Abstract
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer deaths in both sexes combined. Recent years have seen major advances in the diagnostic and treatment options for patients with non-small-cell lung cancer (NSCLC), including the routine use of 2-deoxy-2[18F]-fluoro-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) in staging and response evaluation, minimally invasive endoscopic biopsy, targeted radiotherapy, minimally invasive surgery, and molecular and immunotherapies.
In this review, the central roles of CT and 18F-FDG PET/CT in staging and response in both NSCLC and malignant pleural mesothelioma (MPM) are critically assessed. The Tumour Node Metastases (TNM-8) staging systems for NSCLC and MPM are presented with critical appraisal of the strengths and pitfalls of imaging. Overviews of the Response Evaluation Criteria in Solid Tumours (RECIST 1.1) for NSCLC and the modified RECIST criteria for MPM are provided, together with discussion of the benefits and limitations of these anatomical-based tools. Metabolic response assessment (not evaluated by RECIST 1.1) will be explored. We introduce the Positron Emission Tomography Response Criteria in Solid Tumours (PERCIST 1.0) to include its advantages and challenges. The limitations of both anatomical and metabolic assessment criteria when applied to NSCLC treated with immunotherapy and the important concept of pseudoprogression are addressed with reference to immune RECIST (iRECIST).
Separate consideration is given to the diagnosis and follow up of solitary pulmonary nodules with reference to the British Thoracic Society guidelines and Fleischner guidelines and use of the Brock (CT-based) and Herder (addition of 18F-FDG PET/CT) models for assessing malignant potential. We discuss how these models inform decisions by the multidisciplinary team, including referral of suspicious nodules for non-surgical management in patients unsuitable for surgery. We briefly outline current lung screening systems being used in the UK, Europe and North America.
Emerging roles for MRI in lung cancer imaging are reviewed. The use of whole-body MRI in diagnosing and staging NSCLC is discussed with reference to the recent multicentre Streamline L trial. The potential use of diffusion-weighted MRI to distinguish tumour from radiotherapy-induced lung toxicity is discussed. We briefly summarise the new PET-CT radiotracers being developed to evaluate specific aspects of cancer biology, other than glucose uptake. Finally, we describe how CT, MRI and 18F-FDG PET/CT are moving from primarily diagnostic tools for lung cancer towards having utility in prognostication and personalised medicine with the agency of artificial intelligence.
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer deaths in both sexes combined. 1 The age-standardised 5-year net survival for lung cancer is in the range 10–20%. 2 Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and includes adenocarcinoma, squamous cell carcinoma and large cell carcinoma. 3 Management of the lung cancer patient is influenced by the histopathological subtype, the molecular characteristics of the tumour and the stage of disease. Recent years have seen major advances in the diagnostic and treatment options for patients with NSCLC, including the routine use of 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in staging and response evaluation, 4 minimally invasive endoscopic biopsy, 5 targeted radiotherapy, 6 minimally invasive surgery, 7 and molecular and immunotherapies. 8 It is incumbent on the radiologist to keep abreast of both therapeutic and imaging advances in order to optimise and accurately interpret imaging examinations in lung cancer patients. 9
Lung cancer diagnosis and staging
Whether identified through screening or a suspected cancer pathway, CT (low-dose CT (LDCT) in screening; typically i.v. contrast-enhanced CT in suspected cases), is the first modality for diagnosis and initial staging of lung cancer. Patients diagnosed with possible lung cancer are then offered 18F-FDG PET/CT for staging completion. Currently, patients with stage II–IV disease are offered MRI brain for identification of cerebral metastases (UK National Institute for Health and Care Excellence (NICE) guidelines). The eighth edition of the Tumour, Node, Metastases (TNM) classification for NSCLC (TNM-8) (Table 1) is used to stage lung cancer. This is informed by sophisticated statistical analysis of an international database of over 100,000 patients. 11 TNM-8 can be applied to preoperative clinical staging, pathological staging, restaging post-therapy and staging of recurrence. 4
Table 1.
TNM-8 descriptors for NSCLC (Adapted from Carter et al, 2018 10 )
| Category | Definition |
|---|---|
| T | |
| Tx | Primary tumour cannot be assessed, or tumour proven by non-radiological means (sputum or bronchial washings) and cannot be visualised with imaging (or bronchoscopy) |
| T0 | No primary tumour |
| Tis | Carcinoma in situ (squamous or adenocarcinoma) |
| T1 | Tumour ≤3 cm in greatest dimension |
| T1a T1b T1c |
Tumour ≤1 cm |
| Tumour >1 cm but ≤2 cm | |
| Tumour >2 cm but ≤3 cm | |
| T2 | Tumour >3 cm but ≤5 cm or tumour involving the main bronchus (regardless of the distance from the carina), the visceral pleura, with partial or complete lung atelectasis or pneumonitis |
| T2a T2b |
Tumour >3 cm but ≤4 cm in greatest dimension |
| Tumour >4 cm but ≤5 cm in greatest dimension | |
| T3 | Tumour >5 cm but ≤7 cm in greatest dimension or one that directly invades: parietal pleura, chest wall (including superior sulcus tumours), phrenic nerve, parietal pericardium, or separate tumour nodule or nodules in the same lobe |
| T4 | Tumour measuring >7 cm in greatest dimension or which invades mediastinum, diaphragm, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina; or separate tumour nodule(s) in a different lobe of ipsilateral lung |
| N | |
| N0 | No regional lymph node metastases |
| N1 | Ipsilateral peribronchial and/or ipsilateral hilar nodes and intrapulmonary nodes, including involvement by direct extension |
| N2 | Ipsilateral mediastinal and/or subcarinal nodes |
| N3 | Contralateral mediastinal, contralateral hilar, scalene, or supraclavicular nodes |
| M | |
| M0 | No distant metastases |
| M1a | Separate tumour nodule(s) in the contralateral lung, malignant pleural effusion, pleural thickening/nodules or masses, malignant pericardial effusion/ pericardial thickening/nodules/masses |
| M1b | Single extrathoracic metastasis in a single organ (including non-regional lymph nodes) |
| M1c | Multiple extrathoracic metastases in a single organ or multiple organs |
NSCLC, non-small cell lung cancer.
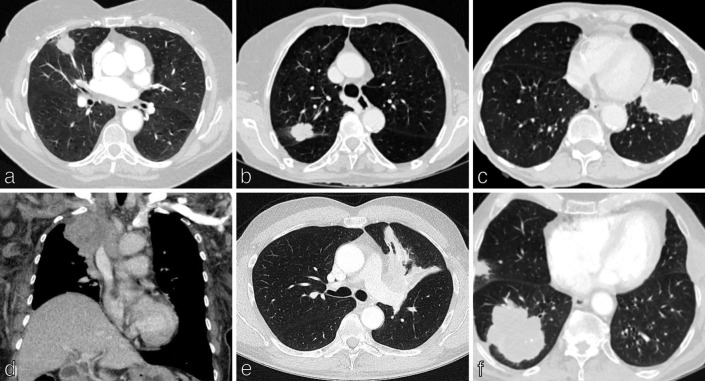
The T element refers to the pre-operative primary tumour with size being important and progressive reduction in patient survival observed for each 1 cm cut point. 11,12 As Table 1 demonstrates, primary lung tumours (T) are categorised according to size from T1 to T4. The size of the primary is measured as the longest diameter in any plane on CT, on lung windows. 13 In part-solid tumours, the size of the solid component determines the T category (Figures 1a - 1c). 13 In addition to size criteria, T2–T4 descriptors assess further features of the primary malignancy which impact treatment options and survival. T2 descriptors include any size tumour which involves the main bronchus, invades the visceral pleura or is associated with atelectasis or pneumonitis (Figure 1e). T3 defines malignancies which invade parietal pleura, chest wall (including superior sulcus tumours), phrenic nerve, parietal pericardium or separate tumour nodule(s) in the same lobe. T4 descriptors include tumours greater than 7 cm and/or which invade mediastinum, diaphragm, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina or separate tumour nodule(s) in different lobe of the same lung (Figures 1d and 1f).
Figure 1.
TNM staging in non-small cell lung cancer: Tumour (T) category. Tumour (T) classification is defined by the size of the primary lung tumour measured in the longest plane on CT on lung windows and by additional characteristics which affect the treatment options and prognosis. (a) Axial CT chest shows a 2.4 cm middle lobe tumour in a 65-year-old woman with emphysema, T1b disease. (b) Axial CT chest shows a 3.7 cm right upper lobe tumour in a 76-year-old woman, T2a disease. (c) Axial CT chest shows a 6.5 cm lingular tumour in a 70-year-old man with emphysema, T3 disease. (d) Coronal CT chest shows an 8 cm right upper lobe tumour in a 63-year-old woman which invades the superior vena cava, T4 disease. (e) Axial CT chest shows a 4.5 cm left upper lobe tumour with atelectasis, T2b disease. (f) Axial CT chest shows an 8 cm right lower lobe tumour with a further 2 cm tumour in separate lobe (right upper) of the ipsilateral lung, T4 disease. TNM, Tumour, Node, Metastases.
Nodal (N) classification is defined by the involvement of intrathoracic lymph nodes and nodes are measured in shortest diameter. N1 refers to disease in ipsilateral peribronchial, hilar and intrapulmonary nodes (including from direct extension). N2 includes metastases in ipsilateral mediastinal and/or subcarinal nodes; patients can have single- or multistation N2 disease. N3 refers to disease in contralateral hilar, ipsi- or contralateral scalene, or supraclavicular lymph nodes. 18F-FDG PET/CT plays a central role in mapping nodal disease in lung cancer and in identifying targets for histological sampling and radiotherapy planning (Figure 2a and 2b). 14 As described later in this review, potential nodal involvement usually requires histological confirmation. Metastatic disease burden is divided into M0, M1a (separate tumour nodule(s) in contralateral lung, malignant pleural effusion, pleural thickening/nodules/masses, malignant pericardial effusion, pericardial thickening/nodules/masses), M1b (single extrathoracic metastasis in a single organ) and M1c (multiple extrathoracic metastases in a single organ or multiple organs) (Figure 2).
Figure 2.
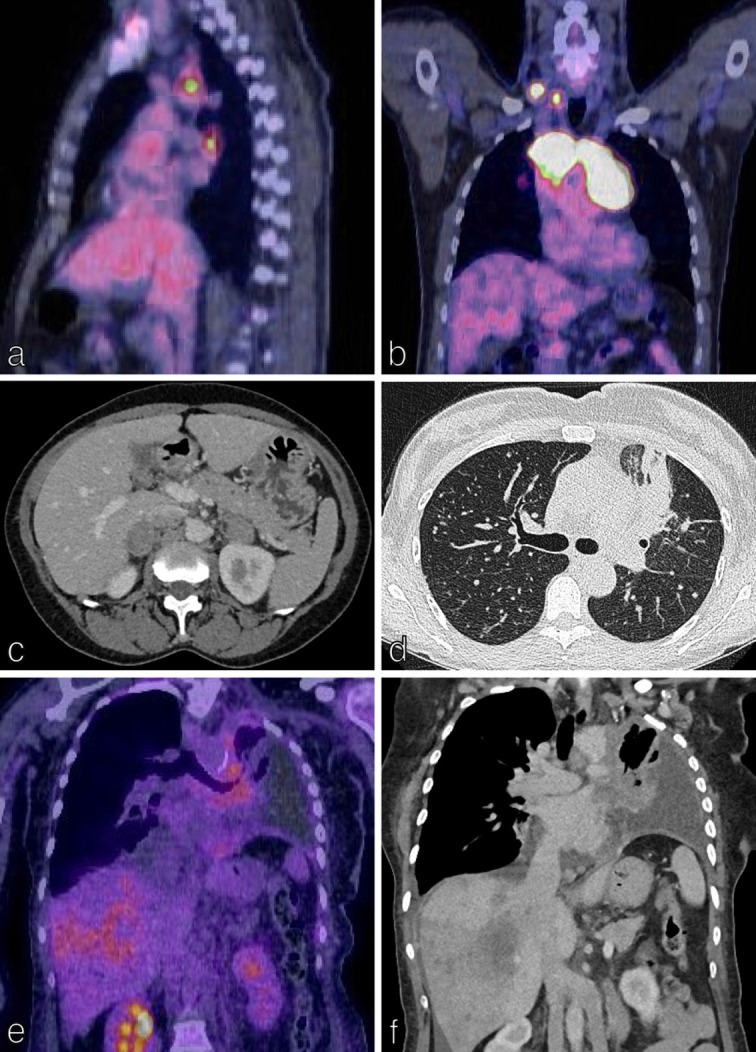
TNM staging in NSCLC-nodal (N) and metastatic (M) categories.Nodal (N) classification is defined by involvement of intrathoracic lymph nodes, measured in shortest diameter. 18F-FDG PET/CT plays a central role in mapping nodal disease in lung cancer and in the identification of distant metastases. (a) N2 disease: fused, saggital 18F-FDG PET/CT shows FDG-uptake in ipsilateral mediastinal lymph nodes in a 55-year-old man with a right upper lobe lung NSCLC (not imaged); nodal metastases were confirmed on EBUS-TBNA. (b) N3 disease: fused, coronal 18F-FDG PET/CT shows FDG-uptake in bilateral mediastinal and right supraclavicular lymph nodes in a 50-year-old man with a 3 cm right lower lobe NSCLC (not imaged); nodal metastases were confirmed by ultrasound-guided biopsy of the right supraclavicular nodes. (c) M1C disease: axial CT of the abdomen shows bilateral adrenal metastases in a 65-year-old man with histologically confirmed NSCLC. (d–f) T4N2M1C disease in a 54-year-old patient with a left lung primary tumour shown on axial CT chest (d), ipsilateral mediastinal lymph nodes shown on coronal 18F-FDG PET/CT (e), left pleural effusion and multiple liver metastases shown on coronal 18F-FDG PET/CT (e) and coronal CT chest (f). EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; NSCLC, non-small cell lung cancer; TNM, Tumour, Node, Metastases.
Special considerations: lung cancer with multiple primary sites of involvement
In patients with more than one primary lung cancer, each malignancy should be staged separately, and this applies to both synchronous and metachronous lung cancers, regardless of location. 10 This can be challenging in clinical practice if the histology of the cancer is unknown and usual practice is to provide a range of stages (e.g. T1a (multiple), or M1a) and ensure that patients are not denied potentially effective treatment. Lung cancer can present as multiple ground-glass/part-solid nodules/masses on CT and these appearances are associated with lepidic adenocarcinoma, adenocarcinoma in situ or minimally invasive adenocarcinoma. 15 Classification is based on the lesion with the highest T-level (based on solid component) and the number of lesions (Figure 3b and c). A subset of lung cancers are defined as consolidative/pneumonic appearing on CT as consolidation and ground-glass opacification, often with air bronchograms (Figure 3a). Most pneumonic-type lung cancers are invasive mucinous adenocarcinomas, can be multicentric with metastases being rare at presentation. 15 In these cases, multiple sites of tumour involvement are designated T3 if they are confined to a single lobe and T4 if they affect different lobes of the same lung and M1a if both lungs are affected.
Figure 3.
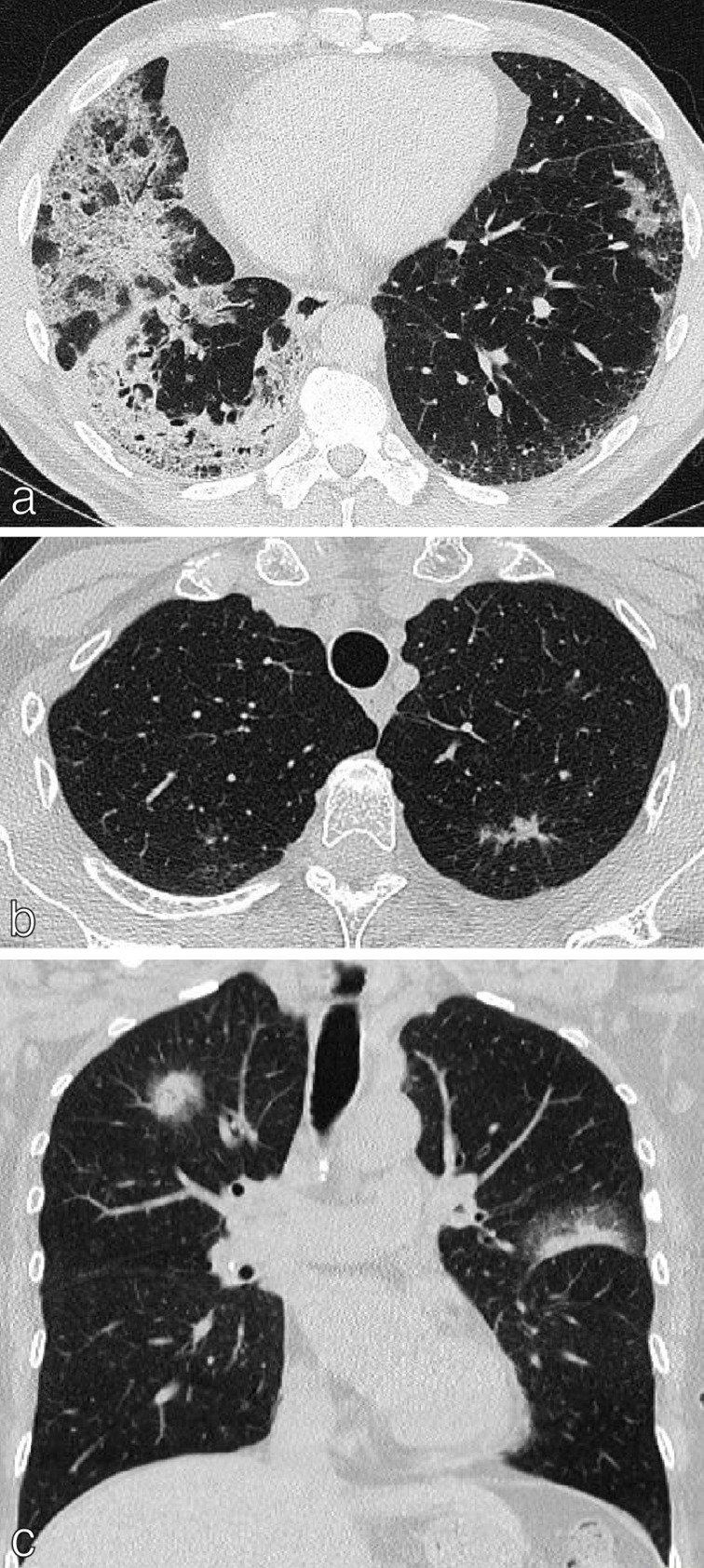
Lung cancer with multiple sites of involvement. (a) Axial CT chest of a 54-year-old ex-smoker presenting with bronchorrhea showing multiple areas of consolidation and ground-glass opacification; histologically confirmed as mucinous adenocarcinoma. (b) Axial CT chest of a 60-year-old female showing a 1.5 cm left upper lobe spiculate nodule histologically confirmed as NSCLC. Multiple ground-glass nodules bilaterally represent AIS/MIA. (c) Coronal CT chest of a 66-year-old female showing solid right upper lobe and left upper lobe tumours with peripheral ground-glass (largest 2.5 cm) plus multiple ground-glass opacities in the lungs; histologically proven as non-mucinous adenocarcinoma. These were staged as multiple synchronous primaries, T1C (multiple) disease. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; NSCLC, non-small cell lung cancer.
CT and 18F-FDG PET/CT in staging NSCLC: strengths and pitfalls
In most cases, CT is the most suitable technique for defining the T category. 12 Assessment of nodal disease on CT considers lymph nodes with short axis greater than 10 mm to be abnormal. However, studies show that this classical criterion has little diagnostic accuracy and that CT alone is suboptimal for nodal staging. 11,16 This is because factors other than size, e.g. density, enhancement, morphology and location are also important predictors of lymph node involvement. 17 Possible distant metastases (e.g. adrenal, liver, bone), identified on CT require further imaging for confirmation.
18F-FDG PET/CT provides both anatomical and metabolic information with particular strengths in the assessment of lymph nodes and unanticipated Stage IV disease. Thus, 18F-FDG PET/CT results can significantly impact on treatment options and reduce the incidence of futile thoracotomies. 18–20 In a meta-analysis of 8699 patients, 18F-FDG PET/CT had significantly higher sensitivity and specificity than CT alone in staging NSCLC. 21 Specifically, the pooled sensitivities and specificities of 18F-FDG PET/CT were 0.72 and 0.91 for mediastinal nodal staging, 0.71 and 0.83 for intrathoracic staging, 0.75 and 0.95 for all extrathoracic metastases, 0.91 and 0.98 for bone metastases. 21 18F-FDG PET/CT plays less of a role in assigning the T-status, although it is superior to CT in enabling radiologists to distinguish tumour from rounded and post-obstructive atelectasis (Figure 4 a-d). 22 Uptake of FDG in lymph nodes (especially in patients with T1 disease) may help radiologists to identify smaller nodes which are involved. This improved sensitivity of 18F-FDG PET/CT in identifying nodal disease is influenced by nodal dimension. 23 18F-FDG PET/CT is the chosen modality for assessing distant metastases (outside of the CNS). It has a higher sensitivity and specificity than bone scintigraphy for imaging bone metastases with a positive predictive value of 98% if there is PET and CT concordance (Figure 4e and f). 24
Figure 4.
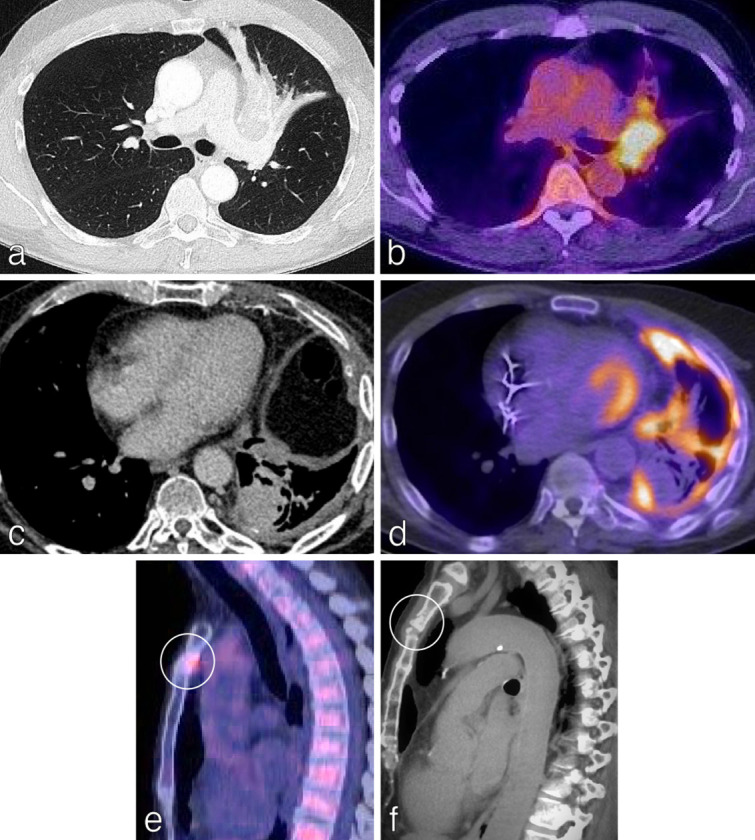
18F-FDG PET/CT is central to the accurate staging of NSCLC.18F-FDG PET/CT is superior to CT in distinguishing tumour from rounded and post-obstructive atelectasis. (a) Axial CT chest of a 62-year-old man demonstrates a left upper lobe mass with atelectasis. (b) 18F-FDG PET/CT in the same patient helps define the bronchogenic primary separate from the atelectasis enabling more accurate T-staging. (c) Axial CT chest of a 68-year-old man demonstrates a mass in the left lower lobe, estimated at 2.5 cm in diameter (T1C). Subtle pleural thickening in the left hemithorax is also present. (d) Axial 18F-FDG PET/CT in the same patient demonstrates that the 2.5 cm mass is not PET-avid and is more consistent with rounded atelectasis. The bronchogenic primary measured only 9 mm. Additionally, there is FDG uptake in the left pleura. The 18F-FDG PET/CT therefore changed the T staging to T1a and the M staging to M1a (avid pleural thickening; confirmed as NSCLC on pleural biopsy). (e) Sagittal 18F-FDG PET/CT imaging for a 60-year-old patient with NSCLC demonstrates avid FDG uptake in the manubrium. (d) An undiagnosed pathological fracture (no history of trauma) was confirmed on the original staging CT, thereby upstaging the disease and changing management.
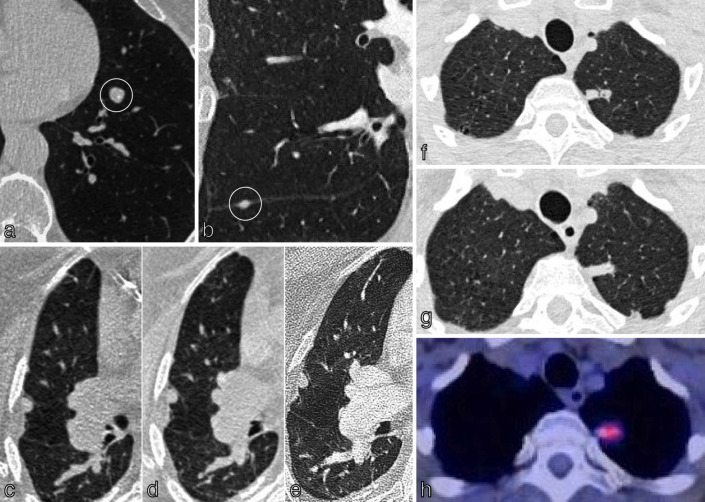
There are important limitations to the use of 18F-FDG PET/CT in staging NSCLC. In assessment of the T-status, false negatives can occur in nodules below 10 mm (T1a), low grade disease (Tis), minimally invasive adenocarcinoma (MIA), mucinous adenocarcinoma and well-differentiated carcinoid tumours (Figure 5a and b). 14 False positives can occur in infection, inflammation and granulomatous disease (Figure 5c and d). Nodal false positives can occur, e.g. in patients with TB or sarcoidosis, 21 whilst nodal false negatives can occur in small volume nodes (below the resolution of PET/CT) or those with low metabolic rate. 25 In terms of adrenal metastases, false positives (e.g. adrenal hyperplasia, functioning adenomas and tuberculosis) and false negatives (e.g. very small volume disease, haemorrhagic or necrotic metastases) must be considered. Importantly, it is not just the presence/absence of 18F-FDG uptake in intrathoracic lymph nodes or adrenal glands that influences staging and treatment. The SUVmax is important and must be interpreted in the context of the avidity of the primary tumour and other imaging and clinical factors. 26 Intracranial metastases are better imaged using MRI due to the intrinsic high FDG uptake in the central nervous system (CNS). 18F-FDG PET/CT findings suggestive for distant disease require either further radiological or histological confirmation and for mediastinal nodes, histological confirmation is needed as it affects management options (surgery/chemotherapy/radiotherapy).
Figure 5.
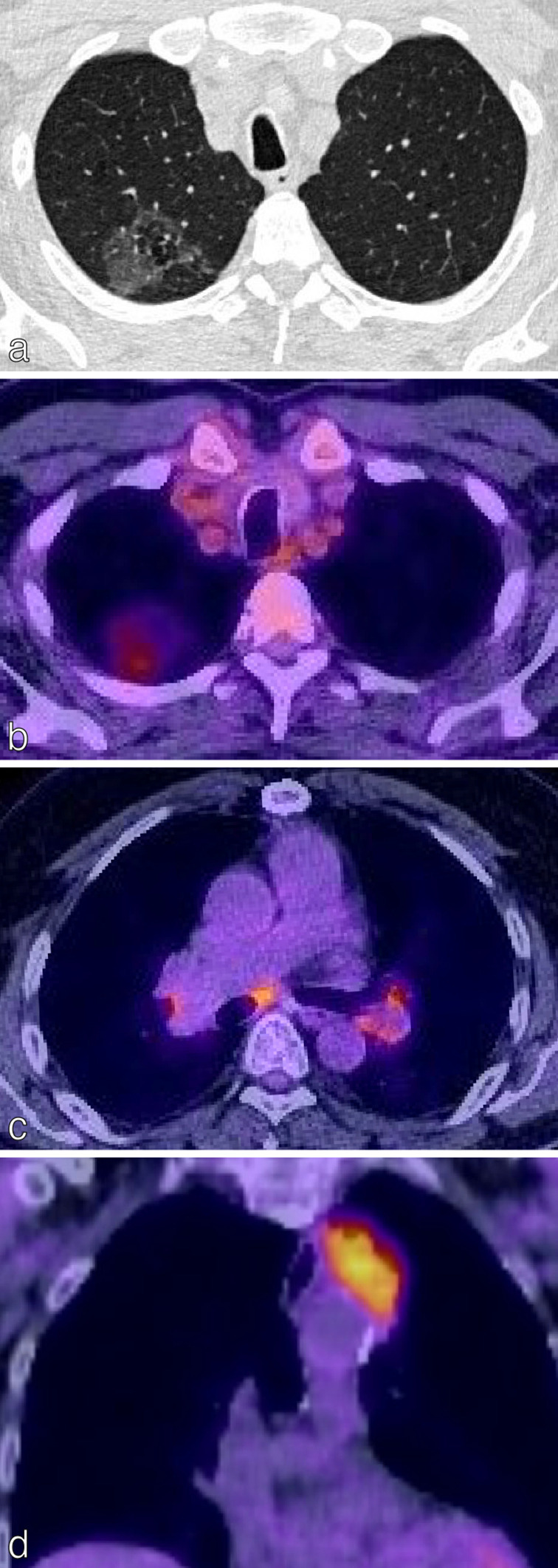
False negatives and false positives on 18F-FDG PET/CT. (a) Axial CT chest of a 72-year-old patient with confirmed adenocarcinoma in situ demonstrates a right upper lobe ground-glass opacity. (b) There was faint FDG uptake in the opacity (SUVmax less than the mediastinal blood pool); resulting in a low HERDER score. This is solely because of the ground-glass composition and emphasises the importance of interpreting FDG avidity carefully in each clinical context. (c) Axial 18F-FDG PET/CT in a patient with a T1c primary NSCLC demonstrates FDG-avidity in bilateral hilar lymph nodes and a subcarinal node; suspected N3 disease. EBUS confirmed granulomatous cells and no malignancy, thereby a false-positive study. (d) Coronal 18F-FDG PET/CT images of the thorax in an 88-year-old female presenting with trauma. The left upper lobe mass adjacent to the mediastinum is highly FDG-avid. CT-guided biopsy confirmed necrotising granulomatous disease with no malignant cells; thus a false positive. 18F-FDG PET, 18-fludeoxyglucose positron emission tomography; EBUS, endobronchial ultrasound; NSCLC, non-small cell lung cancer; SUVmax, maximum standardized uptake value.
Staging malignant pleural mesothelioma (MPM) (TNM-8)
MPM is a rare (incidence: approximately 30 cases/million in USA/UK), but very aggressive intrathoracic cancer with a very poor prognosis. 27,28 It is the most common primary malignancy of the pleura, associated with asbestos exposure in 80% of patients. 29 It consists of multiple nodules that may become confluent, spreading along pleural surfaces. There is often an associated pleural effusion (this may be the only finding on CT), thoracic volume loss and many patients have pleural plaques (Figure 6a–6f). 29 The T component of the TNM classification is challenging to apply in MPM because of its unconventional growth pattern. 30 The T-staging classification is shown in Table 2. Notably, tumour volume is not one of the main descriptors of disease burden even though it affects prognosis. 31 There are significant survival differences between T4 vs T3 disease and T3 vs T2 disease. 32 The N-component is an important prognostic indicator for survival in MPM as patients with nodal disease have significantly lower survival rates that those without. 33 N1 disease refers to ipsilateral bronchopulmonary, hilar or mediastinal nodes and N2 to contralateral intrathoracic and supraclavicular nodes. Distant metastases in MPM are designated M1 disease and is the only M status which determines Stage IV disease (Table 2; Figure 6). 34 Distant metastases are usually rare since patients often die from locally aggressive disease. 33
Figure 6.
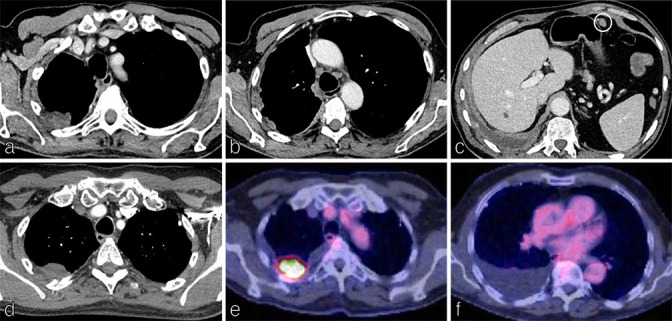
Staging malignant pleural mesothelioma (MPM). Axial CT images of the chest and upper abdomen on soft tissue windows in an 83y man presenting with chest pain shows irregular pleural thickening involving the right pleura (a) and including the mediastinal surface (b) with associated volume loss and a shallow right pleural effusion (c). A metastatic deposit is identified anterior to the stomach (c) making this stage IV disease; confirmed as epitheloid MPM on histology. Axial CT chest on soft tissue windows (d) and corresponding 18F-FDG PET/CT (e, f) demonstrate a focus of irregular soft tissue in the pleura of the right upper lobe with invasion into the adjacent posterior chest wall, rib destruction plus a right pleural effusion, T4 tumour.
Table 2.
Modified eighth TNM descriptors for staging malignant pleural mesothelioma. Adapted from Berzenji et al., 2018
| Category | Definition |
|---|---|
| T | |
| Tx | Primary tumour not assessable |
| T0 | No evidence of a primary tumour |
| T1 | Tumour involving the ipsilateral parietal pleura (including mediastinal and diaphragmatic pleura) with or without involvement of the visceral pleura |
| T2 | Tumour involving each of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic and visceral) with at least one of the following:
|
| T3 | Tumour involving all of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic and visceral) with at least one of the following features:
|
| T4 | Tumour involving all of the ipsilateral pleural surfaces with at least one of the following features:
|
| N | |
| Nx | Regional lymph nodes not assessable |
| N0 | No regional lymph nodes metastases |
| N1 | Metastases in ipsilateral bronchopulmonary, hilar or mediastinal lymph nodes (including the internal mammary, peridiaphragmatic, pericardial fat pad or intercostal lymph nodes) |
| N2 | Metastases in the contralateral bronchopulmonary, hilar or mediastinal lymph nodes or ipsilateral or contralateral supraclavicular lymph nodes |
| M | |
| Mx | Presence of distant metastases not assessable |
| M0 | No evidence of distant metastases |
| M1 | Evidence of distant metastases |
CT has 68% sensitivity and 78% specificity for pleural malignancy but cannot reliably be used to differentiate MPM from pleural metastatic disease. 35 Although not routinely used, MRI, with its superior soft-tissue resolution over CT, improves identification of T3 disease and disease in the diaphragm, pericardium and chest wall. 36 In clinical practice, 18F-FDG PET/CT is routinely used for staging and to guide surgical biopsy. Important limitations of 18F-FDG PET/CT include its inability to differentiate between MPM and pleural metastases, and the fact that inflammatory disorders such as tuberculous pleurisy and prior pleurodesis can give false- positive results. 37 Importantly, pleurodesis-related 18F-FDG PET-CT avidity can “wax and wane” for months to years following the procedure with consequent fluctuations in standard uptake value maximum (SUVmax) due to activated macrophages and granulation tissue. Regardless of the imaging modalities used to stage the disease, thorascopic biopsy is required for definitive diagnosis and staging of MPM. 33
Solitary pulmonary nodules
Pulmonary nodules are focal densities ≤3 cm surrounded by aerated lung. The challenge is to discriminate between benign, inflammatory nodules (the vast majority; even in smokers) and those which represent pre-malignant, pre-invasive or early invasive adenocarcinoma. 38 Identification of early stage adenocarcinoma reduces mortality. 39 The 2015 guidelines from the British Thoracic Society (BTS) and the Fleischner Society guidelines (2017) 40 outline recommendations for the identification and follow-up of suspicious pulmonary nodules with areas of agreement and divergence between the two. In both guidelines, nodule follow-up is not recommended for nodules with clear features of benignity such as popcorn calcification (Figure 7a) or for nodules with “typical” perifissural or subpleural location with triangular or lentiform shape (often intrapulmonary lymph nodes) (Figure 7b) or for nodules <5 mm (<80 mm3) (BTS) or <6 mm (Fleischner). In both guidelines, recommendations for nodules not considered definitely benign are informed by the composition of the nodule (solid, part-solid, ground-glass), followed by nodule size and assessment of risk of malignancy (nodule characteristics and patient risk factors). 41 The guidelines differ in a number of respects, such as the scope and target populations, definition of substantial growth, follow-up intervals and components of the risk assessment. 41
Figure 7.
Identification and follow up of pulmonary nodules. In both the BTS and Fleischner guidelines, nodules with clear features of benignity are not followed up. (a) Axial chest CT of a 66y smoker shows a left lower lobe nodule with calcification and central fat attenuation typical for a hamartoma; no follow up. (b) Coronal chest CT in a 60y smoker shows a lentiform/triangular shaped nodule associated with the horizontal fissure, typical for intrapulmonary lymph node; no follow up. The risk of malignancy in pulmonary nodules can be calculated using the Brock risk prediction tool. Nodules with < 10 % risk malignancy which are 80 mm3 (6mm) are followed up at three months and one year from baseline. (c-e) Axial CT chest imaging of an incidentally detected 9mm right upper lobe solid pulmonary nodule at baseline (c), 3 months (d) and one year (e) demonstrates stability based on the 2D non-automated diameter; plan for follow up 2 years from baseline as assessed under the BTS guidelines. Nodules showing clear growth ( 25 % volume increase/volume doubling time (VDT) of < 400 days) undergo further diagnostic work-up with 18F-FDG PET/CT /biopsy. (f-h) A 17mm left upper lobe nodule assessed at baseline (f) and at year 1 (g). Clear growth was demonstrated with more than 25 % increase in volume. The SUVmax on 18F-FDG PET/CT was 2.83 (moderate) (h) with a Herder score of 89.1 % and subsequent plan for image-guided biopsy and MDT discussion regarding treatment options.
The BTS guidelines are applied to nodules detected both incidentally and in screening. In BTS, CT surveillance is offered to patients with nodules ≥5 to<8 mm (≥80 to < 300 mm3). Automated or semi-automated volumetry is preferred over diameter measurements as it is more reproducible. In nodules ≥300 mm3 or ≥8 mm, the pre-test probability of malignancy is assessed using the Brock Risk Prediction Tool which is derived from a study of two cohorts of current or former smokers undergoing LDCT. 42 Factors including family history of lung cancer, emphysema, larger nodule size, nodule location in the upper lobe, part-solid type and spiculation are considered malignant features. 42 Nodules which are risk stratified as having a <10% risk of malignancy using Brock are followed up on imaging (Figure 7c–e). Nodules with a ≥10% risk of malignancy are referred for 18F-FDG PET/CT (provided they are larger than local PET-CT threshold) with post-test probability of malignancy assessed by the Herder model (Figure 7f–h).
In the Herder model, an ordinal scale is used to define tracer uptake as absent (indiscernible from background lung tissue), faint (less than or equal to mediastinal blood pool), moderate (greater than mediastinal blood pool), or intense (markedly greater than mediastinal blood pool). 43 Although the Herder score was based on raw PET data, rather than the corrected data (as used in MDT discussions), it has been validated in a retrospective study. 44 Following Herder analysis, patients with a malignancy risk of >10–70% are offered image-guided biopsy, excision biopsy or CT surveillance. Patients with risk >70% are offered surgical resection (ideally by video-assisted thorascopic surgery), or non-surgical treatment, often with (although sometimes without) image-guided biopsy. The decision to refer patients with >70% risk of malignancy for non-surgical management (e.g. stereotactic ablative body radiotherapy (SABR), radiofrequency/microwave ablation, conventional radical radiotherapy) without histopathological confirmation, is a multidisciplinary one. The incorporation of clinically diagnosed lung cancer into the BTS guidelines reflects the findings of retrospective cohort studies showing similar outcomes between pathologically proven and clinically diagnosed early stage lung cancers in certain patient groups. 45–47 SABR, in particular, represents a significant advance in modern radiotherapy and provides targeted high doses in few fractions resulting in excellent local control and relatively minimal toxicity in standard-risk patients. 45,48
For those nodules under CT surveillance, the BTS guidelines define significant growth as a ≥25% vol increase. Further diagnostic work-up should be offered for nodules showing clear growth or a volume-doubling time (VDT) of <400 days. Patients with stable solid nodules 1 year after baseline (based on VDT) or 2 years (based on two-dimensional measurements) can be discharged. Part-solid and pure ground-glass nodules are followed-up differently, reflecting their generally slower growth, but paradoxically higher malignant potential.
Lung screening
Targeted lung screening reduces disease-specific mortality and is now recommended by both the UK National Screening Committee (UKNSC) and the US Preventive Services Task Force (USPSTF). 49 In both screening programmes, current and former smokers aged 55–74 years (UKNSC) and 50–80 years (USPSTF) are invited for screening, (biennially in the UK; annually in North America), with LDCT. Reporting of screen-detected lung nodules in the UK and Europe is based on the BTS guidelines. 50 The European position statement on lung cancer screening (2017) 50 recommends the use of volumetry and volumed-doubling time in risk assessment. In North America, the Lung CT Screening Reporting and Data System (Lung-RADS) from the American College of Radiology (ACR) provides a standard method of reporting and managing screening-detected lung nodules. 51 It can reduce the false-positive rate without increasing the rate of false negatives and is now routinely used in this screening population. 52,53 In this tool, the diameter (rather than the volume), of the dominant solid, part-solid or ground-glass nodule is used to risk-stratify nodules into five categories: 1, negative; 2, benign; 3, probably benign; 4A, suspicious; 4B, very suspicious. Suggested clinical management is annual repeat LDCT for categories 1 and 2, 6 month follow-up LDCT for category 3 nodules; either 3 month repeat LDCT or 18F-FDG PET/CT for 4A and chest CT, 18F-FDG PET-CT and/or biopsy for category 4B. 54,55 Notably, in both the BTS and the Lung-RADS systems, the follow-up and management of ground-glass nodules remains challenging, especially when found in younger patients. 55 Special consideration must be given to “incident” nodules detected in lung screening. These are nodules which are were not present at baseline and are therefore potentially fast-growing and have a high cancer risk of 2–8%. 50 In Europe, proposed follow-up protocols for these nodules are therefore different from baseline nodules. 50 It is likely that nodule management protocols will continue to evolve as lung screening programmes are implemented more widely.
Response to treatment
RECIST 1.1
Accurate and timely assessment of response to treatment (tumour shrinkage; time to progression) is vital in order to optimise patient outcomes. 56 RECIST 1.1 (Response Evaluation Criteria in Solid Tumours) uses changes in tumour burden on CT/MRI to assess response to treatment (Figure 8). A maximum of five lesions are monitored for change in size (measured in one dimension; must be >10 mm on CT to be measurable) and/or two lesions per organ. 57 Pathological lymph nodes (nodes with a dimension 15 mm (short axis) and above) are considered measurable and are target lesions. Nodes that shrink to <10 mm short axis are considered normal. Response to treatment is described as: (i) complete response (CR, disappearance of all target lesions), (ii) partial response (PR, ≥30% decrease in tumour size from baseline), (iii) progressive disease (PD, ≥20% increase in tumour size) and (iv) stable disease (SD) (Figure 8). 58 Important caveats to diagnosing recurrence on CT alone include the difficulty in distinguishing between radiation-induced lung toxicity (RILT) and tumour-atelectasis complex formation; this is where other modalities can add benefit. 59
Figure 8.
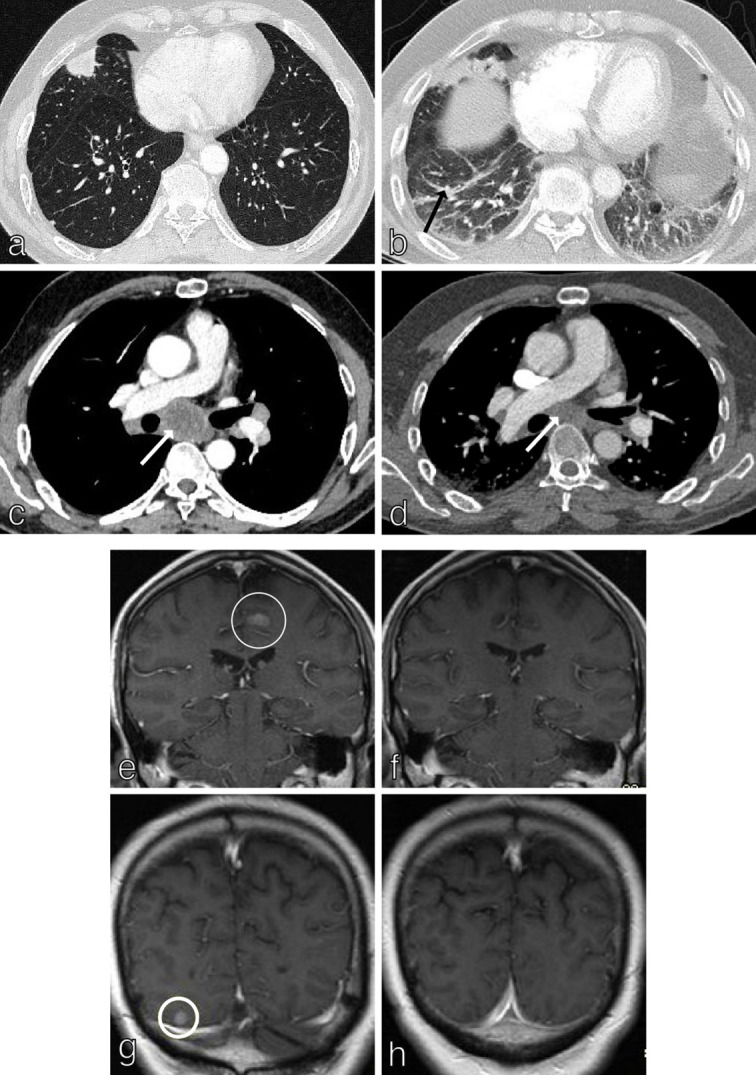
RECIST 1.1 in NSCLC. RECIST 1.1 uses changes in tumour burden on CT/MRI to assess response to treatment. Axial CT chest in a 62y patient with T1cN3M1c (brain, adrenal glands) NSCLC at baseline (a, c) and follow-up (b, d) demonstrates partial response (≥30 % decrease in tumour size from baseline) in a middle lobe primary tumour and subcarinal lymph nodes (white arrows). Treatment-related pneumonitis is evident on the follow-up study (black arrow; b). Contrast enhanced MRI brain in the same patient at baseline (e, g) and follow up (f, h) demonstrates complete response to treatment with disappearance of all target brain metastases.
Modified RECIST—MPM
The modified RECIST criteria were developed in 2004 to enable response evaluation in MPM, given its unique growth pattern. 60 In this system, tumour thickness perpendicular to the chest wall or mediastinum is measured in two positions at three separate levels on axial CT and the sum of these six measurements defines a pleural unidimensional measurement. 60 Treatment response is then assessed by measuring the same areas of pleura. Measurable lesions such as nodes and subcutaneous nodules are assessed as per RECIST. CT remains the main modality to assess MPM but similarly to NSCLC, functional imaging techniques (PET, MRI-DWI, DCE-MRI) play a complementary role. There is great potential for the use of automated measurement of MPM and deep learning in order to address issues such as inter- and intraobserver variability and difficulties in distinguishing between pleural disease and pleural effusion. 61
Assessing response: beyond RECIST 1.1
Functional and metabolic imaging
Although RECIST 1.1 is validated in prospective studies and is used widely for response assessment, there are important limitations to assessing response based solely on size in lung cancer. Accurate and consistent measurement can be difficult in measuring ground-glass nodules, semi-solid nodules or invasive lepidic carcinoma. 58,62 Markers of disease response such as density, functional and metabolic changes are not evaluated by RECIST 1.1. Metabolic responses and necrosis often precede size changes, especially in the context of cytostatic agents and immunotherapy. Techniques such as dual-energy CT, 18F-FDG PET/CT, MRI and diffusion-weighted imaging (DWI)-MRI are increasingly used to provide functional and metabolic information in both assessment and prediction of response to treatment. 58 Analysis of tumour textural heterogeneity (marker for vascularity), tumour density (marker for necrosis) analysed on CT, CT perfusion studies in anti-angiogenic chemotherapy agents and dual-energy CT have been evaluated in response assessment in a bid to move beyond size alone. 63–66 These techniques provide useful predictive information but are not currently in widespread clinical use and in many cases require high quality and reproducible protocols for analysis.
18F-FDG PET/CT has high diagnostic accuracy in detecting tumour recurrence with the added value of detecting metastases post-treatment. 67 However, timing of follow-up is vital as false positives can occur in post-treatment inflammation and radiation-induced lung toxicity (RILT) (Table 3). 56,67 In 2009, PET response criteria (PERCIST 1.0) were developed for systematic and structured assessment of response for use in clinical trials and potentially in the clinic. 68 In PERCIST 1.0, the peak SUV corrected for lean body mass (SULpeak) is measured in the single “hottest” tumour. To be measurable at baseline this must be ≥ 1.5 times the mean SUL plus two times its standard deviation. The mean SUL is usually measured in a 3 cm diameter spherical volume of interest (VOI) in the right side of the liver. 69 Briefly, response is assessed as complete (18F-FDG uptake less than mean SUL of liver), partial (decrease of ≥30% and of at least 0.8 SUL units in the most intense lesion (not necessarily the same lesion)), stable (increase or decrease of SULpeak<30%) or progressive (increase of ≥30% and an increase of at least 0.8 SUL units in a target lesion or development of a new lesion). 69 Challenges remain in the interpretation of response using PERCIST 1.0, including assessing the behaviour of non-target lesions, a lack of definition for unequivocal progression, how to categorise new lesions as well as multiple technical factors such as accurately defining SULpeak, accurately registering serial images from the same patient, selecting the mean SUL in the liver and comprehensively monitoring all factors affecting SUV between studies. 69,70 Automated systems to assess early response may improve on these constraints. 70 PERCIST 1.0 offers great potential; not currently realised at present.
Table 3.
Optimal time intervals proposed for response assessment using 18F-FDG PET/CT Adapted from Kandathil et al., 2019)
| Therapeutic modality | Time interval |
|---|---|
| Chemotherapy | 10 days |
| Surgery | 6 weeks |
| Radiofrequency ablation | 6–8 weeks |
| Immunotherapy | 8–9 weeks |
| Radiotherapy | 3 months |
18F-FDG PET, 18-fludeoxyglucose positron emission tomography.
Response to immunotherapy
Tumours treated with immunotherapy (particularly immune checkpoint inhibitors), demonstrate unique response patterns which are not always assessable using classic RECIST 1.1. For example, an increase in tumour size after starting immunotherapy does not always represent progression but may be a result of T-cell infiltration and thus represent response. 71 Incidences of this “pseudoprogression” in patients receiving PD-1/PDL1 inhibitors in Phase II/III clinical trials can reach 10% (Figure 9). 72 Immune-specific response criteria including iRECIST (immunotherapy RECIST) that allow continued treatment beyond radiological progression have been developed for use in clinical trials. 72 The key change in iRECIST (compared to RECIST1.1) is the concept of resetting the bar if RECIST progression is followed at the next assessment by tumour shrinkage. 73 In iRECIST, response is designated as immune complete response (iCR), immune stable disease (iSD), immune partial response (iPR) and immune unconfirmed progressive disease (iUPD) or immune confirmed progressive disease (iCPD). 73 Definitions of CR, SD, PR and PD are unchanged from RECIST1.1. iUPD can be assigned multiple times provided that iCPD is not confirmed at the next assessment. Progression is confirmed in a target lesion if the next assessment after iUPD (4–8 weeks later) confirms a further increase in the sum of measures of target disease from iUPD, with at least 5 mm increase. 73 This means that patients who are designated as iUPD and not iCPD on follow-up studies can remain on therapy. However, these are not considered superior to RECIST 1.1 given the relatively low incidences of pseudoprogression and the added problem of hyperprogression that has been observed in 9–29% of patients treated with immunotherapy. 72,74,75
Figure 9.
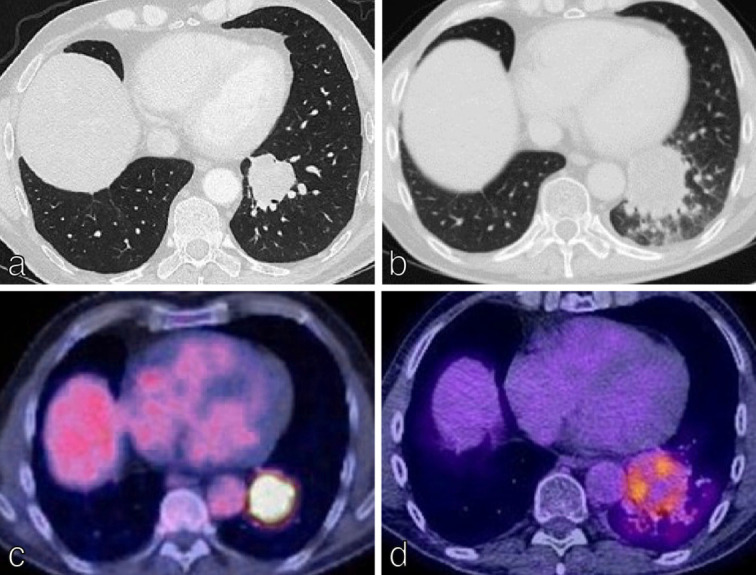
Response to immunotherapy: pseudoprogression. (a-d) Axial CT and corresponding axial 18F-FDG PET/CT images from a 67y man with left lower lobe T2aN2M0 NSCLC commenced on immunotherapy (Durvalumab). CT images show an increase in the size of the lung primary between baseline (a) and follow-up (b) suggesting disease progression. However, the corresponding 18F-FDG PET/CT images (c) and (d) demonstrate partial metabolic response in the tumour. MDT decision was therefore for the patient to remain on current treatment regimen with close surveillance imaging.
Future horizons
As outlined earlier, the current diagnostic pathway for patients with newly diagnosed NSCLC involves a combination of CT, MRI and 18F-FDG PET/CT. In this section, we review some of the new and emerging techniques which offer potential for improved accuracy of staging and response evaluation of NSCLC.
Whole-body MRI (WB-MRI)
A multicentre, prospective study in England (Streamline L trial) compared the diagnostic accuracy and efficiency of WB-MRI with standard staging pathways for metastatic disease in new NSCLC patients (destined for curative treatment). WB-MRI pathways had similar accuracy to standard pathways for T-staging but reduced sensitivities for nodal staging. 76 Diagnostic accuracy was similar to standard pathways for identifying metastatic disease but WB-MRI had improved performance in terms of time and cost efficiencies. Although generally underappreciated, reducing time to treatment is associated with improved survival and reduced patient anxiety. 77
Diffusion-weighted magnetic resonance imaging (DW MRI)
When tumours respond to treatment we expect reduction in their cellularity. This can be measured as an increase in apparent diffusion coefficient (ADC) on DWI MRI. 78 A number of small studies indicate that ADC may be an independent marker of response of NSCLC to radiochemotherapy with potential to assess for early response, before reduction in tumour size. 78 As mentioned earlier, conventional CT or 18F-FDG PET-CT imaging follow-up of NSCLC patients treated with radiotherapy cannot reliably distinguish tumour recurrence, atelectasis and RILT. A number of small studies have shown that DWI offers potential to troubleshoot in such cases, thus reducing the number of patients undergoing unnecessary invasive diagnostic procedures for benign lung change. 56,79 In addition to assessing response to treatment, DWI can potentially distinguish benign from malignant lymph nodes (benign having high ADC values) 80 and could play a role in radiotherapy-planning. 81
New PET tracers
The need to evaluate more specific aspects of cancer biology other than glucose uptake has led to the development of new radiopharmaceuticals for potential future use in PET imaging. 82 18F-fluorothymidine (18F-FLT) is a marker for cellular proliferation and correlates with the activity of thymidine kinase-1 (TK-1). It does not appear to accumulate in inflammatory cells, thereby offering improved specificity over 18F-FDG PET/CT. 83 This tracer also has the potential to identify chemoradioresistant tumours post-treatment. 83 The response of tumours to treatment is influenced by the level of tumour oxygenation with intratumoral hypoxia increasing radio- and chemoresistance. 84 A number of tracers for imaging hypoxia have been developed. 18F-FMISO, first used in cardiac imaging has been shown in clinical studies to have selectivity in NSCLC. 85 Angiogenesis (an important factor in the tumour microenvironment) can be assessed by targeted integrin PET imaging using 18F-Galacto-RGD. 86 Although not currently in clinical use, new PET tracers offer potential for planning individualised therapeutic regimens.
Artificial intelligence (AI), radiomics & deep-learning
CT, MRI and 18F-FDG PET/CT are moving from primarily diagnostic tools for lung cancer towards having utility in prognostication and personalised medicine with the agency of AI. 87 Radiomics represents a non-invasive biomarker that draws on a subset of AI—Machine Learning (ML) to classify quantitative features associated with a given clinical end point. 88 The radiomics workflow involves manual segmentation of a region of interest (ROI) on a scan, from which features are extracted and pre-processed prior to classification 89,90 (Figure 10). Deep-learning is a subset of ML that describes a group of more complex algorithms. When applied to medical imaging, these facilitate automated segmentation, feature-extraction and identification of complex patterns in image data. 91 These approaches have shown promise in lung cancer diagnosis, 88,92–96 including a state-of-the-art deep-learning system for LDCT screening for pulmonary nodules (AUC = 0.944) which demonstrated similar performance to radiologists. 97 Additionally, they demonstrate ability in prognostication and evaluating response to treatment, 98–100 including a model that can estimate treatment failure following stereotactic body radiotherapy and guide individualisation of radiotherapy dose. 101
Figure 10.
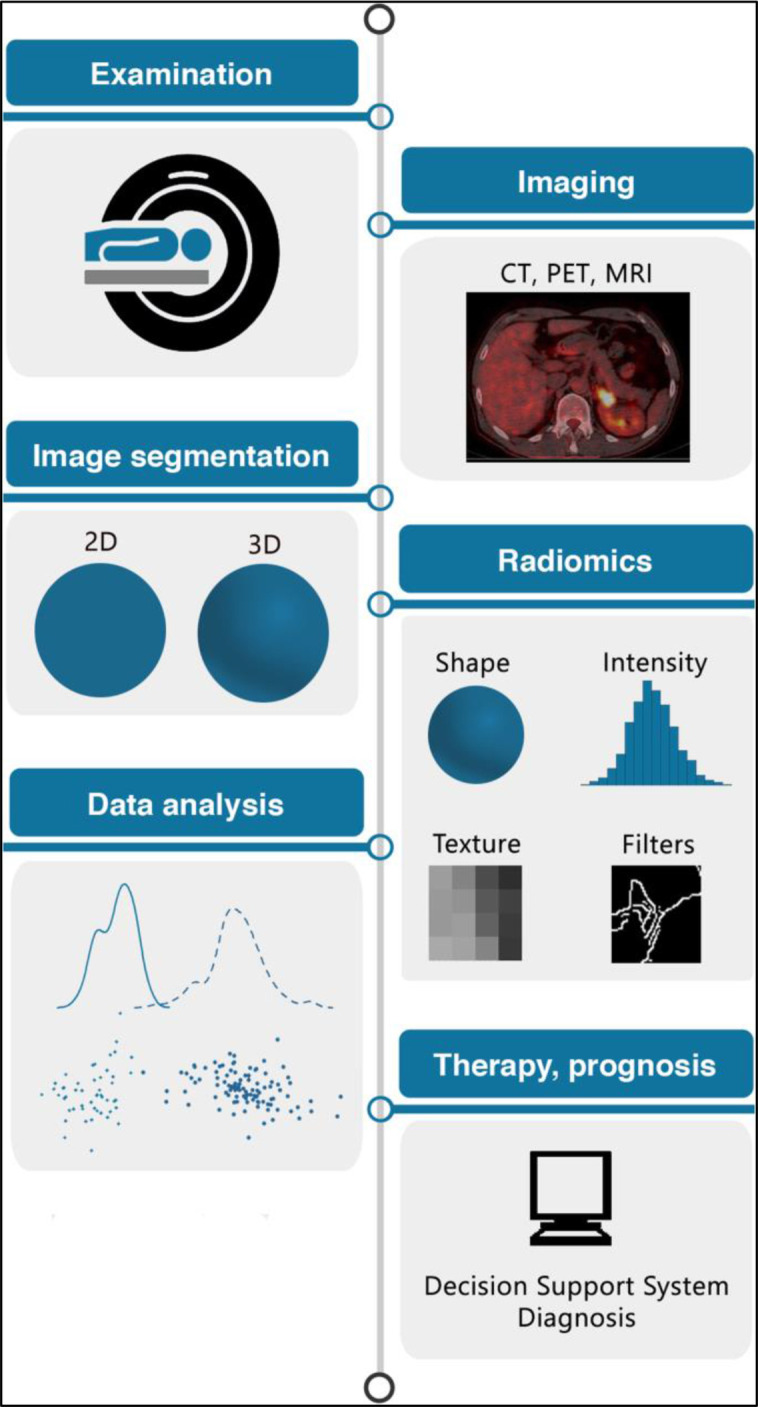
AI: The radiomics workflow. Patients undergo medical imaging, e.g. CT, MRI or PET-CT. Regions of interest (ROI) e.g. a tumour are segmented either in 2D or 3D. Radiomic features are extracted from the ROI. Machine learning is applied to radiomic features to predict a given outcome e.g. benign vs malignant lung nodule. Machine learning models can be developed for disease classification, patient clustering, or risk stratification. Figure adapted with permission from: van Timmerman J et al. DOI: https://doi.org/10.1186/s13244-020-00887-2.
Conclusion
Much progress has been made in the diagnosis and treatment of lung cancer in the past 20 years. Undoubtedly imaging has played a central role in this progress with the routine use of CT and 18F-FDG PET/CT in staging and response evaluation, the use of MRI and DWI for “problem solving”, automated and semi-automated assessment of solitary pulmonary nodules and targeted lung screening of high-risk populations. As we move towards tailored precision medicine based upon individual tumour and patient characteristics, the role of imaging will become ever more vital to ensure continued progress.
Footnotes
Acknowledgements: The authors gratefully acknowledge NHS National Institute of Health Research (NIHR) funding to the Royal Marsden Hospital and Institute of Cancer Research (ICR) London Biomedical Research Centre (BRC). Sumeet Hindocha is funded by the UKRI CDT in AI for Healthcare http://ai4health.io (Grant No. P/S023283/1).
Contributor Information
Cara Owens, Email: c.owens22@imperial.ac.uk, cara.owens@nhs.net.
Sumeet Hindocha, Email: sumeet.hindocha@rmh.nhs.uk.
Richard Lee, Email: richard.lee@rmh.nhs.uk.
Thomas Millard, Email: thomas.millard@nhs.net.
Bhupinder Sharma, Email: bhupinder.sharma@rmh.nhs.uk.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–75. doi: 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–60. doi: 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 4. Kandathil A, Kay FU, Butt YM, Wachsmann JW, Subramaniam RM. Role of FDG PET/CT in the eighth edition of TNM staging of non-small cell lung cancer. Radiographics 2018; 38: 2134–49. doi: 10.1148/rg.2018180060 [DOI] [PubMed] [Google Scholar]
- 5. Folch E, Costa DB, Wright J, VanderLaan PA. Lung cancer diagnosis and staging in the minimally invasive age with increasing demands for tissue analysis. Transl Lung Cancer Res 2015; 4: 392–403. doi: 10.3978/j.issn.2218-6751.2015.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Ruysscher D, Faivre-Finn C, Nestle U, Hurkmans CW, Le Péchoux C, Price A, et al. European organisation for research and treatment of cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol 2010; 28: 5301–10. doi: 10.1200/JCO.2010.30.3271 [DOI] [PubMed] [Google Scholar]
- 7. Ikeda N. Updates on minimally invasive surgery in non-small cell lung cancer. Curr Treat Options Oncol 2019; 20: 1–8: 16. doi: 10.1007/s11864-019-0614-9 [DOI] [PubMed] [Google Scholar]
- 8. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018; 553: 446–54. doi: 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- 9. Sharma B, Martin A, Stanway S, Johnston SRD, Constantinidou A. Imaging in oncology -- over a century of advances. Nat Rev Clin Oncol 2012; 9: 728–37. doi: 10.1038/nrclinonc.2012.195 [DOI] [PubMed] [Google Scholar]
- 10. Carter BW, Lichtenberger JP III, Benveniste MK, de Groot PM, Wu CC, Erasmus JJ, et al. Revisions to the TNM staging of lung cancer: rationale, significance, and clinical application. RadioGraphics 2018; 38: 374–91. doi: 10.1148/rg.2018170081 [DOI] [PubMed] [Google Scholar]
- 11. Rami-Porta R, Bolejack V, Giroux DJ, Chansky K, Crowley J, Asamura H, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014; 9: 1618–24. doi: 10.1097/JTO.0000000000000334 [DOI] [PubMed] [Google Scholar]
- 12. Rami-Porta R, Call S, Dooms C, Obiols C, Sánchez M, Travis WD, et al. Lung cancer staging: a concise update. Eur Respir J 2018; 51: 1–17. doi: 10.1183/13993003.00190-2018 [DOI] [PubMed] [Google Scholar]
- 13. Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016; 11: 1204–23. doi: 10.1016/j.jtho.2016.03.025 [DOI] [PubMed] [Google Scholar]
- 14. Greenspan BS. Role of PET/CT for precision medicine in lung cancer: perspective of the Society of nuclear medicine and molecular imaging. Transl Lung Cancer Res 2017; 6: 617–20. doi: 10.21037/tlcr.2017.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lambe G, Durand M, Buckley A, Nicholson S, McDermott R. Adenocarcinoma of the lung: from BAC to the future. Insights Imaging 2020; 11: 1–10. doi: 10.1186/s13244-020-00875-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gelberg J, Grondin S, Tremblay A. Mediastinal staging for lung cancer. Can Respir J 2014; 21: 159–61. doi: 10.1155/2014/890108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ganeshalingam S, Koh DM. Nodal staging. Cancer Imaging 2009; 9: 104–11. doi: 10.1102/1470-7330.2009.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JHAM, Schreurs AJM, Stallaert RALM, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the plus multicentre randomised trial. Lancet 2002; 359: 1388–93. doi: 10.1016/s0140-6736(02)08352-6 [DOI] [PubMed] [Google Scholar]
- 19. Schimmer C, Neukam K, Elert O. Staging of non-small cell lung cancer: clinical value of positron emission tomography and mediastinoscopy. Interact Cardiovasc Thorac Surg 2006; 5: 418–23. doi: 10.1510/icvts.2006.129478 [DOI] [PubMed] [Google Scholar]
- 20. Kubota K, Matsuno S, Morioka N, Adachi S, Koizumi M, Seto H, et al. Impact of FDG-PET findings on decisions regarding patient management strategies: a multicenter trial in patients with lung cancer and other types of cancer. Ann Nucl Med 2015; 29: 431–41. doi: 10.1007/s12149-015-0963-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu Y, Li P, Zhang H, Shi Y, Wu H, Zhang J, et al. Diagnostic value of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for the detection of metastases in non-small-cell lung cancer patients. Int J Cancer 2013; 132: E37–47. doi: 10.1002/ijc.27779 [DOI] [PubMed] [Google Scholar]
- 22. Beer L, Jajodia A, Prosch H. Pearls and pitfalls in lung cancer staging. 2020; 2: 20200019: 20200019. doi: 10.1259/bjro.20200019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lv Y-L, Yuan D-M, Wang K, Miao X-H, Qian Q, Wei S-Z, et al. Diagnostic performance of integrated positron emission tomography/computed tomography for mediastinal lymph node staging in non-small cell lung cancer: a bivariate systematic review and meta-analysis. J Thorac Oncol 2011; 6: 1350–58. doi: 10.1097/JTO.0b013e31821d4384 [DOI] [PubMed] [Google Scholar]
- 24. Taira AV, Herfkens RJ, Gambhir SS, Quon A. Detection of bone metastases: assessment of integrated FDG PET/CT imaging. Radiology 2007; 243: 204–11. doi: 10.1148/radiol.2431052104 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt-Hansen M, Baldwin DR, Hasler E, Zamora J, Abraira V, Roqué I Figuls M. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev 2014; 2014(. doi: 10.1002/14651858.CD009519.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Divisi D, Barone M, Crisci R. Current role of standardized uptake valuemax-derived ratios in N2 fluorine-18 fluorodeoxyglucose positron-emission tomography non-small cell lung cancer. J Thorac Dis 2018; 10: 503–7. doi: 10.21037/jtd.2017.11.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schumann SO, Kocher G, Minervini F. Epidemiology, diagnosis and treatment of the malignant pleural mesothelioma, a narrative review of literature. J Thorac Dis 2021; 13: 2510–23. doi: 10.21037/jtd-20-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg 2012; 1: 491–96. doi: 10.3978/j.issn.2225-319X.2012.11.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nickell LT Jr, Lichtenberger JP 3rd, Khorashadi L, Abbott GF, Carter BW. Multimodality imaging for characterization, classification, and staging of malignant pleural mesothelioma. Radiographics 2014; 34: 1692–1706. doi: 10.1148/rg.346130089 [DOI] [PubMed] [Google Scholar]
- 30. Berzenji L, Van Schil PE, Carp L. The eighth TNM classification for malignant pleural mesothelioma. Transl Lung Cancer Res 2018; 7: 543–49. doi: 10.21037/tlcr.2018.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Perrot M, Dong Z, Bradbury P, Patsios D, Keshavjee S, Leighl NB, et al. Impact of tumour thickness on survival after radical radiation and surgery in malignant pleural mesothelioma. Eur Respir J 2017; 49: 1–7. doi: 10.1183/13993003.01428-2016 [DOI] [PubMed] [Google Scholar]
- 32. Rusch VW, Giroux D, Kennedy C, Ruffini E, Cangir AK, Rice D, et al. Initial analysis of the International association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012; 7: 1631–39. doi: 10.1097/JTO.0b013e31826915f1 [DOI] [PubMed] [Google Scholar]
- 33. Armato SG, Nowak AK. Revised modified response evaluation criteria in solid tumors for assessment of response in malignant pleural mesothelioma (version 1.1). J Thorac Oncol 2018; 13: 1012–21. doi: 10.1016/j.jtho.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 34. Rusch VW, Chansky K, Kindler HL, Nowak AK, Pass HI, Rice DC, et al. The IASLC mesothelioma staging project: proposals for the M descriptors and for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for mesothelioma. J Thorac Oncol 2016; 11: 2112–19. doi: 10.1016/j.jtho.2016.09.124 [DOI] [PubMed] [Google Scholar]
- 35. Hallifax RJ, Haris M, Corcoran JP, Leyakathalikhan S, Brown E, Srikantharaja D, et al. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax 2015; 70: 192–93. doi: 10.1136/thoraxjnl-2014-206054 [DOI] [PubMed] [Google Scholar]
- 36. Heelan RT, Rusch VW, Begg CB, Panicek DM, Caravelli JF, Eisen C. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol 1999; 172: 1039–47. doi: 10.2214/ajr.172.4.10587144 [DOI] [PubMed] [Google Scholar]
- 37. Kruse M, Sherry SJ, Paidpally V, Mercier G, Subramaniam RM. FDG PET/CT in the management of primary pleural tumors and pleural metastases. AJR Am J Roentgenol 2013; 201: W215–26. doi: 10.2214/AJR.13.10572 [DOI] [PubMed] [Google Scholar]
- 38. Callister MEJ, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015; 70 Suppl 2: ii1–54. doi: 10.1136/thoraxjnl-2015-207168 [DOI] [PubMed] [Google Scholar]
- 39. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the fleischner society 2017. Radiology 2017; 284: 228–43. doi: 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 41. Nair A, Devaraj A, Callister MEJ, Baldwin DR. The fleischner Society 2017 and British thoracic Society 2015 guidelines for managing pulmonary nodules: keep calm and carry on. Thorax 2018; 73: 806–12. doi: 10.1136/thoraxjnl-2018-211764 [DOI] [Google Scholar]
- 42. McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013; 369: 910–19. doi: 10.1056/NEJMoa1214726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005; 128: 2490–96. doi: 10.1378/chest.128.4.2490 [DOI] [PubMed] [Google Scholar]
- 44. Al-Ameri A, Malhotra P, Thygesen H, Plant PK, Vaidyanathan S, Karthik S, et al. Risk of malignancy in pulmonary nodules: a validation study of four prediction models. Lung Cancer 2015; 89: 27–30. doi: 10.1016/j.lungcan.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 45. Nagata Y, Matsuo Y, Takayama K, Norihisa Y, Mizowaki T, Mitsumori M, et al. Current status of stereotactic body radiotherapy for lung cancer. Int J Clin Oncol 2007; 12: 3–7. doi: 10.1007/s10147-006-0646-6 [DOI] [PubMed] [Google Scholar]
- 46. Haidar YM, Rahn DA 3rd, Nath S, Song W, Bazhenova L, Makani S, et al. Comparison of outcomes following stereotactic body radiotherapy for non-small cell lung cancer in patients with and without pathological confirmation. Ther Adv Respir Dis 2014; 8: 3–12. doi: 10.1177/1753465813512545 [DOI] [PubMed] [Google Scholar]
- 47. Louie AV, Senan S, Patel P, Ferket BS, Lagerwaard FJ, Rodrigues GB, et al. When is a biopsy-proven diagnosis necessary before stereotactic ablative radiotherapy for lung cancer?: a decision analysis. Chest 2014; 146: 1021–28. doi: 10.1378/chest.13-2924 [DOI] [PubMed] [Google Scholar]
- 48. Sebastian NT, Xu-Welliver M, Williams TM. Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC): contemporary insights and advances. J Thorac Dis 2018; 10: S2451–64. doi: 10.21037/jtd.2018.04.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dyer SC, Bartholmai BJ, Koo CW. Implications of the updated lung CT screening reporting and data system (lung-RADS version 1.1) for lung cancer screening. J Thorac Dis 2020; 12: 6966–77. doi: 10.21037/jtd-2019-cptn-02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oudkerk M, Devaraj A, Vliegenthart R, Henzler T, Prosch H, Heussel CP, et al. European position statement on lung cancer screening. Lancet Oncol 2017; 18: e754–66. doi: 10.1016/S1470-2045(17)30861-6 [DOI] [PubMed] [Google Scholar]
- 51. Martin MD, Kanne JP, Broderick LS, Kazerooni EA, Meyer CA. Lung-RADS: pushing the limits. Radiographics 2017; 37: 1975–93. doi: 10.1148/rg.2017170051 [DOI] [PubMed] [Google Scholar]
- 52. Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, et al. Performance of lung-RADS in the National lung screening trial: a retrospective assessment. Ann Intern Med 2015; 162: 485–91. doi: 10.7326/M14-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR lung-RADS in a clinical CT lung screening program. J Am Coll Radiol 2016; 13: R25–9. doi: 10.1016/j.jacr.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 54. Kastner J, Hossain R, Jeudy J, Dako F, Mehta V, Dalal S, et al. Lung-RADS version 1.0 versus lung-RADS version 1.1: comparison of categories using nodules from the National lung screening trial. Radiology 2021; 300: 199–206. doi: 10.1148/radiol.2021203704 [DOI] [PubMed] [Google Scholar]
- 55. Mayo JR, Lam S. Doing too much or not enough: striking a balance. Radiology 2021; 300: 207–8. doi: 10.1148/radiol.2021210774 [DOI] [PubMed] [Google Scholar]
- 56. Jagoda P, Fleckenstein J, Sonnhoff M, Schneider G, Ruebe C, Buecker A, et al. Diffusion-weighted MRI improves response assessment after definitive radiotherapy in patients with NSCLC. Cancer Imaging 2021; 21: 15. doi: 10.1186/s40644-021-00384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 58. Coche E. Evaluation of lung tumor response to therapy: current and emerging techniques. Diagn Interv Imaging 2016; 97: 1053–65. doi: 10.1016/j.diii.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 59. Dunlap NE, Yang W, McIntosh A, Sheng K, Benedict SH, Read PW, et al. Computed tomography-based anatomic assessment overestimates local tumor recurrence in patients with mass-like consolidation after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 84: 1071–77. doi: 10.1016/j.ijrobp.2012.01.088 [DOI] [PubMed] [Google Scholar]
- 60. Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004; 15: 257–60. doi: 10.1093/annonc/mdh059 [DOI] [PubMed] [Google Scholar]
- 61. Anderson O, Kidd AC, Goatman K, Weir A, Voisey J, Dilys V, et al. Estimating the false positive prediction rate in automated volumetric measurements of malignant pleural mesothelioma. Communication in Computer and Information Science 2021; 116–39. [Google Scholar]
- 62. Mozley PD, Bendtsen C, Zhao B, Schwartz LH, Thorn M, Rong Y, et al. Measurement of tumor volumes improves RECIST-based response assessments in advanced lung cancer. Translational Oncology 2012; 5: 19–25. doi: 10.1593/tlo.11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 2012; 22: 796–802. doi: 10.1007/s00330-011-2319-8 [DOI] [PubMed] [Google Scholar]
- 64. Ravanelli M, Farina D, Morassi M, Roca E, Cavalleri G, Tassi G, et al. Texture analysis of advanced non-small cell lung cancer (NSCLC) on contrast-enhanced computed tomography: prediction of the response to the first-line chemotherapy. Eur Radiol 2013; 23: 3450–55. doi: 10.1007/s00330-013-2965-0 [DOI] [PubMed] [Google Scholar]
- 65. Yabuuchi H, Kawanami S, Iwama E, Okamoto I, Kamitani T, Sagiyama K, et al. Prediction of therapeutic effect of chemotherapy for NSCLC using dual-input perfusion CT analysis: comparison among bevacizumab treatment, two-agent platinum-based therapy without bevacizumab, and other non-bevacizumab treatment groups. Radiology 2018; 286: 685–95. doi: 10.1148/radiol.2017162204 [DOI] [PubMed] [Google Scholar]
- 66. Otrakji A, Digumarthy SR, Lo Gullo R, Flores EJ, Shepard J-AO, Kalra MK. Dual-Energy CT: spectrum of thoracic abnormalities. RadioGraphics 2016; 36: 38–52. doi: 10.1148/rg.2016150081 [DOI] [PubMed] [Google Scholar]
- 67. Kandathil A, Carson R, Subramaniam R. Lung cancer recurrence: 18 F-FDG PET/CT in clinical practice. Nuc Med Mol Imag 2019; 1136–2019. [Google Scholar]
- 68. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 Suppl 1: 122S–50S. doi: 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O JH, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology 2016; 280: 576–84. doi: 10.1148/radiol.2016142043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shang J, Ling X, Zhang L, Tang Y, Xiao Z, Cheng Y, et al. Comparison of recist, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2016; 43: 1945–53. doi: 10.1007/s00259-016-3420-7 [DOI] [PubMed] [Google Scholar]
- 71. Jiang X, Dudzinski S, Beckermann KE, Young K, McKinley E, J McIntyre O, et al. MRI of tumor T cell infiltration in response to checkpoint inhibitor therapy. J Immunother Cancer 2020; 8: 1–12. doi: 10.1136/jitc-2019-000328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of response and progression to immunotherapy. American Society of Clinical Oncology Educational Book 2018; :: 169–78. doi: 10.1200/EDBK_200643 [DOI] [PubMed] [Google Scholar]
- 73. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. IRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–52. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017; 23: 1920–28. doi: 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 75. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23: 4242–50. doi: 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Taylor SA, Mallett S, Ball S, Beare S, Bhatnagar G, Bhowmik A, et al. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed non-small-cell lung cancer: the prospective streamline L trial. The Lancet Respiratory Medicine 2019; 7: 523–32. doi: 10.1016/S2213-2600(19)30090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Navani N, Nankivell M, Lawrence DR, Lock S, Makker H, Baldwin DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015; 3: 282–89. doi: 10.1016/S2213-2600(15)00029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Regier M, Derlin T, Schwarz D, Laqmani A, Henes FO, Groth M, et al. Diffusion weighted MRI and 18F-FDG PET/CT in non-small cell lung cancer (NSCLC): does the apparent diffusion coefficient (ADC) correlate with tracer uptake (SUV)? Eur J Radiol 2012; 81: 2913–18. doi: 10.1016/j.ejrad.2011.11.050 [DOI] [PubMed] [Google Scholar]
- 79. Munoz-Schuffenegger P, Kandel S, Alibhai Z, Hope A, Bezjak A, Sun A, et al. A prospective study of magnetic resonance imaging assessment of post-radiation changes following stereotactic body radiation therapy for non-small cell lung cancer. Clin Oncol (R Coll Radiol) 2019; 31: 720–27. doi: 10.1016/j.clon.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 80. Shen G, Hu S, Deng H, Kuang A. Performance of DWI in the nodal characterization and assessment of lung cancer: a meta-analysis. AJR Am J Roentgenol 2016; 206: 283–90. doi: 10.2214/AJR.15.15032 [DOI] [PubMed] [Google Scholar]
- 81. Fleckenstein J, Jelden M, Kremp S, Jagoda P, Stroeder J, Khreish F, et al. The impact of diffusion-weighted MRI on the definition of gross tumor volume in radiotherapy of non-small-cell lung cancer. PLoS ONE 2016; 11: e0162816. doi: 10.1371/journal.pone.0162816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Theodoropoulos AS, Gkiozos I, Kontopyrgias G, Charpidou A, Kotteas E, Kyrgias G, et al. Modern radiopharmaceuticals for lung cancer imaging with positron emission tomography/computed tomography scan: a systematic review. SAGE Open Medicine 2020; 8: 205031212096159. doi: 10.1177/2050312120961594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bollineni VR, Kramer GM, Jansma EP, Liu Y, Oyen WJG. A systematic review on [(18)F]FLT-PET uptake as A measure of treatment response in cancer patients. Eur J Cancer 2016; 55: 81–97. doi: 10.1016/j.ejca.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 84. Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26: 225–39. doi: 10.1007/s10555-007-9055-1 [DOI] [PubMed] [Google Scholar]
- 85. Vera P, Bohn P, Edet-Sanson A, Salles A, Hapdey S, Gardin I. Simultaneous positron emission tomography (PET) assessment of metabolism with 18F-fluoro-2-deoxy-d-glucose (FDG), proliferation with 18F-fluoro-thymidine (FLT), and hypoxia with 18fluoro-misonidazole (F-miso) before and during radiotherapy in patients with non-small-cell lung cancer (NSCLC): A pilot study. Radiother Oncol 2011; 98: 109–16. doi: 10.1016/j.radonc.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 86. Li L, Zhao W, Sun X, Liu N, Zhou Y, Luan X, et al. 18f-RGD PET/CT imaging reveals characteristics of angiogenesis in non-small cell lung cancer. Transl Lung Cancer Res 2020; 9: 1324–32. doi: 10.21037/tlcr-20-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hunter B, Hindocha S, Lee RW. The role of artificial intelligence in early cancer diagnosis. Cancers (Basel) 2022; 14(): 1524. doi: 10.3390/cancers14061524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Correction: corrigendum: decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014; 5: 1–8. doi: 10.1038/ncomms5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14: 749–62. doi: 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 90. van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging 2020; 11: 91. doi: 10.1186/s13244-020-00887-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hosny A, Aerts HJ, Mak RH. Handcrafted versus deep learning radiomics for prediction of cancer therapy response. Lancet Digit Health 2019; 1: e106–7. doi: 10.1016/S2589-7500(19)30062-7 [DOI] [PubMed] [Google Scholar]
- 92. Wu G, Woodruff HC, Shen J, Refaee T, Sanduleanu S, Ibrahim A, et al. Diagnosis of invasive lung adenocarcinoma based on chest CT radiomic features of part-solid pulmonary nodules: a multicenter study. Radiology 2020; 297: 451–58. doi: 10.1148/radiol.2020192431 [DOI] [PubMed] [Google Scholar]
- 93. Nishio M, Sugiyama O, Yakami M, Ueno S, Kubo T, Kuroda T, et al. Computer-Aided diagnosis of lung nodule classification between benign nodule, primary lung cancer, and metastatic lung cancer at different image size using deep convolutional neural network with transfer learning. PLoS One 2018; 13(): e0200721. doi: 10.1371/journal.pone.0200721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Binczyk F, Prazuch W, Bozek P, Polanska J. Radiomics and artificial intelligence in lung cancer screening. Transl Lung Cancer Res 2021; 10: 1186–99. doi: 10.21037/tlcr-20-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ciompi F, Chung K, van Riel SJ, Setio AAA, Gerke PK, Jacobs C, et al. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci Rep 2017; 7: 46479. doi: 10.1038/srep46479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Avanzo M, Stancanello J, Pirrone G, Sartor G. Radiomics and deep learning in lung cancer. Strahlenther Onkol 2020; 196: 879–87. doi: 10.1007/s00066-020-01625-9 [DOI] [PubMed] [Google Scholar]
- 97. Ardila D, Kiraly AP, Bharadwaj S, Choi B, Reicher JJ, Peng L, et al. End-To-End lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med 2019; 25: 954–61. doi: 10.1038/s41591-019-0447-x [DOI] [PubMed] [Google Scholar]
- 98. Xu Y, Hosny A, Zeleznik R, Parmar C, Coroller T, Franco I, et al. Deep learning predicts lung cancer treatment response from serial medical imaging. Clin Cancer Res 2019; 25: 3266–75. doi: 10.1158/1078-0432.CCR-18-2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Baek S, He Y, Allen BG, Buatti JM, Smith BJ, Tong L, et al. Deep segmentation networks predict survival of non-small cell lung cancer. Sci Rep 2019; 9: 17286. doi: 10.1038/s41598-019-53461-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chetan MR, Gleeson FV. Radiomics in predicting treatment response in non-small-cell lung cancer: current status, challenges and future perspectives. Eur Radiol 2021; 31: 1049–58. doi: 10.1007/s00330-020-07141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lou B, Doken S, Zhuang T, Wingerter D, Gidwani M, Mistry N, et al. An image-based deep learning framework for individualising radiotherapy dose: a retrospective analysis of outcome prediction. The Lancet Digital Health 2019; 1: e136–47. doi: 10.1016/S2589-7500(19)30058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]