ABSTRACT
Next year marks one-quarter of a century since the discovery of the so-called COPI-independent pathway, which operates between the Golgi apparatus and the endoplasmic reticulum (ER) in eukaryotic cells. Unlike almost all other intracellular trafficking pathways, this pathway is not regulated by the physical accumulation of multisubunit proteinaceous coat molecules, but instead by the small GTPase Rab6. What also sets it apart from other pathways is that the transport carriers themselves often take the form of tubules, rather than conventional vesicles. In this review, we assess the relevant literature that has accumulated to date, in an attempt to provide a concerted description of how this pathway is regulated. We discuss the possible cargo molecules that are carried in this pathway, and the likely mechanism of Rab6 tubule biogenesis, including how the cargo itself may play a critical role. We also provide perspective surrounding the various molecular motors of the kinesin, myosin and dynein families that have been implicated in driving Rab6-coated tubular membranes long distances through the cell prior to delivering their cargo to the ER. Finally, we also raise several important questions that require resolution, if we are to ultimately provide a comprehensive molecular description of how the COPI-independent pathway is controlled.
KEYWORDS: membrane traffic, retrograde transport, Rab6, Golgi apparatus, membrane tubules, COPI-independent transport
Introduction
The Golgi apparatus sits at the heart of the endomembrane system in animal cells and is vital for performing post-translational modifications to proteins and coordinating trafficking in the secretory pathway. In order to balance this flux of material in the anterograde direction, the Golgi apparatus also facilitates retrograde transport of molecules towards the endoplasmic reticulum (ER). This allows the return of escaped ER-resident proteins, and the recycling of Golgi-resident proteins such as glycosylation enzymes for periodic quality control.
Golgi-to-ER retrograde transport is predominantly mediated by vesicular structures that are surrounded by a proteinaceous assembly termed coat protein complex I (COPI). The mechanisms of action of COPI-dependent retrograde traffic are relatively well understood (for a recent review, see [1]). In brief, to form a COPI-coated vesicle, the small GTPase Arf1 is activated by the guanine nucleotide exchange factor (GEF) GBF1 and recruited to the Golgi membrane. Arf1 in turn recruits coatomer, the structural element of COPI, from the cytosol. Following further rounds of Arf1 and coatomer addition, sufficient curvature of the membrane eventually causes the vesicle to bud from the Golgi. Subsequently, Arf GTPase activating proteins (ArfGAPs) dissociate Arf1 from the vesicle membrane. This ultimately leads to its uncoating, and following arrival of the vesicle at the ER facilitates the interaction of the vesicle with tethering proteins. This is followed by interactions between vesicle- and target-associated soluble N-ethylmaleimide-sensitive factor activating protein receptors (SNAREs), leading to membrane fusion and the delivery of the cargo to the ER.
Rab GTPases are a family of small GTP-binding proteins, intimately associated with membrane trafficking events. Rab proteins mediate membrane trafficking events through interactions with effector proteins. There are around 70 known Rabs in humans, and each Rab localizes to distinct membrane compartments in cells [2]. At the Golgi apparatus, a number of different Rab proteins have been identified – Rab1, Rab2, Rab6, Rab8 and Rab33b are most commonly associated with the Golgi, although it is clear that a wider number of Rabs play crucial roles, particularly at the ER–Golgi interface [3]. Rab proteins exist in an inactive, GDP-bound state, but are converted to an active, GTP-bound form by the activity of GEFs. It is in this active state that Rab proteins can interact with their effector proteins to coordinate membrane trafficking events.
Rab6 was first identified in 1989 [4] and has since been shown to play a prominent role in the regulation of the Golgi apparatus and associated transport pathways [5,6]. The Rab6 gene family consists of the widely expressed RAB6A, further broken into two equally occurring splice variants, RAB6A and RAB6A’, the neuronal-cell-type specific isoform RAB6B, the primate-specific retrogene RAB6C and the distantly related RAB41 [7,8]. Rab6 localizes predominantly to the trans-Golgi apparatus and, like many Rab proteins, Rab6 is intimately involved in coordinating membrane trafficking events in conjunction with motor proteins and cytoskeletal elements, with its focus being on pathways emerging from the Golgi [9]. Although Rab6 plays a predominant role in facilitating the anterograde transport of post-Golgi secretory carriers [10], it is also widely recognized for a role in retrograde trafficking. It regulates pathways from the endosomal system into the Golgi [11], and in 1999 was shown to not only facilitate, but in fact be the limiting factor in a novel Golgi-to-ER transport pathway that functions without the need for conventional coat proteins. As such, this pathway is usually described as the COPI-independent retrograde pathway [12,13]. Rab6-positive transport carriers are often vesicular in nature, however importantly many Rab6-positive retrograde transport carriers take the form of long, extended tubular structures that stretch from the membranes of the Golgi apparatus towards the ER. Of the Rab6 isoforms, Rab6A and Rab6A’ are most frequently associated with such structures, and are the most well studied of all the family members. These particular variants differ by only three amino acids, and while there has been some evidence suggesting that they play non-overlapping roles in membrane trafficking [14], this remains to be fully explored. Rab6B, while primarily expressed in neuronal cells, has also recently been reported to be expressed in murine macrophages [15]. The crystal structure of Rab6B was solved a number of years ago, revealing differences between this family member and Rab6A’, specifically in the interswitch region of the proteins [16]. The functional consequences of these differences, with regard to how the proteins interact with their effectors, have not yet been determined. While the role of Rab6B in neuronal cell trafficking pathways continues to be explored, its involvement in COPI-independent tubular transport is still unclear. The Rab6C protein is only expressed in a very limited number of human tissues and is associated with the centrosome and cell cycle progression, rather than membrane traffic [17]. The most recently characterized family member, Rab41 [18], is associated with the Golgi, however it is thought to be involved in structural maintenance of this organelle, rather than in Golgi-to-ER transport mechanisms [19]. As such, from this point, we will use the general term ‘Rab6’ to refer to Rab6A and Rab6A’, unless otherwise specified.
In contrast to our knowledge of the transport pathways regulated by the COPI coat, our understanding of Rab6-dependent tubular transport is substantially more scant. One major challenge in advancing our knowledge of this pathway comes from the morphology of the carriers themselves. Not only are they highly dynamic and short-lived, but they are undoubtedly challenging to biochemically purify [6]. As such, researchers have utilized and developed a variety of methods which, when applied to cells, enhance tubulation of membranes derived from the Golgi apparatus, thereby increasing the abundance of these carriers to better enable their study. Perhaps, most commonly employed is the fungal metabolite, brefeldin A (BFA). BFA functions by competitively and reversibly inhibiting GBF1, the Arf1 GEF, which is a key molecule involved in the formation of COPI-coated vesicles. When COPI vesicle formation is inhibited, the Golgi apparatus becomes destabilized and its contents are redistributed throughout the cell. Golgi-resident enzymes show dramatic redistribution to the ER within dynamic Rab6-coated tubules, and Golgi matrix proteins are predominantly redistributed to ER-exit sites (ERES), also via tubules [20–23]. The mechanics of these processes are independent of COPI, and thus researchers often employ BFA as an initial method to study COPI-independent transport and validate findings through alternative techniques.
It is now almost a quarter of a century since this pathway was first revealed, but progress towards a consolidated mechanism of action of tubule-mediated Rab6-dependent retrograde transport has been slow. There remain a large number of unanswered questions, which need to be addressed if we are to fully understand how cell homeostasis is achieved. For example, what is the preferred cargo of a Rab6-dependent retrograde tubule? What determines whether a Golgi-derived, Rab6-positive transport carrier is anterograde- versus retrograde-destined? Why is the morphology of some carriers tubular, and for others vesicular? Which molecules are involved in the different mechanical steps of tubulation, and what are the temporal and spatial triggers for the recruitment and release of these molecules?
In this review, we will explore recent advancements in our understanding of Rab6-dependent tubular transport, and we will consider the outstanding questions that researchers in this field are seeking to answer.
Cargo selection and tubule biogenesis
Strikingly, there is little knowledge on the mechanics of Rab6-dependent carrier formation. It has been widely hypothesized that the biogenesis of a membrane tubule, similar to a vesicle, will commence with the budding of the donor membrane at a specific site in the presence of suitable cargo. While the molecules involved in the formation of a COPI-coated vesicle, and the triggers for their recruitment, are well known, there have been relatively few molecules associated with the initiation of a Rab6-coated retrograde tubule.
In the early secretory pathway, cargo accumulation at a specific location in a donor membrane compartment is known to be a critical driver of carrier initiation [24]. Unfortunately, the cargo of Rab6-coated Golgi-to-ER carriers remains to be fully identified, slowing our ability to test whether the same mechanism is employed in the generation of retrograde carriers. Thus, the identification of the preferred cargo of Rab6-dependent transport carriers will be one pivotal step in piecing together how these carriers are formed. Despite this pathway being retrograde in nature, it has been shown that export signal-deficient secretory molecules can also become cargo of this pathway. In work from 2014, Fossati and colleagues showed that when the Golgi export signal is mutated in the anterograde model cargo vesicular stomatitis virus glycoprotein (VSV-G), the protein is recycled back to the ER in a Rab6-dependent manner. What remains to be discerned is whether it is the absence of the export signal, or the nature of the mutated motif, that identifies this protein as a Rab6-associated cargo [25].
In all of the major models explaining Golgi function, the anterograde movement of secretory cargo is countered by the retrograde transport of Golgi-resident proteins to ensure their continued localization to specific Golgi cisternae and prevent their loss along the secretory pathway, with the different models relying on the importance of retrograde transport to different extents [26]. In this regard, any Golgi-resident protein could be considered as a potential cargo for the Rab6-dependent retrograde pathway. It is well known that there is a wide variety of proteins that reside at the Golgi, allowing it to perform its many functions. These include ion transporters, sugar transporters, matrix proteins and resident enzymes, namely glycosylation enzymes, phosphorylation enzymes and kinases [27]. Given the fundamental role of the Golgi in protein glycosylation, the presence of distinct sugar transporters in the different cisternae is logical. These transmembrane proteins are predominantly from the SLC35 family [28], with specific members involved in the import of sugars such as galactose, fucose and xylose [29]. Similarly, the high metabolic state of the organelle means that it also has a requirement for zinc, copper and manganese ions, and therefore needs relevant ion transporters to be present in the Golgi membrane [30]. Manganese in particular is known to be an essential component for a number of glycosylation enzymes to function correctly. Invariably, these two classes of transporters, as well as the glycosylation enzymes themselves, may become defective. As such, appropriate quality control mechanisms in the Golgi must exist, to ensure their removal and/or correction. Recent work employing a modified B4GALT1 glycosyltransferase, which could be induced into a misfolded state, observed a proportion of the protein to relocate to the ER and subsequently associate with molecular chaperones [31]. Although this study did not examine the transport mechanisms used, it highlights the necessity for a functional Golgi-to-ER route to maintain cell homeostasis. Indeed, the glycosylation enzymes of the Golgi apparatus arguably have the most evidence to place them as the primary cargo of Rab6-dependent retrograde carriers, and they have long been used as a marker of Golgi-to-ER redistribution. Early work, blocking anterograde flow at the level of the ER, resulted in truncated variants of GalNAc-T2 and GALT accumulating in the ER [32]. Although the constructs used in these experiments only contained the Golgi targeting domains of the respective enzymes, it highlighted for the first time the existence of a functional Golgi-to-ER pathway that could potentially be exploited by Golgi residents.
More recent work from the Lippincott-Schwartz lab has solidified the hypothesis that Golgi-resident glycosylation enzymes are the primary preferred cargo of Rab6-positive tubular retrograde carriers [23]. Using a technique in which Golgi enzymes engineered with FKBP-rapamycin binding (FRB) domains become trapped in the ER by FK506-binding protein of 12 kDa (FKBP12)-tagged ER-resident proteins upon treatment with rapamycin, Sengupta and colleagues confirmed (in a BFA-independent manner) that Golgi-resident enzymes cycle from the Golgi apparatus to the ER in tubules that require GTP-bound Rab6A. Notably, these retrograde tubules also require the activity of calcium-independent phospholipase A2 (iPLA2) for formation, as bromoenol lactone (BEL)-derived inhibition of iPLA2 significantly reduced the proportion of Golgi-resident enzymes that reached the ER upon treatment with rapamycin. iPLA2 had previously been reported to be involved in the formation of tubular transport carriers arising from the Golgi, and its involvement in the formation of Rab6-coated retrograde tubules had previously been hypothesized [6,33].
The tubules observed by Sengupta and colleagues [23] were seen to traffic along ‘straight or curvilinear tracks’ from the Golgi towards the cell periphery, where they then disappeared. The authors of this work proposed that this is due to their fusion with the ER. This would be suggestive that these tubules transit directly from the Golgi to the ER, bypassing the ERGIC. However, other work has implicated ERGIC-resident proteins as being necessary for Rab6-mediated Golgi-to-ER transport [34]. Specifically, on RNAi-mediated silencing of lipid phosphate phosphatase-3 (LPP3), cells showed an absence of Rab6-positive membrane tubules containing the marker cargo Shiga toxin B-fragment (STxB), and the toxin failed to be delivered to the ER following stimulation of the pathway. A catalytically inactive variant of the LPP3 protein also strongly reduced the redistribution of overexpressed wild-type Rab6, further implicating this ERGIC-resident in Rab6-dependent transport. The ambiguity surrounding the involvement, or lack thereof, of the ERGIC in Rab6-dependent transport will need to be addressed.
While the retrograde recycling of Golgi-resident glycosylation enzymes has been explored in some depth, there is also evidence to support the retrograde recycling of Golgi-resident matrix proteins [35]. Golgi matrix proteins are understood to constitute the primary structural scaffold of the Golgi apparatus, as it has been shown that they can assemble into a juxtanuclear Golgi ribbon even in the absence of Golgi cargo and resident enzymes [36]. Curiously, the destination of matrix proteins following stimulation of the COPI-independent pathway varies greatly between individual matrix proteins. For example, cis-Golgi localizing matrix proteins such as GRASP65 and GM130 (GOLGA2) have been shown to accumulate at ERES following BFA-mediated disruption of the Golgi stack [22]. It is thought that this localization facilitates the rapid ER export of these proteins and the reassembly of the Golgi stack upon BFA washout. In striking contrast, Golgin-160 (GOLGA3), a trans-Golgi localizing matrix protein, has been shown to localize to centrosomes following BFA treatment [37]. Unlike the direct role of GM130 and GRASP65 in Golgi formation, it is understood that Golgin-160 mediates recruitment of dynein to the Golgi and the positioning of the Golgi at the microtubule organizing centre (MTOC), thus conferring it with a more indirect role in maintaining Golgi homeostasis. Giantin, the largest of the matrix proteins, has been shown to relocate to the ER following treatment of cells with BFA, although it does not diffuse through the ER in the same manner that Golgi-resident enzymes seem to [38,39]. Like GM130 and GRASP65, it also plays a central role in coordinating the higher architecture of the Golgi apparatus [39]. The retrograde cycling of matrix proteins is yet to be linked with Rab6-dependent trafficking, and the highly distinct destinations of this family of proteins following stimulation of COPI-independent transport paint them as unlikely preferred cargo candidates for a dedicated, Rab6-dependent Golgi-to-ER pathway.
In the case of coated vesicle generation, the accumulation of cargo and cargo receptors at the donor membrane is a necessary and mechanistic prerequisite for transport carrier formation to avoid the wasteful generation of empty vesicles. For tubular carriers, however, this is less clear. Early work from our own laboratory has shown that at the ER–Golgi interface there is an intrinsic link between cargo levels and the abundance of tubular-transport intermediates (TTIs) [40]. In these experiments, cells were microinjected with increasing concentrations of DNA encoding for GFP-tagged model cargo that cycled between the ER and the Golgi, namely KDEL receptor (GFP-KDEL-R) or ERGIC-53 (GFP-ERGIC-53). The increasing concentrations of DNA resulted in increased cargo expression levels in the cells, and it was shown that the presence of TTIs was more prevalent in cells expressing a higher amount of ER-Golgi cargo. Although these experiments did not directly assess whether these were retrograde-destined tubules, they did demonstrate the link between cargo levels and the capacity of cells to generate tubular carriers.
In keeping with this hypothesis, in recent years further evidence suggests that it is the physical accumulation of the principal proposed cargo of Rab6-dependent retrograde carriers, Golgi-resident enzymes, that acts as a trigger for membrane tubulation. Perhaps, the most convincing evidence in support of this comes from a series of experiments performed by Petrosyan and Cheng [41]. Utilizing the human pancreatic cell line PANC-1 stably expressing a Myc-epitope-tagged construct of the bovine isoform of the Golgi-resident enzyme core 2 N-acetylglucosaminyltransferase mucus-type (C2GnT-M), they first showed through RNA interference (RNAi) that non-muscle myosin 2A (NM2A) is necessary for BFA-dependent Golgi destabilization. NM2A had previously been shown to form a complex with Rab6A at the Golgi apparatus, so its involvement in BFA-induced tubulation is a promising sign of its involvement in Rab6-dependent retrograde traffic [41,42]. The same authors then showed that the activity and association of NM2A at Golgi membranes is substantially more pronounced in cells with a larger volume of C2GnT-M, as determined through fluorescence intensity measurements. Further to this, in LnCAP prostate cancer cells, the depletion of two endogenous enzymes showing naturally high expression levels, core 2 N-acetylglucosaminyltransferase leukocyte-type (C2GnT-L) and β-galactoside α-2,3 sialyltransferase 1 (ST3GAL1), delayed BFA-induced Golgi destabilization and reduced the association of Rab6A with NM2A, as shown through co-immunoprecipitation. Interestingly, this observation could be replicated in cells depleted of the coatomer subunit, beta-COP, as a substitute for BFA treatment. Together, these experiments strongly implicate cargo accumulation at the Golgi membrane as a trigger for recruitment of COPI-independent, Rab6-dependent biogenesis machinery, and implicate NM2A as a potential effector protein involved in this process.
One possibility to bear in mind when considering the biogenesis of Rab6-dependent membrane tubules is that NM2A itself may provide the energy necessary to drive membrane deformation and the extension of a tubular carrier from the Golgi apparatus. While this has not been directly assessed in the context of Rab6-dependent Golgi-to-ER carriers, there is evidence that NM2A is necessary for the formation of VSV-G-containing vesicles arising at the TGN [43]. In this early work, cells were depleted of NM2A with antibodies against the protein, and it was observed that vesicle release from the TGN in these cells was reduced to 50% efficiency. This vesicle biogenesis-deficient phenotype could be rescued through the introduction of purified NM2A to the cells. Perhaps, these experiments could be replicated with a model cargo of the Rab6-dependent Golgi-to-ER pathway, such as Shiga toxin, to assess whether this role of NM2A also applies to retrograde transport.
Another candidate as an effector mediating the biogenesis of Rab6-dependent carriers is TATA element modulatory factor/androgen receptor coactivator of 160 kDa (TMF/ARA160) [44]. First discovered in 1992 as a DNA-binding factor, TMF has since been more appropriately classed as a golgin and Golgi matrix protein, which binds to Rab6 and influences the morphology of the Golgi [45,46]. It contains a conserved coiled-coil motif at its C-terminus that is sufficient for binding to Rab6A, Rab6A’ and Rab6B [46]. In 2007, Yamane and colleagues identified TMF localizing specifically to the budding structures at the edges of Golgi cisternae in HeLa and NRK cells. This pattern was replicated in cells expressing FLAG-tagged human TMF. TMF was found to localize to tubules containing the Golgi enzyme GalNAc-T2 and Rab6 under BFA treatment, although interestingly the TMF and Rab6 signals did not overlap perfectly. Curiously, the retrograde transport of Shiga toxin 1 (STx1) was severely impacted in cells depleted of TMF; Cy3-labelled STx1 did not even localize to the Golgi apparatus and instead was found localizing to punctate structures, which were identified as late endosomes/lysosomes [44]. It is hard to discern precisely whether TMF has a role in the biogenesis of Rab6-coated Golgi-to-ER retrograde tubules, if the primary model cargo of this pathway does not reach its starting point (the Golgi) in cells depleted of TMF.
What is also unclear is whether TMF interacts with NM2A, and if so, how it does so. Investigations into these proteins may provide insight into the finer details of the mechanics of tubule biogenesis, and perhaps even the identification of a tubule initiation complex. Interestingly, TMF was shown to play a vital role in facilitating the Golgi localization of GalNAc-T2 in a manner dependent on the cytoplasmic tail of the enzyme – notably the same region that has been shown to also interact with NM2A [44,47]. RNAi-induced depletion of TMF led to a decrease of GalNAc-T2-GFP at the Golgi apparatus. Yamane and colleagues therefore proposed that the interaction between TMF and GalNAc-T2 prevents this cargo from being incorporated into retrograde carriers, thus resulting in its retention at the Golgi. It can then further be argued that dissociation of TMF from GalNAc-T2 is a requirement for the incorporation of GalNAc-T2 into a retrograde carrier. It is important to note that direct interaction between TMF and the cytoplasmic region of GalNAc-T2 could not be detected, thus there are likely to be additional, as yet unidentified molecules, involved in facilitating this association. Further studies exploring the relationship between TMF, NM2A, and Golgi glycosyltransferases are needed to clarify the nature of the relationships between these molecules in the context of Rab6-dependent retrograde transport.
In keeping with the hypothesis suggested by Yamane and colleagues regarding the role of a Golgi matrix protein in negatively regulating membrane trafficking, another scenario driving tubule formation worth consideration is what the authors of this review refer to as the ‘de-scaffolding’ of the Golgi membrane. In this scenario, in order for a membrane tubule carrier to form, a highly localized patch of the Golgi cisternal membrane must be cleared of the structural matrix proteins and golgins that give the organelle its integrity. This would generate a ‘Golgi exit site’ (GoES), a structure whose existence has not yet been validated experimentally but has been hypothesized by others [48]. There is evidence in support of this hypothesis, such as the findings from Yamane and colleagues [44] implying a need for the dissociation of TMF for the incorporation of GalNAc-T2 into a retrograde carrier. It has also long been known that during BFA-induced Golgi tubulation, matrix proteins are the first to dissociate from the Golgi and be rapidly transported to secondary destinations via tubules, then followed by the transport of Golgi resident enzymes to the ER [22].
Interestingly, Arf1 has been reported to facilitate the formation of tubules at the Golgi apparatus in a largely COPI-independent manner [49]. Using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology to introduce a HALO tag to endogenous Arf1 in HeLa cells, Bottanelli and colleagues showed that the Golgi apparatus produces Arf1-coated tubules in the absence of any tubule-enhancing agent. Cargo studies showed that while ca. 80% of these tubules were positive for the anterograde model cargo VSV-G, ca. 20% were positive for the model retrograde cargo KDEL-R. KDEL-R-positive tubules showed non-continuous localization of COPI at regular intervals along their length; however, these regions were notably not enriched in Arf1. The authors speculate that this is likely due to the mechanism through which Arf1 is hypothesized to induce membrane tubulation – the insertion of the two myristoylated amphipathic helices of an Arf1 homodimer into the outer leaf of a bilayer membrane increases the surface area of the outer leaf relevant to the inner leaf, leading to the development of a tubular conformation to facilitate this surface area increase [49,50]. The tight packing of Arf1 dimers in these tubular membrane structures prevents the binding of Arf1 effectors such as coatomer, thus explaining the association of COPI to regions along the tubule that are not enriched in Arf1. Importantly, Bottanelli and colleagues note and acknowledge that although KDEL-R was observed to be present in a sub-population of Arf1-positive tubules, it is unlikely that these tubules are the primary mode of retrograde transport of KDEL-R or KKXX-containing proteins due to the propensity of these proteins to bind to the components of coatomer in COPI-coated vesicles. The primary cargo of Arf1-positive retrograde tubules is likely to be lipids or proteins that are not carried by COPI-coated vesicles. There has been no role of Arf1 yet reported in the mechanics of Rab6-positive tubules, however this work presents exciting and valuable insight into the general mechanics behind membrane tubulation that may indeed translate across to COPI-independent, tubule-mediated retrograde transport.
Tubule motility and elongation
The motor protein(s) and cytoskeletal elements that provide momentum to Rab6-coated tubules remain shrouded in mystery. Various candidates have been proposed in the last quarter of a century since the first discovery of the Rab6-dependent pathway, although there has not yet been irrefutable confirmation or consensus on any. The major challenge thus far seems to be that we are yet to identify a bona fide motor protein with the exclusive role of providing mechanical propulsion to Rab6-coated Golgi-to-ER carriers – any candidates identified thus far have also been implicated in mediating additional steps in the wider mechanism of action of Rab6-dependent retrograde transport. For any long-range transport, it is probable that the motor proteins will be associated with microtubules rather than actin filaments. It is also likely that it will be a plus-end directed motor protein that will facilitate the transport of tubule-encapsulated cargo from the Golgi to the ER, due to the extensive evidence in the literature demonstrating that Rab6-coated retrograde tubules are capable of travelling to regions of the ER at the cell periphery. This implicates the kinesin family of motor proteins. The challenge with this is that kinesins also regulate post-Golgi anterograde transport, where Rab6 has also been shown to play a role [51]. Resolving which kinesins regulate which Rab6-associated pathway, or indeed if there is any overlap between the kinesins of the pathways, will be no easy task.
In 1998, it was suggested that kinesin family protein 20A (KIF20A), also known as Rabkinesin-6, acted as a molecular motor to drive Rab6-dependent retrograde transport carriers due to the presence of Rab6-binding domains at its carboxy terminus [52]. This hypothesis has since been challenged, as endogenous KIF20A localizes weakly to the Golgi in interphase cells and instead shows a 10-fold higher localization to the nucleus during mitosis, suggesting a primary role for this protein in mitosis [53,54]. Further to this, the overexpression of KIF20A does not induce the relocation of Golgi enzymes to the ER as seen with the overexpression of GTP-locked Rab6 [21]. During mitosis, KIF20A has been shown to be essential for cytokinesis and provides stability to the mitotic spindle through its ability to bind microtubules at its C-terminus and to promote the association of important mitotic proteins with microtubules [54–56]. In their 2017 work, Miserey-Lenkei and colleagues proposed that in interphase cells, KIF20A may play a similar role by binding nucleating microtubules at sites of Golgi fission to act as tracks for emerging Rab6-dependent carriers [56]. Indeed, they showed that KIF20A serves to limit the diffusion of Rab6 throughout the Golgi membranes and encourage its preferential localization to specific fission sites at the edges of the cisternae. Importantly, however, these experiments only confirmed the involvement of KIF20A in anterograde Rab6-dependent transport, and its involvement in Rab6-dependent retrograde transport is yet to be confirmed.
Kinesin-1 has been shown to have an involvement in anterograde Rab6-dependent transport, but it is unlikely that it also regulates Golgi-to-ER retrograde transport [42,51]. In a 2010 publication from the Goud group, it was shown that depletion of KIF5B (the kinesin-1 heavy chain), in tandem with the inhibition of NM2A, produced tubules which were on average 43% shorter than those in cells where KIF5B had not been depleted [42]. They argued that kinesin-1 extends tubules by pulling them along microtubule tracks before the tubules undergo fission from the Golgi induced by NM2A. Under the conditions of KIF5B depletion this extension is limited, thus resulting in shorter tubules. Despite this sign of involvement in Golgi membrane tubulation, in work from the Storrie group exploring partner proteins of ER-associated tethers in Rab6-dependent retrograde traffic, epistatic KIF5B depletion did not rescue the phenotype induced by depletion of the tether candidates ZW10, RINT1 and COG3, suggesting that it does not have a role in Rab6-dependent Golgi-to-ER retrograde traffic [57].
Kinesin-2, a heterotrimer composed of KIF3A, KIF3B and KAP3, has also been investigated in the context of BFA-stimulated retrograde tubular transport [58]. The Xenopus ortholog of KIF3B, Xklp3, localizes to Golgi membranes in immunofluorescence microscopy experiments, but membrane fractionation suggests that it also localizes to the ER. Following 5-min treatment with BFA, KIF3B was shown to localize to punctate structures, as opposed to tubules, containing the resident enzyme GalNAc-T2 – a phenotype more reminiscent of redistributed matrix proteins rather than resident enzymes [22,58]. Retrograde transport of matrix proteins has not yet been shown to be Rab6-dependent. The Golgi-to-ER redistribution of Golgi enzymes induced by the overexpression of the GTP-restricted Rab6A mutant (Rab6A[Q27L]) has been shown to be independent of kinesin-2, and other studies on kinesin-2 indicate a role in COPI-dependent Golgi-to-ER recycling [59,60]. In this latter work, it was shown that in cells depleted of the kinesin-2 non-motor subunit KAP3, KDEL-R failed to localize to the Golgi and instead localized throughout the ER. Experiments employing VSV-G validated that this phenotype was not a result of aberrant anterograde transport, but rather COPI-dependent recycling. In addition, cells depleted of KAP3 and treated with Shiga-like toxin 1 (SLT-1) displayed no change in SLT-1-induced toxicity, confirming that kinesin-2 does not have a role in Rab6-dependent Golgi-to-ER retrograde transport.
The minus-end directed microtubule motor complex dynein and its adaptors have also been suggested to play a role in Rab6-dependent retrograde traffic, despite arguably travelling in the wrong direction along microtubules for these carriers [59,61]. Initial work identifying the dynein adaptor proteins BICD1 and BICD2 as effectors of Rab6A demonstrated that, following low-temperature incubation at 20°C, BicD1, Rab6A and STxB colocalised with high specificity along tubular carriers emerging from the cell periphery and eventually fusing with the Golgi apparatus in HeLa cells [61]. These carriers did not colocalize with endosomal markers such as early endosome antigen 1 (EEA1), secretory pathway markers such as VSV-G, or ER-Golgi shuttling molecules such as ERGIC-53 and COPI. Matanis and colleagues proposed that active dynein and dynactin are necessary for Rab6-dependent Golgi-to-ER transport, although the exact reasons are unclear – one possible explanation is that they may retrieve upstream machinery that is necessary for Rab6-dependent Golgi-to-ER transport, or alternatively they may play a role in facilitating intra-Golgi transport [61]. In later work by Young and colleagues, it was also shown that the overexpression of the C-terminal fragment of the dynein adaptor BicD, which lacks the dynein-binding N-terminal domain, competitively displaces functional BicD from Rab6, and substantially impairs the Golgi-to-ER redistribution of Golgi enzymes induced by overexpression of the GTP-restricted form of Rab6A [59].
There have not been many other developments in our understanding of the relationship between Rab6 and dynein or the associated dynein effector complex, dynactin, in recent years, so we refer readers to another review from our group which discusses our knowledge of the Rab6–dynein interaction in more depth [5]. Since the publication of this previous review, the role of BicD in Rab6-dependent retrograde transport has been partially clarified. Initially understood to act as an adaptor between Rab6 and the dynein motor complex, it has been demonstrated by numerous works that the role of BicD is more likely to be in facilitating the fusion of Rab6-dependent tubules at the ER, although the precise mechanism remains to be clarified [57,61,62]. This is discussed in more depth later in this review. It also cannot be ignored that there is the possibility that BicD2 may facilitate both tubule motility and tubule fusion – by acting as an adaptor for dynein to transport the tubule to the ER, and by acting as a carrier-associated tether for fusion machinery at the ER. Further studies will be needed to clarify this.
Interestingly, it has recently been reported that the Rab6-dynein axis, in conjunction with the Rab6 effector and modulator of dynein activity LIS1, regulates post-Golgi secretory trafficking in radial glial cells, although the subcellular organization of these cells varies substantially from standard animal model cell lines [63]. Radial glial cells are highly polarized, and maintenance of this polarization is mediated by the PAR, Crumbs and Scribble complexes. The secretory pathway in radial glial cells also plays a vital role in maintaining cell polarity due to its role in sorting and facilitating the transport of CRB, a component of the Crumbs complex, which is a determinant of the apical domain of radial glial cells. Using subcellular live-cell microscopy on embryonic mouse brain slices, Brault and colleagues first demonstrated that apical transport along microtubules in radial glial cells is minus-end directed, and noted that Rab6-positive vesicles displayed bi-directional movement along microtubules throughout radial glial cells. Importantly, it was shown that the dynein inhibitor dynarrestin drastically inhibited the movement of Rab6-positive vesicles into the apical processes of these cells, and later it was shown that knockdown of LIS1, or the double knockdown of Rab6A and Rab6B, prevented CRB3 from localizing to the apical membrane. This leads to the delamination of the apical membrane of the radial glial cells, which can in turn lead to microcephaly. Despite the drastic organizational differences between radial glial cells and other animal cell lines previously used to study Rab6-dependent transport, it is important to note the conservation of Rab6-dependent transport mechanics throughout cell types, and also interesting to consider the role of Rab6B in facilitating transport and cell polarization in neural cell types.
The implications of numerous members of the dynein machinery being effectors of Rab6 cannot be ignored (Table 1). One possible explanation for this is that Rab6-dependent Golgi-to-ER carriers may not necessarily depend on dynein for their motor activity, but rather dynein and/or its machinery must be associated with the carrier in order for an associated kinesin to exert its processive motor activity. Alternatively, dynein may be necessary for Rab6-dependent retrograde transport in highly polarized cells such as neurons, but less important in non-polarized cells.
Table 1.
List of Rab6 effectors and interacting proteins.
| Protein | Comment | Proposed role in Golgi-to-ER transport | Reference |
|---|---|---|---|
| Bicaudal D1 (BicD1) | Interacts with C-terminal of GTP-locked Rab6A; thought to play a role in Rab6-dependent endocytic transport but its role in Golgi-ER transport, if any, is less well defined |
Motility? | [61,64] |
| Bicaudal D2 (BicD2) | Interacts with C-terminal of GTP-locked Rab6A; thought to play roles in both the motility of Rab6-dependent Golgi-ER carriers and the fusion of these carriers with the ER membrane |
Motility; fusion | [61,64] |
| Dynein light chain roadblock-type 1 (DYNLRB1) | Identified as an effector of Rab6A, Rab6A’ and Rab6B through co-immunoprecipitation, yeast two-hybrid and pull down studies |
Motility | [65] |
| GAPCenA/RabGAP1 | Rab6 GAP; localizes to centrosomes and understood to be involved in microtubule nucleation |
None | [66] |
| GCC185 | Contains a Rab-binding domain upstream of its C-terminal GRIP domain | None | [67] |
| Giantin | N-terminal of Giantin interacts directly with the N-terminal of GTP-locked Rab6A; interaction between Rab6 and Giantin decreases upon pharmacologically induced Golgi destabilization |
Biogenesis? | [47,68] |
| Golgin-97 | Contains Rab6-interacting domain at C-terminal | None | [69] |
| Golgin-245 | Contains Rab6-interacting domain at C-terminal | None | [69] |
| Golgin, Rab6 interacting (GORAB; aka SCYL1BP1) | Identified as an interactor with both Rab6A and Rab6B in yeast two-hybrid studies; later identified as COPI scaffolding factor |
None | [70,71] |
| KIF1C | Binds to Rab6A at both the C-terminus and the motor domain; binding of Rab6A at the motor domain inhibits the interaction of KIF1C with microtubules |
None | [72] |
| KIF5B | Shown to facilitate Rab6-dependent exocytosis, not yet been shown to be involved in Rab6-dependent Golgi-ER transport |
Motility* | [51] |
| KIF20A | Binds to Rab6 via the C-terminus, plays a role in the fission of Rab6-dependent anterograde carriers |
Motility?*; fission* | [52] |
| LIS1 | GTP-locked Rab6A releases LIS1 from dynein idling complex – no direct binding reported | Motility? | [73] |
| Mint3/APBA3 | The phosphotyrosine-binding (PTB) domain interacts with GTP-locked Rab6A; confirmed through yeast two-hybrid studies and FRET |
None | [74,75] |
| Non-muscle myosin 2A (NM2A) | Coiled-coil heavy chain region shown to interact with GTP-locked Rab6A and Rab6A’ through yeast-two-hybrid studies; important for biogenesis of Rab6-dependent Golgi-ER carriers and fission of Rab6-dependent anterograde carriers |
Biogenesis; fission* | [42] |
| Non-muscle myosin 2B (NM2B) | Coiled-coil heavy chain region interacts with GTP-locked Rab6A and Rab6A’, identified through yeast two-hybrid studies |
None | [42] |
| OCRL1 | Identified as a Rab6 interactor through yeast two-hybrid studies; contains a Rab-binding domain also capable of binding to Rab1B, Rab5A and Rab8A in addition to Rab6A |
None | [76] |
| p150glued | Identified as an interactor of Rab6 through yeast-two-hybrid studies; capable of interacting with wild-type Rab6 but preferentially interacts with GTP-locked Rab6 |
Motility? | [64] |
| Rab6IP1/ DENND5 |
Identified as a Rab6 effector through yeast-two-hybrid studies and pull-down experiments; binds to Rab6 through a central RUN domain |
None | [77,78] |
| Rab6IP2/ELKS | Contains a Rab6-binding domain at the C-terminus; in-vitro studies show preferential binding to Rab6B and Rab6A’ over Rab6A; potentially involved in Rab6-dependent endosome-Golgi transport |
None | [79] |
| TGN38 | Cytoplasmic, carboxy-terminal domain binds to a complex of Rab6 with p62; is thought to play a role in the budding of exocytic vesicles but has not been studied in the context of Rab6-dependent Golgi-ER traffic |
None | [80] |
| TMF/ARA160 | Contains a coiled-coil motif that is capable of binding to Rab6A, Rab6A’ and Rab6B; thought to play an as yet undefined role in Rab6-dependent Golgi-ER carrier biogenesis | Biogenesis | [46] |
*Identified as playing a role in Rab6-dependent anterograde transport.
?Possible role in Rab6-dependent Golgi-to-ER transport, yet to be experimentally validated.
An alternative explanation for the association of Rab6 with dynein could be provided when considering the role of Rab6 in trafficking from endosomal compartments to the Golgi apparatus. Our understanding of the mechanical involvement of Rab6 in this particular transport step requires much development, however there is early evidence pointing to a role in the early endosome-to-TGN retrograde transport step. Inhibition of Rab6A’ through overexpression of the dominant-negative GDP-locked mutant, Rab6A’T27N, resulted in accumulation of STxB in transferrin receptor-positive early/recycling endosomes, and a failure to traffic to the TGN [11]. Additionally, in work characterizing the mammalian retromer complex, it was shown that two retromer sorting nexins (SNX) SNX5 and SNX6 interact with the Rab6 effector and dynactin subunit p150glued [81]. Another retromer SNX, SNX1, was also shown to interact specifically with Rab6-interacting protein 1 (Rab6IP1). Retromer-dependent transport is dynein-driven, and the carriers are tubular-vesicular in nature [82] – the overlap between Rab6 and dynein in this pathway could potentially point to an involvement of Rab6 in a more diverse range of tubule-dependent transport than exclusively Golgi-to-ER tubular transport. Resolution of this question, along with resolution of the motor protein(s) responsible for driving the specific Golgi-to-ER transport step of Rab6-coated carriers is of vital importance, in order to be able to fully understand this pathway and its role in cells.
Tubule fission
Membrane fission as an event can be described as either active, in which the hydrolysis of nucleotide triphosphates generates energy to facilitate membrane fission, or passive, in which lipids and proteins of a membrane spontaneously rearrange in a manner which favours membrane fission over membrane continuity [83]. The fission, be it active or passive, of Rab6-positive tubules from Golgi membranes remains poorly understood. In recent years, a number of discoveries have been made regarding the molecular mechanisms of active fission of Rab6-positive vesicles from the Golgi, and it is plausible that there may be overlap between these molecules and those involved in Rab6-positive tubule fission. A key question that remains to be addressed is that of the temporal triggers for tubule fission, and specifically whether any delay in carrier fission would result in elongation of the emerging bud and the production of a tubule. A variety of different mechanisms to drive membrane fission exist in cells, and a wide range of molecular machinery has now been identified [83].
Some of the first work to identify players involved in the fission of retrograde tubules came from a series of experiments investigating the involvement of actin in retrograde transport [84]. While microtubules tend to be the cytoskeletal structure most frequently implicated in long-range membrane trafficking events, a number of studies have also shown the involvement of actin in directing transport carriers, including BFA-induced transport tubules [85]. Durán and colleagues showed that by pharmacologically inhibiting the activity of the actin-associated motor protein, NM2A, using the broad-spectrum myosin inhibitor 2,3-butanedione 2-monoxime (BDM) and the myosin light-chain kinase inhibitor ML-7, BFA-induced redistribution of α-mannosidase II was substantially delayed [84]. BDM- and ML-7-based inhibition of myosins still allowed the formation of tubules under cells treated with BFA and these tubules persisted longer than untreated cells, suggesting that myosins, and NM2A in particular, play a role in facilitating the fission event.
ML-7 is also known to inhibit protein kinase A (PKA) and protein kinase C (PKC), and it was notable that the inhibition of BFA-induced destabilization reported by Durán and colleagues in [84] was shown not to result from this activity of ML-7. Curiously, work from the Mardones group argued the opposite [86]. In a series of elegant microscopy experiments utilizing BFA, they first showed that HeLa and NRK cells treated with two other inhibitors of PKA, H-89 and myr-PKI-A, produced long tubules which were incapable of detaching from the Golgi apparatus. They then showed that following incubation of cells with both BFA and myr-PKI-A, the temporary withdrawal of myr-PKI-A in the continuous presence of BFA was sufficient to induce the fission of accumulated tubules from the Golgi. Together these data suggest that PKA is an important protein in facilitating the fission of retrograde tubules from the Golgi, although notably it is still unclear as to what the targets of phosphorylation of PKA are in this process. A tempting hypothesis to consider is that PKA may phosphorylate Golgi matrix proteins, thus influencing their function at the Golgi membrane. Very recent work by Jia and colleagues supports this hypothesis, by showing that the Golgi phosphoprotein 1 (GOLPH1; also known as Acyl CoA Binding Domain containing 3 (ACBD3)) functions as an A-kinase anchoring protein (AKAP) and recruits PKA to the Golgi apparatus [87].
Work from the Goud group has also implicated NM2A in the fission of Rab6-positive carriers from the Golgi apparatus [42]. NM2A was initially identified as playing a promiscuous and prominent role in the fission of Rab6-positive carriers through fluorescence microscopy studies investigating the dynamics of GFP-Rab6A and GFP-Rab6A’. NM2A was inhibited through the action of blebbistatin and ML-7, and the Rho kinase inhibitor Y27632 was also included. Parallel studies incorporating RNAi-based depletion of NM2A were also undertaken. Interestingly, NM2A was shown to play a role in both the anterograde transport of the model secretory cargo, VSV-G, and the retrograde transport of STxB. Conflictingly, the experiments reported by Durán and colleagues [84] (discussed above) showed no effect on the anterograde transport of VSV-G in cells treated with ML-7 alone, although their results on the failed ER localization of STxB are in agreement with those reported by the Goud group.
Another study from the Goud group identified the kinesin KIF20A (Rabkinesin-6) as being an additional protein with a vital role in NM2A-mediated fission of Rab6-positive carriers from the Golgi [56]. In these experiments, regions of the Golgi membrane in which Rab6-dependent vesicle production was enriched were observed, and named ‘Golgi fission hotspots’ by the authors of this work. Importantly, when the NM2A inhibitor blebbistatin was employed, Rab6-positive tubules, as opposed to vesicles, were formed, in the same regions of the Golgi as the Rab6-positive vesicles. KIF20A was identified as a mutual interactor of NM2A and Rab6, and its inhibition through a variety of mechanisms also resulted in the formation of long, continuous tubules attached to the Golgi membrane – a phenotype indicative of defective fission, similar to that observed in NM2A inhibition experiments. Although the findings in this series of experiments are of great importance to understanding the wider role of Rab6 at the Golgi apparatus, only VSV-G anterograde transport was assessed, and currently there is no understanding of whether KIF20A also plays a role in Rab6-/NM2A-mediated retrograde transport.
It is worth noting that the Golgi fission hotspots observed at the edges of Golgi cisternae [56] bear remarkable similarity to the budding structures to which TMF has been shown to localize [44]. Together, these two studies support the hypothesis of the presence of GoES or tubulation complexes at the Golgi apparatus, in which machinery regulating multiple stages of tubulation is recruited, with varying temporal triggers. This hypothesis will require further investigation. Perhaps most curious is the apparent multifaceted role of NM2A in Rab6-dependent transport. NM2A has been shown to be involved in two steps of tubulation with strikingly opposing functions – the development of a membrane tubule, as suggested by Petrosyan and Cheng [41], and the fission of a membrane tubule [56]. It is plausible that Rab6 and NM2A may form a ‘master regulator’ complex of sorts – together, these two proteins might regulate a multitude of tubulation events, in combination with the recruitment of additional, temporally and/or spatially triggered effectors.
An alternative explanation for the involvement of NM2A in two mechanically distinct steps can be provided when considering the hypothesis that tubules, as opposed to vesicles, are formed as a means of transporting large volumes of retrograde cargo. If NM2A does indeed interact directly with Golgi-to-ER cargo such as glycosylation enzymes and act as a bridge for Rab6 and its associated mechanical effectors, perhaps it is the absence of NM2A from the membranes that triggers membrane scission. In this scenario, transport-ready Golgi-resident enzymes would accumulate, thus recruiting NM2A and Rab6 [41,47,88]. Membrane deformation would occur, allowing the formation of a Rab6-coated transport carrier. Due to a high volume of cargo, NM2A is then continually recruited to the point of exit of the Golgi enzymes, lengthening the transport carrier and developing a tubule, rather than a vesicle. It is only when the cargo has entered into the transport carrier fully, and NM2A remains associated with the enzymes in the tubule, as opposed to on the Golgi membranes, that membrane scission can occur. In this hypothesis, NM2A is involved in both tubule biogenesis and tubule fission – its presence at tubule budding hotspots promotes tubulation, and its exit from these hotspots promotes membrane scission.
While tempting, this hypothesis is not without its flaws. For example, there would surely need to be a means in place to prevent the continuous recruitment of NM2A by the cytosolic tails of Golgi enzymes and thus their perpetual retrograde transport, thereby allowing them to remain in the Golgi to perform post-translational modifications on anterograde-moving cargo. One possible solution to this dilemma is the Golgi membrane ‘de-scaffolding’, alluded to previously. As well as interacting with membrane trafficking associated proteins, Rab6 has also been shown to interact with a number of different Golgi matrix proteins, responsible for maintaining the structural integrity of the organelle [89]. Given the pivotal role that Rab6 has been shown to play in regulating Golgi-associated transport pathways, it begs the question whether the interactions between Rab6 and matrix proteins are purely to facilitate the maintenance of Golgi structural integrity, or whether these structural proteins might also be involved in facilitating transport. Giantin is a large 376 kDa Golgi matrix protein that exists as a coiled-coil homodimer, and which has a Rab6-binding region at its N-terminus [68,90]. In work from the Petrosyan group exploring Golgi restoration following ethanol treatment, it was observed that ethanol treatment on HeLa and LNCaP cells resulted in the fragmentation of the Golgi apparatus, the de-dimerization of giantin and an associated loss of its binding to Rab6 [47]. Strikingly, the loss of the association between Rab6 and giantin corresponded with an increase in the association between Rab6 and phosphorylated NM2A [47]. In later studies exploring the role of giantin in the reformation of the Golgi apparatus following BFA washout, the same group also showed that as the Golgi reforms, Rab6 associates more with giantin, and its association with NM2A decreases [39]. The reformation of the Golgi apparatus was also associated with the dimerization of giantin, suggesting that Rab6 may have a preference for binding to dimerized giantin as opposed to giantin monomers, although this has not been directly explored. One possible explanation for this is that giantin, when in its coiled-coil, dimerized state, appears to exist in a ‘hooked’ conformation, as opposed to a rigid conformation extending into the cytoplasm [39]. This would bring its Rab6-binding region at the N-terminus in close proximity to the Golgi membrane, where active Rab6 resides. Although tubule biogenesis was not directly explored in these studies, together they present an enticing hypothesis – it is the stoichiometry of the association of Rab6 with either matrix scaffold proteins or with motor proteins that determines whether the Golgi membrane remains intact or becomes destabilized and ‘de-scaffolded’, thus creating a temporal and spatial opportunity for the biogenesis and formation of a transport carrier (Figure 1). Similar hypotheses have also been presented by others in the field [8]. Further studies on the relationship between Rab6 and its different classes of effectors will be needed to explore this possibility.
Figure 1.
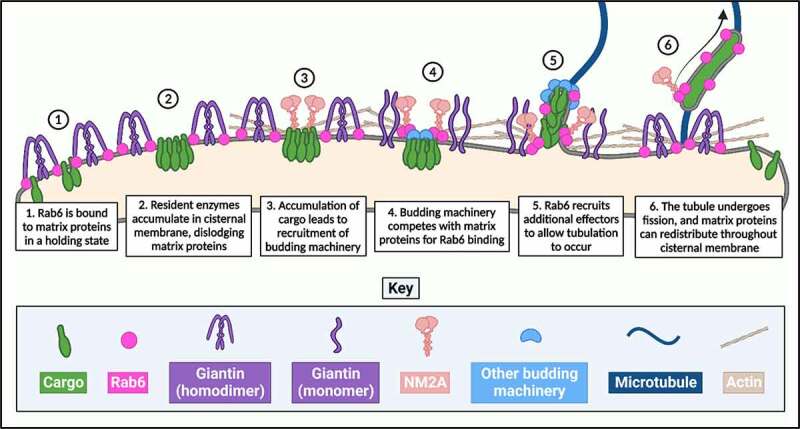
De-scaffolding of the Golgi apparatus. Schematic diagram outlining the likely order of ‘de-scaffolding’ of the Golgi membrane in order to generate a Rab6-positive tubular carrier.
Passive fission of Rab6-positive tubules is also an appealing theory to consider [83]. While the factor(s) driving the initial membrane curvature and budding to produce a Rab6-coated tubule remain to be identified, it is plausible that if these factors behave in a similar manner to Arf1, as described by Bottanelli and colleagues [49], passive fission may indeed be the default fission mechanism for Rab6-coated carriers. Under the models of passive fission, recruitment of curvature-inducing agents at a high density to the membrane drives the continuous expansion of the membrane until it closes in on itself to form a transport carrier such as a vesicle [83,91]. This model applies to Arf1-induced membrane curvature to drive the synthesis of COPI-coated vesicles [50], but creates a conundrum – how, then, can Arf1-coated tubules simultaneously exist? What prevents the closing-in of an Arf1-coated membrane, to allow it to extend and exist as a tubule as opposed to a vesicle? One answer could be the volume of cargo that is incorporated into the transport carrier. Under circumstances in which there is a high volume of cargo to be transported, the high density of this cargo in the membrane of the transport carrier would logically prevent the enhanced association of curvature-inducing agents. This might allow the carrier to expand until such a point where most of the cargo has been incorporated into the carrier and there is sufficient surface area at the membrane to only then allow the density of the curvature-inducing agent to surpass that of the cargo. As discussed above, there is much evidence to support the hypothesis that it is large volumes of cargo such as Golgi-resident enzymes that drive the formation of Rab6-coated retrograde tubules. It falls to reason that this large volume of cargo may also be the driving force behind the extension, and inhibition of passive fission, of the membrane.
Tubule fusion
The fusion of a transport carrier with its target membrane is mediated by a combination of target-associated tether proteins, capturing the incoming transport carrier, and SNARE proteins, which facilitate membrane homogenization and fusion [92]. Since the initial discovery of the Rab6-dependent pathway, a number of different potential molecules involved in facilitating the fusion of Rab6-dependent carriers with the ER have been proposed.
Zeste White 10 (ZW10) in complex with RINT-1 have been identified as strong candidates for target membrane-associated Golgi-to-ER tether proteins in the Rab6-mediated pathway by the Storrie group [57,93]. The ZW10 and RINT-1 complex associates with the SNARE protein syntaxin-18 at the ER membranes, and depletion of either ZW10 or RINT-1 results in the fragmentation of the Golgi ribbon into a series of punctate, juxtanuclear Golgi fragments [93–95]. Epistatic knockdown or silencing of Rab6 reverses these phenotypes, as well as the fragmented Golgi phenotype induced by depletion of COG3, a component of the known ER tether conserved oligomeric Golgi (COG) complex, providing strong evidence that these proteins play a role in Rab6-mediated transport [93]. In another series of epistatic depletion experiments exploring other potential partner proteins of ZW10/RINT-1 and COG3 in Rab6-mediated retrograde transport, Majeed and colleagues [57] identified NM2A and KIF20A as proteins likely to lie upstream of ZW10/RINT-1, but not COG3, in Golgi-derived transport. Given the high likelihood of involvement of NM2A and KIF20A in the Rab6-dependent, COPI-independent pathway, as discussed previously in this review, it is reasonable to suggest that the ZW10/RINT-1 complex likely plays a role.
The dynein adaptor proteins BicD1 and BicD2 were also identified as candidates lying upstream of both ZW10/RINT-1 and COG3 [57]. BicD was first identified as playing a role in COPI-independent retrograde transport as early as 2002 [61]. In this early work, it was noted that BicD1 and BicD2 bind to the C-terminus of Rab6A, and both BicD variants colocalize with Rab6 on vesicular and tubular structures (although BicD1 more so than BicD2). Notably, cells incubated at low temperatures, a common tubule enhancing treatment, demonstrated the formation of BicD1-positive tubules at the cell periphery, which progressed towards the Golgi apparatus with increased time at the low temperature. It was also shown that BicD1 colocalised with both Rab6 and STxB on these tubules. Inhibition of BicD2 through the overexpression of the C-terminus of BicD2 led to the accumulation of large vesicle-like structures containing both Rab6 and GalTase at the cell periphery – importantly neither at the Golgi nor the ER. Together, these findings suggest a role of BicD at multiple stages of Rab6-dependent retrograde transport.
In 2015, work from the Murata group further solidified the role of BicD in Rab6-dependent retrograde transport [62]. BicD2 was confirmed as an interactor of Rab6 through cytosolic reconstitution experiments, and it was also shown that the presence of BicD2 in the cytosol is necessary for the Golgi-localization of Rab6. Interestingly, it was the knockdown of BicD2, but not BicD1, which prevented the stabilization of Rab6 at the Golgi apparatus. In the context of the results obtained from Matanis and colleagues [61], perhaps BicD1 and BicD2 play non-overlapping roles in Rab6-dependent retrograde transport – BicD1 in early, pre-Golgi Rab6-dependent retrograde transport, and BicD2 in post-Golgi Rab6-dependent retrograde transport. In keeping with this idea, Matsuto and colleagues observed that cells depleted of BicD2 and treated with BFA demonstrated elevated tubule persistence times and a delay in the appearance of the redistribution of the Golgi enzyme GalT to the ER – phenotypes indicative of perturbed tubule fusion at the ER [62]. The mechanics through which BicD2 mediates tubules fusion remain to be elucidated.
Recent work by Shomron and colleagues [96] suggests a role of Rab6 and BicD2 in the positioning of ERES sites in the perinuclear region at the cell centre. In these experiments, the overexpression of the GDP-restricted Rab6 mutant (Rab6A’[T27N]) reduced the clustering of both Sec23-positive and Sec16-positive ERES in close proximity to the Golgi apparatus, and this phenotype was replicated in cells overexpressing the N-terminal fragment of BicD2, which lacks the Rab6-binding region. VSV-G accumulation at the Golgi was also impaired upon overexpression of Rab6A’[T27N] or the N-terminus of BicD2. These findings suggest that it is Rab6 in cooperation with BicD2 which plays a role in the positioning of ERES. While this work does not directly explore Rab6-dependent retrograde traffic, it cannot be ignored that there are likely to be indirect links. If the principal function of the Rab6-dependent pathway is indeed the recycling of Golgi-resident molecules such as enzymes, as is the current understanding, it would be reasonable to suggest that this recycling of cargo would be targeted at destinations of the ER close to ERES to facilitate their swift secretion and re-entry to the Golgi apparatus. Furthermore, as Rab6 is predominantly localized to the trans-Golgi and TGN, and not the ER, arguably the only explanation for its involvement in the positioning of ERES is linked to its role in Golgi-to-ER retrograde transport.
Our own recent work has implicated Sec1 family domain containing protein 1 (SCFD1), also known as SLY1, in the fusion of Rab6-dependent tubular carriers at the ER [97]. SCFD1 interacts with syntaxin-17 at the ER, in conjunction with COG4. COG4 forms lobe A of the COG complex in conjunction with COG1, COG2 and COG3, with lobe B being formed by COG5–8 [98]. In a systematic RNAi screen investigating molecules involved in mediating the fusion of Rab6-dependent retrograde tubules at the ER, syntaxin-5 (STX5), vesicle associated membrane protein 4 (VAMP4) and SCFD1 were identified as candidates whose depletion resulted in delayed ER redistribution of Rab6 in a COPI-independent pathway. Further experiments on the impact of SCFD1 depletion in conjunction with Shiga-like toxin B chain (SLTxB) retrograde transport assays demonstrated that depletion of SCFD1 prevented SLTxB from relocalising to the ER, and instead localized to punctate structures which colocalised with Rab6. In cells depleted of SCFD1, and treated with BFA, live-cell microscopy revealed substantially impeded tubulation of the Golgi apparatus – tubules emerging from the Golgi apparatus were shorter than those in control cells, and EGFP-Rab6 failed to redistribute to the ER over the entire imaging period. The role of SCFD1 and its associated partner molecules at the ER, namely syntaxin-17 and COG4, will require further investigation, although it is very promising that the COG complex, lobe A specifically, has been implicated in Rab6-dependent retrograde transport in two independent works – pointing to an important role of this complex in the pathway [57,97].
In addition to the physical fusion machinery, other highly localized biochemical reactions are also likely to be required for membrane fusion to occur. Tenorio and colleagues [86] showed that PKA activity is important for facilitating tubule fission, as discussed above. In the same work, they showed that tubule fusion at the ER is also significantly abrogated in cells treated with pharmacological inhibitors of PKA. Following on from their experiments in which myr-PKI-A was temporarily withdrawn from cells in the continuous presence of BFA, cells were then re-incubated with myr-PKI-A, and fixed at different time points. The cut tubules observed following the initial withdrawal of myr-PKI-A were still present and, interestingly, these cut tubules of ca. 5 µm in length persisted as long as 15 min following myr-PKI-A reintroduction. There was no re-distribution of resident enzymes to the ER, or of matrix proteins to ERES. The persistence of these cut tubules shows that not only is PKA activity important for the initial fission of the tubules from the Golgi membrane but also fusion at the ER membranes. This work is also suggestive that tubule fission occurs in advance of tubule fusion at the ER membrane, and therefore that free tubules may exist. We are still left with more questions than answers, however. What are the downstream targets of PKA – could they be molecular motors such as NM2A? Also, is tubule fission from the Golgi a necessary prerequisite to tubule fusion at the ER, or can a tubule fuse with the ER prior to its scission from the Golgi thereby creating transient connections between the two organelles? This latter question has never been explored in the context of Golgi-to-ER retrograde transport, however recent work from the Stephens lab has suggested that continuous anterograde-directed membrane connections between the ER and Golgi may exist as a mechanism for the transport of large secretory molecules, such as pro-collagen [99,100]. Conventional ER-to-Golgi COPII carriers tend to range between 60 and 90 nm in size, whereas the secretory cargo pro-collagen is ca. 300 nm in length, falling well outside the limits of COPII vesicles. In a series of live-cell experiments employing a GFP-tagged pro-collagen construct (herein referred to as GFP-COL), the secretion of which could be induced and controlled using the Retention Using Selective Hooks (RUSH) system, McCaughey and colleagues observed that GFP-COL tended not to localize to large membrane-bound intermediates between the ER and the Golgi as reported by other groups, but instead gradually emptied from the ER, and just as gradually appeared in the Golgi apparatus. To explain these findings, they hypothesized that there may be a short-range trafficking loop connecting juxtanuclear ER membranes with membranes of the ER-Golgi intermediate compartment (ERGIC). In this scenario, due to the limited spatial separation between the ERGIC and some ER membranes, and the size of the large secretory cargo such as pro-collagen, transport carriers arising from the ER may fuse with the ERGIC before carrier scission at the ER has occurred. It would be interesting to explore the possibility of continuous membrane joins between the Golgi and ER in the context of tubule-mediated retrograde transport.
Conclusions and future directions
Despite almost one-quarter of a century having elapsed since the discovery of the Rab6-dependent Golgi-to-ER transport pathway, there are evidently still many unresolved questions with respect to how it is regulated. In this review, we have attempted to reconcile the available evidence for how this pathway is mechanistically controlled to produce a consolidated view (Figure 2). Our lack of knowledge of the mechanics of this pathway is in stark comparison to our level of understanding of how coated vesicular carriers, such as COPI, COPII and clathrin-coated vesicles, are formed and utilized within cells. There are a number of factors explaining why our knowledge of the molecular composition of Rab6 carriers is relatively sparse.
Figure 2.
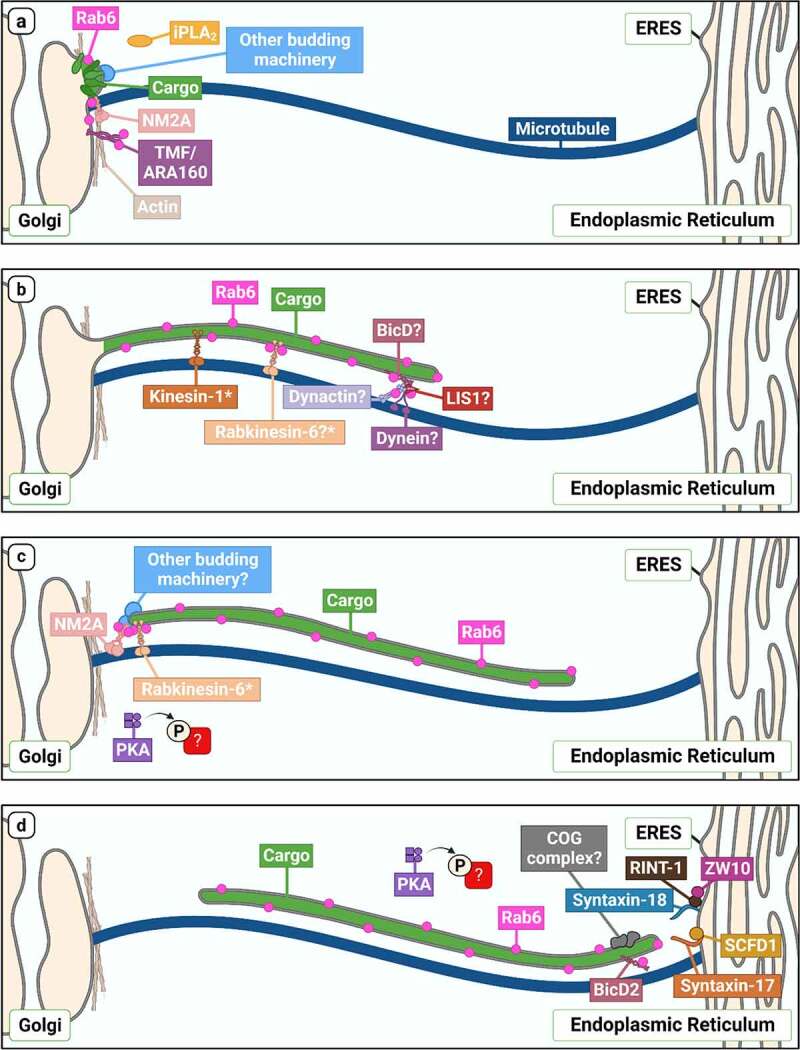
An overview of Rab6-dependent Golgi-to-ER transport. Schematic outlining our current understanding of molecules involved in the different steps of Rab6-dependent Golgi-to-ER transport (a: biogenesis, b: motility and elongation, c: tubule fission, d: tubule fusion at the ER). Proteins speculated but not explicitly confirmed to play a role in this pathway are indicated with a ‘?’, and proteins confirmed to play a role in mediating anterograde Rab6-dependent transport, but have not been investigated in the context of retrograde transport, are marked with an asterisk.
Firstly, initial identification of the core components of coated vesicular carriers came from elegant biochemical isolation approaches, in which the vesicles were purified in high abundance and their composition determined. Given that there seems to be no conventional coat on Rab6 tubular carriers, they are likely to be highly labile such that biochemical isolation methods would be futile. Secondly, we are still unclear about their physiological function. While a variety of studies clearly show their presence in both perturbed and homoeostatic cells, and some involvement in recycling Golgi glycosylation enzymes back to the ER, to date, the specific range of cargo types associated with them has remained elusive. Thirdly, the molecules that have thus far been linked to Rab6 tubule function are not solely involved in this pathway. It may indeed be the case that this pathway does not utilize any unique machinery, but rather ‘borrows’ a subset of molecules from other transport pathways.
Resolving these points is essential if we are to shed light on this pathway. Not only is this important from an academic perspective, in particular addressing the curious question of why the cell needs to be able to generate long membrane tubules rather than conventional vesicles, but also from a physiological one. The pathway was discovered, almost by chance, through experiments investigating the trafficking pathways taken by a number of protein toxins [101]. What has emerged is that it seems to be the primary pathway parasitized by the Shiga and E. coli Shiga-like toxins in order to reach the ER and then dislocate their catalytic subunits into the cytoplasm [102]. Given the unique nature of the pathway, effectively linking the plasma membrane and endosomal system with the primary organelle in the early secretory pathway, it is not unsurprising that it is also now being viewed in the context of a novel pathway for therapeutic delivery [103]. If that potential is to be realized, it is imperative that it does not take us another quarter of a century to precisely define how its molecular regulation is achieved.
Acknowledgments
The authors gratefully acknowledge all the members of the Cell Screening Laboratory for comments on this article. Work at the Cell Screening Laboratory is funded by the UCD College of Science. L.G.D. is supported by a Government of Ireland Postgraduate Scholarship from the Irish Research Council (IRC), under grant [GOIPG/2022/1944]. The figures were created with BioRender.com.
Funding Statement
The work was supported by the Irish Research Council [GOIPG/2022/1944].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Béthune J, Wieland FT.. Assembly of COPI and COPII vesicular coat proteins on membranes. Annu Rev Biophys. 2018;47:63–83. doi: 10.1146/annurev-biophys-070317-033259 [DOI] [PubMed] [Google Scholar]
- [2].Homma Y, Hiragi S, Fukuda M. Rab family of small GTPases: an updated view on their regulation and functions. FEBS J. 2021;288(1):36–55. doi: 10.1111/febs.15453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Galea G, Simpson JC. High-content analysis of Rab protein function at the ER-Golgi interface. Bioarchitecture. 2015;5(3–4):44–53. doi: 10.1080/19490992.2015.1102826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zahraoui A, Touchot N, Chardin P, et al. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989;264(21):12394–12401. doi: 10.1016/S0021-9258(18)63872-4 [DOI] [PubMed] [Google Scholar]
- [5].Goud B, Zahraoui A, Tavitian A, et al. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990;345(6275):553–556. doi: 10.1038/345553a0 [DOI] [PubMed] [Google Scholar]
- [6].Heffernan LF, Simpson JC. The trials and tubule-ations of Rab6 involvement in Golgi-to-ER retrograde transport. Biochem Soc Trans. 2014;42(5):1453–1459. doi: 10.1042/bst20140178 [DOI] [PubMed] [Google Scholar]
- [7].Liu S, Storrie B. How Rab proteins determine Golgi structure. Int Rev Cell Mol Biol. 2015;315:1–22. doi: 10.1016/bs.ircmb.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goud B, Liu S, Storrie B. Rab proteins as major determinants of the Golgi complex structure. Small GTPases. 2018;9(1–2):66–75. doi: 10.1080/21541248.2017.1384087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Valente C, Polishchuk R, De Matteis MA. Rab6 and myosin II at the cutting edge of membrane fission. Nat Cell Biol. 2010;12(7):635–638. doi: 10.1038/ncb0710-635 [DOI] [PubMed] [Google Scholar]
- [10].Fourriere L, Kasri A, Gareil N, et al. RAB6 and microtubules restrict protein secretion to focal adhesions. J Cell Bio. 2019;218(7):2215–2231. doi: 10.1083/jcb.201805002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mallard F, Tang BL, Galli T, et al. Early/Recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Bio. 2002;156(4):653–664. doi: 10.1083/jcb.200110081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Girod A, Storrie B, Simpson JC, et al. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol. 1999;1(7):423–430. doi: 10.1038/15658 [DOI] [PubMed] [Google Scholar]
- [13].White J, Johannes L, Mallard F, et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Bio. 1999;147(4):743–760. doi: 10.1083/jcb.147.4.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Del Nery E, Miserey-Lenkei S, Falguières T, et al. Rab6a and Rab6A′ GTPases play non-overlapping roles in membrane trafficking. Traffic. 2006;7(4):394–407. doi: 10.1111/j.1600-0854.2006.00395.x [DOI] [PubMed] [Google Scholar]
- [15].Domínguez Cadena LC, Schultz TE, Zamoshnikova A, et al. Rab6b localizes to the Golgi complex in murine macrophages and promotes tumor necrosis factor release in response to mycobacterial infection. Immunol Cell Biol. 2021;99(10):1067–1076. doi: 10.1111/imcb.12503 [DOI] [PubMed] [Google Scholar]
- [16].Garcia-Saez I, Tcherniuk S, Kozielski F. The structure of human neuronal Rab6B in the active and inactive form. Acta Crystallogr D. 2006;62(7):725–733. doi: 10.1107/s0907444906015319 [DOI] [PubMed] [Google Scholar]
- [17].Young J, Ménétrey J, Goud B. RAB6C is a retrogene that encodes a centrosomal protein involved in cell cycle progression. J Mol Biol. 2010;397:69–88. doi: 10.1016/j.jmb.2010.01.009 [DOI] [PubMed] [Google Scholar]
- [18].Liu S, Hunt L, Storrie B, et al. Rab41 is a novel regulator of Golgi apparatus organization that is needed for ER-to-Golgi trafficking and cell growth. PLoS One. 2013;8(8):e71886. doi: 10.1371/journal.pone.0071886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu S, Majeed W, Kudlyk T, et al. Identification of Rab41/6d effectors provides an explanation for the differential effects of Rab41/6d and Rab6a/a’ on Golgi Organization. Front Cell Dev Biol. 2016;4:13. doi: 10.3389/fcell.2016.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lippincott-Schwartz J, Yuan LC, Bonifacino JS, et al. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martinez O, Antony C, Pehau-Arnaudet G, et al. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc Natl Acad Sci, USA. 1997;94(5):1828–1833. doi: 10.1073/pnas.94.5.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mardones GA, Snyder CM, Howell KE. Cis-Golgi matrix proteins move directly to endoplasmic reticulum exit sites by association with tubules. Mol Biol Cell. 2006;17(1):525–538. doi: 10.1091/mbc.e05-05-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sengupta P, Satpute-Krishnan P, Seo AY, et al. ER trapping reveals Golgi enzymes continually revisit the ER through a recycling pathway that controls Golgi organization. Proc Natl Acad Sci, USA. 2015;112(49):E6752–6761. doi: 10.1073/pnas.1520957112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Verissimo F, Halavatyi A, Pepperkok R, et al. A microtubule-independent role of p150glued in secretory cargo concentration at endoplasmic reticulum exit sites. J Cell Sci. 2015;128:4160–4170. doi: 10.1242/jcs.172395 [DOI] [PubMed] [Google Scholar]
- [25].Fossati M, Colombo SF, Borgese N. A positive signal prevents secretory membrane cargo from recycling between the Golgi and the ER. Embo J. 2014;33(18):2080–2097. doi: 10.15252/embj.201488367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boncompain G, Perez F. The many routes of Golgi-dependent trafficking. Histochem Cell Biol. 2013;140(3):251–260. doi: 10.1007/s00418-013-1124-7 [DOI] [PubMed] [Google Scholar]
- [27].Berninsone PM, Hirschberg CB. Nucleotide sugar transporters of the Golgi apparatus. Curr Opin Struct Biol. 2000;10(5):542–547. doi: 10.1016/s0959-440x(00)00128-7 [DOI] [PubMed] [Google Scholar]
- [28].Hadley B, Litfin T, Day CJ, et al. Nucleotide sugar transporter SLC35 family structure and function. Computat Struct Biotechnol j. 2019;17:1123–1134. doi: 10.1016/j.csbj.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maszczak-Seneczko D, Wiktor M, Skurska E, et al. Delivery of nucleotide sugars to the mammalian Golgi: A very well (un)explained story. Int J Mol Sci. 2022;23(15):8648. doi: 10.3390/ijms23158648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gao J, Gao A, Zhou H, et al. The role of metal ions in the Golgi apparatus. Cell Biol Int. 2022;46(9):1309–1319. doi: 10.1002/cbin.11848 [DOI] [PubMed] [Google Scholar]
- [31].Hellerschmied D, Serebrenik YV, Shao L, et al. Protein folding state-dependent sorting at the Golgi apparatus. Mol Biol Cell. 2019;30(17):2296–2308. doi: 10.1091/mbc.E19-01-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Storrie B, White J, Röttger S, et al. Recycling of golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Bio. 1998;143(6):1505–1521. doi: 10.1083/jcb.143.6.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Figueiredo P, Drecktrah D, Katzenellenbogen JA, et al. Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc Natl Acad Sci, USA. 1998;95(15):8642–8647. doi: 10.1073/pnas.95.15.8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gutiérrez-Martínez E, Fernández-Ulibarri I, Lázaro-Diéguez F, et al. Lipid phosphate phosphatase 3 participates in transport carrier formation and protein trafficking in the early secretory pathway. J Cell Sci. 2013;126:2641–2655. doi: 10.1242/jcs.117705 [DOI] [PubMed] [Google Scholar]
- [35].Marra P, Maffucci T, Daniele T, et al. The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nat Cell Biol. 2001;3(12):1101–1113. doi: 10.1038/ncb1201-1101 [DOI] [PubMed] [Google Scholar]
- [36].Seemann J, Jokitalo E, Pypaert M, et al. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 2000;407(6807):1022–1026. doi: 10.1038/35039538 [DOI] [PubMed] [Google Scholar]
- [37].Yadav S, Puthenveedu MA, Linstedt AD. Golgin160 recruits the dynein motor to position the Golgi apparatus. Dev Cell. 2012;23(1):153–165. doi: 10.1016/j.devcel.2012.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu Z, Joseph D, Bugnard E, et al. Golgi complex reorganization during muscle differentiation: visualization in living cells and mechanism. Mol Biol Cell. 2001;12(4):795–808. doi: 10.1091/mbc.12.4.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Frisbie CP, Lushnikov AY, Krasnoslobodtsev AV, et al. Post-ER stress biogenesis of Golgi is governed by giantin. Cells. 2019;8(12):1631. doi: 10.3390/cells8121631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Simpson JC, Nilsson T, Pepperkok R. Biogenesis of tubular ER-to-Golgi transport intermediates. Mol Biol Cell. 2006;17(2):723–737. doi: 10.1091/mbc.e05-06-0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Petrosyan A, Cheng PW. A non-enzymatic function of Golgi glycosyltransferases: mediation of Golgi fragmentation by interaction with non-muscle myosin IIA. Glycobiology. 2013;23(6):690–708. doi: 10.1093/glycob/cwt009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Miserey-Lenkei S, Chalancon G, Bardin S, et al. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol. 2010;12(7):645–654. doi: 10.1038/ncb2067 [DOI] [PubMed] [Google Scholar]
- [43].Müsch A, Cohen D, Rodriguez-Boulan E. Myosin II is involved in the production of constitutive transport vesicles from the TGN. J Cell Bio. 1997;138(2):291–306. doi: 10.1083/jcb.138.2.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yamane J, Kubo A, Nakayama K, et al. Functional involvement of TMF/ARA160 in Rab6-dependent retrograde membrane traffic. Exp Cell Res. 2007;313(16):3472–3485. doi: 10.1016/j.yexcr.2007.07.010 [DOI] [PubMed] [Google Scholar]
- [45].Garcia JA, Ou SH, Wu F, et al. Cloning and chromosomal mapping of a human immunodeficiency virus 1 “TATA” element modulatory factor. Proc Natl Acad Sci USA. 1992;89(20):9372–9376. doi: 10.1073/pnas.89.20.9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fridmann-Sirkis Y, Siniossoglou S, Pelham HR. TMF is a golgin that binds Rab6 and influences Golgi morphology. BMC Cell Biol. 2004;5(1):18. doi: 10.1186/1471-2121-5-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Petrosyan A, Casey CA, Cheng PW. The role of Rab6a and phosphorylation of non-muscle myosin IIA tailpiece in alcohol-induced Golgi disorganization. Sci Rep. 2016;6(1):31962. doi: 10.1038/srep31962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Spang A. Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5(6):a013391–a013391. doi: 10.1101/cshperspect.a013391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bottanelli F, Kilian N, Ernst AM, et al. A novel physiological role for ARF1 in the formation of bidirectional tubules from the Golgi. Mol Biol Cell. 2017;28(12):1676–1687. doi: 10.1091/mbc.E16-12-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Beck R, Sun Z, Adolf F, et al. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc Natl Acad Sci, USA. 2008;105(33):11731–11736. doi: 10.1073/pnas.0805182105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Grigoriev I, Splinter D, Keijzer N, et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13(2):305–314. doi: 10.1016/j.devcel.2007.06.010 [DOI] [PubMed] [Google Scholar]
- [52].Echard A, Jollivet F, Martinez O, et al. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279(5350):580–585. doi: 10.1126/science.279.5350.580 [DOI] [PubMed] [Google Scholar]
- [53].Hill E, Clarke M, Barr FA. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. Embo J. 2000;19(21):5711–5719. doi: 10.1093/emboj/19.21.5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fontijn RD, Goud B, Echard A, et al. The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol Cell Biol. 2001;21(8):2944–2955. doi: 10.1128/mcb.21.8.2944-2955.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gruneberg U, Neef R, Honda R, et al. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Bio. 2004;166(2):167–172. doi: 10.1083/jcb.200403084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Miserey-Lenkei S, Bousquet H, Pylypenko O, et al. Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions. Nat Commun. 2017;8(1):1254. doi: 10.1038/s41467-017-01266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Majeed W, Liu S, Storrie B. Distinct sets of Rab6 effectors contribute to ZW10–and COG-dependent Golgi homeostasis. Traffic. 2014;15(6):630–647. doi: 10.1111/tra.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Le Bot N, Antony C, White J, et al. Role of xklp3, a subunit of the Xenopus kinesin II heterotrimeric complex, in membrane transport between the endoplasmic reticulum and the Golgi apparatus. J Cell Bio. 1998;143(6):1559–1573. doi: 10.1083/jcb.143.6.1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Young J, Stauber T, Del Nery E, et al. Regulation of microtubule-dependent recycling at the Trans -Golgi Network by Rab6A and Rab6A’. Mol Biol Cell. 2005;16(1):162–177. doi: 10.1091/mbc.e04-03-0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stauber T, Simpson JC, Pepperkok R, et al. A role for kinesin-2 in COPI-dependent recycling between the ER and the Golgi complex. Curr Biol. 2006;16(22):2245–2251. doi: 10.1016/j.cub.2006.09.060 [DOI] [PubMed] [Google Scholar]
- [61].Matanis T, Akhmanova A, Wulf P, et al. Bicaudal-D regulates COPI-independent Golgi–ER transport by recruiting the dynein–dynactin motor complex. Nat Cell Biol. 2002;4(12):986–992. doi: 10.1038/ncb891 [DOI] [PubMed] [Google Scholar]
- [62].Matsuto M, Kano F, Murata M. Reconstitution of the targeting of Rab6A to the Golgi apparatus in semi-intact HeLa cells: A role of BICD2 in stabilizing Rab6A on Golgi membranes and a concerted role of Rab6A/BICD2 interactions in Golgi-to-ER retrograde transport. Biochim Biophys Acta. 2015;1853(10):2592–2609. doi: 10.1016/j.bbamcr.2015.05.005 [DOI] [PubMed] [Google Scholar]
- [63].Brault JB, Bardin S, Lampic M, et al. RAB6 and dynein drive post-Golgi apical transport to prevent neuronal progenitor delamination. EMBO Rep. 2022;23(10):e54605. doi: 10.15252/embr.202254605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Short B, Preisinger C, Schaletzky J, et al. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr Biol. 2002;12(20):1792–1795. doi: 10.1016/s0960-9822(02)01221-6 [DOI] [PubMed] [Google Scholar]
- [65].Wanschers B, van de Vorstenbosch R, Wijers M, et al. Rab6 family proteins interact with the dynein light chain protein DYNLRB1. Cell Motil Cytoskeleton. 2008;65(3):183–196. doi: 10.1002/cm.20254 [DOI] [PubMed] [Google Scholar]
- [66].Cuif MH. Characterization of GAPCenA, a GTPase activating protein for Rab6, part of which associates with the centrosome. Embo J. 1999;18(7):1772–1782. doi: 10.1093/emboj/18.7.1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Burguete AS, Fenn TD, Brunger AT, et al. Rab and Arl GTPase family members cooperate in the localization of the golgin GCC185. Cell. 2008;132(2):286–298. doi: 10.1016/j.cell.2007.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rosing M, Ossendorf E, Rak A, et al. Giantin interacts with both the small GTPase Rab6 and Rab1. Exp Cell Res. 2007;313(11):2318–2325. doi: 10.1016/j.yexcr.2007.03.031 [DOI] [PubMed] [Google Scholar]
- [69].Barr FA. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr Biol. 1999;9(7):381–384. doi: 10.1016/s0960-9822(99)80167-5 [DOI] [PubMed] [Google Scholar]
- [70].Hennies HC, Kornak U, Zhang H, et al. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet. 2008;40(12):1410–1412. doi: 10.1038/ng.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Witkos TM, Chan WL, Joensuu M, et al. GORAB scaffolds COPI at the trans-Golgi for efficient enzyme recycling and correct protein glycosylation. Nat Commun. 2019;10(1):127. doi: 10.1038/s41467-018-08044-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lee PL, Ohlson MB, Pfeffer SR. Rab6 regulation of the kinesin family KIF1C motor domain contributes to Golgi tethering. Elife. 2015;4: doi: 10.7554/eLife.06029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yamada M, Kumamoto K, Mikuni S, et al. Rab6a releases LIS1 from a dynein idling complex and activates dynein for retrograde movement. Nat Commun. 2013;4(1):2033. doi: 10.1038/ncomms3033 [DOI] [PubMed] [Google Scholar]
- [74].Teber I, Nagano F, Kremerskothen J, et al. Rab6 interacts with the mint3 adaptor protein. Biol Chem. 2005;386:671–677. doi: 10.1515/bc.2005.078 [DOI] [PubMed] [Google Scholar]
- [75].Thyrock A, Stehling M, Waschbüsch D, et al. Characterizing the interaction between the Rab6 GTPase and Mint3 via flow cytometry based FRET analysis. Biochem Biophys Res Commun. 2010;396(3):679–683. doi: 10.1016/j.bbrc.2010.04.161 [DOI] [PubMed] [Google Scholar]
- [76].Hyvola N, Diao A, McKenzie E, et al. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. Embo J. 2006;25(16):3750–3761. doi: 10.1038/sj.emboj.7601274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Miserey-Lenkei S, Waharte F, Boulet A, et al. Rab6-interacting protein 1 links Rab6 and Rab11 function. Traffic. 2007;8(10):1385–1403. doi: 10.1111/j.1600-0854.2007.00612.x [DOI] [PubMed] [Google Scholar]
- [78].Recacha R, Boulet A, Jollivet F, et al. Structural basis for recruitment of Rab6-interacting protein 1 to Golgi via a RUN domain. Structure. 2009;17(1):21–30. doi: 10.1016/j.str.2008.10.014 [DOI] [PubMed] [Google Scholar]
- [79].Monier S, Jollivet F, Janoueix-Lerosey I, et al. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic. 2002;3(4):289–297. doi: 10.1034/j.1600-0854.2002.030406.x [DOI] [PubMed] [Google Scholar]
- [80].Jones SM, Crosby JR, Salamero J, et al. A cytosolic complex of p62 and rab6 associates with TGN38/41 and is involved in budding of exocytic vesicles from the trans-Golgi network. J Cell Bio. 1993;122(4):775–788. doi: 10.1083/jcb.122.4.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wassmer T, Attar N, Harterink M, et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17(1):110–122. doi: 10.1016/j.devcel.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Buser DP, Spang A. Protein sorting from endosomes to the TGN. Front Cell Dev Biol. 2023;11:1140605. doi: 10.3389/fcell.2023.1140605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Renard HF, Johannes L, Morsomme P. Increasing diversity of biological membrane fission mechanisms. Trends Cell Biol. 2018;28(4):274–286. doi: 10.1016/j.tcb.2017.12.001 [DOI] [PubMed] [Google Scholar]
- [84].Durán JM, Valderrama F, Castel S, et al. Myosin motors and not actin comets are mediators of the actin-based Golgi-to-endoplasmic reticulum protein transport. Mol Biol Cell. 2003;14(2):445–459. doi: 10.1091/mbc.e02-04-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Valderrama F, Durán JM, Babià T, et al. Actin microfilaments facilitate the retrograde transport from the Golgi complex to the endoplasmic reticulum in mammalian cells. Traffic. 2001;2(10):717–726. doi: 10.1034/j.1600-0854.2001.21006.x [DOI] [PubMed] [Google Scholar]
- [86].Tenorio MJ, Luchsinger C, Mardones GA, et al. Protein kinase a activity is necessary for fission and fusion of Golgi to endoplasmic reticulum retrograde tubules. PLoS One. 2015;10(8):e0135260. doi: 10.1371/journal.pone.0135260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jia J, Tang S, Yue X, et al. An A-kinase anchoring protein (ACBD3) coordinates traffic-induced PKA activation at the Golgi. J Biol Chem. 2023;299(5):104696. doi: 10.1016/j.jbc.2023.104696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Petrosyan A, Ali MF, Verma SK, et al. Non-muscle myosin IIA transports a Golgi glycosyltransferase to the endoplasmic reticulum by binding to its cytoplasmic tail. Int J Biochem Cell Biol. 2012;44(7):1153–1165. doi: 10.1016/j.biocel.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Barnekow A, Thyrock A, Kessler D. Chapter 5 rab proteins and their interaction partners. In: Jeon KW, editor. Int. Rev. Cell Mol. BiolVol. 274. San Diego, USA: Elsevier; 2009. pp. 235–274. doi: 10.1016/S1937-6448(08)02005-4 [DOI] [PubMed] [Google Scholar]
- [90].Seelig HP, Schranz P, Schröter H, et al. Macrogolgin—A New 376 kD Golgi complex outer membrane protein as target of antibodies in patients with rheumatic disease and HIV Infections. J Autoimmun. 1994;7(1):67–91. doi: 10.1006/jaut.1994.1006 [DOI] [PubMed] [Google Scholar]
- [91].Boucrot E, Pick A, Çamdere G, et al. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell. 2012;149(1):124–136. doi: 10.1016/j.cell.2012.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang T, Li L, Hong W. SNARE proteins in membrane trafficking. Traffic. 2017;18(12):767–775. doi: 10.1111/tra.12524 [DOI] [PubMed] [Google Scholar]
- [93].Sun Y, Shestakova A, Hunt L, et al. Rab6 regulates both ZW10/RINT-1– and conserved oligomeric Golgi Complex-dependent Golgi trafficking and homeostasis. Mol Biol Cell. 2007;18(10):4129–4142. doi: 10.1091/mbc.e07-01-0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hirose H, Arasaki K, Dohmae N, et al. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. Embo J. 2004;23(6):1267–1278. doi: 10.1038/sj.emboj.7600135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Arasaki K, Taniguchi M, Tani K, et al. RINT-1 regulates the localization and entry of ZW10 to the syntaxin 18 complex. Mol Biol Cell. 2006;17(6):2780–2788. doi: 10.1091/mbc.e05-10-0973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shomron O, Hirschberg K, Burakov A, et al. Positioning of endoplasmic reticulum exit sites around the Golgi depends on BicaudalD2 and Rab6 activity. Traffic. 2021;22(3):64–77. doi: 10.1111/tra.12774 [DOI] [PubMed] [Google Scholar]
- [97].Heffernan LF, Suckrau PM, Banerjee T, et al. An imaging-based RNA interference screen for modulators of the Rab6-mediated Golgi-to-ER pathway in mammalian cells. Front Cell Dev Biol. 2022;10:1050190. doi: 10.3389/fcell.2022.1050190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Willett R, Ungar D, Lupashin V. The Golgi puppet master: COG complex at center stage of membrane trafficking interactions. Histochem Cell Biol. 2013;140(3):271–283. doi: 10.1007/s00418-013-1117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].McCaughey J, Stevenson NL, Cross S, et al. ER-to-Golgi trafficking of procollagen in the absence of large carriers. J Cell Bio. 2019;218(3):929–948. doi: 10.1083/jcb.201806035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].McCaughey J, Stephens DJ. ER-to-Golgi Transport: A sizeable problem. Trends Cell Biol. 2019;29(12):940–953. doi: 10.1016/j.tcb.2019.08.007 [DOI] [PubMed] [Google Scholar]
- [101].Jackson ME, Simpson JC, Girod A, et al. The KDEL retrieval system is exploited by Pseudomonas exotoxin A, but not by Shiga-like toxin-1, during retrograde transport from the Golgi complex to the endoplasmic reticulum. J Cell Sci. 1999;112(4):467–475. doi: 10.1242/jcs.112.4.467 [DOI] [PubMed] [Google Scholar]
- [102].Sandvig K, Kavaliauskiene S, Skotland T. The protein toxins ricin and Shiga toxin as tools to explore cellular mechanisms of internalization and intracellular transport. Toxins (Basel). 2021;13(6):377. doi: 10.3390/toxins13060377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Luginbuehl V, Meier N, Kovar K, et al. Intracellular drug delivery: Potential usefulness of engineered Shiga toxin subunit B for targeted cancer therapy. Biotechnol Adv. 2018;36(3):613–623. doi: 10.1016/j.biotechadv.2018.02.005 [DOI] [PubMed] [Google Scholar]


