Graphical Abstract
Graphical Abstract.
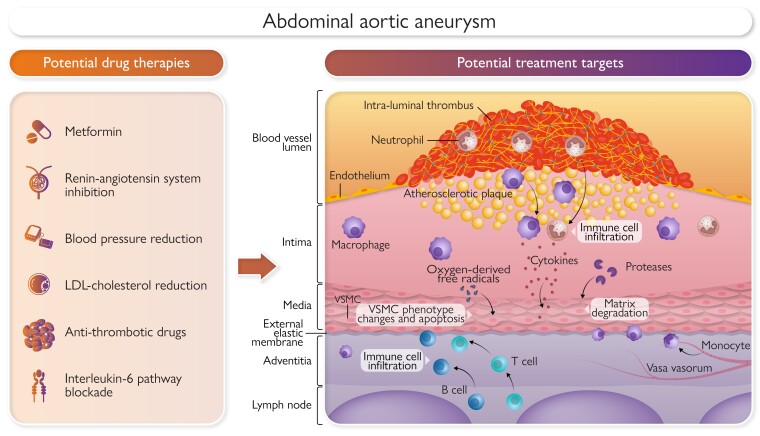
Potential medical therapies to limit abdominal aortic aneurysm growth or rupture. VSMC, vascular smooth muscle cell; LDL, low density lipoprotein.
Keywords: Abdominal aortic aneurysm, AAA rupture, IL-6, Metformin, RAS, Single-nucleotide polymorphism
Abstract
Abdominal aortic aneurysm (AAA) causes ∼170 000 deaths annually worldwide. Most guidelines recommend asymptomatic small AAAs (30 to <50 mm in women; 30 to <55 mm in men) are monitored by imaging and large asymptomatic, symptomatic, and ruptured AAAs are considered for surgical repair. Advances in AAA repair techniques have occurred, but a remaining priority is therapies to limit AAA growth and rupture. This review outlines research on AAA pathogenesis and therapies to limit AAA growth. Genome-wide association studies have identified novel drug targets, e.g. interleukin-6 blockade. Mendelian randomization analyses suggest that treatments to reduce low-density lipoprotein cholesterol such as proprotein convertase subtilisin/kexin type 9 inhibitors and smoking reduction or cessation are also treatment targets. Thirteen placebo-controlled randomized trials have tested whether a range of antibiotics, blood pressure–lowering drugs, a mast cell stabilizer, an anti-platelet drug, or fenofibrate slow AAA growth. None of these trials have shown convincing evidence of drug efficacy and have been limited by small sample sizes, limited drug adherence, poor participant retention, and over-optimistic AAA growth reduction targets. Data from some large observational cohorts suggest that blood pressure reduction, particularly by angiotensin-converting enzyme inhibitors, could limit aneurysm rupture, but this has not been evaluated in randomized trials. Some observational studies suggest metformin may limit AAA growth, and this is currently being tested in randomized trials. In conclusion, no drug therapy has been shown to convincingly limit AAA growth in randomized controlled trials. Further large prospective studies on other targets are needed.
Introduction
Abdominal aortic aneurysm (AAA) is a focal expansion of the abdominal aorta usually diagnosed by maximum aortic diameter of ≥30 mm on imaging.1 Abdominal aortic aneurysm is usually asymptomatic unless it ruptures which is fatal without successful surgical repair. It was reported that in 2017 there were 167 200 deaths and 3 million disability-adjusted life years worldwide attributable to AAA, although due to the low rates of post-mortems, it is likely these estimates are inaccurate.2 Efforts to reduce AAA-related burden have focused on early identification and improved surgical treatment. Randomized trials found that ultrasound screening in men aged ≥65 years to identify cases for elective repair reduced aneurysm-related mortality, but the effect on all-cause mortality is controversial with one analysis showing a small significant reduction and a more recent one not confirming this.3,4 Ultrasound screening of high-risk groups has been introduced in a number of countries, including Sweden, the UK, and the USA.3,5 With declining smoking rates, the age-adjusted prevalence of AAA has fallen in some countries, leading to questions about how screening can be delivered in the most cost-effective and ethical way.6 The widespread use of abdominal imaging has led to many asymptomatic AAA being identified incidentally, and advances in artificial intelligence are facilitating automatic referral to vascular surgeons and surveillance programs.7,8 There have been tremendous advances in minimally invasive devices for endovascular aneurysm repair (EVAR), but durability of this treatment remains a problem, and rupture can still occur.9
A key remaining priority is the development of therapies to limit AAA growth and rupture.10 A survey of 191 participants with an AAA from Oxford UK found that their top three aneurysm research questions were ‘discovering why an AAA develops’ (selected by 83%), ‘discovering new medications that can make AAA shrink back to normal size’ (selected by 71%), and ‘discovering new medications that can stop an AAA from growing further’ (selected by 64%).11 A survey of 277 vascular surgeons working in Africa, Asia, Australasia, Europe, Middle East, North America, and South America found that their top three AAA research priorities were ‘new tests that can predict if an AAA will be fast growing’ (selected by 23%), ‘new medications that can stop an AAA from growing further’ (selected by 23%), and ‘new medications that can make an AAA shrink back to normal size’ (selected by 20%).12 Effective new AAA drugs or intra-arterial therapies could treat early-stage aneurysms, act as adjuvant treatment after EVAR, and be used in people unfit for surgical repair. This article outlines the evidence supporting current management of AAA and research being undertaken to address these top priorities of better understanding of AAA pathogenesis and developing drug therapies (Graphical Abstract).
Abdominal aortic aneurysm pathogenesis
While research on AAA pathogenesis has been ongoing for decades using animal models, in vitro, and human observational studies, the recent findings from genetic studies have led to major advances in our understanding of AAA. This review focuses mainly on recent clinical genetic, observational studies, and trials. Readers are referred to previous reviews on AAA animal models and laboratory studies.13,14 Animal studies testing the effects of drugs on established aneurysms have been included since this experimental design simulates the clinical need for drug therapies to limit growth and rupture of small AAA.15
Traditional risk factors including sex disparity
Past studies suggest higher AAA prevalence in Caucasians compared with black and Asian races.16 Racial inequality in access to AAA screening and treatment may account for the differences in prevalence reported.17,18 Older age, male sex, hypertension, smoking, coronary heart disease, peripheral artery disease, and family history of aneurysm increase the risk of AAA (Table 1).19–23 Diabetes is negatively associated with AAA.28 Men have a 3- to 16-fold increased AAA risk when using a ≥ 30 mm definition.21 Given that women have a smaller body size than men, this criterion underdiagnoses AAA in women.29 Most animal research on sex disparity in AAA pathogenesis has focused on sex hormones or sex chromosomes.30–33 Multiple clinical studies have associated low serum testosterone with increased risk of AAA in older men.34,35 Both low and high estradiol have been associated with AAA.35,36 Women with both a history of smoking (≥20 pack year) and premature menopause have an increased risk of AAA.37 Sex differences in extracellular matrix remodeling, the renin–angiotensin system (RAS), inflammatory pathways, and oxidative stress control have been reported in studies within animal models, cells in vitro, and human populations (see previous review).38 One small study reported that the AAA wall strength was lower in samples from women compared with men; although the difference was not significant, this finding could contribute to the reported higher rate of small AAA rupture in females compared with males.39,40
Table 1.
Risk factors for abdominal aortic aneurysm diagnosis
| Risk factor | Relative risk | 95% CIs |
|---|---|---|
| Male sexa | 5.93 | 4.26, 8.25 |
| Hypertension | 1.66 | 1.49, 1.85 |
| Per 20 mmHg higher systolic blood pressure | 1.14 | 1.06, 1.23 |
| Per 20 mmHg higher diastolic blood pressure | 1.28 | 1.12, 1.46 |
| Current smoking | 4.87 | 3.93, 6.02 |
| Former smoking | 2.10 | 1.76, 2.50 |
| Per 10 pack years | 1.78 | 1.54, 2.06 |
| Family history of aortic aneurysma | 3.80 | 3.66, 3.95 |
| Coronary heart diseasea | 2.29 | 1.75, 3.01 |
| Peripheral artery diseasea | 2.50 | 2.12, 2.95 |
| Diabetes | 0.58 | 0.51, 0.66 |
Odds ratios or relative risk and 95% CIs based on data from previous systematic reviews and large population screening or case-control studies.19–27
Inherited risk
Population screening and case-control studies show that a family history of AAA increases the risk of AAA by between two- and four-fold.24–27 Twin studies suggest that the heritability of AAA is very high at between 70% and 77%.41,42 This strongly implicates genetic and epigenetic mechanisms in AAA pathogenesis, which is of importance since genetic determinants of disease are being successfully used to identify drug targets and determine which drugs are most effectively applied through personalized management.43
Genome-wide association studies (GWAS) have identified multiple common single-nucleotide polymorphisms (SNP) determining the risk of AAA (Table 2).44–49 Case-control studies have implicated multiple non-coding RNAs in AAA.64 A systematic review of 15 studies identified miR-15a, miR-15b, miR-21, and miR-15 to be the most consistently differentially expressed micro-RNA in the aorta and blood of people with AAA and controls.64 One study identified 20 micro-RNAs associated with fast-growing AAA, but this could not be replicated in another population.65,66 A number of long non-coding RNAs, such as H19, have also been implicated in AAA pathogenesis.67
Table 2.
Single-nucleotide polymorphisms associated with the risk of abdominal aortic aneurysm validated in genome-wide association studies
| rs number | Nearest gene | OR (95% CI) | Proposed mechanisms |
|---|---|---|---|
| rs11591147 | PCSK9 | 1.58 (1.38, 1.82) | Degradation of the LDL receptor, thereby controlling circulating levels of LDL-C and inhibit VSMC proliferation and apoptosis45,50 |
| rs118039278 | LPA | 1.28 (1.21, 1.35) | Lipoprotein(a) concentrations45,51 |
| rs73149487 | ABHD16B | 1.26 (1.16, 1.36) | Endocannabinoid homeostasis45 |
| rs4007642 | CDKN2BAS1 | 1.21 (1.17, 1.25) | Long non-coding RNA controlling apoptosis and inflammation43,44,52 |
| rs964184 | ZNF259/APOA5 | 1.18 (1.14, 1.23) | Triglyceride, cholesterol, and glucose concentrations45,53 |
| rs429358 | APOE | 1.17 (1.12, 1.21) | Cholesterol transport45 |
| rs646776 | PSRC1-CELSR2-SORT1 | 1.15 (1.11, 1.20) | Cholesterol homeostasis45,48 |
| rs73015016 | LDLR | 1.15 (1.09, 1.21) | Cholesterol homeostasis44,45,47 |
| rs8124182 | MMP9 | 1.15 (1.10, 1.20) | Matrix remodeling44,45 |
| rs12730935 | IL-6R | 1.14 (1.10, 1.18) | Inflammation45,54–56 |
| rs7994761 | LINC00540 | 1.14 (1.09, 1.19) | Unknown44,45 |
| rs4936098 | ADAMTS8 | 1.13 (1.10, 1.16) | VSMC proliferation and AMPK activity45,57 |
| rs7025486 | DAB2IP | 1.12 (1.08, 1.16) | Cell apoptosis49,58 |
| rs55958997 | CHRNA3 | 1.12 (1.09, 1.16) | Nicotine dependance45 |
| rs4401144 | CTAGE1 | 1.11 (1.08, 1.14) | T cell function45 |
| rs12125521 | SMYD2 | 1.10 (1.06, 1.14) | Histone methylation44,45,59 |
| rs11172113 | LRP1 | 1.10 (1.06, 1.14) | VSMC MMP-9 clearance45,46,60 |
| rs2836411 | ERG | 1.10 (1.06, 1.14) | Inflammation44,45,61 |
| rs7255 | AC012065.7 | 1.10 (1.07, 1.13) | Long non-coding RNA expression45,62 |
| rs3176336 | CDKN1A | 1.10 (1.07, 1.13) | Cell cycle progression45 |
| rs10808546 | RP11-136O12.2/TRIB1 | 1.10 (1.07, 1.13) | Long non-coding RNA45 |
| rs1412445 | LIPA | 1.10 (1.07, 1.13) | Lipid homeostasis45,63 |
| rs10023907 | MEPE | 1.09 (1.06, 1.12) | Calcium-binding phosphoprotein involved in bone mineralisation45 |
| rs35254673 | CRISPLD2 | 1.09 (1.06, 1.13) | Unknown45 |
Single-nucleotide polymorphisms were selected based on genome-wide significance (P < 5 × 108) during discovery in one of nine previously published AAA genome-wide association studies and validation to be significantly associated with AAA in at least one other cohort including the largest and most recent study from the Million Veteran Program.44–46,48,49 The ORs and 95% CIs shown are all taken from the Million Veterans Program study to allow comparison of effect sizes.45 CDKN2BAS1, CDKN2B antisense RNA-1; IL-6R, interleukin-6 receptor; LDLR, low-density lipoprotein receptor; PSRC1-CELSR2-SORT1, proline/serine-rich coiled coil 1—cadherin, EGF LAG seven-pass G-type receptor 2-sortilin; MMP9, matrix metallopeptidase 9; SMYD2, SET And MYND domain containing 2; ERG, erythroblast transformation-specific related gene; LINC00540, long intergenic non–protein coding RNA-540; DAB2IP, disabled homolog 2 interacting protein; AC012065.7, clone-based Vega gene; CDKN1A, cyclin-dependent kinase inhibitor 1A; RP11-136O12.2/TRIB1, RP11-136O12.2 tribbles pseudokinase 1; LIPA, lysosomal acid lipase; ZNF259/APOA5, zinc finger protein/apolipoprotein A5; ADAMTS8, ADAM metallopeptidase with thrombospondin type 1 motif 8; CTAGE1, cutaneous T cell lymphoma-associated antigen 1; APOE, apolipoprotein E; PCSK9, proprotein convertase subtilisin/kexin type 9; LPA, lipoprotein(a); CHRNA3: cholinergic receptor nicotinic alpha 3 subunit; ABHD16B, abhydrolase domain containing 16B; VSMC, vascular smooth muscle cells; AMPK, AMP-activated protein kinase; LDL-C, low-density lipoprotein cholesterol; MEPE, matrix extracellular phosphoglycoprotein; CRISPLD2, cysteine-rich secretory protein LCCL domain containing 2.
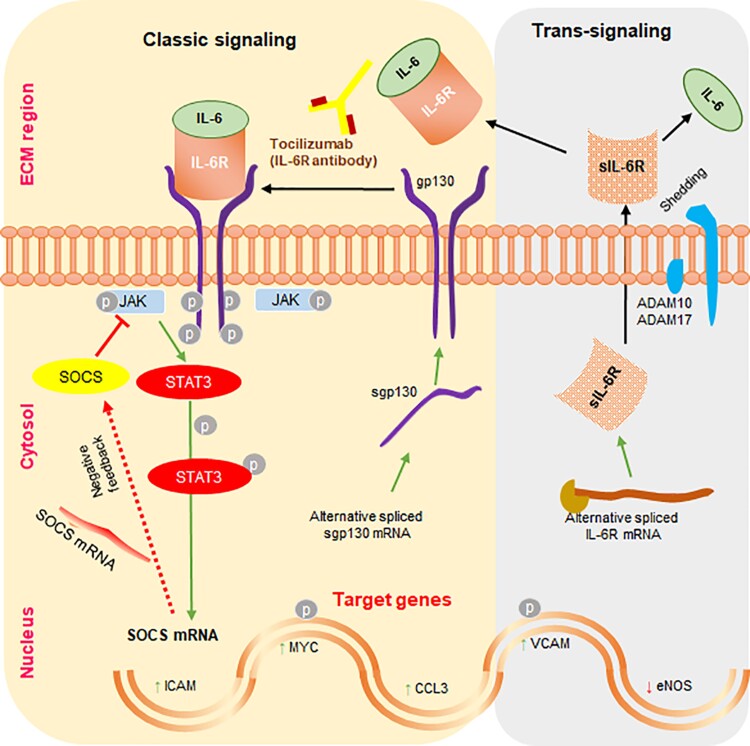
The Million Veteran Program (MVP) analyzed 18 million DNA sequence variants in 12 614 AAA cases and 272 030 controls and identified multiple genetic loci determining AAA risk, such as a SNP within the intron of the interleukin (IL)-6 receptor (IL-6R) gene (Table 2).45 An independent analysis of 5 other studies (4524 cases and 15 710 controls) found that a coding SNP in the IL-6R (rs2228145) gene was associated with AAA and requirement for aneurysm repair or rupture.54 Administration of IL-6 to lymphoblast cells with varying IL-6R genotypes in vitro had significantly different effects on IL-6 target genes of signal transducer and activator of transcription 3 (STAT3) and intercellular adhesion molecule (ICAM)-1, suggesting the functional importance of the SNPs (Figure 1).54 A meta-analysis of four mouse studies (n = 69) found that a variety of different ways of blocking the IL-6 pathway (e.g. a decoy protein blocking IL-6 trans-signaling called sgp130; see Figure 1) significantly limited aortic expansion.55,56,68–70 Furthermore, a systematic review identified 7 studies (n = 300) which all found significantly higher levels of IL-6 in human AAA compared with control aortic tissue samples,68 while a meta-analysis of 15 studies (1001 AAA cases and 1129 controls) found significantly higher circulating IL-6 concentrations in AAA participants compared with controls.68 A transcriptomic analysis suggested activation of IL-6 signaling via the JAK-STAT pathway in human AAA (Figure 1).55 Overall, these findings suggest that the IL-6 pathway plays an important role in AAA pathogenesis and represents a potential drug target. This is particularly relevant as monoclonal antibodies targeting the IL-6 pathway (e.g. tocilizumab) are in clinical use for treating rheumatoid arthritis and appear to be safe to use.71
Figure 1.
Illustration of the interleukin-6 pathway. Shown are both the classical and trans-signalling interleukin-6 pathways. ADAM, a disintegrin and metalloprotease; CCL3, chemokine (C-C motif) ligand 3; eNOS, endothelial nitric oxide synthase; sgp130, soluble glycoprotein 130; ICAM, intracellular adhesion molecule; sIL-6R, soluble interleukin-6 receptor; JAK, Janus kinase; MYC, myelocytomatosis oncogene; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription 3; VCAM, vascular cellular adhesion molecule; ECM, extracellular matrix; P, phosphorylation.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) controls the degradation of the low-density lipoprotein (LDL) receptor, with monoclonal antibodies neutralizing PCSK9 in widespread use to reduce circulating LDL cholesterol (LDL-C). Single-nucleotide polymorphisms in the gene encoding PCSK9 have been associated with AAA (Table 2).45 Gain-of-function mutations in PCSK9 promote angiotensin II–induced aneurysm formation in mice associated with increase in circulating cholesterol.72 PCSK9 also inhibits vascular smooth muscle cell proliferation and promotes apoptosis.50 In keeping with a key role of dyslipidemia in AAA, SNPs in the genes encoding the LDL receptor, apolipoprotein E (Apo E), and lipoprotein(a) have also been associated with AAA (Table 2).44,45,47,51 Mendelian randomization uses the genetic determination of risk factors to examine their causal impact and is thought to avoid much of the bias of traditional observational studies.43 Multiple Mendelian randomization analyses suggest causative roles for raised LDL-C and lipoprotein(a) and reduced high-density lipoprotein cholesterol (HDL-C) in AAA pathogenesis.45,73,74 Mendelian randomization analyses also suggest causal roles for high pulse pressure75 and diastolic blood pressure,45 smoking initiation and heaviness,45 and the CD40 inflammatory pathway,76 but not raised systolic blood pressure45 or C-reactive protein77 in AAA. Counterintuitively, increased IL-1 signaling has been suggested to increase the risk of AAA, possibly by stimulating an increase in LDL-C which may in part explain the lack of benefit of IL-1 blocking antibody in a small trial in AAA participants (ClinicalTrials.gov Identifier: NCT02007252).68,78
Other SNPs identified in GWAS studies have supported roles for matrix metalloproteinase (MMP)-9,44,46,60 lipid transport and uptake,44,45,47,48,63 epigenetic mechanisms such as non-coding RNAs and histone methylation,44,45,52,59,62 inflammation,44,52,61 and cell senescence, proliferation, and apoptosis,44,45,49,52,57,58 in AAA pathogenesis.45,53 The genetic risk alleles have been used to develop polygenic risk scores (PRS) in order to identify high-risk people needing screening for AAA.45 The MVP combined 29 genetic risk alleles to develop an AAA PRS for which a one standard deviation score increase independently predicted AAA risk [odds ratio (OR) 1.27, 95% confidence interval (CI) 1.22, 1.32, P < 0.001].45 The PRS identified people with a risk of AAA between 1.7% and 9.4% depending on ancestry with a lower effect size in African compared with European populations, possibly due to over-representation of European participants (59% of the sample).45
Current abdominal aortic aneurysm management and need for medical management to limit growth and rupture
Current management
Randomized controlled trials suggest that early elective open surgical repair (OSR) or EVAR of asymptomatic 40–55 mm AAA does not reduce mortality.79 One trial suggested that EVAR did not significantly reduce mortality in people with large AAA unfit for OSR although this evidence maybe outdated due to advances in EVAR.80 One trial found that EVAR and OSR had similar 30-day mortality for ruptured AAA.81 Quality of life was better in the EVAR group, and 3-year mortality was lower after EVAR in participants with proven rupture.81 The risk of small aneurysm rupture is higher, and outcome of surgery is worse in women compared with men.40,82 Based on this evidence, current European Society for Vascular Surgery guidelines recommend monitoring of small AAA (<55 mm in men; < 50 mm in women) through imaging surveillance, elective surgical repair of large aneurysms in fit patients, and EVAR of ruptured AAA, if morphologically suitable.1 The intervention threshold for women is not evidence-based and remains a research priority.1
Small abdominal aortic aneurysm and large abdominal aortic aneurysm that do not undergo surgical repair
The risk of rupture of small AAA in men is <1%/year.79 The 3-year rupture rate of unrepaired 50–54 mm AAA in women was 3.4% in a recent study.83 Most small AAA grow in size over time, with the UK small aneurysm trial finding that 70% of 40–55 mm AAA grew to 55 mm within 5 years.79 Large aneurysms have a higher risk of rupture (estimated as 2.2%, 6.0%, and 18.4% over 3 years for 55–60 mm, 61–70 mm, and >70 mm AAA, respectively).83 Rupture rate of large AAA is significantly greater in women than men, with a recent study reporting women with unrepaired 61–70 mm AAA had a 3-year incidence of rupture of 12.8% compared with 4.5% in men.83 There is no evidence that the growth rate of small AAA has changed over time.40 Thus, medical treatments that can slow AAA growth and reduce the risk of small aneurysm rupture are needed. The remainder of this review summarizes previous research performed to test and develop medical therapy to limit AAA growth and rupture.
Previous clinical trials and observational studies testing drugs to limit abdominal aortic aneurysm growth
Table 3 summarizes the results of the 13 completed placebo-controlled trials testing drug therapies to limit AAA growth, and the findings of both observational and randomized trials are described in this section.84–96
Table 3.
Previous randomized placebo-controlled trials testing drugs to limit abdominal aortic aneurysm growth
| Trial | Drug tested (daily dose) | Sample size | AAA growth (mm) | AAA events | ||
|---|---|---|---|---|---|---|
| Active | Placebo | Active | Placebo | |||
| Blood pressure lowering | ||||||
| TEDY84 | Telmisartan (40 mg) | 210 | 1.7 (1.8) | 1.8 (1.8) | 11 (10.3%) | 8 (7.8%) |
| AARDVARK85 | Perindopril (10 mg) | 227e. | 1.8 (1.7) | 1.7 (1.8) | 10 (13.3%) | 9 (11.4%) |
| AARDVARK85 | Amlodipine (5 mg) | 227e | 1.8 (1.7) | 1.7 (1.8) | 7 (9.6%) | 9 (11.4%) |
| PAT86 | Propranolol (240 mg) | 552 | 2.2 (2.8) | 2.6 (3.1) | 57 (20.6%) | 73 (26.5%) |
| Propranolol87 | Propranolol (80 mg) | 54 | 3.1 (2.5) | 2.8 (2.4) | 7 (23.3%) | 5 (20.8%) |
| Antibiotics | ||||||
| Azithromycin88 | Azithromycin (variablea) | 247 | 2.3 (1.8) | 2.2 (1.9) | 16 (13.1%) | 13 (10.4%) |
| Roxithromycin 189 | Roxithromycin (300 mg for 28 days) | 92 | 1.6 (1.5)b | 2.8 (2.5) | 5 (11.6%) | 7 (14.3%) |
| Roxithromycin 290 | Roxithromycin (300 mg for 28 days annually) | 84 | 1.6 (1.5) | 2.5 (2.5) | 12 (28.6%) | 14 (40.5%) |
| Doxycycline 191 | Doxycycline (150 mg for 3 months) | 32 | 1.5 (2.4) | 3.1 (4.7) | 2 (11.8%) | 3 (20.0%) |
| PHAST92 | Doxycycline (100 mg) | 286 | 2.8 (2.1)c | 2.1 (2.1) | 21 (14.6%) | 24 (16.9%) |
| N-TA3CT93 | Doxycycline (200 mg) | 261 | 3.6 (2.1)d | 3.6 (2.8)d | 13 (9.8%) | 9 (7.0%) |
| Anti-platelet | ||||||
| TicAAA94 | Ticagrelor (180 mg) | 136 | 2.3 (1.7, 2.9) | 2.2 (1.6, 2.7) | 4 (5.8%) | 0 |
| Mast cell inhibitor | ||||||
| AORTA95 | Pemirolast (20 mg) | 326f | 2.6 (2.1, 3.1) | 2.0 (1.6, 2.5) | 5 (6.3%) | 2 (2.4%) |
| Pemirolast (50 mg) | 326f | 2.3 (1.9, 2.8) | 2.0 (1.6, 2.5) | 2 (2.6%) | 2 (2.4%) | |
| Pemirolast (80 mg) | 326f | 2.7 (2.3, 3.2) | 2.0 (1.6, 2.5) | 6 (7.1%) | 2 (2.4%) | |
| Fibrate | ||||||
| FAME-296 | Fenofibrate (145 mg) | 140 | 1.0 (3.7) | 1.4 (3.7) | 0 | 0 |
Shown are mean standard deviation or 95% CIs of AAA growth from ultrasound or numbers (%) of AAA events (repair or rupture). PAT, propranolol aneurysm trial; AAA, abdominal aortic aneurysm; N-TA3CT, Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial; PHAST, Pharmaceutical Aneurysm Stabilization Trial; TEDY, TElmisartan in abDominal aortic aneurYsm trial; AARDVARK, Aortic Aneurysmal Regression of Dilation: Value of ACE-Inhibition on RisK; AORTA, Anti-inflammatory ORal Treatment of AAA; FAME-2, Fenofibrate in the Management of Abdominal Aortic Aneurysm 2; TicAAA, The Efficacy of Ticagrelor on Abdominal Aortic Aneurysm Expansion.
600 mg/d for 3 days then 600 mg/week.
AAA growth significantly slower in the roxithromycin group, P = 0.02.
AAA growth significantly faster in the doxycycline group, P = 0.007.
Growth rate over 2 years rather than annual.
Sample size for all three groups including placebo, amlodipine, and perindopril.
Sample size for all four groups including pemirolast (20 mg), pemirolast (50 mg), pemirolast (80 mg), and placebo.
Antibiotics
Six trials tested the efficacy of tetracycline (doxycycline, n = 3) or macrolide (azithromycin, n = 1; roxithromycin, n = 2) antibiotics in limiting AAA growth.88–93 One reason for testing antibiotics was due to their ability to inhibit MMP activity. Doxycycline, for example, was found to reduce plasma concentrations of MMP-9 in patients with AAA.97 There was also interest in antibiotics because of a postulated role of the bacterium Chlamydophila pneumoniae in AAA in some98,99 but not other studies.100 Furthermore, a 2-week course of doxycycline was reported to reduced AAA wall neutrophils and cytotoxic T cells and down-regulated inflammatory pathways implicated in AAA pathology, including IL-6 expression within a 60-participants randomized controlled trial.101 This preliminary evidence appeared to be supported by a 92-participants trial showing that roxithromycin significantly reduced AAA growth compared with placebo (Table 3).89 These findings were not confirmed in subsequent larger trials (Table 3). The largest antibiotic trial (n = 286) found significantly faster aneurysm growth in the doxycycline compared with placebo group.92 The other four trials found no significant effect of the antibiotics tested (Table 3). A meta-analysis of these trials found no significant effect of antibiotics on AAA growth [standardized mean difference (SMD) −0.11, 95% CI −0.38, 0.16, P = 0.42] and AAA repair or rupture [risk ratio (RR) 0.93, 95% CI 0.69, 1.25; P = 0.61].102
Renin–angiotensin system inhibition
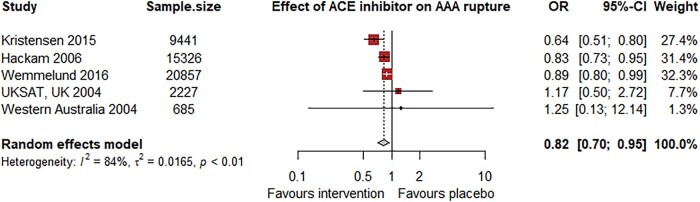
The RAS has been strongly implicated in AAA pathogenesis through studies in animal models and human aortic samples.13,103 A meta-analysis of three mice studies (n = 45 intervention; 36 controls) found that administration of RAS inhibitors (aliskiren, telmisartan, or ramipril) significantly reduced the diameter of established AAA.15 This evidence was supported by the findings of a Canadian population case-control study of patients with ruptured (n = 3379) and intact (n = 11 947) AAA.104 Angiotensin-converting enzyme (ACE) inhibitor prescription was associated with a reduced risk of AAA rupture (OR 0.83, 95% CI 0.73, 0.95).104 Only 1% of the population were prescribed angiotensin receptor blockers (ARB), and this was not associated with reduced risk of AAA rupture (OR 1.24, 95% CI 0.71, 2.18). A Danish registry study of 9441 patients also found a reduced risk of aneurysm-related mortality for those prescribed ACE inhibitors (HR 0.64, 95% CI 0.51, 0.80) or ARBs (HR 0.65, 95% CI 0.48, 0.88).105 Angiotensin-converting enzyme inhibitor (HR 0.86, 95% CI 0.74, 0.99) but not ARB (HR 1.02, 95% CI 0.84, 1.23) prescription was associated with reduced risk of aneurysm repair.105 Another study of 3586 patients with rupture AAA and 17 271 patients with intact AAA reported an association of ACE inhibitor prescription with reduced aneurysm rupture in an unadjusted analysis (OR 0.89, 95% CI 0.84, 0.94), but in a propensity matched case-control sub-analysis, neither ACE inhibitor (OR 1.02, 95% CI 0.88, 1.19) nor ARB (OR 1.02, 95% CI 0.83, 1.26) prescription was associated with aneurysm rupture.106 Analysis of two cohorts with small AAA from Western Australia and the UK found no association between ACE inhibitor prescription and aneurysm rupture (HR 1.25, 95% CI 0.13, 12.14 for n = 685 in Western Australia; 1.17, 95% CI 0.50, 2.72 for n = 2227 in the UK).40 Our meta-analysis of these studies (n = 48 536) suggested ACE inhibitor prescription was associated with reduced risk of AAA rupture (OR 0.82, 95% CI 0.70, 0.95, P = 0.007), but the potential for confounding of these unadjusted observational data needs to be acknowledged (Figure 2).
Figure 2.
Meta-analysis of observational studies assessing the association between angiotensin-converting enzyme inhibitors and abdominal aortic aneurysm rupture. Included were the unadjusted ORs and 95% CIs from previously reported observational studies.40,104–106
In contrast, among cohorts with small AAA, there is no evidence that ACE inhibitors or ARBs limit AAA growth. A meta-analysis of seven cohorts (n = 4826) found no association between ACE inhibitor prescription and AAA growth.40 These observational findings were supported by the result of the Aortic Aneurysmal Regression of Dilation: Value of ACE-Inhibition on RisK (AARDVARK) placebo-controlled trial that randomized 227 patients with 30–54 mm AAA to perindopril (10 mg), amlodipine (5 mg), or placebo.85 Neither perindopril nor amlodipine significantly reduced AAA growth (Table 3).85 Similarly, the TElmisartan in abDominal aortic aneurYsm (TEDY) trial (n = 210) found that 40 mg of the ARB telmisartan did not significantly reduce AAA growth (Table 3).84 Combined, these two trials only included a total of 182 participants allocated to perindopril or telmisartan, and both trials excluded all patients currently eligible or ineligible for ACE inhibitors or ARBs due to co-morbidities such as hypertension, renal disease, or diabetes. The small sample size means they were under-powered to identify a moderate or small treatment effect. Two placebo-controlled trials found that propranolol did not slow AAA growth, but 42% of participants allocated to propranolol in the largest trial had to discontinue drug due to side effects (Table 3).86,87 A meta-analysis of all four blood pressure–lowering placebo-controlled trials found no effect of blood pressure lowering on AAA growth.102
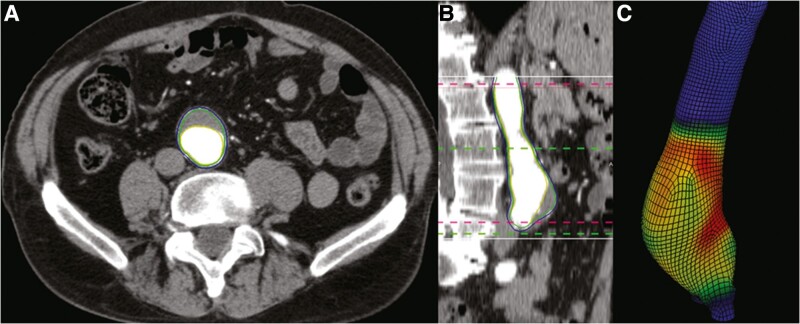
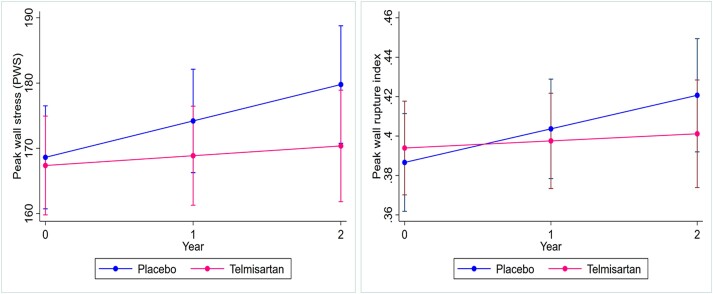
The main aim of interventional treatment is to limit the risk of aneurysm rupture. The risk factors (and likely mechanisms) for AAA growth and rupture are not identical, and thus, it cannot be assumed that the effect of a drug on aneurysm growth and rupture is the same (Tables 1 and 4).40 Of note, higher blood pressure had been associated with increased risk of small aneurysm rupture in some studies but not AAA growth (Table 4).40,102,107 Since the risk of small AAA rupturing is very low and most large aneurysms undergo repair, it is very difficult to design trials adequately powered to test the effect of a treatment on aneurysm rupture.10,83 In all previous placebo-controlled trials, increase in aneurysm size (mainly diameter) was the primary outcome.10 While the trials did record AAA-related events, these were dominated by elective aneurysm repairs (Table 3). Aneurysm diameter is not always an accurate reflection of the risk of rupture since some small AAA do rupture and many large aneurysm remain intact for many years.83 Methods have been developed for reproducible estimation of the biomechanical risk of AAA rupture using aortic peak wall stress (PWS) and peak wall rupture index (PWRI) (Figure 3).108 Previous meta-analyses have reported higher PWS and PWRI in ruptured than intact AAA.110,111 Although the association between PWS/PWRI and aneurysm rupture is attenuated by adjustment for aneurysm diameter, it is possible these alternative surrogate measures may better reflect the risk of AAA rupture and be valuable outcomes in trials of drug therapies. We used data from the TEDY trial to assess the impact of telmisartan and blood pressure reduction on the change in PWS and PWRI over 24 months of follow-up.109 In this sub-analysis of 124 participants from TEDY (n = 65 telmisartan; 59 placebo), telmisartan significantly reduced the increase in PWS and PWRI (Figure 4).109 As expected, allocation to telmisartan was associated with a reduction in blood pressure.109 After adjusted for systolic blood pressure at 1 year, the annual changes in PWS and PWRI were no longer significantly different between groups although in the same direction.109 The exploratory and selective nature of this analysis is acknowledged given the primary analysis for TEDY was negative. It should also be noted that the role of high PWS in aneurysm rupture remains controversial since low wall shear stress has also been associated with AAA growth and rupture.112,113 Collectively, however, these data provide evidence that blocking the RAS does not inhibit small aneurysm growth but may limit the risk of aneurysm rupture.
Table 4.
Risk factors for growth and rupture of small abdominal aortic aneurysm based on a previous individual patient data meta-analysis
| Risk factor | n | Growth | Rupture | |||
|---|---|---|---|---|---|---|
| n | Mean difference | P | HR | P | ||
| Age (per year) | 13 966 | 13 966 | −0.001 (0.006) | 0.82 | 1.04 (1.01, 1.07) | 0.004 |
| Female sex | 8472 | 8472 | 0.142 (0.150) | 0.34 | 3.76 (2.58, 5.47) | <0.001 |
| Body mass index (per kg/m2) | 3439 | 3439 | −0.008 (0.009) | 0.35 | 0.93 (0.88, 0.99) | 0.029 |
| Current smoking | 7486 | 7486 | 0.354 (0.065) | <0.001 | 2.02 (1.33, 3.06) | 0.001 |
| Diabetes | 5697 | 5697 | −0.505 (0.097) | <0.001 | 1.27 (0.45, 3.54) | 0.65 |
| Mean blood pressure (per 10 mmHg) | 5957 | 5957 | 0.013 (0.021) | 0.53 | 1.32 (1.11, 1.56) | 0.001 |
| Pulse pressure (per 10 mmHg) | 5957 | 5957 | −0.027 (0.014) | 0.06 | 1.11 (1.02, 1.22) | 0.019 |
| Cardiovascular disease | 6302 | 6302 | −0.105 | 0.23 | 1.32 (0.77, 2.27) | 0.32 |
The data presented are from an individual patient meta-analysis including 15 475 patients with small AAA attending surveillance programs in the UK, Australia, Norway, the USA, Sweden, and Denmark as previously reported.40 Data available varied for different risk factors; thus, the sample size for each risk factor has been shown (n). Shown are estimated mean difference (standard error of the mean) in AAA growth (mm/year) for each risk factor or hazard ratio (HR; 95% CI) for risk of aneurysm rupture as previously reported.40 Abdominal aortic aneurysm growth estimates were adjusted for the other risk factors shown in the table and initial AAA diameter, while HRs were adjusted for current AAA diameter only.
Figure 3.
Measuring peak wall stress in an abdominal aortic aneurysm. Finite element analysis using the A4 Clinics software estimated peak wall stress using computed tomograms of an abdominal aortic aneurysm. (A) Axial and (B) sagittal views of an AAA. (C) Three-dimensional segmentation of the aneurysm generated using finite element analysis. The red pixels indicate areas of high aortic wall stress. Reproduced with permission.109
Figure 4.
Telmisartan limits increase in peak wall stress (left) and peak wall rupture index (right) within data from the telmisartan in the management of the abdominal aortic aneurysm trial. Reproduced with permission.109
Anti-thrombotic drugs
Most aneurysm have large volumes of intra-luminal thrombus that contains large number of inflammatory cells and high concentrations of proteolytic enzymes and cytokines.13 The volume of intra-luminal thrombus is correlated with the rate of aneurysm growth after adjusting for aortic diameter.114 A meta-analysis found that the volume of intra-luminal thrombus was significantly greater in rupture than intact AAA.115 This association was no longer present in a sub-analysis restricted to participants matched for aortic diameter although this was under-powered (n = 181).115 A meta-analysis of 6 animal studies (n = 138 intervention; 133 control mice) found that administration of anti-platelet drugs (e.g. aspirin or clopidogrel) or anti-coagulants (e.g. fondaparinux or rivaroxaban) significantly reduced the diameter of established AAA, although the effect on aneurysm rupture was non-significant.15 A small human observational study associated aspirin prescription with slower aneurysm growth in 31 patients with 40–49 mm AAA.116 The prescription of therapeutic anti-coagulant drugs was also associated with reduced requirement for aneurysm repair or rupture-related mortality (HR 0.61, 95% CI 0.42, 0.90) in a large 1161 patient study of participants with unrepaired AAA.116,117 Another observational study (n = 3926) found that aspirin prescription was associated with reduced AAA rupture in unadjusted (OR 0.72, 95% CI 0.66, 0.79) but not adjusted (OR 0.97, 95% CI 0.86, 1.08) analyses.118 An analysis of more than 245 million hospital admissions recorded in the US National Inpatient Sample database found an association between anti-platelet prescription and reduced risk of AAA admission (OR 0.70, 95% CI 0.68, 0.72) and rupture (OR 0.67, 95% CI 0.54, 0.82).119 In the only placebo-controlled trial testing an anti-thrombotic drug, the anti-platelet drug ticagrelor did not significantly reduce AAA growth.94 Larger randomized controlled trials are needed to thoroughly test the efficacy of anti-thrombotic drugs in limiting AAA growth and rupture.
Anti-inflammatory drugs
Studies of human and animal model AAA samples show marked infiltrations by a wide range of immune cells.13 A meta-analysis of 10 mice studies (n = 154 intervention; 141 controls) found that administration of a broad range of drugs proposed to have anti-inflammatory effects (e.g. receptor interacting protein 1 inhibitor, celecoxib, c-Jun N-terminal kinase inhibitor, and bradykinin 2 receptor antagonist) significantly reduced the diameter of established AAA and lowered the risk of aneurysm rupture.15 Genomic studies of human aneurysm samples show up-regulation of multiple inflammatory pathways.120,121 A Mendelian randomization analysis found genetic predicted CD40 levels were inversely associated with AAA (OR 0.91, 95% CI 0.84, 0.98).76 Thus, blocking inflammation has been proposed as a treatment to limit AAA growth. There are however multiple challenges to targeting inflammation as a treatment for AAA. Firstly, given the widespread activation of inflammatory cascades, it is likely down-regulating one component will lead to compensatory changes. Secondly, there are potential safety concerns with immune suppression of promoting cancer and serious infections. Thirdly, whether the inflammatory response is a cause or effect of the aneurysm is not resolved. While there is strong evidence from animal studies that both the adaptive and innate immunity promotes aneurysm development and rupture, there is less evidence from clinical studies.13,14 A small study of 20 patients with AAA that had received organ transplantation and were prescribed intensive immune suppression reported they had rapid AAA growth of ∼10 mm/year.122 A retrospective observational study (n = 176) found that prescription of oral steroids was associated with AAA growth greater than median.123 These studies suggest that immune suppression may promote rather than inhibit AAA growth. In contrast, an observational study of 621 patients with AAA measuring between 30 and 50 mm found no significant difference in AAA growth in participants that were and were not prescribed immunosuppressant drugs.124 Concurrent malignancy and AAA is common, and a recent study of 217 patients with small AAA receiving radiotherapy and/or chemotherapy reported that radiotherapy was associated with significantly slower AAA growth.125 Topoisomerase inhibitors and anti-androgens were also associated with non-significant reductions in AAA growth.125 The only placebo-controlled trial to test an anti-inflammatory drug reported no effect of the mast cell degranulation inhibitor pemirolast on growth of small AAA (Table 3).95 Overall, there is no consistent evidence that blocking inflammation inhibits AAA growth, but more targeted approaches may be effective although this remains to be tested.
Lipid-modifying medication
Some observational studies suggest that statin prescription is associated with reduced AAA growth and rupture with one meta-analysis finding a mean reduction in AAA growth of 0.82 mm/year and an OR for rupture risk of 0.63 among statin users.126 This finding appears to be driven by smaller studies since the larger prospective studies have found no significant association between statin prescription and AAA growth or rupture.40,127 The only placebo-controlled trial which has tested the effect of a lipid-modifying medication on AAA growth was the Fenofibrate in the Management of Abdominal Aortic Aneurysm 2 (FAME-2) trial.96 FAME-2 tested the triglyceride-lowering drug fenofibrate. The rationale for this trial was based on animal evidence that suggested fenofibrate down-regulated the bone proteins osteopontin and osteoprotegerin to limit AAA development.128,129 However, in the human clinical trial, fenofibrate did not significantly limit AAA growth or reduce serum osteopontin or osteoprotegerin (Table 3).96 Furthermore, a related placebo-controlled trial of 40 patients undergoing OSR (FAME) found that a short course of fenofibrate did not down-regulate AAA wall concentrations of osteopontin or reduce aortic inflammation.130
Genomic data suggest a key role for LDL-C in AAA pathogenesis and it is possible that intensive LDL-C reduction might limit AAA growth. The counterintuitive association of peripheral artery disease with reduced AAA growth reported in previous observational studies and an analysis of the TEDY trial may have been driven by better control of modifiable risk factors such as LDL-C in these patients.131,132 Trials testing the value of intensive control of LDL-C on AAA growth are needed and could incorporate the impact of this intervention on the incidence of cardiovascular events given myocardial infarction, stroke, and cardiovascular death are very common in these patients. The 5-year incidence of major vascular events was 38% in a recent study of patients with small AAA.133
Challenges and lessons from previous drug trials
All past trials have tested the effects of medications in limiting AAA growth, which is usually slow and subject to measurement error and incomplete data and competing risks, as patients are lost to events such as AAA repair or death.84,85,92,93,95,96 Also patients with AAA are usually elderly, often with multiple co-morbidities, meaning they frequently cannot tolerate drugs with side effects which can lower drug adherence.84,85,92,93,95,96 Only two previous trials included sample sizes over 300 patients, and one of these trials tested three different doses of drugs, and so group sizes were still under 90 participants, and the other has very poor drug adherence.86,95 All these trials likely overestimated the treatment effect of the intervention they tested. Much larger sample sizes of at least 300 patients per group with longer follow-up of ∼5 years are needed to identify a realistic treatment effect on AAA growth. Also, the effects of drugs on very small AAA could be different from those of AAA approaching the threshold for surgical repair but previous trials have not been designed to test this. The primary outcome in one current trial is the clinically relevant end-point of AAA repair or rupture rather than AAA growth, but this design requires a sample size of ∼2000 participants to reliably test a realistic treatment effect.134
Ongoing trials testing metformin
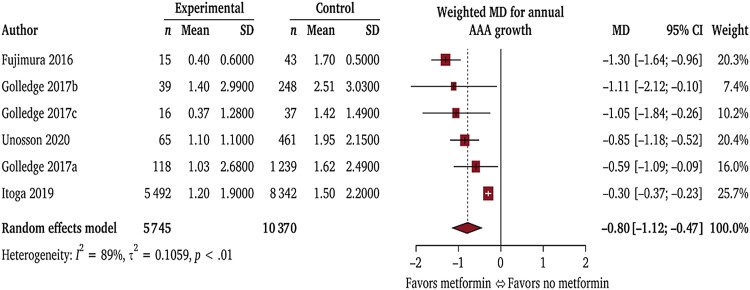
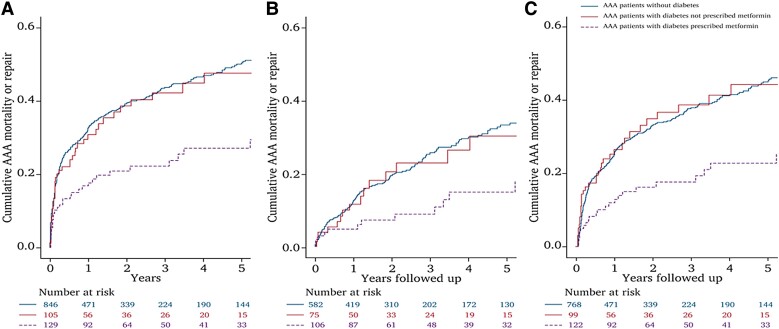
Diabetes is a negative risk factor for AAA diagnosis (Table 1) and growth (Table 4).135 Multiple theories have been proposed to explain these findings, such as hyperglycemia causing aortic wall cross-linking and blocking proteolytic degradation and inhibiting vascular inflammation.136,137 Metformin has also been reported to inhibit AAA development in animal models.138,139 A meta-analysis of 8 observational studies involving 35 240 patients who were and 118 313 who were not prescribed metformin to treat diabetes found metformin was associated with significantly slower aneurysm growth (Figure 5).140 Metformin prescription was associated with a 40% reduced risk of AAA repair or rupture (RR 0.60, 95% CI 0.40, 0.90). An example of the findings from one observational study is shown in Figure 6.141 It should be noted, however, due to the observational nature of these findings, the moderate or high risk of bias of five of the eight studies, and the heterogeneity of studies, the GRADE summary suggested that the certainty of evidence that metformin limited AAA growth was very low.140 The results of four ongoing randomized trials testing the ability of metformin to limit AAA growth and events are therefore needed to clarify whether this drug is an effective treatment for AAA (Table 5).134,142–144
Figure 5.
Metformin prescription is associated with slower annual abdominal aortic aneurysm growth: results of a meta-analysis of observational studies. Mean difference (MD) in annual abdominal aortic aneurysm growth shown in millimetres. Reproduced with permission.140
Figure 6.
Combined incidence of abdominal aortic aneurysm (AAA) repair or mortality from AAA rupture (AAA events) in patients with diabetes prescribed metformin (purple dotted line), those with diabetes not prescribed metformin (red line), and those without diabetes (blue line). Graphs show cumulative proportion of events over 5 years for the whole cohort (A), patients with initial AAA diameter ≤ 50 mm (B), and patients with ≥6 month follow-up (C). In the whole cohort, the incidence of AAA events was less in patients with diabetes prescribed metformin than in patients who had diabetes but were not prescribed metformin (P = 0.003) or patients who did not have diabetes (P < 0.001). The incidence of AAA events was similar in people who had diabetes but were not prescribed metformin and patients who did not have diabetes (P = 0.839). Numbers at risk during different stages of follow-up are shown below the graphs. Reproduced with permission.141
Table 5.
Design of randomized trials testing metformin as a treatment for abdominal aortic aneurysm
| Trial | Registration number | Planned sample size | Design | Daily dose | Control | Primary outcome | Anticipated completion data |
|---|---|---|---|---|---|---|---|
| MAT134 | ACTRN12618001707257 | 1954 to achieve 616 primary outcome events | PCRT; active 6 week run-in; event driven | 1500 mg | Placebo | AAA repair or rupture | 2028 |
| MAGGI142 | NCT04224051 | 500 | Open label | 2000 mg | No metformin | AAA growth measured by orthogonal diameter from CT over 5 years | 2026 |
| LIMIT143 | NCT04500756 | 480 | PCRT | 2000 mg | Placebo | AAA growth measured by orthogonal diameter from CT over 24 months | 2028 |
| Met-AAA144 | NCT03507413 | 170 | PCRT | 2000 mg | Placebo | AAA growth measured by orthogonal diameter from CT over 12 months | 2023 |
MAT, Metformin Aneurysm Trial; MAGGI, Metformin for Abdominal Aortic Aneurysm Growth Inhibition; LIMIT, Limiting Abdominal Aortic Aneurysm With Metformin Trial; PCRT, placebo-controlled randomized trial; CT, computed tomography; Met-AAA, metformin therapy to inhibit progression in non-diabetic patients with abdominal aortic aneurysm.
Other targets for therapies to limit abdominal aortic aneurysm growth and future directions
Other risk factor targets for abdominal aortic aneurysm growth
In an analysis of 18 cohorts including 15 475 people with small aneurysms, the prevalence of current smokers varied between 18% and 60%.40 Current smoking was associated with faster AAA growth and increased risk of aneurysm rupture (Table 4).40 Measurement of AAA diameter in routine practice has considerable measurement error which can be improved by use of core laboratories, standardized measure protocols, and single observer readings as used in some of the randomized trials.84,93,94 In unadjusted analyses, the Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA3CT) reported that current smoking was associated with faster AAA growth, while diabetes, larger body mass index, ARB, and statin prescription were associated with slower AAA growth.145
Developing and testing novel targets to limit abdominal aortic aneurysm growth
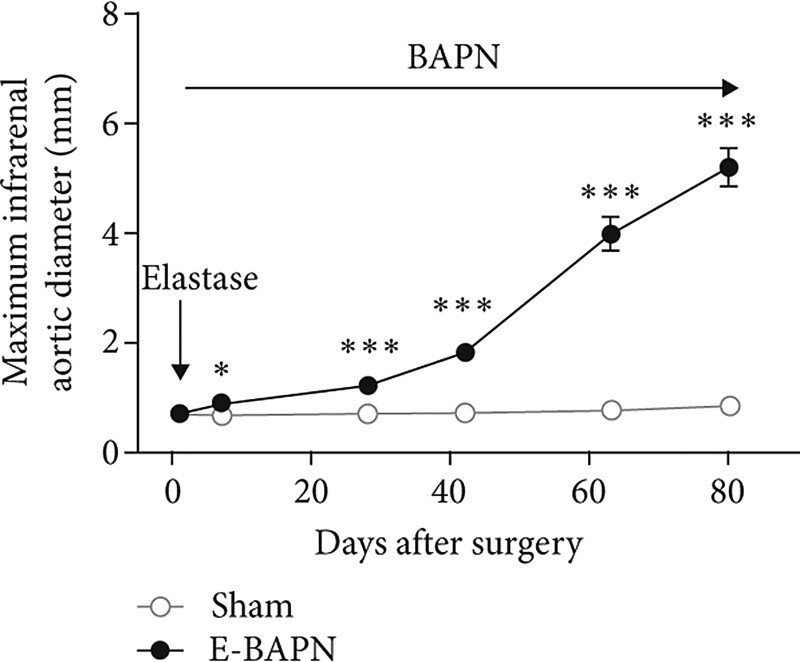
Taking advantage of the enormous and growing tissue and blood biobanks, novel drug targets are expected to be identified from ongoing technical advances in genomics, phenomics, and proteomics.45 The availability of appropriate laboratory models to test potential AAA drugs remains challenging with drugs such as fenofibrate initially shown to be promising in animal studies later found to be ineffective in human clinical trials.96,128–130 Detailed ultrasound investigation suggests that the elastase, angiotensin II, and CaCl2 models have limited severity of and variable abdominal aortic expansion, meaning there is limited ability to first establish a small aneurysm then examine the effect of a drug on AAA growth over an extended time.13–15,146 Furthermore, the most commonly used angiotensin II model does not cause aortic dilatation in all mice and induces aortic dissection rather than true aneurysm formation.147 In contrast, a new model that combines two methods of inducing AAA using application of elastase to the adventitia of the infra-renal aorta and oral administration of a lysyl oxidase inhibitor holds more promise for testing drugs.148 In this model, expansion of aortic diameter occurs over 3 months (Figure 7). The model also illustrates typical human AAA characteristics of true aneurysm formation, intra-luminal thrombus, marked aortic wall inflammation, and a rupture rate of ∼30%.148The use of this and other novel animal models along with rigorous study design similar to clinical trials may identify drugs more likely to translate to patients.
Figure 7.
Illustration of maximum infra-renal abdominal aortic diameter in the adventitial elastase and β-aminopropionitrile mouse model. Previously reported by Phie and colleagues.148
Personalizing drug therapy
When AAA drug therapies are established, ideally, these need to be targeted at patients most likely to benefit. Personalizing AAA drugs requires accurate ways to determine people most likely to benefit due to good drug responsiveness and excellent tolerance. The drugs also need to be targeted at people most at risk of aneurysm-related complications. The use of genomic data may in the future provide a means to personalize drugs by identifying people able to respond to the medications due to their ability to metabolize it and because the drivers of their AAA are sensitive to it. Risk models able to predict AAA growth using established traditional risk factors (Table 4) along with new PRS may also provide a way to better identify those patients most at risk of fast aneurysm growth and rupture. Future drug trials need to consider whether a more targeted approach using genomic and other biomarkers might be a better way of selecting participants for inclusion.
Under-represented groups
Given the worse outcome of AAA in women than men, there is a need for more focus on AAA in women.83 There is also a need to address racial disparity in access to AAA treatment and to better understand the unique features of AAA in different ethnicities.17,18
Conclusion
Current management of AAA is by imaging surveillance of small aneurysm and surgical repair of large, symptomatic, or ruptured aneurysms. Patients and clinicians require effective drug therapies to limit AAA growth and the risks of both AAA repair and rupture. The diabetes drug metformin holds some promise and is being evaluated in ongoing trials. Other areas that could be targeted regarding development of drugs are dyslipidemia, IL-6 signalling, and stabilization of the extracellular matrix.
Acknowledgements
The authors apologize that due to word limits they have been unable to include data from multiple excellent research studies. The authors thank all their colleagues who have contributed to their research and thus the development of the concepts presented in this review.
Contributor Information
Jonathan Golledge, Queensland Research Centre for Peripheral Vascular Disease, College of Medicine and Dentistry, James Cook University, 1 James Cook Drive, Douglas, Townsville, QLD, Australia; Australian Institute of Tropical Health and Medicine, James Cook University, 1 James Cook Drive, Douglas, Townsville, QLD, Australia; Department of Vascular and Endovascular Surgery, Townsville University Hospital, 100 Angus Smith Drive, Douglas, QLD, Australia.
Shivshankar Thanigaimani, Queensland Research Centre for Peripheral Vascular Disease, College of Medicine and Dentistry, James Cook University, 1 James Cook Drive, Douglas, Townsville, QLD, Australia; Australian Institute of Tropical Health and Medicine, James Cook University, 1 James Cook Drive, Douglas, Townsville, QLD, Australia.
Janet T Powell, Department of Surgery & Cancer, Imperial College London, Fulham Palace Road, London, UK.
Phil S Tsao, Department of Cardiovascular Medicine, Stanford University, 450 Serra Mall, Stanford, CA, USA; VA Palo Alto Health Care System, 3801 Miranda Avenue, Palo Alto, CA, USA; Stanford Cardiovascular Institute, Stanford University, 450 Serra Mall, Stanford, CA, USA.
Declarations
Disclosure of Interest
All authors declare no conflict of interest for this contribution.
Data Availability
No new data were generated or analyzed for this manuscript.
Funding
J.G. holds a Senior Clinical Research Fellowship from the Queensland Government and received research grants from the Heart foundation, the National, Health and Medical Research Council, the Medical Research Futures Fund, and Townsville and Hospital Health Services.
References
- 1. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. . Editor’s choice—European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg 2019;57:8–93. 10.1016/j.ejvs.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 2. Wei L, Bu X, Wang X, Liu J, Ma A, Wang T. Global burden of aortic aneurysm and attributable risk factors from 1990 to 2017. Glob Heart 2021;16:35. 10.5334/gh.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US preventive services task force. JAMA 2019;322:2219–2238. 10.1001/jama.2019.17021 [DOI] [PubMed] [Google Scholar]
- 4. Lederle FA. The last (randomized) word on screening for abdominal aortic aneurysms. JAMA Intern Med 2016;176:1767–1768. 10.1001/jamainternmed.2016.6663 [DOI] [PubMed] [Google Scholar]
- 5. Kapila V, Jetty P, Wooster D, Vucemilo V, Dubois L; Canadian Society for Vascular Surgery . Screening for abdominal aortic aneurysms in Canada: 2020 review and position statement of the Canadian Society for Vascular Surgery. Can J Surg 2021;64:E461–E466. 10.1503/cjs.009120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benson RA, Meecham L, Fisher O, Loftus IM. Ultrasound screening for abdominal aortic aneurysm: current practice, challenges and controversies. Br J Radiol 2018;91:20170306. 10.1259/bjr.20170306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khashram M, Tiong LC, Jones GT, Roake JA. The impact of CT colonography on abdominal aortic aneurysm referrals in a tertiary hospital. J Med Imaging Radiat Oncol 2017;61:180–184. 10.1111/1754-9485.12535 [DOI] [PubMed] [Google Scholar]
- 8. Adam C, Fabre D, Mougin J, Zins M, Azarine A, Ardon R, et al. . Pre-surgical and post-surgical aortic aneurysm maximum diameter measurement: full automation by artificial intelligence. Eur J Vasc Endovasc Surg 2021;62:869–877. 10.1016/j.ejvs.2021.07.013 [DOI] [PubMed] [Google Scholar]
- 9. Hensley SE, Upchurch GR Jr. Repair of abdominal aortic aneurysms: JACC focus seminar, part 1. J Am Coll Cardiol 2022;80:821–831. 10.1016/j.jacc.2022.04.066 [DOI] [PubMed] [Google Scholar]
- 10. Golledge J, Moxon JV, Singh TP, Bown MJ, Mani K, Wanhainen A. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med 2020;288:6–22. 10.1111/joim.12958 [DOI] [PubMed] [Google Scholar]
- 11. Lee R, Jones A, Cassimjee I, Handa A. Patients’ opinions regarding research and management of abdominal aortic aneurysms. Int Angiol 2017;36:526–530. 10.23736/S0392-9590.17.03889-5 [DOI] [PubMed] [Google Scholar]
- 12. Lee R, Jones A, Cassimjee I, Handa A; Oxford Abdominal Aortic Aneurysm Study . International opinion on priorities in research for small abdominal aortic aneurysms and the potential path for research to impact clinical management. Int J Cardiol 2017;245:253–255. 10.1016/j.ijcard.2017.06.058 [DOI] [PubMed] [Google Scholar]
- 13. Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol 2019;16:225–242. 10.1038/s41569-018-0114-9 [DOI] [PubMed] [Google Scholar]
- 14. Busch A, Bleichert S, Ibrahim N, Wortmann M, Eckstein HH, Brostjan C, et al. . Translating mouse models of abdominal aortic aneurysm to the translational needs of vascular surgery. JVS Vasc Sci 2021;2:219–234. 10.1016/j.jvssci.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phie J, Thanigaimani S, Golledge J. Systematic review and meta-analysis of interventions to slow progression of abdominal aortic aneurysm in mouse models. Arterioscler Thromb Vasc Biol 2021;41:1504–1517. 10.1161/ATVBAHA.121.315942 [DOI] [PubMed] [Google Scholar]
- 16. Jacomelli J, Summers L, Stevenson A, Lees T, Earnshaw JJ. Editor’s choice—inequalities in abdominal aortic aneurysm screening in England: effects of social deprivation and ethnicity. Eur J Vasc Endovasc Surg 2017;53:837–843. 10.1016/j.ejvs.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Lehman EB, Aziz F. African Americans are less likely to have elective endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2019;70:462–470. 10.1016/j.jvs.2018.10.107 [DOI] [PubMed] [Google Scholar]
- 18. Ribieras AJ, Kang N, Shao T, Kenel-Pierre S, Rey J, Velazquez OC, et al. . Racial disparities in presentation and outcomes for endovascular abdominal aortic aneurysm repair. J Vasc Surg 2023;77:69–77. 10.1016/j.jvs.2022.06.094 [DOI] [PubMed] [Google Scholar]
- 19. Kobeissi E, Hibino M, Pan H, Aune D. Blood pressure, hypertension and the risk of abdominal aortic aneurysms: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 2019;34:547–555. 10.1007/s10654-019-00510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. Sci Rep 2018;8:14786. 10.1038/s41598-018-32100-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. Risk factors for abdominal aortic aneurysm in population-based studies: a systematic review and meta-analysis. Int J Environ Res Public Health 2018;15:2805. 10.3390/ijerph15122805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cornuz J, Sidoti Pinto C, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health 2004;14:343–349. 10.1093/eurpub/14.4.343 [DOI] [PubMed] [Google Scholar]
- 23. Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE Jr, et al. . Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart 2014;9:159–170. 10.1016/j.gheart.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 24. Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. . Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative study group. Ann Intern Med 1997;126:441–449. 10.7326/0003-4819-126-6-199703150-00004 [DOI] [PubMed] [Google Scholar]
- 25. Jamrozik K, Norman PE, Spencer CA, Parsons RW, Tuohy R, Lawrence-Brown MM, et al. . Screening for abdominal aortic aneurysm: lessons from a population-based study. Med J Aust 2000;173:345–350. 10.5694/j.1326-5377.2000.tb125684.x [DOI] [PubMed] [Google Scholar]
- 26. Summers KL, Kerut EK, Sheahan CM, Sheahan MG III. Evaluating the prevalence of abdominal aortic aneurysms in the United States through a national screening database. J Vasc Surg 2021;73:61–68. 10.1016/j.jvs.2020.03.046 [DOI] [PubMed] [Google Scholar]
- 27. Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. . Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010;52:539–548. 10.1016/j.jvs.2010.05.090 [DOI] [PubMed] [Google Scholar]
- 28. Aune D, Schlesinger S, Norat T, Riboli E. Diabetes mellitus and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. J Diabetes Complications 2018;32:1169–1174. 10.1016/j.jdiacomp.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 29. Jones GT, Sandiford P, Hill GB, Williams MJA, Khashram M, Tilyard MW, et al. . Correcting for body surface area identifies the true prevalence of abdominal aortic aneurysm in screened women. Eur J Vasc Endovasc Surg 2019;57:221–228. 10.1016/j.ejvs.2018.08.048 [DOI] [PubMed] [Google Scholar]
- 30. Mukherjee K, Pingili AK, Singh P, Dhodi AN, Dutta SR, Gonzalez FJ, et al. . Testosterone metabolite 6beta-hydroxytestosterone contributes to angiotensin II-induced abdominal aortic aneurysms in apoe(-/-) male mice. J Am Heart Assoc 2021;10:e018536. 10.1161/JAHA.120.018536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alsiraj Y, Thatcher SE, Charnigo R, Chen K, Blalock E, Daugherty A, et al. . Female mice with an XY sex chromosome complement develop severe angiotensin II-induced abdominal aortic aneurysms. Circulation 2017;135:379–391. 10.1161/CIRCULATIONAHA.116.023789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis JP, Salmon M, Pope NH, Lu G, Su G, Meher A, et al. . Pharmacologic blockade and genetic deletion of androgen receptor attenuates aortic aneurysm formation. J Vasc Surg 2016;63:1602–1612e1602. 10.1016/j.jvs.2015.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu XF, Zhang J, Paskauskas S, Xin SJ, Duan ZQ. The role of estrogen in the formation of experimental abdominal aortic aneurysm. Am J Surg 2009;197:49–54. 10.1016/j.amjsurg.2007.11.022 [DOI] [PubMed] [Google Scholar]
- 34. Yeap BB, Hyde Z, Norman PE, Chubb SA, Golledge J. Associations of total testosterone, sex hormone-binding globulin, calculated free testosterone, and luteinizing hormone with prevalence of abdominal aortic aneurysm in older men. J Clin Endocrinol Metab 2010;95:1123–1130. 10.1210/jc.2009-1696 [DOI] [PubMed] [Google Scholar]
- 35. Villard C, Roy J, Bogdanovic M, Eriksson P, Hultgren R. Sex hormones in men with abdominal aortic aneurysm. J Vasc Surg 2021;74:2023–2029. 10.1016/j.jvs.2021.06.020 [DOI] [PubMed] [Google Scholar]
- 36. Ohlsson C, Langenskiold M, Smidfelt K, Poutanen M, Ryberg H, Norlen AK, et al. . Low progesterone and low estradiol levels associate with abdominal aortic aneurysms in men. J Clin Endocrinol Metab 2022;107:e1413–e1425. 10.1210/clinem/dgab867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chou EL, Pettinger M, Haring B, Allison MA, Mell MW, Hlatky MA, et al. . Association of premature menopause with risk of abdominal aortic aneurysm in the Women’s Health Initiative. Ann Surg 2022;276:e1008–e1016. 10.1097/SLA.0000000000004581 [DOI] [PubMed] [Google Scholar]
- 38. Boese AC, Chang L, Yin KJ, Chen YE, Lee JP, Hamblin MH. Sex differences in abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol 2018;314:H1137–H1152. 10.1152/ajpheart.00519.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vande Geest JP, Dillavou ED, Di Martino ES, Oberdier M, Bohra A, Makaroun MS, et al. . Gender-related differences in the tensile strength of abdominal aortic aneurysm. Ann N Y Acad Sci 2006;1085:400–402. 10.1196/annals.1383.048 [DOI] [PubMed] [Google Scholar]
- 40. Sweeting MJ, Thompson SG, Brown LC. Powell JT; RESCAN collaborators . Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 2012;99:655–665. 10.1002/bjs.8707 [DOI] [PubMed] [Google Scholar]
- 41. Joergensen TM, Christensen K, Lindholt JS, Larsen LA, Green A, Houlind K. Editor’s choice—high heritability of liability to abdominal aortic aneurysms: a population based twin study. Eur J Vasc Endovasc Surg 2016;52:41–46. 10.1016/j.ejvs.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 42. Wahlgren CM, Larsson E, Magnusson PK, Hultgren R, Swedenborg J. Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population. J Vasc Surg 2010;51:3–7; discussion 7. 10.1016/j.jvs.2009.08.036 [DOI] [PubMed] [Google Scholar]
- 43. Holmes MV, Richardson TG, Ference BA, Davies NM, Davey Smith G. Integrating genomics with biomarkers and therapeutic targets to invigorate cardiovascular drug development. Nat Rev Cardiol 2021;18:435–453. 10.1038/s41569-020-00493-1 [DOI] [PubMed] [Google Scholar]
- 44. Jones GT, Tromp G, Kuivaniemi H, Gretarsdottir S, Baas AF, Giusti B, et al. . Meta-analysis of genome-wide association studies for abdominal aortic aneurysm identifies four new disease-specific risk loci. Circ Res 2017;120:341–353. 10.1161/CIRCRESAHA.116.308765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klarin D, Verma SS, Judy R, Dikilitas O, Wolford BN, Paranjpe I, et al. . Genetic architecture of abdominal aortic aneurysm in the Million Veteran Program. Circulation 2020;142:1633–1646. 10.1161/CIRCULATIONAHA.120.047544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bown MJ, Jones GT, Harrison SC, Wright BJ, Bumpstead S, Baas AF, et al. . Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet 2011;89:619–627. 10.1016/j.ajhg.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bradley DT, Hughes AE, Badger SA, Jones GT, Harrison SC, Wright BJ, et al. . A variant in LDLR is associated with abdominal aortic aneurysm. Circ Cardiovasc Genet 2013;6:498–504. 10.1161/CIRCGENETICS.113.000165 [DOI] [PubMed] [Google Scholar]
- 48. Jones GT, Bown MJ, Gretarsdottir S, Romaine SP, Helgadottir A, Yu G, et al. . A sequence variant associated with sortilin-1 (SORT1) on 1p13.3 is independently associated with abdominal aortic aneurysm. Hum Mol Genet 2013;22:2941–2947. 10.1093/hmg/ddt141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gretarsdottir S, Baas AF, Thorleifsson G, Holm H, den Heijer M, de Vries JP, et al. . Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet 2010;42:692–697. 10.1038/ng.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guo Y, Tang Z, Yan B, Yin H, Tai S, Peng J, et al. . PCSK9 (proprotein convertase subtilisin/kexin type 9) triggers vascular smooth muscle cell senescence and apoptosis: implication of its direct role in degenerative vascular disease. Arterioscler Thromb Vasc Biol 2022;42:67–86. 10.1161/ATVBAHA.121.316902 [DOI] [PubMed] [Google Scholar]
- 51. Lampsas S, Oikonomou E, Pantelidis P, Theofilis P, Grammatopoulos K, Marathonitis A, et al. . Lipoprotein (a) levels and abdominal aortic aneurysm. A systematic review and meta-analysis. Curr Pharm Des 2022;28:3492–3499. 10.2174/1381612829666221124110920 [DOI] [PubMed] [Google Scholar]
- 52. Li J, Chen J, Zhang F, Li J, An S, Cheng M, et al. . LncRNA CDKN2B-AS1 hinders the proliferation and facilitates apoptosis of ox-LDL-induced vascular smooth muscle cells via the ceRNA network of CDKN2B-AS1/miR-126-5p/PTPN7. Int J Cardiol 2021;340:79–87. 10.1016/j.ijcard.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 53. Kvaloy K, Holmen J, Hveem K, Holmen TL. Genetic effects on longitudinal changes from healthy to adverse weight and metabolic status—the HUNT study. PLoS One 2015;10:e0139632. 10.1371/journal.pone.0139632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harrison SC, Smith AJ, Jones GT, Swerdlow DI, Rampuri R, Bown MJ, et al. . Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J 2013;34:3707–3716. 10.1093/eurheartj/ehs354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kokje VBC, Gabel G, Koole D, Northoff BH, Holdt LM, Hamming JF, et al. . IL-6: a Janus-like factor in abdominal aortic aneurysm disease. Atherosclerosis 2016;251:139–146. 10.1016/j.atherosclerosis.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 56. Paige E, Clement M, Lareyre F, Sweeting M, Raffort J, Grenier C, et al. . Interleukin-6 receptor signaling and abdominal aortic aneurysm growth rates. Circ Genom Precis Med 2019;12:e002413. 10.1161/CIRCGEN.118.002413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Omura J, Satoh K, Kikuchi N, Satoh T, Kurosawa R, Nogi M, et al. . ADAMTS8 Promotes the development of pulmonary arterial hypertension and right ventricular failure: a possible novel therapeutic target. Circ Res 2019;125:884–906. 10.1161/CIRCRESAHA.119.315398 [DOI] [PubMed] [Google Scholar]
- 58. Zhang M, Peng Y, Yang Z, Zhang H, Xu C, Liu L, et al. . DAB2IP down-regulates HSP90AA1 to inhibit the malignant biological behaviors of colorectal cancer. BMC Cancer 2022;22:561. 10.1186/s12885-022-09596-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yi X, Jiang XJ, Fang ZM. Histone methyltransferase SMYD2: ubiquitous regulator of disease. Clin Epigenetics 2019;11:112. 10.1186/s13148-019-0711-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chan CY, Chan YC, Cheuk BL, Cheng SW. Clearance of matrix metalloproteinase-9 is dependent on low-density lipoprotein receptor-related protein-1 expression downregulated by microRNA-205 in human abdominal aortic aneurysm. J Vasc Surg 2017;65:509–520. 10.1016/j.jvs.2015.10.065 [DOI] [PubMed] [Google Scholar]
- 61. Sperone A, Dryden NH, Birdsey GM, Madden L, Johns M, Evans PC, et al. . The transcription factor Erg inhibits vascular inflammation by repressing NF-kappaB activation and proinflammatory gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 2011;31:142–150. 10.1161/ATVBAHA.110.216473 [DOI] [PubMed] [Google Scholar]
- 62. Subhash S, Andersson PO, Kosalai ST, Kanduri C, Kanduri M. Global DNA methylation profiling reveals new insights into epigenetically deregulated protein coding and long noncoding RNAs in CLL. Clin Epigenetics 2016;8:106. 10.1186/s13148-016-0274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zharkova M, Nekrasova T, Ivashkin V, Maevskaya M, Strokova T. Fatty liver and systemic atherosclerosis in a young, lean patient: rule out lysosomal acid lipase deficiency. Case Rep Gastroenterol 2019;13:498–507. 10.1159/000504646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Iyer V, Rowbotham S, Biros E, Bingley J, Golledge J. A systematic review investigating the association of microRNAs with human abdominal aortic aneurysms. Atherosclerosis 2017;261:78–89. 10.1016/j.atherosclerosis.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 65. Wanhainen A, Mani K, Vorkapic E, De Basso R, Bjorck M, Lanne T, et al. . Screening of circulating microRNA biomarkers for prevalence of abdominal aortic aneurysm and aneurysm growth. Atherosclerosis 2017;256:82–88. 10.1016/j.atherosclerosis.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 66. Thanigaimani S, Iyer V, Bingley J, Browne D, Phie J, Doolan D, et al. . Association of serum microRNAs with abdominal aortic aneurysm diagnosis and growth. Eur J Vasc Endovasc Surg 2023;65:573–581. 10.1016/j.ejvs.2022.12.028 [DOI] [PubMed] [Google Scholar]
- 67. Li DY, Busch A, Jin H, Chernogubova E, Pelisek J, Karlsson J, et al. . H19 induces abdominal aortic aneurysm development and progression. Circulation 2018;138:1551–1568. 10.1161/CIRCULATIONAHA.117.032184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thanigaimani S, Ibrahim M, Golledge J. Potential of disease-modifying anti-rheumatic drugs to limit abdominal aortic aneurysm growth. Biomedicines 2022;10:2409. 10.3390/biomedicines10102409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nishihara M, Aoki H, Ohno S, Furusho A, Hirakata S, Nishida N, et al. . The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLoS One 2017;12:e0185923. 10.1371/journal.pone.0185923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pope NH, Salmon M, Johnston WF, Lu G, Lau CL, Upchurch GR Jr, et al. . Interleukin-6 receptor inhibition prevents descending thoracic aortic aneurysm formation. Ann Thorac Surg 2015;100:1620–1626. 10.1016/j.athoracsur.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Castagne B, Viprey M, Martin J, Schott AM, Cucherat M, Soubrier M. Cardiovascular safety of tocilizumab: a systematic review and network meta-analysis. PLoS One 2019;14:e0220178. 10.1371/journal.pone.0220178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lu H, Howatt DA, Balakrishnan A, Graham MJ, Mullick AE, Daugherty A. Hypercholesterolemia induced by a PCSK9 gain-of-function mutation augments angiotensin II-induced abdominal aortic aneurysms in C57BL/6 mice-brief report. Arterioscler Thromb Vasc Biol 2016;36:1753–1757. 10.1161/ATVBAHA.116.307613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ibrahim M, Thanigaimani S, Singh TP, Morris D, Golledge J. Systematic review and meta-analysis of Mendelian randomisation analyses of abdominal aortic aneurysms. Int J Cardiol Heart Vasc 2021;35:100836. 10.1016/j.ijcha.2021.100836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Satterfield BA, Dikilitas O, Safarova MS, Clarke SL, Tcheandjieu C, Zhu X, et al. . Associations of genetically predicted lp(a) (lipoprotein [a]) levels with cardiovascular traits in individuals of European and African ancestry. Circ Genom Precis Med 2021;14:e003354. 10.1161/CIRCGEN.120.003354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burgess S, Chirinos JA, Damrauer SM, Gill D. Genetically predicted pulse pressure and risk of abdominal aortic aneurysm: a Mendelian randomization analysis. Circ Genom Precis Med 2022;15:e003575. 10.1161/CIRCGEN.121.003575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cui X, Xuan T, Chen S, Guo X. Causal associations between CD40/CD40L and aortic diseases: a Mendelian randomization study. Front Genet 2022;13:998525. 10.3389/fgene.2022.998525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qin XY, Juan J, Xiang X, Wei YQ, Zuo SW, Huang T, et al. . Plasma C-reactive protein and abdominal aortic aneurysm: a Mendelian randomization analysis. Chin Med J (Engl) 2018;131:2630–2633. 10.4103/0366-6999.244124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Interleukin 1 Genetics Consortium . Cardiometabolic effects of genetic upregulation of the interleukin 1 receptor antagonist: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol 2015;3:243–253. 10.1016/S2213-8587(15)00034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ulug P, Powell JT, Martinez MA, Ballard DJ, Filardo G. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev 2020;7:CD001835. 10.1002/14651858.CD001835.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess 2018;22:1–132. 10.3310/hta22050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ulug P, Hinchliffe RJ, Sweeting MJ, Gomes M, Thompson MT, Thompson SG, et al. . Strategy of endovascular versus open repair for patients with clinical diagnosis of ruptured abdominal aortic aneurysm: the IMPROVE RCT. Health Technol Assess 2018;22:1–122. 10.3310/hta22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pouncey AL, David M, Morris RI, Ulug P, Martin G, Bicknell C, et al. . Editor’s choice—systematic review and meta-analysis of sex specific differences in adverse events after open and endovascular intact abdominal aortic aneurysm repair: consistently worse outcomes for women. Eur J Vasc Endovasc Surg 2021;62:367–378. 10.1016/j.ejvs.2021.05.029 [DOI] [PubMed] [Google Scholar]
- 83. Lancaster EM, Gologorsky R, Hull MM, Okuhn S, Solomon MD, Avins AL, et al. . The natural history of large abdominal aortic aneurysms in patients without timely repair. J Vasc Surg 2022;75:109–117. 10.1016/j.jvs.2021.07.125 [DOI] [PubMed] [Google Scholar]
- 84. Golledge J, Pinchbeck J, Tomee SM, Rowbotham SE, Singh TP, Moxon JV, et al. . Efficacy of telmisartan to slow growth of small abdominal aortic aneurysms: a randomized clinical trial. JAMA Cardiol 2020;5:1374–1381. 10.1001/jamacardio.2020.3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bicknell CD, Kiru G, Falaschetti E, Powell JT, Poulter NR; AARDVARK Collaborators . An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK). Eur Heart J 2016;37:3213–3221. 10.1093/eurheartj/ehw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Propanolol Aneurysm Trial Investigators . Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002;35:72–79. 10.1067/mva.2002.121308 [DOI] [PubMed] [Google Scholar]
- 87. Lindholt JS, Henneberg EW, Juul S, Fasting H. Impaired results of a randomised double blinded clinical trial of propranolol versus placebo on the expansion rate of small abdominal aortic aneurysms. Int Angiol 1999;18:52–57. [PubMed] [Google Scholar]
- 88. Karlsson L, Gnarpe J, Bergqvist D, Lindback J, Parsson H. The effect of azithromycin and Chlamydophilia pneumonia infection on expansion of small abdominal aortic aneurysms–a prospective randomized double-blind trial. J Vasc Surg 2009;50:23–29. 10.1016/j.jvs.2008.12.048 [DOI] [PubMed] [Google Scholar]
- 89. Vammen S, Lindholt JS, Ostergaard L, Fasting H, Henneberg EW. Randomized double-blind controlled trial of roxithromycin for prevention of abdominal aortic aneurysm expansion. Br J Surg 2001;88:1066–1072. 10.1046/j.0007-1323.2001.01845.x [DOI] [PubMed] [Google Scholar]
- 90. Hogh A, Vammen S, Ostergaard L, Joensen JB, Henneberg EW, Lindholt JS. Intermittent roxithromycin for preventing progression of small abdominal aortic aneurysms: long-term results of a small clinical trial. Vasc Endovascular Surg 2009;43:452–456. 10.1177/1538574409335037 [DOI] [PubMed] [Google Scholar]
- 91. Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, et al. . Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg 2001;34:606–610. 10.1067/mva.2001.117891 [DOI] [PubMed] [Google Scholar]
- 92. Meijer CA, Stijnen T, Wasser MN, Hamming JF, van Bockel JH, Lindeman JH, et al. . Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial. Ann Intern Med 2013;159:815–823. 10.7326/0003-4819-159-12-201312170-00007 [DOI] [PubMed] [Google Scholar]
- 93. Baxter BT, Matsumura J, Curci JA, McBride R, Larson L, Blackwelder W, et al. . Effect of doxycycline on aneurysm growth among patients with small infrarenal abdominal aortic aneurysms: a randomized clinical trial. JAMA 2020;323:2029–2038. 10.1001/jama.2020.5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wanhainen A, Mani K, Kullberg J, Svensjo S, Bersztel A, Karlsson L, et al. . The effect of ticagrelor on growth of small abdominal aortic aneurysms-a randomized controlled trial. Cardiovasc Res 2020;116:450–456. 10.1093/cvr/cvz133 [DOI] [PubMed] [Google Scholar]
- 95. Sillesen H, Eldrup N, Hultgren R, Lindeman J, Bredahl K, Thompson M, et al. . Randomized clinical trial of mast cell inhibition in patients with a medium-sized abdominal aortic aneurysm. Br J Surg 2015;102:894–901. 10.1002/bjs.9824 [DOI] [PubMed] [Google Scholar]
- 96. Pinchbeck JL, Moxon JV, Rowbotham SE, Bourke M, Lazzaroni S, Morton SK, et al. . Randomized placebo-controlled trial assessing the effect of 24-week fenofibrate therapy on circulating markers of abdominal aortic aneurysm: outcomes from the FAME -2 trial. J Am Heart Assoc 2018;7:e009866. 10.1161/JAHA.118.009866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW Jr, Kent KC, et al. . Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (phase II) multicenter study. J Vasc Surg 2002;36:1–12. 10.1067/mva.2002.125018 [DOI] [PubMed] [Google Scholar]
- 98. Karlsson L, Gnarpe J, Naas J, Olsson G, Lindholm J, Steen B, et al. . Detection of viable Chlamydia pneumoniae in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2000;19:630–635. 10.1053/ejvs.1999.1057 [DOI] [PubMed] [Google Scholar]
- 99. Lindholt JS, Ashton HA, Scott RA. Indicators of infection with Chlamydia pneumoniae are associated with expansion of abdominal aortic aneurysms. J Vasc Surg 2001;34:212–215. 10.1067/mva.2001.115816 [DOI] [PubMed] [Google Scholar]
- 100. Nyberg A, Skagius E, Nilsson I, Ljungh A, Henriksson AE. Lack of association between Chlamydophila pneumoniae seropositivity and abdominal aortic aneurysm. Vasc Endovascular Surg 2007;41:246–248. 10.1177/1538574407301429 [DOI] [PubMed] [Google Scholar]
- 101. Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 2009;119:2209–2216. 10.1161/CIRCULATIONAHA.108.806505 [DOI] [PubMed] [Google Scholar]
- 102. Golledge J, Singh TP. Effect of blood pressure lowering drugs and antibiotics on abdominal aortic aneurysm growth: a systematic review and meta-analysis. Heart 2021;107:1465–1471. 10.1136/heartjnl-2020-318192 [DOI] [PubMed] [Google Scholar]
- 103. Sawada H, Lu HS, Cassis LA, Daugherty A. Twenty years of studying AngII (angiotensin II)-induced abdominal aortic pathologies in mice: continuing questions and challenges to provide insight into the human disease. Arterioscler Thromb Vasc Biol 2022;42:277–288. 10.1161/ATVBAHA.121.317058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin-converting enzyme inhibitors and aortic rupture: a population-based case-control study. Lancet 2006;368:659–665. 10.1016/S0140-6736(06)69250-7 [DOI] [PubMed] [Google Scholar]
- 105. Kristensen KE, Torp-Pedersen C, Gislason GH, Egfjord M, Rasmussen HB, Hansen PR. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with abdominal aortic aneurysms: nation-wide cohort study. Arterioscler Thromb Vasc Biol 2015;35:733–740. 10.1161/ATVBAHA.114.304428 [DOI] [PubMed] [Google Scholar]
- 106. Wemmelund H, Hogh A, Hundborg HH, Johnsen SP, Lindholt JS. Preadmission use of renin-angiotensin blockers and rupture of abdominal aortic aneurysm: a nationwide, population-based study. Pharmacoepidemiol Drug Saf 2016;25:141–150. 10.1002/pds.3913 [DOI] [PubMed] [Google Scholar]
- 107. Manapurathe DT, Moxon JV, Krishna SM, Quigley F, Bourke M, Bourke B, et al. . Cohort study examining the association of optimal blood pressure control at entry with infrarenal abdominal aortic aneurysm growth. Front Cardiovasc Med 2022;9:868889. 10.3389/fcvm.2022.868889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Singh TP, Moxon JV, Iyer V, Gasser TC, Jenkins J, Golledge J. Comparison of peak wall stress and peak wall rupture index in ruptured and asymptomatic intact abdominal aortic aneurysms. Br J Surg 2021;108:652–658. 10.1002/bjs.11995 [DOI] [PubMed] [Google Scholar]
- 109. Singh TP, Moxon JV, Gasser TC, Dalman RL, Bourke M, Bourke B, et al. . Effect of telmisartan on the peak wall stress and peak wall rupture index of small abdominal aortic aneurysms: an exploratory analysis of the TEDY trial. Eur J Vasc Endovasc Surg 2022;64:396–404. 10.1016/j.ejvs.2022.07.042 [DOI] [PubMed] [Google Scholar]
- 110. Singh TP, Moxon JV, Gasser TC, Golledge J. Systematic review and meta-analysis of peak wall stress and peak wall rupture index in ruptured and asymptomatic intact abdominal aortic aneurysms. J Am Heart Assoc 2021;10:e019772. 10.1161/JAHA.120.019772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Khosla S, Morris DR, Moxon JV, Walker PJ, Gasser TC, Golledge J. Meta-analysis of peak wall stress in ruptured, symptomatic and intact abdominal aortic aneurysms. Br J Surg 2014;101:1350–1357; discussion 1357. 10.1002/bjs.9578 [DOI] [PubMed] [Google Scholar]
- 112. Boyd AJ, Kuhn DC, Lozowy RJ, Kulbisky GP. Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J Vasc Surg 2016;63:1613–1619. 10.1016/j.jvs.2015.01.040 [DOI] [PubMed] [Google Scholar]
- 113. Bappoo N, Syed MBJ, Khinsoe G, Kelsey LJ, Forsythe RO, Powell JT, et al. . Low shear stress at baseline predicts expansion and aneurysm-related events in patients with abdominal aortic aneurysm. Circ Cardiovasc Imaging 2021;14:1112–1121. 10.1161/CIRCIMAGING.121.013160 [DOI] [PubMed] [Google Scholar]
- 114. Parr A, McCann M, Bradshaw B, Shahzad A, Buttner P, Golledge J. Thrombus volume is associated with cardiovascular events and aneurysm growth in patients who have abdominal aortic aneurysms. J Vasc Surg 2011;53:28–35. 10.1016/j.jvs.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Singh TP, Wong SA, Moxon JV, Gasser TC, Golledge J. Systematic review and meta-analysis of the association between intraluminal thrombus volume and abdominal aortic aneurysm rupture. J Vasc Surg 2019;70:2065–2073 e2010. 10.1016/j.jvs.2019.03.057 [DOI] [PubMed] [Google Scholar]
- 116. Lindholt JS, Sorensen HT, Michel JB, Thomsen HF, Henneberg EW. Low-dose aspirin may prevent growth and later surgical repair of medium-sized abdominal aortic aneurysms. Vasc Endovascular Surg 2008;42:329–334. 10.1177/1538574408315205 [DOI] [PubMed] [Google Scholar]
- 117. Golledge J, Jenkins J, Bourke M, Bourke B, Singh TP. Association of oral anticoagulation prescription with clinical events in patients with an asymptomatic unrepaired abdominal aortic aneurysm. Biomedicines 2022;10:2112. 10.3390/biomedicines10092112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wemmelund H, Jorgensen TM, Hogh A, Behr-Rasmussen C, Johnsen SP, Lindholt JS. Low-dose aspirin and rupture of abdominal aortic aneurysm. J Vasc Surg 2017;65:616–625e614. 10.1016/j.jvs.2016.04.061 [DOI] [PubMed] [Google Scholar]
- 119. Elbadawi A, Omer M, Ogunbayo G, Owens P III, Mix D, Lyden SP, et al. . Antiplatelet medications protect against aortic dissection and rupture in patients with abdominal aortic aneurysms. J Am Coll Cardiol 2020;75:1609–1610. 10.1016/j.jacc.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Biros E, Gabel G, Moran CS, Schreurs C, Lindeman JH, Walker PJ, et al. . Differential gene expression in human abdominal aortic aneurysm and aortic occlusive disease. Oncotarget 2015;6:12984–12996. 10.18632/oncotarget.3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Biros E, Moran CS, Rush CM, Gabel G, Schreurs C, Lindeman JH, et al. . Differential gene expression in the proximal neck of human abdominal aortic aneurysm. Atherosclerosis 2014;233:211–218. 10.1016/j.atherosclerosis.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 122. Tchana-Sato V, Koch JN, Ancion A, Adelin A, Minga Lowampa E, Burelli M, et al. . Abdominal aortic aneurysm in heart transplant recipients: new insights from a 30-year experience at a single center. Ann Vasc Surg 2022;87:478–486. 10.1016/j.avsg.2022.05.038 [DOI] [PubMed] [Google Scholar]
- 123. Tajima Y, Goto H, Ohara M, Hashimoto M, Akamatsu D, Shimizu T, et al. . Oral steroid use and abdominal aortic aneurysm expansion—positive association. Circ J 2017;81:1774–1782. 10.1253/circj.CJ-16-0902 [DOI] [PubMed] [Google Scholar]
- 124. Thanigaimani S, Phie J, Quigley F, Bourke M, Bourke B, Velu R, et al. . Immunosuppressive drugs for nontransplant comorbidities are not associated with abdominal aortic aneurysm growth. JVS Vasc Sci 2023;3:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Becker von Rose A, Kobus K, Bohmann B, Lindquist-Lilljequist M, Eilenberg W, Bassermann F, et al. . Radiation and chemotherapy are associated with altered aortic aneurysm growth in patients with cancer: impact of synchronous cancer and aortic aneurysm. Eur J Vasc Endovasc Surg 2022;64:255–264. 10.1016/j.ejvs.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 126. Salata K, Syed M, Hussain MA, de Mestral C, Greco E, Mamdani M, et al. . Statins reduce abdominal aortic aneurysm growth, rupture, and perioperative mortality: a systematic review and meta-analysis. J Am Heart Assoc 2018;7:e008657. 10.1161/JAHA.118.008657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ferguson CD, Clancy P, Bourke B, Walker PJ, Dear A, Buckenham T, et al. . Association of statin prescription with small abdominal aortic aneurysm progression. Am Heart J 2010;159:307–313. 10.1016/j.ahj.2009.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Krishna SM, Seto SW, Moxon JV, Rush C, Walker PJ, Norman PE, et al. . Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse model. Am J Pathol 2012;181:706–718. 10.1016/j.ajpath.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 129. Golledge J, Cullen B, Rush C, Moran CS, Secomb E, Wood F, et al. . Peroxisome proliferator-activated receptor ligands reduce aortic dilatation in a mouse model of aortic aneurysm. Atherosclerosis 2010;210:51–56. 10.1016/j.atherosclerosis.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Moxon JV, Rowbotham SE, Pinchbeck JL, Lazzaroni SM, Morton SK, Moran CS, et al. . A randomised controlled trial assessing the effects of peri-operative fenofibrate administration on abdominal aortic aneurysm pathology: outcomes from the FAME trial. Eur J Vasc Endovasc Surg 2020;60:452–460. 10.1016/j.ejvs.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 131. Matthews EO, Rowbotham SE, Moxon JV, Jones RE, de Ceniga MV, Golledge J. Meta-analysis of the association between peripheral artery disease and growth of abdominal aortic aneurysms. Br J Surg 2017;104:1765–1774. 10.1002/bjs.10675 [DOI] [PubMed] [Google Scholar]
- 132. Matthews EO, Moxon JV, Singh TP, Thanigaimani S, Jones RE, Gasser TC, et al. . Athero-occlusive disease appears to be associated with slower abdominal aortic aneurysm growth: an exploratory analysis of the TEDY trial. Eur J Vasc Endovasc Surg 2022;63:632–640. 10.1016/j.ejvs.2021.12.038 [DOI] [PubMed] [Google Scholar]
- 133. Nastasi DR, Norman R, Moxon JV, Quigley F, Velu R, Jenkins J, et al. . The potential benefits and costs of an intensified approach to low density lipoprotein cholesterol lowering in people with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2021;62:643–650. 10.1016/j.ejvs.2021.06.031 [DOI] [PubMed] [Google Scholar]
- 134. Golledge J, Arnott C, Moxon J, Monaghan H, Norman R, Morris D, et al. . Protocol for the Metformin Aneurysm Trial (MAT): a placebo-controlled randomised trial testing whether metformin reduces the risk of serious complications of abdominal aortic aneurysm. Trials 2021;22:962. 10.1186/s13063-021-05915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Xiong J, Wu Z, Chen C, Wei Y, Guo W. Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: a meta-analysis. Int J Cardiol 2016;221:484–495. 10.1016/j.ijcard.2016.07.016 [DOI] [PubMed] [Google Scholar]
- 136. Norman PE, Davis TM, Le MT, Golledge J. Matrix biology of abdominal aortic aneurysms in diabetes: mechanisms underlying the negative association. Connect Tissue Res 2007;48:125–131. 10.1080/03008200701331524 [DOI] [PubMed] [Google Scholar]
- 137. Golledge J, Karan M, Moran CS, Muller J, Clancy P, Dear AE, et al. . Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur Heart J 2008;29:665–672. 10.1093/eurheartj/ehm557 [DOI] [PubMed] [Google Scholar]
- 138. He J, Li N, Fan Y, Zhao X, Liu C, Hu X. Metformin inhibits abdominal aortic aneurysm formation through the activation of the AMPK/mTOR signaling pathway. J Vasc Res 2021;58:148–158. 10.1159/000513465 [DOI] [PubMed] [Google Scholar]
- 139. Fujimura N, Xiong J, Kettler EB, Xuan H, Glover KJ, Mell MW, et al. . Metformin treatment status and abdominal aortic aneurysm disease progression. J Vasc Surg 2016;64:46–54e48. 10.1016/j.jvs.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Thanigaimani S, Singh TP, Unosson J, Phie J, Moxon J, Wanhainen A, et al. . Editor’s choice—association between metformin prescription and abdominal aortic aneurysm growth and clinical events: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2021;62:747–756. 10.1016/j.ejvs.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 141. Golledge J, Morris DR, Pinchbeck J, Rowbotham S, Jenkins J, Bourke M, et al. . Editor’s choice—metformin prescription is associated with a reduction in the combined incidence of surgical repair and rupture related mortality in patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2019;57:94–101. 10.1016/j.ejvs.2018.07.035 [DOI] [PubMed] [Google Scholar]
- 142. Wanhainen A, Unosson J, Mani K, Gottsater A; MAAAGI Trial Investigators . The Metformin for Abdominal Aortic Aneurysm Growth Inhibition (MAAAGI) trial. Eur J Vasc Endovasc Surg 2021;61:710–711. 10.1016/j.ejvs.2020.11.048 [DOI] [PubMed] [Google Scholar]
- 143. US National Library of Medicine . Limiting AAA With Metformin (LIMIT) Trial. ClinicalTrials.gov 2020. Available athttps://clinicaltrials.gov/ct2/show/NCT04500756.
- 144. US National Library of Medicine . Metformin Therapy in Non-diabetic AAA Patients (MetAAA). ClinicalTrials.gov. 2018. Available athttps://clinicaltrials.gov/ct2/show/NCT03507413.
- 145. Nordness MJ, Baxter BT, Matsumura J, Terrin M, Zhang K, Ye F, et al. . The effect of diabetes on abdominal aortic aneurysm growth over 2 years. J Vasc Surg 2022;75:1211–1222e1211. 10.1016/j.jvs.2021.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ibrahim N, Bleichert S, Klopf J, Kurzreiter G, Knobl V, Hayden H, et al. . 3D Ultrasound measurements are highly sensitive to monitor formation and progression of abdominal aortic aneurysms in mouse models. Front Cardiovasc Med 2022;9:944180. 10.3389/fcvm.2022.944180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Adelsperger AR, Phillips EH, Ibriga HS, Craig BA, Green LA, Murphy MP, et al. . Development and growth trends in angiotensin II-induced murine dissecting abdominal aortic aneurysms. Physiol Rep 2018;6:e13668. 10.14814/phy2.13668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Phie J, Thanigaimani S, Huynh P, Anbalagan R, Moran CS, Kinobe R, et al. . Colchicine does not reduce abdominal aortic aneurysm growth in a mouse model. Cardiovasc Ther 2022;2022:5299370. 10.1155/2022/5299370 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed for this manuscript.