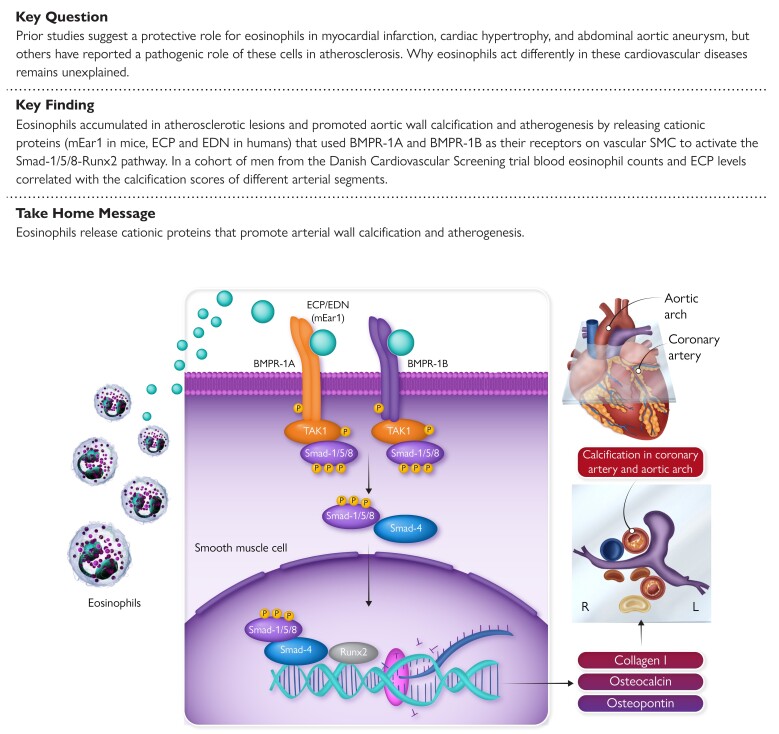
Abstract
Aims
Blood eosinophil count and eosinophil cationic protein (ECP) concentration are risk factors of cardiovascular diseases. This study tested whether and how eosinophils and ECP contribute to vascular calcification and atherogenesis.
Methods and results
Immunostaining revealed eosinophil accumulation in human and mouse atherosclerotic lesions. Eosinophil deficiency in ΔdblGATA mice slowed atherogenesis with increased lesion smooth muscle cell (SMC) content and reduced calcification. This protection in ΔdblGATA mice was muted when mice received donor eosinophils from wild-type (WT), Il4−/−, and Il13−/− mice or mouse eosinophil-associated-ribonuclease-1 (mEar1), a murine homologue of ECP. Eosinophils or mEar1 but not interleukin (IL) 4 or IL13 increased the calcification of SMC from WT mice but not those from Runt-related transcription factor-2 (Runx2) knockout mice. Immunoblot analyses showed that eosinophils and mEar1 activated Smad-1/5/8 but did not affect Smad-2/3 activation or expression of bone morphogenetic protein receptors (BMPR-1A/1B/2) or transforming growth factor (TGF)-β receptors (TGFBR1/2) in SMC from WT and Runx2 knockout mice. Immunoprecipitation showed that mEar1 formed immune complexes with BMPR-1A/1B but not TGFBR1/2. Immunofluorescence double-staining, ligand binding, and Scatchard plot analysis demonstrated that mEar1 bound to BMPR-1A and BMPR-1B with similar affinity. Likewise, human ECP and eosinophil-derived neurotoxin (EDN) also bound to BMPR-1A/1B on human vascular SMC and promoted SMC osteogenic differentiation. In a cohort of 5864 men from the Danish Cardiovascular Screening trial and its subpopulation of 394 participants, blood eosinophil counts and ECP levels correlated with the calcification scores of different arterial segments from coronary arteries to iliac arteries.
Conclusion
Eosinophils release cationic proteins that can promote SMC calcification and atherogenesis using the BMPR-1A/1B-Smad-1/5/8-Runx2 signalling pathway.
Keywords: Eosinophil, Eosinophil cationic protein, Bone morphogenetic protein receptors, Smooth muscle cell, Calcification
Structured Graphical Abstract
Structured Graphical Abstract.
Eosinophils release cationic proteins ECP and EDN in humans (or their orthologue mEar1 in mice) that use BMPR-1A and BMPR-1B as their receptors on vascular SMC. Their ligation of these receptors activates downstream Smad-1/5/8 and Runx2 that induce the expression of osteogenic proteins as a mechanism that promotes arterial wall calcification and atherogenesis. ECP, eosinophilic cationic protein; EDN, eosinophil-derived neurotoxin; mEar1, mouse eosinophil-associated-ribonuclease-1; BMPR, bone morphogenetic protein receptor.
See the editorial comment for this article ‘Eosinophils promote vascular calcification and atherosclerosis: adding another layer of complexity on the path to clarity?', by N. Gerdes, https://doi.org/10.1093/eurheartj/ehad323.
Translational perspective.
Blood eosinophils and their cationic proteins are cardiovascular disease risk factors. This study demonstrates a role for eosinophils and their cationic proteins in atherogenesis by promoting aortic smooth muscle cell osteogenic differentiation. Eosinophil cationic proteins have been recognized as risk factors of several diseases for a half-century. We find here that eosinophil cationic proteins bind to bone morphogenetic protein receptors to stimulate smooth muscle cell calcification. In humans, blood eosinophil counts and cationic protein levels associate with the calcification scores of various arterial segments. Interruption of eosinophil cationic proteins binding on their receptors may block vascular calcification and attenuate atherogenesis.
Introduction
Eosinophils develop in the bone-marrow under the control of transcription factor GATA1.1,2 They are often considered effectors of allergy that reside in the respiratory tract and lymphoid organs,3 circulate in blood, or migrate to the site of inflammation.4,5 Like other granulocytes, eosinophil granules contain cytokines such as interleukin-4 (IL4), IL5, IL13, chemokines, growth factors, and a unique set of cationic proteins such as eosinophilic cationic protein (ECP) and eosinophil-derived neurotoxin (EDN) that are released upon activation6 and mediate cell-cell interaction.3,6 Eosinophil accumulation characterizes eosinophilic myocarditis patients with heart failure.7,8 Yet, clinical studies have yielded conflicting results regarding eosinophil involvement in cardiovascular events. In a Turkish study of 1909 ST-segment elevation myocardial infarction (STEMI) patients, the rate of in-hospital mortality and major adverse cardiac events (MACEs) were significantly higher in patients with low blood eosinophil count at admission (the percentage of eosinophil count in the peripheral blood ≤0.60%) than those with high blood eosinophil count at admission (>0.60%). Therefore, low blood eosinophil count is a biomarker for risk of STEMI patient in-hospital mortality and MACEs.9 A similar study of 5287 Chinese patients who underwent coronary angiography found significantly lower blood eosinophil counts in patients with non-STEMI and STEMI than in those without coronary artery diseases (CADs). Multivariable logistic regression analysis indicated a protective role for blood eosinophil counts in severe CAD [odds ratio (OR) = 0.897, P = 0.000] or in non-STEMI/STEMI (OR = 0.728, P = 0.000).10 However, other studies have drawn the opposite conclusion. From 3742 patients undergoing coronary angiography, blood eosinophil counts associated positively with major cardiovascular risk factors and cardiovascular disease (CVD) prevalence.11 In the UK Biobank, of 478 259 individuals, 1377 died of CVD and 8987 died of other causes after 7 years of follow-up. Blood eosinophil counts were significantly higher in the CVD mortality group than in those of total death (n = 13 482) or alive (n = 464 797).12 A sensitive marker of eosinophil activation, plasma ECP levels, was also significantly higher in patients with angiographic evidence of CAD such as stable angina and non-STEMI than those in patients with normal coronary arteries.13 In patients who received first generation drug-eluting stents, serum ECP levels were significantly higher in those who had MACE than in those without MACE during 18 month follow-up. Pre-intervention ECP levels predict clinical events.14 These clinical studies suggest a pathogenic role for eosinophils in human CVD.
In contrast to these clinical studies, several preclinical studies using eosinophil-deficient mice support a reparative role of eosinophils in the myocardium after infarct or hypertrophic injury and in abdominal aortic aneurysms (AAA). Eosinophil deficiency in ΔdblGATA mice increased LV dilation and cardiac hypertrophy along with enhanced cardiac dysfunction after myocardial infarction (MI) or hypertrophic injury15–17 and enhanced AAA growth.18 Yet, use of the same eosinophil-deficient ΔdblGATA mice yielded conflicting results regarding the role of eosinophils in atherosclerosis. In apolipoprotein E-deficient (Apoe−/−) mice, eosinophil deficiency reduced atherogenesis. Eosinophils were recruited to the lesions after aggregating with platelets, thereby enhancing lesion platelet accumulation and arterial thrombosis.19 In contrast, transplantation of bone-marrow from ΔdblGATA mice did not affect atherogenesis in low-density lipoprotein receptor-deficient (Ldlr−/−) mice.20 Thus, the role of eosinophils in atherosclerosis remains uncertain.
Here, we report a pathogenic role for eosinophils in atherogenesis. Eosinophils released mouse eosinophil-associated-ribonuclease-1 (mEar1), which increased vascular smooth muscle cell (SMC) apoptosis and calcification, while eosinophil-derived IL4 and IL13 did not. We found that mouse mEar1 and human ECP and EDN bound to bone morphogenetic protein receptor-1A (BMPR-1A) and BMPR-1B and activated the Smad-1/5/8-Runt-related transcription factor-2 (Runx2) signalling pathway, as a mechanism that promoted human and mouse vascular SMC calcification. A population-based study of 5864 men and a subpopulation of 394 men from the Danish Cardiovascular Screening (DANCAVAS) trial revealed positive correlations of blood eosinophil counts and ECP levels with the calcification scores in coronary artery, renal artery, iliac artery, aortic valve, mitral valve, abdominal superarenal aorta, ascending and descending aortas, and infrarenal aorta.
Methods
Human studies
The human population included men attending the DANCAVAS trial in Odense, Denmark. The DANCAVAS trial, which has been described in detail elsewhere,21 is a population-based, randomized trial of screening for subclinical CVD in men aged 65∼74. No exclusion criteria were used. One-third was invited for cardiovascular screening examinations including a CT scan at one of the four locations, among which 62.4% men attended. The screening included a low-dose, non-contrast CT scan to detect coronary artery calcification and aortic and iliac aneurysms; brachial and ankle blood pressure index to detect peripheral arterial disease and hypertension; a telemetric assessment of heart rhythm; and a measurement of blood cholesterol and glucose levels. For this study, leucocyte counts, including eosinophils, were measured by flow cytometry on a Sysmex XN-9000 in the clinic from 5864 consecutive men who gave blood samples from January 2015 to August 2018. Of these 5864 men, a random subpopulation of 3494 men was divided into two groups with the highest and lowest CAC scores. From each group, we selected 200 participants and measured their plasma ECP levels using a high sensitivity human ECP ELISA kit (SK00128-06, Aviscera Bioscience, Santa Clara, CA). The investigators measuring the ECP levels were blinded to the CAC scores. The samples between the two groups were analyzed in a random order on the ELISA kit. Six samples were excluded due to incomplete information, leading to an ECP subpopulation of 394 individuals. The calcification score of the coronary artery, aortic segment, renal artery, and iliac artery was calculated using the Agatston Method22 with a SyngoVia (Siemens Healthcare Solutions) workstation and seven trained radiographers, blinded to clinical data. At attendance of screening, each man was first given the informed consent and then a medical history was obtained including previous acute MI, coronary revascularization, chronic obstructive pulmonary disease (COPD), medication history, and symptoms. Up-to-date cardiovascular preventive treatment was recommended in case of subclinical cardiovascular disease. Use of anonymized patient information from the DANCAVAS was approved by the Human Investigation Review Committee at the Brigham and Women’s Hospital, Boston, MA, USA (protocol #2010P001930) with a waiver of informed consent since the study did not involve patient contact or enrolment.
Mice and atherosclerotic lesion characterization
ΔdblGATA (C57BL/6, N12), Apoe−/− (C57BL/6, N12), Il4−/− (C57BL/6, N12), and Myh11CreER(T) (C57BL/6, N > 7) mice were obtained from the Jackson laboratory (Bar Harbor, ME). Il13−/− and BMPR-1Afl/fl mice were reported previously.18,23BMPR-1Bfl/fl (C57BL/6J, N12) mice were produced from Cyagen Biosciences Inc. (Santa Clara, CA). ΔdblGATA and Apoe−/− mice were crossbred to generate the Apoe−/−ΔdblGATA and Apoe−/− littermates. BMPR-1Afl/fl and/or BMPR-1Bfl/fl mice were crossbred with Myh11CreER(T) mice to generate Myh11CreER(T)BMPR-1Afl/fl, Myh11CreER(T)BMPR-1Bfl/fl, and Myh11CreER(T)BMPR-1Afl/fl/1Bfl/fl mice and their littermate BMPR-1Afl/fl, BMPR-1Bfl/fl, and BMPR-1Afl/fl/1Bfl/fl mice. To induce atherosclerotic calcification, 6-week-old male Apoe−/−ΔdblGATA and Apoe−/− control mice were fed with an atherogenic diet (D12108c, Research Diet, New Brunswich, NJ) for 12 weeks. To determine the role of eosinophils in mouse atherosclerotic calcification, eosinophil-deficient Apoe−/−ΔdblGATA mice received adoptive transfer of in vitro cultured eosinophils from C57BL/6 WT mice, followed by the consumption of an atherogenic diet. Our recent study showed that donor eosinophils remained high for 7 days after adoptive transfer in recipient mouse hearts but lowered after 14 days.17 Therefore, each recipient mouse received six doses of 1 × 107 donor eosinophils by intravenous (i.v.) injection starting at day 0 before being fed an atherogenic diet and two weeks thereafter. To test the role of eosinophil-derived IL4 and IL13 and mEar1 in atherosclerosis and aortic calcification, we performed adoptive transfer of eosinophils from Il4−/− and Il13−/− mice to Apoe−/−ΔdblGATA-recipient mice by six doses of i.v. injection of 1 × 107 donor eosinophils or subcutaneous (s.c.) injection of mEar1 twice per week (5 μg/mouse/time, 00128-03, Aviscera Bioscience) or phosphate-buffered saline (PBS) as controls. A total of 9∼11 mice per experimental group reached to the 12-week time point. All mice were sacrificed with carbon dioxide narcosis, followed by cardiac puncture blood collection.
We embedded the aortic root and aortic arch in optimal cutting temperature (OCT) compound (23730571, Thermo Fisher Scientific, Hampton, NH) after harvesting the heart and aorta. We prepared 10 slides per block, each of which contained three 6-μm frozen serial sections through the aortic sinus with all three valve leaflets visible, aortic arch with all three branches (left subclavian artery, left common carotid artery, and brachiocephalic artery) visible. The lesions in the root of the aorta beneath all three-valve leaflets near the ostia of the coronary arteries were analyzed. The lesions in the aortic arches were analyzed as we reported previously.24 To characterize atherosclerotic lesion composition, serial cryostat cross-sections were used for immunostaining to detect SMC (α-actin, 1:750, F3777, Sigma-Aldrich, St. Louis, MO), macrophages (Mac-3, 1:900, 553322, BD Biosciences, Billerica, MA), CD4+ T-cells (CD4, 1:90; 553043, BD Biosciences), CD31+ mircovessels (CD31, 1:1500, 553370, BD Biosciences), MHC class-II (MHC-II) (antigen-presenting cell marker, 1:150, 556999, clone M5/114.15.2, BD Biosciences), elastin (Modified Verhoeff Van Gieson Elastic Stain Kit, HT25A, Sigma-Aldrich), and collagen (0.1% Sirius Red F3BA; 09400, Polysciences Inc., Warrington, PA). Lesion apoptotic cells were determined with the in-situ apoptosis detection kit, according to the manufacturer’s instructions (S7100, Sigma-Aldrich). Collagen content and elastin fragmentation were graded according to the grading keys described previously.25 CD4+ T-cells, CD31+ microvessels and apoptotic-positive cells were counted blindly and quantified as numbers per aortic section. Serial cryostat cross-sections were used for image analysis using an inverted Nikon Eclipse TE2000-U microscope. The relative SMC, macrophage, and MHC-II-positive cell contents within the aortas were quantified by measuring the immunostaining signal positive area using computer-assisted image analysis software (Image-Pro Plus; Media Cybernetics, Bethesda, MD). Investigators were blinded to the sources of samples, the assay, and quantification.
Mouse aortic tissue and cell calcification characterization
The deposition of mineralized calcium in mouse aortic tissues and in vitro cultured SMC was determined by the Alizarin red S (A5533, Sigma-Aldrich) staining. Cryostat sections of aortic tissues or human and mouse SMC were fixed with 4% paraformaldehyde (PFA) for 5 min, rinsed for 5 min, stained with 1% Alizarin red S solution (pH 4.2) for 5 min, rinsed by three changes of PBS (pH 7.4) to remove non-specific staining, dehydrated with acetone, acetone/xylene (1:1), and xylene, and slides were mounted with Permanent Mounting Medium (H-5000, Vector Laboratories, Burlingame, CA). Images were captured and calcium contents were quantified by measuring the Alizarin red-positive area using Image-Pro Plus.
Alkaline phosphatase activity detection
The alkaline phosphatase (ALP) activity in mouse aortic roots and arches were determined using Vector® Red Substrate Kit, ALP (SK-5100, Vector Laboratories) according to the manufacturers’ instructions. Briefly, cryostat sections of aortic roots and arches were rinsed with PBS and stained with an appropriate amount of substrate-working solution at room temperature in the dark for 30 min. Slides were then rinsed with PBS to remove non-specific staining, and the stained matrix was photographed. The ALP activity in mouse aortic tissues was quantified by measuring the positive area using Image-Pro Plus.
Intracellular ALP activity was detected using an ALP activity colorimetric assay kit (K412, BioVision Inc., Mountain View, CA). After osteogenic induction and treatments, the original medium was removed. The cells were added with 50 µL of assay buffer to homogenize and centrifuged at 13 000 g for 3 min to remove insoluble materials. Samples were plated to a 96-well plate, added with 50 µL of 5 mM p-nitrophenyl phosphate solution in each well, and incubated at room temperature in the dark for 60 min. The ALP activity was calculated based on the standard curve and normalized to the total cellular protein content: ALP activity (U/g protein) =A (amount of p-nitrophenyl produced by each sample, μmol)/V (volume of the reaction system, mL)/T (time of reaction, min)/lysate protein concentration (g/mL).
Mouse aortic tissue and cell immunofluorescence staining
To characterize osteogenesis, we performed immunofluorescence triple-staining on acetone-fixed cryosections from mouse atherosclerotic lesions and mouse SMC cultured in eight-chamber culture slide (08-774-26, Thermo Fisher Scientific). Slides were blocked with PBS containing 5% BSA for 1 h at room temperature and then incubated with the following antibodies: mouse α-actin-FITC antibody (1:100, F3777, Sigma-Aldrich), rabbit osteocalcin antibody (1:100, AB10911, Sigma-Aldrich), and rat Runx2 antibody (1:100, 692802, BioLegend, San Diego, CA). The secondary antibodies were Alexa Fluor 555 (1:500, A21432, Thermo Fisher Scientific) or Alexa Fluor 647 (1:300, A21244, Thermo Fisher Scientific). The nuclei were counterstained with DAPI (1:10, R37606, Thermo Fisher Scientific). Slides were mounted using Fluorescence Mounting Medium (S3023, Agilent/Dako, Santa Clara, CA), and images were collected under an Olympus FluoView™ FV1000 confocal microscope. α-Actin, osteocalcin and nuclear Runx2 were quantified by measuring the immunostaining signal-positive or double-stained area using Image-Pro Plus. Ki67 or cleaved caspase-3-positive SMCs were determined by immunofluorescence double-staining with rat monoclonal Ki67 antibody (1:100, 14-5698-82, Thermo Fisher Scientific) or rabbit monoclonal cleaved caspase-3 antibody (Alexa Fluor(R) 594 Conjugate, 1:100, 8172S, Cell Signaling Technology, Danvers, MA), together with mouse monoclonal α-actin-FITC antibody (1:100, F3777, Sigma-Aldrich). Secondary antibody for anti-Ki67 antibody was Alexa Fluor 555 (1:500, A21432, Thermo Fisher Scientific). Ki67 or cleaved caspase-3-positive SMCs in arterial wall were quantified by measuring the double-stained area using Image-Pro Plus. Cleaved caspase-3- and osteopontin-positive SMCs were determined by immunofluorescence triple-staining with rabbit monoclonal cleaved caspase-3 antibody [Alexa Fluor(R) 594 Conjugate, 1:100, 8172S, Cell Signaling Technology], goat polyclonal osteopontin antibody (1:100, MBS421341, MyBioSource, San Diego, CA), together with mouse monoclonal α-actin-FITC antibody (1:100, F3777, Sigma-Aldrich). Secondary antibody for osteopontin antibody was Alexa Fluor 647 (1:500, A21447, Thermo Fisher Scientific). Cleaved caspase-3- and osteopontin-positive SMC in arterial wall were quantified by measuring the triple-stained area using Image-Pro Plus. Cleaved caspase-3-positive macrophages were determined by immunofluorescence double-staining with rabbit monoclonal cleaved caspase-3 antibody [Alexa Fluor(R) 594 Conjugate, 1:100, 8172S, Cell Signaling Technology], together with mouse monoclonal CD68 antibody (1:100, ab955, Abcam, Waltham, MA). Secondary antibody for CD68 antibody was Alexa Fluor 488 (1:500, A11029, Thermo Fisher Scientific). Cleaved caspase-3-positive macrophages in arterial wall were quantified by measuring the double-stained area using Image-Pro Plus. Human atherosclerotic lesions were double stained with rabbit monoclonal tryptase antibody (1:100, 51550S Cell Signaling Technology) or rat monoclonal CD68 antibody (1:100, 137002, BioLegend), together with mouse monoclonal Siglec-8 antibody (1:100, 347102, BioLegend). Secondary antibody for tryptase, CD68, or Siglec-8 antibodies were Alexa Fluor 488 (1:500, A11008), Alexa Fluor 488 (1:500, A11006) or Alexa Fluor 555 (1:500, A21422), all from Thermo Fisher Scientific. Mouse atherosclerotic lesions were double stained with rabbit polyclonal mouse mast cell protease-4 (MMCP4) antibody (1:200)26 or mouse monoclonal CD68 antibody (1:100, ab955, Abcam), together with rat monoclonal Siglec-F antibody (1:100, 552125, BD Pharmingen, San Diego, CA). Secondary antibody for MMCP4, CD68, or Siglec-F antibodies were Alexa Fluor 488 (1:500, A11008), Alexa Fluor 488 (1:500, A11029), or Alexa Fluor 555 (1:500, A21434), all from Thermo Fisher Scientific. Aortic root lesions from Apoe−/−ΔdblGATA mice received adoptive transfer of eosinophils from CD45.1 transgenic mice were double stained with rabbit polyclonal mEar1 antibody (1:100, orb13385, Biorbyt, St Louis, MO) or mouse monoclonal CD45.1 antibody (1:100, 1795-10, SouthernBiotech, Birmingham, AL), together with rat monoclonal Siglec-F antibody (1:100, 552125, BD Pharmingen). Secondary antibody for mEar1, CD45.1 or Siglec-F antibodies were Alexa Fluor 555 (1:500, A21428), Alexa Fluor 488 (1:500, A11029), Alexa Fluor 488 (1:500, A11006), or Alexa Fluor 555 (1:500, A21434), all from Thermo Fisher Scientific.
Mouse plasma lipoprotein and BMP measurement
Blood samples were collected at harvest from Apoe−/−, Apoe−/−ΔdblGATA, and Apoe−/−ΔdblGATA mice treated with mEar1 or received adoptive transfer of eosinophils from WT, Il4−/−, and Il13−/− mice. Plasma samples were stored at −80°C until analysis. Plasma total cholesterol (23-666-200, Pointe Scientific, Canton, MI), triglyceride (23-666-410, Pointe Scientific), and HDL (23-666-301, Pointe Scientific) levels were determined using the enzymatic methods according to the manufacturer. The LDL cholesterol level was determined using the Friedewald formula: Plasma LDL cholesterol concentration (mg/dL) = total cholesterol−HDL cholesterol−(triglycerides/5). Plasma BMP-2 (MBS262739, MyBioSource, San Diego, CA), BMP-4 (MBS260174, MyBioSource), mEar1 (SK00128-03, Aviscera Bioscience), leptin (RD291001200R, Biovendor, Asheville, NC), IL-1β, IL-6, TNF-α, IFN-γ (88-7013, 88-7064, BMS607-3TWO, 88-7314, Thermo Fisher Scientific) levels were determined using ELISA kits according to the manufacturers’ instructions.
Cell culture
Eosinophils were cultured as previously reported.17,18 Briefly, bone-marrow cells were collected from mouse femurs and tibias, centrifuged for 5 min at 300 g, followed by lysing red blood cells (RBCs) by pipetting cell pellet up and down twice in 9 mL sterile H2O and then immediately adding 1 mL of 10 × PBS. Once RBCs were lysed, cells were suspended in 10 mL 1 × PBS and counted. Cells with a concentration of 106 cells/mL were plated in an 80 cm2 flask and cultured in a base media containing mouse stem cell factor (SCF, 100 ng/mL, 250-03, PeproTech, Rocky Hill, NJ) and Flt-3-ligand (Flt3-L, 100 ng/mL, 250-31L, PeproTech) for 2 days. On day 2, half of the media was replaced with fresh medium containing SCF and Flt3-L. On day 4, culture media was replaced with fresh media containing IL5 (10 ng/mL, 405-ML, R&D Systems, Minneapolis, MN). Half of the culture media were changed every 2 days with fresh media containing IL5 until the 14th days. On day 14, cells were collected for the further study. FACS monitored eosinophil purity and expression of eosinophil surface markers (Siglec-F and CD45.1), as we recently described.16
Mouse aortic SMCs were isolated from aortas of WT, Runx2f/f and Runx2f/f; SM22α-Cre mice.24,27 Briefly, mouse abdominal aortas were removed and separated from fat and adventitia followed by digestion with collagenase type II (2 mg/mL, Worthington Biochemical Corp., Lakewood, NJ) at 37°C for 10 min. The adventitia was completely stripped under microscopic visualization, the aortas were opened, and EC were removed by abrasion. The aortas were then chopped and digested in type II collagenase (1 mg/mL; Worthington Biochemical Corp.) for 40–50 min at 37°C. Digestion was stopped by adding 10 mL of Dulbecco's Modified Eagle Medium (DMEM) containing 10% foetal bovine serum (FBS). Cells were resuspended by gentle pipetting, passed through a 70 μm filter, and spun at 300 g for 10 min at 4°C. Cells were cultured in DMEM (11995-065, Gibco, Waltham, MA) supplemented with 10% (vol/vol) FBS and GlutaMAX (4.5 g/L, 35050061, Thermo Fisher Scientific). SMCs were plated onto Corning 60 mm dish. After 2 passages SMCs were transferred onto six-well plates and allowed to grow until they were ready for treatment. Aortic SMCs were isolated from Myh11CreER(T)BMPR-1Afl/fl, Myh11CreER(T)BMPR-1Bfl/fl, and Myh11CreER(T)BMPR-1Afl/fl/1Bfl/fl mice and BMPR-1Afl/fl, BMPR-1Bfl/fl, and BMPR-1Afl/fl/1Bfl/fl control mice. Cells were treated with 0.5 μM of tamoxifen or vehicle (dimethyl sulfoxide, DMSO) for 24 h to induce Cre-mediated recombination.
Human aortic SMCs were isolated and cultured by explant outgrowth from unused portions of non-atherosclerotic carotid arteries from heart transplant donors, as previously described.28 Cells were grown in DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1.25 μg/mL amphotericin B, and 2 mmol/L l-glutamine. Cells were passaged by brief trypsinization and used through passage 5 for the experiments after being growth-arrested for 2 days in serum-free insulin–transferrin medium consisting of DMEM and Ham’s F12 (1:1, vol/vol; 11320-033, Thermo Fisher Scientific) supplemented with 1 μmol/L insulin and 5 μg/mL transferrin. Fresh insulin–transferrin medium was used for the experiments with or without addition of stimuli.
SMC osteogenic differentiation induction and treatment
Human and mouse SMC were plated in 24-well plates with 2 × 104 cells in each well until reaching 60%–70% confluence. Cells were exposed to osteogenic media containing 2.5 mM CaCl2 and 5 mM β-glycerophosphate by continuous culture in medium for 14 days. Osteogenic media was refreshed every 2 days. Human SMC were treated with 500 ng/mL ECP (00128-01-100, Aviscera Bioscience) or EDN (H00006036-Q01, Abnova, Walnut, CA). Control and Runx2 knockout aortic SMC were exposed to osteogenic media with or without mEar1 (500 ng/mL), murine IL4 (100 ng/mL, 214-14, PeproTech) or IL13 (100 ng/mL, 210-13, PeproTech), eosinophil lysate from WT, Il4−/− or Il13−/− mice at 106 eosinophils/mL, and WT eosinophil lysate plus mEar1 antibody (1000 ng/mL, orb13385, Biorbyt). The treatment was refreshed along with the changes of osteogenic media. At the 14th day, the cells were stimulated by the respective treatment for 30 min before collection for further study. To test the effect of calcification on SMC apoptosis, WT SMCs were cultured in control media or osteogenic media and treated with or without 20 μM of PDTC.
Real-time PCR
Total RNA from human and mouse SMC was prepared using the TRIzol™ reagent (15596018, Thermo Fisher Scientific), and cDNA was reverse transcribed using the high-capacity cDNA reverse transcription kit (4368813, Thermo Fisher Scientific). The relative mRNA levels of target genes were quantified using the iTaq UniverSYBR Green SMX 5000 (1725125, Bio-Rad, Hercules, CA) with an ABI PRISM 7900 sequence detector system (Applied Biosystems Co, Foster City, CA). Each reaction was performed in duplicate, and changes in relative gene expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels using the relative threshold cycle method. Primer sequences are listed in Supplementary data online, Table S3.
Immunoblot analysis
Human and mouse SMCs were harvested and lysed in a lysis buffer containing 50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS. Equal amounts of protein from each cell-type preparation were separated on a SDS–PAGE, blotted, and detected with rabbit anti-ALP (1:1000, NBP2-67295, Novus Biologicals, Littleton, CO), goat anti-BMPR-1A (1:1000, PA5-18494, Thermo Fisher Scientific), mouse anti-BMPR-1B (1:1000, MAB505-100, R&D Systems), mouse anti-BMPR-2 (1:1000, MA5-15827, Thermo Fisher Scientific), mouse anti-collagen I (1:1000, PA5-29569, Thermo Fisher Scientific), rabbit anti-osteocalcin (1:1000, AB10911, Sigma-Aldrich), mouse anti-osteopontin (1:1000, 691302, BioLegend), rat anti-Runx2 (1:1000, 692802, BioLegend), rat anti-TGFBR-1 (1:1000, MAB5871, R&D Systems), and rat anti-TGFBR2 (1:1000, MAB532, R&D Systems), and GAPDH (1:2000, 2118S, Cell Signaling Technology) antibodies. Western blot signals were assessed by densitometric analysis using the Image J software.
Nuclear and cytoplasmic extract preparation
The nuclear extraction was prepared using an NE-PER nuclear cytoplasmic extraction reagent kit (78833, Thermo Fisher Scientific). Briefly, human and mouse SMC cultured on a six-well plate were exposed to the above-mentioned treatment and then washed three times with PBS. The cultured cells were lysed in RIPA buffer (50 mM Tris-HCL pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) supplemented with complete protease inhibitor cocktail tablets (04906845001, Thermo Fisher Scientific). Cell debris was removed by centrifugation at 12 000 rpm for 20 min. Then cells were suspended in 200 μL of cytoplasmic extraction reagent-I and incubated on ice for 10 min followed by addition of 11 μL of a second cytoplasmic extraction reagent-II. After centrifugation, the supernatant fraction (cytoplasmic extract) was transferred to a pre-chilled tube. The insoluble pellet fraction, which contains crude nuclei, was re-suspended in 100 μL of a nuclear extraction reagent. After centrifugation, the resulting supernatant, constituting the nuclear extract, was used for immunoblotting to detect Runx2 (1:1000, 692802, BioLegend) and histone-H3 (1:1000, 9715S, Cell Signaling Technology).
Overexpression, transient knockdown and transfection
The mammalian expression vector pcDNA3.0 encoding the BMPR-1A (ID 80873), BMPR-1B (ID 80882), and BMPR-2 (ID 163403) cDNAs were obtained from Addgene (Watertown, MA) to overexpress the BMPRs in 293 T cells. BMPR-1A (ID s281) and BMPR-1B (ID s2042) siRNA was obtained from Invitrogen (Carlsbad, CA) to knockdown the expression of these receptors in human SMC. Transfections of the cDNA and siRNA were performed using Lipofectamine® 2000 Reagent (11668-027, Invitrogen) according to manufacturer’s protocol. The cells were incubated in cDNA/siRNA-lipid complexes for 24 h at 37°C. After 24 h, the media was changed to fresh media and the transfected cells were used for further experiments.
ECP and mEar1 BMPR binding assay and Scatchard plot analysis
Recombinant mouse mEar1, and human ECP and EDN were conjugated with FITC using the FluoroTag™ FITC Conjugation Kit (FITC1-1KT, Sigma-Aldrich). Briefly, mEar1, ECP, or EDN was diluted at 5.0 mg/mL in 0.1 M carbonate-bicarbonate buffer, pH 9.0, and combined with 50 μL FITC solution with different dilutions (5:1, 10:1, 20:1), according to the manufacturer’s instructions. Reaction was carried at room temperature under dark for 2 h. FITC-conjugated mEar1, ECP, or EDN was purified over a Sephadex G-25 M column. Binding assay was performed according to an established protocol as previously described.29 In brief, SMC and 293 T cells were resuspended in a buffer containing 20 mM HEPES and 10 mM EDTA, pH 7.5 and incubated at 4°C for 15 min. Cells were then homogenized and centrifuged at 28 000 rpm at 4°C for 30 min. Cell pellet was resuspended into a storage buffer containing 20 mM HEPES and 1 mM EDTA, pH 7.5, and re-homogenized with polytron PT3100 homogenizer. An equal amount of protein was incubated with FITC-mEar1, FITC-ECP or FITC-EDN in a binding buffer (50 mM Tris-HCL, 5 mM MgCl2, 25 μM EDTA, 0.2% BSA) for 90 min at room temperature in an AcroWell™ 96 Filter Plate (Pall Corp., Port Washington, NY). The plate was then washed four times with 50 mM Tris-HCl and 5 mM MgCl2. The plate was read at 494 nm excitation and 518 nm emission. Scatchard plot analysis was performed using the Prism 9.0 software (GraphPad Software Inc., La Jolla, CA). To test FITC-mEar1, FITC-ECP or FITC-EDN binding affinity on SMC, the cells post osteogenic differentiation were transfected with BMPR-1A and/or BMPR-1B siRNA and treated with or without excessive BMP2 (1000 ng/mL, 767304, BioLegend). To test FITC-mEar1 binding affinity on BMPRs from 293 T cells, the cells were transfected with BMPR expression vectors and treated with or without excessive mEar1 (5 µg/mL) or BMP2 (1 µg/mL). Empty vector-transfected 293 T cells were used as background controls. Data were presented as the mean of three experiments.
Immunoprecipitation
Human and mouse SMCs were lysed in an immunoprecipitation lysis buffer (0.025 M Tris, 0.15 M NaCl, 0.001 M EDTA, 1% NP-40, 5% glycerol; pH 7.4) and pre-cleared with control agarose resin for 1 h according to the manufacturer’s instructions (26149, Thermo Fisher Scientific). Cell lysates (500 μg) were subsequently incubated overnight at 4°C with either rabbit anti-human ECP (10 μg, orb156688, Biorbyt), rabbit anti-human EDN (10 μg, NBP3-03635, Novus Biologicals), rabbit anti-mouse mEar1 (10 μg, orb13385, Biorbyt), or mouse IgG isotype (10 μg, 026502, Thermo Fisher Scientific) antibodies. After captured, washed and eluted, the immunoprecipitates were separated on a 10% SDS–PAGE, immunoblotted with goat anti-mouse BMPR1A (1:1000), mouse anti-BMPR-1B (1:1000), mouse anti-BMPR-2 (1:1000), rat anti-TGFBR-1 (1:1000), and rat anti-TGFBR2 (1:1000) antibodies, to detect the immune complexes. Likewise, cell lysates were incubated with goat anti-BMPR-1A (10 μg) or mouse anti-BMPR-1B (10 μg) antibodies. The immunoprecipitates were separated and immunoblotted with rabbit anti-human ECP (1:1000) or rabbit anti-human EDN (1:1000) antibodies. The same blot was also probed with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:3000, A16104, Thermo Fisher Scientific), rabbit anti-goat IgG (1:3000, A16136, Thermo Fisher Scientific), and goat anti-mouse IgG (1:3000, 31430, Thermo Fisher Scientific) to detect immunoprecipitated IgG from each sample.
Statistical analysis
Blood eosinophil counts and calcification scores were poorly normally distributed. Therefore, non-parametric test was performed for univariate analyses. Median and interquartile range (IQR) were given as descriptive statistics, and baseline characteristics were compared by χ2, Wilcoxon rank sum test, or Kruskal–Wallis test. Associations with a P-value below 0.100 were considered to be potential confounders. Spearman’s correlation analysis was used to assess the correlation between eosinophil counts and calcification score, and Kruskal–Wallis analysis was used for the quartiles of calcification scores and the eosinophil counts. Finally, multivariate test was performed after log-transformation of blood eosinophil counts and calcification scores. Partial correlation analyses were performed after adjusting for the potential confounders identified above. All mouse data were expressed as mean ± standard error of means (SEM). Shapiro–Wilk test was used to determine data distribution normality. One-way analysis of variance test followed by a post hoc Tukey’s test was used for multiple comparisons (≥3 groups) with normally distributed variables. The Kruskal–Wallis test followed by Dunn’s procedure was conducted for multiple comparisons with abnormally distributed variables. SPSS 20.0 and Prism 7 (GraphPad) software were used for statistical analysis. SPSS28 0.1.0 version was used for analysis and P < 0.05 was considered statistically significant.
Study approvals
Discarded and decoded human aortas and patient data were reused according to the protocol #2010P001930 pre-proved by the Human Investigation Review Committee at the Brigham and Women’s Hospital, Boston, MA, USA. No patient informed consent was required. All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was approved by the Brigham and Women's Hospital Standing Committee on Animals (protocol #2016N000442).
Results
Eosinophil accumulate in human and murine atherosclerotic lesions
Immunostaining using the antibody against human sialic-acid-binding immunoglobulin-like lectin (Siglec-8), an inhibitory receptor selectively expressed on human eosinophils,30 detected no eosinophils in normal human aorta, but accumulation of Siglec-8-positive eosinophils in human atherosclerotic lesions (see Supplementary data online, Figure S1A). Studies of mouse atherosclerotic lesions yielded similar results. Frozen sections of aortic roots from Apoe−/− mice that consumed an atherogenic diet or a chow diet (normal) for 12 weeks were used for immunostaining with mouse eosinophil-specific Siglec-F antibody. The normal mouse aortic root showed negligible Siglec-F staining, but Siglec-F-positive eosinophils accumulated in mouse atherosclerotic arteries, mostly in the adventitia (see Supplementary data online, Figure S1B). To exclude the possibility that mast cells or macrophages express Siglec-8 or Siglec-F, we performed immunofluorescence double-staining with Siglec-8 antibody and human mast cell tryptase or macrophage CD68 antibodies in human normal aorta and atherosclerotic arteries, and used Siglec-F antibody together with mouse mast cell chymase (MMCP4)26 and CD68 antibodies for mouse normal aorta and atherosclerotic aorta. As expected, normal human or mouse aortas did not contain mast cells or macrophages. Tryptase- or chymase-positive mast cells and CD68-positive macrophages accumulated in human and mouse atherosclerotic lesions. These cells did not express Siglec-8 or Siglec-F (see Supplementary data online, Figure S2A–D). ECP and mEar1 (mouse eosinophil-associated-ribonuclease-1, a murine homologue of ECP) antibodies were used to confirm human and mouse eosinophil accumulation. All Siglec-8-positive cells in human atherosclerotic lesions and Siglec-F-positive cells in mouse atherosclerotic lesions expressed ECP and mEar1 (see Supplementary data online, Figure S2E and F).
Eosinophil deficiency protects mice from atherosclerosis
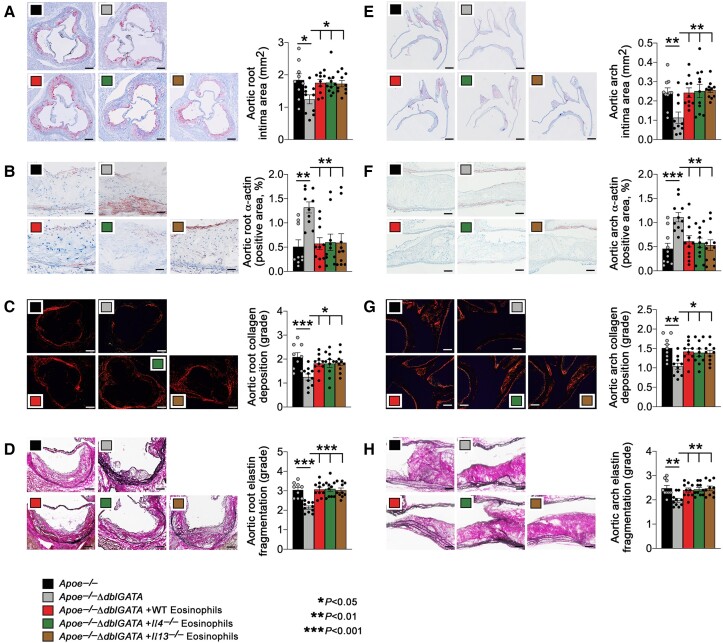
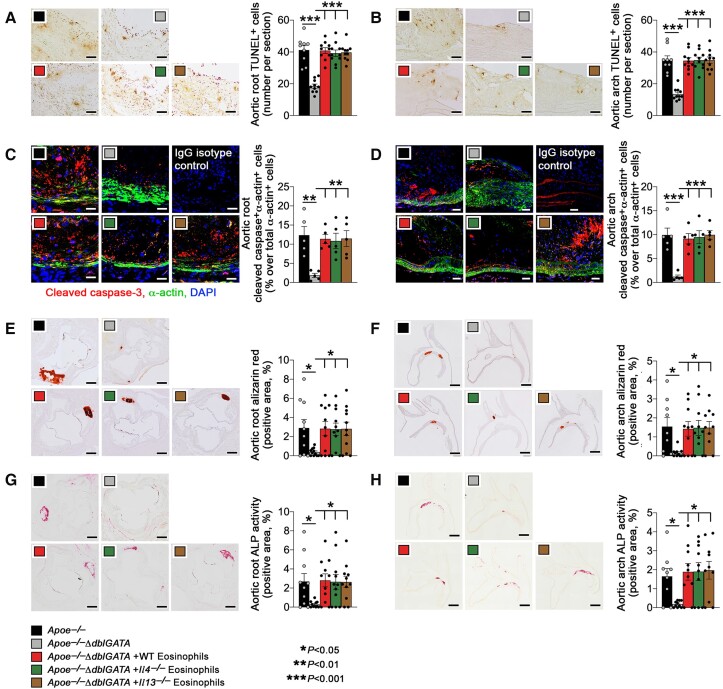
To test the participation of eosinophils in atherogenesis, we produced eosinophil-deficient Apoe−/−ΔdblGATA mice and fed both Apoe−/− and Apoe−/−ΔdblGATA mice an atherogenic diet for 12 weeks. Compared with the Apoe−/− mice, Apoe−/−ΔdblGATA mice showed significantly reduced atherosclerotic lesion size, increased lesion α-actin-positive SMC content, and reduced lesion collagen accumulation and elastica fragmentation in both the aortic root (Figure 1A–D) and aortic arch (Figure 1E–H). Our earlier studies demonstrated a cardiovascular reparative role for eosinophil-derived IL4 and IL13 in myocardial infarcted hearts, pressure overload-induced cardiac hypertrophy, and angiotensin-II infusion-induced AAA.16–18 Yet, adoptive transfer studies showed that donor eosinophils from wild-type (WT), Il4−/− or Il13−/− mice did not differ in reversing the atherosclerotic findings in Apoe−/−ΔdblGATA recipient mice. Reduced atherosclerosis in Apoe−/−ΔdblGATA recipient mice was reversed by all these donor eosinophils (Figure 1A–H). Immunostaining showed that eosinophil deficiency or reconstitution of eosinophils from the same sets of mice did not affect aortic root and arch lesion macrophage or CD4+ T-cell contents, MHC class-II levels, or CD31+ microvessel numbers (see Supplementary data online, Figure S3A–F). Immunofluorescence double-staining with Ki67 and α-actin antibodies indicated that eosinophil deficiency did not affect lesion SMC proliferation (see Supplementary data online, Figure S3G). Nor did eosinophil deficiency or eosinophil reconstitution affect plasma total cholesterol, triglyceride, LDL, or HDL levels (see Supplementary data online, Figure S4A). Elevated aortic root and arch SMC content in Apoe−/−ΔdblGATA mice (Figure 1B and F) but no significant difference in lesion SMC proliferation (see Supplementary data online, Figure S3G) suggest a role for eosinophils in SMC death. Consistent with this hypothesis, we detected significant reduction of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells in aortic root and arch lesions from Apoe−/−ΔdblGATA mice (Figure 2A and B). Immunofluorescence double-staining with the cleaved caspase-3 and α-actin antibodies showed that eosinophil deficiency reduced apoptosis of lesional SMC. Adoptive transfer of eosinophils from WT, Il4−/− or Il13−/− mice reversed the lesion total cell apoptosis or lesion SMC apoptosis as well (Figure 2A–D). Irrelevant isotype-matched IgGs served as controls (Figure 2C and D). The assessment of the caspase-3 + α-actin+ apoptotic SMC contents in the intima from the aortic root or arch using the same immunofluorescence double-staining approach yielded similar results (see Supplementary data online, Figure S4B and C). This study did not detect significant differences in caspase-3 + CD68+ apoptotic macrophage content in either the media or intima from aortic root or arch (see Supplementary data online, Figure S4D–E). Unexpectedly, we detected vascular calcification in most of the aortic root and arch lesions from Apoe−/− mice, but not in lesions from the Apoe−/−ΔdblGATA mice. To quantify these differences in vascular calcification, we stained the lesion calcium deposition with Alizarin red27,31 and measured the vascular calcification marker ALP activity.32 Eosinophil deficiency blunted calcium deposition (Figure 2E and F) and ALP activity (Figure 2G and H) in lesions from aortic root and arch. Such reductions were all recovered by adoptive transfer of eosinophils from WT, Il4−/− or Il13−/− mice (Figure 2E–H), suggesting that eosinophils promote atherogenesis and aortic lesion calcification by mechanisms not involving IL4 or IL13. To confirm that donor eosinophils targeted and survived in the aorta, we transferred donor eosinophils from CD45.1 transgenic mice to Apoe−/−ΔdblGATA mice and then rendered them atherosclerotic and then performed Immunofluorescence double-staining to detect donor eosinophils in the lesions. Siglec-F-positive donor eosinophils in recipient mouse lesions expressed mEar1 (see Supplementary data online, Figure S5A) in addition to CD45.1, the marker of donor cells (Figure S5B).
Figure 1.
Eosinophils accelerate atherogenesis in apoe−/− mice. Apoe−/− and Apoe−/−ΔdblGATA mice were fed with an atherosclerotic diet for 12 weeks. Apoe−/−ΔdblGATA mice were also reconstituted with eosinophils from wild-type, Il4−/−, or Il13−/− mice every 2 weeks. Aortic root intima area, scale: 500 μm (A), α-actin+ smooth muscle cell contents in arterial wall, scale: 200 μm (B), Sirius red collagen staining in grade, scale: 200 μm (C), and elastin fragmentation in grade, scale: 200 μm (D). Aortic arch intima area, scale: 500 μm (E), α-actin+ smooth muscle cell contents in arterial wall, scale: 200 μm (F), Sirius red collagen staining in grade, scale: 200 μm (G), and elastin fragmentation in grade, scale: 200 μm (H). The results are expressed as mean ± standard error of mean of 9–10 mice per group.
Figure 2.
Eosinophils promote arterial calcification and lesion apoptosis in apoe−/− mice. Apoe−/− and Apoe−/−ΔdblGATA mice were fed with an atherosclerotic diet for 12 weeks. Apoe−/−ΔdblGATA mice were reconstituted with eosinophils from WT, Il4−/−, or Il13−/− mice every 2 weeks. (A) Aortic root and (B) arch TUNEL-positive apoptotic cell contents. Scale: 100 μm. (C) Root and (D) arch immunofluorescence staining for cleaved caspase-3 + α-actin+ smooth muscle cell. IgG isotypes were used as experimental controls. Scale: 50 μm. (E) Root and (F) arch Alizarin red-positive calcium deposition. Scale: 500 μm. (G) Root and (H) arch alkaline phosphatase activity-positive area. Scale: 500 μm. The results are expressed as mean ± standard error of mean of 9–10 mice per group.
Eosinophils use cationic protein mEar1 to promote atherogenesis
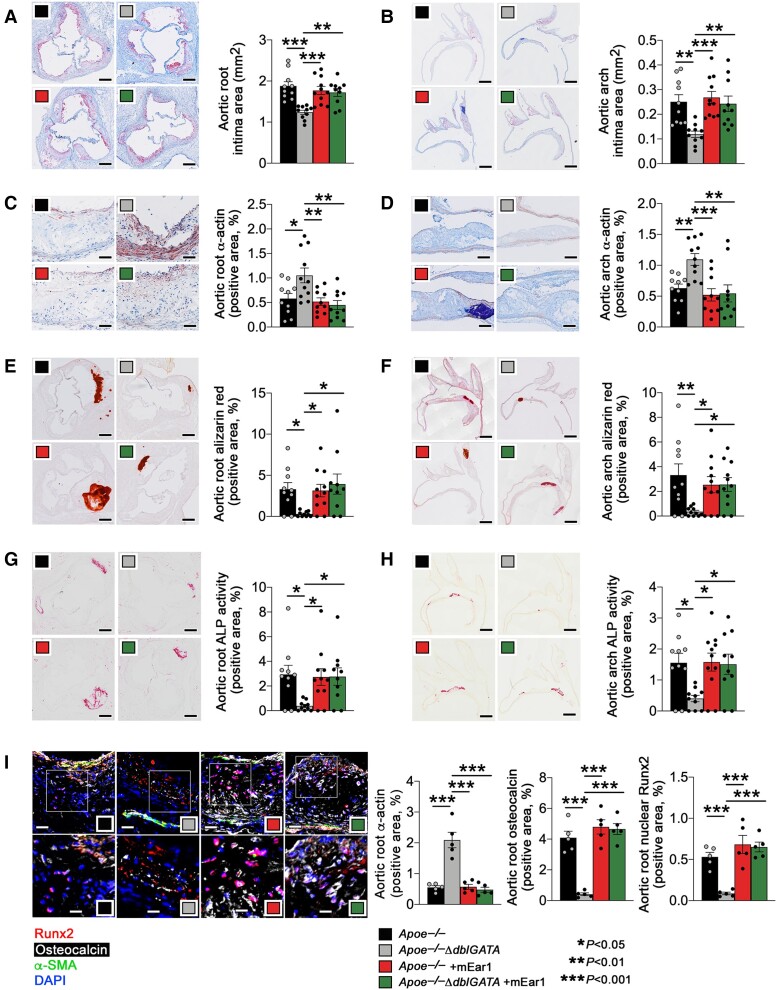
In addition to cytokines (e.g. IL4 and IL13), eosinophils also release cationic proteins such as ECP and EDN3,8 that could account for reduced atherosclerosis and lesion calcification in Apoe−/−ΔdblGATA mice. To test this possibility, we administered recombinant mouse mEar1 to Apoe−/− and Apoe−/−ΔdblGATA mice by subcutaneous injection, followed by consumption of an atherogenic diet. This treatment reduced intimal area, increased SMC content, and lowered Alizarin red-positive area and ALP activity in atherosclerotic lesions in both aortic roots and arches in the Apoe−/−ΔdblGATA mice to the levels observed in Apoe−/− mice. These parameters did not change in Apoe−/− mice with or without mEar1 treatment (Figure 3A–H), suggesting the requirement of higher doses of mEar1. ELISA results showed the recovery of blunted plasma mEar1 in Apoe−/−ΔdblGATA mice to the levels in Apoe−/− mice in either Apoe−/− or Apoe−/−ΔdblGATA mice after mEar1 treatment (see Supplementary data online, Figure S6A). As in the Apoe−/−ΔdblGATA mice, mEar1 did not affect plasma lipids measured (see Supplementary data online, Figure S6B), the adipokine leptin (see Supplementary data online, Figure S6C), or pro-inflammatory cytokines (see Supplementary data online, Figure S6D) in Apoe−/− or Apoe−/−ΔdblGATA mice. Like in the Apoe−/−ΔdblGATA mice that received donor eosinophils from WT, Il4−/−, or Il13−/− mice (Figure 2C and D; see Supplementary data online, Figure S4B–E), mEar1 treatment also increased aortic root and arch intima caspase-3 + α-actin+ apoptotic SMC content in Apoe−/−ΔdblGATA mice (see Supplementary data online, Figure S7A and B). Eosinophil deficiency or mEar1 treatment also did not affect aortic root or aortic arch lesion caspase-3 + CD68+ macrophage content (see Supplementary data online, Figure S7C and D).
Figure 3.
Mouse eosinophil-associated-ribonuclease-1 exacerbates atherogenesis and arterial calcification in apoe−/− mice. Apoe−/− and Apoe−/− ΔdblGATA mice were fed with an atherosclerotic diet and administrated with recombinant mouse eosinophil-associated-ribonuclease-1 (5 μg/mouse/time) twice per week for 12 weeks. (A) Aortic root and (B) arch intima area. Scale: 500 μm. (C) Root and (D) arch α-actin+ smooth muscle cell contents. Scale: 200 μm. (E) Root and (F) arch Alizarin red-positive calcium deposition. Scale: 500 μm. (G) Root and (H) arch alkaline phosphatase activity-positive area. Scale: 500 μm. (I) Aortic root α-actin, osteocalcin, and nuclear Runt-related transcription factor-2 contents by immunofluorescence staining. Scale: 50 μm (top) and 25 μm (bottom). The results are expressed as mean ± standard error of mean of 10–11 (A–H) and 5 mice (I) per group.
The transcription factor Runx2 is a master regulator for vascular SMC osteogenic transition and vascular calcification.27,33,34 The Runx2-regulated bone marker osteocalcin is the most abundant non-collagenous protein in bone35 and associates with vascular calcification.36 Eosinophil deficiency increased aortic root lesion α-actin SMC positive area but blunted lesion osteocalcin-positive area and lesion nuclear Runx2-positive area. Subcutaneous mEar1 treatment reversed these changes in Apoe−/−ΔdblGATA mice, but not in Apoe−/− mice (Figure 3I). Reduced lesion collagen deposition, aortic wall elastica fragmentation, total TUNEL-positive cell, and cleaved caspase-3 + α-actin+ SMC in both aortic roots and arches from Apoe−/−ΔdblGATA mice were also reversed in mice that received mEar1. The mEar1 treatment did not affect these variables in Apoe−/− mice (see Supplementary data online, Figure S8A–H). Nor did mEar1 affect aortic root or arch lesion macrophage and CD4+ T-cell content, MHC class-II-positive area, CD31+ microvessel numbers, and Ki67 + α-actin+ SMC proliferation in Apoe−/− or Apoe−/−ΔdblGATA mice (see Supplementary data online, Figure S9A–G). These observations suggest that eosinophils contributed to atherogenesis and vascular calcification in Apoe−/− mice via the cationic protein mEar1.
Immunofluorescence triple-staining demonstrated blunted calcified apoptotic SMC (caspase-3 + osteopontin + α-actin+), apoptotic SMC (caspase-3 + α-actin+), calcified apoptotic cells (caspase-3 + osteopontin+), and cellular calcification (osteopontin+) in aortic root atherosclerotic lesions in Apoe−/−ΔdblGATA mice. All these blunted phenotypes reverted when mice received subcutaneous mEar1 treatment (see Supplementary data online, Figure S10). Therefore, osteogenic differentiated SMC may be prone to undergoing apoptosis and vice versa. To test this possibility, we cultured mouse aortic SMC under normal and osteogenic media37 and induced cell apoptosis with or without pyrrolidine dithiocarbamate (PDTC). The results showed that osteogenic medium enhanced PDTC-induced apoptosis (increased cleaved caspase-3 and reduced Bcl-2), and PDTC also increased SMC calcification (increased expression of ALP and osteopontin, another sensitive marker of coronary artery calcification38) (see Supplementary data online, Figure S11).
Eosinophil mEar1 promotes vascular SMC osteogenic differentiation
The results from atherogenic diet-fed Apoe−/− or Apoe−/−ΔdblGATA mice suggest that eosinophil mEar1, but not IL4 or IL13, promotes atherogenesis and vascular calcification. We tested a direct effect of eosinophils and mEar1 in SMC osteogenic differentiation using mouse aortic SMC from WT (Runx2f/f) mice. Recombinant mEar1 and eosinophils from WT, Il4−/−, or Il13−/− mice all promoted SMC osteogenic differentiation when cells were cultured in osteogenic media, as determined by Alizarin red staining (Figure 4A and B) and ALP activity (Figure 4C), suggesting that only mEar1 but not IL4 or IL13 directly affected SMC osteogenic differentiation. In support of this conclusion, recombinant IL4 or IL13 did not affect SMC osteogenic differentiation and mEar1 antibody blunted the osteogenic activity of eosinophils from WT mice (Figure 4A–C). Runx2 is required for vascular SMC osteogenic differentiation in vitro and in vivo.33,34 Using aortic SMC from the SMC-specific Runx2-depleted (Runx2f/f; SM22α-Cre) (Runx2 KO) mice,27 we demonstrated that treatments with mEar1, eosinophils from WT, Il4−/− or Il13−/− mice, eosinophils from WT mice together with mEar1 antibody, or recombinant IL4 and IL13, did not affect SMC osteogenic differentiation (Figure 4A–C). Immunoblot analyses yielded the same results. mEar1 and eosinophils from WT, Il4−/−, or Il13−/− mice, but not IL4 or IL13 enhanced the expression of collagen I, ALP, osteocalcin, and osteopontin in SMC from Runx2f/f mice, but not those from Runx2 KO mice (Figure 4D). mEar1 and eosinophils from WT, Il4−/− or Il13−/− mice, but not IL4 or IL13 also increased Runx2 nuclear translocation, although none of the treatments affected the expression of total cellular Runx2 (Figure 4D). Immunofluorescence triple-staining showed that mEar1 and eosinophils from WT, Il4−/−, or Il13−/− mice, but not IL4, IL13, or WT eosinophils treated with mEar1 antibody blunted the smooth muscle α-actin (α-SMA) expression, but increased the expression of osteocalcin and nuclear Runx2 in aortic SMC from control mice, indicating SMC osteogenic differentiation. None of these treatments affected the expression of α-SMA or osteocalcin in SMC from Runx2 KO mice (Figure 4E). At the mRNA level, mEar1 and eosinophils from WT, Il4−/− or Il13−/− mice, but not IL4, IL13, or WT eosinophils treated with mEar1 antibody increased the expression of ALP, collagen I, osteocalcin, and osteopontin in SMC from Runx2f/f mice but not in those from Runx2 KO mice (see Supplementary data online, Figure S12A–D). None of these treatments affected Runx2 expression (see Supplementary data online, Figure S12E). Together, these observations suggest that mEar1-mediated SMC osteogenic differentiation is mediated by Runx2 activation but not its total concentration.
Figure 4.
Mouse eosinophil-associated-ribonuclease-1 aggravates vascular smooth muscle cell calcification via Runt-related transcription factor-2. Wild-type and Runt-related transcription factor-2 KO mouse aortic smooth muscle cell were exposed to osteogenic media with or without mouse eosinophil-associated-ribonuclease-1, interleukin-4, or interleukin-13 proteins, eosinophil lysates from wild-type, Il4−/− or Il13−/− mice, and wild-type eosinophil lysate plus anti-mouse eosinophil-associated-ribonuclease-1 antibody. (A) Representative images of stained dishes (top), photomicrographs (bottom, Scale: 200 μm). Quantification of (B) Alizarin red staining for mineralized calcium and (C) intracellular alkaline phosphatase activity. (D) Immunoblots of collagen I, alkaline phosphatase, osteocalcin, osteopontin, total and nuclear Runt-related transcription factor-2. (E) Immunofluorescence staining of α-actin, osteocalcin, and Runt-related transcription factor-2. Scale: 50 μm. The results are expressed as mean ± standard error of mean of three independent experiments.
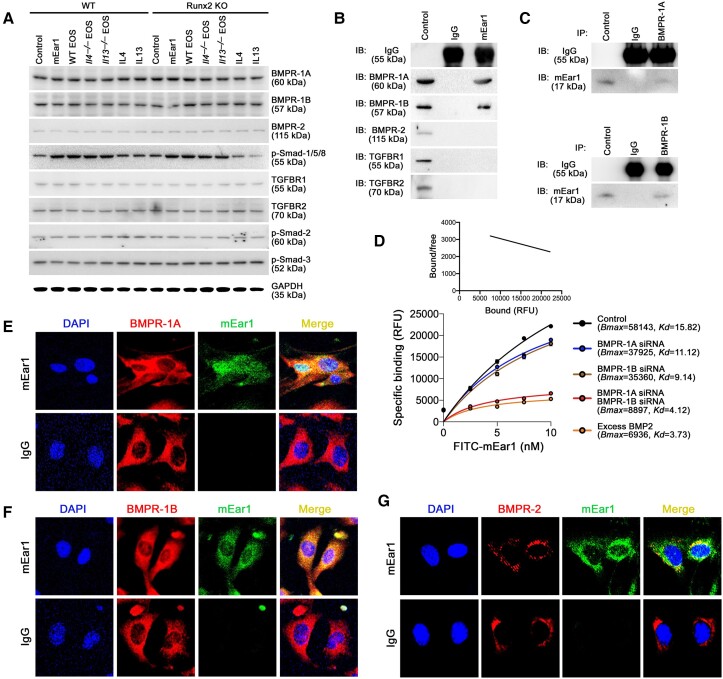
mEar1 binds to the BMP receptors on mouse aortic SMC
TGF-β-TGF-β receptors (TGFBR1/2)-Smad-2/3 pathway and the bone morphogenetic proteins (BMP2/4)-BMPR-1A/1B/2-Smad-1/5/8 pathway promote Runx2 activation during bone development.39 Immunoblot analyses demonstrated that mEar1 and eosinophils from WT, Il4−/−, or Il13−/− mice, or IL4 and IL13 did not affect the expression of BMPR-1A, BMPR-1B, BMPR-2, TGFBR1, and TGFBR2, nor the activation (phosphorylation) of Smad-2 and Smad-3 in aortic SMC from WT or Runx2 KO mice. Only mEar1 and eosinophils from control, Il4−/− or Il13−/− mice, but not IL4, IL13 activated p-Smad-1/5/8 in aortic SMC from WT and Runx2 KO mice (Figure 5A; see Supplementary data online, Figure S13A–C). At the mRNA level, none of these treatments affected the expression of BMPR-1A/1B/2 or TGFBR1/2 (see Supplementary data online, Figure S13D–E). Therefore, mEar1 may use the BMPR-1A/1B/2 rather than TGFBR1/2 as its receptors. To test this hypothesis, we added mEar1 to cultured mouse aortic SMC followed by immunoprecipitation with mEar1 antibody or its isotype IgG. Immunoprecipitates were examined by immunoblotting with different antibodies. The results showed that mEar1 formed immune complexes with BMPR-1A and BMPR-1B. mEar1 did not interact with BMPR-2 or the TGF-β receptors (TGFBR1/2) (Figure 5B). The interaction between mEar1 and BMPR-1A or BMPR-1B was confirmed by immunoprecipitation with BMPR-1A and BMPR-1B antibodies, followed by immunoblotting with mEar1 antibody. The results demonstrated complex formation of mEar1 with BMPR-1A and with BMPR-1B (Figure 5C). Cell surface binding assay using FITC-labelled mEar1 and Scatchard plot analysis showed that mEar1 bound to SMC (Bmax = 58143, Kd = 15.82 nM). Knockdown of BMPR-1A or BMPR-1B with their siRNAs reduced such binding (Bmax = 37925, Kd = 11.12 nM, or Bmax = 35360, Kd = 9.14 nM). Knockdown of both BMPR-1A and BMPR-1B or use of excess authentic BMPR ligand BMP2 fully blocked the FITC-mEar1 binding (Figure 5D). These observations suggest that mEar1 acted like BMP2 and used BMPR-1A and BMPR-1B as its receptors. Immunofluorescence double-staining with mEar1 and BMPR-1A or BMPR-1B antibodies revealed mEar1 colocalization with BMPR-1A (Figure 5E) and BMPR-1B (Figure 5F). Interestingly, under the same conditions, we also detected mEar1 localization with BMPR-2, although on many fewer cells (Figure 5G). Therefore, the absence of mEar1 interaction with BMPR-2 in our immunoprecipitation experiment (Figure 5B) was likely due to the low BMPR-2 expression in mouse aortic SMC (Figure 5A). Together, the results from immunoprecipitation, ligand binding, Scatchard plot analysis, and immunofluorescence staining indicated that mEar1 used BMPR-1A and BMPR-1B as its receptors on mouse aortic SMC. BMP2 and BMP4 are both authentic ligands for BMPR-1A and BMPR-1B.40 Eosinophil deficiency in Apoe−/−ΔdblGATA mice or mEar1 treatment in Apoe−/−ΔdblGATA mice may affect atherogenesis and vascular calcification indirectly by altering the expression of BMP2 or BMP4. Yet, ELISA did not detect any changes in plasma BMP2 or BMP4 levels between Apoe−/− and Apoe−/−ΔdblGATA mice or those received mEar1 (see Supplementary data online, Figure S14).
Figure 5.
Mouse eosinophil-associated-ribonuclease-1 uses bone morphogenetic protein receptors to activate the smad-1/5/8-Runt-related transcription factor-2 signalling pathway. (A) Wild-type and Runt-related transcription factor-2 KO mouse smooth muscle cell were exposed to osteogenic media with or without mouse eosinophil-associated-ribonuclease-1, interleukin-4, interleukin-13, eosinophil lysates from wild-type, Il4−/− or Il13−/− mice. Immunoblots of different bone morphogenetic protein receptors, TGFβRs, and phosphorylated Smad-2, Smad-3, and Smad-1/5/8. (B–G) Wild-type smooth muscle cell were exposed to osteogenic media for 14 days and stimulated with mouse eosinophil-associated-ribonuclease-1 for 30 min before collection. Immunoprecipitation with anti-mouse eosinophil-associated-ribonuclease-1 (B), anti-bone morphogenetic protein receptor-1A (C, top) and anti-bone morphogenetic protein receptor-1B (C, bottom) antibodies, followed by immunoblotting detection of different bone morphogenetic protein receptors, TGFβRs, or mouse eosinophil-associated-ribonuclease-1. (D) FITC-mouse eosinophil-associated-ribonuclease-1 (0∼10.0 nM) binding affinity and Scatchard plot on smooth muscle cell treated with or without bone morphogenetic protein receptor siRNA or excessive BMP2 (1000 ng/mL). Immunofluorescence double-staining of mouse eosinophil-associated-ribonuclease-1 with bone morphogenetic protein receptor-1A (E), bone morphogenetic protein receptor-1B (F) or bone morphogenetic protein receptor-2 (G).
To confirm that mEar1 uses BMPR-1A, BMPR-1B, and possibly BMPR-2 as its receptors, we transfected all three receptors in 293T cells that have only basal levels of expression. Immunoblot analyses confirmed the transfection efficiency. Empty vectors served as negative controls (see Supplementary data online, Figure S15A). Cell surface binding assay and Scatchard plot analysis showed that FITC-mEar1 bound with high affinity to 293T cells that expressed BMPR-1A (Bmax = 54808, Kd = 10.58 nM) and BMPR-1B (Bmax = 59595, Kd = 9.45 nM) (see Supplementary data online, Figure S15B). FITC-mEar1 also bound to 293T cells that was transfected with BMPR-2, but at much lower affinity (Bmax = 18050, Kd = 6.09 nM) (see Supplementary data online, Figure S15C). Much higher binding affinity was detected in 293T cells transfected with both BMPR-1A and BMPR-1B (Bmax = 91814, Kd = 15.49 nM) (see Supplementary data online, Figure S15D). Combined expression of BMPR-1A with BMPR-2 or BMPR-1B with BMPR-2 also increased FITC-mEar1 binding affinity. Empty vector-transfected 293T cells (Control) showed no mEar1 binding (see Supplementary data online, Figure S15D). Excessive unlabelled mEar1 or BMP2 competed the binding of FITC-mEar1 on BMPR-1A- and BMPR-1B- transfected 293T cells (see Supplementary data online, Figure S15B) and also on BMPR-2-transfected 293T cells (see Supplementary data online, Figure S15C).
Deficiency of BMPR-1a or 1B blocks mEar1 activity on SMC osteogenic differentiation
To test a direct role for BMPR-1A or BMPR-1B on mEar1-mediated SMC osteogenic differentiation, we bred the BMPR-1Afl/fl and BMPR-1Bfl/fl mice with Myh11CreER(T) mice and generated Myh11CreER(T)BMPR-1Afl/fl, Myh11CreER(T)BMPR-1Bfl/fl, BMPR-1Afl/fl/1Bfl/fl, and Myh11CreER(T)BMPR-1Afl/fl/1Bfl/fl mice. Aortic SMC from these mice were treated with tamoxifen to induce the depletion of BMPR-1A, BMPR-1B, or both. As expected, mEar1 induced SMC osteogenic differentiation with increased Alizarin red staining, intracellular ALP activity, and expression (mRNA) of collagen I, ALP, osteopontin, and osteocalcin in cells from BMPR-1Afl/fl mice or Myh11CreER(T)BMPR-1Afl/fl mice after vehicle treatment. Such activities of mEar1 were muted in cells from Myh11CreER(T)BMPR-1Afl/fl mice after tamoxifen-induced BMPR-1A depletion (see Supplementary data online, Figure S16A and B). BMPR-1A depletion or mEar1 treatment did not affect the expression (mRNA) of BMPR-1B/2, Runx2, and TGFBR1/2 (see Supplementary data online, Figure S16C). Immunoblot analyses yielded the same conclusions. BMPR-1A depletion or mEar1 treatment did not affect the expression of BMPR-1B/2, total Runx2, TGFBR1/2, and p-Smad-2/3 signalling. mEar1 lost its activities in activating p-Smad-1/5/8 and in inducing the expression of collagen I, ALP, osteopontin, and osteocalcin, and nuclear Runx2 (see Supplementary data online, Figure S16D–E). Aortic SMC from BMPR-1Bfl/fl and Myh11CreER(T)BMPR-1Bfl/fl mice (see Supplementary data online, Figure S17A–E) or from BMPR-1Afl/fl/1Bfl/fl and Myh11CreER(T)BMPR-1Afl/fl/1Bfl/fl mice (Figure 6A–E) yielded the same conclusions as those from BMPR-1Afl/fl mice or Myh11CreER(T)BMPR-1Afl/fl mice. Therefore, SMC osteogenic differentiation requires BMPR-1A and BMPR-1B to mediate mEar1 activity.
Figure 6.
Tamoxifen-induced depletion of bone morphogenetic protein receptor-1A and bone morphogenetic protein receptor-1B in aortic smooth muscle cell blocks mouse eosinophil-associated-ribonuclease-1-induced calcification. Aortic smooth muscle cell were isolated from BMPR-1Afl/fl/1Bfl/fl and Myh11CreER(T)BMPR-1Afl/fl/1Bfl/fl mice and treated with tamoxifen (0.5 µM) or vehicle (DMSO) for 24 h. Cells were then exposed to osteogenic media with or without mouse eosinophil-associated-ribonuclease-1 for 14 days. (A) Representative images of Alizarin red-stained dishes (top) and photomicrographs (bottom, scale: 200 um), and quantifications of Alizarin red staining for mineralized calcium and intracellular alkaline phosphatase activity. (B and C) RT-PCR detected the expression osteogenetic genes (B), bone morphogenetic protein receptor, Runt-related transcription factor-2, and TGF-β receptors (C). (D and E) Immunoblot analysis of bone morphogenetic protein receptor signalling molecules and osteogenetic proteins (D) and TGF-β receptors and signalling molecules (E). lRepresentative images are presented to the left (A, D, E). Data are mean ± standard error of mean from three independent experiments.
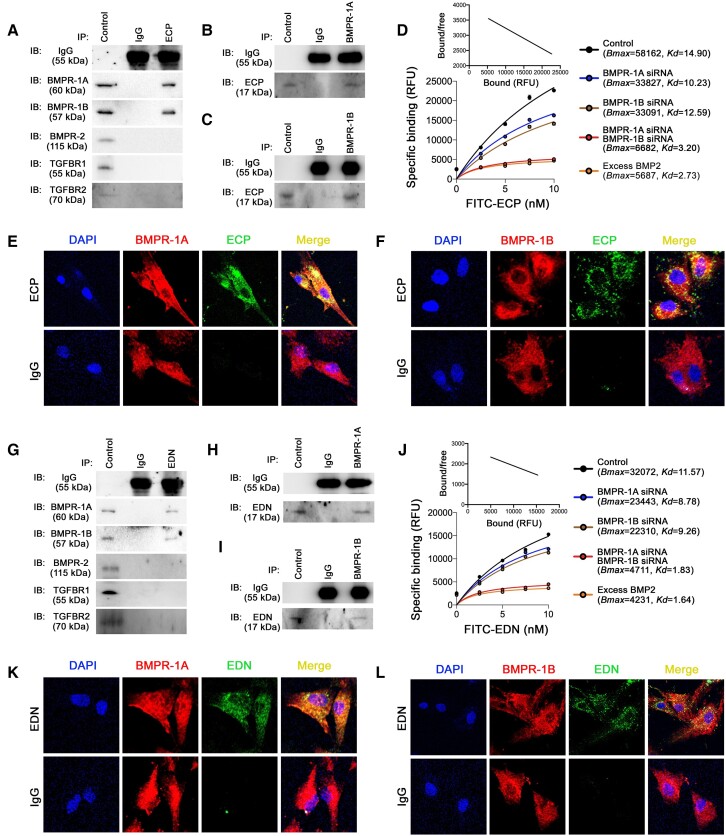
ECP and EDN bind to the BMP receptors on human aortic SMC
mEar1 is a murine homologue of human ECP.41 Human ECP and EDN share similar tertiary sequence structure and surface charges with mEar1.17 mEar1 binding on BMPR-1A/1B and less efficiently on BMPR-2 suggests that human ECP and EDN also use BMPR-1A, BMPR-1B, and possibly BMPR-2 as their receptors on vascular SMC. After human vascular SMCs were treated with ECP followed by immunoprecipitation with ECP antibody, we performed immunoblot analyses with various antibodies and found that human ECP formed complexes with BMPR-1A and BMPR-1B, but not BMPR-2 or TGFBR1/2 (Figure 7A). Reverse immunoprecipitation with BMPR-1A and BMPR-1B antibodies followed by immunoblotting with ECP antibody confirmed the formation of ECP-BMPR-1A and ECP-BMPR-1B immune complexes (Figure 7B and C). Ligand binding assay and Scatchard plot analysis showed that FITC-ECP bound to human aortic SMC (Bmax = 58162, Kd = 14.90). The binding affinity fell after siRNA knockdown of BMPR-1A or BMPR-1B and was abrogated by siRNA knockdown of both BMPR-1A and BMPR-1B or by excess BMP2 (Figure 7D). Immunofluorescence double-staining revealed colocalization of ECP with BMPR-1A and BMPR-1B on human aortic SMC (Figure 7E and F).
Figure 7.
Binding of eosinophil cationic protein and eosinophil-derived neurotoxin on human smooth muscle cell bone morphogenetic protein receptors. Human smooth muscle cell were exposed to osteogenic media for 14 days and stimulated with recombinant eosinophil cationic protein or eosinophil-derived neurotoxin for 30 min before collection. Immunoprecipitation with anti-eosinophil cationic protein (A), anti-bone morphogenetic protein receptor-1A (B) and anti-bone morphogenetic protein receptor-1B (C) antibodies, followed by immunoblotting detection of different bone morphogenetic protein receptors, TGFβRs, and eosinophil cationic protein. (D) FITC-eosinophil cationic protein (0∼10.0 nM) binding affinity and Scatchard plot on smooth muscle cell treated with or without bone morphogenetic protein receptor siRNA or excessive BMP2 (1000 ng/mL). Immunofluorescence double-staining of eosinophil cationic protein and bone morphogenetic protein receptor-1A (E) or bone morphogenetic protein receptor-1B (F). Immunoprecipitation with anti-eosinophil-derived neurotoxin (G), anti-bone morphogenetic protein receptor-1A (H), and anti-bone morphogenetic protein receptor-1B (I) antibodies, followed by immunoblotting detection of different bone morphogenetic protein receptors, TGFBRs, and eosinophil-derived neurotoxin. (J) FITC-eosinophil-derived neurotoxin (0∼10.0 nM) binding affinity and Scatchard plot on smooth muscle cell treated with or without with bone morphogenetic protein receptor siRNA or excessive BMP2 (1000 ng/mL). Immunofluorescence double-staining of eosinophil-derived neurotoxin and bone morphogenetic protein receptor-1A (K) or bone morphogenetic protein receptor-1B (L).
EDN acted directionally similar to ECP, but bound to BMPR-1A and BMPR-1B at much lower affinity. Immunoprecipitation with EDN antibody followed by immunoblotting demonstrated complex formation of EDN with BMPR-1A and BMPR-1B, but not with BMPR-2 or TGFBR1/2 (Figure 7G). Reverse immunoprecipitation with BMPR-1A and BMPR-1B antibodies followed by immunoblotting with EDN antibody confirmed complex formation of EDN with BMPR-1A and BMPR-1B in human aortic SMC (Figure 7H and I). Cell binding assay and Scatchard plot analysis showed that EDN also bound to BMPR-1A and BMPR-1B. This binding fell after BMPR-1A or BMPR-1B knockdown with their cognate siRNAs or was abrogated by siRNA knockdown of both BMPR-1A and BMPR-1B or with excess BMP2 (Figure 7J). Yet, EDN bound to BMPR-1A and BMPR-1B at much lower affinity (Bmax = 32072, Kd = 11.57) than ECP did (Bmax = 58162, Kd = 14.90) (Figure 7D and J). Immunofluorescence double-staining also revealed co-localization of EDN with BMPR-1A and BMPR-1B on human aortic SMC (Figure 7K–L).
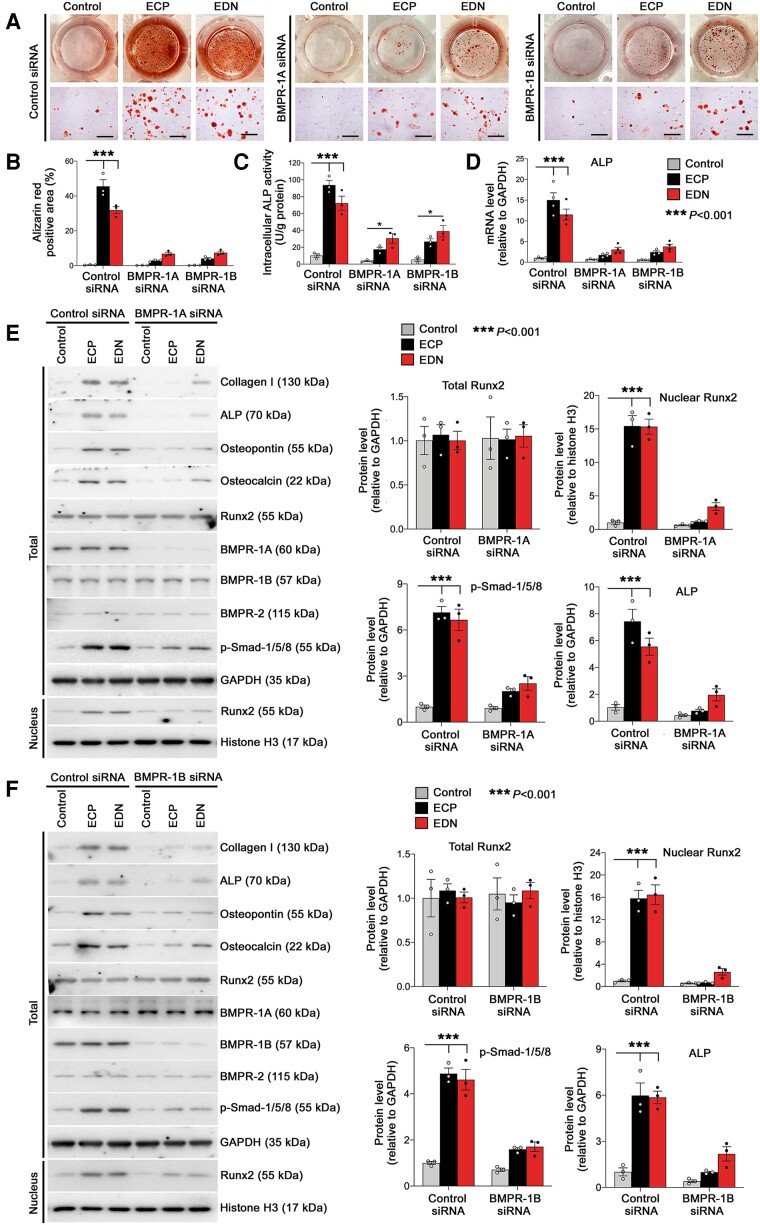
ECP and EDN use BMP receptors to promote human vascular SMC osteogenic transition
Like mEar1, ECP and EDN also used BMPR-1A and BMPR-1B to promote human aortic SMC osteogenic differentiation. Both ECP and EDN enhanced the Alizarin red-positive area, ALP activity, and ALP mRNA levels in human aortic SMC. BMPR-1A siRNA or BMPR-1B siRNA blunted these activities of ECP and EDN (Figure 8A–D). Immunoblot analyses showed that ECP and EDN increased the expression of collagen I, ALP, osteopontin, and osteocalcin, activated p-Smad-1/5/8, and enhanced Runx2 nuclear translocation. Treated with BMPR-1A siRNA(Figure 8E; see Supplementary data online, Figure S18A) or with BMPR-1B siRNA (Figure 8F; see Supplementary data online, Figure S19A) muted all of these activities of ECP and EDN in human SMC. However, ECP or EDN did not affect the expression of Runx2, BMPR-1A/1B, BMPR-2, TGFBR1/2, and their downstream P-Smad-2/3 (Figure 8E and F; see Supplementary data online, Figures S18B and C, S19B and C). At the mRNA level, ECP and EDN also increased the expression of collagen I, osteocalcin, and osteopontin in human aortic SMC. These activities of ECP and EDN were muted after human SMC were treated with BMPR-1A siRNA or BMPR-1B siRNA (see Supplementary data online, Figure S20A). ECP, EDN, BMPR-1A siRNA, or BMPR-1B siRNA did not affect the expression of BMPR-1A/1B, BMPR-2, TGFBR1/2, or Runx2 (see Supplementary data online, Figure S20B–E). Together, these observations suggest that ECP and EDN both use BMPR-1A/1B to promote human vascular SMC osteogenic differentiation.
Figure 8.
Human bone morphogenetic protein receptor silencing ameliorates eosinophil cationic protein- and eosinophil-derived neurotoxin-induced human smooth muscle cell calcification. Human vascular smooth muscle cell were transfected with bone morphogenetic protein receptor-1A, bone morphogenetic protein receptor-1B, or control siRNA and then exposed to osteogenic media with or without recombinant eosinophil cationic protein or eosinophil-derived neurotoxin for 14 days. (A) Representative images of stained dishes (top) and photomicrographs (bottom. Scale: 200 μm.) along with quantification (B) of Alizarin red staining for mineralized calcium, (C) intracellular alkaline phosphatase activity, and (D) alkaline phosphatase gene expression. Immunoblot analysis of different bone morphogenetic protein receptor-Smad-Runt-related transcription factor-2 signalling proteins in human vascular smooth muscle cell treated with or without eosinophil cationic protein or eosinophil-derived neurotoxin after cells were treated with bone morphogenetic protein receptor-1A (E), bone morphogenetic protein receptor-1B (F), and control siRNAs. The results are expressed as mean ± standard error of mean from three to four independent experiments.
Blood eosinophil counts and ECP levels are associated with human aortic artery calcification
Blood eosinophil counts have been associated with coronary artery calcification (CAC) in patients with coronary heart disease (CHD).42,43 We took the advantage of our on-going DANCAVAS trial,21 a population-based randomized clinically controlled screening trial primarily designed to evaluate the health benefits and cost-effectiveness of using computer tomography (CT) scans to measure CAC and to identify aortic/iliac aneurysms and to measure the ankle brachial blood pressure index as part of a multifocal screening and intervention programme for CVD in men aged 65–74. From January 2015 to August 2018, 5864 men had their blood eosinophil counts measured (see Supplementary data online, Table S1). Additionally, a sub-population of 400 participants had measurement of plasma ECP concentrations. After few exclusions from missing information, 394 ECP measurements were included in the analyses (197 with non-existing CAC and 197 with high CAC). In addition to CAC, calcification scores of other arteries were also available, including aortic valve, abdominal suprarenal aorta, mitral valve, ascending aorta, aortic arch, descending aorta, renal artery, infrarenal aorta, and iliac artery. Because both our blood eosinophil counts and calcification scores were heavily left-skewed even after transformation, we performed non-parametric Spearman’s correlation test. The plasma ECP levels were equally left skewed but were close to normally distributed upon logarithmic transformation. Thus, the correlations between unadjusted ECP levels and calcification scores were evaluated using the Spearman’s correlation test, whilst the adjusted correlations between the logarithmic transformed ECP levels and calcification scores were evaluated using the multivariate linear regression analyses. The Spearman’s Rho between blood eosinophil counts and plasma ECP levels was 0.23 (P < 0.001) and the Pearson’s r between the log-transformed eosinophil counts and ECP levels was 0.21 (P < 0.001). Consistent with earlier studies, we showed that the blood eosinophil counts correlated significantly with CAC (P = 0.011). In addition, blood eosinophil counts also correlated with the calcification scores of aortic valve (P = 0.023), aortic arch (P = 0.001), infrarenal aorta (P = 0.014), and iliac artery (P = 0.005), but not with calcification scores from the abdominal suprarenal aorta, mitral valve, ascending and descending aortas, and renal artery (Table 1; see Supplementary data online, Figure S21). Plasma ECP levels also correlated significantly with CAC (P < 0.001) and with the calcification scores of abdominal suprarenal aorta (P < 0.001), mitral valve (P = 0.002), aortic arch (P < 0.001), descending aorta (P = 0.008), renal artery (P = 0.008), infrarenal aorta (P < 0.001), and iliac artery (P = 0.005), but not with calcification of the aortic valve and ascending aorta (Table 1; see Supplementary data online, Figure S22). After adjusting for the potential blood eosinophil association confounders (P < 0.100) in Supplementary data online, Table S1, including smoking, diabetes, COPD, use of anti-platelet, angiotensin-converting enzyme inhibitor, and glucocorticoids, as well as high blood pressure, and obesity, blood eosinophil counts remained correlated significantly with the calcification scores of coronary artery (P = 0.017), aortic arch (P = 0.010), infrarenal artery (P = 0.011), and iliac artery (P = 0.009) (see Supplementary data online, Table S2). After adjusting for the same potential confounders for the logarithmic transformed ECP levels, a significant linear correlation was observed between ECP levels and calcification scores from CAC (P = 0.001), descending aorta (P < 0.001), suprarenal aorta (P = 0.014), infrarenal aorta (P < 0.001), renal artery (P < 0.001), and iliac artery (P = 0.034), respectively. Calcification scores were also compared between the quartiles of blood eosinophil counts from each artery category using the Kruskal–Wallis test. The results showed that the eosinophil count quartiles correlated significantly with the calcification scores in the coronary artery (P = 0.004), ascending aorta (P = 0.038), aortic arch (P = 0.002), infrarenal artery (P = 0.017), and iliac artery (P = 0.008) (Table 2). This was not tested for plasma ECP levels, as the subpopulation undergoing ECP measurements was already stratified according to level of CAC levels (non-existing CAC vs. high levels of CAC). Together, these clinical data support an association of blood eosinophil counts and plasma ECP levels with the calcification scores of arteries from coronary arteries to the iliac arteries.
Table 1.
Non-parametric univariate rho correlations between blood EOS counts or ECP levels and calcification scores from various aortic segments, arteries, and heart valves
| Coronary artery | Aortic valve | Abdominal superarenal aorta | Mitral valve | Ascending aorta | Aortic arch | Descending aorta | Renal artery | Infra-renal aorta | Iliac artery | |
|---|---|---|---|---|---|---|---|---|---|---|
| Blood EOS counts | ||||||||||
| Patient number | 5541 | 5490 | 5864 | 5513 | 5520 | 5525 | 4441 | 4426 | 4440 | 4423 |
| Spearman’s correlation coefficient | 0.034a | 0.031a | 0.015 | 0.014 | −0.001 | 0.043b | 0.005 | 0.017 | 0.037a | 0.042a |
| Lower 95% C.I. | 0.007 | 0.003 | −0.015 | −0.013 | −0.028 | 0.016 | −0.026 | −0.014 | 0.007 | 0.012 |
| Upper 95% C.I. | 0.061 | 0.058 | 0.045 | 0.042 | 0.026 | 0.070 | 0.035 | 0.047 | 0.067 | 0.072 |
| P value | 0.011 | 0.023 | 0.323 | 0.283 | 0.957 | 0.001 | 0.764 | 0.264 | 0.014 | 0.005 |
| Plasma ECP levels | ||||||||||
| Patient number | 394 | 382 | 343 | 390 | 394 | 394 | 344 | 342 | 343 | 340 |
| Spearman’s correlation coefficient | 0.188b | 0.041 | 0.185b | 0.158b | 0.087 | 0.178b | 0.144b | 0.142b | 0.179b | 0.154b |
| P value | <0.001 | 0.423 | <0.001 | 0.002 | 0.084 | <0.001 | 0.008 | 0.008 | <0.001 | 0.005 |
Correlation is significant at the 0.05 level (two-tailed).
Correlation is significant at the 0.01 level (two-tailed).
Table 2.
Comparison of EOS count quartiles and calcification scores in various aortic segments, arteries, and heart valves
| Arteries | Quartiles | n | Mean | SD | 25th | 50th | 75th | P-value |
|---|---|---|---|---|---|---|---|---|
| Coronary artery calcium score | Lowest | 1189 | 449.5 | 850.7 | 8.6 | 104.0 | 473.3 | 0.004 |
| Second | 1562 | 372.1 | 698.0 | 6.7 | 102.5 | 405.7 | ||
| Third | 1352 | 427.8 | 750.6 | 11.1 | 119.5 | 516.2 | ||
| Upper | 1438 | 505.8 | 870.1 | 13.0 | 141.0 | 573.2 | ||
| Aortic valve calcium score | Lowest | 1177 | 98.2 | 320.1 | 0.0 | 5.6 | 69.6 | 0.160 |
| Second | 1556 | 96.0 | 294.7 | 0.0 | 6.2 | 75.0 | ||
| Third | 1339 | 107.5 | 369.7 | 0.0 | 7.1 | 87.6 | ||
| Upper | 1418 | 126.0 | 361.0 | 0.0 | 6.3 | 91.5 | ||
| Mitral valve calcium scores | Lowest | 1185 | 57. 8 | 401.8 | 0.0 | 0.0 | 1.5 | 0.081 |
| Second | 1552 | 61.0 | 467.2 | 0.0 | 0.0 | 0.0 | ||
| Third | 1347 | 50.8 | 275.6 | 0.0 | 0.0 | 0.8 | ||
| Upper | 1429 | 80.6 | 501.6 | 0.0 | 0.0 | 1.6 | ||
| Ascending aorta calcium scores | Lowest | 1183 | 92.0 | 426.9 | 0.0 | 0.0 | 8.0 | 0.038 |
| Second | 1555 | 59.8 | 223.8 | 0.0 | 0.0 | 4.0 | ||
| Third | 1349 | 85.5 | 333.7 | 0.0 | 0.0 | 5.0 | ||
| Upper | 1433 | 106.4 | 413.3 | 0.0 | 0.0 | 10.5 | ||
| Aortic arch calcium scores | Lowest | 1184 | 741.5 | 1621.1 | 25.0 | 215.5 | 728.8 | 0.002 |
| Second | 1555 | 796.6 | 6029.0 | 20.0 | 196.0 | 724.0 | ||
| Third | 1351 | 742.5 | 1293.6 | 20.0 | 226.0 | 867.0 | ||
| Upper | 1435 | 867.3 | 1742.8 | 30.0 | 277.0 | 940.0 | ||
| Descending aorta calcium scores | Lowest | 859 | 919.2 | 2816.4 | 8.0 | 57.0 | 503.0 | 0.180 |
| Second | 1253 | 718.2 | 2110.9 | 7.0 | 48.0 | 464.0 | ||
| Third | 1126 | 637.8 | 1586.0 | 5.0 | 52.5 | 463.0 | ||
| Upper | 1203 | 740.3 | 1797.7 | 6.0 | 68.0 | 608.0 | ||
| Renal artery calcium scores | Lowest | 855 | 46.7 | 189.5 | 0.0 | 0.0 | 7.0 | 0.504 |
| Second | 1253 | 34.6 | 119.8 | 0.0 | 0.0 | 4.0 | ||
| Third | 1118 | 36.3 | 124.8 | 0.0 | 0.0 | 5.0 | ||
| Upper | 1200 | 46.8 | 158.0 | 0.0 | 0.0 | 9.8 | ||
| Infrarenal artery calcium scores | Lowest | 858 | 3223.5 | 4446.0 | 321.0 | 1610.0 | 4514.8 | 0.017 |
| Second | 1253 | 2870.3 | 3736.4 | 311.0 | 1488.0 | 4048.0 | ||
| Third | 1125 | 2996.3 | 3567.9 | 402.0 | 1601.0 | 4389.5 | ||
| Upper | 1204 | 3480.6 | 5396.0 | 427.0 | 1844.5 | 4980.3 | ||
| Iliac artery calcium scores | Lowest | 854 | 2807.3 | 4946.2 | 196.0 | 1034.0 | 3112.5 | 0.008 |
| Second | 1249 | 2347.1 | 3520.4 | 160.5 | 1032.0 | 3025.5 | ||
| Third | 1121 | 2533.0 | 3560.7 | 207.0 | 1111.0 | 3375.0 | ||
| Upper | 1199 | 2953.3 | 4493.6 | 281.0 | 1253.0 | 3876.0 |
Discussion
Earlier studies indicated a reparative role for eosinophils in the myocardium and abdominal aorta by releasing cationic protein mEar1 and cytokines IL4 and IL13 to protect cardiomyocytes, cardiac fibroblasts, SMC, endothelial cells (ECs), macrophages, and monocytes from pathologic activation after cardiac and aortic injury.16–18,44 Here, we report a pathogenic role for eosinophils in atherogenesis by releasing cationic proteins mEar1, or ECP and EDN in humans, to promote SMC apoptosis and osteogenic differentiation and vascular calcification. Although eosinophils are rich in cytokines or growth factors such as IL4, IL5, IL6, IL13, and TGF-β,45 our results using eosinophils from Il4−/− and Il13−/− mice and recombinant IL4 and IL13 did not support a role for these cytokines in SMC osteogenic differentiation, vascular calcification, and atherogenesis. We identified that eosinophil-derived mEar1 directly participated in vascular calcification and atherogenesis in the present study. Marx et al. demonstrated that eosinophil-derived major basic proteins participate in platelet activation and thrombus formation.19 Thus both studies support a contribution of eosinophils to atherothrombosis. This conclusion differs from the observations of Hofheinz et al., who claimed a negligible role of eosinophils in atherosclerosis after transferring bone-marrow from ΔdblGATA mice to atherosclerosis-prone Ldlr−/− mice,20 suggesting that the use of bone-marrow transfer is not specific to eosinophils.
The presence and quantification of CAC define coronary atherosclerosis,46 and represent an anatomic measure of coronary plaque burden.47 CAC is a risk factor for cardiovascular events48 and positively associates with blood eosinophil counts.42,43 In a cross-sectional study of 1363 patients with CHD, patients with significant stenosis had higher blood eosinophil counts and positively correlated with the CAC level (Log[CAC + 1]).42 In a multi-center longitudinal study of 3094 patients from the coronary artery risk development in young adults, eosinophil counts again associated with CAC in 566 patients after 20 years of follow-up.43 These clinical studies support an atherogenic role for eosinophils and their cationic proteins ECP and EDN in promoting aortic wall SMC calcification.
Eosinophil cationic proteins were first reported 50 years ago,41,49 and human ECP has been used as a biomarker for many human diseases, including CVD, cancers, pulmonary diseases, infectious diseases, and even recent COVID-19.13,50–54 Human ECP is cytotoxic and inhibits T-cell responses to antigens and B lymphocytes, activates mast cells, and basophils and increases epithelial cell expression of insulin-like growth factor-1 and intracellular adhesion molecule-155 that in turn stimulates eosinophil release of ECP and EDN.56 ECP also activates fibroblasts to produce proteoglycans.57 We recently reported that mEar1 protects mouse cardiomyocytes from apoptosis or hypertrophy, inhibits fibroblast fibrotic protein expression and P-Smad-2/3 signalling, blocks leukocyte adhesion on ECs, inactivates the NF-κB signalling pathway in macrophages, EC, and SMC, promotes M2 macrophage and Ly6lo monocyte polarization, and triggers eosinophil IL4 secretion.16–18 However, it remains unknown how mEar1/ECP/EDN target these cells, and whether there is a receptor or receptors involved in mediating these mEar1/ECP/EDN activities. Here we discovered that mEar1/ECP/EDN use the BMPR-1A and BMPR-1B on mouse and human aortic SMC to activate the p-Smad-1/5/8-Runx2 signalling pathway and consequent osteogenic differentiation and vascular calcification. Not only SMC but also cardiomyocytes, cardiac fibroblasts, EC, epithelial cells, and immune cells may all use these BMP receptors, a hypothesis that is essential to explore in different diseases where eosinophils participate.
BMP2 and BMP4 are members of the TGF-β family that induce the formation of bone and cartilage.58 When TGF-β binds to TGFBR1/2 heterodimers and activates the Smad-2/3 signalling pathway for fibrotic protein expression,59 BMP-2/4 bind to BMPR-1A/1B, BMPR-2, or BMPR-1A/1B/2 tetramer and activate the Smad-1/5/8 signalling and downstream Runx2.60,61 BMP-2/4 preferentially use BMPR-1A/1B as their receptors, which form a homodimer or heterodimer.62 BMP2/4 bind to BMPR-2 with much lower affinity than BMPR-1A/1B, but the ligand binding affinity increases when BMPR-1A/1B and BMPR-2 form a tetrameric complex.62–65 Here, we showed that mEar1 acted the same as BMP2/4 with much higher affinity to BMPR-1A/1B than to BMPR-2. We were only able to detect such differences in 293 T cells that were transfected with BMPR-1A, BMPR-1B, or BMPR-2, but not in cultured mouse and human aortic SMC. In mouse aortic SMC, we did not detect immune complex formation of mEar1 with BMPR-2. This does not mean that mEar1 does not use BMPR-2 as its receptor. Indeed, immunofluorescence staining did show mEar1 colocalization with BMPR-2. Failure to detect mEar1 interaction with BMPR-2 might be due to the low expression of BMPR-2 in mouse aortic SMC. Human ECP and EDN yielded the same results. The expression of BMPR-2 was much lower than that of BMPR-1A/1B. Therefore, immunoprecipitation with ECP or EDN followed by immunoblot analyses only detected BMPR-1A and BMPR-1B, but not BMPR-2. These results suggest that eosinophil cationic proteins use BMPR-1A/1B as their receptors. They also use BMPR-2 in cells that express this receptor, a hypothesis that was not thoroughly tested in this study.
Together, this study reports a pathogenic role for eosinophils in atherogenesis by releasing cationic proteins to promote vascular calcification. Most importantly, this study discovers that eosinophil cationic proteins mEar1 in mice and ECP and EDN in humans use the BMP receptors BMPR-1A/1B on SMC to activate the Smad-1/5/8 signalling without the involvement of the TGF-β receptors and Smad-2/3 pathway. Eosinophil cationic proteins also use BMPR-2 as their receptor, although this receptor is negligible on mouse and human vascular SMC. Therefore, our study suggests that human ECP and EDN use BMPRs as their receptors to mediate eosinophil pathogenic or reparative activities, depending on the disease and cell types. Blockade of ECP and EDN binding on these BMPRs and silencing of the Smad-1/5/8 signalling pathway may have therapeutic potential to control eosinophil-associated human diseases, including CVD (Structured Graphical Abstract).
Study limitations
Clinical studies based on blood eosinophil counts yielded different conclusions regarding the possible eosinophil involvement in human CVD. We cannot explain why some studies showed low blood eosinophil counts in patients with MACEs, non-STEMI, or acute STEMI,9,10 but others showed high blood eosinophils in patients with established CVD risk factors, record of percutaneous or surgical coronary revascularization, and antihypertensive and antiplatelet therapy, and those with high CVD mortality rate.11,12 Findings that support a reparative role of eosinophils in post-MI cardiac injury, cardiac hypertrophy, and AAA formation,16–18 but a pathogenic function of eosinophils in atherogenesis, also remain unexplained. SMC calcification may not influence experimental MI, cardiac hypertrophy, or AAA. Atherosclerosis is a chronic disease. Diet-induced experimental atherosclerosis took at least three months while left anterior-descending coronary artery ligation-induced MI,16 pressure overload- and β-adrenergic receptor agonist isoproterenol-induced cardiac hypertrophy,17 and peri-vascular CaCl2 injury or angiotensin-II perfusion-induced AAA18 occurred in less than 4 weeks. Such differences in the time course of experimental disease development may contribute to the apparently contradictory roles of eosinophils under different conditions. Our recent unpublished clinical observations support this hypothesis. In patients with acute MI, low blood eosinophil counts at admission strongly predicted short-term all-cause and cardiac deaths after hospital discharge. In contrast, high blood eosinophil counts at 5∼7 days after acute MI onset associate with long-term all-cause mortality.
This study proposed a role for Runx2 in mEar1/ECP/EDN-mediated p-Smad-1/5/8 activation and osteogenic differentiation. Although not tested, Runx2 may not act as the only downstream mechanism of mEar1/ECP/EDN-mediated SMC calcification. Eosinophils or mEar1/ECP/EDN increased SMC expression of ALP, a known enzyme involved in SMC calcification.66 The expression or functions of transcription factor osterix and transcription repressor msh homeobox-2 are also downstream targets of p-Smad-1/5/8 activation,67 which eosinophils and mEar1/ECP/EDN may regulate.
The DANCAVAS trial is a large on-going cardiovascular screening trial of 45 000 men, although we selected only 5864 men with blood eosinophil counts available. This trial includes detailed calcification scores from different arteries and aortic/iliac aneurysm information, but these patients do not have plasma concentrations of the arterial calcification-associated molecules osteocalcin and osteopontin, nor do they have the eosinophil molecules ECP and EDN.21 Here we only measured plasma ECP levels from limited number of subjects (n = 394), which may underestimate the correlation between plasma ECP levels and aortic or arterial calcification scores. The reduced aortic wall calcification in eosinophil-deficient Apoe−/−ΔdblGATA mice may also be secondary from reduced atherogenesis from these mice. Indeed, eosinophils also play other atherogenic roles such as promoting SMC apoptosis, activating platelets, and enhancing atherosclerotic lesion thrombus formation. Nevertheless, the positive associations of blood eosinophil counts or plasma ECP levels with aortic and arterial calcification scores from this study remain consistent with our observations from experimental atherosclerotic mice. Increased atherosclerotic lesion eosinophil contents and plasma mEar1 levels not only correlate with atherosclerotic lesion development, but the present data support their causal role in atherogenesis.
Supplementary Material
Acknowledgements
The authors thank Ms. Chelsea Swallom for editorial assistance and Ms. Amalie Kamstrup Mogensen from the Renal and Cardiovascular Research Unit, Institute for Molecular Medicine, University of Southern Denmark, Denmark for her technical support. This study was supported by the Hainan Province Science and Technology special fund (ZDYF2020214 to J.G.); the National Natural Science Foundation of China (81770487, 91939107 and 82170440 to J.G., and 82170234 to C.Y.); the National Science Fund for Distinguished Young Scholars of Hainan Medical University (JBGS202104 to J.G.); the Key Laboratory of Emergency and Trauma (Hainan Medical University) of Ministry of Education (KLET-201917 to J.G.), the National Heart, Lung, and Blood Institute (HL151627 and HL157073 to G.-P.S., and HL134892 and HL163099 to P.L.), and the National Institute of Neurological Disorders and Stroke (AG063839 to G.-P.S.).
Contributor Information
Zhaojie Meng, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
Shuya Zhang, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA; Hainan Provincial Key Laboratory for Tropical Cardiovascular Diseases Research & Key Laboratory of Emergency and Trauma of Ministry of Education, Institute of Cardiovascular Research of the First Affiliated Hospital, Hainan Medical University, Haikou 571199, Hainan, China.
Wei Li, College of Chinese Medicinal Materials, Jilin Agricultural University, Changchun 130118, Jilin, China.
Yunzhe Wang, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA; Department of Cardiology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China.
Minjie Wang, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
Xin Liu, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
Cong-Lin Liu, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA; Department of Cardiology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China.
Sha Liao, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
Tianxiao Liu, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
Chongzhe Yang, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA; Department of Geriatrics, National Key Clinical Specialty, Guangzhou First People's Hospital, Guangzhou Medical University, Guangzhou 510000, Guangdong, China.
Jes S Lindholt, Department of Cardiothoracic and Vascular Surgery, Odense University Hospital, Odense, Denmark; Elite Research Centre of Individualized Treatment for Arterial Disease, University Hospital, Odense, Denmark.
Lars M Rasmussen, Elite Research Centre of Individualized Treatment for Arterial Disease, University Hospital, Odense, Denmark; Department of Clinical Biochemistry, Odense University Hospital, Odense, Denmark.
Lasse M Obel, Elite Research Centre of Individualized Treatment for Arterial Disease, University Hospital, Odense, Denmark; Department of Clinical Biochemistry, Odense University Hospital, Odense, Denmark.
Jane Stubbe, Cardiovascular and Renal Research unit, Institute for Molecular Medicine, University of Southern Denmark, Odense, Denmark.
Axel C Diederichsen, Elite Research Centre of Individualized Treatment for Arterial Disease, University Hospital, Odense, Denmark; Department of Cardiology, Odense University Hospital, Odense, Denmark.
Yong Sun, Department of Pathology, The University of Alabama at Birmingham, Birmingham, AL 35294, USA; Birmingham VA Medical Center, Research Department, Birmingham, AL 35294, USA.
Yabing Chen, Department of Pathology, The University of Alabama at Birmingham, Birmingham, AL 35294, USA; Birmingham VA Medical Center, Research Department, Birmingham, AL 35294, USA.
Paul B Yu, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
Peter Libby, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
Guo-Ping Shi, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
Junli Guo, Hainan Provincial Key Laboratory for Tropical Cardiovascular Diseases Research & Key Laboratory of Emergency and Trauma of Ministry of Education, Institute of Cardiovascular Research of the First Affiliated Hospital, Hainan Medical University, Haikou 571199, Hainan, China.
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Hirasawa R, Shimizu R, Takahashi S, Osawa M, Takayanagi S, Kato Y, et al. Essential and instructive roles of GATA factors in eosinophil development. J Exp Med 2002;195:1379–1386. 10.1084/jem.20020170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med 1995;182:1169–1174. 10.1084/jem.182.4.1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol 2017;17:746–760. 10.1038/nri.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller M, Sung KL, Muller WA, Cho JY, Roman M, Castaneda D, et al. Eosinophil tissue recruitment to sites of allergic inflammation in the lung is platelet endothelial cell adhesion molecule independent. J Immunol 2001;167:2292–2297. 10.4049/jimmunol.167.4.2292 [DOI] [PubMed] [Google Scholar]
- 5. Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol 1989;142:4428–4434. 10.4049/jimmunol.142.12.4428 [DOI] [PubMed] [Google Scholar]
- 6. Bystrom J, Amin K, Bishop-Bailey D. Analysing the eosinophil cationic protein–a clue to the function of the eosinophil granulocyte. Respir Res 2011;12:10. 10.1186/1465-9921-12-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Löffler W. Endocarditis parietalis fibroplastica mit bluteosinophilie. Ein eigenartiges krankheitsbild. Schweizerische medizinische Wochenschrift (in German) 1936;66:817–820. [PubMed] [Google Scholar]
- 8. Davies JNP. Endomyocardial fibrosis in Africans. East Afr Med J 1948;25:10–17. [Google Scholar]
- 9. Guner A, Zehir R, Kacik M, Usls A, Osken A, Kalkan AK, et al. Eosinophil percentage as a new prognostic marker in patients with ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Interv Med Appl Sci. 2019;11:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao S, Deng Y, Wu J, Zhang L, Deng F, Zhou J, et al. Eosinophils count in peripheral circulation is associated with coronary artery disease. Atherosclerosis 2019;286:128–134. 10.1016/j.atherosclerosis.2019.05.027 [DOI] [PubMed] [Google Scholar]
- 11. Verdoia M, Schaffer A, Cassetti E, Di Giovine G, Marino P, Suryapranata H, et al. Absolute eosinophils count and the extent of coronary artery disease: a single centre cohort study. J Thromb Thrombolysis 2015;39:459–466. 10.1007/s11239-014-1120-3 [DOI] [PubMed] [Google Scholar]
- 12. Welsh C, Welsh P, Mark PB, Celis-Morales CA, Lewsey J, Gray SR, et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK biobank. Arterioscler Thromb Vasc Biol 2018;38:1415–1423. 10.1161/ATVBAHA.118.310945 [DOI] [PubMed] [Google Scholar]
- 13. Niccoli G, Ferrante G, Cosentino N, Conte M, Belloni F, Marino M, et al. Eosinophil cationic protein: a new biomarker of coronary atherosclerosis. Atherosclerosis 2010;211:606–611. 10.1016/j.atherosclerosis.2010.02.038 [DOI] [PubMed] [Google Scholar]
- 14. Niccoli G, Schiavino D, Belloni F, Ferrante G, La Torre G, Conte M, et al. Pre-intervention eosinophil cationic protein serum levels predict clinical outcomes following implantation of drug-eluting stents. Eur Heart J. 2009; 30:1340–1347. 10.1093/eurheartj/ehp120 [DOI] [PubMed] [Google Scholar]
- 15. Toor IS, Rückerl D, Thomson A, Tang K, Newby DE, Rossi AG, et al. Eosinophils have an essential role in cardiac repair following myocardial infarction. Heart 2017;103:A152. 10.1136/heartjnl-2017-311726.236 [DOI] [Google Scholar]
- 16. Liu J, Yang C, Liu T, Deng Z, Fang W, Zhang X, et al. Eosinophils improve cardiac function after myocardial infarction. Nat Commun. 2020;11:6396. 10.1038/s41467-020-19297-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang C, Li J, Deng Z, Luo S, Liu J, Fang W, et al. Eosinophils protect pressure overload- and β-adrenoreceptor agonist-induced cardiac hypertrophy. Cardiovasc Res. 2023;119:195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu CL, Liu X, Zhang Y, Liu J, Yang C, Luo S, et al. Eosinophils protect mice from angiotensin-II perfusion-induced abdominal aortic aneurysm. Circ Res 2021;128:188–202. 10.1161/CIRCRESAHA.120.318182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marx C, Novotny J, Salbeck D, Zellner KR, Nicolai L, Pekayvaz K, et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood 2019;134:1859–1872. 10.1182/blood.2019000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofheinz K, Seibert F, Ackermann JA, Dietel B, Tauchi M, Oszvar-Kozma M, et al. Formation of atherosclerotic lesions is independent of eosinophils in male mice. Atherosclerosis 2020;311:67–72. 10.1016/j.atherosclerosis.2020.08.030 [DOI] [PubMed] [Google Scholar]
- 21. Diederichsen AC, Rasmussen LM, Sogaard R, Lambrechtsen J, Steffensen FH, Frost L, et al. The danish cardiovascular screening trial (DANCAVAS): study protocol for a randomized controlled trial. Trials 2015;16:554. 10.1186/s13063-015-1082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 23. Agarwal S, Loder SJ, Breuler C, Li J, Cholok D, Brownley C, et al. Strategic targeting of multiple BMP receptors prevents trauma-induced heterotopic ossification. Mol Ther 2017;25:1974–1987. 10.1016/j.ymthe.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest 2003;111:897–906. 10.1172/JCI200314915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest 2007;117:3359–3368. 10.1172/JCI31311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med 2007;13:719–724. 10.1038/nm1601 [DOI] [PubMed] [Google Scholar]
- 27. Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, et al. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res 2012;111:543–552. 10.1161/CIRCRESAHA.112.267237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kranzhofer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kubler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1999;19:1623–1629. 10.1161/01.ATV.19.7.1623 [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Sun C, Gerdes N, Liu C, Liao M, Liu J, et al. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat Med 2015;21:820–826. 10.1038/nm.3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem 2000;275:861–866. 10.1074/jbc.275.2.861 [DOI] [PubMed] [Google Scholar]
- 31. Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, et al. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol 2011;31:1387–1396. 10.1161/ATVBAHA.110.222547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathieu P, Voisine P, Pepin A, Shetty R, Savard N, Dagenais F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis 2005;14:353–357. [PubMed] [Google Scholar]
- 33. Cobb AM, Yusoff S, Hayward R, Ahmad S, Sun M, Verhulst A, et al. Runx2 (runt-related transcription factor 2) links the DNA damage response to osteogenic reprogramming and apoptosis of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2021;41:1339–1357. 10.1161/ATVBAHA.120.315206 [DOI] [PubMed] [Google Scholar]
- 34. Lin ME, Chen TM, Wallingford MC, Nguyen NB, Yamada S, Sawangmake C, et al. Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation. Cardiovasc Res 2016;112:606–616. 10.1093/cvr/cvw205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Komori T. What is the function of osteocalcin? J Oral Biosci 2020;62:223–227. 10.1016/j.job.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 36. Millar SA, Patel H, Anderson SI, England TJ, O'Sullivan SE. Osteocalcin, vascular calcification, and atherosclerosis: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2017;8:183. 10.3389/fendo.2017.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 2008;283:15319–15327. 10.1074/jbc.M800021200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berezin AE, Kremzer AA. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis 2013;229:475–481. 10.1016/j.atherosclerosis.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 39. Rutkovskiy A, Stenslokken KO, Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res 2016;22:95–106. 10.12659/MSMBR.901142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lowery JW, de Caestecker MP. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev 2010;21:287–298. 10.1016/j.cytogfr.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olsson I, Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood 1974;44:235–246. 10.1182/blood.V44.2.235.235 [DOI] [PubMed] [Google Scholar]
- 42. Tanaka M, Fukui M, Tomiyasu K, Akabame S, Nakano K, Yamasaki M, et al. Eosinophil count is positively correlated with coronary artery calcification. Hypertens Res 2012;35:325–328. 10.1038/hr.2011.191 [DOI] [PubMed] [Google Scholar]
- 43. Hou L, Lloyd-Jones DM, Ning H, Huffman MD, Fornage M, He K, et al. White blood cell count in young adulthood and coronary artery calcification in early middle age: coronary artery risk development in young adults (CARDIA) study. Eur J Epidemiol 2013;28:735–742. 10.1007/s10654-013-9842-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu JY, Xiong YY, Tang RJ, Jiang WY, Ning Y, Gong ZT, et al. Interleukin-5-induced eosinophil population improves cardiac function after myocardial infarction. Cardiovasc Res 2022;118:2165–2178. 10.1093/cvr/cvab237 [DOI] [PubMed] [Google Scholar]
- 45. Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev 2011;242:161–177. 10.1111/j.1600-065X.2011.01026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. Circulation 2006;114:1761–1791. [DOI] [PubMed] [Google Scholar]
- 47. Mintz GS, Pichard AD, Popma JJ, Kent KM, Satler LF, Bucher TA, et al. Determinants and correlates of target lesion calcium in coronary artery disease: a clinical, angiographic and intravascular ultrasound study. J Am Coll Cardiol 1997;29:268–274. 10.1016/S0735-1097(96)00479-2 [DOI] [PubMed] [Google Scholar]
- 48. Margolis JR, Chen JT, Kong Y, Peter RH, Behar VS, Kisslo JA. The diagnostic and prognostic significance of coronary artery calcification. A report of 800 cases. Radiology 1980;137:609–616. 10.1148/radiology.137.3.7444045 [DOI] [PubMed] [Google Scholar]
- 49. Gleich GJ, Loegering DA, Maldonado JE. Identification of a major basic protein in Guinea pig eosinophil granules. J Exp Med 1973;137:1459–1471. 10.1084/jem.137.6.1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kruckel A, Moreira A, Frohlich W, Schuler G, Heinzerling L. Eosinophil-cationic protein - a novel liquid prognostic biomarker in melanoma. BMC Cancer 2019;19:207. 10.1186/s12885-019-5384-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Amoani B, Adu B, Frempong MT, Sarkodie-Addo T, Nuvor SV, Wilson MD, et al. Levels of serum eosinophil cationic protein are associated with hookworm infection and intensity in endemic communities in Ghana. PLoS One 2019;14:e0222382. 10.1371/journal.pone.0222382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kulkarni NS, Hollins F, Sutcliffe A, Saunders R, Shah S, Siddiqui S, et al. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol 2010;126:61–69 e3. 10.1016/j.jaci.2010.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scacchetti AT, Tagliavini S, Carone C, Bandiera G, Pesci C, Varani M, et al. The role of eosinophilic cationic protein as a new biomarker in SARS-CoV-2 positive patients with different severity of illness. Biochim Clin 2020;44(SUPPL 2):S48. [Google Scholar]
- 54. Katar M, Beyhan M, Demir O. Could eosinophil cationic protein be a useful biomarker in the diagnosis of COVID-19? Sakarya Med J 2021;11:328–336. [Google Scholar]
- 55. Venge P, Bystrom J, Carlson M, Hakansson L, Karawacjzyk M, Peterson C, et al. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy 1999;29:1172–1186. 10.1046/j.1365-2222.1999.00542.x [DOI] [PubMed] [Google Scholar]
- 56. Chihara J, Yamamoto T, Kurachi D, Kakazu T, Higashimoto I, Nakajima S. Possible release of eosinophil granule proteins in response to signaling from intercellular adhesion molecule-1 and its ligands. Int Arch Allergy Immunol 1995;108:52–54. 10.1159/000237204 [DOI] [PubMed] [Google Scholar]
- 57. Hernnas J, Sarnstrand B, Lindroth P, Peterson CG, Venge P, Malmstrom A. Eosinophil cationic protein alters proteoglycan metabolism in human lung fibroblast cultures. Eur J Cell Biol 1992;59:352–363. [PubMed] [Google Scholar]
- 58. Kounis NG, Soufras GD, Tsigkas G, Hahalis G. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost 2015;21:139–143. 10.1177/1076029614531449 [DOI] [PubMed] [Google Scholar]
- 59. Zhang X, Zhou Y, Yu X, Huang Q, Fang W, Li J, et al. Differential roles of cysteinyl cathepsins in TGF-beta signaling and tissue fibrosis. iScience 2019;19:607–622. 10.1016/j.isci.2019.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leopold JA. Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv 2012;5:605–614. 10.1161/CIRCINTERVENTIONS.112.971028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Willems BA, Furmanik M, Caron MMJ, Chatrou MLL, Kusters DHM, Welting TJM, et al. Ucma/GRP inhibits phosphate-induced vascular smooth muscle cell calcification via SMAD-dependent BMP signalling. Sci Rep 2018;8:4961. 10.1038/s41598-018-23353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev 1998;9:49–61. 10.1016/S1359-6101(97)00036-1 [DOI] [PubMed] [Google Scholar]
- 63. Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A 1995;92:7632–7636. 10.1073/pnas.92.17.7632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gomez-Puerto MC, Iyengar PV, Garcia de Vinuesa A, Ten Dijke P, Sanchez-Duffhues G. Bone morphogenetic protein receptor signal transduction in human disease. J Pathol 2019;247:9–20. 10.1002/path.5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hinck AP. Structural studies of the TGF-betas and their receptors - insights into evolution of the TGF-beta superfamily. FEBS Lett 2012;586:1860–1870. 10.1016/j.febslet.2012.05.028 [DOI] [PubMed] [Google Scholar]
- 66. Narisawa S, Harmey D, Yadav MC, O'Neill WC, Hoylaerts MF, Millan JL. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res 2007;22:1700–1710. 10.1359/jbmr.070714 [DOI] [PubMed] [Google Scholar]
- 67. Niu Z, Su G, Li T, Yu H, Shen Y, Zhang D, et al. Vascular calcification: new insights into BMP type I receptor A. Front Pharmacol 2022;13:887253. 10.3389/fphar.2022.887253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.