Abstract
Background
HER2-low has emerged as a new predictive biomarker in metastatic breast cancer. However, its prognostic value in early-stage carcinomas needs to be revisited. We aimed to evaluate the association of HER2-low carcinomas with PAM50 risk groups combined with clinicopathological variables in early breast cancer.
Methods
We conducted a retrospective analysis of 332 patients with early-stage breast cancer that underwent PAM50 signature analysis between 2015 and 2021at Hospital Universitario 12 de Octubre (Madrid, Spain). Clinical and pathological variables were collected from medical records. After adjusting for potential confounders, we estimated Odds Ratio (OR) and 95% confidence interval for high-risk PAM50 subgroup, comparing HER2-low versus HER2-zero carcinomas by multivariable logistic regression. P values below 0.05 were deemed statistically significant.
Results
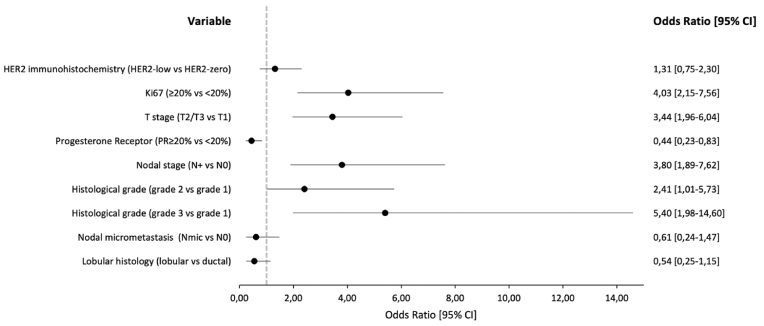
192 (57%) patients were classified as HER2-low carcinomas. Median follow-up was 34 months. Adjusted OR for high-risk PAM50 when comparing HER2-low versus HER2-zero carcinomas was 1.31 (95% CI: 0.75–2.30, p = 0.33). The multivariable model detected significant associations for Ki-67% (≥20% vs. <20%: OR = 4.03, 95% CI: 2.15–7.56, p < 0.001), T staging category (T2/T3 vs. T1: OR = 3.44, 95% CI: 1.96–6.04, p < 0.001), progesterone receptor (PR ≥ 20% vs. <20%: OR = 0.44, 95% CI: 0.23–0.83, p = 0.01), nodal staging category (N+ vs. N0: OR = 3.8, 95% CI: 1.89–7.62, p < 0.001) and histological grade (grade 2 vs. 1: OR = 2.41, 95% CI: 1.01–5.73, p = 0.04; grade 3 vs 1: OR = 5.40, 95%CI: 1.98–14.60, p = 0.001).
Conclusions
In this early-stage breast cancer cohort, HER2-low was not associated with a high-risk PAM50 compared to HER2-zero carcinomas. Ki-67 ≥ 20%, T2/T3, histological grade 2/3, N+ and PR<20% were significantly associated to a high-risk PAM50.
Keywords: Breast cancer, HER2-Low, PAM50, Prosigna, HER2-Zero, HER2
Highlights
-
•
HER2-low is not associated with a high-risk PAM50 compared to HER2-zero.
-
•
High-risk PAM50 correlates to high-risk clinical and pathological tumor characteristics.
-
•
HER2-low has a predictive role in advanced BC, although its prognostic value is controversial.
-
•
HER2-low does not seem to be a distinct biological subtype in early breast cancer.
1. Introduction
HER2-positive breast cancer (BC) is defined as the presence of HER2 3+ by immunohistochemistry (IHC) or IHC 2+ and ErbB2 genetic amplification by in situ hybridization (ISH) [1]. Recently, a new nomenclature has been proposed for those tumors typically defined as HER2 negative, but with an IHC assay reported as 1+ or 2+, with negative ISH, that has been categorized as HER2-low carcinomas [2]. Multiple studies focused on both early and metastatic BC have sought to define the prognostic value of this potential biomarker by comparing it with other clinical and pathological prognostic features; however, the evidence obtained has often been controversial and inconsistent [[3], [4], [5]]. The predictive capacity of HER2-low expression has also been widely explored and, contrary to its prognostic results, it has shown promising results in terms of efficacy of the new antibody-drug conjugate (ADC) Trastuzumab-Deruxtecan, even in the absence of HER2 expression by IHC [[6], [7], [8]].
BC intrinsic subtyping, defined as the classification of tumors based on different gene expression profiles, has emerged as a biomarker with clinical, prognostic and predictive implications. In this sense, genomic platforms like Prosigna are currently used as an added analytical tool for predicting prognosis and risk of recurrence (ROR) in early-stage hormone receptor positive (HR+) BC, facilitating counseling and treatment tailoring, based on algorithms combining gene expression and pathological characteristics [[9], [10], [11], [12], [13]]. The clinical validity of these platforms has been explored in different studies, such as the ABCSG-8 trial for Prosigna, which showed in postmenopausal patients with HR+ early BC a distant recurrence-free interval probability at 10 years of up to 96.7% when they were categorized as low risk, added to a significantly better prognosis in terms of ROR at 10 years of the Luminal A compared to the Luminal B intrinsic subtype [12,14,15]. However, few studies have looked for the correlation between HER2 expression levels, especially HER2-low, and gene expression platforms information [16,17]. In our study, we aimed to determine the association of HER2-low expression by IHC and the results in the Prosigna/PAM50 gene expression platform in a cohort of early-stage HR+ BC.
2. Methods
This single-center retrospective observational study included a cohort of patients with early-stage luminal (HR+, HER2-) BC with available Prosigna/PAM50 genetic profile information performed as per clinical practice to guide adjuvant treatment [18,19], at Hospital Universitario 12 de Octubre, Madrid (Spain), between 2015 and 2021. Medical records and pre-specified patient demographic, clinical and pathological information (age, menopausal status, Ki-67%, histological grade and type, hormone receptor status, T and N staging categories) were collected. Ki-67 was evaluated following a one pathology counting method of at least 500 cells stained using a MIB-1 antibody, based on recommendations of The International Ki-67 in Breast Cancer Working Group [20]. A Ki-67 cut-off of 20% was chosen to differentiate a low versus high Ki-67, as a value considering clinical consensus, scientific reports and daily clinical practice [21,22]. Similarly, based on clinical practice guidelines, a progesterone receptor (PR) value of 20% was chosen as a cut-off to differentiate low versus high PR expression [23]. HER2 IHC was determined with a Leica Bond Oracle IHC system and its CB11 anti-HER2 antibody; while ISH was determined using an LSI HER2/CEP17 probe in a Leica HER2 FISH system. HER2 IHC values from pathology reports were recorded. Tumors with IHC 1+ or 2+ with negative ISH were classified as HER2-low, while those with HER2 IHC 0 were categorized as HER2-zero. Risk group (low, intermediate and high), risk of recurrence (ROR) score and intrinsic subtype (Luminal A, Luminal B, HER2-enriched, Basal-like) were retrieved from Prosigna/PAM50 assay. The study was approved by the Institutional Ethics Committee.
Statistical analysis was carried out with STATA v16.1 software. Data were collected in contingency tables and were classified and summarized as continuous or categorical variables as appropriate. The mean and standard deviation (SD) of continuous variables were estimated. Chi-2 test was used for categorical variable comparisons between the HER2-low and HER2-zero categories. After adjusting for potential confounding variables in a multivariable logistic regression model, we estimated the odds ratio (OR) and 95% confidence intervals of high-risk PAM50 subgroup, comparing HER2-low versus HER2-zero categories. Clinical and pathological information (i.e., Ki-67%, T and N staging categories, progesterone receptor expression, histological grade) were also included as independent variables in the regression model. Finally, those results with p < 0.05 were deemed as statistically significant.
3. Results
The study cohort comprised a total of 332 patients, of which 192 (57%) were categorized as HER2-low and 140 (43%) as HER2-zero. The mean age was 57 years. The median follow-up was 34 months (2–75 months). In the HER2-low category, the predominant intrinsic subtype was Luminal A with 114 (60%) patients, followed by Luminal B with 73 (38.4%) patients, with a very low proportion of HER2-Enriched (HER2-E) (1%) and Basal-like subtypes (0.6%); Similar trends and proportions of intrinsic subtypes were found for the HER2-zero group (Table 1). The adjusted OR for high-risk PAM50 subgroup for HER2-low category compared to HER2-zero was 1.33 (95% CI: 0.75–2.30, p = 0.33) (Fig. 1). On the other hand, multivariable analysis showed significant associations of high-risk PAM50 subgroup with Ki-67% (≥20% vs. <20%: OR = 4.03, 95% CI: 2.15–7.56, p < 0.001), T staging category (T2/T3 vs. T1: OR = 3.44, 95% CI: 1.96–6.04, p < 0.001), progesterone receptor expression (PR ≥ 20% vs. <20%: OR = 0.44, 95% CI: 0.23–0.83, p = 0.01), nodal staging category (N+ vs. N0: OR = 3.8, 95% CI: 1.89–7.62, p < 0.001) and histological grade (grade 2 vs. 1: OR = 2.41, 95% CI: 1.01–5.73, p = 0.04; grade 3 vs. 1: OR = 5.40, 95% CI: 1.98–14.60, p = 0.001) (Fig. 1). There were no associations between high-risk PAM50 subgroup and nodal micrometastasis, neither lobular histology. Finally, median ROR was similar for HER2-low (44) and HER2-zero (45) categories.
Table 1.
Main clinical and pathological characteristics studied.
| HER2-low (n = 192) | HER2-zero (n = 140) | |
|---|---|---|
| Age, years (SD) | 57 (14) | 58 (14) |
| PAM50 subtype, n (%) | ||
| Luminal A | 114 (60.0) | 78 (55.7) |
| Luminal B | 73 (38.4) | 59 (42.1) |
| HER2-enriched | 2 (1.0) | 2 (1.4) |
| Basal-like | 1 (0.6) | 1 (0.8) |
| Risk group, n (%) | ||
| Low | 56 (29.5) | 37 (26.4) |
| Intermediate | 62 (32.6) | 55 (39.3) |
| High | 72 (37.9) | 48 (34.3) |
| ROR, mean (SD) | 44 (21) | 45 (19) |
| Ki-67, n (%) | ||
| Low (<20%) | 96 (51.0) | 59 (42.5) |
| High (≥20%) | 92 (49.0) | 80 (57.5) |
| PR, n (%) | ||
| <20% | 59 (30.7) | 30 (21.6) |
| ≥20% | 133 (69.3) | 109 (78.4) |
| Histology, n (%) | ||
| Ductal | 144 (75.8) | 108 (77.1) |
| Lobular | 40 (21.0) | 25 (17.9) |
| Others | 6 (3.2) | 7 (5.0) |
| Histological Grade, n (%) | ||
| Low/1 | 32 (17.0) | 24 (17.4) |
| Intermediate/2 | 116 (61.7) | 84 (60.9) |
| High/3 | 40 (21.3) | 30 (21.7) |
| T stage, n (%) | ||
| T1 | 109 (56.8) | 82 (58.6) |
| T2 | 77 (40.1) | 52 (37.1) |
| T3 | 6 (3.1) | 6 (4.3) |
| N stage, n (%) | ||
| N0 | 113 (58.9) | 83 (59.3) |
| Nmic | 29 (15.1) | 21 (15.0) |
| N+ | 50 (26.0) | 36 (25.7) |
| Menopausal status, n (%) | ||
| Premenopausal | 56 (29.5) | 43 (31.8) |
| Postmenopausal | 135 (70.5) | 96 (68.2) |
Fig. 1.
Multivariable adjusted Odd Ratios of High-Risk PAM50 subgroup.
4. Discussion
Our study analyzed the clinical and pathological characteristics of HER2-low and its correlation with gene expression signature Prosigna/PAM50 in an early BC cohort. We found no significant association between a high-risk PAM50 subgroup and HER2-low expression in patients with early-stage luminal BC (OR 1.31, 95% CI: 0.75–2.30, p = 0.33), however, there was a quantitative trend toward a high-risk PAM50 in HER2-low tumors. On the other hand, irrespective of HER-2 status, the multivariable analysis found a correlation between high-risk clinical and pathological tumor characteristics (i.e., Ki-67 ≥ 20%, T2/T3, histological grade 2–3, N+ and progesterone receptor expression <20%) with a high-risk Prosigna/PAM50.
Similarly, other studies focused on the prognostic role of HER2-low have shown conflicting results. A study by Won et al. focused on localized disease showed a significant correlation between HER2-low and lower tumor staging categories (T) (p = 0.041), higher histological grades (p = 0.01), as well as a non-significant trend towards lower nodal stages (N) (p = 0.213) in HR+ tumors. The same analysis found no differences in OS between HER2-low and HER2-zero (p = 0.086) [3]. Another study by Tan et al. demonstrated an improvement in terms of relapse-free interval (RFS) (HR: 0.88, 95% CI: 0.82–0.93, p = 0.001) and OS (HR: 0.82, 95% CI: 0.76–0.89, p = 0.001) in HER2-low compared to HER2-zero, independent of the HR status [5]. On the other hand, Denkert et al., found that HER2-low tumors presented a lower pathological complete response (PCR) compared to HER2-zero HR+ tumors (17.5% vs. 23.6%, p = 0.024), consistent with less aggressive clinical and pathological features (lower histological grade, nodal stage and Ki-67%). However, the same study demonstrated higher disease-free intervals (DFS) and OS at 3-year cutoffs in HER2-low compared to HER2-zero (83.4% vs. 76.1%; p = 0.0084 and 91.6% vs. 85.8%; p = 0.0016, respectively) in the general cohort, which did not extrapolate to the HR+ cohort [4]. A descriptive study by Schettini et al., correlated HER2-low tumors with high-risk clinical and pathological features, including larger tumor size (T) (p = 0.007), greater lymph node involvement (N) (p = 0.01) and higher histological grade (p = 0.049). The same study analyzed PAM50 intrinsic subtypes and their correlation with HER2 expression, showing that luminal tumors were more frequent in HER2-low 2+, HER2-low and HR+ tumors, while HER2-E and basal-like intrinsic subtypes were more common in HER2-zero and triple-negative tumors. Likewise, in the case of HR+ tumors, luminal A intrinsic subtype was more represented than luminal B when comparing HER2-low (58.9% vs. 2.8%) versus HER2-zero (8% vs. 34.9%) [24]. Agostinetto et al. sought to characterize the molecular profile of HER2-low tumors and their relationship with the PAM50 intrinsic subtype. They found that HER2-low tumors were represented by a higher proportion of intrinsic HER2-E regardless of their HR status. Similarly, the HER2-low/HR+ subtype was the most represented among luminal A and B tumors. The study found no significant differences in survival intervals when comparing HER2-low with other non-HER2-low subtypes. However, it detected significant differences in DFS and progression free survival (PFS) between HER2-low HR+ tumors and their HR- counterparts, attributed to a higher expression of luminal-related genes in the former [25].
While these and other studies focus on the correlation of HER2-low with pathological aggressiveness features and survival intervals, our study and others, have studied the prognostic value of HER2-low by correlating it with high-risk profiles of gene expression platforms. In this line, with similar objectives but a different methodology to ours, the study by Mutai et al. sought to determine the prognostic potential of HER2-low in localized BC in terms of clinical characteristics, survival intervals, and its correlation with the prognostic information of the Oncotype DX genetic signature. The study found no differences between clinical aggressiveness features (i.e., N, histological grade, Ki-67%) or survival intervals (OS at 10 years of 91% vs. 88%, p = 0.10; DFS at 10 years of 87% vs. 82%, p = 0.09) of HER2-low compared to HER2-zero. On the other hand, when accounting for the risk assigned by Oncotype DX, there were no differences in OS, DFS, or distant disease free survival (DDFS) in patients with low genomic risk; however, in the high genomic risk subgroup, there was a significant improvement in OS (89% vs. 68%, p = 0.01) and DDFS (86% vs. 59%, p = 0.002) in HER2-low versus HER2-zero tumors [16]. Another study, also focused on Oncotype DX recurrence score (RS), found no significant correlation between RS and the survival outcome in HER2-low patients, unlike HER2-zero patients. The authors also evaluated the prognostic role of different RS gene modules in HER2-low and HER2-zero patients, and found that proliferation-related genes could not predict survival outcomes in HER2-low patients, compared to the HER2-zero counterpart. However, the HER2-low subgroup showed an increased HER2 module genes expression compared to HER2-zero, which predicted a worse DFS in the former group (HR: 1.89, 95% CI: 1.02–3.48, p = 0.04) [17]. Compared to these, although there was a quantitative trend, our study did not find any statistically significant correlation between HER2-low and a high-risk profile for the Prosigna/PAM50 gene expression signature. Possible explanations for these contrasting results could be given by the inter-assay heterogeneity and characteristics of different gene signature platforms [[26], [27], [28]], the lower number of participants studied in our analysis, the biological differences of the different populations taken into account on each study [[29], [30], [31]] and finally, our analysis methodology of a direct comparison between HER2-low status and the genetic signature results not taking survival intervals into account.
HER2-low breast carcinomas have a biological crosstalk between estrogen receptor (ER) and HER2. This correlates to an increased ER protein expression [17,32], increased expression of luminal-related genes (e.g., BCL2, BAG1, FOXA1, ESR1, PGR, GPR160, and AR), as well as a higher proportion of luminal A and B PAM50 signatures in HER2-low compared to HER2-zero tumors [24]. These leads to the hypothesis that hormone receptor and luminal-related genes are key determinants and dominate the underlying biology of HER2-low tumors, and that such interaction between both the HR and HER2 pathway may end up promoting HER2/ErbB2 protein overexpression with consequently increased proliferation, angiogenesis and invasiveness properties in HER2-low tumors, as a mechanism of tumor adaptation, treatment resistance and endocrine therapy disruption [2,17,33]. This ER-driven HER2 expression has been described by several authors who have shown that ErbB2 expression is higher in the HER2-low/HR+ than in the HER2-low/HR- subgroup [24,25], and has been validated in other studies like the one by Phinel et al., who showed that in HER2 negative tumors (low and zero), there was a significant positive correlation between HER2 RNA levels and ER status (r = 0.43, p < 0.0001), being 1.75-fold higher in ER+ versus ER- tumors [32]. In clinical practice, these could translate into a more aggressive tumor behavior, with HER2-low tumors often associated with lymph node positivity, ductal histology, higher tumor grading and higher proliferation characteristics [34].
This study has some limitations that should be taken into account. First, this is a study in which the Prosigna/PAM50 genetic signature was performed in a merely clinical context to define prognosis, ROR, and to tailor and define the need of adjuvant chemotherapy treatment in localized HR+ tumors, so other tumor subgroups (i.e., triple-negative BC) are not represented in our sample and have not been included in the analysis, unlike other published studies. Second, Prosigna was performed in HR+ tumors, as per clinical and expert recommendations indications, so very low-risk and high-risk stages may not be properly represented. Third, this is a retrospective study with a relatively small sample size compared to other studies with similar context and objectives, which could justify our central hypothesis's lack of statistical power. Fourth, as a retrospective study, we did not review the pathology specimens but only relied on the pathology report of HER2-IHC. This entails significance, given the importance of correctly differentiating a HER2-zero IHC from a HER2-low, after interobserver, laboratory and technique variability could derive in discordant results and labeling, with some studies showing low concordance between HER2 IHC scores 0 and 1+ [35,36]. This is relevant, especially in cohorts like ours, going back before the HER2-low era when a correct distinction between HER2-zero and HER2-low did not confer any known clinical implications. Finally, although diagnosis, recurrence and death dates were collected, calculations and comparisons of DFS and OS intervals were not possible, given the low relapse rate in the study sample.
Our study has some strengths to be highlighted. To our best knowledge, this is the first study that seeks to define the prognostic value of HER2-low, contrasting it with the information of a different genetic signature recommended by American and European guidelines, the Prosigna/PAM50 [18,23,37]. On the other hand, although having a smaller sample size compared to other studies contrasting genomic platforms, we were able to collect a representative patient cohort that allowed us to perform a multivariate analysis with statistically significant associations of a high-risk PAM50 adjusted to the HER2 status, which represents another study strength.
In conclusion, in our cohort HER2-low status was not associated with the high-risk PAM50 subgroup in early-stage luminal BC. Classical clinical and pathological features were statistically associated with the PAM50 high-risk category. Our results do not support the use of HER2-low as a prognostic biomarker in early luminal BC.
Conflicts of interest
PT: declares consulting and advisory fees from AstraZeneca, Daiichi-Sankyo, Novartis and Seagen, also declares honoraria for non-CME services received directly from commercial interest from Pfizer, Novartis, Lilly, Astra Zeneca, Daiichi-Sankyo and Seagen, outside of the submitted work. AM: declares honoraria from Astra Zeneca, Glaxo Smith Kline, PharmaMar and Clovis, outside of the submitted work. LM: declares honoraria and advisory and consultancy fees, as well as travel and accommodation expenses from AstraZeneca and Gilead, outside of the submitted work. EC: declares honoraria from AstraZeneca, Lilly, Pfizer, Daiichi-Sankyo, as well as travel and accommodation expenses from Pfizer and Roche, outside of the submitted work. RSB: declares honoraria from Novartis, AstraZeneca, MSD, Lilly, GSK, Clovis, Seagen and Pfizer outside of the submitted work. ST, MA, MRC, LL and YR: declare no conflicts of interest.
Author contributions
ST: Conceived and designed the analysis, collected data and wrote the manuscript; RSB: Conceived and designed the analysis, collected data, performed data analysis and interpretation, and reviewed the manuscript; MA, PT, MRC: Conceived and designed the analysis, collected data, and reviewed the manuscript; LL, YR, LM, EC: Conceived and designed the analysis, reviewed the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
N/A.
References
- 1.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Orthod. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 2.Tarantino P., Hamilton E., Tolaney S.M., Cortes J., Morganti S., Ferraro E., et al. HER2-Low breast cancer: pathological and clinical landscape. J Clin Orthod. 2020;38:1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 3.Won H.S., Ahn J., Kim Y., Kim J.S., Song J.-Y., Kim H.-K., et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022;24:22. doi: 10.1186/s13058-022-01519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denkert C., Seither F., Schneeweiss A., Link T., Blohmer J.-U., Just M., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22:1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 5.Tan R.S.Y.C., Ong W.S., Lee K.-H., Lim A.H., Park S., Park Y.H., et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022;20:105. doi: 10.1186/s12916-022-02284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modi S., Park H., Murthy R.K., Iwata H., Tamura K., Tsurutani J., et al. Antitumor activity and safety of Trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: results from a phase ib study. J Clin Orthod. 2020;38:1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022 doi: 10.1056/NEJMoa2203690. NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortés J., Kim S.-B., Chung W.-P., Im S.-A., Park Y.H., Hegg R., et al. Trastuzumab deruxtecan versus Trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 9.Prat A., Pineda E., Adamo B., Galván P., Fernández A., Gaba L., et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Jensen M.-B., Lænkholm A.-V., Nielsen T.O., Eriksen J.O., Wehn P., Hood T., et al. The Prosigna gene expression assay and responsiveness to adjuvant cyclophosphamide-based chemotherapy in premenopausal high-risk patients with breast cancer. Breast Cancer Res. 2018;20:79. doi: 10.1186/s13058-018-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lænkholm A.-V., Jensen M.-B., Eriksen J.O., Roslind A., Buckingham W., Ferree S., et al. Population-based study of prosigna-PAM50 and outcome among postmenopausal women with estrogen receptor-positive and HER2-negative operable invasive lobular or ductal breast cancer. Clin Breast Cancer. 2020;20:e423–e432. doi: 10.1016/j.clbc.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Vieira A.F., Schmitt F. An update on breast cancer multigene prognostic tests—emergent clinical biomarkers. Front Med. 2018;5:248. doi: 10.3389/fmed.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puppe J., Seifert T., Eichler C., Pilch H., Mallmann P., Malter W. Genomic signatures in luminal breast cancer. Breast Care. 2020;15:355–365. doi: 10.1159/000509846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lal S., McCart Reed A.E., de Luca X.M., Simpson P.T. Molecular signatures in breast cancer. Methods. 2017;131:135–146. doi: 10.1016/j.ymeth.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Gnant M., Filipits M., Greil R., Stoeger H., Rudas M., Bago-Horvath Z., et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]
- 16.Mutai R., Barkan T., Moore A., Sarfaty M., Shochat T., Yerushalmi R., et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021;60:62–69. doi: 10.1016/j.breast.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M., Chen W., Liu D., Chen W., Shen K., Wu J., et al. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: features of HER2-low breast cancer. Breast Cancer. 2022;29:844–853. doi: 10.1007/s12282-022-01364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andre F., Ismaila N., Henry N.L., Somerfield M.R., Bast R.C., Barlow W., et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update—integration of results from TAILORx. J Clin Orthod. 2019;37:1956–1964. doi: 10.1200/JCO.19.00945. [DOI] [PubMed] [Google Scholar]
- 19.National comprehensive cancer network. NCCN Breast Cancer guidelines; 2022. [n.d] [Google Scholar]
- 20.Dowsett M., Nielsen T.O., A'Hern R., Bartlett J., Coombes R.C., Cuzick J., et al. Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. JNCI Journal of the National Cancer Institute. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarantino P., Burstein H.J., Lin N.U., Krop I.E., Winer E.P., Schnitt S.J., et al. Should Ki-67 be adopted to select breast cancer patients for treatment with adjuvant abemaciclib? Ann Oncol. 2022;33:234–238. doi: 10.1016/j.annonc.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Burstein H.J., Curigliano G., Thürlimann B., Weber W.P., Poortmans P., Regan M.M., et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32:1216–1235. doi: 10.1016/j.annonc.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 24.Schettini F., Chic N., Brasó-Maristany F., Paré L., Pascual T., Conte B., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. Npj Breast Cancer. 2021;7:1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agostinetto E., Rediti M., Fimereli D., Debien V., Piccart M., Aftimos P., et al. HER2-Low breast cancer: molecular characteristics and prognosis. Cancers. 2021;13:2824. doi: 10.3390/cancers13112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartlett J.M.S., Bayani J., Marshall A., Dunn J.A., Campbell A., Cunningham C., et al. Comparing breast cancer multiparameter tests in the OPTIMA prelim trial: No test is more equal than the others. JNCI J Natl Cancer Inst. 2016;108:djw050. doi: 10.1093/jnci/djw050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowsett M., Sestak I., Lopez-Knowles E., Sidhu K., Dunbier A.K., Cowens J.W., et al. Comparison of PAM50 risk of recurrence score with onco type DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Orthod. 2013;31:2783–2790. doi: 10.1200/JCO.2012.46.1558. [DOI] [PubMed] [Google Scholar]
- 28.Sestak I., Buus R., Cuzick J., Dubsky P., Kronenwett R., Denkert C., et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor–positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:545. doi: 10.1001/jamaoncol.2017.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holowatyj A.N., Cote M.L., Ruterbusch J.J., Ghanem K., Schwartz A.G., Vigneau F.D., et al. Racial differences in 21-gene recurrence scores among patients with hormone receptor–positive, node-negative breast cancer. J Clin Orthod. 2018;36:652–658. doi: 10.1200/JCO.2017.74.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoskins K.F., Danciu O.C., Ko N.Y., Calip G.S. Association of race/ethnicity and the 21-gene recurrence score with breast cancer–specific mortality among US women. JAMA Oncol. 2021;7:370. doi: 10.1001/jamaoncol.2020.7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J.-W., Zabidi M.M.A., Ng P.-S., Meng M.-Y., Hasan S.N., Sandey B., et al. The molecular landscape of Asian breast cancers reveals clinically relevant population-specific differences. Nat Commun. 2020;11:6433. doi: 10.1038/s41467-020-20173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinhel I., Hills M., Drury S., Salter J., Sumo G., A'Hern R., et al. ER and HER2 expression are positively correlated in HER2 non-overexpressing breast cancer. Breast Cancer Res. 2012;14:R46. doi: 10.1186/bcr3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godoy-Ortiz A., Sanchez-Muñoz A., Chica Parrado M.R., Álvarez M., Ribelles N., Rueda Dominguez A., et al. Deciphering HER2 breast cancer disease: biological and clinical implications. Front Oncol. 2019;9:1124. doi: 10.3389/fonc.2019.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hein A., Hartkopf A.D., Emons J., Lux M.P., Volz B., Taran F.-A., et al. Prognostic effect of low-level HER2 expression in patients with clinically negative HER2 status. European Journal of Cancer. 2021;155:1–12. doi: 10.1016/j.ejca.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Sajjadi E., Guerini-Rocco E., De Camilli E., Pala O., Mazzarol G., Venetis K., et al. Pathological identification of HER2-low breast cancer: tips, tricks, and troubleshooting for the optimal test. Front Mol Biosci. 2023;10 doi: 10.3389/fmolb.2023.1176309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez A.I., Liu M., Bellizzi A., Brock J., Fadare O., Hanley K., et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 2022;8:607. doi: 10.1001/jamaoncol.2021.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andre F., Ismaila N., Allison K.H., Barlow W.E., Collyar D.E., Damodaran S., et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Orthod. 2022;40:1816–1837. doi: 10.1200/JCO.22.00069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.