Abstract
The human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2) appear to have originated by cross-species transmission of simian immunodeficiency virus (SIV) from asymptomatically infected African primates. Few of the SIVs characterized to date efficiently infect human primary lymphocytes. Interesting, two of the three identified to infect such cultures (SIVsm and SIVcpz) have appeared in human populations as genetically related HIVs. In the present study, we characterized a novel SIV isolate from an East African monkey of the Cercopithecus genus, the l’hoest monkey (C. l’hoesti), which we designated SIVlhoest. This SIV isolate efficiently infected both human and macaque lymphocytes and resulted in a persistent infection of macaques, characterized by high primary virus load and a progressive decline in circulating CD4 lymphocytes, consistent with progression to AIDS. Phylogenetic analyses showed that SIVlhoest is genetically distinct from other previously characterized primate lentiviruses but clusters in the same major lineage as SIV from mandrills (SIVmnd), a West African primate species. Given the geographic distance between the ranges of l’hoest monkeys and mandrills, this may indicate that SIVmnd arose through cross-species transmission from close relatives of l’hoest monkeys that are sympatric with mandrills. These observations lend support to the hypothesis that the primate lentiviruses originated and coevolved within monkeys of the Cercopithecus genus. Regarded in this light, lentivirus infections of primates not belonging to the Cercopithecus genus may have resulted from cross-species transmission in the not-too-distant past.
The human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2) are lentiviruses that appear to have originated by cross-species transmission from African primates (22, 25, 45). Five distinct types of lentiviruses from nonhuman primates have previously been molecularly characterized: SIVsm from sooty mangabeys (Cercocebus atys) (7, 19, 37, 44), SIVagm from the four species within the African green monkey superspecies (Cercopithecus aethiops) (3, 4, 8, 10, 11, 20, 28, 30, 38), SIVsyk from Sykes monkeys (Cercopithecus mitis) (9, 21), SIVmnd from mandrills (Mandrillus sphinx) (49), and SIVcpz from chimpanzees (Pan troglodytes) (26, 27, 43, 50). It is currently believed that these characterized viruses may represent just a small part of a very large family of primate lentiviruses. Indeed, serologic surveys of other African primates have identified a number of other monkey species that have SIV-specific antibodies (18, 33, 36, 40, 42). For example, SIV has recently been isolated from a red-capped mangabey (Cercocebus torquatus torquatus); partial characterization of this virus suggests that it may represent a distinct (sixth) lineage, although analysis of the complete genome will be necessary to establish the exact phylogenetic relationship between SIVrcm and other primate lentiviruses (17).
Further study of the lentiviruses infecting nonhuman primates is important because it may provide insight into the origins and evolution of HIV in humans. The phylogenetic relationships among SIVsm and HIV-2 isolates clearly implicate SIVsm as the proximal source of the HIV-2 epidemic in West Africa (13, 14, 19, 37), but the origins of HIV-1 have not been identified with certainty. A small number of pet chimpanzees have been found to be infected with a virus (SIVcpz) closely related to HIV-1 (26, 27, 43, 50), but the lack of serologic evidence of SIVcpz infection in feral chimpanzee populations sheds doubt on whether this virus constitutes a natural infection in this species (45). It is intriguing that SIVs which are capable of infecting human peripheral blood mononuclear cells (PBMC) are in a minority and at least for two of these (SIVsm and SIVcpz), related HIVs (HIV-2 and HIV-1, respectively) have been demonstrated in humans. The ability to infect the CD4+ lymphocytes of humans may thus be a prerequisite for cross-species transmission to humans. While the ability of these SIV strains to utilize human coreceptors is clearly one mechanism, the accessory proteins Vpr and Vif also limit the ability of some SIVs to replicate in human PBMC (46, 47).
Among the other species of African primates identified by serologic surveys as harboring SIV are a number of species from the genus Cercopithecus, commonly called the guenons (32, 34, 41). Cercopithecus monkeys are a diverse group of 25 species and as many as 70 subspecies of forest-dwelling monkeys that are distributed throughout subsaharan Africa (32, 34, 41). The most commonly known members are the four species of African green monkeys (sabaeus monkeys, C. sabaeus; grivets, C. aethiops; vervets, C. pygerythrus; and tantalus monkeys, C. tantalus). Each of these four species harbors closely related SIV strains, leading to the hypothesis that the primate lentiviruses have coevolved with their host species (22, 25, 45). The only other member of the guenons from which a novel SIV has been characterized is the Sykes monkey from which SIVsyk (9, 21) was isolated. Despite the fact that both Sykes and African green monkeys are members of the same genus, SIVsyk and SIVagm are no more closely related to one another than to any of the other characterized primate lentiviruses. Other Cercopithecus monkeys for which SIV seropositivity has been observed include DeBrazza monkeys (C. neglectus) (40), red-tailed monkeys (C. ascanius schmidtii) (20), Hamlyn’s monkeys (C. hamlyni) (40), and l’hoest monkeys (C. l’hoesti l’hoesti; 40). The close phylogenetic relationships among these monkeys and their widespread distribution across Africa, often in distinct habitats, suggest that the study of SIV in these species will enrich our understanding of the evolution of the primate lentiviruses in general and of the origins of the AIDS epidemic in humans in particular.
Molecular characterization of SIV strains from many of the African monkeys has been hampered by the lack of availability of samples from feral or wild-caught animals and the difficulty in isolating the virus. An alternative source of samples are wild-caught captive populations, such as those found at primate centers or zoo collections, although it is difficult to extrapolate that seropositivity in these populations is indicative of similar infection in free-living feral populations. In a serologic survey of a troop of l’hoest monkeys (C. l’hoesti l’hoesti) in the Portland Zoo, one wild-caught male with antibodies cross-reactive with SIVmac was identified. Here we describe the isolation of SIV from PBMC of this seropositive l’hoest monkey by cocultivation with the human T-cell line, Molt4 Clone 8 (M4C8). This isolate, designated SIVlhoest, has been characterized and compared to other known SIV sequences.
MATERIALS AND METHODS
Virus isolation and infectivity studies.
Virus was isolated from PBMC of a male l’hoest monkey by coculture of phytohemagglutinin-stimulated PBMC with M4C8 cells, using production of reverse transcriptase (RT) activity in the culture supernatant as a measure of viral replication. Virus stocks were prepared from these infected cells by filtration through a 0.45-μm-pore-size filter and cryopreserved in the vapor phase of liquid nitrogen for use in subsequent infectivity studies. These culture supernatants were used to infect M4C8 and CEMss cells to establish a cell line for isolation of total genomic DNA for subsequent cloning studies and for preparation of cell-free virus stocks for infectivity studies in vitro and in macaques.
Animal infectivity studies.
Four juvenile (simian retrovirus- and simian T-cell leukemia virus type 1-free) pigtailed macaques (Macaca nemestrina) were inoculated intravenously with 1 ml of the uncloned SIVlhoest virus stock described above. Macaques were handled in accordance with the guidelines of the NIH Animal Care and Use Committee. Animals were subsequently monitored by virus isolation, coculture of PBMC (at 1, 2, 3, 4, 8, 12, and 16 weeks), limiting dilution infectivity assay of plasma (at 1, 2, 3, and 4 weeks), limiting dilution coculture of disrupted lymph node cells with CEMss cells (at 1, 2, 4, and 16 weeks), and in situ hybridization (ISH) of lymph node biopsies for SIVlhoest viral RNA expression. Antibody responses were monitored by Western blot analysis, using SIVlhoest virus pelleted through sucrose as the viral antigen and published Western blot procedures (24). Lymphocyte subsets (CD4, CD8, CD2, and CD20) were analyzed at the same intervals as virus isolation assays from PBMC, and hematological alterations were monitored by performing complete blood counts.
PCR amplification and plasmid cloning.
Total cellular DNA was extracted from infected CEMss cells at 10 to 15 days postinfection. Degenerate primers (LV1 and LV2 for the first round and LV3 and DDMY for the second round of amplification) designed to PCR amplify a small portion of all lentivirus pol sequences (15) were used to amplify a portion of pol from total cellular DNA. The amplification conditions were as follows: one cycle at 94°C for 2 min, 37°C for 2 min, and 72°C for 3 min, followed by 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min. The resulting 120-bp fragment was cloned into the TA plasmid vector (Invitrogen) and sequenced. A reverse primer was designed based on this sequence and by using a conserved forward primer situated in the primer binding site of SIVs (30), a 2.4-kb fragment was amplified using the following PCR amplification conditions: 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min. This fragment was cloned into the plasmid vector pGEM-7Zf and subsequently utilized as a probe for Southern blot hybridization and screening of a bacteriophage lambda library.
Bacteriophage lambda cloning.
A variety of restriction enzymes were evaluated by Southern blot hybridization of total DNA from infected CEMss cells using the 2.4-kb gag-pol virus fragment described above as a probe. Based on this analysis, SstI was chosen for full-length cloning since the proviral DNA did not appear to contain any SstI sites. Total cellular DNA was digested to completion with SstI, fractionated over a 20 to 60% sucrose gradient to obtain 9- to 20-kb fragments, and ligated into SstI- and XhoI-cleaved arms of λGem12 (Promega, Madison, Wis.). Ligation products were packaged in vitro (Gigapack Gold III; Stratagene), titrated, and plated on bacteria (Escherichia coli K802). A total of 1.5 × 106 recombinant plaques were screened, using a horseradish peroxidase-labeled 2.4-kb gag-pol fragment and a direct detection method and following the manufacturer’s instructions (ECL Direct Detection; Amersham). One positive clone (λSIVlhoest-P7) was detected and plaque purified. To facilitate sequence analysis, three subgenomic clones were generated by digestion of the recombinant lambda clone with SstI and BamHI and ligation into a pGEM-7Zf+ vector. The complete SIVlhoest provirus, including flanking cellular sequences (a 14-kb SstI fragment), was also subcloned into pGEM-7Zf to facilitate subsequent transfection studies. Both strands of the virus were sequenced by a combination of manual dideoxy sequencing using T4 sequenase (USB) and automated fluorescent sequencing (Taq amplification/termination; Perkin Elmer Applied Biosystems) in an ABI 377. Nucleotide sequence analysis was performed by using the GeneWorks (Oxford Molecular) and the Intelligenetics programs (Oxford Molecular).
Sequence comparisons.
The predicted protein sequences encoded by SIVlhoest were compared to the following representatives of the major primate lentivirus lineages: HIV-1 subtype A (isolate U455; GenBank accession no. M62320), subtype B (BRU; K02013), and group O (ANT70; L20587); SIVcpz strains Gab (X52154) and Ant (U42720); SIVsm (PBj; M31325); HIV-2 subtype A (ROD; M15390) and subtype B (UC1; L07625); SIVagm from vervets (ver155; M29975), grivets (gri-1; M58410), and tantalus monkeys (tan-1; U58991); SIVsyk (173; L06042); and SIVmnd (GB1; M27470). Protein sequences were aligned using CLUSTAL X (48) with minor subsequent adjustments using SEAVIEW (12). Sites that could not be aligned unambiguously, as well as all sites for which there was a gap in any of the sequences, were excluded from the analyses.
The extent of sequence difference, along the genome, between SIVlhoest and other viruses was examined in a diversity plot in which protein sequences were concatenated with segments encoded by overlapping genomic regions represented only once: for example, in the region of the Gag-Pol overlap, the amino terminus of the Pol protein was excluded. The fractional amino acid sequence difference was calculated for a window size of 200 residues, moved in steps of 10 residues.
The phylogenetic relationship of SIVlhoest to other primate lentivirus sequences was estimated from aligned Gag, Pol, and Env sequences and from subregions within these alignments to check for evidence that SIVlhoest might have a mosaic genome resulting from recombination during its ancestry. In the absence of such evidence, a summary phylogeny was derived from a concatenated Gag-Pol-Env alignment (the amino terminus of the Pol protein was again excluded) totaling 1,909 amino acids. Relationships were estimated by the neighbor-joining and maximum likelihood methods. The neighbor-joining method, with Kimura protein distances and 1,000 bootstrap replicates, was implemented within the CLUSTAL X package (48). The maximum likelihood method was implemented with PROTML (1) using the JTT model. The order of sequence input was shuffled five times, with the same best tree being found each time.
Generation of ISH probes.
PCR was used to amplify five 1.5- to 2-kb fragments of the SIVlhoest provirus from the complete plasmid clone by using the following primers, where the restriction sites (SstI and Csp45I) introduced to facilitate cloning are underlined: 1F, (nucleotide [nt] 1018) 5′-ttagagctcttgtgagaagtgtgtaattctgat; 1R, (nt 2482) 5′-tgattcgaatctgcttttgttggagcactctcc; 2F, (nt 2506) 5′-atcgagctcgagagcactggagacttacaggac; 2R, (nt 4505) 5′-atcttcgaattcctttatgagcaggcacccatc; 3F, (nt 4529) 5′-attgagctctaggaggtaatcaagaggtagacc; 3R, (nt 6487) 5′-catttcgaataattaacactgcatacttaacat; 4F, (nt 6502) 5′-ttagagctcccaatactagcctctgggtcacga; 4R, (nt 8503) 5′-atattcgaataattaacactgcatacttaacat; 5F, (nt 8527) 5′-atcgagctctacttgtaatcatagggttaagag; 5R, (nt 9844) 5′-taattcgaaactagcttgtactttctaacaatg.
These five fragments were cloned into pGEM-7Zf, the subsequent clones were digested with SstI (antisense) and Csp45I (sense), and RNA was transcribed to incorporate digoxigenin using Sp6 and T7 polymerase, respectively. The pooled antisense probe was used to detect viral mRNA expression in lymph node biopsy samples that were fixed in STRECKS fixative using previously described methodology (24).
Transfection and infectivity studies.
Virus stocks were generated by transfection of 5 to 10 μg of either the lambda or plasmid clone into 293 cells by a calcium phosphate-mediated procedure (CellPhect; Stratagene). The infectivity of these filtered supernatants was evaluated by infection of CEMss cells, macaque PBMC, and macaque monocyte-derived macrophages as previously described (24).
Nucleotide sequence accession number.
The complete sequence of SIVlhoest has been submitted to GenBank under accession no. AF075269.
RESULTS
Infectivity and pathogenicity of SIVlhoest in vivo.
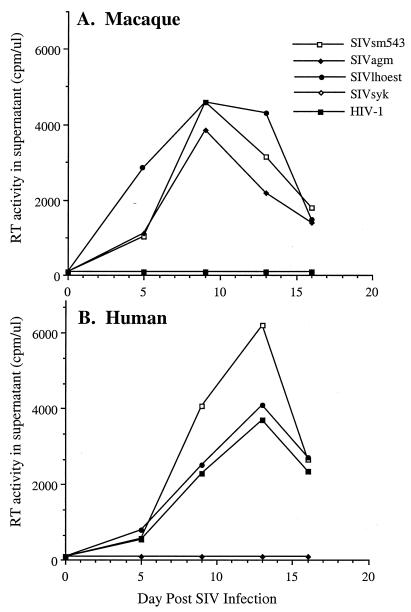
SIVlhoest was clearly distinct from other primate lentiviruses, such as SIVagm, SIVsm, or HIV-1, since the Gag antigens in culture supernatants of persistently infected cells did not cross-react with a commercially available SIVmac p27 antigen capture assay (Coulter Corp.). As a prelude to any in vivo characterization, the infectivity of SIVlhoest for macaque and human PBMC was compared to that of representative members of the SIVsm, SIVsyk, SIVagm, and HIV-1 lineage (Fig. 1). Like SIVsmE543-3, SIVlhoest infected both human and macaque PBMC efficiently. This contrasted with the restricted tropism of SIVsyk for Sykes monkey PBMC (not shown), SIVagm for macaque PBMC, and HIV-1 for human PBMC.
FIG. 1.
In vitro growth characteristics of SIVlhoest and other primate lentiviruses in human and macaque PBMC. The replication of SIVlhoest, SIVagm, SIVsm, SIVsyk, and HIV-1 as assessed by RT activity in culture supernatant is shown graphically for macaque (A) and human (B) PBMC. For this assay, equivalent amounts (based on RT activity of stocks) of the following viruses were used: SIVlhoest-P, SIVagm155-4, SIVsmE543-3, SIVsyk/cm173, and HIV-1IIIB. Since RT activity was never observed in SIVsyk-infected cultures, the symbols are superimposed by values for the negative control.
Infectivity in macaque PBMC is a prerequisite for a robust infection in vivo but clearly is not predictive of pathogenicity for that species. To evaluate the potential pathogenicity of SIVlhoest, a cohort of four pigtailed macaques (M. nemestrina) was inoculated intravenously with uncloned SIVlhoest. Since this latter virus had been passaged twice through human T-cell lines, which might produce attenuation, we also isolated virus from a homogenate of cryopreserved spleen from the same l’hoest monkey by short-term coculture with M4C8 cells (4 days) and subsequent infection of macaque PBMC. This virus was designated SIVlhoest-S to distinguish it from the PBMC isolate; four additional pigtailed macaques were inoculated intravenously with this primary virus isolate.
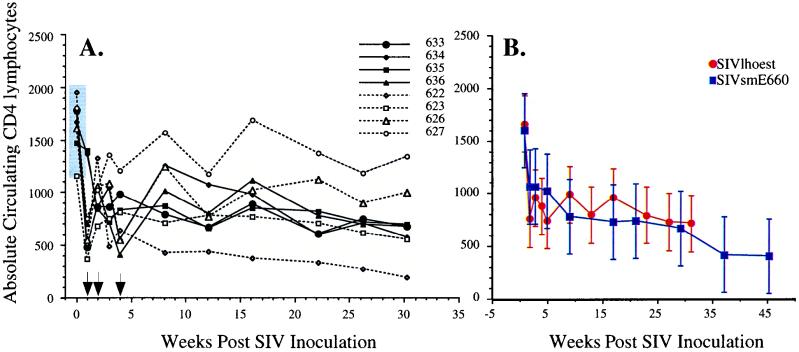
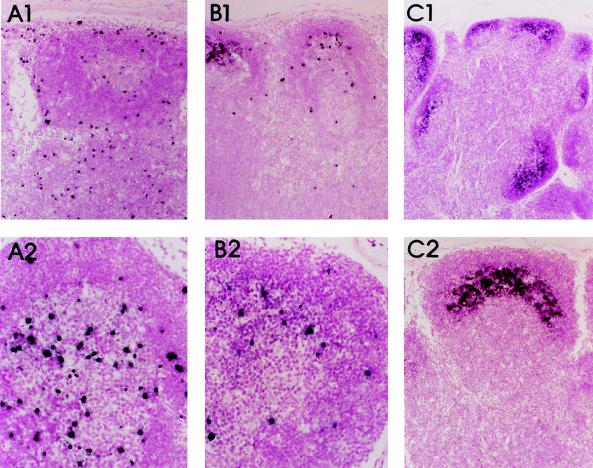
Each of the macaques became persistently infected, as evidenced by isolation of virus from PBMC and lymph node biopsies at multiple time points postinoculation (Table 1). The macaques inoculated with the virus isolate from PBMC (macaques 622, 623, 626, and 627) that was passaged twice in human T-cell lines exhibited less-consistent virus isolation than macaques inoculated with the splenic isolate (macaques 633, 634, 635, and 636), which is suggestive of some degree of viral attenuation of the PBMC isolate. However, one of these (macaques 622) exhibited the most profound and early CD4 depletion of all the inoculated animals. Each macaque exhibited an early decline in all lymphocyte subsets (CD4 subset shown in Fig. 2A), reaching a lowest point at 1 week postinoculation. Lymphopenia was coincident with peak levels of infectious SIV in the plasma (1,000 50% tissue culture infectious doses/ml) and a high proportion of SIV-expressing cells within lymph nodes, as demonstrated by limiting dilution coculture of disrupted lymph node cells with CEMss cells (1 in 1,000 cells). High lymphoid virus expression was confirmed by ISH with SIVlhoest-specific riboprobes (Fig. 3). The plasma viremia resolved by 3 weeks, with declining prevalence of SIV-expressing cells in lymph nodes; this phase was associated with the onset of massive lymphoid hyperplasia and the trapping of virions in a pattern characteristic of the distribution of follicular dendritic cells in germinal centers (6). Subsequent fluorescence-activated cell sorter analysis of lymphocyte subsets revealed a gradual decline (2- to 10-fold) in the numbers of circulating CD4+ lymphocytes, similar to that observed in a cohort of six pigtailed macaques inoculated with a pathogenic uncloned SIVsm isolate (SIVsmE660; Fig. 2B). As was also observed with SIVsmE660 (22), the severity of the CD4 decline in SIVlhoest-infected macaques varied considerably, from mild to profound depletion. The macaque that exhibited the most severe CD4 depletion (macaque 622) has also failed to gain weight and exhibits mild anemia (hematocrit of 28%), thrombocytopenia (<30,000 per μl), and periodic bouts of diarrhea. Two other macaques currently exhibit mild weight loss in association with peripheral CD4 lymphocytes of less than 500 per μl.
TABLE 1.
Sequential virus isolation from PBMC and lymph nodes of macaques inoculated with SIVlhoest isolates
| Isolation of virus at indicated wk postinoculationa
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate source | Macaque | 1 | 2 | 4 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 |
| PBMC | 622 | ++ | ++ | ++ | + | + | ++ | + | − | − | + | + | + |
| 623 | ++ | ++ | ++ | − | − | ++ | − | − | − | − | + | − | |
| 626 | ++ | ++ | ++ | + | − | +− | − | − | − | − | − | − | |
| 627 | ++ | ++ | ++ | + | − | ++ | + | + | + | − | + | − | |
| Spleen | 633 | ++ | ++ | ++ | + | + | ++ | − | + | + | − | + | + |
| 634 | ++ | ++ | ++ | + | + | ++ | + | + | + | + | − | + | |
| 635 | ++ | ++ | ++ | + | + | ++ | + | + | + | + | + | + | |
| 636 | ++ | ++ | ++ | + | + | ++ | − | − | − | − | − | + | |
Virus isolation from PBMC and lymph nodes as detected by RT activity in culture supernatant within a 6-week period of coculture is indicated by a +, and inability to isolate virus is indicated by a −.
FIG. 2.
Characteristics of SIVlhoest infection of pigtailed macaques. (A) Kinetics of sequential alterations in CD4 lymphocytes in the peripheral blood during the first 16 weeks postinoculation in eight inoculated macaques, where the open symbols and dotted lines indicate animals inoculated with the PBMC isolate and solid lines and black symbols indicate animals inoculated with the splenic isolate. (B) Kinetics of mean CD4 lymphocyte numbers with standard deviations are compared between SIVlhoest-inoculated macaques and a cohort of six macaques inoculated with SIVsmE660, demonstrating that SIVlhoest induces a profile in declining CD4 lymphocyte numbers similar to that observed with an AIDS-inducing SIV isolate.
FIG. 3.
Kinetics of virus expression in lymph nodes by ISH. (A1 and A2) ISH of lymph node biopsy sample obtained 1 week postinoculation, demonstrating high virus expression. Magnification, ×6 (top) and ×55 (bottom). (B1 and B2) Lymph node biopsy sample obtained 2 weeks after inoculation, showing a reduction in the numbers of SIV-positive cells relative to that observed at 1 week. Magnification, ×6 (top) and ×55 (bottom). (C1 and C2) ISH of a lymph node biopsy sample obtained 4 weeks after inoculation, showing a further reduction in the numbers of SIV-positive cells, with diffuse hybridization localized in the crescentric distribution of follicular dendritic cells within the germinal center consistent with trapping of immune complexes containing SIV on dendritic cells. Magnification, ×6 (top) and ×55 (bottom).
SIVlhoest is a novel member of the SIVmnd lineage.
In order to characterize SIVlhoest molecularly, we used degenerate primers to amplify a 120-bp fragment of the pol gene from cellular DNA extracted from infected M4C8 cells. This fragment was cloned, and based on the sequence, we designed a reverse primer in pol that was used in combination with a forward primer in the highly conserved primer binding site to amplify and clone a 2.4-kb gag-pol fragment. This fragment was used as a probe in a subsequent Southern blot hybridization to identify restriction enzymes useful for cloning and as a probe to identify proviruses within a bacteriophage lambda library generated by SstI digestion of cellular DNA extracted from infected M4C8 cells. One full-length clone, λSIVlhoest-P7 was obtained and purified. λSIVlhoest-P7 was infectious after transfection of M4C8 or CEMss cells and subsequent infection of macaque and human PBMC. After being subcloned into plasmid vectors, the proviral portion of the clone was sequenced in its entirety (9,957 nt) and compared to the sequences of other known primate lentiviruses.
The genomic organization of SIVlhoest was similar to that of SIVagm, SIVmnd, and SIVsyk. Each of these viruses encodes gag, pol, and env, as well as the accessory genes vif, vpr, tat, rev, and nef, but lacks the additional genes vpu (found only among the members of the HIV-1 and SIVcpz lineage) and vpx (specific to SIVsm, SIVmac, and HIV-2). The long terminal repeat (LTR) of SIVlhoest (789 nt) contained all the characteristic features of other primate lentivirus LTRs, including one NF-κB site and two potential SP-1 binding sites (data not shown). Comparisons of the predicted protein sequences encoded by the eight common genes revealed that SIVlhoest was quite distinct from all other SIV (and HIV) isolates analyzed to date, exhibiting at least 33% amino acid sequence difference from representatives of each of the five lineages of primate lentiviruses (Table 2). For the large genes gag, pol, and env, as well as for vpr and tat, the SIVlhoest proteins were most similar to those of SIVmnd, but for the vif, rev, and nef genes, the distance between the SIVlhoest and SIVmnd proteins was similar to that between SIVlhoest and other SIVs. The similarity between SIVlhoest and SIVmnd is shown in an alignment of the surface unit (SU) portion of the Env protein of these two viruses (Fig. 4). Although scattered substitutions are evident throughout gp120, many of the cysteine residues (asterisks) and potential N-linked glycosylation sites were conserved and regions such as the V3 loop analog and the CD4 binding domain showed remarkable conservation. The sequence of the envelope amplified from the spleen of the l’hoest monkey (lhoest-S) had 95% identity with that of the infectious clone (lhoest-7). These two envelope sequences are representative of the two isolates used to inoculate the macaques. As expected from other primate lentivirus envelopes, the V1 region was the most variable, with characteristic threonine residues and insertion/deletion polymorphism. In contrast, the CD4 binding domain was absolutely conserved and only one substitution was observed in the V3 loop analog.
TABLE 2.
Comparison of amino acid identity among primate lentiviruses, demonstrating that SIVlhoest is distantly related to SIVmnda
| Comparisons of SIVlhoest and major SIV lineages
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Gag | Pol | Vif | Vpr | Tat | Rev | Env | Nef | |
| SIVmnd | 54 | 67 | 38 | 65 | 43 | 32 | 51 | 44 |
| SIVsyk | 48 | 51 | 31 | 21 | 31 | 19 | 35 | 40 |
| SIVagm | 49 | 57 | 41 | 42 | 41 | 32 | 36 | 41 |
| SIVsm | 49 | 58 | 30 | 42 | 35 | 30 | 35 | 43 |
| SIVcpz | 49 | 58 | 33 | 41 | 26 | 19 | 30 | 41 |
Identities were calculated as described in the “Sequence comparisons” section of Materials and Methods.
FIG. 4.
Comparison of the predicted protein sequence of the surface subunit (SU) of the envelope of SIVlhoest and SIVmnd reveals remarkable conservation of cysteine residues and regions such as the V3 loop analog and CD4 binding domain. Conserved cysteines are indicated by ∗, and variable cysteine residues are indicated by a ∗ above the top sequence. Potential N-linked glycosylation sites are underlined. The predicted sequence of gp120 of the SIVlhoest-7 molecularly cloned virus derived from a PBMC isolate is shown on the top (lhoest-7). Substitutions relative to this sequence in the predicted sequence of a clone of envelope amplified directly from the spleen of this monkey (lhoest-S) and the SIVmnd/GB-1 clone are shown aligned below. Dots indicate amino acid identity at a residue, and a dash indicates a gap introduced to optimize alignment. Variable regions analagous to those observed in HIV-1 and other SIVs are indicated, and the cleavage site for the transmembrane glycoprotein (TM) is shown.
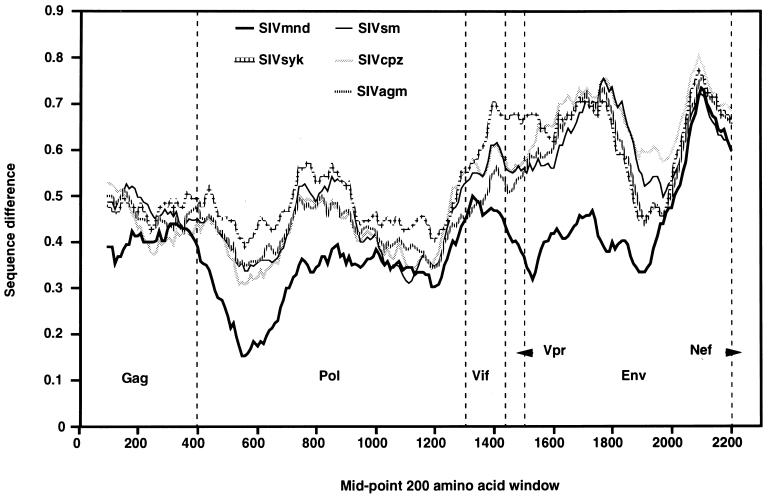
To examine the divergence of SIVlhoest from the other primate lentiviruses in more detail, the extent of sequence difference was determined for moving windows of 200 amino acids. The resulting diversity plot confirmed that overall, SIVlhoest is most similar to SIVmnd but in some segments these two viruses are about as different from each other as they are from other SIVs (Fig. 5). Furthermore, that plot showed that the boundaries of these regions lie within genes, so that each of the major genes (gag, pol, and env) includes segments of both types. This variation in the extent of relative divergence between SIVlhoest and SIVmnd (i.e., relative to the extent of divergence from other SIVs) could indicate that the ancestors of these viruses were generated by recombination of different SIV lineages, as has been found for SIVagm from sabaeus monkeys (29). To examine the evolutionary relationship of SIVlhoest to the other primate lentiviruses, we generated numerous phylogenetic trees derived from alignments of individual gene products and from smaller regions defined by consideration of the diversity plot (data not shown). These all indicated a clustering of SIVlhoest with SIVmnd, though their relative distances varied. Thus, these analyses provided no evidence that recombination has played a significant role in the evolution of SIVlhoest (or SIVmnd) and suggested that the variation in the extent of relative divergence between SIVlhoest and SIVmnd reflects changes in rates of evolution specific to these viruses.
FIG. 5.
Diversity plot comparing SIVlhoest with representatives of each of the five major lineages of primate lentiviruses, i.e., SIVmnd, SIVsyk, SIVsm, SIVcpz, and SIVagm (SIVver). Protein sequence difference is plotted for windows of 200 amino acids moved in steps of 10.
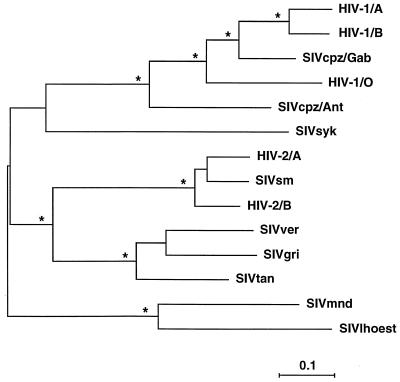
Since the SIVlhoest-SIVmnd clade was maintained across all of the analyses, an overview of the phylogenetic relationships between these viruses was obtained from an analysis of an alignment of concatenated Gag-Pol-Env proteins, in which SIVlhoest appears as a highly divergent member of the same major lineage as SIVmnd (Fig. 6). The interpretation that SIVlhoest and SIVmnd are members of the same clade is dependent on the position of the root of the phylogenetic tree; the tree shown in Fig. 6 has been midpoint rooted. The “precise” position of the root of the primate lentivirus tree is problematic, since the nearest available outgroup sequences, namely, lentiviruses from other mammalian hosts, are quite distantly related. However, analyses using various such outgroups suggest that the rooting shown in Fig. 6 is appropriate. Importantly, none of our analyses using nonprimate lentivirus outgroups ever placed the root in such a position as to disrupt the SIVlhoest-SIVmnd clade. Thus, we conclude that SIVlhoest and SIVmnd are both members, albeit distantly related, of the same major primate lentivirus lineage. Overall, SIVlhoest and SIVmnd are rather more divergent from each other than are SIVs from different species of African green monkey (Table 3). The only other example of such high divergence within a major lineage involves the SIVcpz-HIV-1 lineage, where SIVcpz/Ant is almost as different from SIVcpz/Gab1 (Table 3) and HIV-1 as SIVlhoest and SIVmnd are from each other.
FIG. 6.
Phylogenetic relationship of SIVlhoest to other primate lentiviruses. The tree was derived by maximum likelihood analysis of a concatenated Gag-Pol-Env protein alignment (see text for details). A tree derived by neighbor-joining analysis differed in no significant way. Stars indicate that the clade to the right was found in 100% of bootstrap replicates of the neighbor-joining analysis. Horizontal branch lengths are drawn to scale, with the bar indicating 0.1 amino acid replacement per site.
TABLE 3.
Divergence of SIVlhoest and SIVmnd from members of the African green monkey familya
| % Identity within lineages for indicated protein:
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Gag | Pol | Vif | Vpr | Tat | Rev | Env | Nef | |
| SIVagm | 77 | 70 | 57 | 76 | 57 | 53 | 70 | 73 |
| SIVcpz | 68 | 74 | 57 | 64 | 58 | 54 | 51 | 53 |
Identities were calculated as described in the “Sequence comparisons” section of Materials and Methods.
DISCUSSION
It seems clear that HIV-1 originated through cross-species transmission(s) in the recent past from a naturally infected African primate, but the species involved remains open to question. Therefore, further exploration of the nature of the diversity among primate lentiviruses is necessary to elucidate the origins and evolution of the human viruses. Now that we have characterized SIVlhoest and examined the relationship between it and the previously known primate lentiviruses, it is clear that this particular virus is not the proximal source of HIV-1. However, the phylogenetic position of SIVlhoest is surprising and has implications for our understanding of primate lentivirus evolution. In particular, at first sight, the genetic similarity between SIVlhoest and SIVmnd seems difficult to explain.
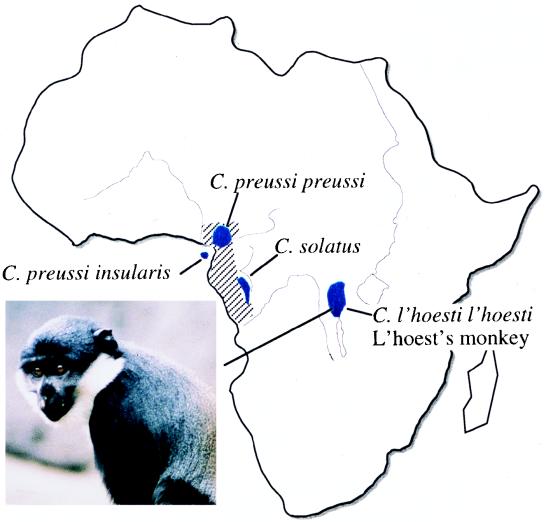
While the phylogeny of the primate lentiviruses indicates that there have been multiple cross-species transmissions (45), there are also indications that some of the viruses have coevolved with their natural host species and thus that the group as a whole may be quite ancient. The four species of African green monkeys (vervets, grivets, and sabaeus and tantalus monkeys) each harbor their own variants of SIVagm, while SIVsyk and now SIVlhoest are rather more divergent viruses from rather more divergent Cercopithecus species. It is tempting to speculate that these Cercopithecus SIVs represent the vertical transmission “backbone” of the primate lentivirus phylogeny, with the other SIVs from more distantly related primates having all resulted from horizontal transmissions. Whether this scenario is accurate, the comparatively close relationship between SIVlhoest and SIVmnd seems most unlikely to be a reflection of host-dependent viral evolution, since the mandrill belongs to a genus (Mandrillus) quite distant from the Cercopithecus genus, and so (at least) one of these viruses is likely the result of cross-species transmission. There is accumulating evidence that such transfer of SIV does occur naturally between different species of monkeys that share a common habitat in the wild. For example, the sabaeus subtype of SIVagm has been identified in wild-caught patas monkeys (Erythorocebus patas) in West Africa (5) and the vervet subtype of SIVagm has been found in a yellow baboon (Papio hamadryas cynocephalus) in Tanzania (29). However, mandrills are restricted to the west coastal region of central Africa around Gabon, whereas l’hoest monkeys inhabit a localized area of central Africa more than 1,000 km to the east (Fig. 7) (32, 34, 42).
FIG. 7.
A schematic view of Africa, showing the ranges occupied by l’hoest monkeys (C. l’hoesti l’hoesti) and their close relatives, preussis monkeys (C. preussi preussi) and suntailed monkeys (C. solatus); the distribution of mandrills (Mandrillus sphinx) is indicated by cross-hatching. A representative l’hoest monkey is shown.
The geographical separation of l’hoest monkeys and mandrills implies that neither could be the immediate source of virus for the other. However, close relatives of l’hoest monkeys are found in regions of west central Africa overlapping the mandrill range (Fig. 7). Preussis monkeys (C. preussi preussi and insularis) from Cameroon and Bioko Island and sun-tailed monkeys (C. solatus) from Gabon are sufficiently similar to l’hoest monkeys that they have been considered by some to be a subspecies of C. lhoesti. Furthermore, at least one of these species, C. solatus, has serologic evidence of SIV infection (25a). We therefore hypothesize that SIVmnd infection of mandrills resulted from cross-species transmission of SIV from one of these relatives of the l’hoest monkey. This direction of the transmission, rather than from mandrill to the l’hoest monkey, is consistent with the Cercopithecus origin of primate lentiviruses suggested above and is supported by the apparently low rate of SIV seroprevalence in wild mandrills. Only two seropositive wild-caught mandrills have been reported, and the only existing clone came from one of two seropositive founders of a colony in Gabon. Although SIVmnd is now prevalent within this colony, all of the circulating viruses appear to be highly related, suggesting subsequent transmission of this virus within the colony (16, 39). It will be interesting to determine whether feral mandrills from other locations also harbor SIVmnd.
Consistent with the proposed ancient relationship between primate lentiviruses and their respective natural hosts, SIVs appear to result in an asymptomatic infection in their natural host species (e.g., SIVsm in sooty mangabeys or SIVagm in African green monkeys) (24). However, AIDS may result upon experimental infection of other primates, particularly macaques (2, 7, 22, 24, 35). In the present study, SIVlhoest-infected macaques demonstrated many of the virologic, immunologic, and clinical characteristics of early infection of macaques with pathogenic isolates of SIVsm, SIVmac, or SIVagm. Such characteristics included (i) high viral expression in lymphoid tissues in the primary phase of infection, (ii) high primary plasma viremia, (iii) subsequent lymphadenopathy and trapping of viral RNA in germinal centers in a follicular dendritic cell pattern, (iv) declining peripheral CD4 lymphocyte numbers, (v) persistent PBMC-associated viremia, and (vi) thrombocytopenia and weight loss in one macaque. Although these animals have not been infected for a sufficient period of time to become symptomatic, the virologic and clinical features of infection are consistent with progression to AIDS. Moreover, SIVlhoest appears to be directly pathogenic for macaques without adaptation by prior macaque passage.
The ability of SIVlhoest to infect human PBMC in vitro at least as efficiently as it infects macaque PBMC suggests that this virus has the potential to infect human populations. Such cross-species transmission to humans has already been observed for the SIVsm isolate that is now circulating among human populations as HIV-2 (13, 14). While the ability of this virus to infect human PBMC may not be predictive of virulence in humans, serologic surveys of humans in regions near the habitat of l’hoest monkeys are necessary to evaluate this possibility. These highly specialized forest dwellers have considerably less contact with humans than species such as sooty mangabeys. However, the continued expansion of human populations and encroachment upon the habitats of primates such as the l’hoest monkey may amplify the risk for such an event in the future.
ACKNOWLEDGMENTS
We thank Russell Byrum and Marisa St. Claire, Bioqual, Inc., Rockville, Md., for assistance in conducting the animal studies; Michael Durham, Oregon Zoo, Portland, Ore., for providing pictures of l’hoest monkeys; and John Oates, Hunter College, New York, N.Y., for useful discussions of primatology.
REFERENCES
- 1.Adachi J, Hasegawa M. MOLPHY (a program package for MOLecular PHYlogenetics), version 2.2. Tokyo, Japan: Institute of Statistical Mathematics; 1994. [Google Scholar]
- 2.Allan J S. Pathogenic properties of simian immunodeficiency viruses in nonhuman primates. Annu Rev AIDS Res. 1991;1:191–206. [Google Scholar]
- 3.Allan J S, Short M, Taylor M E, Su S, Hirsch V M, Johnson P R, Shaw G M, Hahn B H. Natural infection of West African green monkeys with a unique subtype of simian immunodeficiency virus SIVagm. J Virol. 1991;65:2816–2828. doi: 10.1128/jvi.65.6.2816-2828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baier M, Werner A, Cichutek K, Garber C, Muller C, Kraus G, Ferdinand F J, Hartung S, Papas T S, Kurth R. Molecularly cloned simian immunodeficiency virus SIVagm3 is highly divergent from other SIVagm isolates and is biologically active in vitro and in vivo. J Virol. 1989;63:5119–5123. doi: 10.1128/jvi.63.12.5119-5123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibollet-Ruche F, Galat-Luong A, Cuny G, Sarni-Manchado P, Galat G, Durant J P, Pourrut X, Veas F. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopthecus aethiops sabeus) in the wild. J Gen Virol. 1996;77:773–781. doi: 10.1099/0022-1317-77-4-773. [DOI] [PubMed] [Google Scholar]
- 6.Chalifoux L V, Ringler D J, King N W, Seghal P K, Desrosiers R C, Daniel M D, Letvin N L. Lymphadenopathy in macaques experimentally infected with simian immunodeficiency virus (SIV) Am J Pathol. 1987;128:104–110. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Telfer P, Reed P, Gettie A, Zhand L, Ho D D, Marx P. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70:3617–3627. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel M D, Li Y, Naidu Y M, Durda P J, Schmidt D K, Troup C D, Silva D P, MacKey J J, Kestler H W, Seghal P K, et al. Simian immunodeficiency viruses from African green monkeys. J Virol. 1988;62:4123–4128. doi: 10.1128/jvi.62.11.4123-4128.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emau P, McClure H M, Isahakai M, Else J G, Fultz P N. Isolation from African Sykes’ monkeys (Cercopithecus mitis) of a lentivirus related to human and simian immunodeficiency viruses. J Virol. 1991;65:2135–2140. doi: 10.1128/jvi.65.4.2135-2140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fomsgaard A, Hirsch V M, Allan J S, Johnson P R. A highly divergent proviral DNA clone of SIV from a distinct species of African green monkey. Virology. 1991;182:397–402. doi: 10.1016/0042-6822(91)90689-9. [DOI] [PubMed] [Google Scholar]
- 11.Fukasawa M, Miura T, Hasegawa A, Morikawa S, Tsuijmoto H, Miki K, Kitamura T, Hayami M. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature. 1988;333:457–461. doi: 10.1038/333457a0. [DOI] [PubMed] [Google Scholar]
- 12.Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comp Appl Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- 13.Gao F L, Yue L, White A T, Pappas P G, Barchue J, Hanson A J, Green B M, Sharp P M, Shaw G M, Hahn B H. Human infection by genetically diverse SIVsm-related HIV-2 in West Africa. Nature. 1992;358:495–499. doi: 10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- 14.Gao F L, Yue L, White A T, Pappas P G, Barchue J, Hanson A J, Green B M, Sharp P M, Shaw G M, Hahn B H. Genetic diversity of human immunodeficiency viruses type 2: evidence for distinct sequence subtypes with differences in virus biology. J Virol. 1994;68:7433–7447. doi: 10.1128/jvi.68.11.7433-7447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelman I H, Zhang J, Hailman E, Hanafusa H, Morse S S. Identification and evaluation of new primer sets for the detection of lentivirus proviral DNA. AIDS Res Hum Retroviruses. 1992;8:1981–1989. doi: 10.1089/aid.1992.8.1981. [DOI] [PubMed] [Google Scholar]
- 16.Georges-Courbot M C, Moisson P, Leroy E, Pingard A M, Nerrienet E, Dubreuil G, Wickings E J, Debels F, Bedjabaga I, Poaty-Mavoungou V, Hahn N T, Georges A J. Occurrence and frequency of transmission of naturally occurring simian retroviral infections (SIV, STLV and SRV) at the CIRMF primate center, Gabon. J Med Primatol. 1996;25:313–326. doi: 10.1111/j.1600-0684.1996.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 17.Georges-Courbot M C, Lu C Y, Makuwa M, Telfer P, Onanga R, Dubreuil G, Chen Z, Smith S M, Georges A, Gao F, Hahn B H, Marx P A. Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J Virol. 1998;72:600–608. doi: 10.1128/jvi.72.1.600-608.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendry R M, Wells M A, Phelan M A, Schneider A L, Epstein J S, Quinnan G V. Antibodies to simian immunodeficiency virus in African green monkeys in Africa in 1957–1962. Lancet. 1986;ii:455. doi: 10.1016/s0140-6736(86)92156-2. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch V M, Olmsted R A, Murphey-Corb M, Purcell R H, Johnson P R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch V M, McGann C, Dapolito G, Goldstein S, Ogen-Odoi A, Biryawaho B, Lakwo T, Johnson P R. Identification of a new subgroup of SIVagm in tantalus monkeys. Virology. 1993;197:426–430. doi: 10.1006/viro.1993.1606. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch V M, Dapolito G, Goldstein S, McClure H, Emau P, Fultz P N, Isahakia M, Neroot R, Myers G, Johnson P R. A distinct African lentivirus from Sykes’ monkeys. J Virol. 1993;67:1517–1528. doi: 10.1128/jvi.67.3.1517-1528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch V M, Johnson P R. Genetic diversity and phylogeny of primate lentiviruses. In: Morrow J, Haigwood N, editors. HIV molecular organization, pathogenicity and treatment. Amsterdam, The Netherlands: Elsevier Science Publishers; 1993. pp. 221–240. [Google Scholar]
- 23.Hirsch V M, Johnson P R. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch V M, Dapolito G, Johnson P R, Elkins W R, London W T, Montali R J, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch V M, Dapolito G, Goeken R, Campbell B. Phylogeny and natural history of the primate lentiviruses, SIV and HIV. Curr Opin Genet Dev. 1997;5:798–806. doi: 10.1016/0959-437x(95)80014-v. [DOI] [PubMed] [Google Scholar]
- 25a.Hirsch, V. M. Unpublished data.
- 26.Huet T, Cheynier R, Meyerhans A, Roelants G, Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990;345:356–359. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- 27.Janssens W, Fransen K, Peeters M, Heyndrickx L, Motte J, Bedjabaga L, Depaporte E, Piot P, Van Der Groen G. Phylogenetic analysis of a new chimpanzee lentivirus SIVcpz-gab-2 from a wild-captured chimpanzee from Gabon. AIDS Res Hum Retroviruses. 1994;10:1191–1192. doi: 10.1089/aid.1994.10.1191. [DOI] [PubMed] [Google Scholar]
- 28.Jin M J, Rogers J, Phillips-Conroy J E, Allan J S, Desrosiers R C, Shaw G M, Sharp P M, Hahn B H. Mosaic genome structure of simian immunodeficiency virus from West African green monkeys. EMBO J. 1994;13:2935–2947. doi: 10.1002/j.1460-2075.1994.tb06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin M J, Rogers J, Phillips-Conroy J E, Allan J S, Desrosiers R C, Shaw G M, Sharp P M, Hahn B H. Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J Virol. 1994;68:8454–8460. doi: 10.1128/jvi.68.12.8454-8460.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson P R, Fomsgaard A, Allan J, Gravell M, London W T, Olmsted R A, Hirsch V M. Simian immunodeficiency viruses from African green monkeys display unusual genetic diversity. J Virol. 1990;64:1086–1092. doi: 10.1128/jvi.64.3.1086-1092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones D T, Taylor W R, Thornton J M. The rapid generation of mutation data matrices from protein sequences. Comp Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 32.Kingdon J. East African mammals: an atlas of evolution in Africa. Vol. 1. Chicago, Ill: The University of Chicago Press; 1974. [Google Scholar]
- 33.Kodama T, Silva D P, Daniel M D, Phillips-Conroy J E, Jolly C J, Rogers J, Desrosiers R C. Prevalence of antibodies to SIV in baboons in their native habitat. AIDS Res Hum Retroviruses. 1989;5:337–343. doi: 10.1089/aid.1989.5.337. [DOI] [PubMed] [Google Scholar]
- 34.Lernould J-M. Classification and geographical distribution of guenons: a review. In: Gautier-Hion A, Bouliere F, Gautier C, Kingdon J, editors. A primate radiation: evolutionary biology of the African guenons. Cambridge, United Kingdom: Cambridge University Press; 1988. pp. 54–77. [Google Scholar]
- 35.Letvin N L, King N W. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquired Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 36.Lowenstine L J, Pederson N C, Higgins J, Pallis K C, Uyeda A, Marx P, Lerche N W, Munn R J, Gardner M B. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from a sooty mangabeys (Cercocebus atys) Int J Cancer. 1986;38:563–574. doi: 10.1002/ijc.2910380417. [DOI] [PubMed] [Google Scholar]
- 37.Marx P, Li Y, Lerche N W, Sutjipto S, Gettie A, Yee J A, Brotman B, Prince A M, Hanson A, Webster R G, Desrosiers R C. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a West African pet sooty mangabey. J Virol. 1991;65:4480–4485. doi: 10.1128/jvi.65.8.4480-4485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller M C, Saksena N K, Nerrienet E, Chappey C, Herve V M, Durand J P, Legal-Campodonico P, Lang M C, Digoutte J P, Georges A J, Sonigo P, Barre-Sinoussi F. Simian immunodeficiency viruses from central and western Africa: evidence for a new species-specific lentivirus in tantalus monkeys. J Virol. 1993;67:1227–1235. doi: 10.1128/jvi.67.3.1227-1235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nerrienet E, Amouretti X, Muller-Trutwin M C, Poaty-Mavoungou V, Bedjebaga I, Ngyuyen H T, Dubreuil G, Corbet S, Wickings E J, Barre-Sinoussi F, Georges A J, Georges-Corbot M-C. Phylogenetic analysis of SIV and STLV-1 in mandrills (Mandrillus sphinx): indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Res Hum Retroviruses. 1998;14:785–796. doi: 10.1089/aid.1998.14.785. [DOI] [PubMed] [Google Scholar]
- 40.Nicol I, Messinger D, Dibouch P, Bernard J, Desportes I, Jouffre R, Snart R, Nara P, Gallo R C, Zagury D. Use of Old World monkeys for acquired immunodeficiency syndrome research. J Med Primatol. 1989;18:227–236. [PubMed] [Google Scholar]
- 41.Oates J F. The distribution of Cercopithecus monkeys in West African forests. In: Gautier-Hion A, Bouliere F, Gautier C, Kingdon J, editors. A primate radiation: evolutionary biology of the African guenons. Cambridge, United Kingdom: Cambridge University Press; 1988. pp. 78–103. [Google Scholar]
- 42.Ohata Y, Masuda T, Tsujimoto H, Ishikawa T, Kodama T, Morikawa S, Nakai M, Honjo S, Hayami M. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus in various nonhuman primates. Int J Cancer. 1988;41:115–122. doi: 10.1002/ijc.2910410121. [DOI] [PubMed] [Google Scholar]
- 43.Peeters M, Honore C, Huet T, Bedjabaga I, Oussari S, Bussi P, Cooper R W, Delaporte E. Isolation and partial characterization of an HIV-related virus occurring naturally in chimpanzees in Gabon. AIDS. 1989;3:625–630. doi: 10.1097/00002030-198910000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Peeters M, Janssens W, Fransen K, Brandful J, Hendrickx L, Koffi K, Delaporte E, Piot P, Gershy-Damet G M, Van Der Groen G. Isolation of simian immunodeficiency viruses from two sooty mangabey monkeys in Cote d’Ivoire: virological and genetic characterization and relationship to other HIV, type 2 and SIVsm/mac strains. AIDS Res Hum Retroviruses. 1994;10:1289–1294. doi: 10.1089/aid.1994.10.1289. [DOI] [PubMed] [Google Scholar]
- 45.Sharp P M, Robertson D L, Hahn B H. Cross-species transmission and recombination of “AIDS” viruses. Phil Trans R Soc London Ser B. 1995;349:41–47. doi: 10.1098/rstb.1995.0089. [DOI] [PubMed] [Google Scholar]
- 46.Simon J H M, Miller D L, Fouchier R A M, Soares M A, Peden K W C, Malim M H. The regulation of primate lentivirus infectivity by vif is cell species restricted: a role for vif in determining virus host range and cross-species transmission. EMBO J. 1998;17:1259–1267. doi: 10.1093/emboj/17.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stivahtis G L, Soares M A, Vodicka M A, Hahn B H, Emerman M. Conservation and host specificity of Vpr-mediated cell cycle arrest suggest a fundamental role in primate lentivirus evolution and biology. J Virol. 1997;71:4331–4338. doi: 10.1128/jvi.71.6.4331-4338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsujimoto H, Hasegawa A, Maki N, Fukasawa M, Miura T, Speidel S, Cooper R W, Motiyama E N, Gojobori T, Hayami M. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature. 1989;341:539–541. doi: 10.1038/341539a0. [DOI] [PubMed] [Google Scholar]
- 50.Van den Haesevelde M, Peeters M, Jannes G, Janssens W, Van Der Groen G, Sharp P M, Saman E. Sequence analysis of a highly divergent HIV-1 related lentivirus isolated from a wild captured chimpanzee. Virology. 1996;221:346–350. doi: 10.1006/viro.1996.0384. [DOI] [PubMed] [Google Scholar]