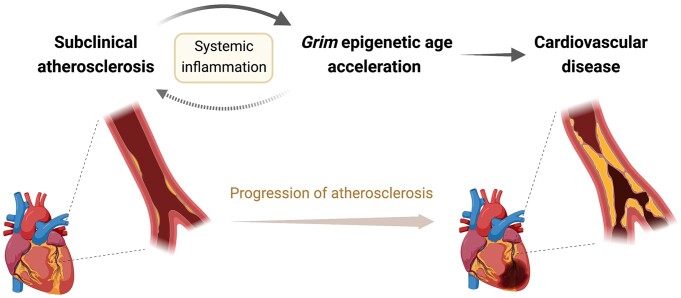
Graphical Abstract
Graphical Abstract.
A potential mediatory role for systemic inflammation in the association between subclinical atherosclerosis and Grim epigenetic age acceleration, identified by multi-omics analyses utilizing participants in the Progression of Early Subclinical Atherosclerosis (PESA) study.
This editorial refers to ‘Subclinical atherosclerosis and accelerated epigenetic age mediated by inflammation: a multi-omics study’, by F. Sanchez-Cabo et al., https://doi.org/10.1093/eurheartj/ehad361.
Ageing and cardiovascular disease
Atherosclerosis and many other cardiovascular diseases are very age dependent, with chronological age a major risk factor for cardiac disease.1,2 Atherosclerotic plaques can accumulate in otherwise healthy individuals with age, remaining clinically silent for years as subclinical atherosclerosis (SA).3 The highest prevalence of SA is in middle-aged individuals. Understanding the interplay of ageing and pathogenesis of SA and uncovering early biomarkers of SA are important, as clinical manifestation of initial symptoms can be lethal.
Biological ageing seems to progress at different rates amongst individuals with the same chronological age, with significant variation in physiological and functional hallmarks of ageing.4 Epigenetic parameters such as DNA methylation patterns vary significantly between individuals of the same chronological age and are demonstrably linked to human ageing.4,5 Rigorous study of the dynamic landscape of epigenetic changes has established ‘epigenetic clocks’, which can provide biological vs. chronological age of an individual, and thus a prediction of health and life span. Further, epigenetic age acceleration (EAA) defines the difference between epigenetic age and chronological age, and is strongly associated with many variables of health, disease, and ageing in humans.5 The emerging association between cardiovascular diseases and EAA highlights the potential usefulness of EAA as a biomarker of cardiovascular disease.6
Association between Grim epigenetic age acceleration and subclinical atherosclerosis
The study by Sanchez-Cabo et al. in the current issue of the European Heart Journal establishes a strong association between SA and EAA, and describes a potential role for proinflammatory mechanisms in this association.7 The authors utilized a subset of participants of the Progression of Early Subclinical Atherosclerosis (PESA) study, which established the prevalence of SA in middle-aged individuals.3,8 Participants were scored on the extent of SA based on the presence of plaques in key territories (aorta, carotid, and ilio-femoral arteries) as well as the Coronary Artery Calcification (CAC) score. Whole blood methylomics, transcriptomics, and plasma proteomics were obtained from 391 asymptomatic participants to estimate EAA.
Sanchez-Cabo et al. report an association between Grim EAA and the presence, extension, and progression of SA.7 The authors calculated epigenetic age based on four different epigenetic clocks: two predictors of life span, Grim and Pheno; and two predictors of chronological age known as Horvath’s and Hannum’s clocks. The presence of SA and extension of SA were both found to be associated with predictors of life span such as Grim EAA and Pheno EAA, independent of traditional cardiovascular risk factors, by 2D/3D vascular ultrasound. By computed tomography measurements, however, only Grim EAA was associated with a positive CAC score and its extension, independent of traditional cardiovascular risk factors. Further, by three different imaging techniques, predictors of chronological age such as Horvath’s and Hannum’s clocks were not significantly associated with the presence or extension of SA. The authors thus show that Grim EAA occurs with an increase in SA, independent of traditional cardiovascular risk factors. To confirm these findings, the authors also conducted an analysis that categorized participants based on Grim EAA, to estimate SA progression and cardiovascular risk among these categories. By this analysis, the authors further show a trend for increasing SA burden with Grim EAA. Remarkably, the association of Grim EAA with the progression of CAC remained significant even after adjustment for cardiovascular risk factors, including smoking. This is despite individuals with Grim EAA having a higher prevalence of cardiovascular risk factors than other groups, and also exhibiting increased cardiovascular age. The association between SA and Grim EAA was further validated using publicly available CD14+ methylomics data from a multiethnic study of atherosclerosis.9
Proinflammatory pathways mediate epigenetic age acceleration
Sanchez-Cabo et al. report a mediatory role for proinflammatory pathways in association between Grim EAA and SA.7 By RNA-seq analysis, the top canonical pathways associated with EAA were immune response pathways, including Th1/2 activation pathways, the STAT3 pathway, interleukin (IL)-10 signalling, and Toll-like receptor signalling. Key biomarkers for increased systemic inflammation, including an increase in total number of platelets and white blood cells, plasma C-reactive protein level, and neutrophil to lymphocyte ratio, were found to be strongly correlated with EAA. The INFLA-Score, a measure of chronic low-grade inflammation, was also significantly associated with Grim EAA.10 Further, the INFLA-Score was found to partially mediate the effect of SA on EAA, but not of EAA on SA. The role of inflammation as a critical mediator of all phases of atherosclerosis is well established.11 The results of Sanchez-Cabo et al. expand upon this, and suggest an effect of SA and chronic inflammation on overall reduced health and life span of individuals.7
Inflammation in subclinical atherosclerosis and cardiovascular disease risk
Early detection at the subclinical stages of atherosclerosis is a critical goal.3 Further, while the link between initiation of SA and inflammation is known,12 an exploration of the direct impact of SA on health and life span of individuals, as well as the mediatory mechanisms underlying it, were not established. The study by Sanchez-Cabo et al. addresses these gaps in knowledge by identifying the robust association between Grim EAA and SA.7 They also describe a mediatory role for systemic inflammation in the effect of SA on Grim EAA. Thus, they show a detrimental impact of SA on human health and life span, alongside increased cardiovascular age and increased Grim EAA both being associated with SA (Graphical Abstract).
Understanding these associations is valuable to establish effective biomarkers for identifying early atherosclerosis, as well as developing therapeutic approaches for preventing progression of atherosclerosis beyond the subclinical stages. The authors highlight the potential for attenuating systemic inflammation to reduce the detrimental effect of SA on epigenetic age. By mediation analysis of omics data, they identified proinflammatory molecules such as IL1B, CLEC10A, and OSM which mediate key mechanisms such as IL10 signalling and inflammasome pathways. OSM was recently reported to have a role in progression of atherosclerosis as well as survival probability in humans.13 The CANTOS study also highlighted the importance of targeting proinflammatory IL-1β signalling with canakinumab to reduce atherosclerotic disease and cardiovascular risk, although a recent study found no reduction in plaque burden with a canakinumab treatment strategy in peripheral artery disease.14,15
This study by Sanchez-Cabo et al. certainly does not exclude other factors beyond known inflammatory pathways in the presence and progression of SA. Also, while the authors identified a strong association between SA and Grim EAA, a causal effect cannot be established due to the study design. Further longitudinal studies designed to assess effect and directionality of SA on health and life span, as well as the viability of modulating inflammatory signalling to prevent progression of SA, are needed. Nevertheless, the findings of Sanchez-Cabo et al. strengthen the role of inflammatory mechanisms in SA and its impact on EAA. The study also provides more mechanistic evidence for strategies aiming at attenuating systemic inflammation for the delay of atherosclerosis progression in cardiovascular disease.
Acknowledgements
The graphical abstract was created with BioRender.com.
Contributor Information
Nivedhitha Velayutham, Department of Stem Cell and Regenerative Biology and the Harvard Stem Cell Institute, Harvard University, Cambridge, MA 02138, USA.
Richard T Lee, Department of Stem Cell and Regenerative Biology and the Harvard Stem Cell Institute, Harvard University, Cambridge, MA 02138, USA; Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Data availability
No new data were generated or analysed in support of this work.
Funding
This work was supported by the National Institutes of Health (HL151684 and HL137710 to R.T.L.).
References
- 1. Kim HW, Shi H, Winkler MA, Lee R, Weintraub NL. Perivascular adipose tissue and vascular perturbation/atherosclerosis. Arterioscler Thromb Vasc Biol 2020;40:2569–2576. 10.1161/ATVBAHA.120.312470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sniderman AD, Furberg CD. Age as a modifiable risk factor for cardiovascular disease. Lancet 2008;371:1547–1549. 10.1016/S0140-6736(08)60313-X [DOI] [PubMed] [Google Scholar]
- 3. Ibanez B, Fernández-Ortiz A, Fernández-Friera L, García-Lunar I, Andrés V, Fuster V. Progression of early subclinical atherosclerosis (PESA) study: jACC focus seminar 7/8. J Am Coll Cardiol 2021;78:156–179. 10.1016/j.jacc.2021.05.011 [DOI] [PubMed] [Google Scholar]
- 4. Xia X, Chen W, McDermott J, Han JJ. Molecular and phenotypic biomarkers of aging. F1000Res 2017;6:860. 10.12688/f1000research.10692.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet 2018;19:371–384. 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 6. Joyce BT, Gao T, Zheng Y, Ma J, Hwang S-J, Liu L, et al. Epigenetic age acceleration reflects long-term cardiovascular health. Circ Res 2021;129:770–781. 10.1161/CIRCRESAHA.121.318965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sánchez-Cabo F, Fuster V, Silla GC, González G, Lorenzo-Vivas E, Alvarez R, et al. Subclinical atherosclerosis and accelerated epigenetic age mediated by inflammation: a multi-omics study. Eur Heart J 2023;44:2698–2709. 10.1093/eurheartj/ehad361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernández-Ortiz A, Jiménez-Borreguero LJ, Peñalvo JL, Ordovás JM, Mocoroa A, Fernández-Friera L, et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J 2013;166:990–998. 10.1016/j.ahj.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 9. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 10. Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, et al. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-sani study. Haematologica 2016;101:1434–1441. 10.3324/haematol.2016.144055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Libby P. Inflammation in atherosclerosis—no longer a theory. Clin Chem 2021;67:131–142. 10.1093/clinchem/hvaa275 [DOI] [PubMed] [Google Scholar]
- 12. Devesa A, Lobo-González M, Martínez-Milla J, Oliva B, García-Lunar I, Mastrangelo A, et al. Bone marrow activation in response to metabolic syndrome and early atherosclerosis. Eur Heart J 2022;43:1809–1828. 10.1093/eurheartj/ehac102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Keulen D, Pouwer MG, Emilsson V, Matic LP, Pieterman EJ, Hedin U, et al. Oncostatin M reduces atherosclerosis development in APOE*3Leiden.CETP mice and is associated with increased survival probability in humans. PLoS One 2019;14:e0221477. 10.1371/journal.pone.0221477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell KS, Yates DP, Kramer CM, Feller A, Mahling P, Colin L, et al. A randomized, placebo-controlled trial of canakinumab in patients with peripheral artery disease. Vasc Med 2019;24:414–421. 10.1177/1358863X19859072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this work.