Abstract
Aim:
To determine the prevalence of dystonia in individuals with periventricular leukomalacia (PVL) and spastic cerebral palsy (CP), but without basal ganglia and thalamic injury (BGTI) on brain magnetic resonance imaging (MRI).
Method:
This was a retrospective study of individuals with spastic CP and PVL on MRI evaluated between 2005 and 2018 in a CP center. Individuals with non-PVL brain lesions on MRI, including BGTI, were excluded. Dystonia was assessed via blinded review of neurological exam videos by pediatric movement disorders specialists.
Results:
Eighty- five participants (45 males, 40 females; mean age at videotaping 12 years [standard deviation 5 years 6 months], range 4– 26 years) met inclusion and exclusion criteria. Of these participants, 50 (59%) displayed dystonia in their exam videos. The most common locations of dystonia were the fingers and hip adductors. The prevalence of dystonia was unaffected by the gestational age or severity of PVL, and was affected by Gross Motor Function Classification System level.
Interpretation:
Dystonia is common in individuals with spastic CP and PVL, even without BGTI on MRI. Our findings suggest vigilance for dystonia in individuals with spastic CP should remain high, even without MRI evidence of BGTI.
The predominant forms of high tone in individuals with cerebral palsy (CP) are spasticity and dystonia. Spasticity-predominant CP is seen in up to 79% to 86% of individuals with CP.1,2 Dyskinetic CP, which is predominated by dystonia, is seen in 6.5% to 16% of individuals with CP.1,2 It should be noted, however, that regardless of the predominant movement type of CP, spasticity and dystonia often coexist.3 It is important to distinguish between spasticity and dystonia because, despite some overlap, management strategies for spasticity and dystonia are different. Inadequate treatment of dystonia can result in significant motor impairment, muscle contractures, and pain.4,5
Spasticity is often associated with white matter injury manifesting as periventricular leukomalacia (PVL), one of the most common brain injury patterns seen in individuals with CP.6 Individuals with PVL often present with spastic bilateral CP, and these symptoms can be symmetrical or asymmetrical, depending on the location of the underlying brain injury. Dystonia, on the other hand, is typically thought to involve abnormalities in the basal ganglia and associated thalamocortical network.7 However, neuroimaging evidence of basal ganglia and thalamic injury (BGTI) is not always necessary to demonstrate the clinical presence of dystonia. For example, primary dystonia is characterized by the absence of significant abnormal structural changes on conventional brain magnetic resonance imaging (MRI).8
In the brains of individuals with PVL- associated CP, there may be BGTI at the microscopic or circuit level even if there is no visible evidence of injury on conventional MRI.9 Therefore, we hypothesize that dystonia may be common in individuals with PVL and spastic CP, even in the absence of evidence of BGTI on imaging. In this study, we performed a blinded, visual assessment of a series of neurological exam videos in a large cohort of ambulatory individuals with spastic CP and MRI- confirmed PVL without BGTI to determine the prevalence of dystonia in this population.
METHOD
This study was approved by the Human Research Protection Office of Washington University School of Medicine (IRB # 201808036). Requirement for informed consent was waived as this was a retrospective study based upon de-identified data from the medical record.
Setting
This was a retrospective, single- center study performed at the Cerebral Palsy Center in St. Louis Children’s Hospital. Patients were referred to the center for evaluation and treatment for their motor impairment and were seen by three board- certified child neurologists and one nurse practitioner who specialized in CP. As part of the routine evaluation, brain MRI, video recording, and neurological examination were performed. Video recordings of gait were taken of patients walking, with and without orthotics, approximately 12 meters down a hallway from the front and sides. They were instructed to walk in a straight line down the hallway at their own pace. Neurological examination of the upper extremity consisted of maintaining the arm extended at shoulder height, pointing to a target (finger- to- nose test), and opening and closing the hands. The total time of the video was about 5 minutes per participant.
Participant selection
Eligible participants were evaluated in the clinic between 1st January 2005 and 31st December 2018, and had an International Classification of Diseases, 9th Revision (ICD-9) or 10th Revision (ICD-10) diagnosis of spastic unilateral or spastic bilateral CP, radiological evidence of PVL on brain MRI, and reviewable video recordings of independent walking and upper extremity examination. Since the evaluation of lower extremity dystonia relied upon gait examination, only ambulatory patients (Gross Motor Function Classification System [GMFCS] levels I–III) were included. Exclusion criteria were insufficient clinical information in the medical record to determine whether inclusion criteria were met, no evidence of PVL on brain MRI, and concurrent central nervous system disorders including BGTI on brain MRI (Figure S1). These exclusion criteria were chosen to identify participants whose brain MRI injury pattern was exclusively PVL. Participants whose MRI was performed within the first 6 months of life (while the brain is still rapidly myelinating causing significant changes in the appearance of brain MRIs) and those who were less than 4 years old at the time of videotaping (when gait and movement patterns are still developing rapidly) were excluded.
Medical record review
Data collected from the medical records included sex, gestational age at birth, date of birth, age at time of MRI, date of video recording, site of upper extremity and lower extremity spasticity at time of video recording, and GMFCS level at time of video recording.
Using the MRI scans and radiological reports, PVL was identified and its severity was graded as follows using a modified Kidokoro classification system:10 (1) punctate T1/T2 signal changes in the periventricular white matter, (2) extensive lesions along the lateral ventricular wall with ventricular enlargement, and (3) cystic lesions in the periventricular white matter.
Video analysis for dystonia
All videos were reviewed by a fellowship- trained pediatric movement disorder specialist (KU) blinded to participant identity and medical record contents for identification and characterization of dystonia. Upper extremity dystonia was assessed by video of the upper extremity neurological examination and lower extremity dystonia was assessed by video of gait. To confirm the accuracy of this video review, a random selection of videos (30 videos of the upper extremity neurological examinations and 34 videos of gait) were additionally assessed by two other pediatric movement disorder specialists (TSP and BRA) in a blinded fashion for the presence of dystonia.
In this study, dystonia was defined as an intermittent abnormal movement or posture of varying amplitude triggered by purposeful movement of the same or another body part. For the upper extremities, dystonia commonly manifested as hyperextension and hyperflexion of the fingers (so- called ‘spooning’), excessive forearm pronation or supination, excessive elbow flexion or extension, and/or excessive shoulder adduction or abduction; for the lower extremities, dystonia commonly manifested as hip adduction (‘leg crossing’ or dystonic ‘scissoring’), external or internal rotation of the legs, and/or inversion or eversion of the feet.11,12 Dystonia was distinguished from spasticity by its variable amplitude and variable presence with purposeful movements. Abnormal body part positioning associated with spasticity was thought to be more constantly present and of largely invariable amplitude.
Statistical analysis
χ2 test and Fisher’s exact test were used to determine the association between the presence of dystonia and categorical variables such as the site of spasticity, gestational age (<33 weeks vs ≥33 weeks), PVL grade, and GMFCS level. To check interrater reliability for video assessment of dystonia, Cohen’s kappa coefficient was calculated for the 64 videos assessed by all three pediatric movement disorders specialists.
A p-value less than 0.05 was considered statistically significant. All analyses were calculated using EZR, version 1.41 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).13 EZR is a modified version of the R commander that adds statistical functions frequently used in biostatistics.
RESULTS
Participant characteristics
Of a total of 1514 individuals diagnosed with spastic CP screened for this study, 85 (45 males, 40 females; mean age at videotaping 12 years [standard deviation 5 years 6 months], range 4–26 years) met the inclusion criteria (Figure S1). A total of 31 individuals with isolated PVL were excluded because they were in GMFCS levels IV or V (and thus could not independently ambulate to allow for assessment of gait dystonia) or had no GMFCS data available at the time of recording. The demographics of the participants are shown in Table 1.
TABLE 1.
Demographic and clinical characteristics of participants
| n (%) | |
|---|---|
| Total | 85 |
| Males | 45 (53) |
| Mean age at videotaping (years) | 12 (range 4–26) |
| Mean age at MRI (months) | 74 (range 7–312) |
| Gestational age, mean (weeks) | 32 (range 25–41) |
| ≥33 | 26 (31) |
| <33 | 50 (59) |
| Unknown | 9 (11) |
| Distribution of spasticitiy UE only |
10 (12) |
| LE only | 46 (54) |
| Both UE and LE | 26 (31) |
| None | 3 (4) |
| GMFCS level I |
18 (21) |
| II | 39 (46) |
| III | 28 (33) |
| Grade of PVL Grade 1 |
23 (27) |
| Grade 2 | 57 (67) |
| Grade 3 | 5 (6) |
Abbreviations: UE, upper extremity; LE, lower extremity; GMFCS, Gross Motor Function Classification System; PVL, periventricular leukomalacia.
Prevalence of dystonia
To ensure reliable assessment of dystonia in participant videos, interrater reliability for upper extremity and lower extremity dystonia was calculated separately across all three movement disorders specialists. Cohen’s kappa was 0.81 for upper extremity dystonia and 0.80 for lower extremity dystonia, indicating almost perfect and substantial agreement respectively for assessment of dystonia in participant videos.
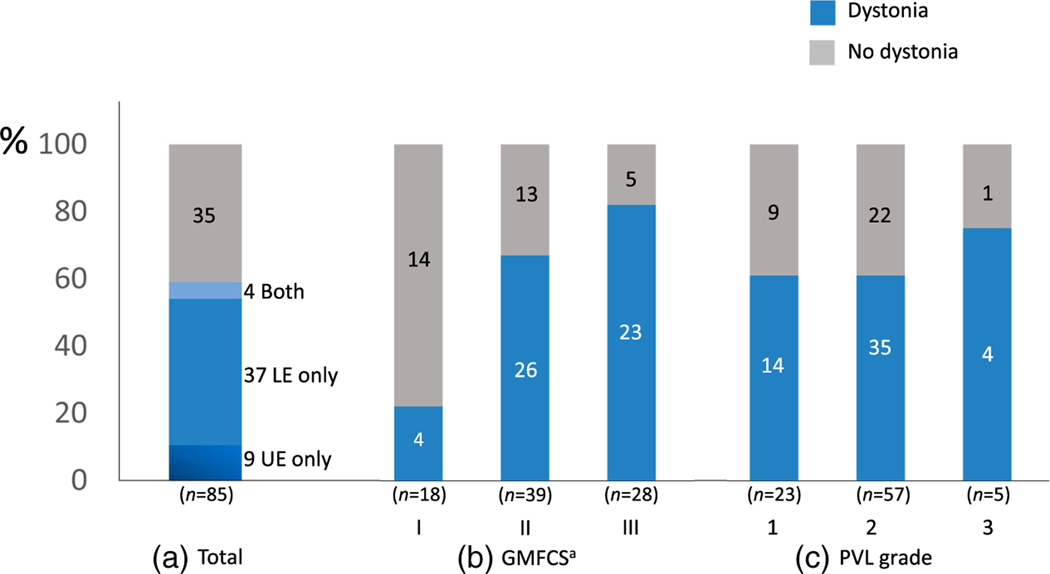
Overall, dystonia was observed in 50 of the 85 evaluated participants (59%) as determined by pediatric movement disorders specialists after video assessment (Figure 1). Of the 85 participants evaluated, nine (11%) had only upper extremity dystonia (Video S1), 37 (44%) had only lower extremity dystonia (Video S2), and four (5%) had both (Figure 1a). Dystonia was more prevalent in individuals with greater gross motor impairment (higher GMFCS level): 4 out of 18 in GMFCS level I, 26 out of 39 in GMFCS level II, and 23 out of 28 in GMFCS level III (p = 0.002, χ2 test, Figure 1b). There was no significant difference in the prevalence of dystonia in those with PVL grades 1, 2, or 3 (Figure 1c). There was also no significant difference in the prevalence of dystonia between males and females (30/45 males vs 23/40 females, p = 0.39) or between those with gestational age at birth of 33 weeks or greater, and those less than 33 weeks (17/26 with gestational age ≥ 33 weeks vs 29/50 with gestational age < 33 weeks, p = 0.53; n = 76 since nine participants with unknown gestational age ≥ 33 weeks vs 29/50 with gestational age < 33 weeks, p = 0.53; n = 76 since nine participants with unknown gestational age were excluded). The body regions most frequently affected by dystonia were the fingers and the hips (Figure 2).
FIGURE 1.
Prevalence of dystonia. Numbers of participants are indicated in each bar plot. (a) Total number of participants with dystonia: 50/85 (59%) had dystonia in the upper extremities (UE), lower extremities (LE), or both. (b) Dystonia was more prevalent in participants with higher Gross Motor Function Classification System (GMFCS) levels (p = 0.002, χ2). (c) There was no significant difference in the prevalence of dystonia related to periventricular leukomalacia (PVL) grade (p = 0.70). ap <0.05.
FIGURE 2.
Number of participants with dystonia by body regions. Fingers and hip adduction accounted for more than 50% of upper extremity dystonia and lower extremity dystonia respectively.
DISCUSSION
These results demonstrate that dystonia in the upper and lower extremities is common in individuals with mild to moderate spastic CP and isolated PVL. That is, even without the presence of BGTI on MRI, dystonia is common in CP. We also found that the presence of dystonia was not related to gestational age at birth, or MRI grade of PVL, but individuals with dystonia tended to have greater gross motor functional impairment than those without dystonia.
Lower extremity dystonia was observed in 48% (41/85) of the participants. Whereas previous studies have shown that the typical site of lower extremity dystonia is the foot, causing abnormal postures such as inversion and extension of the big toe,11 this study revealed that the most frequent form of lower extremity dystonia in this study population manifested as hip adduction. Upper extremity dystonia was observed in 15% (13/85) of the participants, predominantly in the fingers. For example, hyperextension dystonia of the fingers was evident when reaching for an object or raising the arm in front of the chest, and flexion dystonia of the fingers was present when opening and closing the hand. A previous observational study of seven individuals with PVL and spastic bilateral CP revealed the coexistence of upper extremity dystonia.14 We replicated this finding in a larger cohort of participants with isolated PVL and added the fact that dystonia was frequently found in lower extremity as well as upper extremity.
The presence of dystonia was not associated with the gestational age at birth or PVL severity, but GMFCS levels were associated with the presence of dystonia. Intriguingly, a cross-sectional study of individuals with dyskinetic CP found a positive correlation between GMFCS level and severity of dystonia, but not with severity of choreoathetosis.5 The authors suggested more negative effects of dystonia on gross motor function and hand movement. In a study of dystonia severity in children with CP associated with PVL, the severity of dystonia, but not spasticity, was associated with greater motor function impairment (higher GMFCS levels).15 Thus, it is possible that dystonia affects functional capacity, and that proper diagnosis and treatment of dystonia may in turn lead to improvement in functional mobility.
The possible pathophysiology of dystonia in PVL is complex and is likely different from the pathophysiology of spasticity. The pathologies underlying both processes may not necessarily colocalize. Injuries in PVL consist of two types: focal and diffuse injuries. Focal injuries are white matter injuries located dorsally in the external horn of the lateral ventricules involving pyramidal tract fibers, causing muscle weakness and spasticity.16 Diffuse injury, on the other hand, involves a large area of the brain and primarily affects premature oligodendrocytes.17 Animal studies have suggested that abnormalities in oligodendrocyte maturation are associated with the onset of DYT6 dystonia in childhood.18,19 Furthermore, the involvement of the thalamus in the pathogenesis of PVL-associated dystonia has been also discussed. Thalamic lesions may cause dystonia via sensorimotor impairment due to dysfunction of the basal ganglia-cerebellum-thalamus-cortical network and increased activation of thalamocortical excitatory projections to motor and premotor cortices.20–22 An animal study using mouse models of PVL has shown that premature oligodendrocytes and axon terminals in cortico-thalamo-cortical circuits are affected, resulting in damage to thalamocortical circuits.23 Autopsies of individuals with PVL showed gliosis in the medial dorsal and reticular nuclei of the thalamus.24 A case–control study of children with PVL using diffusion tensor imaging showed reduced thalamic volume and damage to afferent and efferent myelinated axons of the thalamus.25 Taken together, these results suggest that diffuse injuries affecting oligodendrocytes and the thalamus may be associated with PVL dystonia. However, these microstructural injuries may be too subtle to be readily apparent on brain MRI.9,26 In other words, individuals whose only obvious injury pattern on brain MRI is PVL may have BGTI that is just not visible on clinical imaging. Therefore, regardless of the injury pattern on brain MRI, one should maintain a high level of vigilance for dystonia in patients with CP.
Several limitations of this study need to be kept in mind. We identified dystonia based on video review alone. While we acknowledge that physical examination is important to optimize diagnostic accuracy, the observation of increased involuntary movements/postures is a key feature of dystonia that can be assessed reliably by video, and video review has the advantage of permitting standardized assessment across physician raters. Because this study was conducted in a tertiary referral facility, the participants may not have represented a true community example. We only examined independently ambulatory people with CP so that we could consistently assess for dystonia during a motor task that was relatively comparable across participants (gait). However, this precludes the generalizability of these results to people with more limited gross motor function, who are more likely to be affected by dystonia.15 Future studies need to assess dystonia in people with CP at all GMFCS levels. Because of the cross- sectional study design, it is not possible to discuss changes in dystonia over time; long-term follow-up would be beneficial to understand the progression of dystonia in people with PVL. Though dystonia was assessed by movement disorders experts reviewing a standardized set of exam videos, the severity of dystonia was not assessed. Symptoms of dystonia might be masked or reduced by severe weakness, spasms, muscle contractures, or pharmacological treatments such as baclofen or botulinum neurotoxin A injections. This suggests that that prevalence of dystonia reported here is likely an underestimate of the true prevalence. Neuroimaging findings were used to identify isolated PVL, but could not completely exclude other brain lesions, particularly because MRI techniques have significantly improved over the long study period and brain lesions may have been more likely to be ‘missed’ on older scans. All participants who underwent MRI scans before 6 months of age were excluded, as myelination significantly progresses within the first 6 months of life. However, we additionally acknowledge that uniform interpretation of MRI scans across a wide age range is a limitation.
Conclusion
In summary, we found that dystonia is common in people with spastic CP and isolated PVL on brain MRI and can occur in both the upper extremity (most commonly the fingers) and lower extremity (most commonly affecting hip adduction). Clinicians need to be aware that dystonia is common in people with PVL even in the absence of BGTI on brain MRI and that, in clinical practice, dystonia may be underestimated or misdiagnosed as spasticity. That is, vigilance for dystonia in people with CP, regardless of brain MRI injury pattern, should remain high.
Future work could investigate what movement patterns may appear later in life and what the progression patterns are. To examine this issue and further understand the pathophysiology of dystonia in people with PVL and its natural history and progression, it is necessary to conduct long-term follow-up studies and to prospectively evaluate cohorts with up-to-date MRI and physical examination assessments across all GMFCS levels.
Supplementary Material
Figure S1: Enrollment flow chart.
Video S2: Person with dystonia in the lower extremity during gait evaluation.
Video S1: Person with dystonia in the upper extremity during upper limb evaluation.
What this paper adds.
Individuals with spastic cerebral palsy and isolated periventricular leukomalacia on magnetic resonance imaging commonly display dystonia.
Common sites of dystonia are in the fingers and hip adductors.
ACKNOWLEDGMENTS
No funding or sponsorship was obtained for producing this manuscript.
Dr Ueda reports no financial disclosure and conflict of interest to disclose. Dr Aravamuthan receives research funding from the National Institute of Neurological Disorders and Stroke and reports consulting fees from Neurocrine Biosciences. Dr Pearson receives research funding from the National Institute of Neurological Disorders and Stroke and reports consulting fees from Teva Pharmaceuticals.
The authors affirm that the work described is consistent with the Journal’s guidelines for ethical publication. All authors acknowledge (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Funding information
National Institute of Neurological Disorders and Stroke; Neurocrine Biosciences; National Institute of Neurological Disorders and Stroke
Abbreviations:
- BGTI
Basal ganglia and thalamic injury
- PVL
Periventricular leukomalacia
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Himmelmann K, Uvebrant P. Function and neuroimaging in cerebral palsy: a population-based study. Dev Med Child Neurol 2011;53: 516–21. [DOI] [PubMed] [Google Scholar]
- 2.Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol 2002;44:633–40. [PubMed] [Google Scholar]
- 3.Rice J, Skuza P, Baker F, Russo R, Fehlings D. Identification and measurement of dystonia in cerebral palsy. Dev Med Child Neurol 2017; 59:1249–55. [DOI] [PubMed] [Google Scholar]
- 4.Lin JP, Lumsden DE, Gimeno H, Kaminska M. The impact and prognosis for dystonia in childhood including dystonic cerebral palsy: a clinical and demographic tertiary cohort study. J Neurol Neurosurg Psychiatry 2014;85:1239–44. [DOI] [PubMed] [Google Scholar]
- 5.Monbaliu E, de Cock P, Ortibus E, Heyrman L, Klingels K, Feys H. Clinical patterns of dystonia and choreoathetosis in participants with dyskinetic cerebral palsy. Dev Med Child Neurol 2016;58:138–44. [DOI] [PubMed] [Google Scholar]
- 6.Drougia A, Giapros V, Krallis N, et al. Incidence and risk factors for cerebral palsy in infants with perinatal problems: a 15- year review. Early Hum Dev 2007;83:541–7. [DOI] [PubMed] [Google Scholar]
- 7.Stoessl AJ, Lehericy S, Strafella AP. Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet 2014;384: 532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonyan K. Neuroimaging Applications in Dystonia. Int Rev Neurobiol 2018;143:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierson CR, Folkerth RD, Billiards SS, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol 2007;114:619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 2014;134:e444–53. [DOI] [PubMed] [Google Scholar]
- 11.Sanger TD, Ferman D. Similarity of Involuntary Postures between Different Children with Dystonia. Mov Disord Clin Pract 2017;4:870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aravamuthan BR, Ueda K, Miao H, Gilbert L, Smith SE, Pearson TS. Gait features of dystonia in cerebral palsy. Dev Med Child Neurol 2021;63:748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda Y. Investigation of the freely available easy-t o- use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pons R, Vanezis A, Skouteli H, et al. Upper Limb Function, Kinematic Analysis, and Dystonia Assessment in Children With Spastic Diplegic Cerebral Palsy and Periventricular Leukomalacia. J Child Neurol 2017;32:936–41. [DOI] [PubMed] [Google Scholar]
- 15.Papadimitriou I, Dalivigka Z, Outsika C, Scarmeas N, Pons R. Dystonia assessment in children with cerebral palsy and periventricular leukomalacia. Eur J Paediatr Neurol 2021;32:8–15. [DOI] [PubMed] [Google Scholar]
- 16.Staudt M, Pavlova M, Böhm S, Grodd W, Krägeloh-Mann I. Pyramidal tract damage correlates with motor dysfunction in bilateral periventricular leukomalacia (PVL). Neuropediatrics 2003;34:182–8. [DOI] [PubMed] [Google Scholar]
- 17.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 2001;50:553–62. [DOI] [PubMed] [Google Scholar]
- 18.Yellajoshyula D, Liang CC, Pappas SS, et al. The DYT6 Dystonia Protein THAP1 Regulates Myelination within the Oligodendrocyte Lineage. Dev Cell. 2017;42:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yellajoshyula D, Pappas SS, Rogers AE, et al. THAP1 modulates oligodendrocyte maturation by regulating ECM degradation in lysosomes. Proc Natl Acad Sci U S A 2021;118:e2100862118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mink JW. Basal ganglia mechanisms in action selection, plasticity, and dystonia. Eur J Paediatr Neurol 2018;22:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desrochers P, Brunfeldt A, Sidiropoulos C, Kagerer F. Sensorimotor Control in Dystonia. Brain Sci 2019;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord 2002;17:S49–S62. [DOI] [PubMed] [Google Scholar]
- 23.Liu XB, Shen Y, Pleasure DE, Deng W. The vulnerability of thalamocortical circuitry to hypoxic-ischemic injury in a mouse model of periventricular leukomalacia. BMC Neurosci 2016;17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ligam P, Haynes RL, Folkerth RD, et al. Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr Res 2009;65:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagasunder AC, Kinney HC, Blüml S, et al. Abnormal microstructure of the atrophic thalamus in preterm survivors with periventricular leukomalacia. AJNR Am J Neuroradiol 2011;32:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buser JR, Maire J, Riddle A, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 2012;71:93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Enrollment flow chart.
Video S2: Person with dystonia in the lower extremity during gait evaluation.
Video S1: Person with dystonia in the upper extremity during upper limb evaluation.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.