Summary:
Current evidence suggests at least one-third of humeral shaft fractures initially managed nonoperatively will fail closed treatment, and this review highlights surgical considerations in those circumstances. Although operative indications are well-defined, certain fracture patterns and patient cohorts are at greater risk of failure. When operative intervention is necessary, internal fixation through an anterolateral approach is a safe and sensible alternative. Determining which patients will benefit most involves shared decision-making and careful patient selection. The fracture characteristics, bone quality, and adequacy of the reduction need to be carefully evaluated for the specific operative risks for individuals with certain comorbid conditions, inevitably balancing the patient's expectations and demands against the probability of infection, nerve injury, or nonunion. As our understanding of the etiology and risk of nonunion and symptomatic malunion of the humeral diaphysis matures, adhering to the principles of diagnosis and treatment becomes increasingly important. In the event of nonunion, respect for the various contributing biological and mechanical factors enhances the likelihood that all aspects will be addressed successfully through a comprehensive solution. This review further explores specific strategies to definitively restore function of the upper extremity with the ultimate objective of an uninfected, stable union.
Keywords: humerus, fracture, nonoperative, internal fixation, malunion, nonunion
1.Introduction
Humerus fractures are relatively common injuries, accounting for up to 3% of all orthopaedic injuries.1 Although these fractures may involve any portion extending from the most proximal through to the most distal extent, this review simply cannot address the comprehensive treatment principles associated with the entire humerus. Instead, this review will focus on isolated fractures of the humeral shaft, extending either proximal or distal without true engagement of the periarticular bone. For acute, isolated, closed humeral shaft fractures, nonoperative treatment with functional bracing has long been widely regarded as the preferred method.2–8 Operative treatment of humeral shaft fractures has been largely reserved for patients with open fractures, neurovascular compromise, polytrauma, concomitant elbow/forearm fractures, and ipsilateral clavicle/shoulder fractures and for those who have failed initial treatment with bracing.1,9,10 As the population ages, diaphyseal fractures of the humerus are becoming even more common. With an incidence of 30 of 100,000 in the third decade of life, this steadily increases to an incidence of 100 of 100,000 by the seventh decade.11 For the past 50 years, the general consensus has held that closed low-energy fractures could be adequately treated through nonoperative means. The original work by Sarmiento4,6 established the paradigm for conservative management with functional bracing. With a published nonunion rate of 2% for closed fractures, 6% for nonoperative fractures, and alignment within 16 degrees of anatomic, bracing became the widely accepted standard of care.4,6 However, over the past 10 years, ongoing research has shed more light on nonoperative management of these fractures, suggesting that the nonunion rate is typically between 15% and 30% in modern trials.12,13 Therefore, having an understanding, approach, and plan to address humeral shaft fractures that fail nonoperative treatment or to manage those diaphyseal humeral nonunions that are likely to occur has become increasingly important.
Satisfactory outcomes have been reported with nonoperative treatment in carefully selected patients with extra-articular distal third fractures of the humeral diaphysis.14 Nevertheless, others consider open reduction, stable internal fixation, and early range of motion the standard of care for grossly displaced fractures of the distal quarter of the humerus to avoid issues with stiffness, nonunion, or malunion. Nonoperative treatment is often the preferred option for elderly patients and those with significant comorbidities precluding surgery or stable nondisplaced fractures that allow an early protected range of motion. Likewise, minimally displaced proximal humerus fractures in all ages are best treated nonoperatively while displaced unstable fractures in younger, healthier patients are more likely treated operatively. However, there is no consensus on the treatment of displaced unstable fractures in elderly patients, although many can clearly be treated nonoperatively.15 Although there is a question as to the benefit of operative intervention for proximal humerus fractures in general,15 for many patients it is apparent that surgical treatment provides better functional outcomes in displaced unstable fractures, and nonoperative management should generally be reserved for low-demand patients with comorbid conditions.
The principal focus here will be on humeral shaft fractures, acknowledging that the majority can be successfully managed with nonoperative measures. However, given that contemporary studies report nonunion rates as high as 20%–33%, primary operative intervention is becoming a more reasonable and frequently selected option.12,13,16–18 In addition, patient tolerance of nonoperative treatment can limit its utility, with studies indicating a 30% crossover to operative treatment due to loss of reduction, nonunion, delayed union, and the inability to tolerate functional bracing.19,20 While primary operative treatment in isolated humeral shaft fractures is still debated, certain scenarios represent absolute indications for surgery including polytrauma, vascular injury, brachial plexus injury, ipsilateral forearm/upper extremity fractures, and bilateral fractures.9,10 Furthermore, certain fracture characteristics may drive the surgeon toward selecting primary operative intervention.18 Ali et al reported that with conservative management, nonunion rates were 12% for middle third humeral shaft fractures and 15% for distal third humeral shaft fractures. However, proximal third humeral shaft fractures exhibited a much higher rate of nonunion at 24%.21 Ring et al22 examined the relationship between fracture pattern and location and demonstrated that proximal third humeral shaft fractures with oblique or spiral patterns have a higher nonunion rate. Other fracture characteristics that may influence the surgeon to consider offering primary operative treatment include transverse fractures, short oblique fractures, distraction at the fracture site, and adjacent arthritis of the shoulder or elbow.7 Age is certainly the leading etiologic factor for diaphyseal humerus fractures, and the majority of these injuries in the elderly result from low-energy trauma. Multiple medical comorbidities, liver failure, preexisting shoulder arthritis, and the use of preinjury nonsteroidal anti-inflammatory medications (NSAIDs) are all nonmodifiable risk factors of nonunion.23 Dementia is another factor that seems to be an independent risk factor of nonunion with nonoperative treatment.24 Furthermore, proximal third humerus fractures with a butterfly fragment have been associated with a higher nonunion rate.25 Alcohol dependency and smoking are recognized modifiable risk factors of nonunion.26 In the nonunion after initial surgical fixation, a history of deep infection increases the likelihood that a nonunion will develop.23,26
Although often framed within the context of isolated indications and contraindications for surgery, as our approach continues to mature it becomes more reasonable to consider the fracture characteristics, bone quality, and the adequacy of the reduction vis-á-vis the specific operative risks for individuals with certain comorbid conditions. This most often balances a given patient's anticipated expectations and demands against the probability of infection, nerve injury, or nonunion. Ultimately, it becomes an exercise in matching each patient with the various therapeutic alternatives available and selecting the option that maximizes the probability of a successful outcome while minimizing the risk of treatment failure. As always, shared decision making is another key to achieving the greatest patient satisfaction, in part further governed by the particular skill set of the responsible surgeon and resources they have available. This review provides a contemporary synopsis of the management of humeral shaft fractures, spanning the breadth from nonoperative treatment through to conventional open reduction and internal fixation for those injuries that fail initial splinting and bracing. It concludes with a discussion in greater detail of the treatment options for those more difficult cases where the fracture fails to unite after either nonoperative or operative management.
2. Nonoperative Care With a Functional Brace
The primary treatment for most diaphyseal humerus fractures is still nonoperative when the alignment is acceptable or can be obtained and maintained with closed reduction and splinting. First and foremost, the healing potential is very high, most likely associated with an excellent circumferential soft-tissue envelope. In addition, deformity of the humeral diaphysis is traditionally tolerated to a high degree and associated with minimal to no functional loss in the short or long term because of multiple factors. The large multiplanar arc of motion through the shoulder effectively compensates for deformity. Compared with the lower extremity, where limb length discrepancy and malalignment can be associated with inferior outcomes, shortening of the humeral diaphysis usually does not result in functional problems or uneven loading of the contiguous joints predisposing to arthritis.
Although not fully based on high levels of evidence, the upper limits of acceptable alignment parameters that have been used in practice for more than half a century with successful outcomes include (a) 20 degrees of sagittal plane angulation, (b) 30 degrees of coronal plane angulation (varus), (c) 3 cm of shortening, and (d) 15 degrees of malrotation.27 Nevertheless, over the past decade, the rate of humeral diaphyseal fractures being managed operatively has increased, from 47% in 2002 to 60% in 2011.18 Earlier, very high rates of union (94%–100%) were typically reported with conventional nonoperative treatment.2–7 However, this has come under increasing scrutiny, and recent studies now reveal diminished rates of spontaneous healing with closed treatment and functional bracing, with reported union rates of 77%–97% in various studies.8,12,13,17,28,29
Nonoperative treatment requires careful management with meticulous technique and assiduous follow-up.6,30 The first phase involves closed reduction and application of a coaptation splint at presentation. The coaptation splint should be applied paying attention to a number of important details: (a) The splint is applied with the patient upright; (b) in the sagittal plane, the medial limb of the splint reaches as far proximal into the axilla as possible, and the lateral limb passes above the deltoid and over the acromion toward the base of the neck; (c) a valgus mold is applied at the level of the fracture; and (d) a collar and cuff is incorporated with the elbow unsupported and the upper arm dangling because of gravity. The second phase involves later transition to a functional bivalved brace 1–2 weeks after injury, and the straps are tightened as the swelling resolves over time. The collar and cuff are used for comfort and discontinued within several weeks. It is critical that active elbow range of motion is started early, as muscular contraction provides circumferential compression and applies hydrostatic pressure to the upper arm, thereby stimulating fracture healing. It is also vital to begin early shoulder range of motion: pendulum exercises first and then gradually increasing the range of motion as the fracture stabilizes. The functional brace is usually worn for 10–12 weeks, until there is no gross motion at the fracture site and signs of radiographic healing are observed. Serial radiographs are monitored at regular intervals, to verify that satisfactory alignment is maintained and to confirm continued progression toward solid union.
The advantages of nonoperative treatment are the limited risks of nerve injury, wound healing problems, infection, scar formation, or the detrimental effects of anesthesia. The disadvantages are the potential for a prolonged recovery, the risk of nonunion, and the increased probability of shoulder and elbow stiffness. Some fracture patterns (transverse and proximal third spiral) are associated with a greater risk of nonunion.5,7,25 The lack of bridging callus and gross motion of the fracture site at 6 weeks is directly correlated with progression to nonunion.31
3. Primary Open Reduction and Internal Fixation
Most humeral shaft fractures can be conveniently exposed through the anterolateral approach. For this approach, the patient is placed in a semiseated position and with the arm draped so that it can be moved freely and manipulated throughout the case. The radial nerve should be identified distally between the brachialis and brachioradialis and protected throughout the case. The surgeon can then dissect down to the fracture site, debride the hematoma and fracture edges, and obtain a provisional reduction. For certain fracture patterns, provisional fixation with lag screws or positional screws may be indicated before applying the plate. For most humeral shaft fractures, a 4.5-mm compression plate is traditionally recommended for fixation with at least 3 bicortical screws proximal and distal to the fracture site.1,9,13
Although this fixation method is appropriate for many fracture patterns, distal third humeral shaft fractures create a scenario where the standard fixation cannot always be reliably applied. During preoperative planning, if there is not adequate space distal to the fracture to place 3 screws through a 4.5-mm plate, a different approach may be needed. In this case, the patient may be placed in the lateral decubitus position with an arm bolster. The humeral shaft can instead be approached through a posterior paratricipital approach. If the dissection needs to be performed proximally, the radial nerve should be identified by tracing back the posterior brachial cutaneous nerve or by developing the interval between the long and lateral heads of the triceps and finding the nerve in the spiral groove. Again, the surgeon should then dissect down to the fracture site, debride hematoma and early callus to clear the fracture margins, and then obtain a provisional reduction. When using the posterior approach for distal third humeral shaft fractures, dual-column plating can often be used with parallel 3.5-mm anatomic distal humerus plates.1,9,13
4. Postoperative Care After Operative Stabilization
Postoperatively, a soft dressing should be applied that allows for immediate range of motion. In the recovery area, the radial nerve function should be assessed and a thorough neurovascular examination should be performed routinely. If a peripheral nerve block is offered by the anesthesiologist for postoperative pain control, this should be held until after this postoperative neurovascular examination has been completed. Patients may begin immediate range of motion as tolerated on the operative extremity. Patients should also be allowed to weight-bear as tolerated through their operatively treated arm. This is especially important in polytrauma patients with lower extremity injuries that necessitate the use of ambulatory aids including walkers and crutches. Tingstad et al32 reported no difference in secondary surgery, malunion rate, or nonunion rate in patients with plated fractures of the humeral shaft comparing those who were non–weight-bearing versus those who were allowed to immediately weight-bear.
5. Complications of Internal Fixation
In addition to the complications that are standard to any orthopaedic surgical intervention, there are others that are specific to humeral shaft fracture surgery. The most common is radial nerve palsy, which is seen in 7% of patients who had no symptoms of neuropraxia or nerve injury preoperatively.33 However, the risk of radial nerve injury is dependent on the approach used to access the humerus. Claessen et al33 reported that only 4% of patients developed nerve palsy after anterolateral exposure, as compared with 11% of patients with the posterior approach and 20% of patients with the lateral approach. Therefore, the anterolateral approach is generally preferred whenever feasible. If a different approach is necessary based on the fracture pattern or location, locating and protecting the nerve throughout the case is recommended to minimize the risk of iatrogenic injury. Although most radial nerve palsies will recover spontaneously, they should be monitored closely. Shao et al34 demonstrated that 88% of patients with radial nerve palsy will recover with a mean onset of recovery at 7 weeks and a mean resolution of symptoms at 6 months. For those patients who do not show signs of improvement by 2 months postinjury/surgery, obtaining a baseline electromyogram and nerve conduction study (EMG/NCS) is recommended. If there is no sign of clinical or EMG/NCS improvement by 4–6 months, surgical exploration and decompression or repair may be indicated. While nonunion is more common after nonoperative treatment, primary surgical intervention does not eliminate this risk. Recent investigations indicate nonunion rates of 4%–10% with surgical treatment,12,13,17 and the evaluation and management of malunions/nonunions are the final topics to consider.
6. Treatment of Symptomatic Malunions
The purpose of the diaphysis of a long bone is to maintain the spatial relationship of the 2 joints above and below. In the upper limb, this facilitates our ability to position the hand in space and so determines the functional capacity of the involved extremity. In 1966, Klenerman27 first reported that 20 degrees of anterior bowing and 30 degrees of varus were well tolerated by patients with humeral shaft fractures. Now, more than 50 years later, we continue to find that sagittal deformity up to 18 degrees and coronal deformity up to 27 degrees has a minimal effect on outcome.35 However, we also now understand that the magnitude of angulation in the humerus can drastically affect the ability of the hand to reach certain positions relative to the head and trunk, and it is most sensitive to malalignment in the sagittal plane and in valgus.36 Furthermore, patients with a slender body habitus are more vulnerable to cosmetic concerns, particularly with coronal plane deformity.
Therefore, the primary goal in the treatment of symptomatic malunion of the diaphyseal humerus is to restore the anatomical relationship of the shoulder and elbow. This includes not only the cardinal planes of varus-valgus and flexion-extension but also the rotational alignment of the proximal humerus about the distal humeral epicondylar axis.
In the case of malunions, the challenge is not quite as great as with nonunions, because the reason for failure is unrelated to biology and instead reflects the resulting mechanics and alignment. As a result, the solution must focus on correction of these specific aspects. The surgeon must adequately measure the center of the deformity in both cardinal planes and identify the rotational plane. This is best understood with a CT scan of the humerus, comparing the version of the humeral head with the humeral epicondylar axis. The normal anatomic retroversion is approximately 30 degrees from the epicondylar axis, but this can be variable and, if in question, is best compared with the contralateral side.
Correcting malunion through an osteotomy appropriately positioned about both the coronal and sagittal apices of deformity can then be planned. This is usually a long oblique biplanar osteotomy at the level of the deformity itself or a dome osteotomy within the metaphysis. Adequate stability is achieved through the use of plate fixation, with compression achieved either through conventional lag techniques or plate tensioning, depending on the osteotomy orientation.
7. Treatment of Symptomatic Nonunions
The approach to treatment of any nonunion must focus on first identifying and then addressing each of the modifiable etiological variables. Optimizing the patient's medical status, support for smoking cessation, and discontinuing offending medications are among the most important interventions. The surgeon should generate a list of the various possible contributing biological and mechanical factors specific to each case and then develop a preoperative plan that provides solutions to address the responsible features that are identified.
Nonunions that have been previously operated on or are the result of an open fracture should be assumed to be infected until proven sterile. This requires special attention from the surgeon to understand the index injury, amount of bone loss, implants used, and prior surgical approach. The first stage in these patients is to remove the failed implants and to obtain appropriate deep biopsy specimens of the area involved. Presuming infection until proven otherwise always entails obtaining tissue for culture and performing a thorough and aggressive irrigation/debridement of the surgical site, often delivering local antibiotics through polymethylmethacrylate beads. A single debridement can be sufficient; however, plans should be made for as many debridements as required until specimens are sterile and the surgical site has healthy, bleeding tissue. The entire zone of infection and the exposed bone must be adequately debrided, including reaming the remainder of the diaphyseal canal and inserting antibiotic beads or an antibiotic-coated rod when necessary.
In cases of a segmental defect after the debridement of infected or devitalized bone, the options are to shorten the limb up to 5 cm37 or instead to maintain limb length and reconstruct the bone defect with a combination of autogenous and allogenous bone graft.38 Methods of bone grafting include the induced membrane technique with staging of a cancellous bone graft, vascularized transfers, as well as intramedullary and extracortical grafting.
Whereas tension band plating with compression across the nonunion site with autograft is the gold standard treatment of long bone nonunions, other variables certainly come to bear to achieve successful rates of union. These include the use of biologic enhancers, decortication of local bone, and adequate strength of implants. An important principle to consider is that if we generally rely on one size of implant to treat a fracture in healthy bone, then it stands to reason that we need a larger implant and stronger construct to treat a nonunion, given the longer expected time to healing (Figs. 1–4). Furthermore, whereas bridge plating and long working lengths with a low strain environment have a role in the treatment of diaphyseal fractures, these principles are less applicable in nonunions when the surgeon desires a rigid but biologically active environment for healing. Percutaneous strain reduction screws and other reverse dynamization techniques are strategies that can be used to optimize both the mechanical environment and the biological conditions simultaneously.39–41
Figure 1.
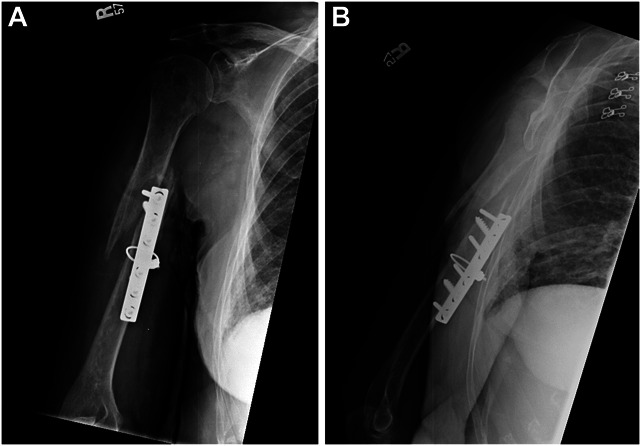
Anteroposterior (A) and lateral (B) radiographs of the humerus in a 76-year-old demented woman who had failed fixation of an acutely treated fracture with ORIF and a cerclage cable. She presented with a nearly functionless, flail extremity and weak wrist extension. She could not assist herself for hygiene, feeding, or basic activities of daily living. Her risk factors included, age, sex, osteoporosis, and malnutrition.
Figure 4.
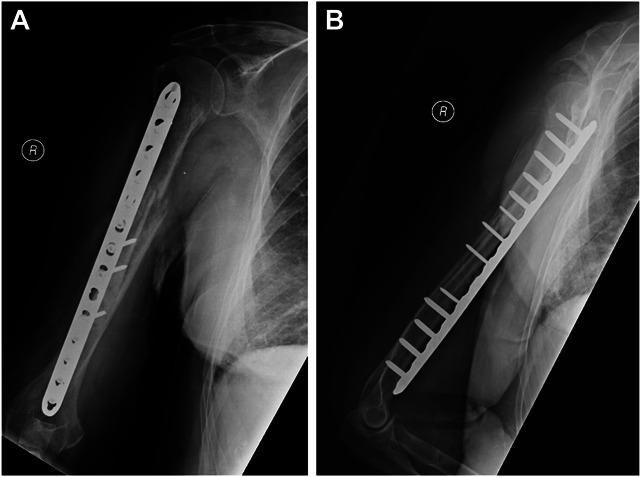
Anteroposterior (A) and lateral (B) radiographs of the humerus confirming that the procedure was successful and the humerus had united 3 months after surgery. She was painless and could reach overhead.
Figure 2.
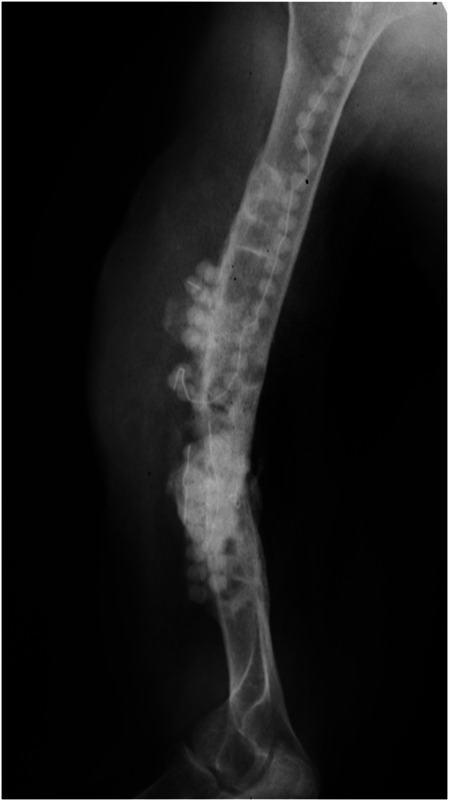
This was treated in a staged fashion, with this radiograph obtained after an initial stage to perform debridement with implant removal, reaming, biopsy, cultures, and placement of PMMA beads to deliver antibiotics locally.
Figure 3.
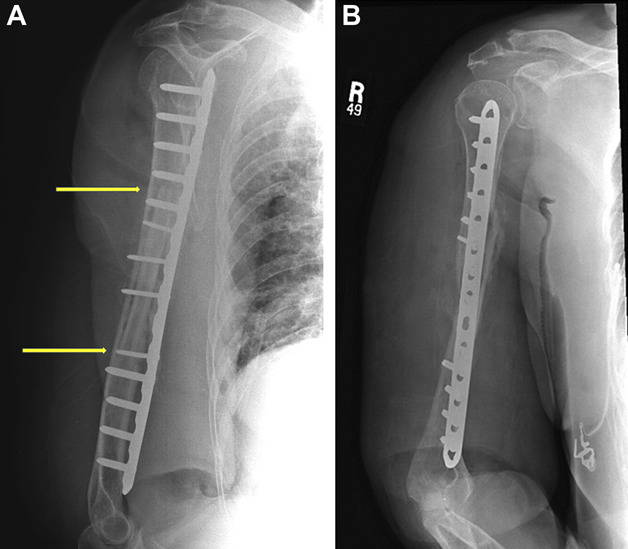
These radiographs, anteroposterior (A) and lateral (B), were obtained after the second stage and definitive stabilization, with solid compression plate fixation to minimize motion. This construct protects nearly the entire diaphysis from refracture and provides compression at the fracture site. Fixation was augmented with an endosteal allograft fibular cortical strut graft spanning the nonunion site (indicated by yellow arrows). Radial nerve exploration revealed that it was entrapped in scar tissue, but was not incarcerated or strangulated by the cable. The second stage included preparation and insertion of the strut graft, followed by compression of the fracture and then compression plating with decortication of the bone locally. Approximately 20 cc of autogenous cancellous bone graft was packed around the nonunion site.
8. Conclusions
Although nonoperative management and functional bracing remain the first choice for the initial treatment of most closed isolated humeral shaft fractures, this still requires skilled care and diligent follow-up. Current evidence suggests that at least one-third of patients initially managed nonoperatively will fail closed treatment, and the burden is on the treating physician to identify patients at risk as early as possible. Operative indications are well-defined, and certain fracture patterns and patient cohorts are known to be at greater risk. Internal fixation through an anterolateral approach is a safe and sensible alternative in those patients for whom operative intervention is preferred. Shared decision making and careful patient selection can help determine which patients will benefit the most. In recognition of our changing appreciation of the rate of nonunion and symptomatic malunion of the humeral diaphysis, a principled approach to diagnosis and treatment becomes increasingly important. There must be adequate respect for all the potential contributing biological and mechanical factors, addressing each of these variables with a comprehensive solution. This is the best strategy to ultimately achieve an uninfected, stable union, restoring function of the arm. Patients who heal from these challenging conditions are often the most appreciative, given they are moving from a useless extremity to a meaningful, functional extremity, restoring their quality of life and eliminating the associated morbidity.
Footnotes
Source of funding: Nil.
Ethical Review Statement: No IRB approval was required for this study.
The authors declare no conflicts of interest.
Contributor Information
Utku Kandemir, Email: utku.kandemir@ucsf.edu.
Emily H. Naclerio, Email: enaclerio516@gmail.com.
Michael D. McKee, Email: michael.mckee@bannerhealth.com.
David J. Weatherby, Email: davidjohnweatherby@gmail.com.
Peter A. Cole, Email: peter.a.cole@healthpartners.com.
Kevin Tetsworth, Email: ktetsworthmd@gmail.com.
References
- 1.Walker M, Palumbo B, Badman B, et al. Humeral shaft fractures: a review. J Shoulder Elbow Surg. 2011;20:833–844. [DOI] [PubMed] [Google Scholar]
- 2.Balfour GW, Mooney V, Ashby ME. Diaphyseal fractures of the humerus treated with a ready-made fracture brace. J Bone Joint Surg Am. 1982;64:11–13. [PubMed] [Google Scholar]
- 3.Zagorski JB, Latta LL, Zych GA, et al. Diaphyseal fractures of the humerus. Treatment with prefabricated braces. J Bone Joint Surg Am. 1988;70:607–610. [PubMed] [Google Scholar]
- 4.Sarmiento A, Horowitch A, Aboulafia A, et al. Functional bracing for comminuted extra-articular fractures of the distal third of the humerus. J Bone Joint Surg Br. 1990;72:283–287. [DOI] [PubMed] [Google Scholar]
- 5.Wallny T, Westermann K, Sagebiel C, et al. Functional treatment of humeral shaft fractures: indications and results. J Orthop Trauma. 1997;11:283–287. [DOI] [PubMed] [Google Scholar]
- 6.Sarmiento A, Zagorski JB, Zych GA, et al. Functional bracing for the treatment of fractures of the humeral diaphysis. J Bone Joint Surg Am. 2000;82:478–486. [DOI] [PubMed] [Google Scholar]
- 7.Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11:143–150. [DOI] [PubMed] [Google Scholar]
- 8.Dielwart C, Harmer L, Thompson J, et al. Management of closed diaphyseal humerus fractures in patients with injury severity score ≥17. J Orthop Trauma. 2017; 31:220–224. [DOI] [PubMed] [Google Scholar]
- 9.McCormack RG, Brien D, Buckley RE, et al. Fixation of fractures of the shaft of the humerus by dynamic compression plate or intramedullary nail. A prospective, randomised trial. J Bone Joint Surg Br. 2000;82:336–339. [DOI] [PubMed] [Google Scholar]
- 10.Chapman JR, Henley MB, Agel J, et al. 2000. Randomized prospective study of humeral shaft fracture fixation: intramedullary nails versus plates. J Orthop Trauma. 14: 162–166. [DOI] [PubMed] [Google Scholar]
- 11.Ekholm R, Adami J, Tidermark J, et al. Fractures of the shaft of the humerus. An epidemiological study of 401 fractures. J Bone Joint Surg Br. 2006;88-A:1469–1473. [DOI] [PubMed] [Google Scholar]
- 12.Harkin FE, Large RJ: Humeral shaft fractures: union outcomes in a large cohort. J Shoulder Elbow Surg. 2017;26:1881–1888. [DOI] [PubMed] [Google Scholar]
- 13.Westrick E, Hamilton B, Toogood P, et al. Humeral shaft fractures: results of operative and non-operative treatment. Int Orthop. 2017;41:385–395. [DOI] [PubMed] [Google Scholar]
- 14.Jawa A, McCarty P, Doornberg J, et al. Extra-articular distal-third diaphyseal fractures of the humerus. A comparison of functional bracing and plate fixation. J Bone Joint Surg Am. 2006;88-A:2343–2347. [DOI] [PubMed] [Google Scholar]
- 23.Olson JJ, Entezari V, Vallier HA. Risk factors for nonunion after traumatic humeral shaft fractures in adults. JSES Int. 2020; 4:734–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberg G, Otremski H, Segev E, et al. Rethinking conservative treatment of humeral diaphyseal fractures in elderly patients with dementia. J Orthop Trauma 2022;36:634–638. [DOI] [PubMed] [Google Scholar]
- 25.Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20:597–601. [DOI] [PubMed] [Google Scholar]
- 26.Zura R, Mehta S, Della Rocca GJ, et al. Biological risk factors for nonunion of bone fracture. JBJF Rev. 2016; 4:e5. [DOI] [PubMed] [Google Scholar]
- 15.Rangan A, Handoll H, Brealey S, et al. , PROFHER Trial Collaborators. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. JAMA. 2015;313:1037–1047. [DOI] [PubMed] [Google Scholar]
- 16.Toivanen JAK, Nieminen J, Laine HJ, et al. Functional treatment of closed humeral shaft fractures. Int Orthop. 2005;29:10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denard A, Richards JE, Obremskey WT, et al. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33:16. [DOI] [PubMed] [Google Scholar]
- 18.Schoch BS, Padegimas EM, Maltenfort M, et al. Humeral shaft fractures: national trends in management. J Orthop Traumatol. 2017;18:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramo L, Sumrein BO, Lepola V, et al. FISH investigators: effect of surgery vs functional bracing on functional outcome among patients with closed displaced humeral shaft fractures: the FISH Randomized Clinical Trial. JAMA. 2020;323:1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serrano R, Mir HR, Sagi HC, et al. Modern results of functional bracing of humeral shaft fractures: a multicenter retrospective analysis. J Orthop Trauma. 2020;34:206–209. [DOI] [PubMed] [Google Scholar]
- 21.Ali E, Griffiths D, Obi N, et al. Nonoperative treatment of humeral shaft fractures revisited. J Shoulder Elbow Surg. 2015;24:210–214. [DOI] [PubMed] [Google Scholar]
- 22.Ring D, Chin K, Taghinia AH, et al. Nonunion after functional brace treatment of diaphyseal humerus fractures. J Trauma. 2007;62:1157–1158. [DOI] [PubMed] [Google Scholar]
- 27.Klenerman L. Fractures of the shaft of the humerus. J Bone Joint Surg Br. 1966;48:105–111. [PubMed] [Google Scholar]
- 28.Matsunaga FT, Tamaoki MJ, Matsumoto MH, et al. Minimally invasive osteosynthesis with a bridge plate versus a functional brace for humeral shaft fractures: a randomized controlled trial. J Bone Joint Surg Am. 2017;99:583–592. [DOI] [PubMed] [Google Scholar]
- 29.Neuhaus V, Menendez M, Kurylo JC, et al. Risk factors for fracture mobility six weeks after initiation of brace treatment of mid-diaphyseal humeral fractures. J Bone Joint Surg Am. 2014;96:403. [DOI] [PubMed] [Google Scholar]
- 30.Zehms CT, Balsamo L, Dunbar R. Coaptation splinting for humeral shaft fractures in adults and children: a modified method. Am J Orthop. 2006;35:452–454. [PubMed] [Google Scholar]
- 31.Driesman AS, Fisher N, Karia R, et al. Fracture site mobility at 6 weeks after humeral shaft fracture predicts nonunion without surgery. J Orthop Trauma. 2017;31:657–662. [DOI] [PubMed] [Google Scholar]
- 32.Tingstad EM, Wolinsky PR, Shyr Y, et al. Effect of immediate weightbearing on plated fractures of the humeral shaft. J Trauma. 2000;49:278–280. [DOI] [PubMed] [Google Scholar]
- 33.Claessen FMAP, Peters RM, Verbeek DO, et al. Factors associated with radial nerve palsy after operative treatment of diaphyseal humeral shaft fractures. J Shoulder Elbow Surg. 2015;24:307–311. [DOI] [PubMed] [Google Scholar]
- 34.Shao YC, Harwood P, Grotz MRW, et al. Radial nerve palsy associated with fracture of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87:1647–1652. [DOI] [PubMed] [Google Scholar]
- 35.Shields E, Sundem L, Childs S, et al. The impact of residual angulation on patient reported functional outcome scores after non-operative treatment for humeral shaft fractures. Injury. 2016;47:914–918. [DOI] [PubMed] [Google Scholar]
- 36.Crespo AM, Konda SR, De Paolis A, et al. Posttraumatic malalignment of the humeral shaft: challenging the existing paradigm. J Orthop Trauma. 2016;30:e48–e52. [DOI] [PubMed] [Google Scholar]
- 37.Brennan ML, Taitsman LA, Barei DP, et al. Shortening osteotomy and compression plating for atrophic humeral nonunions: surgical technique. J Orthop Trauma. 2008;22:643–647. [DOI] [PubMed] [Google Scholar]
- 38.Cole PA. Endosteal allograft plating for the treatment of recalcitrant nonunions, Tech Orthop. 2003;18:344–355. [Google Scholar]
- 39.Bence M, Kothari A, Riddick A, et al. Percutaneous strain reduction screws are a reproducible minimally invasive method to treat long bone nonunion. J Orthop Trauma. 2022;36:e343–e348. [DOI] [PubMed] [Google Scholar]
- 40.Glatt V, Evans CH, Tetsworth K. A concert between biology and biomechanics: the influence of the mechanical environment on bone healing. Front Physiology. 2017;7:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glatt V, Evans CH, Tetsworth K. Reverse dynamisation: a modern perspective on Stephan Perren's strain theory. Eur Cell Mater. 2021;10:41. [DOI] [PubMed] [Google Scholar]


