Abstract
The role of the sympathetic nervous system in cerebral blood flow (CBF) regulation remains unclear. Previous studies have primarily measured middle cerebral artery blood velocity to assess CBF. Recently, there has been a transition toward measuring internal carotid artery (ICA) and vertebral artery (VA) blood flow using duplex Doppler ultrasound. Given that the VA supplies autonomic control centers in the brainstem, we hypothesized that graded sympathetic activation via lower body negative pressure (LBNP) would reduce ICA but not VA blood flow. ICA and VA blood flow were measured during two protocols: protocol 1, low-to-moderate LBNP (−10, −20, −30, and −40 Torr) and protocol 2, moderate-to-high LBNP (−30, −50, and −70 Torr). ICA and VA blood flow, diameter, and blood velocity were unaffected up to −40 LBNP. However, −50 and −70 LBNP evoked reductions in ICA and VA blood flow [e.g., −70 LBNP: percent change (%∆)VA-baseline = −27.6 ± 3.0] that were mediated by decreases in both diameter and velocity (e.g., −70 LBNP: %∆VA-baseline diameter = −7.5 ± 1.9 and %∆VA-baseline velocity = −13.6 ± 1.7), which were comparable between vessels. Since hyperventilation during −70 LBNP reduced end-tidal pressure of carbon dioxide (), this decrease in was matched via voluntary hyperventilation. Reductions in ICA and VA blood flow during hyperventilation alone were significantly smaller than during −70 LBNP and were primarily mediated by decreases in velocity (%∆VA-baseline velocity = −8.6 ± 2.4 and %∆VA-baseline diameter = −0.05 ± 0.56). These data demonstrate that both ICA and VA were unaffected by low-to-moderate sympathetic activation, whereas robust reflex-mediated sympathoexcitation caused similar magnitudes of vasoconstriction in both arteries. Thus, contrary to our hypothesis, the ICA was not preferentially vasoconstricted by sympathetic activation.
NEW & NOTEWORTHY Our study demonstrates that moderate-to-high reflex-mediated sympathetic activation with lower body negative pressure (LBNP) decreases internal carotid artery and vertebral artery blood flow via reductions in both vessel diameter and blood velocity. This vasoconstriction was primarily sympathetically mediated as voluntary hyperventilation alone, to isolate the effect of decreases in end-tidal pressure of carbon dioxide that occurred during LBNP, resulted in a significantly smaller vasoconstriction. In contrast to our hypothesis, these data indicate a lack of heterogeneity between the anterior and posterior cerebral circulations in response to sympathoexcitation.
Keywords: cerebral vasoconstriction, internal carotid artery blood flow, MCA blood velocity, partial pressure of end-tidal CO2, vertebral artery blood flow
INTRODUCTION
The role of the sympathetic nervous system in the regulation of cerebral blood flow (CBF) remains controversial. Some studies have suggested that sympathetic activation plays a role in CBF regulation using steady-state (18) and oscillatory (13) lower body negative pressure (LBNP) at −40 Torr. In contrast, similar reductions in CBF were reported during −40 Torr LBNP before and following ganglionic blockade (40), contesting the role of the sympathetic nervous system in CBF regulation. Importantly, these studies assessed CBF by measuring middle cerebral artery (MCA) blood velocity, with the assumption that the diameter of the MCA remained unchanged. However, recent studies using magnetic resonance imaging have shown this assumption to be incorrect (7, 8, 35), which, in part, may have contributed to the equivocal findings in previous studies. Therefore, assessment of CBF by measuring both blood velocity and artery diameter would be important to elucidate further the role of sympathetic activation in the regulation of CBF.
There is a growing interest in examining the differential regulation of the anterior and the posterior cerebral circulations using duplex Doppler ultrasound. The anterior cerebral circulation is supplied by the internal carotid arteries (ICAs) which branch into the MCA and other intracranial arteries and provide blood flow to a large part of the cerebral cortex. The posterior cerebral circulation is fed by the vertebral arteries (VAs), which fuse to form the basilar artery and supply blood flow to the cerebellum, brainstem, and spinal cord (32). Thus, with the VA supplying blood flow to the cardiac, vasomotor, and respiratory control centers in the brainstem, it plays an important role in the regulation of the autonomic nervous system. Notably, there is emerging evidence suggesting heterogeneity in the regulation of the anterior and posterior cerebral circulations (19, 27–29, 39). For example, hypercapnia induces a larger increase in ICA blood flow compared with VA blood flow, implying lower CO2 reactivity in the posterior cerebral circulation (29). Recently, Ogoh et al. (22) showed that reflex-mediated sympathetic activation via −50 Torr LBNP caused a significant reduction in ICA blood flow, whereas VA blood flow remained unchanged, suggesting that sympathetic activation may differentially control the anterior versus the posterior cerebral circulations. However, −50 Torr LBNP in the aforementioned study also resulted in hypocapnia, and whether the observed decrease in ICA blood flow was in part due to hypocapnia remains unknown. In addition, only low and moderate levels of LBNP were performed in the study. Although −50 Torr LBNP elicits a clear increase in sympathetic nerve activity, there is a progressive increase in sympathetic activation with higher levels of LBNP (6, 26). Whether there is a differential regulation of regional CBF during higher sympathetic activation via LBNP has not been explored.
The purpose of the present study was to characterize comprehensively the effect of graded reflex-mediated sympathetic activation via LBNP on regional CBF. We tested the hypothesis that graded sympathetic activation would reduce ICA blood flow, whereas VA blood flow would remain unchanged. Initial studies were performed to examine the effect of graded low-to-moderate reflex-mediated sympathoexcitation on the anterior and posterior cerebral circulations (protocol 1: −10, −20, −30, and −40 Torr LBNP). Next, we investigated whether higher sympathetic activation resulted in differential vasoconstriction of the anterior and posterior cerebral circulations (protocol 2: −30, −50, and −70 Torr LBNP). Lastly, since hyperventilation during LBNP reduced partial pressure of end-tidal carbon dioxide (), which can independently decrease CBF, a separate protocol was performed to isolate the effect of reduced using voluntary hyperventilation.
METHODS
Study Population
Sixteen young healthy men participated in the study. Two separate experimental protocols were performed: protocol 1 (n = 8): age 25 ± 2 yr, height 178 ± 3 cm, and weight 84 ± 2 kg; and protocol 2 (n = 8): age 21 ± 1 yr, height 180 ± 2 cm, and weight 79 ± 4 kg. Participants were nonsmokers, had no known cardiovascular, metabolic, or neurological diseases, and were not using any prescription or over-the-counter medications. All experiments were performed at least 2 h postprandial in a temperature-controlled room (21°C–22°C). Subjects abstained from strenuous physical activity and alcohol for 24 h and from caffeine for 12 h before the study. Each subject gave written consent before participation. All experimental procedures conformed to the Declaration of Helsinki and were approved by the Institutional Review Board at University of Texas at Arlington (no. 2016–0783).
Experimental Measurements
Upon arrival to the laboratory, subjects laid supine with their lower body enclosed in an LBNP chamber, sealed at the level of the iliac crest. Subjects were instrumented with lead II electrocardiogram (Q710; Quinton, Bothell, WA) to monitor heart rate (HR), finger photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, the Netherlands) for beat-to-beat arterial blood pressure, and automated sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) to validate the absolute blood pressure measurements from the Finometer. Thoracic impedance was measured using noninvasive impedance cardiography (model 304B; Minnesota Impedance Cardiograph, Instrumentation for Medicine, Greenwich, CT), and was measured using a capnograph (Capnocheck Plus, Smith Medical, Dublin, OH). MCA blood velocity (MCAV) was measured using a 2-MHz transcranial Doppler probe (Multigon Industries Inc., Yonkers, NY) placed on the left temporal window. In protocol 1, blood velocity and diameter of the right ICA and VA were measured using a color-coded ultrasound system (Vivid I, GE Medical Systems) with a 13-MHz linear transducer. In protocol 2, blood velocity and diameter of the right ICA and VA were measured using a duplex Doppler ultrasound (GE Logiq P5, Milwaukee, WI) with a 10- to 12-MHz linear transducer. The use of different Doppler ultrasound units in the protocols was based on availability. In both protocols, blood flow in the right ICA was measured 1–1.5 cm distal to the carotid bifurcation, and blood flow in the right VA was measured between the transverse processes of C3 and the subclavian artery. Importantly, the skin was marked for probe placement to ensure all measurements were made at the same location. The left brachial artery was imaged via duplex Doppler ultrasound (GE Logiq P5 or Logiq 7) with a linear transducer (10–12 MHz). For all Doppler measurements, the velocity cursor was set midvessel with a 60° angle of insonation, and sample volume was adjusted to encompass the entire vessel lumen without extending beyond it.
Experimental Protocols
Protocol 1.
After instrumentation, baseline cardiovascular measures and MCAV were recorded continuously for 5 min while the subjects rested quietly. During this time, ICA, VA, and brachial artery blood flow were also recorded for 1 min each. Four levels of LBNP (−10, −20, −30, and −40 Torr) were performed for 5 min each in a randomized order. Once the target LBNP level was achieved and maintained for 1 min, ICA and VA blood flow were recorded for 30–45 s each, and brachial blood flow was measured for 1 min. This time frame was chosen based on our initial studies in which the largest changes in cardiovascular parameters occurred during the ramp up to and during the first minute of LBNP, and all cardiovascular parameters were fairly stable thereafter. Subjects rested for a minimum of 3 min between each trial to allow for all hemodynamic variables to return to baseline values. Following the first two LBNP trials, a second baseline period was performed in which all cardiovascular and CBF measurements were recorded.
Protocol 2.
After instrumentation, baseline cardiovascular data and MCAV were recorded for 5 min during quiet rest. ICA, VA, and brachial artery blood flow were also recorded for 1 min each. Following baseline, 3 levels of LBNP (−30, −50, and −70 Torr) were performed. Because −70 Torr LBNP poses a higher risk of syncope, the LBNP trials in this protocol were performed incrementally rather than randomly to avoid early cessation of the study. In addition, given the higher levels of LBNP used in this protocol, we extended the resting periods to a minimum of 10 min between the trials to ensure recovery of all hemodynamic variables, and baseline data were recorded before each LBNP trial. Each level of LBNP was applied for 3–5 min. Once the target LBNP level was achieved and maintained for 1 min, ICA and VA blood flow were recorded for 30–45 s each. Brachial blood flow was measured for 30–45 s immediately after reaching the target LBNP level. Since −50 and −70 Torr LBNP induced a hyperventilation-mediated decrease in , and CBF is highly sensitive to changes in (2, 3), a hyperventilation alone trial was also performed. The intent of this protocol was to investigate the effect of reduced on ICA and VA blood flow. Subjects returned to the laboratory on a separate day at the same time of day following the same instructions and instrumentation as the LBNP experimental day, except for MCAV. Subjects laid quietly in the supine position, and resting measures of HR, blood pressure, , ICA, and VA blood flow were recorded for 1 min. Then, subjects were instructed to hyperventilate until they reached the same reduction in as during the −70 Torr LBNP trial from their previous visit. was displayed on a monitor at eye level to provide continuous visual feedback to the subject to assist with reaching and maintaining the target . Once reached the target, ICA and VA blood flow were recorded for 30–45 s each.
Data Analysis
Mean blood velocity in the ICA and VA in protocol 1 was analyzed using the pulse wave mode velocity spectrum and averaged over 20–30 s. Mean diameter for ICA and VA in protocol 1 was calculated as [(systolic diameter/3) + (diastolic diameter × 2/3)]. In protocol 2, beat-to-beat blood velocity and diameter of ICA, VA, and brachial artery in both protocols were analyzed using customized edge detection and wall tracking software (LabView, National Instruments, Austin, TX) as previously described (11, 25, 36). Beat-to-beat values were averaged for 30 s to obtain mean values. Mean blood flow in each artery was calculated as = [π (mean diameter/2)2] × mean blood velocity × 60. Within the same protocol, each vessel was imaged by the same sonographer.
Mean arterial pressure (MAP), HR, thoracic impedance, MCA mean blood velocity (MCAVmean), and were recorded continuously at a frequency of 1,000 Hz using Powerlab (ADInstruments, Bella Vista, Australia). Resting values for MAP, HR, thoracic impedance, and MCAVmean were averaged over 2 min during the baseline before each LBNP trial. During LBNP, the cardiovascular parameters were averaged over the last 1 min. was averaged for the duration in which blood flow measurements in the ICA and VA were made. Stroke volume was obtained from the Finometer blood pressure waveform using the Modelflow method which incorporates the age, height, weight, and sex of the subject (15, 16). Cardiac output (CO) was calculated as: HR × stroke volume. MCA conductance index was calculated as: MCAVmean/MAP. Vascular conductance for ICA, VA, and brachial artery were calculated as: blood flow in the respective artery/MAP. Resting cardiovascular and CBF measures obtained at baseline before each LBNP trial were statistically compared within each protocol, and since there were no significant differences, the baselines in protocol 1 and in protocol 2 were averaged to obtain a single baseline for each protocol.
Statistical Analysis
All data are presented as means ± SE, and an α-level of P < 0.05 was used to determine statistical significance. Shapiro-Wilk was performed to test for normal distribution. One-way repeated measures ANOVA was used to evaluate the changes in hemodynamic variables in response to each level of LBNP in both protocols. When a significant main effect was found, Bonferroni post hoc tests were used for pairwise comparisons. For nonnormally distributed data (protocol 1: ICA blood velocity, VA diameter, and MCAV), Friedman test (nonparametric test) was performed on ranks, and pairwise comparisons were made using the Student Newman-Keuls method. Comparisons between ICA and VA for reductions in blood flow, diameter, and blood velocity in response to graded LBNP were performed using two-way repeated measures ANOVA to compare the changes from baseline to LBNP (LBNP effect) and between ICA and VA (vessel effect). When appropriate, Bonferroni post hoc tests were used for pairwise comparisons. Reponses during hyperventilation alone (without LBNP) and −70 Torr LBNP were compared using paired t-tests.
RESULTS
Protocol 1
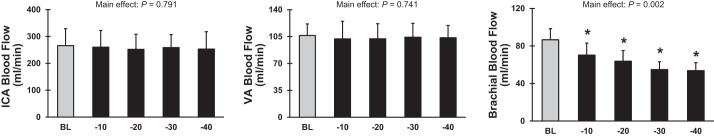
ICA and VA blood flow and vascular conductance remained unchanged during LBNP applied at −10, −20, −30, and −40 Torr (Fig. 1 and Table 1). In contrast, there was a progressive decrease in brachial artery blood flow and vascular conductance with increasing LBNP, demonstrating reflex-mediated sympathetic vasoconstriction. Similarly, diameter and blood velocity in ICA and VA did not change during −10 to −40 Torr LBNP, whereas brachial artery diameter (P = 0.010) and blood velocity (P = 0.058) decreased with increasing LBNP levels. MCAVmean and MCA conductance index did not change in response to any LBNP level up to −40 Torr. There was a significant decrease in MAP and CO and a significant increase in HR in response to −30 and −40 Torr LBNP (Table 2). As expected, graded increases in thoracic impedance were observed from −10 to −40 Torr LBNP (Table 2), indicating graded reductions in central blood volume.
Fig. 1.
Blood flow in the internal carotid artery (ICA), vertebral artery (VA), and brachial artery at baseline (BL) (light shaded bars) and during lower body negative pressure at −10, −20, −30, and −40 Torr (black bars) in protocol 1 (n = 8). *P < 0.05 vs. BL.
Table 1.
Doppler measurements during graded LBNP in protocol 1
| Baseline | −10 LBNP | −20 LBNP | −30 LBNP | −40 LBNP | P Values | |
|---|---|---|---|---|---|---|
| Internal carotid artery | ||||||
| Diameter, mm | 4.80 ± 0.50 | 4.75 ± 0.51 | 4.92 ± 0.57 | 4.77 ± 0.36 | 4.81 ± 0.32 | 0.476 |
| Velocity, cm/s | 24.5 ± 3.9 | 24.5 ± 4.8 | 22.1 ± 3.1 | 24.1 ± 3.3 | 23.0 ± 4.1 | 0.406 |
| Conductance, ml·min−1·mmHg−1 | 2.98 ± 0.27 | 2.86 ± 0.23 | 2.80 ± 0.21 | 2.90 ± 0.18 | 2.89 ± 0.26 | 0.881 |
| Vertebral artery | ||||||
| Diameter, mm | 3.84 ± 0.26 | 3.81 ± 0.30 | 3.77 ± 0.32 | 3.83 ± 0.45 | 3.84 ± 0.29 | 0.558 |
| Velocity, cm/s | 15.4 ± 1.8 | 14.8 ± 2.5 | 15.3 ± 2.8 | 15.3 ± 2.9 | 15.0 ± 2.7 | 0.885 |
| Conductance, ml·min−1·mmHg−1 | 1.19 ± 0.06 | 1.12 ± 0.09 | 1.13 ± 0.07 | 1.18 ± 0.08 | 1.19 ± 0.07 | 0.276 |
| Brachial artery | ||||||
| Diameter, mm | 4.70 ± 0.21 | 4.67 ± 0.20 | 4.59 ± 0.21 | 4.64 ± 0.23 | 4.52 ± 0.18* | 0.010 |
| Velocity, cm/s | 8.0 ± 1.0 | 7.3 ± 1.6 | 6.3 ± 0.8 | 5.5 ± 0.9 | 5.7 ± 1.0 | 0.058 |
| Conductance, ml·min−1·mmHg−1 | 0.96 ± 0.16 | 0.80 ± 0.16* | 0.71 ± 0.15* | 0.57 ± 0.11* | 0.53 ± 0.07* | 0.007 |
| Middle cerebral artery | ||||||
| Velocity, cm/s | 56.6 ± 3.5 | 55.7 ± 3.1 | 57.5 ± 3.3 | 56.4 ± 3.4 | 55.6 ± 3.3 | 0.165 |
| Conductance index, cm·s−1·mmHg−1 | 0.62 ± 0.04 | 0.62 ± 0.04 | 0.64 ± 0.03 | 0.64 ± 0.04 | 0.64 ± 0.04 | 0.170 |
Values are means ± SE. LBNP, lower body negative pressure.
P < 0.05 vs. baseline.
Table 2.
Cardiovascular measurements during graded LBNP in protocol 1
| Baseline | −10 LBNP | −20 LBNP | −30 LBNP | −40 LBNP | P Values | |
|---|---|---|---|---|---|---|
| MAP, mmHg | 93 ± 3 | 91 ± 3 | 90 ± 2 | 87 ± 3* | 88 ± 3* | 0.001 |
| HR, beats/min | 57 ± 3 | 56 ± 3 | 62 ± 4 | 63 ± 4* | 72 ± 5* | 0.001 |
| CO, l/min | 6.5 ± 0.4 | 6.2 ± 0.5 | 6.3 ± 0.5 | 5.8 ± 0.4* | 5.9 ± 0.4* | 0.008 |
| , mmHg | 39.6 ± 0.6 | 39.9 ± 0.6 | 39.7 ± 0.7 | 38.6 ± 0.9 | 37.3 ± 1.1* | 0.003 |
| Impedance, ohms | 26.6 ± 1.2 | 26.8 ± 1.2* | 27.2 ± 1.3* | 27.7 ± 1.4* | 28.7 ± 1.3* | 0.001 |
Values are means ± SE. CO, cardiac output; HR, heart rate; LBNP, lower body negative pressure; MAP, mean arterial pressure; , partial pressure of end-tidal carbon dioxide.
P < 0.05 vs. baseline.
Protocol 2
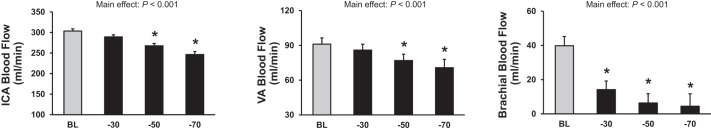
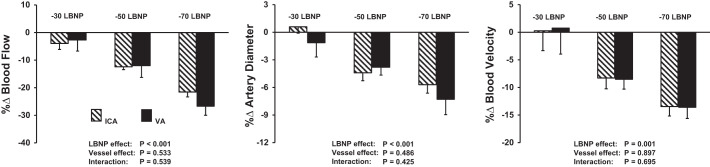
There were graded decreases in ICA and VA blood flow in response to −50 Torr LBNP [percent change (%∆)ICA-baseline: −12.4 ± 1.1 and %∆VA-baseline: −12.0 ± 4.3] and −70 Torr LBNP (%∆ICA-baseline: −21.0 ± 1.8 and %∆VA-baseline: −27.6 ± 3.0; Fig. 2). Reductions in both diameter and blood velocity contributed to this decrease in ICA and VA blood flow during −50 and −70 LBNP (Fig. 3, Table 3). These reductions in blood flow, diameter, and blood velocity were not different between the ICA and VA (vessel effect: P = 0.533, P = 0.486, and P = 0.897, respectively). ICA and VA vascular conductance also decreased significantly in response to −50 and −70 Torr LBNP (Table 3). Brachial artery blood flow and vascular conductance decreased in response to all levels of LBNP in this protocol (P < 0.001). MCAVmean and MCA conductance index decreased during −50 and −70 Torr LBNP. There was a progressive and significant increase in HR with graded LBNP (Table 4). Although MAP decreased with LBNP, this did not reach significance (P = 0.079). CO decreased to a similar level during all LBNP levels, and decreased significantly during −50 and −70 Torr LBNP (Table 4). Of note, responses during −30 Torr LBNP in protocol 2 were similar to those observed in protocol 1 (P = 0.506). Similar to protocol 1, increasing levels of LBNP caused graded increases in thoracic impedance in protocol 2 (P < 0.001).
Fig. 2.
Blood flow in the internal carotid artery (ICA), vertebral artery (VA), and brachial artery at baseline (BL) (light shaded bars) and during lower body negative pressure at −30, −50, and −70 Torr (black bars) in protocol 2 (n = 8). *P < 0.05 vs. BL.
Fig. 3.
Percent changes (%∆) from baseline in the internal carotid artery (ICA) (striped bars) and vertebral artery (VA) (solid black bars) blood flow, artery diameter, and blood velocity in response to −30, −50, and −70 Torr lower body negative pressure (LBNP) in protocol 2 (n = 8).
Table 3.
Doppler measurements during graded LBNP in protocol 2
| Baseline | −30 LBNP | −50 LBNP | −70 LBNP | P Values | |
|---|---|---|---|---|---|
| Internal carotid artery | |||||
| Diameter, mm | 4.92 ± 0.19 | 4.84 ± 0.22 | 4.75 ± 0.18 | 4.64 ± 0.15* | 0.019 |
| Velocity, cm/s | 26.7 ± 1.2 | 25.3 ± 1.1 | 24.9 ± 1.3 | 23.5 ± 1.1* | 0.011 |
| Conductance, ml·min−1·mmHg−1 | 3.41 ± 0.19 | 3.24 ± 0.23 | 3.03 ± 0.20* | 2.85 ± 0.22* | <0.001 |
| Vertebral artery | |||||
| Diameter, mm | 3.64 ± 0.12 | 3.60 ± 0.14 | 3.50 ± 0.12 | 3.38 ± 0.15* | 0.001 |
| Velocity, cm/s | 14.9 ± 0.9 | 14.9 ± 0.9 | 13.7 ± 1.0 | 13.3 ± 0.9*† | 0.011 |
| Conductance, ml·min−1·mmHg−1 | 1.03 ± 0.06 | 1.01 ± 0.05 | 0.90 ± 0.07* | 0.81 ± 0.09*† | <0.001 |
| Brachial artery | |||||
| Diameter, mm | 3.81 ± 0.16 | 3.82 ± 0.17 | 3.77 ± 0.14 | 3.78 ± 0.18 | 0.593 |
| Velocity, cm/s | 6.0 ± 1.2 | 2.2 ± 0.8* | 1.1 ± 0.5* | 0.72 ± 0.3* | <0.001 |
| Conductance, ml·min−1·mmHg−1 | 0.45 ± 0.09 | 0.17 ± 0.05* | 0.08 ± 0.03* | 0.06 ± 0.02* | <0.001 |
| Middle cerebral artery | |||||
| Velocity, cm/s | 55.7 ± 4.0 | 54.1 ± 3.7 | 48.8 ± 3.6* | 43.1 ± 2.9* | <0.001 |
| Conductance index, cm·s−1·mmHg−1 | 0.60 ± 0.04 | 0.62 ± 0.04 | 0.57 ± 0.05* | 0.52 ± 0.04* | <0.001 |
Values are means ± SE. LBNP, lower body negative pressure.
P < 0.05 vs. baseline;
P < 0.05 vs. −30 Torr LBNP.
Table 4.
Cardiovascular measurements during graded LBNP in protocol 2
| Baseline | −30 LBNP | −50 LBNP | −70 LBNP | P Values | |
|---|---|---|---|---|---|
| MAP, mmHg | 88 ± 2 | 85 ± 2 | 85 ± 2 | 84 ± 2 | 0.079 |
| HR, beats/min | 61 ± 3 | 70 ± 5* | 88 ± 5*† | 111 ± 8*‡ | <0.001 |
| CO, l/min | 6.7 ± 0.4 | 6.1 ± 0.4 | 6.0 ± 0.4* | 5.9 ± 0.3* | 0.016 |
| , mmHg | 40.2 ± 0.5 | 41.0 ± 0.5 | 36.0 ± 0.9*† | 34.2 ± 0.9*† | <0.001 |
| Impedance, ohms | 26.9 ± 0.7 | 28.3 ± 0.8* | 29.2 ± 0.9*† | 29.9 ± 0.9*‡ | <0.001 |
Values are means ± SE. CO, cardiac output; HR, heart rate; LBNP, lower body negative pressure; MAP, mean arterial pressure; , partial pressure of end-tidal carbon dioxide.
P < 0.05 vs. baseline;
P < 0.05 vs. −30 Torr LBNP;
P < 0.05 vs. −50 Torr LBNP.
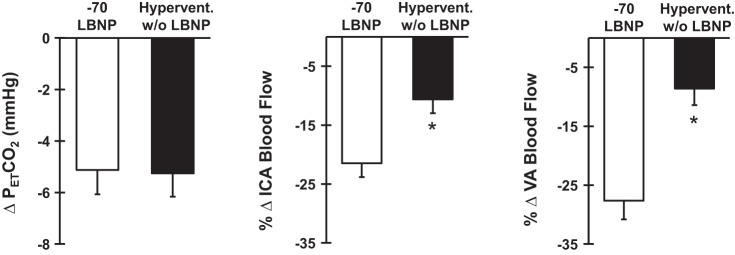
During voluntary hyperventilation alone, there was a significant decrease in blood flow in the ICA (baseline: 370.9 ± 16.9 ml/min, hyperventilation: 330.8 ± 15.5 ml/min; P = 0.013) and VA (baseline: 89.6 ± 4.1 ml/min, hyperventilation: 82.5 ± 5.5 ml/min; P = 0.001). Importantly, this reduction in blood flow was mediated by decreases in blood velocity only (%∆ICA-baseline = −8.2 ± 3.1, P = 0.037 and %∆VA-baseline = −10.8 ± 3.6, P = 0.018), as no significant changes in ICA or VA diameter were observed during hyperventilation alone (%∆ICA-baseline = −1.39 ± 0.8, P = 0.108 and %∆VA-baseline = −0.05 ± 0.56; P = 0.81). Furthermore, the decrease in ICA and VA blood flow during hyperventilation alone was significantly smaller than during reflex-mediated sympathetic activation via −70 Torr LBNP (Fig. 4). Similar responses were identified for ICA (baseline: 4.42 ± 0.23 ml·min−1·mmHg−1, hyperventilation: 3.99 ± 0.21 ml·min−1·mmHg−1; P = 0.002) and VA vascular conductance (baseline: 1.04 ± 0.07 ml·min−1·mmHg−1, hyperventilation: 0.97 ± 0.08 ml·min−1·mmHg−1; P = 0.012).
Fig. 4.
Absolute change in partial pressure of end-tidal carbon dioxide (), percent change (%∆) in the internal carotid artery (ICA), and vertebral artery (VA) blood flow in response to −70 Torr lower body negative pressure (LBNP) (open bars) and hyperventilation without LBNP (Hypervent. w/o LBNP) (closed bars) (n = 8). *P < 0.05 vs. −70 LBNP.
DISCUSSION
The novel finding of this study was that robust reflex-mediated sympathetic activation via LBNP caused vasoconstriction in both the anterior and posterior cerebral circulations, with the extent of vasoconstriction in the ICA and VA being similar. Notably, the reductions in ICA and VA blood flow were mediated by decreases in both artery diameter and blood velocity. Furthermore, when was reduced via voluntary hyperventilation alone (to match the decrease in observed during LBNP), there was a significantly smaller decrease in ICA and VA blood flow that was mediated by decreases in blood velocity only, as diameter remained unchanged. Collectively, these data demonstrated for the first time that robust LBNP-induced sympathetic activation evoked arterial vasoconstriction in both the ICA and VA that were mediated by reductions in both artery diameter and blood velocity. Thus, contrary to our hypothesis, we did not find any evidence for heterogeneity between the vessels supplying the anterior and posterior cerebral vasculatures.
Although it has been well known for a long time that the cerebral circulation is richly innervated by sympathetic nerves (10, 12, 21), the role of the sympathetic nervous system in the regulation of CBF remains controversial (31, 34). To date, only one study has directly investigated the effect of graded sympathetic activation via LBNP on the anterior (ICA) and the posterior (VA) cerebral circulations (22). During −50 Torr LBNP, a stimulus previously shown to moderately increase sympathetic nerve activity (6, 26), ICA blood flow was significantly reduced, whereas VA blood flow remained unchanged. The authors concluded that the vertebral circulation may be unaffected by moderate sympathetic activation. However, whether the VA is affected during higher sympathetic activation remained unknown. In addition, the mechanisms for reduced ICA blood flow during −50 Torr LBNP were unclear since hypocapnia also occurred during LBNP, which would decrease CBF independent of LBNP (22). In the current study, we performed low, moderate, and high levels of LBNP to characterize comprehensively the effect of graded and robust increases in sympathetic activation on cerebral hemodynamics. During −50 Torr LBNP, our findings are consistent with the previous work where ICA blood flow was reduced; however, we also observed a decrease in VA blood flow with reductions in both arterial diameter and blood velocity. The reason for these differences is unclear but could be due to differences in the race of study participants (Caucasian Americans vs. Japanese) or inclusion of both men and women in the previous study. More importantly, we extend these previous findings by eliciting greater sympathetic activation via −70 Torr LBNP during which both ICA and VA underwent vasoconstriction. Therefore, robust sympathoexcitation elicits a clear vasoconstrictor response in both the anterior and posterior cerebral circulations.
The majority of previous studies investigating CBF regulation during sympathetic activation have only measured MCAV to assess changes in CBF. Since this technique only allows for the measurement of blood velocity, changes in the vessel diameter are not directly detected. Furthermore, some studies have used changes in MCAV to draw conclusions about global CBF. This may be an important limitation to consider because CBF regulation in the anterior and posterior cerebral circulations has been shown to be heterogeneous (19, 29, 39), and since the MCA branches off of the ICA, MCAV can only serve as an index of the anterior cerebral circulation. In the current study, we measured ICA blood flow in addition to MCAV and observed that changes in MCAVmean follow the changes in ICA blood flow in response to graded LBNP. Although hypocapnia observed in the current study was much smaller than that previously shown to reduce MCA diameter (5.7 ± 0.8 mmHg vs. ~13 mmHg decrease in ) (7), we cannot rule out vasoconstriction of the MCA. Nevertheless, if sympathetic activation and/or hypocapnia during LBNP elicit decreases in MCA diameter, measuring changes in MCAV alone would only underestimate the reduction in anterior CBF, since a reduction in diameter would have caused an increase in blood velocity.
The cerebral vasculature is highly sensitive to changes in arterial CO2 (2, 3). A decrease in arterial CO2 causes cerebral vasoconstriction and a reduction in CBF, whereas an increase in arterial CO2 results in vasodilation and an increase in CBF (2, 4, 7, 38). In the current study, there was a small but significant decrease in during LBNP, indicating that some of the cerebral vasoconstriction could be due to the reduction in . To isolate the effect of CO2, we matched the reduction in observed during −70 Torr LBNP via voluntary hyperventilation without LBNP. During hyperventilation without sympathetic activation, blood flow in both ICA and VA decreased to a significantly smaller extent than that during −70 Torr LBNP. Importantly, the decrease in ICA and VA blood flow during voluntary hyperventilation was only mediated by reductions in blood velocity, as no changes in diameter occurred. In contrast, reductions in blood flow during −70 Torr LBNP were mediated by significant decreases in diameter of both ICA and VA, in addition to the decreases in blood velocity. These data demonstrate a clear, sympathetically mediated arterial vasoconstriction of the anterior and posterior cerebral vascular beds during sympathetic activation via LBNP. The magnitude of hyperventilation-induced vasoconstriction in the current study was consistent with that observed in previous studies (29), which showed an ~2% decrease in CBF for every mmHg decrease in . Collectively, these data suggest that vasoconstriction in the ICA and VA during −70 Torr LBNP is primarily sympathetically mediated, as hyperventilation alone without LBNP had a much smaller magnitude of vasoconstriction in both vascular beds.
In the present study, we observed a clear progressive decrease in brachial artery blood flow and vascular conductance during all levels of LBNP, which demonstrates effective reflex-mediated sympathetic vasoconstriction. Although we contend that the reflex-mediated sympathetic activation is responsible for the observed changes in ICA and VA, other regulatory mechanisms warrant discussion. CBF is maintained constant over a wide range of systemic pressures because of cerebral autoregulation (17, 24). Cerebral autoregulation is primarily mediated by myogenic and metabolic mechanisms (24). With a decrease in MAP, as seen during −50 and −70 Torr LBNP, autoregulatory mechanisms would cause vasodilation to maintain CBF. Given that vasoconstriction of ICA and VA was observed, it is unlikely that autoregulation plays a role in the observed responses. In fact, if any vasodilation occurred because of autoregulatory mechanisms, it was superseded by LBNP-induced sympathetically mediated vasoconstriction. Another possible mechanism involved in CBF regulation is nitric-oxide (NO)-mediated vasodilation. However, there is controversial evidence, with some studies supporting the role of NO in CBF regulation (30, 33) and others suggesting a negligible role of NO (1, 37, 41). In addition, the cerebral vasculature is also well innervated with cholinergic fibers (10), although the role of the cholinergic system in CBF regulation remains unclear. There is evidence both for (9, 14) and against (5, 20) a role of cholinergic vasodilation in the regulation of CBF. Lastly, in the current study, we also observed a decrease in CO in response to LBNP. However, the reduction in CO was similar at −30, −50, and −70 Torr LBNP (P = 0.910), whereas ICA and VA blood flow only decreased at −50 and −70 LBNP. Therefore, it is unlikely that the reduction in CO can fully explain the decrease in ICA and VA blood flow at the higher levels of LBNP.
It is important to consider some potential limitations of the present study. Although previous studies have shown progressive increases in sympathetic nerve activity with increasing LBNP levels (6, 23, 26), we did not quantify the increase in sympathetic outflow in the current study. Also, we did not attempt to use α-adrenergic receptor blockade to eliminate cerebral vasoconstriction; however, this approach may be problematic for subjects, particularly during high levels of LBNP. Notably, only men were included in the study, and thus the conclusions of the present study cannot be extended to women. Lastly, in future studies, it will be important to consider more sophisticated techniques for examining regional heterogeneity in cerebral perfusion (e.g., magnetic resonance imaging) and the responses to sympathetic activation.
Perspectives and Significance
Sympathetic innervation of cerebral blood vessels has been demonstrated previously (10, 12, 21); however, the influence of sympathetic activation on the anterior and posterior cerebral circulations has remained controversial. Contrary to our hypothesis, we demonstrated that robust sympathoexcitation reduced blood flow in both the anterior and posterior cerebral circulations, which was mediated by decreases in both arterial diameter and blood velocity. In addition, when was reduced via voluntary hyperventilation without sympathetic activation, the reduction in CBF was significantly lower than during −70 Torr LBNP and was mediated only by a decrease in blood velocity, as diameter remained unaffected. These data indicate sympathetically mediated cerebral vasoconstriction during LBNP, which was partly due to arterial diameter constriction of the ICA and VA. However, it is important to note that although CBF is reduced in response to robust sympathoexcitation, the magnitude of this vasoconstriction is much smaller than that which occurs in the skeletal muscle circulation. Indeed, brachial artery blood flow decreased by 85 ± 4% during −70 Torr LBNP, whereas ICA and VA blood flow decreased by 22 ± 2% and 28 ± 3% from baseline, respectively. Therefore, although vasoconstriction is observed in the anterior and posterior cerebral circulations, CBF appears to still be protected over other vascular beds during robust sympathetic activation.
GRANTS
This study was supported by the College of Nursing and Health Innovation at University of Texas at Arlington. J. Kaur, T. C. Barbosa, and P. J. Fadel were supported by NIH Grant R01-HL-127071.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K., S.O., and P.J.F. conceived and designed research; J.K., J.R.V., T.C.B., T.W., B.E.Y., B.Y.S., R.M.B., S.O., and P.J.F. performed experiments; J.K., J.R.V., T.C.B., and T.W. analyzed data; J.K. and P.J.F. interpreted results of experiments; J.K. prepared figures; J.K. drafted manuscript; J.K., J.R.V., T.C.B., B.E.Y., B.Y.S., and P.J.F. edited and revised manuscript; J.K., J.R.V., T.C.B., T.W., B.E.Y., B.Y.S., R.M.B., S.O., and P.J.F. approved final version of manuscript.
REFERENCES
- 1.Adachi K, Takahashi S, Melzer P, Campos KL, Nelson T, Kennedy C, Sokoloff L. Increases in local cerebral blood flow associated with somatosensory activation are not mediated by NO. Am J Physiol Heart Circ Physiol 267: H2155–H2162, 1994. doi: 10.1152/ajpheart.1994.267.6.H2155. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 3.Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Exp Physiol 95: 251–262, 2010. doi: 10.1113/expphysiol.2008.045575. [DOI] [PubMed] [Google Scholar]
- 4.Al-Khazraji BK, Shoemaker LN, Gati JS, Szekeres T, Shoemaker JK. Reactivity of larger intracranial arteries using 7 T MRI in young adults. J Cereb Blood Flow Metab X18762880, 2018. doi: 10.1177/0271678X18762880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busija DW, Heistad DD. Effects of cholinergic nerves on cerebral blood flow in cats. Circ Res 48: 62–69, 1981. doi: 10.1161/01.RES.48.1.62. [DOI] [PubMed] [Google Scholar]
- 6.Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J Physiol 587: 4987–4999, 2009. doi: 10.1113/jphysiol.2009.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- 8.Coverdale NS, Lalande S, Perrotta A, Shoemaker JK. Heterogeneous patterns of vasoreactivity in the middle cerebral and internal carotid arteries. Am J Physiol Heart Circ Physiol 308: H1030–H1038, 2015. doi: 10.1152/ajpheart.00761.2014. [DOI] [PubMed] [Google Scholar]
- 9.D’Alecy LG, Rose CJ. Parasympathetic cholinergic control of cerebral blood flow in dogs. Circ Res 41: 324–331, 1977. doi: 10.1161/01.RES.41.3.324. [DOI] [PubMed] [Google Scholar]
- 10.Edvinsson L. Neurogenic mechanisms in the cerebrovascular bed. Autonomic nerves, amine receptors and their effects on cerebral blood flow. Acta Physiol Scand Suppl 427: 1–35, 1975. [PubMed] [Google Scholar]
- 11.Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. Am J Physiol Heart Circ Physiol 305: H867–H874, 2013. doi: 10.1152/ajpheart.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985) 100: 1059–1064, 2006. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 13.Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke 41: 102–109, 2010. doi: 10.1161/STROKEAHA.109.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. J Physiol 590: 6343–6352, 2012. doi: 10.1113/jphysiol.2012.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212–222, 2001. doi: 10.1093/bja/87.2.212. [DOI] [PubMed] [Google Scholar]
- 16.Jansen JR, Wesseling KH, Settels JJ, Schreuder JJ. Continuous cardiac output monitoring by pulse contour during cardiac surgery. Eur Heart J 11, Suppl I: 26–32, 1990. doi: 10.1093/eurheartj/11.suppl_I.26. [DOI] [PubMed] [Google Scholar]
- 17.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 39: 183–238, 1959. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- 18.Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90: 298–306, 1994. doi: 10.1161/01.CIR.90.1.298. [DOI] [PubMed] [Google Scholar]
- 19.Lewis NC, Messinger L, Monteleone B, Ainslie PN. Effect of acute hypoxia on regional cerebral blood flow: effect of sympathetic nerve activity. J Appl Physiol (1985) 116: 1189–1196, 2014. doi: 10.1152/japplphysiol.00114.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita Y, Hardebo JE, Bouskela E. Influence of cerebrovascular parasympathetic nerves on resting cerebral blood flow, spontaneous vasomotion, autoregulation, hypercapnic vasodilation and sympathetic vasoconstriction. J Auton Nerv Syst 49, Suppl: S9–S14, 1994. doi: 10.1016/0165-1838(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 21.Nelson E, Rennels M. Innervation of intracranial arteries. Brain 93: 475–490, 1970. doi: 10.1093/brain/93.3.475. [DOI] [PubMed] [Google Scholar]
- 22.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Sadamoto T, Shibasaki M. Blood flow in internal carotid and vertebral arteries during graded lower body negative pressure in humans. Exp Physiol 100: 259–266, 2015. doi: 10.1113/expphysiol.2014.083964. [DOI] [PubMed] [Google Scholar]
- 23.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990. [PubMed] [Google Scholar]
- 25.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan KL, Rickards CA, Hinojosa-Laborde C, Cooke WH, Convertino VA. Arterial pressure oscillations are not associated with muscle sympathetic nerve activity in individuals exposed to central hypovolaemia. J Physiol 589: 5311–5322, 2011. doi: 10.1113/jphysiol.2011.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 589: 2847–2856, 2011. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato K, Sadamoto T. Different blood flow responses to dynamic exercise between internal carotid and vertebral arteries in women. J Appl Physiol (1985) 109: 864–869, 2010. doi: 10.1152/japplphysiol.01359.2009. [DOI] [PubMed] [Google Scholar]
- 29.Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, Ogoh S. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol 590: 3277–3290, 2012. doi: 10.1113/jphysiol.2012.230425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz JM, Al-Khazraji BK, Shoemaker JK. Sodium nitroglycerin induces middle cerebral artery vasodilatation in young, healthy adults. Exp Physiol 103: 1047–1055, 2018. doi: 10.1113/EP087022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strandgaard S, Sigurdsson ST. Last word on point: counterpoint: sympathetic nervous activity does/does not influence cerebral blood flow. J Appl Physiol (1985) 105: 1375, 2008. doi: 10.1152/japplphysiol.91088.2008. [DOI] [PubMed] [Google Scholar]
- 32.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of human brain: brainstem and cerebellum. Neurology 47: 1125–1135, 1996. doi: 10.1212/WNL.47.5.1125. [DOI] [PubMed] [Google Scholar]
- 33.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev 61: 62–97, 2009. doi: 10.1124/pr.108.000547. [DOI] [PubMed] [Google Scholar]
- 34.van Lieshout JJ, Secher NH. Last word on point: counterpoint: sympathetic activity does/does not influence cerebral blood flow. J Appl Physiol (1985) 105: 1374, 2008. doi: 10.1152/japplphysiol.91077.2008. [DOI] [PubMed] [Google Scholar]
- 35.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 36.Vranish JR, Young BE, Kaur J, Patik JC, Padilla J, Fadel PJ. Influence of sex on microvascular and macrovascular responses to prolonged sitting. Am J Physiol Heart Circ Physiol 312: H800–H805, 2017. doi: 10.1152/ajpheart.00823.2016. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Kjaer T, Jørgensen MB, Paulson OB, Lassen NA, Diemer NH, Lou HC. Nitric oxide does not act as a mediator coupling cerebral blood flow to neural activity following somatosensory stimuli in rats. Neurol Res 15: 33–36, 1993. doi: 10.1080/01616412.1993.11740103. [DOI] [PubMed] [Google Scholar]
- 38.Wasserman AJ, Patterson JL Jr. The cerebral vascular response to reduction in arterial carbon dioxide tension. J Clin Invest 40: 1297–1303, 1961. doi: 10.1172/JCI104359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590: 3261–3275, 2012. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang R, Levine BD. Autonomic ganglionic blockade does not prevent reduction in cerebral blood flow velocity during orthostasis in humans. Stroke 38: 1238–1244, 2007. doi: 10.1161/01.STR.0000260095.94175.d0. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Wilson TE, Witkowski S, Cui J, Crandall GG, Levine BD. Inhibition of nitric oxide synthase does not alter dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 286: H863–H869, 2004. doi: 10.1152/ajpheart.00373.2003. [DOI] [PubMed] [Google Scholar]