Abstract
Corneal diseases are among the most common causes of blindness worldwide. Regardless of the etiology, corneal opacity- or globe integrity-threatening conditions may necessitate corneal replacement procedures. Several procedure types are currently available to address these issues, based on the complexity and extent of injury. Corneal allograft or keratoplasty is considered to be first-line treatment in many cases. However, a significant proportion of the world’s population are reported to have no access to this option due to limitations in donor preparation. Thus, providing an appropriate, safe, and efficient synthetic implant (e.g., artificial cornea) may revolutionize this field. Nanotechnology, with its potential applications, has garnered a lot of recent attention in this area, however, there is seemingly a long way to go. This narrative review provides a brief overview of the therapeutic interventions for corneal pathologies, followed by a summary of current biomaterials used in corneal regeneration and a discussion of the nanotechnologies that can aid in the production of superior implants.
Keywords: Nanotechnology, Bioengineering, Corneal donor, Keratoplasty, Corneal transplantation, Biopolymer
Introduction
The advent of nanotechnology in the mid-1900s represented the start of a new scientific frontier. Many different nanostructures have been utilized across various fields of science, including applications in medical diagnostics and therapeutics. 1 Nanomaterials (e.g., materials possessing, at a minimum, one external dimension measuring 1–100 nm) can have completely different physicochemical features compared with the same materials in their bulk state, which may be harnessed to produce new, advanced tools. 2 For example, the unique optical, electrical, and magnetic properties of inorganic nanomaterials, such as quantum dots, metallic nanoparticles, and magnetite nanocrystals, have enabled the development of nano-biosensors. 3 Similarly, intelligent drug delivery systems have been made possible by the structural and conformational changes of organic nanomaterials. 4 In regenerative medicine and the development of prostheses, nanomaterials and nanofabrication techniques have unique roles in the production process, surface modification, and bio-activation. 5
One of the major applications of medical nanotechnology is in corneal therapy. Ocular injuries and diseases can severely impact on a patient’s quality of life. According to the World Health Organization, corneal diseases are the fifth-leading cause of blindness worldwide. Their etiologies encompass many infectious and inflammatory disorders, 6 including microbial keratitis,6–10 autoimmune disorders with ocular surface involvement,11–16 genetic defects,17–19 chemical and thermal injuries, 20 ocular inflammation, 21 and surgical complications,22,23 any of which may result in corneal scarring and, potentially, functional blindness. 24 Despite current advances in medical treatment, severe corneal infections, injuries, or systemic diseases often lead to corneal perforation, which may progress to vision loss and often requires surgical reconstruction. Several procedure types are available for corneal repair, depending on the complexity and extent of the corneal injury and the clinical status of other ocular structures.
Keratoplasty, also known as corneal transplantation, refers to any surgical method in which a complete or partial cornea from a human donor is grafted onto the patient’s eye. It is the primary, first-line intervention for repairing a damaged cornea. Yet, although corneal transplants have become a mainstay therapy, there is a severe global shortage of donor corneal tissue. 25 According to a global survey conducted in 2016, about 53% of the world’s population do not have access to corneal transplantation, with only one cornea available for every 70 needed. 26
Some patients are poor candidates for keratoplasty, often due to the nature of the ocular pathologies or the onset of severe complications, such as multiple prior graft rejections. 27 If a keratoplasty is not possible or feasible, an artificial cornea (e.g., keratoprosthesis) may be considered. Keratoprosthesis refers to replacing a damaged cornea with a synthetic implant. 28 Given the frequency and severity of its associated complications, keratoprosthesis has historically been the last resort therapy for patients who are poor candidates for keratoplasty, who have had multiple prior graft rejections, or who have poor visual acuity (20/400 or worse) due to severe corneal opacification. 28 More recently, however, keratoprostheses have begun to be used as the first-line treatment for patients with a high likelihood of graft failures, such as bilateral limbal stem cell deficiency, neurotrophic keratitis, or severe corneal neovascularization, with better outcomes as a result.29–33 This narrative review provides a brief overview on currently available artificial corneas and their drawbacks, followed by a summary of current biomaterials used in corneal regeneration, and a discussion of the nanotechnologies that might aid in the production of superior implants.
Common keratoprostheses and their drawbacks
Most artificial corneas consist of two distinct parts: the optic at the center of the prosthesis, responsible for transmitting light, and the skirt around the periphery to affix the prosthesis to the rest of the eye. Accordingly, high transparency is the main priority of the optic material, while high bioactivity is desired for the skirt material, as it must interact well with the native cells and tissues to integrate properly.
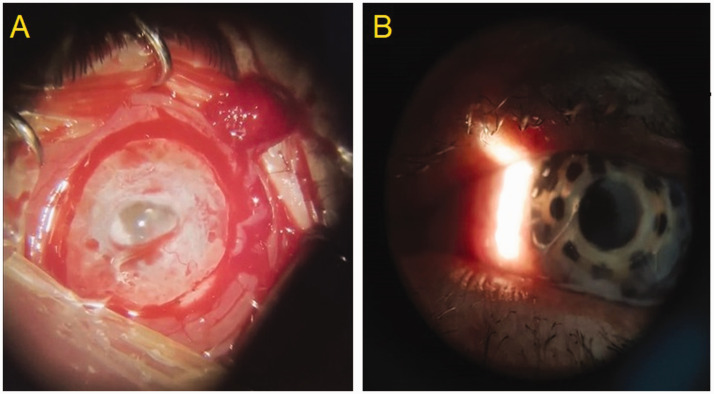
There are several commercially available artificial corneas, including Boston keratoprosthesis types I and II, osteo-odonto-keratoprosthesis, and many others, which are detailed elsewhere. 34 Boston type I, the most common corneal prosthesis worldwide, comprises an optical stem and skirt that is first made of polymethyl methacrylate (PMMA) and then titanium. 35 Despite being an artificial implant, however, the Boston keratoprosthesis still requires donor corneal tissue as a carrier for the prosthesis, and thus, is subject to the same limits of donor availability as traditional keratoplasties (Figure 1).
Figure 1.
Representative images from a patient with severely compromised ocular surface due to chemical burn, showing: (a) the cornea and ocular surface prior to surgery; and (b) the cornea and ocular surface after Boston keratoprosthesis surgery.
Among the complications of Boston keratoprostheses, a retroprosthetic membrane is by far the most common, occurring in 18–55% of cases. 36 Retroprosthetic membranes form due to keratocyte migration from the intersection of the transplanted cornea towards the prosthesis, through the aqueous humor. These displaced keratocytes can opacify the artificial cornea and obstruct the flow of nutrients, resulting in corneal melt, 37 chronic hypotonia, and retinal detachment.38,39 Correction via Nd:YAG laser, pars plana membranectomy, or surgical replacement of the entire prosthesis may be necessary. 40 Other complications include corneal melt caused by autoimmune disorders or chemical injuries,41–44 progressive glaucoma,45,46 infection,47,48 and other vitreoretinal segment complications, such as choroidal or retinal detachment.49,50
Regarding osteo-odonto-keratoprostheses, up to 50% of complications involve the buccal membrane, including overgrowth of the buccal mucosa on the optic, exposure of the lamina, and mucosal ulceration. 51 Physical removal of overgrown mucosa may be required, although mitomycin C may be used in milder cases. 52 Mucosal ulcerations due to dry eye, ischemia, and infection must be treated immediately to prevent an internal infection and erosion of the lamina. Small ulcers can be treated with antibiotic-containing lubricants, while larger ulcers may require surgical methods such as free patch grafts, mucosal rotation, bucket handle flaps, and tarsal pedicles. 53 Even the Pintucci prosthesis, a newer device based on the osteo-odonto-keratoprosthesis that utilizes Dacron (polyethylene terephthalate) instead of a natural lamina, remains susceptible to membrane mucosal necrosis. 54 Glaucoma, 55 vitreous hemorrhage, choroidal detachment, 56 endophthalmitis, 57 and anatomic device failure,58,59 are the other known complications related to osteo-odonto-keratoprostheses.
As seen, the major challenge with keratoprostheses is modulating the bioactivity of its two parts: high bioactivity in the skirt but low bioactivity in the optic. Most of the complications discussed above involve biomembrane formation on the optic or structural failure of the skirt membrane. Other keratoprostheses, such as the Korea Seoul-Type, Fyodorov-Zuev, AlphaCor, and KeraKlear have various complications, but retroprosthetic membrane formation or structural perforation remains chief among them. 34
Material considerations for corneal scaffolds
To mitigate the lack of supply, many attempts have been made to bioengineer a viable corneal implant as an alternative to donor tissues for corneal grafting, 60 with the aim of generating specific layers of the cornea in vitro using biocompatible materials that would then be surgically grafted onto the patient’s eye. These corneal scaffolds might either replace the donor corneal tissue in a keratoplasty or serve as the skirt material of a keratoprosthesis.
A viable cornea must be both mechanically robust and fully transparent. As the outermost barrier of the eye, the cornea shields the inner ocular structures from the external environment. The strength of the cornea stems from the stroma, which accounts for 90% of the corneal thickness and comprises approximately 300 crisscrossing layers of collagen. 61 Other layers consist of collagen, elastin, fibronectin, laminin, and proteoglycans, all organized in distinct patterns and varying densities, which give rise to additional mechanical properties. 62 Some of these layers are deposited as an extracellular matrix by keratocytes, which are differentiated mesenchymal cells that can proliferate and migrate within the stroma.63–65 At the same time, the cornea is also the most powerful light-focusing visual element, accounting for 80% of the eye's refractive power, or about three times the focusing power of the lens. 66 The cornea's transparency is maintained through the dense packing of proteins in a proteoglycan-rich matrix, allowing 80% of visible light to pass through without obstruction. 67 The limbus region maintains the cornea's transparency by preventing neovascularization and ensuring immune privilege. The limbus forms a boundary between the corneal epithelium and the conjunctiva, and its stem cells are essential for repairing the epithelial layer. As a result, the main factors threatening corneal transparency are inflammatory reactions, vascularized areas, and limbal stem cell deficiency. 68
Beyond mechanical and optical properties, the structural organization of the scaffold material plays a key role. A scaffold’s topography has been revealed to influence cellular behavior, including migration, proliferation, and phenotypic differentiation.69–71 Scaffold curvature, for example, has been shown to affect the orientation and phenotypic expression of epithelial cells. 72 Importantly, it should be noted that this structure-bioactivity relationship is bidirectional, as the cells can exert various modulative effects on the polymeric matrix, possibly remodeling it to be different from the native corneal infrastructure. Therefore, consideration of the cells’ ability to assemble and degrade the specific polymers of the scaffold material is also essential.
Thus, there are five primary considerations related to scaffold materials. First, they must have sufficient mechanical strength to maintain stability against the internal ocular pressure and eyelid squeezing. 73 Second, the optical properties of the engineered scaffolds must be similar to the native cornea. 74 Third, the structural arrangement of the scaffold material and its interaction with the cells should be carefully considered. Fourth, the scaffold material must be permeable to nutrients and air to ensure cell viability. 75 Finally, as with any bioengineered implant, basic requirements, such as safety and biocompatibility, must be met.
To date, the identification of a biocompatible material that fully satisfies all requirements has been exceedingly challenging. Various natural and synthetic materials have been investigated in search of an optimal combination of mechanical, optical, and biological properties. Several candidates have been identified for use in corneal regeneration, as discussed below.
Amniotic membrane
Amniotic membranes, obtained from human placentae after Caesarean sections, are commonly used specifically in corneal epithelial regeneration. Amniotic membranes are conducive to treating epithelial defects due to their biocompatibility and anti-inflammatory properties. To improve the mechanical properties and durability of amniotic membranes for storage, the membranes are reinforced with nanofibers and processed by freeze-drying. 76 However, its disadvantages include inaccessibility, structural variations across different donors, rapid degradation, and difficulties related to preparation and storage.77,78
Synthetic polymers
Several synthetic polymers, such as PMMA, ultra-high molecular weight polyethylene (UHMWPE), and polyether ether ketone (PEEK), have been used successfully in bioimplants, however, they remain limited by poor bioactivity. For example, the clinical outcomes of Boston keratoprostheses remain limited by poor adhesion between the PMMA stem and donor tissues. Multiple research groups have attempted surface functionalization of the polymers in response to this shortcoming. In their 2010 study, Pino et al. 79 treated films of UHMWPE and PEEK with concentrated sodium hydroxide and simulated body fluid, which produced an apatite layer around the polymers with an organic ion profile portending good biocompatibility. In 2019, Sharifi et al. 80 reported chemically modifying the surface of PMMA by removing methyl groups and adding L-DOPA. Without affecting the transparency and mechanical properties, the biocompatibility of this new material was enhanced dramatically, with increased cell proliferation and migration compared with pure PMMA for both human corneal fibroblasts (HCF) and human corneal epithelial cells (HCEp). Research efforts remain ongoing to augment the bioactivity of the polymers for improved adhesion to native ocular tissue.
Hydrogels
Hydrogels are crosslinked matrices of heavy-chain polymers. Their many hydrophilic groups enable rapid and effective water retention, which facilitates the maintenance of cells and culture media. Hydrogels can be dried and processed into nanofilms for long-term storage and rehydrated immediately before implantation. 81 Hydrogels can be manufactured using synthetic and naturally-derived materials, and they have been used extensively in tissue engineering studies. It should be mentioned that hydrogels are limited by their relatively short shelf-life. 82
Collagen
Type I collagen is the most abundant molecule in the native cornea. Its ability to support the growth and proliferation of epithelial, stromal, and endothelial cells is already well-established. 83 Despite its advantages, the use of collagen remains a challenge due to its poor mechanical properties and differences in fibril arrangements compared with native cornea. Collagen scaffolds have low mechanical strength due to the lower density of fibrils in the polymer matrix. However, this can be somewhat overcome by the addition of crosslinking agents, such as glutaraldehyde, 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC), 84 or less cytotoxic agents, such as genipin 85 or riboflavin. 74 Additionally, collagen harvested from different parts of different animals can have different structural arrangements that affect the resulting scaffold. 86 More recent efforts have thus pivoted toward using recombinant type I and type III human collagen. 87
Alginate
Alginate, a naturally occurring substance extracted from brown algae, is a polymer consisting of b-d-mannuronic and a-1-guluronic acid monomers. Alginate has poor bioactivity and cell adhesion, however, this can be mitigated by incorporating other biomaterials, such as gelatin. 88
Chitosan
Chitosan is the deacetylated derivative of natural chitin commonly found in the exterior shell of arthropods. It cannot be used to manufacture scaffolds on its own due to poor mechanical properties, however, it has the unique property of being positively charged. Thus, chitosan has been extensively used as a crosslinking agent for many scaffolds, as most biopolymers are composed of negatively charged molecules.89,90
Silk fibroin
A more promising candidate is silk fibroin, which has garnered much interest due to its biocompatibility, mechanical strength, and versatility to form a variety of nanostructures. 91 As it can be easily harvested in large quantities at a low cost from the cocoons of Bombyx mori, many corneal regeneration scaffolds in recent years have been made from this material. 92 To improve their structural integrity, cell adhesion/proliferation and cell migration, fibroin-based scaffolds are commonly blended with several other biomaterials, including arginyl-glycyl-aspartic acid (RGD) peptide, 93 poly-D-lysine (PDL), 94 aloe vera, 95 β-carotene, 96 lysophosphatidic acid, 97 chitosan, and collagen.98,99 Various matrices obtained from silk fibroin are used to design scaffolds for the regeneration of corneal epithelial, stromal, and endothelial cells. 100
Despite the individual limitations of these materials, they become much more feasible as scaffold candidates when strengthened by crosslinking and formed into hydrogels. An alginate hydrogel reinforced with gelatin nanofibers has been shown to have more than a five-fold increase in tensile strength, without corresponding changes in transparency, and a further ten-fold increase in elasticity after crosslinking with EDC. 101 A nanopatterned gelatin methacrylate (GelMA) hydrogel augmented by sequential crosslinking was found to have an eight-fold rise in mechanical strength, and facilitate the growth and implantation of human corneal endothelial cells to a greater extent, compared with standard GelMA. 102 A synthetic, collagen-like peptide hydrogel embedded with a fine fibronectin network was shown to be transparent, biocompatible, noncytotoxic with good bioactivity, and more than a hundred-fold resistant to microbial contamination compared with amniotic membranes. 103
One of the most common challenges facing corneal regeneration scaffolds is the phenotypic transformation of keratocytes into fibroblasts. In 2020, Kong et al. 104 reported a GelMA/microfiber composite hydrogel that could maintain the keratocyte phenotype in stromal regeneration therapies. Upon further analysis, fiber spacing was found to modulate many of its mechanical properties, as it is directly proportional to transparency and swelling index and inversely related to compressibility, tensile strength, and elongation module. 104
Despite the versatility and popularity of hydrogels, their mechanical properties currently remain inadequate for corneal applications. To address this, Parke-Houben et al. 105 described the construction of an interpenetrating polymer network of hydrogels from poly ethylene glycol and poly acrylic acid. Once their surfaces were functionalized with extracellular proteins, scaffolds produced by these composite hydrogels exhibited the ability to support corneal fibroblasts. 105 More recently, Sharifi et al. 106 developed a gelatin glycidyl methacrylate (G-GMA) hydrogel interspersed with graphene-coated spherical micropores that exhibited superior mechanical properties while maintaining biocompatibility and cellular support. 106 Both improved hydrogels show promise as corneal scaffolds or carriers for Boston keratoprostheses.
Nanomaterials for corneal tissue engineering
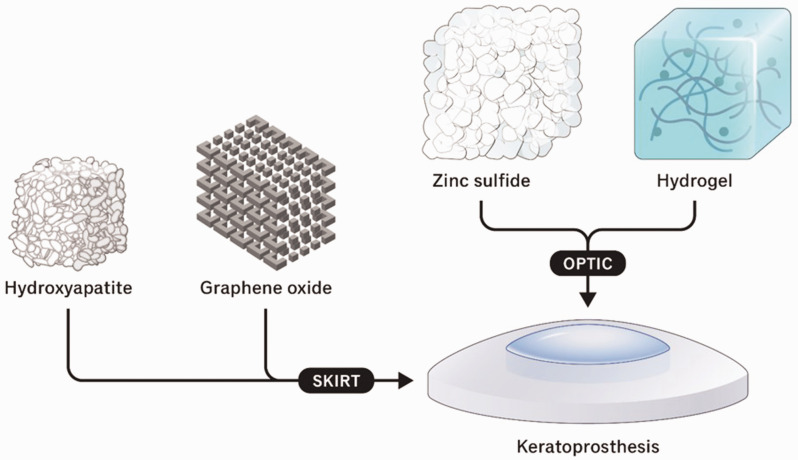
While the materials surveyed above demonstrate potential for use in corneal tissue engineering, each has significant drawbacks, such as poor mechanical strength, differences in molecular organization, inaccessibility, and poor bioactivity. As discussed below and summarized in Figure 2, nanomaterials may enhance existing materials by addressing these shortcomings, resulting in potentially superior corneal scaffolds and keratoprosthesis skirts.
Figure 2.
Overview of nanomaterials used in corneal keratoprosthesis.
Hydroxyapatite
Hydroxyapatite and its derivatives are the major components of human bone and teeth. It is a suitable candidate for tissue engineering applications due to its excellent biocompatibility and its ability to integrate with soft tissues. Hydroxyapatite is widely used as a reinforcing agent for bioactive nanocomposites of different natural and synthetic polymers, such as collagen, polyvinyl alcohol (PVA), keratin, chitosan, hyaluronic acid, and polyethylene alginate. 107 Rees et al. 108 reported excellent interaction and integration of hydroxyapatite nano-minerals with glycosaminoglycan and their core protein of intracellular matrix. Shi et al. 109 showed that a nano-coating of hydroxyapatite on silicon rubber improved its biocompatibility, which may be related to elongation factor 1-beta (EF1β)/γ-actin adjusted cytoskeletal rearrangement.
Research groups have experimented with hydroxyapatite in the production of artificial corneas. An early sample comprised an optic made of PVA and a skirt made of hydroxyapatite-incorporated PVA, which showed good bio-integration into the eye of a rabbit model. 110 Due to the addition of hydroxyapatite, the resultant skirt was more porous, facilitating the growth and migration of stromal fibroblasts toward the prosthesis, as well as nutrient transport and connection to host tissues. 110 A more recent study showed that nano-hydroxyapatite fixed onto a PMMA cylinder via dip-coating had a substantial effect on the construct's biocompatibility and bio-integration rate in a rabbit model. 111 The nano-coated transplant was shown to exhibit significantly milder apoptotic and inflammatory responses than PMMA alone, resulting in superior stability and safety. By comparison, bare PMMA exhibited the formation of new loose structures similar to those observed in bone tissues in response to non-bioactive materials. 111
Zhou et al. 112 designed a porous, PVA/silk fibroin hydrogel matrix crosslinked by genipin and with hydroxyapatite nanoparticles interspersed throughout. The genipin strengthened and stabilized the hydrogel in a dose-dependent manner, and the hydroxyapatite helped improve its bioactivity. Testing against human corneal fibroblasts showed minimal cytotoxicity, with viable proliferating cells well-adhered to the scaffold.
Among the physicochemical and morphological properties of hydroxyapatite nanoparticles, the dispersant ratio and aggregation rate when coated on the base material have been shown to have the greatest impact on the construct’s biocompatibility and bioactivity. A study into the effect of different parameters of size, shape, aggregation rate, and hydroxyapatite/dispersant ratio on the biological activity of an artificial cornea made of poly 2-hydroxyethyl methacrylate (PHEMA) decorated with hydroxyapatite, showed that a lower aggregation rate was associated with higher bioactivity and cell growth when the ratio of hydroxyapatite/dispersant was 1.25. 113 Aside from its advantages, hydroxyapatite has poor mechanical strength.
Graphene oxide
Graphene oxide (GO) and other graphene-family nanomaterials have drawn significant interest in recent years for their potential applications in regenerative medicine and tissue engineering. Similar to hydrogels, GO is hydrophilic, but it also has excellent mechanical strength, adheres well to cells, and is structurally versatile. 114 Indeed, GO has been shown to create a valuable structure for regulating stem cell behavior, differentiation, and proliferation. Despite the successful synthesis of GO materials, the process produces a significant amount of toxic and hazardous gases, including nitrogen dioxide and chlorine dioxide, which can cause explosions. 114
Sintered nanocomposites of titanium dioxide (TiO2) reinforced by different GO structures have been tested. In 2019, Li et al. 115 reported incorporating GO nanoparticles and liquid crystalline GO, in both the natural and reduced state, into TiO2 matrices. These nanocomposites with GO in the reduced state were safer and more biocompatible than pure TiO2. In vitro biocompatibility assays verified that these nanocomposites have no cytotoxicity or apoptotic effects and that stromal fibroblasts can attach well to the constructs. In vivo implantation into the eyes of a rabbit model has also shown favorable results, including decreased inflammation, edema, and neovascularization. 115
Accordingly, GO nanoparticles may enhance biocompatibility of the skirt material. When GO nanoparticles were incorporated into PHEMA during polymerization via a one-pot synthesis, the final material exhibited good bioactivity, growth, and attachment of fibroblasts on its surface, in addition to greater mechanical strength than pure PHEMA. 116
Zinc sulfide
Unlike the skirt, the ideal optic would have low bioactivity to avoid biomembrane formation on the inner prosthesis-eye interface, which is a common challenge for commercial keratoprostheses. As a result, current keratoprostheses require the application of additional repellent materials on the optic.
Early studies have shown zinc sulfide (ZnS) nanoparticles to be particularly helpful in limiting biomembrane formation and fine-tuning the refractive index, which can be adjusted to match the value of a native cornea. Zhang et al. 117 designed a ZnS-incorporated PHEMA/polyacrylic acid (PAA) hydrogel nanocomposite with a refractive index of 1.65 and 1.49 in its dry and hydrated states, respectively, for use as the optic part of an artificial cornea. Although the material was shown to be cytotoxic, it is not in direct contact with corneal cells, and this toxicity effect can be advantageous in preventing biomembrane formation. Later, a hydrogel based on polyvinyl pyrrolidone (PVP) and N, N-dimethyl acrylamide (DMAA) containing ZnS nanoparticles was developed by the same research group. 118 This ZnS/PVP/DMAA hydrogel exhibited good transparency, even in high quantities of ZnS, and it had a variable refractive index of 1.58–1.70 in the dry state and 1.38–1.46 in the hydrated state, which is suitable for corneal applications. Additionally, this hydrogel showed good biocompatibility in vivo, evidenced by successful transplants into rabbit eyes. 118 In both hydrogel prototypes, no cell–protein adhesion on the construct was observed.
Nanofabrication methods for corneal tissue engineering
Beyond material properties, fiber alignment and topography are also important considerations. In this regard, nanofabrication techniques may be helpful, by controlling the specific order and arrangement of the biomaterials during the scaffold construction process. Electrospun nanofibers and 3D-bioprinted scaffolds have demonstrated potential in corneal tissue regeneration applications.119,120
Electrospinning
Electrospinning involves the application of a high electric voltage between the polymer syringe nozzle and the collector, and the electrospun nanofibers' size, shape, layering, and organization can be customized as desired. Its main drawback is the need for advanced devices and proficiency with the technique. 121
One interesting variable is the alignment of the electrospun fibers. In a study into the behavior of epithelial and stromal cells on random or aligned gelatin nanofibers, keratocytes predominated on aligned structures whereas more epithelial cells were found on random-patterned structures. 72 In a 2018 study by Aslan et al., 122 the properties of a hydrothermally crosslinked collagen foam, reinforced by either random or aligned poly-1-lactic acid (PLLA) nanofibers, were compared with a decellularized corneal scaffold as the control. Compared with a pure collagen foam, the electrospun PLLA nanofibers decreased the scaffold’s transparency from 90% to 80% and decreased the degradation rate from 20% to 0.9% within 28 days. Through these studies, randomly patterned fibers were found to be more effective at activating cells than uniformly aligned ones, while the cells on aligned scaffolds were found to be more phenotypically homogeneous. It is possible, therefore, to control the differentiation, proliferation, and growth of each type of corneal cell based on the physical structure of the scaffolds. 72
A micro-stereolithography system was used to manufacture a polyethylene glycol diacrylate ring, whose surface was covered by poly lactic-co-glycolic acid nanofibers via electrospinning. Designed to address limbal stem cell deficiency, this engineered construct could facilitate the attachment, growth, and proliferation of epithelial and limbal stem cells. 123 Electrospun polycaprolactone scaffolds demonstrated sufficient light transmission. Corneal scaffolds made of aligned PVA/collagen composite nanofibers that were electrospun and seeded with keratocytes and corneal epithelial cells exhibited good adherence and orderly growth. 124
3D-bioprinting
To date, the most precise scaffold production method is 3D bioprinting, whereby biomaterials and cells are systematically layered as specified. It should be mentioned that classic 3D printing techniques, such as stereolithography, selective laser sintering, inkjet printing and fused deposition modeling, mainly print objects on a micro-scale. Nevertheless, two-photon polymerization-based 3D printing, which utilizes near-infrared femtosecond laser, can produce objects at nanoscale resolution. Scaffolds produced by 3D bioprinting are highly customizable and reproducible, and the process can be easily automated. However, the opacity of current 3D-bioprinted scaffolds remains a significant limitation of this method. 125
Three-dimensional-bioprinted collagen and recombinant human laminin matrices have been trialed as scaffolds for epithelial limbal stem cells and human adipose tissue-derived stem cells to regenerate epithelial and stromal tissues, respectively. These scaffolds supported cell types in morphology and expression of type I collagen. In vivo studies also demonstrated good biointegrity and facilitated cell migration from the scaffold to the host cornea in a porcine model. 126
Another example of a 3D-bioprinted scaffold was produced from decellularized human corneas, which have almost identical transparency and composition to native corneas. Human turbinate-derived mesenchymal stem cells (hTMSCs) have been shown to differentiate into keratocytes in the scaffold without any cytotoxicity. In vivo studies have also found no adverse effects on host tissues associated with the implantation of this scaffold. 127 A similar study used collagen containing encapsulated corneal keratocytes as bio-ink for 3D bioprinting a corneal scaffold, which exhibited high cell viability at 7 days post-printing. 128
Nanotechnology for gene delivery
Gene therapy, delivering suitable therapeutic genetic materials along with rational regulatory elements into the desired cells to fix or replace defective genetic materials, is a promising option for treating a variety of acquired and inherited corneal disorders. Traditionally, viral vectors have been used to transfer genes into tissues of interest, however, nanotechnology-based gene delivery systems have been shown to offer a wide range of possibilities for gene delivery to the eye. Compared with viral vectors, non-viral vectors offer greater flexibility in customising a system for the purpose of targeting or enhancing the in vivo circulation time. 129 Nanoparticles are a commonly used type of nanomaterial for gene delivery that may be made from various materials, such as lipids, polymers, or metals. Lipid-based nanoparticles, such as liposomes or lipid nanoparticles, are particularly well-suited for gene delivery due to their biocompatibility and ability to fuse with cell membranes. Polymer-based nanoparticles, including polymeric micelles or dendrimers, offer advantages that include tunable size, stability, and controlled release of genetic material. 130 The use of nanomaterials for gene delivery in corneal tissue engineering offers several advantages. First, nanomaterials can protect the genetic material from degradation by enzymes and enhance its stability, thus improving its efficacy. Secondly, nanomaterials can enhance cellular uptake of the genetic material, increasing its bioavailability and reducing the required dosage. Moreover, the surface modifications of nanomaterials may provide targeted delivery to specific corneal cells or tissues, minimizing off-target effects and improving therapeutic outcomes. However, there are also challenges and considerations associated with the use of nanomaterials for gene delivery in corneal tissue engineering. 131 These include the need to optimize the nanomaterial properties to achieve efficient gene delivery, ensuring long-term safety and biocompatibility, and addressing potential immunogenic responses or toxicity concerns. Additionally, the regulatory and clinical translation aspects need to be carefully addressed to ensure the safe and effective use of these technologies in human patients. In summary, the use of nanomaterials for gene delivery in corneal tissue engineering holds promise for developing innovative therapies to treat corneal diseases and injuries. Nanomaterials offer unique properties that may enhance gene delivery efficiency, protect genetic material, and provide targeted delivery. Ongoing research in this field aims to further optimize nanomaterial design and delivery techniques to advance the field of corneal tissue engineering and improve patient outcomes. 132
Conclusion
Regenerative medicine is a rapidly evolving novel concept for the substitution of lost tissues, such as the cornea, however, current cell-based therapies in this field are immature, containing several challenges and defects. Appropriate biomaterials should address multiple points, including cell adhesion, proliferation, and differentiation, maintaining the desired cellular phenotype, proper signaling, and biochemical properties. Hence, the application of nanomedicine/bioengineering in regeneration of the cornea is a promising option to solve these problems. Different types of nanoscaffolds have been introduced, which can successfully facilitate oxygen and nutrient transport, cellular waste removal, and promote cellular attachment, proliferation and differentiation. Notably, these products are modifiable to mimic the natural structure of the cornea. The cornea is susceptible to various pathophysiological disorders. With keratoplasties and current artificial corneas limited by the availability of donor tissues, the importance of viable synthetic implants is growing. Several currently available nanomaterials and nanofabrication methods have been introduced to produce bioengineered artificial corneas. Although specific disadvantages remain, this technology may overcome the limitations of traditional corneal donors. There is still a long way to go, but further research will elucidate the grey areas of current knowledge.
Acknowledgements
The authors would like to thank Ms Lauren Kalinoski (University of Illinois at Chicago) for assistance in preparing the figures.
Footnotes
The authors declare that there are no conflicts of interest.
Funding: This work was supported by UH3 EY031809 (ARD) and a Core Grant for Vision Research P30EY01792, both from the National Eye Institute/National Institutes of Health; Vision Research Program – Congressionally Directed Medical Research Program VR170180 from the Department of Defense; and an Unrestricted Grant to the Department and Physician-Scientist Award, both from Research to Prevent Blindness.
ORCID iDs: Mohammad Soleimani https://orcid.org/0000-0002-6546-3546
Kasra Cheraqpour https://orcid.org/0000-0002-1273-9166
References
- 1.Tolochko N. History of nanotechnology. In: Kharkin V, Bai C, Kapitza S, et al. (eds) Nanoscience and Nanotechnologies. Volume 1. Oxford: Encyclopedia of Life Support Systems (EOLSS) Publishers, 2009. [Google Scholar]
- 2.Cao GZ. Nanostructures and nanomaterials: synthesis, properties and applications. London: Imperial College Press, 2004. [Google Scholar]
- 3.Lammers T, Aime S, Hennink WE, et al. Theranostic nanomedicine. Acc Chem Res 2011; 44: 1029–1038. [DOI] [PubMed] [Google Scholar]
- 4.Patra D, Sengupta S, Duan W, et al. Intelligent, self-powered, drug delivery systems. Nanoscale 2013; 5: 1273–1283. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, Votruba AR, Farokhzad OC, et al. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett 2010; 10: 3223–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldave AJ, Kamal KM, Vo RC, et al. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology 2009; 116: 640–651. [DOI] [PubMed] [Google Scholar]
- 7.Patel NP, Kim T, Rapuano CJ, et al. Indications for and outcomes of repeat penetrating keratoplasty, 1989–1995. Ophthalmology 2000; 107: 719–724. [DOI] [PubMed] [Google Scholar]
- 8.Davila JR, Mian SI. Infectious keratitis after keratoplasty. Curr Opin Ophthalmol 2016; 27: 358–366. [DOI] [PubMed] [Google Scholar]
- 9.Jain R, Bhutia KL, Mohan N, et al. Outcome of therapeutic keratoplasty in hopeless microbial keratitis cases otherwise advised evisceration. Cornea 2018; 37: 151–155. [DOI] [PubMed] [Google Scholar]
- 10.Soleimani M, Tabatabaei SA, Masoumi A, et al. Infectious keratitis: trends in microbiological and antibiotic sensitivity patterns. Eye (Lond) 2021; 35: 3110–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tugal-Tutkun I, Akova YA, Foster CS. Penetrating keratoplasty in cicatrizing conjunctival diseases. Ophthalmology 1995; 102: 576–585. [DOI] [PubMed] [Google Scholar]
- 12.Sayegh RR, Ang LP, Foster CS, et al. The Boston keratoprosthesis in Stevens-Johnson syndrome. Am J Ophthalmol 2008; 145: 438–444. [DOI] [PubMed] [Google Scholar]
- 13.Ciralsky J, Papaliodis GN, Foster CS, et al. Keratoprosthesis in autoimmune disease. Ocul Immunol Inflamm 2010; 18: 275–280. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T. Inflammatory response in dry eye. Invest Ophthalmol Vis Sci 2018; 59: DES192–DES199. [DOI] [PubMed] [Google Scholar]
- 15.Georgoudis P, Sabatino F, Szentmary N, et al. Ocular mucous membrane pemphigoid: current state of pathophysiology, diagnostics and treatment. Ophthalmol Ther 2019; 8: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutt J, Sapra A, Sheth-Dutt P, et al. Stevens-Johnson syndrome: a perplexing diagnosis. Cureus 2020; 12: e7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akpek EK, Harissi-Dagher M, Petrarca R, et al. Outcomes of Boston keratoprosthesis in aniridia: a retrospective multicenter study. Am J Ophthalmol 2007; 144: 227–231.e1. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Khan R, O’Keefe M. Aniridia: current pathology and management. Acta Ophthalmol 2008; 86: 708–715. [DOI] [PubMed] [Google Scholar]
- 19.Vicente A, Byström B, Lindström M, et al. Aniridia-related keratopathy: structural changes in naïve and transplanted corneal buttons. PloS One 2018; 13: e0198822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates A, Zanaboni A. Ocular burns. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2020.
- 21.Duplechain A, Conrady CD, Patel BC, et al. Uveitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2019.
- 22.Patel AP, Wu EI, Ritterband DC, et al. Boston type 1 keratoprosthesis: the New York eye and ear experience. Eye (Lond) 2012; 26: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelkar J, Kelkar A, Chougule Y. Management of pseudophakic bullous keratopathy with ultrathin Descemet stripping automated endothelial keratoplasty and modified Yamanes' technique of scleral fixation. Indian J Ophthalmol 2020; 68: 185–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001; 79: 214–221. [PMC free article] [PubMed] [Google Scholar]
- 25.Karamichos D. Ocular tissue engineering: current and future directions. J Funct Biomater 2015; 6: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 2016; 134: 167–173. [DOI] [PubMed] [Google Scholar]
- 27.Krysik K, Wroblewska-Czajka E, Lyssek-Boron A, et al. Total penetrating keratoplasty: indications, therapeutic approach, and long-term follow-up. J Ophthalmol 2018; 2018: 9580292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaghouti F, Nouri M, Abad JC, et al. Keratoprosthesis: preoperative prognostic categories. Cornea 2001; 20: 19–23. [DOI] [PubMed] [Google Scholar]
- 29.Fadous R, Levallois-Gignac S, Vaillancourt L, et al. The Boston keratoprosthesis type 1 as primary penetrating corneal procedure. Br J Ophthalmol 2015; 99: 1664–1668. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad S, Mathews PM, Lindsley K, et al. Boston type 1 keratoprosthesis versus repeat donor keratoplasty for corneal graft failure: a systematic review and meta-analysis. Ophthalmology 2016; 123: 165–177. [DOI] [PubMed] [Google Scholar]
- 31.Kang KB, Karas FI, Rai R, et al. Five year outcomes of Boston type I keratoprosthesis as primary versus secondary penetrating corneal procedure in a matched case control study. PloS One 2018; 13: e0192381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Wang C, Liu Q, et al. Comparison of the clinical efficacy of Boston keratoprosthesis type I and repetitive penetrating keratoplasty for refractory keratopathy. J Craniofac Surg 2020; 31: e194–e199. [DOI] [PubMed] [Google Scholar]
- 33.Nonpassopon M, Niparugs M, Cortina MS. Boston type 1 keratoprosthesis: updated perspectives. Clin Ophthalmol 2020; 14: 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holland G, Pandit A, Sanchez-Abella L, et al. Artificial cornea: past, current, and future directions. Front Med (Lausanne) 2021; 8: 770780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley JC, Hernandez EG, Schwab IR, et al. Boston type 1 keratoprosthesis: the University of California Davis experience. Cornea 2009; 28: 321–327. [DOI] [PubMed] [Google Scholar]
- 36.Rudnisky CJ, Belin MW, Todani A, et al. Risk factors for the development of retroprosthetic membranes with Boston keratoprosthesis type 1: multicenter study results. Ophthalmology 2012; 119: 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magalhaes FP, Hirai FE, De Sousa LB, et al. Boston type 1 keratoprosthesis outcomes in ocular burns. Acta Ophthalmol 2013; 91: e432–e436. [DOI] [PubMed] [Google Scholar]
- 38.Sivaraman KR, Hou JH, Allemann N, et al. Retroprosthetic membrane and risk of sterile keratolysis in patients with type I Boston Keratoprosthesis. Am J Ophthalmol 2013; 155: 814–822. [DOI] [PubMed] [Google Scholar]
- 39.Dokey A, Ramulu PY, Utine CA, et al. Chronic hypotony associated with the Boston type 1 keratoprosthesis. Am J Ophthalmol 2012; 154: 266–271.e1. [DOI] [PubMed] [Google Scholar]
- 40.Srikumaran D, Munoz B, Aldave AJ, et al. Long-term outcomes of Boston type 1 keratoprosthesis implantation: a retrospective multicenter cohort. Ophthalmology 2014; 121: 2159–2164. [DOI] [PubMed] [Google Scholar]
- 41.Palioura S, Kim B, Dohlman CH, et al. The Boston keratoprosthesis type I in mucous membrane pemphigoid. Cornea 2013; 32: 956–961. [DOI] [PubMed] [Google Scholar]
- 42.Alexander JK, Basak SK, Padilla MDB, et al. International outcomes of the Boston Type I keratoprosthesis in Stevens–Johnson syndrome. Cornea 2015; 34: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 43.Shanbhag SS, Saeed HN, Paschalis EI, et al. Boston keratoprosthesis type 1 for limbal stem cell deficiency after severe chemical corneal injury: a systematic review. Ocul Surf 2018; 16: 272–281. [DOI] [PubMed] [Google Scholar]
- 44.Phillips DL, Hager JL, Goins KM, et al. Boston type 1 keratoprosthesis for chemical and thermal injury. Cornea 2014; 33: 905–909. [DOI] [PubMed] [Google Scholar]
- 45.Aravena C, Yu F, Aldave AJ. Long-term visual outcomes, complications, and retention of the Boston type I keratoprosthesis. Cornea 2018; 37: 3–10. [DOI] [PubMed] [Google Scholar]
- 46.Vajaranant TS, Liu J, Wilensky J, et al. Innovative approaches to glaucoma management of Boston keratoprosthesis type 1. Curr Ophthalmol Rep 2016; 4: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goins KM, Kitzmann AS, Greiner MA, et al. Boston type 1 keratoprosthesis: visual outcomes, device retention, and complications. Cornea 2016; 35: 1165–1174. [DOI] [PubMed] [Google Scholar]
- 48.Nouri M, Terada H, Alfonso EC, et al. Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol 2001; 119: 484–489. [DOI] [PubMed] [Google Scholar]
- 49.Ray S, Khan BF, Dohlman CH, et al. Management of vitreoretinal complications in eyes with permanent keratoprosthesis. Arch Ophthalmol 2002; 120: 559–566. [DOI] [PubMed] [Google Scholar]
- 50.Goldman DR, Hubschman JP, Aldave AJ, et al. Postoperative posterior segment complications in eyes treated with the Boston type I keratoprosthesis. Retina 2013; 33: 532–541. [DOI] [PubMed] [Google Scholar]
- 51.Basu S, Pillai VS, Sangwan VS. Mucosal complications of modified osteo-odonto keratoprosthesis in chronic Stevens-Johnson syndrome. Am J Ophthalmol 2013; 156: 867–873.e2. [DOI] [PubMed] [Google Scholar]
- 52.Avadhanam VS, Herold J, Thorp S, et al. Mitomycin-C for mucous membrane overgrowth in OOKP eyes. Cornea 2014; 33: 981–984. [DOI] [PubMed] [Google Scholar]
- 53.Norris JH, Carpenter D, Al Raqqad N, et al. Indications for orbital decompression for patients undergoing keratoprosthesis surgery. Ophthalmic Plast Reconstr Surg 2012; 28: 346–349. [DOI] [PubMed] [Google Scholar]
- 54.Pintucci S, Pintucci F, Cecconi M, et al. New Dacron tissue colonisable keratoprosthesis: clinical experience. Br J Ophthalmol 1995; 79: 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sadiq SA, Vernon SA. Sublingual timolol–an alternative to topical medication in glaucoma? Br J Ophthalmol 1996; 80: 532–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim LS, Ang CL, Wong E, et al. Vitreoretinal complications and vitreoretinal surgery in osteo-odonto-keratoprosthesis surgery. Am J Ophthalmol 2014; 157: 349–354. [DOI] [PubMed] [Google Scholar]
- 57.Hughes EH, Mokete B, Ainsworth G, et al. Vitreoretinal complications of osteoodontokeratoprosthesis surgery. Retina 2008; 28: 1138–1145. [DOI] [PubMed] [Google Scholar]
- 58.De la Paz MF, De Toledo JÁ, Charoenrook V, et al. Impact of clinical factors on the long-term functional and anatomic outcomes of osteo-odonto-keratoprosthesis and tibial bone keratoprosthesis. Am J Ophthalmol 2011; 151: 829–839.e1. [DOI] [PubMed] [Google Scholar]
- 59.Falcinelli G, Falsini B, Taloni M, et al. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol 2005; 123: 1319–1329. [DOI] [PubMed] [Google Scholar]
- 60.Abdollahiyan P, Oroojalian F, Mokhtarzadeh A. The triad of nanotechnology, cell signalling, and scaffold implantation for the successful repair of damaged organs: an overview on soft-tissue engineering. J Control Release 2021; 332: 460–492. [DOI] [PubMed] [Google Scholar]
- 61.De Stefano VS, Dupps WJ Jr. Biomechanical diagnostics of the cornea. Int Ophthalmol Clin 2017; 57: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pouw AE, Greiner MA, Coussa RG, et al. Cell-matrix interactions in the eye: from cornea to choroid. Cells 2021; 10: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fridenshteĭn A, Petrakova K, Kuralesova A, et al. Precursor cells for osteogenic and hemopoietic tissues. Analysis of heterotopic transplants of bone marrow. Tsitologiia 1968; 10: 557–567 [In Russian]. [PubMed] [Google Scholar]
- 64.Du Y, Funderburgh ML, Mann MM, et al. Multipotent stem cells in human corneal stroma. Stem Cells 2005; 23: 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinnamaneni N, Funderburgh JL. Concise review: stem cells in the corneal stroma. Stem Cells 2012; 30: 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mobaraki MM, Abbasi R, Omidian Vandchali S, et al. Corneal repair and regeneration: current concepts and future directions. Front Bioeng Biotechnol 2019; 7: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res 2010; 91: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajendran V, Netuková M, Griffith M, et al. Mesenchymal stem cell therapy for retro-corneal membrane–a clinical challenge in full-thickness transplantation of biosynthetic corneal equivalents. Acta Biomater 2017; 64: 346–356. [DOI] [PubMed] [Google Scholar]
- 69.Quaranta V. Cell migration through extracellular matrix: membrane-type metalloproteinases make the way. J Cell Biol 2000; 149: 1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy CM, Haugh MG, O'brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010; 31: 461–466. [DOI] [PubMed] [Google Scholar]
- 71.Ahearne M, Fernández‐Pérez J, Masterton S, et al. Designing scaffolds for corneal regeneration. Adv Funct Mater 2020; 30: 1908996. [Google Scholar]
- 72.Yan J, Qiang L, Gao Y, et al. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J Biomed Mater Res A 2012; 100: 527–535. [DOI] [PubMed] [Google Scholar]
- 73.Masterton S, Ahearne M. Mechanobiology of the corneal epithelium. Exp Eye Res 2018; 177: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahearne M, Liu KK, El Haj AJ, et al. Online monitoring of the mechanical behavior of collagen hydrogels: influence of corneal fibroblasts on elastic modulus. Tissue Eng Part C Methods 2010; 16: 319–327. [DOI] [PubMed] [Google Scholar]
- 75.Whitford C, Movchan NV, Studer H, et al. A viscoelastic anisotropic hyperelastic constitutive model of the human cornea. Biomech Model Mechanobiol 2018; 17: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stoppel WL, Ghezzi CE, McNamara SL, et al. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann Biomed Eng 2015; 43: 657–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soleimani M, Cheraqpour K, Koganti R, et al. Concise review: bioengineering of limbal stem cell niche. Bioengineering (Basel) 2023; 10: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toda A, Okabe M, Yoshida T, et al. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci 2007; 105: 215–228. [DOI] [PubMed] [Google Scholar]
- 79.Pino M, Chrzanowski W, Fabel D, et al. Apatite deposition on NaOH‐treated PEEK and UHMWPE films for sclera materials in artificial cornea implants. Adv Eng Mater 2010; 12: B234–B244. [Google Scholar]
- 80.Sharifi R, Mahmoudzadeh S, Islam MM, et al. Covalent functionalization of PMMA surface with L‐3, 4‐dihydroxyphenylalanine (L‐DOPA) to enhance its biocompatibility and adhesion to corneal tissue. Adv Mater Interfaces 2020; 7: 1900767. [Google Scholar]
- 81.Lawrence BD, Marchant JK, Pindrus MA, et al. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009; 30: 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 2011; 8: 607–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robert L, Legeais JM, Robert AM, et al. Corneal collagens. Pathol Biol (Paris) 2001; 49: 353–363. [DOI] [PubMed] [Google Scholar]
- 84.Duan X, Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials 2006; 27: 4608–4617. [DOI] [PubMed] [Google Scholar]
- 85.Avila MY, Gerena VA, Navia JL. Corneal crosslinking with genipin, comparison with UV-riboflavin in ex-vivo model. Mol Vis 2012; 18: 1068–1073. [PMC free article] [PubMed] [Google Scholar]
- 86.Antoine EE, Vlachos PP, Rylander MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng Part B Rev 2014; 20: 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fagerholm P, Lagali NS, Ong JA, et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 2014; 35: 2420–2427. [DOI] [PubMed] [Google Scholar]
- 88.Venkatesan J, Bhatnagar I, Manivasagan P, et al. Alginate composites for bone tissue engineering: a review. Int J Biol Macromol 2015; 72: 269–281. [DOI] [PubMed] [Google Scholar]
- 89.Kim IY, Seo SJ, Moon HS, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 2008; 26: 1–21. [DOI] [PubMed] [Google Scholar]
- 90.Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. European Polymer Journal 2013; 49: 780–792. [Google Scholar]
- 91.Kim EY, Tripathy N, Park JY, et al. Silk fibroin film as an efficient carrier for corneal endothelial cells regeneration. Macromol Res 2015; 23: 189–195. [Google Scholar]
- 92.Mathur AB, Gupta V. Silk fibroin-derived nanoparticles for biomedical applications. Nanomedicine (Lond) 2010; 5: 807–820. [DOI] [PubMed] [Google Scholar]
- 93.Darshan GH, Kong D, Gautrot J, et al. Physico-chemical characterization of Antheraea mylitta silk mats for wound healing applications. Sci Rep 2017; 7: 10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jia L, Ghezzi CE, Kaplan DL. Optimization of silk films as substrate for functional corneal epithelium growth. J Biomed Mater Res B Appl Biomater 2016; 104: 431–441. [DOI] [PubMed] [Google Scholar]
- 95.Kim DK, Sim BR, Khang G. Nature-derived aloe vera gel blended silk fibroin film scaffolds for cornea endothelial cell regeneration and transplantation. ACS Appl Mater Interfaces 2016; 8: 15160–15168. [DOI] [PubMed] [Google Scholar]
- 96.Kim DK, Sim BR, Kim JI, et al. Functionalized silk fibroin film scaffold using β-carotene for cornea endothelial cell regeneration. Colloids Surf B Biointerfaces 2018; 164: 340–346. [DOI] [PubMed] [Google Scholar]
- 97.Choi JH, Jeon H, Song JE, et al. Biofunctionalized lysophosphatidic acid/silk fibroin film for cornea endothelial cell regeneration. Nanomaterials (Basel) 2018; 8: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guan L, Ge H, Tang X, et al. Use of a silk fibroin-chitosan scaffold to construct a tissue-engineered corneal stroma. Cells Tissues Organs 2013; 198: 190–197. [DOI] [PubMed] [Google Scholar]
- 99.Guan L, Tian P, Ge H, et al. Chitosan-functionalized silk fibroin 3D scaffold for keratocyte culture. J Mol Histol 2013; 44: 609–618. [DOI] [PubMed] [Google Scholar]
- 100.Kasoju N, Bora U. Silk fibroin in tissue engineering. Adv Healthc Mater 2012; 1: 393–412. [DOI] [PubMed] [Google Scholar]
- 101.Tonsomboon K, Oyen ML. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J Mech Behav Biomed Mater 2013; 21: 185–194. [DOI] [PubMed] [Google Scholar]
- 102.Rizwan M, Peh GS, Ang HP, et al. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017; 120: 139–154. [DOI] [PubMed] [Google Scholar]
- 103.Haagdorens M, Jangamreddy J, Islam MM, et al. Collagen-like-peptide nano-implants as stem cell loaded substitutes to human cornea transplantation. Invest Ophthalmol Vis Sci 2018; 59: 3452. [Google Scholar]
- 104.Kong B, Chen Y, Liu R, et al. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat Commun 2020; 11: 1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parke-Houben R, Fox CH, Zheng LL, et al. Interpenetrating polymer network hydrogel scaffolds for artificial cornea periphery. J Mater Sci Mater Med 2015; 26: 107. [DOI] [PubMed] [Google Scholar]
- 106.Sharifi S, Sharifi H, Akbari A, et al. Graphene-lined porous gelatin glycidyl methacrylate hydrogels: implications for tissue engineering. ACS Appl Nano Mater 2021; 4: 12650–12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Du M, Song W, Cui Y, et al. Fabrication and biological application of nano-hydroxyapatite (nHA)/alginate (ALG) hydrogel as scaffolds. J Mater Chem 2011; 21: 2228–2236. [Google Scholar]
- 108.Rees SG, Wassell DTH, Waddington RJ, et al. Interaction of bone proteoglycans and proteoglycan components with hydroxyapatite. Biochim Biophys Acta 2001; 1568: 118–128. [DOI] [PubMed] [Google Scholar]
- 109.Shi XH, Wang SL, Zhang YM, et al. Hydroxyapatite-coated sillicone rubber enhanced cell adhesion and it may be through the interaction of EF1β and γ-Actin. PloS One 2014; 9: e111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fenglan X, Yubao L, Xiaoming Y, et al. Preparation and in vivo investigation of artificial cornea made of nano-hydroxyapatite/poly (vinyl alcohol) hydrogel composite. J Mater Sci Mater Med 2007; 18: 635–640. [DOI] [PubMed] [Google Scholar]
- 111.Riau AK, Lwin NC, Gelfand L, et al. Surface modification of corneal prosthesis with nano-hydroxyapatite to enhance in vivo biointegration. Acta Biomater 2020; 107: 299–312. [DOI] [PubMed] [Google Scholar]
- 112.Zhou H, Wang Z, Cao H, et al. Genipin-crosslinked polyvinyl alcohol/silk fibroin/nano-hydroxyapatite hydrogel for fabrication of artificial cornea scaffolds–a novel approach to corneal tissue engineering. J Biomater Sci Polym Ed 2019; 30: 1604–1619. [DOI] [PubMed] [Google Scholar]
- 113.Lin RR, Mao X, Yu QC, et al. Preparation of bioactive nano-hydroxyapatite coating for artificial cornea. Curr Appl Phys 2007; 7: e85–e89. [Google Scholar]
- 114.Maleki M, Zarezadeh R, Nouri M, et al. Graphene oxide: a promising material for regenerative medicine and tissue engineering. Biomol Concepts 2020; 11: 182–200. [DOI] [PubMed] [Google Scholar]
- 115.Li Z, Goh TW, Yam GH, et al. A sintered graphene/titania material as a synthetic keratoprosthesis skirt for end-stage corneal disorders. Acta Biomater 2019; 94: 585–596. [DOI] [PubMed] [Google Scholar]
- 116.Sinha M, Gupte T. Design and evaluation of artificial cornea with core–skirt design using polyhydroxyethyl methacrylate and graphite. Int Ophthalmol 2018; 38: 1225–1233. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Q, Fang Z, Cao Y, et al. High refractive index inorganic–organic interpenetrating polymer network (IPN) hydrogel nanocomposite toward artificial cornea implants. ACS Macro Lett 2012; 1: 876–881. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Q, Su K, Chan-Park MB, et al. Development of high refractive ZnS/PVP/PDMAA hydrogel nanocomposites for artificial cornea implants. Acta Biomater 2014; 10: 1167–1176. [DOI] [PubMed] [Google Scholar]
- 119.Weigel T, Schinkel G, Lendlein A. Design and preparation of polymeric scaffolds for tissue engineering. Expert Rev Med Devices 2006; 3: 835–851. [DOI] [PubMed] [Google Scholar]
- 120.Norman JJ, Desai TA. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann Biomed Eng 2006; 34: 89–101. [DOI] [PubMed] [Google Scholar]
- 121.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine 2006; 1: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aslan B, Guler S, Tevlek A, et al. Evaluation of collagen foam, poly (l‐lactic acid) nanofiber mesh, and decellularized matrices for corneal regeneration. J Biomed Mater Res B Appl Biomater 2018; 106: 2157–2168. [DOI] [PubMed] [Google Scholar]
- 123.Ortega Í, Ryan AJ, Deshpande P, et al. Combined microfabrication and electrospinning to produce 3-D architectures for corneal repair. Acta Biomater 2013; 9: 5511–5520. [DOI] [PubMed] [Google Scholar]
- 124.Wu Z, Kong B, Liu R, et al. Engineering of corneal tissue through an aligned PVA/collagen composite nanofibrous electrospun scaffold. Nanomaterials (Basel) 2018; 8: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu W, Ma X, Gou M, et al. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol 2016; 40: 103–112. [DOI] [PubMed] [Google Scholar]
- 126.Sorkio A, Koch L, Koivusalo L, et al. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018; 171: 57–71. [DOI] [PubMed] [Google Scholar]
- 127.Kim H, Park MN, Kim J, et al. Characterization of cornea-specific bioink: high transparency, improved in vivo safety. J Tissue Eng 2019; 10: 2041731418823382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Isaacson A, Swioklo S, Connon CJ. 3D bioprinting of a corneal stroma equivalent. Exp Eye Res 2018; 173: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh SR, Kompella UB. Nanotechnology for gene delivery to the eye. European Ophthalmic Review 2009; 3: 7–11. [Google Scholar]
- 130.Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res 2008; 48: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitra RN, Zheng M, Han Z. Nanoparticle-motivated gene delivery for ophthalmic application. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2016; 8: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kompella UB, Amrite AC, Ravi RP, et al. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog Retin Eye Res 2013; 36: 172–198. [DOI] [PMC free article] [PubMed] [Google Scholar]