Abstract
Alphavirus expression systems based on suicidal virus particles carrying recombinant replicons have proven to be a very efficient way to deliver genes for heterologous protein expression. However, present strategies for production of such particles have biosafety limitations due to the generation, by RNA recombination, of replication-proficient viruses (RPVs). Here we describe a new packaging system for Semliki Forest virus (SFV) based on a the use of a two-helper system in which the capsid and spike proteins of the C-p62-6K-E1 polyprotein are expressed from two independent RNA molecules. The capsid gene contains a translational enhancer and therefore that sequence was also engineered in front of the spike sequence p62-6K-E1. A sequence coding for the foot-and-mouth disease virus 2A autoprotease was inserted in frame between the capsid translational enhancer and the spike genes. This allows production of the spike proteins at high levels with cotranslational removal of the enhancer sequence and normal biosynthesis of the spike complex. The autoprotease activity of the capsid protein was abolished by mutation, further increasing the biosafety of the system. Cotransfection of cells with both helper RNAs and an SFV vector replicon carrying the LacZ gene led to production of recombinant particles with titers of up to 8 × 108 particles per 106 cells. Extensive analysis failed to demonstrate the presence of any RPVs, emphasizing the high biosafety of the system based on two-helper RNAs.
In recent years, much progress has been made in the development of expression systems for the delivery of foreign genes for vaccination or gene therapy. These systems include the use of viral vectors and the direct delivery of naked nucleic acids (for reviews, see references 8, 33, 35, 37–39, 48, and 52). Viral vectors are superior in terms of efficiency of entry into the target cells, which is usually mediated through specific receptor binding at the cell surface. However, one of the main problems present in many viral systems is the generation of replication-proficient viruses (RPVs) during the production process. This is usually the consequence of recombination taking place between the recombinant genome to be encapsidated into particles and the helper systems used to provide all of the elements necessary for the production of the particles. Contamination with RPVs can be a major problem in the case of vectors based on pathogenic viruses, such as retroviruses (4, 5, 29). Other problems associated with the production of viral vectors are related to low packaging efficiencies and difficulties in the generation of high titers of recombinant particles (20).
Alphaviruses, which are enveloped positive-strand RNA viruses, have recently been exploited as expression vectors (for reviews, see references 11 and 23). Alphaviruses offer several advantageous features, such as a broad host range (including cells of avian, insect, and mammalian origin), high levels of protein expression, and a small genome that is easy to manipulate. Alphaviral vectors have been based on Semliki Forest virus (SFV), Sindbis virus, and Venezuelan equine encephalitis virus (VEE) (26, 36, 51). The genome of alphaviruses is a capped and polyadenylated positive-strand RNA molecule of 11 to 12 kb (45). Productive infection can be initiated either by infection or by transfection of a cell with the viral RNA. The 5′ two-thirds of this RNA can be directly translated to produce the viral replicase. This enzyme will copy the genome into negative strands, which serve as the template for the production of new positive-strand genomes. The replicase also utilizes an internal promoter on the minus strand to produce a subgenomic RNA which corresponds to the last third of the virus genome. This subgenomic RNA encodes the structural proteins of the virus. These proteins are synthesized as a polyprotein precursor (NH2-C-p62-6K-E1-COOH) which is cotranslationally cleaved by the capsid protease and signal peptidase to produce the capsid protein (C) and the envelope proteins p62, 6K, and E1 (13, 19, 25, 31, 32). In SFV and Sindbis virus sequences at the 5′ end of the capsid gene function as a translational enhancer, providing a high expression level of the structural proteins (12, 43). The C protein complexes with new viral genomes to form cytoplasmic nucleocapsid structures, while the spike proteins are translocated to the endoplasmic reticulum (ER), where p62 and E1 dimerize and are routed to the cell surface, where budding occurs (14, 16, 46). During transport to the cell surface p62 is cleaved to its mature form E2 by a host cell protease. This cleavage is necessary for the infectivity of the particles (28, 42).
In the SFV vector system, the production of recombinant particles is based on the cotransfection of cells with two independent RNAs. The first one is the vector replicon, which contains the replicase gene, the subgenomic promoter followed by the heterologous gene, and the 5′ and 3′ ends of the genome required for replication. The second one is the helper RNA, which also contains the 5′ and 3′ replication signals and a subgenomic promoter followed by the genes coding for the structural proteins (helper-1) (23, 26). Both RNAs can be synthesized in vitro from plasmids which contain these sequences under the SP6 promoter or produced within the cell by transcription from a eukaryotic promoter (2, 7, 9). The replicase encoded by the vector molecule will amplify both RNA species. However, only the replicase containing RNA is packaged into viral particles, since the capsid packaging signal is located within the replicase gene, a region which is absent from the helper RNA (22, 50). The particles generated in this way contain a defective genome that while able to replicate cannot result in a productive infection. When such particles are used to infect animal cells, only the replicase and the heterologous gene products are expressed, and since no structural proteins can be produced no new particles are generated. However, a potential problem arises if wild-type virus is formed during packaging reactions due to recombination between the two RNA species. The generation of such wild-type virus occurs with a relatively high frequency in this type of SFV packaging system (SFV-helper-1) (1). In order to circumvent this problem a new SFV helper system was developed in which a mutation abolishing intracellular cleavage of p62 was introduced, rendering the particles conditionally infectious (helper-2) (1). The noninfectious particles can be artificially “activated” by incubation with chymotrypsin in vitro. With this packaging strategy, RPVs have not been detected, increasing considerably the biosafety of the SFV expression system. However, the helper-2 system presents some drawbacks, such as the necessity of activating the particles with chymotrypsin, which makes their possible use in vaccination less convenient and more costly. In addition, recombination between the SFV replicon and the helper-2 RNAs is not prohibited, although the generation of RPVs would also require reversions or suppressor mutations to regenerate a functional p62 cleavage site. Such mutations have been found which either regenerated the p62 cleavage site or restored infectivity without cleavage of p62, probably through conformational changes of the protein (47). When additional nucleotide changes were made to reduce the frequency of reversion, new suppressors were found in a different domain of the protein (47). Although the probability of simultaneously having recombination and suppressor mutations is very low, it cannot be completely excluded. In this study we present an alternative strategy for packaging of SFV recombinant RNAs. By splitting the capsid and spike helper regions into two independent RNAs, recombination becomes negligible. An additional mutation that abolishes the capsid self-cleaving activity (19, 32) has also been introduced into the helper, further increasing the biosafety of the system.
MATERIALS AND METHODS
Plasmid constructs.
The replicon encoding plasmids pSFV-lacZ (26) and pSFV-Camber (originally designated as pSFV-c) (46) have been previously described. pSFV-helper-S1, -S2, and -S3 were constructed as follows. Two complementary synthetic oligonucleotides, 5′-gttgcaggccactccggtggctcccgtcgtcaattttgaccttcttaagcttgcgggagacgtcgagtccaaccctgggccctccgccccgctgattactgcc-3′ and 5′-ggagggcccagggttggactcgacgtctcccgcaagcttaagaaggtcaaaattgacgacgggagccaccggagtggcctgcaac-3′, containing the sequence of foot-and-mouth disease virus (FMDV) 2A protease (40) preceded by a few nucleotides of the 3′ end of the SFV capsid translation enhancer (43), were hybridized and cloned into the unique StuI site of pSFV-helper-1. The small BstXI/BstXI fragment was removed to delete extra capsid sequences. Finally, the small ApaI/XhoI fragment was substituted by PCR products S1, S2, and S3 (synthesized as described below), yielding plasmids pSFV-helper-S1, -S2, and -S3, respectively. PCR products S1, S2, and S3 were synthesized by using pSFV-helper-1 as a template; primer 5′-gaccggacggcattctcaatg-3′ (nucleotides 442 to 420 of p62) as downstream primer; and 5′-cctgggccctccgccccgctgattactgcc-3′, 5′-aaccctgggcccgccccgctgattactgcc-3′, and 5′-aaccctggg cccctgattactgccatgtgtgtcc-3′, which contain the different 2A-p62 fusion sequences, as upstream primers, respectively. pSFV-helper-E was constructed by digesting pSFV-spike (46) with AccI to remove most of the open reading frame (ORF) coding for the replicase. pSFV-helper-C was derived from pSFV-Camber by deletion of both the replicase sequences flanked by AccI and the small NdeI/XhoI fragment within the p62 ORF. In order to generate pSFV-helper-C-S219A and pSFV-helper-1-S219A, a PCR fragment was synthesized by using pSFV-helper-C as a template, and primers 5′-gattgaaaatgactgtatcttcgaagtc-3′ and 5′-ttgtcaaagatgggccggccggcgtctcccggtttgcccgc-3′ as upstream and downstream primers, respectively. The latter contains three mutated nucleotides (underlined sequence), which give rise to a change of serine to alanine in position 219 of the capsid. The PCR fragment obtained in this way was digested with BstBI and FseI and cloned into both pSFV-helper-C and pSFV-helper-1 digested with the same enzymes.
Transfection of cells and in vivo packaging of recombinant RNA.
RNA synthesis was performed as described previously (26), but omitting the bovine serum albumin from the reaction and using 45 U of SP6 polymerase (Pharmacia Biotech, Uppsala, Sweden) per reaction. RNA was transfected into BHK-21 cells by electroporation as described previously (24, 27). To package recombinant RNAs into SFV particles with a single helper, 25 μl of the recombinant and the helper RNA transcription reactions were mixed and added to the cells for electroporation. When recombinant SFV RNA was packaged with the two-helper RNA system, 50 μl of each RNA transcription reaction was used.
Analysis of gene expression.
Metabolic labelling of SFV infected-transfected cells (27), immunofluorescence (42), immunoprecipitation (49), and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (25) were done essentially as described previously. Monoclonal antibodies (MAbs) specific for E1 (E1-1) and E2 (E2-3) were kindly provided by M. Kielian (21). For detection of the capsid protein, specific MAb 36-1-9 was used (17). For detection of β-galactosidase (β-Gal) expression, a commercial MAb was used (Boehringer Mannheim). The MAb specific for the nsp2 subunit of the replicase was kindly provided by W. Bodemer. Supernatants from metabolically labeled transfected cells were clarified three times by low-speed centrifugation and then ultracentrifuged at 100,000 × g through a 20% sucrose cushion in order to selectively pellet the viral particles.
Infection of BHK-21 cells with SFV recombinant particles.
BHK-21 cells were infected with SFV particles as described previously (27). To determine the number of PFU, confluent monolayers of BHK-21 cells were infected with different dilutions of the virus, after 1 h of adsorption medium was removed and overlay medium was added (BHK-21 complete medium and minimal essential medium–1.9% agarose, [1:1]). Cells were incubated for 48 to 72 h at 37°C, fixed with 10% formaldehyde in phosphate-buffered saline, and stained with 0.1% crystal violet in methanol-H2O (20:80).
RESULTS
Expression of capsid and spike proteins from separate helpers.
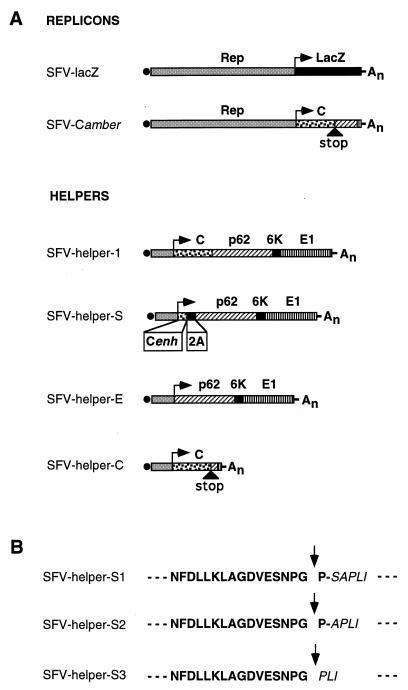
The two-helper system placed the capsid and spike protein encoding genes of helper-1 on separate molecules. Translation termination of the capsid was achieved by placing a stop codon (amber) at the end of the ORF (Fig. 1A). The 5′ end of the capsid gene coding for the first 34 amino acid residues has been shown to contain a translational enhancer (12, 43). Therefore, to express the spike proteins of SFV independently from the capsid at a level similar to that of the wild-type virus, a fragment coding for those residues was placed in front of p62-6K-E1. The sequence of FMDV 2A autoprotease, encoding only 17 amino acids (40), was inserted in frame between the capsid translational enhancer and p62 in order to provide cleavage between the two proteins (Fig. 1A). As the 2A protease requires a proline at the COOH-terminal side of the cleavage site (41), this amino acid will be the first residue of p62. Because the effect of this extra amino acid in p62 was unknown, three different constructs were made. The first one contained an extra proline at the NH2-terminal sequence of p62 (helper-S1). In the second one, the extra proline was substituted for the first serine of p62 (helper-S2), and in the third construct the first two amino acids of p62 were deleted, taking advantage of the fact that the third residue in p62 is a proline (helper-S3) (Fig. 1B).
FIG. 1.
SFV replicons and helpers used in the study. (A) The two replicons SFV-lacZ and SFV-Camber, as well as helper SFV-helper-1, have been described previously (26, 46). In all cases the arrow indicates the position of the subgenomic promoter 26S from which the replicase starts the synthesis of the subgenomic RNAs. The solid black circle to the left of each RNA represents the CAP. Rep, SFV replicase; C, capsid; Cenh, capsid enhancer; stop, termination codon UAG; 2A, FMDV 2A autoprotease; An, poly(A). (B) Amino acid sequence of the FMDV 2A protease-p62 fusion in the different SFV-helper-S. Boldface text indicates the sequence from FMDV 2A protease. Text in italics indicates the sequence from p62. The arrow indicates the putative 2A cleavage site.
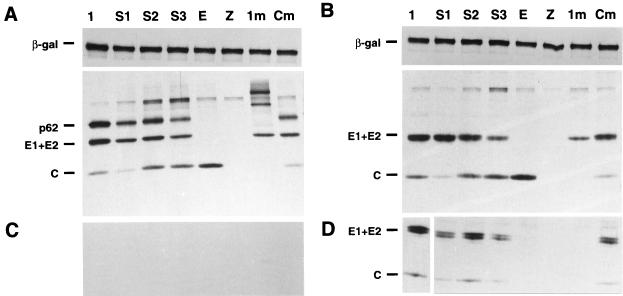
To compare the expression of the spike proteins from the three different SFV-helper-S systems, RNAs were transcribed in vitro from each of the plasmids with SP6 polymerase and used to cotransfect BHK-21 cells together with SFV-Camber RNA (46). This latter RNA is an SFV genome in which the sequences coding for the spike proteins have been deleted and a stop codon has been placed at the end of the capsid gene, providing both the replicase and the capsid protein in trans (Fig. 1A). SFV-helper-E RNA, in which the spike proteins are expressed directly from the SFV 26S promoter without the capsid enhancer was also cotransfected with SFV-Camber RNA to monitor the capsid enhancer activity. Cells were pulsed-labeled and chased for different times (Fig. 2). When the expression was analyzed after a 5-min chase, a band which had the same apparent molecular weight as p62 expressed in cells cotransfected with SFV-Camber plus helper-1 (used as a positive control) could be detected with helper-S1, -S2, and -S3 in lysates of transfected cells, indicating that the protein had been properly cleaved off from the capsid enhancer by the 2A autoprotease. The intensity of the p62 band and of a lower band of approximately 50 kDa corresponding to E1+E2 is very similar in both helper-1- and the helper-S-transfected cells, indicating that the capsid NH2-terminal sequence placed in helper-S is providing the expected translational enhancement. The bands corresponding to p62 and E1+E2 had a much lower intensity when they are expressed from helper-E, which does not contain the translation enhancer (Fig. 2A, lane E). After a 3-h chase most of p62 had been processed to E2 (which comigrates with E1) in all three helper-S systems and helper-1 (Fig. 2B). Moreover, similar amounts of spike proteins (E1+E2) and capsid could be detected in the supernatant of cells transfected with helper-1 and the different helper-S RNAs after a 3-h chase, indicating that in all cases viral particles had been formed with similar efficiency (Fig. 2D).
FIG. 2.
SDS-PAGE analysis of SFV structural proteins in cells cotransfected with SFV-Camber replicon and different SFV-helper RNAs. At 12 h posttransfection, cells were pulse-labeled for 15 min and chased for 5 min (A and C) or 3 h (B and D). Cell lysates were loaded directly onto the gel (A and B). The bands in gels A and B are specific for SFV, since the efficient shutoff of host cell proteins induced by SFV replication only allows SFV-driven genes to be expressed. Virus was sedimented from supernatants as described in Materials and Methods (C and D). SFV-Camber RNA was coelectroporated with the following RNAs: lane 1, SFV-helper-1; lane S1, SFV-helper-S1; lane S2, SFV-helper-S2; lane S3, SFV-helper-S3; lane E, SFV-helper-E.
Production of viral particles with different helpers.
BHK-21 cells were cotransfected with SFV-Camber RNA and helper-1, -S1, -S2, -S3, or -E, respectively. Supernatants were collected 24 h later and titrated on BHK-21 monolayers. Only marginally higher titers were obtained for helper-1 compared to the different helper-S RNAs tested (Table 1), indicating that the small modification in the NH2-terminal end of p62 expressed from the three different helper-S RNAs did not affect the functionality of the protein. These titers also confirmed that the spike proteins were expressed at a similar level from all helper-S RNAs compared to that from helper-1. The titers obtained when the spikes were expressed without the capsid enhancer (helper-E) were reduced 16 to 24 times, reflecting the much lower expression level of the spikes.
TABLE 1.
Titers of SFV recombinant particles packaged with different SFV helper RNAs
| Packaged replicon | SFV helper RNAs | Titer (particles/ 106 cells)a |
|---|---|---|
| SFV-Camber | Helper-1 | 4.8 × 109 |
| Helper-S1 | 2.0 × 109 | |
| Helper-S2 | 2.4 × 109 | |
| Helper-S3 | 1.6 × 109 | |
| Helper-E | 1.0 × 108 | |
| SFV-lacZ | Helper-1 | 1.9 × 109 |
| Helper-S1 + helper-C | 2.2 × 108 | |
| Helper-S2 + helper-C | 2.0 × 108 | |
| Helper-S3 + helper-C | 8.3 × 107 | |
| Helper-E + helper-C | 3.0 × 106 | |
| Helper-1-S219A | 8.0 × 104 | |
| Helper-S1 + helper-C-S219A | 4.7 × 108 |
The titers are the average of at least three independent experiments that yielded very similar results. SFV-Camber and SFV-lacZ particles were titrated with an MAb specific for the SFV replicase and with an MAb specific for β-Gal, respectively.
Packaging of SFV recombinant RNA with a two-helper system.
In order to test the functionality of the SFV two-helper system, BHK-21 cells were cotransfected with RNAs transcribed from pSFV-lacZ, pSFV-helper-C, and each of the pSFV-helper-S plasmids, or pSFV-helper-E. Cells were pulsed-labeled and chased for different times (Fig. 3). After either a 5-min or a 3-h chase, the same pattern of labeled proteins was observed in lysates of cells cotransfected with SFV-lacZ plus SFV-helper-C plus SFV-helper-S RNAs as in cells cotransfected with SFV-lacZ plus SFV-helper-1 RNAs (Fig. 3A and B, six first lanes). When cells were cotransfected with SFV-lacZ plus SFV-helper-C plus SFV-helper-E RNAs, the spike proteins could not be detected, as was expected from previous results. In the supernatants, only the SFV structural proteins could be detected after a 3-h chase, but not β-Gal (not shown) since this protein is not incorporated into particles (Fig. 3C and D, six first lanes). The relative intensity of the bands in the supernatants indicated a higher production of particles in the cells electroporated with helper-1 compared to those cotransfected with helper-S plus helper-C. Very faint bands could be detected with helper-E plus helper-C, indicating a very poor production of particles (Fig. 3D, lane E). In a parallel experiment the supernatants from similarly transfected cells were collected after 24 h and titrated (Table 1). Cells transfected with SFV-lacZ plus SFV-helper-C plus SFV-helper-S RNAs yielded titers which were about 10 times lower than those obtained with SFV-lacZ plus SFV-helper-1; this was probably due to the higher probability of transfecting a cell with two RNAs versus three RNAs. However, in one single experiment, a titer of 8 × 108 particles/106 cells was obtained with SFV-helper-S1 (not included in Table 1), indicating that the system can be further optimized. In general, the titers obtained with helper-S3 were slightly lower than the ones obtained with helper-S1 and -S2. When cells were cotransfected with SFV-lacZ plus SFV-helper-E plus SFV-helper-C RNAs, the titers were reduced almost 1,000-fold with respect to helper-1, as was expected from previous experiments involving two RNAs.
FIG. 3.
SDS-PAGE analysis of SFV structural proteins in cells cotransfected with the SFV-lacZ replicon and different SFV-helper RNAs. At 12 h posttransfection, cells were pulse-labeled for 15 min and chased for 5 min (A and C) or 3 h (B and D). Cell lysates were immunoprecipitated with a β-Gal-specific MAb (A and B, upper panels) or with a cocktail of MAbs specific for the SFV structural proteins (capsid, E2 and E1) (A and B, lower panels). Virus was sedimented from supernatants as described in Materials and Methods (C and D). SFV-lacZ RNA was coelectroporated with the following: lane 1, SFV-helper-1; lane S1, SFV-helper-S1 plus SFV-helper-C; lane S2, SFV-helper-S2 plus SFV-helper-C; lane S3, SFV-helper-S3 plus SFV-helper-C; lane E, SFV-helper-E plus SFV-helper-C; lane Z, no RNA; lane 1m, SFV-helper-1-S219A; lane Cm, SFV-helper-S-1 plus SFV-helper-C-S219A. The upper portions of panels A and B were exposed only one-third of the time compared to the lower panels; in panel D, lane 1 was exposed one-tenth of the time compared to the other lanes. The capsid protein of SFV often smears in SDS-PAGE and is not a good indicator of quantitation.
Frequency of RPVs in the stocks generated with the two-helper system.
To determine whether any RPVs were generated with the two-helper packaging strategy, a procedure involving three sequential steps was used. In the first step, SFV-lacZ RNA was packaged into particles by cotransfecting cells with SFV-lacZ plus SFV-helper-S1 plus SFV-helper-C RNAs as described above. In the second step, these particles were used to infect confluent monolayers of BHK-21 cells at a multiplicity of infection (MOI) of 5 and incubated for 20 h to amplify any RPV present in the stock. Finally, supernatants from the infected cells were titrated in a PFU assay. With this protocol, no PFU could be found in 4.6 × 109 particles from 13 independent SFV-lacZ recombinant stocks, indicating that the frequency of recombination (recombinants) is lower than 4.6 × 10−9. The possibility that the presence of a few RPVs in the stock could be masked by the great number of recombinant particles present in the stock (superinfection exclusion) was ruled out by mixing a small number of wild-type SFV particles with a recombinant stock that had previously been shown not to contain RPVs and then using the mixture to infect BHK-21 cells at different multiplicities. Supernatants were titrated after a 20-h incubation. As shown in Table 2, a few PFUs added to the stock (three or five) could be detected when an MOI of 6 was used to infect the monolayer.
TABLE 2.
Occurrence of PFU in growth media of cells infected with recombinant SFV
| Wild-type SFV added (PFU) | No. of positive expts/total no. of expts with recombinant SFV-lacZ added at an MOI of:
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 6 | |
| 0 | 0/12 | 0/12 | 0/12 | 0/12 |
| 3 | 12/12 | 11/12 | 9/12 | 10/12 |
| 5 | 12/12 | 11/12 | 12/12 | 11/12 |
Introduction of a mutation in SFV-helper-C to increase the safety of the system.
Although no RPVs were detected in SFV-lacZ recombinant particles packaged with the two-helper system, the possibility of generating wild-type genomes through two recombination events still remains. To further improve the safety of the system, the capsid gene encoded by pSFV-helper-C was mutated in order to abolish its cleaving activity, which is dispensable in the two-helper system but necessary if a full-length genome is reconstituted through recombination. The mutation of serine-215 to alanine had earlier been shown to abolish the self-cleaving activity of the capsid in Sindbis virus without altering the structure of the protein (3, 19). The equivalent position in the SFV capsid (Ser-219) (32) was mutated to alanine in both pSFV-helper-C and pSFV-helper-1, generating plasmids pSFV-helper-C-S219A and pSFV-helper-1-S219A, respectively. To test the functionality of the mutated helper, cells were coelectroporated with SFV-lacZ, SFV-helper-S1, and SFV-helper-C-S219A RNAs. Both the pattern of protein expression and the titers of SFV-lacZ particles packaged with this new system were very similar to those obtained with the original SFV-helper-C RNA (Fig. 3, lane Cm, and Table 1), indicating that the mutation in the capsid did not affect the functionality of the protein. To check the effect of this mutation in a system where capsid cleavage is necessary for the processing of the spike proteins, cells were coelectroporated with SFV-lacZ and SFV-helper-1-S219A RNAs. When the pattern of protein expression was analyzed through a pulse-chase experiment, no mature capsid protein could be detected in the cell extracts (Fig. 3A and B, lane 1m), indicating that self-cleavage from the spikes did not occur. Several high-molecular-weight products were detected after a 5-min chase; these presumably corresponded to the polyprotein intermediates. A band of 50 kDa, most likely corresponding to E1, could also be detected; this protein was the last fragment of the polyprotein which may have been translocated to the ER and generated via signal peptidase cleavage. After a 3-h chase, most of the polyprotein intermediates had been degraded, and only the 50-kDa band remained. No viral protein could be detected in the supernatants (Fig. 3D, lane 1m), and the titers after 24 h were about 104 times lower than those obtained with SFV-helper-1 (Table 1), a result possibly reflecting the frequency of reversion of the capsid mutation.
DISCUSSION
The present study shows that SFV recombinant RNA can be efficiently packaged by using two independent helper RNAs which provide in trans the spike and capsid proteins, respectively. This technology offers the following important advantages over previous SFV packaging systems. (i) A high level of biosafety is obtained due to the requirement of at least two recombination events in order to generate full-length genomes. (ii) It does not require the self-cleaving activity of the capsid, which allows the introduction of mutations abolishing this activity, thus further enhancing the safety of the system. (iii) It does not require the activation of particles with chymotrypsin, making the system much easier to handle and less costly than previous SFV helper systems (1, 47).
In order to construct the two-helper system, it was necessary to express the spike proteins without the capsid at levels comparable to those of the wild-type virus. Because the high level of expression of structural proteins in SFV is dependent on the translation-enhancing effect of 5′ sequences in the capsid gene, it was necessary to fuse this enhancing sequence of the capsid with the spike genes. It had been shown previously that as few as 102 nucleotides from the 5′ end of the capsid gene could provide an enhancer effect of about 80% of that achieved with the whole gene when this sequence was fused to the β-Gal gene (43). This minimal enhancing sequence was able to enhance the expression of the SFV spike proteins to levels similar to those obtained with helper-1, as shown in pulse-chase and packaging experiments in which the capsid was supplied in trans by the SFV-Camber replicon. The lack of the capsid enhancer considerably decreased the production of the spikes, with a corresponding decrease in particle titers of up to 102, in experiments involving two or three RNAs. In the normal processing of SFV structural proteins, the COOH-terminal domain of the capsid functions as a serine protease that is able to self-cleave from p62 (32). In order to remove the 34 amino acids encoded by the capsid enhancer from p62, it was necessary to use the 2A autoprotease from FMDV. This is a very short self-cleaving protease that can function with a sequence that is as short as 17 amino acids (40, 41). The protease has previously been used to fuse proteins of different origin, being able to work independently of the sequences placed upstream and downstream of it (30, 34, 40). The cleavage takes place between the two last residues, which means that the last proline of the 2A sequence will remain at the NH2-terminal end of the protein placed downstream. In our study, the 2A protease showed a high cleavage efficiency in all of the different capsid enhancer-p62-6K-E1 fusions that were tested. This cleavage allowed a normal translocation of the p62-6K-E1 polyprotein to the ER, as indicated by the normal signal peptidase processing of the polyprotein into its subunits, and the efficient production of SFV particles. Three different variants of p62 were expressed that contained one extra proline at the amino terminal end, a substitution of the first amino acid by a proline, or a deletion of the two first amino acids, respectively. All of them showed similar expression levels and packaging efficiencies. This indicates that the NH2-terminal end of p62, which upon processing by a host furin-like protease becomes E3 (a small glycosylated peptide of 66 amino acids that in SFV remains in the surface of the virus particles) (6, 15), can be subjected to small modifications without affecting the viability of the virion.
A different strategy to express the spike proteins of an alphavirus at high levels in the context of two-helper RNAs has been used by Frolov et al. (10). They constructed a helper in which the Sindbis spike genes were fused to the capsid gene of Ross River virus containing deletions in the RNA binding domain but which maintained both the translation-enhancing and the self-cleaving activities. Although no recombinants were detected with this system, the heterologous mutated capsids were able to assemble into chimeric particles that were not completely devoid of viral RNA. A bipartite helper packaging system has also been described for VEE (36), but in this case the spike proteins were expressed without the capsid translation enhancer, which apparently is not needed in the VEE context.
The cotransfection of cells with helper-S and helper-C RNAs in combination with a recombinant SFV RNA carrying a foreign gene proved to be a powerful method for the production of recombinant particles. Titers of 2 × 108 SFV-lacZ particles per 106 cells were routinely obtained with the two-helper system, and in some cases up to 8 × 108 particles/106 cells could be generated, indicating that the system can be further optimized. The two-helper system proved to be extremely safe in terms of RPV generation, given the fact that no RPVs could be detected in 4.6 × 109 particles. This is due to the fact that two different recombination events, plus the reversion of the stop codon at the 3′ end of the capsid gene, would be necessary to generate a wild-type genome. There is also the possibility that only two recombination events could be enough to generate a wild-type genome if one of them took place exactly between the last codon of the capsid and the beginning of p62, but in this case it would be essential that the last residue of the capsid, Trp 267, be maintained for retaining the self-cleaving activity of the protein (44). In order to further reduce the probability of generating RPVs, the mutation S219A was introduced into the capsid gene in helper-C to abolish the self-cleaving activity of this protein, an activity which is not required in the two-helper expression system. The serine in position 215 of the capsid of the Sindbis virus had previously shown to be part of a catalytic triad formed by His-141, Asp-147, and Ser-215 (18, 19). When this serine was mutated to isoleucine or alanine, the cleaving activity was completely suppressed (19). Furthermore, crystallographic studies of the Sindbis virus capsid COOH-terminal domain containing either a serine or an alanine at position 215 did not show any significant differences in structure (3). We showed that the SFV capsid containing a similar mutation was incorporated normally in viral particles when expressed from SFV-helper-C-S219A, indicating that the mutation in this position does not affect the functionality of the protein. The maximum frequency of reversion of this mutation can be estimated from the number of particles generated with the SFV-helper-1-S219A, since these can only be generated through a reversion restoring the capsid self-cleaving activity. This frequency can be calculated as follows: SFV-helper-1-S219A titer/SFV-helper-1 titer = 4.2 × 10−5. The frequency of one single recombination event in the SFV-helper-1 system had been previously determined to be less than 10−6 (1). If we assume a similar frequency for each of the two recombination events necessary to generate wild-type virus in the two-helper system, the theoretical frequency of RPV generation with SFV-helper-C-S219A would be less than 10−6 × 10−6 × 4.2 × 10−5 = 4.2 × 10−17. This number is too small for testing experimentally. The RPV generation frequency in the SFV two-helper packaging system was determined to be <4.6 × 10−9 and thus an empirical RPV generation frequency in this system with the S219A mutant would be <4.6 × 10−9 × 4.2 × 10−5 = <1.9 × 10−13. These results indicate that the two-helper system constitutes an efficient and highly safe method for packaging recombinant SFV particles carrying heterologous antigens.
ACKNOWLEDGMENTS
We thank M. Kielian for providing the SFV spike specific MAbs and W. Bodemer for providing the SFV replicase specific MAb. We also thank Steven Brown and Tamarra Cadd for their critical reading of the manuscript.
This work was supported by the Swedish Medical Research Council, the Swedish Council for Engineering Sciences, and the European Union Biotechnology Program. C.S. was supported by an European Commission Research Training Grant in the Biotechnology program.
REFERENCES
- 1.Berglund P, Sjöberg M, Garoff H, Atkins G J, Sheahan B J, Liljeström P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Bio/Technology. 1993;11:916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- 2.Berglund P, Smerdou C, Fleeton M N, Tubulekas I, Liljeström P. Enhancing immune responses using suicidal DNA vaccines. Nat Biotechnol. 1998;16:562–565. doi: 10.1038/nbt0698-562. [DOI] [PubMed] [Google Scholar]
- 3.Choi H-K, Lee S, Zhang Y-P, McKinney B R, Wengler G, Rossman M G, Kuhn R J. Structural analysis of Sindbis virus capsid mutants involving assembly and catalysis. J Mol Biol. 1996;262:151–167. doi: 10.1006/jmbi.1996.0505. [DOI] [PubMed] [Google Scholar]
- 4.Chong H, Starkey W, Vile R G. A replication-competent retrovirus arising from a split-function packaging cell line was generated by recombination events between the vector, one of the packaging constructs, and endogenous retroviral sequences. J Virol. 1998;72:2663–2670. doi: 10.1128/jvi.72.4.2663-2670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong H, Vile R G. Replication-competent retrovirus produced by a ‘split-function’ third generation amphotropic packaging cell line. Gene Ther. 1996;3:624–629. [PubMed] [Google Scholar]
- 6.de Curtis I, Simons K. Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proc Natl Acad Sci USA. 1988;85:8052–8056. doi: 10.1073/pnas.85.21.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiCiommo D P, Bremner R. Rapid, high level protein production using DNA-based Semliki Forest virus vectors. J Biol Chem. 1998;273:18060–18066. doi: 10.1074/jbc.273.29.18060. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly J J, Ulmer J B, Liu M A. DNA vaccines. Life Sci. 1997;60:163–172. doi: 10.1016/s0024-3205(96)00502-4. [DOI] [PubMed] [Google Scholar]
- 9.Dubensky T W, Jr, Driver D A, Polo J M, Belli B A, Latham E M, Ibanez C E, Chada S, Brumm D, Banks T A, Mento S J, Jolly D J, Chang S M. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J Virol. 1996;70:508–519. doi: 10.1128/jvi.70.1.508-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frolov I, Frolova E, Schlesinger S. Sindbis virus replicons and Sindbis virus: assembly of chimeras and of particles deficient in virus RNA. J Virol. 1997;71:2819–2829. doi: 10.1128/jvi.71.4.2819-2829.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frolov I, Hoffman T A, Pragai B M, Dryga S A, Huang H V, Schlesinger S, Rice C M. Alphavirus-based expression vectors: strategies and applications. Proc Natl Acad Sci USA. 1996;93:11371–11377. doi: 10.1073/pnas.93.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frolov I, Schlesinger S. Translation of Sindbis virus mRNA: effects of sequences downstream of the initiating codon. J Virol. 1994;68:8111–8117. doi: 10.1128/jvi.68.12.8111-8117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garoff H, Huylebroeck D, Robinson A, Tillman U, Liljeström P. The signal sequence of the p62 protein of Semliki Forest virus is involved in initiation but not in completing chain translocation. J Cell Biol. 1990;111:867–876. doi: 10.1083/jcb.111.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garoff H, Liljeström P, Metsikkö K, Lobigs M, Wahlberg J. Formation and function of the Semliki Forest virus membrane. In: Brinton M A, Heinz F X, editors. New aspects of positive-strand RNA viruses. Washington, D.C: American Society for Microbiology; 1990. pp. 166–172. [Google Scholar]
- 15.Garoff H, Simons K, Renkonen O. Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology. 1974;61:493–504. doi: 10.1016/0042-6822(74)90285-2. [DOI] [PubMed] [Google Scholar]
- 16.Garoff H, Wilschut J, Liljeström P, Wahlberg J M, Bron R, Suomalainen M, Smyth J, Salminen A, Barth B U, Zhao H, et al. Assembly and entry mechanisms of Semliki Forest virus. Arch Virol. 1994;9:329–338. doi: 10.1007/978-3-7091-9326-6_33. [DOI] [PubMed] [Google Scholar]
- 17.Greiser-Wilke I, Moenning V, Kaaden O R, Figueiredo L T. Most alphaviruses share a conserved epitopic region on their nucleocapsid protein. J Gen Virol. 1989;70:743–748. doi: 10.1099/0022-1317-70-3-743. [DOI] [PubMed] [Google Scholar]
- 18.Hahn C S, Strauss E G, Strauss J H. Sequence analysis of three Sindbis virus mutants temperature-sensitive in the capsid protein autoprotease. Proc Natl Acad Sci USA. 1985;82:4648–4652. doi: 10.1073/pnas.82.14.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn C S, Strauss J H. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J Virol. 1990;64:3069–3073. doi: 10.1128/jvi.64.6.3069-3073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson C P. The vector void in gene therapy. Bio/Technology. 1995;13:222–225. doi: 10.1038/nbt0395-222. [DOI] [PubMed] [Google Scholar]
- 21.Kielian M, Jungerwirth S, Sayad K U, DeCandido S. Biosynthesis, maturation, and acid activation of the Semliki Forest virus fusion protein. J Virol. 1990;64:4614–4624. doi: 10.1128/jvi.64.10.4614-4624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levis R, Weiss B G, Tsiang M, Huang H, Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986;44:137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- 23.Liljeström P. Alphavirus expression systems. Curr Opin Biotechnol. 1994;5:495–500. doi: 10.1016/0958-1669(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 24.Liljeström P, Garoff H. Expression of proteins using Semliki Forest virus vectors. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1994. pp. 16.20.11–16.20.16. [Google Scholar]
- 25.Liljeström P, Garoff H. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J Virol. 1991;65:147–154. doi: 10.1128/jvi.65.1.147-154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liljeström P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 27.Liljeström P, Lusa S, Huylebroeck D, Garoff H. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the 6,000-molecular-weight membrane protein modulates virus release. J Virol. 1991;65:4107–4113. doi: 10.1128/jvi.65.8.4107-4113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobigs M, Garoff H. Fusion function of the Semliki Forest virus spike is activated by proteolytic cleavage of the envelope glycoprotein p62. J Virol. 1990;64:1233–1240. doi: 10.1128/jvi.64.3.1233-1240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez I, Dornburg R. Partial reconstitution of a replication-competent retrovirus in helper cells with partial overlaps between vector and helper cell genomes. Hum Gene Ther. 1996;7:705–712. doi: 10.1089/hum.1996.7.6-705. [DOI] [PubMed] [Google Scholar]
- 30.Mattion N M, Harnish E C, Crowley J C, Reilly P A. Foot-and-mouth disease virus 2A protease mediates cleavage in attenuated Sabin 3 poliovirus vectors engineered for delivery of foreign antigens. J Virol. 1996;70:8124–8127. doi: 10.1128/jvi.70.11.8124-8127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melancon P, Garoff H. Reinitiation of translocation in the Semliki Forest virus structural polyprotein: identification of the signal for the E1 glycoprotein. EMBO J. 1986;5:1551–1560. doi: 10.1002/j.1460-2075.1986.tb04396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melancon P, Garoff H. Processing of the Semliki Forest virus structural polyprotein: role of the capsid protease. J Virol. 1987;61:1301–1309. doi: 10.1128/jvi.61.5.1301-1309.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moelling K. DNA for genetic vaccination and therapy. Cytokines Cell Mol Ther. 1997;3:127–135. [PubMed] [Google Scholar]
- 34.Percy N, Barclay W S, Garcia-Sastre A, Palese P. Expression of a foreign protein by influenza A virus. J Virol. 1994;68:4486–4492. doi: 10.1128/jvi.68.7.4486-4492.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkus M E, Tartaglia J, Paoletti E. Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious diseases. J Leukoc Biol. 1995;58:1–13. doi: 10.1002/jlb.58.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Pushko P, Parker M, Ludwig G V, Davis N L, Johnston R E, Smith J F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 37.Randrianarison-Jewtoukoff V, Perricaudet M. Recombinant adenoviruses as vaccines. Biologicals. 1995;23:145–157. doi: 10.1006/biol.1995.0025. [DOI] [PubMed] [Google Scholar]
- 38.Robbins P D, Tahara H, Ghivizzani S C. Viral vectors for gene therapy. Trends Biotechnol. 1998;16:35–40. doi: 10.1016/S0167-7799(97)01137-2. [DOI] [PubMed] [Google Scholar]
- 39.Robinson H L. Nucleic acid vaccines: an overview. Vaccine. 1997;15:785–787. doi: 10.1016/s0264-410x(96)00249-6. [DOI] [PubMed] [Google Scholar]
- 40.Ryan M D, Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 1994;13:928–933. doi: 10.1002/j.1460-2075.1994.tb06337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan M D, King A M Q, Thomas G P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol. 1991;72:2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- 42.Salminen A, Wahlberg J M, Lobigs M, Liljeström P, Garoff H. Membrane fusion process of Semliki Forest virus. II. Cleavage-dependent reorganization of the spike protein complex controls virus entry. J Cell Biol. 1992;116:349–357. doi: 10.1083/jcb.116.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjöberg E M, Suomalainen M, Garoff H. A significantly improved Semliki Forest virus expression system based on translation enhancer segments from the viral capsid gene. Bio/Technology. 1994;12:1127–1131. doi: 10.1038/nbt1194-1127. [DOI] [PubMed] [Google Scholar]
- 44.Skoging U, Liljeström P. Role of the C-terminal tryptophan residue for the structure-function of alphavirus capsid protein. J Mol Biol. 1998;279:865–872. doi: 10.1006/jmbi.1998.1817. [DOI] [PubMed] [Google Scholar]
- 45.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suomalainen M, Liljeström P, Garoff H. Spike protein: nucleocapsid interactions drive the budding of alphaviruses. J Virol. 1992;66:4737–4747. doi: 10.1128/jvi.66.8.4737-4747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tubulekas I, Liljeström P. Suppressors of cleavage-site mutations in the p62 envelope protein of Semliki Forest virus reveal dynamics in spike structure function. J Virol. 1998;72:2825–2831. doi: 10.1128/jvi.72.4.2825-2831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vile R G, Tuszynski A, Castleden S. Retroviral vectors: from laboratory tools to molecular medicine. Mol Biotechnol. 1996;5:139–158. doi: 10.1007/BF02789062. [DOI] [PubMed] [Google Scholar]
- 49.Wahlberg J M, Garoff H. Membrane fusion process of Semliki Forest virus. I. Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992;116:339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss B, Nitschko H, Ghattas I, Wright R, Schlesinger S. Evidence for specificity in the encapsidation of Sindbis virus RNAs. J Virol. 1989;63:5310–5318. doi: 10.1128/jvi.63.12.5310-5318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong C, Levis R, Shen P, Schlesinger S, Rice C M, Huang H V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243:1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- 52.Yeh P, Perricaudet M. Advances in adenoviral vectors: from genetic engineering to their biology. FASEB J. 1997;11:615–623. doi: 10.1096/fasebj.11.8.9240963. [DOI] [PubMed] [Google Scholar]