Highlights
-
•
This study is the first to describe the safety and immunogenicity of a nanoporous microneedle array for SARS-CoV-2 vaccination with the mRNA-1273 vaccine in healthy participants as a booster vaccination.
-
•
Nanoporous microneedle array for SARS-CoV-2 vaccination with 20 µg mRNA-1273 was safe but failed to induce an anamnestic antibody response, which is probably due to the used vaccine loading technique resulting in a very low amount of loaded vaccine on the microneedle tips.
-
•
As microneedle patch immunisation is a promising vaccination technique; it is worth to evaluate this novel technology further to be better prepared for pandemics in the future.
Keywords: COVID-19, Safety, Immunogenicity, mRNA-1273 vaccine, Ceramic skin patch, Dose-sparing
Abstract
Introduction
Nanoporous microneedle arrays (npMNA) are being developed as skin patches for vaccine delivery. As alternative for needle-based immunisation, they may potentially result in higher vaccine acceptance, which is important for future mass vaccination campaigns to control outbreaks, such as COVID-19, and for public vaccination in general. In this study we investigated the safety and immunogenicity of needle-free intradermal delivery of a fractional third or fourth dose of mRNA-1273 vaccine by npMNA.
Methods
This study was an open-label, randomised-controlled, proof-of-concept study. Healthy adults were eligible if they had received a primary immunisation series against SARS-CoV-2 with two doses of mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) mRNA vaccine. A history of a COVID-19 infection or booster vaccination with mRNA-1273 or BNT162b2 was allowed if it occurred at least three months before inclusion. Participants were randomised in a 1:1 ratio to receive 20 µg mRNA-1273 vaccine, either through npMNA patch applied on the skin (ID-patch group), or through intramuscular (IM) injection (IM-control group). Primary outcomes were reactogenicity up to two weeks after vaccination, and fold-increase of SARS-CoV-2 spike S1-specific IgG antibodies 14 days post-vaccination.
Results
In April 2022, 20 participants were enroled. The geometric mean concentration (GMC) did not increase in the ID-patch group after vaccination, in contrast to the IM-control group (GMC was 1,006 BAU/mL (95% CI 599–1,689), 3,855 (2,800–5,306), and 3,513 (2,554–4,833) at day 1, 15 and 29, respectively). In addition, SARS-CoV-2-specific T cell responses were lower after ID vaccination through npMNA.
Conclusion
Needle-free delivery of 20 µg mRNA-1273 vaccine by npMNA failed to induce antibody and T cell responses. As this is a potentially very useful vaccination method, it is important to determine which adjustments are needed to make this npMNA successful.
Clinical trial registry (on ClinicalTrial.gov)
1. Introduction
Vaccination against COVID-19 is the most powerful tool to protect people from a severe SARS-CoV-2 infection. (Haas et al., 2021; Thompson et al., 2021) Especially early in the pandemic, vaccine stockpiles were insufficient to immunise the larger part of the world's population. Even now only 32% of people in low-income countries had received at least one dose. (Our World in Data, WHO) In addition, SARS-CoV-2 vaccination was hindered by vaccine-hesitancy (Euser et al., 2022; WHO 2019), leading to lower vaccination willingness and vaccine coverage. Reasons for refusal of SARS-CoV-2 vaccines were lack of trust in vaccines in general, doubts about the efficiency of the vaccine, needle anxiety and concerns about perceived side-effects. (Troiano and Nardi, 2021)
Fractional dosing (using a part of the registered dose) can address these problems as it provides several advantages. For instance, the incidence of dose-dependant side effects is lower, which could be an important factor in improving acceptability. Another major benefit is that more people can be immunised with the same vaccine stockpile, leading to less vaccine inequality and to improved overall public health outcomes.
The immunogenicity of fractional vaccine dose could be augmented by intradermal (ID) vaccination. The papillary dermis contains a much higher density of antigen presenting cells (APCs) than the subcutis and muscular tissue. (Nicolas and Guy, 2008) These APCs are key in initiating cellular and humoral immune responses. (Huggenberger and Detmar, 2011) The potency of the skin as a route of delivery has been studied in multiple vaccines, including rabies, hepatitis B, influenza and polio vaccine (Hickling et al., 2011). Recently we demonstrated that a one-tenth and one-fifth dose of the mRNA-1273 vaccine administered ID have shown a robust antibody response at day 43, with a better safety profile compared with full-dose IM vaccination. (Roozen et al., 2022)
Despite the advantages of ID vaccination, there are also some drawbacks. It is technically more demanding to perform than IM injection, requiring a more trained staff. Furthermore, needle-based immunisation has several limitations such as pain, needle stick injuries and poor patient compliance due to needle-phobia. Therefore, the development of needle-free delivery systems, like microneedles, has been identified as an important goal in global health care (Mitragotri, 2005).
Microneedles are minute, needle-like structures, placed on an array (or patch), with a length ranging from 0.10 to 3 mm, but typically 0.10 to 1 mm (Aldawood et al., 2021; Le et al., 2023), that allows to overcome the skin's main barrier, the stratum corneum. (Bal et al., 2010; van der Maaden et al., 2012; van der Maaden et al., 2015) There are 4 different types of microneedles formally described in reviews (solid, coated, dissolving and hollow microneedles (Aldawood et al., 2021)), however several other types exist, such as hydrogel-forming and nanoporous microneedles (Le et al., 2023; van der Maaden et al., 2015; Mansoor et al., 2022). The ceramic nanoporous microneedle array (npMNA MyLife Technologies) is a novel microneedle technology in which the microneedle patch has an interconnected nanofluidic network throughout both the microneedles and the MNA backplate reservoir. This provides the storage capacity for pharmaceutical formulations, such as vaccines. After application of the npMNA patch, vaccine which is pre-loaded onto the npMNA will diffuse from the microneedle coating or from its nanopores into the interstitial fluid of the skin. (van der Maaden et al., 2015) Vaccine delivery through microneedle technology potentially combines several important advantages: dose-sparing, options for dry product forms distributed at ambient temperatures and better acceptability by the public as it does not involve (injection with) needles.
In this study, we determined the safety and immunogenicity of the needle-free ID delivery of a single fractional third or fourth dose of 20 µg mRNA-1273 vaccine (40% of standard booster dose) administered through a npMNA patch (ID-patch), compared to a fractional dose of 20 µg mRNA-1273 delivered IM.
2. Material and methods
2.1. Study design and participants
We conducted a phase 2A, open-label, randomised-controlled, proof-of-concept vaccine study at the Leiden University Medical Centre in the Netherlands. Eligible participants were healthy adults aged 18–50 years who have received a primary vaccination series of mRNA-1273 (SpikeVax, Moderna) or BNT162b2 (Comirnaty, Pfizer-BioNTech) mRNA vaccine against SARS-CoV-2. A mid-turbinate/ throat swab was taken to exclude a concurrent SARS-CoV-2 infection before enrolment and during every on-site visit. Main exclusion criteria included a previous microbiological diagnosis of COVID-19 or COVID-19 revaccination less than 3 months ago, autoimmune disease, immunodeficiency, risk factors for developing severe COVID-19, history of severe allergic reaction, use of systemic or topical corticosteroids, bleeding condition, pregnancy and breastfeeding. For women a urine pregnancy test was performed at screening.
All participants provided written informed consent before participation in the study. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice principles. The protocol was approved by the Medical Ethical Committee Leiden, Den Haag, Delft (NL80101.058.22) and registered in the clinicaltrials.gov. The vaccine manufacturer was not involved in this trial.
2.2. Randomisation and masking
Participants were randomised by block randomisation (block size of 4) in a 1:1 ratio to receive 20 µg mRNA-1273 vaccine, either through application on the skin of the npMNA delivery system (ID-patch group) or through IM injection (IM-control group). Randomisation was done using sealed envelopes. The study was unblinded to the participant, primary investigator, and other site staff, as the administration routes differ.
2.3. Nanoporous microneedle array
The nanoporous ceramic skin patches (npMNA, MyLife Technologies, Leiden, the Netherlands) are small round ceramic discs of alumina (AI2O3) of 9 mm diameter with a homogeneous nanoporous structure. The disc is also called the backplate. One side of the backplate is covered with approximately 105 microneedles. The area between the needles is called the baseplate (Figs. 1 and S1). The backplate and the needles form one monolithic ceramic unit. The npMNA is nanoporous, and acts as an integrated nanofluidic system with an intrinsic nanoporous drug reservoir. The nanopores have an average pore size of 80 nm (+/- 5 nm) and allow for absorbence of the vaccine solution, yet the larger lipid nanoparticles (100–150 nm) will stay on the needle surface and baseplate. The microneedles have a length of 475 µm, of which typically 2/3 will penetrate through the stratum corneum and reach into the dermis. In theory the lipid nanoparticles will diffuse from the needle tips and potentially also the baseplate along the cavities created by the needles into the skin. The npMNAs were pre-coated with sucrose before use. Detailed information about the npMNA is provided in Supplement B.
Fig. 1.
Picture of the skin patch on which the vaccine is loaded
A.The nanoporous ceramic skin patch, made of alumina (AI2O3), is a round disc of 9 mm diameter. One side of the disc is covered with approximately 100 microneedles (left picture), the other side has a flat surface (right picture). The npMNA was fixed on the skin using a translucent dermal tape (right picture).
B. Schematic representation of the side view of the npMNA. The round alumina disc is called the backplate. One side of the 9 mm wide backplate is covered with approximately 100 microneedles, which surface between the needles is called the baseplate, the other side (bottom) has a flat surface.
npMNA= nanoporous microneedle array.
2.4. Procedures
All participants received 20 µg mRNA-1273 vaccine from the same batch (lot number 000191A). The cold chain was preserved by scheduling the daily doses, based on the number of participants vaccinated that day. Vaccination of the participants was performed on two different days.
In the experimental ID-patch group, vaccine was administered by placing four individual nanoporous ceramic skin patches on the ventral side of the non-dominant forearm. Each patch was loaded with 5 µg mRNA-1273, by applying 25 µl of the vaccine solution drop wise onto the needle side of the backplate with a calibrated laboratory pipette by trained personnel (Fig. S1A). As a result of this loading procedure, the mRNA-1273 was applied to the needles as well as the baseplate (Fig. S1B). The npMNA was allowed to absorb the vaccine solution for 15–30 min before application to the skin (Fig. S2A). The npMNA were fixed under occlusive conditions using a translucent dermal tape (Tegaderm™). A high impact spring-loaded applicator (Micropoint, Singapore) with a spring force of 4.4 N was used to apply pressure on the npMNA disks in order to pierce the microneedles through the stratum corneum into the epidermis and dermis. After application, the npMNAs remained in place for one hour by using dermal tape and bandage to allow diffusion of the vaccine into the skin. On the ipsilateral arm one npMNA was placed in the same manner but without vaccine to differentiate between local adverse reactions to the vaccine and to the patch. After each ID application and removal of the npMNA and the tape, the injection site was examined by a physician for erythema, swelling and other local reactions.
Participants in the IM-control group received a fractional dose of 0.1 mL mRNA-1273 vaccine in the deltoid muscle by IM injection (23-gauge needle of 25 mm in length, BD® Eclipse™ Hypodermic Safety Needle).
After vaccination, participants remained on site for 15 or 30 min for observation of acute reactions.
2.5. Monitoring of tolerability and safety
Participants were instructed to record temperature and any local or systemic adverse events (AE) daily in a diary during two weeks after vaccination (Supplement E). Follow-up visits by telephone were scheduled on day 4 ± 1 and at month 6 ± 1 post-vaccination. Two on-site visits were scheduled (D15 and D29±1). During the telephone calls and on-site visits symptom diaries were reviewed for local and systemic side effects and AEs.
Solicited AEs were nature and severity of local reactions at the injection site (redness and swelling, pain and itch at the injection site) and regional lymph nodes, and nature and severity of systemic events (vomiting, diarrhoea, headache, fatigue, chills, muscle pain, joint paint, fever). All AEs were categorised according to a standardised grading scale from 1 to 4 (Supplement D).
Any use of antipyretic or pain medication in the 14 days after vaccination was registered.
At baseline and at each on-site visit a concurrent SARS-CoV-2 infection was excluded by PCR on a mid-turbinate/throat swab. Participant who experienced respiratory symptoms were tested for COVID-19 at the Municipal Health Centre, and if confirmed, follow-up telephone calls occurred until resolution.
2.6. Assessment of antibody and IFN-gamma response
Blood was collected at baseline, two weeks (D15) and four weeks (D29) after vaccination. Serum was tested for SARS-CoV-2 spike S1-specific IgG binding antibodies (IgGSP) using a chemiluminescent microparticle (CMIA) assay (Abbott Alinity i) according to the manufacturer's instructions. (Narasimhan et al., 2021) Anti-SARS-CoV-2 antibody concentrations were reported as international binding antibody units per mL (BAU/mL). According to the manufacturer's instructions the threshold for seropositivity was 50 antibody units per mL (AU/mL) for S1-specific IgG antibodies, which equals 7.1 BAU/mL.
Spike-specific T cell responses were measured at baseline and at day 29 using a commercially available IFN-γ release assay (IGRA) (Quantiferon® SARS-CoV-2, Qiagen, Germantown, MD, USA), according to the manufacturer's instructions. (Qiagen, 2020, Qiagen, 2021) In short, heparinised fresh whole blood was incubated overnight with two different combinations of spike protein peptide (SARS-CoV-2 Ag1 and Ag2). Next, plasma was obtained and IFN-γ production of CD4+ and CD8+ cell in response was determined by enzyme-linked immunosorbent assay (ELISA). IFN-γ values are expressed in IU/mL after subtraction of the values from the unstimulated control (Nil tube). The cut-off value was a value of at least 0.15 IU/mL greater than the background IU/mL value from the SARS-CoV-2 Nil tube.
2.7. mRNA-1273 content npMNA before and after application
The amount of mRNA-1273 vaccine present on the npMNAs was determined before and after application.
For each participant, four npMNAs loaded with vaccine (applied npMNA (Rx)) and one unloaded npMNA (control npMNA (C-)) were applied to each participant under occlusive conditions using Tegaderm™ tape (on two different days; five participants on each day were vaccinated). After removal of the npMNAs from the skin, the Tegaderm™ was still attached to the backside of the backplate. These loaded (Rx) and unloaded npMNAs (C-) with the Tegaderm™ were transferred separately to a tube, containing 4 ml phosphate buffered saline (PBS). In addition, several controls were included (positive control (C+), load control (LC) and load control with Tegaderm™ (LCT)), all prepared in duplicate, which were not applied to the skin (Table S4). The npMNAs not used after loading (LC and LCT) were transferred to the tube, containing 4 ml PBS, immediately after applying the npMNAs to the participant, as it is important for the particle analysis that the samples do not completely dry, because this can affect the particle integrity. The control samples not containing a npMNA (C+) were prepared immediately after loading the npMNAs used for the application on the participants.
The residual mRNA-1273 lipid nanoparticle content on the removed npMNAs (Rx) after vaccination was measured by placing them in release buffer (by in vitro release experiments, see Supplements C) and determining the concentration of the mRNA-1273 lipid nanoparticles released in the buffer using two different methods. One method is based on particle count in dynamic light scattering (DLS) (Malvern Panalytical, Zetasizer Nano ZS90) measurements by preparing 8-point calibration curves of particle count vs. dilutions of the mRNA-1273 lipid nanoparticles with known concentration as stated on the vaccine label (i.e., label claim) used in the study. The LCT control is used to normalise for the final residual content determination to prevent underestimation. The second method of determining residual content of the mRNA-1273 lipid nanoparticles was by measuring the amount of free mRNA (which is present in the vaccine outside the lipid nanoparticles) using a Ribogreen™ assay. Extra details regarding the in vitro experiments and the Ribogreen™ assay are provided in Supplements C.
The total amount delivered to each participant was calculated by subtracting the average amount of vaccine remaining on the 4 npMNAs (results from the DLS and Ribogreen™ assay) from the total amount loaded on these 4 npMNAs. The mean of the LCT1 and LCT2 was determined for each individual participant and was set as 100% and represents a total of 20 µg of mRNA-1273 RNA (5 µg/npMNA).
2.8. Outcome
Primary outcome was the proportion of participants with an antibody booster response defined as 1.75-fold increase in geometrical mean concentration (GMC) two weeks after vaccination. Frequency and severity of local and systemic adverse reactions was also a primary outcome.
Secondary outcomes included SARS-CoV-2 specific T cell response after vaccination measured by IGRA and the average amount in microgram of mRNA-1273 particles and free mRNA released from the patch after application.
2.9. Statistical analysis
To detect a 30% booster response in the ID-patch group and 90% in the IM-control group with a significance level of 0.05 and a power of 80%, a sample size of nine participants per group was required.
All eligible participants who had at least one valid serological test result within an appropriate window after vaccination, were included in the immunogenicity analysis. Participants were excluded if they became SARS-CoV-2 positive during follow-up. Missing diary data is not imputed. Participants were analysed according to the vaccine administration route to which they were randomised.
For SARS-CoV-2 anti-S1 levels and GMCs, 2-side 95% CI were provided. In addition, geometric mean fold rise (GMFRs) and 2-sided 95% CIs were provided, calculated as the mean of the difference of logarithmically transformed test results and transformed back to the original scale.
Results of the functional cellular assays are expressed as percentage of total population. Median IFN-γ responses were plotted. Comparison of the median IFN-γ response between the administration routes used a Mann-Whitney-U test. Summary statistics are presented as medians and interquartile ranges (IQRs), mean and standard deviations (SD), GMC, and 95% confidence interval (CI).
For the safety analyses, all participants who received the study intervention and had safety data were included. Data are summarised using descriptive statistics for any AE for each vaccine group and include counts and percentage of participants with the indicated endpoints and the associated Clopper-Pearson 95% CI. AEs were categorised according to the ICD10 terms.
Statistical analyses were performed using IBM SPSS version 25 (Armonk, New York: IBM Corp) and Graphpad Prism version 9.3.1 for Windows, Graphpad Software, San Diego, California. Values of p<0.05 were considered statistically significant.
3. Results
Between April 14 and May 2, 2022, 25 participants were screened for eligibility. Two participants were excluded due to a positive SARS-CoV-2 PCR at the screening visit. Three other participants served as backup. A total of 20 participants were included and randomly assigned to IM (n = 10) or ID (n = 10) vaccination through npMNA (Fig. 2). All participants completed the scheduled safety visits during the 28 days post vaccination. No participants were lost to follow-up.
Fig. 2.
Flowchart of inclusions. ID= intradermal; IM= intramuscular.
Overall, the median age of participants was 23 years (IQR 20–36) and 50% were women (Table 1).
Table 1.
Characteristics of participants at inclusion.
| Overall (n = 20) |
20 µg IM (n = 10) |
20 µg ID-patch (n = 10) |
|
|---|---|---|---|
| Female, n (%) | 10 (50) | 6 (60) | 4 (40) |
| Age, years Median (IQR) |
23 (20–36) | 22 (19–41) | 24 (21–27) |
| BMI, kg/m2 Mean (SD) |
25.3 (4.9) | 26.7 (3.4) | 23.9 (6.0) |
| Primary series with BNT162b2, n (%) | 16 (80) | 7 (70) | 9 (90) |
| Time between second vaccination and D01, months (SD) | 9.5 (1.6) | 9.3 (2.1) | 9.7 (1.0) |
| Booster vaccination, n (%) | 14 (70) | 7 (70) | 7 (70) |
| Booster vaccination with BNT162b2, n (%) | 14 (100) | 7 (100) | 7 (100) |
| Time between booster vaccination and D01, months (SD) | 4.1 (0.8) | 4.3 (1.0) | 4.0 (0.5) |
| Prior SARS-CoV-2 disease, n (%) | 3 (15) | 2 (20) | 1 (10) |
| Time between disease and D01, months (SD) | 7.0 (4.3) | 8.3 (5.0) | 4.2 (-) |
BMI= body mass index; IM= intramuscular; ID= intradermal; SD= standard deviation.
Sixteen participants (80%) had received BNT162b2 for their primary vaccination series; 14 (70%) had received a booster, all with BNT162b2. The mean interval between the booster dose and day 1 of the study was 4.1 (SD 0.8) months. Of the remaining 6 participants, 3 (15%) were previously infected with SARS-CoV-2, all at least four months ago, and 3 participants did not receive a COVID-19 revaccination nor had a previous COVID-19 diagnosis.
3.1. Safety
No serious AE occurred. All participants in the IM-control and 8 (80%) in the ID-patch group reported at least one AE. In total, 18 participants reported 109 AEs, of which 68 (62.4%) were classified as possibly, probably or definitely related to the vaccination.
All AEs were mild (87%) or moderate (13%) in severity and all were self-limiting (Table S5). The most commonly reported local AE in the IM-control group was pain at the injection site, which occurred in 80% of the participants, in contrast to 10% of those in the ID-patch group (Fig. 3). Other local AEs, only reported in the IM group were muscle stiffness (60%), erythema (40%) and swelling (40%).
Fig. 3.
Local and systemic adverse events related to vaccine administration, subdivided into mild, moderate or severe. All adverse events possibly, probably or definitely related to the vaccination in the following 14 days after the vaccination are reported. Numbers in bars represent percentages of participants reporting this adverse event.
ID= intradermal; IM= intramuscular.
The incidence of reported systemic AEs was higher in the IM-control group than the ID-patch group, with highest incidences of nausea (30% vs 0%, respectively), headache (30% vs 30%), fatigue & malaise (30% vs 30%) and myalgia (30% vs 0%). None of the participants in the IM group and one (10%) in the ID-patch group used antipyretics for related AEs.
3.2. Antibody response
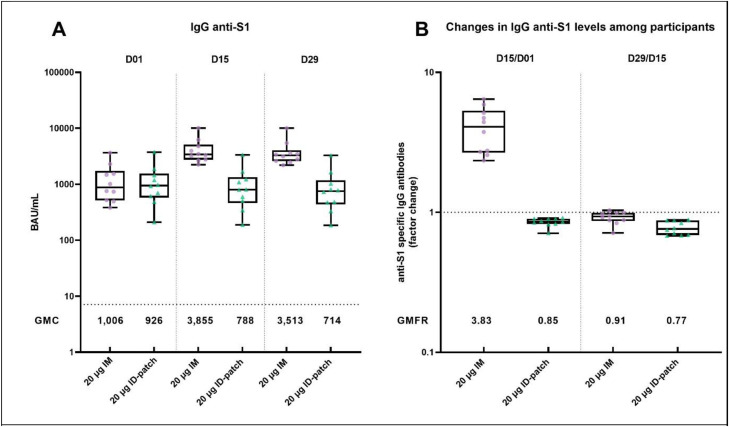
All participants had detectable SARS-CoV-2 spike S1-specific IgG antibodies at baseline (Table 2). GMC of SARS-CoV-2 spike binding antibodies rapidly increased in all participants of the IM-control group at day 15 and slightly declined at day 29 after the vaccination (Fig. 4). The GMC of SARS-CoV-2 S1 IgG antibodies at day 1, 15 and 29 in the IM group were 1006 BAU/mL (95% CI 599–1689), 3855 (2800–5306), and 3513 (2554–4833), respectively (Table 2 and Fig. 4A). All the participants of the IM group had an >1.75-fold increase in GMC two weeks after the vaccination (Fig. 4B). However, none of the participants in the ID-patch group had a booster response at day 15 or 29 after the vaccination.
Table 2.
Geometric mean concentrations and geometric mean fold rise of anti-S1 IgG antibodies in BAU/mL (95% CI).
| 20 µg IM (n = 10) |
20 µg ID-patch (n = 10) |
|||
|---|---|---|---|---|
| GMC (95% CI) | GMFR (95% CI) | GMC (95% CI) | GMFR (95% CI) | |
| Day 1 | 1006 (599–1689) | 926 (527–1628) | ||
| Day 15 | 3855 (2800–5306) | 788 (439–1411) | ||
| D15/D01 | 3.83 (2.94–5.00) | 0.85 (0.80–0.90) | ||
| Day 29 | 3513 (2554–4833) | 714 (400–1274) | ||
| D29/D15 | 0.91 (0.84–0.99) | 0.77 (0.71–0.83) | ||
| D29/D01 | 3.49 (2.68–4.55) | 0.91 (0.85–0.97) | ||
GMFR is calculated as the mean of the difference of logarithmically transformed assay results (late time point-earlier time point) and transformed back to the original scale.
GMC= geometric mean concentration; GMFR= geometric mean fold rise; CI = Confidence Intervals; n = number of participants at day 1; ID=intradermal; IM= intramuscular.
Fig. 4.
SARS-CoV-2 specific antibody response
(A) SARS-CoV-2 S1-specific IgG antibody concentrations in binding antibody units per millilitre (BAU/mL) in the two groups at each timepoint. Horizontal dotted lines represent the cut-off for seropositivity (=7.1 BAU/mL). Horizontal lines represent the geometric mean + 95% CI of the geometric mean.
(B) Per-participant factor change for anti-S1-specific IgG antibodies, calculated by dividing responses on two different days (D15/D01 and D29/D15). The horizontal dotted line represents a factor change of 1 (no increase or decrease). All data are presented as min-to-max box plots with individual values. The whiskers indicate the range. The top and the bottoms of the boxes represent the interquartile range. The horizontal line in each box indicates the median. The GMC's and GMFR are presented in the figures.
Each symbol represents a sample from an individual participant.
|D=day; ID=intradermal; IM=intramuscular; GMC= geometric mean concentrations; GMFR= geometric mean fold rise.
3.3. SARS-CoV-2 spike-specific T cell responses measured by interferon gamma release assay (IGRA)
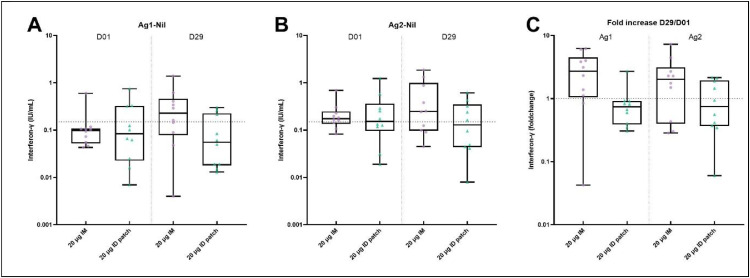
At baseline, 10% and 70% had detectable T cell responses in the IM group after stimulation with Ag1 and Ag2 peptide, respectively. For the ID-patch group, 30% and 50% had detectable responses at baseline after stimulation with Ag1 and Ag2 peptide, respectively (Fig. 5A and 5B). In contrast to the ID-patch group, the booster vaccination led to a recall of T cell responses to both Ag1 and Ag 2 in the IM group (Fig. 5C). The median fold change was 2.73 (IQR, 1.05–4.52) and 0.74 (IQR, 0.39–0.92) in the IM and ID-patch group, respectively, for the Ag1 tubes. After stimulation with Ag2 peptides, the median fold change was 2.02 (IQR, 0.40–3.14) and 0.75 (IQR, 0.37–1.95) in the IM and ID-patch group, respectively.
Fig. 5.
SARS-CoV-2-specific T cell response. (A and B) Interferon-gamma levels in international units per millilitre (IU/mL) in plasma after stimulation of whole blood with SARS-CoV-2 Ag1 peptide (A) and Ag2 peptide (B) at baseline (D01) and 4 weeks post-vaccination (D29) in the 2 different groups.
Dashed horizontal lines indicate the responder cut-off value (=0.15 IU/mL). (C) Per participant fold-changes, calculated by dividing the post-vaccination response (D29) by the pre-vaccination response (D01). The horizontal dotted line represents a factor change of 1 (no increase or decrease). All data are presented as min-to-max box plots with individual values. The whiskers indicate the range. The top and the bottoms of the boxes represent the interquartile range. The horizontal line in each box indicates the median.
Mann-Whitney U tests are performed for figs. A and B to compare vaccine deliveries (IM and ID-patch) for each timepoint. No significant difference between the groups was measured at all timepoints.
D=day; IM= intramuscular; ID= intradermal.
3.4. Residual mRNA-1273 content by particle analysis (DLS)
All samples from the npMNAs that were applied to the participants (R1–4) for the DLS analysis show a lower in vitro release, with a large variation, than the positive controls (Fig. S4 and S5). Analysis by Ribogreen™ assay showed comparable findings (Supplements H).
The released amounts (in or onto the skin) from the npMNA range between 6.2 µg and 18.9 µg per participant (average 13.3 µg) of the intended 20 µg, with an average delivered percentage of 68% ± 23% (SD) (Fig. S6 and Table S6).
4. Discussion
Delivery of a single fractional third or fourth dose of 20 µg mRNA-1273 by means of npMNA patch (ID-patch) failed to induce a SARS-CoV-2 spike S1 IgG binding antibody booster response or increase in interferon-gamma production by spike-specific T cells. The npMNA itself was safe, even though probably no vaccine or a very low dose of vaccine was delivered into the dermis.
Possible explanations for the absence of the immune response are discussed in the following paragraphs.
Insufficient release of mRNA-1273 lipid nanoparticles from the npMNA. An in vitro release experiment was performed prior to the study to describe the performance of the devices and as a reference for the in-vivo release performance. The npMNAs were loaded with 5 µg RNA by applying 25 µl of the mRNA-1273 lipid nanoparticle solution and were placed in 4 mL PBS release buffer. The concentration of the released mRNA-1273 lipid nanoparticles was also measured using both the Ribogreen™ and DLS assay. The drug load and free RNA was allowed to release from all sides and from the entire surface of het npMNAs, not just the needle tip. Therefore, the release was expected to be faster (and more complete) than release through the needle tips only. There was no suggestion that additional RNA was released from the particles compared to the original vaccine solution, which is indicative that the particles were intact upon release from the npMNAs. Also, the particle count of the mRNA-1273 lipid nanoparticles after being applied to the npMNA was comparable to the same amount of the control mRNA-1273 lipid nanoparticle sample which was not exposed to the npMNA. From internal MyLife Technologies communications, a representative npMNA batch loaded with the mRNA-1273 vaccine showed an in-vitro release of 107±13% after 15 min and 115±6% vaccine after 1 hour based on DLS and 97±4% after 15 min and 106 ± 10% after 60 min, based on Ribogreen™. DLS and Ribogreen™ methods showed consistent results.
Wasting of the vaccine during the loading process. 25 µl of the mRNA-1273 lipid nanoparticle solution contained 5 µg mRNA-1273; the concentration of the vaccine label provided by the manufacturer was taken as reference. The vaccine vials were homogenised before use. All participants in both groups received the same dose of the mRNA-1273 vaccine (100 µl, 20 µg). In the IM group, the control group, the administration of 20 µg IM resulted in a robust immune response, thereby confirming the presence of mRNA-1273 in the vaccine solution. The total dosage of 20 µg was loaded on 4 patches in the ID-patch group (5 µg by loading of 25 µl of the same vaccine solution to the patch). In theory, wasting of the vaccine during the loading process could be possible. However, even if half of the vaccine was wasted (which is unlikely, because all patches were loaded very precisely and the vaccine is carefully resuspended), the total loaded dose on the npMNA's should be around the 10 µg mRNA-1273. We have previously shown that even an ID dose of 10 µg is sufficient to generate a robust immune response (Roozen et al., 2022), which was not shown in our ID patch group. In addition, during the loading process and during application using a calibrated pipette suitable for pipetting volumes of 25 µl with an uncertainty of <5%, a visual check has been performed by two different persons to ensure that no material was lost. During the loading process, the npMNA should not be wet and during the application process no drops should fall off a microneedle patch array to classify the loading and application as successful and eligible for use in the study.
Insufficient duration of application of the npMNA onto the skin. In our study, the loaded npMNA patch remained into place on the subjects’ arm for one hour. However, it is possible that in the human body the release of the mRNA-1273 vaccine from the npMNA is achieved after a longer period than one hour, suggesting that an interval between application and removal of the patch of one hour may be too short, resulting in an incomplete release from the npMNA. However, in the study of Rouphael et all, the microneedle patches used for influenza vaccination were applied for only 20 min, resulting in a robust antibody response. (Rouphael et al., 2017) In addition, this possible explanation is contradictory with the findings in our previous study (Roozen et al., 2022) and with our residual content analysis showing a lower in vitro release for all applied npMNAs than the positive controls, indicating that a substantial amount of the vaccine (68% of the 20 µg loaded) is delivered from the patch (Fig. S6). Therefore, the most likely explanation for the lack of response in the ID-patch group in all participants is that the mRNA-1273 vaccine was released from the npMNA but did not reach the papillary dermis, the target site for ID vaccination.
This could be due to too short length of the needles to reach the papillary dermis or an insufficient loading of the npMNA microneedles specifically. The microneedles used in this study are 475 µm of which typically 2/3 (∼317 µm) will penetrate through the stratum corneum. The microneedle patch used for influenza vaccination in the study of Rouphael et al., which resulted in successful vaccine delivery, was slightly longer (650 µm). (Rouphael et al., 2017) The dermis lies beneath the epidermis (50–100/200 µm) (8,26), is 1.5–3 mm and is arranged into two sublayers: the papillary and reticular dermis. The papillary dermis is the upper layer and is comparable in thickness to the epidermis. To reach the papillary dermis, a needle length above 317 µm should thus be sufficient to reach this layer.
Evaluation of the used patch loading procedure (i.e., by placing droplets of mRNA-1273 on the needle side of the npMNA and not necessarily on the needles only) by means of microscopy scanning techniques by MyLife Technologies has shown that approximately only 10% of the load was present on the surface of the needle tips of the LC's while 90% was residing on the baseplate, which is the surface of the npMNA between the needles (Fig. 1B). The mRNA-1273 lipid nanoparticles are likely too large (around 180 nm, comparable for all controls, measured by MyLife Technologies) for diffusion from the baseplate along the cavities created by the needles into the skin.
Compared with fractional IM dose booster, ID booster through npMNA had less local and systemic reactogenicity. No erythema and swelling >2.5 cm was reported, indicating that the use of a skin patch by itself (without vaccine) is a safe strategy to explore dermal vaccination.
Our study has several limitations. First, there was heterogenicity amongst participants, as some had a previous COVID-19 infection and/or received a booster vaccination and other not. As a result, the antibody concentrations at baseline varied, with some participants with a high concentration. The assessment of the GMFR is done to overcome this limitation, in addition to keep a similar interval between the second vaccination and D01 and between the booster vaccination and D01 in both groups. Also, the number of participants receiving a booster vaccination was similar across the groups, all receiving the Pfizer-BioNTech vaccine.
Our study has several strengths. Firstly, this is the first study to compare immunogenicity and safety after a third- or fourth COVID-19 vaccination through a skin patch. This is important, because despite the absence of sufficient immunogenicity, it is important to search for easier ways to apply vaccines, allow the reduction of the amount of vaccine needed, making vaccine hesitancy and logistics no longer a hurdle for people to get vaccinated in general. Secondly, no people were lost to follow up and the data about the safety was complete.
In conclusion, we were unable to show a sufficient immunity after administration of a fractional dose of 20 µg mRNA-1273 as an ID booster (third or fourth) vaccine, administered through a npMNA. The most likely explanation is that the majority of the load is deposited onto the skin and not into the skin, as we have previously shown that ID vaccination with 10 µg mRNA-1273 administered with the Mantoux technique elicits a good antibody response against SARS-CoV-2. (Roozen et al., 2022) As microneedle patch immunisation is a promising vaccination technique. it is worth to evaluate further to be better prepared for pandemics in the future.
CRediT authorship contribution statement
Manon L.M. Prins: Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Project administration, Visualization. Corine Prins: Project administration, Validation, Resources. Jutte J.C. de Vries: Investigation. Leo G. Visser: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition. Anna H.E. Roukens: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported by MyLife Technologies. The funders had no role in the study design. The funders had a role in the analysis and interpretation of the residual content analysis and in writing the article. They had no role in the data collection, analysis of the immunogenicity or reactogenicity, or interpretation; or in the decision to submit the article for publication.
Acknowledgments
The authors would like to thank all participants for participating in this trial.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199175.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.G., Stenehjem E., Grannis S., Ball S.W., Naleway A.L., Ong T.C., et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N. Engl. J. Med. 2021;385(15):1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser S., Kroese F.M., Derks M., de Bruin M. Understanding COVID-19 vaccination willingness among youth: a survey study in the Netherlands. Vaccine. 2022;40(6):833–836. doi: 10.1016/j.vaccine.2021.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano G., Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J.F., Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev. Vaccines. 2008;7(8):1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- Huggenberger R., Detmar M. The cutaneous vascular system in chronic skin inflammation. J. Investig. Dermatol. Symp. Proc. 2011;15(1):24–32. doi: 10.1038/jidsymp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling J.K., Jones K.R., Friede M., Zehrung D., Chen D., Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull. World Health Organ. 2011;89(3):221–226. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen G.V.T., Prins M.L.M., van Binnendijk R., den Hartog G., Kuiper V.P., Prins C., et al. Safety and immunogenicity of intradermal fractional dose administration of the mRNA-1273 vaccine: a proof-of-concept study. Ann. Intern. Med. 2022;175(12):1771–1774. doi: 10.7326/M22-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S. Immunization without needles. Nat. Rev. Immunol. 2005;5(12) doi: 10.1038/nri1728. 905-16. [DOI] [PubMed] [Google Scholar]
- Aldawood F.K., Andar A., Comprehensive D.S.A. Review of microneedles: types, materials, processes, characterizations and applications. Polymers. 2021;13(16):4. doi: 10.3390/polym13162815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Z., Yu J., Quek Y.J., Bai B., Li X., Shou Y., et al. Design principles of microneedles for drug delivery and sampling applications. Mater. Today. 2023;63:137–169. [Google Scholar]
- Bal S.M., Ding Z., van Riet E., Jiskoot W., Bouwstra J.A. Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J. Control Release. 2010;148(3):266–282. doi: 10.1016/j.jconrel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- van der Maaden K., Jiskoot W., Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control Release. 2012;161(2):645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- van der Maaden K., Luttge R., Vos P.J., Bouwstra J., Kersten G., Ploemen I. Microneedle-based drug and vaccine delivery via nanoporous microneedle arrays. Drug Deliv. Transl. Res. 2015;5(4):397–406. doi: 10.1007/s13346-015-0238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor I., Eassa H.A., Mohammed K.H.A., Abd El-Fattah M.A., Abdo M.H., Rashad E., et al. Microneedle-based vaccine delivery: review of an emerging technology. AAPS PharmSci.Tech. 2022;23(4):103. doi: 10.1208/s12249-022-02250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan M., Mahimainathan L., Araj E., Clark A.E., Markantonis J., Green A., et al. Clinical evaluation of the abbott alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays among infected, recovered, and vaccinated groups. J. Clin. Microbiol. 2021;59(7) doi: 10.1128/JCM.00388-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouphael N.G., Paine M., Mosley R., Henry S., McAllister D.V., Kalluri H., et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390(10095):649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiagen . SARS-CoV-2 Starter Set Blood Collection Tubes Instructions for Use (Handbook) 2020. pp. 11–13. [Google Scholar]
- Instructions for Use (Handbook) 2021. pp. 13–21. [Google Scholar]
- Our World in Data. Coronavirus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations (accessed 20 July 2023).
- WHO. Ten threats to global health in 2019.https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed 16 June 2022).
- WHO. Vaccine equity.https://www.who.int/campaigns/vaccine-equity (accessed 9 January 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.