ABSTRACT
The selectivity in selective macroautophagy/autophagy pathways is achieved via selective autophagy receptors (SARs) – proteins that bind a ligand on the substrate to be degraded and an Atg8-family protein on the growing autophagic membrane, phagophore, effectively bridging them. In mammals, the most common ligand of SARs is ubiquitin, a small protein modifier that tags substrates for their preferential degradation by autophagy. Consequently, most common SARs are ubiquitin-binding SARs, such as SQSTM1/p62 (sequestosome 1). Surprisingly, there is only one SAR of this type in yeast – Cue5, which acts as the receptor for aggrephagy and proteaphagy – pathways that remove ubiquitinated protein aggregates and proteasomes, respectively. However, recent studies described ubiquitin-dependent autophagic pathways that do not require Cue5, e.g. the stationary phase lipophagy for lipid droplets or nitrogen starvation-induced mitophagy for mitochondria. What is the role of ubiquitin in these pathways? Here, we propose that ubiquitinated lipid droplets and mitochondria are recognized by alternative ubiquitin-binding SARs. Our analysis identifies proteins that could potentially fulfill this role in yeast. We think that matching of ubiquitin-dependent (but Cue5-independent) autophagic pathways with ubiquitin- and Atg8-binding proteins enlisted here might uncover novel ubiquitin-binding SARs in yeast.
Abbreviations: AIM: Atg8-family interacting motif; CUE: coupling of ubiquitin conjugation to ER degradation; ERMES: endoplasmic reticulum-mitochondria encounter structure; HECT: homologous to the E6-AP carboxyl terminus; LD: lipid droplet; SAR: selective autophagy receptor; SGD: Saccharomyces Genome Database; UBA: ubiquitin-associated; UBX: ubiquitin regulatory X; UIM: ubiquitin-interacting motif.
KEYWORDS: autophagic receptor, Cue5, selective autophagy, selective autophagy receptor, ubiquitin-binding protein, ubiquitin-binding receptor
Tagging of substrates for degradation by selective autophagy pathways can proceed either without or with ubiquitin. Ubiquitin-independent cargo recognition is more prevalent in yeast and can be exemplified by the first autophagic receptor that was identified, Atg19 (autophagy related 19) [1,2]. This protein binds precursor Ape1 (aminopeptidase 1) in the cytoplasm and ensures its selective delivery to the vacuole via the cytoplasm-to-vacuole targeting pathway or via (otherwise) nonselective autophagy. In contrast, the ubiquitin-dependent recognition of substrates is more widespread in more complex eukaryotes and can be represented by the first identified mammalian autophagic receptor, SQSTM1/p62 (sequestosome 1) [3,4]. This receptor binds a protein modifier, ubiquitin, and promotes aggregation of ubiquitinated proteins and their targeting to the lysosome via aggrephagy, the selective autophagy of protein aggregates. Because ubiquitin-binding SQSTM1-like receptors can recognize a variety of ubiquitinated proteins at a surface of different structures, their role in selective autophagy is much broader than that of Atg19-like receptors and involves simultaneous functioning in several autophagic pathways, including (since recently) lipophagy, the selective autophagy of lipid droplets (LDs) [5,6]. However, the two types of receptors share a common step – bridging of a non-ubiquitinated or ubiquitinated substrate with an Atg8-family protein that lines the membrane of the phagophore, the autophagic transport vesicle (autophagosome) precursor. For example, Atg19 bridges precursor Ape1 with Atg8 in yeast [7,8] and SQSTM1 brings together ubiquitinated protein aggregates and MAP1LC3B/LC3B (microtubule associated protein 1 light chain 3 beta) in mammalian cells [3,4].
At present, Cue5 is the only known ubiquitin-binding autophagic receptor in yeast. It drives two autophagic pathways, aggrephagy and proteaphagy (selective autophagy of proteasomes) (Figure 1) [9,10]. In aggrephagy, it satisfies all the requirements of a selective autophagy receptor (SAR) [5]. Specifically, the Saccharomyces cerevisiae Cue5: 1) binds ubiquitinated proteins via its ubiquitin-binding “coupling of ubiquitin conjugation to ER degradation” (CUE) domain, 2) bridges them with the membrane-bound Atg8 through the Atg8-family interacting motif (AIM), 3) is co-degraded with ubiquitinated proteins in the vacuole, and 4) is required for their vacuolar degradation under nitrogen-starvation conditions [9]. Moreover, because of a low affinity of the CUE domain to ubiquitin and its ability to oligomerize, Cue5 can target only the ubiquitinated protein aggregates that, unlike ubiquitinated soluble proteins, are rich in ubiquitin for Cue5 oligomers to bind with high avidity [11]. By its oligomerization, Cue5 resembles mammalian SQSTM1-like receptors even though they do not have this affinity problem with ubiquitin and oligomerize rather to cluster ubiquitinated proteins into protein aggregates [5]. In proteaphagy, Cue5 satisfies requirements of a SAR for ubiquitinated 26S proteasomes (note that proteasomes are ubiquitinated after their inactivation with MG132; they are not ubiquitinated and do not require Cue5 for their autophagic degradation under nitrogen-starvation conditions) [10]. Precisely, the S. cerevisiae Cue5: 1) binds ubiquitinated proteasomes, 2) bridges them with Atg8 using its CUE and AIM domains, which are both essential for proteaphagy, and 3) is required for the vacuolar degradation of ubiquitinated proteasomes. Importantly, inactivated 26S proteasomes must first be aggregated by Hsp42 chaperone into cytoplasmic aggresomes before they can be degraded by the Cue5-mediated process [10]. This makes the Cue5-dependent aggrephagy and proteaphagy very similar, if not the same pathway. It will be interesting to see if MG132 also induces degradation of aggrephagy substrates.
Figure 1.
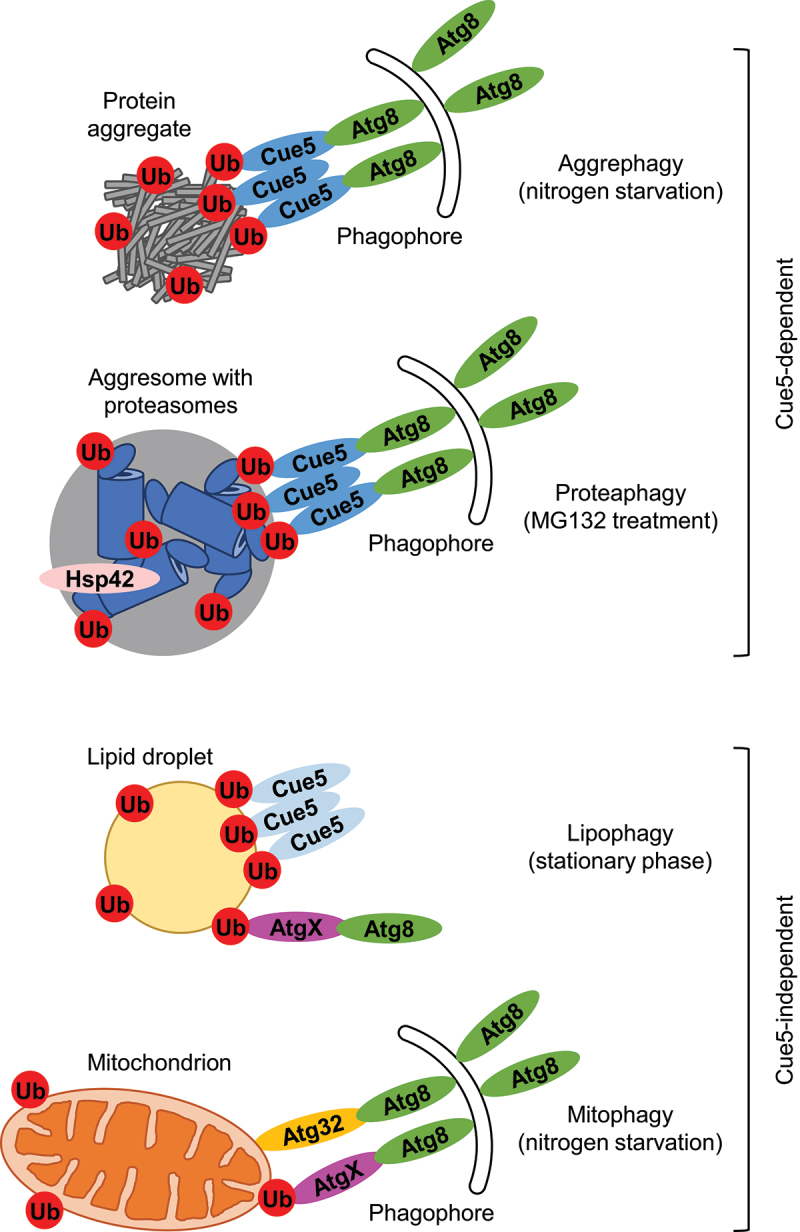
Ubiquitin-dependent selective autophagy pathways in yeast. Cue5 is the only known ubiquitin-binding SAR in yeast that functions in aggrephagy and proteaphagy. However, Cue5-independent pathways of lipophagy and mitophagy suggest the existence of other ubiquitin-binding SARs (depicted as AtgX). For potential identities of AtgX, see Table 1.
Recently, Cue5 was also probed for the involvement in lipophagy [24,25]. Similar to the Cue5-dependent aggrephagy and proteaphagy (but unlike Cue5-independent pathways, including the nitrogen starvation-induced proteaphagy) [9,10], lipophagy does not require the autophagic scaffold protein Atg11 [26–29]. The similarity does not end there, as the Komagataella phaffii Cue5 binds LDs via its CUE domain and is co-degraded with them in the vacuole (note that this happens in the stationary phase of growth, but not under nitrogen-starvation conditions) [25]. However, both K. phaffii and S. cerevisiae Cue5 proteins are dispensable for stationary phase lipophagy [24,25]. This means that in the stationary phase, Cue5 is a co-substrate (and not a SAR) for lipophagy (Figure 1). Because K. phaffii Prl1 (positive regulator of lipophagy 1) is essential for the recruitment of Cue5 to LDs (most likely via LD ubiquitination) and for stationary phase lipophagy, we hypothesized that another ubiquitin-binding protein (AtgX) could be a SAR for stationary phase lipophagy and, as such, would act as the lipophagy effector of Prl1 [25,29]. Here, we make a broader case for the existence and possible identities of such alternative ubiquitin-binding SARs in yeast.
Another argument for the presence of such receptors in yeast came from studies of mitophagy in S. cerevisiae [20,30]. During mitophagy induced by the treatment of cells with rapamycin, the Ubp3-Bre5 deubiquitination complex is recruited from the cytosol to mitochondria where it inhibits mitophagy suggesting that ubiquitination of mitochondria plays an important positive role in their degradation in yeast [30], and not only in mammals [5]. Interestingly, the subsequent study found that ubiquitination of the two mitochondrial outer membrane proteins, Mdm12 and Mdm34, which are components of the endoplasmic reticulum-mitochondria encounter structure (ERMES), is required for efficient mitophagy under a variety of conditions, including rapamycin treatment, stationary phase growth and nitrogen starvation [20]. However, despite a widespread involvement of Mdm12 and Mdm34 ubiquitination in mitophagy, the ubiquitin-binding Cue5 is not a part of the mechanism, at least under nitrogen-starvation conditions [9], indicating that another ubiquitin-binding SAR must fulfill the role of an additional receptor (the main one is a well-characterized Atg19-like receptor, Atg32 [31,32]) (Figure 1).
Because at least two autophagic pathways (lipophagy and mitophagy) are ubiquitin dependent but Cue5 independent, we looked for proteins that, similar to Cue5, could bind both ubiquitin and Atg8. For this, we compared physical interactomes of ubiquitin (179 proteins in Saccharomyces Genome Database [SGD]) and Atg8 (143 proteins in SGD and 92 proteins in reference [22]), and found 16 overlaps, including Cue5 (Table 1). The other 15 proteins are Arc40, Ccr4, Cdc48, Dhh1, Dsk2, Ede1, Ent1, Ent2, Nab2, Nup84, Rsp5, Shp1, Sla1, Ubx5 and Ydj1. Similar to Cue5, many of them are capable of self-interaction. Unlike Cue5, three of them (Ccr4, Dhh1 and Ede1) can bind Atg11. Interestingly, Ccr4 and Dhh1 affect autophagy in two directions: decrease it under growth conditions and increase it during nitrogen starvation by down- and upregulating the expression of different ATG genes, respectively [12,16,17]. However, both Ccr4 and Dhh1, as well as another candidate, Nab2, are very promiscuous proteins with thousands of binding partners (Table 1). Therefore, they are unlikely to serve highly specific roles of SARs.
Table 1.
Proteins that physically interact with both ubiquitin and Atg8 in yeast.
| Protein | Brief description | Ubiquitin-binding domain | Physical interactions |
Autophagy regulator | ||
|---|---|---|---|---|---|---|
| Atg11 | Self | Total | ||||
| Arc40 | Subunit of the Arp2/3 complex | - | - | - | 51 | - |
| Ccr4 | Component of the Ccr4-Not complex | - | Yes | Yes | 3,035 | Yes [12] |
| Cdc48 | AAA ATPase with protein-unfoldase activity | - | - | Yes | 200 | Yes [13–15] |
| Cue5 | Ubiquitin-binding protein | CUE | - | Yes | 29 | Yes [9,10] |
| Dhh1 | DEAD-box helicase/mRNA decapping activator | - | Yes | Yes | 3,608 | Yes [16,17] |
| Dsk2 | Ubiquitin-like polyubiquitin-binding protein | UBA | - | Yes | 43 | Yes [18] |
| Ede1 | Endocytic adaptor | UBA | Yes [19] | Yes | 62 | Yes [19] |
| Ent1 | Epsin-like protein | UIM (x2) | - | - | 20 | - |
| Ent2 | Epsin-like protein | UIM (x2) | - | - | 48 | - |
| Nab2 | Nuclear polyadenylated RNA-binding protein | - | - | Yes | 2,641 | - |
| Nup84 | Subunit of the Nup84 subcomplex of NPC | - | - | Yes | 74 | - |
| Rsp5 | NEDD4 family E3 ubiquitin ligase | HECT | - | Yes | 354 | Yes [9,20,21] |
| Shp1 | UBX domain-containing protein, binds Cdc48 | UBA | - | - | 65 | Yes [14] |
| Sla1 | Cytoskeletal protein binding protein | - | - | Yes | 189 | - |
| Ubx5 | UBX domain-containing protein, binds Cdc48 | UBA, UIM [22] | - | - | 26 | Yes [22] |
| Ydj1 | Type I Hsp40 co-chaperone | - | - | Yes | 133 | Yes [23] |
This analysis was performed based on the data in SGD (accessed on 11/22/2022) and reference [22], unless stated otherwise.
Importantly, 7 proteins, in addition to Cue5, have at least one known ubiquitin-binding domain: (1) Dsk2, Ede1, Shp1 and Ubx5 possess a ubiquitin-associated (UBA) domain, (2) Ent1 and Ent2 each carry two ubiquitin-interacting motifs (UIMs), and (3) Rsp5 has a ubiquitin-transfering “homologous to the E6-AP carboxyl terminus” (HECT) domain (Table 1). Interestingly, all UBA and HECT domain-containing proteins have been already implicated in autophagy. For example, Dsk2 (as well as another candidate, Ydj1) stimulates the formation of the mutated HTT (huntingtin) inclusion body, which is then efficiently degraded by autophagy in yeast [18,23]. Similar to this artificial scenario, Dsk2 and Ydj1 can also promote assembly and autophagic clearance of endogenous substrates. In addition, the Atg11-binding protein Ede1, through its oligomerization and condensation, achieves the formation of endocytic protein deposits at the plasma membrane, and then serves as a SAR for their autophagic degradation [19]. Ede1’s UBA domain seems to be not involved in this process, but we cannot exclude the possibility that it might bring Ede1 to some other substrates for a similar phase separation into condensates that will be degraded by selective autophagy. Next, since another candidate, Cdc48, and its UBA domain-containing cofactor, Shp1, promote both selective and nonselective autophagy [13–15], their functioning as SARs is highly unlikely (all yeast SARs are dispensable for nonselective autophagy). Therefore, the Cdc48-Shp1 pair must play some other, more generic role in autophagic pathways that is not related to the selection of substrates. Finally, Ubx5 is another UBA domain-containing cofactor of Cdc48 that targets dysfunctional Cdc48 for autophagic degradation as a SAR, but is not involved in the autophagic degradation of functional Cdc48, piecemeal microautophagy of the nucleus and nonselective autophagy under nitrogen-starvation conditions [14,22]. Interestingly, the UBA domain of Ubx5 is not required for the selective autophagy of dysfunctional Cdc48, which is bridged with Atg8 via the ubiquitin regulatory X (UBX) and UIM domains of Ubx5, respectively [22]. However, it might be required for selective autophagy of ubiquitinated substrates yet to be identified.
Last, the HECT domain-containing E3 ubiquitin ligase, Rsp5, regulates several autophagic pathways, including Cue5-dependent aggrephagy, proteaphagy, and Cue5-independent mitophagy, but not nonselective autophagy [9,20,21]. In aggrephagy, it is required for ubiquitination of proteins, their recognition by Cue5 and their vacuolar degradation. Interestingly, Rsp5 also ubiquitinates the Cue5 receptor itself, and is co-degraded with it and its aggrephagy substrates [9]. However, because autophagic degradation of Rsp5 heavily relies on Cue5 [9], it is unlikely to act as an alternative aggrephagy SAR. In proteaphagy, Rsp5 ubiquitinates inactivated 26S proteasomes in cytoplasmic aggresomes created by Hsp42 and promotes their vacuolar clearance [21]. Similarly, Rsp5 also ubiquitinates ERMES components, Mdm12 and Mdm34, and supports efficient vacuolar degradation of mitochondria via mitophagy [20]. In the latter two cases, the role of Rsp5 was studied only in the frame of ubiquitination, but because Rsp5 binds hundreds of proteins in addition to ubiquitin and Atg8 (Table 1), it is unlikely to function as a SAR in these or other pathways and bring enough selectivity to the process.
In summary, we think that the topic of ubiquitin-binding autophagic receptors is understudied in yeast. While one such receptor (Cue5) has been identified and implicated in two autophagic pathways (aggrephagy and proteaphagy), our analysis suggests the existence of ubiquitin-dependent, but Cue5-independent, pathways (lipophagy and mitophagy) that could engage other ubiquitin- and Atg8-binding proteins as ubiquitin-binding SARs. Some of these proteins, such as Ede1 and Ubx5, are already known as Atg19-like SARs for some substrates. Therefore, in our opinion, they are poised to function also as ubiquitin-binding SARs for other substrates. It will be interesting to see further developments in this exciting area of research.
Funding Statement
This work was supported by the NIH grant, GM119571, to Taras Y. Nazarko.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Leber R, Silles E, Sandoval IV, et al. Yol082p, a novel CVT protein involved in the selective targeting of aminopeptidase I to the yeast vacuole. J Biol Chem. 2001 Aug 3;276(31):29210–29217. DOI: 10.1074/jbc.M101438200 [DOI] [PubMed] [Google Scholar]
- [2].Scott SV, Guan J, Hutchins MU, et al. Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway. Mol Cell. 2001. Jun;7(6):1131–1141. DOI: 10.1016/S1097-2765(01)00263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bjorkoy G, Lamark T, Brech A, et al. P62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005 Nov 21;171(4):603–614. DOI: 10.1083/jcb.200507002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pankiv S, Clausen TH, Lamark T, et al. P62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007 Aug 17;282(33):24131–24145. DOI: 10.1074/jbc.M702824200 [DOI] [PubMed] [Google Scholar]
- [5].Kirkin V , Rogov VV.. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol Cell. 2019 Oct 17;76(2):268–285. DOI: 10.1016/j.molcel.2019.09.005 [DOI] [PubMed] [Google Scholar]
- [6].Shroff A, Nazarko TY. SQSTM1, lipid droplets and current state of their lipophagy affairs. Autophagy. 2023. Feb;19(2):720–723.DOI: 10.1080/15548627.2022.2094606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim J, Huang WP, Stromhaug PE, et al. Convergence of multiple autophagy and cytoplasm to vacuole targeting components to a perivacuolar membrane compartment prior to de novo vesicle formation. J Biol Chem. 2002 Jan 4;277(1):763–773. DOI: 10.1074/jbc.M109134200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shintani T, Huang WP, Stromhaug PE, et al. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002. Dec;3(6):825–837. DOI: 10.1016/S1534-5807(02)00373-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu K, Psakhye I, Jentsch S. Autophagic clearance of polyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell. 2014 Jul 31;158(3):549–563. DOI: 10.1016/j.cell.2014.05.048 [DOI] [PubMed] [Google Scholar]
- [10].Marshall RS, McLoughlin F, Vierstra RD. Autophagic turnover of inactive 26S proteasomes in yeast is directed by the ubiquitin receptor Cue5 and the Hsp42 Chaperone. Cell Rep. 2016 Aug 9;16(6):1717–1732. DOI: 10.1016/j.celrep.2016.07.015 [DOI] [PubMed] [Google Scholar]
- [11].Lu K, den Brave F, Jentsch S. Receptor oligomerization guides pathway choice between proteasomal and autophagic degradation. Nat Cell Biol. 2017. Jun;19(6):732–739. DOI: 10.1038/ncb3531 [DOI] [PubMed] [Google Scholar]
- [12].Yin Z, Zhang Z, Lei Y, et al. Bidirectional roles of the Ccr4-Not complex in regulating autophagy before and after nitrogen starvation. Autophagy. 2023. Feb;19(2):415–425. DOI: 10.1080/15548627.2022.2036476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ossareh-Nazari B, Bonizec M, Cohen M, et al. Cdc48 and Ufd3, new partners of the ubiquitin protease Ubp3, are required for ribophagy. EMBO Rep. 2010. Jul;11(7):548–554. DOI: 10.1038/embor.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krick R, Bremer S, Welter E, et al. Cdc48/P97 and Shp1/p47 regulate autophagosome biogenesis in concert with ubiquitin-like Atg8. J Cell Biol. 2010 Sep 20;190(6):965–973. DOI: 10.1083/jcb.201002075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Buchan JR, Kolaitis RM, Taylor JP, et al. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell. 2013 Jun 20;153(7):1461–1474. DOI: 10.1016/j.cell.2013.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu G, McQuiston T, Bernard A, et al. A conserved mechanism of TOR-dependent RCK-mediated mRNA degradation regulates autophagy. Nat Cell Biol. 2015. Jul;17(7):930–942. DOI: 10.1038/ncb3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu X, Yao Z, Jin M, et al. Dhh1 promotes autophagy-related protein translation during nitrogen starvation. PLoS Biol. 2019. Apr;17(4):e3000219. DOI: 10.1371/journal.pbio.3000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chuang KH, Liang F, Higgins R, et al. Ubiquilin/Dsk2 promotes inclusion body formation and vacuole (lysosome)-mediated disposal of mutated huntingtin. Mol Biol Cell. 2016 Jul 1;27(13):2025–2036. DOI: 10.1091/mbc.E16-01-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wilfling F, Lee CW, Erdmann PS, et al. A selective autophagy pathway for phase-separated endocytic protein deposits. Mol Cell. 2020 Dec 3;80(5):764–778 e7. DOI: 10.1016/j.molcel.2020.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Belgareh-Touze N, Cavellini L, Cohen MM. Ubiquitination of ERMES components by the E3 ligase Rsp5 is involved in mitophagy. Autophagy. 2017 Jan 2;13(1):114–132. DOI: 10.1080/15548627.2016.1252889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marshall RS, Vierstra RD. A trio of ubiquitin ligases sequentially drives ubiquitylation and autophagic degradation of dysfunctional yeast proteasomes. Cell Rep. 2022 Mar 15;38(11):110535. DOI: 10.1016/j.celrep.2022.110535 [DOI] [PubMed] [Google Scholar]
- [22].Marshall RS, Hua Z, Mali S, et al. ATG8-binding UIM proteins define a new class of autophagy adaptors and receptors. Cell. 2019 Apr 18;177(3):766–781 e24. DOI: 10.1016/j.cell.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Higgins R, Kabbaj MH, Hatcher A, et al. The absence of specific yeast heat-shock proteins leads to abnormal aggregation and compromised autophagic clearance of mutant Huntingtin proteins. PLoS ONE. 2018;13(1):e0191490. DOI: 10.1371/journal.pone.0191490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Robichaud S, Fairman G, Vijithakumar V, et al. Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells. Autophagy. 2021. Nov;17(11):3671–3689. DOI: 10.1080/15548627.2021.1886839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kumar R, Shroff A, Nazarko TY. Komagataella phaffii Cue5 piggybacks on lipid droplets for its vacuolar degradation during stationary phase lipophagy. Cells. 2022 Jan 10;11(2):215. DOI: 10.3390/cells11020215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].van Zutphen T, Todde V, de Boer R, et al. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2014. Jan;25(2):290–301. DOI: 10.1091/mbc.e13-08-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang CW, Miao YH, Chang YS. A sterol-enriched vacuolar microdomain mediates stationary phase lipophagy in budding yeast. J Cell Biol. 2014 Aug 4;206(3):357–366. DOI: 10.1083/jcb.201404115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Seo AY, Lau PW, Feliciano D, et al. AMPK and vacuole-associated Atg14p orchestrate μ-lipophagy for energy production and long-term survival under glucose starvation. Elife. 2017 Apr 10;6:e21690. DOI: 10.7554/eLife.21690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kumar R, Rahman MA, Nazarko TY. Nitrogen starvation and stationary phase lipophagy have distinct molecular mechanisms. Int J Mol Sci. 2020 Nov 29;21(23):9094. DOI: 10.3390/ijms21239094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Muller M, Kotter P, Behrendt C, et al. Synthetic quantitative array technology identifies the Ubp3-Bre5 deubiquitinase complex as a negative regulator of mitophagy. Cell Rep. 2015 Feb 24;10(7):1215–1225. DOI: 10.1016/j.celrep.2015.01.044 [DOI] [PubMed] [Google Scholar]
- [31].Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell. 2009. Jul;17(1):87–97. DOI: 10.1016/j.devcel.2009.06.013 [DOI] [PubMed] [Google Scholar]
- [32].Kanki T, Wang K, Cao Y, et al. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell. 2009. Jul;17(1):98–109. DOI: 10.1016/j.devcel.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]


