Abstract
First trimester entry into prenatal care is recommended for all women, and especially women with pre-pregnancy conditions. Our objective was to determine whether women with pre-pregnancy conditions were at lower risk of entry after the first trimester (delayed entry) into prenatal care than women without a pre-pregnancy health condition. We used data from 10,890 participants in the National Birth Defects Prevention Study who delivered liveborn infants without birth defects. Women reported pre-pregnancy conditions and timing of entry into prenatal care during a computer-assisted telephone interview. Multivariable logistic regression analyses were conducted to evaluate whether having a pre-pregnancy condition was associated with delayed entry into prenatal care compared to women without pre-pregnancy conditions. Approximately 13% of women reported delayed entry into prenatal care, and 18% of women reported a pre-pregnancy condition. Delayed entry into prenatal care was not associated with pre-pregnancy cardiometabolic or neurologic conditions. Women with thyroid conditions were less likely to report delayed entry into prenatal care (prevalence odds ratio (OR), 95% confidence interval (CI): 0.55 [0.32, 0.94]), but women with hematologic and respiratory conditions were more likely to report delayed entry into prenatal care (OR: 1.95 [1.00, 3.82] and 1.27 [0.95, 1.72], respectively), compared to those without any chronic conditions. Future research investigating the success of early prenatal care among women with thyroid conditions could identify ways to reduce delayed prenatal care among women with other pre-pregnancy conditions.
Keywords: Chronic disease, Pregnancy, Prenatal care, Pre-pregnancy health, National Birth Defects Prevention Study
1. Introduction
Most pregnant women obtain prenatal care at some point during pregnancy (Healthy People, 2019; Osterman and Martin, 2018). However, annually there are an estimated one million pregnant women in the United States with entry into prenatal care after the first trimester (delayed entry into prenatal care), or with no prenatal care (Osterman and Martin, 2018; Institute of Medicine, 1988). First trimester (timely) entry into prenatal care allows early identification of pregnancy complications, access to education about and resources for a healthy pregnancy, and development of treatment and monitoring plans for women with pre-pregnancy chronic conditions (American Academy of Pediatrics, American College of Obstetrics and Gynecology, 2017).
Women with pre-pregnancy chronic conditions, such as diabetes, hypertension, epilepsy, and thyroid conditions, are at higher risk of pregnancy complications. Timely entry into prenatal care allows women and their providers to manage treatment for their conditions, including medication management (Honein et al., 2013; Borgelt et al., 2016; Soh and Nelson-Piercy, 2015; Dunlop et al., 2008), and to optimize maternal and fetal health, thus lowering the risk of maternal and neonatal complications during and after pregnancy (Dunlop et al., 2008; Krans et al., 2013; Kupek et al., 2002; Goldfarb et al., 2017). There is some evidence to suggest that women with pre-pregnancy conditions that increase their obstetric risk (e.g., diabetes, epilepsy, hypertension) are more likely to have more intense levels of prenatal care compared to women without obstetric risk factors, which may be driven by increases in the total number of encounters, not necessarily timely entry into prenatal care (Krans et al., 2013; Kupek et al., 2002; Goldfarb et al., 2017; Chen et al., 2007). In contrast, women with increased psychosocial or mental health risks have been found to have less adequate use of prenatal care (Krans et al., 2013); this has been driven by later entry and fewer visits.
Lack of, and delayed, entry into prenatal care has been associated with younger age (Goldenberg et al., 1992), higher parity (Beeckman et al., 2011), not having a regular medical provider (Braveman et al., 2000), being of non-white race/ethnicity (Goldenberg et al., 1992; Bryant et al., 2010; Greenberg, 1983; Taffel, 1978), and having lower educational attainment (Ayoola et al., 2010; Ward et al., 2013). Lack of transportation, lack of childcare, type of employment, or insurance eligibility have also been associated with not obtaining prenatal care (Goldenberg et al., 1992; Joyce et al., 1983; Agopian et al., 2012; Bengiamin et al., 2010). Many of these social and economic factors are also associated with access to healthcare and the development of chronic health conditions (Wang et al., 2020; Admon et al., 2017; Christopher et al., 2016). It is unclear whether an underlying pre-pregnancy condition independently affects timing of entry into prenatal care, and there may be differences by type of pre-pregnancy condition. We hypothesize that, among women with liveborn infants unaffected by birth defects, women with specific pre-pregnancy conditions were less likely to enter prenatal care after the first trimester compared to women without a pre-pregnancy health condition.
2. Methods
2.1. Study participant selection
We used data from the National Birth Defects Prevention Study (NBDPS). The NBDPS is a U.S. population-based multicenter case-control study designed to assess risk factors for major birth defects (Reefhuis et al., 2015). Data were collected from mothers of infants with estimated dates of delivery (EDD) between October 1, 1997 and December 31, 2011. We restricted our analysis to mothers of control infants (Cogswell et al., 2009). These women were randomly selected from liveborn infants without major birth defects from birth certificates or birth hospitals located within the catchment area of ten birth defects surveillance systems (Reefhuis et al., 2015).
2.2. Data collection
Women completed a computer assisted telephone interview, which covered lifestyle factors, behaviors, and maternal health from three months before through the end of pregnancy. Women were interviewed between six weeks and 24 months after EDD (i.e., between 1997 and 2013). During the interview, women were asked about diagnoses of pre-pregnancy diabetes, epilepsy, and hypertension. Additionally, they were asked to report other medical conditions they had. These questions were asked of all women during the interview period, but the form of some questions changed (Appendix A).
2.3. Exposures
A woman was considered exposed to a pre-pregnancy condition if she reported a chronic condition diagnosed before her pregnancy. Women who did not report a pre-pregnancy condition or reported a condition diagnosed during or after pregnancy were considered unexposed. Pre-pregnancy conditions were grouped: any pre-pregnancy condition, cardiometabolic conditions (pre-pregnancy diabetes, heart conditions, high cholesterol, and chronic hypertension), thyroid conditions, respiratory conditions, neurologic conditions (including epilepsy and migraines), hematologic conditions (bleeding and clotting disorders), and mental health conditions. To improve sensitivity, mental health conditions were defined by maternal report of the condition or report of use of an antidepressant, antipsychotic, or antianxiety medication in the three months before conception (Appendix B). Thyroid conditions also included report of thyroid medications in the three months before conception (Appendix B). The remaining conditions (e.g., autoimmune conditions, reproductive conditions, chronic infections, etc.) did not have sufficient sample size to conduct condition-specific analyses (fewer than ten women reporting the condition and delayed entry into prenatal care), but were included in analyses of any pre-pregnancy condition (Appendix C).
2.4. Outcome
Timing of entry into prenatal care was based on maternal report of week, month, or trimester of entry into prenatal care, based on estimated last menstrual period (LMP). Women who reported entering prenatal care after the 13th week, third month, or first trimester were considered to have delayed entry to prenatal care.
2.5. Statistical analysis
Analyses were performed in SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina). Descriptive statistics, comparing women with delayed versus timely entry into prenatal care were estimated. Crude and adjusted prevalence odds ratios (OR) and 95% confidence intervals (CI) were calculated using logistic regression for odds of delayed entry into prenatal care among women with versus without a pre-pregnancy condition. Separate models were run for each pre-pregnancy condition, comparing women with the condition to women without any pre-pregnancy conditions. All adjusted models included maternal pre-pregnancy body mass index (BMI), age at delivery, highest educational level obtained at delivery, employment status (full or part time vs. none) from three months before through the end of pregnancy, maternal race/ethnicity, smoking in the month before conception through the third month of pregnancy, alcohol use in the month before conception through the third month of pregnancy, having a prior live birth, and study site. These confounding variables were selected a priori, based on causal diagrams (Greenland et al., 1999). To align with the American Statistical Association guidelines, we considered ORs meaningful if they indicated an effect size 20% greater than the null (Wasserstein and Lazar, 2016; Wasserstein et al., 2019).
2.6. Bias analyses
Among 11,829 women, those missing information about receipt of prenatal care (n = 70), who reported having a pre-pregnancy condition with unknown timing of diagnosis (N = 568), or missing information about the presence of a pre-pregnancy condition (N = 301) were excluded. Further, women missing information for confounding variables (N = 668) were excluded from multivariable analyses. To assess the impact of missing data, multiple imputation was used. We assessed the impact of selection bias on control participation in the NBDPS using inverse probability of selection weights (Hernan et al., 2004). We additionally conducted a probabilistic bias analysis to assess the impact of differential misclassification of the exposure on the main results (Johnson et al., 2018; Lash et al., 2009). Detailed methods are described in Appendix D.
2.7. Ethics approval
All participating study sites had Institutional Review Board approval to conduct study activities and all participants provided informed consent.
3. Results
There were 11,829 mothers of control infants interviewed for the NBDPS. Of those, 11,759 provided interview information related to use of prenatal care; 568 were subsequently excluded from the main analyses because they were missing (n = 61) or had unknown dates (n = 507) of diagnosis of pre-pregnancy conditions and 301 were excluded due to missing data about timing of entry into prenatal care, resulting in 10,890 women (92.6%). A further 668 women were missing data for confounding variables. These women were retained in the sample for descriptive analyses but excluded from modeling estimates, resulting in 10,222 women whose data were available for the multivariable analysis. Prenatal care was used by 99.0% of women at some point during pregnancy. Among women with information available to determine timing of entry into prenatal care, 86.9% (9466) reported entry during the first three months of pregnancy (Table 1). A greater proportion of women with delayed entry into prenatal care were in the youngest (<20 years and 20–24 years of age) age groups; were of non-white or Hispanic race/ethnicity; had lower educational attainment; reported not being employed; had an unintended/mistimed pregnancy; and reported no periconceptional folic acid (Table 1). Overall, 17.8% of mothers reported a pre-pregnancy condition (Table 2). The most prevalent conditions were cardiometabolic conditions (n = 545 [5.0%]; hypertension was the most common cardiometabolic condition reported, n = 465), mental health conditions (n = 537 [4.9%]), respiratory conditions (n = 424 [3.9%]), and thyroid conditions (n = 289 [2.7%]).
Table 1.
Characteristics of mothers of liveborn infants without birth defects, stratified by timing of entry into prenatal care, National Birth Defects Prevention Study, 1997–2011
| Characteristics | Category | Total | Timely entry into prenatal carea n (row %) |
Delayed entry into prenatal care n (row %) |
|---|---|---|---|---|
|
| ||||
| Total | 10,890 | 9466 (86.9) | 1424 (13.1) | |
| Any pre-pregnancy condition | ||||
| No | 8948 | 7753 (86.6) | 1195 (13.4) | |
| Yes | 1942 | 1713 (88.2) | 229 (11.8) | |
| Maternal age | ||||
| < 20 years | 1022 | 757 (74.1) | 265 (25.9) | |
| 20–24 years | 2440 | 1999 (81.9) | 441 (18.1) | |
| 25–29 years | 3042 | 2720 (89.4) | 322 (10.6) | |
| 30–34 years | 2848 | 2605 (91.5) | 243 (8.5) | |
| 35–39 years | 1280 | 1163 (90.9) | 117 (9.1) | |
| ≥ 40 years | 258 | 222 (86.0) | 36 (14.0) | |
| Maternal race | ||||
| Non-Hispanic white | 6393 | 5857 (91.6) | 536 (8.4) | |
| Non-Hispanic Black | 1160 | 937 (80.8) | 223 (19.2) | |
| Hispanic | 2618 | 2057 (78.6) | 561 (21.4) | |
| Other | 713 | 609 (85.4) | 104 (14.6) | |
| Missing | 6 | 6 | 0 | |
| Maternal pre-pregnancy body mass index | ||||
| Underweight | 566 | 480 (84.8) | 86 (15.2) | |
| Normal weight | 5668 | 4996 (88.1) | 672 (11.9) | |
| Overweight | 2343 | 2066 (88.2) | 277 (11.8) | |
| Obese | 1876 | 1623 (86.5) | 253 (13.5) | |
| Missing | 437 | 301 | 136 | |
| Maternal education | ||||
| Less than high school | 1722 | 1254 (72.8) | 468 (27.2) | |
| High school | 2508 | 2081 (83.0) | 427 (17.0) | |
| Some college or college degree | 6449 | 5948 (92.2) | 501 (7.8) | |
| Missing | 211 | 183 | 28 | |
| Maternal employmentb | ||||
| Employed | 7648 | 6821 (89.2) | 827 (10.8) | |
| Not employed | 3057 | 2483 (81.2) | 574 (18.8) | |
| Missing | 185 | 162 | 23 | |
| Pregnancy intendedness | ||||
| Intended/ambivalent | 7372 | 6690 (90.7) | 682 (9.3) | |
| Unintended/mistimed | 3492 | 2756 (78.9) | 736 (21.1) | |
| Missing | 26 | 20 | 6 | |
| Prior live birth | ||||
| Yes | 6658 | 5727 (86.0) | 931 (14.0) | |
| No | 4229 | 3737 (88.4) | 492 (11.6) | |
| Missing | 3 | 2 | 1 | |
| Prior pregnancy loss | ||||
| Yes | 2609 | 2324 (89.1) | 285 (10.9) | |
| No | 8278 | 7140 (86.3) | 1138 (13.7) | |
| Missing | 3 | 2 | 1 | |
| Maternal smokingc | ||||
| Yes | 1923 | 1614 (83.9) | 309 (16.1) | |
| No | 8797 | 7703 (87.6) | 1904 (21.6) | |
| Missing | 170 | 149 | 21 | |
| Maternal alcohol usec | ||||
| Yes | 4038 | 3636 (90.0) | 402 (10.0) | |
| No | 6648 | 5653 (85.0) | 995 (15.0) | |
| Missing | 204 | 177 | 27 | |
| Periconceptional folic acidd | ||||
| Yes | 5780 | 5421 (93.8) | 359 (6.2) | |
| No | 5065 | 4005 (79.1) | 1060 (20.9) | |
| Missing | 45 | 40 | 5 | |
Timely entry defined as entry in the first three months of pregnancy
Maternal report of employment from three months before conception through the end of pregnancy
Maternal use in the month before conception through the third month of pregnancy
Maternal use in the month before conception through the first month of pregnancy
Table 2.
Unadjusted and adjusted prevelance odds ratios for the association between maternal pre-pregnancy chronic conditions and delayed entry into prenatal care, National Birth Defects Prevention Study 1997–2011.
| Condition | Timely entrya into prenatal care n (%)b | Delayed entry into prenatal care n (%)b | Unadjusted odds ratio (95% confidence interval) | Adjusted odds ratioc (95% confidence interval) |
|---|---|---|---|---|
|
| ||||
| No pre-pregnancy chronic conditionsd | 7753 (81.9) | 1195 (83.9) | 1.0 (reference) | 1.0 (reference) |
| Any pre-pregnancy chronic conditionse | 1713 (18.1) | 229 (16.1) | 0.87 (0.75, 1.01) | 1.04 (0.88, 1.23) |
| Cardiometabolic conditionsf | 462 (5.6) | 83 (6.5) | 1.16 (0.92, 1.48) | 1.15 (0.87, 1.51) |
| Mental health conditionsg | 489 (5.9) | 48 (3.9) | 0.64 (0.47, 0.86) | 0.88 (0.64, 1.22) |
| Respiratory conditionsh | 361 (4.4) | 63 (5.0) | 1.13 (0.86, 1.49) | 1.27 (0.95, 1.72) |
| Thyroid conditionsi | 274 (3.4) | 15 (1.2) | 0.36 (0.21, 0.60) | 0.55 (0.32, 0.94) |
| Neurologic conditionsj | 103 (1.3) | 14 (1.2) | 0.88 (0.50, 1.55) | 0.94 (0.51, 1.75) |
| Hematologic conditionsk | 55 (0.7) | 12 (1.0) | 1.42 (0.76, 2.65) | 1.95 (1.00, 3.82) |
Timely entry defined as entry in the first three months of pregnancy.
Column percents; unexposed for all comparisons were those with no reported pre-pregnancy chronic conditions.
Adjusted models included study site, age (< 20 years, 20–24 years, 25–29 years (ref), 30–34 years,35–39 years, >39 years), pre-pregnancy body mass index (underweight, normal weight (ref), overweight, obese), maternal education (less than high school, high school (ref), some college or college degree), maternal race (non-Hispanic white (ref), non-Hispanic Black, Hispanic, other race), prior live birth, smoking in the month before conception through the third month of pregnancy, alcohol use in the month before conception through the third month of pregnancy, and maternal employment.
Women with no pre-pregnancy chronic conditions were considered unexposed for all models.
Any self-reported chronic health condition; women could report more than one condition.
Includes heart conditions, high cholesterol, high blood pressure, and pre-pregnancy diabetes.
Self-reported mental health condition, or report of an antidepressant, antianxiety, or antipsychotic medication before pregnancy.
Includes asthma, chronic bronchitis, and chronic obstructive pulmonary disease.
Includes Graves disease, Hashimoto’s thyroiditis, report of a “thyroid condition,” “thyroid cancer,” “thyroid removal,” or report of a thyroid medication.
Includes epilepsy, migraines, and multiple sclerosis.
Includes clotting and bleeding disorders.
After adjustment, among mothers of liveborn infants without major birth defects, report of any pre-pregnancy condition was not associated with delayed entry into prenatal care (OR: 1.04, 95% CI: 0.88, 1.23) (Table 2). However, there were differences by specific condition. Women who reported cardiometabolic and neurologic conditions did not have a difference in timing of entry into prenatal care compared to women without a pre-pregnancy condition. Two conditions were associated with increased prevalence of delayed entry into prenatal care: respiratory conditions (OR: 1.27, 95% CI: 0.95, 1.72) and hematologic conditions (OR: 1.95, 95% CI: 1.00, 3.82). Maternal report of a thyroid condition was associated with reduced prevalence of delayed entry into prenatal care (OR: 0.54, 95% CI: 0.32, 0.94).
Analyses to assess the impact of missing data, selection bias, and misclassification bias were reassuring. While point estimates varied for each condition, conditional on the accuracy of the assumptions and values assigned to bias parameters in the models, results were of similar magnitude and in the same direction (or null) as results that adjusted only for confounding (Fig. 1). Results from the bias analyses suggest that differential misclassification of the exposure could have the greatest impact on the estimates, particularly for thyroid, mental health, and neurologic conditions. Null estimates for timing of entry into prenatal care shifted to suggest women who reported a mental health or neurologic condition were less likely to report delayed entry into prenatal care. Further, with differential misclassification of the exposure, the association between respiratory conditions and delayed entry into prenatal care was slightly attenuated, while the association between hematologic and thyroid conditions and delayed entry into prenatal care was slightly strengthened.
Fig. 1.
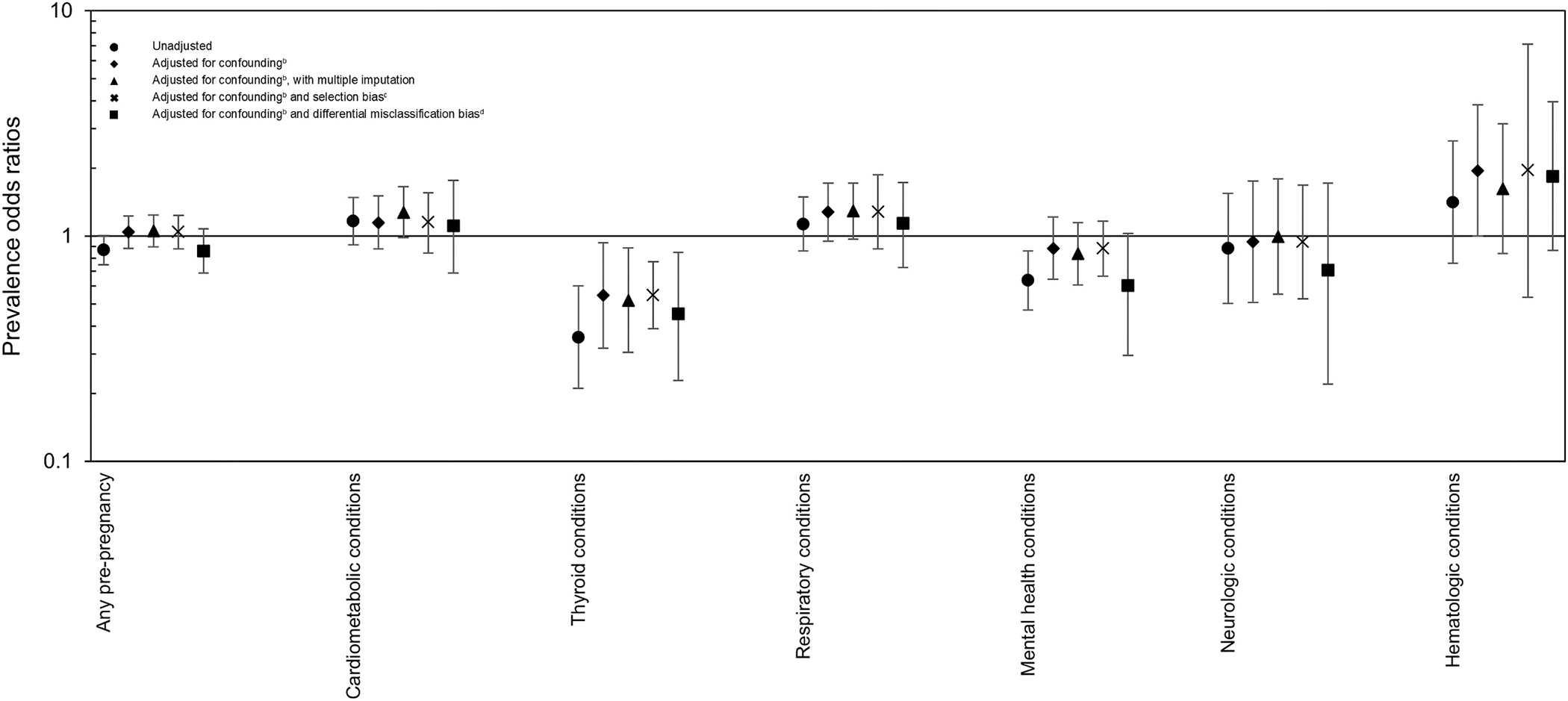
Comparison of odds ratio estimates for association between pre-pregnancy conditions and delayeda entry into prenatal care, adjusting for different biases, National Birth Defects Prevention Study, 1997–2011.
a. Delayed entry into care was entry after the first three months of pregnancy.
b. Adjusted models included study site, age (< 20 years, 20–24 years, 25–29 years (ref), 30–34 years,35–39 years, >39 years), pre-pregnancy body mass index (underweight, normal weight (ref), overweight, obese), maternal education (less than high school, high school (ref), some college or college degree), maternal race (non-Hispanic white (ref), non-Hispanic Black, Hispanic, other race), prior live birth, smoking in the month before conception through the third month of pregnancy, alcohol use in the month before conception through the third month of pregnancy, and maternal employment.
c. Selection bias models used the following initial selection probabilities: 0.57 for women with late entry into prenatal care and a pre-pregnancy chronic condition, 0.61 for women with timely entry with a pre-pregnancy chronic condition.
d. Misclassification bias models used the following parameters for the trapezoidal distributions (min, mode1, mode2, max):
Any chronic condition: Sensitivity among those with late entry into prenatal care: 0.76, 0.78, 0.8, 0.82; Specificity among those with late entry into prenatal care: 0.93, 0.95, 0.97, 0.98; Sensitivity among those with timely entry into prenatal care: 0.78, 0.8, 0.82, 0.85; Specificity among those with timely entry into prenatal care: 0.95, 0.97, 0.99, 1.
Cardiometabolic conditions: Sensitivity among those with late entry into prenatal care: 0.65, 0.66, 0.69, 0.71; Specificity among those with late entry into prenatal care: 0.95, 0.97, 0.98, 0.99; Sensitivity among those with timely entry into prenatal care: 0.67, 0.69, 0.71, 0.75; Specificity among those with timely entry into prenatal care: 0.96, 0.97, 0.99, 1.
Thyroid conditions: Sensitivity among those with late entry into prenatal care: 0.77, 0.79, 0.82, 0.84; Specificity among those with late entry into prenatal care: 0.995, 0.998, 0.9995, 1; Sensitivity among those with timely entry into prenatal care: 0.8, 0.83, 0.85, 0.87; Specificity among those with timely entry into prenatal care: 0.998, 0.999, 0.9999, 1.
Respiratory conditions: Sensitivity among those with late entry into prenatal care: 0.77, 0.79, 0.82, 0.84; Specificity among those with late entry into prenatal care: 0.96, 0.97, 0.98, 0.99; Sensitivity among those with timely entry into prenatal care: 0.8, 0.83, 0.85, 0.87; Specificity among those with timely entry into prenatal care: 0.98, 0.99, 0.995, 1.
Mental health conditions: Sensitivity among those with late entry into prenatal care: 0.77, 0.79, 0.82, 0.84; Specificity among those with late entry into prenatal care: 0.97, 0.98, 0.99, 1; Sensitivity among those with timely entry into prenatal care: 0.8, 0.83, 0.85, 0.87; Specificity among those with timely entry into prenatal care: 0.98, 0.99, 0.995, 1.
Neurologic conditions: Sensitivity among those with late entry into prenatal care: 0.7, 0.73, 0.75, 0.78; Specificity among those with late entry into prenatal care: 0.99, 0.995, 0.996, 1; Sensitivity among those with timely entry into prenatal care: 0.75, 0.78, 0.8, 0.82; Specificity among those with timely entry into prenatal care: 0.995, 0.997, 0.999, 1.
Hematologic conditions: Sensitivity among those with late entry into prenatal care: 0.77, 0.79, 0.82, 0.84; Specificity among those with late entry into prenatal care: 0.997, 0.998, 0.999, 1; Sensitivity among those with timely entry into prenatal care: 0.8, 0.83, 0.85, 0.87; Specificity among those with timely entry into prenatal care: 0.998, 0.999, 0.9995, 1.
4. Discussion
We found that among mothers of liveborn infants without major birth defects, prenatal care was widely used, and most women entered prenatal care during the first trimester of pregnancy regardless of whether they had a pre-pregnancy condition. However, 13% of women with and without pre-pregnancy conditions reported entering prenatal care after the first trimester, which presents a missed opportunity for optimizing maternal and fetal health early in pregnancy. Only women reporting pre-pregnancy thyroid conditions were less likely to have delayed entry into care. In contrast, women reporting hematologic conditions and respiratory conditions were more likely to have delayed entry into care compared to women with no pre-pregnancy conditions although these differences were small. Women reporting mental health, cardiometabolic, and neurologic conditions had similar timing of entry into prenatal care compared to women without a pre-pregnancy condition.
Prenatal care has been shown to have benefits for maternal health (Creanga et al., 2015; Conway and Kutinova, 2006; Yan, 2017). Specifically, it is an opportunity to access pregnancy and other health information, and prenatal care has been associated with improvements in maternal and child health behaviors after pregnancy (Conway and Kutinova, 2006; Yan, 2017; Reichman et al., 2010). The American College of Obstetricians and Gynecologists recommend prenatal care for all pregnant women; for women with chronic medical conditions, preconception and early prenatal care is recommended (American Academy of Pediatrics, American College of Obstetrics and Gynecology, 2017; American College of Obstetricians and Gynecologists, 2019). Prenatal care could differ based on the type of condition. Our results suggest that women with thyroid conditions were less likely to have delayed entry into prenatal care than women without pre-pregnancy conditions. At least two potential explanations for this finding exist. First, thyroid disease is common among women of reproductive age and thyroid disfunction is known to increase the risk of pregnancy losses and other adverse outcomes (Negro and Mestman, 2011; Mannisto et al., 2013). Therefore, women undergoing treatment for thyroid disease diagnosed before pregnancy may be more likely to be counseled on the importance of maintaining thyroid levels before and during pregnancy, and may also be in regular care for their thyroid disorder. Second, thyroid disease is associated with fertility problems (Dosiou, 2020); if women with thyroid disease used fertility treatments for help becoming pregnant, they would likely enter prenatal care early in the first trimester. Other chronic conditions are not necessarily notable risk factors for outcomes such as pregnancy loss, or may be diagnosed many months or years before pregnancy. Therefore, women with these conditions may not receive the same type of counseling, or be in regular treatment, for their condition, reducing awareness of the importance of prenatal care. This could explain why women with hematologic and respiratory conditions were more likely to report delayed entry into prenatal care.
These results should be considered in the context of several limitations. The women included in this study all delivered live-born infants without a major birth defect. Women who participated in the NBDPS and were exposed to pre-pregnancy conditions, or their treatments, may be fundamentally different from the underlying population. It is possible that by restricting the analysis to live births without birth defects, the population analyzed had a lower prevalence of chronic conditions or of severe chronic conditions. Furthermore, because women whose pregnancies ended in miscarriage or stillbirth were not included, we could not assessing timing of entry into prenatal care among women whose pregnancies did not survive to live birth. These restrictions could have impacted the generalizability of the analysis.
The proportion of women with timely entry into prenatal care in this population is higher than in the general population (estimated at about 77% of U.S. women) (Osterman and Martin, 2018), suggesting that the NBDPS control population may not represent the general population of women experiencing pregnancy in the United States. Additionally, while the control participants in the NBDPS have been found to be representative of the source population, differences in participation by race/ethnicity, infant birth weight, and prenatal care were observed, with women with later entry into prenatal care less likely to participate (Cogswell et al., 2009). Because the participation in the study could have been impacted by the presence of a chronic condition and pregnancy loss, the potential selection bias could result in bias both toward and away from the null. However, conditional on the accuracy of the bias models, the results of the selection bias analyses suggest that, among live births, this bias was insubstantial.
Both timing of entry into prenatal care and pre-pregnancy conditions were obtained via retrospective self-report. Women can generally recall the approximate timing of entry into prenatal care (Githens et al., 1993; Hosler et al., 2010). However, maternal self-report of pre-pregnancy conditions can be misclassified, with high specificity and lower sensitivity of self-reported conditions (Krakowiak et al., 2015; Newport et al., 2008; van Gelder et al., 2015). While our probabilistic bias analysis suggested that the main results could have been biased either toward or away from the null, depending on the condition, simulation intervals were wide and changes in point estimates were small.
Some factors known to impact entry into prenatal care, such as prenatal insurance and preconception care, were not available in the NBDPS, potentially resulting in unmeasured confounding. Similarly, information about other contact with the healthcare system, such as specialty care related to a chronic condition, was not collected. Though this study grouped women by type of condition, the overall prevalence of these conditions is low in the population of women of reproductive age. This prevented us from analyzing specific conditions of interest. We defined delayed entry into prenatal care as entry after the first trimester, as this is the time frame that provides an opportunity for accurate dating of pregnancy, pregnancy risk assessment, and development of treatment plans. However, we may have observed additional differences if other definitions of delayed entry (e.g., entry after the second trimester) were used. Finally, data collection for the NDBPS ended with pregnancies ending in 2011. Since 2011, efforts to increase access to prenatal care may have changed and altered the patterns observed here (Health Resources and Services Administration, 2020; Daw and Sommers, 2019; Association of Maternal and Child Health Programs, 2016; Shah et al., 2018), although prevalence of delayed entry into prenatal care has remained high (Osterman and Martin, 2018; Daw et al., 2020).
Despite these limitations, our analysis had several strengths. The NBDPS is a large, population-based study, with extensive information on maternal pre-pregnancy conditions, and exposures and behaviors before and during pregnancy. The control population has generally been found to be representative of the source population (Cogswell et al., 2009). Further, we used the interview data to gather information for specific groups of pre-pregnancy conditions, rather than combining women into one high-risk group. Finally, we conducted multiple bias analyses to assess the potential impact of common systematic errors on the main results.
5. Conclusions
In summary, we found that prenatal care was widely used and delayed entry into prenatal care was similar among women with and without pre-pregnancy conditions. Timely entry into prenatal care is important for all women, but particularly for women with pre-pregnancy conditions, because of the need for treatment and monitoring of their condition. Given the importance of monitoring women with pre-pregnancy conditions before and during pregnancy, identifying modifiable factors associated with pre-pregnancy health and timing of entry into prenatal care may improve maternal and pregnancy outcomes in these populations. Future research investigating the success of early prenatal care among women with thyroid conditions could identify ways to reduce delayed prenatal care among women with other pre-pregnancy conditions.
Supplementary Material
Disclosure of funding
This project was supported through Centers for Disease Control and Prevention (CDC) cooperative agreements under PA #96043, PA #02081, FOA #DD09-001, FOA #DD13-003, and NOFO #DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study (NBDPS) and/or the Birth Defects Study To Evaluate Pregnancy exposureS (BD-STEPS). Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CRediT authorship contribution statement
Regina M. Simeone: Conceptualization, Methodology, Software, Formal analysis, Writing – original draft, Writing – review & editing. Jennita Reefhuis: Conceptualization, Data curation, Methodology, Writing – review & editing. Denise J. Jamieson: Conceptualization, Methodology, Writing – review & editing. Carolyn D. Drews-Botsch: Conceptualization, Methodology, Writing – review & editing. Timothy L. Lash: Conceptualization, Methodology, Writing – review & editing. Sarah C. Fisher: Validation, Writing – review & editing. Meredith M. Howley: Validation, Writing – review & editing. Shannon Evans: Validation, Writing – review & editing. Penelope P. Howards: Conceptualization, Methodology, Writing – review & editing, Supervision.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2022.107272.
Data availability
The authors do not have permission to share data.
References
- Admon LK, Winkelman TNA, Moniz MH, Davis MM, Heisler M, Dalton VK, 2017. Disparities in chronic conditions among women hospitalized for delivery in the United States, 2005–2014. Obstet. Gynecol. 130, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agopian AJ, Lupo PJ, Herdt-Losavio ML, Langlois PH, Rocheleau CM, Mitchell LE, et al. , 2012. Differences in folic acid use, prenatal care, smoking, and drinking in early pregnancy by occupation. Prev. Med. 55, 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics, American College of Obstetrics and Gynecology, 2017. Guidelines for Perinatal Care, 8th ed. AAP, Elk Grove Village (IL). Washington, DC: American College of Obstetricians and Gynecologists. [Google Scholar]
- American College of Obstetricians and Gynecologists, 2019. Pre-pregnancy counseling: Committee Opinion No. 762. Obstet. Gynecol. 133, e78–e89. [DOI] [PubMed] [Google Scholar]
- Association of Maternal & Child Health Programs, 2016. Opportunities to Optimize Access to Prenatal Care through Health Transformation [February 11, 2021]; Available from: http://www.amchp.org/Policy-Advocacy/health-reform/resources/Documents/Pregnancy%20Issue%20Brief_Final%202016.pdf. [Google Scholar]
- Ayoola AB, Nettleman MD, Stommel M, Canady RB, 2010. Time of pregnancy recognition and prenatal care use: a population-based study in the United States. Birth. 37, 37–43. [DOI] [PubMed] [Google Scholar]
- Beeckman K, Louckx F, Putman K, 2011. Predisposing, enabling and pregnancy-related determinants of late initiation of prenatal care. Matern. Child Health J. 15, 1067–1075. [DOI] [PubMed] [Google Scholar]
- Bengiamin MI, Capitman JA, Ruwe MB, 2010. Disparities in initiation and adherence to prenatal care: impact of insurance, race-ethnicity and nativity. Matern. Child Health J. 14, 618–624. [DOI] [PubMed] [Google Scholar]
- Borgelt LM, Hart FM, Bainbridge JL, 2016. Epilepsy during pregnancy: focus on management strategies. Int. J. Women’s Health 8, 505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P, Marchi K, Egerter S, Pearl M, Neuhaus J, 2000. Barriers to timely prenatal care among women with insurance: the importance of pre-pregnancy factors. Obstet. Gynecol. 95, 874–880. [DOI] [PubMed] [Google Scholar]
- Bryant AS, Worjoloh A, Caughey AB, Washington AE, 2010. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am. J. Obstet. Gynecol. 202, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XK, Wen SW, Yang Q, Walker MC, 2007. Adequacy of prenatal care and neonatal mortality in infants born to mothers with and without antenatal high-risk conditions. Aust. N. Z. J. Obstet. Gynaecol. 47, 122–127. [DOI] [PubMed] [Google Scholar]
- Christopher AS, McCormick D, Woolhandler S, Himmelstein DU, Bor DH, Wilper AP, 2016. Access to care and chronic disease outcomes among Medicaid-insured persons versus the uninsured. Am. J. Public Health 106, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell ME, Bitsko RH, Anderka M, Caton AR, Feldkamp ML, Hockett Sherlock SM, et al. , 2009. Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. Am. J. Epidemiol. 170, 975–985. [DOI] [PubMed] [Google Scholar]
- Conway KS, Kutinova A, 2006. Maternal health: does prenatal care make a difference? Health Econ. 15, 461–488. [DOI] [PubMed] [Google Scholar]
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM, 2015. Pregnancy-related mortality in the United States, 2006–2010. Obstet. Gynecol. 125, 5–12. [DOI] [PubMed] [Google Scholar]
- Daw JR, Sommers BD, 2019. The affordable care act and access to care for reproductive-aged and pregnant women in the United States, 2010–2016. Am. J. Public Health 109, 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw JR, Winkelman TNA, Dalton VK, Kozhimannil KB, Admon LK, 2020. Medicaid expansion improved perinatal insurance continuity for low-income women. Health Aff. (Millwood) 39, 1531–1539. [DOI] [PubMed] [Google Scholar]
- Dosiou C, 2020. Thyroid and fertility: recent advances. Thyroid. 30, 479–486. [DOI] [PubMed] [Google Scholar]
- Dunlop AL, Jack BW, Bottalico JN, Lu MC, James A, Shellhaas CS, et al. , 2008. The clinical content of preconception care: women with chronic medical conditions. Am. J. Obstet. Gynecol. 199, S310–S327. [DOI] [PubMed] [Google Scholar]
- van Gelder MM, Schouten NP, Merkus PJ, Verhaak CM, Roeleveld N, Roukema J, 2015. Using web-based questionnaires and obstetric records to assess general health characteristics among pregnant women: a validation study. J. Med. Internet Res. 17, e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githens PB, Glass CA, Sloan FA, Entman SS, 1993. Maternal recall and medical records: an examination of events during pregnancy, childbirth, and early infancy. Birth. 20, 136–141. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Patterson ET, Freese MP, 1992. Maternal demographic, situational and psychosocial factors and their relationship to enrollment in prenatal care: a review of the literature. Women Health 19, 133–151. [DOI] [PubMed] [Google Scholar]
- Goldfarb SS, Smith W, Epstein AE, Burrows S, Wingate M, 2017. Disparities in prenatal care utilization among U.S. versus foreign-born women with chronic conditions. J. Immigr. Minor. Health 19, 1263–1270. [DOI] [PubMed] [Google Scholar]
- Greenberg RS, 1983. The impact of prenatal care in different social groups. Am. J. Obstet. Gynecol. 145, 797–801. [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM, 1999. Causal diagrams for epidemiologic research. Epidemiology. 10, 37–48. [PubMed] [Google Scholar]
- Health Resources & Services Administration, 2020. Women’s Preventive Services Guidelines [Jul 12, 2021]; Available from: https://www.hrsa.gov/womens-guidelines/index.html. [Google Scholar]
- Healthy People, 2019. Maternal, Infant, and Child Health [updated July 1, 2019; July 2, 2019]; Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health. [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JM, 2004. A structural approach to selection bias. Epidemiology. 15, 615–625. [DOI] [PubMed] [Google Scholar]
- Honein MA, Gilboa SM, Broussard CS, 2013. The need for safer medication use in pregnancy. Expert. Rev. Clin. Pharmacol. 6, 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler A, Nayak SG, Radigan AM, 2010. Agreement between self-report and birth certificate for gestational diabetes mellitus: New York state PRAMS. Matern. Child Health J. 14, 786–789. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, 1988. Prenatal Care: Reaching Mothers, Reaching Infants. National Academy Press. [PubMed] [Google Scholar]
- Johnson CY, Howards PP, Strickland MJ, Waller DK, Flanders WD, 2018. National Birth Defects Prevention Study. Multiple bias analysis using logistic regression: an example from the National Birth Defects Prevention Study. Ann. Epidemiol. 28, 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce K, Diffenbacher G, Greene J, Sorokin Y, 1983. Internal and external barriers to obtaining prenatal care. Soc. Work Health Care 9, 89–96. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Tancredi DJ, Hertz-Picciotto I, 2015. Maternal recall versus medical records of metabolic conditiosn from the prenatal period: a validation study. Matern. Child Health J. 19, 1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krans EE, Davis MM, Palladino CL, 2013. Disparate patterns of prenatal care utilization stratified by medical and psychosocial risk. Matern. Child Health J. 17, 639–645. [DOI] [PubMed] [Google Scholar]
- Kupek E, Petrou S, Vause S, Maresh M, 2002. Clinical, provider and sociodemographic predictors of late initiation of antenatal care in England and Wales. BJOG. 109, 265–273. [DOI] [PubMed] [Google Scholar]
- Lash TL, Fox MP, Fink A, K., 2009. Applying Quantitative Bias Analysis to Epidemiologic Data. Springer, New York. [Google Scholar]
- Mannisto T, Mendola P, Grewal J, Xie Y, Chen Z, Laughon SK, 2013. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J. Clin. Endocrinol. Metab. 98, 2725–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro R, Mestman JH, 2011. Thyroid disease in pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 25, 927–943. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Brennan PA, Green P, Ilardi D, Whitfield TH, Morris N, et al. , 2008. Maternal depression and medication exposure during pregnancy: comparison of maternal retrospective recall to prosective documentation. BJOG. 115, 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman MJK, Martin JA, 2018. Timing and adequacy of prenatal care in the United States, 2016. Natl. Vital Stat. Rep. 67, 1–14. [PubMed] [Google Scholar]
- Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, et al. , 2015. The National Birth Defects Prevention Study: a review of the methods. Birth Defects Res. A Clin. Mol. Teratol. 103, 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman NE, Corman H, Noonan K, Schwartz-Soicher O, 2010. Effects of prenatal care on maternal postpartum behaviors. Rev. Econ. Househ. 8, 171–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JS, Revere FL, Toy EC, 2018. Improving rates of early entry prenatal care in an underserved population. Matern. Child Health J. 22, 1738–1742. [DOI] [PubMed] [Google Scholar]
- Soh MC, Nelson-Piercy C, 2015. High-risk pregnancy and the rheumatologist. Rheumatology (Oxford) 54, 572–587. [DOI] [PubMed] [Google Scholar]
- Taffel S, 1978. Prenatal care in the United States, 1969–1975. In: Vital and Health Statistics. Series, vol. 21, pp. 78–1911. [PubMed] [Google Scholar]
- Wang E, Glazer KB, Howell EA, Janevic TM, 2020. Social determinants of pregnancy-related mortality and morbidity in the United States: a systematic review. Obstet. Gynecol. 135, 896–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward T, Mazul M, Ngui E, Bridgewater F, Harley A, 2013. “You learn to go last”: perceptions of prenatal care experiences among African-American women with limited incomes. Matern. Child Health J. 17, 1753–1759. [DOI] [PubMed] [Google Scholar]
- Wasserstein RL, Lazar NA, 2016. The ASA statement on p-values: context, process, and purpose. Am. Stat. 70, 129–133. [Google Scholar]
- Wasserstein RL, Schirm AL, Lazar NA, 2019. Moving to a world beyond “p<0.05”. Am. Stat. 73, 1–19. [Google Scholar]
- Yan J, 2017. The effects of prenatal care utilization on maternal health and health behaviors. Health Econ. 26, 1001–1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.


