‐assessed.
All‐cause mortality
Hypoglycaemic episodes
Cardiovascular events
Renal failure
Length of ICU stay
Length of hospital stay
Socioeconomic effects
Weight gain
Mean blood glucose during the intervention
Risk of bias for a trial across outcomes
Some risk of bias domains, such as selection bias (random sequence generation and allocation sequence concealment), affect the risk of bias across all outcome measures in a trial. In case of a high risk of selection bias, we marked all endpoints investigated in the associated trial as being at high risk. Otherwise, we did not perform a summary assessment of the risk of bias across all outcomes for a study.
Risk of bias for an outcome within a trial and across domains
We assessed the risk of bias for an outcome measure by including all entries relevant to that outcome (i.e. both trial‐level entries and outcome‐specific entries). We considered a low risk of bias to denote a low risk of bias for all key domains, an unclear risk to denote an unclear risk of bias for one or more key domains and a high risk to denote a high risk of bias for one or more key domains.
Risk of bias for an outcome across trials and across domains
To facilitate our assessment of the certainty of the evidence for key outcomes, we assessed the risk of bias across studies and domains for the outcomes included in the summary of findings table. We defined the evidence as being at low risk of bias when most information came from trials at low risk of bias, unclear risk of bias when most information came from studies at a low or unclear risk of bias, and high risk of bias when a sufficient proportion of information came from studies at high risk of bias.
Measures of treatment effect
When at least two included trials were available for a comparison and a given outcome, we tried to express dichotomous data as a risk ratio (RR) with 95% confidence interval (CI). For continuous outcomes measured on the same scale (e.g. weight gain in kg) we estimated the intervention effect using the mean difference (MD) with 95% CI. For continuous outcomes that measured the same underlying concept (e.g. health‐related quality of life), but used different measurement scales, we calculated the standardised mean difference (SMD). If data had been available, we would have expressed time‐to‐event outcomes as a hazard ratio (HR) with 95% CI.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. If more than one comparison from the same trial was eligible for inclusion in the same meta‐analysis, we either combined groups to create a single pair‐wise comparison or appropriately reduced the sample size so that the same participants did not contribute data to the meta‐analysis more than once (splitting the 'shared' group into two or more groups). While the latter approach offers some solutions for adjusting the precision of the comparison, it does not account for correlation arising from the same set of participants being in multiple comparisons (Higgins 2019a).
If we had included cluster‐RCTs, we would have attempted some re‐analyses. We would have attempted to re‐analyse cluster‐RCTs that had not appropriately adjusted for the potential clustering of participants within clusters in their analyses. In these cases, the variance of the intervention effects was inflated by a design effect. Calculation of a design effect involves the estimation of an intracluster correlation coefficient (ICC). We would have obtained estimates of ICCs by contacting trial authors or imputing the ICC values by using either an estimate from other included trials that report ICCs or external estimates from empirical research (e.g. Bell 2013). In these cases, we also planned to examine the impact of clustering using sensitivity analyses.
Dealing with missing data
If possible, we obtained missing data from the authors of the included trials. We carefully evaluated important numerical data such as screened, randomly assigned participants as well as intention‐to‐treat, as‐treated and per‐protocol populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals), and we critically appraised issues concerning missing data and the use of imputation methods (e.g. last observation carried forward).
In trials where the standard deviation (SD) of the outcome was not available at follow‐up, or we could not recreate it, we standardised by the mean of the pooled baseline SD from those trials that reported this information.
Where included trials did not report means and SDs for outcomes, and we did not receive the necessary information from trial authors, we imputed these values by estimating the mean and variance from the median, range and size of the sample (Hozo 2005).
We investigated the impact of imputation on meta‐analyses by performing sensitivity analyses, and we reported for every outcome that trials had imputed SDs.
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we did not report trial results as the pooled effect estimate in a meta‐analysis. We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi2 test with a significance level of α = 0.1 (Deeks 2019). In view of the low power of this test, we also considered the I2 statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). When we identified heterogeneity, we attempted to determine potential reasons for this by examining individual characteristics of the study and subgroups.
Assessment of reporting biases
If we included 10 or more studies that investigated a particular outcome, we used funnel plots to assess small study effects. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to study size, poor methodological design (and hence bias of small studies) and selective non‐reporting (Kirkham 2010). Therefore, we interpreted the results carefully (Sterne 2011).
Data synthesis
We planned to undertake (or display) a meta‐analysis only if we judged the participants, interventions, comparisons and outcomes to be sufficiently similar to ensure a result that was clinically meaningful. Unless good evidence showed homogeneous effects across trials of different methodological quality, we primarily summarised data with a low risk of bias using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration to the whole distribution of effects and presented a confidence interval. We performed statistical analyses according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019).
Subgroup analysis and investigation of heterogeneity
We did not expect that specific characteristics from the included studies could introduce clinical heterogeneity and did not carry out subgroup analyses to explore interactions (Altman 2003).
Sensitivity analysis
We planned to restrict the analyses (when applicable) to the following factors to explore their impact on effect sizes:
Published data: excluding unpublished data.
Risk of bias: excluding studies at an overall high or unclear risk of bias.
Long‐lasting or large studies to establish how much they dominate the results: excluding smaller studies.
Summary of findings and assessment of the certainty of the evidence
Certainty of the evidence
We presented the overall certainty of the evidence for each outcome specified below, according to the GRADE approach, which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (such as directness of results). Two review authors (IS, FB) independently rated the certainty of the evidence for each outcome. We resolved any differences in assessment by discussion or consulting a third review author (DM).
We included an appendix entitled 'Checklist to aid consistency and reproducibility of GRADE assessments' to help with the standardisation of the summary of findings tables (Meader 2014). Alternatively, we used the GRADEpro Guideline Development Tool (GDT) software and presented evidence profile tables as an appendix (GRADEproGDT 2015). We presented results for the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented the results in a narrative format in the summary of findings table. We justified all decisions to downgrade the quality of trials using footnotes and made comments to aid the reader's understanding of the Cochrane Review where necessary.
Summary of findings table
We presented a summary of the evidence in a summary of findings table. This provided key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies, the numbers of participants and studies addressing each important outcome and a rating of overall confidence in effect estimates for each outcome. We created the summary of findings table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019) and Review Manager 5 software (Review Manager 2020).
The intervention presented in the summary of findings table was intensive glucose control. The comparator was conventional glucose control.
We reported the following outcomes, listed according to priority.
All‐cause mortality
Severe hypoglycaemic episodes
Infectious complications
Cardiovascular events
Renal failure
Length of ICU stay
Length of hospital stay
Results
Description of studies
For a detailed description of trials, see Table 2 and the Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies sections.
Results of the search
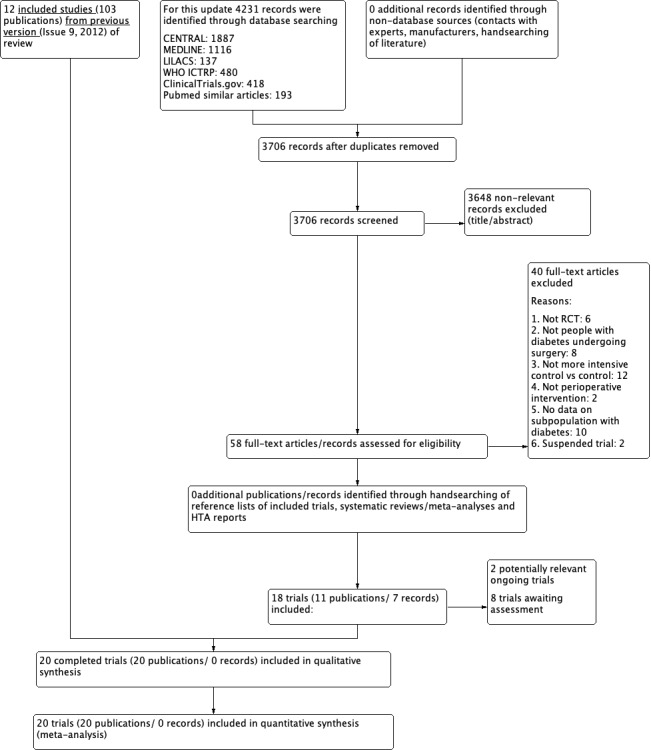
The database search yielded a total of 4231 records. After the removal of duplicates, we had 3706 records for title and abstract screening. We excluded 3648 by title and abstract. We screened a total of 58 records for eligibility. After obtaining full‐text articles, we excluded 40 studies for the following reasons: irrelevance to our research topic, not an RCT, not on participants with diabetes and undergoing surgery, not a more intensive versus conventional intervention, suspended study or outcome data for people with diabetes were not available separately. No unpublished studies were identified. We identified two registered ongoing trials (NCT02032953; NCT04742023), and listed eight records as trials awaiting classification.
Finally, incorporating eight additional studies with the 12 studies from the previous review, in this update 20 trials met our inclusion criteria (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; Hermayer 2012; Lazar 2004; Lazar 2011; Li 2006; NICE SUGAR 2009; Parekh 2016; Rassias 1999; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015).
All included studies were published in English. For a detailed description of the search results and selection procedure, see Figure 1.
1.
Included studies
A detailed description of the included trials is presented elsewhere (see Characteristics of included studies). The following is a succinct overview.
Source of data
The results of nine trials were published as original papers in scientific journals between 1999 and 2016, and additional data were obtained from entries at https://clinicaltrials.gov/. We contacted 26 trial authors to request separate data from people with diabetes; 12 of the authors replied, and 11 of them provided relevant information and the requested data. For the exact data, see Appendix 17.
Comparisons
All included trials compared an intensive glycaemic control with lower perioperative blood glucose values to less intensive glycaemic control with higher perioperative blood glucose values.
There were considerable differences between studies regarding perioperative intensive glycaemic control. The vast majority of the trials used a continuous infusion of insulin in a saline solution (Cao 2010; Chan 2009; De La Rosa 2008; Gandhi 2007; Hermayer 2012; Li 2006; NICE SUGAR 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015). One study used an algorithm for an intravenous bolus of insulin in addition to a continuous infusion of insulin (Lazar 2011), and in one study the choice of bolus therapy or insulin infusion was left to the discretion of the anaesthesia team members (Parekh 2016). In three studies, the study protocol controlled the insulin infusion, but the saline infusion and others were controlled by an anaesthesiologist (Glucontrol 2009; Rassias 1999; Subramaniam 2009). In two of the trials, it was the treatment protocol that determined insulin and glucose infusion (Abdelmalak 2013; Desai 2012). Finally, two studies used a glucose‐insulin‐potassium infusion to control the blood glucose of participants (Duncan 2018; Lazar 2004).
Blood glucose target levels varied between the studies from a very strict control (blood glucose target equal or less than 120 mg/dL) (Abdelmalak 2013; Cao 2010; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; Hermayer 2012; Lazar 2011; NICE SUGAR 2009; Yuan 2015), to less strict control (blood glucose target equal to or less than 200 mg/dL) (Lazar 2004; Li 2006; Wallia 2017). Four studies used an intermediate glucose control (blood glucose target equal to or less than 160 mg/dL) (Chan 2009; Subramaniam 2009; Umpierrez 2015; Wahby 2016). One study targeted blood glucose between strict and intermediate control (blood glucose target between 80 mg/dL and 160 mg/dL) (Parekh 2016), and in one study no blood glucose target was defined: insulin infusion was started only whenever blood glucose levels exceeded 150 mg/dL (Rassias 1999).
In the majority of the trials, blood glucose was measured hourly until blood glucose was stable, following two‐hour measurements. In six studies blood glucose was measured more often, every half hour (Abdelmalak 2013; Gandhi 2007; Lazar 2011; Parekh 2016), every 15 to 30 minutes (Rassias 1999), and every 10 to 15 minutes (Duncan 2018). In one study variable times of measurement were used following the algorithms of the glucommander (Umpierrez 2015), and one study did not mention how often blood glucose measurements were done (Cao 2010).
We can observe variations in the time points during the perioperative period when participants were undergoing the intervention. In nine trials the intervention happened in the intensive care unit (ICU), i.e. in the postoperative period when the treatment was started within 24 hours after the surgery (Cao 2010; De La Rosa 2008; Desai 2012; Glucontrol 2009; Li 2006; NICE SUGAR 2009; Umpierrez 2015; Wallia 2017; Yuan 2015). Another nine studies began after induction of anaesthesia and the intervention was continued postoperatively (Abdelmalak 2013; Chan 2009; Duncan 2018; Hermayer 2012; Lazar 2004; Lazar 2011; Parekh 2016; Subramaniam 2009; Wahby 2016). Intensive glycaemic control was performed only during the surgery in two other studies (Gandhi 2007; Rassias 1999).
We also noticed variations between studies in the control group intervention. In 11 studies the difference from the intervention group was a higher blood glucose target only (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Glucontrol 2009; NICE SUGAR 2009; Umpierrez 2015; Wahby 2016; Wallia 2017). In one trial, the blood glucose target in the control group was less intensive and during surgery an anaesthetist treated blood glucose at their discretion using intravenous insulin (Parekh 2016). In one study, an intravenous insulin bolus was used to control blood glucose instead of continuous insulin infusion; postoperatively, after the intervention was finished, the blood glucose was controlled in the same way as in the intervention group (Gandhi 2007). In three studies, blood glucose in the control group was controlled using subcutaneous insulin bolus (Hermayer 2012; Lazar 2004; Yuan 2015). Two trials used the same glucose target as in the intervention group (Li 2006; Subramaniam 2009); the two groups differed in blood glucose measurements. Participants in the control group received less frequent blood glucose measurements (every two hours instead of every hour in Li 2006 and every four hours instead of every hour in Subramaniam 2009). This difference in measurement determines the administration of insulin and better control of the blood glucose, therefore we considered the intervention more intensive than the control group. In the control groups of these studies, a subcutaneous insulin bolus method was used. One trial did not define blood glucose targets, but the control group received a regular insulin bolus if blood glucose transcended 200 mg/dL, compared with a blood glucose of more than 150 mg/dL in the intervention group (Rassias 1999).
Overview of trial populations
The 20 included studies had a total of 2670 participants with diabetes undergoing surgery that were randomised to the different comparison groups. The total sample sizes ranged from 26 to 6104 participants per study, and when restricted to participants with diabetes only from 13 to 475 participants per study. Randomised participants finishing the trial were between 93% and 100%.
Trial design
All 20 included studies had a parallel and superiority design. All trials made use of a control group where a less intensive blood glucose target was determined or a less intensive way of treatment was implemented. There were four trials with a multicentre design with the number of centres ranging from two to 42 (Duncan 2018; Glucontrol 2009; NICE SUGAR 2009; Umpierrez 2015).
All trials were reported as open‐label. Neither participants nor personnel were blinded. The outcome assessors were reported to be blinded in various trials (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Duncan 2018; Gandhi 2007; Glucontrol 2009). In the rest of the included trials blinding of outcome assessors was not reported.
Included trials were performed between 1996 and 2016. Intervention duration ranged from the duration of surgery to five days postoperative. The majority of the studies did not report the exact duration of the intervention, only that the intervention was stopped after discharge at the ICU. One study did not report any details on the duration of the intervention (Yuan 2015). Follow‐up duration ranged between discharge from ICU stay and five years. Three studies did not report their follow‐up period (Li 2006; Rassias 1999; Yuan 2015). None of the trials had run‐in periods.
Three trials were terminated early, one because of a high rate of unintended protocol violations (Glucontrol 2009), and one because of slow recruitment, the implementation of a more aggressive blood glucose control protocol in the hospital and an increase in minimally invasive surgery techniques (Subramaniam 2009). One trial was stopped after the second interim analysis at which all three interventions crossed the futility boundary for the primary outcome (Abdelmalak 2013)
Settings
Most of the trials were performed in the United States (Abdelmalak 2013; Desai 2012; Gandhi 2007; Hermayer 2012; Lazar 2004; Lazar 2011; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wallia 2017), one in Canada, one in Egypt, one in Brazil, one in Lebanon and one in Colombia, respectively (Chan 2009; De La Rosa 2008; Duncan 2018; Rassias 1999; Wahby 2016). Three studies were performed in China (Cao 2010; Li 2006; Yuan 2015). One study was performed in study centres in Austria, Belgium, France, Israel, The Netherlands, Slovenia and Spain (Glucontrol 2009), and one in study centres in Austria, New Zealand, Canada and America (NICE SUGAR 2009).
Participants
The mean age of the participants included was 63 years, ranging from 56 to 73 years (where age was not available for the surgical population with diabetes, that of the total study population was used). Females represented less than 50% in most trials; only in two of them were men less than 50% (Cao 2010, Yuan 2015), and in another study female representation was around 50% (Chan 2009).
Slightly less than half of the studies exclusively included participants with diabetes (Cao 2010; Hermayer 2012; Lazar 2004; Lazar 2011; Li 2006; Parekh 2016; Rassias 1999; Wahby 2016; Yuan 2015); the other studies included both participants with and without diabetes (Abdelmalak 2013; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; NICE SUGAR 2009; Subramaniam 2009; Umpierrez 2015; Wallia 2017). In five studies, the surgery was a coronary artery bypass graft (Desai 2012; Lazar 2004; Lazar 2011; Li 2006; Wahby 2016), and in two a possible combination with other cardiac procedures (Duncan 2018; Umpierrez 2015). In three studies, the participants were undergoing cardiac surgery (Gandhi 2007; Rassias 1999; Subramaniam 2009); one of these also included major lower extremity amputation (Subramaniam 2009). In five trials, cardiopulmonary bypass surgery was performed (Chan 2009; Duncan 2018; Lazar 2004; Lazar 2011; Rassias 1999). In two studies, participants underwent gastrectomy for gastric tumours (Cao 2010; Yuan 2015). In three studies, the inclusion criteria were to be admitted to the ICU (De La Rosa 2008; Glucontrol 2009; NICE SUGAR 2009); in this regard, De La Rosa 2008 required a minimum stay of two days. Three studies included participants undergoing renal transplantation (Hermayer 2012; Parekh 2016) and liver transplantation (Wallia 2017). One study enrolled participants having elective major non‐cardiac surgery with two or more hours of general anaesthesia (Abdelmalak 2013).
Five trials were from low‐and middle‐income countries (Cao 2010; Chan 2009; De La Rosa 2008; Wahby 2016; Yuan 2015), representing 26% of the total included participants.
Ethnicity was reported in seven studies. In five studies more than 70% of participants were white/Caucasian (Abdelmalak 2013; Desai 2012; Gandhi 2007; Umpierrez 2015; Wallia 2017). Parekh 2016 differentiated Caucasians from Icelanders, representing 17% and 45%, respectively. Also, Hermayer 2012 distinguished between White and African‐Americans (37% and 64%, respectively).
Only six of the 20 included studies reported the duration of diabetes of the participants. In these studies, the duration of the disease was balanced between the control and intervention groups. In the control group versus the intervention group, the mean duration of diabetes (in years) was 10.5 versus 10.6 (Abdelmalak 2013), 5.5 versus 6 (Cao 2010), 18.6 versus 21.1 (Parekh 2016), 10.9 versus 10.8 (Umpierrez 2015), and 6.2 versus 5.8 (Yuan 2015), respectively.
The mean body mass index (BMI) (kg/m2) was well‐balanced between the intervention and control groups. In the intervention group, the mean BMI ranged from 21.1 to 33.5 and in the control groups from 21.6 to 32.1. BMI was not recorded in five studies (Duncan 2018; Hermayer 2012; Lazar 2004; Lazar 2011; Wahby 2016).
The mean HbA1c at baseline was reported in nine studies (Abdelmalak 2013; Cao 2010; Desai 2012; Gandhi 2007; Hermayer 2012; Lazar 2011; Parekh 2016; Umpierrez 2015; Yuan 2015), with a mean value ranging from 6.5% to 8.4% in the intervention group and from 6.7% to 8.3% in the control group. In Cao 2010 these data are extracted from the total trial population.
Fifteen out of 20 studies reported co‐morbidities. Cardiovascular disease is reported in all studies, including hypertension, heart failure, myocardial infarction and atrial fibrillation. In this regard, Umpierrez 2015 only considers arterial hypertension. Cerebrovascular disease is reported in most of the studies (Duncan 2018; Gandhi 2007; Hermayer 2012; Li 2006; Subramaniam 2009; Wahby 2016; Yuan 2015), and peripheral vascular disease in two (Duncan 2018; Li 2006). With regard to respiratory disease, it was reported in six studies (Cao 2010; Duncan 2018; Lazar 2004; Li 2006; NICE SUGAR 2009; Yuan 2015). Data on renal disease were also collected in Cao 2010, De La Rosa 2008, Duncan 2018, Gandhi 2007, Li 2006, NICE SUGAR 2009, Subramaniam 2009, Wahby 2016 and Yuan 2015, and liver disease in Cao 2010, De La Rosa 2008, NICE SUGAR 2009 and Yuan 2015. Other co‐morbidities covered included cancer (De La Rosa 2008), coronary artery bypass grafting (Subramaniam 2009), coagulopathy (NICE SUGAR 2009), and dyslipidaemia (Hermayer 2012; Umpierrez 2015). Abdelmalak 2013 encompasses any type of co‐morbidity.
Thirteen of the 20 studies reported concomitant medication. Ten described the treatment for diabetes (Cao 2010; Duncan 2018; Gandhi 2007; Lazar 2004; Lazar 2011; Li 2006; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Yuan 2015). Other medical regimes are described, such as angiotensin‐converting‐enzyme (ACE) inhibitors and beta‐blockers (Desai 2012; Duncan 2018; Gandhi 2007; Lazar 2011; Subramaniam 2009), steroids (Duncan 2018; NICE SUGAR 2009), aspirin (Desai 2012; Gandhi 2007; Subramaniam 2009) or cox‐2 inhibitors (Duncan 2018), lipid‐lowering medication (Desai 2012; Duncan 2018; Lazar 2011; Subramaniam 2009), diuretics (Li 2006), vasopressors/inotropes (Glucontrol 2009; Li 2006), and antiarrhythmic medication (Duncan 2018; Gandhi 2007).
Several exclusion criteria were applied. The most common exclusion criteria include co‐morbidities, such as kidney and hepatic disease, severe obesity or medication (antibiotics, steroids), which may influence the results. Other criteria mentioned were emergency surgery (Rassias 1999; Wahby 2016), palliative surgery (Cao 2010; Yuan 2015) or off‐pump surgery (Duncan 2018; Gandhi 2007; Wahby 2016), ketoacidosis (De La Rosa 2008; NICE SUGAR 2009), history of hyperglycaemia (Umpierrez 2015), pregnancy (De La Rosa 2008; Umpierrez 2015), or imminent risk of death (Glucontrol 2009; NICE SUGAR 2009; Umpierrez 2015). Three studies did not report exclusion criteria (Lazar 2004; Li 2006; Wallia 2017).
Diagnosis
Four of the 20 trials reported the diagnostic criterion for diabetes. The diagnosis in Yuan 2015 was made according to the American Diabetes Association (ADA) criteria and for Cao 2010 the WHO criteria were followed. NICE SUGAR 2009 reported that the diagnosis of diabetes was withdrawn from the report in the participants' medical history. Li 2006 confirmed newly diagnosed diabetes with a fasting blood glucose level equal to or greater than 200 mg/dL associated with an elevated glycosylated haemoglobin A1c (HbA1c).
Interventions
In all studies, treatment in the intervention group aimed to achieve tight glycaemic control by intravenous insulin infusion. In one study target blood glucose was achieved by adjusting the glucose infusion while maintaining a fixed insulin infusion rate (Duncan 2018). In the other studies, the target blood glucose was achieved by adjusting the insulin infusion rate. The control group was treated with an intravenous insulin infusion adapted to a higher glucose target (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Desai 2012; Gandhi 2007; Glucontrol 2009; Lazar 2011NICE SUGAR 2009; Rassias 1999; Umpierrez 2015; Wallia 2017), or with boluses of subcutaneous insulin (Hermayer 2012; Lazar 2004; Li 2006; Parekh 2016; Subramaniam 2009; Wahby 2016; Yuan 2015).
In the study Abdelmalak 2013 the tight glucose control intervention was part of a factorial design with two additional arms, namely dexamethasone versus placebo and deep versus light anaesthesia.
In studies that set a specific duration of the intervention, this ranged from a few hours to five days. In three studies the intervention happened during surgery in the operating room (Abdelmalak 2013; Gandhi 2007; Rassias 1999), and in seven studies the intervention was performed during the ICU stay (De La Rosa 2008; Desai 2012; Glucontrol 2009; Li 2006; NICE SUGAR 2009; Umpierrez 2015; Wallia 2017). In seven studies the intervention started during surgery and was either extended to ICU (Chan 2009; Duncan 2018; Lazar 2004; Lazar 2011; Subramaniam 2009), or until extubation (Wahby 2016) or discharge (Hermayer 2012). In one study the intervention began before surgery and was prolonged during surgery (Parekh 2016), and in another study, the intervention was dispensed postoperatively (Yuan 2015). In one study the intervention began in the ICU and lasted until oral intake or enteral nutrition was established (Cao 2010).
Outcomes
Eleven of the 20 included studies were registered in ClinicalTrials.gov (Abdelmalak 2013; Chan 2009; Duncan 2018; Gandhi 2007; Glucontrol 2009; Hermayer 2012; Lazar 2011; NICE SUGAR 2009; Parekh 2016; Umpierrez 2015; Wallia 2017). In all of these trials, the outcomes defined in the publication showed consistency with the trial registration. In the publication of De La Rosa 2008, a registration number was provided, but it could not be found, possibly due to an error in some digit in the register.
All the studies specified the primary outcome in the publication, except for Rassias 1999 and Wahby 2016, in which it was not clearly stated. In the case of secondary outcomes, these were not listed in these two studies, nor in Desai 2012 and Yuan 2015.
All‐cause mortality and infection were the most commonly defined primary outcomes in publications. All‐cause mortality was reported in 18 studies as a primary outcome (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; Lazar 2004; Lazar 2011; Li 2006; NICE SUGAR 2009; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015). Infection complications were available in 18 studies (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Lazar 2004; Lazar 2011; Li 2006; NICE SUGAR 2009; Parekh 2016; Rassias 1999; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015). Most of the trials provided information on hypoglycaemic episodes, except three (Lazar 2004; Li 2006; Rassias 1999). All‐cause mortality, infectious complications and hypoglycaemic episodes were well‐defined in all trials that collected these data, with the exception of Wahby 2016: while they provided the number of participants with hypoglycaemia, they did not specify the blood glucose values that defined it.
Renal failure was available in 13 trials (Abdelmalak 2013; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; NICE SUGAR 2009; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Yuan 2015), and cardiovascular events in 14 trials (Abdelmalak 2013; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; Lazar 2004; Lazar 2011; Li 2006; NICE SUGAR 2009; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Yuan 2015). Only two studies assessed weight gain (Lazar 2004; Lazar 2011). Three studies reported the length of ICU stay (De La Rosa 2008; Duncan 2018; Li 2006), four studies reported the length of hospital stay (Cao 2010; Parekh 2016; Subramaniam 2009; Wallia 2017), eight studies reported both ICU and hospital length of stay (Chan 2009; Desai 2012; Gandhi 2007; Glucontrol 2009; Lazar 2004; Lazar 2011; NICE SUGAR 2009; Umpierrez 2015), and five studies did not report information in this regard (Abdelmalak 2013; Hermayer 2012; Rassias 1999; Wahby 2016; Yuan 2015). With the exception of one trial (Wahby 2016), the included trials reported on the mean blood glucose during the intervention. However, Subramaniam 2009 showed a figure with blood glucose concentrations but did not specify the data. Some of the included studies reported other complications within outcomes, such as neurological dysfunction (Chan 2009; Duncan 2018; Wahby 2016) or stroke (Gandhi 2007; Parekh 2016), prolonged mechanical ventilation (Gandhi 2007; Li 2006; Wahby 2016), respiratory failure (Umpierrez 2015), delayed or loss graft function (Hermayer 2012; Parekh 2016), use of inotropic agent support (Li 2006; Wahby 2016), mechanical circulatory support (Duncan 2018), 30‐day readmission (Wallia 2017) and, lastly, delayed gastric emptying, obstruction, bleeding, anastomotic leak and hepatic dysfunction (Yuan 2015).
Outcomes for renal failure and length of stay (ICU and hospital) were well‐defined in the 13 trials. Mean blood glucose during intervention was well‐defined in most of the included trials (Cao 2010; Chan 2009; De La Rosa 2008; Duncan 2018; Gandhi 2007; Glucontrol 2009; Hermayer 2012; Lazar 2004; Lazar 2011; Li 2006; NICE SUGAR 2009; Parekh 2016; Rassias 1999; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015), however Wahby 2016 did not report the related date and, conversely, Abdelmalak 2013 and Desai 2012 reported the data but did not provide specific outcome definition. Adverse events, other than hypoglycaemic episodes, were well‐defined in the outcomes of all included studies.
Only one study, Desai 2012, assessed quality of life using the Short Form‐12 questionnaire. However, these data were not included in the publication. We requested specific data from the author and incorporated these into the results.
In the search results, we found a sub‐study (Cardona 2017) with the same sample as Umpierrez 2015 (minus 14 participants who were excluded because of incomplete financial data), incorporating the economic data related to the participants' hospitalisation.
The duration of follow‐up differed between included trials and depended on the outcome measurements. Therefore, the follow‐up ranged from discharge from the ICU (Glucontrol 2009) to five years (Lazar 2004).
For more information on outcomes see Appendix 11.
Ongoing trials
We found two ongoing RCTs (NCT02032953; NCT04742023). See Characteristics of ongoing studies for a detailed description.
Excluded studies
We excluded 39 studies after evaluating the full text: six studies had the wrong study design (not RCTs); eight studies performed the intervention on participants who did not suffer from diabetes; 12 studies did not evaluate a more intensive glycaemic control versus moderate glycaemic control; two studies did not report on a perioperative intervention; 10 studies were excluded because separate data for participants with diabetes undergoing surgery were not obtained after contacting the authors, and two records were excluded because they were suspended trials. For further details see Characteristics of excluded studies.
Risk of bias in included studies
The risk of bias assessment of the included trials is detailed in the Characteristics of included studies section. Figure 2 and Figure 3 provide an overview of judgements about each domain for individual trials and across all included trials. We discuss the details about the judgements specifically for each domain.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials (blank cells indicate that the particular outcome was not measured in some trials).
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the particular outcome was not measured in some trials)
Allocation
We judged almost half of the included studies to be at low risk of selection bias (Abdelmalak 2013; De La Rosa 2008; Duncan 2018; Gandhi 2007; Glucontrol 2009; NICE SUGAR 2009; Parekh 2016; Umpierrez 2015). Three studies described appropriate randomisation sequences but failed to describe how they ensured concealment (Cao 2010; Wahby 2016; Wallia 2017).
The rest of the included trials did not provide enough details to judge their randomisation sequences. Six studies were simply described as randomised (Hermayer 2012; Lazar 2004; Lazar 2011; Li 2006; Rassias 1999; Yuan 2015). Two used block randomisation but did not specify if it was permuted and, in consequence, allocation to the blocks may be predictable (Desai 2012; Subramaniam 2009). Finally, a trial used randomisation sequences that were susceptible to manipulation (Chan 2009).
Blinding
Regarding performance bias, all‐cause mortality, cardiovascular events and renal failure were outcomes unlikely influenced by lack of blinding. On the other hand, the rest of the outcomes (hypoglycaemic events and other major complications, length of stay and mean blood glucose) may be influenced by lack of blinding, and we judged studies to be at high risk of performance bias as it was not feasible to blind the intervention and, in consequence, the trials were open for participants and trial personnel.
Only six studies blinded the measurement of outcomes and were at low risk of detection bias (Abdelmalak 2013; Cao 2010; Chan 2009; Gandhi 2007; Glucontrol 2009; Lazar 2011). The rest did not blind the outcome assessor (Hermayer 2012; NICE SUGAR 2009; Parekh 2016), or did not provide details (De La Rosa 2008; Desai 2012; Duncan 2018; Lazar 2004; Li 2006; Rassias 1999; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015), and, in consequence, we judged them to be at high risk of detection bias.
Incomplete outcome data
We judged 14 trials at low risk of attrition bias (Cao 2010; Chan 2009; De La Rosa 2008; Duncan 2018; Gandhi 2007; Hermayer 2012; Lazar 2004; Lazar 2011; NICE SUGAR 2009; Rassias 1999; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015).
Two trials did not recruit the number of participants to reach the sample size calculated (Parekh 2016; Subramaniam 2009), one trial modified the planned enrolment (Abdelmalak 2013), and one did not report details on the analyses to deal with this issue.
We judged a further three trials to be at unclear risk of attrition bias. Desai 2012 described cross‐over between treatment arms (approximately for 20% of randomised participants), which led to a per‐protocol analysis. Glucontrol 2009 stopped the study prematurely due to the high rate of unintended protocol violations. Li 2006 reported many protocol deviations and cross‐over between groups that were excluded from final analyses.
Selective reporting
We categorised the risk of outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification (Appendix 10). We suspected that three trials likely assessed the hypoglycaemic episodes but did not report results regarding this outcome (Hermayer 2012; Lazar 2004; Li 2006). An additional trial disclosed mortality as the primary outcome in its registration record but reported the results as a secondary outcome (Cao 2010).
Ten trials reported insufficient information on registration or the outcomes assessed to judge if they were biased in this domain (Chan 2009; De La Rosa 2008; Desai 2012; Hermayer 2012; Lazar 2011; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015). These trials reported results for all the outcomes described in the methods section.
Other potential sources of bias
We did not identify any other source of bias that could impact the internal validity of the included trials.
Effects of interventions
See: Table 1
See Table 1.
Baseline characteristics
For details of baseline characteristics, see Appendix 7 and Appendix 8.
Perioperative intensive glycaemic control versus conventional glycaemic control
Primary outcomes
All‐cause mortality
Twelve of the studies included measured all‐cause mortality as a primary outcome (Abdelmalak 2013; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Glucontrol 2009; Lazar 2011; Li 2006; NICE SUGAR 2009; Umpierrez 2015; Wahby 2016; Yuan 2015); two trials reported death from any cause as a secondary outcome (Cao 2010; Parekh 2016), and four included studies reported this outcome in their study (Gandhi 2007; Lazar 2004; Subramaniam 2009; Wallia 2017).
Intensive glycaemic control resulted in little or no difference in all‐cause mortality compared to the conventional glycaemic control (130/1263 (10.3%) and 117/1288 (9.1%) events, respectively, risk ratio (RR) 1.08, 95% confidence interval (CI) 0.88 to 1.33; I2 = 0%; 2551 participants, 18 studies; Analysis 1.1). We did not identify noticeable asymmetry in the funnel plot (Figure 4). The certainty of the evidence for this outcome was high.
1.1. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 1: All‐cause mortality
4.

Hypoglycaemic episodes
Seventeen studies reported the number of participants with measured hypoglycaemic episodes (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; Hermayer 2012; Lazar 2011; NICE SUGAR 2009; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015), and 11 studies provided measurement of severe hypoglycaemic episodes (Abdelmalak 2013; Desai 2012; Duncan 2018; Glucontrol 2009; Hermayer 2012; NICE SUGAR 2009; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wallia 2017; Yuan 2015).
Intensive glycaemic control may slightly increase severe hypoglycaemic episodes compared to the conventional treatment group (37/927 (4%) and 6/969 (0.6%) events, respectively, RR 4.73, 95% CI 2.12 to 10.55; I2 = 0%; 1896 participants, 11 studies; Analysis 1.3). The certainty of the evidence is low due to risk of bias and imprecision.
1.3. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 3: Hypoglycaemic episodes (severe)
Intensive glycaemic control resulted in a slight increase in hypoglycaemic episodes compared to conventional care (141/1184 (11.9%) and 41/1226 (3.3%) events, respectively, RR 3.36, 95% CI 1.69 to 6.67; I2 = 64%; 2410 participants, 17 studies; Analysis 1.4). The certainty of the evidence is low due to risk of bias and inconsistency. We did not identify noticeable asymmetry in the funnel plot (Figure 5).
1.4. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 4: Hypoglycaemic episodes (any)
5.

Infectious complications
A total of 18 trials included infection as one of their outcomes (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Lazar 2004; Lazar 2011; Li 2006; NICE SUGAR 2009; Parekh 2016; Rassias 1999; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Wallia 2017; Yuan 2015).
Intensive glycaemic control may result in little to no difference in the rate of infectious complications compared to conventional glycaemic control (160/1228 (13%) and 224/1225 (18.3%) events, respectively, RR 0.75, 95% CI 0.55 to 1.04; I2 = 55%; 2453 participants, 18 studies; Analysis 1.7). The certainty of the evidence is low due to risk of bias and inconsistency. We did not identify noticeable asymmetry in the funnel plot (Figure 6).
1.7. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 7: Infectious complications
6.

Secondary outcomes
Cardiovascular events
Twelve studies reported cardiovascular events (Abdelmalak 2013; Cao 2010; Desai 2012; Gandhi 2007; Glucontrol 2009; Lazar 2004; Lazar 2011; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Yuan 2015).
Intensive glycaemic control may result in a decrease in cardiovascular events compared to the conventional treatment group (127/729 (17.4%) and 117/725 (24.4%) events, respectively, RR 0.73, 95% CI 0.55 to 0.97; I2 = 44%; 1454 participants, 12 studies; Analysis 1.9). The certainty of the evidence is low due to risk of bias and imprecision. We did not identify noticeable asymmetry in the funnel plot (Figure 7).
1.9. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 9: Cardiovascular events
7.

Renal failure
A total of 14 studies included renal failure as an outcome in their trials (Abdelmalak 2013; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; Hermayer 2012; NICE SUGAR 2009; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wahby 2016; Yuan 2015). Various definitions were used for renal failure (Appendix 11), mostly depending on creatine levels or the need for dialysis.
Intensive glycaemic control may result in little or no difference in the number of renal failure events compared to the conventional treatment group (137/1029 (13.3%) and 158/1057 (14.9%) events, respectively, RR 0.92, 95% CI 0.69 to 1.22; I2 = 38%; 2086 participants, 14 studies; Analysis 1.11). We did not identify noticeable asymmetry in the funnel plot (Figure 8). The certainty of the evidence is low due to indirectness and imprecision.
1.11. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 11: Renal failure
8.

Length of ICU
Data on the length of ICU stay were available from 11 studies (Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; Lazar 2004; Lazar 2011; Li 2006; NICE SUGAR 2009; Umpierrez 2015).
Intensive glycaemic control likely results in little to no difference in the number of days in the ICU compared to the control group (mean difference (MD) ‐0.10 days, 95% CI ‐0.57 to 0.38; P = 0.69; I2 = 69%; 1687 participants, 11 studies; Analysis 1.13). The certainty of the evidence is low due to inconsistency and risk of bias. We did not identify noticeable asymmetry in the funnel plot (Figure 9).
1.13. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 13: Length of ICU stay
9.

Length of hospital stay
Data on the total length of hospital stay were provided by 12 studies (Cao 2010; Chan 2009; Desai 2012; Gandhi 2007; Glucontrol 2009; Lazar 2004; Lazar 2011; NICE SUGAR 2009; Parekh 2016; Subramaniam 2009; Umpierrez 2015; Wallia 2017).
Intensive glycaemic control may result in little to no difference in the mean length of hospital stay compared to the control group, but the evidence is very uncertain (MD ‐0.79 days, 95% CI ‐1.79 to 0.21; I2 = 77%; 1520 participants, 12 studies; Analysis 1.15). The certainty of the evidence is very low due to risk of bias, inconsistency and imprecision. We did not identify noticeable asymmetry in the funnel plot (Figure 10).
1.15. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 15: Length of hospital stay
10.

Mean blood glucose during intervention
Mean blood glucose during intervention was available from 17 studies (Abdelmalak 2013; Cao 2010; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Hermayer 2012; Lazar 2004; Lazar 2011; Li 2006; NICE SUGAR 2009; Parekh 2016; Rassias 1999; Umpierrez 2015; Wallia 2017; Yuan 2015). One study could not be included in the analysis due to missing SD values (Chan 2009). We obtained data on mean blood glucose during the intervention from one study by approximating mean values at zero and six hours from the figure and the SD from the reported standard error of the mean. We assumed the SD values at 0 and 6 hours in the ICU (not reported) to be approximately equal to the associated time value. We estimated overall mean and SD values in each group by the pooled value computed from the five measures (Lazar 2004). In another trial, we obtained data on mean glucose during the intervention by the pooled value computed from the two measures (values at the end of cardiopulmonary bypass and at 18 hours) (Lazar 2011). In one study we obtained the pooled SD per day from the mean and P value, assuming equal variances in both groups, and we estimated the overall mean and SD values by the pooled value computed from the five days (Li 2006). In another study, we estimated the overall mean by averaging the approximated values obtained from the graphical evolution of glucose levels during interventions. Overall SD was assumed to be comparable to the baseline glucose SD (Rassias 1999). The studies showed important differences regarding this outcome and, in consequence, we could not combine the individual results in a meta‐analysis. Within the included studies, intensive glycaemic control resulted in the reduction of mean blood glucose with a mean lowering of blood glucose effect ranging from 13.42 mg/dL to 91.30 mg/dL.
Health‐related quality of life
Only one study assessed health‐related quality of life with the SF‐12 norm‐based physical component score (Desai 2012). This means that a physical health composite score is composed using the scores of the 12 questions of the questionnaire that range from 0 to 100, indicating the lowest and the highest level of health, respectively. This composite score was compared to a national norm, such as a mean score of 50 (SD 10). In the intervention group the mean value of the physical health component score was 45 (SD 13) and in the control group 48 (SD 9). These data were available from only 12/37 (32.4%) participants in the intervention group and 13/44 (29.5%) participants in the control group.
Socioeconomic effects
The Umpierrez 2015 study population is the only one for which socioeconomic data were available through the publication of a specific sub‐study by Cardona 2017.
In this post hoc cost analysis, the hospitalisation costs, utilisation of resources and perioperative complications were described for a total of 143 participants with diabetes, 72 and 71 participants in the conventional and intensive glycaemic control group, respectively. Costs by category and cost differences between the treatment arms were calculated. Hospital costs and utilisation of resources included pharmacy, radiology, laboratory, consultations and ICU costs. The adjustment ratios were determined from the Medicare Hospital Cost Report published by the Centers of Medicare and Medicaid Services and data available from the participating hospitals. The data were expressed in USD dollars with a mean (min‐max).
The total hospital costs were higher in the conventional glycaemic control group, 42,052 USD (32,858 to 56,421), compared to the intensive glycaemic control group, 40,884 USD (31.216 to 49,992). The utilisation of resources was also higher in the conventional glycaemic control group (2138 USD (1711 to 2968)) compared to the group with intensive glycaemic control (1911 USD (1569 to 2773)). However, these differences in costs between conventional and intensive glycaemic control groups were not statistically significant for hospital costs (P = 0.18) or resource utilisation (P = 0.26).
Weight gain
Only two studies provided data on weight gain. In one study, the weight gain was 11.0 kg (SD 7.5) in the intervention group and 10.6 kg (SD 5.8) in the control group (Lazar 2011). The other study reported the mean maximum weight gain, which was 3.1 kg (SD 0.2) in the intervention group and 6.0 kg (SD 0.4) in the control group (Lazar 2004).
Sensitivity analyses
To assess the impact of risk of bias on the certainty of the evidence for all‐cause mortality, we performed a sensitivity analysis eliminating trials with an unclear risk of selection bias. Pooled data from eight trials with a low risk of selection bias (Abdelmalak 2013; De La Rosa 2008; Duncan 2018; Gandhi 2007; Glucontrol 2009; NICE SUGAR 2009; Parekh 2016; Umpierrez 2015) showed a similar effect to when we assessed the whole set of included trials, with a RR of 1.09 (95% CI 0.88 to 1.36; I² = 0%; 1421 participants, 8 studies; high certainty of evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 2: All‐cause mortality (sensitivity analysis: only published data; low risk of bias)
We also performed sensitivity analyses restricted to data published in the reports of the included trials, with similar results to those in the main analyses.
Sensitivity analysis restricted to published data for all‐cause mortality showed a RR of 0.76 in favour of intensive glycaemic control (95% CI 0.34 to 1.72; I² = 0%; 7 studies, 820 participants; high certainty of evidence; Analysis 1.2). The primary authors from seven trials provided data (Cao 2010; Gandhi 2007; Lazar 2011; Li 2006; Parekh 2016; Wahby 2016; Yuan 2015).
Sensitivity analysis restricted to published data for severe hypoglycaemic events included three studies (Hermayer 2012; Parekh 2016; Yuan 2015). Excluding Parekh 2016 from the pooled analysis due to reporting zero events in both the intervention and control group, analysis of the two remaining trials showed a RR of 1.59 (95% CI 0.05 to 52.32; I² = 73%; 365 participants; low certainty of evidence; Analysis 1.5). For any hypoglycaemic events, sensitivity analysis included seven studies (Cao 2010; Gandhi 2007; Hermayer 2012; Lazar 2011; Parekh 2016; Wahby 2016; Yuan 2015), and resulted in a pooled RR of 2.24 (95% CI 0.56 to 8.94; I² = 68% ; 834 participants; low certainty of evidence; Analysis 1.6).
1.5. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 5: Hypoglycaemic episodes (severe; sensitivity analysis: only published data)
1.6. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 6: Hypoglycaemic episodes (any; sensitivity analysis: only published data)
For infectious complications, sensitivity analysis restricted to published data showed a RR of 0.54 (95% CI 0.38 to 0.75; I² = 24%; 9 studies, 1001 participants; low certainty of evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 8: Infectious complications (sensitivity analysis: only published data)
Sensitivity analysis restricted to published data for cardiovascular events resulted in a RR of 0.80 (95% CI 0.50 to 1.25; I² = 50%; 6 studies, 727 participants; moderate certainty of evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 10: Cardiovascular events (sensitivity analysis: only published data)
Sensitivity analysis for renal failure restricted to published data (Gandhi 2007; Hermayer 2012; Parekh 2016; Wahby 2016; Yuan 2015) showed a RR of 0.79 (95% CI 0.42 to 1.45; I² = 47%; 5 trials, 559 participants; Analysis 1.12).
1.12. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 12: Renal failure (sensitivity analysis: only published data)
Sensitivity analysis restricted to published data for the length of ICU stay (Gandhi 2007; Lazar 2011; Li 2006) resulted in a MD of 0.18 days (95% CI ‐0.07 to 0.43; I² = 0%; 3 trials, 248 participants; Analysis 1.14)
1.14. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 14: Length of ICU stay (sensitivity analysis: only published data)
Sensitivity analysis restricted to published data for hospital stay (Cao 2010; Gandhi 2007; Lazar 2011; Parekh 2016) showed evidence of a reduction of hospital stay in favour of the intensive treatment group (MD ‐1.09 days, 95% CI ‐1.82 to ‐0.35; I² = 10%; 4 trials, 394 participants; low certainty of evidence; Analysis 1.16).
1.16. Analysis.

Comparison 1: Perioperative intensive vs conventional glycaemic control, Outcome 16: Length of hospital stay (sensitivity analysis: only published data)
Discussion
Summary of main results
This review update aimed to investigate the effects of intensive perioperative glycaemic control versus conventional glycaemic control in people with diabetes mellitus. A total of 20 randomised controlled trials and two potentially relevant ongoing trials met the inclusion criteria. The review included studies on 2670 participants with diabetes, 1320 allocated to intensive perioperative glycaemic control and 1350 to conventional glycaemic control.
We were able to demonstrate that intensive glycaemic control may decrease the occurrence of cardiovascular events. This beneficial effect was accompanied by a slight increase in the number of individuals experiencing hypoglycaemic and severe hypoglycaemic episodes in the intensive glycaemic control group. However, we should point out that moderate statistical heterogeneity between studies was present for these outcomes. Overall, we should underline that heterogeneity among studies was present for several outcomes. Additionally, there was heterogeneity regarding the methodology in the design of the intervention, the definition of intensive glycaemic control, and the definition and assessment of study variables (e.g. hypoglycaemia, infection and renal failure).
The outcome of all‐cause mortality was the only one considered to have a high certainty of evidence. We judged the other outcomes to have low‐ or very low‐certainty evidence, and all trials had an unclear or high risk of bias in several of the risk of bias domains.
We could not demonstrate that the intervention led to any clear reductions on important endpoints, such as all‐cause mortality, infectious complications and renal failure. Furthermore, no relevant effects were observed on the length of hospital stay.
Considering the socioeconomic effects, we included data from a sub‐study on the same population from an included study in this review. Data showed positive effects, but no firm conclusions could be drawn.
Overall completeness and applicability of evidence
We performed an extensive search for trials, included publications in any language and placed no restrictions on the outcomes included in the trials. Where needed we tried to obtain additional information. The trials were conducted in five different continents with a large preponderance of studies performed in the USA. Most of the included trials did not define the diagnostic criteria for diabetes mellitus and also few trials provided information about the duration of diabetes of the participants.
The participants included in these trials may not be representative of the general population of people with diabetes. We should point out that 11 studies included participants undergoing cardiovascular surgical interventions (Chan 2009; Desai 2012; Duncan 2018; Gandhi 2007; Lazar 2004; Lazar 2011; Li 2006; Rassias 1999; Subramaniam 2009; Umpierrez 2015; Wahby 2016). Also, it should be kept in mind that, in most trials, participants with the most severe surgical or pre‐surgical conditions were excluded. All these issues preclude the generalisation of the currently available results to many other patients with diabetes or for many types of surgical procedures that have not been included in this review.
Nine studies exclusively included participants with diabetes (Cao 2010; Hermayer 2012; Lazar 2004; Lazar 2011; Li 2006; Parekh 2016; Rassias 1999; Wahby 2016; Yuan 2015), while the other studies included both patients with and without diabetes (Abdelmalak 2013; Chan 2009; De La Rosa 2008; Desai 2012; Duncan 2018; Gandhi 2007; Glucontrol 2009; NICE SUGAR 2009; Subramaniam 2009; Umpierrez 2015; Wallia 2017) and, when applicable, we contacted the authors to obtain separate data on patients with diabetes. We had to exclude some potentially relevant studies because, despite contacting the authors, we were not provided with specific data for the subgroup of participants with diabetes mellitus undergoing surgery (Abdelmalak 2011; Abdelmalak 2019; Albacker 2008; Arabi 2008; Bilotta 2009; Carvalho 2011; Giakoumidakis 2013; He 2007; Kalfon 2014; Kumar 2020; Kurnaz 2017; Mitchell 2006; Mohod 2019; Okabayashi 2014; Rujirojindakul 2014; Santana‐Santos 2019; Wang 2008). Therefore, potentially available relevant information stemming from participants in other RCTs is missing from this review.
The target blood glucose concentrations and the regimens used in the intensive study groups differed among trials. Further, the duration and time of initiation of interventions were also different. Therefore, the available information from trials does not allow the establishment of an optimal glycaemic target, an optimal treatment regimen or an adequate duration of the perioperative intervention.
Finally, the review deals with a heterogeneous group of studies. Our meta‐analyses were limited by the inability to use individual participant data to assess whether distinct clinical characteristics may have influenced the effect estimates of the intervention. Although the analysis shows homogeneity only for all‐cause mortality and length of ICU stay, we have determined to include all outcomes in the meta‐analysis in order to explore the data and gain further insights in the review. The reader should take into account this heterogeneity in interpreting the results.
Quality of the evidence
None of the included studies was classified as having a low risk of bias in all the assessed domains. Concerning the design of the trials, it was not feasible to establish double‐blinding of investigators and participants. This may have influenced the performance of investigators, especially in relation to the hypoglycaemic episodes and assessment of infection. For this reason, we judged most trials to be at high risk of performance bias for these outcomes. On the other hand, we considered the blinding of outcome assessment to be of low or uncertain risk of bias for most variables. This can be considered a lesser concern as all reported outcomes were not subject to individual interpretation. Additionally, many trials had an unclear risk of bias for randomisation and allocation concealment.
The major reasons for downgrading the certainty of the evidence were the impact of the risk of bias (mainly the lack of blinding), inconsistency (due to unexplained statistical heterogeneity within effect estimates from trials included), or imprecision. As a result of this assessment, we were confident about the estimates of effect for all‐cause mortality but not for the other outcomes, which had problems related to the risk of bias, severe inconsistency and imprecision.
Potential biases in the review process
As part of this update, we contacted the authors of the eligible trials to request possible missing data, clarification and separate data on participants with diabetes undergoing surgery if necessary. Some authors were not contacted because all information was available in the published article. A total of 12 trials (Appendix 17) could not be included because the authors did not provide outcome data for a subpopulation of patients with diabetes, and a large amount of possible additional information could not be captured. Additionally, we identified two ongoing studies.
The design of the intervention and the glycaemic targets also varied substantially between trials. Further, the definitions of the trial outcomes varied among trials, and some of the trials did not include data on relevant outcomes such as hypoglycaemic events or infectious complications.
Agreements and disagreements with other studies or reviews
The present review is an update of a previously published systematic review (Buchleitner 2012). The addition of eight new studies in this updated review yielded different and relevant insights, including a decrease in infection rates in participants under intensive perioperative blood glucose control.
Other reviews on strict blood glucose control in the perioperative period have been published. The most recent systematic review dealt with patients with diabetes undergoing cardiac surgery (Jin 2020). Its main conclusion was that intensive blood glucose control is associated with a lower risk of atrial fibrillation and sternal wound infection. This review included six RCTs, three of which were also assessed in the present review (Lazar 2004; Lazar 2011; Wahby 2016). One of the other three included studies was considered to be awaiting classification in our review (Zadeh 2016). The other two studies were excluded from this review due to methodological concerns or not fulfilling the criteria for inclusion in the current review (Asida 2013; Kirdemir 2008).
Another recent meta‐analysis dealt with patients with and without diabetes (Kang 2018), and its main conclusion was that compared to liberal control, perioperative tight glucose control was associated with a significant reduction in short‐term mortality, cardiac surgery mortality, mortality in patients with diabetes and certain postoperative complications (acute kidney injury, sepsis, surgical site infection, atrial fibrillation).
From a clinical point of view, the burden of the potentially associated late complications of diabetes in participants with diabetes mellitus puts these people at a clearly different baseline risk of intra‐ and postoperative morbidity and mortality as compared with participants without diabetes mellitus. Therefore, we strongly believe that trials addressing the main question of the current review should be designed exclusively for participants with diabetes and that systematic reviews should clearly differentiate between participants with and without diabetes.
Finally, the review by Sathya et al in patients with diabetes and under different targets for blood glucose during the perioperative period concluded that a moderately intensive perioperative glycaemic target (150 mg/dL to 200 mg/dL (5.6 mmol/L to 8.3 mmol/L)) was associated with a reduction in postoperative mortality and stroke compared with a liberal target (Sathya 2013). Of the studies included in this review, only three were RCTs (Kirdemir 2008; Lazar 2004; Lazar 2011). The reviews by Kang (Kang 2018) and Sathya (Sathya 2013) further reported an increased risk of hypoglycaemia with strict blood glucose strategies.
Authors' conclusions
Implications for practice.
We analysed 20 randomised controlled trials addressing the effect of perioperative intensive glycaemic management compared to conventional glucose control in people with diabetes mellitus undergoing a surgical procedure. More stringent glycaemic control reduced mean blood glucose with a mean lowering of blood glucose effect ranging from 13.42 mg/dL to 91.30 mg/dL. There is high‐certainty evidence that more stringent perioperative glycaemic control results in little or no difference in overall mortality in people with diabetes undergoing surgery. However, low‐certainty evidence indicates that people managed with more stringent perioperative glucose‐lowering regimens are at a slightly increased risk of developing hypoglycaemic events, including a slightly increased risk of severe hypoglycaemic events. Additionally, low‐certainty evidence indicates that people undergoing more stringent glycaemic perioperative management may have a reduced risk of cardiovascular events. No meaningful differences in length of hospital or intensive care unit stay were found, and there were no apparent differences in the other outcomes of perioperative intensive compared to conventional blood glucose control regimens. Only one study provided data on health‐related quality of life in a small number of individuals, for which no difference was noted. Regarding the socioeconomic effects, one study evaluated hospital cost and resource utilisation; this study did not reveal differences between intensive and conventional glucose control.
It must be pointed out that the algorithms in the intervention and comparison arms varied substantially, including the target blood glucose concentrations. We could not analyse any results regarding the different types of surgical procedures. Apart from the heterogeneity in the design and delivery of the intervention, a relevant degree of heterogeneity was present regarding several study outcomes. Therefore, the interpretation of the findings of this review should consider the heterogeneity that affects the design and conduct of the studies, and their outcomes.
Implications for research.
We are uncertain whether stringent perioperative glycaemic control in people with diabetes results in benefits for every type of surgical procedure. Furthermore, it is not well established which is the optimal blood glucose algorithm and glycaemic target range. Low‐certainty evidence exists for different clinically relevant outcomes, especially for hypoglycaemic events. Data on health‐related quality of life and socioeconomic effects are very scarce. Future studies should focus on these issues.
What's new
| Date | Event | Description |
|---|---|---|
| 1 August 2023 | New search has been performed | This is an update of the Cochrane Review first published in Issue 9, 2012. |
| 1 August 2023 | New citation required and conclusions have changed | We found beneficial evidence for intensive glucose control for cardiovascular events. Intensive perioperative glucose control slightly raised the risk of hypoglycaemic and severe hypoglycaemic episodes. There is high‐certainty evidence that more stringent perioperative glycaemic control results in little or no difference in overall mortality in people with diabetes undergoing surgery. |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 9, 2012
Notes
Portions of the background and methods sections, the appendices, additional tables and Figures 1 to 3 of this review are based on a standard template established by Cochrane Metabolic and Endocrine Disorders.
Acknowledgements
Acknowledgements from the authors
We would like to thank the authors of NICE SUGAR 2009, Duncan 2018, Wallia 2017, Umpierrez 2015, Glucontrol 2009, Chan 2009, Lazar 2011, Subramaniam 2009, Desai 2012, De La Rosa 2008 and Abdelmalak 2013 for providing additional non‐published data on participants with diabetes and undergoing surgery, making it possible for the trials to be included in the review.
Editorial and peer reviewer contributions
Cochrane Metabolic and Endocrine Disorders supported the authors in the development of this review. Bernd Richter and Gudrun Paletta supported this review update in its earlier stages.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Brenda Bongaerts, Institute of General Practice, Medical Faculty of the Heinrich‐Heine‐University Düsseldorf, Düsseldorf, Germany.
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Juan Victor Ariel Franco, Institute of General Practice, Medical Faculty of the Heinrich‐Heine‐University Düsseldorf, Düsseldorf, Germany.
Copy Editor (copy‐editing and production): Jenny Bellorini, c/o Cochrane Central Production Service.
Peer reviewers (provided comments and recommended an editorial decision): Joanne Platt (Information Specialist, Cochrane GNOC), Raghuraman M Sethuraman (Department of Anesthesiology, Sree Balaji Medical College & Hospital, BIHER, Chennai, India), Khalid Maudood Siddiqui, Assistant Professor and consultant, Aga Khan University Hospital Karachi Pakistan; Oscar L Morey‐Vargas, Cleveland Clinic, Ohio and another clinical peer reviewer who chose not to be publicly acknowledged.
Didac Mauricio (author of this review) is a Cochrane CMED Editorial Board member but was not otherwise involved in the editorial process or decision‐making for this article.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies Online) |
|
| MEDLINE (Ovid) |
|
| LILACS |
| ((MH:"Blood Glucose" OR MH:"Insulin" OR ((glucos$ OR glyc$ OR glic$) AND (control$ OR level$ OR nivel$)) OR insulin$) AND (MH:"Perioperative Care" OR perioperativ$ OR postoperativ$ OR intraoperativ$ OR preoperativ$ OR surgery OR surgical OR cirurg$ OR cirug$ OR operative OR quirurg$ OR operac$)) [added filter "Controlled Clinical Trial" from database menu, restricted to 2012 onwards] |
| WHO ICTRP (Standard search) |
| perioperative* AND glucose* OR peri operative* AND glucose* OR perioperative* AND insulin* OR peri operative* AND insulin* OR postoperative* AND glucose* OR post operative* AND glucose* OR postoperative* AND insulin* OR post operative* AND insulin* OR intraoperative* AND glucose* OR intra operative* AND glucose* OR intraoperative* AND insulin* OR intra operative* AND insulin* OR preoperative* AND glucose* OR pre operative* AND glucose* OR preoperative* AND insulin* OR pre operative* AND insulin* OR surger* AND glucose* OR surgica* AND insulin* OR operative* AND glucose* OR operative* AND insulin* [removed ClinicalTrials.gov records, restricted to 2012 onwards] |
| ClinicalTrials.gov (Expert search) |
| (perioperative OR "peri operative" OR postoperative OR "post operative" OR intraoperative OR "intra operative" OR preoperative OR "pre operative" OR surgery OR surgical OR operative) AND ("insulin therapy" OR "insulin infusion" OR "glycemic control" OR "glycaemic control" OR "glucose control") [restricted to 2012 onwards] |
| PubMed "similar articles" search |
| 20878324[PMID] OR 19142552[PMID] OR 18799004[PMID] OR 22137804[PMID] OR 17310047[PMID] OR 19636533[PMID] OR 15006999[PMID] OR 19387173[PMID] OR 10320160[PMID] OR 19318384[PMID] OR 17215967[PMID] OR 21865944[PMID] = 2845 [restricted to 2012 onwards = 912, CRS Web RCT classifier: 75 to 100% likelihood of RCT = 193 records] |
Appendix 2. Assessment of risk of bias
| Risk of bias domains |
|
Random sequence generation (selection bias due to inadequate generation of a randomised sequence) For each included trial, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment (selection bias due to inadequate concealment of allocation prior to assignment) We described for each included trial the method used to conceal allocation to interventions prior to assignment and we assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We also evaluated trial baseline data to incorporate assessment of baseline imbalance into the 'Risk of bias' judgement for selection bias (Corbett 2014). Chance imbalances may also affect judgements on the risk of attrition bias. In the case of unadjusted analyses, we distinguished between trials that we rate as being at low risk of bias on the basis of both randomisation methods and baseline similarity, and trials that we judged as being at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We will reclassify judgements of unclear, low or high risk of selection bias as specified in Appendix 3. Blinding of participants and study personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Incomplete outcome data (attrition bias due to amount, nature or handling of incomplete outcome data) For each included trial and/or each outcome, we described the completeness of data, including attrition and exclusions from the analyses. We stated whether the trial reported attrition and exclusions, and report the number of participants included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator groups). We also noted if the trial reported the reasons for attrition or exclusion and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
Selective reporting (reporting bias due to selective outcome reporting) We assessed outcome reporting bias by integrating the results of the appendix 'Matrix of trial endpoints (publications and trial documents)' (Boutron 2014; Jones 2015; Mathieu 2009), with those of the appendix 'High risk of outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Other bias
|
Appendix 3. Selection bias decisions
| Selection bias decisions for trials reporting unadjusted analyses ‐ comparison of results obtained using method details alone with results using method details and trial baseline informationa | |||
| Reported randomisation and allocation concealment methods | Risk of bias judgement using methods reporting | Information gained from study characteristics data | Risk of bias using baseline information and methods reporting |
| Unclear methods | Unclear risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited or no baseline details | Unclear risk | ||
| Would generate a truly random sample, with robust allocation concealment | Low risk | Baseline imbalances present for important prognostic variable(s) | Unclear riskb |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Low risk | ||
| No baseline details | Unclear risk | ||
| Sequence is not truly random, or allocation concealment is inadequate | High risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Unclear risk | ||
| No baseline details | High risk | ||
| aTaken from Corbett 2014; judgements highlighted in bold indicate situations in which the addition of baseline assessments would change the judgement about risk of selection bias, compared with using methods reporting alone. bImbalance identified that appears likely to be due to chance. cDetails for the remaining important prognostic variables are not reported. | |||
Appendix 4. Descriptions of participants
| Study ID | Provide brief but concise description | |
| Duncan 2018 | Inclusion criteria | Adults between 18 and 90 years old scheduled for elective coronary artery bypass grafting, valve repair or replacement, or a combination of these procedures with cardiopulmonary bypass between August 2007 and April 2015 |
| Exclusion criteria | Off‐pump cardiac surgery, anticipated hypothermic circulatory arrest, elevated baseline cardiac troponin I (greater than 0.5 ng /l–1, Montreal) or troponin T (greater than 0.1 ng/ml–1, Cleveland), kidney disease requiring renal replacement therapy, or active infection requiring ongoing antibiotic therapy | |
| Diagnostic criteria | — | |
| Wallia 2017 | Inclusion criteria | Age 18 to 80 years old, able to give informed consent personally or via family member with appropriate authorisation to do so if patient were unable, expected survival after transplantation of 1 year, BG level 180 mg/dL postoperatively regardless of diabetes status (with or without diabetes), and no previous liver transplantation |
| Exclusion criteria | — | |
| Diagnostic criteria | — | |
| Wahby 2016 | Inclusion criteria | Patients with diabetes planned for CABG surgery |
| Exclusion criteria | Emergency CABG, off‐pump surgery and combined valve and CABG surgery | |
| Diagnostic criteria | — | |
| Parekh 2016 | Inclusion criteria | Adult patients with a diagnosis of DM who were admitted for deceased donor renal transplantation |
| Exclusion criteria | Children and adult candidates enrolled in a concurrent study evaluating the effect of a medication or other intervention on graft function | |
| Diagnostic criteria | — | |
| Yuan 2015 | Inclusion criteria | Adult patients with type 2 DM undergoing gastrectomy for gastric tumours between September 2006 and March 2014 |
| Exclusion criteria | Patients undergoing laparotomy or palliative surgery, unable to tolerate enteral nutrition, as shown by vomiting, diarrhoea, or abdominal distention, or the nasojejunal tube became occluded or was pulled out | |
| Diagnostic criteria | According to the criteria of the ADA | |
| Umpierrez 2015 | Inclusion criteria | Patients aged between 18 and 80 years undergoing primary or a combination of CABG and other cardiac operations such as valve repair or aortic surgery |
| Exclusion criteria | Patients with impaired renal function (serum creatinine ≥ 3.0 mg/dL or glomerular filtration rate, 30 mL/min/1.73 m2), hepatic failure, or history of hyperglycaemic crises and those at imminent risk of death (brain death or cardiac standstill) or pregnancy, or patients or next of kin unable to provide consent | |
| Diagnostic criteria | — | |
| Abdelmalak 2013 | Inclusion criteria | Age ≥ 40 years old. Major non‐cardiac surgical procedures scheduled to take ≥ 2 hours done under general anaesthesia. Written informed consent. |
| Exclusion criteria | Recent intravenous or oral steroid therapy within 30 days (inhaled steroids are permitted). Any contraindications to the proposed interventions, ASA Physical Status > 4, non English‐speaking patients. | |
| Diagnostic criteria | — | |
| Hermayer 2012 | Inclusion criteria | Renal transplant candidates admitted to MUSC who were 18 years of age or greater and who had a DM diagnosis (type 1 and type 2), a fasting BG over 100 mg/dL per admission screening labs, and a random BG over 120 mg/dL per admission screening labs |
| Exclusion criteria | History of an active gastrointestinal bleed 3 months previously, patients who were scheduled to receive a simultaneous pancreas transplant, patients with a history of a functioning pancreatic transplant, patients currently managed on an insulin pump, patients who were unable or unwilling to provide informed consent, and patients who were unable to commit to the study protocol, including the outpatient follow‐up phase of care. | |
| Diagnostic criteria | — | |
| Desai 2012 | Inclusion criteria | All patients with diabetes who underwent first‐time, isolated, non‐emergency CABG, patients without diabetes who underwent first‐time, isolated, non‐emergency CABG who were found to have had 3 consecutive BG readings greater than 150 mg/dL or any 1 BG reading greater than 200 mg/dL perioperatively, which is aligned with the current STS guidelines, patients who were started on an insulin infusion while in the operating room. |
| Exclusion criteria | Patients who underwent open surgery other than isolated CABG, patients who were found not to require an insulin infusion post‐CABG, patients who underwent a concomitant procedure in addition to CABG (e.g. CABG + valve repair). | |
| Diagnostic criteria | — | |
| Lazar 2011 | Inclusion criteria | Patients with diabetes mellitus (controlled by insulin, oral medication, diet, or combination of oral medication and insulin) undergoing CABG surgery on cardiopulmonary bypass |
| Exclusion criteria | Patients with severe hyperglycaemia (serum glucose ≥ 400 mg/dL), which could not be controlled on a stable insulin regime preoperatively if they had chronic renal failure (creatinine level > 2.5 mg/dL), acute renal failure (urine output < 20 mL/hours for 3 hours), and those patients requiring concomitant procedures in addition to CABG surgery | |
| Diagnostic criteria | — | |
| Cao 2010 | Inclusion criteria | Adult patients with type 2 DM who were to undergo open elective gastrectomy for gastric cancer. In all cases, diagnosis of type 2 diabetes and gastric cancer had been confirmed before surgery |
| Exclusion criteria | Age was less than 16 years, severe obesity (BMI > 30 kg/m2) or severe malnutrition (BMI < 15 kg/m2), expected SICU stay after surgery was less than 24 hours, the tumour was unresectable or the patient with late‐stage cancer underwent palliative surgery, pregnancy, took corticosteroids, steroids, growth hormone, or immunosuppressive drugs within 2 weeks prior to the study, patient received neoadjuvant chemotherapy, patient was diagnosed with gastric stump cancer or recurrent gastric cancer | |
| Diagnostic criteria | Type 2 DM was defined according to 1999 WHO criteria | |
| Glucontrol 2009 | Inclusion criteria | Adult patients (older than 18 years) admitted to the participating ICUs |
| Exclusion criteria | Life expectancy lower than 24 hours, and the absence of consent | |
| Diagnostic criteria | — | |
| NICE SUGAR 2009 | Inclusion criteria | At time of patient's admission to the ICU the treating ICU specialist expects the patient will require treatment in the ICU that extends beyond the calendar day following the day of admission. Patient has an arterial line in situ or placement of an arterial line is imminent (within the next hour) as part of routine ICU management. |
| Exclusion criteria | Age < 18 years, imminent death (cardiac standstill or brain death anticipated in less than 24 hours) and the treating clinicians are not committed to full supportive care. Patients admitted to the ICU for treatment of diabetic ketoacidosis or hyperosmolar state. Patient is expected to be eating before the end of the day following admission. Patients who have suffered hypoglycaemia without documented full neurological recovery. Patient thought to be at abnormally high risk of suffering hypoglycaemia. Patient previously enrolled in the NICE‐SUGAR study. Patient who can not provide prior informed consent, there is documented evidence that the patient has no legal surrogate decision marker, and it appears unlikely that the patient will regain consciousness or sufficient ability to provide delayed informed consent. The patient has been in the study ICU or another ICU for longer than 24 hours for this admission. |
|
| Diagnostic criteria | Based on the medical history. | |
| Subramaniam 2009 | Inclusion criteria | People with or without diabetes who were aged 18 years or older, had an ASA physical status of I‐IV, undergoing peripheral vascular bypass surgery, abdominal aortic surgery or major lower extremity amputation (above or below the knee), and were expected to stay in the hospital for at least 48 hours. |
| Exclusion criteria | Brittle diabetes (previously diagnosed by endocrinologist), varicose vein ligation, continuous insulin infusion pumps, planned stent procedures for vascular disease, an ASA physical status of V. | |
| Diagnostic criteria | — | |
| Chan 2009 | Inclusion criteria | Adults from both genders who were older than 21 years of age and who were undergoing open heart cardiac surgery with CPB. |
| Exclusion criteria | Renal failure (creatinine > 1.5 g/dL), neurological dysfunction (diagnosis from medical records), COPD, current use of any type of antibiotic, current use of inotropic support, emergency and urgent surgeries and reoperations. | |
| Diagnostic criteria | — | |
| De La Rosa 2008 | Inclusion criteria | Patients aged 15 years or older admitted to the ICU at the Hospital Pablo Tobón Uribe, Medellín, Colombia, between 12 July 2003 and 21 December 2005 with an expected ICU stay of at least 2 days. |
| Exclusion criteria | Exclusion were pregnancy, diabetic ketoacidosis, hyperosmolar non‐ketotic state, readmission to the ICU during the same hospitalisation, advanced stage cancer (solid or haematological), decision to withhold or withdraw aggressive therapies, and inclusion in another clinical trial. | |
| Diagnostic criteria | — | |
| Gandhi 2007 | Inclusion criteria | Adults undergoing elective cardiac surgery between July 2004 and April 2005. |
| Exclusion criteria | Patients who had off‐pump CPB procedures. | |
| Diagnostic criteria | — | |
| Li 2006 | Inclusion criteria | Patients with DM who were to undergo CABG for the 1st time. |
| Exclusion criteria | — | |
| Diagnostic criteria | In most cases, the diagnosis of diabetes had been made before admission for surgery. Newly diagnosed diabetes was confirmed by a fasting blood glucose level of ≥ 200 mg/dL associated with an elevated level of haemoglobin A1c | |
| Lazar 2004 | Inclusion criteria | Patients with diabetes mellitus undergoing primary or reoperative CABG performed on cardiopulmonary bypass. |
| Exclusion criteria | — | |
| Diagnostic criteria | — | |
| Rassias 1999 | Inclusion criteria | Patients with DM scheduled to undergo elective cardiac surgery with CPB. All patients underwent surgery starting at 8:00 am at the Dartmouth‐Hitchcock Medical Center during 1996 and 1997. |
| Exclusion criteria | Exclusion criteria included emergency surgery, conditions known to cause immunosuppression (other than DM), age under 18 years or inability to provide written informed consent. | |
| Diagnostic criteria | — | |
| —: not reported ADA: American Diabetes Association, ASA: American Society of Anaesthesiology, BG: blood glucose, BMI: body mass index, CABG: coronary artery bypass grafting, CPB: cardiopulmonary bypass, COPD: chronic obstructive pulmonary disease, CVA: cerebrovascular accident, DM: diabetes mellitus, EF: ejection fraction, ICU: intensive care unit, MUSC: Medical University of South Carolina, SICU: surgical intensive care unit, STS: Society of Thoracic Surgeons, TIA: transient ischaemic attacks, WHO: World Health Organization | ||
Appendix 5. Description of interventions
| Study author | Duncan 2018 |
| Brief name | Intraoperative hyperinsulinaemic normoglycaemia or standard glycaemic management |
| Recipient | Patients undergoing cardiac surgery |
| Why | Previous studies have demonstrated that hyperglycaemia is associated with mortality and morbidity in critically ill patients undergoing cardiac surgery. This study determined whether hyperinsulinaemic normoglycaemia reduces 30‐day mortality and morbidity after cardiac surgery. |
| What (materials) | Accu‐Check (Roche Diagnostics, Switzerland) glucose monitor Insulin therapy protocol (Cleveland clinic operating room): Appendix 1 |
| What (procedures) | Intraoperative glycaemic management with hyperinsulinaemic normoglycaemia, a fixed high‐dose insulin and concomitant variable glucose infusion titrated to glucose concentrations of 80 mg/dL to 100 mg/dL; or standard glycaemic management, low‐dose insulin infusion targeting glucose greater than 150 mg/dL |
| Who provided | Research personnel (specific training not reported) |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Operating room. Intensive care unit (upon intensive care unit admission, both groups transitioned to the same standardised postoperative insulin treatment protocol) |
| When and how much | I: a fixed‐dose insulin infusion of 5 mU/kg/min with a concomitant variable glucose (dextrose 20%) infusion supplemented with potassium (40 mEq/L) and phosphate (30 mmol/L) The glucose infusion was initiated at approximately 40 to 60 mL/hr – when serum glucose concentration was approximately 110 mg/dL or less, and manually titrated to target glucose concentrations of 80 to 110 mg/dL every 10 to 15 min throughout surgery. Additional boluses of insulin were given for blood glucose greater than 110 mg/dL. At sternal closure, the insulin infusion was reduced to 1 mU/kg/min and converted to a standard low‐dose insulin infusion upon intensive care unit admission. After intensive care unit arrival, the glucose infusion was decreased by 25% to 50% every 20 min when the blood glucose was greater than 110 mg/dL. When the infusion was at 20 mL/h or less and blood glucose was greater than 110 mg/dL, the infusion was discontinued. Blood glucose concentrations were followed for 45 to 60 minutes after discontinuation of the dextrose infusion to ensure that hypoglycaemia was avoided. C: a conventional low‐dose insulin infusion titrated to blood glucose concentrations measured by arterial blood gas analysis every 30 to 90 min throughout surgery. This low‐dose insulin infusion was initiated for blood glucose concentration greater than 120 mg/dL before initiation of cardiopulmonary bypass or greater than 150 mg/dL during or after cardiopulmonary bypass, at a rate based on patient weight and current glucose concentration. Subsequent adjustments were based on a sliding scale of current blood glucose concentration and the change from the previous measurement. Supplemental boluses of insulin were given with acute increases (greater than 30 mg/dL) in blood glucose. The insulin protocol for patients assigned to standard glucose management is listed in appendix 1. Upon intensive care unit admission, both groups transitioned to the same standardised postoperative insulin treatment protocol in the intensive care unit. |
| Tailoring | The intervention was titrated I: manually titrated to target glucose concentrations of 80 mg/dL to 110 mg/dL every 10‐15 min throughout surgery C: titrated to blood glucose concentrations every 30 to 90 min throughout surgery. Low‐dose insulin infusion was initiated for blood glucose concentrations greater than 120 mg/dL before initiation of cardiopulmonary bypass or greater than 150 mg/dL during or after cardiopulmonar bypass |
| Modification of intervention throughout the trial | The intervention was not modified during the course of the study |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Wallia 2017 |
| Brief name | Glycaemic control reduces infections in post‐liver transplant patients |
| Recipient | Post liver transplant patients |
| Why | Clinical trials have shown morbidity and mortality benefits from intensive inpatient hyperglycaemia management. Very few studies have been performed that evaluate the relationship of glucose levels to outcomes in patients undergoing solid organ transplantation. |
| What (materials) | Insulin infusion according to the Northwestern protocol adjusted if needed. Discharge instructions for patients, which included details regarding home self‐blood glucose monitoring. |
| What (procedures) | Surgical ICU stay: the patients were seen daily by a member of the GMS, and the glucose levels were reviewed at least daily to assess whether insulin protocol adjustments were needed. Hospital stay: the insulin doses were adjusted daily by the GMS team to maintain the premeal glucose levels as close as possible to the respective target of 140 mg/dL and 180 mg/dL. Discharge from hospital: patient's primary care physician, in consultation with the transplantation service and the GMS, generally managed the hyperglycaemia in the first month. This subsequent posthospital care and glycaemic target were at the discretion of these healthcare providers and were not be governed by the in‐hospital protocol. |
| Who provided | The glucose management service team (GMS): experienced nurse practitioners and endocrinologist Experienced transplant ICU and floor nurses |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Surgical intensive care unit (ICU) |
| When and how much | An intravenous insulin infusion was started according to the protocols for insulin infusion. These protocols had been modified from the earlier Northwestern protocol, such that glucose levels of 140 mg/dL and 180 mg/dL were targeted in the 2 groups rather than 110 mg/dL. The patients were seen daily by a member of the GMS, and the glucose levels were reviewed at least daily to assess whether insulin protocol adjustments were needed. Once the patients were stable and had begun to eat, rapid acting insulin was given to cover their food intake in doses of approximately 10% of the basal (glargine) insulin dose. If patients were still otherwise unstable, the insulin infusion was continued. Once patients were stable, the insulin infusion was converted to a basal bolus regimen. A member of the GMS started the basal insulin, usually at a dose that was about 50% to 60% of the basal infusion rate. In this setting, an additional rapid acting insulin analog was given to cover food intake at approximately 10% of the basal insulin dose. At conversion, subcutaneous injection of rapid acting insulin was also administered at 15% of the stable hourly insulin infusion rate given during the previous several hours (324 hours to calculate the total daily dose) to maintain adequate insulin levels as a “bridge” dose. Once patients were fully receiving the subcutaneous insulin regimen, the doses of glargine were generally reduced by about 50% daily (with flexibility from 40% to 60% according to the patient’s clinical status and BG values). The doses of premeal rapid‐acting insulin were maintained, reflecting a decrease in insulin resistance and an increase in meal size as the patient’s clinical status improved. Again, the insulin doses were adjusted daily by the GMS team to maintain the premeal glucose levels as close as possible to the respective target of 140 mg/dL and 180 mg/dL while patients were in the hospital. At discharge from the hospital, the patients received discharge instructions, which included details regarding home selfBG monitoring. If the patients were still hyperglycaemic, a medication regimen was recommended in an effort to maintain the BG goal of 140 mg/dL or 180 mg/dL. |
| Tailoring | I: personalised according to patient glucose levels and insulin protocol was adjusted if needed to maintain the BG goal of 140 mg/dL C: personalised according to patient glucose levels and insulin protocol was adjusted if needed to maintain the BG goal of 180 mg/dL |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | The adherence was assessed, but the results were not reported in this paper |
| Study author | Wahby 2016 |
| Brief name | Tight versus moderate glycaemic control in patients with diabetes undergoing coronary artery bypass graft surgery (CABG) |
| Recipient | Patients with diabetes planned for CABG surgery |
| Why | Perioperative glycaemic control in patients undergoing cardiac surgery was conducted in many studies but remains unclear how tight the glycaemic control should be. |
| What (materials) | Syringe pump, blood glucose meter |
| What (procedures) | Tight glycaemic control during operation to maintain blood glucose levels between 110 mg/dL and 149 mg/dL versus conventional moderate glycaemic control to achieve blood glucose level between 150 mg/dLand 180 mg/dL during operation. Perioperative tight glycaemic control was achieved by continuous insulin infusion using insulin actrapid HM Novonordisk 50 unit in 500 mL saline 0.9% by syringe pump started before anaesthesia induction and continued till patient weaned from mechanical ventilation in ICU. The blood glucose was checked hourly by blood glucose meter. |
| Who provided | — |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Perioperative and ICU |
| When and how much | Continuos insulin infusion started before anaesthesia induction and continued till the patient is extubated in ICU |
| Tailoring | I: Tight glycaemic control during operation to maintain blood glucose levels between 110 mg/dL and 149 mg/dL C: conventional moderate glycaemic control to achieve blood glucose level between 150 mg/dL and 180 mg/dL |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Parekh 2016 |
| Brief name | Effect of moderately intense perioperative glucose control on renal allograft function |
| Recipient | Adult patients with diabetes undergoing deceased donor renal transplant |
| Why | To determine whether moderately intense glucose control, with the primary goal of achieving a blood glucose level between 80 mg/dL and 160 mg/dL at the time of allograft reperfusion, would reduce the incidence of poor graft function and reduce the hyperglycaemia that occur after transplant |
| What (materials) | Accu‐chek Hospital Meters (Roche Diagnostics, Indianapolis, IN, USA). Insulin treatment algorithms in use at University of California San Francisco Medical Center. |
| What (procedures) | I: Preoperatively, recipients in the moderately intense glucose control group were started on an insulin infusion if their blood glucose was greater than 120 mg/dL. To avoid prolonged treatment, the infusion was started no earlier than 4 hours before the anticipated start of the transplant. Intraoperatively, the anaesthesia team was advised to check the blood glucose every 30 to 45 minutes with the goal of keeping it between 80 mg/dL and 160 mg/dL. The decision to target a blood glucose of less than 160 mg/dL was based on our previous work that indicated a threshold effect above that level, resulting in greater rates of DGF and markers of ischaemic injury. The use of bolus therapy or insulin infusion was left to the discretion of the anaesthesia team. C: standard preoperative management consisting of ordering an insulin sliding scale or no intervention unless blood glucose exceeded 200 mg/dL Postoperatively, intervention and control recipients were placed on an insulin infusion (existing protocol already approved by the medical centre for use on standard medical–surgical wards) that targeted blood glucose levels of 100 mg/dL to 180 mg/dL. This infusion was continued for 24 hours postoperatively. After 24 hours of the infusion, the primary transplant team was given complete control over glucose management |
| Who provided | Primary transplant team, anaesthesia team, nephrology team, research team |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Pre‐operatively and during surgery |
| When and how much | Intervention was delivered 1 time during perioperatively period |
| Tailoring | Adapted by insulin scale if blood glucose was > 200 mg/dL (not personalised) |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Yuan 2015 |
| Brief name | Intensive versus conventional glycaemic management strategies in patients with diabetes receiving enteral nutrition after gastrectomy |
| Recipient | Adult patients with diabetes who underwent gastrectomy |
| Why | Hyperglycaemia is a stress response to surgery. Early enteral nutrition after upper gastrointestinal surgical resection has been associated with a significantly shorted length of hospital stay and improved clinical outcomes. Because of the inability to assess the exact amount of glucose absorbed through the intestines, it is difficult to control blood glucose in patients receiving enteral nutrition. This study assessed whether intensive glycaemic control was well‐tolerated, safe and improved clinical outcomes in patients with diabetes receiving enteral nutrition after gastrectomy |
| What (materials) | Intensive group intravenous insulin algorithm and conventional group insulin algorithm |
| What (procedures) | Intensive glycaemic (IG) management: with continuous insulin infusion to a target blood glucose concentration 4.4 mmol/L to –6.1 mmol/L (80 mg/dL to 110 mg/dL) Conventional glycaemic (CG) management: with intermittent bolus insulin to o a target blood glucose concentration < 11.1 mmol/L (< 200 mg/dL) |
| Who provided | — |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Postoperative |
| When and how much | I: started on intravenous infusion of 0.5 to 1 U/h insulin. Blood glucose was monitored hourly (every 2 to 4 h when stable), with the insulin infusion rate adjusted according to an algorithm. C: administered insulin subcutaneously every 4 to 6 h, based on the results of bedside glucose monitoring, with extra injections administered if necessary Postoperative management: patients were infused with 250 mL of normal saline, starting within 12 h after surgery. Patients received feedings of 20 mL/h SP or TPF (Nutricia) through a naso‐jejunal tube beginning on the first postoperative day, with the rate increasing 10 mL/h as tolerated every 12 to 24 h, to a maximum rate of 80 mL/h.8 The average caloric intake was 25 to 30 kcal/kg/day. Between the 8th and 10th days, the naso‐jejunal tube was removed except for special reasons |
| Tailoring | Adapted by blood glucose/insulin algorithm |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Umpierrez 2015 |
| Brief name | GLUCO‐CABG trial. Intensive versus conservative glucose control in patients undergoing CABG |
| Recipient | Patients undergoing primary, elective and emergency CABG |
| Why | The optimal level of glycaemic control needed to improve outcomes in patients undergoing cardiac surgery remains controversial |
| What (materials) | Glucommander computer‐guided continuous insulin infusion device/algorithm |
| What (procedures) | I: continuous insulin infusion (CII) adjusted to maintain a glucose target between 100 mg/dL and 140 mg/dL during ICU admission C: continuous insulin infusion (CII) adjusted to maintain a glucose target between 140 mg/dL and 180 mg/dL during ICU admission After discontinuation of CII, intervention and control participants were transitioned to a single treatment protocol aimed to maintain a glucose target of 140 mg/dL before meals during the hospital stay and during the 90 days after discharge |
| Who provided | — |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Post surgical holding area and ICU of 3 academic medical centres, including Emory University Hospital, Emory Midtown Hospital, and Grady Memorial Hospital in Atlanta, GA |
| When and how much | Postoperative and during ICU stay until patient could eat |
| Tailoring | Adjusted to maintain a glucose target between 100 mg/dL and 140 mg/dL. No tailoring was performed. |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Abdelmalak 2013 |
| Brief name | Potential anti‐inflammatory interventions to reduce perioperative mortality of patients undergoing major non‐cardiac surgery |
| Recipient | Patients undergoing mayor non‐cardiac surgery |
| Why | The inflammatory response to surgery may be an important part of the pathophysiology of adverse outcomes after surgery. Dexamethasone, tight glycaemic control and light anaesthesia may attenuate these inflammatory responses. |
| What (materials) | DeLiT trial intravenous insulin infusion algorithm |
| What (procedures) | Glucose control began shortly after induction of anaesthesia using pre‐designed protocols and continued through the first 2 hours of post‐anaesthesia care unit stay I: blood glucose concentrations were targeted to 80 mg/dL to 110 mg/dL C: blood glucose concentrations were targeted to 180 mg/dL to 200 mg/dL Patients followed the routine of the ICU/hospital ward where they were admitted: hospital ward (70 mg/dL to 150 mg/dL) or critical care unit (80 mg/dL to 120 mg/dL) |
| Who provided | Clinicians |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Operating room |
| When and how much | The intervention was delivered once during the period between induction of anaesthesia and the first 2 postoperative hours Glucose was subsequently managed per routine for the hospital ward (target of 3.9 mmol/L to 8.3 mmol/L 21 (70 mg/dL to 150 mg dL 21)) or critical care unit (target of 4.4 mmol/L 6.7 mmol/L 21 (80 mg/dL to 120 mg dL 21)) to which they were admitted |
| Tailoring | — |
| Modification of intervention throughout the trial | The intervention was not modified during the course of the study |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Hermayer 2012 |
| Brief name | Evaluate the effect of glycaemic control on renal transplantation outcomes: intensive versus standard control |
| Recipient | Renal transplant candidates who were 18 year of age or greater and who had a DM diagnosis (type 1 and type 2), a fasting blood glucose (BG) over 100 mg/dL per admission screening labs, and a random BG over 120 mg/dL per admission screening labs |
| Why | Outcomes from intensive glycaemic control post renal transplant have not been studied |
| What (materials) | Finger stick BG method using the Precision QID monitor; insulin treatment protocol |
| What (procedures) | I: BG level checked in the Operating Room (OR) via indwelling venous cannula or finger‐stick BG just before starting the iv insulin infusion. The iv insulin infusion solution was prepared as a mixture of 250 U regular insulin (Novolin Regular) with 250 mg 0.9% NaCl rendering 1 U insulin/mL saline. The formula was (current BG 60 mg/dL) 0.03 rate of insulin infusion per hour. BG levels were maintained according to study protocol (70 mg/dL to 110 mg/dL). BG levels were checked every 1 to 2 h per study protocol to maintain glycaemic control at 70 mg/dL to 110 mg/dL and were followed by the DMS team. The at least 72‐h time period started at the beginning of surgery and continued until 0700 h on postoperative day 3. Experimental participants in the intensive group had all BG levels checked by the fingerstick BG method using the Precision QID monitor. BG management followed the iv insulin infusion calculator study protocol (70 mg/dL to 110 mg/dL). After diet consumption, BG levels followed the iv insulin infusion calculator study protocol for the remainder of the at least 72 h. The doses of insulin were adjusted daily according to protocol. After the 72‐h period concluded, the intensive group was transitioned to long‐acting and rapid‐acting sc insulin for glycaemic control (70 mg/dL to 140 mg/dL). C: BG levels checked every hour while in the OR via indwelling venous cannula or finger‐stick BG and was treated with rapid‐acting sc insulin as needed to aim for target BG levels (70 mg/dL to 180 mg/dL). Control participants had BG levels checked every 4 h while in the postoperative acute care unit (PACU), treated with long‐acting and rapid‐acting sc insulin to aim for target BG levels (70 mg/dL to 180 mg/dL), and were followed by the DMS team. After control participants were transferred to the transplant unit, BG levels were checked every 4 h and treated with long‐acting (neutral protamine hagedorn insulin or glargine insulin) and rapid‐acting (aspart) sc insulin to maintain glycaemic control at 70 mg/dL to 180 mg/dL and were followed by the DMS team. After diet consumption, BG levels were checked before meals, at bedtime and at 0300 h. The doses of insulin were adjusted daily according to protocol. It was DMS protocol to maintain glycaemic control at 70 mg/dL to 180 mg/dL for the remainder of the at least 72 h. Control participants were placed on a regimen of a minimum of one to two insulin sc injections per protocol at discharge to maintain glycaemic control at 90 mg/dL to 180 mg/dL. |
| Who provided | Transplant unit registered nurses and assigned certified diabetes educators of the Diabetes Management Service (DMS) |
| How (mode of delivery; individual or group)O | Individual, face to face |
| Where | Operating room, postoperative acute care unit, discharge |
| When and how much | |
| Tailoring | Adapted by insulin treatment protocol |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | All study participants received routine nursing care and patient education specific to self‐care management (insulin therapy and BG monitoring) by the transplant unit Registered Nurses and assigned Certified Diabetes Educators |
| Extent of intervention fidelity | — |
| Study author | Desai 2012 |
| Brief name | Strict versus liberal target range for perioperative glucose in patients undergoing CABG |
| Recipient | Patients after first‐time isolated CABG |
| Why | Strict glycaemic control increased the incidence of hypoglycaemic events, but did not result in any significant improvement in clinical outcomes that was achieved with the more moderate control |
| What (materials) | Glucommander (Gluco Tec, Greenville, SC). Glucose Accu‐Chek Advantage with the AccuData GTS/ GTS manufactured by Roche (Basel, Switzerland). |
| What (procedures) | Maintenance of BG levels according to their randomised arm was started in the ICU using the programmed Glucommander to adjust the BG level to patients’ assigned range. Hourly BG monitoring was performed with blood obtained from a patient’s arterial line and analysed by point‐of‐care testing through Glucose Accu‐Chek Advantage with the AccuData GTS/ GTS. BG levels less than 40 mg/dL or greater than 500 mg/dL we sent to the laboratory for further analysis; however, treatment was initiated for low BG if indicated. Patients were maintained on the electronic‐based protocol of intravenous insulin for a minimum of 72 hours perioperatively. I: BG maintained at less than 180 mg/dL (121 mg/dL to 180 mg/dL) C: BG maintained in the range of 90 to less than 120 mg/dL |
| Who provided | The bedside nurses, the anaesthesiologist, nursing staff |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Postoperative ICU |
| When and how much | Maintenance of BG levels according to their randomised arm was started in the ICU. Patients were maintained on the electronic‐based protocol of intravenous insulin for a minimum of 72 hours perioperatively. |
| Tailoring | Adapted |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Lazar 2011 |
| Brief name | Effects of aggressive versus moderate glycaemic control on clinical outcomes in patients with diabetes undergoing CABG |
| Recipient | Patients with diabetes mellitus undergoing CABG |
| Why | There is general consensus that tighter glycaemic control improves outcomes in patients with diabetes undergoing CABG, but the optimal target for serum glucose levels is unknown. Recent trials in both ICU and non‐ICU patients have raised concerns that more aggressive glycaemic control may actually result in increased mortality from cardiovascular disease and increases episodes of hypoglycaemia. |
| What (materials) | Algorithm for moderate and aggressive glycaemic control |
| What (procedures) | After induction of general anaesthesia, a continuous insulin infusion with 100 units of regular insulin in 100 mL of 0.9% normal saline was initiated at 3 mL/hour and titrated to maintain the targeted glucose level on the basis of the algorithm. The study protocol continued during the periods of cardiopulmonary bypass and cardioplegic arrest, after the discontinuation of bypass, and for 18 hours in the ICU. After the 18‐hour ICU period, patients were transitioned off the insulin drip using either short‐ or long‐acting insulin agents and ultimately back to their preoperative diabetic regimens maintaining a fasting glucose level of less than 120 mg/dL and 4 PM glucose levels of less than 180 mg/dL I: maintaining serum glucose 90 mg/dL to 120 mg/dL using continuous intravenous insulin solutions C: maintaining serum glucose 120 mg/dL to 180 mg/dL using continuous intravenous insulin solutions |
| Who provided | Clinicians |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Operating room and ICU |
| When and how much | After induction of general anaesthesia, a continuous insulin infusion with 100 units of regular insulin in 100 mL of 0.9% normal saline was initiated at 3 mL/hour and titrated. After the 18‐hour ICU period, patients were transitioned off the insulin drip using either short‐ or long‐acting insulin agents and ultimately back to their preoperative diabetic regimens maintaining a fasting glucose level of less than 120 mg/dL and 4 PM glucose levels less than 180 mg/dL |
| Tailoring | Titrated: in both moderate and aggressive groups, a continuous insulin infusion of 100 units of regular insulin in 100 mL of 0.9% normal saline was initiated at 3 mL/hour and titrated to the target range using the earlier algorithms |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Cao 2010 |
| Brief name | Intensive versus conventional insulin therapy in patients with type 2 diabetes undergoing D2 gastrectomy for gastric cancer |
| Recipient | Adult patients with type 2 diabetes who were to undergo open elective gastrectomy for gastric cancer |
| Why | The impact of intensive glucose control on short‐term mortality and morbidity in patients with type 2 diabetes undergoing D2 gastrectomy for gastric cancer is unclear. There has been controversy over Portland safety, increased workload and benefits. |
| What (materials) | Bedside glucometer (OneTouch Ultra 2, LifeScan) infusion pump (Smiths Medical Instrument Co., Zhejiang, China) Portland protocol for continuous insulin infusion |
| What (procedures) | I: insulin infusion was started if the blood glucose levels exceeded 6.1 mmol/l and was adjusted to maintain the blood glucose target between 4.4 mmol/L and 6.1 mmol/L. The infusions were continued postoperatively until oral intake or enteral nutrition was established, after which the usual treatment for DM was resumed. C: insulin infusion was started if the blood glucose level exceeded 12.0 mmol/L and was adjusted to maintain the blood glucose target between 10.0 mmol/L and 11.0 mmol/L. The infusions were continued postoperatively until oral intake or enteral nutrition was established, after which the usual treatment for DM was resumed. |
| Who provided | Well‐trained surgeons, diabetologists and SICU nurses who had extensive experience in blood glucose control |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Surgical intensive care unit (SICU) until oral intake or enteral nutrition was established |
| When and how much | Fifty IU of regular insulin in 50 mL of normal saline was administered using an infusion pump. The infusions were continued postoperatively until oral intake or enteral nutrition was established, after which the usual treatment for DM was resumed. |
| Tailoring | Adapted per protocol. In IG treatment, the insulin infusion was started if the blood glucose levels exceeded 6.1 mmol/l and was adjusted to maintain the blood glucose target between 4.4 mmol/L and 6.1 mmol/L. In CG treatment, the insulin infusion was started if the blood glucose level exceeded 12.0 mmol/L and was adjusted to maintain the blood glucose target between 10.0 and 11.0 mmol/L. |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Glucontrol 2009 |
| Brief name | Glucontrol study: tight glucose control by intensive therapy in adult intensive care units |
| Recipient | Adult patients (older than 18 years) admitted to the participating ICUs |
| Why | An optimal target for glucose control in ICU patients remains unclear |
| What (materials) | Specific glucometer (Accu‐Chek Inform, Roche Diagnostics, Mannheim, Germany) Insulin infusion algorithm in electronic supplementary material on the publication |
| What (procedures) | Regular human insulin (Actrapid, Novo‐Nordisk, DK, 1 IU/mL NaCl 0.9%) was administered by continuous intravenous infusion (algorithm in electronic supplemental material) via the pumps available at each site. There was no standardised policy for ICU discharge, nutrition, or for the weaning of mechanical ventilation. After discharge from the ICU or when the patient was on full oral feeding, intravenous insulin was shifted to subcutaneous administration, according to the standard local practice. There was no restriction for any other treatment including nutritional support (enteral or parenteral) or intravenous glucose. The vital outcome of the patients was recorded until discharge from the hospital or until the 28th day after ICU admission if the patient was discharged before this day. In case of readmission for a second ICU stay, only the outcome data of the last stay was used. BG was measured in arterial or central venous samples when indwelling catheter were in place, or in samples drawn from the fingertip. The centres were asked to use a blood gas analyser, or a specific glucometer (Accu‐Chek Inform, Roche Diagnostics, Mannheim, Germany) to measure the glucose concentration and to check BG hourly until the achievement of the target and at least every 4 h thereafter. Built‐in checks of quality parameters were left under the responsibility of the local laboratories. At least one BG value per day was measured by the hospital central laboratory on a morning sample (‘‘morning value’’) and recorded. The other BG values measured by a blood gas analyser or by a glucose reader on plasma samples were recorded and used uncorrected for the adaptation of the insulin infusion rate. I: maintaining blood glucose target at 4.4 mmol/L to 6.1 mmol/L C: maintaining blood glucose target at 7.8 mmol/L to 10.0 mmol/L |
| Who provided | — |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Twenty‐one medico‐surgical ICUs (working group on metabolism and nutrition of the European Society of Intensive Care Medicine) |
| When and how much | Regular human insulin (Actrapid, Novo‐Nordisk, DK, 1 IU/mL NaCl 0.9%) was administered by continuous intravenous infusion (algorithm in electronic supplemental material) via the pumps available at each site. After discharge from the ICU or when the patient was on full oral feeding, intravenous insulin was shifted to subcutaneous administration, according to the standard local practice. |
| Tailoring | Adapted by insulin treatment protocol |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | NICE SUGAR 2009 |
| Brief name | Intensive (i.e. tight) control target of 81 mg/dL to 108 mg/dL or conventional control target of 180 mg/dL or less in critically ill patients |
| Recipient | Patients expected to require treatment in the ICU on 3 or more consecutive days |
| Why | Hyperglycaemia is common in acutely ill patients, including those treated in intensive care units (ICUs). The occurrence of hyperglycaemia, in particular severe hyperglycaemia, is associated with increased morbidity and mortality in a variety of groups of patients. The optimal target range for blood glucose in critically ill patients remains unclear |
| What (materials) | Arterial catheters and blood gas analysers or laboratory analysers. Acute Physiology and Chronic Health Evaluation II (APACHE II) score (range from 0 to 71, with higher scores indicating more severe illness). Sequential Organ Failure Assessment (SOFA, for which scores can range from 0 to 4 for each organ system, with higher scores indicating more severe dysfunction) Insulin infusion algorithms accessed through a secure website (https://studies.thegeorgeinstitute.org/nice/) |
| What (procedures) | I: control target of 81 mg/dLto 108 mg/dL C: control target of 180 mg/dL or less Blood glucose levels were managed as part of the normal duties of the clinical staff at the participating centre guided by treatment algorithms accessed through a secure website. The trial intervention was discontinued once the patient was eating or was discharged from the ICU but was resumed if the patient was readmitted to the ICU within 90 days. It was discontinued permanently at the time of death or 90 days after randomisation, whichever occurred first. |
| Who provided | Clinical staff at each participating centre |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | ICUs of 42 hospitals (38 academic tertiary care hospitals and 4 community hospitals) |
| When and how much | Control of blood glucose was achieved with the use of an intravenous infusion of insulin in saline guided by treatment algorithms accessed through a secure website. |
| Tailoring | Titrated when needed to maintain blood glucose concentrations |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Subramaniam 2009 |
| Brief name | Continuos perioperative insulin infusion decreases major cardiovascular events in patients undergoing vascular surgery |
| Recipient | Patients who were undergoing peripheral vascular bypass surgery, abdominal aortic surgery or major lower extremity amputation (above or below the knee) |
| Why | Evidence suggests that hyperglycaemia is an independent predictor of increased cardiovascular risk. Aggressive glycaemic control in the intensive care decreases mortality. The benefit of glycaemic control in noncardiac surgery is unknown. |
| What (materials) | Continuous insulin infusion protocol and standard intermittent sliding‐scale insulin bolus, both available in the publication (appendix 1 and 2) |
| What (procedures) | I: using a continuous insulin infusion (CII) protocol. The target blood glucose concentration was 100 mg/dL to 150 mg/dL. If blood glucose levels exceeded 150 mg/dL, a continuous insulin infusion was initiated. Adjustments to the insulin infusion were determined by both the current blood glucose concentrations and insulin infusion rates and as specified in the protocol. Most of the target population resumed oral intake at 48 hours, and they were started on their original antidiabetic regimen. C: using a standard intermittent sliding‐scale insulin bolus (IIB) protocol. |
| Who provided | Changes in the insulin infusion rate were made by the anaesthesiologist in the operating room (intraoperative) and by the registered nurse in the postanesthetic care unit and vascular intensive care unit (postoperative) |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | In the operating room and in the postanesthetic care unit/vascular intensive care unit |
| When and how much | I: Start insulin infusion when blood glucose concentration is greater than 150 mg/dL. All patients with diabetes received half of their baseline long‐acting insulin regimen. No oral hypoglycaemic drugs were given throughout the study period. All patients had hourly blood glucose checks. Drug: regular insulin only. Route: by intravenous route only. C: received half of their long‐acting insulin on the morning of surgery. Oral hypoglycaemic drugs were withheld. The long‐acting insulin was reinitiated during the transition period at 48 h. Regular insulin was used intravenously in the operating rooms at the discretion of the treating anaesthesiologist, as is the standard of care, and in the postoperative period was initiated for blood glucose greater than 150 mg/dL. All patients received 4‐hourly blood glucose checks. |
| Tailoring | Adapted per protocol. Continuous insulin infusion group: start insulin infusion when blood glucose concentration is greater than 150 mg/dL. All patients with diabetes received half of their baseline long‐acting insulin regime. No oral hypoglycaemic drugs were given during the study period. All patients had hourly blood glucose checks. Intermittent insulin bolus group: all patients with diabetes received half of their long‐acting insulin on the morning of surgery. Oral hypoglycaemic drugs were withheld. The long‐acting insulin was reinitiated during the transition period at 48 hours. Regular insulin was used intravenously in the operating rooms at the discretion of the treating anaesthesiologist, as is the standard care, and in the postoperative period was initiated for blood glucose greater than 150 mg/dL. All patients received 4‐hourly blood glucose checks |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Chan 2009 |
| Brief name | Intensive perioperative glucose control does not improve outcomes of patients submitted to open‐heart surgery |
| Recipient | Adults from both genders who were older than 21 years of age and who were undergoing open‐heart cardiac surgery with cardiopulmonary bypass |
| Why | Data provided by recently completed trials reveal that we should regard tight glucose control during cardiac surgery as experimental and confine its use to clinical trials. |
| What (materials) | Glucose meter (Accu Check Advantage, Roche, Manheim, Germany). Glucose analyzer (ABL700, Radiometer Medical A/S, Copenhagen, Denmark). Infusion device (B. Braun, Melsungen, Germany). Insulin dose adjusted according to Leuven modified algorithm. |
| What (procedures) | At the beginning of the study, all patients were kept in intraoperative rooms and were started on intravenous glucose (8 g to 12 g/h), which was maintained for the first 24 hours after arrival in the ICU. After 24 hours, patients started a standardised feeding schedule, intended to deliver 20 to 30 nonprotein calories/kg‐1/24 h‐1 with a balanced composition (0.13 g to 0.26 g nitrogen/kg‐1/24 hrs‐1 and 20% to 40% of nonprotein calories as lipids) of enteral feeding. All of the patients were able to receive enteral feeding after surgery. Parenteral nutrition was not prescribed for any patients in the study. I: target glucose level between 80 mg/dL to 130 mg/dL C: target glucose level between 160 mg/dL to 200 mg/dL Adjustment of the insulin dose was based on the measurements of whole blood glucose in undiluted arterial blood every one to four hours, using a glucose analyser (ABL700, Radiometer Medical A/S, Copenhagen, Denmark). The dose was adjusted by the intensive care nurses according to a titration algorithm. These insulin doses were approved by a study physician not involved in the clinical care of the patients. Insulin was given exclusively by continuous intravenous infusion through a central venous catheter using an infusion device (B. Braun, Melsungen, Germany). The standard concentration was 100 IU of Actrapid HM (Novo Nordisk, Copenhagen, Denmark) in 100 mL of 0.9% NaCl. Prepared solutions, which were stable for up to 12 hours when kept at < 25 °C, were not to be used beyond that time. During the intraoperative period and during the first 24 hours after admission to the ICU, measurement of blood glucose was advised every 1 to 2 hours until the targeted level of blood glucose was achieved. Thereafter, blood glucose was measured every 4 hours, unless dramatic decreases or increases in the blood glucose level occurred. In these cases, hourly control was performed after each dose adjustment. Adequate administration of the prescribed nutrients was emphasised. Intravenous glucose‐containing solutions were administered by an infusion pump to avoid fluctuation of blood glucose levels and frequent adjustment of the insulin dose. At the time of planned interruptions of feeding, the insulin dose was proportionately reduced to avoid hypoglycaemia. Hence, in a patient receiving total enteral nutrition, insulin was virtually stopped during the twice daily, 2‐hour interruptions of tube feeding. In some patients, however, including those with diabetes and those requiring insulin before ICU admission, a low maintenance dose was needed during that time. At the time of patient transportation to an investigation or to the operating room for surgery, all intravenous and enteral administration of feeding was halted, and insulin infusion was temporarily discontinued. The blood glucose level was measured to ensure that it was adequate before transport. Whenever a patient was extubated and allowed to initiate a limited intake of oral foods, the intravenous or tube feeding was usually reduced to allow the patient’s appetite to return. The insulin dose was proportionately reduced and often temporarily discontinued. |
| Who provided | Intensivists and ICU nurses |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Intraoperative room, surgery, ICU |
| When and how much | One time during surgery and ICU |
| Tailoring | Titrated. Dose adjustments were always proportionate to the observed change in blood glucose. When blood glucose decreased by > 50%, the dose of insulin was reduced to half, and the blood glucose level was checked within the next hour. When blood glucose was 60 mg/dL to 80 mg/dL, insulin was reduced depending on the previous blood glucose level, and the blood glucose level was checked again within the next hour. When blood glucose was 40 mg/dL to 60 mg/dL, insulin infusion was stopped, an adequate baseline glucose intake was ensured, and the blood glucose level was checked within the next hour. When the blood glucose was < 40 mg/dL, insulin infusion was stopped, an adequate baseline glucose intake was ensured, glucose was administered via 10 g intravenous boluses, and the blood glucose level was checked within the next hour. When blood glucose started to decrease within the normal range in a stable patient, recovery of insulin sensitivity was assumed, and the insulin dose was reduced by 20%. Additional blood glucose controls were advised whenever changes in body temperature or infection occurred |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | De La Rosa 2008 |
| Brief name | Effect of intensive insulin therapy compared with standard therapy in patients hospitalised in a mixed ICU |
| Recipient | Patients aged 15 years or older admitted to the ICU at the Hospital Pablo Tobón Uribe with an expected ICU stay of at least 2 days |
| Why | It remains unclear if intensive insulin therapy is equally efficacious in both medical and surgical patients |
| What (materials) | Continuous infusion pump. A point‐of‐care glucometer (MediSense Optium, Abbot Laboratories MediSense Products Bedford, MA, USA). Insulin therapy protocol in additional files 1 and 2 of the publications |
| What (procedures) | The standard concentration of insulin was 100 units in 100 mL of 0.9% saline solution. I: insulin infusion was started when blood glucose levels exceeded 110 mg/dL, and was adjusted to maintain a glucose level of between 80 mg/dL and 110 mg/dL (4.4 mmol/L to 6.1 mmol/L). Blood glucose levels were measured in undiluted arterial blood. Undiluted samples were obtained by removing at least 4 times the flush‐volume in the line between the sampling point and the arterial puncture site before the actual sample was taken or, when an arterial catheter was not available, in capillary blood with the use of a point‐of‐care glucometer. Glucose levels were determined with a glucometer at admission to ICU. They were repeated every 1, 2 and 4 hours if the patient had insulin infusion, and every 4 and 6 hours if no insulin was required according to the algorithm. C: insulin infusion was started when glucose levels exceeded 215 mg/dL and was adjusted to maintain blood glucose levels between 180 mg/dL and 200 mg/dL (10.0 mmol/L to 11.1 mmol/L) |
| Who provided | ICU nurse |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | ICU |
| When and how much | Glucose levels were determined with a glucometer at admission to ICU. They were repeated every 1, 2 and 4 hours if the patient had insulin infusion, and every 4 and 6 hours if no insulin was required according to the algorithm |
| Tailoring | Titration of insulin. A protocol (original paper) managed by the ICU nurses, was used for the adjustment of the insulin dose. Protocols were consistently followed throughout the patient's whole ICU stay. |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Gandhi 2007 |
| Brief name | Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery |
| Recipient | Adults with and without diabetes who were undergoing on‐pump cardiac surgery |
| Why | Intensive insulin therapy used to maintain normoglycaemia during intensive care after cardiac surgery improves perioperative outcomes. Its effect during cardiac surgery is unknown. |
| What (materials) | Double P Modular System (Roche Diagnostics, Indianapolis, Indiana). Accu‐Check Inform blood glucose monitoring system (glucometer) (Roche Diagnostics). |
| What (procedures) | I: continuous intravenous insulin infusion, 250 units of NovoLin R (Novo Nordisk, Princeton, New Jersey) in 250 mL of 0.45% sodium chloride, when their blood glucose levels exceeded 5.6 mmol/L (> 100 mg/dL). We adjusted the infusions to maintain blood glucose levels between 4.4 (80 mg/dL) and 5.6 mmol/L (100 mg/dL). We adjusted the dose according to a standardised algorithm used by anaesthesiologist. C: did not receive insulin during surgery unless their glucose levels exceeded 11.1 mmol/L (≥ 200 mg/dL). If glucose concentration was between 11.1 (200 mg/dL) and 13.9 mmol/L (250 mg/dL), patients received an intravenous bolus of 4 units insulin every hour until the glucose concentration was less than 11.1 mmol/L (< 200 mg/dL). If the intraoperative glucose concentration was greater than 13.9 mmol/L (> 250 mg/dL), patients received an intravenous infusion of insulin that was continued until the glucose level was less than 8.3 mmol/L (< 150 mg/dL) Postoperative period: intravenous insulin infusion was started in patients in the conventional treatment group on their arrival in the ICU. Thereafter, both study groups were treated identically, with the intravenous insulin infusion rates adjusted by a nursing staff that was not involved with the study according to a standard protocol. During the first 24 hours after surgery, patients were given only clear liquids by mouth; we did not administer subcutaneous insulin or oral diabetic medications during this time. |
| Who provided | Anaesthesiologist in the operating room and nursing staff in the ICU |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Operating room |
| When and how much | I: the infusions were adjusted to maintain blood glucose levels between 4.4 mmol/L (80 mg/dL) and 5.6 mmol/L (100 mg/dL). Dose was adjusted according to a standardised algorithm used by anaesthesiologist. C: patients did not receive insulin during surgery unless their glucose levels exceeded 11.1 mmol/L (> 200 mg/dL). If glucose concentration was between 11.1 (200 mg/dL) and 13.9 mmol/L (250 mg/dL), patients received an intravenous bolus of 4 units insulin every hour until the glucose concentration was less than 11.1 mmol/L (< 200 mg/dL). If the intraoperative glucose concentration was greater than 13.9 mmol/L (> 250 mg/dL), patients received an intravenous infusion of insulin that was continued until the glucose level was less than 8.3 mmol/L (< 150 mg/dL). |
| Tailoring | We adjusted the dose according to a standardised algorithm used by anaesthesiologist |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Li 2006 |
| Brief name | Glucometer‐guided insulin (GGI) versus continuous insulin infusion (CII) in postoperative patients with diabetes |
| Recipient | People with diabetes who were undergoing CABG for the 1st time |
| Why | Postulating that continuous insulin infusion would provide better control of postoperative blood glucose levels |
| What (materials) | Insulin infusion protocol modified from the Portland protocol |
| What (procedures) | I: continuous insulin infusion (CII) group. The CII protocol (Appendix published in the original paper) used in this group was modified from the Portland protocol. Insulin was initiated and the dosage titrated according to the results of glucose testing to maintain the blood glucose between the desired target levels. C: glucometer‐guided insulin (GGI) group received subcutaneous insulin injections (Humulin® R, Eli Lilly and Company; Indianapolis, Ind) every 2 hours in an attempt to maintain blood glucose levels between 150 and 200 mg/dL. The dose of insulin was adjusted on the basis of each patient’s response to the previous insulin injection. When 2 consecutive measurements showed that the target glucose level was attained, the frequency of GGI injections was decreased to once every 4 hours. When the patients began eating, the frequency of injections was changed to 4 times per day (before every meal and at bedtime). Given continuing stability in glucose‐level readings, the patient’s usual preoperative glucose‐control regimen was resumed 5 days after the operation. |
| Who provided | — |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | ICU |
| When and how much | |
| Tailoring | Insulin was titrated according to the results of glucose testing to maintain the blood glucose between the desired target levels |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Lazar 2004 |
| Brief name | Tight glycaemic control (serum glucose 125 mg/dL to 200 mg/dL) with glucose‐insulin‐potassium (GIK) versus standard therapy (serum glucose > 250 mg/dL) in people with diabetes undergoing CABG |
| Recipient | Patients with diabetes mellitus undergoing primary or preoperative CABG performed on cardiopulmonary bypass |
| Why | There is now evidence to suggest that achieving tighter glycaemic control in people with diabetes during acute coronary syndromes improves survival |
| What (materials) | — |
| What (procedures) | I: patients received an infusion through a central line consisting of 500 mL D5W with 80 U of regular insulin and 40 mEq of KCl infused at 30 mL/h, prepared by a research pharmacist. The GIK was started just before anaesthetic induction and continued until cardiopulmonary bypass was instituted. It was then discontinued and restarted after the aorta was unclamped and continued for 12 hours after arrival in the Intensive Care Unit (ICU). Blood glucose and K were monitored every hour. C: Patients in the No‐GIK group received D5W infused at 30 mL/h. Blood glucose and K were also monitored every hour, and the scale shown in original paper (Table 3) was used to administer subcutaneous insulin. After the 18‐hour study period, patients resumed their preoperative diabetic regimens (oral agents or insulin) titrated to keep blood glucose 200 mg/dL |
| Who provided | — |
| How (mode of delivery; individual or group) | Individual, face to face. Follow‐up was obtained by directly by telephone. |
| Where | Operating room and ICU |
| When and how much | The intervention was started just before anaesthetic induction and continued until cardiopulmonary bypass was instituted. It was discontinued and restarted after the aorta was unclamped and continued for 12 hours after arrival in the ICU. |
| Tailoring | Adjustments in the rate of GIK infusion via protocol |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Study author | Rassias 1999 |
| Brief name | Standard insulin therapy (SIT group) versus aggressive insulin therapy (AIT group) in coronary artery bypass surgery of people with diabetes |
| Recipient | Patients with diabetes scheduled to undergo elective cardiac surgery with cardiopulmonary bypass |
| Why | To examine the effect of aggressive insulin therapy on polymorphonuclear neutrophils I (PMN) function in patients with diabetes undergoing cardiac surgery |
| What (materials) | Glucose levels were checked using an automated device (AccuDataTMGTS; Boehringer Mannheim Corporation, Indianapolis, IN) Insulin infusion according to modified from Zerr et al. PMNs were isolated over PolymorphprepTM per the manufacturer's directors. Suspension concentrations were determined using a model JT Coulter Counter (Beckman Coulter, Inc., Fullerton, CA). Insulin administration according to a schedule detailed on the publication |
| What (procedures) | I: glucose levels were checked 1 hour before surgery and then repeated intraoperatively. Patients were started on an insulin infusion according to the protocols modified from Zerr et al. Further insulin was titrated according to the protocol. Postoperative insulin therapy was not controlled by the study protocol and consisted of an IV infusion of insulin as directed by the protocol of Zerr et al C: glucose levels were checked 1 hour before surgery and then repeated intraoperatively. IV regular insulin was administered according to the schedule published in the original paper. For high glucose levels (> 450 mg %), an infusion of insulin was started after an IV bolus of 13 U. The infusion was started at 4 U/h and titrated hourly. |
| Who provided | — |
| How (mode of delivery; individual or group) | Individual, face to face |
| Where | Operating room of Dartmouth‐Hitchcock Medical Cener |
| When and how much | Patients who were taking insulin were given one half of their usual subcutaneous NPH insulin dosage the morning of surgery and started on an infusion of 5% dextrose with 0.45% sodium chloride solution at 50 mL/h. Oral hypoglycemics were not given the morning of surgery in both groups. |
| Tailoring | Insulin was titrated according to the protocol |
| Modification of intervention throughout the trial | — |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
Appendix 6. Study endpoints and timing of outcome measurement
| Study ID | Review's primary and secondary outcomes | Timing of outcome measurement |
| Duncan 2018 | Any kind of infectious complication | Within 30 days of surgery |
| All‐cause mortality | Within 30 days of surgery and 1 year follow‐up | |
| Hypoglycaemic episodes | Within 30 days of surgery | |
| Adverse events other than hypoglycaemic episodes | Within 30 days of surgery | |
| Cardiovascular events | Within 30 days of surgery | |
| Renal failure | Within 30 days of surgery | |
| Length of ICU and hospital stay | Within 30 days of surgery | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | I: every 10 to 15 min throughout surgery C: every 30 to 90 min throughout surgery |
|
| Wallia 2017 | Any kind of infectious complication | From the day of transplant, at 30 days and at 1 year after transplant |
| All‐cause mortality | From the day of transplant to 1 year after transplant | |
| Hypoglycaemic episodes | During the initial post transplant hospital admission | |
| Adverse events other than hypoglycaemic episodes | During the initial post transplant hospital admission | |
| Cardiovascular events | — | |
| Renal failure | — | |
| Length of ICU and hospital stay | Length of ICU and hospital stay after transplantation | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | During intervention (before initiating the insulin infusions and after conversion to subcutaneous insulin). Immediately postoperatively when patients reached surgical ICU. | |
| Wahby 2016 | Any kind of infectious complication | 30 days |
| All‐cause mortality | Within 30 days of operation | |
| Hypoglycaemic episodes | 30 days | |
| Adverse events other than hypoglycaemic episodes | — | |
| Cardiovascular events | 30 days | |
| Renal failure | 30 days | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Hourly | |
| Parekh 2016 | Any kind of infectious complication | Post transplant hospital stay |
| All‐cause mortality | 30 days, 6 months and 1 year | |
| Hypoglycaemic episodes | Every 30 to 45 minutes during operation, frequency of blood glucose checking before and after operation not stated | |
| Adverse events other than hypoglycaemic episodes | 30 days, 6 months and 1 year | |
| Cardiovascular events | 30 days, 6 months and 1 year (stroke) | |
| Renal failure | ||
| Length of ICU and hospital stay | Post transplant hospital stay | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Preoperative, every 30 to 45 minutes during operation; allograft reperfusion: 0 to 6 h, 6 to 12 h and 12 to 24 h | |
| Yuan 2015 | Any kind of infectious complication | Per year (from 2006 to 2014) |
| All‐cause mortality | Per year (from 2006 to 2014) | |
| Hypoglycaemic episodes | — | |
| Adverse events other than hypoglycaemic episodes | — | |
| Cardiovascular events | — | |
| Renal failure | — | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Daily during 7 days postoperatively | |
| Umpierrez 2015 | Any kind of infectious complication | During admission, either during ICU, transition to non‐ICU hospital setting, or 90 days after discharge |
| All‐cause mortality | During admission, either during ICU, transition to non‐ICU hospital setting, or 90 days after discharge | |
| Hypoglycaemic episodes | During admission, either during ICU, transition to non‐ICU hospital setting, or 90 days after discharge | |
| Adverse events other than hypoglycaemic episodes | — | |
| Cardiovascular events | During admission, either during ICU, transition to non‐ICU hospital setting, or 90 days after discharge | |
| Renal failure | During admission, either during ICU, transition to non‐ICU hospital setting, or 90 days after discharge | |
| Length of ICU and hospital stay | After discharge | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | During admission, either during ICU, transition to non‐ICU hospital setting, or 90 days after discharge | |
| Abdelmalak 2013 | Any kind of infectious complication | Within 30 days of surgery |
| All‐cause mortality | 30 days and 1 year | |
| Hypoglycaemic episodes | Hourly during surgery and during the first 2 postoperative hours | |
| Adverse events other than hypoglycaemic episodes | Within 30 days of surgery | |
| Cardiovascular events | Within 30 days of surgery | |
| Renal failure | Within 30 days of surgery | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Intraoperative | |
| Hermayer 2012 | Any kind of infectious complication | — |
| All‐cause mortality | Up to a median of 1.5 years | |
| Hypoglycaemic episodes | — | |
| Adverse events other than hypoglycaemic episodes | — | |
| Cardiovascular events | — | |
| Renal failure | Within the first 7 days after transplant | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | I: on admission and every 1 hour, 1 to 2 hours per study protocol C: every hour during surgery and every 4 hours while in the recovery room and in the transplant unit. After diet consumption BG levels are checked before meals, at bedtime, and at 03:00 am |
|
| Desai 2012 | Any kind of infectious complication | — |
| All‐cause mortality | Within 30 days | |
| Hypoglycaemic episodes | 72 hours perioperatively | |
| Adverse events other than hypoglycaemic episodes | — | |
| Cardiovascular events | During perioperative time period before discharge | |
| Renal failure | — | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | Preoperatively | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Hourly | |
| Lazar 2011 | Any kind of infectious complication | During surgery, 18 h after arrival in the ICU and 30 days follow‐up |
| All‐cause mortality | 30 days after surgery | |
| Hypoglycaemic episodes | Every 30 minutes in the operating room and each hour in the ICU | |
| Adverse events other than hypoglycaemic episodes | During surgery, 18 h after arrival in the ICU and 30 days follow‐up | |
| Cardiovascular events | During surgery, 18 h after arrival in the ICU and 30 days follow‐up | |
| Renal failure | — | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | The evening before surgery and the day after surgery | |
| Mean blood glucose during intervention | Every 30 minutes in the operating room and each hour in the ICU | |
| Cao 2010 | Any kind of infectious complication | Daily and 15 and 28 days after surgery |
| All‐cause mortality | Within 28 days of the operation or during the period of hospital stay | |
| Hypoglycaemic episodes | After surgery to the end of the protocol | |
| Adverse events other than hypoglycaemic episodes | 28 days after surgery | |
| Cardiovascular events | — | |
| Renal failure | — | |
| Length of ICU and hospital stay | 28 days after surgery | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Composite average of all the glucose levels from the period immediately after surgery to the end of the protocol. | |
| Glucontrol 2009 | Any kind of infectious complication | — |
| All‐cause mortality | During ICU stay, during hospitalisation and 28 days | |
| Hypoglycaemic episodes | Daily | |
| Adverse events other than hypoglycaemic episodes | — | |
| Cardiovascular events | Daily | |
| Renal failure | — | |
| Length of ICU and hospital stay | At discharge from the hospital | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Hourly until the achievement of the target and at least every 4 h thereafter | |
| NICE SUGAR 2009 | Any kind of infectious complication | 90 days after randomisation |
| All‐cause mortality | At 28 and 90 days after randomisation | |
| Hypoglycaemic episodes | During intensive care stay | |
| Adverse events other than hypoglycaemic episodes | During intensive care stay | |
| Cardiovascular events | — | |
| Renal failure | 90 days after randomisation | |
| Length of ICU and hospital stay | 90 days after randomisation | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Blood glucose levels measured half‐hourly to 2 hourly | |
| Subramaniam 2009 | Any kind of infectious complication | Post procedural at hospital discharge |
| All‐cause mortality | During hospital stay and 30 days after surgery | |
| Hypoglycaemic episodes | Blood glucose levels at 4 hours intervals starting from 4 hours after the procedure and ending at 48 hours | |
| Adverse events other than hypoglycaemic episodes | During hospital stay | |
| Cardiovascular events | Intra procedural and post procedural at hospital discharge | |
| Renal failure | Before surgery to after surgery | |
| Length of ICU and hospital stay | From the date of surgery to discharge from the hospital | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Blood glucose monitored every 4 hours (intraoperatively) and until 48 hours postoperatively | |
| Chan 2009 | Any kind of infectious complication | At discharge and 30 days after surgery |
| All‐cause mortality | 30 days after surgery | |
| Hypoglycaemic episodes | At discharge and 30 days after surgery | |
| Adverse events other than hypoglycaemic episodes | At discharge and 30 days after surgery | |
| Cardiovascular events | — | |
| Renal failure | At discharge and 30 days after surgery | |
| Length of ICU and hospital stay | At discharge | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | After arrival in the operating room, immediately after standard monitoring, anaesthesia induction and tracheal intubation Then every hour until the end of the operation. Then on ICU, hourly until glucose levels stabilised and then every 2 hours for 36 hours. | |
| De La Rosa 2008 | Any kind of infectious complication | During ICU stay |
| All‐cause mortality | In‐hospital and 28 days | |
| Hypoglycaemic episodes | During ICU stay | |
| Adverse events other than hypoglycaemic episodes | During ICU stay | |
| Cardiovascular events | — | |
| Renal failure | — | |
| Length of ICU and hospital stay | At discharge | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | At admission to ICU and if patient had insulin infusion measures were repeated at 1, 4 and 6 hours and every 4 and 6 hours if no insulin was required | |
| Gandhi 2007 | Any kind of infectious complication | In hospital and post discharge (up to 30 days after surgery) |
| All‐cause mortality | In hospital and post discharge (up to 30 days after surgery) | |
| Hypoglycaemic episodes | Intraoperative every 30 minutes and postoperative every 1 to 2 hours | |
| Adverse events other than hypoglycaemic episodes | In hospital and post discharge (up to 30 days after surgery) | |
| Cardiovascular events | In hospital and post discharge (up to 30 days after surgery) | |
| Renal failure | In hospital and post discharge (up to 30 days after surgery) | |
| Length of ICU and hospital stay | At discharge of ICU and hospital | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Intraoperative every 30 minutes and postoperative every 1 to 2 hours | |
| Li 2006 | Any kind of infectious complication | During 5 days after surgery |
| All‐cause mortality | During 5 days after surgery | |
| Hypoglycaemic episodes | — | |
| Adverse events other than hypoglycaemic episodes | During 5 days after surgery | |
| Cardiovascular events | During 5 days after surgery | |
| Renal failure | During 5 days after surgery | |
| Length of ICU and hospital stay | Length of ICU stay after surgery | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | 24‐hour period during 5 days after surgery | |
| Lazar 2004 | Any kind of infectious complication | 30 days postoperatively and long term follow‐up (5 y) |
| All‐cause mortality | 30 day postoperatively and long term follow‐up (5 y) | |
| Hypoglycaemic episodes | — | |
| Adverse events other than hypoglycaemic episodes | — | |
| Cardiovascular events | Before CABG, immediately on arrival in the ICU and on postoperative days 1, 2, 5 and 7. Long‐term follow‐up (5 years). | |
| Renal failure | — | |
| Length of ICU and hospital stay | Day of discharge | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | Evening before surgery and at 5:00 am the day after surgery | |
| Mean blood glucose during intervention | Monitored every hour | |
| Rassias 1999 | Any kind of infectious complication | Immediately before induction of anaesthesia, 1 hour after surgery and at 8:00am on the first postoperative day |
| All‐cause mortality | — | |
| Hypoglycaemic episodes | — | |
| Adverse events other than hypoglycaemic episodes | — | |
| Cardiovascular events | — | |
| Renal failure | — | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Every 15 to 30 min during intervention (intraoperative period) | |
| —: not reported d: day(s); h: hour(s); mo: month(s); NI: not investigated; wk: week(s); y: year(s); ICU: intensive care unit; CABG: coronary artery bypass grafting; BG: blood glucose; ICU: intensive care unit. | ||
Appendix 7. Baseline characteristics (I)
| Study ID | Intervention(s) and control(s) | Duration of intervention/duration of follow‐up | Description of participants | Study period | Country | Setting | Ethnic groups (%) | Duration of diabetes (mean years (SD)) |
| Duncan 2018 | I: hyperinsulinaemic normoglycaemia | Intervention: during surgery and ICU stay Follow‐up: within 30 days of surgery and 1 year all‐cause mortality |
Adults between 18 and 90 years old scheduled for elective coronary artery bypass grafting, valve repair or replacement, or a combination of these procedures with cardiopulmonary bypass | 2007‐2015 | Canada | Intraoperative | — | — |
| C: standard therapy | — | |||||||
| Wallia 2017 | I: intensive | Intervention: immediately postoperatively until patients were stable and had begun to eat. Mean duration of insulin infusion in hours: 56.5 + 78.6 Follow‐up: 1 year after transplantation |
Adults between 18 and 80 years old with no previous liver transplantation, with a BG level > 80 mg/dL postoperatively (with or without diabetes) and with an expected survival after transplantation of > 1 year | 2010‐2016 | USA | Postoperative | White 69.5a Black 7.3a Hispanic 18.3a Asian 1.2a Other 3.7a |
— |
| C: moderate | White 76.8a Black 11a Hispanic 8.5a Asian 1.2a Other 2.4a |
|||||||
| Wahby 2016 | I: tight glycaemic control | Intervention: during operation until patient weaned from mechanical ventilation Follow‐up: 30 days after discharge |
135 participants with diabetes planned for CABG surgery | 2013‐2015 | Egypt | Perioperative | — | — |
| C: conventional moderate glycaemic control | ||||||||
| Parekh 2016 | I: moderately intense control | Intervention: Preoperatively, intra operatively and 24 h postoperatively Follow‐up: 30 days, 6 months, and 1 year |
Adult people with diabetes undergoing deceased donor renal transplant | 2012‐2014 | USA | Perioperative | Caucasian: 6.9 Hispanic: 37.9 Black: 10.3 Islander: 44.8 |
18.6 (11) |
| C: standard glucose control | Caucasian: 16.7 Hispanic: 43.3 Black: 20 Islander: 20 |
21.1 (9.9) | ||||||
| Yuan 2015 | I: intensive glycaemic (IG) management | Intervention: — Follow‐up: — |
Adult patients with type 2 diabetes mellitus undergoing gastrectomy for gastric tumours | 2006‐2014 | China | Postoperative | — | 6.2 (0.4) |
| C: conventional glycaemic (CG) management | — | 5.8 (0.7) | ||||||
| Umpierrez 2015 | I: intensive group | Intervention: postoperative, during ICU stay Follow‐up: 90 days after hospital discharge |
Patients with and without diabetes undergoing primary, elective, and emergency CABG who experienced perioperative hyperglycaemia | — | USA | Postoperative | White: 68* Black: 27* Other: 5* |
10.9 (8.8)* |
| C: conservative control | White: 71* Black: 24* Other: 5* |
10.8 (10.1)* | ||||||
| Abdelmalak 2013 | I: intensive glucose management | During surgery after induction of anaesthesia and during the first 2 postoperative hours Follow up: 30 days and 1 year (mortality only) |
Mayor non‐cardiac surgery who were ≥ 40 years old and had an ASA physical status ≤4 | 2007‐2010 | USA | Intraoperative and postoperative | White: 94.4* | 10.5 (13.45)* |
| C: conventional glucose management | White: 95.9* | 10.6 (13.48)* | ||||||
| Hermayer 2012 | I: experimental group | Intraoperatively and 72 h postoperatively Follow‐up: median 1, 5 years |
Renal transplant candidates who were 18 years of age or greater and who had a DM diagnosis (type 1 and type 2) | 2008‐ 2010 | USA | Intraoperative and postoperative | African‐America: 64 White: 34 Other: 2 |
— |
| C: control group | African‐American: 57 White: 37 Other: 6 |
— | ||||||
| Desai 2012 | I: liberal blood glucose control | Intervention: patients were maintained on the electronic‐based protocol of intravenous insulin for a minimum of 72 hours Follow‐up: 30 days for all‐cause mortality |
Participants with and without diabetes with BG > 150 mg/dL undergoing CABG | — | USA | Postoperative | White: 82* Asian: 11* African‐American: 5* Hispanic: 2* |
— |
| C: strict blood glucose control | White: 81*
Asian: 16*
African‐American: 3* Hispanic: 0* |
— | ||||||
| Lazar 2011 | I: aggressive glucose control | Intervention: intraoperatively and the initial 18 hours in ICU Follow‐up: 30 days |
Participants with diabetes undergoing CABG surgery | 2009‐2011 | USA | Intraoperative + postoperative | — | — |
| C: moderate glucose control | — | — | ||||||
| Cao 2010 | I: intensive insulin therapy | Intervention: until oral intake or enteral nutrition was established Follow‐up: 28 days |
Adults with type 2 diabetes who were to undergo open elective gastrectomy for gastric cancer | 2005‐2009 | China | Postoperative | — | 5.5 |
| C: conventional insulin therapy | — | 6.0 | ||||||
| Glucontrol 2009 | I: intensive insulin treatment | Intervention: during ICU stay Follow‐up: until discharge from the hospital or until 28 days after ICU admission |
Adult participants admitted to the participating ICUs | 2004 | Austria, Belgium, France, Israel, Slovenia, Spain, The Netherlands | Postoperative | — | — |
| C: intermediate glucose control | — | — | ||||||
| NICE SUGAR 2009 | I: intensive insulin therapy | Intervention: during ICU stay (if not eating) Follow‐up: 90 days |
Participants undergoing medical and surgical treatment admitted to the ICU | 2004‐2008 | Australia, Canada, New Zealand, USA | Postoperative | — | — |
| C: conventional insulin therapy | — | — | ||||||
| Subramaniam 2009 | I: intensive insulin therapy | Intervention: 48 h after the start of surgery Follow‐up: until 30 days |
Adult participants undergoing peripheral vascular bypass surgery, abdominal aortic surgery or major lower extremity amputation | — | USA | Intraoperative + postoperative | — | — |
| C: conventional insulin therapy | — | — | ||||||
| Chan 2009 | I: intensive insulin therapy | Intervention: during surgery and for 36 hours after surgery in the intensive care unit Follow‐up: 30 days for all‐cause mortality |
Adults undergoing cardiac surgery with cardiopulmonary bypass | — | Brazil | Intraoperative + postoperative | — | — |
| C: conventional insulin therapy | ||||||||
| De La Rosa 2008 | I: intensive insulin therapy | Intervention: during ICU stay Follow‐up: 28 days |
Participants ≥ 15 years old admitted to the ICU, non‐cardiovascular surgeries | 2003‐2005 | Colombia | Postoperative | — | — |
| C: conventional insulin therapy | — | — | ||||||
| Gandhi 2007 | I: intensive insulin therapy | Intervention: during the surgery Follow‐up: 30 days |
Adults undergoing on‐pump cardiac surgery | 2004‐2005 | USA | Intraoperative | White: 96a | — |
| C: conventional insulin therapy | White: 96a | — | ||||||
| Li 2006 | I: continuous insulin infusion | Intervention: during 5 days immediately after surgery Follow‐up: — |
Participants with diabetes who underwent CABG | 2001‐2003 | Republic of China, Taiwan | Postoperative | — | — |
| C: glucometer‐guided insulin | — | — | ||||||
| Lazar 2004 | I: tight glycaemic control with GIK | Intervention: during surgery (discontinued and restarted after the aorta was unclamped) and continued for 12 h after arrival in the ICU Follow‐up: 30 days and 5 years |
Participants with diabetes mellitus undergoing primary or reoperative CABG performed on cardiopulmonary bypass | — | USA | Intraoperative + postoperative | — | — |
| C: standard therapy | — | — | ||||||
| Rassias 1999 | I: aggressive insulin therapy | Intervention: during surgery and on the first postoperative day Follow‐up: — |
Participants with diabetes scheduled to undergo elective cardiac surgery with cardiopulmonary bypass | 1996‐1997 | Lebanon, USA | Intraoperative | — | — |
| C: standard insulin therapy | — | — | ||||||
| —: denotes not reported
*denotes data provided by study authors adata from total study population ASA: American Society of Anesthesiologists; BG: blood glucose; CABG: coronary artery bypass graft; CPB: cardiopulmonary bypass; ICU: intensive care unit; GIK: glucose‐insulin‐potassium. | ||||||||
Appendix 8. Baseline characteristics (II)
| Study ID | Intervention(s) and comparator(s) | Sex (female %) | Age (mean years (SD)) | HbA1c (mean % (SD) | BMI (mean kg/m² (SD)) | Co‐medications/Co‐interventions (% of participants) | Co‐morbidities (% of participants) | |
| Duncan 2018 | I: hyperinsulinaemic normoglycaemia | 27a | 66 (11)a | — | 28.5 (5.7)a | ACE inhibitor: (39)a
Antiarrhythmic: (8)a
ß‐blocker: (63)a
Calcium blocker: (19)a
Cox‐2 inhibitor: (2)a
Statin: (69)a
Steroid: (5)a Diabetic medication: (30)a
|
Diabetes: (32)a COPD/asthma: (16)a Pulmonary hypertension: (15)a Stroke: (6)a Hypertension: (67)a Heart failure: (21)a Myocardial infarction: (28)a Dialysis: (1)a Peripheral vascular disease: (7)a Smoking: (29)a ASA physical status:a II: (0) III: (49) IV: (5) V: (0) |
|
| C: standard therapy | 26a | 66 (11)a | — | 28.3 (5.4)a | ACE inhibitor: 37a
Antiarrhythmic: (10)a
ß‐Blocker: (64)a
Calcium blocker: (21)a
Cox‐2 inhibitor: (0)a
Statin: (69)a
Steroid: (3)a Diabetic medication (29):a
|
Diabetes: (34)a
COPD/asthma: (12)a
Pulmonary hypertension:(14)a
Stroke: (15)a
Hypertension: (79)a
Heart failure: (19)a
Myocardial infarction: (24)a
Dialysis: (1)a
Peripheral vascular disease: (6)a
Smoking: (25)a
ASA physical status:a II: (0) III: (49) IV: (51) V: (0) |
||
| Wallia 2017 | I: intensive (140) | 23* | 57.8 (6.06)* | — | 32.0 (6.5)* | — | — | |
| C: moderate (180) | 26* | 60.4 (5.5)* | — | 30.5 (6.85)* | ||||
| Wahby 2016 | I: tight glycaemic control | 18 (26.87) | 54.99 (6.49) | — | — | — | Hypertension: (80.6) Renal dysfunction: (7.5) Cerebrovascular accident: (4.5) Myocardial infarction: (35.8) |
|
| C: conventional moderate glycaemic control | 22 (32.35) | 56.40 (7.79) | Hypertension: (77.9) Renal dysfunction: (4.4) Cerebrovascular accident: (2.9) Myocardial infarction: (32.3) |
|||||
| Parekh 2016 | I: moderately intense control | 10 (33) | 60.9 (9.1) | 6.5 (1.2) | 27.33 (4.19) | Insulin: (56.7) Oral agents: (20) None: (23.3) | — | |
| C: standard glucose control | 10 (33) | 60.7 (11) | 6.7 (1) | 29.23 (6.24) | Insulin: (56.7) Oral agents: (23.3) None: 20 | — | ||
| Yuan 2015 | I: intensive glycaemic (IG) management | 60 (56.6) | 60.5 (13.2) | 8.0 (0.6) | 22.1 (2.5) | Insulin: 71 (67) Oral agents: 25 (23.6) None: 10 (9.4) |
Respiratory disfunction: (5.7) Cardiovascular disfunction: (15.1) Liver disfunction: (2.8) Renal disfunction: (0.9) Cerebrovascular disease: (1.9) | |
| C: conventional glycaemic (CG) management | 65 (61.3) | 61.1 (13.5) | 7.9 (0.5) | 21.6 (2.0) | Insulin: 67 (63.2) Oral agents: 30 (28.3) None: 9 (8.5) |
Respiratory disfunction: (7.5) Cardiovascular disfunction: (14.2) Liver disfunction: (3.8) Renal disfunction: (1.9) Cerebrovascular disease: (1.9) | ||
| Umpierrez 2015 | I: intensive group | 29 (38)* | 65.2 (9.1)* | 8.2 (2.0)* | 32.9 (8.0)* | No antidiabetic agents: (9)a Oral agents: (45)a Insulin alone: (20)a Insulin + oral agents: (26)a | Previous smoking: (42)a Current smoking: (23)a Dyslipidaemia: (86)a Hypertension: (94)a | |
| C: conservative control | 20 (27)* | 62.8 (9.1)* | 7.8 (2.0)* | 32.1 (7.3)* | No antidiabetic agents: (7)a Oral agents: (48)a Insulin alone: (20)a Insulin + oral agents: (25)a | Previous smoking: (51)a Current smoking: (33)a Dyslipidaemia: (81)a Hypertension: (91)a | ||
| Abdelmalak 2013 | I: intensive glucose management | 39* | 68.1 (10.6)* | 8 (2.21)* | 29.2 (6.62)* | — | Any type of co‐morbidities: 94.4* | |
| C: conventional glucose management | 25* | 66.9 (9.85)* | 7.7 (1.48)* | 29.5 (6.66)* | — | Any type of co‐morbidities: 89.9* | ||
| Hermayer 2012 | I: experimental group | 32 | 58 (9.8) | 7 (1.8) | — | — | Hyperlipidaemia: 45 Hypertension: 95 Cardiovascular disease: 39 Previous myocardial infarction:11 Congestive heart failure: 9 Atrial fibrillation: 9 Cerebrovascular events: 11 Vascular disease: 25 |
|
| C: control group | 29 | 56.3 (9.6) | 7.2 (1.7) | — | — | Hyperlipidaemia: 49 Hypertension: 100 Cardiovascular disease: 33 Previous myocardial infarction: 10 Congestive heart failure: 14 Atrial fibrillation: 12 Cerebrovascular events: 12 Vascular disease: 31 |
||
| Desai 2012 | I: liberal blood glucose control | 25* | 62.7 (9.4)* | 7.4 (1.3)* | 33.5 (8.5)* | Aspirin: 94a Beta‐blockers: 93a ACE/ARB inhibitors: 47a Lipid‐lowering medications: 94a |
Hypertension: 86a Congestive heart failure: 9a | |
| C: strict blood glucose control | 14* | 62.6 (10.6)* | 7.9 (1.5)* | 31.2 (7.4)* | Aspirin: 92a Beta‐blockers: 85a ACE/ARB inhibitors: 51a Lipid‐lowering medications: 92a |
Hypertension: 8a Congestive heart failure: 13a | ||
| Lazar 2011 | I: aggressive glucose control | 20 | 63 (9) | 8.4 (1.5) | — | (1) Insulin: 38 (2) Oral diabetic medication: 52 (3) Insulin and oral diabetic medication: 5 (4) hyperlipidaemia medication: 100 (5) B‐blockers: 100 (6) Statins: 100 (7) ACE inhibitors: 60 |
(1) Angina class IV: 33 (2) Congestive heart failure: 13 (3) Myocardial infarction: 57 (4)Hypertension: 93 (5) Left main disease: 15 |
|
| C: moderate glucose control | 38 | 65 (9) | 8.3 (1.5) | — | (1) Insulin: 31 (2) Oral diabetic medication: 48 (3) Insulin and oral diabetic medication: 11 (4) hyperlipidaemia medication: 100 (5) B‐blockers: 100 (6) Statins: 100 (7) ACE inhibitors: 59 |
(1) Angina class IV: 33 (2) Congestive heart failure: 14 (3) Myocardial infarction: 57 (4) Hypertension: 100 (5) Left main disease: 21 |
||
| Cao 2010 | I: intensive insulin therapy | 70 | 58.2 (6.3) | 7.5 (0.7) | 21.1 (2.0) | (1) Insulin treatment: 65.2 (2) Oral agents: 27.2 |
Hypertension: 18.5 Coronary artery disease: 2.2 Cardiac insufficiency: 7.6 Pulmonary disease: 4.3 Renal insufficiency: 1 Liver insufficiency: 4.3 Any preoperative co‐morbidity: 38.0 |
|
| C: conventional insulin therapy | 66 | 59.4 (7.3) | 7.3 (0.6) | 22.2 (2.6) | (1) Insulin treatment: 72.4 (2) Oral agents: 21.8 |
Hypertension: 17.2 Coronary artery disease: 1.1 Cardiac insufficiency: 5.7 Pulmonary disease: 5.7 Renal insufficiency: 2.3 Liver insufficiency: 3.4 Any preoperative co‐morbidity: 35.6 |
||
| Glucontrol 2009 | I: intensive insulin treatment | 31* | 66 (14)* | — | 29.8 (5)* | Vasopressors/inotropes: 37.5a | — | |
| C: intermediate glucose control | 42* | 67 (10)* | — | 28.9 (4.7)* | Vasopressors/inotropes: 40.2a | — | ||
| NICE SUGAR 2009 | I: intensive insulin therapy | 41* | 65.4 (12.2)* | — | 30.8 (7.4)* | Corticosteroid treatment: 34.6a | (1) Respiratory dysfunction: 40.3a (2) Respiratory failure: 51a (3) Coagulatory dysfunction: 31.7a (4) Coagulatory failure: 4.3a (5) Hepatic dysfunction: 29.6a (6) Hepatic failure: 2.5a (7) Cardiovascular dysfunction: 19.4a (8) Cardiovascular failure: 57.3a (9) Renal dysfunction: 35a (10) Renal failure: 8.4a (11) Diabetes: 20.4a |
|
| C: conventional insulin therapy | 31* | 65.4 (12.4)* | — | 30.9 (8.3)* | Corticosteroid treatment: 31.7a | (1) Respiratory dysfunction: 40.9a (2) Respiratory failure: 50.9a (3) Coagulatory dysfunction: 29.2a (4) Coagulatory failure: 4.6a (5) Hepatic dysfunction: 29.8a (6) Hepatic failure: 1.8a (7) Cardiovascular dysfunction: 20.4a (8) Cardiovascular failure: 56.3a (9) Renal dysfunction: 36a (10) Renal failure: 7.7a (11) Diabetes: 19.8a |
||
| Subramaniam 2009 | I: intensive insulin therapy | 41a | 67 (10)a | — | 30 (8)a | (1) Statin: 67a (2) Aspirin: 85a (3) ACE inhibitor: 56a (4) B‐blocker: 73a (5) Insulin: 65a (6) Metformin: 15a (7) Glyburide: 37a |
(1) Diabetes: 54a (2) Hypertension: 81a (3) CAD: 51a (4) CHF: 11a (5) CABG: 21a (6) CRF: 13a (7) Stroke: 8a (8) COPD: 20a |
|
| C: conventional insulin therapy | 46a | 71 (11)a | — | 28 (8)a | (1) Statin: 57a (2) Aspirin: 84a (3) ACE inhibitor: 57a (4) B‐blocker: 80a (5) Insulin: 53a (6) Metformin: 19a (7) Glyburide: 30a |
(1) Diabetes: 53a (2) Hypertension: 78a (3) CAD: 58a (4) CHF: 9a (5) CABG: 30a (6) CRF: 12a (7) Stroke: 9a (8) COPD: 25a |
||
| Chan 2009 | I: intensive insulin therapy | 57a | 57 (12)a | — | 24 (3.4)a | — | — | |
| C: conventional insulin therapy | 43a | 58 (12)a | — | 26 (4.9)a | — | — | ||
| De La Rosa 2008 | I: intensive insulin therapy | 42a | 59.4 (14.6)* | — | 26.6 (5.0)* | — | (1) History of diabetes: 32a (2) History of cirrhosis: 9a (3) History of heart failure: 6a (4) History of kidney failure: 10a (5) History of cancer: 15a |
|
| C: conventional insulin therapy | 38a | 73.5 (12.0)* | — | 21.9 (4.3)* | — | (1) History of diabetes: 29a (2) History of cirrhosis: 7a (3) History of heart failure: 3a (4) History of kidney failure: 16a (5) History of cancer: 9a |
||
| Gandhi 2007 | I: intensive insulin therapy | 28a | 63 (15)a | 7 (2)a | 30 (6)a | Insulin: 22a Oral diabetic medication: 54a Insulin and oral diabetic medication: 16a Angiotensin‐converting enzyme inhibitor: 35a B‐blocker: 52a Antiarrhythmic: 9a Aspirin: 48a |
Diabetes: 20a Chronic renal failure: 1a History of myocardial infarction: 11a Stroke or transient ischaemic attack: 11a ASA II: 2a ASA III: 88a ASA IV: 10a |
|
| C: conventional insulin therapy | 34a | 63 (16)a | 7 (2)a | 29 (6)a | Insulin: 28a Oral diabetic medication: 31a Insulin and oral diabetic medication: 19a Angiotensin‐converting enzyme inhibitor: 39a B‐blocker: 55a Antiarrhythmic: 10a Aspirin: 60a |
Diabetes: 19a Chronic renal failure: 2a History of myocardial infarction: 16a Stroke or transient ischaemic attack: 7a ASA II: 1a ASA III: 88a ASA IV: 11a |
||
| Li 2006 | I: continuous insulin Infusion | 39 | 63.5 | — | 25.1 | Insulin: (5.9) Oral hypoglycaemic agents: (86.3) Diuretics: (17.6) Inotropic agents: (7.8) |
Smoking: (45.1) Hypertension: (84.3) Congestive heart failure: (56.9) Renal insufficiency: (9.8) Stroke: (7.8) Peripheral vascular disease: (3.9) Chronic obstructive pulmonary disease: (5.9) Previous myocardial infarction: (51) Left main stenosis: (25.5) |
|
| C: glucometer‐guided insulin | 36 | 63.7 | — | 25.4 | Insulin: (11.9) Oral hypoglycaemic agents: (78.6) Diuretics: (28.6) Inotropic agents: (4.8) |
Smoking: (40.5) Hypertension: (81.1) Congestive heart failure: (59.5) Renal insufficiency: (14.3) Stroke: (16.7) Peripheral vascular disease: (2.4) COPD: (9.5) Previous myocardial infarction: (61.9) Left main stenosis: (31) |
||
| Lazar 2004 | I: tight glycaemic control with GIK | 42 | 63.7 (1.4) | — | — | IV: nitroglycerin (31.9) IV: heparin (58.3) Insulin: (31.9) Oral diabetic medication: (59.7) |
Congestive heart failure: (27.7) Myocardial infarction: (70.8) Hypertension: (77.7) COPD: (8.3) Left main disease: (19.4) |
|
| C: standard therapy | 33 | 63 (1.5) | — | — | IV: nitroglycerin (33.3) IV: heparin (62.3) Insulin: (27.5) Oral diabetic medication: (59.4) |
Congestive heart failure: (39.1) Myocardial infarction: (71.0) Hypertension: (68.1) Chronic obstructive pulmonary disease:(4.3) Left main disease: (24.6) |
||
| Rassias 1999 | I: aggressive insulin therapy | 23 | 64.3 (9.3) | — | 27.8 (3.4) | — | — | |
| C: standard insulin therapy | 62 | 68.4 (7.5) | — | 28.7 (3.1) | — | — | ||
| —: denotes not reported *denotes data provided by trial authors. aData from total trial population. BMI: body mass index; GIK: glucose‐insulin‐potassium; HbA1c: glycosylated haemoglobin A1c; SD: standard deviation; CAD: coronary artery disease; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CRF: chronic renal failure; CABG: coronary artery bypass grafting; ASA: American Society Anaesthesiologists; IV: intravenous. | ||||||||
Appendix 9. Matrix of study endpoints (publications and trial documents)
| Study ID | |
| Duncan 2018 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00524472 Primary outcome measure(s): any major morbidity/30‐day mortality (time frame: within 30 days post surgery) a composite (any versus none) of the following major postoperative complications occurring: 1. All‐cause postoperative mortality. 2. Failure to wean from cardiopulmonary bypass or postoperative low cardiac index requiring mechanical circulatory support with intra aortic balloon counter pulsation, ventricular assist device, and/or extracorporeal mechanical oxygenation. 3. Serious postoperative infection. 4. Acute postoperative kidney injury requiring renal replacement therapy. 5. New postoperative focal or global neurologic deficit. Secondary outcome measure(s): 1. Post operative atrial fibrillation (time frame: 15 ‐ 30 days post operative). Evidence suggests that maintaining intra‐operative normoglycaemia during cardiac surgery while providing exogenous glucose and high‐dose insulin may decrease post‐operative morbidity or mortality. Using a randomized, controlled design, we propose to test the primary hypothesis that normalization of blood glucose using a hyperinsulinaemic‐normoglycemic clamp technique reduces the risk of a composite of serious adverse outcomes in patients undergoing cardiac surgery 2. Duration of hospitalisation (time frame: starting post operative day one to discharge from hospital, on an average of 8 days), days from date of surgery to hospital discharge 3. Duration of intensive care stay (time frame: ICU stay hours during hospital stay after surgery, on average of 25 hours), hours from date of surgery to discharge from intensive care unit 4. All‐cause mortality (time frame: one year post operative): all‐cause mortality identified during one‐year follow‐up 5. Composite of minor postoperative complications (time frame: within 30 days after surgery): composite of minor postoperative complications, which includes: a) prolonged mechanical ventilation, b) low cardiac index, c) acute kidney injury, d) prolonged hospitalisation, and all‐cause hospital readmission within 30 days Other outcome measure(s): — Trial results available in trial register: yes (last verified: January 19,2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): all‐cause postoperative mortality; failure to wean from cardiopulmonary bypass or postoperative low cardiac index (less than 1.8 l · min–1 · m–2) requiring mechanical circulatory support with intra‐aortic balloon counter‐pulsation, ventricular assist device, and/or extracorporeal mechanical oxygenation; serious postoperative infection including any of the following infectious complications: mediastinitis, sternal wound infection requiring surgical debridement, sepsis, or pneumonia requiring mechanical ventilatory support; acute postoperative kidney injury requiring renal replacement therapy; and (5) new postoperative focal (aphasia, decrease in limb function, hemiparesis) or global (diffuse encephalopathy with greater than 24h of severely altered mental status or failure to awaken postoperatively) neurologic deficit. Secondary outcome measure(s): postoperative atrial fibrillation, defined as the occurrence of new‐onset postoperative atrial fibrillation after cardiac surgery, duration of hospitalisation (days) and intensive care unit stay (days), and 1‐year all‐cause mortality Other outcome measure(s): mechanical ventilation greater than 72h, low cardiac index (cardiac index less than 1.8 l · min–1 · m–2 despite adequate fluid replacement (lack of haemodynamic response to repeated fluid administration of crystalloid or colloid intravascular solutions) and high‐dose inotropic support for greater than 4h), acute kidney injury (increase in creatinine greater than 100%), hospitalisation greater than 30 days, and all‐cause hospital readmission within 30 days | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): composite of 30‐day mortality, mechanical circulatory support, infection, renal or neurologic morbidity Secondary outcome measure(s): — Other outcome measure(s): — | |
| Wallia 2017 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT01211730 Primary outcome measure(s): rejection of liver transplant (within 1 year of transplantation) Secondary outcome measure(s): hypoglycaemia (within first 3 days following transplantation), infection rates (within 1 year following transplantation), re‐hospitalisation rates (within 1 year following transplantation), overall graft survival at 1 year (1 year following transplantation), death within 1 year (between 1 day and 1 year) Other outcome measure(s): — Trial results available in trial register: yes (last verified: January 19,2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): the primary outcome was the number of patients experiencing an episode of rejection within 1 year after transplantation Secondary outcome measure(s): the principal secondary outcomes was the number of patients experiencing an infection within 1 year after transplantation. Additional secondary outcomes were divided into inpatient outcomes (episodes of hypoglycaemia, including symptoms occurring when hypoglycaemic, ICU length of stay, hospital length of stay, and death) and outpatient outcomes (re‐hospitalisation, raft survival, and death) Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — | |
| Wahby 2016 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): operative mortality, renal dysfunction, acute renal failure required postoperative dialysis, postoperative permanent neurological deficit, sternal wound infection, leg infection and need for postoperative inotropic, all patients were followed up regarding duration of mechanical ventilation postoperatively, the occurrence of postoperative atrial fibrillation (AF), and perioperative myocardial infarction | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): operative mortality and postoperative outcome | |
| Parekh 2016 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT01643382 Primary outcome measure(s): incidence of poor graft function after kidney transplant (time frame: 7 days after transplant). Our primary endpoint will be poor initial graft function defined by the occurrence of DGF (defined by a decrease in serum creatinine of < 10%/day for 3 consecutive days after transplant) or slow graft function (serum creatinine > 3 mg/dL 5 days after transplant without dialysis) Secondary outcome measure(s): Secondary endpoints will include wound infection, length of hospital stay, 30 day mortality, hypoglycaemic episodes (glucose <70 mg/dL) and stroke Other outcome measure(s): — Trial results available in trial register: no (last verified: January 19,2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): poor graft function defined by the need for dialysis within seven days of transplant or a failure of the serum creatinine to drop by more than 10% for three consecutive days.The primary safety measure was the number of severe hypoglycaemic events (blood glucose <40 mg/dL) Secondary outcome measure(s): DGF (need for dialysis within seven days of transplant), perioperative death, stroke, and seizure, as well as serum creatinine and estimated GFR, using the modification of diet in renal disease study calculation, at 30 days, six months, and one year Other outcome measure(s): graft‐specific outcomes were biopsy‐proven rejection and graft loss | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): poor graft function (dialysis within seven days of transplant or failure of serum creatinine to fall by 10% for three consecutive days) Secondary outcome measure(s): — Other outcome measure(s): — | |
| Yuan 2015 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): blood glucose concentrations, insulin administration, volume of enteral and parenteral nutrition, and additional intravenous glucose administered were recorded. Serious adverse events included severe hypoglycaemia and severe hyperglycaemia. Outcome measures included postoperative morbidity and mortality rates Secondary outcome measure(s): — Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): blood glucose concentrations, insulin administration, and postoperative morbidity and mortality Secondary outcome measure(s): — Other outcome measure(s): — | |
| Umpierrez 2015 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT01361594 Primary outcome measure(s): number of subjects that were diagnosed for perioperative complications (time frame: within 6 months of hospitalisation. Number of participants that presented at least 1 complication including sternal wound infection, bacteraemia, acute renal failure, respiratory failure, and major cardiovascular events during the current hospitalisation and up to 6 months after hospitalisation Hospital Mortality (time frame: average 1 month during the hospitalisation). Mortality is defined as death occurring during admission, either during ICU or after transition to non‐ICU admission Secondary outcome measure(s): 1. glycaemic control (time frame: average 1 month during the hospitalisation): a. hyperglycaemic events (BG > 200 mg/dL) in ICU and non‐ICU, b. hypoglycaemic events (BG < 70 mg/dL; severe hypoglycaemia (BG < 40 mg/dL) 2. major cardiovascular events (time frame: average 1 month during the hospitalisation): a. acute myocardial infarction: (1) typical increase and gradual decrease (troponin) or (2) more rapid increase and decrease (creatine kinase MB) of biochemical markers of myocardial necrosis with at least one of the following: ischaemic symptoms, development of pathologic Q waves on the electrocardiogram, electrocardiographic changes indicative of ischaemia (ST‐segment elevation or depression), or coronary artery intervention (e.g., coronary angioplasty) b. congestive heart failure c. cardiac arrhythmias: malignant arrhythmia 3. acute renal failure (time frame: average 1 month during the hospitalisation): new‐onset abnormal renal function: serum creatinine > 2.0 mg/dL or an increment level > 50% from baseline 4. respiratory failure, defined as PaO2 Value < 60 mm Hg while breathing Air or a PaCO2 > 50 mm Hg. (time frame: average 1 month during the hospitalisation) Respiratory failure, defined as PaO2 value < 60 mm Hg while breathing air or a PaCO2 > 50 mm Hg. 5. ICU and hospital length of stay, and ICU readmissions (time frame: average 1 month during the hospitalisation) ICU and hospital length of stay, and ICU readmissions 6. surgical wound infection (time frame: average 1 month during the hospitalisation) superficial and deep sternal wound infection 7. pneumonia (CDC Criteria) (time frame: average 1 month during the hospitalisation) pneumonia (CDC criteria) 8. cerebrovascular events (time frame: average 1 month during the hospitalisation) permanent stroke and reversible ischaemic neurologic deficit 9. duration of ventilatory support and ICU readmission (time frame: average 1 month during the hospitalisation) duration of ventilatory support and ICU readmission 10. thirty day mortality (time frame: within 30 days of discharge) thirty day mortality 11. number of hospital readmissions and emergency room visits (time frame: within 30 days after discharge) number of hospital readmissions and emergency room visits 12. incidence of organ failures assessed by the daily SOFA score (time frame: average 1 month during the hospitalisation) incidence of organ failures assessed by the daily SOFA score 13. measures of inflammation (time frame: average 1 month during the hospitalisation) measures of inflammation (C‐reactive protein, TNF‐alpha; IL‐6) and oxidative stress markers 14. major cardiovascular events (time frame: within 3 months after discharge) a. acute myocardial infarction: (1) typical increase and gradual decrease (troponin) or (2) more rapid increase and decrease (creatine kinase MB) of biochemical markers of myocardial necrosis with at least one of the following: (a) ischaemic symptoms, (b) development of pathologic Q waves on the electrocardiogram, (c) electrocardiographic changes indicative of ischaemia (ST‐segment elevation or depression), or (d) coronary artery intervention (e.g., coronary angioplasty). b. congestive heart failure ‐c. cardiac arrhythmias: malignant arrhythmia 15. surgical wound infection (time frame: within 3 months after discharge) superficial and deep sternal wound infection 16. pneumonia (CDC Criteria) (time frame: within 3 months after discharge) pneumonia (CDC criteria) 17. cerebrovascular events (time frame: within 3 months after discharge) permanent stroke and reversible ischaemic neurologic deficit Other outcome measure(s): — Trial results available in trial register: yes (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): primary outcome was differences in a composite of complications, including mortality, wound infection, pneumonia, bacteraemia, respiratory failure, acute kidney injury, and major cardiovascular events Secondary outcome measure(s): the secondary outcome was to compare differences between intensive and conservative glucose control on the following: 1. glycaemic control, including mean daily and fasting glucose concentration, number of hypoglycaemic events (<70 mg/dL) and severe hypoglycaemia (<40 mg/dL), and glycaemic variability 2. individual complications: MACE as defined per the American College of Cardiology–American Heart Association, including acute myocardial infarction, congestive heart failure, and cardiac arrhythmias; acute kidney injury, defined as an increment in creatinine level <50% from baseline; respiratory failure, defined as the need for ventilator assistance for longer than 48 h; pneumonia; cerebrovascular events; surgical wound infections recorded as deep sternal wound infection, defined as chest wound infection involving the sternum or mediastinal tissues and as superficial sternal wound infection as those chest wound infections involving the skin or subcutaneous tissues; mortality was recorded during admission, either during ICU, transition to non‐ICU hospital setting, or 90 days after discharge Other outcome measure(s): we collected information on hospital length of stay, ICU readmissions, reoperations, and number of hospital readmissions and emergency room visits after discharge | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): primary outcome was differences in a composite of complications, including mortality, wound infection, pneumonia, bacteraemia, respiratory failure, acute kidney injury, and major cardiovascular events Secondary outcome measure(s): — Other outcome measure(s): — | |
| Hermayer 2012 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00609986 Primary outcome measure(s): delayed graft function (time frame: 10 days), acute/active rejection (time frame 30 months) Secondary outcome measure(s): severe hypoglycaemia (time frame: 30 months) blood glucose less than 40 mg/dL, severe hyperglycaemia (time frame: 30 month) blood glucose greater than 350 mg/dL Other outcome measure(s): — Trial results available in trial register: yes (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): the surrogate endpoint of DGF was chosen as the primary outcome variable for this study Secondary outcome measure(s): glycaemic control, graft loss and graft survival Other outcome measure(s):— | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): DGF Secondary outcome measure(s): glycaemic control, graft survival, and acute rejection episodes Other outcome measure(s): — | |
| Abdelmalak 2013 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00995501 Primary outcome measure(s): major perioperative morbidity: primary outcome was a collapsed composite endpoint (any versus none) defined as the occurrence of at least one of 15 major complications before hospital discharge, including sepsis, severe surgical site infection, myocardial infarction, heart failure, stroke, unstable ventricular arrhythmias, pulmonary embolism, pneumonia, respiratory failure, dialysis dependent renal failure, large pleural or peritoneal effusions, major bleeding, major wound and surgical site healing complications, vascular graft thrombosis, and 30‐day mortality Secondary outcome measure(s): 1 year mortality, all‐cause mortality Other outcome measure(s): — Trial results available in trial register: yes (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): the primary outcome was a collapsed composite endpoint (any vs none) defined as the occurrence of at least one of the 15 major complications before hospital discharge, including sepsis, severe surgical site infection, myocardial infarction, heart failure, stroke, unstable ventricular arrhythmias, pulmonary embolism, pneumonia, respiratory failure, dialysis dependent renal failure, large pleural or peritoneal effusions, major bleeding, major wound and surgical site healing complications, vascular graft thrombosis, and 30‐day mortality Secondary outcome measure(s): 1 year mortality Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): the primary outcome was a collapsed composite of 15 major complications and 30 days mortality Secondary outcome measure(s): — Other outcome measure(s): — | |
| Desai 2012 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): the primary end points were 2‐fold: superiority hypothesised for glucose control and target management and non‐inferiority hypothesised for complications and outcomes. Superiority end points included time to target glucose range, amount of insulin given, number of readings in target range, and number of patients with hypoglycaemic events (BG < 60 mg/dL and BG < 40 mg/dL). Non‐inferiority end points included perioperative renal failure, deep sternal wound infection, pneumonia, length of stay, atrial fibrillation, and operative mortality (death within 30 days) Secondary outcome measure(s): — Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): patient perioperative outcomes | |
| Lazar 2011 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00460499 Primary outcome measure(s): glycaemic control, postoperative morbidity, inflammatory markers Secondary outcome measure(s): free fatty acid levels Other outcome measure(s): — Trial results available in trial register: no (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): incidence of major adverse events (MAEs), changes in serum glucose, and the incidence of hypoglycaemic episodes Secondary outcome measure(s): time on the ventilator defined as the time of admission to the ICU to the time of extubation, length of ICU, and hospital stay, all of which was standardised using, weight gain defined as the difference between the weight the evening before surgery to 5 am the day after surgery,an inotropic score that ranged from 0 to 4 (0 = no inotropic support; 1 = dopamine at 2 μg/kg for <24 hours; 2 = dopamine > 2 μg/kg for >24 hours; 3 = 2 inotropic agents; 4 = inotropic agents and intra‐aortic balloon support), the amount of insulin administered, and the cardiac index during the study Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): incidence of major adverse events ( 30‐day mortality, myocardial infarction, neurologic events, deep sternal infections, and AF), the level of serum glucose, and the incidence of hypoglycaemic events Secondary outcome measure(s): — Other outcome measure(s): — | |
| Cao 2010 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): the primary outcome was postoperative short‐term complication rate Secondary outcome measure(s): the secondary outcomes included postoperative 28‐day mortality rate, HOMA‐IR score, and HLA‐DR Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — | |
| Glucontrol 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00107601 Primary outcome measure(s): mortality in the intensive care unit (ICU) Secondary outcome measure(s): hospital mortality, 28 day mortality, length of ICU stay, length of hospital stay, number of episodes of hypoglycemia and associated clinical signs, infectious morbidity, incidence of organ failures, number of red‐cell transfusions, number of days spent in ICU without life‐support: vasopressors/inotropes, cardiac mechanical support, mechanical ventilation, renal replacement therapy, daily SOFA (sequential organ failure assessment) score Other outcome measure(s): — Trial results available in trial register: no (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): the primary outcome variable was the all‐cause absolute mortality during the ICU stay Secondary outcome measure(s): secondary outcome variables included hospital and 28‐day mortality, ICU and hospital, Length of stay (LOS), incidence of organ failures assessed by the daily SOFA score, rate of hypoglycaemia and the SOFA score on the day of hypoglycaemia, duration of mechanical ventilation, inotrope/ vasopressor and renal replacement therapy, number of packed red blood cells transfusion, febrile days and days with therapeutic anti‐infective agents Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — | |
| NICE SUGAR 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00220987 Primary outcome measure(s): all‐cause mortality at 90 days Secondary outcome measure(s): all cause mortality at 28 days, length of intensive care unit stay, length of hospital stay, the need for organ support (inotropes, renal replacement therapy and positive pressure ventilation), incidence of blood stream infections, incidence and severity of hypoglycaemia, extended Glasgow outcome score Other outcome measure(s): — Trial results available in trial register: no (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): death from any cause within 90 days after randomization, in an analysis that was not adjusted for baseline characteristics Secondary outcome measure(s): survival time during the first 90 days, cause‐specific death, and durations of mechanical ventilation, renal‐replacement therapy, and stays in the ICU and hospital Other outcome measure(s): death from any cause within 28 days after randomization, place of death (ICU, hospital ward, or other), incidence of new organ failure, positive blood culture, receipt of red‐cell transfusion, and volume of the transfusion | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): death from any cause within 90 days after randomization Secondary outcome measure(s): — Other outcome measure(s): — | |
| Subramaniam 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): the primary endpoint was defined as a composite rate of the following intra pocedural and post porcedural major cardiovascular events at hospital discharge: all‐cause death, myocardial infarction, acute congestive heart failure Secondary outcome measure(s): secondary endpoints included the following efficacy and safety endpoints: (!) blood glucose levets al 4‐h intervals starting from 4 h after the procedure and ending at 48h, (2) rate of hypoglycaemia defined as a glucose level less than 60mg/dL, (3) rate of glucose concentrations greater than 150 mg/dL, (4) graft failure or a need for reintervention (reoperation due to graft failure or lack of peripheral pulses in the postoperative period), (5) surgical site of infection, (6) acute renal insufficiency (a 25% change in creatinine from before surgery to after surgery), (7) hospital duration of stay (from the date of surgery to discharge from the hospital) Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): the primary endpoint was a composite of all‐cause death, myocardial infarction, and acute congestive heart failure Secondary outcome measure(s): the secondary endpoints were blood glucose concentrations, rates of hypoglycaemia (< 60 mg/dL) and hyperglycaemia (> 150 mg/dL), graft failure or reintervention, wound infection, acute renal insufficiency, and duration of stay Other outcome measure(s): — | |
| Chan 2009 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00370643 Primary outcome measure(s): 1. Duration of intubation 2. ICU length 3. Blood transfusion 4. Infection rate 5. Renal dysfunction 6. Neurological dysfunction 7. Hospital length 8. Mortality Secondary outcome measure(s): 1. Length of surgery 2. Length of cardiopulmonary bypass 3. Physical status 4. EuroSCORE 5. Parsonnet 6. Canadian Multicenter index Other outcome measure(s): — Trial results available in trial register: no (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): primary outcomes were clinical outcomes, which included the duration of mechanical ventilation from the operation room until extubation in the intensive care unit (ICU), the length of stay in the ICU, occurrence of infection (diagnosis of pneumonia, urinary tract infection, sepsis, septic shock, wound infection, blood stream infection, catheter infection), occurrence of hypoglycaemia (glucose level <50 mg/dL), renal dysfunction (characterised as an increase in the level of creatinine higher than 50% of the baseline value), neurological dysfunction (diagnosis by hospital neurologist who was blinded to the protocol), red blood cell transfusion during the first 30 days after surgery, the length of stay in the hospital and mortality by 30 days after surgery Secondary outcome measure(s): — Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): primary outcomes were clinical outcomes, including time of mechanical ventilation, length of stay in the intensive care unit, infection, hypoglycaemia, renal or neurological dysfunction, blood transfusion and length of stay in the hospital Secondary outcome measure(s): the secondary outcome was a combined end‐point (mortality at 30 days, infection or length of stay in the intensive care unit of more than 3 days) Other outcome measure(s): — | |
| De La Rosa 2008 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: study ID NCT00966421 does not exist in ClinicalTrials.gov Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: no (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): 28‐day all‐cause mortality Secondary outcome measure(s): ICU mortality; hospital mortality; incidence of infections in the ICU (ventilator‐associated pneumonia, urinary infections, catheter‐related infections and primary bacteraemia); ICU length of stay; days of mechanical ventilation and incidence of severe hypoglycaemia Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): mortality at 28 days Secondary outcome measure(s): — Other outcome measure(s): — | |
| Gandhi 2007 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00282698 Primary outcome measure(s): mortality, sternal wound infections, stroke, cardiac arrhythmias, renal failure Secondary outcome measure(s): length of ICU stay, length of hospital stay, safety of study insulin infusion, efficacy of study insulin infusion Other outcome measure(s): — Trial results available in trial register: no (last verified: January 19, 2021) | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): death, sternal wound infections, prolonged pulmonary ventilation, cardiac arrhythmias (new‐onset atrial fibrillation, heart block requiring permanent pacemaker, or cardiac arrest), stroke, and acute renal failure within 30 days after surgery Secondary outcome measure(s): secondary outcome measures were length of stay in the ICU and hospital Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): the primary outcome was a composite of death, sternal infections, prolonged ventilation, cardiac arrhythmias, stroke, and renal failure within 30 days after surgery Secondary outcome measure(s): the primary outcome was a composite of death, sternal infections, prolonged ventilation, cardiac arrhythmias, stroke, and renal failure within 30 days after surgery Other outcome measure(s): — | |
| Li 2006 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): the primary endpoints were incidences of operative mortality and sternal wound infection Secondary outcome measure(s): the secondary endpoint was the adequacy of blood‐glucose control Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): Secondary outcome measure(s): Other outcome measure(s): the adequacy of postoperative blood glucose control and clinical outcome were evaluated | |
| Lazar 2004 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): to determine whether tight perioperative glycaemic control in patients with diabetes undergoing CABG with a modified GIK solution would optimise myocardial metabolism and improve perioperative outcomes Secondary outcome measure(s): to determine whether the early beneficial effects of tight glycaemic control would result in improved survival, a decreased incidence of ischaemic events, and reduced wound complications Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): to determine whether tight glycaemic control with a modified glucose‐insulin‐potassium solution in patients with diabetes undergoing CABG would improve perioperative outcomes Secondary outcome measure(s): — Other outcome measure(s): — | |
| Rassias 1999 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: NT Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): — Trial results available in trial register: — | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure(s): to examine the effect of aggressive insulin therapy on Polymorphonuclear neutrophils (PMNs) function in people with diabetes undergoing cardiac surgery Secondary outcome measure(s): — Other outcome measure(s): — | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure(s): we tested the effect of an insulin infusion on perioperative neutrophil function in people with diabetes scheduled for coronary artery bypass surgery Secondary outcome measure(s): — Other outcome measure(s): — | |
| —: denotes not reported
aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers). bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary trial). cPrimary and secondary outcomes refer to verbatim specifications in publication/records. Unspecified outcome measures refer to all outcomes not described as primary or secondary outcome measures. EMA: European Medicines Agency; FDA: Food and Drug Administration (US); mo: month(s); NA: not applicable; NT: no trial document available; ICU: intensive care unit ; DGF: delayed graft function; AF: atrial fibrillation; GFR: glomerular filtration rate ; BG: blood glucose, PaO2: partial pressure of oxygen; SOFA: sequential organ failure assessment; CDC: Centers for Disease Control and Prevention; MACE: mayor adverse cardiac events; CABG: coronary artery bypass graft. | |
Appendix 10. High risk of outcome reporting bias according to Outcome Reporting Bias In Trials (ORBIT) classification
| Study ID | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Duncan 2018 | ND | NA | NA | NA | NA |
| Wallia 2017 | ND | NA | NA | NA | NA |
| Wahby 2016 | ND | NA | NA | NA | NA |
| Parekh 2016 | ND | NA | NA | NA | NA |
| Yuan 2015 | ND | NA | NA | NA | NA |
| Umpierrez 2015 | ND | NA | NA | NA | NA |
| Abdelmalak 2013 | ND | NA | NA | NA | NA |
| Hermayer 2012 | Hypoglycaemic events Major adverse events |
— | — | — | Probably |
| Desai 2012 | ND | NA | NA | NA | NA |
| Lazar 2011 | ND | NA | NA | NA | NA |
| Cao 2010 | All‐cause mortality | — | — | Yes | — |
| Glucontrol 2009 | ND | NA | NA | NA | NA |
| NICE SUGAR 2009 | ND | NA | NA | NA | NA |
| Subramaniam 2009 | ND | NA | NA | NA | NA |
| Chan 2009 | ND | NA | NA | NA | NA |
| De La Rosa 2008 | ND | NA | NA | NA | NA |
| Gandhi 2007 | ND | NA | NA | NA | NA |
| Li 2006 | Hypoglycaemic events | — | — | — | Probably |
| Lazar 2004 | Hypoglycaemic events | — | — | — | Probably |
| Rassias 1999 | ND | NA | NA | NA | NA |
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but reports only that result was not significant (Classification 'A', table 2, Kirkham 2010).
bClear that outcome was measured and analysed;trial report states that outcome was analysed but report no results (Classification 'D', table 2, Kirkham 2010).
cClear that outcome was measured but was not necessarily analysed; judgement says likely to have been analysed but not reported due to non‐significant results (Classification 'E', table 2, Kirkham 2010).
dUnclear whether outcome was measured; not mentioned, but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results (Classification 'G', table 2, Kirkham 2010). NA: not applicable; ND: none detected; ORBIT: Outcome Reporting Bias In Trials. | |||||
Appendix 11. Definition of outcome measurement
| Study ID | Review's primary and secondary outcomes | Definition |
| Duncan 2018 | All‐cause mortality | All‐cause mortality identified during initial hospitalisation or during 30‐day follow‐up. All‐cause mortality identified during 1‐year follow‐up |
| Hypoglycaemic episodes, serious/severe | Severe hypoglycaemia was defined as blood glucose less than 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | Moderate hypoglycaemia was defined as blood glucose less than 60 mg/dL | |
| Hypoglycaemic episodes | Severe and moderate hypoglycaemia was defined as blood glucose less than 40 mg/dL and 60 mg/dL, respectively | |
| Adverse events, infection | Infection morbidity: postoperative course complicated by one of the following: 1. Sepsis with evidence of acute organ dysfunction: sepsis is recognised as a clinical syndrome that may be defined by infection highly suspected (clinical syndrome pathognomonic for infection) or proven (by culture, stain, or polymerase chain reaction) and presence of two or more of the following systemic inflammatory response syndrome criteria: heart rate > 90 beats/min–1 (tachycardia); body temperature < 36 °C or > 38 °C (hypothermia or fever); respiratory rate > 20 breaths/min–1 or a PACO2 < 32 mmHg (tachypnoea or hypocapnia due to hyperventilation); leukocyte count < 4000 cells/mm–3 or > 12,000 cells/mm–3, or greater than 10% band forms (immature white blood cells; leukopenia, leukocytosis or bandaemia) 2. Mediastinitis: sternal click, open sternal wound, drainage from mediastinal incision, with fever, and including positive cultures along with elevated leukocyte count and the institution of antimicrobial therapy and re‐exploration with operative note diagnosing mediastinitis or sternectomy with muscle flap grafts to the affected area or diagnosis by physician of mediastinitis 3. Sternal wound infection: sternal wound infection other than mediastinitis, documented with positive cultures, requiring surgical intervention 4. Pneumonia: fever > 38 °C, elevation in leukocyte count, increase in sputum production, infiltrate in chest x‐ray film > 24h, positive sputum culture requiring mechanical ventilation 5. Neurologic deficit: New postoperative focal (aphasia, decrease in limb function, or hemiparesis confirmed by clinical findings and/or computed tomographic scan) or global neurologic deficit (diffuse encephalopathy with greater than 24h of severely altered mental status, and/or failure to awaken postoperatively) |
|
| Other adverse events | Prolonged intubation: endotracheal intubation and mechanical ventilation required for > 72 h postoperatively, measured from arrival in intensive care unit after surgery until weaning from mechanical ventilation and endotracheal extubation. Additional periods of time when reintubation and mechanical ventilation are required are included Low cardiac index: cardiac index < 1.8 l/min–1 m–2 despite adequate fluid replacement and high‐dose inotropic support for > 4 h Hospital readmission: postoperative complications requiring readmission to a hospital for any reason identified during 30‐day follow‐up |
|
| Cardiovascular events | Postoperative mechanical circulatory support: failure to wean from cardiopulmonary bypass or postoperative low cardiac index (CI < 1.8 l/min–1/m–2) conditions requiring circulatory support with intra‐aortic balloon pump, ventricular assist device and/or extracorporeal Postoperative atrial fibrillation: the occurrence of new‐onset postoperative atrial fibrillation after cardiac surgery occurring within 30 days of surgery. |
|
| Renal failure | Renal insufficiency: postoperative increase in baseline creatinine > 100%. Baseline creatinine was defined as the preoperative measurement immediately before surgery Renal morbidity: postoperative requirement for renal dialysis. |
|
| Length of ICU and hospital stay | Duration of hospitalisation: days from day of surgery to hospital discharge Duration of intensive care unit stay: days from day of surgery to discharge from intensive care unit Prolonged hospitalisation: hospitalisation after surgery > 30 days | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Intraoperative time‐weighted mean glucose concentration was calculated across measurements for each patient using the trapezoidal method and equal to the area under the curve divided by the total glucose reading time | |
| Wallia 2017 | All‐cause mortality | Death following liver transplantation between 1 day and 1 year |
| Hypoglycaemic episodes, serious/severe | BG < 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | Moderate hypoglycaemia: BG between 40 mg/dL and 69 mg/dL | |
| Hypoglycaemic episodes | Glucose ≤ 70 mg/dL within the first 3 days following transplantation (including symptoms occurring when hypoglycaemic) | |
| Adverse events, infection | Included any new infection as an inpatient or outpatient from the day of transplant to 1 year after transplant. Infections were defined by as follows: positive culture results, considered an infection by the primary team or infectious disease consultants, and/or empiric treatment was given for ≥ 3 days because of fever and other signs of infection. Infections were further subclassify by the type of infection (bacterial, viral, fungal or a combination) and the site of infection. | |
| Other adverse events | Episode of rejection: the clinical criteria were a twofold or greater increase in transaminases or alkaline phosphatase levels, for which no other explanation was present and that normalised with empiric pulse methylprednisolone dosing of 500 mg/d for 3 days. The biopsy criteria were based on the Banff schema for acute rejection; however, a biopsy diagnosis was not an absolute criterion for the definition of rejection. All cases that did not clearly meet these criteria were adjudicated by 2 blinded reviewers. | |
| Cardiovascular events | — | |
| Renal failure | — | |
| Length of ICU and hospital stay | Days of length of ICU and hospital stay after transplantation | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Mean blood glucose during intervention (before initiating the insulin infusions and after conversion to subcutaneous insulin) | |
| Wahby 2016 | All‐cause mortality | Mortality within 30 days of operation or during hospitalisation due to cause related to operation |
| Hypoglycaemic episodes, serious/severe | — | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Sternal wound infection, leg infection | |
| Other adverse events | Postoperative permanent neurological deficit. Need for postoperative inotropic support that was defined as the use of dopamine 5 mg/kg/min; any dose of epinephrine, norepinephrine, dobutamine or milrinone | |
| Cardiovascular events | The occurrence of postoperative AF, and perioperative myocardial infarction defined as: any patient having fresh ECG changes including new Q‐waves in two precordial leads, new bundle branch block, haemodynamic compromise with new segmental wall motion dysfunction or elevation of CK MB over 100 U/L after undergoing open heart surgery | |
| Renal failure | Renal dysfunction: elevated serum creatinine above 2 mg/dL postoperative or more than 25% of preoperative level, acute renal failure that required postoperative dialysis | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | BG checked hourly | |
| Parekh 2016 | All‐cause mortality | Perioperative death |
| Hypoglycaemic episodes, serious/severe | BG < 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | — | |
| Other adverse events | Delayed graft function, biopsy‐proven rejection, graft loss | |
| Cardiovascular events | Stroke | |
| Renal failure | Need for dialysis within 7 days of transplant or a failure of the serum creatinine to drop by more than 10% for three consecutive days | |
| Length of ICU and hospital stay | Length of hospitalisation in days | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Preoperative, every 30 to 45 minutes intraoperatively, graft reperfusion (0 to 6 hours, 6 to 12 hours, 12 to 24 hours) | |
| Yuan 2015 | All‐cause mortality | Postoperative mortality |
| Hypoglycaemic episodes, serious/severe | BG level ≤ 2.2 mmol/l (≤ 40 mg/dL) | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Infective complications (surgical site, pneumonia, urinary tract, bacteraemia, anastomotic leak) | |
| Other adverse events | Non‐infective complications (bleeding, delayed gastric emptying, obstruction, hepatic dysfunction, renal dysfunction, circulatory insufficiency). Severe hyperglycaemia: BG level ≥ 16.7 mmol/l (≥ 300 mg/dL) | |
| Cardiovascular events | Circulatory insufficency | |
| Renal failure | Renal dysfunction | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | BG monitored hourly and every 2 to 4 hours when stable | |
| Umpierrez 2015 | All‐cause mortality | During admission, either during ICU, transition to non‐ICU hospital setting, or 90 days after discharge |
| Hypoglycaemic episodes, serious/severe | BG < 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | BG < 70 mg/dL | |
| Hypoglycaemic episodes | BG < 70 mg/dL | |
| Adverse events, infection | Surgical wound infections recorded as deep sternal wound infection, defined as chest wound infection involving the sternum or mediastinal tissues and as superficial sternal wound infection as those chest wound infections involving the skin or subcutaneous tissues | |
| Other adverse events | Respiratory failure, defined as the need for ventilator assistance for longer than 48 hours Pneumonia Cerebrovascular events |
|
| Cardiovascular events | MACE as defined per the American College of Cardiology–American Heart Association, including acute myocardial infarction, congestive heart failure, and cardiac arrhythmias | |
| Renal failure | Acute kidney injury defined as: an increment in creatinine level > 50% from baseline | |
| Length of ICU and hospital stay | Days on ICU and hospital | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Mean daily and fasting glucose concentration | |
| Abdelmalak 2013 | All‐cause mortality | 30‐day mortality and 1‐year mortality data were obtained from electronic medical records, the United States Social Security Index, or both and confirmed by direct telephone contact with patient/family |
| Hypoglycaemic episodes, serious/severe | Severe hypoglycaemia defined by a plasma glucose concentration of 2.2 mmol/lL or less (< 40 mg/dL) | |
| Hypoglycaemic episodes, non‐serious/severe | Moderate hypoglycaemia (4.0 mmol/L ≈72.7 mg/dL) | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Severe surgical site infection. Sepsis | |
| Other adverse events | Pulmonary embolism, pneumonia, respiratory failure, large pleural or peritoneal effusions, major bleeding, major wound and surgical site healing complications and vascular graft thrombosis | |
| Cardiovascular events | Myocardial infarction, heart failure, stroke, unstable ventricular arrhythmia | |
| Renal failure | Dialysis dependent renal failure | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | — | |
| Hermayer 2012 | All‐cause mortality | — |
| Hypoglycaemic episodes, serious/severe | BG < 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | BG < 70 mg/dL | |
| Hypoglycaemic episodes | BG < 70 mg/dL | |
| Adverse events, infection | — | |
| Other adverse events | Delayed graft function (d‐10 serum creatinine value over 2.5 mg/dL or the need for dialysis within the first 7 days after transplant), acute rejection episodes (biopsy‐confirmed), hyperglycaemia (BG > 350 mg/dL) | |
| Cardiovascular events | — | |
| Renal failure | ||
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Control group: every hour during surgery and every 4 hours while in the recovery room and in the transplant unit. After diet consumption BG levels are checked before meals, at bedtime and at 0300h Intervention group: on admission and every 1 hour |
|
| Desai 2012 | All‐cause mortality | Operative mortality within 30 days |
| Hypoglycaemic episodes, serious/severe | BG < 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | BG < 60 mg/dL | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Deep sternal wound infection and pneumonia | |
| Other adverse events | — | |
| Cardiovascular events | MACE, AF | |
| Renal failure | Perioperative renal failure | |
| Length of ICU and hospital stay | 1. Length of hours in the ICU 2. Length of days in the hospital |
|
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | — | |
| Lazar 2011 | All‐cause mortality | 30‐day mortality of any cause |
| Hypoglycaemic episodes, serious/severe | — | |
| Hypoglycaemic episodes, non‐serious/severe | BG level < 80 mg/dL | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Deep sternal wound infections (any infection reaching or directly involving the sternum) | |
| Other adverse events | — | |
| Cardiovascular events | 1. MI (both enzyme and electrocardiographic changes) 2. Cerebral vascular accidents (a persistent neurologic deficit lasting 24 hours or more) 3. AF that lasted for longer than 15 minutes |
|
| Renal failure | — | |
| Length of ICU and hospital stay | Mean of days length of stay (ICU and hospital), standardised using "fast‐track" protocols | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | Difference between the weight the evening before surgery to 5 am the day after surgery | |
| Mean blood glucose during intervention | Mean of BG levels measured every 30 minutes during surgery | |
| Cao 2010 | All‐cause mortality | Death from any cause within 28 days of the operation or during the period of hospital stay |
| Hypoglycaemic episodes, serious/severe | BG level of 2.2 mmol/L or less | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | 1. Wound infection (when pus could be expressed from the incision or aspirated from a loculated mass within the wound) 2. Intra‐abdominal infection (positive culture from drainage or puncture fluid with a need for systemic antibiotic treatment and consistent with the image and clinical findings) 3. Sepsis (definition of the ACCP‐SCCM consensus conference on sepsis and organ failure) 4. Urinary tract infection (positive culture urine) 5. Pneumonia (when culture from sputum, pleural fluid or empyema fluid were positive, consistent with the diagnosis and clinical symptoms or a chest radiograph diagnostic of pulmonary filtrates) |
|
| Other adverse events | — | |
| Cardiovascular events | — | |
| Renal failure | — | |
| Length of ICU and hospital stay | Days of post operation hospital stay | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Composite average of all the glucose levels from the period immediately after surgery to the end of protocol | |
| Glucontrol 2009 | All‐cause mortality | 28‐day mortality |
| Hypoglycaemic episodes, serious/severe | — | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | BG concentration below 2.2 mmol/L | |
| Adverse events, infection | — | |
| Other adverse events | Organ failure assessed by SOFA score on the day of hypoglycaemia | |
| Cardiovascular events | Vasopressor or inotropic | |
| Renal failure | Creatinine greater than 2 mg/100 mL | |
| Length of ICU and hospital stay | Mean of days in ICU and hospital stay | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Mean of basal glycaemias recorded by patient | |
| NICE SUGAR 2009 | All‐cause mortality | Death from any cause |
| Hypoglycaemic episodes, serious/severe | BG level ≤ 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Infectious complication (patients with positive blood cultures). | |
| Other adverse events | — | |
| Cardiovascular events | — | |
| Renal failure | Renal SOFA score of < 3 at baseline who had score of 3 or 4 post‐randomisation | |
| Length of ICU and hospital stay | Median of days in ICU and hospital stay | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Average of daily blood glucose measurements (from baseline to 14 days after randomisation) | |
| Subramaniam 2009 | All‐cause mortality | All‐cause death during hospital stay and 30 days after surgery |
| Hypoglycaemic episodes, serious/severe | BG < 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | BG < 60 mg/dL | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Surgical site infection | |
| Other adverse events | Graft failure or reintervention | |
| Cardiovascular events | MI and acute congestive heart failure defined per Standard American College of Cardiology‐American Heart Association | |
| Renal failure | Acute renal insufficiency: a 25% change in creatinine from before surgery to after surgery | |
| Length of ICU and hospital stay | Days in hospital from the date of surgery to the hospital discharge | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | BG concentration values over the first 48 hours post surgery | |
| Chan 2009 | All‐cause mortality | Mortality at 30 days |
| Hypoglycaemic episodes, serious/severe | — | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | BG level ≤ 50 mg/dL | |
| Adverse events, infection | Occurrence of infection (diagnosis of pneumonia, urinary tract infection, sepsis, septic shock, wound infection, blood stream infection, catheter infection) | |
| Other adverse events | Neurological dysfunction (diagnosis by hospital neurologist who was blinded to the protocol) | |
| Cardiovascular events | — | |
| Renal failure | Characterised as an increase in the level of creatinine higher than 50% of the baseline value | |
| Length of ICU and hospital stay | The length of stay in the ICU | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Every 1 to 2 hours during the intraoperative period and during the first 24 hours after admission to ICU | |
| De La Rosa 2008 | All‐cause mortality | 28‐day all‐cause mortality |
| Hypoglycaemic episodes, serious/severe | Incidence of severe hypoglycaemia defined as number of patients with at least 1 episode of blood glucose level less than 40 mg/dL | |
| Hypoglycaemic episodes, non‐serious/severe | BG levels within 41 mg/dL to 59 mg/dL | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Incidence of infections in the ICU (ventilator‐associated pneumonia, urinary infections, catheter‐related infections and primary bacteraemia) | |
| Other adverse events | — | |
| Cardiovascular events | — | |
| Renal failure | Renal replacement therapy | |
| Length of ICU and hospital stay | Length of days in ICU | |
| Health‐related quality of life | — | |
| Socioeconomic effects | Every 1, 2, 4 hours or every 4 and 6 hours, depending on the study group | |
| Gandhi 2007 | All‐cause mortality | Number of deaths in hospital and 30 days after surgery |
| Hypoglycaemic episodes, serious/severe | — | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | BG concentration < 3.3 mmol/L < 60 mg/dL | |
| Adverse events, infection | Deep sternal infection | |
| Other adverse events | Stroke Prolonged intubation (> 24 hours) |
|
| Cardiovascular events | Cardiac arrest Heart block requiring pacemaker New‐onset atrial fibrillation |
|
| Renal failure | Acute renal failure | |
| Length of ICU and hospital stay | Length of stay in ICU and hospital | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Mean BG levels after cardiopulmonary bypass and mean BG levels at the end of the first 24 hours in the ICU | |
| Li 2006 | All‐cause mortality | Any cause of death |
| Hypoglycaemic episodes, serious/severe | — | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Leg and sternal wound infections, both superficial and deep confirmed by wound culture | |
| Other adverse events | New neurological deficits Prolonged ventilation/intubation Use of postoperative inotropic agents |
|
| Cardiovascular events | New‐onset AT | |
| Renal failure | Renal disfunction | |
| Length of ICU and hospital stay | Mean of days of ICU stay | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Composite average of daily glucose levels (average of BG levels within a 24‐hour period) from the period immediately after surgery through the 5th postoperative day | |
| Lazar 2004 | All‐cause mortality | Death, any cause; 30‐day and 5‐year mortality |
| Hypoglycaemic episodes, serious/severe | — | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Infection (pneumonia and wound) | |
| Other adverse events | Atrial fibrillation Myocardial infarction |
|
| Cardiovascular events | Recurrent ischaemic events (episodes of angina with ECG changes or documented MI with enzyme and ECG changes) and need of recatheterisation | |
| Renal failure | — | |
| Length of ICU and hospital stay | Length of stay in the ICU defined as time from ICU arrival to transfer to the floor or step‐down unit | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | Patients weighted the evening before surgery and at 5:00 AM the day after surgery to determine the postoperative weight gain in pounds | |
| Mean blood glucose during intervention | Mean of serum BG monitoring every hour during intervention | |
| Rassias 1999 | All‐cause mortality | — |
| Hypoglycaemic episodes, serious/severe | — | |
| Hypoglycaemic episodes, non‐serious/severe | — | |
| Hypoglycaemic episodes | — | |
| Adverse events, infection | Infection (septic mediastinitis, pneumonia, urinary tract infection) | |
| Other adverse events | — | |
| Cardiovascular events | — | |
| Renal failure | — | |
| Length of ICU and hospital stay | — | |
| Health‐related quality of life | — | |
| Socioeconomic effects | — | |
| Weight gain | — | |
| Mean blood glucose during intervention | Mean intraoperative glucose levels | |
| —: denotes not reported AF: atrial fibrillation; BG: blood glucose; ECG: electrocardiogram; ICU: intensive care unit; MACE: major adverse cardiac events; MI: myocardial infarction; SOFA: sequential organ failure assessment. | ||
Appendix 12. Adverse events (I)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Deaths (N) | Deaths (% of participants) | Participants with at least one adverse event (N) | Participants with at least one adverse event (%) | Participants with infection (N) | Participants with infection (%) |
| Duncan 2018 | I: hyperinsulinaemic normoglycaemia | 226* | 2* | 0.9* | 9* | 4* | 9* | 4* |
| C: standard therapy | 249* | 6* | 2.4* | 17* | 6.8* | 17* | 6.8* | |
| Wallia 2017 | I: intensive (140) | 23* | 4* | 17.4* | 11* | 47.8* | 10* | 43.5* |
| C: moderate (180) | 26* | 1* | 3.8* | 16* | 61.5* | 16* | 61.5* | |
| Wahby 2016 | I: tight glycaemic control | 67 | 2 | 2.99 | 28 | 41.8 | 27 | 40.3 |
| C: conventional moderate glycaemic control | 68 | 4 | 5.88 | 45 | 66.2 | 51 | 94 | |
| Parekh 2016 | I: moderately intense control | 30 | 0 | 0 | 13 | 43.3 | 1 | 3.3 |
| C: standard glucose control | 30 | 2 | 6.66 | 22 | 73.3 | 1 | 3.3 | |
| Yuan 2015 | I: intensive glycaemic (IG) management | 106 | 1 | 0.94 | 7 | 6.6 | 21 | 19.8 |
| C: conventional glycaemic (CG) management | 106 | 1 | 0.94 | 14 | 13.2 | 32 | 30.2 | |
| Umpierrez 2015 | I: intensive group | 77* | 38* | 49* | 30* | 39* | 4* | 5.2* |
| C: conservative control | 75* | 36* | 48* | 31* | 41* | 7* | 9.3* | |
| Abdelmalak 2013 | I: intensive glucose management | 54* | 0* | 0* | 9* | 16.7* | 9* | 16.7* |
| C: conventional glucose management | 49* | 1* | 2* | 4* | 8.2* | 4* | 8.2* | |
| Hermayer 2012 | I: intensive glycaemic control | 44 | — | — | 9 | — | — | — |
| C: standard glycaemic control | 49 | — | — | 12 | 24 | — | — | |
| Desai 2012 | I: liberal blood glucose control | 44* | 1 | 2.3* | 3* | 8.1* | 0* | 0* |
| C: strict blood glucose control | 37* | 1 | 2.7* | 3* | 6.8* | 0* | 0* | |
| Lazar 2011 | I: aggressive glucose control | 40 | 0 | 0 | 12 | 30 | 0 | 0 |
| C: moderate glucose control | 42 | 0 | 0 | 16 | 38 | 0 | 0 | |
| Cao 2010 | I: intensive insulin therapy | 92 | 4 | 4.3 | 7 | 7.6 | 15 | 16.3 |
| C: conventional insulin therapy | 87 | 5 | 5.7 | 16 | 18.4 | 37 | 42.5 | |
| Glucontrol 2009 | I: intensive insulin treatment | 55* | 11* | 20* | 47* | 85.5* | — | — |
| C: intermediate glucose control | 69* | 7* | 10.1* | 44* | 63.8* | — | — | |
| NICE SUGAR 2009 | I: intensive insulin therapy | 213* | 57* | 26.8* | 57* | 26.8* | 32* | 15* |
| C: conventional insulin therapy | 208* | 49* | 23.6* | 49* | 23.6* | 22* | 10.6* | |
| Subramaniam 2009 | I: continuous insulin infusion | 62* | 0* | 0* | 22* | 35.5* | 22* | 35.5* |
| C: standard intermittent sliding‐scale insulin | 64* | 0* | 0* | 16* | 25* | 16* | 25* | |
| Chan 2009 | I: intensive insulin therapy | 10* | 0* | 0* | 1* | 10* | 1* | 10* |
| C: conventional insulin therapy | 22* | 2* | 9.1* | 6* | 27.3* | 6* | 27.3* | |
| De La Rosa 2008 | I: intensive insulin therapy | 11* | 6* | 54.5* | 6* | 54.5* | 3* | 27.3* |
| C: conventional insulin therapy | 2* | 1* | 50* | 1* | 50* | 0* | 0* | |
| Gandhi 2007 | I: intensive insulin therapy | 37 | 2 | 5.4 | 13 | 35 | 3 | 8 |
| C: conventional insulin therapy | 36 | 0 | 0 | 16 | 44 | 1 | 3 | |
| Li 2006 | I: continuous insulin infusion | 51 | 2 | 3.9 | 42 | 82.4 | 3 | 5.9 |
| C: glucometer‐guided insulin | 42 | 1 | 2.4 | 36 | 85.7 | 2 | 4.8 | |
| Lazar 2004 | I: tight glycaemic control with GIK | 72 | 30 days postoperatively: 0 5 years follow‐up: 1 |
2.5 | 12 | 16.6 | 0 | 0 |
| C: standard therapy | 69 | 30 days postoperatively: 0 5 years follow‐up: 6 |
5.3 | 29 | 42 | 9 | 13 | |
| Rassias 1999 | I: aggressive insulin therapy | 13 | — | — | 0 | 0 | 0 | 0 |
| C: standard insulin therapy | 13 | — | — | 3 | 23.1 | 3 | 23.1 | |
| —: denotes not reported * data provided by study authors (patients with diabetes). adata from total study population. I: intervention group; C: control group; CV: cardiovascular; GIK: glucose‐insulin‐potassium; MACE: major cardiovascular event. | ||||||||
Appendix 13. Adverse events (II)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants discontinuing trial due to an adverse event (N) | Participants discontinuing trial due to an adverse event (%) |
| Duncan 2018 | I: hyperinsulinaemic normoglycaemia | 226* | 0* | 0* |
| C: standard therapy | 249* | 0* | 0* | |
| Wallia 2017 | I: intensive | 23* | 0* | 0* |
| C: moderate | 26* | 0* | 0* | |
| Wahby 2016 | I: tight glycaemic control | 67 | 0 | 0 |
| C: conventional moderate glycaemic control | 68 | 0 | 0 | |
| Parekh 2016 | I: moderately intense control | 30 | 0 | 0 |
| C: standard glucose control | 30 | 0 | 0 | |
| Yuan 2015 | I: intensive glycaemic management | 106 | 0 | 0 |
| C: conventional glycaemic management | 106 | 0 | 0 | |
| Umpierrez 2015 | I: intensive group | 77* | 0* | 0* |
| C: conservative control | 75* | 0* | 0* | |
| Abdelmalak 2013 | I: intensive glucose management | 54* | 0* | 0* |
| C: conventional glucose management | 49* | 0* | 0* | |
| Hermayer 2012 | I: intensive glycaemic control | 44 | — | — |
| C: standard glycaemic control | 49 | — | — | |
| Desai 2012 | I: strict blood glucose control | 37* | — | — |
| C: liberal blood glucose control | 44* | — | — | |
| Lazar 2011 | I: aggressive glucose control | 40 | 0 | 0 |
| C: moderate glucose control | 42 | 0 | 0 | |
| Cao 2010 | I: intensive insulin therapy | 92 | — | — |
| C: conventional insulin therapy | 87 | — | — | |
| Glucontrol 2009 | I: intensive insulin treatment | 55* | — | — |
| C: intermediate glucose control | 69* | — | — | |
| NICE SUGAR 2009 | I: intensive insulin therapy | 3054a | 304a | 10a |
| C: conventional insulin therapy | 3050a | 225a | 7.4a | |
| Subramaniam 2009 | I: continuous insulin infusion | 62* | 0* | 0* |
| C: standard intermittent sliding‐scale insulin bolus | 64* | 0* | 0* | |
| Chan 2009 | I: intensive insulin therapy | 55a | 7a | 12.7a |
| C: conventional insulin therapy | 54a | 4a | 7.4a | |
| De La Rosa 2008 | I: intensive insulin therapy | 254a | 0a | 0a |
| C: conventional insulin therapy | 250a | 0a | 0a | |
| Gandhi 2007 | I: intensive insulin therapy | 37 | 0 | 0 |
| C: conventional insulin therapy | 36 | 0 | 0 | |
| Li 2006 | I: continuous insulin infusion | 51 | — | — |
| C: glucometer‐guided insulin | 42 | — | — | |
| Lazar 2004 | I: tight glycaemic control with GIK | 72 | 0 | 0 |
| C: standard therapy | 69 | 0 | 0 | |
| Rassias 1999 | I: aggressive insulin therapy | 13 | 0 | 0 |
| C: standard insulin therapy | 13 | 0 | 0 | |
| —: denotes not reported adata from total study population. *data provided by study authors (patients with diabetes). C: comparator; I: intervention; GIK: glucose‐insulin‐potassium. | ||||
Appendix 14. Adverse events (III)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with a specific adverse event (description) | Participants with at least one specific adverse events (N) | Participants with at least one specific adverse event (%) |
| Duncan 2018 | I: hyperinsulinaemic normoglycaemia | 226* | (1) Death within 30 days
(2) Postoperative mechanical circulatory support
(3) Serious infection morbidity (4) Renal morbidity (5) Neurological deficit |
(1) 2*
(2) —*
(3) 9* (4) 4* (5) —* |
(1) 0.9*
(2) —*
(3) 4* (4)1.8* (5) —* |
| C: standard therapy | 249* | (1) Death within 30 days
(2) Postoperative mechanical circulatory support
(3) Serious infection morbidity (4) Renal morbidity (5) Neurological deficit |
(1) 6*
(2) —*
(3) 17* (4) 11* (5) — * |
(1) 2.4*
(2) —*
(3) 6.8* (4) 4.4* (5) —* |
|
| Wallia 2017 | I: intensive (140) | 23* | (1) Patients experiencing hypoglycaemia (BG ≤ 70 mg/dL) (2) Patients experiencing severe hypoglycaemia (BG ≤40 mg/dL) (3) Patients with any infection to 1 year (4) 30 days readmission | (1) 11* (2) 2* (3) 10* (4) 11* |
(1) 47.8*
(2) 8.7*
(3) 43.5* (4) 47.8* |
| C: moderate (180) | 26* | (1) Patients experiencing hypoglycaemia (BG ≤ 70 mg/dL) (2) Patients experiencing severe hypoglycaemia (BG ≤ 40 mg/dL) (3) Patients with any infection to 1 year (4) 30 days readmission |
(1) 1* (2) 0* (3) 16* (4) 10* |
(1) 3.9* (2) 0* (3) 61.5* (4) 38.5* |
|
| Wahby 2016 | I: tight glycaemic control | 67 | (1) Postoperative hypoglycaemic events (2) Postoperative AF (3) Perioperative MI (4) Acute renal failure (5) Neurological insult (6) Need for inotropic support (7) Prolonged mechanical ventilation (8) Sternal wound infection (9) Leg wound infection |
(1) 3 (2) 13 (3) 3 (4) 2 (5) 4 (6) 28 (7) 9 (8) 14 (9) 13 |
(1) 4.5 (2) 19.4 (3) 4.5 (4) 3 (5) 6 (6) 41.8 (7) 13.4 (8) 20.9 (9) 19.4 |
| C: conventional moderate glycaemic control | 54 | (1) Postoperative hypoglycaemic events (2) Postoperative AF (3) Perioperative MI (4) Acute renal failure (5) Neurological insult (6) Need for inotropic support (7) Prolonged mechanical ventilation (8) Sternal wound infection (9) Leg wound infection |
(1) 1 (2) 25 (3) 4 (4) 8 (5) 5 (6) 45 (7) 19 (8) 27 (9) 24 |
(1) 1.5 (2) 36.8 (3) 5.9 (4) 11.8 (5) 7.4 (6) 66.2 (7) 27.9 (8) 39.7 (9) 35.3 |
|
| Parekh 2016 | I: moderately intense control | 30 | (1) Delayed graft function (2) Dialysis within 7 days (3) Graft loss (4) Perioperative death (5) Wound infection (6) Stroke |
(1) 13 (2) 13 (3) 0 (4) 0 (5) 1 (6) 0 |
(1) 43.3 (2) 43.3 (3) 0 (4) 0 (5) 3.3 (6) 0 |
| C: standard glucose control | 30 | (1) Delayed graft function (2) Dialysis within 7 days (3) Graft loss (4) Perioperative death (5) Wound infection (6) Stroke |
(1) 22 (2) 19 (3) 0 (4) 2 (5) 1 (6) 0 |
(1) 73.3 (2) 63.3 (3) 0 (4) 6.6 (5) 3.3 (6) 0 |
|
| Yuan 2015 | I: intensive glycaemic management | 106 | (1) Severe hypoglycaemia (2) Postoperative deaths (3) Surgical site infection (4) Pneumonia (5) Urinary tract infection (6) Bacteraemia (7) Anastomotic leak (8) Bleeding (9) Delayed gastric emptying (10) Obstruction (11) Hepatic dysfunction (12) Renal dysfunction (13) Circulatory insufficiency |
(1) 2 (2) 1 (3) 5 (4) 6 (5) 7 (6) 3 (7) 2 (8) 0 (9) 5 (10) 1 (11) 6 (12) 0 (13) 3 |
(1) 1.9 (2) 0.9 (3) 4.7 (4) 5.7 (5) 6.6 (6) 2.8 (7) 1.9 (8) 0 (9) 4.7 (10) 0.9 (11) 5.7 (12) 0 (13) 2.8 |
| C: conventional glycaemic management | 106 | (1) Severe hypoglycaemia (2) Postoperative deaths (3) Surgical site infection (4) Pneumonia (5) Urinary tract infection (6) Bacteraemia (7) Anastomotic leak (8) Bleeding (9) Delayed gastric emptying (10) Obstruction (11) Hepatic dysfunction (12) Renal dysfunction (13) Circulatory insufficiency |
(1) 12 (2) 1 (3) 14 (4) 8 (5) 6 (6) 4 (7) 3 (8) 1 (9) 7 (10) 2 (11) 6 (12) 1 (13) 1 |
(1) 11.3 (2) 0.9 (3) 13.2 (4) 7.5 (5) 5.7 (6) 3.8 (7) 2.8 (8) 0.9 (9) 6.6 (10) 1.9 (11) 5.7 (12) 0.9 (13) 0.9 |
|
| Umpierrez 2015 | I: intensive group | 77* | (1) Hospital mortality (2) Pneumonia (3) ARF (4) Respiratory failure (5) BSI (6) Surgical sternal infection (7) MACE |
(1) 5* (2) 1* (3) 16* (4) 9* (5) 0* (6) 3* (7) 30* |
(1) 6* (2) 1* (3) 21* (4) 12* (5) 0* (6) 4* (7) 39* |
| C: conservative control | 75* | (1) Hospital mortality (2) Pneumonia (3) ARF (4) Respiratory failure (5) BSI (6) Surgical sternal infection (7) MACE |
(1) 2* (2) 5* (3) 15* (4) 12* (5) 1* (6) 1* (7) 31* |
(1) 3* (2) 7* (3) 20* (4) 16* (5) 1* (6) 1* (7) 41* |
|
| Abdelmalak 2013 | I: intensive glucose management | 54* | (1) Sepsis, pneumonia, or surgical site infection (2) Cardiovascular events (MI, arrhythmia, emboli, pulmonary oedema, stroke) (3) Renal failure |
(1) 9* (2) 2* (3) 1* |
(1) 16.7* (2) 3.7* (3) 1.9* |
| C: conventional glucose management | 49* | (1) Sepsis, pneumonia, or surgical site infection (2) Cardiovascular events (MI, arrhythmia, emboli, pulmonary oedema, stroke) (3) Renal failure |
(1) 4* (2) 2* (3) 1* |
(1) 8.2* (2) 4.1* (3) 2.0* |
|
| Hermayer 2012 | I: intensive glycaemic control | 44 | (1) Hyperglycaemia (2) Rejection episodes (3) Delayed graft function |
(1) 5 (2) 9 (3) 8 |
(1) 11 (2) ‐ (18) |
| C: standard glycaemic control | 49 | (1) Hyperglycaemia (2) Rejection episodes (3) Delayed graft function |
(1) 12 (2) 2 (3) 12 |
(1) 24 (2) ‐ (3) 24 |
|
| Desai 2012 | I: strict blood glucose control | 37* | (1) Deep sternal wound infection (2) MACE (3) AF (4) Renal failure |
(1) 0* (2) 0* (3) 3* (4) 1* |
(1) 0* (2) 0* (3) 8.1* (4) 2.7* |
| C: liberal blood glucose control | 44* | (1) Deep sternal wound infection (2) MACE (3) AF (4) Renal failure |
(1) 0* (2) 0* (3) 3* (4) 0* |
(1) 0* (2) 0* (3) 6.8* (4) 0* |
|
| Lazar 2011 | I: aggressive glucose control | 40 | (1) 30 days mortality (2) MI (3) CVA (4) Sternal infection (5) Atrial fibrillation |
(1) 0 (2) 3 (3) 0 (4) 0 (5) 12 |
(1) 0 (2) 10 (3) 0 (4) 0 (5) 30 |
| C: moderate glucose control | 42 | (1) 30 days mortality (2) MI (3) CVA (4) Sternal infection (5) Atrial fibrillation |
(1) 0 (2) 0 (3) 1 (4) 0 (5) 16 |
(1) 0 (2) 0 (3) 2 (4) 0 (5) 38 |
|
| Cao 2010 | I: intensive insulin therapy | 92 | (1) Postoperative hospital mortality (2) Postoperative complications (total) (3) Wound infection (4) Intra‐abdominal infection (5) Pneumonia (6) Urinary tract infection (7) Sepsis |
(1) 4 (2) 7 (3) 4 (4) 2 (5) 3 (6) 4 (7) 2 |
(1) 4.3 (2) 7.6 (3) 4.3 (4) 2.2 (5) 3.3 (6) 4.3 (7) 2.2 |
| C: conventional insulin therapy | 87 | (1) Postoperative hospital mortality (2) Postoperative complications (total) (3) Wound infection (4) Intra‐abdominal infection (5) Pneumonia (6) Urinary tract infection (7) Sepsis |
(1) 5 (2) 16 (3) 12 (4) 9 (5) 10 (6) 3 (7) 3 |
(1) 5.7 (2) 18.4 (3) 13.8 (4) 10.3 (5) 11.5 (6) 3.4 (7) 3.4 |
|
| Glucontrol 2009 | I: intensive insulin treatment | 55* | (1) Cardiovascular events (2) Renal failure (3) All‐cause mortality ICU (4) All‐cause mortality hospital |
(1) 33* (2) 47* (3) 4* (4) 7* |
(1) 60* (2) 85.5* (3) 7.3* (4) 12.7* |
| C: intermediate glucose control | 69* | (1) Cardiovascular events (2) Renal failure (3) All‐cause mortality ICU (4) All‐cause mortality hospital |
(1) 22* (2) 44* (3) 9* (4) 11* |
(1) 31.8* (2) 63.8* (3) 13* (4) 15.9* |
|
| NICE SUGAR 2009 | I: intensive insulin therapy | 213* | (1) Infectious complication (2) Renal failure (3) All‐cause mortality (4) Severe hypoglycaemic episodes |
(1) 32* (2) 16* (3) 57* (4) 21* |
(1) 15* (2) 7.5* (3) 26.8* (4) 9.9* |
| C: conventional insulin therapy | 208* | (1) Infectious complication (2) Renal failure (3) All‐cause mortality (4) Severe hypoglycaemic episodes |
(1) 22* (2) 27* (3) 49* (4) 1* |
(1) 10.6* (2) 13* (3) 23.6* (4) 0.5* |
|
| Subramaniam 2009 | I: continuous insulin infusion | 62* | (1) Infectious complication (2) MI (3) CHF (4) Renal failure (5) Death within 30 days |
(1) 22* (2) 0* (3) 2* (4) 17* (5) 0* |
(1) 35.5* (2) 0* (3) 3.2* (4) 27.4* (5) 0* |
| C: standard intermittent sliding‐scale insulin bolus | 64* | (1) Infectious complication (2) MI (3) CHF (4) Renal failure (5) Death within 30 days |
(1) 16* (2) 3* (3) 5* (4) 11* (5) 0* |
(1) 25* (2) 4.7* (3) 7.8* (4) 17.2* (5) 0* |
|
| Chan 2009 | I: intensive insulin therapy | 10* | (1) Infection (2) Neurological dysfunction (3) Renal failure (4) All‐cause mortality |
(1) 1* (2) 1.36* (3) 0.91* (4) 0.91* |
(1) 10* (2) 13.6* (3) 9.1* (4) 9.1* |
| C: conventional insulin therapy | 22* | (1) Infection (2) Neurological dysfunction (3) Renal failure (4) All‐cause mortality |
(1) 6* (2) 0* (3) 0* (4) 0* |
(1) 27.3* (2) 0* (3) 0* (4) 0* |
|
| De La Rosa 2008 | I: intensive insulin therapy | 11* | (1) Infection (2) Renal failure (3) ICU mortality (4) 28 days mortality |
(1) 3* (2) 3* (3) 5* (4) 6* |
(1) 27.3* (2) 27.3* (3) 45.5* (4) 54.5* |
| C: conventional insulin therapy | 2* | (1) Infection (2) Renal failure (3) ICU mortality (4) 28 days mortality |
(1) 0* (2) 0* (3) 1* (4) 1* |
(1) 0* (2) 0* (3) 50* (4) 50* |
|
| Gandhi 2007 | I: intensive insulin therapy | 37 | (1) Death (2) Stroke (3) Deep sternal infection (4) Cardiac arrest (5) Heart block requiring pacemaker (6) New‐onset atrial fibrillation (7) Acute renal failure (8) Prolonged intubation |
(1) 2 (2) 2 (3) 3 (4) 0 (5) 2 (6) 13 (7) 3 (8) 7 (9) 13 |
(1) 5 (2) 5 (3) 8 (4) 0 (5) 5 (6) 35 (7) 8 (8) 19 |
| C: conventional insulin therapy | 36 | (1) Death (2) Stroke (3) Deep sternal infection (4) Cardiac arrest (5) Heart block requiring pacemaker (6) New‐onset atrial fibrillation (7) Acute renal failure (8) Prolonged intubation |
(1) 0 (2) 0 (3) 1 (4) 0 (5) 0 (6) 16 (7) 2 (8) 9 |
(1) 0 (2) 0 (3) 3 (4) 0 (5) 0 (6) 44 (7) 6 (8) 25 |
|
| Li 2006 | I: continuous insulin infusion | 51 | (1) Use of postoperative inotropic agents (2) Prolonged ventilation (3) New‐onset atrial fibrillation (4) Sternal wound infection (5) Leg wound infection (6) Death |
(1) 42 (2) 9 (3) 8 (4) 2 (5) 1 (6) 2 |
(1) 82.4 (2) 17.6 (3) 15.7 (4) 3.9 (5) 2 (6) 3.9 |
| C: glucometer‐guided insulin | 42 | (1) Use of postoperative inotropic agents (2) Prolonged ventilation (3) New‐onset atrial fibrillation (4) Sternal wound infection (5) Leg wound infection (6) Death |
(1) 36 (2) 5 (3) 7 (4) 2 (5) 0 (6) 1 |
(1) 85.7 (2) 11.9 (3) 16.7 (4) 4.8 (5) 0 (6) 2.4 |
|
| Lazar 2004 | I: tight glycaemic control with GIK | 72 | Postoperative (1) 30‐days mortality (2) MI (3) AF (4) Infections (5) Recurrent ischaemia (5 years follow‐up) (6) Recurrent infections (5 years follow‐up) (7) Recatheterisation (5 years follow‐up) |
(1) 0 (2) 0 (3) 12 (4) 0 (5) 4 (6) 1 (7) 4 |
(1) 0 (2) 0 (3) 16.6 (4) 0 (5) 5 (6) 1 (7) 5 |
| C: standard therapy | 69 | Postoperative (1) 30 days mortality (2) MI (3) AF (4) Infections (5) Recurrent ischaemia (5 years follow‐up) (6) Recurrent infections (5 years follow‐up) (7) Recatheterisation (5 years follow‐up) |
(1) 0 (2) 2 (3) 29 (4) 9 (5) 13 (6) 7 (7) 4 |
(1) 0 (2) 2.8 (3) 42 (4) 13 (5) 19 (6) 10 (7) 6 |
|
| Rassias 1999 | I: aggressive insulin therapy | 13 | (1) Infection | (1) 0 | (1) 0 |
| C: standard insulin therapy | 13 | (1) Infection | (1) 3 | (1) 23.1 | |
| —: denotes not reported AF: atrial fibrillation ; ARF: acute renal failure; BG: blood glucose; C: comparator; CHF: congestive heart failure; CVA: cerebrovascular accident; GIK: glucose‐insulin‐potassium; I: intervention; MACE: major adverse cardiac events; MI: myocardial infarction; BSI: blood stream infection | |||||
Appendix 15. Adverse events (IV)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with at least one hypoglycaemic episode (N) | Participants with at least one hypoglycaemic episode (%) | Participants with at least one severe/serious hypoglycaemic episode (N) | Participants with at least one severe/serious hypoglycaemic episode (%) |
| Duncan 2018 | I: hyperinsulinaemic normoglycaemia | 226* | 24* | 10.6* | 1* | 0.4* |
| C: standard therapy | 249* | 1* | 0.4* | 1* | 0.4* | |
| Wallia 2017 | I: intensive (140) | 23* | 11* | 47.8* | 2* | 8.7* |
| C: moderate (180) | 26* | 1* | 3.9* | 0* | 0* | |
| Wahby 2016 | I: tight glycaemic control | 67 | 3 | 4.5 | — | — |
| C: conventional moderate glycaemic control | 68 | 1 | 1.5 | — | — | |
| Parekh 2016 | I: moderately intense control | 30 | 0 | 0 | 0 | 0 |
| C: standard glucose control | 30 | 0 | 0 | 0 | 0 | |
| Yuan 2015 | I: intensive glycaemic management | 106 | — | — | 8 | 7.5 |
| C: conventional glycaemic management | 106 | — | — | 1 | 0.9 | |
| Umpierrez 2015 | I: intensive group | 77* | 7* | 9* | 0* | 0* |
| C: conservative control | 75* | 4* | 5* | 0* | 0* | |
| Abdelmalak 2013 | I: intensive glucose management | 54* | 0* | 0* | 1* | 1.9* |
| C: conventional glucose management | 49* | 0* | 0* | 0* | 0* | |
| Hermayer 2012 | I: intensive glycaemic control | 44 | — | — | 7 | 16 |
| C: standard glycaemic control | 49 | — | — | 2 | 4 | |
| Desai 2012 | I: strict blood glucose control | 37* | 18* | 48,6* | 1* | 2.7* |
| C: liberal blood glucose control | 44* | 15* | 34.1* | 0* | 0* | |
| Lazar 2011 | I: aggressive glucose control | 40 | — | — | 30 | 75 |
| C: moderate glucose control | 42 | — | — | 4 | 9.52 | |
| Cao 2010 | I: intensive insulin therapy | 92 | — | — | 6 | 6.5 |
| C: conventional insulin therapy | 87 | — | — | 1 | 1.1 | |
| Glucontrol 2009 | I: intensive insulin treatment | 55* | — | — | 3* | 5,5* |
| C: intermediate glucose control | 69* | — | — | 1* | 1.4* | |
| NICE SUGAR 2009 | I: intensive insulin therapy | 213* | ‐ | ‐ | 14* | 6.6* |
| C: conventional insulin therapy | 208* | ‐ | ‐ | 1* | 0.5 | |
| Subramaniam 2009 | I: continuous insulin infusion | 62* | 8* | 12.9* | 0* | 0* |
| C: standard intermittent sliding‐scale insulin bolus | 64* | 2* | 3.1* | 0* | 0* | |
| Chan 2009 | I: intensive insulin therapy | 10* | 0* | 0* | — | — |
| C: conventional insulin therapy | 22* | 0* | 0* | — | — | |
| De La Rosa 2008 | I: intensive insulin therapy | 11* | — | — | 0* | 0* |
| C: conventional insulin therapy | 2* | — | — | 1* | 50* | |
| Gandhi 2007 | I: intensive insulin therapy | 37 | 1 | 3 | — | — |
| C: conventional insulin therapy | 36 | 6 | 16.6 | — | — | |
| Li 2006 | I: continuous insulin infusion | 51 | — | — | — | — |
| C: glucometer‐guided insulin | 42 | — | — | — | — | |
| Lazar 2004 | I: tight glycaemic control with GIK | 72 | — | — | — | — |
| C: standard therapy | 69 | — | — | — | — | |
| Rassias 1999 | I: aggressive insulin therapy | 13 | — | — | — | — |
| C: standard insulin therapy | 13 | — | — | — | — | |
| —: denotes not reported *data provided by study authors (patients with diabetes). adata from total study population. C: comparator; I: intervention;GIK: glucose‐insulin‐potassium. | ||||||
Appendix 16. Length of stay
| Study ID |
Length of ICU stay (mean days (SD)) |
Length of hospital stay (mean days (SD)) |
| Duncan 2018 | I: 2.7 (3.9)* C: 2.6 (4.1)* |
— |
| Wallia 2017 | — | I: 16.7 (25.3)* C: 9.3 (10.8)* |
| Wahby 2016 | — | — |
| Parekh 2016 | — | I: 4.1 (1.9) C: 5 (2.4) |
| Yuan 2015 | — | — |
| Umpierrez 2015 | I: 4 (5.4)* C: 5.1 (13)* |
I: 10.3 (6.6)* C: 11.1 (11.8)* |
| Abdelmalak 2013 | — | — |
| Hermayer 2012 | — | — |
| Desai 2012 | I: 1.2 (1.0)* C: 2.4 (5.1)* |
I: 4.52 (1.79)* C: 4.68 (1.75)* |
| Lazar 2011 | I: 2.9 (0.7) C: 2.7 (0.5) |
I: 10.1 (3.5) C: 10.8 (3.5) |
| Cao 2010 | — | I: 8 (4.3) C: 10 (4.3) |
| Glucontrol 2009 | I: 9.5 (11.2)* C: 7.4 (11.5)* |
I: 23.3 (17.8)* C: 19.6 (19.1)* |
| NICE SUGAR 2009 | I: 5 (5.9)* C: 5 (5.2)* |
I: 16 (—)* C: 15 (—)* |
| Subramaniam 2009 | — | I: 7.8 (5.0)* C: 7.4 (4.3)* |
| Chan 2009 | I: 2.8 (1.5)* C: 4.5 (4.4)* |
I: 9 (3)* C: 17 (18)* |
| De La Rosa 2008 | I: 7.2 (5.9)a C: 6.5 (5.0)a |
— |
| Gandhi 2007 | I: 2 (2) days C: 2 (2) days |
I: 8 (6) days C: 8 (3) days |
| Li 2006 | I: 5.7 (—) C: 6.3 (—) |
— |
| Lazar 2004 | I: 0.7 (0.3) C: 1.37 (0.9) |
I: 6.5 (0.1) C: 9.2 (0.3) |
| Rassias 1999 | — | — |
| —: denotes not reported *Data provided by study authors (patients with diabetes). aData from total study population. EGEstimated from graph or table showing longitudinal changes in glucose when the value was not available. EAEstimated from algorithm. C: control group; I: intensive intervention group | ||
Appendix 17. Survey of trial investigators providing information on included trials
| Study ID | Date trial author contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) |
| Hweidi 2021 | 1 August 2022 | No answer | Additional information on the insulin protocol regimen of the control group | |
| Imran‐ul‐hassan 2021 | 1 August 2022 | No answer | Information about study design | |
| * Gupta 2020 | 1 September 2021 | No answer | People with diabetes, separated data | |
| * Kumar 2020 | 28 October 2020 | No answer | People with diabetes, separated data | |
| *Mohod 2019 | 28 October 2020 | No answer | People with diabetes, separated data | |
| *Santana‐Santos 2019 | 28 October 2020 | No answer | People with diabetes, separated data | |
| *Abdelmalak 2019 | 28 October 2020 | No answer | People with diabetes, separated data | |
| Duncan 2018 | 27 May 2019 | 28 May 2019 | People with diabetes, separated data | 13 June 2019 |
| *Kurnaz 2017 | 20 May 2019 | No answer | People with diabetes, separated data | |
| Wallia 2017 | 27 May 2019 | 29 May 2019 | People with diabetes, separated data | 2 June 2019 |
| Wahby 2016 | Not contacted | |||
| Parekh 2016 | Not contacted | |||
| Umpierrez 2015 | 27 May 2019 | 29 May 2019 | People with diabetes, separated data | 24 July 2019 |
| Yuan 2015 | Not contacted | |||
| *Kalfon 2014 | 12 March 2019 | 12 March 2019 | People with diabetes, separated data. | No data provided by author |
| *Okabayashi 2014 | 27 May 2019 | No answer | People with diabetes, separated data | |
| *Rujirojindakul 2014 | 26 May 2019 | No answer | People with diabetes, separated data | |
| *Pezzella 2014 | 27 May 2019 | No answer | People with diabetes, separated data | |
| Abdelmalak 2013 | 23 May 2019 | 23 May 2019 | People with diabetes, separated data | 27 June 2019 |
| *Giakoumidakis 2013 | 27 May 2019 | No answer | People with diabetes, separated data | |
| Hermayer 2012 | Not contacted | |||
| Desai 2012 | 3 June 2019 | No answer | People with diabetes, separated data | Author provided separated data in former review |
| Lazar 2011 | 3 June 2019 | 5 June 2019 | Data on outcomes not reported in the study | Author did not analyse other outcomes |
| Cao 2010 | Not contacted | |||
| Glucontrol 2009 | 3 June 2019 | No answer | People with diabetes, separated data | Author provided separated data in former review |
| NICE SUGAR 2009 | 3 June 2019 | No answer | People with diabetes, separated data | Author provided separated data in former review |
| Subramaniam 2009 | 3 June 2019 | No answer | People with diabetes, separated data | Author provided separated data in former review |
| Chan 2009 | 3 June 2019 | No answer | People with diabetes, separated data | Author provided separated data in former review |
| De La Rosa 2008 | 3 June 2019 | No answer | People with diabetes, separated data | Author provided separated data in former review |
| Gandhi 2007 | 3 June 2019 | No answer | ||
| Li 2006 | 3 June 2019 | No answer | ||
| Lazar 2004 | 3 June 2019 | 5 June 2019 | Data on outcomes not reported in the study | Author did not analyse other outcomes |
| Rassias 1999 | 3 June 2019 | No answer | ||
| * Studies excluded with reason: no outcome data available for subpopulation of patients with diabetes after contacting study authors. | ||||
Appendix 18. Checklist to aid consistency and reproducibility of GRADE assessments
| (1) All‐cause mortality | (2) Hypoglycaemic episodes | (3) Adverse events other than hypoglycaemic episodes | (4) Cardiovascular events | (5) Length of ICU/hospital stay | (6) Health‐related quality of life | (7) Socioeconomic effects | ||
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear/Unclear | Not reported | Not reported |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear/Unclear | |||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | No (↓) | No (↓) | Yes | No (↓)/No (↓) | |||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Unclear | Unclear | Yes | Unclear/ Unclear | |||
| Was an objective outcome used? | Yes | No (↓) | No (↓) | Yes | Yes/Yes | |||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | Yes | Yes/Yes | |||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | Unclear | Unclear | Unclear | Unclear/Unclear | |||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | Yes/Yes | |||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes/Yes | |||
| Inconsistencyb | Point estimates did not vary widely? | Yes | No (↓) | Yes | Yes | Yes | ||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | Some | Substantial | Substantial | Substantial/Substantial | |||
| Was the direction of effect consistent? | Yes | No (↓) | No (↓) | Yes | Yes/ es | |||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | Low | High (↓) | Moderate | Moderate | High (↓)/High (↓) | |||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | Statistically significant (↓) | Statistically significant (↓) | Not statistically significant | Statistically significant (↓)/Statistically significant (↓) | |||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable/Highly applicable | ||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable/Highly applicable | |||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes/Yes | |||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient/Sufficient | |||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes/Yes | |||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) | No (↓) | No (↓) | No (↓) | No (↓)/No (↓) | ||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Low (↓) | Low (↓) | Intermediate | Low (↓)/Intermediate | |||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Large | Moderate | Large | Moderate | Moderate/Moderate | |||
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | Yes | Yes | NA/NA | |||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes/Yes | ||
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes/Yes | |||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes/Yes | |||
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | Yes/Yes | |||
| There was no evidence of funnel plot asymmetry? | Yes | NA | Yes | NA | NA/NA | |||
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | Unclear | Unclear/Unclear | |||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; ICU: intensive care unit; NA: not applicable. | ||||||||
Appendix 19. OLD DATA: Blood glucose matrix
| Study ID | Baseline blood glucose/fasting plasma glucose (mean mg/dL (SD)) | Target blood glucose (mean mg/dL (SD)) | Mean blood glucose during intervention (mean mg/dL (SD) |
| Duncan 2018 | I: 125.3 (59.1) C: 138.6 (52.3) |
I: 80 to 110 C: 120 to 180 | I: 113.5 (24.9)* C: 162.9 (33.1)* |
| Wallia 2017 | I: 223.9 (51.73)* C: 241.7 (59.52)* |
I: 140 C: 180 | I: 152.8 (22)* C: 198.6 (24.4)* |
| Wahby 2016 | I: 164.06 (12.09) C: 166.90 (16.77) | I: 110 to 149 C: 150 to 180 | — |
| Parekh 2016 | I: 125.3 (59.1) C: 138.6 (52.3) | I: < 160 C: < 200 | I: 137.4 (33.8) C: 182.2 (67.7) |
| Yuan 2015 | I: 153.1 (43.2) C: 147.7 (46.8) | I: 80 to 110 mg/dL C: < 200 mg/dL | I: 97.3 (21.6) C: 171.2 (32.4) |
| Umpierrez 2015 | I: 169.7 (28.5)* C: 175.8 (30.8)* | I: 100 to 140 C: 141 to 180 | I: 136.9 (14.4)* C: 161.2 (12.1)* |
| Abdelmalak 2013 | I. 102 (52.06)* C: 150.8 (81.85)* | I: 80 to 110 C: 180 to 200 | I: 113.1 (28.95)* C: 163.7 (38.61)* |
| Hermayer 2012 | — | I: 70 to 110 C: 70 to 180 | I: 122.5 (3.4) C: 177.3 (3.4) |
| Desai 2012 | NA | I: 90 to 120 C: 121 to 180 | I: 119.87 (15.97)* C: 133.29 (18.01)* |
| Lazar 2011 | I: 161 (50) C: 151 (35) | I: 90 to 120 C: 120 to 180 | I: 140 (27) EG C: 163 (21) EG |
| Cao 2010 | I: 112.4 (10.8) C: 126 (12.6) | I: 79.2 to 109.8 C: 180 to 198 | I: 99 (14.4) C: 178 (18) |
| Glucontrol 2009 | — | I: 79 to 110 C: 140 to 180 | I: 1.1 to 30.7 mmol/La C: 1.1 to 33.2 mmol/La |
| NICE SUGAR 2009 | — | I: 81 to 108 C: < 180 | I: 122 (22) C: 164 (23) |
| Subramaniam 2009 | — | I: 100 to 150 C: < 150 | |
| Chan 2009 | NA | I: 80 to 130 C: 160 to 200 | I: 159.4* C: 195.8* |
| De La Rosa 2008 | NA | I: 80 to 110 C: 180 to 200 | I: 138 (47.9)* C: 165.4 (26.1)* |
| Gandhi 2007 | Intraoperative I: 139 (31) C: 141 (53) ICU I: 130 (29) C: 180 (50) |
I: 80 to 100 C: < 200 | Intraoperative I: 132 (29) C: 169 (49) ICU I: 106 (18) C: 105 (25) |
| Li 2006 | I: 193 C: 174 | I: 150 to 200 C: 150 to 200 | I: 203 (50) EG C: 218 (50) EG |
| Lazar 2004 | I: 180 (59) C: 179 (32) | I: 126 to 200 C: 80 to 249 | I: 152 (39) EG C: 244 (50) EG |
| Rassias 1999 | I: 172 (65) C: 152 (51) | I: < 150 EA C: < 200 EA | I: 197 (65) EG C: 220 (51) EG |
| —: denotes not reported *Data provided by study authors. aData from total study population. EGEstimated from graph or table showing longitudinal changes in glucose when value was not available. EAEstimated from algorithm. C: control group; I: intensive intervention group. | |||
Data and analyses
Comparison 1. Perioperative intensive vs conventional glycaemic control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 18 | 2551 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.88, 1.33] |
| 1.2 All‐cause mortality (sensitivity analysis: only published data; low risk of bias) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Only published data | 7 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.34, 1.72] |
| 1.2.2 Low risk of bias | 8 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.88, 1.36] |
| 1.3 Hypoglycaemic episodes (severe) | 11 | 1896 | Risk Ratio (M‐H, Random, 95% CI) | 4.73 [2.12, 10.55] |
| 1.4 Hypoglycaemic episodes (any) | 17 | 2410 | Risk Ratio (M‐H, Random, 95% CI) | 3.36 [1.69, 6.67] |
| 1.5 Hypoglycaemic episodes (severe; sensitivity analysis: only published data) | 3 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.05, 52.32] |
| 1.6 Hypoglycaemic episodes (any; sensitivity analysis: only published data) | 7 | 834 | Risk Ratio (M‐H, Random, 95% CI) | 2.24 [0.56, 8.94] |
| 1.7 Infectious complications | 18 | 2453 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.04] |
| 1.8 Infectious complications (sensitivity analysis: only published data) | 9 | 1001 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.38, 0.75] |
| 1.9 Cardiovascular events | 12 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.97] |
| 1.10 Cardiovascular events (sensitivity analysis: only published data) | 6 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.25] |
| 1.11 Renal failure | 14 | 2086 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.22] |
| 1.12 Renal failure (sensitivity analysis: only published data) | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.42, 1.45] |
| 1.13 Length of ICU stay | 11 | 1687 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.57, 0.38] |
| 1.14 Length of ICU stay (sensitivity analysis: only published data) | 3 | 248 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.07, 0.43] |
| 1.15 Length of hospital stay | 12 | 1520 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.79, 0.21] |
| 1.16 Length of hospital stay (sensitivity analysis: only published data) | 4 | 394 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.82, ‐0.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelmalak 2013.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial | |
| Participants |
Inclusion criteria: age ≥ 40 years old. Major non‐cardiac surgical procedures scheduled to take ≥ 2 hours done under general anaesthesia. Written informed consent. Exclusion criteria: recent intravenous or oral steroid therapy (within 30 days); inhaled steroids are permitted. Any contraindications to the proposed interventions. ASA Physical Status > 4. Non English‐speaking people. Procedures done under regional anaesthesia. Diagnostic criteria: — Setting: Department of General Surgery, Cleveland Clinic Age group: adults Gender distribution: females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): intensive glucose management with target glucose concentrations of 80 mg/dL to 110 mg/dL Comparator(s): conventional glucose management with target glucose concentrations of 180 mg/dL to 200 mg/dL Duration of intervention: intraoperatively (shortly after induction of anaesthesia) and continued through the first 2 postoperative hours Duration of follow‐up: 30 days and 1 year (mortality only) Run‐in period: none Number of study centres: 1 Treatment before trial: — |
|
| Outcomes | Reported outcome(s) in full text of publication: primary outcome was a collapsed composite endpoint (any vs none) defined as the occurrence of at least one of the 15 major complications before hospital discharge, including sepsis, severe surgical site infection, myocardial infarction, heart failure, stroke, unstable ventricular arrhythmias, pulmonary embolism, pneumonia, respiratory failure, dialysis dependent renal failure, large pleural or peritoneal effusions, major bleeding, major wound and surgical site healing complications, vascular graft thrombosis and 30‐day mortality. One‐year mortality data were obtained from electronic medical records, the United States Social Security Index, or both, and confirmed by direct telephone contact with the participant/family. | |
| Study registration |
Trial identifier:NCT00995501; NCT00433251 Trial terminated early: the trial was stopped for futility at 37.7% of planned maximum 970 |
|
| Publication details |
Language of publication: English Funding: commercial funding: financial support for the submitted work from Aspect Medical (now Covidien), Cleveland Clinic Research Project Committee, Anesthesiology Institute (departmental funds), Abbott Laboratories Inc. (limited support; supplied reagents for CRP analysis), W.H.W.T received grant support (money to the institution) in support of other studies from Abbott Laboratories. This is an investigator‐initiated trial independent of the study sponsors. Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "We tested the primary hypotheses that major perioperative morbidity is reduced by: intensive intraoperative glucose control" | |
| Notes | Information exclusively on diabetes participants was provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization codes were generated by the PLAN procedure in SAS statistical software” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization codes […] implemented using a concealed‐allocation web‐based system that was accessed by research physicians just before the planned surgery” |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk |
Quote: “Clinicians were blinded to the dexamethasone but not to the glucose control […]. However, patients […] were fully blinded.” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | Unclear risk |
Quote: “Clinicians were blinded to the dexamethasone but not to the glucose control […]. However, patients […] were fully blinded.” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | Low risk |
Quote: “Clinicians were blinded to the dexamethasone but not to the glucose control […]. However, patients […] were fully blinded.” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk |
Quote: “Clinicians were blinded to the dexamethasone but not to the glucose control […]. However, patients […] were fully blinded.” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk |
Quote: “Clinicians were blinded to the dexamethasone but not to the glucose control […]. However, patients […] were fully blinded.” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | Low risk |
Quote: “Clinicians were blinded to the dexamethasone but not to the glucose control […]. However, patients […] were fully blinded.” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk |
Quote: “Clinicians were blinded to the dexamethasone but not to the glucose control […]. However, patients […] were fully blinded.” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Low risk |
Quote: “[…] investigators responsible for assessing postoperative outcomes were fully blinded.” Comment: described as blinded for outcome assessment |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk |
Quote: “[…] investigators responsible for assessing postoperative outcomes were fully blinded.” Comment: described as blinded for outcome assessment |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Low risk |
Quote: “[…] investigators responsible for assessing postoperative outcomes were fully blinded.” Comment: described as blinded for outcome assessment |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Low risk |
Quote: “[…] investigators responsible for assessing postoperative outcomes were fully blinded.” Comment: described as blinded for outcome assessment |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk |
Quote: “[…] investigators responsible for assessing postoperative outcomes were fully blinded.” Comment: described as blinded for outcome assessment |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk |
Quote: “[…] investigators responsible for assessing postoperative outcomes were fully blinded.” Comment: described as blinded for outcome assessment |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | High risk |
Quote: “[…] investigators responsible for assessing postoperative outcomes were fully blinded.” Comment: described as blinded for outcome assessment |
| Incomplete outcome data (attrition bias) All‐cause mortality | Unclear risk | Comment: the trial modified the planned enrolment obtained from the sample size calculation (970 participants) due to a slow recruitment but also because of concerns from the trial’s Executive Committee on the possible futility of intervention. Recruitment problems also led to a modification of inclusion criteria. The trial finally included 381 participants. |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Unclear risk | Comment: the trial modified the planned enrolment obtained from the sample size calculation (970 participants) due to a slow recruitment but also because of concerns from the trial’s Executive Committee on the possible futility of intervention. Recruitment problems also led to a modification of inclusion criteria. The trial finally included 381 participants. |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: the trial modified the planned enrolment obtained from the sample size calculation (970 participants) due to a slow recruitment but also because of concerns from the trial’s Executive Committee on the possible futility of intervention. Recruitment problems also led to a modification of inclusion criteria. The trial finally included 381 participants. |
| Incomplete outcome data (attrition bias) Cardiovascular events | Unclear risk | Comment: the trial modified the planned enrolment obtained from the sample size calculation (970 participants) due to a slow recruitment but also because of concerns from the trial’s Executive Committee on the possible futility of intervention. Recruitment problems also led to a modification of inclusion criteria. The trial finally included 381 participants. |
| Incomplete outcome data (attrition bias) Renal failure | Unclear risk | Comment: the trial modified the planned enrolment obtained from the sample size calculation (970 participants) due to a slow recruitment but also because of concerns from the trial’s Executive Committee on the possible futility of intervention. Recruitment problems also led to a modification of inclusion criteria. The trial finally included 381 participants. |
| Incomplete outcome data (attrition bias) Infection events | Unclear risk | Comment: the trial modified the planned enrolment obtained from the sample size calculation (970 participants) due to a slow recruitment but also because of concerns from the trial’s Executive Committee on the possible futility of intervention. Recruitment problems also led to a modification of inclusion criteria. The trial finally included 381 participants. |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Unclear risk | Comment: the trial modified the planned enrolment obtained from the sample size calculation (970 participants) due to a slow recruitment but also because of concerns from the trial’s Executive Committee on the possible futility of intervention. Recruitment problems also led to a modification of inclusion criteria. The trial finally included 381 participants. |
| Selective reporting (reporting bias) | Low risk | Comment: the trial register record discloses all the outcomes in the trial report |
| Other bias | Low risk | Nothing detected |
Cao 2010.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: adult people with type 2 DM who were to undergo open elective gastrectomy for gastric cancer Exclusion criteria: age < 16 years; severe obesity (BMI > 30 kg/m²) or severe malnutrition (BMI < 15 kg/m²); expected SICU stay after surgery less than 24 h; tumour was unresectable or the person with late‐stage cancer underwent palliative surgery; pregnancy; took corticosteroids, steroids, growth hormone or immunosuppressive drugs within 2 weeks prior to the study; person received neoadjuvant chemotherapy; person was diagnosed with gastric stump cancer or recurrent gastric cancer Diagnostic criteria: type 2 DM according to 1999 World Health Organization Setting: affiliated hospital of medical college Qingdao University Age group: adults Gender distribution: females and males Country where trial was performed: China Co‐morbidities: gastric cancer. Hypertension (I: 18.5%; C: 17.2%); coronary artery disease (I: 2.2%; C:1.1%); cardiac insufficiency (I: 7.6%; C: 5.7%); pulmonary disease (I: 4.3%; C: 5.7%); renal insufficiency (I: 1%; C: 2.3%); liver insufficiency (I: 4.3%; C:3.4%); any preoperative comorbidity (I: 38.0%; C: 35.6%) Co‐medications: antibiotic prophylaxis: cefamandole 1 g/dose was administered intravenously 30 minutes before the initial surgical incision and then 1 g/8 hours for 2 days postoperatively |
|
| Interventions |
Intervention(s): intensive insulin therapy (maintenance of blood glucose at a level between 4.4 mmol/L and 6.1 mmol/L) Comparator(s): conventional insulin therapy (maintenance of blood glucose at a level between 10 mmol/L and 11.1 mmol/L) Duration of intervention: until oral intake or enteral nutrition was established during postoperative period Duration of follow‐up: 28 days Run‐in period: none Number of study centres: 1 Treatment before trial: Intervention: insulin (65.2%), oral antidiabetic agents (27.2%) Comparator: insulin (72.4 %), oral antidiabetic agents (21.8%) None: I: 7/92 (7.6%), C: 5/87 (5.7%) |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary outcome was postoperative short‐term complication rate. The secondary outcomes included postoperative 28‐day mortality rate, HOMA‐IR score and HLA‐DR | |
| Study registration |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding (this study was partially supported by the Health Science and Technology Development Project of Shandong (2005 HZ024)) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "This study was to compare the effect of intensive insulin therapy (IIT) to conventional insulin therapy (CIT) on postoperative outcomes among type 2 diabetes mellitus (DM) patients who underwent D2 gastrectomy for gastric cancer" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A simple randomization method (300 random numbers were generated through a random number table) […] was used for allocating the patients to different groups […].” |
| Allocation concealment (selection bias) | Unclear risk |
Quote: “A simple randomization method (300 random numbers were generated through a random number table) with concealment was used for allocating […]”. Comment: described as concealed but additional details were unavailable; as the randomisation sequence is susceptible to manipulation, additional details would be required |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk |
Quote: “To avoid the serious harm of severe hypoglycaemia, we decided to use a single‐centre, unblinded design” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk |
Quote: “To avoid the serious harm of severe hypoglycaemia, we decided to use a single‐centre, unblinded design” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk |
Quote: “To avoid the serious harm of severe hypoglycaemia, we decided to use a single‐centre, unblinded design” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk |
Quote: “To avoid the serious harm of severe hypoglycaemia, we decided to use a single‐centre, unblinded design” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk |
Quote: “To avoid the serious harm of severe hypoglycaemia, we decided to use a single‐centre, unblinded design” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk |
Quote: “To avoid the serious harm of severe hypoglycaemia, we decided to use a single‐centre, unblinded design” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk |
Quote: “To avoid the serious harm of severe hypoglycaemia, we decided to use a single‐centre, unblinded design” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Low risk |
Quote: “[…] to minimize the bias, the staff responsible for adjustment of insulin dose and monitoring of blood glucose levels was unaware of clinical decision‐making and important outcome measurements”. “The wound was evaluated daily and 15 and 28 days after surgery by the same surgeon who was blinded to the treatment assignments” |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk |
Quote: “[…] to minimize the bias, the staff responsible for adjustment of insulin dose and monitoring of blood glucose levels was unaware of clinical decision‐making and important outcome measurements”. “The wound was evaluated daily and 15 and 28 days after surgery by the same surgeon who was blinded to the treatment assignments” |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Low risk |
Quote: “[…] to minimize the bias, the staff responsible for adjustment of insulin dose and monitoring of blood glucose levels was unaware of clinical decision‐making and important outcome measurements”. “The wound was evaluated daily and 15 and 28 days after surgery by the same surgeon who was blinded to the treatment assignments” |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Low risk |
Quote: “[…] to minimize the bias, the staff responsible for adjustment of insulin dose and monitoring of blood glucose levels was unaware of clinical decision‐making and important outcome measurements”. “The wound was evaluated daily and 15 and 28 days after surgery by the same surgeon who was blinded to the treatment assignments” |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk |
Quote: “[…] to minimize the bias, the staff responsible for adjustment of insulin dose and monitoring of blood glucose levels was unaware of clinical decision‐making and important outcome measurements”. “The wound was evaluated daily and 15 and 28 days after surgery by the same surgeon who was blinded to the treatment assignments” |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Low risk |
Quote: “[…] to minimize the bias, the staff responsible for adjustment of insulin dose and monitoring of blood glucose levels was unaware of clinical decision‐making and important outcome measurements”. “The wound was evaluated daily and 15 and 28 days after surgery by the same surgeon who was blinded to the treatment assignments” |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Low risk |
Quote: “[…] to minimize the bias, the staff responsible for adjustment of insulin dose and monitoring of blood glucose levels was unaware of clinical decision‐making and important outcome measurements” “The wound was evaluated daily and 15 and 28 days after surgery by the same surgeon who was blinded to the treatment assignments” |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: sample size calculation for 172 participants; 179 participants were randomised, and no dropouts were described |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: sample size calculation for 172 participants; 179 participants were randomised, and no dropouts were described |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 172 participants; 179 participants were randomised, and no dropouts were described |
| Incomplete outcome data (attrition bias) Cardiovascular events | Low risk | Comment: sample size calculation for 172 participants; 179 participants were randomised, and no dropouts were described |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Comment: sample size calculation for 172 participants; 179 participants were randomised, and no dropouts were described |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: sample size calculation for 172 participants; 179 participants were randomised, and no dropouts were described |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 172 participants; 179 participants were randomised, and no dropouts were described |
| Selective reporting (reporting bias) | High risk | Comment: trial register record (ChiCTR‐TRC‐10001126) includes mortality as main outcome, but it was changed in the trial report |
| Other bias | Low risk | Nothing detected |
Chan 2009.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: adults from both genders who were older than 21 years of age and who were undergoing open‐heart cardiac surgery with CPB Exclusion criteria: renal dysfunction, reoperation, use of inotropic support, neurological dysfunction, chronic pulmonary obstructive disease, emergency or urgency Diagnostic criteria: — Setting: Instituto do Coraçao (InCor), Hospital das Clínicas Age group: adults Gender distribution: females and males Country where trial was performed: Brazil Co‐medications: general anaesthesia: sufentanil, atracurium and isoflurane. Others: midazolam, methylprednisolone, second‐generation cephalosporin, lactated Ringer's solution. If needed: red blood cell blood transfusion, aminocaproic acid. |
|
| Interventions |
Intervention(s): intensive protocol, with target glucose level between 80 mg/dL and 130 mg/dL Comparator(s): control group (less intensive), with target glucose level between 160 mg/dL and 200 mg/dL Duration of intervention: during surgery and for 36 hours after surgery in the intensive care unit Duration of follow‐up: 30 days after surgery Run‐in period: none Number of study centres: 1 |
|
| Outcomes | Reported outcome(s) in full text of publication: primary outcomes were clinical outcomes, which included the duration of mechanical ventilation from the operation room until extubation in the intensive care unit (ICU), the length of stay in the ICU, occurrence of infection (diagnosis of pneumonia, urinary tract infection, sepsis, septic shock, wound infection, blood stream infection, catheter infection), occurrence of hypoglycaemia (glucose level < 50 mg/dL), renal dysfunction (characterised as an increase in the level of creatinine higher than 50% of the baseline value), neurological dysfunction (diagnosis by hospital neurologist who was blinded to the protocol), red blood cell transfusion during the first 30 days after surgery, the length of stay in the hospital and mortality by 30 days after surgery | |
| Study registration |
Trial identifier:NCT00370643 Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding (E.J. Zerbini Foundation) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "In light of this suggestion, we aimed to investigate whether different targets of intraoperative and postoperative glucose (80 ‐ 130 mg/dL, 4.4 ‐ 7.2 mEq/L or 160 ‐ 200 mg/dL, 8.8 ‐ 11.1 mEq/L) could affect postoperative clinical outcomes after cardiac surgery with cardiopulmonary bypass" | |
| Notes | Information exclusively on diabetes participants was provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The patients were randomized into two groups through a lottery system" Comment: no details |
| Allocation concealment (selection bias) | Unclear risk | Comment: the system used to generate the randomisation sequence is susceptible to manipulation; details to prevent it were not described |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: the trial was registered as open‐label according the ClinicalTrials.gov record; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: the trial was registered as open‐label according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: the trial was registered as open‐label according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Comment: the trial was registered as open‐label according the ClinicalTrials.gov record; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: the trial was registered as open‐label according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: the trial was registered as open‐label according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Low risk | Quote: “The physicians and nurse who obtained the clinical data were blinded to the randomisation of the group” |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Quote: “The physicians and nurse who obtained the clinical data were blinded to the randomisation of the group” |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Low risk | Quote: “The physicians and nurse who obtained the clinical data were blinded to the randomisation of the group” |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Low risk | Quote: “The physicians and nurse who obtained the clinical data were blinded to the randomisation of the group” |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Quote: “The physicians and nurse who obtained the clinical data were blinded to the randomisation of the group” |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Low risk | Quote: “The physicians and nurse who obtained the clinical data were blinded to the randomisation of the group” |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 109 participants; no dropouts were described according to the report |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 109 participants; no dropouts were described according to the report |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 109 participants; no dropouts were described according to the report |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 109 participants; no dropouts were described according to the report |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 109 participants; no dropouts were described according to the report |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 109 participants; no dropouts were described according to the report |
| Selective reporting (reporting bias) | Unclear risk | Comment: study was not powered for the primary outcomes. All primary outcomes mentioned in the study protocol (NCT00370643) are reported in the results. Secondary outcomes in the protocol are not the same as the one reported in the publication. In the publication it is described in the abstract but not in the methods, and not reported ‐ just stated that it is not different between the two groups. |
| Other bias | Low risk | Nothing detected |
De La Rosa 2008.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: participants aged 15 years or older admitted to the ICU between 12 July 2003 and 21 December 2005 with an expected ICU stay of at least 2 days Exclusion criteria: pregnancy, diabetic ketoacidosis, hyperosmolar non‐ketotic state, readmission to the ICU during the same hospitalisation, advanced stage cancer (solid or haematological), decision to withhold or withdraw aggressive therapies and inclusion in another clinical trial Diagnostic criteria: on the basis of their medical history Setting: mixed ICU in the Hospital Pablo Tobón Uribe Age group: adolescents and adults Gender distribution: females and males Country where trial was performed: Colombia |
|
| Interventions |
Intervention(s): intensive insulin therapy Comparator(s): standard insulin therapy Duration of intervention: during ICU stay Duration of follow‐up: 28 days Run‐in period: none Number of study centres: 1 Treatment before trial: Intervention: insulin: 2.1%, oral antidiabetic agents: — Comparator: insulin: 3.6%, oral antidiabetic agents: — |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary outcome was 28‐day all‐cause mortality. Secondary outcomes were: ICU mortality; hospital mortality; incidence of infections in the ICU (ventilator‐associated pneumonia, urinary infections, catheter‐related infections and primary bacteraemias); ICU length of stay; days of mechanical ventilation and incidence of severe hypoglycaemia | |
| Study registration |
Trial identifiers: NCT 43740413031; 094‐2 in 000966421 Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: Instituto Colombiano para el desarrollo de la Ciencia y la Tecnología 'Francisco Jose de Caldas' (COLCIENCIAS), Grant: 4374‐04‐13013. (Bogota, Colombia) and Hospital Pablo Tobon Uribe (Medellin, Colombia) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "...we conducted a randomised clinical trial to assess the efficacy and safety of intensive insulin therapy compared with standard glucose control in patients hospitalised for medical problems, surgical non‐cardiovascular procedures or trauma in a mixed medical/surgical ICU" | |
| Notes | Trial ID provided in abstract not correct | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned into study groups with 1:1 ratio according to a computer‐generated random number list with permuted blocks of six. They were stratified by diabetes diagnosis" |
| Allocation concealment (selection bias) | Low risk | Quote: "The procedure was managed in the central pharmacy in charge of group assignment. Personnel involved in the treatment and investigation were unaware of the randomised schedule and the block size" Comment: central allocation |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: defined as non‐blinded; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: defined as non‐blinded; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: defined as non‐blinded; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | High risk | Comment: defined as non‐blinded; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: defined as non‐blinded; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: defined as non‐blinded; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Quality of life | High risk | Comment: defined as non‐blinded; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: defined as non‐blinded; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: the researchers described as blinded the assessment for infection acquired at the intensive care unit, but details were not provided for the rest of the outcomes |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: the researchers described as blinded the assessment for infection acquired at the intensive care unit, but details were not provided for the rest of the outcomes; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk | Comment: the researchers described as blinded the assessment for infection acquired at the intensive care unit, but details were not provided for the rest of the outcomes |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: the researchers described as blinded the assessment for infection acquired at the intensive care unit, but details were not provided for the rest of the outcomes |
| Blinding of outcome assessment (detection bias) Renal failure | Unclear risk | Comment: the researchers described as blinded the assessment for infection acquired at the intensive care unit, but details were not provided for the rest of the outcomes |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Unclear risk | Comment: the researchers described as blinded the assessment for infection acquired at the intensive care unit, but details were not provided for the rest of the outcomes |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: the researchers described as blinded the assessment for infection acquired at the intensive care unit, but details were not provided for the rest of the outcomes |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: sample size calculation for 504 participants that were finally randomised. No dropouts described in the trial report |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: sample size calculation for 504 participants that were finally randomised. No dropouts described in the trial report |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 504 participants that were finally randomised. No dropouts described in the trial report |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: sample size calculation for 504 participants that were finally randomised. No dropouts described in the trial report |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Comment: sample size calculation for 504 participants that were finally randomised. No dropouts described in the trial report |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: sample size calculation for 504 participants that were finally randomised. No dropouts described in the trial report |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 504 participants that were finally randomised. No dropouts described in the trial report |
| Selective reporting (reporting bias) | Unclear risk | Comment: the trial publication reports an incorrect study ID (NCT00096421; https://clinicaltrials.gov/ct2/show/NCT00096421). The outcomes described in the methods were reported in the trial's publication results section. |
| Other bias | Low risk | Nothing detected |
Desai 2012.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: all diabetic participants who underwent first‐time, isolated nonemergency CABG. Nondiabetic participants who underwent first‐time, isolated, nonemergency CABG who were found to have had 3 consecutive BG readings greater than 150 mg/dL or any 1 BG reading greater than 200 mg/dL perioperatively, which is aligned with the current STS guidelines. Participants who were started on an insulin infusion while in the operating room. Exclusion criteria: participants who underwent open surgery other than isolated CABG. Participants who were found not to require an insulin infusion post‐CABG. Participants who underwent a concomitant procedure in addition to CABG (e.g. CABG + valve repair). Diagnostic criteria: — Setting: Inova Fairfax Hospital Age group: adults Gender distribution: females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): strict strategy (BG 90 mg/dL to 120 mg/dL) Comparator(s): liberal strategy (121 mg/dL to 180 mg/dL) Duration of intervention: during ICU stay Duration of follow‐up: — Run‐in period: none Number of study centres: 1 Treatment before trial: — |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary endpoints were 2‐fold: superiority hypothesised for glucose control and target management and non‐inferiority hypothesised for complications and outcomes. Superiority endpoints included time to target glucose range, amount of insulin given, number of readings in target range and number of participants with hypoglycaemic events (BG < 60 mg/dL and BG < 40 mg/ dL). Non‐inferiority endpoints included perioperative renal failure, deep sternal wound infection, pneumonia, length of stay, artrial fibrillation and operative mortality (death within 30 days). | |
| Study registration |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "The purpose of this study was to test the hypothesis that a liberal blood glucose strategy (121 to 180 mg/dL) is not inferior to a strict blood glucose (90 to 120 mg/dL) for outcomes in patients after first‐time isolated coronary artery bypass grafting and is superior for glucose control and target blood glucose management" | |
| Notes | Information exclusively on diabetes participants was provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "A block randomisation process was used to randomly assign patients..." Comment: the trial report does not describe in detail how the random sequence was generated. As blocks are not defined as ‘permuted’ allocation to the blocks may be predictable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial report does not describe how the random sequence was concealed. As blocks are not defined as ‘permuted’ allocation to the blocks may be predictable. |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: open trial, to nurse staff was aware of treatment group allocation; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: open trial, nurse staff was aware of treatment group allocation |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk | Comment: open trial, nurse staff was aware of treatment group allocation |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: open trial, nurse staff was aware of treatment group allocation |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Comment: open trial, nurse staff was aware of treatment group allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Unclear risk | Comment: open trial, nurse staff was aware of treatment group allocation |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: open trial, nurse staff was aware of treatment group allocation |
| Incomplete outcome data (attrition bias) All‐cause mortality | Unclear risk | Comment: sample size calculation for 108 participants. 189 participants were randomised, but the authors described cross‐over between treatment arms (approximately for 20% of participants) that was not specified and led to a per‐protocol analysis |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Unclear risk | Comment: sample size calculation for 108 participants. 189 participants were randomised, but the authors described cross‐over between treatment arms (approximately for 20% of participants) that was not specified and led to a per‐protocol analysis |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: sample size calculation for 108 participants. 189 participants were randomised, but the authors described cross‐over between treatment arms (approximately for 20% of participants) that was not specified and led to a per‐protocol analysis |
| Incomplete outcome data (attrition bias) Cardiovascular events | Unclear risk | Comment: sample size calculation for 108 participants. 189 participants were randomised, but the authors described cross‐over between treatment arms (approximately for 20% of participants) that was not specified and led to a per‐protocol analysis |
| Incomplete outcome data (attrition bias) Renal failure | Unclear risk | Comment: sample size calculation for 108 participants. 189 participants were randomised, but the authors described cross‐over between treatment arms (approximately for 20% of participants) that was not specified and led to a per‐protocol analysis |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Unclear risk | Comment: sample size calculation for 108 participants. 189 participants were randomised, but the authors described cross‐over between treatment arms (approximately for 20% of participants) that was not specified and led to a per‐protocol analysis |
| Incomplete outcome data (attrition bias) Infection events | Unclear risk | Comment: sample size calculation for 108 participants. 189 participants were randomised, but the authors described cross‐over between treatment arms (approximately for 20% of participants) that was not specified and led to a per‐protocol analysis |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Unclear risk | Comment: sample size calculation for 108 participants. 189 participants were randomised, but the authors described cross‐over between treatment arms (approximately for 20% of participants) that was not specified and led to a per‐protocol analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: the researchers reported that the trial was registered at ClinicalTrials.gov, but they did not specify the study ID. The outcomes described in the methods section were reported in the publication's results section |
| Other bias | Low risk | Nothing detected |
Duncan 2018.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: adults between 18 and 90 years old scheduled for elective coronary artery bypass grafting, valve repair or replacement, or a combination of these procedures with cardiopulmonary bypass between August 2007 and April 2015
Exclusion criteria: off‐pump cardiac surgery, anticipated hypothermic circulatory arrest, elevated baseline cardiac troponin I (greater than 0.5 ng/ ml–1, Montreal) or troponin T (greater than 0.1 ng/ml–1, Cleveland), kidney disease requiring renal replacement therapy or active infection requiring ongoing antibiotic therapy Age group: adults Gender distribution: females and males Setting: Cleveland Clinic, Cleveland, Ohio & Royal Victoria Hospital, Montreal Countries where trial was performed: USA and Canada Diagnostic criteria: — Co‐morbidities: Diabetes: I: 226 (32%), C: 249 (34%) COPD/asthma: I: 107 (16%), C: 85 (12%) Pulmonary hypertension: I: 101 (15%), C: 102 (14%) Stroke: I: 41 (6%), C: 32 (5%) Hypertension: I: 533 (77%), C: 561 (79%) Heart failure: I: 146 (21%), C: 137 (19%) Myocardial infarction: I: 193 (28%), C: 173 (24%) Dialysis: I: 4 (1%), C: 4 (1%) Peripheral vascular disease: I: 51 (7%), C: 42 (6%) Smoking: I: 197 (29%), C: 180 (25%) ASA physical status II: I: 2 (0%), C: 0 (0%) ASA physical status III: I: 334 (49%), C: 349 (49%) ASA physical status IV: I: 348 (51%), C: 363 (51%) ASA physical status V: I: 2 (0%), C: 1 (0%) Co‐medications: ACE inhibitor, antiarrhythmics, ß‐blockers, calcium blockers, cox‐2 inhibitors, statins, steroids, diabetic medications (sulfonylureas or meglitinides, biguanides, thiazolidinediones, insulin) |
|
| Interventions |
Intervention(s): hyperinsulinaemic normoglycaemia, a fixed high‐dose insulin and concomitant variable glucose infusion titrated to glucose concentrations of 80 mg/dL to 110 mg/dL Comparator(s): standard glycaemic management, low‐dose insulin infusion targeting glucose greater than 150 mg/dL (the standard protocol includes a blood glucose goal of 70 mg/dL to 150 mg/dL; see appendix 1; it is a complex protocol where supplemental boluses are given if acute increases (greater than 30 mg/dL) occur) Duration of intervention: duration of surgery and ICU stay Duration of follow‐up: within 30 days of surgery and 1‐year all‐cause mortality Run‐in period: — Number of study centres: 2 Treatment before trial: diabetic medication I: 194 (30%), C: 193 (29%). Sulfonylureas or meglitinides I: 52 (8%), C: 58 (9%). Biguanides (metformin) I: 126 (20%), C: 124 (19%). Thiazolidinediones I: 16 (3%), C:11 (2%). Insulin I: 70 (11%), C: 68 (10%). Titration period: I: every 10 to 15 minutes throughout surgery, C: every 30 to 90 minutes throughout surgery |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary outcome was a collapsed composite (any vs none) of the following major postoperative complications occurring within 30 days of surgery: (1) all‐cause postoperative mortality; (2) failure to wean from cardiopulmonary bypass or postoperative low cardiac index (less than 1.8 l/min–1/m–2) requiring mechanical circulatory support with intra‐aortic balloon counter‐pulsation, ventricular assist device and/or extracorporeal mechanical oxygenation; (3) serious postoperative infection including any of the following infectious complications: mediastinitis, sternal wound infection requiring surgical debridement, sepsis or pneumonia requiring mechanical ventilatory support; (4) acute postoperative kidney injury requiring renal replacement therapy; and (5) new postoperative focal (aphasia, decrease in limb function, hemiparesis) or global (diffuse encephalopathy with greater than 24 hours of severely altered mental status or failure to awaken postoperatively) neurologic deficit. The secondary outcomes included postoperative atrial fibrillation, defined as the occurrence of new‐onset postoperative atrial fibrillation after cardiac surgery, duration of hospitalisation (days) and intensive care unit stay (days), and 1‐year all‐cause mortality. | |
| Study registration | Trial identifier:NCT00524472 Trial terminated early: no | |
| Publication details |
Language of publication: English Funding: Dr. Duncan received funding from Fresenius Kabi (Bad Homburg vor der Höhe, Germany) for research unrelated to the current investigation. Dr. Abd‐Elsayed is a consultant for Medtronic (Minneapolis, Minnesota), Halyard (Atlanta, Georgia), Axsome (New York, New York), and SpineLoop (Newport Beach, California), and has shares in Ultimaxx Health (Frisco, Texas). Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "This investigation tested the hypothesis that intraoperative hyperinsulinaemic normoglycaemia improves a composite of 30‐day postoperative mortality and serious cardiac, renal, neurologic, and infectious complications in patients recovering from cardiac surgery" | |
| Notes | Information exclusively on diabetes participants was provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by the Plan procedure in SAS software, version 9.4 (SAS Institute Inc., USA), a web‐based system" |
| Allocation concealment (selection bias) | Low risk |
Quote: "Allocation was initially concealed in sealed, sequentially numbered envelopes, and later in a web‐based system, both accessed shortly before induction of anesthesia" Comment: envelopes were not described as opaque, but presumably concealment was ensured through the web‐based system |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk |
Quote: "It was not feasible to blind anesthesia and surgical personnel to the intraoperative glucose management strategy; however, primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation" Comment: the trial was registered as blinded for participants, according the ClinicalTrials.gov record; outcome measure unlikely influenced by lack of blinding for personnel |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | Unclear risk |
Quote: "it was not feasible to blind anesthesia and surgical personnel to the intraoperative glucose management strategy; however, primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation" Comment: the trial was registered as blinded for participants, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding for personnel |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | Low risk |
Quote: "it was not feasible to blind anesthesia and surgical personnel to the intraoperative glucose management strategy; however, primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation" Comment: the trial was registered as blinded for participants, according the ClinicalTrials.gov record; outcome measure unlikely influenced by lack of blinding for personnel |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk |
Quote: "It was not feasible to blind anesthesia and surgical personnel to the intraoperative glucose management strategy; however, primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation" Comment: the trial was registered as blinded for participants, according the ClinicalTrials.gov record; outcome measure unlikely influenced by lack of blinding for personnel |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk |
Quote: "It was not feasible to blind anesthesia and surgical personnel to the intraoperative glucose management strategy; however, primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation" Comment: the trial was registered as blinded for participants, according the ClinicalTrials.gov record; outcome measure unlikely influenced by lack of blinding for personnel |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk |
Quote: "it was not feasible to blind anesthesia and surgical personnel to the intraoperative glucose management strategy; however, primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation" Comment: the trial was registered as blinded for participants, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding for personnel |
| Blinding of participants and personnel (performance bias) Infection events | Unclear risk |
Quote: "it was not feasible to blind anesthesia and surgical personnel to the intraoperative glucose management strategy; however, primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation" Comment: the trial was registered as blinded for participants, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding for personnel |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk |
Quote: “[…] primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation”. Comment: the trial was registered as blinded for participants, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding for personnel |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation Quote: "We could not blind anesthesia or surgical personnel to intraoperative glycaemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment" |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk |
Quote: “[…] primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation”. Quote: "We could not blind anesthesia or surgical personnel to intraoperative glycaemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment"; outcome measure unlikely influenced by potential lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk |
Quote: “[…] primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation”. Quote: "We could not blind anesthesia or surgical personnel to intraoperative glycaemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment" |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Low risk |
Quote: “[…] primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation”. Quote: "We could not blind anesthesia or surgical personnel to intraoperative glycaemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment" |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk |
Quote: “[…] primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation”. Quote: "We could not blind anesthesia or surgical personnel to intraoperative glycaemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment"; outcome measure unlikely influenced by potential lack of blinding |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk |
Quote: “[…] primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation”. Quote: "We could not blind anesthesia or surgical personnel to intraoperative glycaemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment"; outcome measure unlikely influenced by potential lack of blinding |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | High risk |
Quote: “[…] primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation”. Quote: "We could not blind anesthesia or surgical personnel to intraoperative glycaemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment" |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk |
Quote: “[…] primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation”. Quote: "We could not blind anesthesia or surgical personnel to intraoperative glycaemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: sample size calculation for 2790 participants, and a series of interim analyses were predefined to assess efficacy and futility of the primary outcome. Recruitment was continued until reaching one of these predefined thresholds. The efficacy boundary was reached at the third interim analysis |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: sample size calculation for 2790 participants, and a series of interim analyses were predefined to assess efficacy and futility of the primary outcome. Recruitment was continued until reaching one of these predefined thresholds. The efficacy boundary was reached at the third interim analysis |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 2790 participants, and a series of interim analyses were predefined to assess efficacy and futility of the primary outcome. Recruitment was continued until reaching one of these predefined thresholds. The efficacy boundary was reached at the third interim analysis |
| Incomplete outcome data (attrition bias) Cardiovascular events | Low risk | Comment: sample size calculation for 2790 participants, and a series of interim analyses were predefined to assess efficacy and futility of the primary outcome. Recruitment was continued until reaching one of these predefined thresholds. The efficacy boundary was reached at the third interim analysis |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: sample size calculation for 2790 participants, and a series of interim analyses were predefined to assess efficacy and futility of the primary outcome. Recruitment was continued until reaching one of these predefined thresholds. The efficacy boundary was reached at the third interim analysis |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Comment: sample size calculation for 2790 participants, and a series of interim analyses were predefined to assess efficacy and futility of the primary outcome. Recruitment was continued until reaching one of these predefined thresholds. The efficacy boundary was reached at the third interim analysis |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 2790 participants, and a series of interim analyses were predefined to assess efficacy and futility of the primary outcome. Recruitment was continued until reaching one of these predefined thresholds. The efficacy boundary was reached at the third interim analysis |
| Selective reporting (reporting bias) | Low risk |
Quote: registered at ClinicalTrials.gov (NCT00524472) on 31 August 2007 Comment: the trial register record discloses all the outcomes in the trial report |
| Other bias | Low risk | Nothing detected |
Gandhi 2007.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: adults undergoing elective cardiac surgery Exclusion criteria: participants who had off‐pump cardiopulmonary bypass procedures Diagnostic criteria: — Setting: St. Mary's Hospital Age group: adults Gender distribution: females and males Country where trial was performed: USA Co‐morbidities: chronic renal failure: I: 1%, C: 2%; history of myocardial infarction: I: 11%, C: 16%; stroke or transient ischaemic attack: I: 11%, C: 7% |
|
| Interventions |
Intervention(s): intensive insulin therapy Comparator(s): conventional insulin therapy Duration of intervention: during the surgery Duration of follow‐up: 30 days Run‐in period: none Number of study centres: 1 Treatment before trial: Intervention: insulin 22%, oral diabetic medication 54%, both: 16% Comparator: insulin 28%, oral diabetic medication 31%, both: 19% |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary outcome variable was a composite of death, sternal wound infections, prolonged pulmonary ventilation, cardiac arrhythmias (new‐onset atrial fibrillation, heart block requiring permanent pacemaker or cardiac arrest), stroke and acute renal failure within 30 days after surgery. Secondary outcome measures were length of stay in the ICU and hospital | |
| Study registration |
Trial identifier:NCT00282698 Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: Novo Nordisk, Princeton, New Jersey, and Mayo Foundation and Mayo Clinic College of Medicine, Rochester, Minnesota funded the study Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "...we conducted a randomized, controlled trial at 1 center to determine whether maintenance of near normoglycaemia during cardiac surgery by using intraoperative intravenous insulin infusion reduced perioperative death and morbidity when added to rigorous postoperative glycaemic control " | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was computer generated with permuted blocks of 4, with stratification according to surgeon, surgical procedure (...), and diabetes” |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation assignments were concealed in opaque, sealed, tamper‐proof envelopes that were opened sequentially by study personnel after participants signed the patient consent form" |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: described as an open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Comment: described as an open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Comment: described as an open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | Low risk | Comment: described as an open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Low risk | Quote: “Personnel who assessed outcomes were not aware of patient treatment assignment or of the study hypothesis” |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Quote: “Personnel who assessed outcomes were not aware of patient treatment assignment or of the study hypothesis” |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Low risk | Quote: “Personnel who assessed outcomes were not aware of patient treatment assignment or of the study hypothesis” |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Low risk | Quote: “Personnel who assessed outcomes were not aware of patient treatment assignment or of the study hypothesis” |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Quote: “Personnel who assessed outcomes were not aware of patient treatment assignment or of the study hypothesis” |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Quote: “Personnel who assessed outcomes were not aware of patient treatment assignment or of the study hypothesis” |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Low risk | Quote: “Personnel who assessed outcomes were not aware of patient treatment assignment or of the study hypothesis” |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Low risk | Quote: “Personnel who assessed outcomes were not aware of patient treatment assignment or of the study hypothesis” |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: sample size calculation for 177 participants per treatment group that was adjusted to 200 participants to ensure a sufficient number of outcomes. 400 participants were randomised, with minimal dropouts that were balanced between groups |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: sample size calculation for 177 participants per treatment group that was adjusted to 200 participants to ensure a sufficient number of outcomes. 400 participants participants randomised, with minimal dropouts that were balanced between groups |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 177 participants per treatment group that was adjusted to 200 participants to ensure a sufficient number of outcomes. 400 participants were randomised, with minimal dropouts that were balanced between groups |
| Incomplete outcome data (attrition bias) Cardiovascular events | Low risk | Comment: sample size calculation for 177 participants per treatment group that was adjusted to 200 participants to ensure a sufficient number of outcomes. 400 participants were randomised, with minimal dropouts that were balanced between groups |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: sample size calculation for 177 participants per treatment group that was adjusted to 200 participants to ensure a sufficient number of outcomes. 400 participants were randomised, with minimal dropouts that were balanced between groups |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Comment: sample size calculation for 177 participants per treatment group that was adjusted to 200 participants to ensure a sufficient number of outcomes. 400 participants were randomised, with minimal dropouts that were balanced between groups |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: sample size calculation for 177 participants per treatment group that was adjusted to 200 participants to ensure a sufficient number of outcomes. 400 participants were randomised, with minimal dropouts that were balanced between groups |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 177 participants per treatment group that was adjusted to 200 participants to ensure a sufficient number of outcomes. 400 participants were randomised, with minimal dropouts that were balanced between groups |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes published in the protocol (NCT00282698) are reported in the publication. In the publication, primary outcomes from the protocol are considered a composite, and not all secondary outcomes described as secondary in the publication |
| Other bias | Low risk | Nothing detected |
Glucontrol 2009.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: adult participants (older than 18 years) admitted to the participating ICUs Exclusion criteria: life expectancy lower than 24 hours, and the absence of consent Diagnostic criteria: — Setting: medical and surgical ICU Age group: adults Gender distribution: females and males Countries where trial was performed: Austria, Belgium, France, Israel, The Netherlands, Slovenia, Spain |
|
| Interventions |
Intervention(s): intensive insulin therapy (4.4 mmol/L to 6.1 mmol/L) Comparator(s): intermediate target (7.8 mmol/L to 10.0 mmol/L) Duration of intervention: during ICU stay Duration of follow‐up: 28 days Run‐in period: none Number of study centres: 21 Treatment before trial: — |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary outcome variable was the all‐cause absolute mortality during the ICU stay. Secondary outcome variables included hospital and 28‐day mortality, ICU and hospital, length of stay (LOS), incidence of organ failures assessed by the daily SOFA score, rate of hypoglycaemia and the SOFA score on the day of hypoglycaemia, duration of mechanical ventilation, inotrope/vasopressor and renal replacement therapy, number of packed red blood cells transfusion (PRBC), febrile days and days with therapeutic anti‐infective agents | |
| Study registration |
Trial identifier:NCT00107601 Trial terminated early: yes (due to a high rate of unintended protocol violations) |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding. Supported by a grant from the ‘Communauté Française Wallonie‐Bruxelles’ (Belgium) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "The present study was undertaken to test the hypothesis that IIT improves survival of patients treated in medico‐surgical intensive care units (ICU), as compared with a glucose control target of 7.8 ‐ 10.0 mmol/L, lower than in the Leuven trials [...] Specifically, this study was designed to detect whether IIT was associated with a 4% decrease of the absolute ICU mortality" | |
| Notes | Information exclusively on diabetes participants was provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The central computerised randomisation (blocks of eight patients) was stratified by centre and concealed" |
| Allocation concealment (selection bias) | Low risk |
Quote: “The central computerised randomization (blocks of eight patients) was stratified by centre and concealed” Comment: probably adequate concealment |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Quote: “Inherently related to the intervention under investigation, the study was not blinded, ...”; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Quote: “Inherently related to the intervention under investigation, the study was not blinded, ...”; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Quote: “Inherently related to the intervention under investigation, the study was not blinded, ...”; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Quote: “Inherently related to the intervention under investigation, the study was not blinded, ...”; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Quote: “Inherently related to the intervention under investigation, the study was not blinded, ...”; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Quote: “Inherently related to the intervention under investigation, the study was not blinded, ...”; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Quote: “Inherently related to the intervention under investigation, the study was not blinded, ...”; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Quote: "The central data manager and the statistician were blinded to treatment assignment"; “The assessment of outcome and the statistical analysis were performed blindly” |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Low risk | Quote: "The central data manager and the statistician were blinded to treatment assignment"; “The assessment of outcome and the statistical analysis were performed blindly” |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Low risk | Quote: “The central data manager and the statistician were blinded to treatment assignment"; “The assessment of outcome and the statistical analysis were performed blindly” |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Quote: “The central data manager and the statistician were blinded to treatment assignment"; “The assessment of outcome and the statistical analysis were performed blindly” |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Quote: “The central data manager and the statistician were blinded to treatment assignment"; “The assessment of outcome and the statistical analysis were performed blindly” |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Low risk | Quote: “The central data manager and the statistician were blinded to treatment assignment"; “The assessment of outcome and the statistical analysis were performed blindly” |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Low risk | Quote: “The central data manager and the statistician were blinded to treatment assignment"; “The assessment of outcome and the statistical analysis were performed blindly” |
| Incomplete outcome data (attrition bias) All‐cause mortality | Unclear risk | Quote: “...the trial was stopped early due to a high rate of unintended protocol violations.” “Specifically, the proportion of BG values in the assigned range calculated from the BG readings available at the time of the interim analysis [...] were deemed as a high rate of unintended protocol violation rate” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 1101 participants |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Unclear risk | Quote: “...the trial was stopped early due to a high rate of unintended protocol violations.” “Specifically, the proportion of BG values in the assigned range calculated from the BG readings available at the time of the interim analysis [...] were deemed as a high rate of unintended protocol violation rate” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 1101 participants |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Quote: “...the trial was stopped early due to a high rate of unintended protocol violations.” “Specifically, the proportion of BG values in the assigned range calculated from the BG readings available at the time of the interim analysis [...] were deemed as a high rate of unintended protocol violation rate” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 1101 participants |
| Incomplete outcome data (attrition bias) Cardiovascular events | Unclear risk | Quote: “...the trial was stopped early due to a high rate of unintended protocol violations.” “Specifically, the proportion of BG values in the assigned range calculated from the BG readings available at the time of the interim analysis [...] were deemed as a high rate of unintended protocol violation rate” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 1101 participants |
| Incomplete outcome data (attrition bias) Renal failure | Unclear risk | Quote: “...the trial was stopped early due to a high rate of unintended protocol violations.” “Specifically, the proportion of BG values in the assigned range calculated from the BG readings available at the time of the interim analysis [...] were deemed as a high rate of unintended protocol violation rate” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 1101 participants |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Unclear risk | Quote: “...the trial was stopped early due to a high rate of unintended protocol violations.” “Specifically, the proportion of BG values in the assigned range calculated from the BG readings available at the time of the interim analysis [...] were deemed as a high rate of unintended protocol violation rate” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 1101 participants |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Unclear risk | Quote: “...the trial was stopped early due to a high rate of unintended protocol violations.” “Specifically, the proportion of BG values in the assigned range calculated from the BG readings available at the time of the interim analysis [...] were deemed as a high rate of unintended protocol violation rate” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 1101 participants |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes described in the methods are reported in the results. Study powered for the primary outcome. Outcomes in the publication same as in the protocol (NCT00107601) |
| Other bias | Low risk | Nothing detected |
Hermayer 2012.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: renal transplant candidates admitted to Medical University of South Carolina (MUSC) who were 18 years of age or greater and who had a DM diagnosis (type 1 and type 2), a fasting blood glucose (BG) over 100 mg/dL per admission screening labs, and a random BG over 120 mg/dL per admission screening labs. These participants were willing and able to provide informed consent and were awaiting a living or cadaveric kidney transplant. Exclusion criteria: people with a history of an active gastrointestinal bleed 3 months previously, people who were scheduled to receive a simultaneous pancreas transplant, people with a history of a functioning pancreatic transplant, people currently managed on an insulin pump, people who were unable or unwilling to provide informed consent, and people who were unable to commit to the study protocol, including the outpatient follow‐up phase of care Diagnostic criteria: — Setting: diabetes management service (DMS) at the Medical University of South Carolina Age group: adults Gender distribution: females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): intensive intravenous insulin (blood glucose (BG) 70 mg/dL to 110 mg/dL) Comparator(s): subcutaneous insulin (BG 70 mg/dL to 180 mg/dL) Duration of intervention: intraoperatively and 72 hours postoperatively Duration of follow‐up: median 1.5 years Run‐in period: none Number of study centres: 1 Treatment before trial: — |
|
| Outcomes | Reported outcome(s) in full text of publication: primary endpoint was delayed graft function (DGF). Secondary endpoints were glycaemic control, graft survival and acute rejection episodes | |
| Study registration | Trial identifier: NCT00609986 | |
| Publication details |
Language of publication: English Funding: supported by the American Diabetes Association Clinical Investigator Award 7‐07‐CR‐22 Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "To compare the effects of blood sugar control, using iv insulin (BG target 70 –110 mg/dL) or sc insulin (BG target 70 –180 mg/dL) in patients with impaired glucose tolerance or DM undergoing renal transplantation" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “This study was a randomized, unblinded, controlled clinical trial” Comment: described as randomised but no additional details provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: “This study was a randomized, unblinded, controlled clinical trial” Comment: described as randomised but no additional details provided |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Quote: “This study was a randomized, unblinded, controlled clinical trial"; "staff and study personnel were not blinded as to the patient’s treatment group assignments" Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Quote: “This study was a randomized, unblinded, controlled clinical trial"; "staff and study personnel were not blinded as to the patient’s treatment group assignments" Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Unclear risk | Quote: “This study was a randomized, unblinded, controlled clinical trial"; "staff and study personnel were not blinded as to the patient’s treatment group assignments" Comment: described as an open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Quote: “This study was a randomized, unblinded, controlled clinical trial"; "staff and study personnel were not blinded as to the patient’s treatment group assignments" Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | High risk | Quote: “This study was a randomized, unblinded, controlled clinical trial"; "staff and study personnel were not blinded as to the patient’s treatment group assignments" Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | High risk | Quote: “This study was a randomized, unblinded, controlled clinical trial"; "staff and study personnel were not blinded as to the patient’s treatment group assignments" Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Renal failure | Unclear risk | Quote: “This study was a randomized, unblinded, controlled clinical trial"; "staff and study personnel were not blinded as to the patient’s treatment group assignments" Comment: described as an open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | High risk | Quote: “This study was a randomized, unblinded, controlled clinical trial"; "staff and study personnel were not blinded as to the patient’s treatment group assignments" Comment: described as an open trial; outcome measure likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: sample size calculation for 90 participants. Although 104 participants were randomised 11 participants did not receive surgery and were subsequently excluded from analyses, and data for 93 participants were finally analysed |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 90 participants. Although 104 participants were randomised 11 participants did not receive surgery and were subsequently excluded from analyses, and data for 93 participants were finally analysed |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: sample size calculation for 90 participants. Although 104 participants were randomised 11 participants did not receive surgery and were subsequently excluded from analyses, and data for 93 participants were finally analysed |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 90 participants. Although 104 participants were randomised 11 participants did not receive surgery and were subsequently excluded from analyses, and data for 93 participants were finally analysed |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol or register record unavailable, but all the outcomes described in the methods were reported. A surrogate endpoint was chosen as the primary outcome |
| Other bias | Low risk | Nothing detected |
Lazar 2004.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: participants with diabetes mellitus undergoing primary or reoperative CABG performed on cardiopulmonary bypass Exclusion criteria: — Diagnostic criteria: — Setting: Boston Medical Center Age group: adults Gender distribution: females and males Country/countries where trial was performed: USA Co‐morbidities: congestive heart failure: I: 28%, C: 39%; myocardial infarction: I: 71%, C: 71%; hypertension: I: 78%, C: 68%; chronic obstructive pulmonary disease: I: 8%, C: 4%; left main disease: I: 19%, C: 25% Co‐medications: inotropic agents, ß‐blockers. Some participants treated with nitroglycerin and heparin (see table 3). Inotropic use during or after surgery: no information on their use in study groups. |
|
| Interventions |
Intervention: tight glycaemic control (serum glucose 125 mg/dL to 200 mg/dL) with glucose‐insulin‐potassium (GIK) Comparator: standard therapy (serum glucose < 250 mg/dL) using intermittent subcutaneous insulin (no‐GIK) Duration of intervention: beginning before anaesthesia and continuing for 12 hours after surgery Duration of follow‐up: 2 years and extended follow‐up of 5 years Run‐in period: none Number of study centres: 1 Treatment before trial: Intervention: insulin: 32%; oral diabetic medication: 60%; diet: 5%; nitroglycerin: 32%; IV heparin: 58% Comparator: insulin: 27%, oral diabetic medication: 59%, diet: 13%. IV nitroglycerin: 33, IV heparin: I: 62% |
|
| Outcomes | Reported outcome(s) in full text of publication: to determine whether tight perioperative glycaemic control in diabetic CABG participants with a modified GIK solution would optimise myocardial metabolism and improve perioperative outcomes. They also sought to determine whether the early beneficial effects of tight glycaemic control would result in improved survival, a decreased incidence of ischaemic events, and reduced wound complications. | |
| Study registration |
Trial identifier: protocol E 327/A65 from the Boston University Medical Center Institutional Review Board Trial terminated early (for benefit/because of adverse events): no |
|
| Publication details |
English publication in a peer‐reviewed journal, supported by non‐commercial funding Language of publication: English Funding: non‐commercial funding. Supported by a clinical research award form the American Diabetes Association Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "This study was therefore undertaken to determine whether tight perioperative glycaemic control in diabetic CABG patients with a modified GIK solution would optimise myocardial metabolism and improve perioperative outcomes. We also sought to determine whether the early beneficial effects of tight glycaemic control would result in improved survival, a decreased incidence of ischemic events, and reduced wound complications" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to …” Comment: no additional details provided on randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to …” Comment: no additional details provided on concealment |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Weight gain | High risk | Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Weight gain | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 141 participants; no attrition |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 141 participants; no attrition |
| Incomplete outcome data (attrition bias) Cardiovascular events | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 141 participants; no attrition |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Unclear risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 141 participants; no attrition |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 141 participants; no attrition |
| Incomplete outcome data (attrition bias) Weight gain | Low risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 141 participants; no attrition |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Unclear risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 141 participants; no attrition |
| Selective reporting (reporting bias) | Unclear risk |
Quote: "One hundred forty‐one patients were enrolled into the study and completed the protocol without any study‐related complications" Comment: no protocol available and no power calculation was done. All outcomes stated in the methods are reported in the results. Hypoglycaemic episodes would be expected to be an outcome due to the characteristics of the intervention, but was not. However, authors state that there were not study‐related complications |
| Other bias | Low risk | Nothing detected |
Lazar 2011.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: participants with diabetes mellitus undergoing CABG surgery on cardiopulmonary bypass Exclusion criteria: severe hyperglycaemia (serum glucose > 400 mg/dL), which could not be controlled on a stable insulin regimen preoperatively, chronic renal failure (creatinine level ≥ 2.5 mg/dL), acute renal failure (urine output < 20 mL/hour for 3 hours), and those participants requiring concomitant procedures in addition to CABG surgery Diagnostic criteria: — Setting: Boston Medical Center Age group: adults Gender distribution: females and males Country where trial was performed: USA Co‐morbidities: angina class IV: I: 33%, C: 33%; CHF: I: 13%, C: 14%; MI: I: 57%; C: 57%; HTN: I: 93%, C: 100%; left main disease: I: 15%; C: 21% Co‐medications: cardioprotective medication (β‐blockers, statins, ACE inhibitors) |
|
| Interventions |
Intervention(s): aggressive group (serum glucose 90 mg/dL to 120 mg/dL) Comparator(s): moderate group (serum glucose 120 mg/dL to 180 mg/dL) Duration of intervention: during surgery and the next 18 hours in the ICU Duration of follow‐up: 30 days Run‐in period: none Number of study centres: 1 Treatment before trial: Intervention: β‐blockers: 100%; statins: 100%; ACE inhibitors: 60%; hyperlipidaemia treatment: 100%; insulin: 38%; oral diabetic agents: 52%, diet control: 5%; insulin and oral diabetic agents: 5% Comparator: β‐blockers: 100%; statins: 100%; ACE inhibitors: 59%; hyperlipidaemia treatment: 100%; insulin: 31%; oral diabetic agents: 48%, diet control: 10%; insulin and oral diabetic agents: 11% |
|
| Outcomes | Reported outcome(s) in full text of publication: to determine whether more aggressive glycaemic control would result in more optimal clinical outcomes and less morbidity than can be achieved with moderate control in participants with diabetes mellitus undergoing CABG surgery | |
| Study registration |
Trial identifier:NCT00460499 Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: — Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "This study sought to determine whether aggressive glycaemic control (90 ‐ 120 mg/dL) would result in more optimal clinical outcomes and less morbidity than moderate glycaemic control (120 ‐ 180 mg/dL) in diabetic patients undergoing coronary artery bypass graft (CABG) surgery" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a prospective, randomized single center trial..." Comment: no additional details provided on randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “This was a prospective, randomized single center trial …” Comment: no additional details provided on randomisation sequence concealment |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk |
Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk |
Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk |
Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk |
Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk |
Quote: “Clinicians were not blinded to the treatment groups, ...”. Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk |
Quote: “Clinicians were not blinded to the treatment groups, ...”. Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk |
Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk |
Quote: “Clinicians were not blinded to the treatment groups, ...” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Low risk | Comment: the trial report did not provide any detail on blinded assessment, but the trial registration record disclosed that outcome assessment was masked |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: the trial report did not provide any detail on blinded assessment, but the trial registration record disclosed that outcome assessment was masked |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Low risk | Comment: the trial report did not provide any detail on blinded assessment, but the trial registration record disclosed that outcome assessment was masked |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: the trial report did not provide any detail on blinded assessment, but the trial registration record disclosed that outcome assessment was masked |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Comment: the trial report did not provide any detail on blinded assessment, but the trial registration record disclosed that outcome assessment was masked |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Comment: the trial report did not provide any detail on blinded assessment, but the trial registration record disclosed that outcome assessment was masked |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Low risk | Comment: the trial report did not provide any detail on blinded assessment, but the trial registration record disclosed that outcome assessment was masked |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Low risk | Comment: the trial report did not provide any detail on blinded assessment, but the trial registration record disclosed that outcome assessment was masked |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Quote: “All patients completed the protocol, and none were lost to follow‐up.” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 82 participants |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Quote: “All patients completed the protocol, and none were lost to follow‐up.” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 82 participants |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Quote: “All patients completed the protocol, and none were lost to follow‐up.” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 82 participants |
| Incomplete outcome data (attrition bias) Cardiovascular events | Low risk | Quote: “All patients completed the protocol, and none were lost to follow‐up.” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 82 participants |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Quote: “All patients completed the protocol, and none were lost to follow‐up.” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 82 participants |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Quote: “All patients completed the protocol, and none were lost to follow‐up.” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 82 participants |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Quote: “All patients completed the protocol, and none were lost to follow‐up.” Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 82 participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: one of the primary outcomes reported in the trial publication (incidence of major adverse events) was not disclosed at its trial register record |
| Other bias | Unclear risk |
Quote: "Supported by a research grant by Eli Lilly..." Comment: Eli Lilly is a manufacturer of insulin. Also in the protocol an estimated enrolment of 400 is reported, however the study only enrolled 82 participants |
Li 2006.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: participants with diabetes mellitus undergoing CABG for the first time Exclusion criteria: none Diagnostic criteria: in most cases before admission for surgery. Newly diagnosed diabetes was confirmed by a fasting blood glucose level of ≥ 200 mg/dL associated with an elevated level of haemoglobin A1c Setting: Mackay Memorial Hospital Age group: adults Gender distribution: females and males Country where trial was performed: Republic of China/Taiwan Co‐morbidities: hypertension: I: 84%, C: 88%; congestive heart failure: I: 57%, C: 59%; renal insufficiency: I: 10%, C: 14%; stroke: I: 8%, C: 17%; peripheral vascular disease: I: 4%, C: 2%; chronic obstructive pulmonary disease: I: 6%, C: 9%; previous myocardial infarction: I: 51%, C: 62%; left main stenosis: I: 25%, C: 31% |
|
| Interventions |
Intervention(s): continuous insulin infusion (CII) Comparator(s): glucometer‐guided insulin (GGI) Duration of intervention: 5 days postoperative Duration of follow‐up: — Run‐in period: none Number of study centres: 1 Treatment before trial: diabetic control: insulin: I: 5.9%, C: 11.9%, oral hypoglycaemic agents: I: 86.3%, C: 78.6%; diuretics: I: 17.6%, C: 28.6%; inotropic agents: I: 7.8%, C: 4.8% |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary endpoints were incidence of operative mortality and sternal wound infection of operative mortality and sternal wound infection; the secondary endpoint was the adequacy of blood glucose control | |
| Study registration |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "Postulating that continuous insulin infusion would provide better control of postoperative blood glucose levels, we designed this prospective, randomized study to test that hypothesis" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “[…] the patients were randomly assigned to 1 of 2 groups for diabetic control” Comment: no additional details provided on randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] the patients were randomly assigned to 1 of 2 groups for diabetic control” Comment: no additional details provided on randomisation sequence concealment |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: open trial. The researchers report that as consequence of the trial not being blinded, 7 participants were withdrawn due to clinicians' concerns about participants’ glucose levels; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: open trial. The researchers report that as consequence of the trial not being blinded, 7 participants were withdrawn due to clinicians' concerns about participants’ glucose levels; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Comment: open trial. The researchers report that as consequence of the trial not being blinded, 7 participants were withdrawn due to clinicians' concerns about participants’ glucose levels; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: open trial. The researchers report that as consequence of the trial not being blinded, 7 participants were withdrawn due to clinicians' concerns about participants’ glucose levels; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: open trial. The researchers report that as consequence of the trial not being blinded, 7 participants were withdrawn due to clinicians' concerns about participants’ glucose levels; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: open trial. The researchers report that as consequence of the trial not being blinded, 7 participants were withdrawn due to clinicians' concerns about participants’ glucose levels; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Incomplete outcome data (attrition bias) All‐cause mortality | Unclear risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 100 participants, but the researchers described many protocol deviations (see blinding of participants and personnel) and cross‐overs between groups that were excluded from final analyses |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 100 participants, but the researchers described many protocol deviations (see blinding of participants and personnel) and cross‐overs between groups that were excluded from final analyses |
| Incomplete outcome data (attrition bias) Cardiovascular events | Unclear risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 100 participants, but the researchers described many protocol deviations (see blinding of participants and personnel) and cross‐overs between groups that were excluded from final analyses |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Unclear risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 100 participants, but the researchers described many protocol deviations (see blinding of participants and personnel) and cross‐overs between groups that were excluded from final analyses |
| Incomplete outcome data (attrition bias) Infection events | Unclear risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 100 participants, but the researchers described many protocol deviations (see blinding of participants and personnel) and cross‐overs between groups that were excluded from final analyses |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Unclear risk | Comment: the researchers did not report a sample size calculation for this trial. The trial randomised 100 participants, but the researchers described many protocol deviations (see blinding of participants and personnel) and cross‐overs between groups that were excluded from final analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available; the trial report presented results on all outcome measures that were pre‐specified in the methods section as relevant. No power calculation was made for the primary outcome |
| Other bias | Low risk | Nothing detected |
NICE SUGAR 2009.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: medical and surgical participants admitted to the ICU of 42 hospitals, expected to require treatment in the ICU on 3 or more consecutive days Exclusion criteria: imminent death, diabetic ketoacidosis or hyperosmolar state, in ICU for more than 24 hours, expected to be eating before 3rd day after admission, no informed consent, enrolled in the NICE‐SUGAR previously, suffered hypoglycaemia without full neurological recovery Diagnostic criteria for diabetes: on the basis of their medical history Setting: 38 academic tertiary care hospitals and 4 community hospitals Age group: adults Gender distribution: females and males Countries where trial was performed: Australia, New Zealand, Canada and USA |
|
| Interventions |
Intervention(s): intensive glucose control Comparator(s): conventional glucose control Duration of intervention: until participant was eating or discharged from ICU Duration of follow‐up: 90 days Run‐in period: none Number of study centres: 42 hospitals Treatment before trial (insulin): I: 29.8%, C: 27.3% |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary outcome measure was death from any cause within 90 days after randomisation. Secondary outcome measures were survival time during the first 90 days, cause‐specific death and durations of mechanical ventilation, renal‐replacement therapy, and stays in the ICU and hospital. Tertiary outcomes were death from any cause within 28 days after randomisation, place of death (ICU, hospital ward or other), incidence of new organ failure, positive blood culture, receipt of red‐cell transfusion and volume of the transfusion | |
| Study registration |
Trial identifier:NCT00220987 Trial terminated early: no (certain participants yes) |
|
| Publication details |
Language of publication: English Funding: supported by grants from the Australian National Health and Medical Research Council, the Health Research Council of New Zealand, and the Canadian Institutes for Health Research. Dr. Finfer reports receiving reimbursement for travel to present research results at scientific meetings from Eli Lilly, Cardinal Health, and CSL Bioplasma and for serving on steering committees for studies sponsored by Eli Lilly and Eisai (paid to the George Institute for International Health); he also reports that the George Institute for International Health, an independent, not‐for‐profit institute affiliated with the University of Sydney, has received research funding from Servier, Novartis, Eisai, Merck Sharp & Dohme, Pfizer Australia, Fresenius Kabi Deutschland, and Sanofi‐Aventis Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "We designed the normoglycaemia in Intensive Care Evaluation‐Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) trial to test the hypothesis that intensive glucose control reduces mortality at 90 days" | |
| Notes | Information exclusively on diabetes participants was provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned to a treatment group by the clinicians treating them or by local study coordinators, with the use of a minimization algorithm” Quote: (from the protocol): “The George Institute for International Health will take responsibility for the web‐based randomisation. This will be available 24 hours a day.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were randomly assigned to a treatment group by the clinicians treating them or by local study coordinators, with the use of a minimization algorithm accessed through a secure Web site. The treatment assignments were concealed before randomization” |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk |
Quote: “The treatment assignments were concealed before randomization, but subsequently, clinical staff were aware of them” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk |
Quote: “The treatment assignments were concealed before randomization, but subsequently, clinical staff were aware of them” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk |
Quote: “The treatment assignments were concealed before randomization, but subsequently, clinical staff were aware of them” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk |
Quote: “The treatment assignments were concealed before randomization, but subsequently, clinical staff were aware of them” Comment: outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk |
Quote: “The treatment assignments were concealed before randomization, but subsequently, clinical staff were aware of them” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk |
Quote: “The treatment assignments were concealed before randomization, but subsequently, clinical staff were aware of them” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk |
Quote: “The treatment assignments were concealed before randomization, but subsequently, clinical staff were aware of them” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | High risk |
Quote “The primary outcome measure is mortality and therefore not subject to ascertainment bias” Comment: the trial report did not provide any detail on blinded assessment; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk |
Quote: “The primary outcome measure is mortality and therefore not subject to ascertainment bias” Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | High risk |
Quote “The primary outcome measure is mortality and therefore not subject to ascertainment bias” Comment: the trial report did not provide any detail on blinded assessment; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | High risk |
Quote “The primary outcome measure is mortality and therefore not subject to ascertainment bias” Comment: the trial report did not provide any detail on blinded assessment; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk |
Quote “The primary outcome measure is mortality and therefore not subject to ascertainment bias” Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | High risk |
Quote “The primary outcome measure is mortality and therefore not subject to ascertainment bias” Comment: the trial report did not provide any detail on blinded assessment; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | High risk |
Quote “The primary outcome measure is mortality and therefore not subject to ascertainment bias” Comment: the trial report did not provide any detail on blinded assessment; outcome measure likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: sample size calculation for 6100 participants. 6014 participants were randomised, but the treatment was discontinued in 10% of participants assigned to intensive glucose control and 7% of controls, with balanced reasons between groups |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: sample size calculation for 6100 participants. 6014 participants were randomised, but the treatment was discontinued in 10% of participants assigned to intensive glucose control and 7% of controls, with balanced reasons between groups |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 6100 participants. 6014 participants were randomised, but the treatment was discontinued in 10% of participants assigned to intensive glucose control and 7% of controls, with balanced reasons between groups |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: sample size calculation for 6100 participants. 6014 participants were randomised, but the treatment was discontinued in 10% of participants assigned to intensive glucose control and 7% of controls, with balanced reasons between groups |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Comment: sample size calculation for 6100 participants. 6014 participants were randomised, but the treatment was discontinued in 10% of participants assigned to intensive glucose control and 7% of controls, with balanced reasons between groups |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: sample size calculation for 6100 participants. 6014 participants were randomised, but the treatment was discontinued in 10% of participants assigned to intensive glucose control and 7% of controls, with balanced reasons between groups |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 6100 participants. 6014 participants were randomised, but the treatment was discontinued in 10% of participants assigned to intensive glucose control and 7% of controls, with balanced reasons between groups |
| Selective reporting (reporting bias) | Low risk | Comment: study was powered for the primary outcome. All outcomes described in the methods and protocol (NCT00220987) are reported in the results |
| Other bias | Low risk | Nothing detected |
Parekh 2016.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: adults with a diagnosis of diabetes mellitus who were admitted for deceased donor renal transplantation Exclusion criteria: children and adult candidates enrolled in a concurrent study evaluating the effect of a medication or other intervention on graft function Diagnostic criteria: — Setting: University of California San Francisco Medical Center Age group: adults Gender distribution: females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): moderately intense glucose control: insulin infusion if blood glucose > 120 mg/dL no earlier than 4 hours before the anticipated start of the transplant. Operation: glucose goal between 80 mg/dL and 160 mg/dL. Postoperative: insulin infusion with glucose goal of 100 mg/dL to 180 mg/dL during 24 hours. Comparator(s): standard control preoperative: insulin sliding scale when serum blood glucose > 200 mg/dL. Operation: intravenous insulin if the glucose was above 200 mg/dL. Postoperative: standard sliding scale for 24 hours. Duration of intervention: pre‐ and postoperative 24 hours Duration of follow‐up: up to 1 year Run‐in period: none Number of study centres: 1 Treatment before study (medication for diabetes): I: 76.6%, C: 80% |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary outcome for the trial was poor graft function defined by the need for dialysis within 7 days of transplant or a failure of the serum creatinine to drop by more than 10% for 3 consecutive days. Secondary outcomes were DGF (delayed graft function; need for dialysis within 7 days of transplant), perioperative death, stroke and seizure, as well as serum creatinine and estimated glomerular filtration rate (GFR), using the Modification of Diet in Renal Disease (MDRD) study calculation, at 30 days, 6 months and 1 year. Graft‐specific outcomes were biopsy‐proven rejection and graft loss. | |
| Study registration |
Trial identifier:NCT01643382 Trial terminated early: yes Quote: "the trial was then stopped after 60 patients or 75% of the planned total recruitment as the criterion for stopping the trial based on the primary outcome was met" |
|
| Publication details |
Language of publication: English Funding: supported by the American Diabetes Association Clinical Investigator Award 7‐07‐CR‐22 Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "determine whether moderately intense glucose control during allograft reperfusion would reduce the incidence of poor graft function" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization was generated per computer algorithm” |
| Allocation concealment (selection bias) | Low risk | Quote: “[…] the randomization scheme was hidden in an electronic file until each patient was enrolled” |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Unclear risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All‐cause mortality | Unclear risk | Comment: sample size calculation for 80 participants. The trial only randomised 60 patients according a pre‐specified rule to stop the trial as the primary outcome criterion was met, but the report only describes criteria for stopping early for safety |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Unclear risk | Comment: sample size calculation for 80 participants. The trial only randomised 60 patients according a pre‐specified rule to stop the trial as the primary outcome criterion was met, but the report only describes criteria for stopping early for safety |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: sample size calculation for 80 participants. The trial only randomised 60 patients according a pre‐specified rule to stop the trial as the primary outcome criterion was met, but the report only describes criteria for stopping early for safety |
| Incomplete outcome data (attrition bias) Cardiovascular events | Unclear risk | Comment: sample size calculation for 80 participants. The trial only randomised 60 patients according a pre‐specified rule to stop the trial as the primary outcome criterion was met, but the report only describes criteria for stopping early for safety |
| Incomplete outcome data (attrition bias) Renal failure | Unclear risk | Comment: sample size calculation for 80 participants. The trial only randomised 60 patients according a pre‐specified rule to stop the trial as the primary outcome criterion was met, but the report only describes criteria for stopping early for safety |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Unclear risk | Comment: sample size calculation for 80 participants. The trial only randomised 60 patients according a pre‐specified rule to stop the trial as the primary outcome criterion was met, but the report only describes criteria for stopping early for safety |
| Incomplete outcome data (attrition bias) Infection events | Unclear risk | Comment: sample size calculation for 80 participants. The trial only randomised 60 patients according a pre‐specified rule to stop the trial as the primary outcome criterion was met, but the report only describes criteria for stopping early for safety |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Unclear risk | Comment: sample size calculation for 80 participants. The trial only randomised 60 patients according a pre‐specified rule to stop the trial as the primary outcome criterion was met, but the report only describes criteria for stopping early for safety. |
| Selective reporting (reporting bias) | Low risk | Comment: the trial register record only disclosed the primary outcome that was reported |
| Other bias | Low risk | Nothing detected |
Rassias 1999.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: participants with diabetes scheduled to undergo elective cardiac surgery with cardiopulmonary bypass Exclusion criteria: emergency surgery, conditions known to cause immunosuppression (other than diabetes mellitus), age < 18 years or inability to provide written informed consent Diagnostic criteria: — Setting: Dartmouth‐Hitchcock Medical Center Age group: adults Gender distribution: females and males Countries where trial was performed: USA, Lebanon Co‐medication: — |
|
| Interventions |
Intervention(s): aggressive insulin therapy Comparator(s): standard insulin therapy Duration of intervention: during surgery and on the first postoperative day Duration of follow‐up: — Run‐in period: none Number of study centres: 1 Treatment before trial: Intervention: half of their usual subcutaneous NPH insulin dosage the morning of surgery and started on an infusion of 5% dextrose with 0.45% sodium chloride solution at 50 mL/h. Oral hypoglycaemics were not given. Comparator: half of their usual subcutaneous NPH insulin dosage the morning of surgery and started on an infusion of 5% dextrose with 0.45% sodium chloride solution at 50 mL/h. Oral hypoglycaemics were not given. |
|
| Outcomes | Reported outcome(s) in full text of publication: glucose levels and leukocyte function (polymorphonuclear neutrophils) | |
| Study registration |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "We conducted a clinical study to examine the effect of aggressive insulin therapy on PMN function in diabetic cardiac surgery patients" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were prospectively randomized into one of two treatment groups..." Comment: described as randomised but no additional details provided about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: described as randomised but no additional details provided about the sequence concealment |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk |
Quote: “The anesthesia and surgery team was not blinded as to the choice of insulin therapy.” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk |
Quote: “The anesthesia and surgery team was not blinded as to the choice of insulin therapy.” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk |
Quote: “The anesthesia and surgery team was not blinded as to the choice of insulin therapy.” Comment: outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 26 participants, who were finally randomised. The researchers did not describe any dropouts |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: sample size calculation for 26 participants, who were finally randomised. The researchers did not describe any dropouts |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 26 participants, who were finally randomised. The researchers did not describe any dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol or register record unavailable. The researchers reported a unique outcome from which they calculated the sample size |
| Other bias | Low risk | Nothing detected |
Subramaniam 2009.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial with randomisation ratio of 1:1 | |
| Participants |
Inclusion criteria: adult participants (≥ 18 years) with an ASA physical status of I‐IV, undergoing peripheral vascular bypass surgery, abdominal aortic surgery or major lower extremity amputation (above or below the knee) and were expected to stay in the hospital for at least 48 hours Exclusion criteria: participants with brittle diabetes (as previously diagnosed by endocrinologist), varicose vein ligation, continuous insulin infusion pumps, planned stent procedures for vascular disease, or an ASA physical status of V were excluded from the study Diagnostic criteria: — Setting: Beth Israel Deaconess Medical Center Age group: adults Gender distribution: females and males Country where trial was performed: USA Co‐morbidities: hypertension (I: 81%, C: 78%); coronary artery disease (I: 51%, C: 58%); congestive heart failure (I: 11%, C: 9%); coronary artery bypass grafting (I: 30%, C: 21%); chronic renal failure (I: 13%, C: 12%); stroke (I: 8%, C: 9%); chronic obstructive pulmonary disease (I: 20%, C: 25%) Co‐medications: metoprolol |
|
| Interventions |
Intervention(s): continuous insulin infusion Comparator(s): standard intermittent sliding‐scale insulin bolus Duration of intervention: intervention began at the start of surgery and continued for 48 hours Duration of follow‐up: until 30 days Run‐in period: none Number of study centres: 1 Treatment before trial: insulin: I: 65%, C: 53%; metformin: I: 15%, C: 19%; glyburide: I: 37%, C: 30%; statin: I: 67%, C: 57%; aspirin: I: 85%, C: 84%; ACE inhibitor: I: 56%, C: 57%; β‐blocker: I: 73%, C: 80% |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary endpoint was defined as a composite rate of the following intraprocedural and postprocedural major cardiovascular events at hospital discharge: all‐cause of death, myocardial infarction, acute congestive heart failure | |
| Study registration |
Trial identifier: — Trial terminated early: yes (slow recruitment, increasing numbers of minimally invasive stent procedures and the planned hospital‐wide implementation of a more aggressive perioperative glucose management strategy) |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "In the current study, we tested the hypothesis that a strategy of tight perioperative blood glucose control using a continuous insulin infusion in patients undergoing vascular surgery decreases major cardiovascular events (MACEs) when compared with conventional management" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were randomly assigned, using a 1:1 block randomisation scheme..." Comment: described as randomised but no additional details provided about the sequence generation. As blocks are not defined as ‘permuted’ allocation to the blocks may be predictable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: described as randomised but no additional details provided about the sequence concealment. As blocks are not defined as ‘permuted’ allocation to the blocks may be predictable. |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: the trial was described as unblinded and researchers reported that some parts of the management protocol could be delivered at the discretion of anaesthesiologists; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: the trial was described as unblinded and researchers reported that some parts of the management protocol could be delivered at the discretion of anaesthesiologists; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: the trial was described as unblinded and researchers reported that some parts of the management protocol could be delivered at the discretion of anaesthesiologists; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Comment: the trial was described as unblinded and researchers reported that some parts of the management protocol could be delivered at the discretion of anaesthesiologists; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Comment: the trial was described as unblinded and researchers reported that some parts of the management protocol could be delivered at the discretion of anaesthesiologists; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: the trial was described as unblinded and researchers reported that some parts of the management protocol could be delivered at the discretion of anaesthesiologists; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: the trial was described as unblinded and researchers reported that some parts of the management protocol could be delivered at the discretion of anaesthesiologists; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: the trial was described as unblinded and researchers reported that some parts of the management protocol could be delivered at the discretion of anaesthesiologists; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Comment: the trial report did not provide any detail on blinded assessment; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: the trial report did not provide any detail on blinded assessment |
| Incomplete outcome data (attrition bias) All‐cause mortality | Unclear risk | Comment: sample size calculation for 1986 participants with a planned interim analysis after the enrolment of 452. However, the trial stopped after the inclusion of 236 patients due to problems with recruitment and changes in the research hospital regarding perioperative glucose management protocols |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Unclear risk | Comment: sample size calculation for 1986 participants with a planned interim analysis after the enrolment of 452. However, the trial stopped after the inclusion of 236 patients due to problems with recruitment and changes in the research hospital regarding perioperative glucose management protocols |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: sample size calculation for 1986 participants with a planned interim analysis after the enrolment of 452. However, the trial stopped after the inclusion of 236 patients due to problems with recruitment and changes in the research hospital regarding perioperative glucose management protocols |
| Incomplete outcome data (attrition bias) Cardiovascular events | Unclear risk | Comment: sample size calculation for 1986 participants with a planned interim analysis after the enrolment of 452. However, the trial stopped after the inclusion of 236 patients due to problems with recruitment and changes in the research hospital regarding perioperative glucose management protocols |
| Incomplete outcome data (attrition bias) Renal failure | Unclear risk | Comment: sample size calculation for 1986 participants with a planned interim analysis after the enrolment of 452. However, the trial stopped after the inclusion of 236 patients due to problems with recruitment and changes in the research hospital regarding perioperative glucose management protocols |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Unclear risk | Comment: sample size calculation for 1986 participants with a planned interim analysis after the enrolment of 452. However, the trial stopped after the inclusion of 236 patients due to problems with recruitment and changes in the research hospital regarding perioperative glucose management protocols |
| Incomplete outcome data (attrition bias) Infection events | Unclear risk | Comment: sample size calculation for 1986 participants with a planned interim analysis after the enrolment of 452. However, the trial stopped after the inclusion of 236 patients due to problems with recruitment and changes in the research hospital regarding perioperative glucose management protocols |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Unclear risk | Comment: sample size calculation for 1986 participants with a planned interim analysis after the enrolment of 452. However, the trial stopped after the inclusion of 236 patients due to problems with recruitment and changes in the research hospital regarding perioperative glucose management protocols |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol or register record unavailable, but all the outcomes described in the methods section were reported |
| Other bias | Low risk | Nothing detected |
Umpierrez 2015.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: aged between 18 and 80 years undergoing primary or a combination of CABG and other cardiac operations such as valve repair or aortic surgery who experienced perioperative hyperglycaemia, defined as a blood glucose > 140 mg/dL
Exclusion criteria: people with impaired renal function (serum creatinine ≥ 3.0 mg/dL or glomerular filtration rate < 30 mL/min/1.73 m2), hepatic failure, or history of hyperglycaemic crises and those at imminent risk of death (brain death or cardiac standstill) or pregnancy, or individual or next of kin unable to provide consent Diagnostic criteria: — Setting: Emory University Hospital, Emory Midtown Hospital, and Grady Memorial Hospital in Atlanta Age group: adults, elderly people Gender distribution: females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): continuous insulin infusion (CII) adjusted to maintain a glucose target between 100 mg/dL and 140 mg/dL in the ICU Comparator(s): CII adjusted to maintain a glucose level between 141 mg/dL and 180 mg/dL in the ICU Duration of intervention: until discontinuation of CII (at ICU discharge) Duration of follow‐up: 90 days after hospital discharge Run‐in period: none Number of study centres: 3 Treatment before trial: — Intervention: no antidiabetic agents 7 (9%); oral agents only 32 (45%); insulin alone 15 (20%); insulin + oral agents 20 (26%) Comparator: no antidiabetic agents 5 (7%); oral agents only 34 (48%); insulin alone 14 (20%); insulin + oral agents 18 (25%) |
|
| Outcomes | Reported outcome(s) in full text of publication: the primary outcome was to determine differences between intensive and conservative glucose control on a composite of hospital mortality and perioperative complications, including sternal wound infection (deep and superficial), bacteraemia, respiratory failure, pneumonia, acute kidney injury, and MACE (acute myocardial infarction, congestive heart failure and cardiac arrhythmias). The secondary outcome was to compare differences between intensive and conservative glucose control. | |
| Study registration |
Trial identifier:NCT01361594 Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: a clinical research grant from the American Diabetes Association (7‐03‐CR‐35) and a grant from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources (UL1 RR025008) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "The optimal level of glycaemic control needed to improve outcomes in cardiac surgery patients remains controversial" | |
| Notes | Information exclusively on diabetes participants was provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A research pharmacist following a computer‐generated block randomization table coordinated randomization and treatment assignment” |
| Allocation concealment (selection bias) | Low risk | Quote: “A research pharmacist following a computer‐generated block randomization table coordinated randomization and treatment assignment” Comment: likely the pharmacist participated in the trial independently |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: described as an open‐label trial; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: described as an open‐label trial |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk | Comment: described as an open‐label trial |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: described as an open‐label trial |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Comment: described as an open‐label trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Unclear risk | Comment: described as an open‐label trial |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: described as an open‐label trial |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: sample size calculation for 296 participants, adjusted to an expected low attrition rate (< 10%). Although 305 participants were randomised, 3 participants withdrew prior to the intervention initiation, and data for 302 participants were finally analysed |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: sample size calculation for 296 participants, adjusted to an expected low attrition rate (< 10%). Although 305 participants were randomised, 3 participants withdrew prior to the intervention initiation, and data for 302 participants were finally analysed |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 296 participants, adjusted to an expected low attrition rate (< 10%). Although 305 participants were randomised, 3 participants withdrew prior to the intervention initiation, and data for 302 participants were finally analysed |
| Incomplete outcome data (attrition bias) Cardiovascular events | Low risk | Comment: sample size calculation for 296 participants, adjusted to an expected low attrition rate (< 10%). Although 305 participants were randomised, 3 participants withdrew prior to the intervention initiation, and data for 302 participants were finally analysed |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: sample size calculation for 296 participants, adjusted to an expected low attrition rate (< 10%). Although 305 participants were randomised, 3 participants withdrew prior to the intervention initiation, and data for 302 participants were finally analysed |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Comment: sample size calculation for 296 participants, adjusted to an expected low attrition rate (< 10%). Although 305 participants were randomised, 3 participants withdrew prior to the intervention initiation, and data for 302 participants were finally analysed |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: sample size calculation for 296 participants, adjusted to an expected low attrition rate (< 10%). Although 305 participants were randomised, 3 participants withdrew prior to the intervention initiation, and data for 302 participants were finally analysed |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 296 participants, adjusted to an expected low attrition rate (< 10%). Although 305 participants were randomised, 3 participants withdrew prior to the intervention initiation, and data for 302 participants were finally analysed |
| Selective reporting (reporting bias) | Unclear risk | Comment: all the outcomes in the trial register record were disclosed in the trial report, but a composite outcome of major morbidity and 30‐day mortality was used as primary outcome |
| Other bias | Low risk | Nothing detected |
Wahby 2016.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: diabetic people planned for coronary artery bypass graft (CABG) surgery Exclusion criteria: emergency CABG, off‐pump surgery and combined valve and CABG surgery Diagnostic criteria: — Setting: Tanta University Hospital and National Heart Institute Age group: — Gender distribution: females and males Country where trial was performed: Egypt |
|
| Interventions |
Intervention(s): tight glycaemic control during operation to maintain blood glucose level between 110 mg/dL and 149 mg/dL Comparator(s): conventional moderate glycaemic control to achieve blood glucose level between 150 mg/dL and 180 mg/dL during surgery Duration of intervention: perioperative Duration of follow‐up: 30 days after surgery Run‐in period: none Number of study centres: 1 Treatment before trial: — |
|
| Outcomes | Reported outcome(s) in full text of publication: operative mortality (defined as mortality within 30 days of operation or during hospitalisation due to cause related to operation), renal dysfunction (elevated serum creatinine above 2 mg/dL postoperative or more than 25% of preoperative level), acute renal failure required postoperative dialysis, postoperative permanent neurological deficit, sternal wound infection, leg infection and need for postoperative inotropic support that was defined as the use of dopamine 5 mg/kg/min; any dose of epinephrine, norepinephrine, dobutamine or milrinone. All participants were followed up regarding duration of mechanical ventilation postoperatively. Prolonged mechanical ventilation was defined as cumulative duration of 24 h or more of endotracheal intubation starting from transfer of the participant to cardiac surgery ICU after completion of operation. The occurrence of postoperative atrial fibrillation (AF) and perioperative myocardial infarction were recorded. Perioperative myocardial infarction was defined as any participant having fresh ECG changes including new Q‐waves in two precordial leads, new bundle branch block, haemodynamic compromise with new segmental wall motion dysfunction or elevation of CK MB over 100 U/L after undergoing open heart surgery. | |
| Study registration |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: — Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "we aimed to detect the effect of perioperative tight glycaemic control versus moderate glycaemic control on the outcome of diabetic patients undergoing CABG surgery" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned into 2 groups according to computer allocated generation table (graph pad software)” |
| Allocation concealment (selection bias) | Unclear risk | Comment: described as randomised but no additional details provided about the sequence concealment |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: open to personnel. The groups of interest were managed according a protocol with substantial differences that could reveal the participants' allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: open to personnel. The groups of interest were managed according a protocol with substantial differences that could reveal the participants’ allocation; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: open to personnel. The groups of interest were managed according a protocol with substantial differences that could reveal the participants’ allocation; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | Low risk | Comment: open to personnel. The groups of interest were managed according a protocol with substantial differences that could reveal the participants’ allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Comment: open to personnel. The groups of interest were managed according a protocol with substantial differences that could reveal the participants’ allocation; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: open to personnel. The groups of interest were managed according a protocol with substantial differences that could reveal the participants’ allocation; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: open to personnel. The groups of interest were managed according a protocol with substantial differences that could reveal the participants’ allocation; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: open trial |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk | Comment: open trial |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: open trial |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Low risk | Comment: open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Comment: open trial; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: open trial |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Cardiovascular events | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Infection events | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: probably no attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol or register record unavailable. The outcomes of interest were described ambiguously |
| Other bias | Low risk | Nothing detected |
Wallia 2017.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: age 18 to 80 years old; able to give informed consent personally or via a family member with appropriate authorisation to do so if individual unable; expected survival after transplantation of 1 year; BG level 180 mg/dL postoperatively regardless of diabetes status (with or without diabetes); no previous liver transplantation Exclusion criteria: inability of person or family member to give informed consent, not expected to survive for > 1 year following liver transplantation, previous liver transplantation, acute liver failure, living related donor Diagnostic criteria: — Setting: Northwestern Memorial Hospital/Northwestern University Feinberg School of Medicine Age group: adults Gender distribution: females and males Country where trial was performed: USA Co‐morbidities: — Co‐medications: — |
|
| Interventions |
Intervention(s): intensive (insulin treatment to target blood glucose at 140 mg/dL) Comparator(s): moderate (insulin treatment to target blood glucose at 180 mg/dL) Duration of intervention: immediately postoperatively until participants were stable and had begun to eat; mean duration of insulin infusion in hours: 56.5 + 78.6 Duration of follow‐up: 1 year Run‐in period: none Number of study centres: 1 Treatment before trial: — |
|
| Outcomes | Reported outcome(s) in full text of publication: the number of participants experiencing an episode of rejection within 1 year after transplantation. The number of participants experiencing an infection within 1 year after transplantation. The number of participants experiencing an infection within 1 year after transplantation. Outcomes for inpatient: episodes of hypoglycaemia, including symptoms occurring when hypoglycaemic, ICU length of stay, hospital length of stay, death. Outcomes for outpatients: rehospitalisation, graft survival, death. | |
| Study registration |
Trial identifier:NCT01211730 Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: The American Diabetes Association Junior Faculty Award 1‐13‐JF‐54 Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "we designed a prospective, randomised, single‐center, comparative effectiveness trial comparing 2 levels of glycaemic control (intensive, 140 mg/dL, vs moderate, 180 mg/dL) in patients who had undergone liver transplantation to evaluate whether intensive glucose management in the inpatient setting improved the outcomes after liver transplantation" | |
| Notes | Information exclusively on diabetic participants was provided by trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization into the 2 groups was performed using a computer‐generated random number program (GraphPad Software, Inc., San Diego, CA)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: the randomisation was generated according a correct procedure, but details on allocation concealment were not provided in the report |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: the trial was registered as an open study, according the ClinicalTrials.gov record; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: the trial was registered as an open study, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: the trial was registered as an open study, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Length of ICU and hospital stay | High risk | Comment: the trial was registered as an open study, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: the trial was registered as an open study, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: the trial was registered as an open study, according the ClinicalTrials.gov record; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: the report does not explicitly describe that outcomes were measured in a blinded fashion |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: the report does not explicitly describe that outcomes were measured in a blinded fashion. However, the adjudication of the primary outcome followed pre‐specified criteria, and unclear cases were adjudicated by blinded reviewers; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk | Comment: the report does not explicitly describe that outcomes were measured in a blinded fashion |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: the report does not explicitly describe that outcomes were measured in a blinded fashion |
| Blinding of outcome assessment (detection bias) Length of ICU and hospital stay | Unclear risk | Comment: the report does not explicitly describe that outcomes were measured in a blinded fashion |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | Unclear risk | Comment: the report does not explicitly describe that outcomes were measured in a blinded fashion |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: sample size calculation for 136 participants. The trial randomised 164 participants and only 3 participants died early, but data were analysed for the entire sample |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: sample size calculation for 136 participants. The trial randomised 164 participants and only 3 participants died early, but data were analysed for the entire sample |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: sample size calculation for 136 participants. The trial randomised 164 participants and only 3 participants died early, but data were analysed for the entire sample |
| Incomplete outcome data (attrition bias) Length of ICU and hospital stay | Low risk | Comment: sample size calculation for 136 participants.The trial randomised 164 participants and only 3 participants died early, but data were analysed for the entire sample |
| Incomplete outcome data (attrition bias) Infection events | Unclear risk | Comment: sample size calculation for 136 participants. The trial randomised 164 participants and only 3 participants died early, but data were analysed for the entire sample |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | Low risk | Comment: sample size calculation for 136 participants. The trial randomised 164 participants and only 3 participants died early, but data were analysed for the entire sample |
| Selective reporting (reporting bias) | Unclear risk | Comment: the trial register record disclosed all outcomes in the trial report, but the publication states the existence of a “principal secondary outcome” not differentiated in the trial register |
| Other bias | Low risk | Nothing detected |
Yuan 2015.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: adults with type 2 diabetes mellitus (DM) undergoing gastrectomy for gastric tumours between September 2006 and March 2014 Exclusion criteria: participants were excluded if (1) a withdrawal request was made by the participant or surrogate; (2) the participant underwent laparotomy or palliative surgery; (3) the participant was unable to tolerate enteral nutrition, as shown by vomiting, diarrhoea or abdominal distention; or (4) the nasojejunal tube became occluded or was pulled out Diagnostic criteria: type 2 DM was diagnosed according to the criteria of the American Diabetes Association and gastric tumours were diagnosed endoscopically or by imaging modalities before surgery Setting: First Affiliated Hospital of Zhengzhou University Age group: adults Gender distribution: females and males Country where trial was performed: China |
|
| Interventions |
Intervention(s): intensive glycaemic (IG) management, with continuous insulin infusion to a target blood glucose concentration 4.4 mmol/L to 6.1 mmol/L (80 mg/dL to 110 mg/dL) Comparator(s): conventional glycaemic (CG) management, with intermittent bolus insulin to a target blood glucose concentration < 11.1 mmol/L (< 200 mg/dL) Duration of intervention: — Duration of follow‐up: — Run‐in period: none Number of study centres: 1 Treatment before trial: Intervention: insulin 71 (67%), oral antidiabetic agents 25 (23.6%), none 10 (9.4%) Comparator: insulin 67 (63.2%), oral antidiabetic agents 30 (28.3%), none 9 (8.5%) |
|
| Outcomes | Reported outcome(s) in full text of publication: outcomes included blood glucose concentrations, insulin administration, and postoperative morbidity and mortality | |
| Study registration |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: supported by the National Nature Science Foundation of China, Grant No. 81201955 Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote: "This study assessed whether intensive glycaemic control was well‐tolerated, safe, and improved clinical outcomes in diabetic patients receiving enteral nutrition after gastrectomy" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly assigned …” Comment: described as randomised but no additional details provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomly assigned …” Comment: described as randomised but no additional details provided |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: open to personnel. Participants in the control group were managed according a protocol (e.g. insulin administration every 4 to 6 hours) that revealed to which group each participant was allocated; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Hypoglycaemic episodes | High risk | Comment: open to personnel. Participants in the control group were managed according a protocol (e.g. insulin administration every 4 to 6 hours) that revealed to which group each participant was allocated; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Adverse events other than hypoglycaemic episodes | High risk | Comment: open to personnel. Participants in the control group were managed according a protocol (e.g. insulin administration every 4 to 6 hours) that revealed to which group each participant was allocated; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Cardiovascular events | High risk | Comment: open to personnel. Participants in the control group were managed according a protocol (e.g. insulin administration every 4 to 6 hours) that revealed to which group each participant was allocated; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Renal failure | Low risk | Comment: open to personnel. Participants in the control group were managed according a protocol (e.g. insulin administration every 4 to 6 hours) that revealed to which group each participant was allocated; outcome measure unlikely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Infection events | High risk | Comment: open to personnel. Participants in the control group were managed according a protocol (e.g. insulin administration every 4 to 6 hours) that revealed to which group each participant was allocated; outcome measure likely influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Mean blood glucose during intervention | High risk | Comment: open to personnel. Participants in the control group were managed according a protocol (e.g. insulin administration every 4 to 6 hours) that revealed to which group each participant was allocated; outcome measure likely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Infection events | Unclear risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: open‐label study; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Hypoglycaemic episodes | Unclear risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) Adverse events other than hypoglycaemic episodes | Unclear risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) Cardiovascular events | Unclear risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) Renal failure | Low risk | Comment: open‐label study; outcome measure unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Mean blood glucose during intervention | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) All‐cause mortality | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Hypoglycaemic episodes | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Adverse events other than hypoglycaemic episodes | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Cardiovascular events | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Renal failure | Low risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Infection events | Unclear risk | Comment: probably no attrition |
| Incomplete outcome data (attrition bias) Mean blood glucose during intervention | High risk | Comment: probably no attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol or register record unavailable. The outcomes of interest were described ambiguously |
| Other bias | Low risk | Nothing detected |
—: not reported
ACE: angiotensin‐converting‐enzyme; ADA: American Diabetes Association; ASA: American Society of Anesthesiologists; BG: blood glucose; BMI: body mass index; C: comparator; CABG: coronary artery bypass graft; CHF: congestive heart failure; CII: continuous insulin infusion; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass; DM: diabetes mellitus; ECG: electrocardiogram; GGI: glucometer‐guided insulin; HLA‐DR: human leukocyte antigen – DR isotype; HOMA‐IR: homeostatic model assessment for insulin resistance; HTN: hypertension; I: intervention; ICU: intensive care unit; MI: myocardial infarction; NPR: neutral protamine Hagedorn; PMN: polymorphonuclear cells; SICU: surgical intensive care unit; SOFA: sequential organ failure assessment; STS: Society of Thoracic Surgeons
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelmalak 2019 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Agus 2014 | No surgical diabetic patients |
| Agus 2017 | No surgical diabetic patients |
| Asida 2013 | Not a RCT |
| Cao 2013 | No surgical diabetic patients |
| Chuah 2015 | No perioperative intervention |
| Cinotti 2014 | No surgical diabetic patients |
| Duncan 2015 | No intensive glucose control vs standard or conventional glucose control |
| Ellenberger 2018 | No intensive glucose control vs standard or conventional glucose control |
| Giakoumidakis 2013 | Not a RCT |
| Gupta 2020 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Hsu 2012 | No perioperative intervention |
| Huang 2013 | No intensive glucose control vs standard or conventional glucose control |
| Kalfon 2014 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Krishna 2019 | No intensive glucose control vs standard or conventional glucose control |
| Kumar 2020 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Kurnaz 2017 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Laiq 2015 | No intensive glucose control vs standard or conventional glucose control |
| LÜ 2019 | Not a RCT |
| Makino 2019 | No intensive glucose control vs standard or conventional glucose control |
| Mikaeili 2012 | No surgical diabetic patients |
| Mohod 2019 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Mularski 2012 | Not a RCT |
| NCT00394303 | Suspended trial |
| NCT00487162 | Suspended trial |
| NCT03526536 | Not a RCT |
| Okabayashi 2014 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Pasquel 2020 | No intensive glucose control vs standard or conventional glucose control with different blood glucose targets |
| Pezzella 2014 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Polderman 2017 | No intensive glucose control vs standard or conventional glucose control |
| Punke 2014 | No intensive glucose control vs standard or conventional glucose control |
| Qu 2012 | No surgical diabetic patients |
| Ramírez‐Cáceres 2019 | No intensive glucose control vs standard or conventional glucose control |
| Rujirojindakul 2014 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Santana‐Santos 2019 | No outcome data available for subpopulation of diabetic patients after contacting authors |
| Schroeder 2012 | No intensive glucose control vs standard or conventional glucose control |
| Tohya 2018 | No surgical diabetic patients |
| Wang 2017 | No surgical patient population |
| Welsh 2016 | No intensive glucose control vs standard or conventional glucose control |
| Xue 2018 | Not a RCT |
RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Hweidi 2021.
| Methods |
Type of study: interventional Allocation: randomised Masking: single‐blind Primary purpose: treatment |
| Participants |
Conditions: diabetic patients undergoing coronary artery bypass graft (CABG) surgery Gender: females and males Age groups: adult patients Enrolment: 144 participants |
| Interventions |
Intervention: intraoperative blood glucose level of 110 mg/dL to 149 mg/dL Comparator: maintained an intraoperative blood glucose level of 150 mg/dL to 180 mg/dL |
| Outcomes | Primary outcome: surgical site infection |
| Reason for awaiting classification | We contacted the authors because of the need for extra information on the insulin protocol regimen received by the control group, currently without response |
| Study details | — |
| Official title and purpose of study | The effect of intraoperative glycaemic control on surgical site infections among diabetic patients undergoing coronary artery bypass graft (CABG) surgery |
| Notes | — |
Imran‐ul‐hassan 2021.
| Methods |
Type of study: observational Allocation: randomised Intervention model: parallel assignment Masking: not reported Primary purpose: treatment |
| Participants |
Conditions: diabetic patients following open heart surgery Gender: females and males Age groups: 18 years to 60 years Enrolment: 60 participants |
| Interventions |
Intervention: glucose level between 80 mg/dL and 110 mg/dL was targeted using continuous infusion of insulin in saline Comparator: no insulin |
| Outcomes | Primary outcome: pulmonary problem, cardiac problem, renal problem, neurological problem, surgical problem, early mortality |
| Reason for awaiting classification | We contacted the authors because of the need for extra information on the study design, currently without response |
| Study details | — |
| Official title and purpose of study | Outcome of strict peri‐operative glycemic control in diabetic patients following open heart surgery |
| Notes | — |
NCT00899483.
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: double (participant, outcomes assessor) Primary purpose: treatment |
| Participants |
Conditions: type II diabetes mellitus patients, undergoing elective and urgent coronary artery bypass surgery Gender: females and males Age groups: 18 years to 80 years Enrolment: 100 participants |
| Interventions |
Intervention: administered with glucose potassium insulin solution to achieve glycaemia 4.0 mmol/L to 6.0 mmol/L Comparator: normal departmental practice using dextrose insulin infusion |
| Outcomes |
Primary outcome: the difference in the mean left ventricular end‐systolic volume index (LVESVI) after CABG and the amount of new permanent injury detected in the late CMRI study Secondary outcome: glycaemic control |
| Reason for awaiting classification | Unknown |
| Study details | — |
| Official title and purpose of study | Can enhanced glycemic control in type II diabetics improve myocardial protection during coronary artery bypass grafting? |
| Notes | — |
NCT01528189.
| Methods |
Type of study: interventional Allocation: randomised Type of trial: endpoint classification: efficacy study Intervention model: single group assignment Masking: double (participant, outcomes assessor) Primary purpose: treatment |
| Participants |
Conditions: elective liver, pancreatic or colorectal surgery Gender: females and males Age groups: adult/senior Enrolment: 540 |
| Interventions |
Intervention: hyperinsulinaemic normoglycaemic clamp Comparator: standard glucose management |
| Outcomes |
Primary outcome: surgical site infection (30 days after surgery) Secondary outcome: surgical morbidity in the 30 days following the operation |
| Reason for awaiting classification | Unknown |
| Study details | — |
| Official title and purpose of study | Effect of high dose insulin on infectious complications following major surgery |
| Notes | — |
NCT02746432.
| Methods |
Type of study: interventional Allocation: randomised Type of trial: endpoint classification: efficacy study Intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
Conditions: colorectal cancer Gender: both Age groups: adult/senior Enrolment: 144 |
| Interventions |
Intervention: hyper‐insulinaemic euglycaemic clamp Comparator: routine intraoperative saline infusion |
| Outcomes |
Primary outcomes: the anti‐inflammatory effects of intraoperative hyper‐insulinaemic euglycaemic therapy in people undergoing colorectal cancer surgery (1 month); effect on inflammatory profile, namely levels of TNF‐alpha, IL‐8, IL‐6, IL‐10, IL‐1B, IL‐18, IFNy, MIp1‐alpha, MMP‐8, TGF‐beta, CRP Secondary outcomes: the immunomodulatory effect of intraoperative hyper‐insulinaemic euglycaemic infusion change (1 month) on CD4, CD8, T‐cell, quantity and activity |
| Reason for awaiting classification | Unknown |
| Study details | — |
| Official title and purpose of study | Insulin therapy reduce post‐operative inflammatory response after curative colorectal cancer resection: randomization controlled trial |
| Notes | — |
NCT03314272.
| Methods |
Type of study: interventional Allocation: randomised Type of trial: endpoint classification: efficacy study Intervention model: parallel assignment Masking: single (participants) Primary purpose: treatment |
| Participants |
Conditions: diabetes Gender: both Age groups: adult/older adult Enrolment: 59 |
| Interventions |
Intervention: automated protocol consisting of an insulin infusion pump (Space Glucose Control System) Comparator: sliding scale protocol |
| Outcomes |
Primary outcomes: percentage of patients who will have a serum glucose level between 140 mg/dL and 180 mg/dL during surgery. The reduction of this percentage to less than 10% with the fully automated algorithm is clinically significant. Percentage of patients with hyperglycaemic events; percentage of patients with hypoglycaemic events Secondary outcomes: not provided Other outcomes: not provided |
| Reason for awaiting classification | Publication pending |
| Study details | — |
| Official title and purpose of study | Automated vs conventional perioperative glycemic control in diabetic patients undergoing cardiopulmonary bypass surgery |
| Notes | — |
NCT03474666.
| Methods |
Type of study: interventional Allocation: randomised Intervention model: insulin initially as continuous infusion/subcutaneous for first 24 to 48 hours followed by subcutaneous administration once participants eating until hospital discharge Masking: none Primary purpose: treatment |
| Participants |
Conditions: liver transplantation, surgical wound infection Gender: females and males Age groups: adult Enrolment: 41 patients |
| Interventions |
Intervention: insulin initially as continuous infusion/subcutaneous for first 24 to 48 hours followed by subcutaneous administration once participants eating until hospital discharge Comparator: subcutaneous insulin as institutional protocol |
| Outcomes |
Primary outcome: surgical site infection Secondary outcome: hyperglycaemia, hypoglycaemia, duration of mechanical ventilation, ICU stay, ward stay, death, surgical site infection or death, hospital length of stay |
| Reason for awaiting classification | Publication pending |
| Study details | — |
| Official title and purpose of study | Glycemic control and surgical site infection incidence among liver transplantation recipients |
| Notes | Terminated (the data safety monitoring board recommended stopping the study) |
Zadeh 2016.
| Methods |
Type of study: interventional Allocation: randomised Type of trial: endpoint classification: efficacy study Intervention model: single group assignment Masking: open‐label Primary purpose: treatment |
| Participants |
Conditions: elective liver, pancreatic or colorectal surgery Gender: females and males Age groups: adult/senior Enrolment: 75 |
| Interventions |
Intervention: modified tight glucose control Comparator: conventional glucose control |
| Outcomes |
Primary outcome: mortality, sternal wound infection, duration of mechanical ventilation, cardiac arrhythmias (atrial fibrillation, cardiac arrest, heart block which requires pacemaker), cerebrovascular attack and acute renal failure Secondary outcome: length of ICU stay |
| Reason for awaiting classification | Concerns about the trustworthiness of the data: The same population sample was used in 2 publications (in 2016 and in 2020) by the same authors. While it was stated in the 2020 publication that the study was conducted in 2017‐2018, the results are almost identical, with only minor differences, to those reported in the 2016 publication: the study conducted in 2016 was defined as an open‐label trial, whereas the design in the 2020 publication was defined as double‐blind, however no description of blinding was provided. Both articles are identical, except for an additional variable that was added in the 2020 study. We contacted the editors of both journals raising our concerns and asking them to investigate this matter. |
| Study details | — |
| Official title and purpose of study | A study on the outcomes of modified tight glucose control for the management of glycemic control in diabetic patients undergoing cardiac surgery |
| Notes | — |
CABG: coronary artery bypass graft; CRP: C‐reactive protein; ICU: intensive care unit; MMP: matrix metalloproteinase; TNF: tumour necrosis factor; TGF: transforming growth factor
Characteristics of ongoing studies [ordered by study ID]
NCT02032953.
| Study name | Enhancing the anabolic effect of perioperative nutrition with insulin while maintaining normoglycaemia |
| Methods |
Type of study: interventional Allocation: randomised Type of trial: endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants |
Conditions: colorectal surgery for non‐metastatic colorectal adenocarcinoma Gender: both Age groups: adult/senior Enrolment: 24 |
| Interventions |
Intervention 1: insulin with travasol 35% Intervention 2: insulin with travasol 20% Comparator: insulin |
| Outcomes | Net protein balance (6 hours after surgery) |
| Starting date |
Trial start date: December 2013 Trial completion date: estimated April 2022 |
| Contact information | Responsible party/principal investigator: Thomas Schricker, McGill University Health Centre/Research Institute of the McGill University Health Centre |
| Study identifier | NCT number: 02032953 |
| Official title and purpose of study | Enhancing the anabolic effect of perioperative nutrition with insulin while maintaining normoglycemia |
| Notes | — |
NCT04742023.
| Study name | Post‐operative complications and graft survival with conventional versus continuous glucose monitoring in patients with diabetes mellitus undergoing renal transplantation |
| Methods |
Type of study: interventional Allocation: randomised Type of trial: endpoint classification: efficacy study Intervention model: single group assignment Masking: single (participant) Primary purpose: treatment |
| Participants |
Conditions: diabetes mellitus Gender: both male/ female Age groups: adult/senior Enrolment: estimated 40 |
| Interventions |
Intervention: insulin and continuous glucose monitor application Comparator: insulin and continuous glucose monitor placebo applied |
| Outcomes |
Primary outcome: average daily glucose Secondary outcomes: number of hyperglycaemic episodes, number of hypoglycaemic episodes, total insulin use |
| Starting date |
Trial start date: February 2021 Trial completion date: estimated July 2022 |
| Contact information | Responsible party/principal investigator: Northwell Health |
| Study identifier | NCT04742023 |
| Official title and purpose of study | Post‐operative complications and graft survival with conventional versus continuous glucose monitoring in patients with diabetes mellitus undergoing renal transplantation |
| Notes | — |
C: control group; CDC: Centers for Disease Control and Prevention; I: intervention group; ICU: intensive care unit; SOFA: sequential organ failure assessment
Differences between protocol and review
In this review, we made changes to the outcomes:
We included the secondary outcome all‐cause mortality in our primary outcomes.
The original primary outcome microvascular complications was not assessed.
The secondary outcome length of hospital and length of ICU stay were split into two separate outcomes.
We considered the primary outcomes hypoglycaemic episodes and severe hypoglycaemic episodes as two different outcomes.
For this update, we made the following changes regarding the outcomes included in Table 1, in order to show those more important for decision‐making:
We maintained the primary outcomes.
We divided length of stay to reflect differentiated considerations regarding certainty of evidence for the stay at the ICU and the hospital.
We omitted health‐related quality of life because the data informing this outcome were anecdotal.
We changed the title of the review from "Perioperative glycaemic control for diabetic patients undergoing surgery" to "Perioperative glycaemic control for people with diabetes undergoing surgery" as 'people with diabetes' is currently a preferred term.
We considered sensitivity analyses for only published data and in the case of all‐cause mortality additionally for studies with a low risk of bias only.
We conducted no subgroup analyses.
Contributions of authors
All review authors revised and approved the final review update draft.
Filip Bellon (FB): searching for trials, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Ivan Solá (IS): protocol development, quality assessment, data interpretation.
Gabriel Gimenez (GG): searching for trials, trial selection, data interpretation.
Marta Hernández (MH): searching for trials, trial selection.
Maria‐Inti Metzendorf: searching for trials.
Esther Rubinat (ER): searching for trials, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Dídac Mauricio (DM): searching for trials, acquisition of trial reports, trial selection, data extraction, data interpretation, review of drafts and future review updates.
Sources of support
Internal sources
-
Iberoamerican Cochrane Center, Spain
The Iberoamerican Cochrane Center provided methodological support and editorial liaison for the different versions of the review.
External sources
-
Agencia d'Avaluacio de Tecnologia i Recerca Mediques, Departament de Salut, Generalitat de Catalunya, Spain
This review was financially supported by a grant from Agencia d'Avaluacio de Tecnologia i Recerca Mediques, Departament de Salut, Generalitat de Catalunyaa (Project 278/01/2008)
-
Agencia de Calidad del Sistema Nacional de Salud, Ministerio de Sanidad y Consumo, Spain
The original version of the review received partial funding from the Agencia de Calidad del Sistema Nacional de Salud, Ministerio de Sanidad y Consumo
-
University of Lleida, Jade Plus and La Caixa Bank Foundation, Spain
FB was funded by the predoctoral staff in training programme University of Lleida, Jade Plus and La Caixa Bank Foundation 2019
Declarations of interest
The authors declare that they do not have any conflicts of interest.
These authors contributed equally to this work and should be considered as having joint senior authorship.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdelmalak 2013 {published and unpublished data}
- Abdelmalak BB, Bonilla, Mascha EJ, Maheshwari A, Tang WH, You MJ, et al. Dexamethasone, light anaesthesia, and tight glucose control(DeLiT) randomized controlled trial. British Journal of Anaesthesia 2013;111(2):209-21. [DOI] [PubMed] [Google Scholar]
- NCT00433251. The effects of corticosteroids, glucose control, and depth-of-anaesthesia on perioperative inflammation and morbidity form major non-cardiac surgery (dexamethasone, light anesthesia and tight glucose control (DeLiT Trial)). clinicaltrials.gov/ct2/show/NCT00433251 (first received 15 October 2009).
- NCT00995501. The effects of coroticosteroids, glucose control, and depth-of-anesthesia on perioperative inflammation and morbidity from major non-cardiac surgery (dexamethasone, light anesthesia and tight glucose control (DeLiT Trial)). clinicaltrials.gov/ct2/show/NCT00995501 (first received 15 October 2009).
Cao 2010 {published data only}
- Cao SG, Ren JA, Shen B, Chen D, Zhou YB, Li JS. Intensive versus conventional insulin in type 2 diabetes patients undergoing D2 gastrectomy for gastric cancer: a randomized controlled trial. World Journal of Surgery 2011;35(1):85-92. [DOI] [PubMed] [Google Scholar]
Chan 2009 {published and unpublished data}
- Chan RP, Galas FR, Hajjar LA, Bello CN, Piccioni MA, Auler JO Jr. Intensive perioperative glucose control does not improve outcomes of patients submitted to open-heart surgery: a randomized controlled trial. Clinics (Sao Paulo) 2009;64(1):51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00370643. Glucose control in open heart surgery. clinicaltrials.gov/ct2/show/NCT00370643 (first received 1 September 2006).
De La Rosa 2008 {published and unpublished data}
- De La Rosa GC, Donado JH, Restrepo AH, Quintero AM, González LG, Saldarriaga NE, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Critical Care 2008;12(5):R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Desai 2012 {published data only}
- Desai SP, Henry LL, Holmes SD, Hunt SL, Martin CT, Hebsur S, et al. Strict versus liberal target range for perioperative glucose in patients undergoing coronary artery bypass grafting: a prospective randomized controlled trial. Journal of Thoracic and Cardiovascular Surgery 2012;143(2):318-25. [DOI] [PubMed] [Google Scholar]
- Pezzella AT, Holmes SD, Pritchard G, Speir AM, Ad N. Impact of perioperative glycemic control strategy on patient survival after coronary bypass surgery. Annals of Thoracic Surgery 2014;98:1281-5. [DOI: 10.1016/j.athoracsur.2014.05.067] [PMID: ] [DOI] [PubMed] [Google Scholar]
Duncan 2018 {published and unpublished data}
- Duncan AE, Daniel I, Sessler MD, Hiroaki S, Sato T, Keisuke N, et al. Hyperinsulinemic normoglycemia during cardiac surgery reduces a composite of 30-day mortality and serious in-hospital complications. A randomized clinical trial. Anesthesiology 2018;128:1125-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00524472. Outcomes study of hyperinsulinemic glucose control in cardiac surgery. clinicaltrials.gov/ct2/show/NCT00524472 (first received 3 September 2007).
- Saager L, Duncan AE, Yared JP, Hesler BD, You J, Deogaonkar A, et al. Intraoperative tight glucose control using hyperinsulinemic normoglycemia increases delirium after cardiac surgery. Anesthesiology 2015;122(6):1214-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schricker T, Sato H, Beaudry T, Codere T, Hatzakorzian R, Pruessner JC. Intraoperative maintenance of normoglycemia with insulin and glucose preserves verbal learning after cardiac surgery. PloS One 2014;9(6):e99661. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandhi 2007 {published data only}
- Gandhi GY, Nuttall GA, Abel M, Mullany CJ, Schaff HV, O'Brien PC, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery. Annals of Internal Medicine 2007;146(4):233-43. [DOI] [PubMed] [Google Scholar]
- NCT00282698. Outcomes with tight control of hyperglycemia in cardiac surgery patients. clinicaltrials.gov/ct2/show/NCT00282698 (first received 27 January 2006).
Glucontrol 2009 {published and unpublished data}
- NCT00107601. Glucontrol study: comparing the effects of two glucose control regimens by insulin in intensive care unit patients. clinicaltrials.gov/ct2/show/NCT00107601 (first received 6 April 2005).
- Penning S, Chase JG, Preiser JC, Pretty CG, Signal M, Melot C, et al. Does the achievement of an intermediate glycemic target reduce organ failure and mortality? a post hoc analysis of the glucontrol trial. Journal of Critical Care 2014;29(3):374-9. [DOI: 10.1016/j.jcrc.2014.01.013] [DOI] [PubMed] [Google Scholar]
- Preiser J-C, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Medicine 2009;35(10):1738-48. [DOI] [PubMed] [Google Scholar]
Hermayer 2012 {published data only}
- Hermayer KL, Egidi MF, Finch NJ, Baliga P, Lin A, Kettinger L, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. Journal of Clinical Endocrinology and Metabolism 2012;97:4399–406. [DOI] [PubMed] [Google Scholar]
Lazar 2004 {published data only (unpublished sought but not used)}
- Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004;109(12):1497-502. [DOI] [PubMed] [Google Scholar]
Lazar 2011 {published data only}
- Lazar HL, McDonnell MM, Chipkin S, Fitzgerald C, Bliss C, Cabral H. Effects of aggressive versus moderate glycemic control on clinical outcomes in diabetic coronary artery bypass graft patients. Annals of Surgery 2011;254(3):458-64. [NCT00460499] [DOI] [PubMed]
- NCT00460499. Improving outcomes in diabetic patients during CABG surgery by omptimizing glycemic control. clinicaltrials.gov/ct2/show/NCT00460499 (first received 16 April 2007).
Li 2006 {published data only (unpublished sought but not used)}
- Li JY, Sun S, Wu SJ. Continuous insulin infusion improves postoperative glucose control in patients with diabetes mellitus undergoing coronary artery bypass surgery. Texas Heart Institute Journal 2006;33(4):445-51. [PMC free article] [PubMed] [Google Scholar]
NICE SUGAR 2009 {published and unpublished data}
- Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al, NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. New England Journal of Medicine 2009;360(13):1283-97. [DOI] [PubMed] [Google Scholar]
- NCT00220987. Normoglycaemia in intensive care evaluation and survival using glucose algorithm regulation (NICE-SUGAR STUDY). clinicaltrials.gov/ct2/show/NCT00220987 (first received 22 September 2005).
Parekh 2016 {published data only}
- NCT01643382. Hyperglycemia in renal transplantation (HiRT). clinicaltrials.gov/ct2/show/NCT01643382 (first received 18 July 2012).
- Parekh J, Roll GR, Wisel S, Rushakoff R, Hirose R. EEffect of moderately intense perioperative glucose control on renal allograft function: a pilot randomized controlled trial in renal transplantation. Clinical Transplantation 2016;30:1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rassias 1999 {published data only}
- Rassias AJ, Marrin CAS, Arruda J, Whalen PK, Beach M, Yeager MP. Insulin infusion improves neutrophil function in diabetic cardiac surgery patients. Anesthesia and Analgesia 1999;88(5):1011-6. [DOI] [PubMed] [Google Scholar]
Subramaniam 2009 {published and unpublished data}
- Subramaniam B, Panzica PJ, Novack V, Mahmood F, Matyal R, Mitchell JD, et al. Continuous perioperative insulin infusion decreases major cardiovascular events in patients undergoing vascular surgery: a prospective, randomized trial. Anesthesiology 2009;110(5):970-7. [DOI] [PubMed] [Google Scholar]
Umpierrez 2015 {published and unpublished data}
- NCT01361594. Intensive insulin therapy in patients undergoing coronary artery bypass surgery (CABG). clinicaltrials.gov/ct2/show/NCT01361594 (first received 31 December 2014).
- Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary arterybypass graft surgery: GLUCO-CABG trial. Diabetes Care 2015;38(9):1665–72. [DOI: 10.2337/dc15-0303] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wahby 2016 {published data only}
- Wahby EA, Abo Elnasr MM, Eissa MI, Mahmoud SM. Perioperative glycemic control in diabetic patients undergoingcoronary artery bypass graft surgery. Journal of the Egyptian Society of Cardio-Thoracic Surgery 2016;24:143-9. [DOI: ] [Google Scholar]
Wallia 2017 {published and unpublished data}
- NCT01211730. Study of glycemic control on liver transplantation outcomes. clinicaltrials.gov/ct2/show/NCT01211730 (first received 29 September 2010).
- Wallia A, Schmidt K, Oakes DJ, Pollack T, Welsh N, Kling-Colson S, et al. Glycemic control reduces infections in post–liver transplant patients: results of a prospective, randomized study. Journal of clinical Endocrinology and Metabolism 2017;102:451–9. [DOI: 10.1210/jc.2016-3279] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuan 2015 {published data only}
- Yuan J, Liu T, Zhang X, Si Y, Ye Y, Zhao C, et al. Intensive versus conventional glycemic control in patients with diabetes during enteral nutrition after gastrectomy. Journal of Gastrointestinal Surgery 2015;19:1553-8. [DOI: 10.1007/s11605-015-2871-7] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelmalak 2019 {published and unpublished data}
- Abdelmalak BB, You J, Kurz A, Kot M, Bralliar T, Remzi FH, et al. The effects of dexamethasone, light anesthesia, and tight glucose control on postoperative fatigue and quality of life after major noncardiac surgery: a randomized trial. Journal of Clinical Anesthesia 2019;55:83-91. [DOI: ] [DOI] [PubMed] [Google Scholar]
Agus 2014 {published and unpublished data}
- NCT00443599. SPECS: safe pediatric euglycemia in cardiac surgery (SPECS). clinicaltrials.gov/ct2/show/results/NCT00443599 (first received 6 March 2007).
Agus 2017 {published and unpublished data}
- Agus MS, Wypij D, Hirshberg EL, Srinivasan V, Faustino EV, Luckett PM, et al, HALF-PINT Study Investigators and the PALISI Network*. Tight glycemic control in critically Ill children. New England Journal of Medicine 2017;376(8):729-41. [DOI: 10.1056/NEJMoa1612348] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01565941. Heart and lung failure - pediatric insulin titration trial (HALF-PINT). clinicaltrials.gov/ct2/show/NCT01565941 (first received 29 March 2012).
Asida 2013 {published data only}
- Asida SM, Atalla MMM, Gad GS, Aisa KM, Mohamed Hs. Effect of perioperative control of blood glucose level patient’s outcome after anesthesia for cardiac surgery. Egyptian Journal of Anaesthesia 2013;29:71-6. [Google Scholar]
Cao 2013 {published data only}
- Cao Y, Yang T, Yu S, Sun G, Gu C, Yi D. Relationships of adiponectin with markers of systemic inflammation and insulin resistance in infants undergoing open cardiac surgery. Mediators of Inflammation 2013;2013:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuah 2015 {published data only}
- Chuah LL, Miras AD, Papamargaritis D, Jackson SN, Olbers T, le Roux CW. Impact of perioperative management of glycemia in severely obese diabetic patients undergoing gastric bypass surgery. Surgery for Obesity and Related Diseases 2015;11(3):578-84. [DOI] [PubMed] [Google Scholar]
Cinotti 2014 {published data only}
- Cinotti R, Ichai C, Orban JC, Kalfon P, Feuillet F, Roquilly A, et al. Effects of tight computerized glucose control on neurological outcome in severely brain injured patients: a multicenter sub-group analysis of the randomized-controlled open-label CGAO-REA study. Critical Care (London, England) 2014;18(5):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duncan 2015 {published data only}
- Duncan AE, Kateby Kashy B, Sarwar S, Singh A, Stenina-Adognravi O, Christoffersen S, et al. Hyperinsulinemic normoglycemia does not meaningfully improve myocardial performance during cardiac surgery: a randomized trial. Anesthesiology 2015;123(2):272-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellenberger 2018 {published data only}
- Ellenberger C, Sologashvili T, Kreienbuhl L, Cikirikcioglu M, Diaper J, Licker M. Myocardial protection by glucose-insulin-potassium in moderate- to high-risk patients undergoing elective on-pump cardiac surgery: a randomized controlled trial. Anesthesia and Analgesia 2018;126(4):1133-41. [DOI] [PubMed] [Google Scholar]
Giakoumidakis 2013 {published data only}
- Giakoumidakis K, Eltheni R, Patelarou E, Theologou S, Patris V, Michopanou N, et al. Effects of intensive glycemic control on outcomes of cardiac surgery. Heart and Lung 2013;42(2):146-51. [DOI] [PubMed] [Google Scholar]
Gupta 2020 {published data only}
- Gupta R, Bajwa SJS, Abraham J, Kurdi M. The efficacy of intensive versus conventional insulin therapy in reducing mortality and morbidity in medical and surgical critically ill patients: A randomized controlled study. Anesthesia Essays and Researches 2020;14(2):295-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2012 {published data only}
- Hsu CW, Sun SF, Lin SL, Huang HH, Wong KF. Moderate glucose control results in less negative nitrogen balances in medical intensive care unit patients: a randomized, controlled study. Critical Care (London, England) 2012;16(2):R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang QX, Lou FC, Wang P, , Liu Q, Wang K, Zhang L, et al. Basal insulin therapy strategy is superior to premixed insulin therapy in the perioperative period blood glucose management. Chinese Medical Journal 2013;126(21):4030-6. [PubMed] [Google Scholar]
Kalfon 2014 {published data only}
- Kalfon P, Giraudeau B, Ichai C, Guerrini A, Brechot N, Cinotti R, et al, Group, Cgao-Rea Study. Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intensive Care Medicine 2014;40(1):171-81. [DOI] [PubMed] [Google Scholar]
Krishna 2019 {published data only}
- Parthasarathy Arun, Handattu Mahabalesware Krishna. Comparative study between insulin bolus regimen and glucose insulin infusion regimen on effectiveness of intraoperative blood glucose control in patients with type 2 diabetes mellitus undergoing non-cardiac surgery. Journal of Anaesthesiology 2019;27(2):110-14. [DOI: ] [Google Scholar]
Kumar 2020 {published and unpublished data}
- Kumar SS, Pelletier SJ, Shanks A, Thompson A, Sonnenday CJ, Picton P. Intraoperative glycemic control in patients undergoing Orthotopic liver transplant: a single center prospective randomized study. BMC Anesthesiology 2020;20(3):1-11. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurnaz 2017 {published data only}
- Kurnaz P, Sungur Z, Camci E, Sivrikoz N, Orhun G, Senturk M, et al. The effect of two different glycemic management protocols on postoperative cognitive dysfunction in coronary artery bypass surgery. Revista Brasileira de Anestesiologia 2017;67(3):258-65. [DOI] [PubMed] [Google Scholar]
Laiq 2015 {published data only}
- Laiq N, Khan S, Ahmed H, Aslam S, Khan RA. The effects of glycaemic control in cardiac patients undergoing CABG surgery. Journal of Medical Sciences (Peshawar, Print) 2015;23(1):21-5. [Google Scholar]
LÜ 2019 {published data only}
- Lü S, Huang X, Mao J, Li Lifen, Zhao Z. Application of intensive blood glucose management in HCC patients associated with diabetes during perioperative period of radiofrequency ablation. Journal of Interventional Radiology 2019;28:582-5. [DOI: 10.3969 / j.issn.1008-794X.2019.06.018] [Google Scholar]
Makino 2019 {published data only}
- Makino H, Tanaka A, Asakura K, Koezuka R, Tochiya M, Ohata Y, et al. Addition of low-dose liraglutide to insulin therapy is useful for glycaemic control during the peri-operative period: effect of glucagon-like peptide-1 receptor agonist therapy on glycaemic control in patients undergoing cardiac surgery (GLOLIA study). Diabetic Medicine 2019;36(12):1621-8. [DOI: 10.1111/dme.14084] [DOI] [PubMed] [Google Scholar]
Mikaeili 2012 {published data only}
- Mikaeili H, Yazdchi M, Barazandeh F, Ansarin K. Euglycemic state reduces the incidence of critical illness polyneuropathy and duration of ventilator dependency in medical intensive care unit. Bratislavske Lekarske Listy 2012;113(10):616-9. [DOI] [PubMed] [Google Scholar]
Mohod 2019 {published and unpublished data}
- Mohod V, Ganeriwal V, Bhange J. Comparison of intensive insulin therapy and conventional glucose management in patients undergoing coronary artery bypass grafting. Journal of Anesthesiology, Clinical Pharmacology 2019;35(4):493-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mularski 2012 {published data only}
- Mularski KS, Yeh CP, Bains JK, Mosen DM, Hill AK, Mularski R A. Pharmacist glycemic control team improves quality of glycemic control in surgical patients with perioperative dysglycemia. Permanente Journal 2012;16(1):28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00394303 {published data only}
- NCT00394303. Tight intra-operative glucose control during coronary artery bypass surgery. http://clinicaltrials.gov/show/NCT00394303 (accessed 30 July 2012).
NCT00487162 {published data only}
- NCT00487162. The association between peri-operative hyperglycemia and major morbidity and mortality. http://clinicaltrials.gov/show/NCT00487162 (accessed 30 July 2012).
NCT03526536 {published data only}
- NCT03526536. Pilot: Insulin sensitivity/management in hyperglycemic patients in perioperative period ESRD/Non-ESRD. https://clinicaltrials.gov/ct2/show/NCT03526536 (first received 16 May 2018). [CLINICALTRIALS.GOV: NCT03526536]
Okabayashi 2014 {published data only}
- Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Tokumaru T, Liyama T, et al. Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care 2014;37(6):1516-24. [DOI] [PubMed] [Google Scholar]
Pasquel 2020 {published data only}
- Pasquel FJ, Lansang MC, Khowaja A, Urrutia MA, Cardona S, Albury B, et al. A randomized controlled trial comparing glargine U300 and glargine U100 for the inpatient management of medicine and surgery patients with type 2 diabetes: Glargine U300 Hospital Trial. Diabetes Care 2020;43:1242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pezzella 2014 {published data only}
- A Thomas Pezzella, Sari D Holmes, Graciela Pritchard, Alan M Speir, Niv Ad. Impact of Perioperative Glycemic Control Strategy on Patient Survival After Coronary Bypass Surgery [Impact of Perioperative Glycemic Control Strategy on Patient Survival After Coronary Bypass Surgery]. The Annals of Thoracic Surgery May 22 2014;98:1281-1285. [DOI: 10.1016/j.athoracsur.2014.05.067] [PMID: ] [DOI] [PubMed] [Google Scholar]
Polderman 2017 {published data only}
- Polderman JA, Ma XL, Eshuis WJ, Hollmann MW, DeVries JH, Preckel B, et al. Efficacy of continious intravenous glucose monitoring in perioperative glycaemic control: a randomized controlled study. British Journal of Anaesthesia 2017;118(2):264-75. [DOI] [PubMed] [Google Scholar]
Punke 2014 {published data only}
- Punke MA, Goepfert MS, Kluge S, Reichenspurner H, Goetz AE, Reuter DA. Perioperative glycemic control with a computerized algorithm versus conventional glycemic control in cardiac surgical patients undergoing cardiopulmonary bypass with blood cardioplegia. Journal of Cardiothoracic and Cascular Anesthesia 2014;28(5):1273-7. [DOI] [PubMed] [Google Scholar]
Qu 2012 {published data only}
- Qu CZ, Zhang RY, Xiang K F. Blood glucose control in the perioperative stage of cardiac value replacement influences levels of blood lactic acid. Chinese Journal of Tissue Engineering Research 2012;16(53):9955-9. [Google Scholar]
Ramírez‐Cáceres 2019 {published data only}
- César Ramírez-Cáceres, Estrella Uzcátegui Paz, Ricardo Lozano Hérnandez. Protocol for fast postoperative improvement (FPOI) in gastrointestinal surgery [Protocolo de rapida mejoria posoperatoria ( RAMPO) en cirugía gastrointestinal ]. cirugia y cirujanos 2018;87:151-7. [DOI] [PubMed] [Google Scholar]
Rujirojindakul 2014 {published data only}
- Rujirojindakul P, Liabsuetrakul T, McNeil E, Chanchayanon T, Wasinwong W, Oofuvong M, et al. Safety and efficacy of intensive intraoperative glycaemic control in cardiopulmonary bypass surgery: a randomised trial. Acta Anaesthesiologica Scandinavica 2014;58(5):588-96. [DOI] [PubMed] [Google Scholar]
Santana‐Santos 2019 {published and unpublished data}
- Eduesley Santana-Santos, Patricia Hatanaka Kanke, Rita de Cássia Almeida Vieira, Larissa Bertacchini de Oliveira, Renata Eloah de Lucena Ferretti-Rebustin, et al. Impact of intensive glycemic control on acute renal injury: a randomized clinical trial. Acta Paul Enferm 2019;32(6):592-9. [DOI: ] [Google Scholar]
Schroeder 2012 {published data only}
- Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A, et al. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes/Metabolism Research and Reviews 2012;28(1):71-5. [DOI] [PubMed] [Google Scholar]
Tohya 2018 {published data only}
- Tohya A, Kohjitani A, Ohno S, Yamashita K, , Manabe Y, Sugimura M. Effects of glucose-insulin infusion during major oral and maxillofacial surgery on postoperative complications and outcomes. JA Clinical Reports 2018;4(1):9. [DOI: 10.1186/s40981-018-0148-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang Y, Li JP, Song YL, Zhao QH. Intensive insulin therapy for preventing postoperative infection in patients with traumatic brain injury: a randomized controlled trial. Medicine 2017;96(13):e6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welsh 2016 {published data only}
- Welsh N, Derby T, Gupta S, Fulkerson C, Oakes DJ, Schmidt K, et al. Inpatient hypoglycemic events in a comparative effectiveness trial for glycemic control in a high-risk population. Endocrine Practice 2016;22(9):1040-7. [DOI: 10.4158/EP151166.OR] [DOI] [PubMed] [Google Scholar]
Xue 2018 {published data only}
- Xue B, Ruan S, Xie P, Yan K, Gu Z, Meng N, et al. Prospective analysis of glycemic variability in patients with severe traumatic brain injury: modified Leuven's adjustment process versus conventional adjustment process. J Int Med Res 2018;46(4):1505-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hweidi 2021 {published data only}
- Hweidi IM, Zytoon AM, Hayajneh AA, Al Obeisat SM, Hweidi AI. The effect of intraoperative glycemic control on surgical site infections among diabetic patients undergoing coronary artery bypass graft (CABG) surgery. Heliyon 2021;7(12):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imran‐ul‐hassan 2021 {published data only (unpublished sought but not used)}
- Syed Imran-ul-hassan, Sharyar, Muhammad Rashid, Maryam Liaqat, Javairia Saleem, Taiba Zulfiqar1. Outcome of strict peri-operative glycemic control in diabetic patients following open heart surgery. Med. Forum 2021;32(7):43-7. [Google Scholar]
NCT00899483 {published data only}
- NCT00899483. Can enhanced glycaemic control in type II diabetics improve myocardial protection during coronary artery bypass grafting?. https://clinicaltrials.gov/ct2/show/NCT00899483 (first received 12 May 2009). [CLINICALTRIAL.GOV: NCT00899483]
NCT01528189 {published data only}
NCT02746432 {published data only}
NCT03314272 {published data only}
NCT03474666 {published data only}
- NCT03474666. Glycemic control and surgical site infection incidence among liver transplantation recipients. https://clinicaltrials.gov/ct2/show/NCT03474666 (first received 22 March 2018). [CLINICALTRIALS.GOV: NCT03474666]
Zadeh 2016 {published data only}
- Zadeh FJ, Azemati S. Adjusted tight control blood glucose management in diabetic patients undergoing on pump coronary artery bypass graft. A randomized clinical trial. Journal of Diabetes & Metabolic Disorders 2020;1:1-8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh FJ, Ghazi Nour M. A study on the outcomes of modified tight glucose control for the management of glycemic control in diabetic patients undergoing cardiac surgery. Journal of Pharmacy Research 2016;10(11):764-70. [Google Scholar]
References to ongoing studies
NCT02032953 {published data only}
- NCT02032953. Enhancing the anabolic effect of perioperative nutrition with insulin while maintaining normoglycemia. https://clinicaltrials.gov/ct2/show/NCT02032953 (first received 10 January 2014).
NCT04742023 {published data only}
- NCT04742023. Post-operative complications and graft survival with conventional versus continuous glucose monitoring in patients with diabetes mellitus undergoing renal transplantation. https://clinicaltrials.gov/ct2/show/NCT04742023 (first received 5 February 2021).
Additional references
Abdelmalak 2011
- Abdelmalak B, Maheshwari A, Kovaci B, Marscha EJ, Cywinski JB, Kurz A, et al. Validation of the DeLIT Trial intravenous insulin infusion algorithm for intraoperative glucose control in noncardiac surgery: a randomized controlled trial.. Canadian Journal of Anaesthesia 2011;58(7):606-16.. [DOI: 10.1007/s12630-011-9509-3] [DOI] [PubMed] [Google Scholar]
ADA 2003
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5-20. [DOI] [PubMed] [Google Scholar]
ADA 2010
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 2021
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 1 January 2021;44 (supplement(Supplement_1):15-33. [DOI: ] [Google Scholar]
AHRQ 2001
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. AHRQ Publication No. 01-E058 edition. Rockville, MD: Agency for Healthcare Research and Quality, 2001. [PMC free article] [PubMed] [Google Scholar]
Albacker 2008
- Albacker T, Carvalho G, Schricker T, Lachapelle K. High-dose insulin therapy attenuates systemic inflammatory response in coronary artery bypass graTing patients.. Annals of Thoracic Surgery 2008;86(1):20-7. [DOI: 10.1016/j.athoracsur.2008.03.046] [DOI] [PubMed] [Google Scholar]
Alberti 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539-53. [DOI] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arabi 2008
- Yaseen M Arabi, Ousama C Dabbagh, Hani M Tamim, Abdullah A Al-Shimemeri, Ziad A Memish, Samir H Haddad, Sofia J Syed, Hema R Giridhar, Asgar H Rishu, Mouhamad O Al-Daker, Salim H Kahoul, Riette J Britts, Maram H Sakkijha. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Critical Care Medicine December 2008;36(12):3190-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bell 2013
- Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: variance components and intra-cluster correlation of commonly used psychosocial measures. Psycho-oncology 2013;22:1738-47. [DOI] [PubMed] [Google Scholar]
Bilotta 2009
- Bilotta F, Caramia R, Paoloni FP, Delfini R, Rosa G. Safety and efficacy of intensive insulin therapy in critical neurosurgical patients.. Anesthesiology 2009;110(3):611-9. [DOI: 10.1097/ALN.0b013e318198004b] [DOI] [PubMed] [Google Scholar]
Boutron 2014
- Boutron I, Altman DG, Hopewell S, Vera-Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32(36):4120-6. [DOI] [PubMed] [Google Scholar]
Buch 2011
- Buch MH, Aletaha D, Emery P, Smolen JS. Reporting of long-term extension studies: lack of consistency calls for consensus. Annals of the Rheumatic Diseases 2011;70(6):886-90. [DOI] [PubMed] [Google Scholar]
Cardona 2017
- Saumeth Cardona, Francisco J pasquel, Maya Fayman, Limin Peng, Sol Jacobs, Priyathama Vellanki, Jef Weaver, Michael Halkos, Robert A Guyton, Vinod H Thouorani, Guillermo E Umpierrez. Hospitalization costs and clinical outcomes in CABG patients treated with intensive insulin therapy. Journal of Diabetes and its Complications 20 January 2017;31:742-747. [DOI: ] [DOI] [PubMed] [Google Scholar]
Carvalho 2011
- George Carvalho, Patricia Pelletier, Turki Albacker, Kevin Lachapelle, Denis R Joanisse, Roupen Hatzakorzian, Ralph Lattermann, Hiroaki Sato, André Marette, Thomas Schricker. Cardioprotective effects of glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing coronary artery bypass grafting. The Journal of Clinical Endocrinology & Metabolism May 2011;96(5):1469-77. [DOI: 10.1210/jc.2010-1934.] [DOI] [PubMed] [Google Scholar]
Cochrane 2022
- Cochrane. How CENTRAL is created. www.cochranelibrary.com/central/central-creation (accessed 17 June 2022).
CONSORT 2016
- Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285(15):1987-1991. [DOI] [PubMed] [Google Scholar]
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5(1):79-85. [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. In: Deeks JJ, Higgins JPT, Altman DG, editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. [Google Scholar]
Drayton 2022
- Drayton DJ, Birch RJ, D'Souza-Ferrer C, Ayres M, Howell SJ, Ajjan RA. Diabetes mellitus and perioperative outcomes: a scoping review of the literature. British Journal of Anaesthesia May 2022;128(5):817-828. [DOI: doi: 10.1016/j.bja.2022.02.013] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furnary 1999
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Annals of Thoracic Surgery 1999;67(2):352-60; discussion 360-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gandhi 2005
- Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clinic Proceedings 2005;80(7):862-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Golden 1999
- Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999;22(9):1408-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool. Hamilton (ON): McMaster University and Evidence Prime,, 2015. gradepro.org..
He 2007
- He W, Zhang TY, Zhou H, Li T, Zhao JY, Zhao D, et al. Impact of intensive insulin therapy on surgical critically ill patients. Chinese Journal of Surgery 2007;45(15):1052-4. [PMID: ] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect.. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. [Google Scholar]
Higgins 2019b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. . In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hoffmann 2017
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ 2017;358:j2998. [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
International Hypoglycaemia Study Group 2019
- International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinology May 2019;7(5):385-296. [DOI: 10.1016/S2213-8587(18)30315-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jin 2020
- Jin X, Wang J, Ma Y, Li X, An P, Wang J, Mao W, Mu Y, Chen Y, Chen K. Association between Perioperative Glycemic control strategy and mortality in patients with diabetes undergoing cardiac surgery: A Systematic review and Meta-Analysis. Front Endocrinol December 2020;17:513-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2015
- Jones CW, Keil LG, Holland WC, Caughey MC, Platts-Mills TF. Comparison of registered and published outcomes in randomized controlled trials: a systematic review. BMC Medicine 2015;13:282. [DOI: 10.1186/s12916-015-0520-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2018
- Kang ZQ, Huo JL, Zhai XJ. Effects of perioperative tight glycaemic control on postoperative outcomes: a meta-analysis. Endocr Connect. 1 December 2018;12:316-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirdemir 2008
- Kirdemir P, Yildirim V, Kiris I, Gulmen S, Kuralay E, Ibrisim E, et al. Does continuous insulin therapy reduce postoperative supraventricular tachycardia incidence after coronary artery bypass operations in diabetic patients. Journal of Cardiothorac Vascular Anesthesia 2008;22:383-387. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al, Cochrane Information Retrieval Methods Group. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Res Synth Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977-84. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Megan 2012
- Megan B, Pickering RM, Weatherall M. Design, objectives, execution and reporting of published open-label extension studies. Journal of Evaluation in Clinical Practice 2012;18(2):209-15. [DOI] [PubMed] [Google Scholar]
Mitchell 2006
- Imogen Mitchell, Emma Knight, Jelena Gissane, Rohit Tamhane, Rao Kolli, I Anne Leditschke, Rinaldo Bellomo, Simon Finfer, Australian and New Zealand Intensive Care Society Clinical Trials Group. A phase II randomised controlled trial of intensive insulin therapy in general intensive care patients. Critical Care Resuscitation December 2006 ;8(4):289-93. [PMID: ] [PubMed] [Google Scholar]
Page 2020
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews [The PRISMA 2020 statement: an updated guideline for reporting systematic reviews]. BMJ 2021;372(71):1-9. [DOI: doi: 10.1136/bmj.n71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pomposelli 1998
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. Journal of Parenteral and Enteral Nutrition 1998;22(2):77-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Sathya 2013
- Sathya B, Davis R, Taveira T, Whitlatch H, Wu WC. Intensity of peri-operative glycaemic control and postoperative outcomes in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract October 2013;102:8-15. [DOI] [PubMed] [Google Scholar]
Scherer 2007
- Scherer RW, Langenberg P, von EE. Full publication of results initially presented in abstracts. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: MR000005. [DOI: 10.1002/14651858.MR000005.pub3] [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. . In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0 . Cochrane, 2019. [Google Scholar]
Seaquist 2013
- Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: a report of a workgroup of the Diabetes Association and the Endocrine Society. Diabetes Care May 2013;36(5):1384-1395. [DOI: 10.2337/dc12-2480] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smiley 2006
- Smiley DD, Umpierrez GE. Perioperative glucose control in the diabetic or nondiabetic patient. Southern Medical Journal 2006;99(6):580-9; quiz 590-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Van den Berghe 2001
- Greet Van den Berghe, Pieter Wouters, Frank Weekers, Charles Verwaest, Frans Bruyninckx, Miet Schetz, Dirk Vlasselaers, Patrick Ferdinande, Peter Lauwers, Roger Bouillon. Intensive Insulin Therapy in Critically Ill Patients. N Engl J Med 8 November 2001 ;345:1359-1367. [DOI: 10.1056/NEJMoa011300] [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang QG, Lu LF, Zhou YB, Cao SG, Wang DS, Lv L. Effects of intensive insulin therapy on insulin resistance and serum proteins aTer radical gastrectomy.. Chinese Journal of Gastrointestinal Surgery 2008;11(5):444-7. [PMID: ] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. In: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization, 1999:1-59. [Google Scholar]
WHO 2021
- World Health Organization. Improving diabetes outcomes for all, a hundred years on from the discovery of insulin: report of the Global Diabetes Summit. Vol. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization , 2021. [LICENCE: CC BY-NC-SA 3.0 IGO.] [Google Scholar]
Wong 2006
- Wong SSL, Wilczynski NL, Haynes RB. Comparison of top-performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and EMBASE. J Med Libr Assoc 2006;94(4):451-5. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336(7644):601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Buchleitner 2012
- Buchleitner AM, Martínez-Alonso M, Hernández M, Solà I, Mauricio D. Perioperative glycaemic control for diabetic patients undergoing surgery. Cochrane Database of Systematic Reviews 29 February 2012, Issue 9. Art. No: CD007315. [DOI: 10.1002/14651858.CD007315.pub2] [DOI] [PubMed] [Google Scholar]


