Abstract
Background and Objectives
Transcutaneous point-of-care ultrasound (POCUS) is a good tool to monitor the trachea in many clinical practices. The aim of our study is to verify the feasibility of POCUS-guided submucosal injection as a potential drug delivery method for the treatment of tracheal stenosis.
Materials and methods
The inner wall of the trachea was monitored via a bronchoscope during the POCUS-guided submucosal injection of methylene blue in fresh ex vivo porcine trachea to evaluate the distribution of methylene blue. The feasibility and eficacy of POCUS-guided submucosal injection were evaluated in a tracheal stenosis rabbit model. Animals were divided into sham group, tracheal stenosis group, and treatment group. Ten days after the scraping of the tracheal mucosa or sham operation, POCUS-guided submucosal injection of paclitaxel or saline was performed. Seven days after the submucosal injection, the trachea was assessed by cervical computed tomography (CT) scan and ultrasound.
Results
The distribution of methylene blue in trachea proved the technical feasibility of POCUS-guided submucosal injection. CT evaluation revealed that the tracheal stenosis index and the degree of tracheal stenosis increased significantly in the stenosis group, while POCUS-guided submucosal injection of paclitaxel partially reversed the tracheal stenosis. POCUS-guided submucosal injection of paclitaxel also decreased the lamina propria thickness and collagen deposition in the stenosed trachea.
Conclusion
POCUS-guided submucosal paclitaxel injection alleviated tracheal stenosis induced by scraping of the tracheal mucosa. POCUS-guided submucosal injection might be a potential method for the treatment of tracheal stenosis.
Key words: ultrasonography, airway imaging, drug delivery, tracheal stenosis, submucosal instillation
Introduction
Postintubation tracheal stenosis (PITS), a complication of an inflated endotracheal/tracheostomy tube cuff, is a common type of benign subglottic tracheal stenosis. Once established, it may have a significant impact on the patient’s quality of life. The rate of tracheal stenosis related to prolonged intubation varies between 0.6% and 21%.[1] The gold standard treatment for PITS is surgery involving tracheal resection and primary end-to-end anastomosis with reconstruction.[2,3] However, postsurgical recurrences were observed in 24.2% of cases.[4] In addition to surgical treatment, there are other methods such as bronchoscopic dilatation, laser ablation, electrocautery, and stent application.[5] For most cases initially treated by interventional bronchoscopy, serial dilations are effective in some complex PITS cases.[4] However, recurrence after serial dilation was observed in 25.0% of cases during long-term follow-up.[4] With regard to stent implantation, mucostasis and the formation of granulation tissue are the most common adverse events associated with stent insertion.[5] Therefore, repeated monitoring of the severity of stenosis, prevention of restenosis, less-invasive procedures, and economic costs would be key to the management of tracheal stenosis. It was reported that intralesional injection of paclitaxel or mitomycin C using flexible bronchoscope after the endoscopic treatment of the stenotic lesion may prevent recurrence of stenosis and reduce the rate of subsequent formation of granulation tissue and scarring without obvious side effects.[6, 7, 8, 9]
Point-of-care ultrasound (POCUS) is ultrasound (US) performed by the treating physician at the patient’s bedside as an extension of the physical examination to improve diagnostic precision and guide further management.[10,11] Initially used for procedural guidance, POCUS is now used for diagnostics and monitoring of the lung, heart, abdomen, and deep vein thrombosis.[12,13] POCUS has been used to confirm and assist endotracheal tube (ETT) and tracheostomy tube placement and for the assessment of subglottic airway in children.[14, 15, 16] It has been revealed that US measurements of the subglottic airway are strongly correlated with the evaluation of endoscopy[14,15] and magnetic resonance imaging (MRI) measurements,[17] especially in evaluating the abnormal pediatric airway. However, the evidence for POCUS in evaluating tracheal stenosis in adults is still insufficient. In addition, transcutaneous POCUS-guided injection of the adjuvant medication for tracheal stenosis has not been reported before. Therefore, we designed this study to explore the potential feasibility of transcutaneous POCUS-guided submucosal injection as a novel drug delivery method for the treatment of tracheal stenosis in ex vivo tracheal and in vivo tracheal stenosis animal models.
Materials and methods
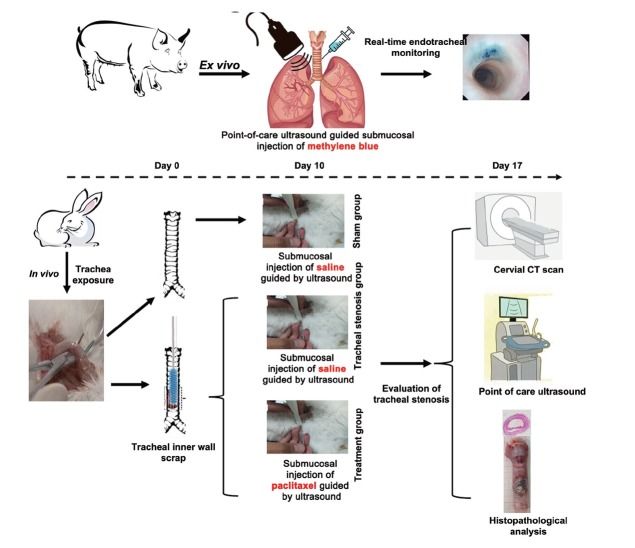
The scheme of the ex vivo and in vivo experiments is shown in Figure 1.
Figure 1.
Scheme of the study design. CT: computed tomography.
Ethics statement
The protocol of this study was approved by the Animal Care and Use Committee of the University (Approval No. CMU2019075), and all experiments were performed in accordance with the ARRIVE guidelines and the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Ex vivo evaluation of submucosal methylene blue injection via transcutaneous US
To achieve the inner view of the tracheal mucosa during the POCUS-guided submucosal medicine injection and locate the gross distribution of the submucosal medicine injection, three fresh ex vivo porcine trachea and lungs were collected and employed and a flexible video-bronchoscope (BF-1T290; Olympus, Tokyo, Japan) monitored the whole procedure and recorded the change in the inner wall of the trachea during the POCUS-guided submucosal injection of 1 mL methylene blue (2 mg/mL; Jumpcan Pharmaceutical Group Co., Ltd., Taixing, China) just through one-point injection via the tracheal ring space. POCUS was performed with Apolio i800 US device (Canon Medical Systems Corporation) with a linear probe (i18LX5).
Experimental animals
A total of 18 male Japanese white rabbits (weighing 2.5–3.0 kg, 6–7 months old) were purchased from Konde Industry Co., Ltd. (Qingdao, China). They were bred in the specific pathogen free (SPF) class barrier system and maintained in a temperature-controlled room (18℃–22℃) with 12-h light/dark cycles and allowed to eat and drink freely. The rabbits were randomly divided into three groups (n = 6): the sham group, tracheal stenosis group (TS group), and treatment group (tracheal stenosis treated with POCUS-guided submucosal paclitaxel injection). Ten days after the establishment of tracheal stenosis or sham operation, paclitaxel or saline was administered into the submucosal layer guided with POCUS, which was performed with an Aixplorer US scanning system (SuperSonic Imagine, Aixen-Provence, France) with a 20-MHz linear probe (L20-6). Seven days after the establishment of tracheal stenosis or sham operation,[18, 19, 20] tracheal stenosis was assessed by cervical computed tomography (CT) and US, and then all experimental rabbits were euthanized (Figure 1).
Establishment of tracheal stenosis model
The tracheal stenosis model was established as previously described[21,22] in the TS group and treatment group. The rabbits had to fast for 8 h before modeling. Each rabbit was intramuscularly anesthetized with 3 mg/kg xylazine for the induction of anesthesia. During the procedure, each rabbit was placed in the supine position. Propofol was infused to maintain anesthesia at a rate of 102 mg/kg per hour.[23] To enhance analgesia, 2 mL of 1% lidocaine hydrochloride was injected into the subcutaneous area of the anterior neck. A polypropylene brush 6.35 mm in diameter (Key Surgical Inc., USA) was inserted into the distal trachea from the incision, and the tracheal mucosa was circumferentially scraped by pushing and pulling the brush 10 times to induce the injury and the later stenosis.[24] Scraping was carried out at an abrasion distance of 1.5 cm from the incision point. During the procedure and 24 h after the operation, 40 mg/mL of ampicillin sodium solution (1 mL) was intramuscularly injected as the prophylactic anti-infection treatment.
Submucosal drug delivery by POCUS-guided injection and ultrasonography measurement of the trachea
All US scans were performed by a single investigator (Bin Jiang) who was experienced in US and had been trained by performing 20 laryngeal ultrasonographic examinations before the beginning of the study, based on previous studies.[25, 26, 27]
Transcutaneous US was performed in the supine position, with the head in slight extension. Ultrasonography measurements were performed with a linear probe placed on the midline of the anterior neck. To avoid any confusion between the cricoid cartilage and a tracheal ring, the ultrasonography procedure began with the location of the true vocal folds (paired hyperechoic linear structures with respiratory and swallowing mobility). To locate the site of tracheal stenosis, a transverse midline scan was performed over the thyroid cartilage; then, starting from the level of the thyroid cartilage, one tracheal ring was placed caudally to locate the stenosis with thickening of the anterior tracheal wall. A midsagittal plane scan revealed irregularity of the cricothyroid membrane and tissue/air border.
Then, a transverse midline scan was performed over the stenosis site to measure the anterior tracheal wall thickness (ATWT). According to a previous study,[28, 29, 30] the inner surface of the trachea was linearly hyperechoic and was known as the air-mucosal interface (A-M interface). The posterior part of the trachea was the reverberation artifact. ATWT was defined as the distance from the hyperechoic front wall to the A-M interface. ATWT and ITD were measured by ImageJ (National Institutes of Health, Bethesda, USA).
Ten days after the scraping-induced injury, 0.6 mg (1 mg/mL) paclitaxel (Yangtze River Pharmaceutical Group, Beijing, China) was administered by POCUS-guided submucosal injection in the treatment group, while in the sham and model groups, 0.6 mL saline was administered as a control. ATWT was recorded by transcutaneous US and compared among different groups 7 days after drug delivery (the scheme is shown in Figure 1). In the treatment group, ATWT before paclitaxel administration and 7 days after administration was compared individually.
CT measurement
A high-resolution CT (Discovery CT750 HD; General Electric Company, Boston, USA) scan was taken using the same parameters (axial slice increment, 1 mm; pixel size, 0.502 mm) 7 days after drug delivery. All high-resolution CT imaging was performed at our institution using standard CT neck and CT chest radiologic protocols. Measurements of the tracheal diameter were taken via CT neck/chest in the anterior-posterior (AP) dimension as D1 and in the transverse dimension as D2 in the axial plane.[30] Three-dimensional (3D) reconstruction was generated from high-resolution CT using Mimics 14.01 (Materialise, Inc., Plymouth, MI, USA) software. The corresponding stenosis airway levels were determined on the 3D model.[31] Cross sections were taken at the specified levels of the 3D model with the narrowest lumen, and the diameters and cross-section areas were measured within the Mimics software.
Comparison of the severity of tracheal stenosis in different groups was performed by calculating the stenosis index[28, 29, 30] and the degree of tracheal stenosis.[20] The stenosis index was based on the diameters measured by the CT scan, and the degree of tracheal stenosis was based on the cross-sectional area (CSA) of tracheal stenosis. The tracheal stenosis index (SI%) was calculated as SI = [1 – (d1 + d2)/(D1 + D2)] × 100%, and the degree of tracheal stenosis (SD%) was calculated as SD= (1 – minimum lumen area/nearest normal area) × 100%. In the treatment group, SI% and SD% before paclitaxel administration and 7 days after paclitaxel administration were compared individually.
Morphologic and histologic examinations
After the evaluation of the trachea by US and CT scans, all rabbits were sacrificed for morphologic and histologic examinations. The tracheal segment was excised, fixed in formaldehyde 4% solution, and paraffin embedded. The paraffin sections (4 μm) were prepared along the cross section of the trachea and used for subsequent hematoxylin and eosin (HE) staining and Masson staining. Lamina propria (LP) thickness at the thickest part of the tracheal stenosis was measured in the HE-stained sections according to the published literature using ImageJ software (http:// rsb.info.nih.gov/ij).[29,32,33]
Statistical analysis
Differences between groups were determined by the Mann-Whitney-Wilcoxon test using GraphPad Prism 9 software (GraphPad Software, Boston, CA, USA). Data were expressed as the mean ± standard error of mean (SEM) and were derived from at least three independent experiments. Two-tailed Student’s t test was used to calculate P values. P < 0.05 was considered as statistically significant.
Results
Real-time monitoring of POCUS-guided submucosal methylene blue injection via bronchoscopic examination
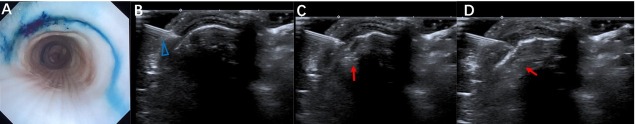
Real-time bronchoscopic monitoring was conducted for the process of submucosal methylene blue injection (Figure 2A) to the ex vivo porcine trachea as guided by POCUS (Figure 2B–2D). The location and distribution of the submucosal injection guided by POCUS was clearly shown with the shape of a semicircle between the adjacent tracheal rings. It also demonstrated the whole process of the submucosal injection and the dynamic thickening of the submucosal layer following the submucosal injection. The location of the injection point of the needle presented with more intense staining of methylene blue compared to the peripheral area, with immersion of the dye.
Figure 2.
Correspondence of the bronchoscopic view of the inner tracheal wall with the submucosal injection of methylene blue guided by transcutaneous POCUS (A) Bronchoscopic manifestation of ex vivo porcine trachea after submucosal injection of methylene blue guided by transcutaneous POCUS. (B–D) The process of submucosal injection of methylene blue guided by POCUS. The blue triangle indicates the injection needle. The red arrow indicates the thickening of the submucosal layer with the medication injection. POCUS: point-of-care ultrasound.
Sonographic manifestations of POCUS-guided submucosal drug administration for tracheal stenosis
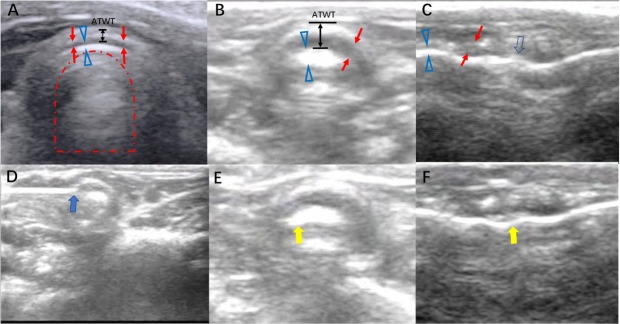
Transcutaneous US could locate and measure the anterior part of the tracheal ring by identifying the tracheal cartilage as the hypoechoic shadow and the underlying tissue–air border as the hyperechoic shadow (A-M interface) (Figure 3A and 3B). The inner trachea air column was presented as an air artifact in the trachea, as reflected by the mirror effect of the A-M interface. The outer edge of the trachea presented a hyperechoic strip with a clear smooth boundary.
Figure 3.
Sonogram of rabbit trachea and the procedure of real-time guidance of transcervical ultrasound for submucosal paclitaxel administration for tracheal stenosis (A) Sonogram of the normal trachea by transcervical ultrasound in the transverse plane. Red arrow, as a hypoechoic shadow, indicates the tracheal ring; hollow blue triangle, as a hyperechoic shadow, indicates the tissue–air border, named the A-M interface; red dotted line arch indicates the air artifact in the trachea; black double arrow line indicates the ATWT. (B) Sonogram of tracheal stenosis by transcervical ultrasound in the transverse plane. (C) Sonogram of tracheal stenosis by midsagittal plane scan. The hollow blue arrow indicates an unsmooth A-M interface, corresponding to the irregularity of the inner side of the trachea. (D) Injection of paclitaxel by a 25G needle into the submucosal site of the trachea (blue solid arrow). (E, F) After the submucosal injection of paclitaxel, transcervical ultrasound revealed a change in the A-M interface (yellow solid arrow), indicating the administration of paclitaxel to the submucosal layer of trachea. A-M: air-mucosal; ATWT: anterior tracheal wall thickness.
Transverse transcutaneous US revealed obvious thickening of the ATWT, increased echogenicity of the A-M interface, and irregularity of the air artifact due to the change in the A-M interface in the stenosis group rabbits. The midsagittal plane scan helped to locate the range of the stenosis by the length of irregularity of the cricothyroid membrane and tissue/air border (Figure 3C).
Due to the delineation of the layered structure of the anterior wall of the trachea, paclitaxel for the inhibition of the proliferation of granulation tissue was administered with identification of the A-M interface and tracheal cartilage. Paclitaxel was injected transcutaneously to the exact location of the hyperechoic region of the A-M interface, as shown in Figure 3D. During administration of paclitaxel, POCUS monitoring revealed changes in the A-M interface and the distribution of the injected medication in the layered structure (Figure 3E and 3F).
Histologic evaluation of the treatment effect of POCUS-guided submucosal paclitaxel injection
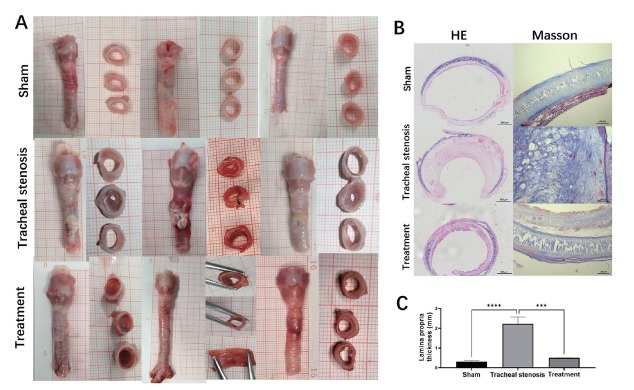
Morphologic gross manifestation revealed thickening and granulation tissue of the trachea in the TS group (Figure 4A). HE staining indicated obvious thickening of the anterior wall of the trachea in the TS group, and Masson staining indicated increased collagen deposition due to the repair of injury and decreased collagen deposition, which was reversed by submucosal injection of paclitaxel (Figure 4B). After local administration of paclitaxel by submucosal injection, the anterior wall thickening was partially reversed. Since LP is the location of fibroblasts and myofibroblasts,[34] we measured the LP thickness. Thickening of the LP was compared, and the treatment effect of submucosal injection of paclitaxel was revealed by the decreased thickness of the LP in HE staining (Figure 4C).
Figure 4.
Representative morphologic evaluation of the trachea in different groups. (A) Morphologic gross manifestation of the trachea in different groups. (B) Histologic evaluation of the trachea in different groups by HE staining and Masson staining. (C) Comparison of LP thickness in different groups in HE staining. Values are expressed as the mean ± SEM (n = 5 rabbits/group). ***P < 0.001 and ****P < 0.0001. HE: hematoxylin and eosin; LP: lamina propria; SEM: standard error of the mean.
Evaluation of the severity of tracheal stenosis by CT and POCUS
To quantify the severity of tracheal stenosis, we calculated the SI% based on the diameters measured on CT scans and SD% based on the CSA measured on the reconstruction of CT scans (Figure 5) and found that on comparing different groups, SI% and SD% increased significantly in the stenosis group, while submucosal injection of paclitaxel partially reversed SI% and SD%. In the treatment group, after the treatment, SI% and SD% decreased obviously, compared to the values before treatment.
Figure 5.
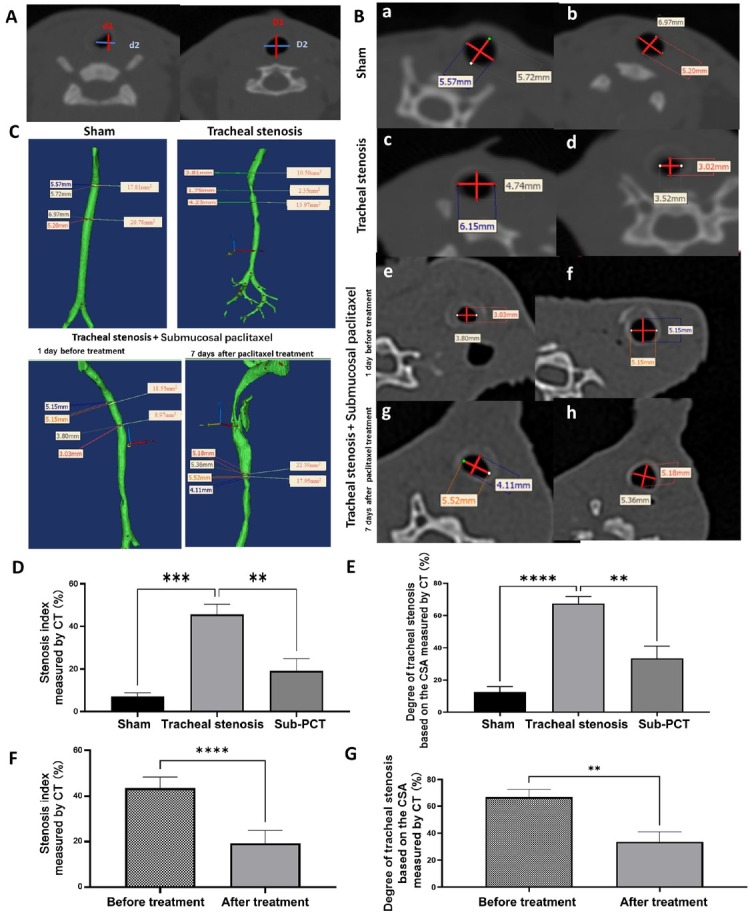
Comparison of diameters of the trachea on CT. (A) Mediastinum window of cervical CT showing the measurement of tracheal diameter at the location of the stenosis and the nearest normal tracheal ring. d1 and D1: the anteroposterior dimension; d2 and D2: transverse dimension. (B) Mediastinum window of cervical CT. a, c, e, and g show scans of the target site of the trachea, while b, d, f, and h show scans of the site of the nearest normal tracheal ring below the target site. Representative axial computed CT neck images in the tracheal stenosis group. Measurements of the tracheal diameter were taken in the AP and transverse dimensions; the cross-sectional area was determined with software by drawing an outline around the airway space at each level. (C) Three-dimensional reconstruction showing the CSA and diameters of the narrowest lumen. (D) Comparison of the severity of tracheal stenosis in different groups. Stenosis index based on the diameters measured by CT scan. (E) Comparison of SD% in different groups. (F) Comparison of the severity of tracheal stenosis in the treatment group before and after treatment. (G) Comparison of SD% in the treatment group before and after treatment. Values are expressed as the mean ± SEM (n = 5 rabbits/group). **P < 0.01, ***P < 0.001, and ****P < 0.0001. AP: anterior-posterior; CSA: cross-sectional area; CT: computed tomography; SEM: standard error of the mean; SD%: degree of tracheal stenosis.
Corresponding to the thickness changes in HE staining, ATWT measured by POCUS presented similar changes, showing increased thickness in the TS model group, while it was significantly decreased in the treatment group (Figure 6).
Figure 6.
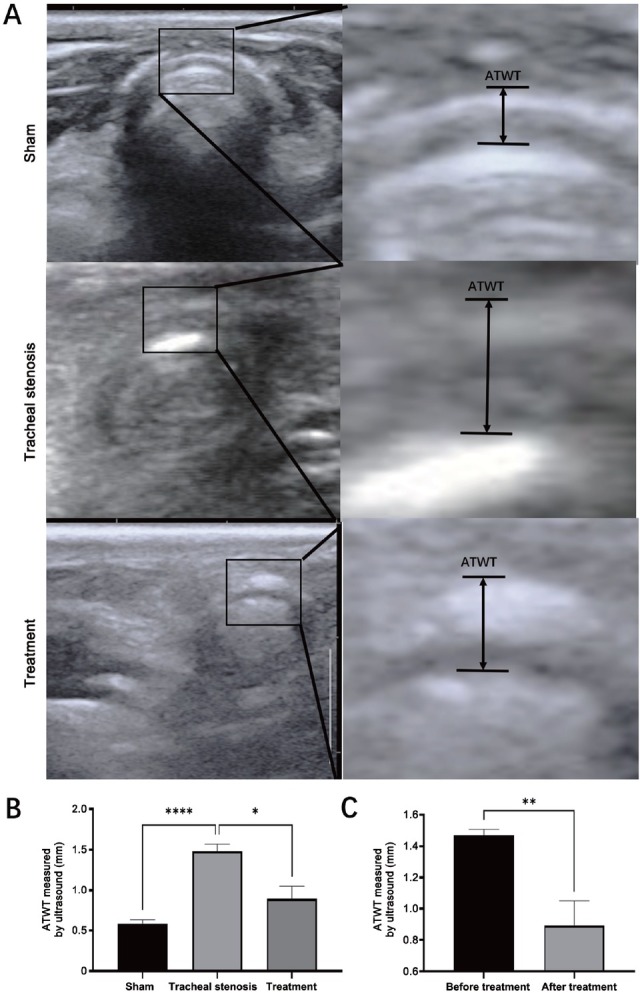
Comparison of ATWT measured by cervical ultrasound in the transverse plane. (A) Sonogram of the trachea by cervical ultrasound in the transverse plane and measurement of ATWT. (B) Comparison of ATWT in the sham group, tracheal stenosis group, and treatment group. (C) Comparison of the changes of AWAT before and after treatment. Values are expressed as the mean ± SEM (n = 5 rabbits/group). *P < 0.05, **P < 0.01, and ****P < 0.0001. ATWT: anterior tracheal wall thickness; SEM: standard error of the mean.
Correlation of ATWT by POSCUS with histologic LP thickness and stenosis severity on CT
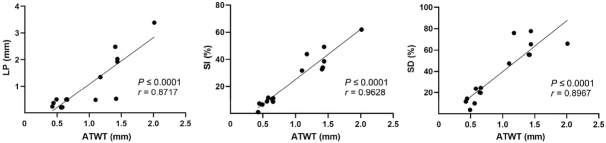
ATWT measured by POCUS was correlated with the histologic LP thickness (r = 0.8717, P < 0.0001), SI% (r = 0.9628, P < 0.0001), and SD% (r = 0.8967, P < 0.0001), indicating the significance of ATWT in identifying the severity of tracheal stenosis (Figure 7).
Figure 7.
Correlation of the thickness of anterior wall of trachea by transcutaneous ultrasound with LP thickness on histology and stenosis severity (SI%, SD%) on CT. CT: computed tomography; LP: lamina propria; SD%: degree of tracheal stenosis; SI%: tracheal stenosis index; ATWT: anterior tracheal wall thickness.
Discussion
Based on this study, POCUS was employed to guide the submucosal injection of paclitaxel to the thickened anterior tracheal wall in tracheal stenosis, especially to the location of the epithelium and LP, where the granulation tissue proliferates and fibroblasts activate. In addition, ATWT by POCUS was employed as the measurement index for the quantification of the stenosis wall. Although POCUS has been integrated into airway management as it allows for rapid and noninvasive assessments, most of the assessments focused on the assessment of airway difficulties, prediction of ETT size and placement, localization of the cricothyroid membrane for emergency airway access and tracheal rings for US-guided tracheostomy, and identification of vocal cord dysfunction and pathology before induction of anesthesia.[35, 36, 37, 38] The application of POCUS in evaluation of subglottic tracheal stenosis was only reported in case reports.[16] Furthermore, the use of POCUS as guidance for the submucosal administration of medicine for the treatment of tracheal stenosis has not been reported before. In this study, we were able to achieve our aim of evaluating the effect of POCUS guiding paclitaxel administration in a rabbit model of scraping injury stimulating tracheal stenosis, and the paclitaxel administration site was monitored ex vivo and identified for the first time.
Submucosal injections with different medications have been reported to be effective in the treatment of tracheal stenosis, which could help to reduce the surgical burden on tracheal stenosis patients and may obviate the need for future airway intervention. For example, mitomycin C appears to be an effective and safe adjuvant treatment in the endoscopic management of tracheal stenosis, with a statistically significant increase, from < 20% to 75%, in the success rate of endoscopic treatment of acquired upper airway stenosis.[7] Another study indicated that serial in-office intralesional steroid injection is safe and well tolerated in adults with subglottic and proximal tracheal stenosis.[39] One of our previous case reports illustrated an adjuvant submucosal triamcinolone injection performed with a hybrid knife that delivered triamcinolone more efficiently into the submucosal layer for the treatment and prevention of the recurrence of tracheal stenosis.[8] Wang et al.[40] conducted a survey about the therapeutic effect of paclitaxel on benign airway stenosis, and the Results showed that local application of paclitaxel inhibits airway scar formation after airway injury in a rabbit model. In a subsequent study, paclitaxel was applied to the airway mucosa at the site of stenosis using a newly developed local instillation catheter for benign cicatricial airway stenosis as the adjuvant treatment after the primary treatment of balloon dilation, cryotherapy, and/or high-frequency needle-knife treatment, with a durable remission rate of 85.7% and a combined effective rate of 96.4%.[9] Most of the previous studies were based on the bronchoscopic delivery of the medication, which is invasive and usually serial. Our study, for the first time, with POCUS guidance, administered paclitaxel as a novel and less-invasive method that could target the involved anterior tracheal wall as an intralesional injection. The advantages of this method are as follows: in contrast to the intralesional injection during bronchoscopic intervention, which can penetrate the mucosa from the bronchoscope but provides no identification of the deeper structure visually, the layer structure of the tracheal wall lesion could be differentiated clearly by the delineation of hypoechoic shadow of the cartilage and hyperechoic shadow of the A-M interface; the whole process of the submucosal injection would be monitored by POCUS with the dynamic thickening of the local layer as the expansion of the medication in the loose tissue; direct injection to the granulation tissue, which was shown as the structural disorder and local thickening of ATWT by US; due to the relatively low invasiveness of the procedure and the point-of-care monitoring, multiple and stepwise treatment could be performed based on the local condition of the recovery status, but with less radioactive exposure compared to sequential CT scans. In addition, ATWT measured by US provides the condition of recovery as quantification of the tracheal wall. It has been proven that in young healthy adults, ultrasonography appears to be a reliable tool in assessing the diameter of the subglottic upper airway.[27] Thus, intralesional injection to the anterior tracheal wall guided by POCUS may give clinicians the chance to reduce the rate of subsequent formation of granulation tissue, while possibly reducing scarring or the need to undergo repeated bronchoscopic interventions or stent implantations for PITS.
Paclitaxel has become a clinical first-line chemotherapeutic agent with broad anticancer spectrum and exerts an antitumor effect by stabilizing microtubules and inhibiting cell cycle progression.[41,42] Previously, studies[43, 44, 45] indicated that paclitaxel inhibits airway scar formation by inhibiting the proliferation of human tracheal fibroblasts and inducing cell apoptosis. In this study, we also found paclitaxel could reverse collagen deposition in the anterior wall of the trachea. However, the specific regulatory mechanism of paclitaxel remains to be further explored.
During the submucosal injection process, POCUS could observe the dynamic changes of the local mucosa and the posterior inner trachea air column presents as an air artifact in the trachea. Thus, POCUS could monitor swelling of the mucosa during and after submucosal injection of the medication and aid in potentially monitoring safety of the submucosal injection, as shown in the ex vivo porcine trachea experiment and in the in vivo rabbit airway stenosis model.
US may be useful to evaluate patients with subglottic stenosis, but there are still some limitations in our study. First, this is a study based on the rabbit tracheal stenosis model and porcine trachea and lung ex vivo. The thyroid glands lie in front of the trachea, sometimes just in front of the tracheal stenosis. However, the nature and size of the thyroid glands of rabbits are different from those of humans. Thus, there might be some minor differences in the images of the trachea, and they influence the POCUS-guided submucosal injection procedure. If possible, exploratory clinical research should be designed and carried out to determine the feasibility of the POCUS-guided submucosal injection in PITS patients. Second, tracheal stenosis only sited in the posterior wall is not an indication for POCUS-guided submucosal injection because POCUS is not able to scan the posterior wall. Third, we evaluated only the scraping injury inducing subglottic stenosis. The sonographic changes of other etiologies of subglottic stenosis should be evaluated in the future.
In summary, POCUS-guided submucosal paclitaxel injection alleviates tracheal stenosis induced by direct scraping of the tracheal mucosa of rabbits. POCUS-guided submucosal injection might work as a potential drug delivery method for the treatment of PITS.
Footnotes
Author Contribution
Hou G, Zhang Q, and Deng M participated in the study design and drafting of the article and revising it. Deng M, Wang M, Jiang B, Yan L, and Bian Y participated in data acquisition, analysis, and interpretation. All the authors approved the final version to be published. Hou G is the “guarantor” and takes responsibility for the integrity of the whole work.
Source of Funding
This work was supported by National High Level Hospital Clinical Research Funding (2022-NHLHCRF-LX-01), the Elite Medical Professionals project of China-Japan Friendship Hospital (No. ZRJY2021-BJ08), the Nonprofit Central Research Institute Fund of the Chinese Academy of Medical Sciences (No. 2020-PT320-001).
Data Sharing Statement
Data are available upon reasonable request made to the corresponding author.
Ethics Approval
The protocol of this study was approved by the Animal Care and Use Committee of China Medical University (Approval No. CMU2019075), and all experiments were performed in accordance with the ARRIVE guidelines and the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
None declared.
References
- 1.Ulusan A, Sanli M, Isik A, İ Celik, Tuncozgur B, Elbeyli L. A retrospective 22-patient series from a single center. Surgical treatment of postintubation tracheal stenosis. 2018;41:356–62. doi: 10.1016/j.asjsur.2017.03.001. Asian J Surg. [DOI] [PubMed] [Google Scholar]
- 2.Grillo H, Mathisen D, Ashiku S, Wright C, Wain J. Ann Otol Rhinol Laryngol. Successful treatment of idiopathic laryngotracheal stenosis by resection and primary anastomosis. 2003;112:798–800. doi: 10.1177/000348940311200909. [DOI] [PubMed] [Google Scholar]
- 3.Özdemir C, Kocatürk C, Sökücü S, Sezen B, Kutluk A, Bilen S. A Multidisciplinary Team Approach. Ann Thorac Cardiovasc Surg. Endoscopic and Surgical Treatment of Benign Tracheal Stenosis. 2018;24:288–95. doi: 10.5761/atcs.oa.18-00073. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freitas C, Martins N, Novais-Bastos H, Morais A, Fernandes G, Magalhães A. A 20-year experience. Pulmonology. The role of interventional bronchoscopy in the management of post-intubation tracheal stenosis. 2021;27:296–304. doi: 10.1016/j.pulmoe.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Dalar L, Karasulu L, Abul Y, Özdemir C, Sökücü S, Tarhan M. Choices for Simple and Complex Tracheal Stenosis. Ann Thorac Surg. Bronchoscopic Treatment in the Management of Benign Tracheal Stenosis. 2016;101:1310–7. doi: 10.1016/j.athoracsur.2015.10.005. et al. [DOI] [PubMed] [Google Scholar]
- 6.Tiran B, Parluk T, Kleinhendler E, Man A, Fomin I, Schwarz Y. A Case Series. Isr Med Assoc J. Fiberoptic Bronchoscopic Submucosal Injection of Mitomycin C for Recurrent Bening Tracheal Stenosis. 2020;22:757–60. [PubMed] [Google Scholar]
- 7.Perepelitsyn I, Shapshay S. Otolaryngol Head Neck Surg. Endoscopic treatment of laryngeal and tracheal stenosis-has mitomycin C improved the outcome? 2004;131:16–20. doi: 10.1016/S0194-5998(04)01136-2. [DOI] [PubMed] [Google Scholar]
- 8.Yin Y, Ma W, Li W, Ma H, Kang J, Herth F. A Case Report. Ear Nose Throat J. Hybrid Knife, a Novel Drug Delivery Tool for Treatment of Tracheal Stenosis. 2022;101 doi: 10.1177/0145561320946649. et al. NP92–5. [DOI] [PubMed] [Google Scholar]
- 9.Qiu X, Zhang J, Wang J, Wang Y, Xu M. J Huazhong Univ Sci Technolog Med Sci. Application of paclitaxel as adjuvant treatment for benign cicatricial airway stenosis. 2016;36:817–22. doi: 10.1007/s11596-016-1668-6. [DOI] [PubMed] [Google Scholar]
- 10.Cid-Serra X, Hoang W, El-Ansary D, Canty D, Royse A, Royse C. A Systematic Review. Ultrasound Med Biol. Clinical Impact of Point-of-Care Ultrasound in Internal Medicine Inpatients. 2022;48:170–9. doi: 10.1016/j.ultrasmedbio.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Thind GS, Fox S, Gupta M, Chahar P, Jones R, Dugar S. Cleve Clin J Med. Point-of-care ultrasonography for the hospitalist. 2021;88:345–59. doi: 10.3949/ccjm.88a.20141. [DOI] [PubMed] [Google Scholar]
- 12.Barrosse-Antle ME, Patel K H, Kramer J A, Baston C M. Chest. Point-of-Care Ultrasound for Bedside Diagnosis of Lower Extremity DVT. 2021;160:1853–63. doi: 10.1016/j.chest.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Yamada H, Ito H, Fujiwara M. current situation, problems, and future prospects. J Med Ultrason. Cardiac and vascular point-of-care ultrasound. 2001;2022;49:601–8. doi: 10.1007/s10396-021-01166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell J, Cohen A, Graff J, Fleck R, O’hara S, Alarcon A De. Otolaryngol Head Neck Surg. Pilot Study to Assess the Use of Ultrasound in Evaluating the Abnormal Pediatric Airway. 2020;162:950–3. doi: 10.1177/0194599820912034. et al. [DOI] [PubMed] [Google Scholar]
- 15.Lambert E, Tran H, Ongkasuwan J. Otolaryngol Head Neck Surg. Comparison of Endoscopic and Ultrasonographic Measurements of the Subglottic Airway in Children. 2020;163:1264–9. doi: 10.1177/0194599820936249. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Cho S, Ji S, Jang Y, Kim E, Kim H. A Case Report. A A Pract. Use of Airway Ultrasound in Infants With Unexpected Subglottic Stenosis During Anesthesia Induction. 2021;15:e01369. doi: 10.1213/XAA.0000000000001369. et al. [DOI] [PubMed] [Google Scholar]
- 17.Rymut S, Harker A, Corey D, Burgess J, Sun H, Clancy J. Am J Physiol Lung Cell Mol Physiol. Reduced microtubule acetylation in cystic fibrosis epithelial cells. 2013;305:L419–31. doi: 10.1152/ajplung.00411.2012. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enyuan Q, Mingpeng X, Luoman G, Jinghua G, Yu L, Wentao L. Ther Adv Respir Dis. Erythromycin combined with corticosteroid reduced inflammation and modified trauma-induced tracheal stenosis in a rabbit model. 2018;12:1753466618773707. doi: 10.1177/1753466618773707. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Z, Wei P, Gan L, Li W, Zeng T, Qin C. Mol Med Report. Protective effects of different anti‑inflammatory drugs on tracheal stenosis following injury and potential mechanisms. 2021;23:314. doi: 10.3892/mmr.2021.11953. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, Wang J, Zeng Y. More simplicity and accessibility. Acta Cir Bras. A modified rabbit model of tracheal stenosis and a household endoscope. 2020;35:e351104. doi: 10.1590/ACB351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagishi Y, Morimoto Y, Fujita M, Ozeki Y, Maehara T, Kikuchi M. The Laryngoscope. Rabbit model of airway stenosis induced by scraping of the tracheal mucosa. 2005;115:1087–92. doi: 10.1097/01.MLG.0000163105.86513.6D. [DOI] [PubMed] [Google Scholar]
- 22.Nakagishi Y, Morimoto Y, Fujita M, Morimoto N, Ozeki Y, Maehara T. Chest. Photodynamic therapy for airway stenosis in rabbit models. 2008;133:123–30. doi: 10.1378/chest.07-1410. et al. [DOI] [PubMed] [Google Scholar]
- 23.Terada Y, Ishiyama T, Asano N, Kotoda M, Ikemoto K, Shintani N. BMC Res Notes. Optimal doses of sevoflurane and propofol in rabbits. 2014;7:820. doi: 10.1186/1756-0500-7-820. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mcilwain W, Wistermayer P, Swiss T, Marko S, Ieronimakis N, Rogers D. Int J Pediatr Otorhinolaryngol. Reproducing severe acute subglottic stenosis in a rabbit model. 2017;103:142–6. doi: 10.1016/j.ijporl.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Ye R, Cai F, Guo C, Zhang X, Yan D, Chen C. an in vitro experimental study. Assessing the accuracy of ultrasound measurements of tracheal diameter. 2021;21:177. doi: 10.1186/s12871-021-01398-3. et al. BMC Anesthesiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gómez-Tamayo J, Puerta-Guarín J, Rojas-Camejo C, Caicedo J, Calvache J. J Ultrasound. Inter-rater and intra-rater reliability of the airway diameter measured by sonography. 2018;21:35–40. doi: 10.1007/s40477-017-0276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakhal K, Delplace X, Cottier J, Tranquart F, Sauvagnac X, Mercier C. Anesth Analg. The feasibility of ultrasound to assess subglottic diameter. 2007;104:611–4. doi: 10.1213/01.ane.0000260136.53694.fe. et al. [DOI] [PubMed] [Google Scholar]
- 28.Wei P, Huang Z, Gan L, Li Y, Qin C, Liu G. Am J Transl Res. Nintedanib ameliorates tracheal stenosis by activating HDAC2 and suppressing IL-8 and VEGF in rabbit. 2020;12:4739–48. [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Y, Zhou L, Zhang T, Qin C, Wei P, Luo L. Life Sci. Anti-fibrosis activity of quercetin attenuates rabbit tracheal stenosis via the TGF-β/AKT/ mTOR signaling pathway. 2020;250:117552. doi: 10.1016/j.lfs.2020.117552. et al. [DOI] [PubMed] [Google Scholar]
- 30.Zaghi S, Alonso J, Orestes M, Kadin N, Hsu W, Berke G. Ann Otol Rhinol Laryngol. Idiopathic Subglottic Stenosis: A Comparison of Tracheal Size. 2016;125:622–6. doi: 10.1177/0003489416642783. [DOI] [PubMed] [Google Scholar]
- 31.Calloway H, Kimbell J, Davis S, Retsch-Bogart G, Pitkin E, Abode K. Laryngoscope. Comparison of endoscopic versus 3D CT derived airway measurements. 2013;123:2136–41. doi: 10.1002/lary.23836. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antón-Pacheco J, Usategui A, Martínez I, García-Herrero C, Gamez A, Grau M. Laryngoscope. TGF-β antagonist attenuates fibrosis but not luminal narrowing in experimental tracheal stenosis. 2017;127:561–7. doi: 10.1002/lary.26402. et al. [DOI] [PubMed] [Google Scholar]
- 33.Hillel A, Namba D, Ding D, Pandian V, Elisseeff J, Horton M. JAMA Otolaryngol Head Neck Surg. An in situ, in vivo murine model for the study of laryngotracheal stenosis. 2014;140:961–6. doi: 10.1001/jamaoto.2014.1663. [DOI] [PubMed] [Google Scholar]
- 34.Dorris E, Russell J, Murphy M. Eur Respir Rev. Post-intubation subglottic stenosis: aetiology at the cellular and molecular level. 2021;30:200218. doi: 10.1183/16000617.0218-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alessandri F, Antenucci G, Piervincenzi E, Buonopane C, Bellucci R, Andreoli C. Ultrasound as a new tool in the assessment of airway difficulties. An observational study. 2019;36:509–15. doi: 10.1097/EJA.0000000000000989. et al. Eur J Anaesthesiol. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb M, Holladay D, Burns K, Nakitende D, Bailitz J. Am J Emerg Med. Ultrasound for airway management: An evidence-based review for the emergency clinician. 2020;38:1007–13. doi: 10.1016/j.ajem.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 37.You-Ten K, Siddiqui N, Teoh W, Kristensen M. Can J Anaesth. Point-of-care ultrasound (POCUS) of the upper airway. 2018;65:473–84. doi: 10.1007/s12630-018-1064-8. [DOI] [PubMed] [Google Scholar]
- 38.Austin D, Chang M, Bittner E. Chest. Use of Handheld Point-of-Care Ultrasound in Emergency Airway Management. 2021;159:1155–65. doi: 10.1016/j.chest.2020.09.083. [DOI] [PubMed] [Google Scholar]
- 39.Bertelsen C, Shoffel-Havakuk H, O’dell K, Johns M, Reder L. JAMA Otolaryngol Head Neck Surg. Serial In-Office Intralesional Steroid Injections in Airway Stenosis. 2018;144:203–10. doi: 10.1001/jamaoto.2017.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Zhang J, Chen N, Zhang Y, Xu M, Yue Y. Chin J Tuberc Respir Dis. The pilot study of the effect of paclitaxel by local application on scar formation after airway injury in rabbits. 2013;36:202–6. [PubMed] [Google Scholar]
- 41.Yu DL, Lou ZP, Ma FY, Najafi M. Int Immunopharmacol. The interactions of paclitaxel with tumour microenvironment. 2022;105:108555. doi: 10.1016/j.intimp.2022.108555. [DOI] [PubMed] [Google Scholar]
- 42.Mahabady M K, Mirzaei S, Saebfar H, Gholami M H, Zabolian A, Hushmandi K. Mechanisms of initiation, progression, and drug sensitivity. Noncoding RNAs and their therapeutics in paclitaxel chemotherapy. 2022;237:2309–44. doi: 10.1002/jcp.30751. et al. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Zhang J, Wang J, Y-H Pei, Xu M, Zhang X. Zhonghua Jie He He Hu Xi Za Zhi. The inhibition effect of paclitaxel on the proliferation of human pulmonary fibroblasts. 2010;33:17–20. et al. [PubMed] [Google Scholar]
- 44.Wang LH, Zhang J, Chen N, Zhang YY, Xu M, Yue YM. Zhonghua Jie He He Hu Xi Za Zhi. The pilot study of the effect of paclitaxel by local application on scar formation after airway injury in rabbits. 2013;36:202–6. [PubMed] [Google Scholar]
- 45.Chen N, Zhang J, Xu M, Wang T, Wang YL, Pei YH. Zhonghua Jie He He Hu Xi Za Zhi. The influence of mitomycin C and paclitaxel on the proliferation and apoptosis of human pulmonary fibroblast. 2013;(36):655–60. [PubMed] [Google Scholar]