Abstract
Background
Splenic rupture because of metastasis from a distant organ is extremely rare.
Case Presentation
An 80‐year‐old man presented with left flank pain. A computed tomography (CT) demonstrated a poorly enhanced enlarged spleen with bulky thrombus in the splenic vein without extravasations. A CT on the following day showed increased intraperitoneal hemorrhage; therefore, an emergency laparotomy was performed. The spleen was enlarged and ruptured with lacerations on its surface. Macroscopic examination showed congestion with a thrombus in the splenic vein around the hilum. Pathology revealed signet‐ring cell carcinoma. On the third postoperative day, a massive cerebral infarction in the left middle cerebral artery was revealed. Endoscopic examination demonstrated normal gastric mucosa except for some erosions, for which biopsies were performed, and two of five specimens encompassed signet‐ring cell carcinoma in the lamina propria.
Conclusion
Occult cancer could result in a drastic manifestation of its metastasis accompanying systemic thrombotic events.
Keywords: brain infarction, occult cancer, signet‐ring cell carcinoma, splenic rupture, splenic vein thrombosis
Splenic rupture because of metastasis from a distant organ is rare, and it becomes much rarer when the primary focus is occult cancer. Meanwhile, it is known that cancer might lead to systemic thrombotic events. Here, we report an 80‐year‐old male patient who developed a spontaneous splenic rupture because of occult signet‐ring cell carcinoma of the stomach and simultaneously manifested systemic thrombotic events including a coincidental bulky splenic vein thrombosis and subsequent major brain infarction.
INTRODUCTION
Spontaneous splenic rupture is a far more uncommon entity than traumatic spleen injury. Infectious diseases, hematologic disorders, and sarcoidosis are the possible causes of splenic rupture. 1 Furthermore, metastasis to the spleen from distant organs is not very frequent. 2 Meanwhile, patients with cancer are generally at high risk for thrombosis because of a predisposition to hypercoagulability. 3 In the medical field, when two or three rare events coincide, the scarcity and significance of the case become much more noteworthy and would attract scientific interest. Here, we present a case of spontaneous splenic rupture associated with occult signet‐ring cell carcinoma diagnosed by biopsies from the stomach in upper gastrointestinal endoscopy, in which gastric mucosa seemed normal except for some erosion‐like lesions and bulky thrombus in the splenic vein combined with postoperative significant brain infarction because of embolus in the cerebral artery. 3
CASE PRESENTATION
An 80‐year‐old man who had a mild loss of appetite for 1 month presented with vomiting and left flank pain. He denied any traumatic events. He was conscious and had a fever of 100.0 F. His abdomen was mildly distended and tender on his left flank. Blood test results showed a white blood cell count of 16,700/μL, a red blood cell count of 378 × 104/μL, a hemoglobin concentration of 11.2 g/dL, platelets were 15.6 × 104 /μL, serum fibrinogen was 449 mg/dL, D‐dimer was 20.3 μg/mL, C reactive protein was 5.68 mg/dL, ferritin was 1058 ng/mL, and soluble interleukin 2 receptor was 1040 U/L. A computed tomography (CT) demonstrated a poorly enhanced and enlarged spleen with perisplenic bloody ascites accompanied by bulky thrombosis in the splenic vein near the hilum of the spleen. Interventional radiology (IVR) of the celiac trunk showed attenuated staining of the spleen with no extravasations (Figure 1A,B). A magnetic resonance imaging (MRI) of the abdomen provided no significant additional information. On the next day of his admission, the patient showed tachycardia, and the second CT showed increased intraabdominal hemorrhage. He underwent an emergency laparotomy for suspected splenic rupture due to an unknown cause. Intraoperative findings revealed a large amount of bloody ascites and clots around the spleen. The spleen was enlarged with mild adhesion to the surrounding tissues, and the capsule of the spleen was torn with bloody exudation. Because the upper tip of the spleen tightly adhered to the serosa of the anterior gastric wall, the spleen was extracted with a part of the gastric wall, and its defect was closed by absorbable braided sutures. The bulky thrombosis in the CT was not detected by palpation or inspection. The macroscopic findings demonstrated that the size of the spleen was 140 × 75 × 50 mm, and its surface had a few lacerations, seemingly caused by a burst because of increased inner pressure. The main splenic vein and its branches around the hilum were remarkably dilated and congested with thrombosis. The pathological findings demonstrated degenerated cells containing Alcian blue‐positive mucus. Most of the cells lacked nuclei, but some had eccentrically located nuclei. Immunostaining revealed CAM5.2(+), AE1/3(+), MUC‐2(+), MUC‐5 AC(+), and MUC‐6(−). Atypical cells suggested metastatic signet‐ring cell carcinoma with remarkable degeneration, presumably because of congestion or infarction. The thrombus in the splenic vein was composed of fibrin, blood clots, and fibrous connective tissues with no carcinomatous cells (Figure 2). On the third postoperative day, the patient manifested sudden loss of consciousness and underwent a CT and an MRI of the brain. These modalities revealed extensive infarction of the left cerebrum and emergency IVR removed the embolus in the left mid‐cerebral artery (Figure 1C,D). A cardiac ultrasound demonstrated no endocarditis or intra‐atrial thrombus. The consciousness gradually improved and almost fully recovered except for mild residual paralysis of the right upper extremity. To pursue the primary focus of metastatic signet‐ring cell carcinoma, upper gastrointestinal endoscopy demonstrated that no tumorous lesions were macroscopically seen except for some small erosive lesions in the stomach. Biopsy was performed for the erosion‐like lesions, and its pathology showed positive for signet‐ring cell carcinoma in two specimens from the corpus in the large curvature of the stomach out of five specimens. Carcinoma cells were observed mainly in the lamina propria (Figure 3). The patient was diagnosed with occult signet‐ring cell carcinoma with a thrombus in the splenic vein, possibly contributing to splenic rupture and thrombus in the mid‐cerebral artery, leading to massive brain infarction.
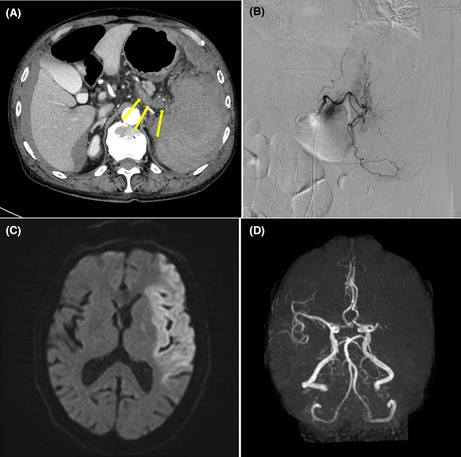
FIGURE 1.
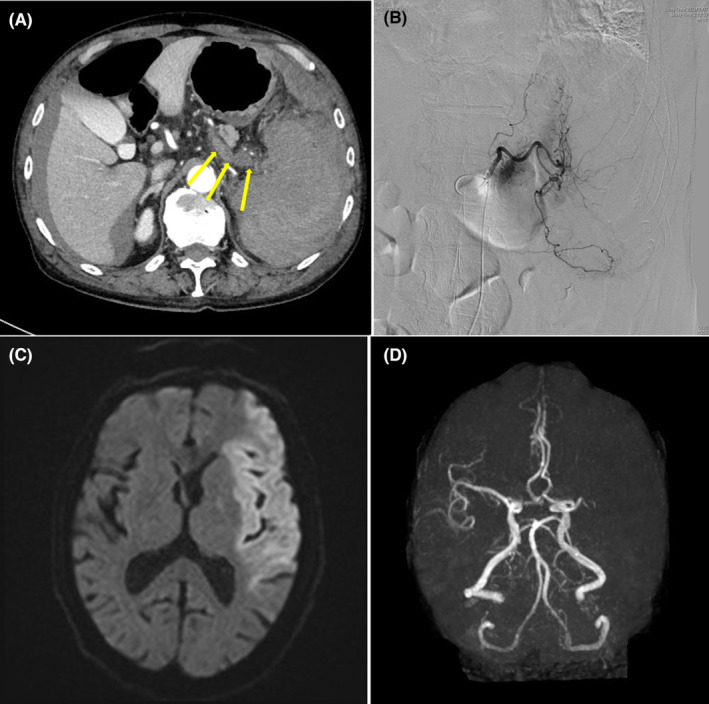
(A) An axial section of the computed tomography of the abdomen shows a poorly unenhanced and enlarged spleen with its destructed structure, accompanied by bulky thrombosis in the splenic vein near the hilum of the spleen (arrows), in addition to perihepatic and perisplenic bloody ascites. (B) Interventional radiology of the celiac trunk shows diffusely attenuated staining of the spleen with no extravasations. (C) A diffusion‐weighted magnetic resonance imaging of the brain demonstrates an extensive high‐intensity area in the left middle cerebral artery (MCA) region, suggesting acute brain infarction. (D) A magnetic resonance angiography depicts disruption of left MCA at the M1 level and no distal blood flow. Emergency interventional radiology has successfully been performed to remove the thrombus from the area.
FIGURE 2.
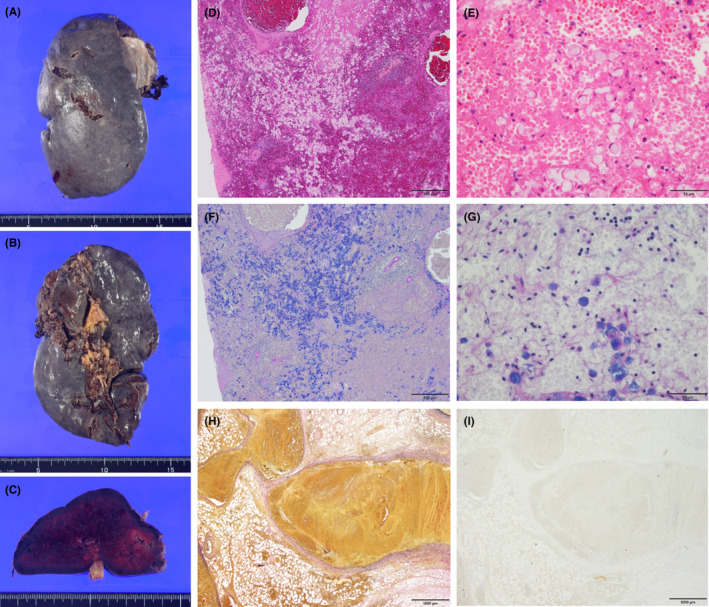
(A) The surface, (B) the hilar side, and (C) a cut surface of the resected spleen are shown. The size of the spleen is 140 × 75 × 50 mm, and its surface has a few lacerations. The main splenic vein and its branches around the hilum are remarkably dilated and congested with thrombosis. (D,E) The hematoxylin and eosin‐stained specimen from the spleen in ×4 (D) and ×40 (E) magnification. (F,G) The Alcian blue‐PAS stained specimen from the spleen in ×4 (F) and ×40 (G) magnification. Most of the cells are devoid of nuclei, but some of them have eccentrically located nuclei. Atypical cells suggest metastatic signet‐ring cell carcinoma with remarkable degeneration, presumably because of congestion or infarction. (H,I) The elastin van Gieson stained (H) and AE1/AE3 stained (I) specimen of the thrombus in the splenic vein in ×2 magnification, which is composed of fibrin, blood clots, and fibrous connective tissues with no carcinomatous cells.
FIGURE 3.
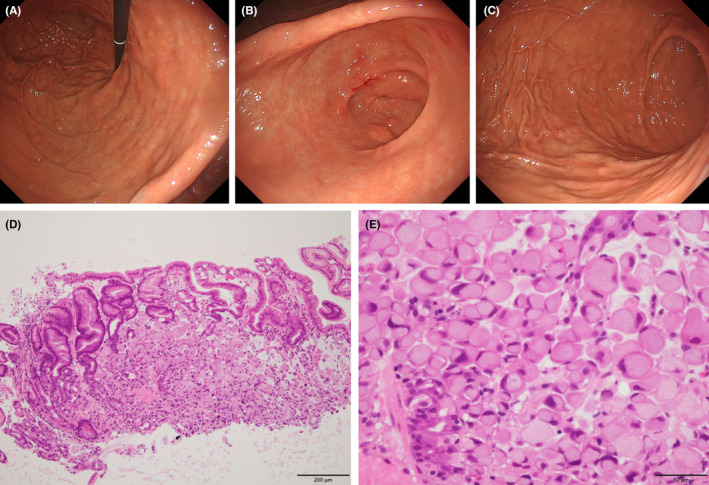
Endoscopic examination reveals normal gastric mucosa in (A) the lesser curvature of the gastric body, (B) the antrum, and (C) the greater curvature of the gastric body except for some elevated erosive lesions. A biopsy was performed for the erosive lesions of the gastric mucosa. (D,E) Two of five specimens showed signet‐ring carcinoma cells in the mucosa of the stomach, mainly in the lamina propria. (×10 [D] and ×40 [E] magnifications with hematoxylin and eosin‐stained).
DISCUSSION
The scenario of this case is unique in that splenic metastasis of non‐hematologic malignancy is manifested in the form of splenic rupture and in that the source of origin is occult gastric carcinoma, which had been undiagnosed. In addition, there are a couple of controversial points. First, what was the cause of the splenic rupture, thrombosis, or metastasis? Second, what type of metastasis occurred from occult gastric cancer to the spleen, hematogenous/lymphatic metastasis, or direct invasion?
The spleen is an organ subject to hematologic disorders, vascular tumors, and infection. 4 Meanwhile, splenic metastasis of non‐hematologic tumors is rare; moreover, spontaneous splenic rupture because of metastasis is much more uncommon, Berge et al. 2 reported that the frequency of cases with splenic metastasis was 7.1% in uniformly post‐mortem series. There have been several case reports of non‐traumatic splenic rupture highly likely caused by distant metastasis from other organs. Regarding the primary source of splenic metastasis, the most common sources are the breast, lung, colorectum, ovary, and melanoma. 5 In general, splenic metastasis results from the growth of early blood‐borne metastasis after a period of clinical latency, several years after diagnosing the primary tumor. 4 In our case, splenic rupture with massive splenic metastasis of signet‐ring cell carcinoma preceded recognition of occult gastric cancer. Additionally, based on all the imaging and clinical findings, no organs other than the stomach could be the primary site based on imaging and clinical findings.
In 2002, Yamanouchi et al. 6 reported a case of solitary splenic metastasis from gastric cancer, in which stage IIIA gastric cancer was treated by a distal gastrectomy, and splenic metastasis was identified by elevated serum carcinoembryonic antigen level and subsequent imaging studies 3 years after the primary resection. There have been some reports of cases that metastasized from early gastric cancer to the spleen, where splenic metastasis was detected by follow‐up CT several years after the surgery. As in such scenarios, detection or elicitation of splenic metastasis is, in common, preceded by diagnosis and treatment of the original organ. Of course, there are gastric cancer cases with splenic metastasis simultaneously diagnosed at the discovery of the original organ. However, all seven cases in the report of Zhu et al. 7 in 2013 are advanced gastric cancer with lymph node metastasis. In the meantime, there have been a few cases of spontaneous splenic rupture accompanied by metastasis from gastric cancer. One case is about a 54‐year‐old who underwent distal gastrectomy for stage IIIB advanced gastric cancer and presented with left upper quadrant pain 17 months after the first diagnosis and surgery. Emergency laparotomy and subsequent pathological analysis revealed that signet‐ring carcinoma massively infiltrated the spleen, and malignant cells were identified in the splenic veins. 8 As to lymph node invasion from metastasis of the signet‐ring cell carcinoma in this case, especially number 10 lymph nodes, the specimen did not contain enough lymph node samples to evaluate the status of lymph node metastasis because the operation was emergency and predominantly intended to control the bleeding from the ruptured spleen.
Cases with distant metastasis from occult gastric cancer are extremely rare. There is only a report from Lee et al. 9 in 2016 regarding a Krukenberg tumor, a rare metastatic ovarian tumor originating from gastrointestinal carcinoma, initially diagnosed by a huge adnexal mass. Upper gastrointestinal endoscopy and colonoscopy were performed to detect the origin, and they revealed normal findings, including gastric mucosa. Random biopsies from the antrum and the body of the stomach eventually demonstrated signet‐ring cell carcinoma. Our case is the second one in which random diagnostic biopsies from the stomach followed the manifestation of distant metastasis. However, spontaneous splenic rupture due to metastasis of occult gastric cancer is the first reported case.
The mechanism of splenic rupture, in this case, should be controversial. Possible mechanisms are increased volume of its parenchyma because of massive invasion of metastatic signet‐ring cells, congestion, and the elevated inner pressure of its parenchyma because of bulky splenic vein thrombosis. The absence of apparent infarction in pathological examination makes thromboembolism with subsequent infarction and rupture unlikely. However, a visible thrombus in the main splenic vein suggests acute thrombosis may have contributed to a sudden increase in intrasplenic pressure, causing a rupture in a weak spleen. 1
When the conservative treatment is chosen for the spontaneous splenic rupture because of metastasis, the mortality rate is reported to be as high as 100%. 1 , 8 The short‐term prognosis of splenic rupture relates to the application of prompt surgical intervention, although the prognosis of the primary tumor determines life expectancy. 8
Both two obvious thromboses in the main splenic vein and the mid‐cerebral artery may be related to hypercoagulability peculiar to cancer patients. Trousseau's syndrome is known as a thrombotic event in those patients, which is related to chronic disseminated intravascular coagulopathy causing microangiopathy, verrucous endocarditis, and arterial emboli. 3 This syndrome is currently ascribed to various clinical situations ranging from these classic descriptions to any coagulopathy that occurs in any malignancy, including arterial and venous thrombosis. 10 To define this case as Trousseau's syndrome, the possibility of tumor embolism must be excluded. In this case, the pathological examination revealed that the splenic embolus was composed of fibrin, blood clots, and fibrous connective tissues, not containing cancer cells. However, the embolus in the cerebral artery removed by IVR was not sent to pathological examination; therefore, there are no clues that exclude the possibility that the embolus was composed of a tumor cell cluster, although the images of MRI and angiography are typical of thrombosis in the cerebral artery. It could not be clarified that two almost simultaneous thrombotic events occurred because of occult gastric cancer with splenic metastasis, although such an inference can be utterly appropriate.
CONCLUSION
Occult cancer could lead to a drastic manifestation of its metastasis, such as splenic rupture and systemic thrombotic events. Emergency physicians should also be versed in oncological pathophysiology.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol: Not applicable.
Informed consent: Informed consent for publication was obtained from the patient.
Registry and registration no. of the study/trial: Not applicable.
Animal studies: Not applicable.
ACKNOWLEDGMENTS
We thank Jean Dominique Morancy, MD, MPH, MBA, for proofreading the manuscript.
Asada S, Mori S, Takemoto A, Tamura K, Ito G, Otomo Y. Spontaneous splenic rupture due to occult signet‐ring cell gastric cancer accompanied by a bulky splenic vein thrombosis and postoperative brain infarction: A case report. Acute Med Surg. 2023;10:e879. 10.1002/ams2.879
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Goldstone SE, Gold MS. Spontaneous splenic rupture. In: Dutcher JP, Wiernik PH, editors. Handbook of hematologic and oncologic emergencies. Boston, MA: Springer US; 1987. p. 221–227. [Google Scholar]
- 2. Berge T. Splenic metastases. Frequencies and patterns. Acta Pathol Microbiol Scand A. 1974;82(4):499–506. [PubMed] [Google Scholar]
- 3. Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110(6):1723–1729. 10.1182/blood-2006-10-053736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Compérat E, Bardier‐Dupas A, Camparo P, Capron F, Charlotte F. Splenic metastases: clinicopathologic presentation, differential diagnosis, and pathogenesis. Arch Pathol Lab Med. 2007;131(6):965–969. 10.5858/2007-131-965-smcpdd [DOI] [PubMed] [Google Scholar]
- 5. Lam KY, Tang V. Metastatic tumors to the spleen: a 25‐year clinicopathologic study. Arch Pathol Lab Med. 2000;124(4):526–530. 10.5858/2000-124-0526-mttts [DOI] [PubMed] [Google Scholar]
- 6. Yamanouchi K, Ikematsu Y, Waki S, Kida H, Nishiwaki Y, Gotoh K, et al. Solitary splenic metastasis from gastric cancer: report of a case. Surg Today. 2002;32(12):1081–1084. 10.1007/s005950200218 [DOI] [PubMed] [Google Scholar]
- 7. Zhu YP, Mou YP, Ni JJ, Zhou YC, Jiang JW, Jiang ZN, et al. Isolated splenic metastases from gastric carcinoma: a case report and literature review. World J Gastroenterol. 2013;19(31):5199–5203. 10.3748/wjg.v19.i31.5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smart P, Cullinan M, Crosthwaite G. Spontaneous splenic rupture secondary to metastatic gastric carcinoma: case report and review. ANZ J Surg. 2002;72(2):153–155. 10.1046/j.1445-2197.2002.02323.x [DOI] [PubMed] [Google Scholar]
- 9. Lee SH, Lim KH, Song SY, Lee HY, Park SC, Kang CD, et al. Occult gastric cancer with distant metastasis proven by random gastric biopsy. World J Gastroenterol. 2016;22(16):4270–4274. 10.3748/wjg.v22.i16.4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–233. 10.1111/jth.12075 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


