Abstract
The current treatment algorithm for chronic thromboembolic pulmonary hypertension (CTEPH) as depicted in the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines on the diagnosis and treatment of pulmonary hypertension (PH) includes a multimodal approach of combinations of pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA) and medical therapies to target major vessel pulmonary vascular lesions, and microvasculopathy. Today, BPA of >1700 patients has been reported in the literature from centers in Asia, the US, and also Europe; many more patients have been treated outside literature reports. As BPA becomes part of routine care of patients with CTEPH, benchmarks for safe and effective care delivery become increasingly important. In light of this development, the ESC Working Group on Pulmonary Circulation and Right Ventricular Function has decided to publish a document that helps standardize BPA to meet the need of uniformity in patient selection, procedural planning, technical approach, materials and devices, treatment goals, complications including their management, and patient follow-up, thus complementing the guidelines. Delphi methodology was utilized for statements that were not evidence based. First, an anatomical nomenclature and a description of vascular lesions are provided. Second, treatment goals and definitions of complete BPA are outlined. Third, definitions of complications are presented which may be the basis for a standardized reporting in studies involving BPA. The document is intended to serve as a companion to the official ESC/ERS guidelines.
Keywords: Chronic thromboembolic pulmonary hypertension, Balloon pulmonary angioplasty, Chronic thromboembolic pulmonary disease
Graphical Abstract
Graphical Abstract.
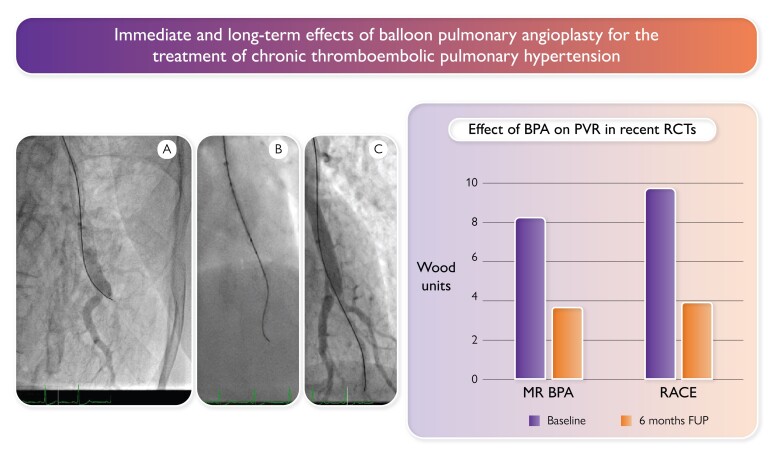
Immediate and long-term effect of balloon pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension.1 The anterior–posterior projection radiographs on the left show a patient example where left A8 was crossed with a wire (panel A), dilated with a 2.0-mm balloon (panel B), and reopened, with immediate filling of a second, bifurcating pulmonary artery (panel C). The bar graph on the right shows PVR at baseline and at 6 months in the absence of concomitant medication with pulmonary vasodilators in two recent randomized controlled trials (MR BPA2 and RACE3). BPA, balloon pulmonary angioplasty; PVR, pulmonary vascular resistance; RCT, randomized controlled trial; FUP, follow-up.
Introduction
The 2022 European Society of Cardiology (ESC) guidelines on the diagnosis and treatment of pulmonary hypertension (PH), developed together with the European Respiratory Society (ERS), provide recommendations on the optimal management of group 4.1 PH, labelled as chronic thromboembolic pulmonary disease (CTEPD) with [chronic thromboembolic pulmonary hypertension (CTEPH)] or without PH.4 CTEPH is thought to result from pulmonary thromboembolism and is characterized by organized vascular occlusions of the pulmonary arteries. While pulmonary endarterectomy (PEA) is guideline recommended as the treatment of choice for suitable patients with CTEPH, interventional treatment by balloon pulmonary angioplasty (BPA) is now also guideline recommended in the therapeutic algorithm of CTEPH.4 Guidelines recommend BPA where PEA is not feasible and medical therapy does not ameliorate symptoms,4 meaning inoperable CTEPH and PH after PEA.4–12 While existing data suggest prognostic benefit, this has yet to be formally demonstrated.13–15 On the other hand, this is true also for medical treatments that are used together with BPA today.
Our Clinical Consensus Statement is focused on technical, structural, and logistic requirements for performing BPA, patient selection and preparation, treatment goals, procedural details, complications and their management, BPA outcomes and patient follow-up, cost of BPA, and patient needs. It focusses mainly on CTEPH, with less evidence for CTEPD without PH, and not on other pathologies of the pulmonary arteries.16 While the best available current evidence is summarized in this document, questionnaires were utilized for statements that were not evidence based to arrive at a group opinion by surveying the panel of participating experts. Responses were aggregated and shared with the group who incorporated the results in their individual paragraphs.
The present document serves as a practical guide to performing BPA in Europe, according to the refinement of the technique by Japanese interventionists.
Clinical consensus statement methods
We systematically searched PubMed, OVID, and EBSCO to identify randomized controlled trials and prospective controlled or retrospective observational studies that reported on the efficacy and safety of BPA without any language restrictions. The last search was performed on 21 December 2022, without any publication date limitations. The search string was created for PubMed and modified accordingly for the other databases and is included in the Supplementary data online, Materials. The additional search of reference lists of newly identified studies or discussions in the authors’ group did not identify other studies that met the eligibility criteria. Non-original studies (reviews, editorials, and commentaries), conference abstracts, meta-analyses, and case reports were excluded. The literature search criteria underlying this document can be found in the Supplementary data online, Materials. Furthermore, ClinicalTrials.gov was manually searched to find ongoing or unpublished clinical trials.
For questions without high-quality evidence, a survey was prepared. The survey was distributed to the authors to prepare consensus-based text. The minimum threshold for consensus was 60% because BPA is an emerging procedure, and at the time of the writing process, individual practices, particularly at the technical level and choice of materials, have varied depending on local availability and conditions of reimbursement. We have added filled questionnaires in the Supplementary data online, Materials. Some questions were discussed during online meetings and the answers were formulated together, e.g. the etiology of lung injury (LI). Areas of knowledge gaps were identified.
History of BPA
After the first published BPA case report in 1988 of a 30-year-old man with PH after pulmonary embolism,17 Feinstein et al.18 described 18 patients in 2001 with inaccessible or ‘nonsurgical’ CTEPH who underwent BPA. Reperfusion pulmonary edema occurred in 11 patients, and 30-day mortality was 5.5%. Because safety issues were compromising efficacy outcomes at these early treatment days, Japanese interventionists refined BPA to bring it to today’s standards,7 mainly by a more cautious approach with multiple sessions, use of smaller balloons in the beginning, and coronary equipment.
Technical, structural, and logistic requirements for performing BPA
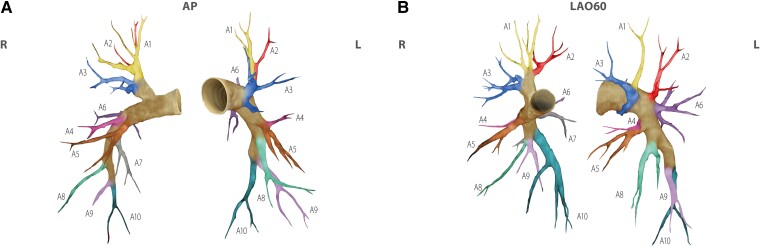
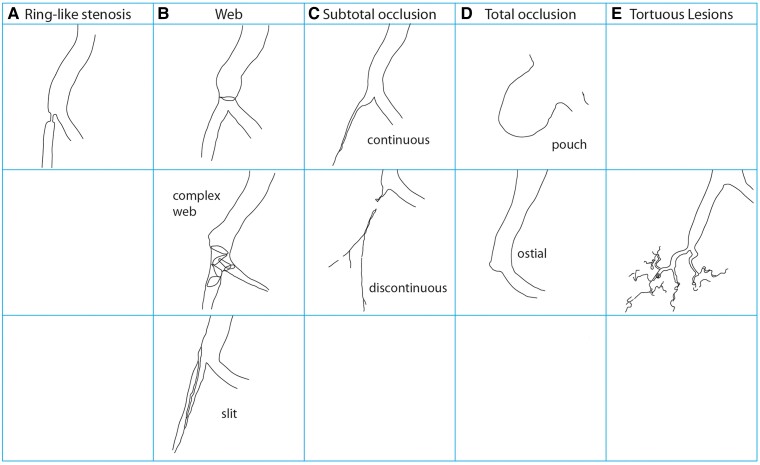
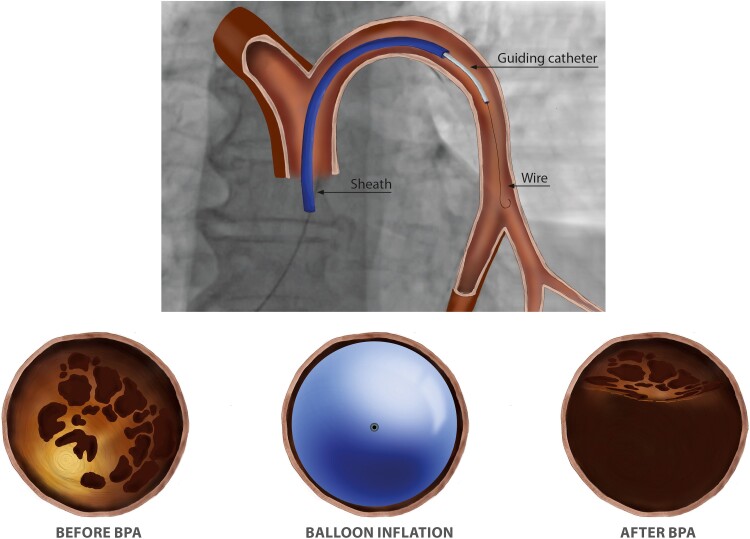
BPA is performed in a catheter laboratory, or in a hybrid room, preferably with biplane cineangiography.19 BPA is a complex procedure requiring a detailed understanding of three-dimensional pulmonary vascular anatomy (Figure 1). Segment labels employ the letter A and numbers, in accordance with labeling of the bronchial tree.20 Operators need to be aware of anatomical variability. The left upper lobe is particularly prone to variation with between two and seven pulmonary arterial branches, the most constant is the left lower lobe with only four described variants.21 Left A7 appears variable, and may come off A8 (Figure 1). In addition, characteristics of lesion types must be emphasized. CTEPH lesions tend to be close to vessel bifurcations. Type A ring-like stenosis lesions result in a concentric stenosis, as if a ring was put on the vessel. Type B web lesions are hazy or abrupt narrowing opacities of the vessel that may appear in various configurations, for example as complex webs or slits. Type C and D lesions represent occlusions with tapered subtotal lesions (type C) that appear almost completely occluded but have continuous or discontinuous subtle and slow blood flow distal to the obstruction. Type D total occlusion lesions appear as pouches or ostial occlusions. Type E tortuous lesions represent web lesions or occlusions in highly tortuous small vessels distal to subsegmental arteries, surrounded by cotton wool–like stains of capillary arteries (Figure 2). The principle of BPA (Figure 3) is to dilate intraluminal fibrotic obstructions and to open occlusions by penetrating proximal fibrous intimal caps with wires, stretching the vessel with balloons and compressing organized thrombi.22,23 Once flow is established, distal vascular territories are reperfused and gain cross sectional diameter over the following 4–6 weeks. No restenosis has been observed to date.
Figure 1.
Anatomical classification of the pulmonary vascular tree, and segment names. Anatomy is in accordance with a computed tomogram of a healthy Danish volunteer. Segments are color coded. Panel A: anterior–posterior projection. Panel B: left anterior oblique (LAO) 60° projection. Segment A7 on the left side is variable; if present, it typically occurs in a common branch with A8.
Figure 2.
Vascular lesions of CTEPH. Lesions are common at bifurcations. Except for tortuous lesions that affect vessels < 2 mm in diameter and pouches that are predominantly proximal, all lesions may occur at any level of vessel size.
Figure 3.
The principle of BPA. The top of the figure shows the setting of BPA, with a guiding catheter telescoped through an 80 or 90 cm sheath. The bottom of the figure shows cross sections of a vessel before BPA with the obstructing webs (left), with the balloon inflated (middle), and the final result (right). In contrast to coronary intervention, the balloon tears and compresses intraluminal webs and bands without injuring the medial layer of the vessel wall.
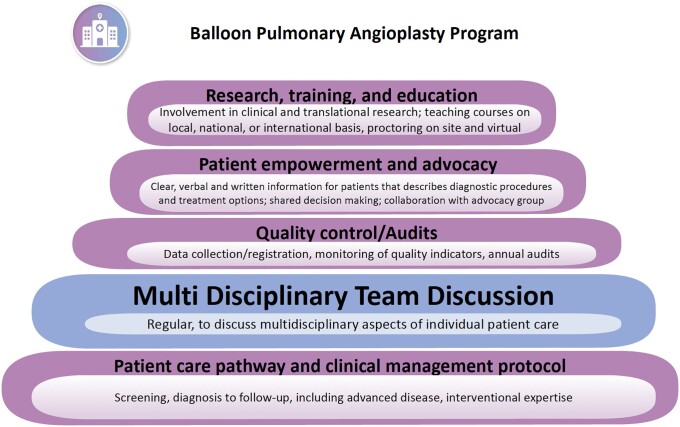
The 2022 ESC/ERS guidelines recommend that all patients with CTEPH are reviewed by a multidisciplinary CTEPH team capable of multimodality management,4 to decide the initial treatment after review of all relevant data (Figure 4). A CTEPH team should consist of a PEA surgeon, a BPA interventionist, a PH specialist, a radiologist experienced in thoracic imaging, an intensive care specialist, a nurse specialist, and a data manager. The team should meet regularly to review new referrals, follow-up cases, and complications.4
Figure 4.
Building blocks of a BPA program.
Centers should have BPA activities at a minimum of 100 procedures/year as described in the guidelines, ideally concentrating care and expertise in high-volume centers.4 Centers should be able to demonstrate excellent safety outcomes such as a 30-day mortality of <2% and serious adverse event (SAE) rates of <5% per session. According to a recent discussion of the RACE3 and MRBPA2 studies, a BPA–SAE is defined as an event that results in death, is life-threatening, requires hospitalization24 or extended hospitalization for treatment, causes permanent or marked impairment or dysfunction, and/or requires medical or surgical intervention to prevent one of the above. All task force members believed it was important that any center planning to set up a BPA service be proctored by an expert center. All BPA centers should seek an annual audit to review the achievement of BPA goals and survival.4,16,25,26 The task force members agreed that onsite cardiothoracic surgery, extra-corporeal membrane oxygenation (ECMO), coils/gelfoam, and covered stents must be available as bail-out options when performing BPA.
Patient selection and preparation
Patient-related factors influencing patient selection are as follows:
To be eligible for BPA, patients must have New York Heart Association (NYHA) class II or greater symptoms, most likely due to CTEPD.4
Patient cooperation during BPA should be ascertained, because lying flat for the duration of the intervention and the ability for a proper breath hold are mandatory.
Contrast allergy, renal, and thyroid dysfunction are handled according to general guideline recommendations.27,28
Patients who reject PEA despite the recommendation of a CTEPH team have a poor prognosis and are advised to be evaluated for BPA.29 There are now reports to support BPA as a treatment option in patients with operable disease. Darocha et al.30 reported the same efficacy and safety of BPA in technically operable patients; however, they excluded patients with large central clots and complete proximal main or lobar branch occlusions. Nishihara et al.’s31 report is including these lesions.
Only case reports exist on rescue BPA in patients who are in right heart failure.
Sex differences in the treatment of CTEPH have been evaluated in few studies.32,33 PEA was performed more frequently in men while more females were classified as inoperable.34 Women have more frequently distal technically inaccessible disease35 and tend to reject PEA,36 although mortality was similar between both sexes.32,34–37 In Japan, female CTEPH patients are elderly with less deep vein thrombosis, less acute embolic episodes, lower arterial oxygen tension, and more peripheral thrombi, and female Japanese CTEPH patients derive less improvement through PEA.35
Advanced age, frailty, 38 and comorbidities remain important causes of non-operability. BPA in elderly patients improves functional class, hemodynamics, and biomarkers, and the rate of procedural complications and peri-procedural mortality is low.39,40 Treatment goals should be individualized in patients with multiple co-morbidities where standard risk–benefit assessments may not hold.
Disease-related factors influencing patient selection are as follows:
Screening and imaging for other causes of PH must have been done for patients undergoing BPA, according to guidelines,4 including a coronary angiogram in patients at risk for coronary artery disease.41,42 After confirming the diagnosis, imaging is performed for the assessment of lesion distribution and lesion characteristics (Figure 2). Computed tomography pulmonary angiography (CT-PA) with multiplanar reconstruction is most commonly used for this purpose today. With a higher spatial resolution and transarterial contrast enhancement, cone beam CT of the pulmonary arteries shows peripheral lesions in more detail than CT-PA and can be used instead.43 In addition to cross-sectional imaging, most centers perform diagnostic and planning digital subtraction angiography of the pulmonary arteries (DSA-PA) during deep inspiratory breath holds in orthogonal projections.44 DSA-PA supplements the CT-PA with dynamic information of the parenchymal perfusion and allows for assessment of lobar, segmental, or subpleural perfusion defects. Direct segment-by-segment invasive PA contrast injection including intravascular imaging with intravascular ultrasound (IVUS) and/or optical coherence tomography (OCT) may be appropriate in cases of diagnostic uncertainty, or in special instances.
The severity of baseline hemodynamics predicts BPA–related complications.25,45 Roughly 40% of patients have significant pulmonary microvasculopathy that can be assessed by the PA occlusion technique.46
BPA after PEA has been found more difficult and less safe. Residual PH after PEA has been reported in 17%–31%.47,48 Clinically relevant residual PH conferring worse long-term survival after PEA mainly occurs when the mean pulmonary arterial pressure is >38 mmHg.47 Selection of candidates for BPA after PEA includes a complete re-assessment of the patient with symptomatic PH 3–6 months after PEA using high-quality imaging techniques such as CT-PA, DSA-PA, and right heart catheterization. Recently, single-center series with small numbers of patients have reported hemodynamics and functional class improved at follow-up, suggesting BPA as a complementary therapeutic strategy for residual or recurrent PH after PEA.9,11,49 However, less hemodynamic improvement than that in primary BPA occurred.49 Polish authors describe very hard occlusions after PEA, challenging to dilate,11 potentially resulting in hemoptysis,50 requiring embolization.51
Patients with typical CTEPH vascular lesions but without PH at rest have been labeled as CTEPD without PH4 and are unfortunately less commonly referred to CTEPH centers.52 Two series have been published: Wiedenroth et al.53 report improved functional and hemodynamic status in 9/10 treated patients with one to five BPA sessions (average four sessions/patient), and Inami et al.54 found improved functional status in all 15 patients after BPA, and less need for nasal oxygen after four BPA sessions (see Supplementary data online, Table S1). Few complications and no periprocedural deaths were reported. Systematic data regarding prognostic impact and therapeutic goals are lacking. According to the 2022 ESC/ERS guidelines,4 both PEA or BPA should be considered in selected symptomatic patients with CTEPD without PH. For the identification of these patients, results of echocardiography, lung function testing, BNP/NT-proBNP, chest radiography, and cardiopulmonary exercise testing are considered in a multiparametric approach.4,55
Patient preparation for BPA is as follows:
Pretreatment with vasodilator drugs targets peripheral vascular remodeling, improves hemodynamics, and has been reported to reduce BPA–related complications,3 which has led to the guideline IIa recommendation of medical therapy prior to BPA.4 Prospective studies investigating efficacy and safety of medical treatment before BPA are needed, and ongoing (ClinicalTrials.gov Identifier NCT04780932).
The use of non-vitamin K antagonist oral anticoagulants (NOACs) vs. vitamin K antagonists in CTEPH may be associated with more clotting, but those data are not from a randomized study.56 Because bridging anticoagulation is associated with increased bleeding,57 the writing group members agreed on continuing vitamin K antagonists and titration to an INR of <3.0 prior to BPA.
Treatment goals
The gold standard of mechanical treatments of CTEPH is PEA, where the goal is to remove the obstructing material from the PA lumen as completely as possible in a single session. In contrast, BPA is delivered as multiple staged interventions, and obstructing material remains in place.38,58,59 Within the writing group, there was universal agreement that an ideal BPA treatment should result in symptom relief at rest and during exercise, treating all lesions whenever possible. Further goals are to improve quality of life, normalize pulmonary hemodynamics60 and exchange gas with a normal resting oxygen saturation. To assess individual treatments goals, a multiparametric approach utilizing exercise capacity, hemodynamics including pulmonary vascular resistance (PVR), biomarkers, World Health Organization classification, cardiopulmonary exercise testing, and quality of life assessments is practical. Because of the prognostic threshold of a mean pulmonary artery pressure (mPAP) of 30 mmHg,61 the minimum hemodynamic goal of BPA is a final mPAP < 30 mmHg. Technical aspects (type, localization, and number of accessible lesions), advanced age, comorbidities, and patient’s expectations may influence individual treatment goals. The writing group rejected a prespecified timeframe to complete BPA outside a study protocol but agreed that the interventional schedule should be adapted to patient and logistics. For example, two initial sessions are done within 1–3 days in a single hospital stay, and subsequent sessions planned after 1–3 months each.
Procedural details
BPA is performed in conscious patients under local anesthesia.
A right heart catheterization is useful before each BPA session, including thermodilution or Fick cardiac output (CO) and pressure assessments (right atrial pressure, PA pressure, and PA wedge pressure), off supplemental oxygen, and predominantly via the femoral vein with generous availability of ultrasound-guided venous puncture. Arterial pressure and saturation may be measured noninvasively.
Standard angiographic projections are anterior–posterior (AP) and left anterior oblique (LAO) 60° (Figure 1).
Contrast media are Iodixanol 320 or Iomeprol 50/50 mixed with saline, hand injected.
Two strategies of anticoagulation during BPA are practiced: 2000–5000 IU of unfractionated heparin, or full anticoagulation at an activated clotting time of 250 s as in coronary intervention.
Patients receive supplemental oxygen to maintain a saturation > 92% during BPA. Oxygen can selectively dilate pulmonary arteries.62
Each procedure is typically performed by two interventionists working together, with the goal to spend 30–60 min radiation time in one session.
Sheath size is 7F-9F with a telescoped 6F guiding catheter (mother and child technique, Figure 3); some prefer 8F/6F or 6F/6F or 8F/8F. Standard access to the lung segments is by multipurpose guiding catheter (MPA-1) or Judkins right (JR). Many different 0.014′ wires are used, usually hydrophilic wires, but some prefer hydrophobic silicone–coated wires (Miracle-3) that are shaped according to the need. In contrast to Japan (B-pahm), there are no dedicated BPA wires in Europe.
Semi-compliant balloons are used at 6 atm on average, clearly below rated burst pressures, including large sizes of 8–10 mm diameter over-the-wire balloons via 0.035′ guidewires.
Microcatheters and guide extension catheters are commonly used, while covered stents, coils, and gelfoam need to be available to manage complications. Occasionally, implantation of a stent is required to maintain the patency of a dilated lesion in a vessel with large intimal flaps.63,64
Initially, ring and web lesions are addressed, with a preference for the right basal artery, and CTOs are addressed when hemodynamics have improved. Immediate BPA success is suspected when brisk venous return post BPA is observed. If mPAP ≥ 40 mmHg and PVR ≥ 7 WU, only 2–2.5 mm balloons are safe. Only a minority practices pressure wire–guided angioplasty.65 Routine endovascular imaging with IVUS or OCT is not standard of care.
Complications and their management
Complications decrease with operator experience.25 It is essential to establish consensus on a classification of complications and on the reporting (Table 1). Complications should be reported both by procedure and by patient.
Table 1.
Complications of BPA
| Type of complication | Symptom/severity | Management | How to avoid | ||
|---|---|---|---|---|---|
| Thoracic complications | Hemoptysis during the procedure | • Mild (<50 mL/episode) • Moderate (100–300 mL) • Severe (more than 300 mL or hemoptysis of any volume leading to respiratory failure)66 |
⧠ No treatment ⧠ Reversal of anticoagulant ⧠ Prolonged balloon inflation ⧠ Embolization (e.g. injection of solubilized gelatine) ⧠ Others |
Use of guide extension for better visualization Careful manipulation of the guidewire (knuckle technique) Avoid over-dilatation |
|
| Vascular injury | Wire perforation | With or without hemoptysis | ⧠ Same as for hemoptysis | Careful manipulation of the guidewire (knuckle technique) | |
| Pulmonary artery rupture | ⧠ No treatment ⧠ Prolonged balloon inflation ⧠ Embolization (e.g. injection of solubilized gelatine) ⧠ Covered stent |
Avoid over-dilatation and vigorous contrast injection | |||
| Pulmonary artery wall dissection | • Non occlusive • Occlusive |
⧠ No treatment ⧠ Prolonged Balloon inflation ⧠ Embolization (e.g. injection of solubilized gelatine) ⧠ Stenting |
Use of guide extension for better visualization Careful manipulation of the guiding catheter Avoid over-dilatation |
||
| Lung injury | ⧠ Early < 3 h ⧠ Late > 3 h |
• Asymptomatic • Mild (nasal oxygen) • Moderate (Non-invasive ventilation) • Severe (mechanical ventilation ± VA ECMO) |
⧠ No treatment ⧠ Nasal oxygen ⧠ NIPPV ⧠ Mechanical ventilation ± VA ECMO |
Limited number of dilated sites Under-sizing of BPA balloon Pressure wire technique Pre-treatment with medical therapy in case of severe hemodynamic compromise |
|
| Others | Pulmonary artery thrombosis | Adjust anticoagulation Perform CDT |
Heparin per procedure | ||
| Pulmonary infection | Treat according to antibiogram | ||||
| Non-thoracic complications | Contrast allergy | • Mild (cutaneous)• Moderate (bronchospasm)• Severe (anaphylactic shock)67 | General management | Pre-medication with cortisone and antihistamines | |
| Associated with RHC | • Conduction disturbance• Supra-ventricular arrythmia• Ventricular arrythmia• Pericardial tamponade | Be careful in case of pre-existing left bundle branch block | |||
| Contrast nephropathy 68 | • AKI• AKD after AKI (staging based on GFR) | ⧠ No dialysis ⧠ Dialysis |
Use of diluted contrast media, use the least possible amount of contrast | ||
| Access site | • Hematoma requiring transfusion• False aneurysm• AV fistulas | ⧠ Manual compression ⧠ Side branch embolization ⧠ Covered stent ⧠ Surgery |
Ultrasound-guided puncture 4F micro-puncture |
||
NIPPV, non-invasive positive pressure ventilation; RHC, right heart catheterization; CDT, catheter-directed treatment for acute pulmonary thrombus; VA ECMO, veno-arterial ECMO; AKI, acute kidney injury; AKD, acute kidney disease after AKI.
Definition of complications
Non-specific complications such as allergic reaction, access-site complications, kidney injury, and infections do not exceed 1% in the literature.69
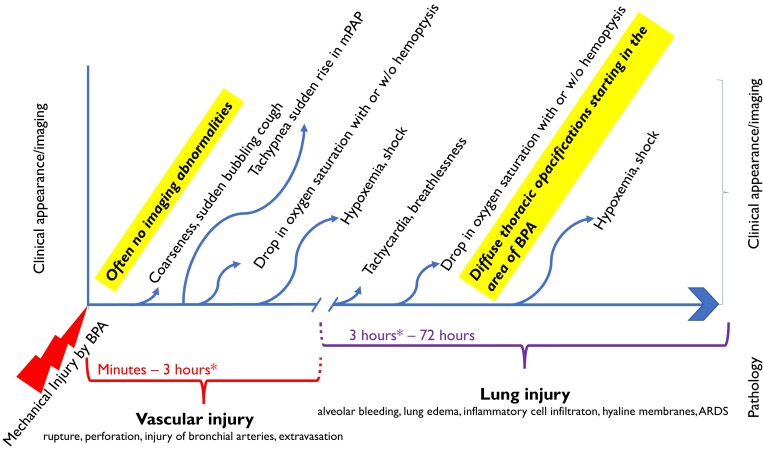
Specific complications or ‘thoracic complications’ are those related to the properties of the BPA substrate, the pulmonary arteries, and the underlying pathology (Table 1). Grades of injury after BPA are depicted in Figure 5. BPA–related vascular injury was shown to be an independent predictor of LI after BPA (odds ratio, 20.1)70 but does not influence the overall outcome of BPA.71 Reperfusion edema usually develops several hours after the procedure, with clinical signs of desaturation and foamy sputum. The group agreed that LI predominantly originates from vascular injury70 and that LI as pure reperfusion injury may be rare.
Figure 5.
Grades of injury after BPA. Time is displayed on the x-axis and clinical appearance, pathology, and imaging on the y-axis. Three hours after the start of the BPA procedure (asterisk) is arbitrary to represent the peri-procedural time frame that may vary.
Sudden cough, drop in oxygen saturation and hemoptysis are the most common symptoms of a complication during BPA. Most frequently mild to moderate, hemoptysis can resolve quickly, but it requires immediate attention and search for a cause.
-
1.1. Vascular injury due to vascular complications is observed during the procedure (Table 1 and Figure 5). Distal perforation by the angioplasty guidewire is commonly accompanied by hemoptysis with onset of bleeding with a few minutes of delay from the injury. Management depends on its mechanism and how hypoxemia is acutely tolerated by the patient.
The first step in persistent hemoptysis is halting the procedure
and starting an angiographic search for the injury
Oxygen therapy must be adapted to desaturation, and
prolonged inflation of the balloon at the suspected angioplasty site, or
immediate wedging of the guiding catheter, or if hemoptysis persists,
heparin anticoagulation is reversed with protamine.
Distal embolization can be performed, preferably using resorbable material (absorbable gelatin sponges, e.g. Serescue; Astellas Pharma Inc., Tokyo, Japan or CuraSpon®),
or a covered or uncovered stent can be placed as bailout in case of rupture. In addition,
continuous positive airway pressure is useful, sometimes
intubation
or ECMO may be needed.
PA dissection at the angioplasty site or by tip of the guide catheter is benign in the majority of cases. In the large vessel segments, PA dissection may reflect contrast medium entering deep layers of thrombus. Arterial rupture is uncommon, and thrombosis at the dilated site is rarely reported.72
1.2. LI is the main severe complication (Table 1 and Figure 5). It is defined by new ground-glass opacities, consolidation, and pleural effusion70 in the territory of dilated vessels, with or without hemoptysis, with or without hypoxemia.73 LI is commonly detected if routine chest scan is carried out; however, clinical consequences are limited to relatively few cases. Ikeda et al.74 performed CT scanning within 15 min of completion of 119 BPA sessions, identifying signs of LI in 10% of simple lesion BPA, and in 40% of occlusion lesion BPA. Most often, LI appears > 3 h after BPA with deterioration of the respiratory state (Figure 5). This is why at least one overnight stay after BPA is felt to be safe. In asymptomatic patients, further imaging is not needed before leaving the hospital. There are different degrees of severity: mild (with a need for oxygen inhalation via prongs), moderate (with a need for high-flow oxygen via face mask), and severe (requiring non-invasive positive pressure ventilation or mechanical ventilation +/− ECMO) (Table 1). Mortality rate varies from 0% to 10%, with an average of ∼2% (see Supplementary data online, Table S1). Deaths are directly attributable to pulmonary injury in more than half of the cases; less commonly, death occurs due to right heart failure or sepsis.
2. Predictors of specific complications
Lesion types (Figure 2)75 predict complications: Complication rates are <3% for ring-like stenosis and web lesions, up to 15.5% for subtotal occlusions, and up to 40% for tortuous lesions.75 As more chronic total occlusions are addressed, new information on procedural complications will be observed, although it does not appear that CTO interventions increase complication rates.45,76
Hemodynamic parameters such as a high mPAP before BPA consistently represent independent risk factors of severe LI.25,45,70,71,77 In the RACE study,3 piecewise logistic regression identified mean PAP > 45 mmHg as predictive factor associated with BPA–related adverse events and SAE in 88 patients who had BPA at any time. Therefore, a threshold mPAP increasing the incidence of LI is around 45 mmHg.
There is a BPA learning curve, with experienced centers observing a significant reduction in adverse event rates with more practice.71 In the French registry, complication rates fell from 11.2% per session in the first 1006 sessions to 7.7% in the more recent 562 sessions.25
3. How to avoid BPA complications
Refinements of the BPA technique have decreased complications. The following practical rules have been developed based on the Japanese experience,7 particularly in case of mPAP ≥ 40 mmHg: (ii) treat simple lesions, rings, and webs first; (ii) verify proper guidewire placement; (iii) undersize balloons (balloon:artery ratio 1:0.5–0.8, or 2–2.5 mm balloons for the first session); and (iv) consider using the pressure wire technique to assess distal pressure (should remain <30 mmHg).41,65 IVUS and/or OCT can be helpful to determine exact vessel diameter and identify dissections, thrombus, and intraluminal calcification.8
Given the data that complications are more severe among patients with very high pulmonary pressures, there is a logic to optimize medical therapy prior to BPA. In the RACE study,3 less severe hemodynamic compromise was recorded at the time of BPA in patients pretreated with riociguat than in patients treated first with BPA. Consequently, the incidence of BPA–related SAEs was lower in patients who were pretreated with riociguat [adverse events in 5 (14%) of 36 pretreated patients vs. 22 (42%) of 52 non-pretreated patients]. IMPACT-CTEPH is formally addressing this question by assessing the efficacy of macitentan on top of standard riociguat on pre-BPA PVR %baseline change in patients with inoperable CTEPH (ClinicalTrials.gov Identifier NCT04780932).
Despite the relatively high contrast volume used during the entire BPA treatment cycle, BPA can be safely performed in patients with chronic kidney disease, and renal function may be improved with an increase in CO.78,79 Radiation exposure (Air Kerma, effective dose, fluoroscopy time, and Kerma area product) needs to be rigorously collected for each patient.
BPA outcomes and patient follow-up
Long-term follow-up is guideline recommended for CTEPH4
BPA leads to a significant decrease of right ventricular afterload (see Supplementary data online, Table S1, and Graphical Abstract). The initial case series from the US reported a 23% decrease in total PVR, a 21% decrease in mPAP, and a 5% increase in CO after BPA.18 BPA interventionists from Europe and US reported an approximately 42%–45% decrease in PVR with a 18%–29% decrease in mPAP after refined BPA.12,25,80–85 More recent European series reported a decrease of PVR by 34%–60% and of mPAP by 23%–44%.30,45,76,86–91 The Japanese registry reported a 66% decrease in PVR and a 48% decrease in mPAP.92 The results obtained in Japan tend to be better than those in other countries,7,65 which may be due to the more extensive experience of operators, patient selection in light of less PEA activity, and the different structure of vascular lesions with a less inflammatory thrombotic phenotype.93 The most recent randomized controlled trials in two expert BPA centers reported a 65% decrease of PVR, a 40% decrease of mPAP, and a 10% increase of CO after BPA.2,3 While vasodilators mainly increase CO, BPA decreases mPAP, resulting in lower PVR. Therefore, BPA success has to be reported together with ongoing vasodilator treatments. Hemodynamic improvement is more pronounced when complete pulmonary revascularization is achieved, including treatment of chronic total occlusions.45,76 For patients with central clots, the decrease in PVR that can be achieved with BPA may be like in patients with distal lesions, but this needs to be confirmed in larger prospective studies.30
Hemodynamic improvement after BPA correlates with significant improvements in clinical status, right ventricular function,94 and patient-reported quality of life.95,96 In Feinstein’s series, follow-up (mean, 36 months) mPAP, NYHA functional class, and 6-minute walking distance (6MWD) had improved, and all vessels previously dilated were patented at angiographic reassessment.18 Clinical benefit of BPA was demonstrated by improvements in functional class, 6MWD, and an increase in peak oxygen consumption and a decrease in VE/VCO2, and PETCO2.97,98 A significant reduction in NT-proBNP levels occurs,39,41,53,80,85,88,90 an improvement in right ventricular function by echo,99 and cardiac magnetic resonance imaging,79,100–102 electrical reverse remodeling by ECG,103 and an increase in pulmonary vascular compliance.53,104 BPA in elderly patients is equally effective.39,105 3- and 5-year survival rates were reported as 92%–95%25,45,92 and 88%–90%,12,30 respectively.
The efficacy of BPA procedures as an adjunctive treatment after PEA has been evaluated in several small studies indicating that BPA is feasible,106 but its effectiveness appears to be lower, and the risk of complications is higher than in untreated patients.9–11,50,51 Significant symptomatic improvement after BPA was demonstrated in CTEPD patients without PH at rest.54,107 Perfusion scans or DSA perfusion is helpful to identify residual lesions. Regardless of the result of BPA, guidelines recommend that patients should be regularly followed-up with functional class, NT-proBNP, echo, and 6MWD, including invasive assessment with right heart catheterization 3–6 months after last BPA.4
Post BPA treatments
After BPA, oral anticoagulation is continued life-long. Existing information on the type of anticoagulation does not suggest a clear advantage of either NOACs or vitamin K antagonists.56 In antiphospholipid syndrome, vitamin K antagonists are prescribed.108 3–6 months after BPA or at any time after BPA if symptoms recur, a right heart catheterization is guideline recommended to assess the need for continuation or termination or initiation of medical treatments approved for CTEPH. For the decision to continue or terminate or initiate medical treatments including riociguat, a multiparametric approach can be used, taking into account patient needs and symptoms during their regular life after BPA, the number of remaining lesions, exercise capacity, hemodynamics, arterial saturation, and response to ongoing or previous medical treatments.
Cost of BPA
No cost effectiveness studies have been done so far, and there are no comparative studies with PEA. One study analyzed the treatment cost in France.109 Authors found that the first BPA session was performed 1.1 years (IQR 0.3–2.92) after the first PH hospitalization. A mean of three stays per patient with BPA sessions was reported. The total hospital cost attributable to BPA was €21 245 ± 12 843 per patient.
Patient needs
BPA is a novel treatment and patients wish to hear about the concept, the expected number of procedures, the speed of improvements, the likelihood of an ongoing need for medical treatments, and the risk of hemoptysis or a SAE during the procedures. While more severe hemodynamics at baseline classify a higher risk category,25,45 no systematic BPA risk scores have yet been established.
What helps patients gain confidence is that they could watch the procedure on a monitor and see the blood vessels opening up after each step of the treatment. While physicians’ guidance during BPA is valuable, a structured brochure or video informing the patient about important details of BPA beforehand is desirable.
Patients report that they improve immediately after the first BPA procedure and that they can breathe better and deeper. Further improvements thereafter were also noted to be significant.
Patients want to speak to their physician and set goals together. For example, if the patient is an elderly lady, she may be content with less procedures, compared with a young man who wants to compete in sports and needs even the smallest pulmonary vessel open.
Conclusions and future steps
The 2022 ESC/ERS guidelines have upgraded their recommendation for BPA from IIb-C in 2015 to I-B. While this reflects increased understanding of its role in CTEPH treatment over the past 10 years, many open issues remain. For example, there is a need for appropriate training standards in high-volume centers, standardized technique to be globally adopted, and development of dedicated interventional equipment. Patient selection remains a significant challenge despite the use of multidisciplinary CTEPH teams, with eligibility for BPA decided on the basis of a consensus among team members. With scarce information on long-term survival, treatment goals need clarification, as it is uncertain to which degree mPAP and PVR lowering leads to improved outcomes. Many patients have mixed anatomical lesions with lobar, segmental, and microvascular involvement, but presently, there is no standardized approach to combination therapy with PEA, BPA, and pharmacological therapy. Future research should include whether PH-targeted medication prior to BPA is beneficial, whether combination treatments are successful, whether medication should be continued after completion of BPA and whether PEA and BPA should be performed simultaneous,19or sequentially. Unmet needs are risk scores for patients undergoing BPA, to determine critical target lesions and a hemodynamic threshold necessary to normalize prognosis, to determine whether post PEA patients have equivalent outcomes as primary BPA candidates. The International BPA Registry (NCT03245268) and the Japan Multicenter Registry for BPA will play important roles to answer some of the open questions.
Supplementary Material
Contributor Information
Irene M Lang, Department of Internal Medicine II, Cardiology, and Comprehensive Center of Cardiovascular Medicine CCVM, Medical University of Vienna, Währinger Gürtel 18-20, Vienna A-1090, Austria.
Arne K Andreassen, Department of Cardiology, Oslo University Hospital Rikshospitalet, Pb 4950 Nydalen, 0424 Oslo, Norway.
Asger Andersen, Aarhus University Hospital, Palle Juul Jensens Boulevard 99 8200 Aarhus N, Denmark.
Helene Bouvaist, Cardiology Department, Grenoble - Alpes University Hospital, 38043 Grenoble, France.
Gerry Coghlan, Royal Free Hospital, London, Pond Street, Middlesex, London, NW3 2QG.
Pilar Escribano-Subias, Hospital Universitario 12 de octubre, Avda de Cordoba SN, 28041 Madrid, Spain.
Pavel Jansa, General University Hospital, U Nemocnice 2, 128 08 Prague 2, Czech Republic.
Grzegorz Kopec, Pulmonary Circulation Centre Jagiellonian University Medical College, John Paul II Hospital in Krakow, Pradnicka Str. 80, 31-202 Krakow.
Marcin Kurzyna, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology Centre of Postgraduate Medical Education, EHC Otwock, Borowa 14/18, Otwock 05-400, Poland.
Hiromi Matsubara, Department of Cardiology, National Hospital Organization Okayama Medical Center, 1711-1 Tamasu, Kita-ku, Okayama 701–1192, Japan.
Bernhard Christian Meyer, Medizinische Hochschule Hannover - Institut für Diagnostische und Interventionelle Radiologie, Carl-Neuberg-Str. 1, 30625 Hannover.
Massimiliano Palazzini, Dipartimento DIMEC (Dipartimento di Scienze Mediche e Chirurgiche), Università di Bologna, 40126 Bologna, Italy.
Marco C Post, Department of Cardiology, St. Antonius Hospital, Koekoekslaan 1, 3435 CM, Nieuwegein, The Netherlands; Department of Cardiology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX, Utrecht, The Netherlands.
Piotr Pruszczyk, Department of Internal Medicine and Cardiology, Medical University of Warsaw, Zwirki I Wigury 61, 02-091 Warsaw, Poland.
Lorenz Räber, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern Freiburgstrasse 18 3010 Bern, Switzerland.
Marek Roik, Department of Internal Medicine and Cardiology, Medical University of Warsaw, Zwirki I Wigury 61, 02-091 Warsaw, Poland.
Stephan Rosenkranz, Dept. of Cardiology and Cologne Cardiovascular Research Center, Heart Center at the University Hospital Cologne, Kerpener Str. 62, 50937 Köln, Germany.
Christoph B Wiedenroth, Department of Thoracic Surgery, Kerckhoff Heart and Thorax Centre, Benekestrasse 2-8, 61231 Bad Nauheim, Germany.
Carlo Redlin-Werle, Department of Internal Medicine II, Cardiology, and Comprehensive Center of Cardiovascular Medicine CCVM, Medical University of Vienna, Währinger Gürtel 18-20, Vienna A-1090, Austria.
Philippe Brenot, Interventional Radiology Department, Marie Lannelongue Hospital, Le Plessis Robinson 92350, France.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
I.M.L. has relationships with drug companies including AOP-Health, Actelion-Janssen, MSD, United Therapeutics, Medtronic, Neutrolis, and Ferrer. In addition to being an investigator in trials involving these companies, relationships include consultancy service, research grants to the institution, and membership of scientific advisory boards. A.K.A. reports grants, consulting fees, and honoraria from Janssen and Nordic Infucare. A.K.A. is a consultant of Magneto Thrombectomy Solutions and Inari Medical, received teaching honoraria from Angiodynamics and Janssen Pharmaceuticals, and is a proctor for EPS vascular, ABBOTT, and Gore. H.B. has nothing to declare. G.C. has received funding for studies and as a speaker from Janssen, from VicorePharma Ltd. for consultancy, and from MSD as a speaker. P.E.-S. reports grants from Janssen; speaker fees from Janssen, MSD, FERRER, and AOP-Health; board activities with Janssen, MSD, FERRER, and AOP-Health; and support for meetings from Janssen and MSD. P.J. has received fees and grants from Janssen Pharmaceutical Companies of AOP Orphan and MSD. G.K. has received fees for lectures and/or consultations from AOP Health, Bayer, Ferrer, Janssen, and MSD. M.K. reports no relevant conflicts with regard to this work. H.M. reports grants from Nippon Shinyaku and fees from Bayer, Janssen, Mochida, Nippon Shinyaku, Kaneka Medix, and Nipro and participated in DSMBs from Bayer and Janssen. B.C.M. reports no relevant conflicts with regard to this work. M.P. received payments for lectures from Janssen. M.C.P. received speaker fees from Johnson & Johnson and MSD and Research grants from St. Antonius hospital research fund, ZonMw, and Johnson & Johnson. P.P. received consulting fees from Bayer, MSD, and Pfizer; payments from MSD, Bayer, and Pfizer; and meeting support from Pfizer. L.R. received research grants to the institution from Abbott, Biotronik, BostonScientific, Heartflow, Sanofi, and Regeneron; consulting fees from Abbott, Amgen, AstraZeneca, Canon, Medtronic, NovoNordisc, Occlutech, and Sanofi; and payments or honoraria from Abbott, Amgen, AstraZeneca, Canon, Medtronic, NovoNordisc, Occlutech, and Sanofi. M.R. has received honoraria from Boston Sci. S.R. reports grants from Actelion, Astra, Bayer, and Janssen; consulting fees from Abbott, Acceleron, Actelion, Altavant, AOP-Heath, Astra, Bayer, Ferrer, Gossamer, Janssen, MSD, and UT; and honoraria from Abbott, Actelion, AOP-Heath, Astra, Bayer, Boehringer-Ingelheim, Edwards, Ferrer, Janssen, MSD, OMT, Vifor, and UT; C.B.W. has received speaker fees from Actelion, MSD, and OrphaCare and honoraria from Actelion, AOP Orphan Pharmaceuticals AG, Bayer AG, BTG, MSD, and Pfizer. C.R.-W. has nothing to declare. P.B. has nothing to declare.
Data Availability
All data are incorporated into the online supplementary material.
Funding
All authors declare no funding for this contribution.
References
- 1. Kennedy MK, Kennedy SA, Tan KT, de Perrot M, Bassett P, McInnis MC, et al. . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2023;46:5–18. 10.1007/s00270-022-03323-8 [DOI] [PubMed] [Google Scholar]
- 2. Kawakami T, Matsubara H, Shinke T, Abe K, Kohsaka S, Hosokawa K, et al. . Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open-label, randomised controlled trial. Lancet Respir Med 2022;10:949–960. 10.1016/S2213-2600(22)00171-0 [DOI] [PubMed] [Google Scholar]
- 3. Jaïs X, Brenot P, Bouvaist H, Jevnikar M, Canuet M, Chabanne C, et al. . Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med 2022;10:961–971. 10.1016/S2213-2600(22)00214-4 [DOI] [PubMed] [Google Scholar]
- 4. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. . 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618–3731. 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 5. Fukuda K, Date H, Doi S, Fukumoto Y, Fukushima N, Hatano M, et al. . Guidelines for the treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017). Circ J 2019;83:842–945. 10.1253/circj.CJ-66-0158 [DOI] [PubMed] [Google Scholar]
- 6. Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, et al. . Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:756–762. 10.1161/CIRCINTERVENTIONS.112.971390 [DOI] [PubMed] [Google Scholar]
- 7. Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:748–755. 10.1161/CIRCINTERVENTIONS.112.971077 [DOI] [PubMed] [Google Scholar]
- 8. Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, Aoki T, et al. . Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012;76:485–488. 10.1253/circj.CJ-11-1217 [DOI] [PubMed] [Google Scholar]
- 9. Shimura N, Kataoka M, Inami T, Yanagisawa R, Ishiguro H, Kawakami T, et al. . Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2015;183:138–142. 10.1016/j.ijcard.2015.01.034 [DOI] [PubMed] [Google Scholar]
- 10. Yanaka K, Nakayama K, Shinke T, Shinkura Y, Taniguchi Y, Kinutani H, et al. . Sequential hybrid therapy with pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Am Heart Assoc 2018;7:e008838. 10.1161/JAHA.118.008838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Araszkiewicz A, Darocha S, Pietrasik A, Pietura R, Jankiewicz S, Banaszkiewicz M, et al. . Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2019;278:232–237. 10.1016/j.ijcard.2018.10.066 [DOI] [PubMed] [Google Scholar]
- 12. Poch DS, Mahmud E, Patel M, Papamatheakis D, Fernandes T, Kerr K, et al. . Patient selection for balloon pulmonary angioplasty: six-year results from a high volume PTE surgical center. Pulm Circ 2022;12:e12148. 10.1002/pul2.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siennicka A, Darocha S, Banaszkiewicz M, Kędzierski P, Dobosiewicz A, Błaszczak P, et al. . Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team. Ther Adv Respir Dis 2019;13:1753466619891529. 10.1177/1753466619891529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taniguchi Y, Jaïs X, Jevnikar M, Boucly A, Weatherald J, Brenot P, et al. . Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2019;38:833–842. 10.1016/j.healun.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 15. Miwa H, Tanabe N, Jujo T, Kato F, Anazawa R, Yamamoto K, et al. . Long-Term outcome of chronic thromboembolic pulmonary hypertension at a single Japanese pulmonary endarterectomy center. Circ J 2018;82:1428–1436. 10.1253/circj.CJ-17-1242 [DOI] [PubMed] [Google Scholar]
- 16. Humbert M, Galié N, Meszaros G. Competency requirements for ERN-lung PH centres. 2021. https://ern-lung.eu/inhalt/wp-content/uploads/2020/10/PH-MCC.pdf.
- 17. Voorburg JA, Cats VM, Buis B, Bruschke AV. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest 1988;94:1249–1253. 10.1378/chest.94.6.1249 [DOI] [PubMed] [Google Scholar]
- 18. Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001;103:10–13. 10.1161/01.CIR.103.1.10 [DOI] [PubMed] [Google Scholar]
- 19. Wiedenroth CB, Liebetrau C, Breithecker A, Guth S, Lautze HJ, Ortmann E, et al. . Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2016;35:591–596. 10.1016/j.healun.2015.10.030 [DOI] [PubMed] [Google Scholar]
- 20. NOMENCLATURE Of broncho-pulmonary anatomy; an international nomenclature accepted by the thoracic society. Thorax 1950;5:222–228. 10.1136/thx.5.3.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kandathil A, Chamarthy M. Pulmonary vascular anatomy & anatomical variants. Cardiovasc Diagn Ther 2018;8:201–207. 10.21037/cdt.2018.01.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimokawahara H, Ogawa A, Mizoguchi H, Yagi H, Ikemiyagi H, Matsubara H. Vessel stretching is a cause of lumen enlargement immediately after balloon pulmonary angioplasty: intravascular ultrasound analysis in patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2018;11:e006010. 10.1161/CIRCINTERVENTIONS.117.006010 [DOI] [PubMed] [Google Scholar]
- 23. Räber L, Ueki Y, Lang IM. Balloon pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. EuroIntervention 2019;15:e814–e815. 10.4244/EIJ-D-18-01212 [DOI] [PubMed] [Google Scholar]
- 24. Jansa P, Ambrož D, Aschermann M, Černý V, Dytrych V, Heller S, et al. . Hospitalisation is prognostic of survival in chronic thromboembolic pulmonary hypertension. J Clin Med 2022;11:6189. 10.3390/jcm11206189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brenot P, Jais X, Taniguchi Y, Garcia Alonso C, Gerardin B, Mussot S, et al. . French Experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1802095. 10.1183/13993003.02095-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martínez-Santos P, Velázquez-Martín MT, Barberá JA, Fernández Pérez C, López-Meseguer M, López-Reyes R, et al. . Chronic thromboembolic pulmonary hypertension in Spain: a decade of change. Rev Esp Cardiol (Engl Ed) 2021;74:384–392. 10.1016/j.rec.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 27. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2018;40:87–165. 10.1093/eurheartj/ehy394 [DOI] [Google Scholar]
- 28. Bednarczuk T, Brix TH, Schima W, Zettinig G, Kahaly GJ. 2021 European Thyroid Association guidelines for the management of iodine-based contrast media-induced thyroid dysfunction. Eur Thyroid J 2021;10:269–284. 10.1159/000517175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quadery SR, Swift AJ, Billings CG, Thompson AAR, Elliot CA, Hurdman J, et al. . The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur Respir J 2018;52:1800589. 10.1183/13993003.00589-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darocha S, Araszkiewicz A, Kurzyna M, Banaszkiewicz M, Jankiewicz S, Dobosiewicz A, et al. . Balloon pulmonary angioplasty in technically operable and technically inoperable chronic thromboembolic pulmonary hypertension. J Clin Med 2021;10:1038. 10.3390/jcm10051038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishihara T, Shimokawahara H, Ogawa A, Naito T, Une D, Mukai T, et al. . Comparison of the safety and efficacy of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension patients with surgically accessible and inaccessible lesions. J Heart Lung Transplant 2023;42:786–794. 10.1016/j.healun.2023.01.003 [DOI] [PubMed] [Google Scholar]
- 32. Barco S, Klok FA, Konstantinides SV, Dartevelle P, Fadel E, Jenkins D, et al. . Sex-specific differences in chronic thromboembolic pulmonary hypertension. Results from the European CTEPH Registry. J Thromb Haemost 2020;18:151–161. 10.1111/jth.14629 [DOI] [PubMed] [Google Scholar]
- 33. Cruz-Utrilla A, Cristo-Ropero MJ, Calderón-Flores M, Velázquez M, López-Gude MJ, Revilla Ostolaza Y, et al. . Sex differences in chronic thromboembolic pulmonary hypertension. Treatment options over time in a national referral center. J Clin Med 2021;10:4251. 10.3390/jcm10184251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kerr KM, Elliott CG, Chin K, Benza RL, Channick RN, Davis RD, et al. . Results from the United States Chronic Thromboembolic Pulmonary Hypertension Registry: enrollment characteristics and 1-year follow-up. Chest 2021;160:1822–1831. 10.1016/j.chest.2021.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shigeta A, Tanabe N, Shimizu H, Hoshino S, Maruoka M, Sakao S, et al. . Gender differences in chronic thromboembolic pulmonary hypertension in Japan. Circ J 2008;72:2069–2074. 10.1253/circj.CJ-08-0377 [DOI] [PubMed] [Google Scholar]
- 36. Guth S, D'Armini AM, Delcroix M, Nakayama K, Fadel E, Hoole SP, et al. . Current strategies for managing chronic thromboembolic pulmonary hypertension: results of the worldwide prospective CTEPH Registry. ERJ Open Res 2021;7:00850-2020. 10.1183/23120541.00850-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Velázquez MM, Maneiro Melón N, Albarrán González-Trevilla A, Sarnago Cebada F, Huertas Nieto S, Cruz-Utrilla A, et al. . Balloon pulmonary angioplasty can be an effective and safe therapeutic option in non-surgical elderly patients. Front Cardiovasc Med 2022;9:1001518. 10.3389/fcvm.2022.1001518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ikeda N, Iijima R, Hara H, Hiroi Y, Nakamura M. Preprocedural frailty is strongly associated with symptoms after balloon pulmonary angioplasty. Glob Health Med 2022;4:45–51. 10.35772/ghm.2021.01019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roik M, Wretowski D, Łabyk A, Irzyk K, Lichodziejewska B, Dzikowska-Diduch O, et al. . Refined balloon pulmonary angioplasty-A therapeutic option in very elderly patients with chronic thromboembolic pulmonary hypertension. J Interv Cardiol 2017;30:249–255. 10.1111/joic.12387 [DOI] [PubMed] [Google Scholar]
- 40. Yamagata Y, Ikeda S, Nakata T, Yonekura T, Koga S, Muroya T, et al. . Balloon pulmonary angioplasty is effective for treating peripheral-type chronic thromboembolic pulmonary hypertension in elderly patients. Geriatr Gerontol Int 2018;18:678–684. 10.1111/ggi.13224 [DOI] [PubMed] [Google Scholar]
- 41. Roik M, Wretowski D, Labyk A, Kostrubiec M, Irzyk K, Dzikowska-Diduch O, et al. . Refined balloon pulmonary angioplasty driven by combined assessment of intra-arterial anatomy and physiology–multimodal approach to treated lesions in patients with non-operable distal chronic thromboembolic pulmonary hypertension–technique, safety and efficacy of 50 consecutive angioplasties. Int J Cardiol 2016;203:228–235. 10.1016/j.ijcard.2015.10.116 [DOI] [PubMed] [Google Scholar]
- 42. Roik M, Wretowski D, Kostrubiec M, Dzikowska-Diduch O, Łabyk A, Irzyk K, et al. . High prevalence of severe coronary artery disease in elderly patients with non-operable chronic thromboembolic pulmonary hypertension referred for balloon pulmonary angioplasty. Postepy Kardiol Interwencyjnej 2016;12:355–359. 10.5114/aic.2016.63637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hinrichs JB, von Falck C, Hoeper MM, Olsson KM, Wacker FK, Meyer BC, et al. . Pulmonary artery imaging in patients with chronic thromboembolic pulmonary hypertension: comparison of cone-beam CT and 64-row multidetector CT. J Vasc Interv Radiol 2016;27:361–368. 10.1016/j.jvir.2015.11.046 [DOI] [PubMed] [Google Scholar]
- 44. Ang L, McDivit Mizzell A, Daniels LB, Ben-Yehuda O, Mahmud E. Optimal technique for performing invasive pulmonary angiography for chronic thromboembolic pulmonary disease. J Invasive Cardiol 2019;31:E211–e219. [PubMed] [Google Scholar]
- 45. Gerges C, Friewald R, Gerges M, Shafran I, Sadushi-Koliçi R, Skoro-Sajer N, et al. . Efficacy and safety of percutaneous pulmonary artery subtotal occlusion and chronic total occlusion intervention in chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2021;14:e010243. 10.1161/CIRCINTERVENTIONS.120.010243 [DOI] [PubMed] [Google Scholar]
- 46. Gerges C, Gerges M, Friewald R, Fesler P, Dorfmuller P, Sharma S, et al. . Microvascular disease in chronic thromboembolic pulmonary hypertension: hemodynamic phenotyping and histomorphometric assessment. Circulation 2020;141:376–386. 10.1161/CIRCULATIONAHA.119.041515 [DOI] [PubMed] [Google Scholar]
- 47. Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, et al. . Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom National Cohort. Circulation 2016;133:1761–1771. 10.1161/CIRCULATIONAHA.115.019470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D'Armini AM, Snijder R, et al. . Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation 2016;133:859–871. 10.1161/CIRCULATIONAHA.115.016522 [DOI] [PubMed] [Google Scholar]
- 49. Palazzini M, Saia F, Taglieri N, Guarino D, Rotunno M, Galiѐ N, et al. . Balloon pulmonary angioplasty after pulmonary thromboendarterectomy. Ann Cardiothorac Surg 2022;11:192–194. 10.21037/acs-2021-pte-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andersen A, Hansen JV, Dragsbaek SJ, Maeng M, Andersen MJ, Andersen G, et al. . Balloon pulmonary angioplasty for patients with chronic thromboembolic pulmonary hypertension previously operated by pulmonary endarterectomy. Pulm Circ 2022;12:e12115. 10.1002/pul2.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ito R, Yamashita J, Sasaki Y, Ikeda S, Suzuki S, Murata N, et al. . Efficacy and safety of balloon pulmonary angioplasty for residual pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2021;334:105–109. 10.1016/j.ijcard.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 52. Coghlan JG. Balloon pulmonary angioplasty: does it have a role in CTED? Pulm Circ 2018;8:2045893218754887. 10.1177/2045893218754887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wiedenroth CB, Ghofrani HA, Adameit MSD, Breithecker A, Haas M, Kriechbaum S, et al. . Sequential treatment with riociguat and balloon pulmonary angioplasty for patients with inoperable chronic thromboembolic pulmonary hypertension. Pulm Circ 2018;8:2045894018783996. 10.1177/2045894018783996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Inami T, Kataoka M, Kikuchi H, Goda A, Satoh T. Balloon pulmonary angioplasty for symptomatic chronic thromboembolic disease without pulmonary hypertension at rest. Int J Cardiol 2019;289:116–118. 10.1016/j.ijcard.2019.04.080 [DOI] [PubMed] [Google Scholar]
- 55. Held M, Kolb P, Grün M, Jany B, Hübner G, Grgic A, et al. . Functional characterization of patients with chronic thromboembolic disease. Respiration 2016;91:503–509. 10.1159/000447247 [DOI] [PubMed] [Google Scholar]
- 56. Humbert M, Simonneau G, Pittrow D, Delcroix M, Pepke-Zaba J, Langleben D, et al. . Oral anticoagulants (NOAC and VKA) in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2022;41:716–721. 10.1016/j.healun.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 57. Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, et al. . Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015;373:823–833. 10.1056/NEJMoa1501035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kataoka M, Inami T, Kawakami T, Fukuda K, Satoh T. Balloon pulmonary angioplasty (percutaneous transluminal pulmonary angioplasty) for chronic thromboembolic pulmonary hypertension: a Japanese perspective. JACC Cardiovasc Interv 2019;12:1382–1388. 10.1016/j.jcin.2019.01.237 [DOI] [PubMed] [Google Scholar]
- 59. Coghlan JG, Rothman AM, Hoole SP. Balloon pulmonary angioplasty: state of the art. Interv Cardiol 2020;16:e02. 10.15420/icr.2020.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shinkura Y, Nakayama K, Yanaka K, Kinutani H, Tamada N, Tsuboi Y, et al. . Extensive revascularisation by balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension beyond haemodynamic normalisation. EuroIntervention 2018;13:2060–2068. 10.4244/EIJ-D-17-00157 [DOI] [PubMed] [Google Scholar]
- 61. Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81:151–158. 10.1378/chest.81.2.151 [DOI] [PubMed] [Google Scholar]
- 62. Shigetoshi M, Hatanaka K, Ogawa A, Tabuchi I, Shimokawahara H, Munemasa M, et al. . Oxygen inhalation can selectively dilate pulmonary arteries in patients with chronic thromboembolic pulmonary hypertension before balloon angioplasty. J Cardiol 2022;79:265–269. 10.1016/j.jjcc.2021.09.003 [DOI] [PubMed] [Google Scholar]
- 63. Darocha S, Pietura R, Banaszkiewicz M, Pietrasik A, Kownacki Ł, Torbicki A, et al. . Balloon pulmonary angioplasty with stent implantation as a treatment of proximal chronic thromboembolic pulmonary hypertension. Diagnostics (Basel) 2020;10:363. 10.3390/diagnostics10060363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cao Y, Singh V, Jiang N, Wei R, Jiang K, Wang H. Stenting for pulmonary artery stenosis resistant to balloon pulmonary angioplasty in chronic thrombo-embolic pulmonary hypertension: a bail out strategy. Eur Heart J Case Rep 2021;5:ytab071. 10.1093/ehjcr/ytab071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Inami T, Kataoka M, Shimura N, Ishiguro H, Yanagisawa R, Fukuda K, et al. . Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: a breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv 2014;7:1297–1306. 10.1016/j.jcin.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 66. Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 2017;23:307–317. 10.5152/dir.2017.16454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chiu TM, Chu SY. Hypersensitivity reactions to iodinated contrast Media. Biomedicines 2022;10:1036. 10.3390/biomedicines10051036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lameire NH, Levin A, Kellum JA, Cheung M, Jadoul M, Winkelmayer WC, et al. . Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int 2021;100:516–526. 10.1016/j.kint.2021.06.028 [DOI] [PubMed] [Google Scholar]
- 69. Nef HM, Achenbach S, Birkemeyer R, Bufe A, Dörr O, Elsässer A, et al. . Manual der arbeitsgruppe interventionelle kardiologie (AGIK) der deutschen gesellschaft für kardiologie—herz- und kreislaufforschung e. V. (DGK). Der Kardiologe Volume 2021;15:542–584. 10.1007/s12181-021-00504-6 [DOI] [Google Scholar]
- 70. Ejiri K, Ogawa A, Fujii S, Ito H, Matsubara H. Vascular injury is a Major cause of lung injury after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2018;11:e005884. 10.1161/CIRCINTERVENTIONS.117.005884 [DOI] [PubMed] [Google Scholar]
- 71. Wiedenroth CB, Deissner H, Adameit MSD, Kriechbaum SD, Ghofrani HA, Breithecker A, et al. . Complications of balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: impact on the outcome. J Heart Lung Transplant 2022;41:1086–1094. 10.1016/j.healun.2022.05.002 [DOI] [PubMed] [Google Scholar]
- 72. Minatsuki S, Takahara M, Kiyosue A, Kodera S, Hatano M, Ando J, et al. . Characteristics and in-hospital outcomes of patients undergoing balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: a time-trend analysis from the Japanese nationwide registry. Open Heart 2021;8:e001721. 10.1136/openhrt-2021-001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, et al. . Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1801915. 10.1183/13993003.01915-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ikeda N, Kubota S, Okazaki T, Iijima R, Hara H, Hiroi Y, et al. . The predictors of complications in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Catheter Cardiovasc Interv 2019;93:E349–E356. 10.1002/ccd.28133 [DOI] [PubMed] [Google Scholar]
- 75. Kawakami T, Ogawa A, Miyaji K, Mizoguchi H, Shimokawahara H, Naito T, et al. . Novel angiographic classification of each vascular lesion in chronic thromboembolic pulmonary hypertension based on selective angiogram and results of balloon pulmonary angioplasty. Circ Cardiovasc Interv 2016;9:e003318. 10.1161/CIRCINTERVENTIONS.115.003318 [DOI] [PubMed] [Google Scholar]
- 76. Stępniewski J, Magoń W, Waligóra M, Jonas K, Bochenek M, Przybylski R, et al. . Hemodynamic effects of balloon pulmonary angioplasty for the treatment of total and subtotal pulmonary artery occlusions in inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2022;361:71–76. 10.1016/j.ijcard.2022.05.029 [DOI] [PubMed] [Google Scholar]
- 77. Inami T, Kataoka M, Shimura N, Ishiguro H, Yanagisawa R, Taguchi H, et al. . Pulmonary edema predictive scoring index (PEPSI), a new index to predict risk of reperfusion pulmonary edema and improvement of hemodynamics in percutaneous transluminal pulmonary angioplasty. JACC Cardiovasc Interv 2013;6:725–736. 10.1016/j.jcin.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 78. Darocha S, Banaszkiewicz M, Pietrasik A, Siennicka A, Piorunek M, Grochowska E, et al. . Changes in estimated glomerular filtration after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Cardiorenal Med 2020;10:22–31. 10.1159/000502254 [DOI] [PubMed] [Google Scholar]
- 79. Kriechbaum SD, Wiedenroth CB, Hesse ML, Ajnwojner R, Keller T, Sebastian Wolter J, et al. . Development of renal function during staged balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Scand J Clin Lab Invest 2019;79:268–275. 10.1080/00365513.2019.1601765 [DOI] [PubMed] [Google Scholar]
- 80. Andreassen AK, Ragnarsson A, Gude E, Geiran O, Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013;99:1415–1420. 10.1136/heartjnl-2012-303549 [DOI] [PubMed] [Google Scholar]
- 81. Kurzyna M, Darocha S, Pietura R, Pietrasik A, Norwa J, Mańczak R, et al. . Changing the strategy of balloon pulmonary angioplasty resulted in a reduced complication rate in patients with chronic thromboembolic pulmonary hypertension. A single-centre European experience. Kardiol Pol 2017;75:645–654. 10.5603/KP.a2017.0091 [DOI] [PubMed] [Google Scholar]
- 82. Olsson KM, Wiedenroth CB, Kamp JC, Breithecker A, Fuge J, Krombach GA, et al. . Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J 2017;49:1602409. 10.1183/13993003.02409-2016 [DOI] [PubMed] [Google Scholar]
- 83. Jansa P, Heller S, Svoboda M, Pad'our M, Ambrož D, Dytrych V, et al. . Balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension: impact on clinical and hemodynamic parameters, quality of life and risk profile. J Clin Med 2020;9:3608. 10.3390/jcm9113608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Godinas L, Bonne L, Budts W, Belge C, Leys M, Delcroix M, et al. . Balloon pulmonary angioplasty for the treatment of nonoperable chronic thromboembolic pulmonary hypertension: single-center experience with low initial complication rate. J Vasc Interv Radiol 2019;30:1265–1272. 10.1016/j.jvir.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 85. Anand V, Frantz RP, DuBrock H, Kane GC, Krowka M, Yanagisawa R, et al. . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: initial single-center experience. Mayo Clin Proc Innov Qual Outcomes 2019;3:311–318. 10.1016/j.mayocpiqo.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wiedenroth CB, Rolf A, Steinhaus K, Adameit MSD, Kriechbaum SD, Haas M, et al. . Riociguat and balloon pulmonary angioplasty improve prognosis in patients with inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2023;42:134–139. 10.1016/j.healun.2022.08.011 [DOI] [PubMed] [Google Scholar]
- 87. Hoole SP, Coghlan JG, Cannon JE, Taboada D, Toshner M, Sheares K, et al. . Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: the UK experience. Open Heart 2020;7:e001144. 10.1136/openhrt-2019-001144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. van Thor MCJ, Lely RJ, Braams NJ, Ten Klooster L, Beijk MAM, Heijmen RH, et al. . Safety and efficacy of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension in The Netherlands. Neth Heart J 2020;28:81–88. 10.1007/s12471-019-01352-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Darocha S, Roik M, Kopeć G, Araszkiewicz A, Furdal M, Lewandowski M, et al. . Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: a multicentre registry. EuroIntervention 2022;17:1104–1111. 10.4244/EIJ-D-21-00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Karyofyllis P, Demerouti E, Giannakoulas G, Anthi A, Arvanitaki A, Athanassopoulos G, et al. . Balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension in Greece: data from the Hellenic Pulmonary Hypertension Registry. J Clin Med 2022;11:2211. 10.3390/jcm11082211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Piliero N, Thony F, Guillien A, Rousseau J, Finas M, Vautrin E, et al. . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: evaluation of haemodynamic effects, complication rates and radiation exposure over time. Arch Cardiovasc Dis 2022;115:295–304. 10.1016/j.acvd.2022.02.010 [DOI] [PubMed] [Google Scholar]
- 92. Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, et al. . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes 2017;10:e004029. 10.1161/CIRCOUTCOMES.117.004029 [DOI] [PubMed] [Google Scholar]
- 93. Chausheva S, Naito A, Ogawa A, Seidl V, Winter MP, Sharma S, et al. . Chronic thromboembolic pulmonary hypertension in Austria and Japan. J Thorac Cardiovasc Surg 2019;158:604–614.e2. 10.1016/j.jtcvs.2019.01.019 [DOI] [PubMed] [Google Scholar]
- 94. Hug KP, Gerry Coghlan J, Cannon J, Taboada D, Toshner M, Sheares K, et al. . Serial right heart catheter assessment between balloon pulmonary angioplasty sessions identify procedural factors that influence response to treatment. J Heart Lung Transplant 2021;40:1223–1234. 10.1016/j.healun.2021.06.011 [DOI] [PubMed] [Google Scholar]
- 95. Darocha S, Pietura R, Pietrasik A, Norwa J, Dobosiewicz A, Pilka M, et al. . Improvement in quality of life and hemodynamics in chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Circ J 2017;81:552–557. 10.1253/circj.CJ-16-1075 [DOI] [PubMed] [Google Scholar]
- 96. Sakamoto H, Goda A, Tobita K, Takeuchi K, Kikuchi H, Inami T, et al. . EmPHasis-10 health-related quality of life and exercise capacity in chronic thromboembolic pulmonary hypertension after balloon angioplasty. J Am Heart Assoc 2022;11:e026400. 10.1161/JAHA.122.026400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Aoki T, Sugimura K, Terui Y, Tatebe S, Fukui S, Miura M, et al. . Beneficial effects of riociguat on hemodynamic responses to exercise in CTEPH patients after balloon pulmonary angioplasty—a randomized controlled study. Int J Cardiol Heart Vasc 2020;29:100579. 10.1016/j.ijcha.2020.100579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wiedenroth CB, Rieth AJ, Kriechbaum S, Ghofrani HA, Breithecker A, Haas M, et al. . Exercise right heart catheterization before and after balloon pulmonary angioplasty in inoperable patients with chronic thromboembolic pulmonary hypertension. Pulm Circ 2020;10:2045894020917884. 10.1177/2045894020917884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kanar BG, Mutlu B, Atas H, Akaslan D, Yıldızeli B. Improvements of right ventricular function and hemodynamics after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Echocardiography 2019;36:2050–2056. 10.1111/echo.14503 [DOI] [PubMed] [Google Scholar]
- 100. Li W, Yang T, Quan RL, Chen XX, An J, Zhao ZH, et al. . Balloon pulmonary angioplasty reverse right ventricular remodelling and dysfunction in patients with inoperable chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. Eur Radiol 2021;31:3898–3908. 10.1007/s00330-020-07481-6 [DOI] [PubMed] [Google Scholar]
- 101. Yamasaki Y, Abe K, Kamitani T, Hosokawa K, Kawakubo M, Sagiyama K, et al. . Balloon pulmonary angioplasty improves right atrial reservoir and conduit functions in chronic thromboembolic pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2020;21:855–862. 10.1093/ehjci/jeaa064 [DOI] [PubMed] [Google Scholar]
- 102. Roller FC, Schüssler A, Hasse A, Kriechbaum S, Richter M, Guth S, et al. . Effects of BPA on right ventricular mechanical dysfunction in patients with inoperable CTEPH—a cardiac magnetic resonance study. Eur J Radiol 2022;147:110111. 10.1016/j.ejrad.2021.110111 [DOI] [PubMed] [Google Scholar]
- 103. Piłka M, Darocha S, Banaszkiewicz M, Florczyk M, Wieteska M, Dobosiewicz A, et al. . The evolution of electrocardiographic signs of right ventricular overload after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Pol Arch Intern Med 2019;129:451–459. 10.20452/pamw.14877 [DOI] [PubMed] [Google Scholar]
- 104. Magoń W, Stępniewski J, Waligóra M, Jonas K, Podolec P, Kopeć G. Pulmonary artery elastic properties after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Can J Cardiol 2019;35:422–429. 10.1016/j.cjca.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 105. Nagai T, Ikeda N, Iijima R, Hara H, Nakamura M. Impact and safety of balloon pulmonary angioplasty for elderly patients. Pulm Circ 2022;12:e12009. 10.1002/pul2.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nishiyama M, Inoue Y, Sasaki H, Seike Y, Aoki T, Ueda J, et al. . Long-term outcomes of combined pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Gen Thorac Cardiovasc Surg 2023;71:91–298. 10.1007/s11748-022-01872-w [DOI] [PubMed] [Google Scholar]
- 107. Wiedenroth CB, Olsson KM, Guth S, Breithecker A, Haas M, Kamp JC, et al. . Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic disease. Pulm Circ 2018;8:2045893217753122. 10.1177/2045893217753122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pengo V, Denas G, Zoppellaro G, Jose SP, Hoxha A, Ruffatti A, et al. . Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365–1371. 10.1182/blood-2018-04-848333 [DOI] [PubMed] [Google Scholar]
- 109. Cottin V, Bensimon L, Raguideau F, Chaize G, Hakmé A, Levy-Bachelot L, et al. . Hospital costs of balloon pulmonary angioplasty (BPA) procedure and management for CTEPH patients: an observational study based on the French national hospital discharge database (PMSI). PLoS One 2021;16:e0260483. 10.1371/journal.pone.0260483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the online supplementary material.