INTRODUCTION:
Management of nonalcoholic steatohepatitis (NASH) is a currently unmet clinical need. Digital therapeutics (DTx) is an emerging class of medicine that delivers evidence-based therapeutic interventions. This study was aimed at investigating the efficacy of DTx in patients with NASH.
METHODS:
We conducted a multicenter, single-arm, 48-week trial in 19 patients with biopsy-confirmed NASH. All patients received a DTx intervention with a newly developed smartphone application. The primary endpoint was change in the nonalcoholic fatty liver disease activity score (NAS) without worsening of liver fibrosis. The secondary endpoints included improvement of the NAS by ≥2 points without worsening of liver fibrosis, change in the body weight, and regression of fibrosis.
RESULTS:
After the 48-week DTx intervention, improvement of the NAS was observed in 68.4% (13/19) of patients. The mean change in the NAS from baseline to the end of the intervention was −2.05 ± 1.96 (P < 0.001 when compared with the threshold of −0.7). A decrease in the NAS by ≥ 2 points was achieved in 11 (57.9%). The average weight loss at the end of the intervention was 8.3% (P < 0.001). Reduction of the fibrosis stage was observed in 58.3% when the analysis was limited to patients with stage F2/3 fibrosis. There were no serious adverse events that could be considered as being related to the DTx intervention.
DISCUSSION:
DTx for NASH was found to be highly efficacious and well-tolerated. Further evaluation of the DTx intervention for NASH in a phase 3 trial is warranted.
KEYWORDS: nonalcoholic steatohepatitis, smartphone, digital therapeutics, application
Study Highlights.
WHAT IS KNOWN
✓ Management of nonalcoholic steatohepatitis (NASH) is a currently unmet clinical need.
✓ Digital therapeutics (DTx) is a rapidly emerging therapeutic modality in which a computer software is used to provide evidence-based interventions to prevent, manage, and treat diseases.
WHAT IS NEW HERE
✓ DTx intervention using newly developed smart phone application resulted in histological improvement in patients with NASH, supported by weight loss.
✓ When the analysis was limited to patients with fibrosis stage F2/3, fibrosis stage reduction was observed in 58.3% of the patients.
✓ DTx intervention seems to be a promising treatment strategy for NASH patients.
INTRODUCTION
With obesity having become a global epidemic, nonalcoholic fatty liver disease (NAFLD) has grown from a relatively unknown disease to becoming the most common cause of chronic liver disease in the world, with a worldwide prevalence of 25% (1). Nonalcoholic steatohepatitis (NASH) is a progressive form of NAFLD that is characterized by the accumulation of fat in the liver, hepatic lobular inflammation, hepatocellular ballooning, and insulin resistance (2,3). Because NASH can progress to cirrhosis or even hepatocellular carcinoma (1), immediate medical attention and effective treatment of NASH are clearly needed; however, no pharmacotherapies have been approved yet for this condition (4).
Lifestyle modifications and weight loss are the only recommended modalities for the treatment of NASH (5). Previous studies have shown that intensive lifestyle modifications successfully led to a 7%–10% reduction of the body weight in patients with NASH and that such weight reduction was associated with a significant improvement of the histological activity of NASH (6). However, many patients with NASH have difficulty in sustaining the lifestyle changes. Furthermore, not all hospitals can provide lifetime intervention services because of the excessive cost involved and/or insufficient availability of trained counselors. Therefore, a better treatment paradigm is needed to overcome these potential concerns.
Digital therapeutics (DTx) is a rapidly emerging therapeutic modality in which a computer software is used to provide evidence-based interventions to prevent, manage, and treat diseases. Various trials of DTx using smartphone applications (app) have been conducted for chronic conditions such as diabetes mellitus (7), nicotine addiction (8,9), hypertension (10,11), alcohol addiction (12), and even cancers (13). We considered that a smartphone app–based DTx intervention could also be a potentially useful novel solution for patients with NASH.
In this study, we present the novel interactive smartphone app, NASH App, which has been designed to support patients achieve sustainable and consistent lifestyle modifications for the control of NASH (Figure 1). This system provides counseling sessions, educational videos, and advice on specific action goals to enable the patients to improve their lifestyles. In this study, we report the results of our multicenter single-arm trial performed to evaluate the clinical efficacy of DTx using NASH App.
Figure 1.
Overview of the NASH app digital therapeutics system. NASH, nonalcoholic steatohepatitis.
METHODS
Study design
This study was a multicenter, single-arm, 48-week trial performed to evaluate the efficacy of a smartphone app (NASH App) –based intervention for patients with NASH. All patients were asked to download the NASH App on their phone, while the attending physicians were provided with the NASH App Console, a web-based patient management console, for 48 weeks. This project was approved by the Ethics Committee of the University of Tokyo (2018035SP), and all patients provided written informed consent. The trial was performed in accordance with the ethics principles of the Declaration of Helsinki and was registered in the University Hospital Information Network Clinical Trial Registry (UMIN000029835) and the Japan Registry of Clinical Trials (jRCT) (No. jRCTs032180372). All authors had accessed to the study data and had reviewed and approved the final manuscript. The sponsor (CureApp) assisted with the trial design and performed site monitoring and data management under a third-party audit.
Participants
Eligible patients were aged 20 years or older, consumed no or only a moderate amount of alcohol (daily intake less than 20 g in women and less than 30 g in men), and had a body mass index of ≥25 kg/m2 according to the obesity criteria of the Japan Society for the Study of Obesity (14) at the initial screening. Additional key inclusion criteria were the presence of histologic evidence of NASH and an NAFLD activity score (NAS) of ≥4 and a score for ballooning of at least 1 on liver biopsy performed within 90 days before obtaining informed consent from the patient and the availability of the NASH App on the patient's phone. Key exclusion criteria were underlying chronic liver disease due to other etiology than NASH, platelet count less than 60,000 per microliter and/or prothrombin time less than 50%, histological evidence of liver cirrhosis (F4) on the Brunt fibrosis scale (15,16), and confounding concomitant drug use (vitamin E, pioglitazone, obeticholic acid, or selonsertib).
NASH app: DTx system for NASH
The NASH App system is a new interactive smartphone app designed to assist patients make intensive and sustainable lifestyle modifications to control NASH. The patients downloaded the NASH App on their smartphone and activated the app by entering a prescription code. They were then asked to enter their baseline profile, including their age, sex, diet/exercise lifestyle, and social characteristics guided by the chatbot-based virtual nurse in the app. Based on the way of thinking and weaknesses of habit of the user or data automatically transmitted to the cloud server through daily weight measurements, the system creates and proposes a lifestyle improvement program to control NASH tailored to each individual based on an internal algorithm developed with the cooperation of healthcare professionals. The NASH App system consists of 3 “steps” that facilitate the implementation and adherence to individualized lifestyle modifications to achieve a 7% weight loss, which has been shown to be associated with the improvement of NASH (6): step 1, input and education: a personalized interactive education program, including lectures and advice by virtual nurses and input of patient information for promoting personalized lifestyle improvement after step 2; step 2, app-based support interventions: specific instructions to implement lifestyle modifications based on the patient characteristics; based on the patient information extracted in step 1, the algorithm embedded in the NASH App system proposes the necessary behavioral goals for each patient (e.g., for a patient in whom motivating eating behavior is expected to be problematic based on the type analysis, the behavioral goals are set with the goal of passing on the urge to eat); and step 3, self-planning and evaluation: encouragement to maintain the lifestyle modifications described in step 2 to maximize the degree of improvement of NASH. The algorithms of the NASH App system are primarily based on the “Japanese guidelines for the management of obesity disease” (17) and the “guidelines for NAFLD/NASH established by the Japanese Society of Gastroenterology and the Japan Society of Hepatology” (18).
Intervention and procedure
Throughout the 48-week intervention period, the patients received intensive and consistent lifestyle modification support through the NASH App in addition to regular outpatient care as recommended by the Japanese Society of Gastroenterology and the Japan Society of Hepatology (18). During the trial, patients worked on step 1 to step 3 of the NASH App. Based on their personal data from the NASH App Console, the physicians provided support to the patients, promoted daily app usage, and provided education related to NASH to the patients. The results of a liver biopsy performed within 90 days before obtaining informed consent for this study were used as the baseline histological variables, and an additional biopsy was performed at the end of week 48. The liver biopsy performed at the end of the trial period was also assessed by the same expert pathologists as the earlier liver biopsy.
Outcomes
The primary efficacy endpoint was change in the NAS without worsening of the liver fibrosis (worsening of liver fibrosis was defined as an increase in the fibrosis stage by at least 1 level). The secondary endpoints included improvement of the NAS by at least 2 points or disappearance of hepatocyte ballooning, changes in other histological findings (fibrosis, steatosis, lobular inflammation, and ballooning), reduction of the body weight by at least 7%, which has been shown to be necessary for the improvement of NASH (6), changes in the levels of liver enzymes, changes in the body weight, and lipid and glucose parameters. Safety endpoints included the incidence of adverse events after enrollment in the study. The following measures were used to evaluate the usage of the app and adherence to the recommendations (1): mobile app engagement rate, (2) weight measurement rate, and (3) habit improvement rate. The mobile app engagement rate was calculated as the number of days that the app was used divided by the total duration (in days) of participation in the trial. The weight measurement rate was the number of days on which weight measurements were taken divided by the duration of the trial (48 weeks). The habit improvement rate was the number of behavioral goals achieved divided by the total number of action goals.
Statistical analysis
The sample size calculation was based on the reported change in the NAS in the placebo group in a previous study (19). In this study, the mean change in the NAS in the placebo group was −0.7, with an SD of 1.8. According to another study, the mean change in the NAS in patients with NASH who achieved a weight loss of 7% or greater at 48 weeks was −3.45(6). Assuming that 50% of patients achieved a weight loss of at least 7% and that the remaining 50% followed the previously reported distribution of change in the NAS in the placebo group (19), the estimated NAS change due to the DTx intervention in this study was set at −2.10. The intervention was considered as being effective if the upper limit of the 95% confidence interval of the NAS change in the study subjects was lower than −0.70. Therefore, with a threshold of −0.70, an expected value of −2.10 and an SD of NAS change of 1.80, a 2-sided significance level of 5%, and a statistical power of 80%, the sample size required was calculated as 16. Assuming a dropout rate of 20%, the target sample size was set at 20 patients.
Continuous variables are presented as mean ± SD or median and interquartile range and were compared with the use of the t test or the Wilcoxon rank sum test, as appropriate. Categorical variables were expressed as numbers and proportions (%). For the comparison between the parameter at the baseline and after 48 weeks of intervention, we used paired t test or the Wilcoxon matched pairs test when appropriate. Correlations among the continuous variables was determined by calculation of the Pearson correlation coefficient. All statistical analyses were performed using the R 4.1.0 software (http://www.r-project.org).
RESULTS
Patients
We prospectively screened 29 patients who underwent liver biopsy for the evaluation of NAFLD between March 1, 2018, and November 30, 2020; finally, 19 patients were included in the analyses (Figure 2).
Figure 2.

Patient flow.
The baseline characteristics of the patients are summarized in Table 1. The mean age of patients was 52.16 years, and male patients accounted for 52.6% (10/19) of all the patients. The mean body mass index of the patients was 32.04 kg/m2. A total of 7 (36.8%) patients had fibrosis stage F1, 6 (31.6%) had stage F2, and 6 (31.6%) had stage F3; the mean NAS was 5.00.
Table 1.
Baseline clinical characteristics (n = 19)

Efficacy endpoints
Figure 3a shows the changes in the NAS from baseline to 48 weeks in each patient. Improvement of the NAS was observed in 68.4% (13 patients) of all patients, and the mean NAS change from the baseline to 48 weeks (primary endpoint) was −2.05 ± 1.96 (95% confidence interval −3.00 to −1.11) (Figure 3b). The Wilcoxon rank sum test showed a significant improvement in the NAS when compared with the hypothetical control group (P < 0.001) (19).
Figure 3.
Changes in the NAS from baseline to 48 weeks. (a) Change in the NAS in each patient. (b) Mean and SD of change in the NAS. NAS, nonalcoholic fatty liver disease score. *P value from the Wilcoxon rank sum test. NAS, nonalcoholic fatty liver disease activity score.
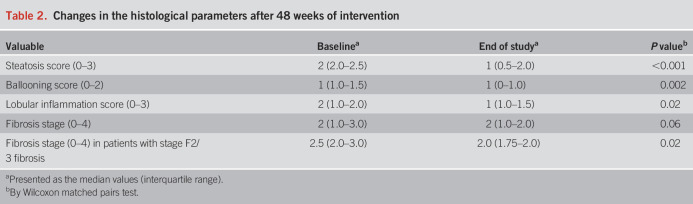
Table 2 and Supplementary Digital Content (see Supplementary Table 1, http://links.lww.com/AJG/C839) shows the histological changes set as secondary endpoints, from baseline to week 48. Decrease in the NAS by at least 2 points from baseline to week 48 without worsening of liver fibrosis was achieved in 57.9% (11/19), and resolution of steatohepatitis (defined as disappearance of hepatocyte ballooning) (20) was observed in 42.1% (8/19) of all patients. Significant histological improvement was achieved for severity of hepatic steatosis, ballooning, and lobular inflammation scores. When limited to patients with stage F2/3 fibrosis, there was a significant histological improvement in fibrosis stage (Table 2). The scores for steatosis, lobular inflammation, and ballooning decreased in 57.9% (11/19), 47.4% (9/19), and 52.6% (10/19) patients, respectively, after 48 weeks of DTx intervention when compared with the baseline. Reduction of the fibrosis stage was observed in 7 of all the 19 patients (36.8%), but when the analysis was limited to patients with stage F2/3 fibrosis, reduction of the fibrosis stage was observed in 58.3% (see Supplementary Table 1, http://links.lww.com/AJG/C839). On the contrary, no patient with stage F1 fibrosis showed reduction of the fibrosis stage. None of the patients who showed improvement of liver fibrosis showed worsening of the NASH (i.e., no worsening of steatosis, ballooning, or lobular inflammation).
Table 2.
Changes in the histological parameters after 48 weeks of intervention
Figure 4a shows the changes in the body weight at 24 and 48 weeks after the start of the DTx intervention in each patient, with the weight at baseline set as 100%. Figure 4b shows the body weight changes expressed as the percent change (%) for each patient from baseline to 24 weeks and 48 weeks after the DTx intervention. After 48 weeks of the DTx intervention, an average weight loss of 8.3% was achieved (P < 0.001 when compared with baseline). Significant weight loss at 48 weeks was observed in both the patient groups with stage F1 (P = 0.001) and F2/3 (P = 0.003) fibrosis. Body weight reduction of at least 7% was achieved in 47.4% of patients (9/19) at 24 weeks and 52.6% of patients (10/19) at 48 weeks. A significant correlation was observed between the actual rate of weight loss observed at 48 weeks and the amount of change in the NAS (ρ = −0.813, P < 0.001) (see Supplementary Figure 1, http://links.lww.com/AJG/C838).
Figure 4.
Changes in the body weight at 24 and 48 weeks, with the weight at baseline set at 100%. (a) Change in the body weight in each patient, with the weight at baseline set at 100%. (b) Mean and SD of body weight change, with the weight at baseline set at 100%.
There were significant reductions in the serum levels of aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, and alkaline phosphatase and triglycerides. Although the difference did not reach a statistically significant level, improvements were also seen in parameters related to insulin resistance, such as blood glucose, fasting insulin, and homeostasis model assessment–insulin resistance (Table 3).
Table 3.
Changes in the physical and biochemical parameters after 24 and 48 weeks of intervention
The mobile app engagement rate was 74.7% at 12 weeks, 71.1% at 24 weeks, and 67.1% at 48 weeks. The corresponding weight measurement rates were 53.3%, 53.5%, and 48.1%, respectively. A significant correlation was observed between the weight measurement rate calculated by dividing the number of days on which weight measurements were made by the duration of trial (48 weeks) and the amount of change in the NAS (ρ = −0.470, P = 0.04) (see Supplementary Figure 2, http://links.lww.com/AJG/C838).
The mean habit improvement rate calculated by dividing the number of behavioral goals achieved divided by the total number of action goals was 19.1%. A negative correlation was observed between the habit improvement rate and the amount of change in the NAS (ρ = −0.456, P = 0.0499) (see Supplementary Figure 3, http://links.lww.com/AJG/C838).
Safety endpoints
During the study, a total of 6 patients (31.6%) reported adverse event(s) (see Supplementary Table 2, http://links.lww.com/AJG/C839). There were 2 serious adverse events: one patient was admitted to hospital for testicular cancer and the other for variant angina. None of the adverse events in this study were considered to be related to the DTx system, with the exception of myalgia in one case.
DISCUSSION
Although weight reduction through therapeutic lifestyle modifications is recommended as the initial treatment for NASH (6), no interventional approach for promoting weight reduction has been established yet. To the best of our knowledge, this study is the first trial of a DTx intervention for patients clinically diagnosed as having NASH, using histological outcomes as endpoints.
In this study, we set the hypothetical control group established based on the placebo group in a previous report (19), in which the mean improvement of NAS was 0.7. The previous meta-analysis of placebo responses in NAFLD trial showed an improvement of the NAS of 0.72 points (21), suggesting that the control group established in this study may be considered acceptable. The DTx intervention in this study yielded significant improvement of the NAS compared with the control group. Furthermore, significant improvements in all the histological endpoints and liver enzyme levels were achieved after 48 weeks of DTx intervention using the NASH App. Decrease of the NAS by ≥2 points has been shown to be associated with regression of liver fibrosis (22). The NAS decreased by a mean of 2.05 points from the baseline to 48 weeks, and improvement of the NAS by ≥2 points without worsening of liver fibrosis was observed in 57.9% of all patients in this study. Patients with moderate or advanced liver fibrosis are at an increased risk of cirrhosis (23), which justifies the need for therapeutic intervention in patients with NASH and advanced liver fibrosis. Improvement of the fibrosis stage was found in 58.3% of patients with F2 and F3 at baseline in this study. In the previous meta-analysis, 25% of placebo-treated patients with NAFLD had an improvement of the NAS by ≥ 2 points and 21% had an improvement in liver fibrosis score (21). NASH is a major predictor of progressive liver fibrosis (22,24), and fibrosis is the primary predictor of medical complications and death in patients with NASH (25). Regression of fibrosis in NASH is associated with a decreased risk of liver-related events (26) and is a surrogate endpoint for regulatory approval (27). Although significant weight loss was observed, no reduction in the fibrosis stage was observed in patients with stage F1 fibrosis. There is less room for improvement in patients with stage F1 fibrosis, and the patients may not have achieved the histologic improvement associated with weight loss within this short study period of 48 weeks. In addition, we did not obtain information in this study on liver fibrosis in long-term follow-up cases after weight loss. Further studies are needed to clarify the effects of weight loss induced by lifestyle interventions on hepatic fibrosis, including the impact of differences in the fibrosis stage at the baseline.
Smartphones are ubiquitous, highly personalized, and easy-to-use devices. The widespread use of smartphones, which can process and communicate data in real time, makes them an ideal platform for therapeutic interventions. Digital interventions have been shown to be highly effective for some conditions such as type 2 diabetes mellitus or depression (7,28). In a randomized controlled trial conducted in New Zealand, a computerized cognitive behavioral therapy intervention for patients with depression yielded a significantly higher remission rate when compared with traditional face-to-face therapy sessions with trained counselors or psychologists (28). In addition, recently, 2 randomized controlled trials of a novel DTx smartphone app, CureApp-SC, for nicotine dependence (9), and HERB Mobile system, for essential hypertension (11), showed a significant clinical efficacy of DTx systems. DTx is expected to play a revolutionary role in supplementing and satisfying unmet needs in real-world clinical practice.
Promrat et al (6) examined the effects of lifestyle interventions using an intensive, state-of-the-art weight loss intervention. In their study, the control group showed an average body weight loss of 0.5%, while those who received the intervention for 48 weeks focused on diet, exercise, and behavioral modifications showed an average body weight loss of 8.8%, indicating the effectiveness of the specialized lifestyle intervention. Although a simplistic comparison would be difficult due to the difference in the profile of the study subjects, in our study, the 48-week DTx intervention led to an average weight loss of 8.3%, which was comparable with that in the specialized intervention group in the study reported by Promat et al (6). DTx interventions may be no less effective than intensive human interventions provided in specialized facilities.
Because the cost of computerized therapeutics has been shown to be substantially lower than that of traditional therapeutics (29), mobile apps and discount programs have begun to emerge in response to the problem of rising medical costs (30). Previous studies have shown a cost-saving effect of mobile phone–supported interventions for smoking cessation (31), diabetes mellitus (32), and hypertension (33). Obesity-related medical costs are currently on the rise (34). Computerized cognitive behavioral DTx intervention for NASH could lead to reductions in obesity-related medical costs in the future, through cognitive reconstruction of the patients, and amelioration of the liver condition.
This study had a few limitations. The major limitation was the single-arm design, which precluded us from assessing the efficacy and safety of the DTx intervention using a control group. The second limitation was the lack of a central evaluation system. Although the liver biopsies performed at the end of the 48-week trial were assessed by the same expert hepatopathologists as the earlier biopsies, a central evaluation by a small number of pathologists would have been desirable. In addition, the study pathologist was not completely blinded to intervention or the order of the procedure. However, the findings of the histological assessments were supported by changes in the body weight, a more objective endpoint leading to histological improvement, and were considered as being credible. A future randomized controlled trial in a larger population may be warranted to confirm the beneficial effect of the DTx intervention on NASH. Third, pioglitazone was the only antidiabetic medication included in the exclusion criteria. Sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 analogs, which could potentially affect the study outcomes (35,36), were not included in the exclusion criteria, but patients taking these drugs were not enrolled in this study. However, the influence of other antidiabetic medications (i.e., metformin in 1 patient) (37) cannot be completely ruled out. Fourth, although the physicians browsed the personal data of the patients at the outpatient department and provided feedback to the patients based on the data, the time spent by the physician browsing the data at the outpatient department was not collected as a variable. Therefore, the app program was not individualized according to the degree of physician involvement, and we were unable to analyze the impact of the time spent by the provider in this study. However, we assume that the attending physician saw the console at the outpatient department and may therefore not have spent much time on it. And finally, because the NASH App system is designed to promote the improvement of NASH through setting and managing behavioral goals for each patient, we did not collect detailed information on the physical activity, caloric intake, or diet composition. Therefore, the impact of these factors on the improvement of NASH cannot be evaluated in this study.
In conclusion, the results of this prospective study suggest the potential effectiveness of a 48-week DTx intervention using the NASH App for patients with NASH. Thus, DTx seems to be a promising therapeutic strategy, and we believe that further evaluation of DTx intervention for NASH in a phase 3 trial is warranted.
CONFLICTS OF INTEREST
Guarantor of the article: Masaya Sato, MD, PhD.
Specific author contributions: The 15 authors are justifiably credited with authorship, according to the authorship criteria. M.S. conception, design, drafting of the manuscript, acquisition of the data, and final approval given; M.A., T.S., T.I., M.Y., S.M., J.I., and T.N.: acquisition of the data, final approval given; R.T.: critical revision of manuscript, supervision, final approval given; N.Y.: acquisition of the data, final approval given; T.U., T.O., and M.F.: supervision, final approval given; E.H.: data analysis, critical revision of manuscript, and final approval given; K.K.: critical revision of manuscript, supervision, and final approval given.
Financial support: This study was supported by CureApp Inc. (Tokyo, Japan).
Potential competing interests: E.H. has a consultation contract as a biostatistician with CureApp Inc. No other authors have conflict of interest to declare.
Data availability: The data that support the findings of this study will be made available by the corresponding author upon reasonable request.
ACKNOWLEDGEMENT
CureApp Inc. provided funding and contributed to the development of the NASH App system. CureApp Inc. assisted with the trial design and performed site monitoring and data management under a third-party audit. This document has been edited by a professional English language editor.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C838, http://links.lww.com/AJG/C839
ClinicalTrials.gov number, UMIN000029835 https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000033976, the Japan Registry of Clinical Trials (jRCT) (No. jRCTs032180372).
Contributor Information
Masatoshi Akamatsu, Email: m-akamatsu@jreast.co.jp.
Toshihide Shima, Email: shima0301d@suita.saiseikai.or.jp.
Tadashi Ikegami, Email: ikegamit@tokyo-med.ac.jp.
Mikio Yanase, Email: myanase@hosp.ncgm.go.jp.
Shintaro Mikami, Email: me_car_3@yahoo.co.jp.
Jun Imamura, Email: jun.imamu@cick.jp.
Takuma Nakatsuka, Email: NAKATSUKAT-INT@h.u-tokyo.ac.jp.
Ryosuke Tateishi, Email: ryo.tate@gmail.com.
Naoko Yamauchi, Email: yamauchi-tky@umin.ac.jp.
Tetsuo Ushiku, Email: usikut-tky@umin.ac.jp.
Takeshi Okanoue, Email: okanoue@suita.saiseikai.or.jp.
Mitsuhiro Fujishiro, Email: mtfujish@gmail.com.
Eisuke Hida, Email: e-hida@bsds.med.osaka-u.ac.jp.
Kazuhiko Koike, Email: kkoike.tky@gmail.com.
REFERENCES
- 1.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology 2020;158(7):1851–64. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004;40(6):1387–95. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig J, Viggiano TR, McGill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55(7):434–8. [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: Summary of an AASLD single topic conference. Hepatology 2003;37(5):1202–19. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002;123(5):1705–25. [DOI] [PubMed] [Google Scholar]
- 6.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinn CC, Shardell MD, Terrin ML, et al. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes care 2011;34(9):1934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masaki K, Tateno H, Kameyama N, et al. Impact of a novel smartphone app (CureApp smoking cessation) on nicotine dependence: Prospective single-arm interventional pilot study. JMIR Mhealth Uhealth 2019;7(2):e12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masaki K, Tateno H, Nomura A, et al. A randomized controlled trial of a smoking cessation smartphone application with a carbon monoxide checker. NPJ Digit Med 2020;3(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kario K, Nomura A, Harada N, et al. A multicenter clinical trial to assess the efficacy of the digital therapeutics for essential hypertension: Rationale and design of the HERB-DH1 trial. J Clin Hypertens (Greenwich) 2020;22(9):1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kario K, Nomura A, Harada N, et al. Efficacy of a digital therapeutics system in the management of essential hypertension: The HERB-DH1 pivotal trial. Eur Heart J 2021;42(40):4111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunami T, So R, Ishii H, et al. A randomized controlled trial of the web-based drinking diary program for problem drinking in multi workplace settings. J Occup Health 2022;64(1):e12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtdirk F, Mehnert A, Weiss M, et al. Results of the optimune trial: A randomized controlled trial evaluating a novel internet intervention for breast cancer survivors. PLoS One 2021;16(5):e0251276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzawa Y. New criteria for “obesity disease” in Japan. Circ J 2002;66(11):987–92. [DOI] [PubMed] [Google Scholar]
- 15.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94(9):2467–74. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41(6):1313–21. [DOI] [PubMed] [Google Scholar]
- 17.Japan Society for the Study of Obesity. Guidelines for the Management of Obesity Disease. Chiyoda-ku, Tokyo: Life Science Publishing; 2016. (in Japanese). [Google Scholar]
- 18.Watanabe S, Hashimoto E, Ikejima K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol 2015;50(4):364–77. [DOI] [PubMed] [Google Scholar]
- 19.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015;385(9972):956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. The Lancet 2016;387(10019):679–90. [DOI] [PubMed] [Google Scholar]
- 21.Han MAT, Altayar O, Hamdeh S, et al. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17(4):616–29.e26. [DOI] [PubMed] [Google Scholar]
- 22.Brunt EM, Kleiner DE, Wilson LA, et al. Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: Results from the nonalcoholic steatohepatitis clinical research network treatment trials. Hepatology 2019;70(2):522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomba R, Adams LA. The 20% rule of NASH progression: The natural history of advanced fibrosis and cirrhosis caused by NASH. Hepatology 2019;70(6):1885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Wilson LA, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2019;2(10):e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65(5):1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Anstee QM, Trauner M, et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology 2022;75(5):1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Food, Drug Administration. Noncirrhotic Nonalcoholic Steatohepatitis with Liver Fibrosis: Developing Drugs for Treatment. Guidance for Industry, 2018. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/noncirrhotic-nonalcoholic-steatohepatitis-liver-fibrosis-developing-drugs-treatment). Accessed September 1, 2022. [Google Scholar]
- 28.Merry SN, Stasiak K, Shepherd M, et al. The effectiveness of SPARX, a computerised self help intervention for adolescents seeking help for depression: Randomised controlled non-inferiority trial. BMJ 2012;344(3):e2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCrone P, Knapp M, Proudfoot J, et al. Cost-effectiveness of computerised cognitive-behavioural therapy for anxiety and depression in primary care: Randomised controlled trial. Br J Psychiatry 2004;185(1):55–62. [DOI] [PubMed] [Google Scholar]
- 30.Dobkin HE, Zirwas M. Price discrepancies among mobile medication applications for common dermatologic prescriptions: Observational cost analysis. J Am Acad Dermatol 2017;77(6):1181–2. [DOI] [PubMed] [Google Scholar]
- 31.Guerriero C, Cairns J, Roberts I, et al. The cost-effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur J Health Econ 2013;14(5):789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobis S, Lehr D, Ebert DD, et al. Efficacy and cost-effectiveness of a web-based intervention with mobile phone support to treat depressive symptoms in adults with diabetes mellitus type 1 and type 2: Design of a randomised controlled trial. BMC Psychiatry 2013;13:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomura A, Tanigawa T, Kario K, et al. Cost-effectiveness of the digital therapeutics for essential hypertension. Hypertens Res 2022;45(10):1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford ES, Li C, Zhao G, et al. Trends in obesity and abdominal obesity among adults in the United States from 1999-2008. Int J Obes (Lond) 2011;35(5):736–43. [DOI] [PubMed] [Google Scholar]
- 35.Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: A randomized controlled trial (E-LIFT trial). Diabetes Care 2018;41(8):1801–8. [DOI] [PubMed] [Google Scholar]
- 36.Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384(12):1113–24. [DOI] [PubMed] [Google Scholar]
- 37.Doycheva I, Loomba R. Effect of metformin on ballooning degeneration in nonalcoholic steatohepatitis (NASH): When to use metformin in nonalcoholic fatty liver disease (NAFLD). Adv Ther 2014;31(1):30–43. [DOI] [PubMed] [Google Scholar]