ABSTRACT
Ovitraps can detect Aedes vectors at an early stage and can serve as an alarm indicator for outbreak prediction. This study aimed to summarize the available literature about the ovitrap system and to determine its feasibility, required resources and costs when installing and maintaining this vector surveillance system in the municipality of Los Patios, Colombia. A scoping review to assess the role of ovitraps as a tool for Aedes vector surveillance was conducted. The subsequent fieldwork consisted of mapping the municipality, manufacturing, and installing 40 ovitraps in 10 blocks, revising them weekly for 4 weeks by two half-time employed vector control technicians, and carrying out a cost analysis. A total of 38 studies were included in this review showing that ovitraps had a better performance than other entomological surveillance methods and a positive correlation with other entomological and disease variables. From the field results over 4 weeks, a high proportion of positive ovitraps (80%, 90%, 75%, 97.5%) and positive blocks (100%) as well as a good acceptance by house owners (76.9%), were identified. Operational indicators such as average installation time of the ovitraps (10h15 m), weekly reading and reinstallation (on average 7h27 m) and the cost of the intervention (COL$1,142,304.47/US$297) were calculated. Literature shows that ovitraps are sensitive to detect the presence of Aedes mosquitoes, providing data efficiently and timely for outbreak prediction. The field testing showed it is an affordable and feasible method in the context of a Colombian municipality and similar endemic areas.
KEYWORDS: Ovitrap, vector surveillance, vector-borne disease, dengue fever, chikungunya, Zika
Background
Aedes mosquitoes transmit diseases like Dengue fever, Chikungunya, and Zika, mainly in urban and suburban areas. Aedes aegypti is the main vector of these diseases worldwide, followed by Aedes albopictus [1,2]. They originated in Africa and Asia but have expanded almost worldwide in tropical and subtropical areas through commerce and travel [3,4].
Dengue fever is the most rapidly expanding arboviral disease in the world. About 2.5 billion people live in dengue endemic countries, and it is estimated that 50 million dengue infections occur annually [2].
In the region of the Americas there are cyclical outbreaks occurring every three to five years [2]. In 2016, the Andean subregion reported more than 200,000 cases, to which Colombia contributed 48% (about 100,000 cases) and had the highest fatality rate. Since the 2000s, 76% of these cases came from just 10 States (departamentos) in Colombia, one of them being the State of Norte de Santander [5].
In Colombia, the four dengue serotypes are endemic (DENV1, DENV2, DENV3, DENV4). Cucuta, the capital city of Norte de Santander State and the adjacent municipality of Los Patios had the highest number of dengue cases from 1999 to 2010, with more than 30,000 infections [5,6].
Colombia’s dengue containment strategy includes the three major components: disease surveillance, vector surveillance, and monitoring of environmental and social risks [6,7]. The notification of Aedes borne diseases is compulsory. As in most other endemic countries of the region, vector surveillance is done through larval surveys which require resource intensive house to house inspections and are conducted inconsistently and unsystematically. The collection of adult mosquitoes are only done in scientific studies with sticky traps or lethal traps [5].
Vector surveillance is critical to identify areas of high vector densities, newly infested areas and periods of increased mosquito populations [2,8].
Oviposition traps or ‘ovitraps’ were developed as a reliable, rapid, noninvasive and economic method for detecting areas in which female Ae. aegypti lay their eggs [2,8,9]. They usually consist of a 1 L bucket (plastic or glass) with a wide-mouth and a black outside; they are equipped with a rough filter paper on the inside or a hardboard or wooden paddle that facilitates the landing of the mosquito. They are partially filled with clean water [10]. The ovitrap must be examined once a week to check the presence and number of Aedes eggs [1,2].
The ovitrap surveillance system may also be capable of predicting the adult vector density in different geographical regions, which denotes its importance [11,12]. Countries that have implemented the ovitrap surveillance system as a public health policy have developed their own guidelines with precise specifications and standardization of their systems, such as Mexico since the year 2013 [13,14], and partially Brazil [15]. Results serve for vector and disease control and scientific investigations.
Mexico adopted the ovitrap system as a permanent strategy for entomological surveillance in response to the Aedes vector adaptability to extreme weather conditions [14]. The CDC (Centers for Disease Control and Prevention) is promoting the ovitrap system [4], but there is no systematic review of the literature or feasibility studies.
As the ovitrap system seems to have advantage over the classical larval surveys for vector surveillance (due to its low cost, easy installation and monitoring, its potential as an alarm indicator for arbovirus outbreaks) the following study was conducted to summarize the published evidence and to confirm the feasibility and low costs in a field study in Colombia.
Methods
The study started with a scoping review following the Arksey and O’Malley’s guidelines [16] to assess the role of ovitraps as a tool for Aedes vector surveillance. The review was followed by a spatial analysis (mapping), a feasibility study and a cost analysis of introducing the ovitrap system for vector surveillance in a municipality of Colombia.
Scoping review
A scoping review was done to assess the evidence of what is known about the value of ovitraps as an Aedes surveillance method (including the prediction of dengue disease outbreaks), and what are its merits and limitations compared to other entomological surveillance techniques.
The search strategy included different categories (sample and phenomenon of interest) in online databases: PubMed, LILACS, Science Direct and Google Scholar. Table 1 shows the ‘SPIDER’ search strategy which was followed [17].
Table 1.
SPIDER search strategy.
| Sample | Phenomenon of interest A | Phenomenon of Interest B |
|---|---|---|
| “ovitrap*” OR “oviposition trap*” | “surveillance” OR “vigilant* OR “monitor*” | “Aedes” OR “mosquito*” OR “vector*” |
Inclusion criteria were scientific articles focusing on ovitraps for surveillance from any country and any date. All full text papers had to be in English or Spanish language. Exclusion criteria were articles with information on lethal ovitraps designed for vector control, autocidal gravid ovitraps, ovitraps for vector control or reduction. Articles not published in English or Spanish were also excluded.
Relevance screening was carried out in three stages. First, titles and abstracts were evaluated according to the inclusion and exclusion criteria. Second, full text articles and articles without abstract availability in the previous stages were evaluated. Third, additional articles were identified through manual searches of the reference lists of all screened articles that met the inclusion criteria.
Data management of the scoping review
Information from the included studies in the scoping review were extracted and recorded in a Data Charting Form. The following information was extracted: title, author, year of publication, location, study methods focused on the way ovitraps were used, study results and conclusions.
No formal assessment of the methodological quality of the included articles was performed in this review [16,18], however, the quality of the papers was defined by the inclusion and exclusion criteria. A narrative description is presented in the ‘Results’ Section.
Feasibility study: site and timeline
Los Patios is a municipality located in the Norte de Santander State (departamento) of Colombia as part of the metropolitan area of Cucuta (7°50′17″N 72°50′47″E), which borders with Venezuela. The municipality of Los Patios has a total area of about 133 square kilometers, an elevation of 410 m above sea level, and approximately 95.000 inhabitants [19]. It has a tropical savanna climate with a mean annual temperature of 27°C and an annual precipitation of around 550 mm mostly during the rainy season from April to November making it endemic for diseases transmitted by the Aedes vector, especially dengue fever. According to the vector control office data provided, the neighborhoods of Patio Centro and La Sabana, are considered as dengue hotspots [20]. Therefore, they were chosen for testing the ovitrap system.
The field work was conducted between July and August 2021.
Manufacturing the ovitraps
This study followed the Mexican ovitrap surveillance system model. The main difference is that ovitraps have a porous fabric with a rough surface inside the bucket called ‘papel pellón’, where the eggs stick on for being counted later, instead of having a wooden paddle [14].
For the study, 40 black plastic 1 L jars bought in local shops were used for manufacturing the ovitraps. The holes in opposite sides of the jar were not drilled as suggested by the Mexican Guide [14], but were opened with a soldering iron, which was easier to handle. Instead of the papel pellón fabric, another type of fabric called ‘entretela’ (a thick filter paper) was used, which has a rough surface and does not soften when exposed to water. It was cut in pieces to cover the interior surface of the black jar and was fixed with metal clips (see Figure 1).
Figure 1.

Ovitraps for the intervention in Los Patios.
Legend 1: Image shows ovitraps made by vector control staff in Los patios.
Spatial analysis, mapping, and installation of ovitraps
The spatial analysis consisted of mapping the targeted blocks and their ways to reach them. Maps were taken from Google Earth and the blocks were highlighted considering their access routes (see Figure 2). The team adopted the design of the Mexican ovitrap model and intervened in 10 blocks (roughly 100 m × 100 m surface area per block, see Figure 3). A house in the middle of every block side was selected to install the ovitraps. The head of the family received a verbal explanation by the vector control technicians, a copy of the information sheet about the study, an explanatory leaflet about the ovitrap surveillance system and had to sign the informed consent form. After the installation of 40 ovitraps, addresses were entered, and coordinates were obtained using Google Maps. Finally, all the coordinates were entered manually in Google Earth and a map with geocoded positions of the ovitraps was automatically created (see Figure 4).
Figure 2.

Spatial analysis of the map of Los Patios.
Legend 2: Map was highlighted with blue pen to indicate selected blocks. Each square is a block of houses with an extension of roughly 100x100 m.
Figure 3.

Schematic distribution of 40 ovitraps in 10 blocks following the Mexican ovitrap mod.
Legend 3: Each grey square represents a block of houses and each red dot represents an ovitrap. Each blanc square represents a block where no ovitraps were installed.
Figure 4.

Geolocation of ovitraps in Los Patios.
Legend 4: Map of Google Earth showing the geolocation of the 40 ovitraps in Los Patios (20 ovitraps installed in the La Sabana neighborhood, yellow color, and 20 ovitraps installed in the Patio Centro neighbourhood, blue color).
From the 40 ovitraps, one per house, 37 were placed in the backyard, and three in the front yard, away from direct sunlight, between plants, and not more than 1.5 m above the ground (see Figure 5). One ovitrap was placed on each cardinal point (north, south, east, west) of the block. The subsequent target block of houses was the third block on each side. This procedure was repeated until 40 ovitraps were installed. Two entomological technicians installed 20 ovitraps each and filled in a form with the information on every ovitrap (exact location, date and hour of installation or reading, with or without eggs).
Figure 5.

Ovitrap installation sites.
Legend 5: Ovitraps placed <1.5 m above the ground during the intervention, in a front yard (left) and in a backyard (right).
Weekly data collection and ovitrap reinstallation
The two vector control technicians – employed for 50% of their time – visited 20 houses/ovitraps each, every seven days after each installation, for 4 weeks. During the visit, they collected the entretela fabrics, which were immediately examined for eggs (positivity), cleaned the jars and fixed new pieces of fabric in them. All findings relevant to the addressed indicators were noted in paper forms. Egg counts were not done, based on the findings by Schultes et al. [21] and others (see results section). However, the egg counting would not delay the vector collection as it is usually done in an entomological laboratory.
Outcome indicators
Two out of five indicators from the Mexican Methodological Guide for Entomological Surveillance with Ovitraps were measured: Percentage of positive ovitraps and percentage of positive blocks. The average number of eggs per positive block, the average number of eggs per block and the average number of eggs per positive block were not determined due to the weak correlation between egg counts and vector density, as argued later in the discussion section. However, four other indicators were obtained: Percentage of rejection by homeowners when installing the ovitraps, percentage of ovitraps relocated during the study, percentage of examined ovitraps per week, percentage of intact (untouched) ovitraps.
Data transmission/online forms
Three forms were developed for the fieldwork: Form #1 ‘Installation and registration’, Form #2 ‘Weekly recollection and reading’ and Form #3 ‘Time use and distribution’ (the last one for the cost calculation). An additional vector control technician was assigned for uploading the information gathered after every weekly recollection and ovitrap reinstallation.
Cost analysis
To differentiate costs that are already covered by the state vector control services, from those that would imply additional investment or expenditures, the following information was obtained: costs of daily income from vector control staff, costs for transportation, and equipment for manufacturing and maintaining the ovitraps. Indirect costs, such as maintenance of the vehicle, were not considered. The cost analysis was done in Colombian pesos (COL$), and then converted to US Dollars. The official conversion rate at the time of the study was USD 1 = COL$ 3846.15
Results
Scoping review
A total of 607 papers were initially retrieved. After the application of the inclusion and exclusion criteria and eliminating duplicates, 38 publications were included for the synthesis of the review (Additional file 1). Figure 6 shows the selection process of the papers.
Figure 6.
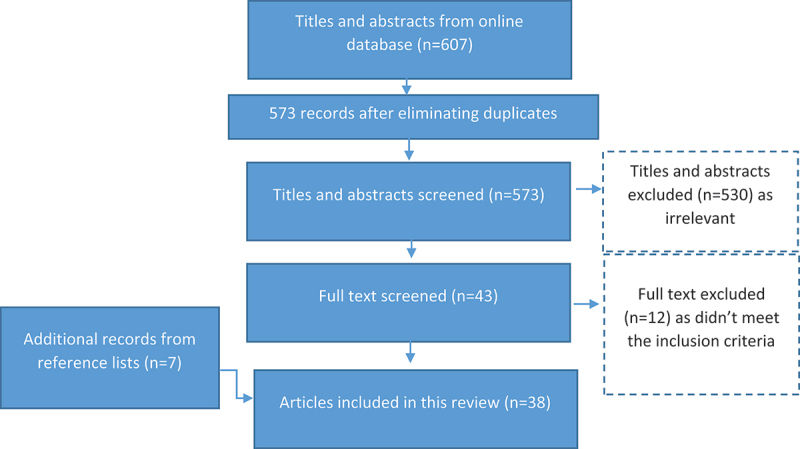
The PRISMA flow diagram.
Legend 6: Search results and selection process of studies
The scoping review showed the following
Performance of ovitraps as an entomological surveillance method
Ovitraps showed in general a better performance in comparison with other entomological surveillance methods. Most of the studies demonstrated ovitraps to be more sensitive than larval surveys to detect the presence of Aedes vectors [22–30]. From these studies, two showed its utility in detecting Aedes when the mosquito population was low [26,28]; three studies showed a higher sensitivity when ovitrap data were complemented with larval surveys [22,24,30]. Furthermore, two studies compared BG sentinel traps, backpack aspirators, resting boxes, ovitraps and sticky traps; they showed that ovitraps were superior in detecting Aedes presence [26,31]. In contrast, two studies using adult traps and double sticky traps had better results than ovitraps [32,33]. Three studies did not find a significant difference between ovitraps and other entomological surveillance methods [23,25,34].
Correlation between ovitrap indexes and adult mosquito presence and abundance
Some articles found a positive correlation between ovitraps indexes and female adult mosquito presence and density. A study found a positive correlation between positive ovitrap index and adult female indoor presence [35]. Four articles found a positive correlation between egg density index and the abundance of adult female Aedes [36–39] and another one, a positive correlation ovitrap index and adult density [40].
Correlation between ovitrap indexes and dengue incidence
Ovitraps could not only be correlated with the vector abundance, as described previously, but also with an increased incidence of dengue fever. Four articles found a correlation between egg density index and dengue fever incidence [11,37,41,42]. Two studies found a positive correlation between the positive ovitraps index and dengue fever [12,43]. For instance, a study in Brazil by de Albuquerque et al. [12], found a correlation between positive ovitraps and confirmed dengue cases within four to six weeks, independent from the egg counts per ovitrap. Only one found no correlation between ovitraps indexes and dengue fever [44].
Ovitraps as alarm indicator for outbreaks
One study showed that the proportion of positive ovitraps (and less the egg density index) was an alarm indicator for dengue outbreaks with a high positive predictive value and sensitivity of outbreak prediction [45].
Correlation between ovitraps indexes and environmental factors
Many articles found a correlation between ovitrap indexes and environmental parameters. Twelve articles found a correlation of the proportion of positive ovitraps with the increase of temperature and/or rainfall [11,37,39,40,46–52]. Two articles found a negative correlation with the decrease in temperature [53,54]. These findings reconfirm that these two parameters are probably the main factors creating favorable conditions for vector breeding. Other parameters correlated with ovitrap indexes were humidity [40,42,43,49,54] and water vapor pressure [47].
Correlation between ovitrap index and socioeconomic factors
Studies showed a positive correlation between ovitrap data and housing conditions: total egg count correlated with house age [55], the presence of uncovered water containers with a positive ovitrap index [54] and tree density with egg density index [56]. Others found a negative correlation of the egg density index with the mean per capita income of the neighborhood [55] and low vegetation [54].
Fieldwork results
On the day of the ovitrap installation, 23.1% (12 of 40 ovitraps) of the selected households refused to have an ovitrap, so it had to be placed in the next house. Reluctancy can be attributed to the Covid-19 fear in the populace. In the end, all 40 ovitraps were successfully installed.
During the study, 5% of the ovitraps (2 of the 40 ovitraps) had to be relocated because they could not be accessed by the vector control technicians during their weekly visits for two consecutive weeks, as recommended by the Mexican Guide [14].
During the first three weeks, the percentage of ovitraps examined was 97.5% (39 of the 40 ovitraps) and 100% (40 ovitraps) during the last week. During the first week, one ovitrap disappeared. During the following two weeks, two ovitraps had to be relocated since they could not be accessed by the vector control technicians. In the last week, all ovitraps could be accessed.
The percentage of intact ovitraps, which means they were untouched by people or animals, did not vary much during the four weeks of observation. In the first week of intervention, 7.5% (3 from 40 ovitraps) were manipulated. During the rest of the intervention, intact ovitraps were found in almost all the houses: 97.5% in weeks 2 and 3 (39 from 40 ovitraps), and 100% in week 4 (40 ovitraps).
The percentage of positive ovitraps varied during the study. In the first week 80% (32 from 40 ovitraps) were positive, in the second week 90% (36 from 40 ovitraps), in the third week 75% (30 from 40 ovitraps) and in the fourth week 97.5% (39 from 40 ovitraps). This points to a high vector density in the study area.
During the four weeks of observation, all the intervened blocks had one or more positive ovitrap (out of 4 per block) confirming these neighborhoods were hotspots for dengue transmission (see Table 2).
Table 2.
Results collected by measuring the indicators of this study.
| Process indicators |
Results indicators |
|||||
|---|---|---|---|---|---|---|
| Form #1 |
Form #2 |
|||||
| Acceptance percentage | Relocation percentage | Examined ovitraps percentage | Intact ovitraps percentage | Positive ovitraps percentage | Positive blocks percentage | |
| 77% | 5% | 98% | 93% | 80% | 100% | Week 1 |
| 98% | 98% | 90% | 100% | Week 2 | ||
| 98% | 98% | 75% | 100% | Week 3 | ||
| 100% | 100% | 98% | 100% | Week 4 | ||
Operational indicators: use of time and human resources
The Mexican Guide describes that each entomological technician should cover 40 ovitraps or 10 blocks each day [14]. We decided to work with two vector control technicians, each of them employed for 4 h per day instead of one for 8 h. Therefore, every vector control technician served 20 households with ovitraps. This allowed us also to compare their time and performance. Another entomological technician digitalized the information. A driver participated by transporting one of the technicians to and from the targeted area. Results of the time distribution are presented in Table 3.
Table 3.
Time distribution by technicians as measured with Form #3.
| Time distribution results | |||||
|---|---|---|---|---|---|
| Activity/Held by | Technician 1 | Technician 2 | Total hours | Activity/Held by | Technician 3 |
| Manufacture of 40 ovitraps | 1 h | 1 h | 2 h | 1st Data transmission | 8 h |
| Installation of 40 ovitraps | 3 h 40 m | 4 h 35 m | 8 h 15 m | 2nd Data transmission | 4 h |
| 1st Reinstallation | 4 h 05 m | 3 h 35 m | 7 h 40 m | 3rd Data transmission | 2 h |
| 2nd Reinstallation | 3 h 45 m | 3 h 30 m | 7 h 15 m | 4th Data transmission | 2 h |
| 3rd Reinstallation | 4 h 08 m | 3 h 45 m | 7 h 53 m | ||
| 4th Reinstallation | 3 h 40 m | 3 h 20 m | 7 h | ||
Installation and weekly revision of ovitraps
The first installation of the 40 ovitraps by 2 technicians was carried out in 8 h and 15 min (3h40 and 4h35 respectively). The weekly revision – cleaning and changing the fabric – took 7 h and 40 min (4h05 and 3h35, respectively) the first week, the second 7h15 (3h45 – 3h30), the third 7h53 (4h08 – 3h45) and 7h00 (3h40 – 3h20) in the last week.
Data transmission
The data transmission was done by one technician and consisted in digitalizing the paper forms. It took 8 h for form 1, four hours for forms 2 and 3 in the first week, and 2 h in the second, third and fourth week. Additional files show these forms in more detail [see Additional file 2, 3, 4].
Cost for installing and revising 40 ovitraps
The cost analysis was broken down to fixed (one time) installation costs and the weekly recurrent costs of revision, maintenance and data collection.
The cost was calculated using working hours, as the technicians and the driver that assisted them, worked only for a few hours in the project. The preparation and installation of 40 ovitraps took a total of 10 h. The fuel costs were calculated using fuel prices from the time of the fieldwork (COL$7,100/Gallon), and as such are subject to fluctuations.
In summary, the cost of preparing and installing an ovitrap including materials, human resources and transportation, amounted to US$7.4 (COL$28,557.61) per ovitrap. The total costs for installing and reviewing 40 ovitraps was US$297 (COL$1.142.304.47) for the 4 weeks intervention (see Table 4).
Table 4.
Summary of installation and recurrent costs for 40 ovitraps over four weeks.
| Installation costs US Dollars | Maintenance and Recurrent costs US Dollars | Total US Dollars | Total Colombian Pesos | Observation | |
|---|---|---|---|---|---|
| Materials | $ 32.72 | $ 53.71 | $ 86.43 | COL$ 332406.50 | Training/Materials/Supplies and Bio safety equipment |
| Personnel | $ 43.17 | $ 166.21 | $ 209.38 | COL$ 805320.71 | Drivers and Technicians |
| Transport | $ 0.24 | $ 0.95 | $ 1.19 | COL$ 4577.26 | Fuel |
| Cost per ovitrap | $ 1.90 | $ 5.52 | $ 7.42 | COL$ 28557.61 | |
| Total costs 40 ovitraps | $ 76.12 | $ 220.88 | $ 297.00 | COL$ 1142304.47 |
Estimated resource requirements for the entire municipality
It was estimated that, for covering the complete municipality of Los Patios, 348 ovitraps would be needed.
The cost for installing and reading 348 ovitraps in this municipality for 12 months including materials, human resources and transportation, using official drivers (from the vector control unit) was estimated to be US$6,404.14 (COL$1.142.304.47).
The total expenditure for one year would be slightly lower if the technicians use public transport rather than the driver and the official vehicle (estimated cost US$6,062.19 - COL$23,316,123.63). However, public transport is less reliable (waiting for buses, their frequency, the distance and number of stops and the availability of space in public transport for safely transporting the ovitraps) which may result in higher costs. As such it is left to the institution in charge to decide which of these two scenarios would be appropriate (see Tables 5, 6).
Table 5.
Summary of costs for 348 ovitraps in one year, using official drivers of the institution.
| Installation costs US Dollars | Maintenance and Recurrent costs US Dollars | Total US Dollars | Total Colombian Pesos |
Observation | |
|---|---|---|---|---|---|
| Materials | $ 238.64 | $ 370.45 | $ 609.08 | COL$ 2342623.63 | Training/Materials/Supplies |
| Personnel | $ 364.22 | $ 5,420.49 | $ 5,784.71 | COL$ 22248874.50 | Drivers and Technicians |
| Transport | $ 2.06 | $ 8.29 | $ 10.35 | COL$ 39822.14 | Fuel |
| Cost per ovitrap | $ 1.74 | $ 16.66 | $ 18.40 | COL$ 70779.66 | |
| Total costs 348 ovitraps | $ 604.92 | $ 5,799.23 | $ 6,404.14 | COL$ 24631320.27 |
Table 6.
Summary of costs for 348 ovitraps in one year, using public transportation.
| Installation costs US Dollars | Maintenance and Recurrent costs US Dollars | Total US Dollars | Total Colombian Pesos | Observation | |
|---|---|---|---|---|---|
| Materials | $ 236.04 | $ 370.45 | $ 606.48 | COL$ 2332623.63 | Training/Materials/Supplies |
| Personnel | $ 184.60 | $ 4,771.91 | $ 4,956.51 | COL$ 19063500 | Technicians |
| Transport | $ 10.40 | $ 488.80 | $ 499.20 | COL$ 1920000 | Public transportation |
| Cost per ovitrap | $ 1.24 | $ 16.18 | $ 17.42 | COL$ 67000.36 | |
| Total costs 348 ovitraps | $ 431.04 | $ 5,631.16 | $ 6,062.19 | COL$ 23316123.63 |
Discussion
Ovitraps and other vector surveillance methods
The preferred method of entomological surveillance over the last decades has been the larval survey establishing the House Index, Container Index and Breteau Index. This method has been installed during the eradication campaign of Ae. aegypti in the Americas by the Pan American Health Organization (PAHO). Its main purpose was to establish and monitor vector infested and non-infested areas [21]. How much the larval indices reflect vector densities is still in debate. In later years larval surveys were complemented by pupal surveys, being the main purpose to establish which types of water containers produce the highest number of Aedes pupae (as a proxy measure of adult mosquitoes as about 79% of pupae develop to adult mosquitoes [22]). There is also evidence that pupal indices reflect vector densities [23].
Larval surveys are time consuming and labor intensive. Usually, they are limited to highly infested areas, and they are carried out in an unsystematic way. Therefore, Mexico and other countries switched over to vector surveillance using the ovitrap system, which has been described in this paper. It has the advantage of being applied in a very systematic way and always in the same houses enabling a continuous assessment of high, medium and low-risk areas for Aedes borne diseases. Chaverri et al. have also highlighted their utility as a cheap and reliable sampling unit for longitudinal ‘semi-field’ studies and to collect medical relevant species, in a setting of tropical rainforest in Costa Rica [57].
Feasibility and acceptance
As stated in the results section, for covering the municipality of Los Patios, 348 ovitraps would be needed, with two vector control technicians working full-time (42 h per week as stated in by the Colombian law), taking into consideration that each of them would oversee the installation, reading and sending of information of 40 ovitraps per day. This means 400 ovitraps per week, 200 per technician. This means that the cost for the vector control service would be around US$6000 per year. However, as the staff costs are anyway covered by the vector control program, only the costs for transport and for preparing the ovitraps would be around US$1100 per year, or US$620 using vehicles from the institution.
The percentage of rejection of ovitraps by households and the percentage of relocation were low. The advantage is that this surveillance system is not as invasive as other methods because, by installing the ovitraps in the front or the backyard, the vector control staff does not need to enter the houses as in larval surveys [58,59]. Also, ovitraps do not require special care so they do not interfere with peoples’ daily activities [58]. This method is not time-consuming, the installation, reinstallation and the interpretation of the positivity takes only some minutes. Based on the studies by de Albuquerque et al. [12] showing that dengue incidence was correlated with ovitrap positivity but not with egg density and by Hussain-Alkhateeb et al. [45] that mentions that the increase of ovitrap positivity is an early alarm indicator for dengue outbreaks but not the egg density, we think that the time-consuming egg count can be often avoided in routine vector surveillance, which saves time and resources. Other types and species of mosquitoes as described by Ortega-Morales et al., have been also identified as a result of the application of the ovitrap surveillance system in Mexico, such as: Ae. epactius, Ae. gabriel Schick, Ae. podographicus Dyar & Knab, Ae. albopictus, Haemagogus equinus Theobald, Culex restrictor Dyar & Knab, Culex coronator sensu lato Dyar & Knab, Culex mollis Dyar & Knab, Culex quinquefasciatus Say, Limatus durhamii Theobald and Wyeomyia guatemala Dyar & Knab. The majority of the mentioned above do not have a clear importance as vectors for human diseases, nevertheless they could be a guide to direct other preventive measures such as fumigating [13].
The spatial analysis can be easily done, accessing maps via applications like Google Earth or Google Maps, which can be edited online or printed and then highlighted.
Conclusion
Despite the report of successful application in countries like Mexico or Brazil, and its promotion by governmental health agencies as the CDC of the United States, there is no systematic review of the literature or feasibility studies about the ovitrap system. This study demonstrated that the Mexican ovitrap surveillance system is affordable and feasible to apply and to manage in urban municipalities endemic for Dengue fever and other Aedes borne diseases such as chikungunya and Zika. Therefore, our detailed description is just in time.
Supplementary Material
Acknowledgements
We would like to thank the team of vector control in Los Patios. Roque Suárez who led and supervised the field project, Gustavo García who digitalized the information and always offered support on-field, Emerson Núñez and Carlos Nieto, who made the installation and the weekly reinstallation of the ovitraps.
Funding Statement
Research team was partially funded by Engagement Global GmbH.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
EW and AK contributed to the concept and design of the study. EW, MAC, RCS and AK did the interviewer training, and drafted the paper. All authors read and approved the study and the procedures. EW and AK prepared the submission to the ethical committee and prepared the logistics. The study selection from the scoping review was done independently by JMVM and AK. Disagreements on the inclusion or exclusion of literature were solved through discussions with MAC. EW, MAC, RCS and JY prepared the field study, contacted the local leaders, organized the logistics. DM contributed with the cost analysis. EW, MAC, AK and GDL formatted, revised and corrected the article until its final version. All authors read and commented on the draft paper and approved the final version.
Ethics approval and consent to participate
The participation in this study was voluntary, anonymous and did not represent a risk for the participants or their families. Participants had to be at least 18 years old, sign an informed consent and were informed they could withdraw from the study at any time. The study was explained in Spanish by a vector control technician to participants. Participants did not receive any kind of compensation for taking part in the study. Ovitraps had an individual code for identification. Sensible data (addresses) was erased after the intervention was finished. Researchers confirm that all methods were carried out in accordance with relevant guidelines and regulations. The ethics committee of the Albert-Ludwigs-Universität Freiburg, Germany approved the project on 15.04.2021 with reference number 21–1235, and the intervention was authorized by the local health authorities in Colombia.
Data availability statement
All data generated or analyzed during this study are included in this published article [additional file 1, additional file 2, additional file 3, additional file 4].
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20477724.2022.2146049.
References
- [1].World Health Organization . A global brief on vector-borne diseases; 2014. [cited 2021 Feb 2]. Available from: https://apps.who.int/iris/bitstream/handle/10665/111008/WHO_DCO_WHD_2014.1_eng.pdf?sequence=1&isAllowed=y
- [2].World Health Organization . Dengue guidelines for diagnosis, treatment, prevention and control: new edition; 2009. [cited 2021 Jan 5]. Available from: https://apps.who.int/iris/handle/10665/44188 [PubMed]
- [3].Kraemer MUG, Reiner RC, Brady OJ, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4(5):854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Centers for disease control and prevention: surveillance and control of Aedes aegypti and Aedes albopictus in the United States; 2018. [cited 2021 Jan 5]. Available from: https://www.cdc.gov/mosquitoes/pdfs/mosquito-control-508.pdf
- [5].Zambrano PD. In: Protocolo de vigilancia en Salud Pública. Instituto Nacional de Salud; 2017. [cited 2021 Jan 25]. Available from: https://www.ins.gov.co/Noticias/Dengue/7.%20Dengue%20PROTOCOLO.pdf
- [6].Padilla JCP, Rojas DP, Gómez R. DISTRIBUCIÓN DEL RIESGO DE TRANSMISIÓN DE DENGUE EN LOS DEPARTAMENTOS Y MUNICIPIOS DE LA REGIÓN CENTRO-ORIENTE. In: Hernández CA, editor. Dengue en Colombia: Epidemiología de la Reemergencia a la Hiperendemia. 1st ed. Bogotá; 2012. p. 140–142. 978-958-46-0661-7. [Google Scholar]
- [7].Pérez Ortiz OG, Argote CC. Identificación de los Factores de Riesgo Determinantes en la Transmisión del Dengue en el Municipio de Cúcuta en los Años 2007 a 2011. INBIOM. 2016;2(1):7–28. [Google Scholar]
- [8].World Health Organization . Efficacy-testing of traps for control of Aedes Spp. Mosquito vectors; 2018. [cited 2021 Jan 20]. Available from: https://apps.who.int/iris/bitstream/handle/10665/275801/WHO-CDS-NTD-VEM-2018.06-eng.pdf?sequence=1&isAllowed=y
- [9].Jakob WL, Bevier GA. Application of ovitraps in the U.S. Aedes aegypti eradication program. Mosquito News. 1969;29(1):55–62. [Google Scholar]
- [10].Frank JH, Lynn HC. Standardizing oviposition traps for Aedes aegypti and Culex quinquefasciatus: time and medium. J Flo Anti-Mosq Assoc. 1982;53(1):22–27. [Google Scholar]
- [11].Pessanha JEM, Brandão ST, Almeida MCM, et al. Ovitrap surveillance as dengue epidemic predictor. J Health Biol Sci. 2014;2(2):51–56. [Google Scholar]
- [12].de Albuquerque BC, Pinto RC, Sadahiro M, et al. Relationship between local presence and density of Aedes aegypti eggs with dengue cases: a spatial analysis approach. Trop Med Int Health. 2018;23(11):1269–1279. [DOI] [PubMed] [Google Scholar]
- [13].Ortega-Morales AI, Moreno-García M, González-Acosta C, et al. Mosquito surveillance in Mexico: the use of ovitraps for Aedes aegypti, Ae. albopictus, and non-target species. Florida Entomologist. 2018;101(4):623–627. [Google Scholar]
- [14].Secretaría de Salud de México: guía Metodológica para la Vigilancia Entomológica con Ovitrampas; 2017. [cited 2021 Jul 29]. Available from: https://www.gob.mx/cms/uploads/attachment/file/37865/guia_vigilancia_entomologica_ovitrampas.pdf
- [15].Ministério da Saúde: Diretrizes Nacionais para a Prevenção e Controle de Epidemias de Dengue; 2009. [cited 2021 Aug 1]. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/publicacoes-svs/dengue/diretrizes_nacionais_prevencao_controle_dengue.pdf/view.
- [16].Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- [17].Cooke A, Smith D, Booth A. Beyond PICO. Qual Health Res. 2012;22(10):1435–1443. [DOI] [PubMed] [Google Scholar]
- [18].Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Departamento Administrativo Nacional de Estadísticas de Colombia . Censo de Población y Vivienda - CNPV 2018. [Dataset]; 2018. [cited 2021 Aug 3]. Available from: http://microdatos.dane.gov.co/index.php/catalog/643/related_materials
- [20].de Los Patios A. Informe de gestión de la secretaria de salud municipio de Los Patios. In: Informes consejo. Informe IV Trimestre de 2014 de la Secretaría de Salud; 2014. [cited 2022 Sep 20]. Available from: https://www.lospatios-nortedesantander.gov.co/Conectividad/InformesConcejo/Informe%20IV%20Trimestre%20de%202014%20de%20la%20Secretar%C3%ADa%20de%20Salud.pdf
- [21].Schultes OL, Morais MHF, Cunha MDCM, et al. Spatial analysis of dengue incidence and Aedes aegypti ovitrap surveillance in Belo Horizonte, Brazil. Trop Med Int Health. 2021;26(2):237–255. [DOI] [PubMed] [Google Scholar]
- [22].Nascimento KLC, da Silva JFM, Zequi JAC, et al. Comparison between larval survey index and positive ovitrap index in the evaluation of populations of Aedes (Stegomyia) aegypti (Linnaeus, 1762) North of Paraná, Brazil. Environ Health Insights. 2020;14:1178630219886570. Accessed 12 Mar. 2020. https://www.ncbi.nlm.nih.gov/pubmed/31933523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Codeço CT, Lima AWS, Araújo SC, et al. Surveillance of Aedes aegypti: comparison of house index with four alternative traps [online]; 2015. [cited 2020 Jul 3]. Available from: https://www.arca.fiocruz.br/handle/icict/11485 [DOI] [PMC free article] [PubMed]
- [24].Rozilawati H, Tanaselvi K, Nazni WA, et al. Surveillance of Aedes albopictus Skuse breeding preference in selected dengue outbreak localities, peninsular Malaysia. Trop Biomed. 2015;32(1):49–64. [PubMed] [Google Scholar]
- [25].Resende MC, de Silva IM, Ellis BR, et al. A comparison of larval, ovitrap and MosquiTRAP surveillance for Aedes (Stegomyia) aegypti. Memórias Inst Oswaldo Cruz. 2013;108(8):1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Melo DPO, Scherrer LR, Eiras ÁE. Dengue fever occurrence and vector detection by larval survey, ovitrap and MosquiTRAP: a space-time clusters analysis. PLoS One. 2012;7(7):e42125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morato VCG, Teixeira M, da G, et al. Infestation of Aedes aegypti estimated by oviposition traps in Brazil. Revista de Saúde Pública. 2005;39(4):553–558. [DOI] [PubMed] [Google Scholar]
- [28].Rawlins S, Martinez R, Wiltshire S, et al. A comparison of surveillance systems for the dengue vector Aedes aegypti in port of Spain, Trinidad. J Am Mosq Control Assoc. 1998;14(2):131–136. [PubMed] [Google Scholar]
- [29].Furlow BM, Young WW. Larval surveys compared to ovitrap surveys for detecting Aedes aegypti and Aedes triseriatus. Mosquito News. 1970;30(3):468–470. [Google Scholar]
- [30].Fay RW, Eliason DA. A preferred oviposition site as a surveillance method for Aedes aegypti. Mosquito News. 1966;26(4):531–535. [Google Scholar]
- [31].Yalwala S, Clark J, Oullo D, et al. Comparative efficacy of existing surveillance tools for Aedes aegypti in Western Kenya. J Vector Ecol. 2015;40(2):301–307. [DOI] [PubMed] [Google Scholar]
- [32].Chadee DD, Ritchie SA. Efficacy of sticky and standard ovitraps for Aedes aegypti in Trinidad, West Indies. J Vector Ecol. 2010;35(2):395–400. [DOI] [PubMed] [Google Scholar]
- [33].Gao Q, Cao H, Fan J, et al. Field evaluation of Mosq-ovitrap, ovitrap and a CO2-light trap for Aedes albopictus sampling in Shanghai, China. PeerJ. 2019;7:e8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wright JA, Larson RT, Richardson AG, et al. Comparison of BG-Sentinel® trap and oviposition cups for Aedes aegypti and Aedes albopictus Surveillance in Jacksonville, Florida, USA. J Am Mosq Control Assoc. 2015;31(1):26–31. [DOI] [PubMed] [Google Scholar]
- [35].Manrique-Saide P, Coleman P, McCall PJ, et al. Multi-scale analysis of the associations among egg, larval and pupal surveys and the presence and abundance of adult female Aedes aegypti (Stegomyia aegypti) in the city of Merida, Mexico. Med Vet Entomol. 2014;28(3):264–272. [DOI] [PubMed] [Google Scholar]
- [36].Carrieri M, Angelini P, Venturelli C, et al. Aedes albopictus (Diptera: Culicidae) population size survey in the 2007 chikungunya outbreak area in Italy. II: estimating epidemic thresholds. J Med Entomol. 2012;49(2):388–399. [DOI] [PubMed] [Google Scholar]
- [37].Barrera R, Amador M, MacKay AJ. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis. 2011;5(12):e1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Manica M, Rosà R, Della Torre A, et al. From eggs to bites: do ovitrap data provide reliable estimates of Aedes albopictus biting females? PeerJ. 2017;5:e2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dibo MR, Chierotti AP, Ferrari MS, et al. Study of the relationship between Aedes (Stegomyia) aegypti egg and adult densities, dengue fever and climate in Mirassol, state of São Paulo, Brazil. Memórias Inst Oswaldo Cruz. 2008;103(6):554–560. [DOI] [PubMed] [Google Scholar]
- [40].Ahmad Qureshi EM, Tabinda AB, Vehra S. Seasonal and spatial quantitative changes in Aedes aegypti under distinctly different ecological areas of Lahore, Pakistan. J Pak Med Assoc. 2017;67(12):1797–1802. [PubMed] [Google Scholar]
- [41].Ahmad R, Suzilah I, Wan Najdah WMA, et al. Factors determining dengue outbreak in Malaysia. PLoS One. 2018;13(2):e0193326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Betanzos-Reyes ÁF, Rodríguez MH, Romero-Martínez M, et al. Association of dengue fever with Aedes spp. abundance and climatological effects. Salud Pública de México. 2017;60(1):12. [DOI] [PubMed] [Google Scholar]
- [43].Mohiddin A, Jaal Z, Lasim AM, et al. Assessing dengue outbreak areas using vector surveillance in north east district, Penang Island, Malaysia. Asian Pac J Trop Dis. 2015;5(11):869–876. [Google Scholar]
- [44].Wijegunawardana NDAD, Gunawardene YINS, Chandrasena TGAN, et al. Evaluation of the effects of Aedes vector indices and climatic factors on dengue incidence in Gampaha District, Sri Lanka. Biomed Res Int. 2019. Jan 31;2019:2950216. DOI: 10.1155/2019/2950216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hussain-Alkhateeb L, Kroeger A, Olliaro P, et al. Early warning and response system (EWARS) for dengue outbreaks: recent advancements towards widespread applications in critical settings. PLoS One. 2018;13(5):e0196811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Heinisch MRS, Diaz-Quijano FA, Chiaravalloti-Neto F, et al. Seasonal and spatial distribution of Aedes aegypti and Aedes albopictus in a municipal urban park in São Paulo, SP, Brazil. Acta Trop. 2019;189:104–113. [DOI] [PubMed] [Google Scholar]
- [47].Estallo EL, Ludueña-Almeida FF, Introini MV, et al. Weather variability associated with Aedes (Stegomyia) aegypti (Dengue vector) oviposition dynamics in Northwestern Argentina. PLoS One. 2015;10(5):e0127820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Estallo EL, Ludueña-Almeida FF, Visintin AM, et al. Prevention of Dengue outbreaks through Aedes aegypti oviposition activity forecasting method. Vector-Borne Zoonotic Dis. 2011;11(5):543–549. [DOI] [PubMed] [Google Scholar]
- [49].Rohani A, Suzilah I, Malinda M, et al. Aedes larval population dynamics and risk for dengue epidemics in Malaysia. Trop Biomed. 2011;28(2):237–248. [PubMed] [Google Scholar]
- [50].Carvalho-Leandro D, de Ribeiro ALM, Rodrigues JSV, et al. Temporal distribution of Aedes aegypti Linnaeus (Diptera, Culicidae), in a hospital in Cuiabá, State of Mato Grosso, Brazil. Revista Brasileira de Entomologia. 2010;54(4):701–706. [Google Scholar]
- [51].Wee LK, Weng SN, Raduan N, et al. Relationship between rainfall and Aedes larval population at two insular sites in Pulau Ketam, Selangor, Malaysia. Southeast Asian J Trop Med Public Health. 2013;44(2):157–166. [PubMed] [Google Scholar]
- [52].Surendran SN, Kajatheepan A, Sanjeefkumar KFA, et al. Seasonality and insecticide susceptibility of dengue vectors: an ovitrap based survey in a residential area of northern Sri Lanka. Southeast Asian J Trop Med Public Health. 2007;38(2):276–282. 319. [PubMed] [Google Scholar]
- [53].Vezzani D, Velázquez SM, Schweigmann N. Seasonal pattern of abundance of Aedes aegypti (Diptera: Culicidae) in Buenos Aires city, Argentina. Memórias Inst Oswaldo Cruz. 2004;99(4):351–356. [DOI] [PubMed] [Google Scholar]
- [54].Hayden MH, Uejio CK, Walker K, et al. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, U.S./Sonora, MX Border. Ecohealth. 2010;7(1):64–77. [DOI] [PubMed] [Google Scholar]
- [55].Walker KR, Joy TK, Ellers-Kirk C, et al. Human and environmental factors affecting Aedes aegypti distribution in an Arid urban environment. J Am Mosq Control Assoc. 2011;27(2):135–141. [DOI] [PubMed] [Google Scholar]
- [56].Cheah WL, Chang MS, Wang YC. Spatial, environmental and entomological risk factors analysis on a rural dengue outbreak in Lundu District in Sarawak, Malaysia. Trop Biomed. 2006;23(1):85–96. [PubMed] [Google Scholar]
- [57].Chaverri LG, Dillenbeck C, Lewis D, et al. Mosquito species (Diptera: Culicidae) diversity from ovitraps in a Mesoamerican tropical rainforest. J Med Entomol. 2018;55(3):646–653. [DOI] [PubMed] [Google Scholar]
- [58].Dibo MR, Chiaravalloti-Neto F, Battigaglia M, et al. Identification of the best ovitrap installation sites for gravid Aedes (Stegomyia) aegypti in residences in Mirassol, state of São Paulo, Brazil. Memórias Inst Oswaldo Cruz. 2005;100(4):339–343. [DOI] [PubMed] [Google Scholar]
- [59].Pemola Devi N, Jauhari RK, Mondal R. Ovitrap surveillance of Aedes mosquitoes (Diptera: Culicidae) in selected areas of Dehradun District, Uttarakhand, India. Global J Med Res Dis. 2013;13(5):53–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [additional file 1, additional file 2, additional file 3, additional file 4].


