Summary
Background
The assessment of severity of aortic regurgitation (AR) by transthoracic echocardiography (TTE) remains challenging in routine practice. Contemporary guidelines recommend cardiovascular magnetic resonance imaging (CMR) in patients with significant disease and suboptimal TTE images. The objective of this study was to assess the role of CMR in the evaluation of severity of AR and to compare both modalities in the quantification of regurgitation and left ventricular volumes.
Methods
Fifty consecutive patients who had isolated chronic AR and who underwent TTE and CMR within an interval of less than three months between May 2009 and June 2020 were included. The main indication for CMR was difficulties in quantifying AR, either because of lack of multiparametric analysis (only one method possible) or because of discrepancies in the different methods by TTE.
Results
In 25 patients, precise grading of AR was not possible by echocardiography. Among them, CMR finally detected seven patients with mild AR, 11 with moderate AR, and seven with severe AR. For the 25 patients who had AR quantification by TTE, there was concordance between TTE and CMR in only seven patients (28%), and the AR was re-graded by CMR in 18 patients, including eight patients with severe AR by TTE and moderate AR by CMR. The concordance between TTE and CMR was weakly significant (intraclass correlation coefficient = 0.39, 95% confidence interval: 0.003– 0.67, p = 0.02). There was a moderate correlation between left ventricular volumes measured by TTE and by CMR (left ventricular end-diastolic volume: r = 0.57; p = 0.01; left ventricular end-systolic volume: r = 0.47, p = 0.01) but regurgitant volumes were not correlated (r = 0.04; p = 0.8). No TTE parameter of quantification was correlated with regurgitant volume measured by CMR.
Conclusion
The concordance of AR quantification by CMR and TTE was weak. CMR re-graded some patients with severe AR by TTE into moderate AR. This should motivate practitioners to systematically assess all significant AR by CMR in order to improve quantification and optimize clinical management.
Keywords: aortic regurgitation, quantification, multimodality, cardiac magnetic resonance
Reliable assessment of severity of valvular regurgitation is crucial in the prognosis and clinical management of patients with aortic regurgitation (AR).1 Transthoracic echocardiography (TTE) is the primary clinical imaging modality to assess severity of AR, but AR quantification remains challenging in routine practice.2
The current recommended echocardiographic assessment of AR uses both quantitative and semi-quantitative criteria. The proximal isovelocity surface area (PISA) method, using two-dimensional (2D) colour Doppler echocardiography is the most widely used approach to estimate the regurgitant volume (RVol) and effective regurgitant orifice area (EROA).3-5 However, this method has several limitations: difficulty in correctly identifying the flow convergence zone, confined flow convergence zones (patients with cusp perforation or commissural leaks), and obtuse flow convergence angles such as those with aneurysmal dilation of the ascending aorta.3
Contemporary guidelines2,6 recommend cardiovascular magnetic resonance imaging (CMR) in patients with significant disease and suboptimal TTE images, which acknowledges CMR’s superior capacity to quantify AR volume and regurgitant fraction by direct measurement of aortic blood flow and to accurately compare right ventricular (RV) and left ventricular (LV) stroke volumes.7,8 The objective of this study was to assess the role of CMR in the evaluation of severity of AR in current practice and to compare both modalities in the quantification of regurgitation and LV volumes.
Methods
This study was performed in the Cardiology Department of Nancy University Hospital Centre (Brabois Hospital). Three analysis and archiving databases were used: DxCare for clinical data, Echopac version R3 for echocardiographic data and Syngovia for CMR data.
All consecutive patients who had isolated chronic AR and who underwent TTE and CMR within an interval of less than three months, from May 2009 to June 2020, were included (Fig. 1). Patients with primary cardiomyopathy and those with other significant valvular disease and atrial fibrillation were excluded.
Echocardiographic examinations were performed using commercially available scanners (Vivid 7, Vivid 9 or Vivid 95, General Electric-Vingmed, Horten, Norway) using a 2.5-MHz phased-array cardiac probe with subjects in the left lateral recumbent position. An experienced sonographer acquired a complete 2D standard echocardiography, including apical fourand two-chamber LV views. All images were acquired at a frame rate of 50 to 70 frames/s for 2D views. Before each acquisition, images were optimised for endocardial visualisation.
Fig. 1.
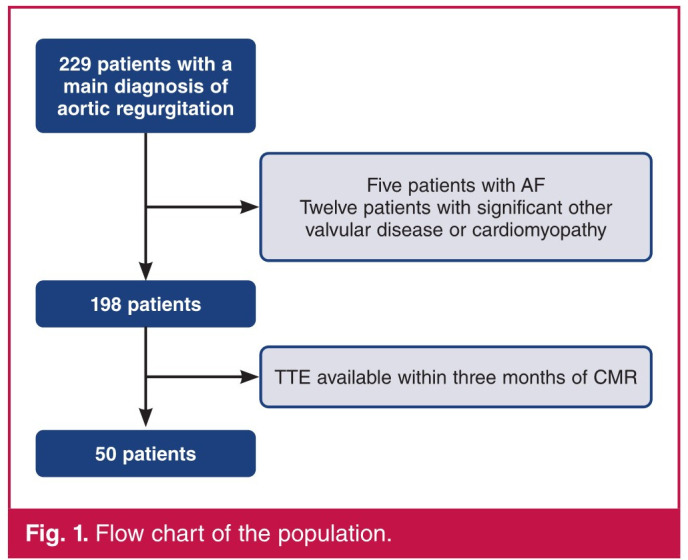
Flow chart of the population.
LV diameters were measured in time–motion mode or in 2D mode when the time–motion line was not perpendicular to the LV longitudinal axis. The left ventricle was considered severely dilated by TTE for a left ventricular end-diastolic diameter (LVEDDTTE) > 70 mm. LV volumes were calculated from apical four- and two-chamber views according to Simpson’s biplane method.9 AR severity was assessed by an integrated approach using four quantification methods: vena contracta width, PISA, diastolic flow reversal velocity in the descending aorta, and pression half-time (PHT).
The vena contracta width was obtained from the parasternal long-axis view. A narrow colour sector scan coupled with the zoom mode was used to improve measurement accuracy. Using a Nyquist limit of 50–60 cm/s, a vena contracta width < 3 mm correlates with mild AR, whereas a width > 6 mm indicates severe AR.
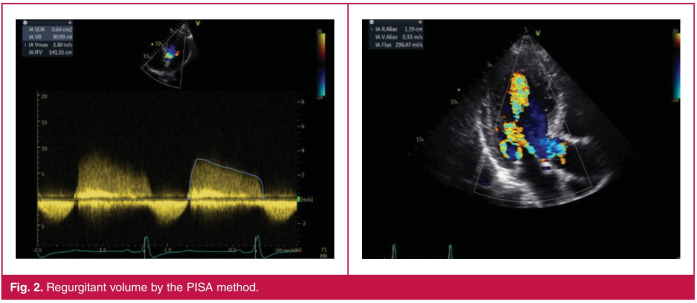
Depending on the orientation of the jet, PISA was measured in the apical five-chamber or parasternal long-axis view with the lower Nyquist limit set at 30 cm/s. Peak AR jet velocity and integral velocity time were determined using continuous-wave Doppler across the aortic valve. The PISA radius was measured from a stop frame as the distance between the regurgitant orifice and the first aliasing in early diastole (closest to the peak of regurgitant velocity) (Fig. 2). Grading of the severity of AR classified regurgitation as mild when EROA was < 10 mm2 or RVol was < 30 ml, and moderate or severe when EROA was ≥ 30 mm2 or RVol was ≥ 60 ml.
Fig. 2.
Regurgitant volume by the PISA method.
End-diastolic velocity flow reversal in the descending aorta (EDVDA) was measured in the upper descending aorta at the aortic isthmus level using a suprasternal view with pulsed Doppler. The sample volume was placed at the origin of the left subclavican artery and it was aligned as much as possible along the major axis of the aorta. The Doppler filter was decreased to its lowest setting to allow detection of low velocities (< 10 cm/s). End-diastolic velocity measured at peak R wave exceeding 20 cm/s indicated severe AR.3
PHT was obtained from the AR flow curve obtained for an apical five-chamber view.3 A PHT of < 200 ms indicated severe AR, whereas a value of > 500 ms was in favour of mild AR. CMR imaging was performed on a 3T system (General Electric Signa HDxt) with an eight-phased-array cardiac coil, electrocardiogram triggered and breath-holding in expiration. After a series of scouting images to determine the position and orientation of the left ventricle within the thorax, Cine Fiesta sequences for cardiac morphology and function were performed with a steady-state free precession technique in 10 to 15 parallel short-axis views. Each slice (slice thickness: 8 mm, gap: 0 mm) was obtained during one breath-hold of 10 to 15 seconds.
CMR and TTE left ventricular ejection fractions (LVEF) were based on endocardial tracing of the LV chamber from the images on different axis views. On each image, end-diastole was defined as the frame in the cardiac cycle in which the cardiac volume was largest. End-systole was defined just before the opening of the mitral valve leaflet or the frame in the cardiac cycle in which the cardiac volume was smallest. Papillary muscles were included in the cavity for the tracing.
Quantitative determination of LVEF was calculated using left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) estimates as follows:
according to the guidelines.10,11 Both Simpson’s method and the area–length method were applied on CMR.
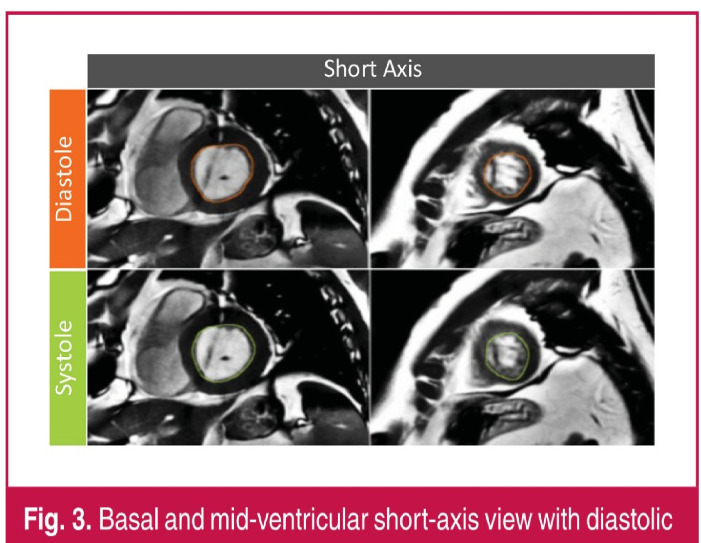
On short-axis view (Fig. 3), the outline of the endocardial border of the left ventricle was traced manually on all slices of each phase by one experienced cardiologist or one radiologist using standard software (Mass Research software, version V2013-EXP, Leiden University Medical Center). Volumes were computed by Simpson’s method of disk summation where the sum of the cross-sectional areas was multiplied by the slice thickness. On CMR, the left ventricle was considered dilated for a LVEDVCMR > 246 ml.
Fig. 3.
Basal and mid-ventricular short-axis view with diastolic and systolic contours.
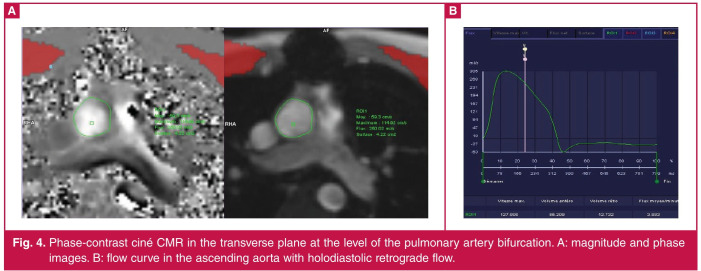
Phase-contrast cardiac magnetic resonance (PC-CMR) images were also acquired in order to compute the stroke volume. On PC images, the lumen of the ascending aorta was segmented automatically and corrected manually throughout the cardiac cycle (Fig. 4). Blood flow within the vessel has been computed by summing the regions of interest.12 The average flow velocity (cm/s) was multiplied by the area of the vessel (cm2) to obtain flow (ml/s) at each point. Stroke volume (ml) was obtained by dividing cardiac output (l/min) by heart rate (bpm).13
Fig. 4.
Phase-contrast ciné CMR in the transverse plane at the level of the pulmonary artery bifurcation. A: magnitude and phase images. B: flow curve in the ascending aorta with holodiastolic retrograde flow.
AR grading was defined as follows: mild, AR volumes < 30 ml (regurgitation fraction < 30%); moderate, AR volumes 30–59 ml (regurgitation fraction 30–49%); severe, AR volumes ≥ 60 ml (regurgitation fraction ≥ 50 %).6
Statistical analysis
The statistical analysis was performed using SPSS for Windows (SPSS version 17, Chicago, Illinois). Continuous variables are expressed as means ± standard deviations. Categorical variables are expressed as percentages. Differences between CMR and TTE were compared using the Student’s t-test. Pearson’s correlation coefficients were calculated by linear regression for continuous variables. A p-value < 0.05 was considered significant.
The intraclass correlation coefficient (ICC) for a two-way random-effects model with absolute agreement was calculated to assess the concordance between TTE and CMR for quantification of AR severity. ICCs were categorised as excellent (ICC ≥ 0.75), good (ICC 0.6–0.74), fair (ICC 0.4–0.59) or poor (ICC < 0.4).14,15
Results
From May 2009 to June 2020, 198 patients had both a CMR and TTE showing AR. After checking the delay between both examinations (< 3 months) and after exclusion of patients with AF and those with other significant valvular disease and primary cardiomyopathy, AR was the sole and main diagnosis in 50 (25.2%) patients, who constituted our population.
The clinical data are summarised in Table 1. Out of the 50 patients, 13 (26%) had bicuspid aortic valve and eight (16%) had aortic valvular prosthesis. The mean time between TTE and CMR was 44.2 ± 19.5 days.
Table 1. Clinical data.
| Patient characteristics | Number (%) or mean + SD |
| Age (years) | 52.1 + 16.1 |
| Gender (male) | 38 (76) |
| Systolic blood pressure (mmHg) | 135.1 + 20.1 |
| Diastolic blood pressure (mmHg) | 72.2 + 10.4 |
| Heart rate (bpm) | 69.8 + 13.0 |
| Body surface area (m ² | 1.9 + 0.2 |
| Body mass index (kg/m²) | 25.7 + 4.6 |
| Hypertension | 27 (54) |
| Diabetes mellitus | 2 (4) |
| Dyslipidaemia | 6 (12) |
| Coronary artery disease | 5 (10) |
| NYHA class | |
| I | 38 (76) |
| II | 6 (12) |
| III | 5 (10) |
| IV | 1 (2) |
NYHA, New York Heart Association.
Regarding echocardiography, the PISA method was possible in 19 patients (38%). According to this method, seven patients (14%) had mild AR, eight (16%) had moderate AR and four (8%) had severe AR. The EDVDA measurement was used in 12 patients (24%) and severe AR was detected in eight patients (16%). The vena contracta was measured in 28 patients (56%) and we classified 10 patients (20%) with severe AR, two (4%) with mild AR and 16 (36%) with moderate AR. PHT was measured in 21 patients (42%) and nine (18%) had mild AR, none had severe AR and 12 (24%) had moderate AR.
Table 2 shows the distribution of patients by number of TTE quantification methods. Only one method of quantification had been possible in 20 (40%) patients. Among the 30 patients who had more than one method of quantification, five (10%) had discrepancies in results. Therefore, for 25 (50%) cases, the operator had difficulty in assessing AR severity and considered TTE as inconclusive for the quantification of AR. Finally, with TTE, AR was considered mild in eight (16 %) patients, moderate in seven (14%), severe in 10 (20%) and inconclusive in 25 (50%) patients.
The main indication for use of CMR was difficulties in quantifying AR, either because of lack of multiparametric analysis (only one method possible) or because of discrepancies in the different methods by TTE. The indication for use of CMR was inconclusive TTE in 25 (50%) patients, aortic bicuspid valve in 13 (26%), valvular aortic prosthesis in eight (16%) and ascending aortic assessment in 17 (34%) patients.
Table 2. TTE and CMR data.
| TTE and CMR data | Number (%) or mean + SD |
| LVEDV TTE (ml/m²) | 95.9 + 27.4 |
| LVESV TTE (ml/m²) | 41.1 + 21.1 |
| LVEDD TTE (mm) | 61.4 + 7.8 |
| LVESD TTE (mm) | 43.2 + 9.1 |
| LVEF) TTE | 54.1 + 10.5 |
| RVo1 (ml) | 53.3 + 21.6 |
| EDVDA (cm/s) | 18.1 + 6.4 |
| PHT (ms) | 460.6 + 153.1 |
| Vena contracta (mm) | 5.3 + 2.4 |
| Inconclusive quantification by TTE | 25 (50) |
| Number of quantification methods by TTE | |
| One method | 20 (40) |
| Two methods | 14 (28) |
| Three methods | 8 (16) |
| Four methods | 8 (16) |
| Disagreement between TTE methods | 5 (10) |
| LVEDV CMR (ml/m²) | 133.3 + 38.1 |
| LVESV CMR (ml/m²) | 65.1 + 25.5 |
| RF CMR (%) | 35.5 + 14.1 |
| RVolcME CMR (ml) | 29.7 + 17.1 |
| LVEF CMR (%) | 51.8 + 8.6 |
| Late gadolinium enhancement | 5 (10) |
TTE, transthoracic echocardiography; CMR, cardiovascular magnetic resonance imaging; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; RVol, regurgitant volume; EDVDA, end-diastolic velocity in the descending aorta; PHT, pressure half-time; RF, regurgitant fraction.
Among the 25 patients (50%) with non-conclusive TTE results, CMR was also indicated in six patients (12%) for aortic bicuspid valve, three (6%) for aortic prosthesis and four patients (8%) for ascending aortic assessment. Among all patients, AR quantification by CMR was as follows: 14 patients (28%) had mild AR, 26 (52%) had moderate AR and 10 had (20%) severe AR. Among the 25 patients (50%) with inconclusive TTE, CMR finally detected 14% with mild AR (seven patients), 22% with moderate AR (11 patients) and 14% with severe AR (seven patients).
Among the 25 patients (50%) who had AR graded by TTE, quantification of AR was concordant with both methods in seven patients (14%). Compared to CMR, AR was underestimated in six (12%) patients (five considered mild by TTE and moderate by CMR, and one considered moderate by TTE and severe by CMR). AR was overestimated in 12 (24%) patients (eight considered severe by TTE and moderate by CMR, and four considered moderate by TTE and mild by CMR) (Table 3). Therefore, AR was re-graded by CMR in 18 (36%) patients. The concordance between the two AR quantification modalities (TTE and CMR) was weakly significant (ICC = 0.39, 95% CI: 0.003–0.67, p = 0.02).
Among all patients, six (12%) had a LVEDDTTE > 70 mm. Out of these six patients, AR grade using TTE was determined as follows: severe in three (6%) patients, inconclusive in two (4%) and mild in one patient (2%). The three patients who had severe AR using TTE were classified as severe by CMR in two patients and moderate in one. The two with inconclusive AR were classified as moderate in one patient and severe in the other using CMR. Lastly, the only patient with mild AR on TTE was classified as moderate on CMR.
Table 3. Comparison of AR severity on TTE and CMR.
| CMR | |||
| AR severity, n (%) | Mild | Moderate | Severe |
| TTE | |||
| Mild | 3 (6) | 5 (10) | 0 (0) |
| Moderate | 4 (8) | 2 (4) | 1 (2) |
| Severe | 0 (0) | 8 (16) | 2 (4) |
| Inconclusive | 7 (14) | 11 (22) | 7 (14) |
AR, aortic regurgitation; TTE, transthoracic echocardiography; CMR, cardiovascular magnetic resonance imaging
Twenty-five patients (50%) had inconclusive AR quantification on TTE. Among them, seven had severe AR on CMR and five had subsequent aortic valvular replacement. Two patients with severe AR on CMR had medical therapy and close follow up. All patients with possible AR quantification using TTE, and severe AR on CMR, had aortic valve replacement (Fig. 5). Among the six patients with LVEDDTTE > 70 mm, three had aortic valvular replacement (two patients with severe AR on TTE and CMR, and one with inconclusive AR on TTE and severe AR on CMR).
LV volume measurements were performed on all patients, both by TTE and CMR (Table 2). LV volumes were lower with TTE than with CMR: LVEDVTTE vs LVEDVCMR (95.9 ± 27.4 vs 133.3 ± 38.1 ml/m2, p < 0.01) and LVESVTTE vs LVESVCMR (65.0 ± 25.5 vs 41.1 ± 21.1 ml/m2, p < 0.01). On the other hand, LVEFTTE was higher than LVEFCMR (54.1 ± 10.5 vs 51.8 ± 8.6%, p = 0.03).
Fig. 5.
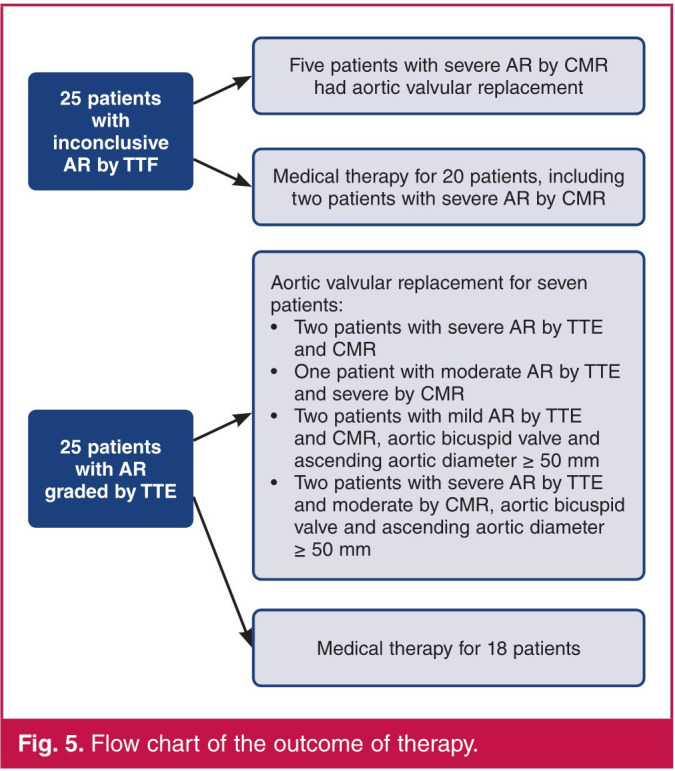
Flow chart of the outcome of therapy.
In our study there was a moderate correlation between LVEDVTTE and LVEDVCMR (r = 0.57, p = 0.01) and LVESVTTE and LVESVCMR (r = 0.47, p = 0.01). On the other hand, among the 19 patients who had the right ventricle measured by PISA during TTE, RVolTTE (53.3 ± 21.6 ml) and RVolCMR (29.7 ± 17.1 ml) were significantly different (p = 0.002) and not correlated (r = 0.04, p = 0.8). None of the TTE quantification parameters were correlated to RVolCMR (Table 4).
Table 4. Correlation coefficients between TTE and CMR parameters.
| LVEDVCMR | LVESVCMR | RVolCMR | |
| LVEDVTTE | 0.57* | 0.48 | 0.33* |
| LVESVTTE | 0.42* | 0.47* | 0.24 |
| LVEDDTTE | 0.54* | 0.53 | 0.39* |
| LVESDTTE | 0.44* | 0.45* | 0.27 |
| RVolTTE | 0.16 | 0.22 | 0.04 |
| Vena contracta | 0.04 | 0.05 | 0.06 |
| PHT | 0.05 | 0.16 | 0.16 |
| EDVDA | 0.11 | 0.13 | 0.22 |
TTE, transthoracic echocardiography; CMR, cardiovascular magnetic resonance imaging; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; RVol, regurgitant volume; EDVDA, enddiastolic velocity in the descending aorta; PHT, pressure half-time. *p < 0.05.
Concerning LV remodelling, 15 patients (30%) with no severe LV dilatation according to TTE thresholds (≤ 70 mm) had severe LV dilatation according to CMR thresholds (> 246 ml). Two patients (4%) had severe LV dilatation by TTE (> 70 mm) and no severe dilatation by CMR (≤ 246 ml). Quantification of LV dilatation was concordant for 33 (66%) patients, with 29 (58%) with non-dilated left ventricles and four (8%) with dilated left ventricles (Table 5).
Table 5. Comparison of LVEDVCMR and LVEDDTTE according to poor prognosis thresholds 6,28.
| LVEDVCMR | ||
| 24 6ml | > 246 ml | |
| LVEDDTTETTE | ||
| 70 mm | 29 (58) | 15 (30) |
| > 70mm | 2 (4) | 4 (8) |
LVEDV, left ventricular end-diastolic volume; LVEDD, left ventricular enddiastolic diameter. p = 0.1.
Discussion
This study highlights the following findings:
For half of the patients who had both CMR and TTE in the clinical management of AR, there were difficulties in quantifying AR, either because of lack of multiparametric analysis (only one method possible) or because of discrepancies in the different methods. In those patients, CMR finally detected seven (14%) patients with mild AR, 11 (22%) with moderate AR and seven (14%) with severe AR.
In 25 patients (50%) who had a quantification by TEE, AR was re-graded by CMR in 18 (36%) patients.
Quantification of LV dilatation was concordant by both methods in only 33 (66%) patients.
LV volumes were greater with CMR than TTE, with a moderate correlation. RVolCMR and RVolTTE were not correlated.
Contemporary guidelines recommend CMR in patients with significant disease and suboptimal TTE images.2 In our cohort, in half of the cases, difficulties prevented an objective quantification of AR using the usual TTE parameters. In inconclusive TTE cases, 20 (40%) patients had no multiparametric analysis. For five (10%) patients, there was a disagreement between TTE parameters. This can be explained by the following reasons.
The PISA method, which is recommended by the current guidelines, is based on a fluid dynamic concept and uses hemispheric assumptions of flow convergence region.16-18
However, the 2D PISA method is inherently limited because of the geometric assumptions of PISA shape that are necessary to calculate regurgitant volume. The pressure half-time depends on systemic vascular resistance and LV compliance.19
The concept of vena contracta is indeed based on the assumption that the regurgitant orifice is almost circular. The orifice is however often elliptical or irregular, which changes the width of the vena contracta in different views. Furthermore, intermediate vena contracta values (3–6 mm) need confirmation by a more quantitative method, when feasible.3 In our study, 36% of patients had intermediate vena contracta values.
Considering the excellent reproducibility of AR grading by CMR20 in prospectively recruited patients,21,22 our findings provide further support for the contemporary recommendation to proceed with CMR in cases of suboptimal TTE assessment.2
RVolCMR and RVolTTE were not correlated in our study. These results contrasted with those of Choi et al.,19 who found a weak correlation for eccentric jets. These variations can be explained by differences in methodology. In our cohort, PISA was not assessed according to jet eccentricity and only 38% of patients were assessed by this method.
Some authors have identified limitations of the PISA method. PISA is based on the assumption of hemispherical and homogenous flow convergence, which may not be present in vivo.23 Another pitfall includes timing of measurement. PISA provides an instantaneous peak flow rate whereas regurgitation is of a dynamic nature,24 with duration of regurgitation,25 and machine settings can vary.26 These limitations also apply to the PISA-derived estimated EROA and RVol, although these variables are more susceptible to errors, which are squared in the formulae.6 Cawley et al. found in a prospective study, poor reproducibility of RVol assessment by the PISA method and reported superiority of CMR with low interobserver variability.21
With regard to the different TTE methods of quantification, our study showed that PISA was not feasible in 62% of patients. These rates were above those found in recent research.27 These disparities can be explained by the method of selection of our patients; indeed, most of them had poor echogenicity and inconclusive quantification of AR, as reported by the operators.
In patients with a possible TTE quantification, CMR allowed a re-grading of AR in 36% of patients: 10% of patients from mild AR with TTE to moderate AR with CMR, 8% from moderate AR with TTE to mild AR with CMR, 2% from moderate AR with TTE to severe AR with CMR, and 16% from severe AR with TTE to moderate AR with CMR.
Our study found that the highest rate of re-grading was achieved in severe AR with TTE, which was finally classified as moderate AR on CMR. This finding was also made in a recent study that found an overestimation of severe AR using TTE. MRI re-graded severe AR on TTE in 34% of cases.27 Despite low proportions of severe AR, Gelfand et al. showed that more than half of the cases were re-graded by CMR.14
The American Society of Echocardiography/Society for Cardiovascular Magnetic Resonance recommends thresholds for AR grading with CMR by AR volume or AR fraction equivalent to TTE cut-off values.6 This can cause a mismatch in AR grade by TTE and CMR. Considering the clinical importance of this differentiation, CMR-specific cut-off values appear better suited to provide reasonable overlap with TTE grading.14,28,29 Polte et al.30 found, with CMR, AR volume > 40 ml, and with TTE, AR fraction > 30% to be the best discriminator between patients with severe AR qualifying for guideline-recommended surgery,1 and patients with moderate AR.
In our study LVEFTTE was significantly higher than LVEFCMR. Conversely, on CMR, LV volumes (LVEDVCMR and LVESVCMR) were significantly higher than LV volumes (LVEDVTTE and LVESVTTE) on TTE. CMR also allows an additional approach to the assessment of LV dilatation, which is not always consistent with TTE (Table 5).
Underestimation of LV volumes by TTE is attributable to three factors: unreliable assessment of the LV apex, contouring of the inner edge of the LV trabeculations as endocardial borders, and use of geometric formulae for LVEF by TTE. Variability in echocardiographic measurement and underestimation of LV volumes may have important clinical implications if TTE results in underestimation of regurgitant severity or fails to recognise early LV remodelling in patients with chronic regurgitation.21 This could be resolved through the use of three-dimensional (3D) TTE.
In our study, LVEDDTTE was correlated with RVolCMR. Our results showed that LVEDDTTE can be used, in accordance with contemporary guidelines, as a criterion for theoretical operability for LV remodelling. Contemporary guidelines recommend (class IIa; level B) surgery in asymptomatic patients with LVEDD > 70 mm and LVESD > 50 mm, based on TTE data.31
Concerning the clinical impact, five patients (10%) with inconclusive AR by TTE and severe AR by CMR had aortic valvular replacements. These results are important and show that AR quantification should be performed very carefully, and although it is performed by experienced TTE operators, it remains difficult and sometimes inaccurate. AR severity using TTE should be confirmed by CMR for reliable quantification and efficient therapeutic decision making.
The main limitation of this study was its retrospective approach, which considerably limited TTE inter- and intraobserver variability analysis. The sample of patients who underwent CMR was relatively small. Moreover, even if the time delay between carrying out TTE and CMR was relatively short compared to the ongoing AR, some changes could have occurred, both in AR grade and in RV remodelling, between the two examinations.
A prospective, multicentre study with current methods of quantification in 3D TTE and CMR, such as holodiastolic retrograde flow in the descending aorta, should be performed in order to assess AR accurately and to overcome significant interobserver variability.19,27
Conclusion
CMR remains, in clinical practice, an insufficiently performed investigation for AR quantification. In our study, one in four patients underwent it over a decade. The re-grading of a number of severe AR cases on TTE into moderate AR on CMR is not insignificant and should motivate practitioners to systematically assess all severe AR on TTE with CMR in order to improve quantification and to proceed to an optimal clinical management.
Contributor Information
Marie-Paule Bernadette N’Cho-Mottoh, Email: nchomottoh@yahoo.fr.
Marie-Paule Bernadette N’Cho-Mottoh, Institut de Cardiologie d’Abidjan, Abidjan, Ivory Coast.
References
- 1.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H. et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33(19):2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA. et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 3.Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E. et al. European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: Aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr. 2010;11(3):223–244. doi: 10.1093/ejechocard/jeq030. [DOI] [PubMed] [Google Scholar]
- 4.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA. American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. et al. J Am Soc Echocardiogr. 2003;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 5.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C. European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease. et al. Eur J Echocardiogr. 2010;11(4):307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- 6.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA. et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance . J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Didier D, Ratib O, Lerch R, Friedli B. Detection and quantification of valvular heart disease with dynamic cardiac MR imaging. Radiographics. 2000;20:1279–1299. doi: 10.1148/radiographics.20.5.g00jl111279. [DOI] [PubMed] [Google Scholar]
- 8.Dall’Armellina E, Hamilton C, Hundley G. Assessment of blood flow and valvular heart disease using phase-contrast cardiovascular magnetic resonance. Echocardiography. 2007;24:207–216. doi: 10.1111/j.1540-8175.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA. Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. et al. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.American College of Radiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology; North American Society for Cardiac Imaging; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology. ACCF/ACR/SCCT/ SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. A report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group. J Am Coll Radiol. 2006;3(10):751–771. doi: 10.1016/j.jacr.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 11.American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG. et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2614–2662. doi: 10.1016/j.jacc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hundley WG, Li HF, Hillis LD, Meshack BM, Lange RA, Willard JE. et al. Quantitation of cardiac output with velocity-encoded, phasedifference magnetic resonance imaging. Am J Cardiol. 1995;75(17):1250–1255. doi: 10.1016/s0002-9149(99)80772-3. [DOI] [PubMed] [Google Scholar]
- 13.Firmin DN, Nayler GL, Kilner PJ, Longmore DB. The application of phase shifts in NMR for flow measurement. Magn Reson Med. 1990;14(2):230–241. doi: 10.1002/mrm.1910140209. [DOI] [PubMed] [Google Scholar]
- 14.Gelfand EV, Hughes S, Hauser TH, Yeon SB, Goepfert L, Kissinger KV. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. et al. J Cardiovasc Magn Reson. 2006;8:503–507. doi: 10.1080/10976640600604856. [DOI] [PubMed] [Google Scholar]
- 15.Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann G. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol. 2015;84:840–848. doi: 10.1016/j.ejrad.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Pouleur AC, le Polain de Waroux JB, Goffinet C, Vancraeynest D, Pasquet A, Gerber BL, Vanoverschelde JL. Accuracy of the flow convergence method for quantification of aortic regurgitation in patients with central versus eccentric jets. Am J Cardiol. 2008;102(4):475–480. doi: 10.1016/j.amjcard.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Schwammenthal E, Chen C, Giesler M, Sagie A, Guerrero JL, Vazquez de Prada JA. et al. New method for accurate calculation of regurgitant flow rate based on analysis of Doppler color flow maps of the proximal flow field. Validation in a canine model of mitral regurgitation with initial application in patients. J Am Coll Cardiol. 1996;27(1):161–172. doi: 10.1016/0735-1097(95)00428-9. [DOI] [PubMed] [Google Scholar]
- 18.Tribouilloy CM, Enriquez-Sarano M, Fett SL, Bailey KR, Seward JB, Tajik AJ. Application of the proximal flow convergence method to calculate the effective regurgitant orifice area in aortic regurgitation. J Am Coll Cardiol. 1998;32(4):1032–1039. doi: 10.1016/s0735-1097(98)00356-8. [DOI] [PubMed] [Google Scholar]
- 19.Choi J, Hong GR, Kim M, Cho IJ, Shim CY, Chang HJ. et al. Automatic quantification of aortic regurgitation using 3D full volume color doppler echocardiography: a validation study with cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2015;31(7):1379–1389. doi: 10.1007/s10554-015-0707-x. [DOI] [PubMed] [Google Scholar]
- 20.Uretsky S, Supariwala A, Nidadovolu P, Khokhar SS, Comeau C, Shubayev O. et al. Quantification of left ventricular remodeling in response to isolated aortic or mitral regurgitation. J Cardiovasc Magn Reson. 2010;12(1):32. doi: 10.1186/1532-429X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cawley PJ, Hamilton-Craig C, Owens DS, Krieger EV, Strugnell WE, Mitsumori L. et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging. 2013;6(1):48–57. doi: 10.1161/CIRCIMAGING.112.975623. [DOI] [PubMed] [Google Scholar]
- 22.Dulce MC, Mostbeck GH, O’Sullivan M, Cheitlin M, Caputo GR, Higgins CB. Severity of aortic regurgitation: interstudy reproducibility of measurements with velocity-encoded cine MR imaging. Radiology. 1992;185(1):235–240. doi: 10.1148/radiology.185.1.1523315. [DOI] [PubMed] [Google Scholar]
- 23.Iwakura K, Ito H, Kawano S, Okamura A, Kurotobi T, Date M. et al. Comparison of orifice area by transthoracic three-dimensional Doppler echocardiography versus proximal isovelocity surface area (PISA) method for assessment of mitral regurgitation. Am J Cardiol. 2006;97(11):1630–1637. doi: 10.1016/j.amjcard.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 24.Schwammenthal E, Chen C, Benning F, Block M, Breithardt G, Levine RA.. Dynamics of mitral regurgitant flow and orifice area. Physiologic application of the proximal flow convergence method: clinical data and experimental testing. Circulation. 1994;90(1):307–322. doi: 10.1161/01.cir.90.1.307. [DOI] [PubMed] [Google Scholar]
- 25.Topilsky Y, Michelena H, Bichara V, Maalouf J, Mahoney DW, Enriquez-Sarano M. Mitral valve prolapse with mid-late systolic mitral regurgitation: pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation. 2012;125(13):1643–1651. doi: 10.1161/CIRCULATIONAHA.111.055111. [DOI] [PubMed] [Google Scholar]
- 26.Utsunomiya T, Ogawa T, Doshi R, Patel D, Quan M, Henry WL, Gardin JM. Doppler color flow ‘proximal isovelocity surface area’ method for estimating volume flow rate: effects of orifice shape and machine factors. J Am Coll Cardiol. 1991;17(5):1103–1111. doi: 10.1016/0735-1097(91)90839-2. [DOI] [PubMed] [Google Scholar]
- 27.Kammerlander AA, Wiesinger M, Duca F, Aschauer S, Binder C, Zotter Tufaro C. et al. Diagnostic and prognostic utility of cardiac magnetic resonance imaging in aortic regurgitation. J Am Coll Cardiol Cardiovasc Imaging. 2019;12(8 Pt 1):1474–1483. doi: 10.1016/j.jcmg.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Ley S, Eichhorn J, Ley-Zaporozhan J, Ulmer H, Schenk JP, Kauczor HU, Arnold R. Evaluation of aortic regurgitation in congenital heart disease: value of MR imaging in comparison to echocardiography. Pediatr Radiol. 2007;37(5):426–436. doi: 10.1007/s00247-007-0414-4. [DOI] [PubMed] [Google Scholar]
- 29.Kutty S, Whitehead KK, Natarajan S, Harris MA, Wernovsky G, Fogel MA. Qualitative echocardiographic assessment of aortic valve regurgitation with quantitative cardiac magnetic resonance: a comparative study. Pediatr Cardiol. 2009;30(7):971–977. doi: 10.1007/s00246-009-9490-6. [DOI] [PubMed] [Google Scholar]
- 30.Polte CL, Gao SA, Johnsson AA, Lagerstrand KM, Bech-Hanssen O. Characterization of chronic aortic and mitral regurgitation undergoing valve surgery using cardiovascular magnetic resonance. Am J Cardiol. 2017;119(12):2061–2068. doi: 10.1016/j.amjcard.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ. ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. et al. Eur Heart J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]