Abstract
Background:
Poor growth and metabolic disturbances remain concerns for children living with HIV (CLHIV). We describe the impact of viral load (VL) on growth and lipid outcomes in South African CLHIV <12 years initiating World Health Organization recommended first-line antiretroviral therapy (ART) from 2012 to 2015.
Methods:
Z scores for length-for-age (LAZ), weight-for-age (WAZ) and body mass index-for-age were calculated. Lipids (total cholesterol, low-density lipoprotein and high-density lipoprotein) were measured. Hemo-globin A1C ≥5.8 was defined as at risk for type 2 diabetes. Mixed effects models were used to assess the association of VL at ART initiation with Z scores and lipids over time.
Results:
Of 241 CLHIV, 151 (63%) were <3 years initiating LPV/r-based ART and 90 (37%) were ≥3 years initiating EFV-based ART. Among CLHIV <3 years, higher VL at ART initiation was associated with lower mean LAZ (ß: −0.30, P=0.03), WAZ (ß: −0.32, P=0.01) and low-density lipoprotein (ß: −6.45, P=0.03) over time. Among CLHIV ≥3, a log 10 increase in pretreatment VL was associated with lower mean LAZ (ß: −0.29, P=0.07) trending towards significance and lower WAZ (ß: −0.32, P=0.05) as well as with more rapid increases in LAZ (ß: 0.14 per year, P=0.01) and WAZ (ß: 0.19 per year, P=0.04). Thirty percent of CLHIV were at risk for type 2 diabetes at ART initiation.
Conclusions:
CLHIV initiating ART <3 years exhibited positive gains in growth and lipids, though high viremia at ART initiation was associated with persistently low growth and lipids, underscoring the need for early diagnosis and rapid treatment initiation. Future studies assessing the long-term cardiometabolic impact of these findings are warranted.
Keywords: body mass index, cholesterol, viral load
Antiretroviral therapy (ART) has extended survival of children living with HIV (CLHIV). However, disturbances in growth and metabolism are prevalent and pose a threat to long-term cardiometabolic health. Metabolic complications, including dyslipidemia,1,2 fat redistribution3–5 and insulin resistance6,7 have been extensively documented and may be the result of complex interactions between HIV, ART, age, race, socio-economics, diet and lifestyle.8
Suboptimal growth during childhood, including failure to thrive, wasting and stunting, is associated with greater risk for morbidity and mortality.9 Among CLHIV, failure to attain normal rates of growth is an independent risk factor for death.10 Suboptimal growth, such as stunting, has implications for long-term health and has been associated with adverse effects on cognitive functioning and decreased work capacity.11 The positive impact of ART on growth, particularly weight gain, is well established for CLHIV.12–21 Furthermore, younger age at ART initiation is associated with better growth trajectories.15,18,22
North American and European studies of CLHIV receiving ART have found high prevalence of unfavorable alterations in lipid and glucose metabolism, well-established risk factors for cardio-vascular disease,23–30 with significant proportions having elevated total cholesterol (TC) and low-density lipoprotein (LDL), and abnormal glucose and insulin homeostasis.23,28,31 Several studies from sub-Saharan Africa demonstrate similar findings.23–27 Since low total and LDL cholesterol in ART-naïve CLHIV is common, elevations after ART initiation may reflect an initial return to healthy metabolism. However, few studies have assessed longitudinal changes in lipids or examined anthropometric outcomes in children under 3 years of age. Hence, we report changes in growth and lipids among CLHIV after initiation of ART regimens for up to 2 years of follow-up. We also describe the association between pretreatment viral load (VL) on growth and metabolic outcomes in a cohort of South African children 0 to 12 years of age.
METHODS
Study Population and Setting
“Enhanced Surveillance and 2 Year Outcomes of Children Enrolled on ART in Public Health Facilities in the Eastern Cape Province, South Africa” was a prospective cohort study of CLHIV conducted between 2012 and 2015, to examine clinical, immunologic, virologic, metabolic, psychosocial and behavioral outcomes. Ethical and administrative reviews were received from the Columbia University Medical Center Institutional Review Board, University of Cape Town Human Research Ethics Committee, CDC Center for Global Health Associate Director for Science Office, East London Hospital Complex Research Ethics Committee, Walter Sisulu University Health Research Ethics Committee and Eastern Cape Department of Health.
The study enrolled 397 CLHIV at the time of ART-eligibility at 5 health care facilities who were followed for 12 to 24 months. Participants ages 1 month to 12 years who were eligible for ART based on the South African Pediatric HIV guidelines, had documented HIV infection, had no prior history of ART (other than prophylaxis for prevention of mother-to-child transmission) were included. Trained study nurses conducted anthropometric measures and obtained additional study-specific blood samples at enrollment and at visits 6, 12 and 24 months during study follow-up. Children attended quarterly study visits coscheduled with routine care. Children who missed study visits were tracked through phone calls and home visits.
During the study period, first-line ART recommended in the South African National Consolidated Guidelines for children <3 years of age was a protease inhibitor-based regimen (abacavir [ABC] + lamivudine [3TC] + lopinavir/ritonavir [LPV/r]), and for children ≥3 years, a nonnucleoside reverse transcriptase inhibitor-based regimen (ABC+3TC+efavirenz). Children who started ART at <3 years on ABC+3TC+LPV/r were maintained on the same regimen as they aged.
This analysis was restricted to measures taken prior to 12th birthday to avoid confounding by puberty which is known to affect metabolic outcomes.29
Primary Outcomes
The primary outcomes included anthropometric measures (length-for-age Z score [LAZ], weight-for-age Z score [WAZ], and body mass index Z score [BMIZ], and lipids [TC, LDL and high-density lipoprotein-C (HDL)]). Outcomes were assessed as continuous variables and evaluated over time and as an annual rate of change. Anthropometrics and lipids (TC, LDL, HDL) were measured at enrollment 6, 12 and 24 months. Z scores were calculated using the LMS method (Lambda for the skew, Mu for the median, and Sigma for the generalized coefficient of variation) with World Health Organization (WHO) standards.30 Recumbent lengths were measured for children under age 2 years and standing heights were measured for children age 2 years and older. At each visit the nurses took 3 measurements for each body part. The LAZ for children under age 2 years and HAZ for children age 2 years and older were calculated using WHO references and have been described as LAZ throughout this manuscript. Because weight-for-length WHO standards only are available for children under age 5 years, we used BMI z-scores whose reference standards are available across all ages. Lipids were not evaluated using Z scores due to the absence of standard pediatric references. In addition, growth and lipids were evaluated as dichotomous outcomes where underweight was defined as WAZ ≤−2, stunting as LAZ ≤−2, hypercholesterolemia as TC >95th percentile, low HDL as HDL <5th percentile and high LDL as >95th percentile using US standards available for children age 4 years and older.36–38 In addition, risk for type 2 diabetes (T2D) as measured by hemoglobin A1C (A1C) among children over age 4 years was examined as a dichotomous variable; CLHIV with a baseline A1C ≥5.8 were categorized as being at risk for T2D.31 The analysis of A1C was restricted to this age group due to lack of norms or standards for use of A1C in children under age 4 years. The “pretreatment” timepoint for all primary outcomes was defined as the first date of measurement within 160 days prior to and 7 days following ART initiation. All laboratory testing was performed by the National Health laboratories at Livingstone, Dora Nginza Frere and Cecilia Makiwane Hospitals.
Primary Exposure of Interest
Pretreatment VL (Cobas 6800/8800, Roche) was measured from plasma obtained from venous blood specimens 160 days prior to ART initiation and up to 7 days following ART initiation and was log-transformed to approximate a normal distribution.
Covariates
Age, sex, caregiver, and socioeconomic status were collected through caregiver interviews. A continuous household wealth index variable was calculated as a linear composite of 3 measures (tap water, toilet and electricity within the home) normalized by means and SDs. Those with a higher household wealth index had more household assets. Skinfold ratios were calculated by dividing the sum of subscapular and side periumbilical skinfolds by the sum of biceps and triceps skinfolds to include a measure of baseline body fat distribution as a covariate.
Statistical Analysis
CLHIV initiating LPV/r-based ART at less than age 3 years (CLHIV <3) were analyzed separately from CLHIV initiating efavirenz-based ART at ≥ age 3 years (CLHIV ≥3). Longitudinal trends of primary outcomes over time were assessed using locally weighted smoothing (LOESS) plots. Average annual rates of change since ART initiation were calculated using univariate mixed effects models for each anthropometric and lipid outcome. Multivariate mixed effects linear regression models were fitted using an unstructured covariance matrix to assess the association of pretreatment VL with the average rate of change in each anthropometric measure and lipid subfraction when controlling for age, sex, caregiver, wealth index, BMIZ and skinfold ratio. We introduced an interaction term between VL and time to test for differences in the rate of change of each anthropometric and lipid outcome as a function of VL. Logistic regression models with generalized estimating equations were used to assess factors associated with frank T2D at enrollment, including age at ART initiation, sex, baseline log VL, caregiver, wealth index, BMIZ and skinfold ratio. All statistical analyses were performed in R version 3.6.3.
RESULTS
After excluding visits occurring after the 12th birthday (which resulted in exclusion of 13 patients), 88 children who did not have at least 6 months of follow-up after ART initiation, 13 who had irregular (per guidelines) or missing ART regimen data and 42 missing a pre-ART VL, a total of 241 CLHIV were included in the analysis: 151 CLHIV <3 and 90 CLHIV ≥3 years.(see Figure, Supplemental Digital Content 1; http://links.lww.com/INF/E466 and table, Supplemental Digital Content 2; http://links.lww.com/INF/E467) At enrollment, younger CLHIV <3 and older CLHIV ≥3 had a median age at ART initiation of 10 months and 8 years, log VL of 6.1 and 5.2, and CD4 percentage of 19% and 15%, respectively. Among CLHIV <3, 95% had a biological mother who was still alive, 14% were born preterm, and 80% had been breast-fed. Among CLHIV ≥3, 76% had a biological mother who was still alive, 10% were born preterm and 69% had been breast-fed. There were no differences in enrollment characteristics between CLHIV who were excluded (due to lack of follow-up for at least 6 months) and those who were included in the analytic sample for longitudinal analysis.
Anthropometric Outcomes
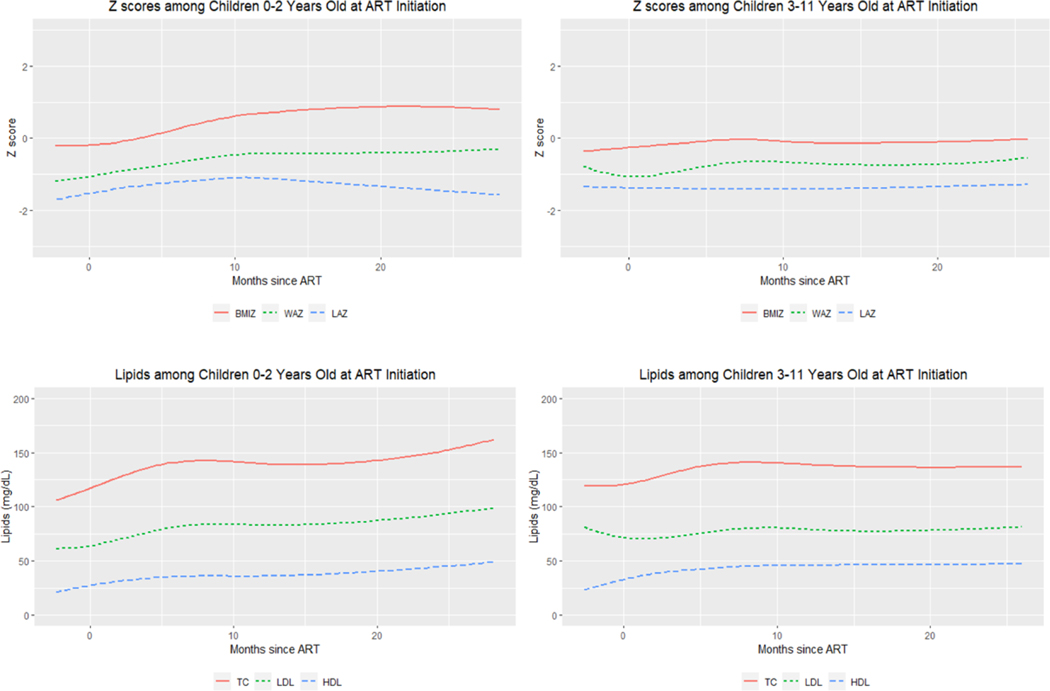
At enrollment, CLHIV <3 had median LAZ, WAZ, and BMIZ of −2.6, −1.6 and −0.3, respectively, while CLHIV ≥3 had median LAZ, WAZ, and BMIZ of −1.7, −1.3 and −0.2, respectively (Table 1). Sixty-three percent of CLHIV <3 and 38% of CLHIV ≥3 were stunted (LAZ ≤−2) at ART initiation, while 45% of CLHIV <3 and 34% of CLHIV ≥3 were underweight (WAZ ≤−2). LOESS plots of Z scores indicate an incremental increase across all Z scores over time in the younger CLHIV <3. Among the older CLHIV ≥3, incremental increases in only WAZ were observed over time (Figure 1). On average, the rate of increase was 0.20 (95% confidence interval [CI]: 0.01–0.39), 0.65 (95% CI: 0.47–0.82) and 0.85 SD per year (95% CI: 0.58–1.1) for LAZ, WAZ and BMIZ, respectively, among children <3 years. CLHIV ≥3 had a positive WAZ growth rate of 0.23 SD per year (95% CI: 0.04–0.42) but no significant changes over time in LAZ (95% CI: −0.05 to 0.17) or BMIZ (95% CI: −0.19 to 0.27).
TABLE 1.
Growth and Metabolic Measures at ART Initiation and 24 Months After Initiation
| Metabolic Outcome | ||||
|---|---|---|---|---|
| Anthropometries | CLHIV <3 Years at ART Initiation (ABC/3TC/LPV/r) |
CLHIV ≥3 Years at ART Initiation (ABC/3TC/EFV) |
||
| At ART Initiation |
24 Months Post ART Initiation |
At ART Initiation |
24 Months Post ART Initiation |
|
| n=137 | n=65 | n=71 | n=44 | |
|
| ||||
| Height | ||||
| LAZ | −2.6 (−3.7 to −1.4) | −1.9 (−2.9 to −0.6) | −1.7 (−2.5 to −1.0) | −1.2 (−2.2 to −0.8) |
| Stunting (LAZ ≤−2) | 81 (63%)* | 31 (48%) | 27 (38%) | 14 (32%) |
| Weight | ||||
| WAZ | −1.6 (−3.1 to −0.5) | −0.5 (−1.0 to 0.5) | −1.3 (−2.2 to −0.3) | −0.5 (−1.3 to −0.1) |
| Underweight (WAZ ≤−2) | 62 (45%) | 6 (9%) | 24 (34%)† | 1 (3%)‡ |
| BMIZ | −0.3 (−1.6 to 1.0) | 1.2 (0.3 to 2.5) | −0.2 (−1.5 to 0.7) | 0.1 (−0.9 to 0.6) |
| Lipids | n=147 | n=66 | n=85 | n=47 |
| TC, mg/dL | 119 (85 to 137) | 160 (140 to 189) | 116 (93 to 147) | 143 (119 to 163) |
| Hypercholesterolemia (>95th pereentile)§ | --- | --- | 3 (4%)¶ | 1 (2%)ǁ |
| LDL (mg/dL) | 60 (34 to 81) | 96 (70 to 114) | 69 (45 to 90) | 80 (64 to 96) |
| High LDL (>95th percentile)** | --- | --- | 3 (5%)†† | 3 (8%)‡‡ |
| HDL, mg/dL | 24 (18 to 32) | 44 (37 to 50) | 29 (23 to 37) | 46 (41 to 52) |
| Low HDL (<fifth percentile)§ | --- | --- | 49 (64%)§§ | 8 (20%)ǁ |
| Diabetes | n=79 | n=37 | ||
| HgAlC ≥5.8§ | --- | 24 (30%) | 7 (19%) | |
Continuous variables are expressed in median (interquartile range); categorical variables are expressed in number (percent).
n=128.
n=70.
n=29.
Only those ≥4 years of age at ART initiation.
n=78.
n=41.
Only those ≥5 years of age at ART initiation.
n=66.
n=36.
n=76.
FIGURE 1.
LOESS plots of longitudinal changes in growth and lipids after ART initiation among South African children living with HIV initiating ART, PESS Study (N=241).
ART=antiretroviral therapy, BMIZ=Body Mass Index Z-score, HDL=high density lipoprotein, LAZ=Length-for-age Z-score, LDL=low density lipoprotein, TC=total cholesterol, WAZ=Weight-for-age Z-score
Among CLHIV <3, a log 10 increase in pretreatment VL was associated with persistently lower means over time in LAZ (ß: −0.30, P=0.03) and WAZ (ß: −0.32, P=0.01) but was not significantly associated with a difference in slopes for any of the outcomes after adjusting for age at ART initiation, sex, caregiver status, and wealth index (Table 2). Among CLHIV ≥3, a log 10 increase in pretreatment VL was associated with lower mean LAZ (ß: −0.29, P=0.07) trending towards significance and lower WAZ (ß: −0.32, P=0.05) as well as with more rapid increases in LAZ (ß: 0.14 per year, P=0.01) and WAZ (ß: 0.19 per year, P=0.04). No associations were found between pretreatment VL and BMIZ among CLHIV.
TABLE 2.
Effect of Log Viral Load at ART Initiation on Mean Anthropometric and Lipid Subfraction Outcomes Over Time in Two Age Groups After Initiating Antiretroviral Therapy
| CLHIV <3 Years at ART Initiation (ABC/3TC/LPV/r) | CLHIV ≥3 Years at ART Initiation (ABC/3TC/EFV) | |||
|---|---|---|---|---|
|
| ||||
| Antdropometric outcome* | Estimate†‡ | P | Estimate†‡ | P |
| LAZ | −0.30 (−0.57 to −0.03) | 0.03 | −0.29 (−0.60 to 0.02)§ | 0.07§ |
| WAZ | −0.32 (−0.58 to −0.07) | 0.01 | −0.32 (−0.64 to 0.01)§ | 0.05§ |
| BMIZ | −0.12 (−0.37 to 0.13) | 0.34 | −0.05 (−0.33 to 0.23) | 0.70 |
| Lipid subfraction outcome¶ | Estimate†‡ | P | Estimate†‡ | P |
| TC | −6.17 (−12.9 to 0.52) | 0.07 | −3.06 (−9.97 to 3.85) | 0.38 |
| LDL | −6.45 (−12.1 to −0.77) | 0.03 | −4.32 (−10.5 to 1.81) | 0.16 |
| HDL | −0.76 (−3.07 to 1.56) | 0.52 | 0.68 (−1.70 to 3.06) | 0.57 |
Adjusted for age, sex, log HIV viral load, caregiver, and wealtd index.
Estimate represents tde mean difference in outcome per unit log viral load increase.
95% confidence intervals in parentdeses.
Model included interaction term of (log HIV viral load)×(time).
Adjusted for age, sex, log HIV viral load, caregiver, wealtd index, BMIZ, and skinfold ratio.
Lipid Outcomes
At enrollment, CLHIV <3 had median TC, LDL and HDL of 119, 60 and 24 mg/dL, respectively (Table 1). CLHIV ≥3 had median TC, LDL and HDL of 116, 69 and 29 mg/dL, respectively. Lipid outcomes were assessed for a subsample of children based on available limits: TC and HDL for children ≥ age 4 years and LDL for children ≥ age 5 years. Among children ≥ age 4 years, 4% had hypercholesterolemia and 64% had low HDL, while 5% of children ≥ age 5 years had elevated LDL. LOESS plots indicate increases across all lipids over time (Figure 1). Mean change in TC, LDL and HDL was +12.9 (95% CI: 7.8–18.4), +10.1 (95% CI: 6.0–14.2) and +7.2 mg/dL per year (95% CI: 5.4–8.9), respectively, among CLHIV <3. Among CLHIV ≥3, mean change in HDL was +5.6 mg/dL (95% CI: 3.1–8.0) per year while there were no significant changes over time in TC (95% CI: −0.8 to 11.4) or LDL (95% CI: −0.9 to 6.2).
Among CLHIV <3, higher VL at ART initiation was associated with persistently lower mean differences over time in TC (ß: −6.17, P=0.07) trending towards significance and LDL (ß: −6.45, P=0.03), even after adjustment. No associations were found between VL and lipids among older CLHIV ≥3. Models including an interaction term between log HIV VL and time when it was significant (P<0.05), did not reveal significant associations between VL and slope changes in any of the outcomes after adjustment (Table 2).
Diabetes Risk Outcomes
A1C was analyzed among a subgroup of 79 CLHIV ≥ age 4 years of age. Overall, at enrollment, 30% of children ≥ age 4 years were at risk for T2D. After 24 months of ART treatment, 19% of children ≥ age 4 years of age were at risk for T2D (Table 1). Multivariable models did not reveal any significant factors associated with a risk for frank T2D at enrollment (data not shown).
DISCUSSION
Despite a high prevalence of stunting and underweight at enrollment, we found gains over time after ART initiation in WAZ, LAZ and BMIZ among CLHIV <3 and for WAZ in CLHIV ≥3 in this observational cohort of South African CLHIV. In addition, for both age cohorts, higher VL at ART initiation was associated with persistently lower LAZ and WAZ over time. Alterations in growth are among the most prevalent comorbidities associated with HIV infection during childhood.32–34 The high rates of stunting and underweight which we observed in CLHIV before ART initiation are consistent with other studies in sub-Saharan Africa, which report similar high rates of stunting (73%) and underweight (50%) before ART initiation, and after 5 years on ART (20% of CLHIV remained stunted), with height improving over a longer time horizon than weight.35 A West African study of CLHIV who were stunted at baseline also found that the median time to crossing over from a Z score ≤−2 to a Z score >−2 was longer for LAZ (17.7 months) than for WAZ (11.7 months) and that children less than age 5 years were more likely to experience catch-up growth compared with older children.36 Our findings that statural growth lagged compared with ponderal growth and that younger children achieved better growth overall are in keeping with previous studies in which children without HIV had irreversible stunting if not reversed prior to age 2.37 In addition, we found that high VL at ART initiation was associated with persistently poor growth for height and weight. This finding is consistent with other recent studies which observed significant associations between viremia (but not CD4) and height velocity38 and points to the potentially central role of HIV replication in poor growth.39,40
In our study, TC, LDL and HDL increased over time in CLHIV <3. Among CLHIV ≥3, TC and LDL remained stable and HDL increased over time. The prevalence of dyslipidemia in children/adolescents with HIV has been reported to be between 11% and 73%, depending on specific study definitions of dyslipidemia, sample size, and study locations.28,41–46 The NEVEREST study, a randomized trial comparing maintenance on LPV/r-based ART or switch to NVP-based ART in CLHIV who initiated ART before age 2 years, reported overall rates of 14% elevated TC and 12% elevated LDL.23 A study of CLHIV age greater than 6 years in Nigeria reported dyslipidemia in 12% compared with 5% in an uninfected comparison group.47 Increased rates of low HDL have also been reported in both ART-naive and experienced CLHIV compared with NHANES standards.28 Indeed, 64% of the CLHIV ≥4 years in our study had low HDL at ART initiation and 20% had low HDL after 24 months of treatment. These rates are consistent with a cross-sectional study among CLHIV in Uganda and Zimbabwe 3 years after ART initiation which revealed that 55.1% had abnormally low HDL.48
Hypercholesterolemia was found in only 4% (before ART) versus 2% (after 24 months) among the CLHIV ≥4 years in our cohort. The lower rates of high TC and LDL may be explained in part by the fact that our CLHIV were younger and had more severe HIV disease, including higher viremia at ART initiation, which we observed to be an independent predictor of persistently low LDL and TC over time in CLHIV <3. Other studies have demonstrated that severe HIV disease is associated with lower levels of TC and LDL.28,45 One study reported CDC HIV infection class C to be associated with low TC and low LDL after adjusting for confounders,28 while another reported CD4% <15% to be protective against hypercholesterolemia.45 Severely immunocompromised individuals may exhibit overall poor cholesterol synthesis prior to initiating ART. Ongoing viremia (HIV RNA level ≥400 copies/mL) has also been reported to be protective against hypercholesterolemia.44,45 Taken together, our results are consistent with other studies which have observed lower lipid levels in severe or uncontrolled HIV disease prior to ART initiation due to dampened metabolism, and an initial increase in these lipids after ART is initiated and HIV disease is controlled, reflecting a return to metabolic homeostasis.
Wide ranges (6.5%–52%) of insulin resistance (IR) prevalence rates are documented in CLHIV.49,50 The US Pediatric HIV/AIDS Cohort Study observed 15% IR in a population of children/adolescents with perinatally acquired HIV receiving a heterogeneous background of ART.51 Impaired glucose tolerance, diagnosed by oral glucose tolerance testing was found in 6.7%. A French study of 130 CLHIV on ART found no T2D or impaired fasting glucose in those who underwent oral glucose tolerance testing, with only one child having impaired glucose tolerance.52 Another small study compared HIV-uninfected controls with children/adolescents with HIV and found higher fasting insulin and glucose in HIV-infected children.50 Other studies in South Africa of older children/adolescents with HIV have reported high rates of IR by the Homeostatic Model Assessment of Insulin Resistance (10%–24%).26,27 Differences in sample size, ART regimen, ethnicity/race and techniques for measuring IR likely explain the wide variability in rates of IR. While IR and T2D are not equivalent, IR is strongly associated with risk for the development of T2D. Few studies have evaluated A1C in CLHIV from resource-constrained settings, making it difficult to interpret the high baseline prevalence of risk for frank T2D (A1C ≥5.8) in our cohort. However, the Pediatric HIV/AIDS Cohort Study study observed that of youth with perinatally acquired HIV with IR, 7% also had an A1C >6%.51 Because A1C testing has not been fully validated in young children, our results should be interpreted with caution, but CLHIV with perinatally acquired HIV may benefit from T2D monitoring later in young adulthood.
A log 10 increase in pretreatment VL was associated with persistently lower growth and lipids over time among CLHIV <3. Efforts to initiate ART as early as possible in infancy after diagnosis of HIV infection have greatly expanded in the last decade. Nonetheless, CLHIV with high levels of viremia at ART initiation may require additional monitoring and potential interventions for growth. Suboptimal growth in childhood has implications for long-term health and has been associated with impaired cognitive development, (64) reduced educational achievement, poor pregnancy and birth outcomes (for females) and decreased work capacity.11 Given the far-reaching potential consequences of stunting, multiple interventions may be advisable. Recent studies have shown that improved infant and young child feeding has reduced the prevalence of stunting among HIV-exposed children. (64) The increases in lipids over 24 months after ART initiation among CLHIV <3 which we observed may reflect an overall slow return to normal metabolic functioning after ART initiation. Further research is needed to understand the impact of this trend in lipids on the long-term cardiometabolic health of CLHIV.
A strength of our study was the inclusion of infants and very young children, and the representative nature of the study population; we recruited all ART-naive children initiating treatment at participating health facilities reflecting children initiating ART in high burden settings. Also, our cohort received WHO-recommended ART regimens which makes the findings highly relevant as other countries adopt these guidelines. The study was limited by the lack of an HIV-uninfected comparison group. Furthermore, we could not evaluate the effects of differing ART regimens on our outcomes, and we were not able to control for birth anthropometrics or breastfeeding duration.
In conclusion, CLHIV initiating ART <3 years exhibit positive gains in growth and lipids after ART initiation. However, high viremia at ART initiation is associated with persistently lower growth and as a result, early infant diagnosis and ART initiation is critical for optimizing growth trajectories in young CLHIV. Short-term gains in lipids among these children may reflect overall normalization of lipid metabolism in those with severe HIV disease, but the long-term impact especially on cardiovascular outcomes of lipid increases associated with lifelong ART in this population warrants attention and continued monitoring.
Supplementary Material
Acknowledgments
This work was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement Numbers 5U62PS223540 and 5U2GPS001537.
Footnotes
The authors have conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com)
REFERENCES
- 1.Carr A, Samaras K, Thorisdottir A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. [DOI] [PubMed] [Google Scholar]
- 2.Tassiopoulos K, Williams PL, Seage GR 3rd, et al. ; International Maternal Pediatric Adolescent AIDS Clinical Trials 219C Team. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson DL, Patel K, Siberry GK, et al. ; Pediatric HIV/AIDS Cohort Study. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: outcomes from the Pediatric HIV/AIDS Cohort Study. Am J Clin Nutr. 2011;94:1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arpadi SM, Cuff PA, Horlick M, et al. Lipodystrophy in HIV-infected children is associated with high viral load and low CD4+ −lymphocyte count and CD4+ −lymphocyte percentage at baseline and use of protease inhibitors and stavudine. J Acquir Immune Defic Syndr. 2001;27:30–34. [DOI] [PubMed] [Google Scholar]
- 5.Dzwonek AB, Lawson MS, Cole TJ, et al. Body fat changes and lipodystrophy in HIV-infected children: impact of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:121–123. [DOI] [PubMed] [Google Scholar]
- 6.De Wit S, Sabin CA, Weber R, et al. ; Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31:1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. [DOI] [PubMed] [Google Scholar]
- 8.Wohl DA, McComsey G, Tebas P, et al. Current concepts in the diagnosis and management of metabolic complications of HIV infection and its therapy. Clin Infect Dis. 2006;43:645–653. [DOI] [PubMed] [Google Scholar]
- 9.Caulfield LE, de Onis M, Blössner M, et al. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–198. [DOI] [PubMed] [Google Scholar]
- 10.Berhane R, Bagenda D, Marum L, et al. Growth failure as a prognostic indicator of mortality in pediatric HIV infection. Pediatrics. 1997;100:E7. [DOI] [PubMed] [Google Scholar]
- 11.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7(suppl 3):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchacz K, Cervia JS, Lindsey JC, et al. Impact of protease inhibitor-containing combination antiretroviral therapies on height and weight growth in HIV-infected children. Pediatrics. 2001;108:E72. [DOI] [PubMed] [Google Scholar]
- 13.Guillén S, Ramos JT, Resino R, et al. Impact on weight and height with the use of HAART in HIV-infected children. Pediatr Infect Dis J. 2007;26:334–338. [DOI] [PubMed] [Google Scholar]
- 14.Verweel G, van Rossum AM, Hartwig NG, et al. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics. 2002;109:E25. [DOI] [PubMed] [Google Scholar]
- 15.Nachman SA, Lindsey JC, Moye J, et al. ; Pediatric AIDS Clinical Trials Group 377 Study Team. Growth of human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2005;24:352–357. [DOI] [PubMed] [Google Scholar]
- 16.Kabue MM, Kekitiinwa A, Maganda A, et al. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008;22:245–251. [DOI] [PubMed] [Google Scholar]
- 17.Shiau S, Arpadi S, Strehlau R, et al. Initiation of antiretroviral therapy before 6 months of age is associated with faster growth recovery in South African children perinatally infected with human immunodeficiency virus. J Pediatr. 2013;162:1138–45, 1145.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath CJ, Chung MH, Richardson BA, et al. Younger age at HAART initiation is associated with more rapid growth reconstitution. AIDS. 2011;25:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutcliffe CG, van Dijk JH, Munsanje B, et al. Weight and height z-scores improve after initiating ART among HIV-infected children in rural Zambia: a cohort study. BMC Infect Dis. 2011;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musoke PM, Mudiope P, Barlow-Mosha LN, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigel R, Phiri S, Chiputula F, et al. Growth response to antiretroviral treatment in HIV-infected children: a cohort study from Lilongwe, Malawi. Trop Med Int Health. 2010;15:934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner F, Kind C, Aebi C, et al. Growth in human immunodeficiency virus type 1-infected children treated with protease inhibitors. Eur J Pediatr. 2001;160:611–616. [DOI] [PubMed] [Google Scholar]
- 23.Arpadi S, Shiau S, Strehlau R, et al. Metabolic abnormalities and body composition of HIV-infected children on Lopinavir or Nevirapine-based antiretroviral therapy. Arch Dis Child. 2013;98:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sztam KA, Jiang H, Jurgrau A, et al. Early increases in concentrations of total, LDL, and HDL cholesterol in HIV-infected children following new exposure to antiretroviral therapy. J Pediatr Gastroenterol Nutr. 2011;52:495–498. [DOI] [PubMed] [Google Scholar]
- 25.Strehlau R, Coovadia A, Abrams EJ, et al. Lipid profiles in young HIV-infected children initiating and changing antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Innes S, Abdullah KL, Haubrich R, et al. High prevalence of dyslipidemia and insulin resistance in HIV-infected prepubertal African Children on Antiretroviral Therapy. Pediatr Infect Dis J. 2016;35:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frigati LJ, Jao J, Mahtab S, et al. Insulin resistance in South African youth living with perinatally acquired HIV receiving antiretroviral therapy. AIDS Res Hum Retroviruses. 2019;35:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chantry CJ, Hughes MD, Alvero C, et al. ; PACTG 1010 Team. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008;122:e129–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brix N, Ernst A, Lauridsen LLB, et al. Timing of puberty in boys and girls: a population-based study. Paediatr Perinat Epidemiol. 2019;33:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Head Circumference-for-Age, Arm Circumference-for-Age, Triceps Skinfold-for-Age and Subscapular Skinfold-for-Age: Methods and Development Geneva: World Health Organization, 2007. [Google Scholar]
- 31.Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011;34:1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bobat R, Coovadia H, Moodley D, et al. Growth in early childhood in a cohort of children born to HIV-1-infected women from Durban, South Africa. Ann Trop Paediatr. 2001;21:203–210. [DOI] [PubMed] [Google Scholar]
- 33.Henderson RA, Miotti PG, Saavedra JM, et al. Longitudinal growth during the first 2 years of life in children born to HIV-infected mothers in Malawi, Africa. Pediatr AIDS HIV Infect. 1996;7:91–97. [PubMed] [Google Scholar]
- 34.Moye J Jr, Rich KC, Kalish LA, et al. Natural history of somatic growth in infants born to women infected by human immunodeficiency virus. Women and Infants Transmission Study Group. J Pediatr. 1996;128:58–69. [DOI] [PubMed] [Google Scholar]
- 35.Feucht UD, Van Bruwaene L, Becker PJ, et al. Growth in HIV-infected children on long-term antiretroviral therapy. Trop Med Int Health. 2016;21:619–629. [DOI] [PubMed] [Google Scholar]
- 36.Jesson J, Koumakpaï S, Diagne NR, et al. ; Paediatric WADA IeDEA Collaboration. Effect of age at antiretroviral therapy initiation on catchup growth within the first 24 months among HIV-infected children in the IeDEA West African pediatric cohort. Pediatr Infect Dis J. 2015;34:e159–e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhutta ZA, Das JK, Rizvi A, et al. ; Lancet Nutrition Interventions Review Group, the Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382:452–477. [DOI] [PubMed] [Google Scholar]
- 38.Stagi S, Galli L, Cecchi C, et al. Final height in patients perinatally infected with the human immunodeficiency virus. Horm Res Paediatr. 2010;74:165–171. [DOI] [PubMed] [Google Scholar]
- 39.Pollack H, Glasberg H, Lee E, et al. Impaired early growth of infants perinatally infected with human immunodeficiency virus: correlation with viral load. J Pediatr. 1997;130:915–922. [DOI] [PubMed] [Google Scholar]
- 40.Arpadi SM, Cuff PA, Kotler DP, et al. Growth velocity, fat-free mass and energy intake are inversely related to viral load in HIV-infected children. J Nutr. 2000;130:2498–2502. [DOI] [PubMed] [Google Scholar]
- 41.Amaya RA, Kozinetz CA, McMeans A, et al. Lipodystrophy syndrome in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2002;21:405–410. [DOI] [PubMed] [Google Scholar]
- 42.European Paediatric Lipodystrophy Group. Antiretroviral therapy, fat redistribution and hyperlipidaemia in HIV-infected children in Europe. AIDS. 2004;18:1443–1451. [DOI] [PubMed] [Google Scholar]
- 43.Dapena M, Jiménez B, Noguera-Julian A, et al. Metabolic disorders in vertically HIV-infected children: future adults at risk for cardiovascular disease. J Pediatr Endocrinol Metab. 2012;25:529–535. [DOI] [PubMed] [Google Scholar]
- 44.Farley J, Gona P, Crain M, et al. ; Pediatric AIDS Clinical Trials Group Study 219C Team. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38:480–487. [DOI] [PubMed] [Google Scholar]
- 45.Brewinski M, Megazzini K, Hance LF, et al. ; NISDI Pediatric Study Group 2010. Dyslipidemia in a cohort of HIV-infected Latin American children receiving highly active antiretroviral therapy. J Trop Pediatr. 2011;57:324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazra R, Cohen RA, Gonin R, et al. ; NISDI Pediatric Study Group 2011. Lipid levels in the second year of life among HIV-infected and HIV-exposed uninfected Latin American children. AIDS. 2012;26:235–240. [DOI] [PubMed] [Google Scholar]
- 47.Ige OO, Yilgwan CS, Ebonyi AO, et al. Serum lipid and glucose profiles in HIV-positive Nigerian children. J Virus Erad. 2017;3:157–162. [PMC free article] [PubMed] [Google Scholar]
- 48.Bwakura-Dangarembizi M, Musiime V, Szubert AJ, et al. ; ARROW Trial Team. Prevalence of lipodystrophy and metabolic abnormalities in HIV-infected African children after 3 years on first-line antiretroviral therapy. Pediatr Infect Dis J. 2015;34:e23–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee B, Aurpibul L, Sirisanthana V, et al. Low prevalence of insulin resistance among HIV-infected children receiving nonnucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy in Thailand. HIV Med. 2009;10:72–78. [DOI] [PubMed] [Google Scholar]
- 50.Rosso R, Parodi A, d’Annunzio G, et al. Evaluation of insulin resistance in a cohort of HIV-infected youth. Eur J Endocrinol. 2007;157:655–659. [DOI] [PubMed] [Google Scholar]
- 51.Geffner ME, Patel K, Miller TL, et al. ; Pediatric HIV/AIDS Cohort Study. Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the pediatric HIV/AIDS cohort study. Horm Res Paediatr. 2011;76:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beregszaszi M, Dollfus C, Levine M, et al. Longitudinal evaluation and risk factors of lipodystrophy and associated metabolic changes in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:161–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.