Abstract
Objective:
To investigate the correlation between the risk of breast cancer for high-risk females and the density and background parenchymal enhancement (BPE) on contrast-enhanced spectral mammography (CESM).
Methods:
Females at high-risk, without breast cancer history and received CESM from July 2016 to December 2017 were retrospectively enrolled. The longest follow-up time was 4.5 years, and patients who developed breast cancer with maximized follow-up time were classified as cancer cohort, while females who did not develop breast cancer were categorized as control cohort. These two cohorts were one-to-one matched in age, family and/or genetic history of breast cancer, menopausal status and BRCA status. The density and BPE at CESM imaging were assessed. Conditional logistic regression was applied to evaluate the relationship between imaging features and breast cancer risk.
Results:
During the follow-up interval, 90 women at high-risk without history of breast cancer were newly diagnosed. Compared with minimal BPE, increasing BPE levels were associated with the risk of breast cancer among high-risk females in a time interval of 4.5 years (mild: odds ratio [OR]=3.2, p = 0.001; moderate: OR = 4.0, p = 0.002; marked: OR = 11.2, p < 0.001). In addition, females with mild, moderate or marked BPE were four times more likely to be diagnosed with breast cancer than females with minimal BPE in a time interval of 4.5 years (OR = 4.0, p < 0.001).
Conclusion:
Qualitative CESM BPE assessment may be useful in the prediction of breast cancer risk among high-risk females.
Advances in knowledge:
• Qualitative CESM BPE assessment may be useful in the prediction of breast cancer risk among high-risk women during the follow-up period of 4.5 years.
• The significance of breast density as an independent risk factor is not fully established for high-risk women during the follow-up period of 4.5 years.
Introduction
Breast cancer is one of the most common malignant tumors among females, Its incidence has slightly increased by 0.3% annually for the past years, 1,2 and the age of onset tends to be younger. 3 Therefore, early detection, diagnosis, and treatment of breast cancer are important factors to reduce mortality, improve cure rate and prognosis, especially for high-risk population. Unfortunately, traditional risk prediction models, including Tyrer–Cuzick and Gail models, are not satisfactory. 4
Research on breast density and breast cancer risk is still an endless stream. Most findings confirmed that dense breasts are associated with a high risk of breast cancer. 5–7 Boyd et al conducted three nested case–control studies on 1112 matched cases in the census population. The results confirmed that the “masking effect” may not fully explain the fivefold breast cancer risk caused by dense breasts. 8 Breast density is accepted as an independent risk factor, but role of density measurement and risk assessment in screening setting are not fully established. Therefore, studying the relationship between breast density and breast cancer risk is necessary.
Background parenchymal enhancement (BPE) is the enhancement of normal fibroglandular tissue in the breast after contrast medium injection. 9–11 Many researchers started studying breast BPE, paid attention to the degree, scope, and probability of BPE in various populations, and explored whether BPE is related to breast cancer. The results showed that BPE is an independent predictor of breast cancer risk, particularly in high-risk population. 12–15 Most studies evaluated breast BPE through MRI.
Contrast-enhanced spectral mammography (CESM) is a new technique of digital mammography that reflects the uptake of iodine contrast agent in breast lesions to a certain extent and indirectly reflects blood supply. CESM includes low-energy and recombined contrast-enhanced image. This technique enhance the detection and diagnosis of breast cancer, thus bringing a new breakthrough for the diagnosis of breast tumor. 16 The sensitivity and specificity of CESM are also higher than those of conventional mammography. 17 Its sensitivity for breast cancer detection is comparable with that of MRI. 18,19 Same with MRI, 20 CESM is also the recommended examination method for breast cancer screening. According to American College of Radiology, 21 the indications for CESM include determination of extent of disease in newly diagnosed breast cancer, response to neoadjuvant chemotherapy, problem solving, intermediate and high-risk screening, and alternative examination when MRI examination is not applicable.
However, to our knowledge, only few studies focused on BPE based on CESM. Sogani et al 22 reported substantial agreement between readers for BPE as detected on CESM and MRI images. Savaridas et al 23 found that CESM BPE has better interradiologist agreement than MRI. Sorin, Vera et al 24 found that females with increased BPE had increased odds for breast cancer. However, Yu, Liangliang et al 25 found that CESM BPE was not correlated with benign or malignant breast lesions for non-high-risk females. Therefore, further research of BPE on CESM images is necessary.
In this study, we attempted to investigate whether breast cancer risk in high-risk females is related to CESM density and BPE. Because breast density and BPE levels are associated with hormone, we also compared imaging characteristics between patients diagnosed with ER-positive and ER-negative cancers.
Methods and materials
Patients
This retrospective study was approved by the Institutional Review Board of Yantai Yuhuangding Hospital, and patient informed consent was waived. Females with high-risk of breast cancer were collected from July 1, 2016 to December 31, 2017 using the Hospital Information System. According to the Tyrer–Cuzick risk model, females with a lifetime risk of breast cancer ≥20% are defined as high-risk. The inclusion criteria were as follows: (a) all females 18 years of age or older, (b) no history of breast cancer prior to index CESM imaging, (c) examination time is the second week of menstrual cycle and menstrual cycle is regular, and (d) has not undergone radiotherapy or hormone therapy before examination. The exclusion criteria were as follows: (a) confirmed breast cancer prior to index CESM imaging, (b) has undergone radiotherapy or hormone therapy before examination, (c) contraindications for CESM (including pregnancy, contrast medium allergy, and renal impairment), and (d) breast implants (the subtraction algorithm is not suitable). The study profile is displayed in Figure 1.
Figure 1.
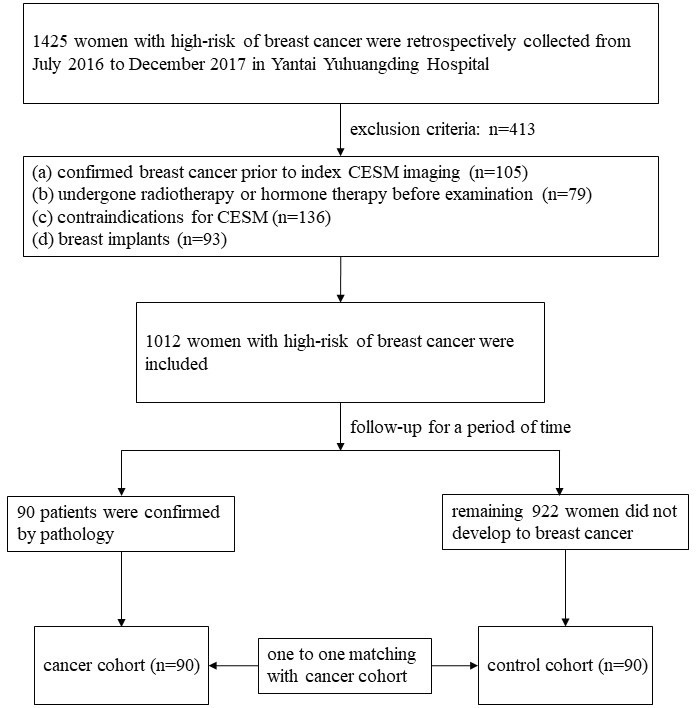
Study profile. CESM, contrast-enhanced spectral mammography.
A total of 1012 women were identified. According to pathology, the 90 enrolled females developed breast cancer within the maximized follow-up time and thus were classified as cancer cohorts. Remaining 922 women did not develop breast cancer within the maximized follow-up time. 90 women were randomly selected from the 922 women and were classified as control cohorts. The randomly selected was conducted by using R 3.5.1. The two cohorts have corresponding age, family and/or genetic history of breast cancer, menopausal status and BRCA gene according to one-to-one matching.
CESM image acquisition protocol
All breast examinations were performed using CESM (GE Healthcare, Senographe DS Senobright) and the imaging protocols were consistent. Since the iodine contrast agent needed to be injected intravenously, all patients were evaluated the risks for contrast reaction before the official injection. After confirming that there was no contrast reaction to iodine contrast agent, the Omnipaque 350 (GE Healthcare, Inc., Princeton, NJ) at a dose of 1.5 ml/kg was injected into the upper arm vein by using a high-pressure syringe at a flow rate of 3 ml s−1. The unilateral mammary gland was compressed to take the mediolateral oblique view and the craniocaudal view images for high-low energy exposure after the injection was completed for approximately 2 min. In the same manner, the mediolateral oblique view and the craniocaudal view images of the other side of the breast were acquired. Imaging for each patient was completed within 7 min. Low and high energy exposures were continuously obtained within 1.5 s of one compression. Each image was acquired on the workstation with two image types, namely, a low-energy image and a recombined image. The low-energy exposure images were used to determine breast density, and the recombined images were used to determine BPE.
Image interpretation
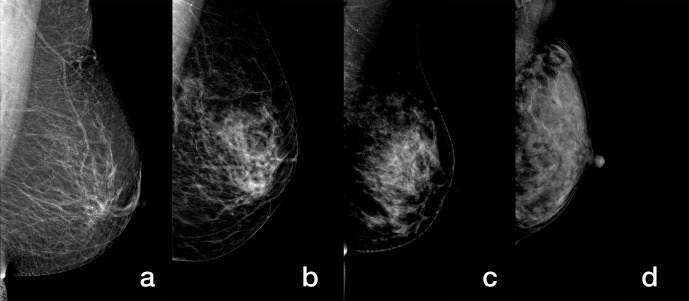
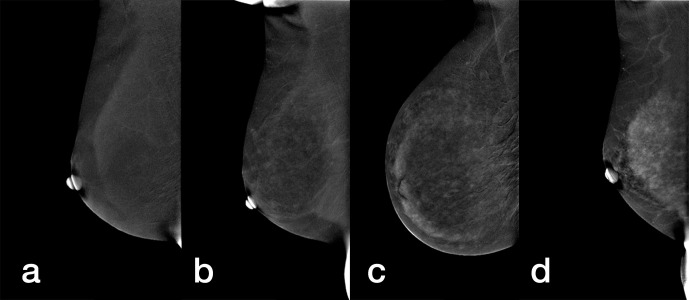
All images were reviewed by two radiologists with at least 10 years of experience in breast imaging diagnosis who assessed breast density and BPE of CESM images according to the BI-RADS system. 26 Prior to image review, the radiologists examined a standardized set of 25 cases that demonstrated density and BPE categories on CESM images. All four views were used for image interpretation. The breast density on the low energy CESM image and the amount of BPE on the recombined CESM image were assessed. The radiologists were blinded as to which cases were cancers and which were controls. Breast density was classified into four categories: A (the breasts are almost entirely fatty), B (there are scattered areas of fibroglandular density), C (the breasts are heterogeneously dense), and D (the breasts are extremely dense) (Figure 2). BPE amount was sorted into four categories: minimal (<25%), mild (25%–50%), moderate (51%–75%), and marked (>75%) (Figure 3). For the same patient, when there was asymmetry in density or BPE in the four views, the radiologists retained the highest category. All disagreements were resolved through consultation. Agreement of breast density and BPE of CESM images from two reviewers was assessed using Kendall’s W coefficient of concordance. If no agreement could be reached, it would be reviewed by a radiologist with 20 years of experience in breast imaging diagnosis.
Figure 2.
Mediolateral oblique low-energy images of CESM demonstrate different breasts with (a) A, (b) B, (c) C, and (d) D density. CESM, contrast-enhanced spectral mammography.
Figure 3.
Mediolateral oblique recombined images of CESM demonstrate different breasts with (a) minimal, (b) mild, (c) moderate, and (d) marked BPE. BPE, background parenchymal enhancement; CESM, contrast-enhanced spectral mammography.
Statistical analysis
All statistical tests were conducted using SPSS 20.0. In the matched study, conditional logistic regression analysis was used to compare the CESM density and amount of BPE. Odds ratios (OR) value and 95% confidence interval (CI) were calculated. OR >1 was used as risk factor, and its high value indicates a high risk degree. Factors that significantly differ across patients and control subjects were further analyzed using receiver operating characteristic (ROC) curve to identify optimal thresholds to maximize both sensitivity and specificity. Logistic regression and Fisher’s exact test were used to evaluate the difference in breast tissue imaging characteristics between ER positive and ER negative patients with breast cancer. p < 0.05 was considered significant.
Results
Patient characteristics
The longest follow-up time was 4.5 years. Table 1 exhibits the patients’ characteristics between the two cohorts. 90 patients were enrolled in the cancer cohort, including 46 invasive cancers and 44 ductal carcinoma in situ. The mean follow-up time, age, family and/or genetic history of breast cancer, menopausal status and BRCA status of the two cohorts were matched.
Table 1.
Patient characteristics and indications of the cancer cohort and matched control cohort
| Variable | Cancer cohort (n = 90) | Control cohort (n = 90) | p-value |
|---|---|---|---|
| Age (y)a | 48.1 ± 9.9 | 47.8 ± 9.7 | 0.923 |
| Follow-up interval (y)a b | 3.8 ± 0.6 | 3.9 ± 0.5 | 0.879 |
| Indication for high-risk screening CESM | 1 | ||
| BRCA1 mutation | 12(14) | 12(14) | |
| BRCA2 mutation | 14(16) | 14(16) | |
| Family and/or genetic history of breast cancer | 64(70) | 64(70) | |
| Menopausal status | 0.766 | ||
| Pre-menopausal | 48(53) | 45(50) | |
| Post-menopausal | 42(47) | 45(50) | |
| DCIS | 44(49) | NA | |
| ER positive | 22(50) | NA | |
| ER negative | 22(50) | NA | |
| Invasive breast cancer | 46(51) | NA | |
| ER positive | 26(57) | NA | |
| ER negative | 20(43) | NA | |
CESM, contrast-enhanced spectral mammography; DCIS, ductal carcinoma in situ; NA, not applicable.
Note: Unless otherwise indicated, data are numbers of patients and data in parentheses are percentages. All patients were matched for age and BRCA mutation status.
Data are mean ± standard deviation.
Data are period between CESM and cancer diagnosis in the cancer cohort and follow-up time in control cohort.
Association between qualitative imaging features and developing breast cancer
Overall agreement (Kendall’s W) values between the readers were 0.788 for breast density and 0.819 for BPE. In the cancer cohort, no significant association was observed between breast cancer risk and breast density (p > 0.05; Table 2) during the follow-up period of 4.5 years. However, the amount of BPE was significantly associated with breast cancer risk (p < 0.05) during the follow-up period of 4.5 years. ROC curve showed an optimal threshold of BPE greater than minimal can maximize sensitivity and specificity at 72 and 71%, respectively, in discriminating patients with cancer and control subjects (Figure 4). By using this threshold, we found that a significantly higher percentage of females in the cancer cohort had either mild, moderate, or marked BPE (78% [70 of 90 women]) than did females in the control cohort (47% [42 of 90 women], p = 0.003).
Table 2.
Comparison of imaging characteristics between the cancer and control cohorts
| Characteristic | Cancer cohort (n = 90) |
Control cohort (n = 90) |
Odds ratioa | p-value |
|---|---|---|---|---|
| BPE | ||||
| Minimal | 20 (22) | 48 (53) | Reference | |
| Mild | 36 (40) | 27 (30) | 3.2 (1.55, 6.59) | 0.001 |
| Moderate | 20 (22) | 12 (13) | 4.0 (1.65, 9.70) | 0.002 |
| Marked | 14 (16) | 3 (4) | 11.2 (2.90, 43.27) | < 0.001 |
| BPE (dichotomous) | ||||
| Minimal | 20 (22) | 48 (53) | Reference | |
| Mild, moderate, or marked | 70 (78) | 42 (47) | 4 (2.09, 7.64) | < 0.001 |
| Density | ||||
| A | 2 (2) | 4 (4) | Reference | |
| B | 22 (25) | 32 (36) | 1.4 (0.23, 8.17) | 0.726 |
| C | 48 (53) | 44 (49) | 2.2 (0.38, 12.51) | 0.381 |
| D | 18 (20) | 10 (11) | 3.6 (0.56, 23.24) | 0.178 |
| Density | ||||
| A, B | 24 (27) | 36 (40) | Reference | |
| C, D | 66 (73) | 54 (60) | 1.8 (0.98, 3.44) | 0.059 |
BPE, background parenchymal enhancement.
Note.Unless otherwise indicated, data are numbers of subjects, with percentages in parentheses.
Data in parentheses are 95% confidence intervals.
Figure 4.
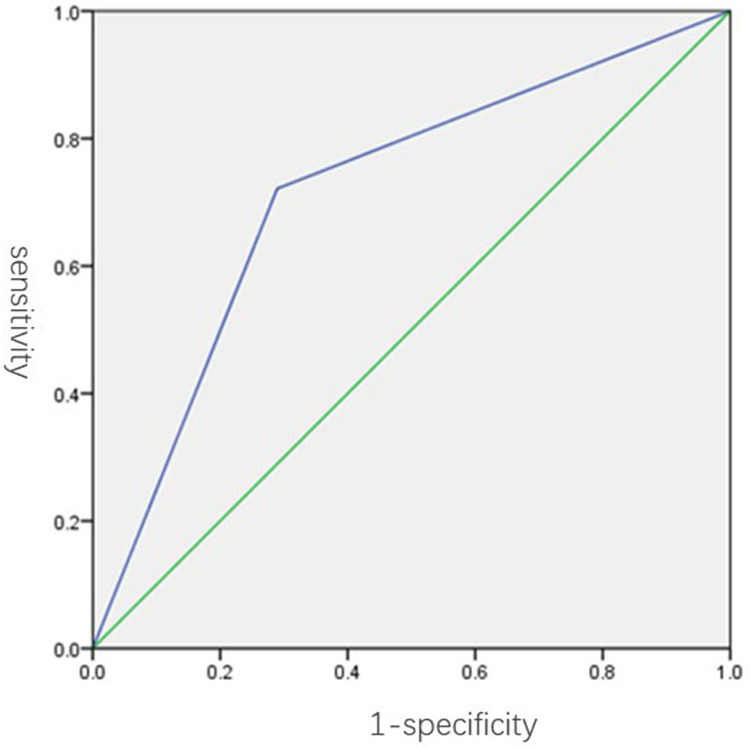
ROC curve shows accuracy of BPE assessment in the discrimination of patients with cancer (n = 90) and control subjects (n = 90). The AUC was 0.72 (95% confidence interval: 0.61, 0.82). An optimal BPE threshold of greater than minimal was identified to maximize sensitivity and specificity, with a resulting diagnostic performance of 72% sensitivity and 71%, specificity. AUC, area under the curve; BPE, background parenchymal enhancement; ROC, receiver operating characteristic.
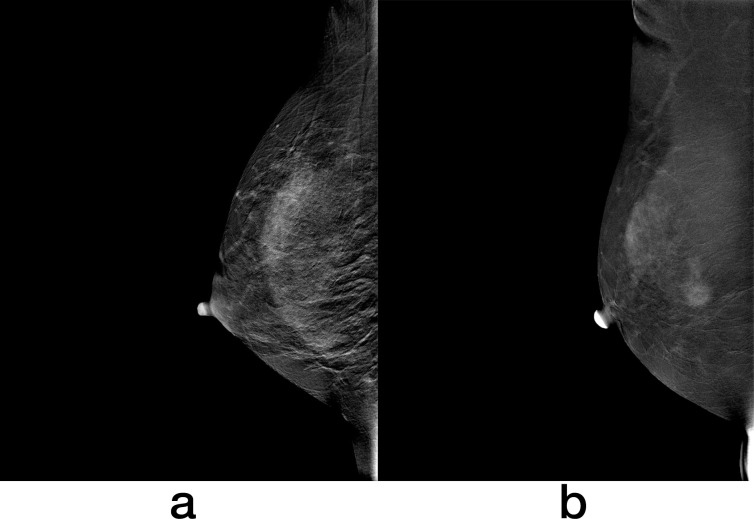
Compared with minimal BPE, increasing BPE levels were associated with the risk of developing breast cancer among high-risk females during the follow-up period of 4.5 years (mild: OR = 3.2, p = 0.001; moderate: OR = 4.0, p = 0.002; and marked: OR = 11.2, p < 0.001). In addition, females with mild, moderate or marked BPE were four times more likely to be diagnosed with breast cancer than females with minimal BPE during the follow-up period of 4.5 years (OR = 4.0; p < 0.001). As shown in Figure 5, a female with moderate BPE has developed breast cancer.
Figure 5.
Recombined images of CESM in a 41-year-old female with a family history of breast cancer shows moderate BPE (a). This patient was found to have invasive ductal carcinoma 171 days after index CESM (b). BPE, background parenchymal enhancement; CESM, contrast-enhanced spectral mammography.
When the breast density was divided into the fatty breasts (density A and B) and the dense breast (density C and D), there was no significant difference between increasing breast density level and the risk of developing breast cancer among high-risk females during the follow-up period of 4.5 years (OR = 1.8; p = 0.059).
Associations of imaging parameters with ER status of breast cancer
In the cancer cohort, breast density and BPE were not significantly associated with ER status (p > 0.05; Table 3).
Table 3.
Comparison of imaging characteristics between patients diagnosed with ER-positive and ER-negative cancers
| Characteristic | ER-positive (n = 48) |
ER-negative (n = 42) |
Odds ratioa | p-value |
|---|---|---|---|---|
| BPE | ||||
| Minimal | 10 (21) | 10 (24) | Reference | |
| Mild | 19 (39) | 17 (40) | 1.1 (0.37, 3.34) | 0.842 |
| Moderate | 11 (23) | 9 (21) | 1.2 (0.35, 4.24) | 0.752 |
| Marked | 8 (17) | 6 (14) | 1.3 (0.34, 5.27) | 0.682 |
| Density | ||||
| A | 1 (2) | 1 (2) | Reference | |
| B | 14 (29) | 8 (19) | 1.8 (0.10, 31.96) | 0.706 |
| C | 25 (52) | 23 (55) | 1.1 (0.06, 18.4) | 0.954 |
| D | 8 (17) | 10 (24) | 0.8 (0.04, 14.89) | 0.881 |
BPE, background parenchymal enhancement.
Note. Unless otherwise indicated, data are numbers of subjects, with percentages in parentheses.
Data in parentheses are 95% confidence intervals.
Discussion
In this study, we investigated the correlation of breast density and BPE on CESM and the risk of developing breast cancer in a high-risk group with the longest follow-up time of 4.5 years. Our results suggest that females with mild, moderate or marked BPE were four times more likely to be diagnosed with breast cancer than females with minimal BPE during the follow-up period of 4.5 years. However, the significance of breast density as an independent risk factor is not fully established for high-risk females during the same follow-up period. Our research has an advantage. The BRCA gene and family and/or genetic history of breast cancer, two independent biomarkers of breast cancer risk, were matched between the cancer and control cohorts. This experimental design increases the reliability of the results.
Previous studies showed that the increased BPE levels on MR or CESM images were associated with an increased risk of breast cancer. 13,23,24 In the present work, we reported similar results that the increased BPE levels on CESM images were associated with an increased risk of breast cancer. Unlike the previous studies, the subjects in this study were high-risk females. This finding may improve the effectiveness of breast cancer risk models.
Previous studies showed that BPE level is affected by hormone level. 27–31 For pre-menopausal people, the enhancement of mammary gland tissue is the most apparent during the first and fourth weeks but the weakest during the second week. 32 Therefore, all participants underwent CESM examination during their second week of menstrual cycle. BPE is reduced by endocrine therapy (including selective estrogen receptor modulator or aromatase inhibitor). King et al 33 reported that BPE is substantially reduced in breast cancer patients treated with tamoxifen and cysts. King et al 34 also evaluated the effect of aromatase inhibitors on BPE and reported similar conclusions. According to literature, the decrease in local blood vessels in the breast after radiotherapy also decreases BPE. 35 Therefore, all high-risk patients included in our study did not undergo endocrine therapy or radiotherapy. In addition, no significant associations between BPE and ER status were reported in the cancer cohort. This finding suggests that BPE can be used as a risk factor for hormone sensitive and non-hormone sensitive breast cancers.
Many studies indicated the correlation between breast density and breast cancer. 36 This correlation is not limited to the statistical data of epidemiology and extends to many different research fields, including genetics, tumor etiology, and tumor therapy. In many cases of breast cancer, the tumor tissue is located in the area with high breast density years before the diagnosis. This phenomenon strongly indicates a biological correlation between dense tissue and breast cancer risk. 37 Although most studies showed that breast density is associated with breast cancer risk, previous works on mainstream journals revealed that an increased mammographic breast density is not associated with high breast cancer risk in females with BRCA mutations. 38 This finding is similar to our results. The difference may be attributed to the non-identical research participants. Passaperuma K’s research subjects are females with BRCA gene mutation, and our research participants are high-risk females. At present, it is controversial to regard breast density as an independent risk factor for breast cancer. Further evaluating the relationship between breast density and breast cancer risk is necessary.
Our study has several limitations. First, this study is single-centered with a small data set. As a new technology, a multicentered research with a large sample size is necessary to obtain high-level evidence for clinical applications. Second, the proportion of ductal carcinoma in situ in the cancer cohort was relatively high, and this situation was inconsistent with the real clinical environment. Future research should try to solve this problem, such as using large sample research. Third, the longest follow-up period was only 4.5 years. If the follow-up time was longer, it was unknown whether the results would be changed. Future studies will be followed up for a longer period of time. Fourth, the relationship between BPE on CESM and breast cancer risk in the general population of females is still unknown. We will continue our research in future works. Fifth, since this study was a retrospective study and the patients’ BMI was not recorded in the medical record system, the correlation between breast density and BMI could not be obtained. In the future, a prospective study will be designed to explore whether BMI can affect breast density.
Conclusion
In summary, our study suggests that qualitative CESM BPE assessment may be useful in the prediction of breast cancer risk among high-risk females, while the significance of breast density as an independent risk factor is not fully established for high-risk females during the follow-up period of 4.5 years. Large sample size, long follow-up time and multicentered retrospective study should be performed to improve efficiency and provide high level evidence for clinical application in subsequent studies.
Footnotes
Acknowledgments: We thank the study participants and referring technicians for their participation in this study.
Funding: The study was supported by the National Natural Science Foundation of China (82001775 and 62176140), the Natural Science Foundation of Shandong Province of China (ZR2021MH120, ZR2022MH274, ZR2021MH161 and ZR2021MH398), the Special Fund for Breast Disease Research of Shandong Medical Association (YXH2021ZX055), and Taishan Scholars (tsqn202211378).
The authors Cong Xu and Meiping Jiang contributed equally to the work.
Data Sharing Statement: Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
Contributor Information
Cong Xu, Email: 616574369@qq.com.
Meiping Jiang, Email: 1355906601@qq.com.
Fan Lin, Email: bzmclinfan@163.com.
Kun Zhang, Email: zkzkzkhp@126.com.
Haizhu Xie, Email: xhz000417@sina.com.
Wei Lv, Email: yjgclw7166@163.com.
Haixia Ji, Email: m13562588533@163.com.
Ning Mao, Email: maoning@pku.edu.cn.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019; 69: 438–51. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 3. Schover LR. Premature ovarian failure and its consequences: Vasomotor symptoms, sexuality, and fertility. J Clin Oncol 2008; 26: 753–58. doi: 10.1200/JCO.2007.14.1655 [DOI] [PubMed] [Google Scholar]
- 4. Wald NJ, Hackshaw AK, Frost CD. When can a risk factor be used as a worthwhile screening test? BMJ 1999; 319: 1562–65. doi: 10.1136/bmj.319.7224.1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: Development and validation of a new predictive model. Ann Intern Med 2008; 148: 337–47. doi: 10.7326/0003-4819-148-5-200803040-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, et al. Quantitative classification of mammographic densities and breast cancer risk: Results from the Canadian National breast screening study. JNCI Journal of the National Cancer Institute 1995; 87: 670–75. doi: 10.1093/jnci/87.9.670 [DOI] [PubMed] [Google Scholar]
- 7. Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, Carney PA, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst 2006; 98: 1204–14. doi: 10.1093/jnci/djj331 [DOI] [PubMed] [Google Scholar]
- 8. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007; 356: 227–36. doi: 10.1056/NEJMoa062790 [DOI] [PubMed] [Google Scholar]
- 9. Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: Normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997; 203: 137–44. doi: 10.1148/radiology.203.1.9122382 [DOI] [PubMed] [Google Scholar]
- 10. Morris EA. Diagnostic breast MR imaging: Current status and future directions. Magn Reson Imaging Clin N Am 2010; 18: 57–74. doi: 10.1016/j.mric.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 11. Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA. Background parenchymal enhancement on baseline screening breast MRI: Impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol 2011; 196: 218–24. doi: 10.2214/AJR.10.4550 [DOI] [PubMed] [Google Scholar]
- 12. Arasu VA, Miglioretti DL, Sprague BL, Alsheik NH, Buist DSM, Henderson LM, et al. Population-Based assessment of the association between magnetic resonance imaging background parenchymal enhancement and future primary breast cancer risk. J Clin Oncol 2019; 37: 954–63. doi: 10.1200/JCO.18.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dontchos BN, Rahbar H, Partridge SC, Korde LA, Lam DL, Scheel JR, et al. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on Mr images, and mammographic density associated with breast cancer risk? Radiology 2015; 276: 371–80. doi: 10.1148/radiol.2015142304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pike MC, Pearce CL. Mammographic density, MRI background parenchymal enhancement and breast cancer risk. Ann Oncol 2013; 24 Suppl 8: viii37–41. doi: 10.1093/annonc/mdt310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011; 260: 50–60. doi: 10.1148/radiol.11102156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luczyńska E, Heinze-Paluchowska S, Dyczek S, Blecharz P, Rys J, Reinfuss M. Contrast-Enhanced spectral mammography: Comparison with conventional mammography and histopathology in 152 women. Korean J Radiol 2014; 15: 689–96. doi: 10.3348/kjr.2014.15.6.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luczyńska E, Heinze-Paluchowska S, Dyczek S, Blecharz P, Rys J, Reinfuss M. Contrast-Enhanced spectral mammography: Comparison with conventional mammography and histopathology in 152 women. Korean J Radiol 2014; 15: 689–96. doi: 10.3348/kjr.2014.15.6.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dromain C, Thibault F, Muller S, Rimareix F, Delaloge S, Tardivon A, et al. Dual-Energy contrast-enhanced digital mammography: Initial clinical results. Eur Radiol 2011; 21: 565–74. doi: 10.1007/s00330-010-1944-y [DOI] [PubMed] [Google Scholar]
- 19. Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital mammography: Feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013; 266: 743–51. doi: 10.1148/radiol.12121084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mann RM, Athanasiou A, Baltzer PAT, Camps-Herrero J, Clauser P, Fallenberg EM, et al. Breast cancer screening in women with extremely dense breasts recommendations of the European Society of breast imaging (EUSOBI). Eur Radiol 2022; 32: 4036–45. doi: 10.1007/s00330-022-08617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee CH, Sung JS, Phillips J, Lewin JM, Newell MS. Contrast Enhanced Mammography (CEM) (A supplement to ACR BI-RADS® Mammography 2013). American College of Radiology. 2022. Available from: https://www.acr.org/-/media/ACR/Files/RADS/BI-RADS/BIRADS_CEM_2022.pdf
- 22. Sogani J, Morris EA, Kaplan JB, D’Alessio D, Goldman D, Moskowitz CS, et al. Comparison of background parenchymal enhancement at contrast-enhanced spectral mammography and breast MR imaging. Radiology 2017; 282: 63–73. doi: 10.1148/radiol.2016160284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savaridas SL, Taylor DB, Gunawardana D, Phillips M. Could parenchymal enhancement on contrast-enhanced spectral mammography (CESM) represent a new breast cancer risk factor? correlation with known radiology risk factors. Clin Radiol 2017; 72: S0009-9260(17)30403-8. doi: 10.1016/j.crad.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 24. Sorin V, Yagil Y, Shalmon A, Gotlieb M, Faermann R, Halshtok-Neiman O, et al. Background parenchymal enhancement at contrast-enhanced spectral mammography (CESM) as a breast cancer risk factor. Academic Radiology 2020; 27: 1234–40. doi: 10.1016/j.acra.2019.10.034 [DOI] [PubMed] [Google Scholar]
- 25. Yu L, Wang Y, Xing D, Gong P, Chen Q, Lv Y. Background parenchymal enhancement on contrast-enhanced spectral mammography does not represent an influencing factor for breast cancer. Medicine (Baltimore) 2020; 99: e23857. doi: 10.1097/MD.0000000000023857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sippo DA, Warden GI, Andriole KP, Lacson R, Ikuta I, Birdwell RL, et al. Automated extraction of BI-RADS final assessment categories from radiology reports with natural language processing. J Digit Imaging 2013; 26: 989–94. doi: 10.1007/s10278-013-9616-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuhl C. The current status of breast MR imaging. Part I. choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007; 244: 356–78. doi: 10.1148/radiol.2442051620 [DOI] [PubMed] [Google Scholar]
- 28. Müller-Schimpfle M, Ohmenhaüser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: Influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997; 203: 145–49. doi: 10.1148/radiology.203.1.9122383 [DOI] [PubMed] [Google Scholar]
- 29. Delille J-P, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: Perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 2005; 11: 236–41. doi: 10.1111/j.1075-122X.2005.21499.x [DOI] [PubMed] [Google Scholar]
- 30. Kajihara M, Goto M, Hirayama Y, Okunishi S, Kaoku S, Konishi E, et al. Effect of the menstrual cycle on background parenchymal enhancement in breast MR imaging. Magn Reson Med Sci 2013; 12: 39–45. doi: 10.2463/mrms.2012-0022 [DOI] [PubMed] [Google Scholar]
- 31. Scaranelo AM, Carrillo MC, Fleming R, Jacks LM, Kulkarni SR, Crystal P. Pilot study of quantitative analysis of background enhancement on breast Mr images: Association with menstrual cycle and mammographic breast density. Radiology 2013; 267: 692–700. doi: 10.1148/radiol.13120121 [DOI] [PubMed] [Google Scholar]
- 32. Zhao S, Zhang X, Zhong H, Qin Y, Li Y, Song B, et al. Background parenchymal enhancement on contrast-enhanced spectral mammography: Influence of age, breast density, menstruation status, and menstrual cycle timing. Sci Rep 2020; 10(): 8608. doi: 10.1038/s41598-020-65526-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. King V, Goldfarb SB, Brooks JD, Sung JS, Nulsen BF, Jozefara JE, et al. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology 2012; 264: 670–78. doi: 10.1148/radiol.12112669 [DOI] [PubMed] [Google Scholar]
- 34. Li J, Dershaw DD, Lee CH, Joo S, Morris EA. Breast MRI after conservation therapy: Usual findings in routine follow-up examinations. AJR Am J Roentgenol 2010; 195: 799–807. doi: 10.2214/AJR.10.4305 [DOI] [PubMed] [Google Scholar]
- 35. Giess CS, Yeh ED, Raza S, Birdwell RL. Background parenchymal enhancement at breast MR imaging: Normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 2014; 34: 234–47. doi: 10.1148/rg.341135034 [DOI] [PubMed] [Google Scholar]
- 36. Pereira SMP, McCormack VA, Hipwell JH, Record C, Wilkinson LS, Moss SM, et al. Localized fibroglandular tissue as a predictor of future tumor location within the breast. Cancer Epidemiol Biomarkers Prev 2011; 20: 1718–25. doi: 10.1158/1055-9965.EPI-11-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Passaperuma K, Warner E, Hill KA, Gunasekara A, Yaffe MJ. Is mammographic breast density a breast cancer risk factor in women with BRCA mutations? J Clin Oncol 2010; 28: 3779–83. doi: 10.1200/JCO.2009.27.5933 [DOI] [PubMed] [Google Scholar]
- 38. Passaperuma K, Warner E, Hill KA, Gunasekara A, Yaffe MJ. Is mammographic breast density a breast cancer risk factor in women with BRCA mutations? J Clin Oncol 2010; 28: 3779–83. doi: 10.1200/JCO.2009.27.5933 [DOI] [PubMed] [Google Scholar]