Abstract
Adenoviruses offer great potential as gene therapy agents but are limited by the strong inflammatory response that occurs in response to the recombinant virus. Since the degree of inflammation correlates in part with the potential of the viral vector for replication, we constructed a preterminal protein (pTP) deletion mutant adenovirus type 5 vector, Ad5dl308ΔpTPβ-gal, that is replication incompetent due to deletion of the pTP gene and that has the E1 genes replaced by the Escherichia coli lacZ reporter gene under the control of the cytomegalovirus major immediate-early promoter. This virus was compared with a first-generation, replication-defective adenovirus vector, Ad5dl308β-gal, that is isogenic except that it contains a wild-type pTP gene. To examine transduction efficiency and induction of inflammation, we developed a novel system involving intradermal injection of BALB/c mouse ears. Mouse ears can be accurately measured to determine the degree of edema as an indirect measurement of inflammation. Edema and inflammation were induced in a dose- and time-dependent manner by both viruses and correlated well. LacZ activity correlated inversely with edema and inflammation. The pTP-defective vector Ad5dl308ΔpTPβ-gal transduced mouse ears much more efficiently and induced edema and inflammatory cell infiltration approximately 10-fold less efficiently than the first-generation vector Ad5dl308β-gal. The diminished inflammatory response and increased efficiency of transduction observed with Ad5dl308ΔpTPβ-gal indicate its promise as a gene therapy agent for other tissues. The results also demonstrate that the mouse ear model offers potential for the study of adenovirus-induced inflammation because of the ready access of the ears, the relative ease of continuous measurement, and the sensitivity to adenovirus transducing vectors.
Adenovirus offers significant advantages as a vector for gene delivery, including growth to high titers, stability, allowance of a fairly large segment of foreign DNA to be introduced, and the ability to transduce a wide variety of tissues, including nonmitotic tissues. However, replication-defective adenovirus vectors induce in naive animals a substantial inflammatory response that limits the expression of transduced genes as well as readministration of the vectors (45; for a review of inflammation, see reference 17).
In first-generation adenovirus transducing vectors, replacement of the E1 region with foreign DNA generally has been used to make the virus replication defective. Complementation for the missing E1 functions may be provided by a variety of cell lines, such as 293 (25) and 911 (16). Loss of E1 functions reduces but does not eliminate the ability of the virus to replicate its DNA (44). Replication of adenovirus DNA (reviewed in references 26, 28, 46, and 48) is absolutely dependent on three adenovirus gene products, the DNA binding protein encoded by E2A (37), preterminal protein (pTP) encoded by E2B (42), and DNA polymerase encoded by E2B (2).
The majority of studies of the ability of recombinant adenoviruses to transduce tissues in vivo have been conducted with mice. Mice are not permissive for the growth of human adenoviruses, but substantial replication of adenovirus DNA occurs after infection with the wild-type virus (7, 51). Indirect evidence suggesting that viral DNA replication occurs after transduction of mouse tissues in vivo by replication-defective adenovirus vectors comes from the fact that the use of an adenovirus transducing vector with lacZ in place of the E1 region and a temperature-sensitive allele of the E2A gene led to a reduction in inflammation in mouse liver relative to the results obtained with a first-generation vector (15). Use of vectors made replication incompetent by deletion of the E2A gene (22, 31), the DNA polymerase gene (2), or all adenovirus genes (33) led to efficient expression of the transduced gene and reduced inflammation. In addition, E4 deletion mutant adenoviruses exhibited prolonged transduced gene expression in vivo (9, 13, 18).
Adenovirus transducing vector-induced inflammation has been observed for a large number of tissues (e.g., 1, 8, 12–14, 27, 30, 34, 36, 50, 53). Adenovirus introduced into the tail vein of mice transduces a variety of tissues, with the great majority of the transducing activity being found in the liver (45). Because of the ease of introduction of the virus into the liver of mice, this method has become common for studying adenovirus transducing vector-induced inflammation. Alternative methods of studying inflammation induced by adenovirus vectors offer complementation and possible extension of the liver studies. In particular, mouse ears offer a promising experimental target. Immunological responses in mouse ears have been examined in great detail in delayed-type hypersensitivity studies (reviewed in reference 24). Mouse ears are readily injected, and effects of manipulation can be studied noninvasively. Inflammation can be observed visually, and edema, which exhibits a good correlation with inflammation (4, 35, 38), can be determined by measurement of mouse ear thickness.
A variety of inbred mouse strains have been used in studies of transduction by adenovirus. Among the most commonly used strains, C57BL6 mice exhibit relatively low and BALB/c mice exhibit relatively high levels of inflammation (5, 32). Thus, BALB/c mice offer a sensitive model.
In this study, we report the development of a system involving injection of adenovirus transducing vectors into BALB/c mouse ears to study inflammation, edema, and transduced gene expression. We demonstrate that there is a good correlation between edema and inflammation and an inverse correlation between inflammation and transduced gene expression. Examination of dose responses to a vector made replication incompetent through deletion of the pTP gene demonstrates that this virus induces inflammation at a level at least 10-fold lower than a first-generation vector and that the pTP deletion mutant vector transduces much more efficiently (>10-fold).
MATERIALS AND METHODS
Construction of 293 cells expressing pTP.
293 cells (25) from the American Type Culture Collection, which grow somewhat more slowly, adhere slightly better, and produce higher titers of virus than the 293 strain that we previously used in the construction of 293 cell lines that express pTP (29, 41), were stably transfected with a plasmid carrying the tetracycline-VP16 transactivator fusion protein (tTA) (23) and hygromycin resistance. Cell lines were selected, cloned, and tested as previously described (29). The clone that exhibited the highest tTA activity in the absence of tetracycline and the best ratio of activity in the absence to activity in the presence of tetracycline was stably transfected with a plasmid carrying a modified genomic pTP construct (29) under the control of the tTA-dependent promoter. Cell lines were selected, cloned, and tested by Western blotting for pTP expression and for the ability to support the growth of the pTP deletion mutant Ad5dl308ΔpTP (42). The best cell line, 293-pTP 2C1, expressed pTP constitutively and produced better yields of pTP deletion mutant viruses than the previously described cell lines (29, 41).
Viruses.
The viruses used in this study were the first-generation vector Ad5dl308Bstβ-gal (herein referred to as Ad5dl308β-gal), which carries Escherichia coli LacZ under the control of the cytomegalovirus (CMV) major immediate-early promoter (43), and the pTP-defective (pTP−) virus Ad5dl308ΔpTPβ-gal. Ad5dl308ΔpTPβ-gal was constructed by overlap recombination between Ad5dl308ΔpTP and a plasmid carrying the left end of the adenovirus type 5 (Ad5) chromosome in which the E1 region between bp 358 and 3318 was replaced by a cassette of the E. coli lacZ gene under the control of the CMV major immediate-early promoter (20) in 293-pTP 2C1 cells. Ad5dl308β-gal was grown in 293 cells, and Ad5dl308ΔpTPβ-gal was grown in 293-pTP 2C1 cells. After substantial cytopathic effects were apparent, infected cells were harvested, concentrated by centrifugation, and lysed by four cycles of freezing-thawing. Cell debris was pelleted, and virus was purified by consecutive banding on a CsCl step gradient consisting of 1 ml of CsCl at 1.4 g/ml and 2 ml of CsCl at 1.25 g/ml in phosphate-buffered saline (PBS) with an SW40 rotor at 36,000 rpm for 50 min, followed by an isopycnic gradient consisting of 1.35 g of CsCl per ml in PBS and centrifugation for 3 h at 65,000 rpm with a VTi65 rotor. Virions were dialyzed against buffer containing 10 mM Tris-HCl (pH 7.6), 135 mM NaCl, 1 mM MgCl2, and 10% glycerol, frozen rapidly in small aliquots, and stored at −70°C until use. Particle titers were determined by measuring the sample absorbance at 260 nm and multiplying by 1012. Particle/PFU ratios for both viruses were approximately 100.
Transducing activity.
The transducing unit concentration in the virus stocks was determined by transducing HeLa-pTP cells (41) with various dilutions of the purified stocks, incubating at 37°C for 5 days to permit high-level expression of LacZ, washing with PBS, fixing for 5 min at room temperature with 4% paraformaldehyde in PBS, washing twice with PBS, and incubating in the presence of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40). After incubation for 24 h, blue cells were counted. Since viruses defective for E1 functions are capable of low-level replication in the absence of complementation (44), HeLa-pTP cells were used in this assay in an attempt to equalize replication between Ad5dl308ΔpTPβ-gal and Ad5dl308β-gal. To assay for total transducing activity, HeLa cells were transduced at multiplicities of 10, 25, 50, and 100 PFU/cell, incubated at 37°C for 12 h (prior to the onset of replication of Ad5dl308β-gal at the highest multiplicity used; data not shown), washed with PBS, harvested by scraping in PBS, pelleted, and resuspended in 100 μl of PBS. LacZ activity was released by three rounds of rapid freezing and thawing of the resuspended cells. Cell debris was pelleted by centrifugation at 8,000 × g for 10 min at 4°C. Ten microliters of each sample, including mock-transduced control cells, was incubated in the presence of 1 mg of ortho-nitrophenyl galactopyranoside per ml in 300 μl of Z buffer (39) at 30°C until yellow color developed. The reaction was terminated by the addition of 200 μl of 1 M Na2CO3, and the absorbance at 420 nm was measured. Relative raw activities were determined by dividing the A420 by the time of incubation, the background present in the mock-transduced extract was subtracted to yield final activities, and activities were plotted as a function of multiplicity of infection to determine the LacZ activities directed by the virus stocks.
Injection of mouse ears with adenovirus transducing vectors.
Female 5- to 7-week-old BALB/c mice were purchased from Harlan Sprague-Dawley. All mice received pelleted food and water ad lib.
Prior to injection of adenovirus or buffer, mice were anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg of body weight). Baseline ear thickness was then quantitated in the approximately top one-third section of the ear, including the site to be injected, by use of an engineer’s micrometer. The dorsal surface of the ear was then injected intradermally with the appropriate virus in 10 μl of 10 mM Tris-HCl (pH 7.6)–135 mM NaCl–1 mM MgCl2–10% (vol/vol) glycerol by use of a U-100 insulin syringe (Becton Dickinson and Co.) fitted with a 28-gauge needle. All groups of mice contained at least three animals, and both ears were injected with the same amount of the same virus. Controls received buffer only. At 24, 48, 72, and 96 h postinjection, ear thickness was remeasured as for the determination of the baseline; the increase over the baseline was expressed in units of 10−4 inches. Ears were also examined visually for the appearance of inflammation.
Histological analysis.
Mice were sacrificed 4 to 14 days after injection. Ears were collected, fixed for 1 h at 4°C in 2% paraformaldehyde in PBS, washed repeatedly with PBS, and incubated for 24 h in the presence of X-Gal (40) at 37°C to determine the level of transduced gene expression. Ears were then washed and stored at 4°C in 4% paraformaldehyde in PBS. For sectioning, ears were cryoprotected in 30% sucrose in PBS at 4°C for 24 h, the regions of the ears that were positive for transgene expression (the site of injection and the base of the ear) were embedded in OCT (Fisher Scientific) and frozen, and 30-μm sections were cut. Sections were stained with hematoxylin and eosin (Gill’s 2× hematoxylin). Sections were then examined microscopically for the presence of an X-Gal product and for inflammatory infiltrate.
RESULTS
A novel model of adenovirus transducing vector-induced inflammation.
Inflammation induced by recombinant adenovirus transducing vectors has been analyzed with a number of systems, most frequently the liver of mice (e.g., 27, 45). Mouse ears offer an alternative that has certain advantages: mouse ears have been used extensively for studies of delayed-type hypersensitivity and thus offer a relatively well-understood immunological model system (24); edema, which exhibits a fairly good correlation with inflammation (4, 35, 38), can be readily measured in ears without sacrificing the mouse, so good kinetic data can be accumulated from individual mice; inflammation in mouse ears can be ascertained by visual inspection; and data can be individually collected from both ears.
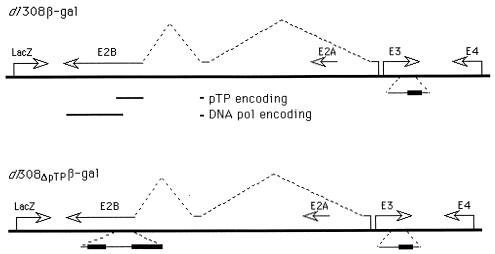
We set out to test whether a replication-incompetent adenovirus vector with a deletion of the pTP gene would be a better transducing agent than a first-generation, replication-defective vector as well as to determine whether injection of adenovirus transducing vectors into mouse ears would offer a sensitive and reproducible model for determination of the degree of inflammation. For comparison, we used transducing vectors in which the E1 region is replaced by the readily detectable lacZ reporter gene from E. coli under the control of the CMV major immediate-early promoter (Fig. 1). The vectors are isogenic except for the pTP gene: the E1 region is replaced by the lacZ gene under the control of the CMV promoter; there is a partial deletion as well as an insertion of foreign DNA within the E3 region, leaving intact the region encoding gp19; gp19 efficiently blocks cell surface expression of the class I major histocompatibility complex (3, 10), although different mouse class I major histocompatibility types are differentially affected (11, 21, 47); the pTP gene of vector Ad5dl308ΔpTPβ-gal is deleted, making the virus incapable of replication in the absence of complementation for pTP (42), while the first-generation vector Ad5dl308β-gal is wild type for pTP and thus is capable of low-level replication in the absence of complementation; and all other viral genes are wild type.
FIG. 1.
Test viruses. Reporter viruses used to test for the induction of inflammation are presented schematically. The viruses are isogenic except for the pTP gene. (Top) The first-generation, replication-defective virus Ad5dl308β-gal (43) chromosome is presented as a thick line, and early-region transcription units are indicated by arrows. The splicing pattern for pTP is indicated by the broken lines above the chromosome. The region of deletion or replacement within E3 (19) is indicated by the thick line in the enlargement below the chromosome. The regions encoding pTP and DNA polymerase (DNA pol) are indicated below the chromosome. (Bottom) Ad5dl308ΔpTPβ-gal is presented as for Ad5dl308β-gal; the regions deleted from the pTP gene are indicated by the thick lines in the enlargements below the chromosome.
Viruses were normalized by particle concentration for use in injections. The concentration of transducing units was determined with HeLa-pTP cells, in which low-level replication of both viruses occurs; it was 4% higher in the stock of Ad5dl308β-gal than in the stock of Ad5dl308ΔpTPβ-gal. The LacZ activity induced in HeLa cells transduced by Ad5dl308ΔpTPβ-gal was found to be 2.5-fold higher than that induced by Ad5dl308β-gal.
Mouse ears were injected intradermally with the recombinant Ad5 vectors. BALB/c mice were chosen for this study because they exhibit a high degree of inflammation in response to adenovirus vectors (5, 32). Visual observations of the ears were made as a preliminary determination of inflammation. The thickness of the ears was measured prior to injection and at 24-h intervals through 96 h after injection to determine the level of edema. The results are presented for different amounts of viruses as a function of time in Fig. 2.
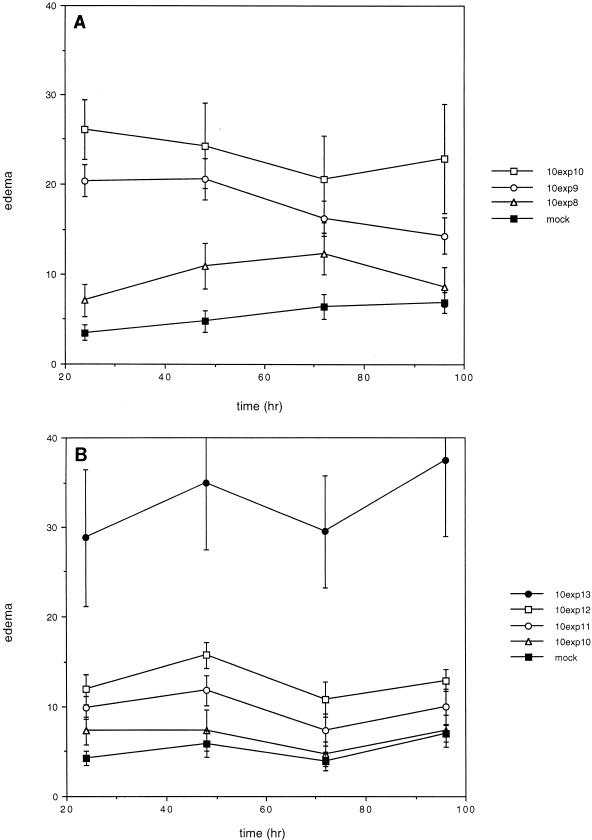
FIG. 2.
Edema induced in mouse ears by injection of adenovirus transducing vectors. Transduced and mock-transduced mouse ears were measured before injection and every 24 h through 96 h after injection. The change in ear thickness is plotted in units of 10−4 inches as a function of time for each concentration (exp, exponent) of each virus; standard errors are indicated. (A) Ears transduced with Ad5dl308β-gal. (B) Ears transduced with the pTP− virus Ad5dl308ΔpTPβ-gal.
Significant dose-dependent edema was observed after injection of Ad5dl308β-gal, a first-generation vector (Fig. 2A). Visual inspection indicated the presence of inflammation at the two highest virus doses. In contrast, injection of the pTP− virus Ad5dl308ΔpTPβ-gal led to significant edema only at a dose 10-fold higher than the highest dose of Ad5dl308β-gal used (Fig. 2B), and inflammation was not apparent upon visual inspection.
The amount of edema, especially at the highest doses of virus, varied fairly widely, as indicated by the sizes of the error bars. It is possible that this result was due to variability in the injection process. However, since variability was significantly greater at the highest virus doses used (1.4 × 109 and 1.4 × 1010 particles of Ad5dl308β-gal and 1.4 × 1011 particles of Ad5dl308ΔpTPβ-gal) and ear measurements for individual ears were consistent over the course of the experiment, it is likely that this variability reflected real differences in inflammatory responses among individual mice.
Transduced gene expression.
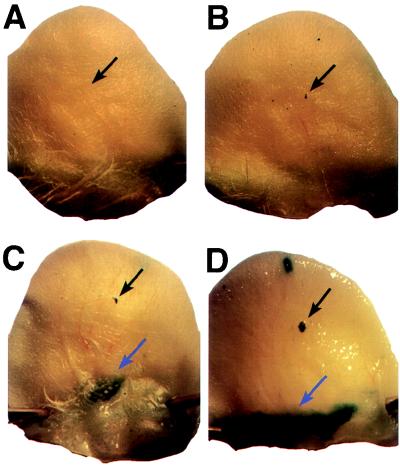
Mice were sacrificed from 4 to 14 days after injection of virus. Ears were collected and stained for the presence of β-galactosidase activity (Fig. 3). Virus-dependent β-galactosidase activity occurred in a small region at the site of injection and in a larger region at the base of the ear. A low level of β-galactosidase activity independent of virus was apparent, but this activity was readily distinguished from virus-dependent activity. X-Gal staining at the site of injection and particularly at the base of the ear was much more intense in ears injected with Ad5dl308ΔpTPβ-gal than in ears injected with the same amounts of Ad5dl308β-gal. Mice injected with Ad5dl308β-gal rarely exhibited staining at the base of the ear at day 4, and no staining was apparent at day 7 or later. Transduced gene expression at the base of the ear was lost with time in ears injected with Ad5dl308ΔpTPβ-gal. At day 4, staining was intense, at day 7, staining was still strong, but at day 14, little or no staining was apparent upon gross examination. These data are consistent with the edema exhibited by the ears (Fig. 2): a relatively large amount of edema was seen with little transduced gene expression by day 4 in ears injected with 1.4 × 109 or 1.4 × 1010 particles of Ad5dl308β-gal; in contrast, a high level of transduced gene expression was observed in ears injected with 1.4 × 109 or more particles of Ad5dl308ΔpTPβ-gal while significant edema was apparent only in ears injected with 1.4 × 1011 particles.
FIG. 3.
Mouse ears transduced by adenovirus vectors. Whole mounts of mouse ears injected with buffer (A), 1.4 × 1010 particles of Ad5dl308β-gal (B), 1.4 × 1010 particles of the pTP− virus Ad5dl308ΔpTPβ-gal (C), and 1.4 × 1011 particles of the pTP− virus Ad5dl308ΔpTPβ-gal (D) 4 days after injection are shown. The injection sites, which exhibited LacZ expression when virus was injected, are indicated by black arrows. Major sites of transduction near the base of the ear are indicated by blue arrows. X-Gal staining dependent on the presence of LacZ was also occasionally observed in a small region near the top of the ear (B and D).
Histological examination of transduced ears.
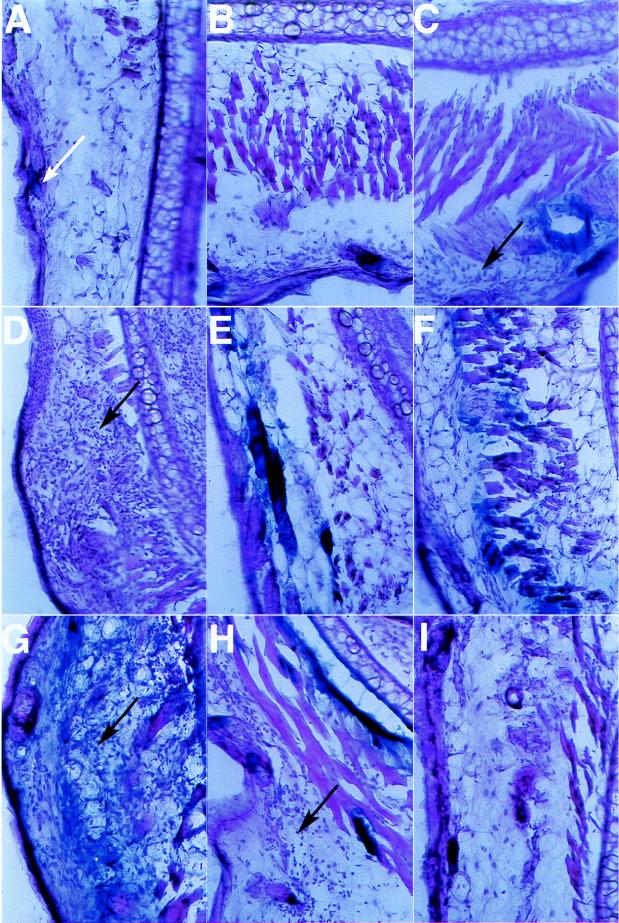
Ears were sectioned and examined for inflammatory infiltrate by staining with hematoxylin and eosin (Fig. 4). Mock-injected ear sections demonstrated a mild infiltrate of inflammatory cells, presumably in response to the slight tissue damage that occurred during injection with the glycerol-containing buffer. Ears injected with Ad5dl308β-gal exhibited the presence of an inflammatory infiltrate in a dose-dependent manner 4 days after injection: at 1.4 × 1010 particles, a tremendous number of inflammatory cells was present (Fig. 4D), while injection with 1.4 × 109 particles led to a smaller but still substantial infiltrate (Fig. 4C) and injection with 1.4 × 108 particles did not cause a significant increase in the inflammatory infiltrate above background (Fig. 4, compare panels A and B). By 7 days after injection, the number of inflammatory cells present had decreased for mice injected with 1.4 × 1010 particles but had increased in ears injected with 1.4 × 109 particles (data not shown). In contrast, a substantial inflammatory infiltrate was apparent 4 days after injection with 1.4 × 1011 particles of Ad5dl308ΔpTPβ-gal, while injection with 1.4 × 1010 particles led to little or no increase over levels obtained with mock injection (Fig. 4F and G). The concentration of inflammatory cells had decreased by day 7 in ears injected with 1.4 × 1011 particles of Ad5dl308ΔpTPβ-gal (Fig. 4H), while it had increased in ears injected with 1.4 × 1010 particles (data not shown). This evidence demonstrates a dose- and time-dependent inflammatory response to both viruses but indicates that the replication-incompetent pTP− virus Ad5dl308ΔpTPβ-gal induced an inflammatory infiltrate that was delayed and more than 10-fold reduced relative to that induced by Ad5dl308β-gal at 4 days after injection.
FIG. 4.
Histology of transduced ears. Frozen 30-μm sections from ears injected with various amounts of Ad5dl308β-gal or Ad5dl308ΔpTPβ-gal 4 days after injection (except for panels H and I) were cut from the regions of transduction and stained with hematoxylin and eosin. (A) Buffer control; the white arrow indicates background staining of a cell surrounding the base of a hair. (B) 1.4 × 108 particles of Ad5dl308β-gal. (C) 1.4 × 109 particles of Ad5dl308β-gal. (D) 1.4 × 1010 particles of Ad5dl308β-gal. (E) 1.4 × 109 particles of Ad5dl308ΔpTPβ-gal. (F) 1.4 × 1010 particles of Ad5dl308ΔpTPβ-gal. (G) 1.4 × 1011 particles of Ad5dl308ΔpTPβ-gal. (H) 1.4 × 1011 particles of Ad5dl308ΔpTPβ-gal 7 days after injection. (I) 1.4 × 1011 particles of Ad5dl308ΔpTPβ-gal 14 days after injection. Regions of stronger inflammatory infiltration (C, D, G, and H) are indicated by black arrows.
A comparison of LacZ activity demonstrated an even greater advantage of the pTP− virus Ad5dl308ΔpTPβ-gal over Ad5dl308β-gal. Staining at the site of injection was observed in a dose-dependent manner for both viruses. However, staining at the major site of transduction near the base of the ear was apparent 4 days or more after injection almost exclusively in ears transduced with Ad5dl308ΔpTPβ-gal. While intense staining was routinely apparent near the base of the ear 4 days after injection with 1.4 × 109 to 1.4 × 1011 particles of Ad5dl308ΔpTPβ-gal (Fig. 4E to G), Fig. 4C represents the highest level of expression seen in any of the sections from any of the ears injected with any concentration of Ad5dl308β-gal. LacZ staining was reduced, although still substantial, at day 7 and was reduced to a low level at day 14 in ears transduced with 1.4 × 1011 particles of Ad5dl308ΔpTPβ-gal (Fig. 4I and J). In ears injected with smaller amounts of Ad5dl308ΔpTPβ-gal, LacZ staining was apparent 7 days but not 14 days after injection (data not shown). In contrast, LacZ staining at the base of the ear was not observed beyond day 4 in any of the ears injected with Ad5dl308β-gal.
The kinetic data derived from measurements of edema are consistent with both LacZ expression and histology. Substantial swelling due to injection of Ad5dl308β-gal was apparent at doses of 1.4 × 109 and 1.4 × 1010 particles, doses at which inflammation 4 days after injection was either strong or very strong; in contrast, among ears injected with Ad5dl308ΔpTPβ-gal, substantial edema was apparent only at a dose of 1.4 × 1011 particles, the only dose at which a strong inflammatory infiltrate was apparent 4 days after injection.
DISCUSSION
We have developed a novel system for the analysis of adenovirus transducing vector-induced inflammation that involves intradermal injection of the virus into the ears of BALB/c mice. Using this system, we demonstrated that a second-generation adenovirus transducing vector made replication incompetent by deletion of the pTP gene is substantially improved relative to a first-generation replication-defective vector.
Transduced mouse ears injected with the first- and second-generation viruses were compared for edema, inflammatory infiltrate, and transduced gene expression as a function of virus vector dose. The pTP− virus Ad5dl308ΔpTPβ-gal induced edema approximately 10-fold less efficiently in the first 4 days (Fig. 2) and an inflammatory cell infiltrate more than 10-fold less efficiently at day 4 (Fig. 4) than Ad5dl308β-gal. This evidence suggests a fairly good, approximately linear relationship between edema and inflammation.
The amount of transduced gene expression varied as a function of time. Ears transduced with the first-generation virus Ad5dl308β-gal exhibited little LacZ expression 4 days and none 7 days after injection. Ears transduced with the pTP− virus Ad5dl308ΔpTPβ-gal exhibited high levels of LacZ expression at day 4 that declined by day 7 and further declined such that very little expression was apparent at day 14 and only in ears injected with the largest amount of the vector (Fig. 4 and data not shown). The amount of activity directed by Ad5dl308ΔpTPβ-gal in vitro was higher by 2.5-fold than that directed by Ad5dl308β-gal when assayed prior to the onset of viral replication. Since Ad5dl308β-gal but not Ad5dl308ΔpTPβ-gal is capable of replicating in mouse ears, it is likely that the difference in activities apparent in vitro overstates the difference in vivo. Regardless, given the dramatic difference apparent in vivo, it is clear that at least the great majority of the difference in LacZ activity induced by Ad5dl308ΔpTPβ-gal relative to Ad5dl308β-gal resulted directly from improvement of the vector.
The loss of LacZ expression in ears injected with 1.4 × 1010 or fewer particles of Ad5dl308ΔpTPβ-gal occurred after substantial inflammatory infiltrates became apparent, suggesting a causal relationship. The difference in transduced gene expression between ears injected with Ad5dl308ΔpTPβ-gal and those injected with Ad5dl308β-gal was much higher than 10-fold (Fig. 3 and 4), suggesting that a more complex, possibly exponential relationship may exist between inflammation and transduced gene expression.
Transduced gene expression was routinely observed in a small area at the site of injection and in a large area near the base of the ear. In addition, transduced gene expression was observed occasionally near the tip of the ear. The localization of X-Gal product at the site of injection suggested that the process of injection contributed to LacZ expression at this site. It is likely that the expression of the adenovirus receptor (6) and coreceptor (49) is restricted to the basal-lateral surface near the site of injection (e.g., 52) and that injury caused by injection exposes the basal-lateral cell surface, permitting transduction. LacZ expression at the site of injection persisted for a longer time than it did near the base of the ear for ears injected with Ad5dl308β-gal (e.g., Fig. 2B) in spite of the fact that inflammatory cell infiltrates were observed throughout much of the ear, including the area near the site of injection (data not shown).
The process of injection of mouse ears induced modest inflammation, as indicated by the presence of mononuclear cells in buffer-injected ears (Fig. 4A). This inflammation may have contributed to the rapid induction of inflammation observed with the injection of viruses. As such, the injected-mouse-ear model may be a good system for studying the ability of adenoviruses to transduce tissues that are affected by inflammatory diseases, such as cystic fibrosis.
The mouse ear system offers advantages for analyzing adenovirus-induced inflammation: ears are accessible and relatively thin, so edema can be readily monitored, permitting kinetic data to be developed for individual mice; inflammation can be observed visually, permitting transduced gene expression and inflammation to be correlated; adenovirus efficiently transduces specific cells within the ear; there is a substantial body of literature on the use of mouse ears for studying delayed-type hypersensitivity (reviewed in reference 24) that can be applied to ears transduced by adenovirus; BALB/c mice exhibit a strong inflammatory response to adenovirus vectors, making analysis relatively sensitive and the time course of the experiment relatively rapid; and the accessibility of ears offers promise for the development of approaches to determine molecular aspects important in the induction of inflammation.
ACKNOWLEDGMENTS
We thank Elizabeth Ullyat and Shawna Tolman for expert technical support and James Stephens and Henry Claman for helpful discussions.
This work was supported by NIH grant HL58344 and a grant from the Gene Therapy Program of the University of Colorado Cancer Center to J.S., NIH grant AI12993 to J.W.M., and NIH grant NS01741 to R.L.S. G.H.C. was supported by NIH training grant NS07321.
REFERENCES
- 1.Adesanya M R, Redman R S, Baum B J, O’Connell B C. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum Gene Ther. 1996;7:1085–1093. doi: 10.1089/hum.1996.7.9-1085. [DOI] [PubMed] [Google Scholar]
- 2.Amalfitano A, Hauser M A, Hu H, Serra D, Begy C R, Chamberlain J S. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson M, Paabo S, Nilsson T, Peterson P A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985;43:215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 4.Asherson G L, Ptak W. Contact and delayed hypersensitivity in the mouse. I. Active sensitization and passive transfer. Immunology. 1968;15:405–416. [PMC free article] [PubMed] [Google Scholar]
- 5.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 6.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 7.Blair G E, Dixon S C, Griffiths S A, Zajdel M E. Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res. 1989;14:339–346. doi: 10.1016/0168-1702(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard K T, Boekelheide K. Adenovirus-mediated gene transfer to rat testis in vivo. Biol Reprod. 1997;56:495–500. doi: 10.1095/biolreprod56.2.495. [DOI] [PubMed] [Google Scholar]
- 9.Brough D E, Lizonova A, Hsu C, Kulesa V A, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol. 1996;70:6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgert H G, Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985;41:987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- 11.Burgert H G, Maryanski J L, Kvist S. “E3/19K” protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci USA. 1987;84:1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrnes A P, Rusby J E, Wood M J, Charlton H M. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66:1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- 13.Dedieu J F, Vigne E, Torrent C, Jullien C, Mahfouz I, Caillaud J M, Aubailly N, Orsini C, Guillaume J M, Opolon P, Delaere P, Perricaudet M, Yeh P. Long-term gene delivery into the livers of immunocompetent mice with E1/E4-defective adenoviruses. J Virol. 1997;71:4626–4637. doi: 10.1128/jvi.71.6.4626-4637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkon K B, Liu C C, Gall J G, Trevejo J, Marino M W, Abrahamsen K, Song X, Zhou J L, Old L J, Crystal R G, Falck-Pedersen E. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelhardt J F, Ye X, Doranz B, Wilson J M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallaux F J, Kranenburg O, Cramer S J, Houweling A, Van Ormondt H, Hoeben R C, Van Der Eb A J. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 17.Gallin J I. Inflammation. In: Paul W E, editor. Fundamental immunology. 2nd ed. New York, N.Y: Raven Press; 1989. pp. 721–733. [Google Scholar]
- 18.Gao G, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gingras M C, Arevalo P, Aguilar-Cordova E. Potential salmon sperm origin of the E3 region insert of the adenovirus 5 dl309 mutant. Cancer Gene Ther. 1996;3:151–154. [PubMed] [Google Scholar]
- 20.Gomez-Foix A M, Coats W S, Baque S, Alam T, Gerard R D, Newgard C B. Adenovirus-mediated transfer of the muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J Biol Chem. 1992;267:25129–25134. [PubMed] [Google Scholar]
- 21.Gooding L R, Wold W S. Molecular mechanisms by which adenoviruses counteract antiviral immune defenses. Criti Rev Immunol. 1990;10:53–71. [PubMed] [Google Scholar]
- 22.Gorziglia M I, Kadan M J, Yei S, Lim J, Lee G M, Luthra R, Trapnell B C. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol. 1996;70:4173–4178. doi: 10.1128/jvi.70.6.4173-4178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 25.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 26.Hay R T, Freeman A, Leith I, Monaghan A, Webster A. Molecular interactions during adenovirus DNA replication. In: Doerfler W, Böhm P, editors. Molecular repertoire of adenoviruses II. Vol. 199. Berlin, Germany: Springer; 1995. pp. 31–48. [DOI] [PubMed] [Google Scholar]
- 27.Jooss K, Ertl H C, Wilson J M. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly T J., Jr . Adenovirus replication. In: Ginsberg H S, editor. The adenoviruses. New York, N.Y: Plenum Press; 1984. pp. 271–308. [Google Scholar]
- 29.Langer S, Schaack J. Construction of 293 cell lines that inducibly express high levels of precursor terminal protein. Virology. 1996;221:172–179. doi: 10.1006/viro.1996.0363. [DOI] [PubMed] [Google Scholar]
- 30.Lei D, Lehmann M, Shellito J E, Nelson S, Siegling A, Volk H D, Kolls J K. Nondepleting anti-CD4 antibody treatment prolongs lung-directed E1-deleted adenovirus-mediated gene expression in rats. Hum Gene Ther. 1996;7:2273–2279. doi: 10.1089/hum.1996.7.18-2273. [DOI] [PubMed] [Google Scholar]
- 31.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, Schultz H, Stoeckel F, Pavirani A, Mehtali M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michou A I, Santoro L, Christ M, Julliard V, Pavirani A, Mehtali M. Adenovirus-mediated gene transfer: influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 1997;4:473–482. doi: 10.1038/sj.gt.3300412. [DOI] [PubMed] [Google Scholar]
- 33.Morsy M A, Gu M C, Zhao J Z, Holder D J, Rogers I T, Pouch W J, Motzel S L, Klein H J, Gupta S K, Liang X, Tota M R, Rosenblum C I, Caskey C T. Leptin gene therapy and daily protein administration: a comparative study in the ob/ob mouse. Gene Ther. 1998;5:8–18. doi: 10.1038/sj.gt.3300565. [DOI] [PubMed] [Google Scholar]
- 34.Muhlhauser J, Jones M, Yamada I, Cirielli C, Lemarchand P, Gloe T R, Bewig B, Signoretti S, Crystal R G, Capogrossi M C. Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Ther. 1996;3:145–153. [PubMed] [Google Scholar]
- 35.Phanuphak P, Moorhead J W, Claman H C. Tolerance and contact sensitivity to DNFB in mice. I. In vivo detection by ear swelling and correlation with in vitro cell stimulation. J Immunol. 1974;112:115–123. [PubMed] [Google Scholar]
- 36.Raper S E, DeMatteo R P. Adenovirus-mediated in vivo gene transfer and expression in normal rat pancreas. Pancreas. 1996;12:401–410. doi: 10.1097/00006676-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Rice S A, Klessig D F. Isolation and analysis of adenovirus type 5 mutants containing deletions in the gene encoding the DNA-binding protein. J Virol. 1985;56:767–778. doi: 10.1128/jvi.56.3.767-778.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson J H, Naysmith J D. A comparison of four methods for measuring cutaneous delayed-type hypersensitivity reactions to protein antigens in the mouse. Scand J Immunol. 1976;5:299–304. doi: 10.1111/j.1365-3083.1976.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Sanes J R, Rubenstein J L, Nicolas J F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaack J, Guo X, Ho W Y-W, Karlok M, Chen C-Y, Ornelles D. Adenovirus type 5 precursor terminal protein-expressing 293 and HeLa cell lines. J Virol. 1995;69:4079–4085. doi: 10.1128/jvi.69.7.4079-4085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaack J, Guo X, Langer S. Characterization of a replication-incompetent adenovirus type 5 mutant deleted for the preterminal protein gene. Proc Natl Acad Sci USA. 1996;93:14686–14691. doi: 10.1073/pnas.93.25.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaack J, Langer S, Guo X. Efficient selection of recombinant adenoviruses using vectors that express β-galactosidase. J Virol. 1995;69:3920–3923. doi: 10.1128/jvi.69.6.3920-3923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenk T, Jones N, Colby W, Fowlkes D. Functional analysis of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harbor Symp Quant Biol. 1979;44:367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- 45.Smith T A, Mehaffey M G, Kayda D B, Saunders J M, Yei S, Trapnell B C, McClelland A, Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat Genet. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- 46.Stillman B W. The replication of adenovirus DNA with purified proteins. Cell. 1983;35:7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka Y, Tevethia S S. Differential effect of adenovirus 2 E3/19K glycoprotein on the expression of H-2Kb and H-2Db class I antigens and H-2Kb- and H-2Db-restricted SV40-specific CTL-mediated lysis. Virology. 1988;165:357–366. doi: 10.1016/0042-6822(88)90580-6. [DOI] [PubMed] [Google Scholar]
- 48.Van der Vliet P C. Adenovirus DNA replication. In: Doerfler W, Böhm P, editors. The molecular repertoire of adenoviruses II. Vol. 199. Berlin, Germany: Springer-Verlag KG; 1995. pp. 1–30. [Google Scholar]
- 49.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 51.Younghusband H B, Tyndall C, Bellett A J. Replication and interaction of virus DNA and cellular DNA in mouse cells infected by a human adenovirus. J Gen Virol. 1979;45:455–467. doi: 10.1099/0022-1317-45-2-455. [DOI] [PubMed] [Google Scholar]
- 52.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zsengeller Z K, Boivin G P, Sawchuk S S, Trapnell B C, Whitsett J A, Hirsch R. Anti-T cell receptor antibody prolongs transgene expression and reduces lung inflammation after adenovirus-mediated gene transfer. Hum Gene Ther. 1997;8:935–941. doi: 10.1089/hum.1997.8.8-935. [DOI] [PubMed] [Google Scholar]