Abstract
Background:
Pneumonia is one of the leading causes of death in under-5 children in India. This led the Ministry of Health & Family Welfare (MoHFW) in India to decide for the nationwide roll-out of the Pneumococcal Conjugate Vaccine (PCV). However, the introduction of PCV became more complex in the face of unprecedented challenges set forth by the COVID-19 pandemic. The study aims to assess enablers and barriers to the introduction of PCV in India during the pandemic.
Methodology:
Qualitative research approach involving key-informant interviews from John Snow India (JSI), the lead technical agency that supported MoHFW in the PCV expansion was employed to delineate the enablers and barriers. Principle of saturation was employed to derive the sample size. Thematic analysis using inductive approach was based on the modified World Health Organization (WHO) framework for new vaccine introduction impact on the Immunization and Health Systems, using NVIVO 12 qualitative data analysis software.
Results:
A total of 11 key informants (4 national-level program managers and 7 state technical officers) were telephonically interviewed. The study found social acceptance, lower cost of the vaccine, and intensive communication activities as potential enablers. Other enablers for PCV introduction included a robust vaccine supply-chain system, ample cold-chain space availability, and strong political commitment, despite the ongoing second wave. Further, the identified barriers included poor physical access, insufficient social mobilization, and limited advocacy along with a stretched workforce.
Conclusion:
The study delineated several enablers and barriers to introducing PCV in the country during the pandemic. The existing barriers in the PCV roll-out prompted the need to address these gaps, making key program-based recommendations to improve future new vaccine introductions during the pandemic.
Keywords: Pneumococcal conjugate vaccine (PCV), routine immunization (RI), vaccine preventable disease (VPD), World Health Organization (WHO), Ministry of Health and Family Welfare (MoHFW) in India
Introduction
The Covid-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) had significantly and widely impacted the country’s functioning. 1 The rapid increase in infectivity rates ushered in a slew of restrictions to curb the spread of infection.1,2 Although, such measures were effective in reducing the chain of COVID-19 transmissions, but led to the unintentional disruption of regular healthcare services such as routine immunization (RI) for children, which is the backbone of the healthcare system and the mainstay for reducing under-5 mortality rates in the country owing to its potential of preventing the dreadful infectious diseases.3-5 According to Shet et al, 6 33.4% of pediatricians in India reported almost complete suspension of vaccination services during the COVID-19 pandemic.
In such a situation, Pneumococcal infection, a vaccine-preventable disease (VPD), continued to risk the lives of those under the age of 5 in India (John Hopkins, 2020) 7 . This was further worsened by the increased vulnerability of children in these times of complete disarray of health services. According to a WHO 8 factsheet, pneumonia accounted for approximately 14% of annual deaths in children under 5 years in India, with pneumococcal pneumonia accounting for 50% of deaths. India has been one of the leading Asian countries with the highest number of deaths due to pneumococcal infection caused by Streptococcus pneumoniae in children under the age of 5, accounting for 23% of the global burden and 36% of the World Health Organization (WHO) regional burden.9,10 However, the situational analysis by the Union Health Ministry suggested that approximately half of the people (47%) who had succumbed to COVID-19 infection were less than 60 years of age with only 1% falling in the age group less than 17 years. 11 On the contrary, the estimated deaths due to pneumococcal disease in the country under the age of 5 were much more and a subject of major concern. 12 As pneumococcal infection resulted in massive death tolls, WHO recommended the inclusion of Pneumococcal Conjugate Vaccine (PCV) in immunization programs in countries across the world. 13
Responding to the above call, India introduced PCV in 2017 in a phased manner across 5 high-burden Indian states, Bihar, Himachal Pradesh, Madhya Pradesh, Rajasthan, and Uttar Pradesh.10,14 However, the Government of India, decided in the 2021 to 2022 budget to rapidly expand the PCV under the UIP throughout the country. 15
The process of a new vaccine introduction has never been an easy task to accomplish. The entire process, from decision-making to vaccine implementation and monitoring is a strenuous and labor-intensive job.16-18 In India, the expansion of PCV became more challenging amidst the unprecedented situation posed by the COVID-19 pandemic. The restrictions in movement, the intensive focus of public health experts, the medical community, and the government on effectively controlling and managing the pandemic, led to challenges in implementing the key activities required for the expansion of PCV in India.
The current study was conducted with the aim to assess key enablers and barriers to the introduction of PCV in India during the COVID-19 pandemic. The results of the study would enable the policymakers to make informed decisions and strategically plan the introduction of new vaccines in the face of such unforeseen challenges.
Methodology
This is a qualitative study involving semi-structured key-informant interviews. Figure 1 summarizes the research design.
Figure 1.
Research design.
Abbreviation: WHO, World Health Organization.
Selection of participants
The participants comprised personnel from John Snow India (JSI). JSI has been the lead technical agency for providing support to the Ministry of Health and Family Welfare (MoHFW), Government of India and the states for the expansion of PCV. JSI served as a connecting link between multiple partner agencies and the government and was involved in the last mile program implementation. Therefore, the study included state and national team members of the JSI team involved in the implementation of the new vaccine (PCV) during the COVID-19 pandemic. While a total of 20 participants were approached for the study, 11 consenting participants comprising of 4 national-level program managers and 7 state technical officers involved in the PCV introduction were included in the study. Principle of saturation was followed to achieve the sample size wherein the recruitment of more participants was stopped once it was observed that the study reached theoretical saturation.
Data collection
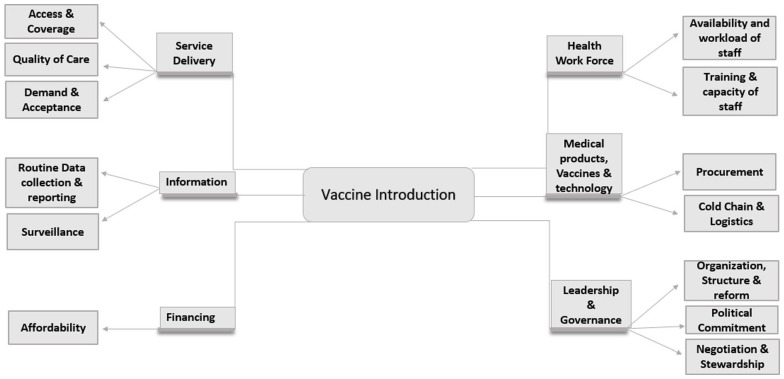
Data collection spanned across a period c.10 day from 24th May 2021 to 2nd June 2021. A semi-structured interview guide was developed based on the WHO Health System Strengthening Framework that comprised of 6 building blocks and 22 themes. However, for the purpose of the study, modified WHO framework was employed that comprised of 6 building blocks and 13 themes as shown in Figure 2. To drive the participants to narrate and clarify their responses, probing questions were raised, wherever required. To evaluate the effectuality of the interview guide, a pilot study was carried out with the national level program manager for PCV introduction project. The pilot study was conducted only on one participant as it generated ample information and revelations. The participant proposed a few suggestions which were incorporated into the interview guide to finalize the tool. Following the pilot test of the research tool, it was curtailed to 25 questions from the originally devised 31 open-ended questions along with several probes.
Figure 2.
Modified WHO health system framework.
Interviews were conducted virtually in English, using Google meet, and for a duration of approximately 45 minutes to 1 hour. With the consent of the participants obtained through circulation of Google forms, the interviews were audio-recorded and later cautiously transcribed in-verbatim by the researcher who was also the interviewer and the principal author of the study. Transcription was done soon after the completion of the interview to generate accurate transcripts for effective interpretation and analysis. During the transcription process, the anonymity of the participants was ensured by providing them pseudo names (participant 1, participant 2, and so on). In order to ensure the anonymity of the participants, all the identifiable information was coded during the transcription process. The data set obtained after the transcription process was further validated by another researcher and organized for analysis. All the data can be made available on request.
Data analysis
Thematic analysis of the interview transcripts was performed through an inductive thematic approach based on the modified WHO framework using NVIVO 12 qualitative data analysis software. The analysis involved thorough and repeated reading of the transcripts followed by coding of the text. The texts were coded and re-coded under different themes. The codes were then corroborated into the relevant sub-themes which were later placed under appropriate themes and subsequent building blocks. While the transcripts were reviewed thoroughly for identifying differences in opinions of the interviewees, no statement of conflicting opinion was found.
Ethical considerations
The ethical clearance to conduct the study was obtained from the Institutional Ethical Board of Gurugram University, Gurugram with issue number GUG/2021/88 and written, signed, and dated informed consent was taken from the participants of the study digitally through an email. The consent form mentioned the purpose of the study and confidentiality protection for all the study participants.
Results
A total of 11 key informants were approached and interviewed in the present study; out of which 4 were national-level program managers and 7 were state technical officers for PCV introduction. Factors facilitating and impeding the introduction of PCV during the pandemic were explored through interviews and data was analyzed in accordance with the modified WHO framework, as shown in Table 1.
Table 1.
Enablers/barriers to a new vaccine introduction during the pandemic.
| Building block | Theme | Different responses received | Sample quotes | B/E | Frequency of responses |
|---|---|---|---|---|---|
| Service Delivery | Access and Coverage | Limited physical access due to COVID-19 pandemic | “In the Containment zones, people couldn’t go out and no one was allowed from outside as well. So, if the people were not taking their children to the immunization sites, the coverage gets reduced by a lot of numbers.” | B | 11 |
| Social acceptance (confidence, low complacency, and convenience)* of new vaccines increased | “Any new vaccine introduction done right at this point of time would be easy and most probably accepted by all the people as half of the work is already done by the media by making a positive environment in the community.” | E | 10 | ||
| Reluctance to vaccine uptake due to misinformation regarding COVID-19 vaccines side-effects | “The new vaccine being launched for the children would not be effective and might lead to side effects.” | B | 1 | ||
| Quality of care | RI sessions got disrupted | “Many of the health staff were diverted to the COVID-19-related work. So, some of the RI sessions got postponed or canceled.” | B | 8 | |
| Feared beneficiaries did not go for vaccination. | “People were scared to go out anywhere, even to go out to facilities, to seek care, medical care due to fear of contracting COVID infection.” | B | 3 | ||
| Demand and Acceptance | Social mobilization was severely impacted | “VHND, which is conducted at Anganwadi is a good platform where families, beneficiaries, and even Sarpanch go. This was severely impacted because of COVID pandemic.” | B | 11 | |
| Health Workforce | Availability and workload of staff | Sick vaccinators led to a shortage | “In some places, the ANMs got infected, so in those areas, there was no one for routine vaccination.” | B | 7 |
| Vaccinators are overburdened due to COVID-19 immunization | “I went for the COVID vaccination on a holiday and I found all the ANMs, health supervisors, the ASHAs, everyone was working and so the workload has increased many folds.” | B | 11 | ||
| Training and capacity of staff | Capacity building of workforce via virtual trainings in short-time | “In one session, we can have 1000 participants who can participate from different districts simultaneously and we can partner participants from different states simultaneously.” | E | 9 | |
| Information | Routine data collection and reporting | Inappropriate data recording & reporting | “Data management requires proper reporting and recording of the things in a timely manner, there should be enough time with the person who is doing this else the things could lapse. As single person was engaged in many things, it suffered.” | B | 11 |
| Surveillance | Poor surveillance due to restricted field activities | “During PCV launch, the COVID situation everywhere was bad, field activities took a back seat.” | B | 8 | |
| PCV is safe with no fear of AEFI | “In the training for the PCV, we had a separate session for the AEFI. But PCV is very safe, so not much of AEFI issues.” | E | 11 | ||
| Medical products, vaccines, and logistics | Procurement | Country has a strong vaccine supply chain system | “The supply chain system was running very well because that is being monitored by the eVIN, that’s a very robust system.” | E | 11 |
| Cold chain and logistics | Ample cold chain space for PCV | “Due to the COVID-19 pandemic, a lot of cold chain equipment has been supplied to the state. So, in a way, it has actually strengthened the cold chain system of the country.” | E | 11 | |
| Financing | Affordability | Availability of cost-free PCV under RI | “Government is giving PCV free of cost and also this is a very costly vaccine in the private sector, which common people cannot easily afford.” | E | 11 |
| Healthy markets, fostering developing country manufacturers | “PCV expansion with indigenous product has helped in smooth launch.” | E | 11 | ||
| Leadership & Governance | Organization, structure, and reform | Governance and accountability | “Earlier getting this checklist done was like time-consuming. But now it was easier with online checklists in a way that, yes, we shared the files with the states and they filled it.” | E | 9 |
| Political commitment | Skillful governance | “Expert guidance from a passionate and prudent person in the Ministry had really supported PCV expansion at that time.” | E | 11 | |
| Strong political commitment to PCV even during pandemic | “The states were quite proactive in participating in training and introducing PCV. So, it was quite a smooth introduction as compared to the previous vaccine introductions in UIP.” | E | 11 | ||
| Negotiation and Stewardship | Inter-agency coordinating committees (ICC’s) | “UNICEF supported us for translation basically of the FAQs booklets in regional languages of reflected state, WHO took sessions in the training, not only the national training but at district and state level also, UNDP supported us in vaccine delivery to the new session site or to the state vaccine stores. So, the partners are supporting us in all the ways.” | E | 11 |
Abbreviations: AEFI, Adverse Events Following Immunization; ANMs, Auxiliary Nurse-Midwifery; ASHAs, Accredited Social Health Activists; COVID-19, coronavirus disease; eVIN, electronic Vaccine Intelligence Network; FAQs, Frequently Asked Questions; PCV, Pneumococcal Conjugate Vaccine; RI, Routine Immunization; UIP, Universal Immunization Programme; UNDP, United Nations Development Programme; UNICEF, United Nations International Children’s Emergency Fund; VHND, Village Health & Nutrition Day; WHO, World Health Organization.
Confidence refers to the trust in effectiveness and safety of the vaccine; Low Complacency means the risk of disease preventable by vaccination is perceived as high; Convenience refers to the influence of certain factors (such as, availability, affordability, willingness to pay, geographical accessibility, degree of satisfaction with vaccination services, etc.) on the decision to get vaccinated.
Figure 3 summarizes the results of the interview transcripts in the form of a word cloud depicting the frequency of their appearance in the word cloud.
Figure 3.
Word Cloud for all interview transcripts.
Based on the analysis and interpretation of the data, the researchers arrived at various themes including service delivery, health workforce, information, medical products, vaccines & logistics, financing, and leadership & governance.
Service delivery
Access and coverage
All the key informants responded that the pandemic was a major barrier to access primarily due to the lockdowns and containment zones restricting the movement of beneficiaries to the session sites. “In the Containment zones, people couldn’t go out and no one was allowed from outside as well. So, if the people were not taking their children to the immunization sites, the coverage gets reduced by a lot of numbers,” said participant 10.
However, the study participants stated that the pandemic had increased the social acceptability of PCV since the community was well-informed about the importance of immunization.
Quality of care
Delays in service delivery were informed by many due to the involvement of the health workforce in COVID-19 duties. Further, most participants reported that hampered RI sessions and a shift in outreach session sites could affect PCV coverage. “Many of the health staff were diverted the COVID-19-related work. So, some of the RI sessions got postponed or canceled,” stated participant 6.
Furthermore, some participants anticipated that beneficiaries were reluctant to visit the session sites for fear of becoming infected with COVID-19, which could jeopardize vaccine delivery.
Demand and acceptance
Information, education, and communication (IEC) materials such as banners, posters and leaflets, social media platforms, electronic media such as radio jingles and local news channels, and print media like newspapers were cited as effective measures for generating community awareness and demand regarding a new vaccine. On the other hand, Accredited Social Health Activists (ASHAs) who are the community mobilizers, Anganwadi Workers (AWWs), and Auxiliary Nurse-Midwife (ANMs) who are the chief vaccinators were unanimously identified as the most effective mobilizers. However, during the pandemic, interpersonal communication (IPC) and social mobilization by ASHAs and AWWs were severely affected. “Village Health & Nutrition Day (VHND), which is conducted at Anganwadi is a good platform where families, beneficiaries, and even Sarpanch go. This was severely impacted because of COVID pandemic,” mentioned participant 8.
Health workforce
Availability and workload of staff
Most participants admitted that sickness absenteeism caused by COVID-19 infection resulted in the shortage of manpower for RI. Further, the pandemic also resulted in an overburdened, exhausted, and strained health workforce. “I went for the COVID vaccination on a holiday and I found all the ANMs, health supervisors, the ASHAs, everyone was working and so the workload has increased many folds,” stated participant 2.
Training and capacity of staff
Capacity building for a trained healthcare workforce is one of the crucial components of new vaccine introduction. However, due to restrictions on gatherings, in-person training for PCV introduction were not possible in many places. Most of the training happened online through virtual platforms with flexible timings allowing for increased participation. However, in areas with irregular connectivity, the training was conducted in a hybrid mode (online and in-person) or in-person. All the participants felt that the hybrid sessions facilitated the capacity building of a large healthcare cohort simultaneously, thereby, saving a lot of time and was extremely cost-effective. “In one session, we can have 1000 participants who can participate from different districts simultaneously and we can add participants from different states simultaneously,” mentioned participant 6.
Information
Routine data collection and reporting
Almost all participants stated that improper data recording and reporting as a result of the involvement of data handlers in COVID-19 duties resulted in compromised data (either inaccurate or sparse), for a new vaccine. “Data management requires proper reporting and recording of the things in a timely manner, there should be enough time with the person who is doing this else the things could lapse. As single person was engaged in many things, it suffered,” stated participant 6.
Surveillance
It was seen that while PCV is a very safe vaccine with rare or no chances of AEFI, AEFI preparedness was done before the roll-out during the training sessions. Most of the participants found a significant impact of the pandemic on the existing surveillance system due to restricted field movements, however, they demonstrated confidence in the safety of PCV was clearly communicated. “In the training for the PCV, we had a separate session for the AEFI. But PCV is very safe, so not much of AEFI issues,” said participant 2.
Medical products, vaccines, and logistics
Procurement
All the participants generally felt that the country has a very robust vaccine supply chain system, therefore stock management was not an issue. Moreover, PCV is an indigenously manufactured vaccine, hence there were no delays, shortages, or hurdles anticipated in its supply. “The supply chain system was running very well because that is being monitored by the electronic Vaccine Intelligence Network (eVIN), that’s a very robust system,” said participant 11.
Cold chain and logistics
Prior to the introduction of a new vaccine, an analysis of cold chain space is performed as a part of the preparedness assessment to rule out any shortages in terms of space availability. Further, all participants agreed that the augmentation of cold chain space during the pandemic resulted in ample cold chain space for PCV. Participant 11 mentioned that, “due to the COVID-19 pandemic, a lot of cold chain equipment has been supplied to the state. So, in a way, it has actually strengthened the cold chain system of the country.”
Financing
Affordability
The impact of domestic financing and external funding for the PCV introduction was viewed positively. All the participants opined that the government’s decision to provide PCV free of cost under RI aided in the successful roll-out of the vaccine. Besides, the technical support grant from the Bill & Melinda Gates Foundation, was stated as an additional facilitator to the smooth roll-out of PCV. As a result of the affordable, safe, and domestically manufactured vaccine with sufficient financing, the nationwide expansion got accelerated. Participant 3 asserted that, “Government is giving PCV free of cost and also this is a very costly vaccine in the private sector, which common people cannot easily afford.”
Leadership and governance
Organization, structure, and reform
Before the introduction of any new vaccine in the country, a comprehensive preparedness assessment is done to assess the health system’s ability to withstand the introduction of a new vaccine. Despite the fact that the pandemic posed a challenge to remote functioning, it led to the digitization of preparedness assessment through the development of the online tool-PROMPT (PCV roll-out monitoring and preparedness tool). As a result, majority of the participants stated that obtaining the filled checklist through the online mode was quite simple, effortless, and less time-consuming. “Earlier getting this checklist done was like time-consuming. But now it was easier with online checklists in a way that, yes, we shared the files with the states and they filled it,” stated participant 9.
Political commitment
Although the COVID-19 vaccination held the priority for the government, state, and district officials, study participants found states to be extremely proactive, supportive, and eager for the PCV launch. Reflecting on the interest of states in achieving overall good health status, participants emphasized the importance of a strong political commitment to PCV roll-out during the pandemic. “The states were quite proactive in participating in training and introducing PCV. So, it was quite a smooth introduction as compared to the previous vaccine introductions in UIP,” stated participant 11.
Further, all participants appreciated the skilled, experienced, and mighty stewardship provided directly by the Ministry’s key immunization actor, that enabled the smooth roll-out of PCV countrywide.
Negotiation and stewardship
Every participant acknowledged the numerous contributions of the international partner agencies such as WHO, United Nations International Children’s Emergency Fund (UNICEF), and United Nations Development Programme (UNDP) in the PCV introduction process from conducting the training to developing the training material, obtaining the assessment checklist, and ensuring communications with the state officials. The participants also confirmed that UNICEF, WHO, and UNDP had designated resource persons in place at the regional and block levels, ensuring smooth coordination in remote areas. “UNICEF supported us for translation basically of the FAQs booklets in regional languages of reflected state, WHO took sessions in the training, not only the national training but at district and state level also, UNDP supported us in vaccine delivery to the new session site or to the state vaccine stores. So, the partners are supporting us in all the ways,” mentioned participant 10.
Discussion
To the best of our knowledge, this is the first study to enumerate the facilitators and barriers to PCV introduction during the pandemic in India. The findings of this research entailed numerous factors that contributed either as a barrier or an enabler to the PCV introduction in the country. Such factors are, therefore, discussed under the section heads of enablers and barriers to the PCV introduction during the pandemic.
Enablers to PCV introduction during pandemic
An already strengthened health system not only set the stage but also enabled the successful introduction of PCV despite an ongoing pandemic. A strong political commitment and a robust vaccine supply chain system were identified as the key enablers for PCV launch in the country amid the pandemic. Existing research on the programmatic evaluation of eVIN revealed the pivotal role of the digital platform in effective and efficient vaccine supply chain management as well as supervision. 19 Additionally, during the pandemic, the cold chain space across the country was ramped up to accommodate COVID-19 vaccines, simultaneously allowing for the smooth introduction of PCV in India. According to a UNICEF report, the increased provision of ice-lined refrigerators (ILRs), walk-in coolers (WICs), deep freezers, vaccine carriers, and logistics to India for enabling COVID-19 vaccination roll-out and ensuring the seamless continuation of RI services confirmed this finding. 20 Previous studies also highlight that the strong-willed decision makers and program implementers pave a smooth path for the vaccine implementation by effectively engaging the community.21-23
Further, the availability of an affordable and indigenous product further gave it a boost in terms of adoption in such dire situations. According to several research studies, a vaccine’s cost-effectiveness can lead to its widespread use.24,25 Additionally, the acceptability of PCV among the beneficiaries contributed to the smooth implementation. According to a study, public interest in PCV which is used to prevent pneumonia, escalated globally, due to media campaigning for COVID vaccination that had generated a lot of awareness for the need and benefits of immunization.26,27 Communication through effective web-based content was identified as an enabler. Earlier studies have also demonstrated that digital media is an effective tool for disseminating credible information to caregivers, thereby, generating the demand for healthcare. 28
Further, the hybrid mode of training used for capacity building of the health workforce for PCV facilitated the generation of a large cohort of health care workforce in a short span, allowing the smooth and efficient new vaccine roll-out during difficult times. Recent studies have provided insights into alternative delivery modalities for continuing medical training amid the pandemic, such as virtual methods, highlighting the advantages and disadvantages of the same.28-30
Studies identify vaccine safety as the leading cause of vaccine hesitancy among people,31,32 thereby reiterating the acceptance for the PCV considering its safety profile and limited concern for associated AEFIs. Existing literature, on the other hand, indicated the presence of a strong pharmacovigilance system for monitoring AEFI issues associated with any vaccine, if reported. 33
Digital intervention tools (PROMPT) aided in obtaining the completed preparedness assessment checklist facilitated the smooth roll-out even more. An earlier study has extensively discussed the promising advancement of digitalization in prompt tracking and reporting of the pandemic consequences. 34 In addition to these factors, major contributions of international partner agencies such as UNICEF, WHO, and UNDP in the PCV roll-out and implementation, were regarded as significant enablers. The existing literature showed that partner organizations have consistently provided tremendous coordinated support during the introduction of new vaccines in the country.17,35,36
Barriers to PCV introduction during pandemic
Though India’s overall health system is highly resilient to sustain and support the new vaccine introduction in the future, certain shortcomings were identified. Physical restriction on movement of beneficiaries and vaccinators for immunization, and limited accessibility of the novel vaccine was highlighted as a barrier to coverage during the pandemic. There was also a disruption in the timeliness of RI services. Few studies have also suggested complete mayhem in the delivery of essential health services including suspension or disorderliness of the RI services for children in India, implying the need for a robust plan to keep the RI continued during the pandemic or even other health emergencies.6,24 On the other hand, interrupted social mobilization and suspension of awareness generation initiatives during the pandemic were also a barrier to boosting PCV coverage. In the midst of the COVID-19 pandemic, another study found a reduction in awareness generation in the community owing to the suspended VHND sessions and community campaigns.24,28
While the current study concluded that the pandemic strained the health workforce, sickness absenteeism due to higher infectivity rates further contributed to the manpower shortage for vaccination. This is consistent with other studies highlighting the strenuous engagement of the health care personnel in pandemic duties and the deficiency of the workforce as a result of COVID-19 infection, which has hampered the RI services.37-39
Another possible impediment to the successful deployment of PCV during the pandemic was poor data synthesis and compilation. A study has highlighted the impact of the COVID-19 pandemic on data gathering operations for national statistics programs, leading to their partial or complete discontinuation.39,40 The severity of the COVID-19 pandemic also resulted in a decline in the surveillance of VPDs. 41
To ensure the uninterrupted roll-out of a new vaccine during a crisis in the future, prompted the need to address the existing gaps. Certain concrete strategies aimed at overcoming such barriers have been recommended such as implementing a slot system for beneficiaries at session sites, organizing refresher training for continuous knowledge enhancement of the health workforce, deploying strong community engagement strategies, involving religious heads and leaders in awareness generation, counseling beneficiaries and adopting COVID appropriate protocols at session sites, digitizing monitoring in remote areas, and encouraging public-private collaborative alliances to alleviate the delays in service delivery enforced during the pandemic. Furthermore, unlike the PROMPT tool, it is imperative to develop a portal that includes a Mother and Child Protection Card (MCP) of RI eligible beneficiaries with unique Id’s, as well as an in-built feature to redirect an automated message to the beneficiaries who are due for a vaccine and allowing the ANMs and ASHAs to track the due beneficiaries for immunization. Thus, a digital healthcare component to focus on electronic record keeping and data visualization can be added to the existing WHO health system framework for digitalization and can be a savior in such health system emergencies.
Limitations to the study include its limited generalizability as a result of the involvement of fewer participants in the study. Even though the key informants provided a detailed description of the research subject, the findings cannot be generalized to a larger group. Besides, the limited ability of the participants to recall their narrated experiences over some time results in faded or altered perceptions which might influence the accuracy of the study.
Conclusion
The present study sought to delineate the enablers and barriers to the introduction of PCV in the country during the pandemic. Increased social acceptability, effective media communications, and lower cost of the vaccine were identified as potential enablers. Other enablers identified were a strong vaccine supply chain system, adequate cold chain space availability, unwavering political commitment, and incredible contributions made by partner organizations during the launch process. On the other hand, barriers to the PCV introduction included limited physical access, disrupted RI sessions, interrupted social mobilization activities, overburdened workforce, inadequate data recording and reporting, and poor surveillance due to pandemic restrictions.
To address the barriers identified in the study, certain program-based recommendations to improve future new vaccine introductions during the pandemic have been suggested. Since this is the first-ever study on the subject, future large-scale studies to compare the impact on coverage of PCV launched during the pandemic era to another vaccine launched during the pre-pandemic era are needed. In addition, a mixed-method study is suggested to validate the hypothesis generated by the present study.
Acknowledgments
The authors wish to express their sincere gratitude to all the participants for their invaluable contributions in willingly sharing their experiences. The authors would like to acknowledge and appreciate the medical writing support provided by Ms. Pooja S. Banerjee. This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation [INV-030655]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Appendix
Accredited Social Health Activists (ASHA): A trained female community health activist that serves as an interface between the community and the public health system. ASHA is primarily a woman resident of the village married/widowed/divorced, preferably in the age group of 25 to 45 years.
Anganwadi Workers (AWW): The Anganwadi worker is a community based front line worker and is most important functionary of the ICDS scheme. She plays a crucial role in promoting child growth and development as she is an agent of social change, mobilizing community support for better care of young children.
Village Health & Nutrition Day (VHND): The VHND is to be organized once every month at the Anganwadi centre (AWC) in the village. The AWC is identified as the hub for service provision in the RCH-II, NHM, and also as a platform for inter-sectoral convergence. VHND is also to be seen as a platform for interfacing between the community and the health system.
Auxiliary Nurse-Midwife (ANM): The Auxiliary nurse midwife (ANM) are respected members of their communities and established providers of maternal and child health care within the community and at the facility level. The cadre was created to focus on maternal and child health. Their scope of work encompasses family planning, immunization, infectious disease prevention and care, in addition to care during pregnancy and childbirth.
Routine Immunization (RI): Routine immunization (RI) is a vital component of the health system owing to its potential of preventing the dreadful infectious diseases. In other words, routine immunization is the backbone of the healthcare system and the mainstay for reducing under-5 mortality rate throughout the country. It is one of the most cost-effective public health interventions and largely responsible for the reduction of vaccine-preventable diseases.
Ministry of Health and Family Welfare (MoHFW): A Ministry under the Government of India charged with health policy formulation and implementation of public health programmes in India.
Containment Zones: A geographical area, well-demarcated with perimeter and enforceability, based on factors like mapping of cases and contacts, and geographical dispersion of cases and contacts.
Electronic Vaccine Intelligence Network (eVIN): The Electronic Vaccine Intelligence Network (eVIN) is an innovative technological solution aimed at strengthening immunization supply chain systems across the country. This is being implemented under National Health Mission (NHM) by Ministry of Health and Family Welfare. eVIN aims to provide real-time information on vaccine stocks and flows, and storage temperatures across all cold chain points in the country. This robust system has been used with the requisite customization during the COVID pandemic for ensuring continuation of the essential immunization services and protecting our children and pregnant mothers against vaccine preventable diseases.
Interview Guide
| Interview guide | ||||
|---|---|---|---|---|
| S. no. | Building block | Theme | Questions | Probing questions |
| 1 | Service Delivery | Access & Coverage | What factors according to you could affect the coverage of a new vaccine like PCV during pandemic? | How? Cover Social and cultural acceptability |
| Do you think the pandemic has influenced the acceptability of a new vaccine amongst the beneficiaries? | If yes, How can it do so? If No, Why? | |||
| Do you think Covid-19 vaccination drive has affected the PCV uptake at government session sites? | If yes, How can it do so? If No, Why? | |||
| If yes, How can it do so? If No, Why? | ||||
| If yes, How can it do so? If No, Why? | ||||
| Quality of Care | Has the pandemic hampered the delivery of PCV under RI? | If yes, how and why? How can it be overcome? If No, how and why? | ||
| What were the challenges in the delivery of this new vaccine during pandemic? | How can it be overcome? | |||
| Demand and Acceptance | According to you, which are the most effective methods of Social mobilization, advocacy, and communication? Why? | Has the pandemic impacted any of these activities? | ||
| According to you, which are the most effective methods to generate awareness among parents/caregivers during the pandemic? | ||||
| 2 | Health Workforce | Availability/distribution of staff | Could you comment on the health workforce situation for PCV introduction in these times? | Elaborate on the workload |
| How according to you pandemic has been in a challenge in execution of routine immunization? | ||||
| Training and capacity of staff | How has Covid-19 affected training for introduction of PCV? | Challenges Benefits Positives/Negative | ||
| How do you think the challenges could be overcome? | ||||
| 3 | Information | Routine data collection and reporting | Were there any challenges in terms of database management during Covid-19 for PCV? | If Yes, how can the challenges be solved? |
| If No, what strategies have been implemented for it? | ||||
| Surveillance | Has there been any challenge in vaccine surveillance system or impact monitoring during pandemic or do you anticipate such a challenge? | If Yes, how can the challenges be solved? | ||
| If No, what strategies have been implemented for it? | ||||
| 4 | Medical products, vaccines, and technologies | Procurement | Were there any challenges for PCV supply chain management? | From state to district level. |
| Cold chain & logistics | Were there any challenges foreseen at regional and state level in cold chain space availability for a new vaccine? | If YES, what would you do to overcome this challenge? | ||
| If NO challenge, what is your approach to avoid this issue? | ||||
| 5 | Financing & Sustainability | Affordability | As PCV vaccine has indigenous manufacturers and government support for scale-up, do you think these factors contributed to its affordability and accessibility under the routine immunization services in India? | Were there any other factors that further contributed to the scale up of PCV? |
| 6 | Leadership and Governance | Political commitment | Did the central and state government support in PCV introduction amid the dreadful pandemic? | If yes, how? |
| Organization, structure, and reform | How has the planning for the introduction of a new vaccine during a pandemic been different than other times? How convinced were the state Director H& FW & SIO- to issue letter for getting an assessment checklist? |
Challenges/Opportunities | ||
| Negotiation and Stewardship | Could you comment on the role of partner organizations and agencies in PCV introduction? | Any special efforts made by them during COVID-19 for the launch? | ||
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Bill & Melinda Gates Foundation under the grant number [INV-030655].
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RH conceptualized the study, designed the methodology, analyzed the findings, and drafted the first draft of the manuscript. RM, SSK, and TP guided in the development of PA, AK, AR, and SQ verified the findings and reviewed & edited the manuscript, ADR supervised and guided throughout the study from inception to culmination. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate: Necessary approvals for the study were taken in accordance with the Helsinki guidelines. The ethics approval for the study was issued by the Institutional Committee of Gurugram University, Gurugram with issue number GUG/2021/88. The methodology of the study was duly approved and adhered. Informed consent was obtained from all subjects involved in the study. In line with ethical principles of social research, appropriate information about the research was provided to the study participants. Throughout the research, anonymity and confidentiality of the participants was protected.
ORCID iD: Rhythm Hora  https://orcid.org/0000-0002-0370-6864
https://orcid.org/0000-0002-0370-6864
References
- 1. Harris RC, Chen Y, Côte P, et al. Impact of COVID-19 on routine immunisation in South-East Asia and Western Pacific: disruptions and solutions. Lancet Reg Health West Pac. 2021;10:100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nageshwaran G, Harris RC, Guerche-Seblain CE. Review of the role of big data and digital technologies in controlling COVID-19 in Asia: public health interest vs. privacy. Digit Health. 2021;7:20552076211002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao Y, Wang L, Lin B, Mao H, Zhang M. Overview of the COVID-19. In: Zhang M, Lin B, eds. Diagnostic Imaging of Novel Coronavirus Pneumonia. Springer; 2020:1-7. [Google Scholar]
- 4. Mansour Z, Arab J, Said R, et al. Impact of COVID-19 pandemic on the utilization of routine immunization services in Lebanon. PLoS One. 2021;16:e0246951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson R. COVID-19 disrupts vaccine delivery. Lancet Infect Dis. 2020;20:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shet A, Dhaliwal B, Banerjee P, et al. COVID-19-related disruptions to routine vaccination services in India: a survey of paediatric providers. BMJ Paediatr Open. 2021;5:e001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hopkins John. 2020. Pneumonia & Diarrhea Progress Report 2020- John Hopkins. IVAC_PDPR_2020.pdf. https://www.jhsph.edu/ivac/wp-content/uploads/2020/11/IVAC_PDPR_2020.pdf [Google Scholar]
- 8. WHO. Pneumonia. Published 2021. Accessed June 30, 2022. https://www.who.int/news-room/fact-sheets/detail/pneumonia
- 9. Pandey A, Galvani AP. The burden of childhood pneumonia in India and prospects for control. Lancet Child Adolesc Health. 2020;4:643-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varghese R, Veeraraghavan B, Jeyaraman Y, Kumar G, Arora NK, Balasubramanian S. Pneumococcal conjugate vaccine rollout in India: expectations and challenges. Indian J Med Microbiol. 2019;37:141-146. [DOI] [PubMed] [Google Scholar]
- 11. Health. Nearly half the people who have died of COVID-19 in India are younger than 60. The Wire Science. Published 2020. Accessed May 28, 2023. https://science.thewire.in/health/india-covid-19-mortality-comorbidities-age-health-ministry/
- 12. UNICEF. Fighting for breath: a call to action to stop children dying from pneumonia. UNICEF DATA. Published 2019. Accessed May 1, 2023. https://data.unicef.org/resources/fighting-for-breath-a-call-to-action-to-stop-children-dying-from-pneumonia/
- 13. MoHFW. National operational guidelines: introduction of pneumococcal conjugate vaccine (PCV), India 2017. Published 2017. Accessed June 30, 2022. https://nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/Guildelines_for_immunization/Operational_Guidelines_for_PCV_introduction.pdf
- 14. Nagar R, Ambiya MS, Dalal S, et al. Real-time monitoring of the rollout of pneumococcal conjugate vaccines in rural India using a digital tracking platform. Gates Open Research. 2021;5:16. [Google Scholar]
- 15. MoHFW. Budget_Speech.pdf. Published 2021. Accessed June 30, 2022. https://www.indiabudget.gov.in/budget2021-22/doc/Budget_Speech.pdf
- 16. Malik A, Haldar P, Ray A, et al. Introducing rotavirus vaccine in the Universal Immunization Programme in India: from evidence to policy to implementation. Vaccine. 2019;37:5817-5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koshal SS, Ray A, Mehra R, et al. Partnering for rotavirus vaccine introduction in India: a retrospective analysis. Vaccine. 2021;39:6470-6476. [DOI] [PubMed] [Google Scholar]
- 18. Uddin J, Sarma H, Bari TI, Koehlmoos TP. Introduction of new vaccines: decision-making process in Bangladesh. J Health Popul Nutr. 2013;31:211-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gurnani V, Singh P, Haldar P, et al. Programmatic assessment of electronic Vaccine Intelligence Network (eVIN). PLoS One. 2020;15:e0241369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. UNICEF Report. UNICEF India COVID-19 pandemic monthly external situation report No. 8. 2021. Accessed June 30, 2022. https://www.unicef.org/media/94026/file/India-COVID19-SitRep-31-January-2021.pdf
- 21. Dutta T, Agley J, Meyerson BE, Barnes PA, Sherwood-Laughlin C, Nicholson-Crotty J. Perceived enablers and barriers of community engagement for vaccination in India: using socioecological analysis. PLoS One. 2021;16:e0253318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dutta T. Decision-makers’ conceptualization and fostering of community engagement for improved adoption and uptake of existing and emerging vaccines in India. ProQuest. Published 2019. Accessed May 28, 2023. https://www.proquest.com/openview/8a44e03baf38b2a8653a899b8786acf0/1?pq-origsite=gscholar&cbl=18750&diss=y [Google Scholar]
- 23. Gatera M, Bhatt S, Ngabo F, et al. Successive introduction of four new vaccines in Rwanda: high coverage and rapid scale up of Rwanda’s expanded immunization program from 2009 to 2013. Vaccine. 2016;34:3420-3426. [DOI] [PubMed] [Google Scholar]
- 24. Olusanya OA, Bednarczyk RA, Davis RL, Shaban-Nejad A. Addressing parental vaccine hesitancy and other barriers to childhood/adolescent vaccination uptake during the Coronavirus (COVID-19) pandemic. Front Immunol. 2021;12:663074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodrigues CMC, Plotkin SA. Impact of vaccines; Health, economic and social perspectives. Front Microbiol. 2020;11:1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunt G. Record 16.5 million flu vaccines to protect Australians. Australian Government Department of Health. Published April 19, 2020. Accessed August 16, 2021. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/record-165-million-flu-vaccines-to-protect-australians [Google Scholar]
- 27. Paguio JA, Yao JS, Dee EC. Silver lining of COVID-19: heightened global interest in pneumococcal and influenza vaccines, an infodemiology study. Vaccine. 2020;38:5430-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh V. The effect of COVID-19 pandemic on immunization services in India - possible challenges and way forward. Epidemiol Int. 2020;5:53-60. [Google Scholar]
- 29. Hau HM, Weitz J, Bork U. Impact of the COVID-19 pandemic on student and resident teaching and training in surgical oncology. J Clin Med. 2020;9:3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sneyd JR, Mathoulin SE, O’Sullivan EP, et al. Impact of the COVID-19 pandemic on anaesthesia trainees and their training. Br J Anaesth. 2020;125:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmad S, Singh S, Rai S, Wasim S. Adverse event following immunization (AEFI) and COVID-19 vaccination: a review. Int J Curr Microbiol Appl Sci. 2021;10:555-565. [Google Scholar]
- 32. Dubé E, MacDonald NE. How can a global pandemic affect vaccine hesitancy? Expert Rev Vaccines. 2020;19:899-901. [DOI] [PubMed] [Google Scholar]
- 33. Meher BR. Need of vibrant vaccine pharmacovigilance during current global COVID-19 pandemic: more than ever. J Pharm Bioallied Sci. 2021;13:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murray CJL, Alamro NMS, Hwang H, Lee U. Digital public health and COVID-19. Lancet Public Health. 2020;5:e469-e470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MoHFW. COVID19VaccineOG111Chapter16.pdf. 2020. Accessed June 30, 2022. https://www.mohfw.gov.in/pdf/COVID19VaccineOG111Chapter16.pdf
- 36. UNICEF. UNICEF responding to COVID-19 in India. Published 2021. Accessed August 17, 2021. https://www.unicef.org/coronavirus/unicef-responding-covid-19-india
- 37. Dinleyici EC, Borrow R, Safadi MAP, van Damme P, Munoz FM. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum Vaccin Immunother. 2021;17:400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:591-593. [DOI] [PubMed] [Google Scholar]
- 39. The Lancet. 2021: the beginning of a new era of immunisations? Lancet. 2021;397:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perucci F, Carletto G. The impact of COVID-19 on National Statistical Offices and the Future of Official Statistics. Published online 2021:29. [Google Scholar]
- 41. Bello IM, Lebo E, Shibeshi ME, et al. Implementation of integrated supportive supervision in the context of coronavirus 19 pandemic: its effects on routine immunization and vaccine preventable surveillance diseases indicators in the East and Southern African countries. Pan Afr Med J. 2021;38:164. [DOI] [PMC free article] [PubMed] [Google Scholar]