Abstract
Poliovirus infects susceptible cells through the poliovirus receptor (PVR), which functions to bind virus and to change its conformation. These two activities are thought to be necessary for efficient poliovirus infection. How binding and conformation conversion activities contribute to the establishment of poliovirus infection was investigated. Mouse L cells expressing mouse high-affinity Fcγ receptor molecules were established and used to study poliovirus infection mediated by mouse antipoliovirus monoclonal antibodies (MAbs) (immunoglobulin G2a [IgG2a] subtypes) or PVR-IgG2a, a chimeric molecule consisting of the extracellular moiety of PVR and the hinge and Fc portion of mouse IgG2a. The antibodies and PVR-IgG2a showed the same degree of affinity for poliovirus, but the infectivities mediated by these molecules were different. Among the molecules tested, PVR-IgG2a mediated the infection most efficiently, showing 50- to 100-fold-higher efficiency than that attained with the different MAbs. A conformational change of poliovirus was induced only by PVR-IgG2a. These results strongly suggested that some specific interaction(s) between poliovirus and the PVR is required for high-level infectivity of poliovirus in this system.
Poliovirus (PV), the causative agent of poliomyelitis, is a human enterovirus belonging to the family Picornaviridae, which includes human rhinovirus and foot-and-mouth disease virus (FMDV). These viruses serve as useful models for studying the uncoating process of nonenveloped viruses. A poliovirion consists of a single-stranded RNA genome and a nonenveloped capsid. The precise three-dimensional structure of the virion particle was elucidated from crystallographic studies (18). The nonenveloped capsid consists of 12 pentamers, each of which is composed of five protomers. Each protomer is made up of the three surface proteins, VP1, VP2, and VP3, and the internal protein VP4. Structural investigations suggest that each protomer carries a single attachment site for the PV receptor (PVR) molecule, termed a canyon (41); five protomers for each of 12 pentamers equals 60 PVR binding-site canyons per virion.
PVR, a member of the immunoglobulin (Ig) superfamily with unknown natural functions (26, 34), is thought to be the only molecule that is involved in the PV uncoating step, although CD44H, an isoform of the lymphocyte homing receptor, is reported to have a possible association with PVR on the cell surface (4, 9, 46). In the extracellular domains of PVR, the first Ig-like domain is essential for PV binding (27, 44), and amino acid residues important for PV binding were identified in this domain (1, 3, 36). At temperatures above 35°C, the interaction between PV and PVR leads to a conformational change of the virion that manifests itself as altered 135S and 80S particles (2, 23, 32, 52, 54). The 135S particle, sometimes called the A particle, has lost the internal protein VP4 (32), and the 80S particle has lost the RNA genome and VP4. The rate of virion alteration is higher in higher concentrations of the soluble receptor, as has been reported for human rhinovirus and PV in vitro (2, 19). This process is not dependent on an acidic environment (12, 52), nor is PV infectivity blocked by bafilomycin A1, suggesting that PV infection proceeds independently of an acidic environment in cultured cells (38).
The importance of the 135S particle in the uncoating process has been emphasized by using several experimental approaches: through measurement of the kinetics of virion alteration and release of genomic RNA (13), through reduction of PV infectivity by some antipicornavirus drugs that prevent conformational alteration of the virion particle (8, 22, 24, 45), because of the affinity of the 135S particle for liposomes (10), and because the 135S particle is infectious for nonpermissive cells (5). These lines of evidence strongly suggest that the 135S particle is an important intermediate in the process of viral uncoating. On the other hand, a strong argument can be made against the importance of the 135S particle. Cold-adapted PV mutants, which can infect and replicate at 25°C, have been isolated (7). Uncoating of these mutants, therefore, must proceed at 25°C, a temperature that would not support the virion conformational alteration of the 135S particle. This observation suggested that some uncoating pathway other than that via the 135S particle may exist. In fact, the 80S particle is not always derived from the 135S particle (2). Thus, the PV uncoating pathway is still controversial with regard to its dependency on the 135S particle route. A high particle-to PFU ratio for PV generally presents an obstacle to studying the uncoating process (42).
In general, PVR binding to PV is accompanied by uncoating activity (1), although a PVR mutant which permits PV type 3 (PV3) binding but not PV infection exists (14). This inability to separate binding from uncoating makes determination of the importance of PVR binding separate from other activities, such as activity that alters virion conformation, somewhat tricky. In this study, we established mouse L cells that express the mouse high-affinity Fcγ receptor (FcRI) and showed that PV infection proceeded in the cells in the presence of anti-PV monoclonal antibodies (MAbs) (IgG2a subtype). Furthermore, PVR-IgG2a, a chimeric molecule consisting of an extracellular moiety of PVR and the hinge and Fc portion of mouse IgG2a, mediates PV infection even more efficiently than those MAbs. Our results suggested that PV binding to the cell surface is the minimum requirement for the establishment of viral infection and that some specific interaction(s) between PV and PVR is required to enhance the efficiency of PV infection.
MATERIALS AND METHODS
Cells and viruses.
Mouse L cells were used for preparation of transformant cell lines, and African green monkey kidney (AGMK) cells were used for plaque assay and preparation of PV. These cells and mouse L-cell transformants, i.e., Lα, LmFcRI, and LpCI-neo cells, were propagated in Dulbecco modified Eagle’s medium (DMEM) supplemented with 5% newborn calf serum (NCS). Lα and LmFcRI cells express human PVRα and the mouse high-affinity Fcγ receptor α subunit (FcRI), respectively. LpCI-neo cells were used as a control for LmFcRI cells and carried only the plasmid vector pCI-neo. These transformant cells were used for virus infection and virus binding assays. Suspension-cultured HeLa S3 cells were grown in RPMI 1640 medium supplemented with 5% NCS and used for preparation of PV. Sf9 cells were propagated as a monolayer or suspension culture in TC-100 medium supplemented with 10% fetal calf serum. For purification of recombinant PVR-IgG2a protein, Sf9 cells infected with recombinant baculovirus were cultured in serum-free medium (EX-CELL 400; JRH Biosciences).
The Mahoney strain of PV1 was recovered from AGMK cells transfected with an RNA transcript of an infectious cDNA clone, PV1(M)OM (47). This PV1 suspension was used as an inoculum to prepare [35S]methionine-labeled or unlabeled PV1 as described previously (2). Recombinant baculovirus designed to express PVR-IgG2a was constructed by using Bac-to-Bac Baculovirus Expression Systems (Gibco BRL).
DNA procedure.
A cDNA of mouse FcRI was prepared from mRNAs of J774 cells by reverse transcription-PCR (RT-PCR) by using SuperScript II reverse transcriptase (Gibco BRL) and EX-Taq polymerase (Pharmacia Biotech) with the sense primer 5′TGGCACGCGTGCCATGATTCTTACCAGCTTTGGAGA3′ and the antisense primer 5′CTCTCCCGGGAGAGTTGCATGCCATGGTCCCACA3′ (the MluI and SmaI sites are underlined). The PCR product, after digestion with MluI and SmaI, was inserted into the corresponding sites of mammalian expression vector pCI-neo (Promega), in which expression of FcRI cDNA was under the control of the cytomegalovirus (CMV) promoter. The resulting plasmid was designated pmFcRI-neo. The nucleotide sequence of the cDNA was coincident with that registered in GenBank. Mouse L cells were transfected with plasmid pmFcRI-neo or pCI-neo by using DOTAP Lipofectin reagent (Boehringer Mannheim), and cells resistant to 0.6 mg of G418 (Sigma) per ml were chosen. Cells carrying pmFcRI-neo and pCI-neo were designated LmFcRI and LpCI-neo cells, respectively.
PVR-IgG2a is a chimeric molecule in which the extracellular moiety of human PVR is joined to the hinge and Fc region of mouse IgG2a. For construction of PVR-IgG2a cDNA, a cDNA of the hinge and Fc region of mouse IgG2a was prepared from hybridoma cells expressing mouse IgG2a by RT-PCR, using the sense primer 5′GCTCCGGATCCGGAGCCCAGAGGGCCCACAAT3′ and the antisense primer 5′ATGATCTAGATCATTTACCCGGAGTCCGGGAG3′ (the BamHI and XbaI sites are underlined). The PCR product was joined to the cDNA of PVR containing all three Ig-like domains (PVR330 in reference 2) at the BamHI site and, after digestion with BglII (at the 5′ side of PVR cDNA) and XbaI (at the 3′ side of IgG2a), cloned into the corresponding site of plasmid vector pCI-neo. The resulting plasmid was treated with BglII and NotI to obtain PVR-IgG2a cDNA, and the cDNA fragment was inserted in the BamHI and NotI sites of plasmid pFastBac of Bac-to-Bac Baculovirus Expression Systems (Gibco BRL). According to the nucleotide sequence of PVR-IgG2a cDNA, five extra amino acid residues, SSPDP, are inserted at the junction of PVR and IgG2a.
Purification.
Crude PVR-IgG2a was prepared from culture supernatants of recombinant baculovirus-infected cells at 70 h postinfection (p.i.) and applied to a protein A-Sepharose 4B column (Pharmacia Biotech). PVR-IgG2a was eluted with 0.1 M sodium (pH 5.0), neutralized by the addition of 1 M Tris-HCl (pH 7.4), desalted over a PD-10 column (Pharmacia Biotech) in phosphate-buffered saline (PBS) (10 mM phosphate buffer [pH 7.0], 137 mM NaCl, and 2.6 mM KCl), and stored at −80°C. The purity of the protein was examined by subjecting PVR-IgG2a samples with or without 1% β-mercaptoethanol to sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis followed by silver staining (Silver Stain II kit; Wako) or Western blot analysis with anti-PVR MAb 5D1 (a generous gift from J. Aoki). About 0.4 mg of purified PVR-IgG2a was obtained from 5 × 108 Sf9 cells infected with the recombinant baculovirus. The concentration of PVR-IgG2a, regarded as a dimer form like an IgG, was determined by measuring the absorbance at 280 nm as described previously (37) and was further confirmed by Western blot analysis with a rabbit anti-mouse IgG Fc antibody (O.E.M. Concepts) followed by quantification with a Molecular Imager (Bio-Rad) as described previously (2).
PV1 was prepared from cytoplasmic extracts of suspension-cultured HeLa S3 cells infected with PV1 at 7 h p.i. and purified as described previously (2). The 135S particle was prepared from purified PV1 in two different ways. One method involved soluble PVR. Purified PV1 particles (3.2 × 10−9 M) were mixed with 1.32 × 10−6 M soluble PVR (PVR330 in reference 2) and incubated at 37°C for 1 h. Conversion of 160S intact PV1 particles to 135S particles was confirmed by sucrose density gradient centrifugation. The other method involved use of a hypotonic buffer containing 20 mM Tris-HCl (pH 7.5), 2 mM CaCl2, and 0.1% Tween 20 (5). Purified PV1 particles in the hypotonic buffer were heated at 50°C for 3 min (5). Complete conversion of the PV1 particles was confirmed as described above. After either of these two treatments, bovine serum albumin (BSA) was added to the mixtures to a final concentration of 1%, and the samples were stored at −80°C.
MAbs.
Mouse anti-PV1 MAbs 7m012, 7m039, Mah 45i, and Mah 49e were used as possible mediators for PV1 infection of LmFcRI cells. All of the MAbs are IgG2a. The first two MAbs are able to bind PV1 but do not neutralize PV1. The latter two neutralize PV1 and have different epitopes. Anti-PVR MAbs p286 and p403 (53), which have the ability to block PV infection, were employed to inhibit conformational change of PV1 by PVR-IgG2a. These MAbs were purified from mouse ascites fluid by the method used for purification of PVR-IgG2a. The concentration of purified IgG2a was determined by measuring absorbance at 280 nm, where 1.35 optical density units was regarded as 1.0 mg of protein (15).
PV infection assay.
PV1 infection mediation activities of anti-PV1 MAbs and PVR-IgG2a were examined by using PV1 at concentrations of 1.3 × 10−12 to 1.3 × 10−10 M (9.6 × 107 to 9.6 × 109 virions) and mediators at concentrations of 1.3 × 10−10 to 1.3 × 10−7 M. The cells (4.0 × 104) were cultured on an eight-chamber glass slide (Nunc). After addition of the mixture of PV1 and anti-PV1 MAb or PVR-IgG2a, the cells were incubated at 37°C for 1 h, washed twice with PBS, and further incubated at 37°C for 7 h in DMEM containing 5% NCS. At 8 h p.i., cells were fixed with 3% paraformaldehyde in PBS and subjected to indirect immunofluorescence study. The infectivity was calculated by counting cells carrying fluorescein isothiocyanate (infected cells) and propidium iodide (total cells) by using NIH Image 1.60.
PV binding assay.
Anti-PV1 MAbs or PVR-IgG2a (6.5 × 10−10 M) was mixed with [35S]methionine-labeled PV1 (adjusted to 1.3 × 10−12 to 1.3 × 10−10 M by the addition of unlabeled PV1) in PBS containing 1% BSA and incubated at 4°C overnight in the presence of 5 μl of protein G-Sepharose FF gel (Pharmacia Biotech) in a total volume of 120 μl. The mixture was centrifuged at 8,200 × g for 10 s, and the supernatant was removed. After two washes with ice-cold PBS, the radioactivity associated with the gel fraction was measured in a liquid scintillation counter.
PV1 binding to LmFcRI cells mediated by anti-PV1 MAbs or PVR-IgG2a was examined as described below. In the presence or absence of anti-PV1 MAbs or PVR-IgG2a, 2 × 105 LmFcRI or LpCI-neo cells were incubated with [35S]methionine-labeled PV1 (3.0 × 10−11 M) at 37°C for 1 h. The cells were washed five times with DMEM containing 5% NCS and then collected by adding 0.2 N NaOH and 1% SDS. The radioactivity associated with the cells was measured in a liquid scintillation counter.
Rosette assay.
Bovine erythrocytes coated with rabbit IgG (Funakoshi) were incubated with LmFcRI or LpCI-neo cells at 37°C for 4 h (6, 43), and the cells were observed for rosette formation with a microscope.
Indirect immunofluorescence.
To detect FcRI molecules on the surfaces of LmFcRI cells, the cells were treated with mouse IgG2a (21 μg/ml) at 4°C for 45 min and then with goat anti-mouse IgG conjugated with fluorescein isothiocyanate as previously described (6). For PV infection assay, the fixed cells were incubated with rabbit anti-PV1 hyperimmune serum (1:100 dilution in PBS containing 1% BSA) at 37°C for 1 h. After three washes with PBS, goat antirabbit antibody conjugated with fluorescein isothiocyanate (1:200 dilution) (MBL) and 5 μg of propidium iodide (Sigma) per ml were added to the cells and further incubated at 37°C for 20 min. The samples were observed with a confocal scanning laser microscope (MRC-1024; Bio-Rad).
Gel filtration.
Purified PVR-IgG2a was analyzed by gel filtration on Superdex 200 PC3.2/30 (Pharmacia Biotech) in PBS with a SMART System (Pharmacia Biotech). A low- and high-molecular-weight gel filtration calibration kit (Pharmacia Biotech) was used to estimate molecular masses according to the instructions of the manufacturer.
Sucrose density gradient centrifugation.
PV1 (8.3 × 104 cpm, adjusted to 1.3 × 10−10 M by the addition of unlabeled PV1) and anti-PV1 MAbs or PVR-IgG2a were incubated with LmFcRI cells at 37°C for 1 h, and then the cell culture supernatants were layered on a 5 to 25% sucrose density gradient and centrifuged at 41,000 rpm for 45 min at 4°C in a Beckman SW55Ti rotor as reported previously (11). The radioactivity in each fraction was measured in a liquid scintillation counter.
RESULTS
Fc receptor-mediated infection of PV.
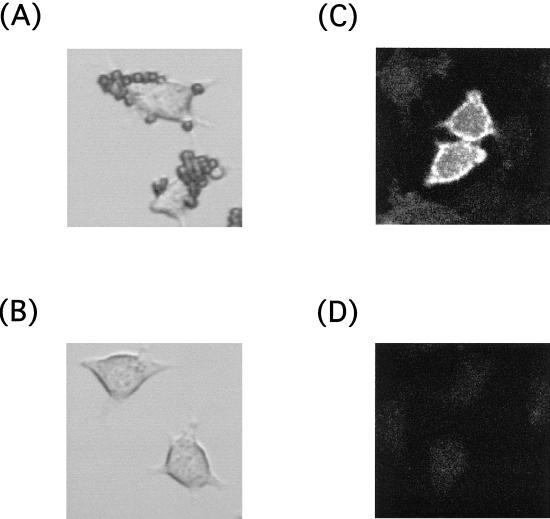
Abortive infection has been reported to result from infection with antibody-complexed PV that is mediated by the low-affinity Fc receptor (33). Here, we examined the high-affinity Fc receptor to determine its contribution to establishment of PV infection. For this purpose, mouse L cells stably expressing mouse FcRI under the control of the CMV promoter were established and designated LmFcRI cells as described in Materials and Methods. The expression of mouse FcRI was confirmed by detection of the mouse FcRI mRNA by RT-PCR (data not shown), by performing a rosette assay with bovine erythrocytes sensitized with rabbit IgG (Fig. 1A and B), and by using indirect immunofluorescence of mouse IgG2a bound to LmFcRI cells (Fig. 1C and D). Rosetting of IgG-sensitized erythrocytes was seen on all of the LmFcRI cells (Fig. 1A) but not on control LpCI-neo cells (Fig. 1B). Similarly, fluorescence was detected only on LmFcRI cells (Fig. 1C) and not on LpCI-neo cells (Fig. 1D). This fluorescence was not visible when mouse IgG2a was omitted (data not shown). These results strongly suggested that FcRI molecules exist on the surfaces of LmFcRI cells.
FIG. 1.
Expression of mouse FcRI on LmFcRI cells. LmFcRI cells (A and C) or LpCI-neo cells (B and D) were analyzed by rosette assay (A and B) or indirect immunofluorescence (C and D) to measure the expression of mouse FcRI on LmFcRI cells, as described in Materials and Methods.
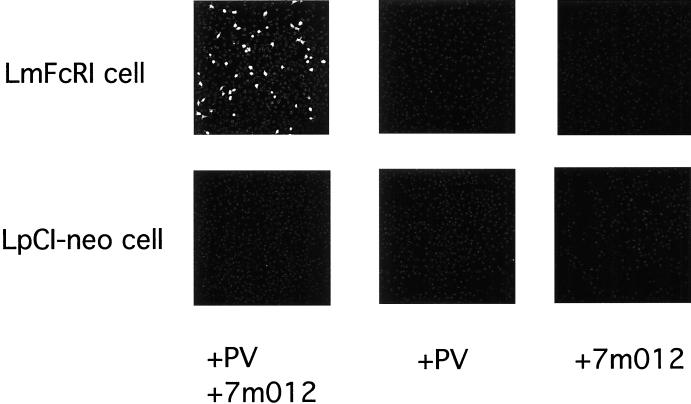
LmFcRI cells or control LpCI-neo cells were challenged with PV1 in the presence or absence of anti-PV1 binding MAb 7m012 (Fig. 2). At 8 h p.i., cells were stained by using rabbit anti-PV1 hyperimmune serum. As shown in Fig. 2, PV1 antigens were detected in LmFcRI cells challenged with PV1 in the presence of MAb 7m012 but not in LpCI-neo cells under the same conditions. PV1 antigen detection appeared to be a result of the presence of MAb 7m012, and infectious PV1 was recovered from LmFcRI cell cultures that showed PV1 antigens (data not shown). Thus, PV1 infection seemed to depend on both the anti-PV1 MAb and the high-affinity Fc receptor.
FIG. 2.
PV1 infection of LmFcRI cells mediated by anti-PV1 MAb. LmFcRI cells or LpCI-neo cells (4.0 × 104 cells) were incubated in the presence of PV1 (9.6 × 109 virions, 1.3 × 10−10 M), anti-PV1 MAb 7m012 (1.3 × 10−8 M), or both, as described in Materials and Methods. Indirect immunofluorescence was performed at 8 h p.i.
Purification of PVR-IgG2a.
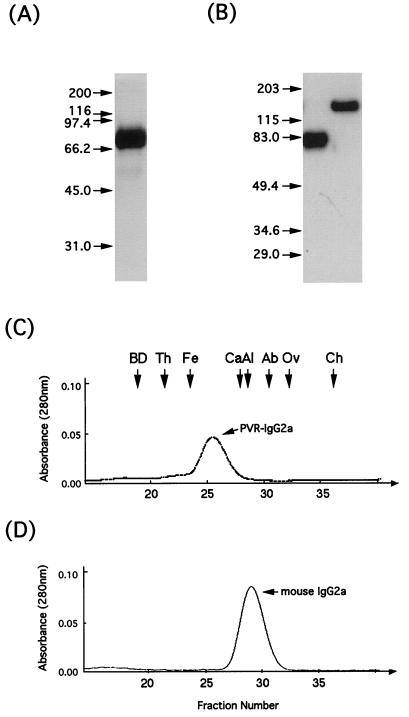
To examine the PVR effect on the efficiency of PV1 infectivity, PVR-IgG2a, a chimera of the extracellular moiety of PVR and the hinge and Fc portion of mouse IgG2a, was prepared as described in Materials and Methods, and its PV1 infection mediation activity was compared with those of anti-PV1 MAbs. The purified extracellular portion of the PVR can change the PV1 conformation from an intact 160S particle to 135S and 80S particles (2). Migration of PVR-IgG2a on SDS-polyacrylamide gels, as visualized by silver staining and Western blotting, occurred as a single band (Fig. 3A). Although the calculated molecular mass of PVR-IgG2a was 60 kDa, PVR-IgG2a migrated to a position of 78 or 145 kDa under reducing or nonreducing conditions, respectively (Fig. 3B). These data indicate that the PVR-IgG2a was highly purified, that the PVR-IgG2a obtained is glycosylated, and that PVR-IgG2a, like IgG, exists as a homodimer form that is linked together by disulfide bonds under nonreducing conditions.
FIG. 3.
Purified recombinant PVR-IgG2a. Purified recombinant PVR-IgG2a was analyzed by polyacrylamide gel electrophoresis followed by silver staining (A) or Western blotting (B) to detect the protein, as described in Materials and Methods. Purified recombinant PVR-IgG2a (C) and, as a control, mouse IgG2a (D) were also analyzed by gel filtration. For Western blot analysis (B), PVR-IgG2a was examined under reducing (left lane) and nonreducing (right lane) conditions. Positions of molecular mass markers (in kilodaltons) are indicated by arrows on the left of panels A and B and at the top of panel C. BD, blue dextran 2000; Th, thyroglobulin (669 kDa); Fe, ferritin (440 kDa); Ca, catalase (232 kDa); Al, aldolase (158 kDa); Ab, albumin (67 kDa); Ov, ovalbumin (43 kDa); Ch, chymotrypsinogen A (25 kDa).
The molecular mass of PVR-IgG2a also was determined by gel filtration (see Materials and Methods). As shown in Fig. 3C, PVR-IgG2a was detected as a single peak at 284 kDa, with a Stoke’s radius of 56.2 Å (Fig. 3C). Because mouse IgG2a, which is a dimer form in PBS, was eluted at 120 kDa (Fig. 3D), it is possible that PVR-IgG2a exists as a tetramer in PBS.
PV1 infection mediated by anti-PV1 MAbs and PVR-IgG2a.
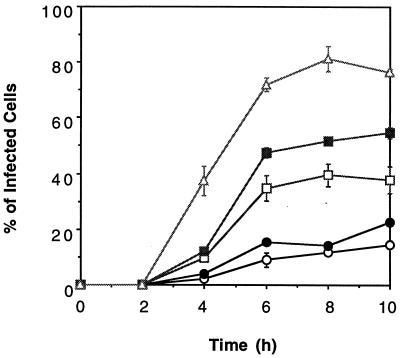
Anti-PV1 binding MAbs (7m012 and 7m039), anti-PV1 neutralizing MAbs (Mah45i and Mah49e), and PVR-IgG2a were used as mediators for establishment of PV1 infection. All of those molecules mediated PV1 infection in LmFcRI cells but not in LpCI-neo cells (data not shown). LmFcRI cells were infected with PV1 in the presence of these mediators under the conditions described in the legend to Fig. 4, and indirect immunofluorescence of the infected LmFcRI cell cultures was observed at various times after infection (Fig. 4). With all of the mediators, the populations of cells that carry the viral antigen were first seen at 4 h p.i. and appeared to reach a plateau at 8 h p.i. (Fig. 4). Accordingly, the PV infection mediation activities of these molecules were compared further at 8 h p.i. as described below.
FIG. 4.
Time course of PV1 infection mediated by anti-PV1 MAbs or PVR-IgG2a. LmFcRI cells (4.0 × 104) were challenged with PV1 (9.6 × 109 virions, 1.3 × 10−10 M) in the presence of anti-PV1 MAb (1.3 × 10−8 M for 7m012 [open circles] and 7m039 [closed circles] and 1.3 × 10−9 M for Mah45i [open squares] and Mah49e [closed squares]) or PVR-IgG2a (6.5 × 10−9 M) (open triangles), and the cells were fixed at the times indicated, followed by indirect immunofluorescence as described in Materials and Methods. The data represent means from three independent experiments, and error bars indicate standard deviations.
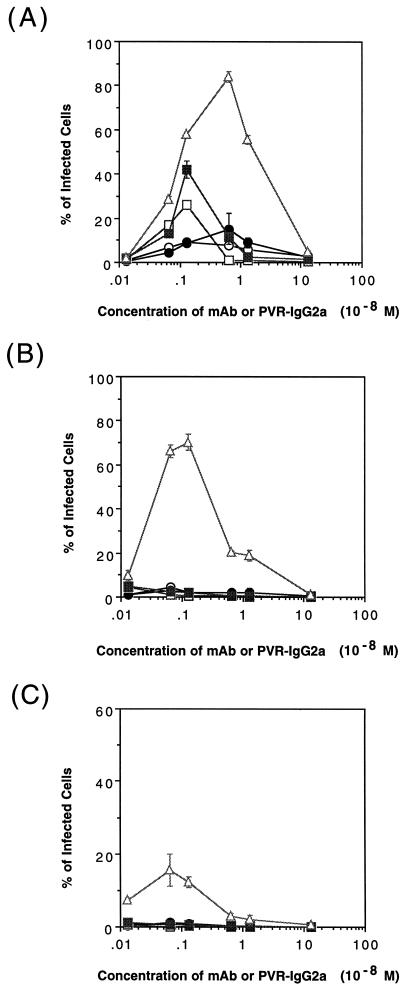
A comparison of the PV infection mediation activities of anti-PV1 MAbs and PVR-IgG2a was performed as described in Materials and Methods. Experimental conditions that gave maximum infectivity were different for individual mediators (Fig. 5). Under the optimal conditions for mediating PV1 infection, the neutralizing MAbs showed subneutralizing activities against PV1, and Mah45i and Mah49e showed a PV1 titer that was reduced, respectively, by about 2 and 1 log10 units (data not shown). PV1 infectivity also varied for the mediators, and PVR-IgG2a was the most effective mediator under any conditions used. With PVR-IgG2a, approximately 80, 70, and 18% of the cells were infected under optimal conditions in the presence of 9.6 × 109, 9.6 × 108, and 9.6 × 107 virions, respectively (Fig. 5). These data indicated that 3.0 × 105, 3.4 × 104, and 1.3 × 104 virions per cell were necessary for PV1 infection mediated by PVR-IgG2a under optimal conditions, as shown in Fig. 5A, B, and C, respectively. Considering the virion number per cell, the MAb-mediated PV1 infections were 50- to 100-fold lower than that mediated by PVR-IgG2a (Fig. 5B and C). PV1 infection of mouse Lα cells, which express PVR molecules on the cell surface, requires about 1.7 × 103 virions per cell (data not shown); consequently, the amount of virus per cell calculated from the data shown in Fig. 5 was significantly greater than that required for PV1 infection of Lα cells.
FIG. 5.
Infectivity of PV1 mediated by the anti-PV1 MAbs or PVR-IgG2a. LmFcRI cells (4.0 × 104) were challenged with PV1 in the presence of anti-PV1 MAbs (7m012 [open circles], 7m039 [closed circles], Mah45i [open squares], and Mah49e [closed squares]) or PVR-IgG2a (open triangles) at the indicated concentrations. The concentrations of PV1 used were 1.3 × 10−10 M (9.6 × 109 virions), 1.3 × 10−11 M (9.6 × 108 virions), and 1.3 × 10−12 M (9.6 × 107 virions) (A, B, and C, respectively). At 8 h p.i., indirect immunofluorescence was performed to detect viral antigens. The data represent means from three independent experiments, and error bars indicate standard deviations.
Affinity of anti-PV1 MAbs and PVR-IgG2a for PV1.
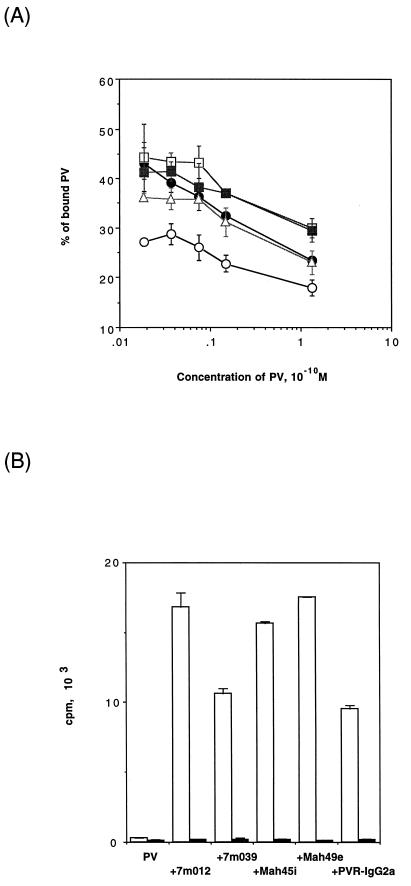
The efficiency of PV1 infection may be determined by the PV1-binding activities of anti-PV1 MAbs and PVR-IgG2a. To test this possibility, the binding of these molecules to PV1 was measured by using protein G-Sepharose, as described in Materials and Methods. As shown in Fig. 6A, for PV1 concentrations in the range of 1.3 × 10−12 to 1.3 × 10−10 M, 20 to 50% of the PV1 was retained in the gel by binding to any of the test molecules. The data suggested that there was not much difference in the MAb- and PVR-IgG2a-mediated affinities of PV1 to protein G (Fig. 6A). PV1 binding to LmFcRI cells mediated by MAbs and PVR-IgG2a was also examined (Fig. 6B). LpCI-neo cells were used as a control. The amount of PV1 bound to LmFcRI cells represented about 5 to 10% of the total PV1. Thus, through these molecules similar amounts of PV1 bound to the cell surface, yet the relative affinities of PV1 for the cells mediated by MAbs and PVR-IgG2a appeared to be different from those for the protein G gel (Fig. 6). These differences may reflect a difference in the binding mode (i.e., divalent or monovalent binding to a virion) or in the orientation of MAbs bound to the virion, which would influence the accessibilities of the Fc binding proteins. In any event, the results indicated that the observed difference in PV1 infectivities mediated by MAbs and PVR-IgG2a arose from some interaction other than their binding to PV.
FIG. 6.
PV1 binding to protein G or cells mediated by anti-PV1 MAbs or PVR-IgG2a. [35S]methionine-labeled PV1 and the anti-PV1 MAbs or PVR-IgG2a were incubated with a protein G gel (A) or cells (LmFcRI cells [open bars] or LpCI-neo cells [closed bars]) (B), and radioactivities associated with the protein G gel or cells were measured as described in Materials and Methods. Symbols in panel A are the same as in Fig. 5. The data represent means from three independent experiments, and error bars indicate standard deviations.
Conformational change of the PV particle.
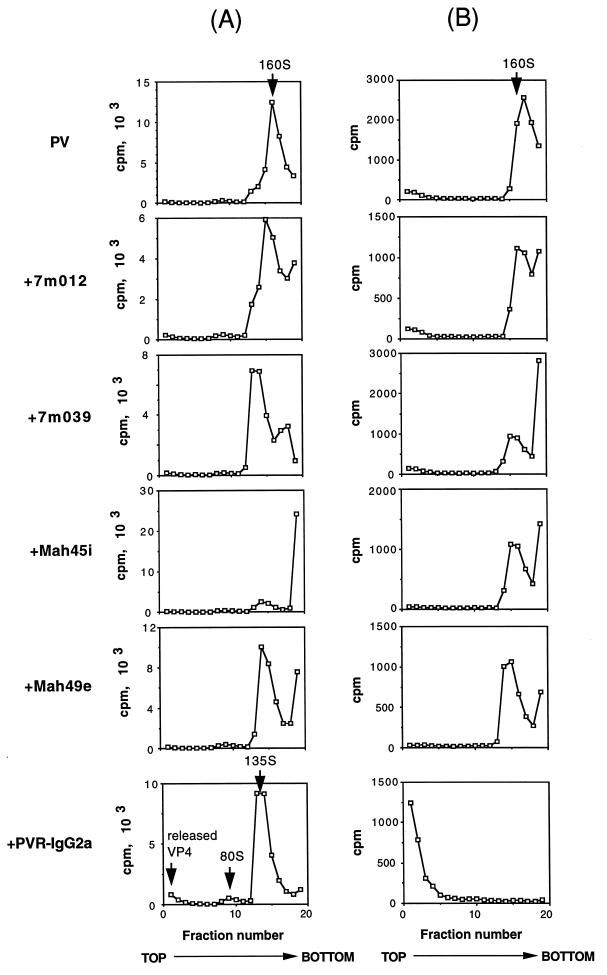
Uncoating of PV1, that is, release of the RNA genome, is essential for establishment of the viral infection. Uncoating should be accompanied by a PV1 conformational change from the intact 160S particle to 135S, 80S, or other forms of PV1-related particles. The MAbs used in this study may have an activity that induces the conformational change of the PV1 particle. Accordingly, the conformational change of PV1 was examined after treatment of PV1 at 37°C for 1 h under the conditions which gave maximum infectivity, as shown in Fig. 5A, and the forms of PV1-related materials recovered from the cell culture supernatants were analyzed by sucrose density gradient centrifugation (Fig. 7A). As expected, PVR-IgG2a changed the PV1 particle conformation from 160S to 135S and 80S, and VP4 released from the virion was observed at the top of the gradient. Some MAbs changed the 160S intact virion particle to materials with lower sedimentation coefficients. These changes, however, appeared not to be due to conformational changes to the 135S particle, because free VP4 was not detected at the top of the gradient. The shift of the peak may have been caused merely by binding of MAbs to PV1. Part of the PV1-related materials sedimented at the bottom of the gradient, which probably was the result of aggregate formation caused by binding of PV1 to the MAb. This phenomenon was especially obvious for PV1 treated with MAb Mah45i (Fig. 7A).
FIG. 7.
Virion conformational alteration induced by anti-PV1 MAbs or PVR-IgG2a. (A) The viral conformational change was examined at the optimal concentration of MAbs or PVR-IgG2a for mediation of PV1 infection, as shown in Fig. 5A: 1.3 × 10−8 M for 7m012 and 7m039, 1.3 × 10−9 M for Mah45i and Mah49e, and 6.5 × 10−9 M for PVR-IgG2a. Arrows indicate positions of intact particles (160S), altered particles (80S and 135S), and released VP4 (top fraction). (B) Each of the peak fractions in panel A was incubated in the presence of 1% SDS at room temperature for 20 min and then subjected to sucrose density gradient centrifugation.
To confirm the above results, the peak fraction of each gradient was treated with 1% SDS and then subjected to the sucrose density gradient analysis again. Intact PV1 particles are resistant to 1% SDS, and 135S particles are not (13). As shown in Fig. 7B, the sedimentation coefficients of PV1 treated with MAbs were 160S, and therefore, the treatment did not affect the conformation of the virion particle itself. Probable decomposition of aggregated virions was observed in the case of PV1 treated with Mah45i. The peak fraction produced by PVR-IgG2a shifted to the top fraction with this treatment (Fig. 7B), indicating that the PV1-related material in this fraction was the 135S particle. Thus, a conformational change in PV1 to the 135S particle was observed only for PV1 treated with PVR-IgG2a. Anti-PVR MAbs p286 and p403 inhibited the PVR-IgG2a-mediated conformational change in PV1 to the 135S particle, and the virion retained full infectivity in HeLa cells after the incubation (data not shown), suggesting that PVR-IgG2a binds PV1 in the same way as natural PVR.
Infectivity of 135S particles in LmFcRI cells.
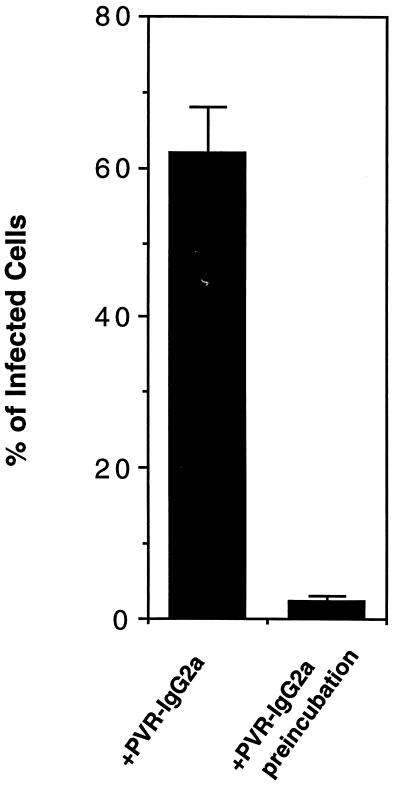
The establishment of PV1 infection mediated by PVR-IgG2a may be due to the induction of 135S particle formation by PVR-IgG2a (5). Accordingly, the infectivity of the 135S particle in LmFcRI cells was examined. The 135S particles were prepared by two methods as described in Materials and Methods. However, infectivity of the 135S particle (equivalent to about 9 × 109 virions) prepared by either of the two methods was not detectable by indirect immunofluorescence with LmFcRI cells (data not shown). The PVR-IgG2a-induced 135S particle may still retain PVR-IgG2a molecules on the particle in a solution, and such complexes may show infectivity in LmFcRI cells. However, the infectivity of 9.6 × 109 virions of PV1 to LmFcRI cells in the presence of 1.3 × 10−8 M PVR-IgG2a was decreased by 30-fold by preincubation with the same concentration of PVR-IgG2a at 37°C for 1 h (Fig. 8). These results suggested that PV1 infection of LmFcRI cells mediated by PVR-IgG2a depends on a PVR-mediated process but not on infectivity of the 135S particle in cell culture supernatants.
FIG. 8.
Direct infection of LmFcRI cells with 135S particles. PV1 (9.6 × 109 virions, 1.3 × 10−10 M) was preincubated with or without PVR-IgG2a (1.3 × 10−8 M), and the infectivities for LmFcRI cells were examined in the presence of 1.3 × 10−8 M PVR-IgG2a. The data represent means from two independent experiments, and error bars indicate standard deviations.
DISCUSSION
An enhancement of viral infectivity that was dependent on antibody was first reported for some viruses which belonged to the Flaviviridae (16), Getah virus of the Togaviridae (16), and rabbitpox virus (Poxviridae) (17). These were followed by reports for feline infectious peritonitis virus (Coronaviridae) (51), rabies virus (Rhabdoviridae) (25), and mouse CMV (Herpesviridae) (21) (reviewed in reference 39) and later FMDV (Picornaviridae) (33). In contrast to the efficient antibody-dependent infection of FMDV, PV1 infection of CHO cells expressing the low-affinity Fcγ receptor (FcRII-B2) was not mediated by anti-PV1 antibody (33). Thus, some other functions of PVR beyond its binding activity were possibly essential for PV1 infection, perhaps at the step of uncoating.
In this study, we established mouse L cells that stably expressed mouse high-affinity Fcγ receptor (FcRI), and we showed that PV1 could infect originally nonsusceptible mouse L cells through the Fc receptor in the presence of anti-PV1 MAbs. Mouse FcRI is the high-affinity Fcγ receptor among the three classes of receptors and is specific to the monomeric mouse IgG2a molecule, with a Kd of 2.0 × 10−8 M (35, 43, 50), whereas FcRII is the low-affinity Fcγ receptor with a specificity covering a broad range of IgGs, including IgG1,-2a, and -2b, to form immune complexes (49). FcRI and FcRII are also different in structure and in the way that they recognize IgG. FcRI contains three extracellular Ig-like domains, and FcRII contains two extracellular domains (30, 40, 43). The specificity and high affinity of the mouse FcRI to monomeric IgG2a are regulated by the third extracellular domain (20). Although how these two Fc receptors play roles in different ways in PV1 infection is not known, a difference in antibody affinity for the receptor possibly determines the efficiency of PV1 infection. A low affinity between the Fc receptor and an antibody would decrease the chances of PV1 staying on the cell surface and might result in inefficient uncoating.
The PV infectivity in LmFcRI cells depends on the concentration of the anti-PV1 MAbs and PVR-IgG2a, and different concentrations of PV1 require different concentrations of MAbs or PVR-IgG2a for optimum conditions (Fig. 7). Thus, the optimum conditions for infection appeared to depend on the combination and concentration of each of the components involved in PV1 infection. A conformational change of PV1 was only observed with PVR-IgG2a (Fig. 7), and PVR-IgG2a was the most effective of the tested mediator molecules (Fig. 5). The affinity between PV1 and MAbs was of the same order as the affinity between PV1 and PVR-IgG2a (Fig. 6), so the observed difference in PV1 infectivity between these molecules seemed to be rooted in another interaction; perhaps it involves virion alteration activity rather than the binding activity.
Both binding and neutralizing antibodies mediated PV1 infection, albeit inefficiently. This binding, therefore, satisfies at least the minimum interaction required for PV1 infection of cells in this system. This observation suggests that a very subtle conformational alteration is involved in the uncoating process, like virion breathing of PV1 (29, 31), although it is also possible that the presumed recycling of Fc receptors could bring PV1 into the cell and that some of them somehow release RNA into the cytoplasm via an abnormal route. The PVR may affect PV1 structure in a way that results in an interaction with the cell surface membrane to begin the uncoating process. Infectivity of the 135S particle was not detected in this study, suggesting that an essential interaction between PV1 and the cell surface probably occurs during the process of conformational change to the 135S particle. This notion is supported by the following; infectosome formation was proposed for the uncoating of human rhinovirus (28), ion channel formation by PV1 may be a result of a direct interaction of the capsid protein with the plasma membrane lipid bilayer (48), and the cold-adapted phenotype of PV1 mutants suggests that virion conformational alteration to the 135S particle is not an essential event for the uncoating process (7). It should be noted that the assay used for Fig. 7 is not sensitive enough to detect the alteration of only 1% of the virion particles. Therefore, we were not able to exclude the possibility that MAbs induced similar conformational changes to PVR-IgG2a at very low efficiencies.
Comparison of PV1 infectivity mediated by PVR-IgG2a with that mediated by native PVR expressed on the cell surface showed that the efficiency of PVR-IgG2a-mediated PV1 infection in LmFcRI cells is 10- to 200-fold lower than that observed in Lα cells (Fig. 5A and C). The difference in the infectivity observed between PVR-IgG2a-mediated infection and PVR-mediated infection may arise in part from abortive production of the altered particles. Direct infection of LmFcRI cells with the 135S particle was not observed (data not shown). Production of 135S particles in an improper location would result in abortive infection. Indeed, in PVR-IgG2a-mediated infection, the virion alteration could occur in solution, apart from the cells, whereas in the case of PVR-mediated infection, virion alteration would be membrane limited, occurring within or at the surface of the cellular membrane. The distance between PV bound to the PVR moiety and the cellular lipid bilayer is possibly different in the two infection systems. This also would cause different efficiencies of PV infection. Virus production activities may not be affected by using mediators such as MAbs and PVR-IgG2a, because the virus titer per infected LmFcRI cell appeared to be similar to that per infected Lα cell.
PVR-IgG2a apparently assumes a tetrameric form in solution, in contrast to the dimer form of IgG antibodies. The PVR moiety of the PVR-IgG2a molecule may have an affinity for itself, although soluble PVR apparently did not show a dimeric form (2). This structural difference between PVR-IgG2a and anti-PV1 MAbs may influence the interaction with the Fc receptor on the cell surface, although the affinities between the cells and PV1 mediated by these molecules seemed to be similar (Fig. 6B). Elucidation of the molecular mechanisms of PV1 uncoating will help us to understand the early events of PV1 infection. The PV1 infection system established in this study should provide new insights into the uncoating process of this nonenveloped virus.
ACKNOWLEDGMENTS
We are grateful to N. Kamoshita, S. Kuge, and K. Shiroki for helpful suggestions and discussions. We thank Y. Sasaki and K. Iwasaki for expert technical assistance and E. Suzuki and M. Watanabe for help in preparation of the manuscript.
This work was supported in part by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan and the Ministry of Health and Welfare of Japan and by the Science and Technology Agency of Japan.
REFERENCES
- 1.Aoki J, Koike S, Ise I, Sato-Yoshida Y, Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J Biol Chem. 1994;269:8431–8438. [PubMed] [Google Scholar]
- 2.Arita M, Koike S, Aoki J, Horie H, Nomoto A. Interaction of poliovirus with its purified receptor and conformational alteration in the virion. J Virol. 1998;72:3578–3586. doi: 10.1128/jvi.72.5.3578-3586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhardt G, Harber J, Zibert A, deCrombrugghe M, Wimmer E. The poliovirus receptor: identification of domains and amino acid residues critical for virus binding. Virology. 1994;203:344–356. doi: 10.1006/viro.1994.1493. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard M J, Racaniello V R. CD44 is not required for poliovirus replication. J Virol. 1997;71:2793–2798. doi: 10.1128/jvi.71.4.2793-2798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry S, Chow M, Hogle J M. The poliovirus 135S particle is infectious. J Virol. 1996;70:7125–7131. doi: 10.1128/jvi.70.10.7125-7131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis W, Harrison P T, Hutchinson M J, Allen J M. Two distinct regions of FC gamma RI initiate separate signalling pathways involved in endocytosis and phagocytosis. EMBO J. 1995;14:432–441. doi: 10.1002/j.1460-2075.1995.tb07019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dove A W, Racaniello V R. Cold-adapted poliovirus mutants bypass a postentry replication block. J Virol. 1997;71:4728–4735. doi: 10.1128/jvi.71.6.4728-4735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox M P, Otto M J, McKinlay M A. Prevention of rhinovirus and poliovirus uncoating by WIN 51711, a new antiviral drug. Antimicrob Agents Chemother. 1986;30:110–116. doi: 10.1128/aac.30.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freistadt M S, Eberle K E. CD44 is not required for poliovirus replication in cultured cells and does not limit replication in monocytes. Virology. 1996;224:542–547. doi: 10.1006/viro.1996.0561. [DOI] [PubMed] [Google Scholar]
- 10.Fricks C E, Hogle J M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990;64:1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greve J M, Forte C P, Marlor C W, Meyer A M, Hoover-Litty H, Wunderlich D, McClelland A. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J Virol. 1991;65:6015–6023. doi: 10.1128/jvi.65.11.6015-6023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gromeier M, Wetz K. Kinetics of poliovirus uncoating in HeLa cells in a nonacidic environment. J Virol. 1990;64:3590–3597. doi: 10.1128/jvi.64.8.3590-3597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guttman N, Baltimore D. A plasma membrane component able to bind and alter virions of poliovirus type 1: studies on cell-free alteration using a simplified assay. Virology. 1977;82:25–36. doi: 10.1016/0042-6822(77)90029-0. [DOI] [PubMed] [Google Scholar]
- 14.Harber J, Bernhardt G, Lu H H, Sgro J Y, Wimmer E. Canyon rim residues, including antigenic determinants, modulate serotype-specific binding of polioviruses to mutants of the poliovirus receptor. Virology. 1995;214:559–570. doi: 10.1006/viro.1995.0067. [DOI] [PubMed] [Google Scholar]
- 15.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. p. 673. [Google Scholar]
- 16.Hawkes R A. Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust J Exp Biol Med Sci. 1964;42:465–482. doi: 10.1038/icb.1964.44. [DOI] [PubMed] [Google Scholar]
- 17.Hawkes R A, Lafferty K J. The enchancement of virus infectivity by antibody. Virology. 1967;33:250–261. doi: 10.1016/0042-6822(67)90144-4. [DOI] [PubMed] [Google Scholar]
- 18.Hogle J M, Chow M, Filman D J. Three-dimensional structure of poliovirus at 2.9 Å resolution. Science. 1985;229:1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- 19.Hoover-Litty H, Greve J M. Formation of rhinovirus-soluble ICAM-1 complexes and conformational changes in the virion. J Virol. 1993;67:390–397. doi: 10.1128/jvi.67.1.390-397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulett M D, Osman N, McKenzie I F, Hogarth P M. Chimeric Fc receptors identify functional domains of the murine high affinity receptor for IgG. J Immunol. 1991;147:1863–1868. [PubMed] [Google Scholar]
- 21.Inada T, Chong K T, Mims C A. Enhancing antibodies, macrophages and virulence in mouse cytomegalovirus infection. J Gen Virol. 1985;66:871–878. doi: 10.1099/0022-1317-66-4-871. [DOI] [PubMed] [Google Scholar]
- 22.Ismail-Cassim N, Chezzi C, Newman J F. Inhibition of the uncoating of bovine enterovirus by short chain fatty acids. J Gen Virol. 1990;71:2283–2289. doi: 10.1099/0022-1317-71-10-2283. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan G, Freistadt M S, Racaniello V R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990;64:4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K H, Willingmann P, Gong Z X, Kremer M J, Chapman M S, Minor I, Oliveira M A, Rossmann M G, Andries K, Diana G D, Dutko F J, Mckinlay M A, Pevear D C. A comparison of the anti-rhinoviral drug binding pocket in HRV14 and HRV1A. J Mol Biol. 1993;230:206–227. doi: 10.1006/jmbi.1993.1137. [DOI] [PubMed] [Google Scholar]
- 25.King A A, Sands J J, Porterfield J S. Antibody-mediated enhancement of rabies virus infection in a mouse macrophage cell line (P388D1) J Gen Virol. 1984;65:1091–1093. doi: 10.1099/0022-1317-65-6-1091. [DOI] [PubMed] [Google Scholar]
- 26.Koike S, Horie H, Ise I, Okitsu A, Yoshida M, Iizuka N, Takeuchi K, Takegami T, Nomoto A. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 1990;9:3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koike S, Ise I, Nomoto A. Functional domains of the poliovirus receptor. Proc Natl Acad Sci USA. 1991;88:4104–4108. doi: 10.1073/pnas.88.10.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee W M, Monroe S S, Rueckert R R. Role of maturation cleavage in infectivity of picornaviruses: activation of an infectosome. J Virol. 1993;67:2110–2122. doi: 10.1128/jvi.67.4.2110-2122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis J K, Bothner B, Smith T J, Siuzdak G. Antiviral agent blocks breathing of the common cold virus. Proc Natl Acad Sci USA. 1998;95:6774–6778. doi: 10.1073/pnas.95.12.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis V A, Koch T, Plutner H, Mellman I. A complementary DNA clone for a macrophage-lymphocyte Fc receptor. Nature. 1986;324:372–375. doi: 10.1038/324372a0. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Yafal A G, Lee Y M, Hogle J, Chow M. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J Virol. 1994;68:3965–3970. doi: 10.1128/jvi.68.6.3965-3970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonberg-Holm K, Gosser L B, Kauer J C. Early alteration of poliovirus in infected cells and its specific inhibition. J Gen Virol. 1975;27:329–342. doi: 10.1099/0022-1317-27-3-329. [DOI] [PubMed] [Google Scholar]
- 33.Mason P W, Baxt B, Brown F, Harber J, Murdin A, Wimmer E. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology. 1993;192:568–577. doi: 10.1006/viro.1993.1073. [DOI] [PubMed] [Google Scholar]
- 34.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 35.Miller K L, Duchemin A M, Anderson C L. A novel role for the Fc receptor gamma subunit: enhancement of Fc gamma R ligand affinity. J Exp Med. 1996;183:2227–2233. doi: 10.1084/jem.183.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison M E, He Y J, Wien M W, Hogle J M, Racaniello V R. Homolog-scanning mutagenesis reveals poliovirus receptor residues important for virus binding and replication. J Virol. 1994;68:2578–2588. doi: 10.1128/jvi.68.4.2578-2588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pace C N, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez L, Carrasco L. Entry of poliovirus into cells does not require a low-pH step. J Virol. 1993;67:4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porterfield J S. Antibody-dependent enhancement of viral infectivity. Adv Virus Res. 1986;31:335–355. doi: 10.1016/s0065-3527(08)60268-7. [DOI] [PubMed] [Google Scholar]
- 40.Ravetch J V, Luster A D, Weinshank R, Kochan J, Pavlovec A, Portnoy D A, Hulmes J, Pan Y C, Unkeless J C. Structural heterogeneity and functional domains of murine immunoglobulin G Fc receptors. Science. 1986;234:718–725. doi: 10.1126/science.2946078. [DOI] [PubMed] [Google Scholar]
- 41.Rossmann M G, Arnold E, Erickson J W, Frankenberger E A, Griffith J P, Hecht H J, Johnson J E, Kamer G, Luo M, Mosser A G, Rueckert R R, Sherry B, Vriend G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985;317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 42.Rueckert R R. Picornaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 609–654. [Google Scholar]
- 43.Sears D W, Osman N, Tate B, McKenzie I F, Hogarth P M. Molecular cloning and expression of the mouse high affinity Fc receptor for IgG. J Immunol. 1990;144:371–378. [PubMed] [Google Scholar]
- 44.Selinka H C, Zibert A, Wimmer E. Poliovirus can enter and infect mammalian cells by way of an intercellular adhesion molecule 1 pathway. Proc Natl Acad Sci USA. 1991;88:3598–3602. doi: 10.1073/pnas.88.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepard D A, Heinz B A, Rueckert R R. WIN 52035-2 inhibits both attachment and eclipse of human rhinovirus 14. J Virol. 1993;67:2245–2254. doi: 10.1128/jvi.67.4.2245-2254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shepley M P, Racaniello V R. A monoclonal antibody that blocks poliovirus attachment recognizes the lymphocyte homing receptor CD44. J Virol. 1994;68:1301–1308. doi: 10.1128/jvi.68.3.1301-1308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiroki K, Ishii T, Aoki T, Kobashi M, Ohka S, Nomoto A. A new cis-acting element for RNA replication within the 5′ noncoding region of poliovirus type 1 RNA. J Virol. 1995;69:6825–6832. doi: 10.1128/jvi.69.11.6825-6832.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tosteson M T, Chow M. Characterization of the ion channels formed by poliovirus in planar lipid membranes. J Virol. 1997;71:507–511. doi: 10.1128/jvi.71.1.507-511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unkeless J C, Scigliano E, Freedman V H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 50.van de Winkel J G, Capel P J. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 51.Weiss R C, Scott F W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis. 1981;4:175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yafal A G, Kaplan G, Racaniello V R, Hogle J M. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology. 1993;197:501–505. doi: 10.1006/viro.1993.1621. [DOI] [PubMed] [Google Scholar]
- 53.Yang W-X, Terasaki T, Shiroki K, Ohka S, Aoki J, Tanabe S, Nomura T, Terada E, Sugiyama Y, Nomoto A. Efficient delivery of circulating poliovirus to the central nervous system independently of poliovirus receptor. Virology. 1997;229:421–428. doi: 10.1006/viro.1997.8450. [DOI] [PubMed] [Google Scholar]
- 54.Zibert A, Selinka H C, Elroy-Stein O, Wimmer E. The soluble form of two N-terminal domains of the poliovirus receptor is sufficient for blocking viral infection. Virus Res. 1992;25:51–61. doi: 10.1016/0168-1702(92)90099-u. [DOI] [PubMed] [Google Scholar]