Abstract
Background
Nephrolithiasis is a common urological disease worldwide. Extracorporeal shock wave lithotripsy (ESWL) has been used for the treatment of renal stones since the 1980s, while retrograde intrarenal surgery (RIRS) and percutaneous nephrolithotomy (PCNL) are newer, more invasive treatment modalities that may have higher stone‐free rates. The complications of RIRS and PCNL have decreased owing to improvement in surgical techniques and instruments. We re‐evaluated the best evidence on this topic in an update of a Cochrane Review first published in 2014.
Objectives
To assess the effects of extracorporeal shock wave lithotripsy compared with percutaneous nephrolithotomy or retrograde intrarenal surgery for treating kidney stones.
Search methods
We performed a comprehensive search in CENTRAL, MEDLINE, Embase, and ClinicalTrials.gov with no restrictions on language or publication status. The latest search date was 6 December 2022.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐RCTs that compared ESWL with PCNL or RIRS for kidney stone treatment.
Data collection and analysis
Two review authors independently classified studies, extracted data, and assessed risk of bias. Our primary outcomes were treatment success rate at three months (defined as residual fragments smaller than 4 mm, or as defined by the study authors), quality of life (QoL), and complications. Our secondary outcomes were retreatment rate, auxiliary procedures rate, and duration of hospital stay. We performed statistical analyses using a random‐effects model and independently rated the certainty of evidence using the GRADE approach.
Main results
We included 31 trials involving 3361 participants (3060 participants completed follow‐up). Four trials were only available as an abstract. Overall mean age was 46.6 years and overall mean stone size was 13.4 mm. Most participants (93.8%) had kidney stones measuring 20 mm or less, and 68.9% had lower pole stones.
ESWL versus PCNL
ESWL may have a lower three‐month treatment success rate than PCNL (risk ratio [RR] 0.67, 95% confidence interval [CI] 0.57 to 0.79; I2 = 87%; 12 studies, 1303 participants; low‐certainty evidence). This corresponds to 304 fewer participants per 1000 (397 fewer to 194 fewer) reporting treatment success with ESWL. ESWL may have little or no effect on QoL after treatment compared with PCNL (1 study, 78 participants; low‐certainty evidence). ESWL probably leads to fewer complications than PCNL (RR 0.62, 95% CI 0.47 to 0.82; I2 = 18%; 13 studies, 1385 participants; moderate‐certainty evidence). This corresponds to 82 fewer participants per 1000 (115 fewer to 39 fewer) having complications after ESWL.
ESWL versus RIRS
ESWL may have a lower three‐month treatment success rate than RIRS (RR 0.85, 95% CI 0.78 to 0.93; I2 = 63%; 13 studies, 1349 participants; low‐certainty evidence). This corresponds to 127 fewer participants per 1000 (186 fewer to 59 fewer) reporting treatment success with ESWL. We are very uncertain about QoL after treatment; the evidence is based on three studies (214 participants) that we were unable to pool. We are very uncertain about the difference in complication rates between ESWL and RIRS (RR 0.93, 95% CI 0.63 to 1.36; I2 = 32%; 13 studies, 1305 participants; very low‐certainty evidence). This corresponds to nine fewer participants per 1000 (49 fewer to 48 more) having complications after ESWL.
Authors' conclusions
ESWL compared with PCNL may have lower three‐month success rates, may have a similar effect on QoL, and probably leads to fewer complications. ESWL compared with RIRS may have lower three‐month success rates, but the evidence on QoL outcomes and complication rates is very uncertain. These findings should provide valuable information to aid shared decision‐making between clinicians and people with kidney stones who are undecided about these three options.
Keywords: Humans; Middle Aged; Kidney Calculi; Kidney Calculi/surgery; Lithotripsy; Lithotripsy/adverse effects; Lithotripsy/methods; Nephrolithotomy, Percutaneous; Nephrolithotomy, Percutaneous/adverse effects; Retreatment; Treatment Outcome
Plain language summary
Is shock wave treatment better than surgical procedures for removing kidney stones?
What are kidney stones?
Stones can form in the kidneys, bladder, ureters (the tubes that carry urine from the kidney to the bladder), or urethra (the tube through which urine leaves the body) when there is not enough fluid in the urine to dilute the minerals or other substances it contains. If the stones form in the kidneys, we call them kidney stones. People who drink too little water, have a poor diet, are overweight, have certain medical conditions, or use certain medicines are more likely to get kidney stones. Kidney stones can cause pain, kidney infection, and kidney failure (when a kidney cannot work on its own).
How are kidney stones treated?
Treatment of kidney stones includes extracorporeal shock wave lithotripsy (ESWL), percutaneous nephrolithotomy (PCNL), and retrograde intrarenal surgery (RIRS). ESWL uses shock waves from outside the body to break a stone inside the kidney into tiny pieces without cutting the skin. The broken stone fragments are small enough to pass out in the urine. PCNL is a surgical method of removing kidney stones that involves inserting a small tube through the skin to the kidney, breaking up the stones using different instruments (such as laser and ultrasound), and removing the fragments through the tube. RIRS is another surgical method, which involves placing a small viewing tube through the urethra and ureter into the kidney, then crushing or evaporating the stone or grabbing and removing it with small pincers.
What did we want to find out?
We wanted to find out how ESWL compares to PCNL and RIRS in terms of treatment success, quality of life, complications, length of hospital stay, and other outcomes that are important to people with kidney stones.
What did we do?
We only included randomized controlled trials (studies that randomly assign the people taking part to an experimental group or a comparator group) that compared ESWL to PCNL or RIRS. We compared and summarized their results and rated our confidence in the evidence.
What did we find?
We found 31 studies that included 3361 people (1360 people were treated by ESWL, 786 by PCNL, and 925 by RIRS). The biggest study included 649 people and the smallest study included 30 people. The studies were conducted in countries around the world; most were set in Europe (12 studies). Medical device companies funded two of the studies. The average stone size was 13.4 mm.
Main results
Compared with PCNL, ESWL may have lower treatment success; for every 1000 people treated, only 619 treated with ESWL might have no stones after three months, compared to 923 people treated with PCNL. ESWL and PCNL may have similar effects on quality of life: on a scale of 0 to 100, where a meaningful difference is 10 points, people treated with ESWL might score 1.5 points lower than those treated with PCNL. ESLW probably leads to fewer complications than PCNL: for every 1000 people treated, 134 treated with ESWL probably have complications, compared to 216 people treated with PCNL.
Compared with RIRS, ESWL may have lower treatment success: for every 1000 people treated, 721 treated with ESWL might have no stones after three months, compared to 848 people treated with RIRS. We are very uncertain about the effect of ESWL compared to RIRS on quality of life and unwanted effects.
What are the limitations of the evidence?
Depending on the outcome, our confidence in the evidence was moderate to very low. This was mainly because some studies had flawed methods, because there were important differences in results across studies that we could not explain, and because some studies included few people. Therefore, our results are likely to change if further evidence becomes available.
How up to date is this evidence?
This review updates our previous review published in 2014. We included evidence published up to 6 December 2022.
Summary of findings
Summary of findings 1. ESWL compared to PCNL for kidney stones.
| ESWL compared to PCNL for kidney stones | ||||||
| Patient or population: people (>14 years) with kidney stones Setting: single or multicenter, inpatients or outpatients Intervention: ESWL Comparison: PCNL | ||||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | What happens? | |
| Risk with PCNL | Risk difference with ESWL | |||||
|
Treatment success rate at 3 months MCID: 5% absolute difference |
1303 (12 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | RR 0.67 (0.57 to 0.79) | Study population | ESWL may result in a reduction in treatment success rate at 3 months compared with PCNL. | |
| 923 per 1000 | 304 fewer per 1000 (397 fewer to 194 fewer) | |||||
|
Quality of life MCID for SF‐36 overall health score: 10 (scale 0 to 100; higher scores indicate better health status) Follow up: 6 months |
78 (1 RCT) |
⊕⊕⊝⊝ Lowa,d | — | Mean 8.2 points (SD 18.1) | MD 1.50 points lower (9.53 lower to 6.53 higher)e | ESWL may have little or no effect on QoL compared with PCNL. |
|
Complications MCID: 3% absolute difference Follow up: 12 months |
1385 (13 RCTs) | ⊕⊕⊕⊝ Moderatea |
RR 0.62 (0.47 to 0.82) | Study population | ESWL likely results in a reduction in complications compared with PCNL. | |
| 216 per 1000 | 82 fewer per 1000 (115 fewer to 39 fewer) | |||||
|
Retreatment rate MCID: 3% absolute difference Follow up: 12 months |
1174 (10 RCTs) | ⊕⊕⊝⊝ Lowa,b,c |
RR 15.53 (6.62 to 36.39) | Study population | ESWL may result in an increase in retreatment rate compared with PCNL. | |
| 28 per 1000 | 401 more per 1000 (155 more to 967 more) | |||||
|
Auxiliary procedures rate MCID: 3% absolute difference Follow up: 12 months |
1044 (8 RCTs) | ⊕⊕⊕⊝ Moderatea |
RR 4.17 (2.67 to 6.52) | Study population | ESWL likely results in an increase in auxiliary procedures rate compared with PCNL. | |
| 41 per 1000 | 130 more per 1000 (69 more to 227 more) | |||||
|
Duration of hospital stay MCID: 1 day absolute difference Follow up: 3 months |
841 (6 RCTs) | ⊕⊕⊕⊝ Moderatea,c | — | The mean hospital stay for PCNL ranged from 1.9 to 7.4 days | MD 3.36 days fewer (4.74 fewer to 1.98 fewer) | ESWL likely results in reduction in hospital stay compared with PCNL. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ESWL: extracorporeal shock wave lithotripsy; MCID: minimum clinically important difference; MD: mean difference; PCNL: percutaneous nephrolithotomy; QoL: quality of life; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation; SF‐36: 36‐Item Short Form Health Survey. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for study limitations (selection bias and reporting bias). b Downgraded one level for publication bias (funnel plot asymmetry). c We noted a high degree of inconsistency but did not downgrade given its perceived lack of clinical importance. d Downgraded one level for imprecision (confidence interval crosses presumed MCID). e Data from Albala 2001.
Summary of findings 2. ESWL compared to RIRS for kidney stones.
| ESWL compared to RIRS for kidney stones | ||||||
| Patient or population: people (>14 years) with kidney stones Setting: single or multicenter, inpatients or outpatients Intervention: ESWL Comparison: RIRS | ||||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | What happens? | |
| Risk with RIRS | Risk difference with ESWL | |||||
|
Treatment success rate at 3 months MCID: 5% absolute difference |
1349 (13 RCTs) | ⊕⊕⊝⊝ Lowa,b |
RR 0.85 (0.78 to 0.93) | Study population | ESWL may result in a reduction in treatment success rate at 3 months compared with RIRS. | |
| 848 per 1000 | 127 fewer per 1000 (186 fewer to 59 fewer) | |||||
|
Quality of life MCID varies by instrument. Follow up: 6 months |
214 (3 RCTs) |
⊕⊝⊝⊝ Very lowa,d,e | — |
|
Not estimable | The evidence is very uncertain about the effect of ESWL on QoL compared with RIRS. |
|
Complications MCID: 3% absolute difference Follow up: 12 months |
1305 (13 RCTs) | ⊕⊝⊝⊝ Very Lowa,c,d | RR 0.93 (0.63 to 1.36) | Study population | The evidence is very uncertain about the complications of ESWL compared with RIRS. | |
| 133 per 1000 | 9 fewer per 1000 (49 fewer to 48 more) | |||||
|
Retreatment rate MCID: 3% absolute difference Follow up: 12 months |
1030 (9 RCTs) | ⊕⊕⊕⊝ Moderatea |
RR 6.78 (3.82 to 12.04) | Study population | ESWL likely results in an increase in re‐ treatment rate compared with RIRS. | |
| 60 per 1000 | 345 more per 1000 (168 more to 658 more) |
|||||
|
Auxiliary procedures rate MCID: 3% absolute difference Follow up: 12 months |
836 (5 RCTs) | ⊕⊕⊝⊝ Lowa,c |
RR 1.98 (1.14 to 3.47) | Study population | ESWL may result in an increase in auxiliary procedures rate compared with RIRS. | |
| 106 per 1000 | 104 more per 1000 (15 more to 262 more) |
|||||
|
Duration of hospital stay MCID: 1 day of absolute difference Follow up: 3 months |
591 (3 RCTs) | ⊕⊕⊕⊝ Moderatea,f |
— | The mean hospital stay for RIRS ranged from 1.2 days to 3.22 days. | MD 1.69 fewer days (2.36 fewer to 1.02 more) | ESWL likely results in a reduction in hospital stay compared with RIRS. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ESWL: extracorporeal shock wave lithotripsy; MCID: minimum clinically important difference; MD: mean difference; QoL: quality of life; RCT: randomized controlled trial; RIRS: retrograde intrarenal surgery; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for study limitations (selection bias and reporting bias). b Downgraded one level for inconsistency (clinically important, unexplained heterogeneity with I2 > 60%). c Downgraded one level for imprecision (wide confidence interval). d Downgraded one level due to insufficient sample size. e Downgraded one level due to heterogeneity; a different direction of the intervention effects estimates across study. f We noted a high degree of inconsistency but did not downgrade given its perceived lack of clinical importance.
Background
Description of the condition
Urolithiasis (stones in the urinary tract) is a common condition, affecting approximately 2% to 3% of the general population around the world. The global prevalence of urolithiasis has increased since the 1970s (Raheem 2017), and current prevalence estimates vary from 1% to 5% in Asia, 5% to 9% in Europe, and 7% to 13% in North America (Sorokin 2017). The epidemiology of urolithiasis differs according to geographic location, age, sex, and race. It affects two to three times more men than women, and white people have the highest incidence compared with Asians, Hispanics, and African Americans (Pearle 2007). Many medical conditions are considered risk factors for stone formation, including diabetes mellitus, hypertension, obesity, and metabolic syndrome. Kidney stones (nephrolithiasis) can cause pain, hematuria, urinary tract infection, decreased kidney function, and even end‐stage renal disease in serious cases. About 50% of people with previous urinary stones have a recurrence within five years (Fink 2013). The ideal treatment goal is complete stone removal without complications. The three most widely used treatment methods are extracorporeal shock wave lithotripsy (ESWL), percutaneous nephrolithotomy (PCNL), and retrograde intrarenal surgery (RIRS), all of which are minimally invasive. Guidelines from the European Association of Urology (EAU) and the American Urological Association (AUA) recommend these modalities as standard treatments of renal stones (AUA guideline 2016; EAU guideline 2023). The advent of minimally invasive techniques has led to a decrease in open surgical nephrolithotomy since the 1990s.
Description of the intervention
Clinicians have used ESWL since the 1980s to disintegrate stones in any location of the upper urinary tract through high‐energy shock waves. The shock waves are delivered by an external machine called a lithotriptor, which uses an electrohydraulic (spark gap), electromagnetic, or piezoelectric generator. This energy disintegrates the stone into tiny fragments, which can spontaneously pass through the urinary system after treatment. This procedure can be performed under intravenous analgesia or regional anesthesia. Sometimes several consecutive sessions are needed to eliminate all the stones; this will depend on the size, location, and composition of the stone, and on patient contour (Turna 2007; Weld 2007).
Contraindications to ESWL include uncontrolled coagulopathies, uncontrolled urinary tract infection, urinary tract obstruction distal to the stone, uncontrolled hypertension, and pregnancy. Severe skeletal malformations, severe obesity, and aortic or renal artery aneurysms also limit the use of ESWL. The complications of ESWL include 'steinstrasse' (obstruction due to fragments becoming lodged in the ureter); renal, liver, or spleen hematoma; urinary tract infection; and cardiac dysrhythmia (AUA guideline 2016; EAU guideline 2023). Morbid cardiac events and bowel perforation are rare complications (Maker 2004; Zanetti 1999).
Standard PCNL involves surgically removing stones from the kidney through a small incision in the skin. This surgical procedure must be performed under general anesthesia. A small skin incision is made, and a needle is punctured into the kidney at a previously planned location to create a tract under fluoroscopy or ultrasound guidance. A nephroscope is then passed into the kidney through the tract after dilation to 30 Fr. The stones are fragmented by laser, ultrasonic, or electrohydraulic lithotriptor and removed through the nephroscope. Finally, a nephrotomy tube or double J stent is placed to drain fluid from the kidney (Matlaga 2011). The main advantage of PCNL is the higher success rate for larger stones, as it is not dependent on the stone burden or composition. The AUA guidelines recommended PCNL as the first‐line therapy in symptomatic people with a total stone burden greater than 20 mm (AUA guideline 2016). However, compared with ESWL, standard PCNL is more invasive and has higher associated morbidity such as fever, sepsis, blood transfusion, thoracic complications, organ injury, and even death (Seitz 2012). Advances in the field have led to the development of minimally invasive PCNL techniques (i.e. mini PCNL, ultra‐mini PCNL), which use a smaller tract. In general, mini PCNL is performed through a 14 to 20 Fr tract, and ultra‐mini PCNL uses only an 11 to 13 Fr tract (Wright 2016). This has reduced the complications associated with standard PCNL while maintaining the high success rate (EAU guideline 2023); as a result, minimally invasive PCNL is gaining popularity.
In RIRS, a flexible ureteroscope is advanced through the urethra, bladder, and ureter to the kidney under fluoroscopy guidance. This procedure is normally performed under general anesthesia. The stones can be seen through the scope, then treated with a laser lithotriptor (holmium:yttrium aluminum garnet; Ho:YAG) and grasping devices. Stent placement is advisable in people who are at risk of complications such as ureteral trauma, bleeding, ureteral perforation, urinary tract infection, and pregnancy (EAU guideline 2023). The current AUA guidelines recommend RIRS for removal of symptomatic non‐lower pole stones with a total burden of 20 mm or less (AUA guideline 2016). Most complications after RIRS are minor. The incidence of serious complications, ureteral avulsion, and stricture is less than 1% (EAU guideline 2023). However, only people with special expertise in this field should perform the procedure (Matlaga 2011).
How the intervention might work
ESWL produces a high‐energy acoustic shock wave with a very brief time span from an external source and focuses on the stone within the body. After traveling through living tissue without energy loss, this shock wave crushes the stone by strong fragmentation forces at the front and rear surface, erosion and spallation at the rear surface, and cavitation by generating a needlelike water jet from air bubbles on the stone surface (Weiss 2012). The stone fragments are then left to pass out spontaneously in the urine.
In PCNL and RIRS, surgeons can visualize the stone with a scope and break the stone directly with a lithotriptor. After fragmentation, they can remove the tiny stone fragment with grasping devices and view the operative outcome at the end of the procedure. Therefore, the treatment success rate should be better than ESWL.
Why it is important to do this review
At present, ESWL is generally accepted as a minimally invasive treatment option for treating kidney stones smaller than 10 mm in the lower pole and stones measuring 20 mm to 25 mm in other parts. However, some factors limit the use of this procedure, such as stone size, composition, and location. PCNL and RIRS have been widely used since the early 2000s because they achieve a higher stone‐free rate. However, these techniques are more invasive and may require a longer hospital stay after treatment. Better ureteroscopes and high‐powered lasers may have improved the safety outcomes of RIRS. For PCNL, smaller tract sizes, improved lithotriptors, and a shift away from nephrostomy tube placement have reduced morbidity and the duration of hospital stays, while efficacy outcomes remain excellent. In addition, changes have been made to ESWL machines to improve their efficacy. Research has shown than urologists are increasingly opting for the surgical techniques over ESWL (Chung 2019a). Therefore, it is necessary to evaluate the benefits of ESWL for renal stone treatment compared to PCNL (especially minimally invasive PCNL) and RIRS.
There have been many randomized controlled trials (RCTs) and systematic reviews published since the last version of this Cochrane Review in 2014 (Srisubat 2014). However, most systematic reviews have focused only on lower pole stones smaller than 20 mm, and none have adhered to all the methodological standards of Cochrane, including a priori protocol publication, GRADE rating of the evidence, and generation of summary of findings tables.
Objectives
To assess the effects of extracorporeal shock wave lithotripsy compared with percutaneous nephrolithotomy or retrograde intrarenal surgery for treating kidney stones.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs that compared ESWL with PCNL or RIRS, regardless of their publication status or language of publication.
Types of participants
We included studies of people with renal stones of any size and at any location in the kidney. We excluded studies in children (under 14 years) and pregnant women. The management of kidney stones in children and pregnant women may require special measures and is beyond the scope of this review.
We included studies in which only a subset of participants was relevant to this review, provided separate data were available for the subset.
Types of interventions
The experimental intervention was ESWL. The two possible comparator interventions were PCNL (all techniques) and RIRS. Any concomitant interventions had to be the same in the experimental and comparator groups to establish fair comparisons.
We did not evaluate PCNL versus RIRS; another Cochrane Review deals with this comparison (Soderberg 2019).
Types of outcome measures
Measurement of our prespecified outcomes was not an eligibility criterion for this review.
Primary outcomes
Treatment success rate at three months
Quality of life (QoL)
Complications
Secondary outcomes
Retreatment rate
Auxiliary procedures rate
Duration of hospital stay
Method and timing of outcome measurement
-
Treatment success rate at three months
Defined as stone‐free status or clinically insignificant residual fragments (measuring less than 4 mm, or as reported by studies) on X‐ray, ultrasonography, or computed tomography (CT) after last treatment
Minimum clinically important difference (MCID): 5% absolute difference (Oestreich 2020)
-
QoL
Final value or change from baseline, assessed with a validated questionnaire (e.g. 36‐Item or 8‐Item Short Form Health Survey [SF‐36 or SF‐8] or the Wisconsin Stone Quality of Life Questionnaire).
MCID: six points for the mental component score (MCS), 14 points for the physical component score (PCS), or 10 points for the overall health score in the SF‐36 (Jayadevappa 2017)
-
Complications
Defined as overall complication rate
MCID: 3% absolute difference
-
Retreatment rate
Defined as a second session of the same treatment modality
MCID: 3% absolute difference (Oestreich 2020)
-
Auxiliary procedures rate
Defined as use of a different modality of treatment to achieve stone‐free status
MCID: 3% absolute difference (Oestreich 2020)
-
Duration of hospital stay
Defined as time that participants required inpatient hospital care, adjusted to the same unit before analysis
MCID: one day
We considered outcomes measured up to and including three months after randomization as short‐term outcomes, those measure from three to six months as medium‐term outcomes, and those measured after six months as long‐term outcomes. We assessed complications, retreatment rate, and auxiliary procedures rate as long‐term, quality of life as medium term, and hospital as short‐term. Treatment success rate at 3 months was assessed at a single time point only.
We assumed an intervention had achieved a clinically meaningful benefit when the mean difference (MD) or risk ratio (RR) was equal to or larger than the MCID. We identified the MCIDs for treatment success, QoL (SF‐36), retreatment, and auxiliary procedures in previous systematic reviews (Jayadevappa 2017; Oestreich 2020). The MCIDs for complications and duration of hospital stay were based on the review authors' clinical experience.
Search methods for identification of studies
We performed a comprehensive search with no restrictions on the language of publication or publication status. We updated our search strategies and searched within three months prior to the anticipated publication of the review.
Electronic searches
We searched the following sources from inception to 30 June 2020 with electronic search strategies approved by Cochrane Urology Group's Information Specialist, then updated the search on 6 December 2022 with revised search strategies (Appendix 1).
MEDLINE via OVID (from 1946)
EMBASE via OVID (from 1947)
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
We searched for ongoing studies in the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/).
Searching other resources
To identify any other eligible trials or ancillary publications, we checked the reference lists of included trials, clinical practice guidelines, reviews, relevant meta‐analyses, and health technology assessment reports. We also contacted the authors of included trials to identify any further studies that we might have missed. Where applicable, we contacted drug/device manufacturers for ongoing or unpublished trials. The abstract proceedings from the most relevant meetings (e.g. the AUA, the EAU, and the Endourological Society) were included in our electronic searches, so we did not search them separately for unpublished studies.
Data collection and analysis
Selection of studies
We manually identified and removed duplicate records. Two review authors (VS, SP) independently scanned the titles and abstracts of the remaining records and eliminated those that were clearly ineligible. Two review authors (VS, BL) read through the full‐text articles of all potentially relevant records, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, and ongoing studies, in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We resolved any discrepancies through consensus or recourse to a third review author (AS or SP). If we were unable to resolve a disagreement, we designated the study as awaiting classification and contacted the study authors for clarification. We documented reasons for the exclusion of studies that readers may reasonably have expected to be included in the review in the Characteristics of excluded studies table. We presented a PRISMA flow diagram showing the study selection process (Page 2021).
Data extraction and management
We developed a dedicated data extraction form, which we piloted in the previous version of this review.
Four review authors (VS, AS, SP, BL) independently extracted the following information from the included studies.
Study design
Study dates
Study settings and country
Duration of follow‐up
Participant inclusion and exclusion criteria
Participant details and baseline demographics (e.g. age, sex, stone location, stone size)
Numbers of participants by study and by study arm
Details of relevant experimental and comparator interventions (e.g. type of anesthesia, flexible ureteroscope, and lithotriptor)
Definitions of relevant outcomes, method and timing of outcome measurement, and any relevant subgroups
Study funding sources
Declarations of interest
We extracted outcome data relevant to this Cochrane Review as needed to calculate summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals to populate a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations (SDs) or the data necessary to calculate these measures.
We resolved disagreements by discussion. We provided information, including trial identifier, from potentially relevant ongoing studies in the Characteristics of ongoing studies table. We attempted to contact the corresponding authors of included studies to obtain key missing data as needed. Another review author (PP) spot‐checked the data for accuracy while analyzing and writing the review findings.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we prioritized the publication that reported the longest follow‐up for our primary or secondary outcomes.
Assessment of risk of bias in included studies
Three review authors (VS, AS, SP) independently assessed the risk of bias of each included study. We resolved disagreements by consensus or by consulting a fourth review author (BL). Another review author (PP) checked for the accuracy of the risk of bias assessment before we incorporated the results into the analyses.
We used the Cochrane risk of bias tool (RoB 1), which covers the following domains (Higgins 2011a).
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias
For each study, we judged the risk of bias for each domain as 'low', 'high', or 'unclear', using the guidance described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We illustrated the results in risk of bias summary figures and provided justifications for our decisions in the Characteristics of included studies table.
For selection bias (random sequence generation and allocation concealment), we evaluated the risk of bias at the trial level. For performance bias (blinding of participants and personnel), we considered that all outcomes were susceptible to performance bias and assessed them as one group. For detection bias (blinding of outcome assessment), we grouped outcomes according to whether they were susceptible or not susceptible to detection bias (subjective or objective). We considered treatment success rate at three months and QoL to be subjective, and the remaining outcomes to be objective. We assessed attrition bias (incomplete outcome data) on a per‐outcome basis and presented the judgment for each outcome separately, considering total attrition rates over 20% indicative of high risk of attrition bias. For reporting bias (selective reporting), we evaluated the risk of bias at the trial level.
We further summarized the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome, in accordance with the approach for summary assessments of risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Measures of treatment effect
We expressed dichotomous data as RRs with 95% confidence intervals (CIs). We expressed continuous data as MDs with 95% CIs unless different studies used different measures to assess the same outcome, in which case we expressed data as standardized mean differences (SMDs) with 95% CIs. If there were insufficient data to enable standard meta‐analysis, we reported the study results narratively.
Unit of analysis issues
The unit of analysis was the individual participant. If studies had more than two intervention arms, we handled these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We contacted study authors to request missing data and recorded details of any attempts to contact study authors in the notes section of the Characteristics of included studies table. We converted reported data into the required format (e.g. unit of hospital stay from hours to days, complication rates to the number of participants with complications, and standard error [SE] to SD).
Assessment of heterogeneity
We identified statistical heterogeneity (inconsistency) by visually inspecting forest plots to determine the amount of overlap of CIs, and by calculating the I2 statistic, which quantifies the proportion of variation due to heterogeneity rather than due to chance (Deeks 2019; Higgins 2003). We interpreted the I2 statistic as follows (Deeks 2019).
0% to 40%: may not be important
30% to 60%: may indicate moderate heterogeneity
50% to 90%: may indicate substantial heterogeneity
75% to 100%: considerable heterogeneity
When we identified substantial heterogeneity (I2 > 60%), we attempted to determine the sources of heterogeneity among the results of studies by conducting subgroup analyses.
In the event of considerable heterogeneity unexplained by subgroup analyses, we examined the direction of effect. Where all studies showed the same direction of effect, we considered the heterogeneity clinically non‐relevant, and we performed a random‐effects meta‐analysis to produce an overall summary. Where the direction of effect differed across studies, we considered the heterogeneity clinically relevant, and we provided a narrative description of the results of each study.
Assessment of reporting biases
We attempted to obtain study protocols to assess selective outcome reporting. If 10 or more studies contributed data to an outcome, we used funnel plots to assess small‐study effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodologic design (and hence bias of small trials), and publication bias. Therefore, we interpreted the results carefully.
Data synthesis
We used the random‐effects model for meta‐analysis. In addition, we performed statistical analyses according to the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We used the Mantel‐Haenszel method for dichotomous outcomes and the inverse variance method for continuous outcomes. Where we considered it was not possible to combine studies, we summarized the results in a narrative manner. We used Review Manager 5.4 software to perform the analyses (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity and planned to carry out subgroup analyses with investigation of interactions for all primary outcomes.
Stone size (20 mm or less versus more than 20 mm)
Stone location (lower pole versus non‐lower pole)
The subgroup analyses by stone size and location were based on observations of potential subgroup effects demonstrated in previous studies (Abdelhamid 2016; Celik 2015; Yamashita 2018).
We used the test for subgroup differences in Review Manager 5.4 to compare subgroup analyses if there were sufficient studies (Review Manager 2020).
Sensitivity analysis
We planned to perform a sensitivity analysis to explore the influence of risk of bias on effect size. To do this, we planned to restrict the analysis to studies at low risk of bias in all domains.
Summary of findings and assessment of the certainty of the evidence
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which considers risk of bias, inconsistency, imprecision, publication bias, and directness of results (Guyatt 2008). Two review authors (VS and PP) independently rated the certainty of evidence for each outcome as 'high', 'moderate', 'low', or 'very low' using GRADEpro GDT. We resolved any discrepancies by consensus or by recourse to a third review author if necessary (AS or SP). For each comparison, we presented a summary of the evidence for the main outcomes in a summary of findings table, which provides: key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Scholtes 2012). If meta‐analysis was not feasible, we assessed the certainty of evidence using the approach of Murad and colleagues and presented the results in a narrative summary of findings table (Murad 2017).
We included the following outcomes in each summary of findings table.
Treatment success rate at three months
QoL
Complications
Retreatment rate
Auxiliary procedures rate
Duration of hospital stay
Results
Description of studies
Results of the search
Our comprehensive literature search identified 4030 records, and we found four applicable records through other sources (Naguib 2016; Saleh 2019; Salem 2013; Sohu 2019). After removing duplicates, we screened the titles and abstracts of 2057 records and excluded 2000. During full‐text screening, we excluded a further 17 records and listed four trials as ongoing (see Characteristics of ongoing studies). We included the remaining 31 studies in this review. Figure 1 shows the study selection process in a PRISMA flow diagram.
1.
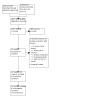
Study flow diagram.
Included studies
We presented details of all included studies in the Characteristics of included studies table, Table 3 (ESWL versus PCNL), and Table 4 (ESWL versus RIRS).
1. Characteristics of included studies (ESWL versus PCNL).
| Ref. ID | Institution (Country) | Setting | Inclusion criteria/size, location, multiplicity |
Definition of treatment success |
Timing of follow up | Modality | Interventions | No. of participants randomized | No. of dropouts | Mean age (years) | Mean stone size (mm) |
Technique/ instrument used |
| AbdelRazek 2021 | Egypt | Single‐center | 10–30 mm | Stone‐free, stone fragments ≤ 3 mm | 3 months | CT scan | ESWL | 58 | 4 | 59.04 | 22 | EML |
| PCNL | 54 | 4 | 53.96 | 23.1 | Standard PCNL/US lithotriptor | |||||||
| Ahmed 2021 | Egypt | Single‐center | 10–20 mm, non‐lower pole, ≥ 1000 HU, single | Stone‐free, stone fragments ≤ 4 mm | 3 months | CT scan | ESWL | 36 | 3 | 40.97 | 17.97 | EML |
| PCNL | 36 | 2 | 42.71 | 18.29 | Mini PCNL/Ho:YAG laser | |||||||
| Albala 2001 | USA | Multicenter | ≤ 30 mm, lower pole, single | Stone‐free, stone fragments ≤ 3 mm | 3 months | NR | ESWL | 68 | 16 | NR | 13.59 | EHL, EML, piezoelectric |
| PCNL | 60 | 5 | NR | 14.43 | Standard PCNL/US, EHL, laser | |||||||
| Bozzini 2017 | Europe | Multicenter | 10–20 mm, lower pole, single | Stone‐free, stone fragments ≤ 3 mm | 3 months | CT scan | ESWL | 217 | 23 | 53.3 | 13.78 | NR |
| PCNL | 206 | 25 | 54.8 | 15.23 | Standard or mini PCNL/30 w holmium laser | |||||||
| Carlsson 1992 | Sweden | Multicenter | 4–30 mm | Stone‐free, stone fragments ≤ 5 mm | 4 weeks and 1 year | NR | ESWL | 28 | NR | 49 | 13 | EHL |
| PCNL | 21 | 48.2 | 12 | Standard PCNL/US lithotriptor | ||||||||
| Deem 2011 | USA | Single‐center | 10–20 mm | Stone‐free | 3 months | CT scan | ESWL | 15 | 3 | 52.25 | 12.16 | EHL |
| PCNL | 20 | 0 | 47.2 | 12.85 | Standard PCNL/combine US and pneumatic lithotriptor | |||||||
| Gadelkareem 2020 | Egypt | Single‐center | 20–30 mm, renal pelvic stone, single, ≤ 1000 HU | Stone‐free, stone fragments ≤ 4 mm | 3 months | CT scan | ESWL | 40 | 0 | 44.18 | 24.6 | EML |
| PCNL | 40 | 0 | 43.25 | 25.2 | Standard PCNL/NR | |||||||
| Kumar 2015b | India | Single‐center | 10–20 mm, lower pole, radiolucent | Stone‐free, stone fragments ≤ 4 mm | 3 months | CT scan | ESWL | 52 | 10 | 33.1 | 13.2 | EML |
| PCNL | 53 | 12 | 33.7 | 13.3 | Mini PCNL/pneumatic lithoclast | |||||||
| McCahy 2020 | Australia | Single‐center | 10–20 mm | Stone‐free | Varied | CT scan | ESWL | 10 | 0 | Median: 60 | Median: 13.5 | EML |
| PCNL | 10 | 0 | Median: 57 | Median: 14 | Standard PCNL/combine US and pneumatic lithotriptor | |||||||
| Naguib 2016 | Egypt | Single‐center | 10–20 mm, lower pole, single | Stone‐free | NR | NR | ESWL | 20 | 0 | NR | 15 | EML |
| PCNL | 20 | 0 | NR | 14 | Mini PCNL/pneumatic lithotriptor and Ho‐YAG laser | |||||||
| Roy 2021 | India | Single‐center | 10‐20 mm, non‐lower pole, single | Stone‐free, stone fragments ≤ 4 mm | 3 weeks | US KUB and X‐ray KUB | ESWL | 54 | 0 | 39.54 | 14.17 | EML |
| PCNL | 51 | 0 | 37.39 | 14.8 | Mini PCNL/pneumatic lithotriptor | |||||||
| Saleh 2019 | Iran | Single‐center | < 20 mm | Stone‐free | NR | NR | ESWL | 20 | 0 | 43.25 | 14.3 | NR |
| PCNL | 20 | 0 | 43.85 | 14.35 | Mini PCNL/pneumatic lithotriptor | |||||||
| Sohu 2019 | Pakistan | Single‐center | ≥ 20 mm | Stone‐free or CIRS | 3 months | US | ESWL | 30 | 0 | NR | NR | NR |
| PCNL | 30 | 0 | NR | NR | NR | |||||||
| Soliman 2021 | Egypt | Single‐center | 10–20 mm, lower pole, radiopaque | Stone‐free, stone fragments ≤ 3 mm | 3 months | Plain KUB and US | ESWL | 75 | 0 | 37.75 | 15.5 | EHL |
| PCNL | 75 | 0 | 40.55 | 15.7 | Mini PCNL/pneumatic lithoclast | |||||||
| Terribile 2019 | Italy | Single‐center | 10–20 mm, lower pole, single | Stone‐free | 3 months | CT scan | ESWL | 33 | NR | NR | 12.98 | NR |
| PCNL | 30 | NR | NR | 14.32 | NR | |||||||
| Yuruk 2010 | Turkey | Single‐center | ≤ 20 mm, lower pole | Stone‐free | 3 months and 1 year | CT scan | ESWL | 33 | 2 | 44.5 | 139.4 mm2 | EML |
| PCNL | 33 | 2 | 44.1 | Mean stone surface area: 153.3 mm2 | Standard PCNL/combine US and pneumatic lithotriptor | |||||||
| Zhang 2019 | PR China | Single‐center | 10–20 mm, lower pole, single | Stone‐free, stone fragments ≤ 3 mm | 3 months | CT scan | ESWL | 60 | 0 | 50.51 | Mean stone surface area: 14.88 mm2 | EML |
| PCNL | 60 | 0 | 48.92 | 15.48 | Ultra‐mini PCNL/30 w Ho:YAG laser |
CIRS: clinically insignificant residual stone; CT: computed tomography; ESWL: extracorporeal shock wave lithotripsy; EHL: electrohydraulic lithotriptor; EML: electromagnetic lithotriptor; Ho:YAG: holmium:yttrium aluminum garnet; HU: Hounsfield units; KUB: kidneys, ureters, and bladder; NR: not reported; PCNL: percutaneous nephrolithotomy; US: ultrasound; YAG: yttrium aluminum garnet.
2. Characteristics of included studies (ESWL versus RIRS).
| Reference ID | Institution (Country) | Setting | Inclusion criteria/size, location, multiplicity | Definition of treatment success | Timing of follow up | Modality | Interventions | No. of patients randomized | No. of dropouts | Mean age (years) | Mean stone size (mm) |
Technique/ instrument used |
| Atis 2021 | Turkey | Single‐center | 10–20 mm | Stone‐free, stone fragments ≤ 4 mm | 1 day and 1 month | Xray, US or CT scan | ESWL | 60 | 24 | 47.22 | 14.5 | EML |
| RIRS | 60 | 15 | 47.2 | 15.29 | f‐URS/Ho:YAG laser | |||||||
| Bosio 2019 | Italy | Single‐center | 6–20 mm | Stone‐free, stone fragments ≤ 5 mm | 3 months | Xray, US | ESWL | 68 | NR | 51 | 10.8 | NR |
| RIRS | 70 | NR | 53 | 11.5 | NR | |||||||
| Bozzini 2017 | Europe | Multicenter | 10– 20 mm, lower pole, single | Stone‐free, stone fragments ≤ 3 mm | 3 months | CT scan | ESWL | 217 | 23 | 53.3 | 13.78 | NR |
| RIRS | 226 | 19 | 55.8 | 14.82 | f‐URS/Ho:YAG laser | |||||||
| Fankhauser 2021 | Switzerland | Single‐center | > 5 mm, single or multiple stones | Stone‐free | 3 months | CT scan | ESWL | 21 | 0 | 50 | 7.6 | EML |
| RIRS | 23 | 0 | 47 | 8.1 | f‐URS/Ho:YAG laser‐basket | |||||||
| Javanmard 2015 | Iran | Single‐center | 10–20 mm, BMI >30 | Stone‐free, stone fragments ≤ 3 mm | 3 months | CT scan | ESWL | 25 | 0 | 36.1 | 16.3 | EML |
| RIRS | 21 | 0 | 33.2 | 17.1 | f‐URS/Ho:YAG laser‐double J stent | |||||||
| Kumar 2015a | India | Single‐center | ≤ 2 cm, lower pole, single | Stone‐free, stone fragments ≤ 3 mm | 3 months | NR | ESWL | 97 | 7 | 37.7 | 12.1 | EML |
| RIRS | 98 | 8 | 35.6 | 12.3 | f‐URS/Ho:YAG laser‐double J stent | |||||||
| Kumar 2015b | India | Single‐center | 10–20 mm, lower pole, radiolucent | Stone‐free, stone fragments ≤ 4 mm | 3 months | CT scan | ESWL | 52 | 10 | 33.1 | 13.2 | EML |
| RIRS | 53 | 10 | 33.4 | 13.1 | f‐URS/100 w Ho:YAG laser | |||||||
| McCahy 2020 | Australia | Single‐center | 10–20 mm | Stone‐free | Vary | CT scan | ESWL | 10 | 0 | Median: 60 | Median: 13.5 | EML |
| RIRS | 11 | 0 | Median: 59 | Median: 14.5 | f‐URS/100 w Ho:YAG laser | |||||||
| Pearle 2005 | USA | Multicenter | ≤ 10 mm, lower pole, single | Stone‐free, stone fragments ≤ 4 mm | 3 months | Xray or CT scan | ESWL | 32 | 6 | 52.5 | Mean stone surface area: 42.2 mm2 | EHL, EML, piezoelectric |
| RIRS | 35 | 3 | 49.3 | Mean stone surface area: 35.9 mm2 | f‐URS/stone retrieval or intracorporeal lithotripsy | |||||||
| Ravier 2015 | France | Single‐center | 5–20 mm, single | Stone‐free, stone fragments ≤ 3 mm | 3 months | CT scan | ESWL | 16 | 5 | 52.8 | 10.7 | EHL |
| RIRS | 14 | 8 | 50.4 | 9.3 | f‐URS/stone retrieval or Ho:YAG laser | |||||||
| Salem 2013 | Egypt | Single‐center | ≤ 20 mm, lower pole | Stone‐free | 3 months | Xray | ESWL | 30 | NR | 35.5 | 11.3 | NR |
| RIRS | 30 | NR | 44.2 | 11.5 | NR | |||||||
| Schoenthaler 2022 | Germany | Single‐center | ≤ 20 mm, lower and non‐lower pole | Stone‐free | 2 weeks and 3 months | CT scan | ESWL | 15 | 0 | NR | 9.4 | NR |
| RIRS | 15 | 0 | NR | 9.93 | NR | |||||||
| Sener 2014 | Turkey | Single‐center | ≤ 10 mm, lower pole, single | Stone‐free | 1 week and 3 months | X‐ray, US or CT scan | ESWL | 70 | 0 | 42.9 | 8.2 | EHL |
| RIRS | 70 | 0 | 45.4 | 7.8 | f‐URS/Ho:YAG laser | |||||||
| Sener 2015 | Turkey | Single‐center | ≤ 10 mm, lower pole, single | Stone‐free | 3 months and 1 year | CT scan | ESWL | 50 | 0 | 34.5 | 7.9 | EHL |
| RIRS | 50 | 0 | 36.84 | 8.2 | f‐URS/Ho:YAG laser | |||||||
| Singh 2014 | India | Single‐center | 10–20 mm, lower pole, radiopaque | Stone‐free | 1 month | X‐ray, US | ESWL | 35 | 0 | 34.5 | 16.45 | EML |
| RIRS | 35 | 0 | 37.65 | 15.05 | f‐URS/Ho:YAG laser | |||||||
| Svihra 2021 | Slovakia | Single‐center | ≤ 20 mm, ≤ 1000 HU | Stone‐free, stone fragments ≤ 1 mm | 6 months | CT scan | ESWL | 39 | 7 | Median: 60 | Median: 9.0 | NR |
| RIRS | 39 | 5 | Median: 57.5 | Median: 9.0 | NR | |||||||
| Terribile 2019 | Italy | Single‐center | 10–20 mm, lower pole, single | Stone‐free | 3 months | CT scan | ESWL | 33 | NR | NR | 12.98 | NR |
| RIRS | 35 | NR | NR | 13.88 | NR | |||||||
| Vilches 2015 | Chile | Single‐center | ≤ 15 mm, lower pole, single | Stone‐free, stone fragments ≤ 3 mm | 2 months | CT scan | ESWL | 32 | 1 | 45.6 | 9.6 | EML and NSAIDs and tamsulosin |
| RIRS | 31 | 7 | 43.7 | 9.7 | f‐URS/30 w Ho:YAG laser‐double J stent | |||||||
| Zhang 2019 | PR China | Single‐center | 10–20 mm, lower pole, single | Stone‐free, stone fragments ≤ 3 mm | 3 months | CT scan | ESWL | 60 | 0 | 50.51 | 14.88 | EML |
| RIRS | 60 | 0 | 50.05 | 14.63 | f‐URS/100 w Ho:YAG laser‐double J stent |
BMI: body mass index; CT: computed tomography; EHL: electrohydraulic lithotriptor; EML: electromagnetic lithotriptor; ESWL: extracorporeal shock wave lithotripsy; f‐URS: flexible ureteroscope; HU: Hounsfield units; NR: not reported; NSAIDs: nonsteroidal anti‐inflammatory drugs; RIRS: retrograde intrarenal surgery; US: ultrasound.
Source of data
Four of the 31 studies were published as abstract proceedings (Bosio 2019; Naguib 2016; Salem 2013; Terribile 2019). Thirty studies were published in English, and Ravier 2015 was published in French. We used Google Translate to translate the article to English (translate.google.com/). We emailed the corresponding authors of several studies to request missing information (Ahmed 2021; Atis 2021; Bosio 2019; ChiCTR‐INR‐17013906; Deem 2011; ISRCTN98970319; Javanmard 2015; Kumar 2015b; McCahy 2020; NCT02522676; NCT02658942; NCT04856722), but we received only three replies (Ahmed 2021; Bosio 2019; NCT02658942). The notes section of the Characteristics of included studies table provides details of correspondence with study authors.
Study design and setting
All studies were parallel RCTs conducted between 1992 and 2022. Two early studies did not report the study duration (Carlsson 1992; Pearle 2005). There were 27 single‐center trials and four multicenter trials (Albala 2001; Bozzini 2017; Carlsson 1992; Pearle 2005).
The 31 included RCTs randomized 3361 participants, of whom 3060 completed the trials. The mean age of participants across trials was 46.6 years, and the mean stone size was 13.4 mm (range 7.3 mm to 48.3 mm). One study did not report the mean stone size (Ravier 2015). Kidney stone size was 20 mm or less in 93.8% of participants, and 68.9% of participants had lower pole stones. Most lower pole stones measured less than 20 mm; only a subgroup of 14 participants in one study had lower pole stones larger than 20 mm (Albala 2001). Kidney stones were in other locations of the kidney in 782 participants from 13 trials, and stone sizes varied from 4 mm to partial staghorn stone (Sohu 2019). Only 108 patients in one study had radiolucent stones (Kumar 2015b); the remaining participants had radiopaque stones. One trial with 48 participants evaluated the treatment results in participants with obesity (Javanmard 2015). One trial included 104 people with renal insufficiency (AbdelRazek 2021).
Interventions and comparisons
Eleven studies evaluated ESWL versus PCNL and 13 studies evaluated ESWL versus RIRS. Five studies evaluated ESWL versus RIRS versus PCNL (Bozzini 2017; Kumar 2015b; McCahy 2020; Terribile 2019; Zhang 2019); from each of these studies, we extracted the ESWL and PCNL data for our first comparison, and the ESWL and RIRS data for our second comparison (i.e. we included the same ESWL data from these studies in both comparisons). One study evaluated ESWL versus PCNL versus observation (Yuruk 2010), and another study evaluated ESWL versus RIRS versus observation (Sener 2015); we did not include the observation arms in our analyses. Seven studies evaluated novel PCNL techniques, including mini PCNL and ultra‐mini PCNL (Ahmed 2021; Kumar 2015b; Naguib 2016; Roy 2021; Saleh 2019; Soliman 2021; Zhang 2019). The length of follow‐up varied from one week to one year.
Outcomes
Treatment success rate at three months was the primary outcome in 21 studies. Some studies evaluated treatment success rate after one week (Deem 2011), three weeks (Roy 2021), one month (Bosio 2019; Carlsson 1992; Singh 2014), two months (Vilches 2015), four months (Carlsson 1992), and one year (Carlsson 1992; Yuruk 2010). Three trials did not report the timing of treatment success rate (Saleh 2019; McCahy 2020; Naguib 2016).
QoL was the primary outcome in two studies (Atis 2021; Svihra 2021), and a secondary outcome in three studies (Albala 2001; Deem 2011; Pearle 2005). Albala 2001, Atis 2021, and Pearle 2005 used the SF‐36 questionnaire to evaluate QoL; and Deem 2011 used the SF‐8. Svihra 2021 used the Wisconsin Stone‐Quality of Life Questionnaire (WISQOL) to evaluate health‐related QoL and quality‐adjusted life‐years (QALYs).
For complications, 26 trials provided adequate data for analysis, and five did not (Atis 2021; Bosio 2019; Deem 2011; Sohu 2019; Svihra 2021).
Sixteen studies reported retreatment rate (Ahmed 2021; Albala 2001; Bozzini 2017; Deem 2011; Fankhauser 2021; Gadelkareem 2020; Javanmard 2015; Kumar 2015a; Kumar 2015b; Naguib 2016; Pearle 2005; Ravier 2015; Roy 2021; Singh 2014; Soliman 2021; Zhang 2019), 12 studies reported auxiliary procedure rate (Ahmed 2021; Albala 2001; Bozzini 2017; Gadelkareem 2020; Kumar 2015a; Kumar 2015b; Naguib 2016; Pearle 2005; Roy 2021; Sener 2015; Soliman 2021; Yuruk 2010), and seven studies reported mean duration of hospital stay (Ahmed 2021; Bozzini 2017; Carlsson 1992; Kumar 2015b; Singh 2014; Soliman 2021; Zhang 2019).
Funding and conflicts of interest
Two studies received funding from medical device companies (Albala 2001; Pearle 2005), while 22 studies claimed no relevant financial interests. The remaining seven studies did not mention their source of funding (AbdelRazek 2021; Ahmed 2021; Bosio 2019; Kumar 2015b; Ravier 2015; Terribile 2019; Yuruk 2010). Authors declared conflicts of interest in four studies (Albala 2001; Deem 2011; Pearle 2005; Schoenthaler 2022). It was unclear whether there was a conflict of interest in seven studies, and twenty studies reported no conflicts of interest.
Excluded studies
We excluded 17 records (17 studies) during full‐text review. For details, see the Characteristics of excluded studies table. Ten studies were not RCTS (El‐Nahas 2012; Eterovic 2005; Hassan 2015; Koo 2011; Liou 2001; Mays 1988; Resorlu 2013; Romeu 2021; Turna 2007; You 2006), two enrolled children (NCT04317443; Zeng 2012), one was a review article (Preminger 2006), one was not for kidney stones (ChiCTR2000031520), one did not compare ESWL with PCNL or RIRS (Meretyk 1997), and one was a time series (Charig 1986). We also found one trial that had been aborted without any results (NCT02658942). Contact details are provided in the notes section of the Characteristics of excluded studies table.
Studies awaiting classification
No studies are awaiting classification.
Ongoing studies
We found four ongoing studies without usable outcome data (ChiCTR‐INR‐17013906; ISRCTN98970319; NCT02522676; NCT04856722). We attempted to contact all corresponding authors of ongoing trials to obtain the study status or results, but we received no replies. For details, see the Characteristics of ongoing studies table.
Risk of bias in included studies
For graphical depictions of the risk of bias assessment results, see Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies. Intentionally left blank for blinding of objective or subjective outcomes assessment (detection bias) where the study did not investigate the outcome.
3.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study. Intentionally left blank for blinding of objective or subjective outcomes assessment (detection bias) where the study did not investigate the outcome.
Allocation
Random sequence generation
Nineteen studies were at low risk of bias for random sequence generation; of these, four used a block randomization method (Ahmed 2021; Deem 2011; McCahy 2020; Pearle 2005), one used a coin toss (Roy 2021), and the remaining 14 studies described using a computer‐generated random sequence. Twelve studies were at unclear risk because the method used to generate the allocation sequence was unclear (AbdelRazek 2021; Albala 2001; Bosio 2019; Carlsson 1992; Naguib 2016; Saleh 2019; Salem 2013; Schoenthaler 2022; Sohu 2019; Terribile 2019; Yuruk 2010; Zhang 2019).
Allocation concealment
Only Fankhauser 2021 described an adequate concealment of allocation prior to assignment; we judged this study at low risk of bias. The remaining studies were at unclear risk because they provided insufficient information on the method of allocation concealment.
Blinding
Performance bias
All studies were at high risk of bias for this domain as they did not blind participants or personnel (the different natures of the interventions made blinding difficult).
Detection bias
Objective outcomes
Twenty‐eight studies reported objective outcomes (retreatment rate, auxiliary procedures rate, complications, or duration of hospital stay). We considered all studies at low risk of bias because these outcomes did not require assessors' judgment, and blinding would not have affected the results.
Subjective outcomes
Twenty‐three studies assessed subjective outcomes (treatment success rate at three months or QoL). We rated one study at low risk of detection bias for subjective outcomes because it provided information about blinding assessors (Ahmed 2021). The remaining studies provided insufficient information about blinding outcome assessment (unclear risk).
Incomplete outcome data
We considered outcomes with more than 20% missing data at high risk of attrition bias. We rated this domain per outcome, as follows.
Treatment success rate at three months: 15 studies reported low levels of attrition (AbdelRazek 2021; Ahmed 2021; Bozzini 2017; Deem 2011; Fankhauser 2021; Gadelkareem 2020; Javanmard 2015; Kumar 2015a; Schoenthaler 2022; Sener 2014; Sener 2015; Sohu 2019; Soliman 2021; Yuruk 2010; Zhang 2019), and four studies reported high levels of attrition (Albala 2001; Kumar 2015b; Pearle 2005; Ravier 2015). For two studies, it was unclear whether all randomized participants were included in the analysis (Salem 2013; Terribile 2019). The remaining studies did not report treatment success rate.
QoL: two studies reported low levels of attrition (Deem 2011; Svihra 2021), and two studies reported high levels of attrition (Albala 2001; Atis 2021). In Pearle 2005, it was unclear whether all randomized participants were included in the analysis. The remaining studies did not report QoL.
Complications: 19 studies reported low levels of attrition (AbdelRazek 2021; Ahmed 2021; Bozzini 2017; Carlsson 1992; Deem 2011; Fankhauser 2021; Gadelkareem 2020; Javanmard 2015; Kumar 2015a; McCahy 2020; Pearle 2005; Roy 2021; Sener 2014; Sener 2015; Singh 2014; Soliman 2021; Vilches 2015; Yuruk 2010; Zhang 2019), and two studies reported high levels of attrition (Albala 2001; Kumar 2015b). In three studies, it was unclear whether all randomized participants were included in the analysis (Bosio 2019; Salem 2013; Terribile 2019). The remaining studies did not report complications.
Retreatment rate: 10 studies reported low levels of attrition (Ahmed 2021; Bozzini 2017; Javanmard 2015; Kumar 2015a; Naguib 2016; Roy 2021; Singh 2014; Sohu 2019; Soliman 2021; Zhang 2019), and three studies reported high levels of attrition (Albala 2001; Kumar 2015b;Ravier 2015). In two studies, it was unclear whether all randomized participants were included in the analysis (Pearle 2005; Terribile 2019). The remaining studies did not report retreatment rate.
Auxiliary procedures rate: 10 studies reported low levels of attrition (Ahmed 2021; Bozzini 2017; Kumar 2015a; Naguib 2016; Roy 2021; Sener 2014; Sener 2015; Singh 2014; Sohu 2019; Soliman 2021), and two studies reported high levels of attrition (Albala 2001; Kumar 2015b). In two studies, it was unclear whether all randomized participants were included in the analysis (Pearle 2005; Terribile 2019). The remaining studies did not report auxiliary procedures rate.
Duration of hospital stay: nine studies reported low levels of attrition (Ahmed 2021; Albala 2001; Bozzini 2017; Carlsson 1992; Pearle 2005; Saleh 2019; Singh 2014; Soliman 2021; Zhang 2019). The remaining studies did not report duration of hospital stay.
Selective reporting
To assess reporting bias, we checked whether outcome reporting and analyses corresponded with a prospective protocol. We identified prospective protocols for four studies (Ahmed 2021; Bosio 2019; Fankhauser 2021; Ravier 2015). Ravier 2015 was at high risk of bias because it did not report several predefined outcomes, and the other three studies were at low risk because they reported all predefined outcomes. We found no preregistered protocols for the remaining 27 studies, which we considered at unclear risk of reporting bias.
Other potential sources of bias
Most studies (27 of 31) were at low risk of other sources of bias. We considered the four studies published as abstract only at unclear risk because they provided insufficient information on baseline characteristics, the intervention procedures, or both aspects (Bosio 2019; Naguib 2016; Salem 2013; Terribile 2019)
Effects of interventions
Comparison 1: extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL)
See: Table 1.
Primary outcomes
Treatment success rate at three months
ESWL may have a lower overall treatment success rate than PCNL (RR 0.67, 95% CI 0.57 to 0.79; I2 = 87%; 12 studies, 1303 participants; low‐certainty evidence; Analysis 1.1). Assuming 923 per 1000 participants undergoing PCNL achieve treatment success at this time point, this corresponds to 304 fewer participants per 1000 (397 fewer to 194 fewer) achieving treatment success with ESWL. We downgraded the certainty of the evidence for serious study limitations and publication bias concerns (funnel plot asymmetry; see Figure 4). We did not downgrade for inconsistency despite the high I2 value because it did not appear clinically relevant.
1.1. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 1: Treatment success rate at three months
4.

Funnel plot of comparison: 1 ESWL versus PCNL, outcome: 1.1 Treatment success rate at three months after treatment (overall).
Quality of life
ESWL compared with PCNL may have little or no effect on QoL (1 study, 78 participants; low‐certainty evidence). Albala 2001 reported that the mean difference of overall health score between baseline and three months in ESWL and PCNL was 6.7 points (SD 18) in the ESWL group and 8.2 points (SD 18.1) in the PCNL group (MD −1.5 points, 95% CI −9.53 to 6.53; Analysis 1.4). We downgraded the certainty of the evidence for serious study limitations and imprecision.
1.4. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 4: Quality of life
Deem 2011 measured QoL before the intervention and at one week and three months after the intervention using two domains of the SF‐8 (physical health score and mental health score). The findings were presented in bar charts. We used WebPlotDigitizer to extract the mean scores in both groups (apps.automeris.io/wpd/). For physical health, mean scores in the ESWL group (12 participants) were 41.55 before the intervention, 42.10 at one week, and 48.95 at three months; and mean scores in the PCNL group (20 participants) were 39.79 before the intervention, 34.77 at one week, and 48.77 at three months. For mental health, mean scores in the ESWL group were 39.68 before the intervention, 40.88 at one week, and 39.19 at three months; and mean scores in the PCNL group were 47.01 before the intervention, 49.90 at one week, and 49.06 at three months. There was no difference between ESWL and PCNL at three months after treatment; however, we could not meta‐analyze the data because Deem 2011 did not provide SDs.
Complications
ESWL likely has a lower overall complication rate than PCNL (RR 0.62, 95% CI 0.47 to 0.82; I2 = 18%; 13 studies, 1385 participants; moderate‐certainty evidence; Analysis 1.5). Assuming 216 per 1000 participants with complications in the PCNL group, this corresponds to 82 fewer participants per 1000 (115 fewer to 39 fewer) having complications with ESWL. We downgraded the certainty of the evidence for serious study limitations. We did not downgrade for publication bias because the funnel plot was fairly symmetrical funnel (Figure 5).
1.5. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 5: Complications
5.

Funnel plot of comparison: 1 ESWL versus PCNL, outcome: 1.5 Complications.
Secondary outcomes
Retreatment rate
ESWL may have a higher overall retreatment rate than PCNL (RR 15.53, 95% CI 6.62 to 36.39; I2 = 61%; 10 studies, 1174 participants; low‐certainty evidence; Analysis 1.8). Assuming 28 per 1000 participants treated by PCNL need retreatment, this corresponds to 401 more participants per 1000 (155 more to 976 more) for ESWL. We downgraded the certainty of the evidence for serious study limitations and publication bias concerns (funnel plot asymmetry; see Figure 6). We did not downgrade for inconsistency despite the high I2 value because it did not appear clinically relevant.
1.8. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 8: Retreatment rate
6.

Funnel plot of comparison: 1 ESWL versus PCNL, outcome: 1.8 Retreatment rate.
Auxiliary procedures rate
ESWL probably requires more auxiliary procedures than PCNL (RR 4.17, 95% CI 2.67 to 6.52; I2 = 0%; 8 studies, 1044 participants; moderate‐certainty evidence; Analysis 1.9). Assuming 41 per 1000 participants treated by PCNL need auxiliary procedures, this corresponds to 130 more participants per 1000 (69 more to 227 more) for ESWL. We downgraded the certainty of the evidence for serious study limitations.
1.9. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 9: Auxiliary procedures rate
Duration of hospital stay
Hospital stays are probably shorter for ESWL than for PCNL (MD −3.36 days, 95% CI −4.74 to −1.98; I2 = 99%; 6 studies, 841 participants; moderate‐certainty evidence; Analysis 1.10). The mean hospital stay in the ESWL group ranged from 0.12 days to 4.10 days. We downgraded the certainty of the evidence for serious study limitations. We did not downgrade further for very substantial inconsistency because all studies demonstrated a clinically important reduction in duration of hospital stay (at least one day).
1.10. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 10: Duration of hospital stay
Preplanned subgroup analysis by stone size (20 mm or less versus greater than 20 mm)
Treatment success rate
The test for subgroup differences did not meet statistical significance (P = 0.59; Analysis 1.2). Differences between results for participants with stones measuring 20 mm or less (RR 0.65, 95% CI 0.56 to 0.76; I2 = 74%; 9 studies, 1045 participants) and those with stones larger than 20 mm (RR 0.45, 95% CI 0.11 to 1.79; I2 = 95%; 3 studies; 154 participants) may be attributable to chance.
1.2. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 2: Treatment success rate at three months by stone size
Quality of life
We found no data to perform the subgroup analysis based on QoL.
Complications
The test for subgroup differences did not meet statistical significance (P = 0.72; Analysis 1.6). Differences between results for participants with stones measuring 20 mm or less (RR 0.57, 95% CI 0.39 to 0.83; I2 = 24%; 10 studies, 1085 participants) and those with stones larger than 20 mm (RR 0.45, 95% CI 0.12 to 1.60; I2 = 90%; 2 studies, 148 participants) may be attributable to chance.
1.6. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 6: Complications by stone size
Preplanned subgroup analysis by stone location (lower pole versus non‐lower pole)
Treatment success rate
The test for subgroup differences did not meet statistical significance (P = 0.63; Analysis 1.3). Differences between results for participants with lower pole stones (RR 0.70, 95% CI 0.61 to 0.80; I2 = 68%; 7 studies, 960 participants) and those with non‐lower pole stones (RR 0.49, 95% CI 0.12 to 2.04; I2 = 97%; 3 studies, 179 participants) may be attributable to chance.
1.3. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 3: Treatment success rate at three months by stone location
Quality of life
We found no data to perform the subgroup analysis based on QoL.
Complications
The test for subgroup differences met statistical significance (P = 0.02; Analysis 1.7), showing a potential difference between results for participants with lower pole stones (RR 0.46, 95% CI 0.33 to 0.64; I2 = 0%; 8 studies; 1009 participants) and those with non‐lower pole stone (RR 0.81, 95% CI 0.57 to 1.17; I2 = 0%; 3 studies; 252 participants). However, there were considerably more trials and participants contributing data to the first subgroup, so findings of this subgroup analysis should be interpreted with caution.
1.7. Analysis.

Comparison 1: Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL), Outcome 7: Complications by stone location
Sensitivity analysis
Because all included studies were at high or unclear risk of bias in at least one domain, we were unable to perform the preplanned sensitivity analyses.
Comparison 2: extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS)
See Table 2
Primary outcomes
Treatment success rate at three months
ESWL may have a lower overall treatment success rate than RIRS (RR 0.85, 95% CI 0.78 to 0.93; I2 = 63%; 13 studies, 1349 participants; low‐certainty evidence; Analysis 2.1). Assuming 848 per 1000 participants undergoing RIRS are successful at this time point, this corresponds to 127 fewer participants per 1000 (186 fewer to 59 fewer) reporting treatment success with ESWL. We downgraded the certainty of the evidence for serious study limitations and serious inconsistency. The funnel plot did not suggest publication bias (Figure 7).
2.1. Analysis.

Comparison 2: Extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS), Outcome 1: Treatment success rate at three months
7.

Funnel plot of comparison: 2 ESWL versus RIRS, outcome: 2.1 Treatment success rate at three months.
Quality of life
Three included studies measured QoL using different tools at different time points (Atis 2021; Pearle 2005; Svihra 2021). We could not meta‐analyze the data from these studies owing to insufficient data, lack of measures of variability, and different timing of assessment. We are very uncertain about the effect of ESWL compared to RIRS on QoL. We downgraded the certainty of the evidence for serious study limitations, inconsistency, and imprecision.
Atis 2021 used the SF‐36 to assess QoL one day and one month after treatment. At day one, the RIRS group (45 participants) had lower scores than the ESWL group (36 participants) in role functioning/physical (33.15 versus 61.11, P = 0.008), role functioning/emotional (49.99 versus 70.42, p = 0.047), energy/fatigue (48.94 versus 60.27, P = 0.011), social functioning (57.56 versus 74.86, P = 0.003), and pain (51.77 versus 67.88, P = 0.003). However, at one month, only emotional well‐being and pain scores were lower in the RIRS groups compared to the ESWL group (52.88 versus 67.77, P = 0.012 for emotional well‐being; 53.44 versus 71.25, P = 0.011 for pain).
Pearle 2005 measured QoL at one month using the SF‐36. The ESWL group (32 participants) compared to the RIRS group (35 participants) required fewer days to drive (mean 1.9 days, SD 1.7 versus mean 5.3 days, SD 6.1; P = 0.001), to return to non‐strenuous activity (mean 3.2 days (SD 3.0) versus mean 7.9 days (SD 9.8); P = 0.021), to return to work (mean 3.3 days (SD 2.7) versus mean 8.5 days (SD 8.3); P = 0.003), and until 100% recovered (mean 8.1 days (SD 10.8) versus mean 15.6 days (SD 1.6); P = 0.006).
Svihra 2021 evaluated the change in the Wisconsin Stone Quality of Life (WISQOL) score and calculated QALYs. QoL was better in the RIRS group (34 participants) compared to the ESWL group (32 participants) in all WISQOL domains and according to QALYs.
Complications
We are very uncertain about the difference in complication rate between ESWL and RIRS (RR 0.93, 95% CI 0.63 to 1.36; I2 = 32%; 13 studies, 1305 participants; very low‐certainty evidence; Analysis 2.3). Assuming 133 per 1000 participants reporting complications in the RIRS group, this corresponds to nine fewer participants per 1000 (49 fewer to 48 more) reporting complications with ESWL. We downgraded the certainty of the evidence for serious study limitations and very serious imprecision. The funnel plot did not suggest publication bias (Figure 8).
2.3. Analysis.

Comparison 2: Extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS), Outcome 3: Complications
8.

Funnel plot of comparison: 2 ESWL versus RIRS, outcome: 2.3 Complications.
Secondary outcomes
Retreatment rate
ESWL probably has a higher overall retreatment rate than RIRS (RR 6.78, 95% CI 3.82 to 12.04; I2 = 46%; 9 studies, 1030 participants; moderate‐certainty evidence; Analysis 2.5). Assuming 60 per 1000 participants treated by RIRS need retreatment, this corresponds to 345 more participants per 1000 (168 more to 658 more) for ESWL. We downgraded the certainty of the evidence for serious study limitations.
2.5. Analysis.

Comparison 2: Extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS), Outcome 5: Retreatment rate
Auxiliary procedures rate
ESWL may require more auxiliary procedures than RIRS to achieve stone‐free status (RR 1.98, 95% CI 1.14 to 3.47; I2 = 53%; 5 studies, 836 participants; low‐certainty evidence; Analysis 2.6). Assuming 106 per 1000 participants treated by RIRS need auxiliary procedures, this corresponds to 104 more participants per 1000 (15 more to 262 more) for ESWL treatment. We downgraded the certainty of the evidence for serious study limitation and imprecision.
2.6. Analysis.

Comparison 2: Extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS), Outcome 6: Auxiliary procedures rate
Duration of hospital stay
Hospital stays are probably shorter for ESWL than RIRS (MD −1.69 days, 95% CI −2.36 to −1.02; 3 studies, 591 participants; I2 = 99%; moderate‐certainty evidence; Analysis 2.7). The mean hospital stay for ESWL ranged from 0.12 to 1.08 days. We downgraded the certainty of the evidence for serious study limitations. Despite the presence of considerable heterogeneity (I2 = 99%), we did not downgrade for inconsistency because it was not clinically relevant.
2.7. Analysis.

Comparison 2: Extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS), Outcome 7: Duration of hospital stay
Preplanned subgroup analysis by stone size (20 mm or less versus greater than 20 mm)
We found no data to perform the subgroup analysis based on stone size for any outcome.
Preplanned subgroup analysis by stone location (lower pole versus non‐lower pole)
Treatment success rate
The test for subgroup differences did not meet statistical significance (P = 0.63; Analysis 2.2). Differences between results for participants with lower pole stones (RR 0.86, 95% CI 0.78 to 0.94; I2 = 67%; 10 studies, 1222 participants) and those with non‐lower pole stones (RR 0.80, 95% CI 0.64 to 1.02; I2 = 0%; 2 studies, 66 participants) may be attributable to chance.
2.2. Analysis.

Comparison 2: Extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS), Outcome 2: Treatment success rate at three months by stone location
Quality of life
We found no data to perform the subgroup analysis based on QoL.
Complications
The test for subgroup differences did not meet statistical significance (P = 0.45; Analysis 2.4). Differences between results for participants with lower pole stones (RR 0.83, 95% CI 0.55 to 1.26; I2 = 39%; 9 studies, 1177 participants) and those with non‐lower pole stones (RR 1.40, 95% CI 0.38 to 5.18; I2 not applicable; 1 study, 46 participants) may be attributable to chance.
2.4. Analysis.

Comparison 2: Extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS), Outcome 4: Complications by stone location
Sensitivity analysis
Because all included studies were at high or unclear risk of bias in at least one domain, we were unable to perform the preplanned sensitivity analyses.
Discussion
Summary of main results
The success of treatment at three months may be lower for ESWL than for PCNL. There may be little or no difference between ESWL and PCNL in terms of their effect on QoL. ESWL compared with PCNL probably leads to fewer complications but may have a higher rate of retreatment and likely requires more auxiliary procedures. People who have ESWL probably stay in hospital less time than those who have PCNL. Further subgroup analyses revealed that treatment success rate may not differ by stone size (less than 20 mm versus 20 mm or greater) or stone location (lower pole versus non‐lower pole). The significantly different rate of complications according to location of kidney stones should be interpreted with caution. We were unable to perform subgroup analyses for QoL or sensitivity analyses including only studies at low risk of bias.
RIRS may have better treatment success after three months than ESWL. We are very uncertain about the effects of ESWL compared to RIRS on QoL and complications. Retreatment is probably more common after ESWL than after PCNL, and the use of auxiliary procedures may also be more common in ESWL. Hospital stays after ESWL are probably shorter than after RIRS. We found no evidence to suggest that treatment success or complications differ by stone location (lower pole versus non‐lower pole). We were unable to perform subgroup analyses for any primary outcomes by stone size (less than 20 mm versus 20 mm or greater), or for QoL by stone location, and we were unable to perform sensitivity analyses including only studies at low risk of bias.
Overall completeness and applicability of evidence
This Cochrane Review represents the most rigorous and up‐to‐date systematic review to evaluate the effectiveness and safety of ESWL compared to PCNL or RIRS. The 31 included RCTs were conducted in different geographical locations and enrolled people with a variety of stone sizes and locations. Therefore, we believe this body of evidence is broadly applicable to current clinical practice. However, there were some limitations related to overall completeness and applicability of evidence, as follows.
All studies comparing ESWL and RIRS included people with kidney stones measuring 20 mm or less. Therefore, the results for this comparison may not be applicable to people with kidney stones larger than 20 mm.
There were differences across studies in outcome measurement methods, follow‐up periods, pretreatment interventions (such as DJ stent insertion), and types of ESWL, all of which influence treatment outcomes.
Carlsson 1992 was conducted during the early period of ESWL and PCNL technology, and the mean duration of hospital stay for people in the ESWL group was 4.1 days. This probably does not reflect current practice, as ESWL is now considered an outpatient procedure.
The published data spans almost three decades, from 1992 to 2021. During this time, endoscopic surgery (PCNL and RIRS) has advanced considerably, but ESWL has remained relatively unchanged. Unlike with ESWL, considerable practice is necessary to achieve expertise in endoscopic techniques. In most included trials, expert surgeons with substantial personal experience in high‐volume centers conducted PCNL and RIRS. Therefore, our findings may not apply to non‐specialized practices.
Quality of the evidence
We downgraded the certainty of the evidence by one, two, or three levels to moderate, low, or very low. The most common reasons for downgrading were as follows.
Study limitations: some studies did not report randomization techniques, and concealment of allocation was frequently unclear (selection bias). No studies blinded participants or personnel (performance bias), and most did not describe blinding of outcome assessment (detection bias). Loss to follow‐up was high or unclear in several studies (attrition bias). Most lacked a registered protocol (reporting bias).
Publication bias (asymmetrical funnel plots)
Imprecision: there were wide CIs around pooled effect size estimates for some outcomes as a result of low event rates and sample sizes.
Potential biases in the review process
We conducted this review in accordance with rigorous Cochrane standards, following a published protocol (Srisubat 2008). Nevertheless, the following issues may have introduced bias.
We performed a comprehensive literature search for eligible studies without any language and publication status restriction. Nevertheless, we may have missed relevant studies, in particular 'negative' studies or studies published in non‐indexed journals or presented at local meetings only.
We were able to create only five funnel plots (treatment success rate for both comparisons, complications for both comparisons, and retreatment rate for comparison 1); too few studies contributed data to the remaining outcomes for us to formally access publication bias.
We combined data from studies with different lengths of follow‐up and studies that did not report length of follow‐up.
Four included studies were only available as an abstract (Bosio 2019; Naguib 2016; Salem 2013; Terribile 2019). We attempted to contact the corresponding authors of some studies to request additional information on several occasions, but we received only three replies (Ahmed 2021; Bosio 2019; NCT02658942). This may represent a source of bias, and the information collected directly from corresponding authors may be affected by selective reporting bias.
We used MCIDs to assess the magnitudes of effect (Hultcrantz 2017), but no validated MCID was available for complications or duration of hospital stay. We established these thresholds based on our experience; using different MCID levels may have altered the conclusions of this review.
Our protocol was outdated and may have lacked specifics such as stone size, location, and treatment techniques (i.e. standard PCNL, mini PCNL, and ultra‐mini PCNL). This may be a source of bias.
Agreements and disagreements with other studies or reviews
We identified eight previous systematic reviews on a similar topic to ours. All were network meta‐analyses (NMAs) comparing the efficacy of ESWL versus PCNL versus RIRS for the treatment of kidney stones.
Lee 2015 evaluated the effectiveness and safety of ESWL versus ESWL plus adjunctive therapy versus uteroscopy versus PCNL in the treatment of lower pole renal stones measuring 20 mm or less. There was no published review protocol. Lee 2015 assessed study quality using the Jadad scale, which is no longer considered appropriate, and there was no rating of the certainty of evidence, which we consider a critical part of any systematic review. The NMA suggested that ESWL was the least effective treatment method, whereas PCNL was the most effective. ESWL was associated with a higher rate of retreatment and auxiliary procedures, but the shortest hospital stay.
Zhang 2015 included RCTs and non‐RCTs and focused on the efficacy and safety of PCNL, RIRS, and ESWL for lower pole renal stones. It used the Jadad scale to assess methodological quality and did not assess the certainty of evidence. The NMA showed than PCNL had a higher stone‐free rate than ESWL and RIRS, but there were no differences in complications among the three treatments.
Donaldson 2015 included seven RCTS (691 participants) and focused on the treatment of lower pole renal stones measuring less than 20 mm. It assessed risk of bias and certainty of evidence (GRADE), but there was no published protocol. PCNL and RIRS performed better than ESWL for all stone sizes (stone‐free rate during three months): the RR for PCNL versus ESWL was 2.04 (95% CI 1.50 to 2.77), and the RR for RIRS versus ESWL was 1.31 (95% CI 1.08 to 1.59).
Junbo 2019 focused on lower pole renal stones measuring 10 mm to 20 mm. It included only eight studies and pooled across study designs. Stone‐free rate was greater after PCNL than after RIRS or ESWL, and there were no differences in complication rate.
Chung 2019b compared the success and stone‐free rates of ESWL, PCNL, and RIRS by pooling data across 35 studies with different study designs. It did not assess the certainty of evidence. ESWL had the lowest success rate of the three treatment modalities.
Kim 2020 compared the efficacy of PCNL, RIRS, and ESWL. It included both RCTs and non‐RCTs and did not assess the certainty of evidence. PCNL and RIRS had a significant advantage over ESWL in terms of stone‐free rate, retreatment rate, and auxiliary procedure rate.
Kallidonis 2020 aimed to determine the most appropriate approach for the management of lower pole stones measuring 20 mm or less. It had no published protocol and included 15 RCTs. It assessed the quality of included studies with Cochrane's risk of bias tool and assessed the certainty of evidence with GRADE. PCNL and RIRS had a greater stone‐free rate than ESWL, but ESWL had a lower complication rate.
Tsai 2020 evaluated six different interventions, including RIRS, PCNL, mini PCNL, micro PCNL, ESWL, and conservative observation, in the treatment of lower pole stones. It included 13 RCTs involving 1832 participants. There was no published study protocol. It used the Cochrane risk of bias tool to assess methodological quality and the GRADE approach to assess the certainty of evidence. ESWL was the least effective intervention and had higher retreatment rates compared with PCNL, mini PCNL, and RIRS.
In summary, many existing systematic reviews found that ESWL had lower success rates and fewer complications compared with PCNL or RIRS, especially in lower pole stones. The distinguishing features of our review are the prepublished protocol, the use of rigorous Cochrane methods, and the GRADE assessment. In addition, our interpretation focused on clinically relevant (rather than statistically significant) findings and provided absolute effect size estimates for all dichotomous outcomes. We also evaluated QoL, an outcome not included in any previous systematic review. However, the certainty of evidence for this outcome was low to very low.
Authors' conclusions
Implications for practice.
The findings of this review indicate that extracorporeal shock wave lithotripsy (ESWL) may have a lower treatment success rate compared with percutaneous nephrolithotomy (PCNL) and retrograde intrarenal surgery (RIRS). ESWL probably leads to shorter hospital stays compared with both other interventions, and is probably associated with fewer complications compared with PCNL; however, it may be necessary to perform more than one EWSL session or other procedures to achieve the treatment goal. Stone size and stone location appear to have no effect on the treatment success rate of ESLW compared with PCNL. Stone location appears to have no effect on the treatment success rate of ESLW compared with RIRS; however, there is little evidence on treatment of large kidney stones (greater than 20 mm) for this comparison. There may be little or no difference between the effect of ESWL versus PCNL on quality of life, and we are unsure of the relative effect of ESWL on quality of life compared with RIRS.
ESWL may still be a viable treatment option for people who are at high risk for surgery or anesthesia. PCNL or RIRS may be a preferable treatment option for people who can tolerate anesthesia and invasive procedures.
Implications for research.
There is a need for larger randomized controlled trials evaluating the effectiveness of ESWL versus PCNL or RIRS. In particular, future research could focus on the following areas.
Any new technology for the non‐invasive elimination of the residual fragments that commonly occur after ESWL treatment.
Non‐lower pole kidney stones and large stones.
Quality of life after treatment
Studies should meet higher methodological standards, which include publication of an a priori protocol (Roberts 2015).
What's new
| Date | Event | Description |
|---|---|---|
| 1 August 2023 | New search has been performed | Search updated; new studies included. Current Cochrane standards applied (revision of outcomes, GRADE assessments and summary of findings tables included). Conclusions changed. |
| 1 August 2023 | New citation required and conclusions have changed | Changes to all sections and elements of the review including summary of findings tables. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 4, 2009
Acknowledgements
We would like to extend our sincere thanks to Phillip Dahm and the Cochrane Urology Editorial Teams based in Minneapolis, MN, USA, and the Korean Satellite of Cochrane Urology in Wonju, South Korea for their help and support. We also thank the peer reviewers (Carrie Price, Michael Borofsky, Michael Lipkin, Roger Sur, and Wei Qiang) for their invaluable input. We thank our copy editor Julia Turner, contact editor Jae Hung Jung, Eu Chang Hwang, Iling Kim, and Yeeun Kim for their support.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| MEDLINE (Ovid) |
|
| Embase (Ovid) |
|
| Cochrane Central |
|
| ClinicalTrials.gov | kidney AND lithotripsy |
Data and analyses
Comparison 1. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Treatment success rate at three months | 12 | 1303 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.57, 0.79] |
| 1.2 Treatment success rate at three months by stone size | 11 | 1199 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.54, 0.76] |
| 1.2.1 Stone size ≤ 20 mm | 9 | 1045 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.56, 0.76] |
| 1.2.2 Stone size > 20 mm | 3 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.11, 1.79] |
| 1.3 Treatment success rate at three months by stone location | 10 | 1139 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.56, 0.79] |
| 1.3.1 Lower pole stone | 7 | 960 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.61, 0.80] |
| 1.3.2 Non‐lower pole stone | 3 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 2.04] |
| 1.4 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Complications | 13 | 1385 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.47, 0.82] |
| 1.6 Complications by stone size | 12 | 1233 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.38, 0.79] |
| 1.6.1 Stone size ≤ 20 mm | 10 | 1085 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.39, 0.83] |
| 1.6.2 Stone size > 20 mm | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.12, 1.60] |
| 1.7 Complications by stone location | 11 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.46, 0.76] |
| 1.7.1 Lower pole stone | 8 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.33, 0.64] |
| 1.7.2 Non‐lower pole stone | 3 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.57, 1.17] |
| 1.8 Retreatment rate | 10 | 1174 | Risk Ratio (M‐H, Random, 95% CI) | 15.53 [6.62, 36.39] |
| 1.9 Auxiliary procedures rate | 8 | 1044 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [2.67, 6.52] |
| 1.10 Duration of hospital stay | 6 | 841 | Mean Difference (IV, Random, 95% CI) | ‐3.36 [‐4.74, ‐1.98] |
Comparison 2. Extracorporeal shock wave lithotripsy (ESWL) versus retrograde intrarenal surgery (RIRS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Treatment success rate at three months | 13 | 1349 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.93] |
| 2.2 Treatment success rate at three months by stone location | 11 | 1288 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.93] |
| 2.2.1 Lower pole stone | 10 | 1222 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.94] |
| 2.2.2 Non‐lower pole stone | 2 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.64, 1.02] |
| 2.3 Complications | 13 | 1305 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.63, 1.36] |
| 2.4 Complications by stone location | 10 | 1223 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.58, 1.27] |
| 2.4.1 Lower pole stone | 9 | 1177 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.55, 1.26] |
| 2.4.2 Non‐lower pole stone | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.38, 5.18] |
| 2.5 Retreatment rate | 9 | 1030 | Risk Ratio (M‐H, Random, 95% CI) | 6.78 [3.82, 12.04] |
| 2.6 Auxiliary procedures rate | 5 | 836 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.14, 3.47] |
| 2.7 Duration of hospital stay | 3 | 591 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.36, ‐1.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AbdelRazek 2021.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study duration: January 2017 to June 2–020 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: Egypt Inclusion criteria
Exclusion criteria
Sample size: 112 Number: ESWL group: 58; PCNL group: 54 Mean age: ESWL: 59.4 (SD 6.7) years; PCNL: 53.96 (SD 6.6) years Sex (M/F): ESWL: 30/24; PCNL: 34/16 Mean stone size: ESWL 22 (SD 5.1) mm; PCNL: 23.1 (SD 5.8) mm |
|
| Interventions | ESWL
PCNL
Co‐intervention: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this study, 104 patients with renal insufficiency and renal stones presented to the clinic and randomized into two groups." Comment: the sequence generation process (method of randomizations) was not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were randomized at the clinic using the closed‐envelope method into two groups." Comment: insufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 104/112; ESWL: 54/58; PCNL: 50/54 Dropouts: 7.14% (8/112) 4/58 patients in ESWL and 3/54 patients in PCNL groups did not receive allocated intervention. 0/58 patients in ESWL and 1/54 patients in PCNL groups were lost to follow‐up. Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 104/112; ESWL: 54/58; PCNL: 50/54 Dropouts: 7.14% (8/112) 4/58 patients in ESWL and 3/54 patients in PCNL groups did not receive allocated intervention. 0/58 patients in ESWL and 1/54 patients in PCNL groups were lost to follow‐up. Comment: low dropout rate (< 20%) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Ahmed 2021.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study duration: February– December 2020 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: Egypt Inclusion criteria
Exclusion criteria
Sample size: 67 Number: ESWL group: 33; mini‐PCNL group: 34 Mean age: ESWL: 40.97 (SD 13.55) years; mini‐PCNL: 42.71 (SD 9.05) years Sex (M/F): ESWL: 6/18; mini‐PCNL: 16/18 Mean stone size: ESWL 17.97 (SD 2.41) mm; mini‐PCNL: 18.29 (SD 2.52) mm |
|
| Interventions | ESWL
mini‐PCNL
Co‐intervention: none |
|
| Outcomes | Primary outcomes
Secondary outcomes
Subgroups: not reported |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: 8 June 2021 (data inquiry) Contact status: replied on 8 June 2021 Preregistered protocol: yes; clinicaltrials.gov/ct2/show/NCT04346134 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A prospective, open‐label, patients‐randomized, parallel‐group superiority trial was carried out." Quote: "Stratified by site, blocked randomization with a 1:1 ratio was used, and patients were assigned to undergo either mini‐PNL or ESWL." Comment: sequence generation process clearly described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocator and outcome assessors who had not been involved in the interventional procedures were blinded to the study." Comment: insufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors who had not been involved in the interventional procedures were blinded. |
| Blinding of subjective outcomes assessment (detection bias) | Low risk | Comment: outcome assessors who had not been involved in the interventional procedures were blinded. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 67/72; ESWL: 33/36; mini‐PCNL: 34/36 Dropouts: 6.94% (5/72) Quote: "Two patients in the mini‐PCNL group and three in the ESWL group did not complete the postoperative follow‐up schedule." Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 67/72; ESWL: 33/36; mini‐PCNL: 34/36 Dropouts: 6.94% (5/72) Quote: "Two patients in the mini‐PCNL group and three in the ESWL group did not complete the postoperative follow‐up schedule." Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 67/72; ESWL: 33/36; mini‐PCNL: 34/36 Dropouts: 6.94% (5/72) Quote: "Two patients in the mini‐PCNL group and three in the ESWL group did not complete the postoperative follow‐up schedule." Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 67/72; ESWL: 33/36; mini‐PCNL: 34/36 Dropouts: 6.94% (5/72) Quote: "Two patients in the mini‐PCNL group and three in the ESWL group did not complete the postoperative follow‐up schedule." Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Participants analyzed/randomized: total: 67/72; ESWL: 33/36; mini‐PCNL: 34/36 Dropouts: 6.94% (5/72) Quote: "Two patients in the mini‐PCNL group and three in the ESWL group did not complete the postoperative follow‐up schedule." Comment: low dropout rate (< 20%) |
| Selective reporting (reporting bias) | Low risk | Comment: published protocol was available and all pre‐specified outcomes of interest for the review were reported. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Albala 2001.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study duration: to 23 June 23 1998 Setting: multicenter Follow‐up: 3 months after treatment |
|
| Participants | Country: USA Inclusion criteria
Exclusion criteria
Sample size: 128 Number: ESWL group: 68; PCNL group: 60 Mean age: NR Sex (M/F): NR Mean stone size: ESWL: 13.59 mm; PCNL: 14.43 mm |
|
| Interventions | ESWL
PCNL
Co‐intervention: none |
|
| Outcomes | Primary outcomes
Secondary outcomes
Subgroups: stone size |
|
| Funding sources | Supported by a grant from Microvasive | |
| Declarations of interest | Financial interests or other relationships with Olympus and Boston Scientific; American Medical Systems, Inc., VidaMed and SURx, Inc; Boston Scientific, Circan ACMI, Ethicon and Applied Medical; Applied Medical; HT Medical, Mission, Olympus and Microvasive; Alza Pharmaceuticals. | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was organized as a prospective, randomized, multicenter clinical trial." Comment: the sequence generation process (method of randomization) was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors |
| Incomplete outcome data (attrition bias): treatment success rate at three months | High risk | Participants analyzed/randomized: total: 107/160; ESWL: 52/NR; PCNL: 55/NR Dropouts: 33.1% (53/160); Quote: "Follow‐up at 3 months was available for 107 of 121 (88%) eligible patients." Comment: high dropout rate (> 20%). |
| Incomplete outcome data (attrition bias): quality of life | High risk | At least 77 (48.1%) patients did not complete the QoL questionnaire (77/160). Comment: high dropout rate (> 20%). |
| Incomplete outcome data (attrition bias): complications | High risk | Dropouts: 27.5% (44/160). Comment: high dropout rate (>20%). |
| Incomplete outcome data (attrition bias): retreatment rate | High risk | Dropouts: 23.8% (38/160). Comment: high dropout rate (> 20%). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | High risk | Dropouts: 23.8% (38/160). Comment: high dropout rate (>20%). |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Dropouts: 20% (32/160); Quote: "Of the 32 patients randomized but no longer enrolled in the study 21 withdrew after randomization, stones moved or passed before surgery in 7, and 4 violated protocol." Comment: low dropout rate (20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Atis 2021.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: August 2016–August 2018 Setting: single center Follow‐up: 1 day after and 1 month after treatment |
|
| Participants | Country: Turkey Inclusion criteria
Exclusion criteria:
Sample size: 81 Number: ESWL: 36; RIRS: 45 Mean age: ESWL 47.22 years; RIRS: 47.20 years Sex (M/F): ESWL: 27/14; RIRS: 23/22 Mean stone size: ESWL: 14.50 (SD2.1) mm; RIRS: 15.29 (SD 2.0) mm |
|
| Interventions | ESWL
RIRS
All participants asked to complete the Turkish version of the SF‐36 Co‐intervention: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: 5 November 2020 (data inquiry) Contact status: no reply at time of review publication Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using computerized pseudorandom numbers, randomization was performed, and patients were assigned to RIRS or ESWL group on 1:1 basis." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of outcome assessors or data assessors. |
| Incomplete outcome data (attrition bias): quality of life | High risk | Participants analyzed/randomized: total: 81/120; ESWL: 36/60; RIRS: 45/60. Dropouts: 32.5% (39/120). Quote "Because of treatment failure, 39 patients were excluded from the study." Comment: high dropout rate (> 20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Bosio 2019.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study duration: April 2012–April 2018 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: Italy Inclusion criteria
Exclusion criteria
Sample size: 138 Number: ESWL: 68; RIRS: 70 Mean age: ESWL (51 (SD 14.6) years; RIRS: 53 (SD 12.8) years Sex (M/F): ESWL: 25/43; RIRS: 29/41 Mean stone size: ESWL: 10.8 (SD 3.3) mm; RIRS: 11.5 (SD 3.4) mm |
|
| Interventions | ESWL
RIRS
Co‐intervention: none |
|
| Outcomes |
Subgroups: none |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes | Language of publication: English Type of publication: abstract Date of contact attempt with study authors: 26 October 2020 (data inquiry) Contact status: replied on 5 November 2020 Preregistered protocol: yes; clinicaltrials.gov/ct2/show/NCT02645058 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We designed a prospective clinical trial with balanced randomization (1:1) between the 2 arms of treatment." Comment: the sequence generation process (method of randomizations) was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias): complications | Unclear risk | Comment: insufficient information in abstract; inadequately addressed the number of randomized participants. |
| Selective reporting (reporting bias) | Low risk | Comment: published protocol was available and all prespecified outcomes of interest for this review were reported. |
| Other bias | Unclear risk | Comment: no information on procedures of ESWL and RIRS in abstract. |
Bozzini 2017.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: January 2010–June 2014 Setting: multicenter Follow‐up: 3 months after treatment |
|
| Participants | Country: Europe Inclusion criteria
Exclusion criteria
Sample size: 582 Number: ESWL: 194; RIRS: 207; PCNL: 181 Mean age: ESWL: 53.3 (SD 14.8) years; RIRS: 55.8 (SD 16.1) years; PCNL: 54.8 (SD 17.2) years Sex (M/F): ESWL: 97/97; RIRS: 101/104; PCNL: 83/98 Mean stone size: ESWL: 13.78 (SD 3.1) mm; RIRS: 14.82 (SD 2.7) mm; PCNL: 15.23 (SD 3.3) mm |
|
| Interventions | ESWL
RIRS
PCNL
Co‐intervention: none |
|
| Outcomes | Primary outcome
Secondary outcomes
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization allocation sequence has been done with the online free Random Allocation Software. No equal size block was done to avoid the risk of a lower number of patients than the one needed per group." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcome assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 582/649; ESWL: 194/217; PCNL: 181/206; RIRS: 207/226 Dropouts: 10.3% (67/649) Quote: "21 lost at follow‐up and 2 not randomized to group A"; "18 lost at follow‐up and 7 not randomized to group B"; "16 lost at follow‐up and 3 not randomized to group C." Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 582/649; ESWL: 194/217; PCNL: 181/206; RIRS: 207/226 Dropouts: 10.3% (67/649) Quote: "21 lost at follow‐up and 2 not randomized to group A"; "18 lost at follow‐up and 7 not randomized to group B"; "16 lost at follow‐up and 3 not randomized to group C." Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 582/649; ESWL: 194/217; PCNL: 181/206; RIRS: 207/226 Dropouts: 10.3% (67/649) Quote: "21 lost at follow‐up and 2 not randomized to group A"; "18 lost at follow‐up and 7 not randomized to group B"; "16 lost at follow‐up and 3 not randomized to group C." Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 582/649; ESWL: 194/217; PCNL: 181/206; RIRS: 207/226 Dropouts: 10.3% (67/649) Quote: "21 lost at follow‐up and 2 not randomized to group A"; "18 lost at follow‐up and 7 not randomized to group B"; "16 lost at follow‐up and 3 not randomized to group C." Comment: low dropout rate (< 20%) |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Participants analyzed/randomized: total: 582/649; ESWL: 194/217; PCNL: 181/206; RIRS: 207/226 Dropouts: 10.3% (67/649) Quote: "21 lost at follow‐up and 2 not randomized to group A"; "18 lost at follow‐up and 7 not randomized to group B"; "16 lost at follow‐up and 3 not randomized to group C." Comment: low dropout rate (< 20%) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Carlsson 1992.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study duration: NR Setting: 2 centers Follow‐up: 4 months and 1 year after treatment |
|
| Participants | Country: Sweden Inclusion criteria
Exclusion criteria
Sample size: 49 Number: ESWL: 28; PCNL: 21 Mean age: ESWL: 49.0 years; PCNL: 48.2 years Sex (M/F): ESWL: 20/8; PCNL: 12/9 Stone size (largest stone in each participant): ESWL: range 13.5–27.0 mm; PCNL: range 12.7–25.0 mm |
|
| Interventions | ESWL
PCNL
Co‐intervention: none |
|
| Outcomes | Primary outcomes
Secondary outcomes
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Published priori protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Of these patients 25 were randomized to have PNL at the Karolinska Hospital and 30 have ESWL at Linkaping University Hospital." Comment: sequence generation process (method of randomizations) not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias): complications | Low risk | Dropouts: 10.9% (6/55). Quote: "Four patients in the PNL group did not have operation and were therefore excluded (two their stones naturally, one moved to another area and one preferred to have ESWL. In the ESWL group, one patient became pregnant and one developed malignant disease." Comment: low dropout rate (< 20%). |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Dropouts: 10.9% (6/55). Quote: "Four patients in the PNL group did not have operation and were therefore excluded (two their stones naturally, one moved to another area and one preferred to have ESWL. In the ESWL group, one patient became pregnant and one developed malignant disease." Comment: low dropout rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Deem 2011.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study duration: November 2008 to March 2010 Setting: single center Duration of follow‐up: 1 week and 3 months |
|
| Participants | Country: USA Inclusion criteria
Exclusion criteria
Sample size: 32 Number: ESWL: 12; PCNL: 20 Mean age: ESWL: 52.25 (SD 14.07) years; PCNL: 47.20 (SD 14.88) years Sex (M/F): ESWL: 6/6; PCNL: 11/9 Mean stone size: ESWL: 12.16 (SD 1.40) mm; PCNL: 12.85 (SD 2.00) mm |
|
| Interventions | ESWL
PCNL
Co‐intervention: none |
|
| Outcomes | Primary outcome
Secondary outcomes
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | Dr Julio Davalos is a consultant for Boston Scientific and Karl Storz Endoscopy America. | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: 24 December 2022 (data inquiry) Contact status: no reply at time of review publication Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomized to either a PNL or ESWL for treatment of their stone by block randomization using statistical software SAS." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information of blinding of subjective outcome assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/enrolled: total: 32/35; ESWL: 12/15; PCNL: 20/20. Dropouts: 8.57% (3/35). Quote: "Thirty‐five patients met all criteria for inclusion and were enrolled in the study. Thirty‐two patients completed the entire protocol, including follow‐up visits." Comment: low dropout rate (< 20%). |
| Incomplete outcome data (attrition bias): quality of life | Low risk | Participants analyzed/enrolled: total: 32/35; ESWL: 12/15; PCNL: 20/20. Dropouts: 8.57% (3/35). Quote: "Thirty‐five patients met all criteria for inclusion and were enrolled in the study. Thirty‐two patients completed the entire protocol, including follow‐up visits." Comment: low dropout rate (< 20%). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/enrolled: total: 32/35; ESWL: 12/15; PCNL: 20/20. Dropouts: 8.57% (3/35). Quote: "Thirty‐five patients met all criteria for inclusion and were enrolled in the study. Thirty‐two patients completed the entire protocol, including follow‐up visits." Comment: low dropout rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: No available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Fankhauser 2021.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study duration: 2011–2016 Setting: single‐center Follow‐up: 3 months |
|
| Participants | Country: Switzerland Inclusion criteria
Exclusion criteria
Sample size: 44 Number: ESWL: 21; RIRS: 23) Mean age: ESWL: 50 (SD 13.2) years; RIRS: 47 (SD 14.7) years Sex (M/F): ESWL: 16/5; RIRS: 16/7 Mean stone size: ESWL: 7.6 (SD 1.9) mm; RIRS: 8.1 (SD 2.4) mm |
|
| Interventions | ESWL
RIRS
Co‐intervention: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Published priori protocol: yes; clinicaltrials.gov/ct2/show/NCT01514032 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Balanced permutated block randomization occurred in blocks of six. The sequence of randomization was computer generated and was performed by the university hospital pharmacy using DatInf Randlist software v.1.2 (DatInf GmbH, Tübingen, Germany)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization data were kept strictly confidential in sealed envelopes that were accessible only to the primary and senior investigator." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 44/44; ESWL: 21/21; RIRS: 23/23. Dropouts: 0% (0/44). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 44/44; ESWL: 21/21; RIRS: 23/23. Dropouts: 0% (0/44). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Low risk | Comment: published protocol was available and all prespecified outcomes of interest for this review were reported. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Gadelkareem 2020.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study duration: May 2017–April 2019 Setting: single center Follow‐up: 3 months |
|
| Participants | Country: Egypt Inclusion criteria
Exclusion criteria
Sample size: 80 Number: ESWL: 40; PCNL: 40) Mean age: ESWL: 44.18 (SD 12.08) years; PCNL: 43.25 (SD 15.16) years Sex (M/F): ESWL: 26/14; PCNL: 24/16 Mean stone size: ESWL: 24.6 (SD 2.3) mm; PCNL: 25.2 (SD 3.9) mm |
|
| Interventions | ESWL
PCNL
Co‐intervention: none |
|
| Outcomes | Primary outcome
Secondary outcomes
Subgroup: NR |
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to the potential modality using a computer‐generated randomization method (JMP, version 12.0.1; SAS Institute, Cary, NC)." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Two authors were responsible to patient’s assignment and its revealing to the operator at the day of operation." Comment: insufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 80/80; ESWL: 40/40), PCNL: 40/40. Dropouts: 0% (0/80). Quote: "Lost to follow up (n=0)" Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 80/80; ESWL: 40/40), PCNL: 40/40. Dropouts: 0% (0/80). Quote: "Lost to follow up (n=0)" Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Javanmard 2015.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: March 2010–March 2014 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: Iran Inclusion criteria
Exclusion criteria
Sample size: 46 Number: ESWL: 25; RIRS: 21 Mean age: ESWL: 36.1 (SD 13.1) years; RIRS: 33.2 (SD 11.4) years Sex (M/F): ESWL: 15/10; RIRS: 13/8 Mean stone size: ESWL: 16.3 (SD 2.4) mm; RIRS: 17.1 (SD 1.9) mm |
|
| Interventions | ESWL
RIRS
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: 15 June 2022 Contact status: no reply at time of review publication Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomization was carried out using computerized random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 46/46; ESWL: 25/25; RIRS: 21/21. Dropouts: 0% (0/46). Quote: "Patients were randomly assigned to one of two groups according to the method of treatment: ESWL (group 1) and RIRS (group 2) (25 patients in group 1 and 21 patients in group 2)" and "A total of 46 patients with renal pelvic calculi and BMI greater than 30 were included in the present study." Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 46/46; ESWL: 25/25; RIRS: 21/21. Dropouts: 0% (0/46). Quote: "Patients were randomly assigned to one of two groups according to the method of treatment: ESWL (group 1) and RIRS (group 2) (25 patients in group 1 and 21 patients in group 2)" and "A total of 46 patients with renal pelvic calculi and BMI greater than 30 were included in the present study." Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 46/46; ESWL: 25/25; RIRS: 21/21. Dropouts: 0% (0/46). Quote: "Patients were randomly assigned to one of two groups according to the method of treatment: ESWL (group 1) and RIRS (group 2) (25 patients in group 1 and 21 patients in group 2)" and "A total of 46 patients with renal pelvic calculi and BMI greater than 30 were included in the present study." Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Kumar 2015a.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: December 2011–January 2012 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: India Inclusion criteria
Exclusion criteria
Sample size: 180 Number
Mean age:
Sex (M/F):
Mean stone size:
|
|
| Interventions | ESWL
RIRS
Co‐interventions: none |
|
| Outcomes |
Subgroups: stone size |
|
| Funding sources | NR | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The eligible patients were randomized into two groups: group A (ESWL group) and group B (RIRS group) using a computer‐generated randomization table." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 180/195; ESWL: 90/97; RIRS: 90/98. Dropouts: 7.69% (15/195). Quote: "Fifteen patients (seven from ESWL group and eight from RIRS group) were lost to follow up." Comment: low dropout rate (< 20%). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 180/195; ESWL: 90/97; RIRS: 90/98. Dropouts: 7.69% (15/195). Quote: "Fifteen patients (seven from ESWL group and eight from RIRS group) were lost to follow up." Comment: low dropout rate (< 20%). |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 180/195; ESWL: 90/97; RIRS: 90/98. Dropouts: 7.69% (15/195). Quote: "Fifteen patients (seven from ESWL group and eight from RIRS group) were lost to follow up." Comment: low dropout rate (< 20%). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 180/195; ESWL: 90/97; RIRS: 90/98. Dropouts: 7.69% (15/195). Quote: "Fifteen patients (seven from ESWL group and eight from RIRS group) were lost to follow up." Comment: low dropout rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Kumar 2015b.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: January 2012–May 2013 Setting: single center Duration of follow‐up: 3 months after treatment |
|
| Participants | Country: India Inclusion criteria
Exclusion criteria
Sample size: 108 Number: ESWL: 42; RIRS: 43; Miniperc: 41 Mean age: ESWL: 33.1 (SD 1.3) years; RIRS: 33.4 (SD 1.4) years; Miniperc: 33.7 (SD 1.6) years Sex (M/F): ESWL: 22/21; RIRS: 20/23; Miniperc: 20/21 Mean stone size: ESWL: 13.2 (SD 1.2) mm; RIRS: 13.1 (SD 1.1) mm; Miniperc: 13.3 (SD 1.3) mm |
|
| Interventions | ESWL
RIRS
Miniperc
Co‐interventions: none |
|
| Outcomes | Primary outcome
Secondary outcome
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: 5 November 2020 Contact status: no reply at time of review publication Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomized to ESWL, RIRS or miniperc using a computer generated randomization table of equal numbers." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | High risk | Participants analyzed/randomized: total: 126/158; ESWL: 42/52; RIRS: 43/53; Miniperc: 41/53. Dropouts: 20.3% (32/158). Quote: "Three, 2 and 4 patients, respectively, were excluded from final analysis due to a matrix stone diagnosis." The flow diagram 'Patient allocation and distribution' shows that 23 patients (7 patients in ESWL, 8 patients in RIRS and 8 patients in Miniperc groups) were lost to follow‐up. Comment: high dropout rate (> 20%). |
| Incomplete outcome data (attrition bias): complications | High risk | Participants analyzed/randomized: total: 126/158; ESWL: 42/52; RIRS: 43/53; Miniperc: 41/53. Dropouts: 20.3% (32/158). Quote: "Three, 2 and 4 patients, respectively, were excluded from final analysis due to a matrix stone diagnosis." The flow diagram 'Patient allocation and distribution' shows that 23 patients (7 patients in ESWL, 8 patients in RIRS and 8 patients in Miniperc groups) were lost to follow‐up. Comment: high dropout rate (> 20%). |
| Incomplete outcome data (attrition bias): retreatment rate | High risk | Participants analyzed/randomized: total: 126/158; ESWL: 42/52; RIRS: 43/53; Miniperc: 41/53. Dropouts: 20.3% (32/158). Quote: "Three, 2 and 4 patients, respectively, were excluded from final analysis due to a matrix stone diagnosis." The flow diagram 'Patient allocation and distribution' shows that 23 patients (7 patients in ESWL, 8 patients in RIRS and 8 patients in Miniperc groups) were lost to follow‐up. Comment: high dropout rate (> 20%). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | High risk | Participants analyzed/randomized: total: 126/158; ESWL: 42/52; RIRS: 43/53; Miniperc: 41/53. Dropouts: 20.3% (32/158). Quote: "Three, 2 and 4 patients, respectively, were excluded from final analysis due to a matrix stone diagnosis." The flow diagram 'Patient allocation and distribution' shows that 23 patients (7 patients in ESWL, 8 patients in RIRS and 8 patients in Miniperc groups) were lost to follow‐up. Comment: high dropout rate (> 20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
McCahy 2020.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: NR Setting: single‐center Follow‐up: NR |
|
| Participants | Country: Australia Inclusion criteria
Exclusion criteria
Sample size: 31 Number: ESWL: 10; RIRS 11; PCNL: 10 Median age: ESWL: 60 years; RIRS: 59 years; PCNL: 57 years Sex (M/F): ESWL: 7/3; RIRS: 8/3; PCNL: 7/3 Median stone size: ESWL: 13.5 mm; RIRS: 14.5 mm; PCNL: 14 mm |
|
| Interventions | ESWL
RIRS
PCNL
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: 5 November 2020 Contact status: no reply at time of review publication Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated list of random treatment allocations was used to assign patients to treatment in a 1:1:1 ratio. Block randomisation was employed to ensure approximate balance of treatment allocation within each stratum." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 31/31; ESWL: 10/10; RIRS: 11/11; PCNL: 10/10. Dropouts: 0% (0/31). Comment: all randomized patients completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Naguib 2016.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study date: May 2013–September 2015 Setting: single‐center Follow‐up: NR |
|
| Participants | Country: Egypt Inclusion criteria
Exclusion criteria
Sample size: 40 Number: ESWL: 20; mini‐PCNL: 20 Mean age: ESWL: NR; mini‐PCNL: NR Sex (M/F): ESWL: NR; mini‐PCNL: NR Mean stone size: ESWL: 15 (SD 3.3) mm; mini‐PCNL: 14 (SD 3.1) mm |
|
| Interventions | ESWL
Mini‐PCNL
Co‐interventions: none |
|
| Outcomes |
Subgroups: none |
|
| Funding sources | None | |
| Declarations of interest | NR | |
| Notes | Language of publication: English Type of publication: abstract Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated in 2 groups: group A [20 patients] treated by mini‐PCNL and in group B [20 patients] were treated by ESWL." Comment: random sequence generation process (method of randomizations) not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly allocated in 2 groups: group A [20 patients] treated by mini‐PCNL and in group B [20 patients] were treated by ESWL." Comment: insufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 40/40; ESWL: 20/20; mini‐PCNL: 20/20. Dropouts: 0% (0/40). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 40/40; ESWL: 20/20; mini‐PCNL: 20/20. Dropouts: 0% (0/40). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Unclear risk | Comment: insufficient information in intervention procedures and baseline characteristics in abstract. |
Pearle 2005.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study date: NS Setting: multicenter Follow‐up
|
|
| Participants | Country: USA Inclusion criteria
Exclusions criteria
Sample size: 67 Number: ESWL: 32; RIRS: 35 Mean age: ESWL: 52.5 (SD 12.3) years; RIRS: 49.3 (SD 14.2) years Sex (M/F): ESWL: 19/13; RIRS: 17/18 Mean stone surface area: ESWL: 42.2 (SD 21.2) mm2; RIRS: 35.9 (SD 18.4) mm2 |
|
| Interventions | ESWL
RIRS
Co‐intervention: none |
|
| Outcomes | Primary outcome
Secondary outcomes
Subgroups: NR |
|
| Funding sources | Boston Scientific, Natick, Massachusetts provided administrative support. | |
| Declarations of interest | Financial interest or other relationship with the following companies.
|
|
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A total of 78 patients from 19 participating institutions were randomized in blocks of 10 according to a random numbers table to undergo ESWL or URS." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcome or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | High risk | Participants analyzed/randomized: total: 58/78; ESWL: 26/NR; RIRS: 32/NR. Dropouts: 25.6% (20/78). Quote: "Of 78 randomized patients 11 dropped out of the study before treatment, leaving 67 treated on protocol, including ESWL in 32 and URS in 35. Reasons for withdrawal were stone movement out of the lower pole prior to treatment, patient refusal to undergo the specified procedure, insurance issues, medical reasons and identification of a renal mass." Comment: high dropout rate (> 20%). |
| Incomplete outcome data (attrition bias): quality of life | Unclear risk | Number of analyzed participants not reported for this outcome. |
| Incomplete outcome data (attrition bias): complications | Low risk | Dropouts: 19.2% (15/78). Comment: low dropout rate (< 20%). |
| Incomplete outcome data (attrition bias): retreatment rate | Unclear risk | Number of analyzed participants not reported for this outcome. |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Unclear risk | Number of analyzed participants not reported for this outcome. |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Dropouts: 14.1% (11/78)
Comment: low dropout rate (< 20%) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Ravier 2015.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: May 2012–February 2014 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: France Inclusion criteria
Exclusion criteria
Sample size: 30 Number: ESWL: 16; RIRS: 14 Mean age: ESWL: 52.8 (SD 4.3) years; RIRS: 50.4 (SD 4) years Sex (M/F): ESWL: 11/5; RIRS: 9/5 Mean stone size: ESWL: 10.7 (SD 1.0) mm; RIRS: 9.3 (SD 0.73) mm |
|
| Interventions | ESWL
RIRS
Co‐interventions: none |
|
| Outcomes | Primary outcome
Secondary outcomes
Subgroups: NR |
|
| Funding sources | Sponsor: Hospices Civils de Lyon | |
| Declarations of interest | None | |
| Notes | Language of publication: French Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: yes; Clinicaltrials.gov NCT01604304. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by secure online software with stratification by calculation size (5 to 10 mm, 11 to 15 mm and 16 to 20 mm), by block of 2." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | High risk | Participants analyzed/randomized: total: 17/30; ESWL: 11/16; RIRS: 6/14. Dropouts: 43.3% (13/30). Quote: "Of the 30 patients included in the study, 8 were excluded from the analysis either because they were lost to sight before treatment (2 patients) or because they withdrew their consent before treatment (4 patients) or because the stone was spontaneously expelled before treatment (2 patients). After the treatment, 4 patients were lost to follow‐up, including 3 who did not have a follow‐up visit because they had no visible residual fragments at the end of the USSR. One patient was randomized to the LEC arm and had a USSR by personal wish (Figure 2)." Comment: high dropout rate (> 20%). |
| Incomplete outcome data (attrition bias): retreatment rate | High risk | Participants analyzed/randomized: total: 17/30; ESWL: 11/16; RIRS: 6/14. Dropouts: 43.3% (13/30). Quote: "Of the 30 patients included in the study, 8 were excluded from the analysis either because they were lost to sight before treatment (2 patients) or because they withdrew their consent before treatment (4 patients) or because the stone was spontaneously expelled before treatment (2 patients). After the treatment, 4 patients were lost to follow‐up, including 3 who did not have a follow‐up visit because they had no visible residual fragments at the end of the USSR. One patient was randomized to the LEC arm and had a USSR by personal wish (Figure 2)." Comment: high dropout rate (> 20%). |
| Selective reporting (reporting bias) | High risk | Comment: published protocol was available, but only primary outcome was reported while secondary outcomes were missing. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Roy 2021.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: September 2017–August 2019 Setting: single‐center Follow‐up: 3 weeks after treatment |
|
| Participants | Country: India Inclusion criteria
Exclusion criteria
Sample size: 105 Number: ESWL: 4; mini‐PCNL: 51 Mean age: ESWL: 39.54 (SD 11.41) years; mini‐PCNL: 37.39 (SD 11.31) years Sex (M/F): ESWL: 35/19; mini‐PCNL: 35/16 Mean stone size: ESWL: 14.17 (SD 2.68) mm; mini‐PCNL: 14.80 (SD 2.74) mm |
|
| Interventions | ESWL
Mini‐PCNL
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned by a coin toss method after diagnosis to either receive ESWL or be subjected to mini‐PCNL." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 105/105; ESWL: 54/54; mini‐PCNL: 51/51. Dropouts: 0% (0/105). Comment: all randomized patients completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 105/105; ESWL: 54/54; mini‐PCNL: 51/51. Dropouts: 0% (0/105). Comment: all randomized patients completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 105/105; ESWL: 54/54; mini‐PCNL: 51/51. Dropouts: 0% (0/105). Comment: all randomized patients completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Saleh 2019.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: May 2012–February 2014 Setting: single‐center Follow‐up: NR |
|
| Participants | Country: Iran Inclusion criteria
Exclusion criteria
Sample size: 40 Number: ESWL: 20; mini‐PCNL: 20 Mean age: ESWL: 43.25 (SD 2.81) years; mini‐PCNL: 43.85 (SD 2.91) years Sex (M/F): ESWL: 12/8; PCNL: 6/14 Mean stone size: ESWL: 14.30 (SD 0.83) mm; mini‐PCNL: 14.35 (SD 0.98) mm |
|
| Interventions | ESWL
PCNL
Co‐intervention: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | Support from Ahvaz Joundishapour University of Medical Sciences. | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this study, 40 consecutive patients with <2 cm lower calyx stone were randomly allocated into two groups of patients with mPCNL and ESWL." Comment: sequence generation process (method of randomizations) not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "In this study, 40 consecutive patients with <2 cm lower calyx stone were randomly allocated into two groups of patients with mPCNL and ESWL." Comment: insufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Participants analyzed/randomized: total: 40/40; ESWL: 20/20; mini‐PCNL: 20/20. Dropouts: 0% (0/40). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Salem 2013.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: May 2010–May 2012 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: Egypt Inclusion criteria
Exclusion criteria
Sample size: 60 Number: ESWL: 0; RIRS: 30 Mean age: ESWL: 35.5 years; RIRS: 44.2 years Sex (M/F): NR Mean stone size: ESWL: 11.3 mm; RIRS: 11.5 mm |
|
| Interventions | ESWL
RIRS
Co‐intervention: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | None | |
| Declarations of interest | NR | |
| Notes | Language of publication: English Type of publication: abstract Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective randomized study was done from May 2010 to May 2012. It included patients with radiopaque unilateral, single or multiple, LC ≤20mm. Patients were divided into 2 groups." Comment: sequence generation process (method of randomizations) was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Unclear risk | Comment: insufficient information in abstract. |
| Incomplete outcome data (attrition bias): complications | Unclear risk | Comment: insufficient information in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Unclear risk | Comment: insufficient information about intervention procedures and baseline characteristics in abstract. |
Schoenthaler 2022.
| Study characteristics | ||
| Methods | Study design: parallel RCT, pilot study Study dates: October 2015–August 2018 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: Germany Inclusion criteria
Exclusion criteria
Sample size (renal stone population): 30 Number (renal stone population): ESWL: 15; RIRS:15 Mean age (renal stone population): ESWL: NR; RIRS: NR Sex (M/F; renal stone population): ESWL: NR; RIRS: NR Mean stone size (renal stone population): ESWL (SD 9.40) mm; RIRS (SD 9.93) mm |
|
| Interventions | ESWL
RIRS
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | None | |
| Declarations of interest | Arkadiusz Miernik is an advisor for Dornier MedTech Europe, RichardWolf, KarlStorz, Lisa laser OHG, and Boston Scientific. | |
| Notes | This study is a pilot study and includes both ureteric stones and renal stones. We included it to our review because it reported adequate information on stone‐free rate Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We conducted a single‐centre, parallel‐group, pilot randomised controlled trial (RCT) comparing shockwave lithotripsy (SWL) and ureteroscopy (URS)." Comment: sequence generation process (method of randomizations) was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 30/30: ESWL: 15/15); RIRS: 15/15. Dropouts: 0% (0/30). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Sener 2014.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: August–October 2012 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: Turkey Inclusion criteria
Exclusion criteria
Sample size: 140 Number: ESWL: 70; RIRS: 70 Mean age: ESWL: 42.9 (SD 5.6) years; RIRS: 45.4 (SD 6.4) years Sex (M/F): ESWL: 31/39; RIRS: 41/29 Mean stone size: ESWL: 8.2 (SD 1.2) mm; RIRS:7.8 (SD 1.3) mm |
|
| Interventions | ESWL
RIRS
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | Declared no funding source | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were randomized using an online randomization tool; www.randomizer.org/index.htm." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 140/140; ESWL: 70/70; RIRS: 70/70. Dropouts: 0/140. Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 140/140; ESWL: 70/70; RIRS: 70/70. Dropouts: 0/140. Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 140/140; ESWL: 70/70; RIRS: 70/70. Dropouts: 0/140. Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Sener 2015.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: December 2011–February 2014 Setting: single‐center Follow‐up: 3 months after treatment for intervention groups |
|
| Participants | Country: Turkey Inclusion criteria
Exclusion criteria
Sample size: 150 Number: ESWL: 50; RIRS: 50; observation: 50 Mean age: ESWL: 34.5 (SD 11.04) years; RIRS: 36.84 (SD 11.70) years; observation: 32.52 (SD 13.29) years Sex (M/F): ESWL: 37/13; RIRS: 35/15; observation: 29/21 Mean stone size: ESWL: 7.9 (SD 0.7) mm; RIRS: 8.2 (SD 1.2) mm; observation: 7.9 (SD 0.7) mm |
|
| Interventions | ESWL
RIRS
Observation
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After informed consent was obtained from the patients for all options, randomization was applied using an online randomization tool; www.randomizer.org/index.htm." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 150/150: ESWL: 50/50; RIRS: 50/50; observation: 50/50. Dropouts: 0% (0/150). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 150/150: ESWL: 50/50; RIRS: 50/50; observation: 50/50. Dropouts: 0% (0/150). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 150/150: ESWL: 50/50; RIRS: 50/50; observation: 50/50. Dropouts: 0% (0/150). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Singh 2014.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: March 2011–January 2013 Setting: single‐center Follow‐up: 3 weeks and 1 month after treatment |
|
| Participants | Country: India Inclusion criteria
Exclusion criteria
Sample size: 70 Number: ESWL: 35; RIRS: 5 Mean age: ESWL: 34.5 (SD 13.07) years; RIRS: 37.65 (SD 11.8) years Sex (M/F): ESWL: 20/15; RIRS: 22/13 Mean stone size: ESWL: 16.45 (SD 2.28) mm; RIRS: 15.05 (SD 3.56) mm |
|
| Interventions | ESWL
RIRS
Co‐interventions: none |
|
| Outcomes | Primary outcome
Secondary outcomes
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using computer‐generated pseudorandom numbers so that patients could be assigned to RIRS or ESWL group on a 1:1 basis." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 70/70; ESWL: 35/35; RIRS: 35/35. Dropouts: 0% (0/70). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 70/70; ESWL: 35/35; RIRS: 35/35. Dropouts: 0% (0/70). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 70/70; ESWL: 35/35; RIRS: 35/35. Dropouts: 0% (0/70). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Participants analyzed/randomized: total: 70/70; ESWL: 35/35; RIRS: 35/35. Dropouts: 0% (0/70). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Sohu 2019.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: May–November 2013 Setting: single‐center Follow‐up: 1 week and 3 months after treatment |
|
| Participants | Country: Pakistan Inclusion criteria
Exclusion criteria
Sample size: 60 Number: ESWL: 30; PCNL: 30 Mean age: 45.83 (SD 7.28) years for all participants (not reported for each group) Sex (M/F): 38/22 in whole sample (not reported for each group) Mean stone size: 48.26 ± 15.67 mm in whole sample (not reported for each group) |
|
| Interventions | ESWL
PCNL
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed and divided into Group A and B will undergo PCNL and ESWL respectively." Comment: sequence generation process (method of randomizations) not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 60/60; ESWL: 30/30; PCNL: 30/30 Dropouts: 0% (0/60). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 60/60; ESWL: 30/30; PCNL: 30/30 Dropouts: 0% (0/60). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 60/60; ESWL: 30/30; PCNL: 30/30 Dropouts: 0% (0/60). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Soliman 2021.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: January 2014–June 2015 Setting: single‐center Follow‐up: 3 months |
|
| Participants | Country: Egypt Inclusion criteria
Exclusion criteria
Sample size: 150 Number: ESWL: 75; miniperc: 75 Mean age: ESWL: 37.75 (SD 11.25) years; miniperc: 40.55 (SD 10.55) years Sex (M/F): ESWL: 41/34; miniperc: 34/41 Mean stone size: ESWL: 1.55 (SD 0.28) mm; miniperc: 1.57 (SD 0.26) mm |
|
| Interventions | ESWL
Miniperc
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | No funding sources | |
| Declarations of interest | All author declared no conflict of interest | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were divided randomly into two groups using computer‐generated randomization in an equal manner." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcome assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 150/150; ESWL: 75/75); miniperc: 75/75. Dropouts: 0% (0/150). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 150/150; ESWL: 75/75); miniperc: 75/75. Dropouts: 0% (0/150). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 150/150; ESWL: 75/75); miniperc: 75/75. Dropouts: 0% (0/150). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Low risk | Participants analyzed/randomized: total: 150/150; ESWL: 75/75); miniperc: 75/75. Dropouts: 0% (0/150). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Participants analyzed/randomized: total: 150/150; ESWL: 75/75); miniperc: 75/75. Dropouts: 0% (0/150). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Svihra 2021.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: January 2018–June 2019 Setting: single‐center Follow‐up: ≥ 24 weeks |
|
| Participants | Country: Slovakia Inclusion criteria
Exclusion criteria
Sample size: 66 Number: ESWL: 32: RIRS: 34 Median age: ESWL: 60.0 years; RIRS: 57.5 years Sex (M/F): ESWL: 23/9; RIRS: 17/17 Median stone size: ESWL: 9.0 (range 8.0–10.0); RIRS: 9.0 (range 7.0–14.0) |
|
| Interventions | ESWL
RIRS
Co‐intervention: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomized controlled trial with a 1:1 allocation compared the effect of RIRS and ESWL treatment. The computer generated odd (RIRS) and even numbers (ESWL) for 78 patients in the intervention part of the study." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A randomized controlled trial with a 1:1 allocation compared the effect of RIRS and ESWL treatment." Comment: insufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcome or data assessors. |
| Incomplete outcome data (attrition bias): quality of life | Low risk | Participants analyzed/randomized: total: 68/78; ESWL: 32/39; RIRS: 34/39. Dropouts: 15.4% (12/78). Quote: "In the intervention part of the study, 66 out of 78 patients (84.6%) successfully fulfilled the conditions (follow‐up of at least 24 weeks after the intervention with completion of the questionnaire in the 24th week, Table 1)." Comment: low dropout rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Terribile 2019.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: NR Setting: single‐center Follow‐up: 10 days and 3 months after treatment |
|
| Participants | Country: Italy Inclusion criteria
Exclusion criteria
Sample size: 98 Number: ESWL: 33; PCNL: 30; RIRS: 35 Mean age: NR Sex (M/F): NR Mean stone size: ESWL: 12.98 mm; PCNL: 14.32 mm; RIRS: 13.88 mm |
|
| Interventions | ESWL
RIRS
PCNL
Co‐interventions: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes | Language of publication: English Type of publication: abstract Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized into three groups: Group A: ESWL (33 pts); Group B: RIRS (35 pts); Group C: PCNL (30 pts)." Comment: sequence generation process (method of randomizations) not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcome assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Unclear risk | Comment: insufficient information in abstract. |
| Incomplete outcome data (attrition bias): complications | Unclear risk | Comment: insufficient information in abstract. |
| Incomplete outcome data (attrition bias): retreatment rate | Unclear risk | Comment: insufficient information in abstract. |
| Incomplete outcome data (attrition bias): auxiliary procedures rate | Unclear risk | Comment: insufficient information in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Unclear risk | Comment: insufficient information on intervention procedures and baseline characteristics in abstract. |
Vilches 2015.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: January 2009–November 2010 Setting: single‐center Follow‐up: 2 months after treatment |
|
| Participants | Country: Chile Inclusion criteria
Exclusion criteria
Sample size: 55 Number: ESWL: 31; RIRS: 24 Mean age: ESWL: 45.6 (SD 13.7); RIRS: 43.7 (SD 9.2) Sex (M/F): ESWL: 18/13; RIRS: 15/9 Mean stone size: ESWL: 9.6 (SD 0.6) mm; RIRS: 9.7 (SD 0.5) mm |
|
| Interventions | ESWL
RIRS
Co‐intervention: none |
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | None | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of patients to either ESWL or RIRS was performed by using RANDI 0.9 randomization soft‐ware, which is available on the Internet (available at: http://dschri mpf.github.io/randi3/)." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 55/63; ESWL: 31/32; RIRS: 24/31. Dropouts: 12.7% (8/63).
Comment: low dropout rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Yuruk 2010.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: April 2007–August 2008 Setting: single‐center Follow‐up: 3 and 12 months |
|
| Participants | Country: Turkey Inclusion criteria
Exclusion criteria
Sample size: 62 Number: ESWL: 31; PCNL: 31; observation: 32 Mean age: ESWL: 44.5 (SD 9.4) years; PCNL: 44.1 (SD 12.3) years: observation: 44.0 (SD 12.2) years Sex (M/F): ESWL: 16/15; PCNL: 15/16; observation: 19/13 Mean stone surface area: ESWL: 139.4 (SD 65.1) mm2; PCNL: 153.3 (SD 39.5) mm2; observation: 136.7 (SD 51.4) mm2 |
|
| Interventions | ESWL
PCNL
Observation
|
|
| Outcomes |
Subgroups: NR |
|
| Funding sources | NR | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 99 patients with asymptomatic lower caliceal stones were prospectively randomized into ESWL, PNL and observation groups." Comment: sequence generation process (method of randomizations) not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcomes assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 94/99; ESWL: 31/33; PCNL: 31/33; observation: 32/33. Dropouts: 5.1% (5/99). Quote: "Five patients withdrew from the study due to the lack of follow‐up data. The table lists final patient demographics and stone characteristics." Comment: low dropout rate (< 20%). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 94/99; ESWL: 31/33; PCNL: 31/33; observation: 32/33. Dropouts: 5.1% (5/99). Quote: "Five patients withdrew from the study due to the lack of follow‐up data. The table lists final patient demographics and stone characteristics." Comment: low dropout rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Zhang 2019.
| Study characteristics | ||
| Methods | Study design: parallel RCT Study dates: March 2015–March 2017 Setting: single‐center Follow‐up: 3 months after treatment |
|
| Participants | Country: Peoples Republic of China Inclusion criteria
Exclusion criteria
Sample size: 180 Number: ESWL: 60; UMP: 60; RIRS: 60 Mean age: ESWL: 50.51 (SD 12.59) years; UMP: 48.92 (SD 11.08) years; RIRS: 50.05 (SD 11.91) years Sex (M/F): ESWL: 35/25; UMP: 37/23; RIRS: 34/26 Mean stone size: ESWL: 14.88 (SD 2.86) mm; UMP: 15.48 (SD 2.45) mm; RIRS: 14.63 (SD 2.67) mm |
|
| Interventions | ESWL
UMP
RIRS
Co‐interventions: none |
|
| Outcomes | Primary outcome
Secondary outcome
Subgroups: NR |
|
| Funding sources | Grants from the Shanghai Pudong New Area Bureau of Health (no.PW2016B‐10) and the Shanghai Leading Academic Discipline Project allocated to the Shanghai Pudong New Area Gongli Hospital (no. ZK2015A11) | |
| Declarations of interest | None | |
| Notes | Language of publication: English Type of publication: full text Date of contact attempt with study authors: none Contact status: NA Preregistered protocol: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients were randomly divided into 3 groups: Group A, managed by UMP; Group B, managed by FURS and Group C managed by ESWL." Comment: sequence generation process (method of randomizations) not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded. |
| Blinding of objective outcomes assessment (detection bias) All outcomes | Low risk | Comment: outcomes unlikely to be influenced by lack of blinding. |
| Blinding of subjective outcomes assessment (detection bias) | Unclear risk | Comment: no information on blinding of subjective outcome assessors or data assessors. |
| Incomplete outcome data (attrition bias): treatment success rate at three months | Low risk | Participants analyzed/randomized: total: 180/180; ESWL: 60/60; UMP: 60/60; RIRS: 60/60. Dropouts: 0% (0/180). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): complications | Low risk | Participants analyzed/randomized: total: 180/180; ESWL: 60/60; UMP: 60/60; RIRS: 60/60. Dropouts: 0% (0/180). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): retreatment rate | Low risk | Participants analyzed/randomized: total: 180/180; ESWL: 60/60; UMP: 60/60; RIRS: 60/60. Dropouts: 0% (0/180). Comment: all randomized participants completed the study (no missing outcome data). |
| Incomplete outcome data (attrition bias): duration of hospital stay | Low risk | Participants analyzed/randomized: total: 180/180; ESWL: 60/60; UMP: 60/60; RIRS: 60/60. Dropouts: 0% (0/180). Comment: all randomized participants completed the study (no missing outcome data). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no available protocol. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
BMI: body mass index; DJ: double‐J; eGFR: estimated glomerular filtration rate; EQ: efficiency quotient; ESWL: extracorporeal shock wave lithotripsy; GFR: glomerular filtration rate; Ho:YAG: holmium:yttrium aluminum garnet; HU: Hounsfield units; KUB: kidney, ureter, and bladder; NA: not applicable; NR: not reported; PCNL: percutaneous nephrolithotomy; QoL: quality of life; RCT: randomized controlled trial; RIRS: retrograde intrarenal surgery; SCr: serum creatinine; SD: standard deviation; SF‐8: 8‐Item Short Form Health Survey; SF‐36: 36‐Item Short Form Health Survey; UMP: ultra‐mini percutaneous nephrolithotomy; URS: ureteroscope; US: ultrasound; UTI: urinary tract infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Charig 1986 | Time‐series of treatments including surgery, PCNL and ESWL. |
| ChiCTR2000031520 | Studies in migrated stone during laparoscopic pyelolithotomy and ureterolithotomy. |
| El‐Nahas 2012 | Not an RCT or quasi‐RCT. |
| Eterovic 2005 | Not an RCT or quasi‐RCT. |
| Hassan 2015 | Not an RCT or quasi‐RCT. |
| Koo 2011 | Not an RCT or quasi‐RCT. |
| Liou 2001 | Not an RCT or quasi‐RCT. |
| Mays 1988 | Not an RCT or quasi‐RCT. |
| Meretyk 1997 | Comparison between ESWL monotherapy and ESWL combined with PCNL. |
| NCT02658942 | Terminated ongoing study. We contacted the study authors on 31 January 2021 for clarification on trial status and received a response the same day. |
| NCT04317443 | Treatment in children. |
| Preminger 2006 | Review article. |
| Resorlu 2013 | Not an RCT or quasi‐RCT. |
| Romeu 2021 | Not an RCT or quasi‐RCT. |
| Turna 2007 | Not an RCT or quasi‐RCT. |
| You 2006 | Not an RCT or quasi‐RCT. |
| Zeng 2012 | Treatment in infants. |
ESWL: extracorporeal shock wave lithotripsy; PCNL: percutaneous nephrolithotomy; RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐INR‐17013906.
| Study name | Soton ureteroscope versus extracorporeal shock wave lithotripsy: a prospective randomized controlled trial in patients with 1‐2cm pelvis stones |
| Methods | Country: People's Republic of China Setting: single‐center Design: parallel RCT |
| Participants | Sample size: 100 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | Planned starting date: 1 March 2018 Estimated study completion date: 28 February 2019 |
| Contact information | Study leader: Lv Jian‐lin; ljlxx01@163.com; +86 13770631917 |
| Notes | Date of contact attempt with study authors: 27 June 2021 (inquiry about trial status) Contact status: no reply at time of review publication |
ISRCTN98970319.
| Study name | The clinical and cost‐effectiveness of surgical interventions for stones in the lower pole of the kidney: the percutaneous nephrolithotomy, flexible ureterorenoscopy and extracorporeal shockwave lithotripsy for lower pole kidney stones randomized controlled trial (PUrE RCT) protocol |
| Methods | Country: UK Setting: multicenter Design: parallel RCT |
| Participants | Sample size: 1044 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | Planned starting date: May 2016 Estimated study completion date: February 2023 |
| Contact information | Sam McClinton MD; pure@abdn.ac.uk |
| Notes | Date of contact attempt with study authors: 7 June 2022 (inquiry about trial status) Contact status: study author response 23 February 2023; study completed. No data available yet but report in progress. |
NCT02522676.
| Study name | Evaluation of Different Treatment Modalities for Lower Pole and Renal Pelvis Stones |
| Methods | Country: Turkey Setting: single‐center Design: parallel RCT |
| Participants | Sample size: 300 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | Planned starting date: June 2020 Estimated study completion date: December 2023 |
| Contact information | Murat Akand, M.D. Selcuk University, School of Medicine, Department of Urology |
| Notes | Date of contact attempt with study authors: 21 February 2023 (Cochrane Editorial Group) Contact status: 24 February 2023: study not conducted due to loss of funding |
NCT04856722.
| Study name | Mini‐percutaneous nephrolithotomy, retrograde intrarenal surgery, and extracorporeal shock wave lithotripsy for treatment of medium‐sized, high‐density, non‐lower pole, renal stones |
| Methods |
|
| Participants | Simple size: 180 Inclusion criteria
Exclusion Criteria:
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes:
|
| Starting date | Planned starting date: 5 April 2021 Estimated study completion date: July 2022 |
| Contact information | Abul‐fotouh Ahmed, MD; abulfotouhahmed@yahoo.com |
| Notes | Date of contact attempt with study authors: 7 June 2022 (inquiry for trial status) Contact status: no reply at time of review publication |
CT: computed tomography; EQ‐5D‐5L: EuroQol Five‐Dimension, Five‐Level Questionnaire; ESWL: extracorporeal shock wave lithotripsy; HU: Hounsfield unit; KUB: kidney, ureter, and bladder; NA: not applicable; NGAL: neutrophil gelatinase‐associated lipocalin; NR: not reported; NRS: numeric rating scale; PCNL: percutaneous nephrolithotomy; RCT: randomized controlled trial; RIRS: retrograde intrarenal surgery; SF‐12: 12‐Item Short Form Health Survey; UTI: urinary tract infection.
Differences between protocol and review
This review was based on a dated protocol (Srisubat 2008) and two previously published reviews (Srisubat 2009; Srisubat 2014). Cochrane methods have advanced considerably since then. As a result, we made the following changes.
The review now focuses on patient‐important outcomes. We dropped efficiency quotient, operative time, and cost‐effectiveness.
To interpret results, we used a minimally contextualized approach that made reasonable assumptions about clinically important differences drawn (whenever possible) from the literature, or informed by the review authors' content expertise.
We rated the certainty of evidence using GRADE and included summary of findings tables.
Contributions of authors
VS: drafted the protocol, wrote the review, screened studies, extracted data, performed data entry and analyses, assessed risk of bias, and graded the certainty of the evidence.
AS: helped draft the protocol, extracted data, and assessed risk of bias.
SP: helped draft the protocol, screened studies for inclusion, extracted data, and assessed risk of bias.
BL: screened studies for inclusion and extracted data.
PP: performed data entry and analyses, assessed risk of bias, graded the certainty of the evidence, and wrote the review.
Sources of support
Internal sources
-
Cochrane Thailand, Thailand
For working spaces, and strengthening methodological skills to complete the review.
External sources
-
No external source of support, Thailand
No external source of support
Declarations of interest
VS: none AS: none SP: none BL: none PP: none
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
AbdelRazek 2021 {published data only}
- AbdelRazek M, Abolyosr A, AbdelKader MS, Hassan AM, Hamed AA, Alsagheer G. Percutaneous nephrolithotomy versus extracorporeal shock wave lithotripsy for renal insufficiency. World Journal of Urology 2021;39(12):4477-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ahmed 2021 {published data only}
- Ahmed AF, Abdelazim H, ElMesery M, El-Feky M, Gomaa A, Tagreda I, et al. Mini-percutaneous nephrolithotomy is a safe alternative to extracorporeal shockwave lithotripsy for high-density, renal stones: a prospective, randomised trial. BJU international 2021;128(6):744-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Albala 2001 {published data only}
- Albala DM, Assimos DG, Clayman RV, Denstedt JD, Grasso M, Gutierrez-Aceves J, et al. Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results. Journal of Urology 2001;166(6):2072-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Atis 2021 {published data only}
- Atis G, Culpan M, Ucar T, Sendogan F, Kazan HO, Yildirim A. The effect of shock wave lithotripsy and retrograde intrarenal surgery on health-related quality of life in 10-20 mm renal stones: a prospective randomized pilot study. Urolithiasis 2021;49(3):247-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Culpan M, Atis G, Sendogan F, Ucar T, Yildirim A, Caskurlu T. The effect of shock wave lithotripsy and retrograde intrarenal surgery on health-related quality of life in 10-20mm renal stones: a prospective randomized study. European Urology Supplements 2019;18(1):e363-4. [Google Scholar]
Bosio 2019 {published data only}
- Bosio A, Ajessandria E, Agosti S, Vitiello F, Vercelli E, Gontero P. RIRS versus ESWL in the treatment of kidney stones, preliminary results of a RCT. In: European Urology Supplements. Vol. 18. 2019:e2763.
- Bosio A, Alessandria E, Agosti S, Vitiello F, Vercelli E, Dalmasso E, et al. Flexible ureterorenoscopy versus shock wave lithotripsy for kidney stones < 2 cm: results from a single centre randomized controlled trial. European Urology Open Science 2020;20:S71. [DOI] [PubMed] [Google Scholar]
- Bosio A, Alessandria E, Agosti S, Vitiello F, Vercelli E, Gontero P. Is flexible ureterorenoscopy superior to eswl in the treatment of 6-20 mm renal stones? Preliminary results of a RCT. Journal of Urology 2020;203:e360. [Google Scholar]
- Bosio A, Alessandria E, Dalmasso E, Agosti S, Vitiello F, Vercelli E, et al. Flexible ureterorenoscopy versus shockwave lithotripsy for kidney stones ≤2 cm: a randomized controlled trial flexible ureterorenoscopy versus shockwave lithotripsy for kidney stones ≤2 cm: a randomized controlled trial [8(6):1816-1822]. European Urology Focus 2022;8(6):1816-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bozzini 2017 {published data only}
- Bozzini G, Verze P, Arcaniolo D, Dal Piaz O, Buffi NM, Guazzoni G, et al. A prospective randomized comparison among SWL, PCNL and RIRS for lower calyceal stones less than 2 cm: a multicenter experience: a better understanding on the treatment options for lower pole stones. World Journal of Urology 2017;35(12):1967-75. [DOI] [PubMed] [Google Scholar]
Carlsson 1992 {published data only}
- Carlsson P, Kinn AC, Tiselius HG, Ohlsen H, Rahmqvist M. Cost effectiveness of extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy for medium-sized kidney stones. A randomised clinical trial. Scandinavian Journal of Urology & Nephrology 1992;26(3):257-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deem 2011 {published data only}
- Deem S, Defade B, Modak A, Emmett M, Martinez F, Davalos J. Percutaneous nephrolithotomy versus extracorporeal shock wave lithotripsy for moderate sized kidney stones. Urology 2011;78(4):739-43. [DOI] [PubMed] [Google Scholar]
Fankhauser 2021 {published data only}
- Fankhauser CD, Weber D, Müntener M, Poyet C, Sulser T, Hermanns T. Effectiveness of flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for renal calculi of 5–15 mm: results of a randomized controlled trial. European Urology Open Science 2021;25:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadelkareem 2020 {published data only}
- Gadelkareem RA, Abdelsalam YM, Ibraheim MA, Reda A, Sayed MA, El-Azab AS. Is percutaneous nephrolithotomy the modality of choice versus extracorporeal shockwave lithotripsy for a 20 to 30 mm single renal pelvic stone with ≤1000 Hounsfield Unit in adults? A prospective randomized comparative study. Journal of Endourology 2020;34(11):1141-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Javanmard 2015 {published data only}
- Javanmard B, Razaghi MR, Ansari Jafari A, Mazloomfard MM. Flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for the treatment of renal pelvis stones of 10-20 mm in obese patients. Journal of Lasers in Medical Sciences 2015;6(4):162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2015a {published data only}
- Kumar A, Vasudeva P, Nanda B, Kumar N, Das MK, Jha SK. A prospective randomized comparison between shock wave lithotripsy and flexible ureterorenoscopy for lower caliceal stones < 2 cm: a single-center experience. Journal of Endourology 2015;29(5):575-9. [DOI] [PubMed] [Google Scholar]
Kumar 2015b {published data only}
- Kumar A, Kumar N, Vasudeva P, Jha SK, Kumar R, Singh H. A prospective, randomized comparison of shock wave lithotripsy, retrograde intrarenal surgery and miniperc for treatment of 1 to 2 cm radiolucent lower calyceal renal calculi: a single center experience. Journal of Urology 2015;193:160-4. [DOI] [PubMed] [Google Scholar]
McCahy 2020 {published data only}
- McCahy PJ, Hong M, Paul E, Berman I, Shahbaz S. Shock-wave lithotripsy, ureterorenoscopy and percutaneous nephrolithotomy for 1–2 cm renal stones: a randomised pilot study. Journal of Clinical Urology 2020;13(6):413-8. [DOI: 10.1177/2051415820935663] [DOI] [Google Scholar]
Naguib 2016 {published data only}
- Naguib M, Eliwa, Seleem M, Abdulwahab K, Elsayed E, Abdulmaksood M, et al. Outcome of mini-PCNL versus extracorporeal shock wave lithotripsy in treatment of single lower calyceal stone 10-20mm with favorable lower calyceal anatomy: a prospective randomized study. In: Journal of Urology. Vol. 195. 2016:e507-8.
Pearle 2005 {published data only}
- Pearle MS, Lingeman JE, Leveillee R, Kuo R, Preminger GM, Nadler RB, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. Journal of Urology 2008;179(Suppl 5):S69-73. [DOI] [PubMed] [Google Scholar]
- Pearle MS, Lingeman JE, Leveillee R, Kuo R, Preminger GM, Nadler RB, et al. Prospective, randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. Journal of Urology 2005;173(6):2005-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ravier 2015 {published data only}
- Ravier E, Abid N, Ruffion A, Fassi-Fehri H, Buron C, Ganne C, et al. Effectiveness of flexible ureteroscopy versus extracorporeal shock wave lithotripsy for kidney stones treatment. Progres en Urologie 2015;25(5):233-9. [DOI] [PubMed] [Google Scholar]
Roy 2021 {published data only}
- Roy P, Sarkar D, Jalan V, Pal D. Comparative study of extracorporeal shock wave lithotripsy versus mini percutaneous nephrolithotomy for the treatment of nonlower calyceal 10–20 mm size kidney stone. Urological Science 2021;32(2):83-8. [DOI: 10.4103/UROS.UROS_134_20] [DOI] [Google Scholar]
Saleh 2019 {published data only}
- Saleh M, Khazaeli D, Dadfar M. Comparison of success rate and complications of mini PCNL with ESWL in treatment of <2cm lower pole kidney stones. International Journal of Medical Reviews and Case Reports 2019;3(12):832. [Google Scholar]
Salem 2013 {published data only}
- Salem A, Saad I, Emran A, Abdelhakiem M, Abdelrazzak O, Abdelkader M. Laser lithotripsy versus ESWL for lower calyceal renal stones. In: Journal of Urology. Vol. 189. 2013:e751.
Schoenthaler 2022 {published data only}
- Schoenthaler M, Hein S, Wilhelm K, Pohlmann PF, Praus F, Walther T, et al. Feasibility of an updated randomised controlled trial on surgical urolithiasis treatments: the pilot trial for the German Endoscopic versus Shock Wave Therapy Study (GESS). European Urology Focus 2022;8(1):271-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sener 2014 {published data only}
- Sener NC, Imamoglu MA, Bas O, Ozturk U, Goktug HN, Tuygun C, et al. Prospective randomized trial comparing shock wave lithotripsy and flexible ureterorenoscopy for lower pole stones smaller than 1 cm. Urolithiasis 2014;42(2):127-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sener 2015 {published data only}
- Sener NC, Bas O, Sener E, Zengin K, Ozturk U, Altunkol A, et al. Asymptomatic lower pole small renal stones: shock wave lithotripsy, flexible ureteroscopy, or observation? A prospective randomized trial. Urology 2015;85(1):33-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Singh 2014 {published data only}
- Singh BP, Prakash J, Sankhwar SN, Dhakad U, Sankhwar PL, Goel A, et al. Retrograde intrarenal surgery vs extracorporeal shock wave lithotripsy for intermediate size inferior pole calculi: a prospective assessment of objective and subjective outcomes. Urology 2014;83:1016-22. [DOI] [PubMed] [Google Scholar]
Sohu 2019 {published data only}
- Sohu S, Shaikh AA, Shaikh AA, Shaikh AB. Comparison of outcome of extracorporeal shockwave lithotripsy versus percutaneous lithotripsy in partial staghorn renal stone. Rawal Medical Journal 2019;44(2):311-13. [Google Scholar]
Soliman 2021 {published data only}
- Soliman T, Sherif H, Sebaey A, Mohey A, Elmohamady BN. Miniperc vs shockwave lithotripsy for average-sized, radiopaque lower pole calculi: a prospective randomized study. Journal of Endourology 2021;35(6):896-901. [PMID: ] [DOI] [PubMed] [Google Scholar]
Svihra 2021 {published data only}
- Svihra J Jr, Sopilko I, Svihrova V, Student V, Luptak J. Is health-related quality of life of patients after single-use flexible ureteroscopy superior to extracorporeal shock wave lithotripsy? A randomised prospective study. Urolithiasis 2021;49(1):73-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Terribile 2019 {published data only}
- Terribile M, Arcaniolo D, Bottone F, Stizzo M, Amicuzi U, Oliva F, et al. Efficacy and safety of SWL, RIRS and PCNL in lower caliceal stones. In: European Urology Supplements. Vol. 18. 2019:e2971.
Vilches 2015 {published data only}
- Vilches RM, Aliaga A, Reyes D, Sepulveda F, Mercado A, Moya F, et al. Comparison between retrograde intrarenal surgery and extracorporeal shock wave lithotripsy in the treatment of lower pole kidney stones up to 15 mm. Prospective, randomized study. Actas Urologicas Espanolas 2015;39(4):236-42. [DOI] [PubMed] [Google Scholar]
Yuruk 2010 {published data only}
- Yuruk E, Binbay M, Sari E, Akman T, Altinyay E, Baykal M, et al. A prospective, randomized trial of management for asymptomatic lower pole calculi. Journal of Urology 2010;183(4):1424-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2019 {published data only}
- Zhang H, Hong TY, Li G, Jiang N, Hu C, Cui X, et al. Comparison of the efficacy of ultra-mini PCNL, flexible ureteroscopy, and shock wave lithotripsy on the treatment of 1-2 cm lower pole renal calculi. Urologia Internationalis 2019;102(2):153-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Charig 1986 {published data only}
- Charig CR, Webb DR, Payne SR, Wickham JE. Comparison of treatment of renal calculi by open surgery, percutaneous nephrolithotomy, and extracorporeal shockwave lithotripsy. British Medical Journal (Clinical research ed.) 1986;292(6524):879-82. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR2000031520 {published data only}
- ChiCTR2000031520. Prospective randomized comparison of transabdominal rigid ureteroscopy versus postoperative extracorporeal shock wave lithotripsy (ESWL) in stone migration during laparoscopic pyelolithotomy and ureterolithotomy. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000031520 (first received 3 March 2020).
El‐Nahas 2012 {published data only}
- El-Nahas AR, Ibrahim HM, Youssef RF, Sheir KZ. Flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for treatment of lower pole stones of 10-20 mm. BJU International 2012;110(6):898-902. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eterovic 2005 {published data only}
- Eterović D, Situm M, Juretić-Kuscić L, Dujić Z. A decrease in blood pressure following pyelolithotomy but not extracorporeal lithotripsy. Urological Research 2005;33(2):93-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hassan 2015 {published data only}
- Hassan M, El-Nahas AR, Sheir KZ, El-Tabey NA, El-Assmy AM, Elshal AM, et al. Percutaneous nephrolithotomy vs. extracorporeal shockwave lithotripsy for treating a 20-30 mm single renal pelvic stone. Arab Journal of Urology 2015;13(3):212-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koo 2011 {published data only}
- Koo V, Young M, Thompson T, Duggan B. Cost-effectiveness and efficiency of shockwave lithotripsy vs flexible ureteroscopic holmium:yttrium-aluminium-garnet laser lithotripsy in the treatment of lower pole renal calculi. BJU International 2011;108(11):1913-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liou 2001 {published data only}
- Liou LS, Streem SB. Long-term renal functional effects of shock wave lithotripsy, percutaneous nephrolithotomy and combination therapy: a comparative study of patients with solitary kidney. The Journal of Urology 2001;166(1):36; discussion 36-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mays 1988 {published data only}
- Mays N, Challah S, Patel S, Palfrey E, Creeser R, Vadera P, et al. Clinical comparison of extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy in treating renal calculi. BMJ (Clinical research ed.) 1988;297(6643):253-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meretyk 1997 {published data only}
- Meretyk S, Gofrit ON, Gafni O, Pode D, Shapiro A, Verstandig A, et al. Complete staghorn calculi: random prospective comparison between extracorporeal shock wave lithotripsy monotherapy and combined with percutaneous nephrostolithotomy. The Journal of Urology 1997;157(3):780-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT02658942 {published data only}
- NCT02658942. Flexible ureteroscopy versus ESWL in the management of lower calyceal stones. clinicaltrials.gov/show/NCT02658942 (first received 20 January 2016).
NCT04317443 {published data only}
- NCT04317443. Comparison of SMP and ESWL for the treatment of renal stones ≥20 mm in children. clinicaltrials.gov/show/NCT04317443 (first received 23 March 2020).
Preminger 2006 {published data only}
- Preminger GM. Management of lower pole renal calculi: shock wave lithotripsy versus percutaneous nephrolithotomy versus flexible ureteroscopy. Urological Research 2006;34(2):108-11. [DOI] [PubMed] [Google Scholar]
Resorlu 2013 {published data only}
- Resorlu B, Unsal A, Ziypak T, Diri A, Atis G, Guven S, et al. Comparison of retrograde intrarenal surgery, shockwave lithotripsy, and percutaneous nephrolithotomy for treatment of medium-sized radiolucent renal stones. World Journal of Urology 2013;31(6):1581-6. [DOI] [PubMed] [Google Scholar]
Romeu 2021 {published data only}
- Romeu G, Marzullo-Zucchet LJ, Díaz J, Villarroya S, Budía A, Ordaz DG, et al. Comparing extracorporeal shock wave lithotripsy and ureteroscopy laser lithotripsy for treatment of urinary stones smaller than 2 cm: a cost-utility analysis in the Spanish clinical setting. World Journal of Urology 2021;39(9):3593-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Turna 2007 {published data only}
- Turna B, Raza A, Moussa S, Smith G, Tolley DA. Management of calyceal diverticular stones with extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy: long-term outcome. BJU International 2007;100(1):151-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
You 2006 {published data only}
- You YD, Kim JM, Kim ME. Comparison of the cost and effectiveness of different medical options for treating lower calyceal stones less than 2cm: extracorporeal shock wave lithotripsy versus percutaneous nephrolithotomy. Korean Journal of Urology 2006;47(7):703-7. [Google Scholar]
Zeng 2012 {published data only}
- Zeng G, Jia J, Zhao Z, Wu W, Zhao Z, Zhong W. Treatment of renal stones in infants: comparing extracorporeal shock wave lithotripsy and mini-percutaneous nephrolithotomy. Urological Research 2012;40(5):599-603. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐INR‐17013906 {published data only}
- ChiCTR-INR-17013906. Soton ureteroscope versus extracorporeal shock wave lithotripsy: a prospective randomized controlled trial in patients with 1-2cm pelvis stones. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-INR-17013906 (first received 13 December 2017).
ISRCTN98970319 {published data only}
- ISRCTN98970319. PUrE: percutaneous nephrolithotomy, flexible ureterorenoscopy and extracorporeal shockwave lithotripsy for lower pole kidney stones. www.isrctn.com/ISRCTN98970319 (first received 11 November 2015).
NCT02522676 {published data only}
- NCT02522676. Evaluation of different treatment modalities for lower pole and renal pelvis stones. clinicaltrials.gov/show/NCT02522676 (first received 13 August 2015).
NCT04856722 {published data only}
- NCT04856722. Mini- percutaneous nephrolithotomy, retrograde intrarenal surgery, and extracorporeal shock wave lithotripsy for treatment of medium-sized, high-density, non-lower pole, renal stones. clinicaltrials.gov/show/NCT04856722 (first received 23 April 2021).
Additional references
Abdelhamid 2016
- Abdelhamid M, Mosharafa AA, Ibrahim H, Selim HM, Hamed M, Elghoneimy MN, et al. A prospective evaluation of high-resolution ct parameters in predicting extracorporeal shockwave lithotripsy success for upper urinary tract calculi. Journal of Endourology 2016;30(11):1227-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
AUA guideline 2016
- Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical management of stones: American Urological Association/Endourological Society Guideline, PART II. Journal of Urology 2016;196(4):1161-9. [DOI] [PubMed] [Google Scholar]
Celik 2015
- Celik S, Bozkurt O, Kaya FG, Egriboyun S, Demir O, Secil M, et al. Evaluation of computed tomography findings for success prediction after extracorporeal shock wave lithotripsy for urinary tract stone disease. International Urology and Nephrology 2015;47(1):69-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chung 2019a
- Chung KJ, Kim JH, Min GE, Park HK, Li S, Del Giudice F, et al. Changing trends in the treatment of nephrolithiasis in the real world. Journal of Endourology 2019;33(3):248-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chung 2019b
- Chung DY, Kang DH, Cho KS, Jeong WS, Jung HD, Kwon JK, et al. Comparison of stone-free rates following shock wave lithotripsy, percutaneous nephrolithotomy, and retrograde intrarenal surgery for treatment of renal stones: A systematic review and network meta-analysis. PLOS One 2019;14(2):e0211316. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated August 2019). Available from www.training.cochrane.org/handbook/archive/v6.
Donaldson 2015
- Donaldson JF, Lardas M, Scrimgeour D, Stewart F, MacLennan S, Lam TB, et al. Systematic review and meta-analysis of the clinical effectiveness of shock wave lithotripsy, retrograde intrarenal surgery, and percutaneous nephrolithotomy for lower-pole renal stones. European Urology 2015;67(4):612-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
EAU guideline 2023
- Skolarikos A, Jung H, Neisius A, Petrik A, Somani B, Tailly T, et al. EAU guidelines on urolithiasis. EUA guidelines office, Arnhem, The Netherlands, 2023. [Google Scholar]
Fink 2013
- Fink HA, Wilt TJ, Eidman KE, Garimella PS, MacDonald R, Rutks IR, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Annals of Internal Medicine 2013;158(7):535-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed before 8 June 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available from gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ (Clinical research ed.) 2008;336(7651):995-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated August 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook/archive/v6. [DOI] [PMC free article] [PubMed]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Hultcrantz 2017
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. Journal of Clinical Epidemiology 2017;87:4-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jayadevappa 2017
- Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health-related quality of life-a systematic review. Journal of Clinical Epidemiology 2017;89:188-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Junbo 2019
- Junbo L, Yugen L, Guo J, Jing H, Ruichao Y, Tao W. Retrograde intrarenal surgery vs. percutaneous nephrolithotomy vs. extracorporeal shock wave lithotripsy for lower pole renal stones 10-20 mm: a meta-analysis and systematic review. Urology Journal 2019;16(2):97-106. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kallidonis 2020
- Kallidonis P, Ntasiotis P, Somani B, Adamou C, Emiliani E, Knoll T, et al. Systematic review and meta-analysis comparing percutaneous nephrolithotomy, retrograde intrarenal surgery and shock wave lithotripsy for lower pole renal stones less than 2 cm in maximum diameter. Journal of Urology 2020;204(3):427-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2020
- Kim CH, Chung DY, Rha KH, Lee JY, Lee SH. Effectiveness of percutaneous nephrolithotomy, retrograde intrarenal surgery, and extracorporeal shock wave lithotripsy for treatment of renal stones: a systematic review and meta-analysis. Medicina (Kaunas, Lithuania) 2020;57(1):E26. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015
- Lee SW, Chaiyakunapruk N, Chong HY, Liong ML. Comparative effectiveness and safety of various treatment procedures for lower pole renal calculi: a systematic review and network meta-analysis. BJU International 2015;116(2):252-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Maker 2004
- Maker V, Layke J. Gastrointestinal injury secondary to extracorporeal shock wave lithotripsy: a review of the literature since its inception. Journal of the American College of Surgeons 2004;198(1):128-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Matlaga 2011
- Matlaga BR, Lingeman JE. Surgical management of upper urinary tract calculi. In: Kavoussi LR, Novick AC, Partin AW, Peters CA, editors(s). Campbell-Walsh Urology. 10th edition. Philadelphia: Saunders Elsevier, 2011. [Google Scholar]
Murad 2017
- Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evidence-Based Medicine 2017;22(3):85-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oestreich 2020
- Oestreich MC, Vernooij RW, Sathianathen NJ, Hwang EC, Kuntz GM, Koziarz A, et al. Alpha-blockers after shock wave lithotripsy for renal or ureteral stones in adults. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013393. [DOI: 10.1002/14651858.CD013393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearle 2007
- Pearle MS, Lotan Y. Urinary lithiasis: etiology, epidemiology, and pathogenesis. In: Campbell MF, Wein AJ, Kavoussi LR, editors(s). Campbell-Walsh Urology. 9th edition. Philadelphia: Saunders Elsevier, 2007. [Google Scholar]
Raheem 2017
- Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of urolithiasis: trends in prevalence, treatments, and costs. European Urology Focus 2017;3(1):18-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Roberts 2015
- Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E. The knowledge system underpinning healthcare is not fit for purpose and must change. BMJ 2015;350:h2463. [DOI] [PubMed] [Google Scholar]
Scholtes 2012
- Scholtes VA, Nijman TH, Beers L, Devereaux PJ, Poolman RW. Emerging designs in orthopaedics: expertise-based randomized controlled trials. The Journal of Bone and Joint Surgery. American Volume 2012;94 Suppl 1:24-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Seitz 2012
- Seitz C, Desai M, Häcker A, Hakenberg OW, Liatsikos E, Nagele U, et al. Incidence, prevention, and management of complications following percutaneous nephrolitholapaxy. European Urology 2012;61(1):146-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Soderberg 2019
- Soderberg L, Ding M, Parker R, Borofsky M, Pais V, Dahm P. Percutaneous nephrolithotomy versus retrograde intrarenal surgery for treatment of renal stones in adults. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD013445. [DOI: 10.1002/14651858.CD013445] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorokin 2017
- Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World Journal of Urology 2017;35(9):1301-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsai 2020
- Tsai SH, Chung HJ, Tseng PT, Wu YC, Tu YK, Hsu CW, et al. Comparison of the efficacy and safety of shockwave lithotripsy, retrograde intrarenal surgery, percutaneous nephrolithotomy, and minimally invasive percutaneous nephrolithotomy for lower-pole renal stones: a systematic review and network meta-analysis. Medicine 2020;99(10):e19403. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turna 2007
- Turna B, Raza A, Moussa S, Smith G, Tolley DA. Management of calyceal diverticular stones with extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy: long-term outcome. BJU International 2007;100(1):151-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weiss 2012
- Weiss OJ. Physics and Technique of Shock Wave Lithotripsy. In: Urolithiasis. Springer, London, 2012:301-11. [Google Scholar]
Weld 2007
- Weld KJ, Montiglio C, Morris MS, Bush AC, Cespedes RD. Shock wave lithotripsy success for renal stones based on patient and stone computed tomography characteristics. Urology 2007;70(6):1043-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wright 2016
- Wright A, Rukin N, Smith D, De la Rosette J, Somani BK. 'Mini, ultra, micro' – nomenclature and cost of these new minimally invasive percutaneous nephrolithotomy (PCNL) techniques. Therapeutic Advances in Urology 2016;8(2):142-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamashita 2018
- Yamashita S, Kohjimoto Y, Iwahashi Y, Iguchi T, Nishizawa S, Kikkawa K, et al. Noncontrast computed tomography parameters for predicting shock wave lithotripsy outcome in upper urinary tract stone cases. Biomed Research International 2018;2018:9253952. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zanetti 1999
- Zanetti G, Ostini F, Montanari E, Russo R, Elena A, Trinchieri A, et al. Cardiac dysrhythmias induced by extracorporeal shockwave lithotripsy. Journal of Endourology 1999;13(6):409-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2015
- Zhang W, Zhou T, Wu T, Gao X, Peng Y, Xu C, et al. Retrograde intrarenal surgery versus percutaneous nephrolithotomy versus extracorporeal shockwave lithotripsy for treatment of lower pole renal stones: a meta-analysis and systematic review. Journal of Endourology 2015;29(7):745-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Srisubat 2008
- Srisubat A, Potisat S, Lojanapiwat B, Setthawong V, Laopaiboon M. Extracorporeal shock wave lithotripsy (ESWL) for kidney stones. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD007044. [DOI: 10.1002/14651858.CD007044] [DOI] [Google Scholar]
Srisubat 2009
- Srisubat A, Potisat S, Lojanapiwat B, Setthawong V, Laopaiboon M. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD007044. [DOI: 10.1002/14651858.CD007044.pub2] [DOI] [PubMed] [Google Scholar]
Srisubat 2014
- Srisubat A, Potisat S, Lojanapiwat B, Setthawong V, Laopaiboon M. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD007044. [DOI: 10.1002/14651858.CD007044.pub3] [DOI] [PubMed] [Google Scholar]


