Abstract
Background
Myeloid cells are critical for iron and immune homeostasis. Ferritin heavy chain (FTH1) is essential for intracellular iron storage. Myeloid FTH1 is important in the pathogenesis of many inflammatory diseases. However, the role of myeloid FTH1 in colitis and colitis-associated cancer has not been determined.
Methods
Myeloid FTH1 deficient and wild-type mice were treated with dextran sodium sulfate (DSS) or azoxymethane (AOM)-DSS to compare their susceptibility to acute colitis or colitis-associated cancer.
Results
Myeloid FTH1-deficient mice fed with a high-iron diet were less susceptible to DSS-induced acute colitis than wild type mice. Mechanistic studies showed that myeloid FTH1 deficiency resulted in lower expression of an iron uptake protein divalent metal transporter 1 (DMT1) and active phosphorylated signal transducer and activator of transcription 3 (STAT3) in the colon tissues. Our studies also showed that pharmacological STAT3 reactivation restored the susceptibility of myeloid FTH1-deficient mice to DSS-induced acute colitis. Consistently, myeloid FTH1-deficient mice fed with a high-iron diet had reduced DMT1, phosphorylated STAT3 and inflammation in their colon tissues, and were less susceptible to colitis-associated colorectal cancer.
Conclusions
Our study demonstrated that myeloid FTH1 is required for colitis and colitis-associated colorectal cancer via maintaining of DMT1-iron-STAT3 signaling activation under excess iron condition.
Keywords: colitis, colorectal cancer, myeloid cells, FTH1, STAT3
Key Messages.
What is already known?
Myeloid FTH1 depletion protects mice from inflammatory diseases such as sepsis and kidney inflammation.
What is new here?
Myeloid FTH1 depletion reduces DSS-induced colitis and colitis-associated cancer formation under a high iron condition through inhibition of DMT1-iron-STAT3 signaling.
How can this study help patient care?
Our finding indicates myeloid FTH1 is a new therapeutic target for colitis and colitis associated cancer.
Introduction
Colorectal cancer is the third leading cancer in terms of both case number and mortality in the United States.1 Large cohort epidemiological and animal studies have shown that iron is important in the process of colon tumorigenesis.2 However, the detailed mechanisms are not completely understood.3 We have shown that intestine epithelial hypoxia inducible factor (HIF)-2α promotes colon tumorigenesis via increasing the expression of an iron uptake protein divalent metal transporter 1 (DMT1) and iron accumulation in colon tumors.4 Colon epithelial cell-specific knockout of DMT1 protects mice from colon tumorigenesis via reducing the Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signaling activation.5 However, the role of immune cells in iron-driven colon tumorigenesis is currently understudied.
Myeloid cells are a group of immune cells referring to macrophages, dendritic cells, and neutrophils.6 They play critical roles in iron homeostasis and inflammatory diseases.7 Macrophages maintain systemic iron homeostasis via recycling iron from senescent red blood cells.8 Neutrophils are important in nutrition immunity via secreting siderophores such as lipocalin 2 to sequester iron and thus deprive iron from pathogens during inflammation.7 Intestinal inflammation is a high-risk factor for colorectal cancer.9,10 Patients with inflammatory bowel disease have increasing colorectal cancer risk with diseased time: 2% at 10 years, 8% at 20 years, and 18% at 30 years.10 In addition to disease duration, the risk of colorectal cancer is also higher in patients with extensive area of inflammation in the intestine.10
Iron deficiency is one of the most prevalent nutritional deficiency disorders affecting women in not only developing countries but also developed countries.11 Roughly, 31% of women in United States are affected by iron deficiency.12 Iron deficiency anemia is one of the most common manifestations in patients with inflammatory bowel disease due to chronic blood loss.13,14 In colorectal cancer, 48.1% of patients show iron deficiency, and 66.1% of the iron-deficient patients develop anemia.15 Iron supplementation has been widely used to replenish iron stores in patients with inflammatory bowel disease and colorectal cancer.16 However, excessive iron supplementation may aggravate colitis and promote colorectal cancer.17
Ferritin complex is important in iron hemostasis by storing iron in a bioavailable, nontoxic form,18 and it is composed of 24 ferritin heavy chain (FTH1) and ferritin light chain subunits.19 Ferritin heavy chain is implicated in inflammation in various tissues. For example, myeloid FTH1-deficient mice are protected from unilateral ureteral obstruction-induced kidney inflammation.20 Myeloid FTH1-deficient mice fed with a high fat diet also show reduced inflammatory cytokine expression in adipose tissue.21 Moreover, myeloid FTH1 deficiency inhibits lipopolysaccharide-induced endotoxemia and cecal ligation and puncture-induced sepsis, with reduced expression of both pro-inflammatory cytokines (TNF, IFN-g, IL-6, IL-12, Cxcl1, IL-1b, and IL-2) and anti-inflammatory cytokine (IL-10 and IL-4).22 Ferritin heavy chain dysregulation is also implicated in various cancers. For example, the serum ferritin levels are increased in Hodgkin’s lymphoma patients.23 Estrogen-induced epigenetic silencing of the FTH1 gene reduces liver cancer cell growth and survival.24 High levels of ferritin have also been detected in serum and tumoral extracts of gastrointestinal neoplasms.25 Immunohistochemical findings suggest that ferritin is produced mainly by stromal cell reaction more than by the epithelial cells in colonic neoplasms.25 However, the role of myeloid FTH1 in colitis and colorectal cancer is not clear.
In this study, we found that myeloid FTH1 deletion reduces dextran sodium sulfate (DSS)-induced acute colitis through inhibiting DMT1/iron-driven STAT3 signaling. The STAT3 reactivation via JAK2 activator Coumermycin A1 (C-A1) restored the susceptibility of myeloid FTH1-deficient mice to DSS-induced acute colitis. Consistent with the colitis data, myeloid FTH1-deficient mice show reduced tumor formation, lower DMT1, and lower inflammatory cytokine levels in colon tumor tissues. Our results indicate that myeloid FTH1 is important in intestinal inflammation and colorectal cancer development under excess iron condition.
Materials and Methods
Antibody and Reagent
Actin (sc-8432) and F4/80 (sc-52664) were from Santa Cruz Biotechnology (Dallas, TX). Divalent metal transporter 1 (D3V8G), Ki-67 (12202T) and p-STAT3 Y705 (9145S) were from Cell Signaling Technology (Danvers, MA). Secondary antibodies against rabbit (7074S) or mouse IgG (7076S) were from Cell Signaling Technology (Danvers, MA). The JAK2 activator Coumermycin A1 (C-A1) (C9451) was from Promega (Madison, WI).
Mice
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of New Mexico Health Sciences Center. All Fth1 flox/flox mice and Lysozyme M (LysM)-Cre mice (both are C57BL/6J background) were purchased from Jackson Laboratory. These 2 mouse lines were crossed to generate myeloid cell-specific FTH1-deficient mice (LysM Cre+ Fth1 Floxed, KO) or control littermates (LysM Cre- Fth1 Floxed, WT). We used both male and female mice in our study because we have not observed any sex difference in our studies.
Induction of Colitis
Wild type or KO mice (6-8 weeks old) were fed with a chow diet (200 mg/kg iron, 2920X, ENVIGO, USA) or a high-iron diet (1000 mg/kg iron, 1000Fe, ENVIGO, USA); and 2% to 3% of (w/v) DSS (Thermo Scientific) was dissolved in sterile, distilled water ad libitum for 7 days followed by 2 days of normal drinking water. Body weights were monitored daily. To activate STAT3, mice were treated daily with JAK2 activator C-A1 by intraperitoneal (i.p.) injection (0.2 mg/kg) since DSS treatment.
Induction of Colorectal Cancer
Mice 6-8-weeks old were fed with a chow diet or a high-iron diet (ENVIGO, USA) and treated with azoxymethane (AOM; 10 mg/kg body weight, i.p.). One day after the AOM injection, mice were treated with 1.5% DSS for 7 days followed by 14 days of regular water. After treating with 1.5% DSS for another 7 days, mice were changed to water for 28 days before killed for tumor counting and collection. Tumor size in diameter (R) was measured by a caliper in both width (W) and length (L) directions and calculated as, R = (W+L)/2. Tumor load (V) was calculated as a summary of all tumors in a mouse: V = 4/3 × 3.14 × R3/8.
Isolation of Peritoneal Neutrophils
Mice were intraperitoneally injected with 1 mL of 3% thioglycolate solution (10 μL/g). Four hours later, mice were killed and 5 mL sterile PBS was injected into the peritoneal cavity. After massaged gently for 60 seconds, the peritoneal fluid was collected and centrifuged for 15 minutes at 200g to recover the neutrophils.
Bone Marrow-derived Macrophage
Bone marrow cells were collected from tibias and femurs by flushing them with 5 mL of cold PBS containing 1% penicillin-streptomycin. After filtered through a 70 μm–Nylon cell strainer, the solution was centrifuged at 450 g for 10 minutes at 4°C. Bone marrow cells were resuspended and cultured in completed DMEM with 50 ng/mL recombinant murine M-CSF (VWR) for a week before collection.
Western Blot Analysis
Animal tissues were lysed and homogenized in radioimmunoprecipitation assay (RIPA) buffer and incubated on ice for 15 minutes. After incubation, tissue extracts were centrifuged at 4°C for 15 minutes. Supernatant was collected, and then protein concentration was measured with Bradford assay. Same amounts (30-50 μg) of protein were loaded for SDS-PAGE gel analysis. After running for 1 hour at 120V, proteins were transferred to nitrocellulose membranes for 1 hour at 100 V. After blocking with 5% milk, membranes were incubated with primary antibodies overnight at 4°C. After the membranes were incubated with second antibodies, they were developed with Chemidoc Image machine (BioRad).
Histology and Immunofluorescence Staining
Colon tissues were collected and intestinal contents were removed. After being fixed with 10% formalin at room temperature for 48 hours, colons were embedded in paraffin blocks. Paraffin slides (5 µm) were stained with hematoxylin and eosin (H&E) and analyzed by a gastrointestinal pathologist (D.M.) in a blinded manner for histology. For the colitis model, inflammation was scored based on the following scheme: grade 1, scattered neutrophils in lamina propria; grade 2, scattered neutrophils plus lymphocytes or plasma cells in lamina propria; and grade 3, dense mixed inflammatory infiltrates involving mucosa plus submucosa. Crypt damage score was based on the following scheme: grade 1, severely crypt attenuation; grade 2, basal crypt atrophy; grade 3, near complete crypt atrophy/drop off; and grade 4, full thickness mucosal drop out. The percentage of involved colons were also scored. Then the final score was achieved by multiplying the percentage with the summary score of inflammation and crypt injury. For tumorigenesis model, we classified tissues as tubular adenoma with low grade (LG, score 1) vs high grade (HG, score 2) dysplasia, and the involved lesion percentage was estimated. The final pathological score was achieved by multiplying the lesion percentage with the grade score. For immunofluorescence analysis after antigen unmasking, tissues were incubated overnight with primary antibody at 4°C. After washing with PBS containing 0.1% Tween-20 for 5 minutes 3 times, slides were incubated with secondary antibody, and images were taken with a fluorescence microscope (EVOS, Thermo Fisher Scientific).
3,3ʹ-Diaminobenzidine (DAB) Enhanced Perl’s Iron Staining
For diaminobenzidine (DAB)-enhanced Perl’s iron staining, sections were incubated in the mixture of 2% hydrochloric acid and 1% ferrocyanide solution (1:1) for 30 minutes. After incubation, the sections were washed in tap water for 5 minutes. Then the slides were incubated with DAB solution (0.033% H2O2 in 0.05% DAB in PBS pH 7.4) for 20 minutes. After washing in tap water for 5 minutes, the slides were sealed with Coverslip using Permount Mounting Medium (Fisher Scientific, Hampton, NH).
Real-time Quantitative Polymerase Chain Reaction Analysis
Animal tissue RNAs were extracted using an IBI reagent Kit (IB47602, IBI Scientific, Dubuque, IA). After reverse transcription, gene expression was analyzed by SYBR green quantitative polymerase chain reaction (qPCR) with a LightCycler 480 instrument (Roche Diagnostics, Indianapolis, IN). All Gene expression was normalized to 18 seconds. Primer and its sequence are listed in Supplement Table 1.
Statistical Analysis
All experiments were repeated 3 times. Data were expressed as mean +/- SD. Student t test, 1-way or 2-way analysis of variance (ANOVA) was applied and P < .05 was considered statistically significant.
Results
Spleen Iron Content Is Reduced in Myeloid Cell-specific FTH1 Knockout Mice
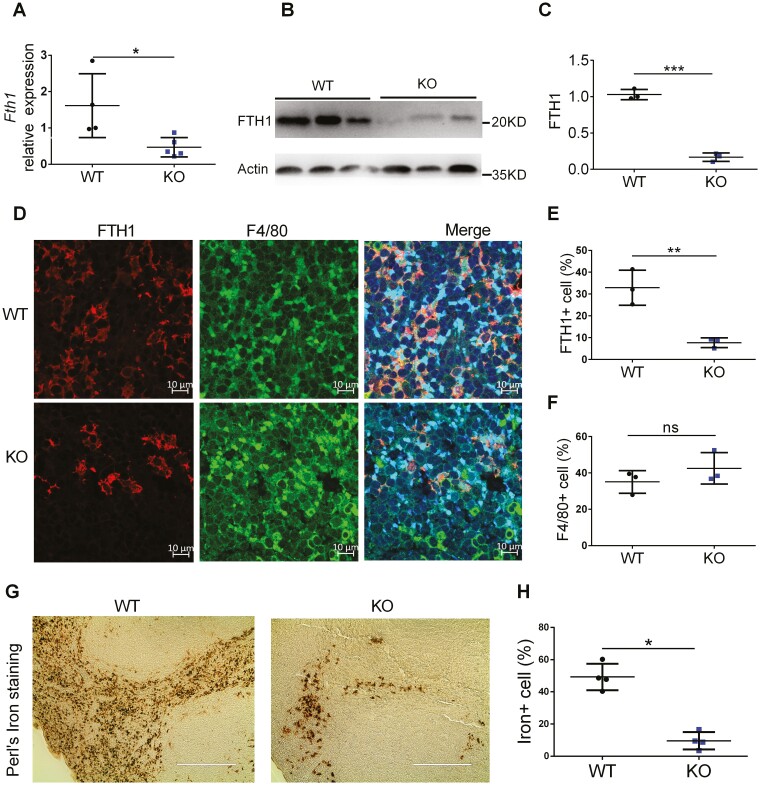
To verify FTH1 deletion in myeloid cells, we first analyzed the RNA and protein levels of FTH1 in spleen tissues of Fth1flox/flox (WT) and LysM-Cre Fth1flox/flox (KO) mice. Compared with WT mice, KO mice showed significantly reduced Fth1 gene expression at both RNA (Figure 1A) and protein levels (Figure 1B, 1C). We also performed costaining of FTH1 and macrophage marker F4/80 in the spleen (Figure 1D) by immunofluorescence (IF) staining. Quantification of IF results indicated that FTH1 expression in KO mice was greatly reduced (Figure 1E), whereas F4/80 showed similar expression (Figure 1F) in WT and KO mice. Perls’ iron staining showed that spleen iron levels were greatly decreased in KO mice (Figure 1G, H). Furthermore, we isolated peritoneal neutrophils from thioglycolate-elicited ascites (Figure S1A) and macrophages from bone marrow (Figure S1B); and confirmed the expression of FTH1 protein was depleted in the KO mice. Our results indicate that FTH1 expression is significantly reduced in myeloid cells from the KO mice.
Figure 1.
Characterization of myeloid cell-specific FTH1 knockout mice. Analysis of FTH1 expression in spleen tissues from WT and KO mice by (A) qPCR, (B) Western blot and (C) quantification of FTH1 protein. D, Confocal microscopy analysis of FTH1 and F4/80 co-staining in spleen tissues of WT and KO mice (Bar = 10 μm), (E) FTH1 quantification and (F) F4/80 quantification. G, Perl’s iron staining of spleen tissues of WT and KO mice (Bar = 200 μm) and (H) quantification. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. not significant. Unpaired Student t test.
Myeloid Cell-specific FTH1 Knockout Mice Are Protected From DSS-Induced Colitis Under the High Iron Condition
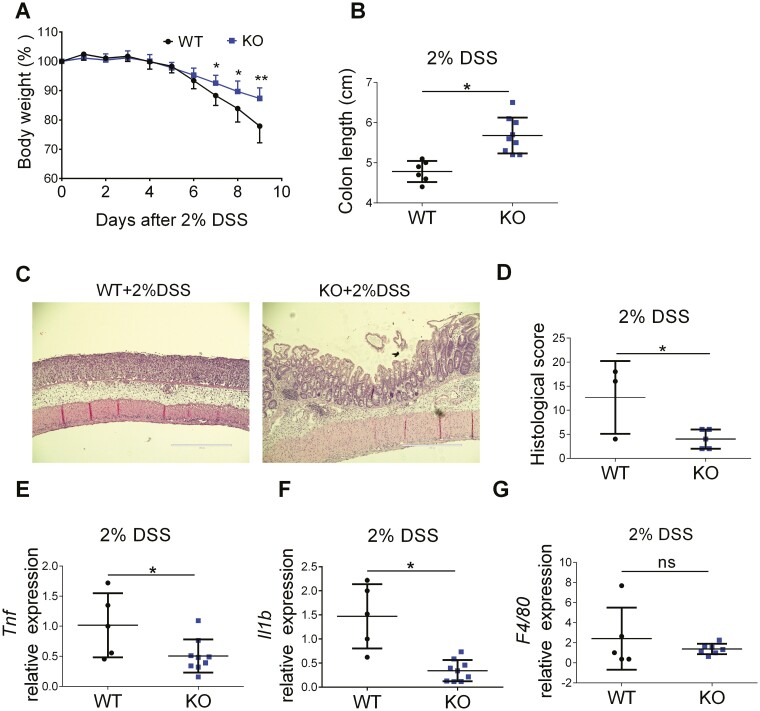
To study the role of myeloid cell-specific FTH1 in intestinal inflammation, we treated WT and FTH1 KO mice with 3% DSS for 7 days to induce acute colitis. We found there was no difference in body weight loss (Figure S2A), colon length (Figure S2B), or colon tissue histology (Figure S2C) between WT and FTH1 KO mice, suggesting no difference in colitis severity at this condition. We then fed WT and FTH1 KO mice with a normal iron diet (40 ppm Fe) and a high-iron diet (1000 ppm Fe) before induction of colitis with 2% DSS. The colon length of WT mice was significantly reduced by 1000 ppm iron compared with 40 ppm iron (Figure S2D), indicating iron supplementation aggravates colitis in DSS-treated WT mice. Under the high-iron diet condition, we consistently found FTH1 KO mice were more resistant to DSS treatment, with less body weight loss (Figure 2A) and longer colon length (Figure 2B) compared with WT mice. Hematoxylin and eosin staining (Figure 2C) and histopathology scoring (Figure 2D) confirmed KO mice had less intestinal inflammation. Immunofluorescence staining showed reduced FTH1 expression in the colonic submucosa from KO mice (Figure S3A and S3B). We further analyzed the cytokine expression in DSS-treated colon samples and found that KO mice showed lower expression of pro-inflammatory cytokines TNF (Figure 2E) and IL-1b (Figure 2F), as well as lower expression of anti-inflammatory cytokines IL-10 (Figure S3C) and IL-22 (Figure S3D), which might be secondary to decreased tissue damage in KO mice. Consistent with our observation in the spleen, there was no change in the expression of macrophage maker F4/80 (Figure 2G). Furthermore, macrophage polarization markers (iNos and Arg1) and neutrophil markers (Ly6g and Mpo) were not changed (Figure S3E-S3H), which indicates no macrophage polarization or neutrophil number change in our model. Together, these data show that FTH1 knockout in myeloid cells protects mice from DSS-induced acute colitis under a high-iron diet.
Figure 2.
Myeloid cell-specific FTH1 knockout mice are protected from DSS-induced colitis. A, Body weight and (B) colon length of WT and KO mice treated with high-iron diet (1000Fe, 1000mg/kg) and 2% DSS for 7 days followed by 2 days of regular water. C, H&E staining (Bar = 400 μm), (D) histological scoring, (E) TNF, (F) IL-1b and (G) F4/80 expression in DSS-treated colon tissues of WT and KO mice. *P < 0.05, **P < 0.01, n.s. not significant. Unpaired Student t test.
Myeloid Cell-specific FTH1 Knockout Mice Show Repressed STAT3 Activation During Colitis
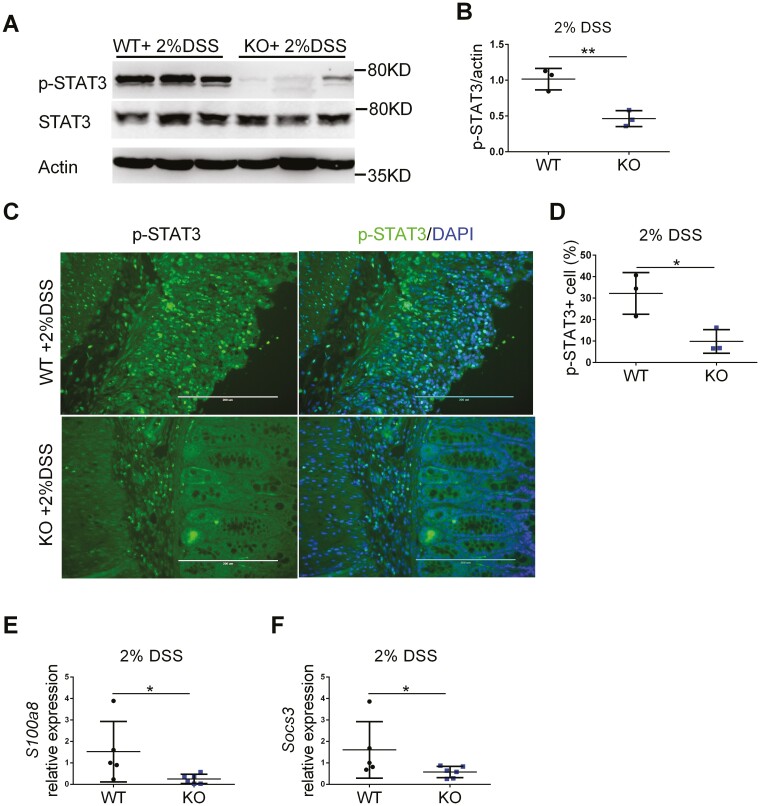
Ferritin expression has the highest correlation value with STAT3 expression and is required for STAT3 activation and tumorigenesis in glioblastoma.26 To study if STAT3 signaling is involved in FTH1 KO mice resistance to DSS-induced acute colitis under high-iron treatment, we analyzed STAT3 signaling in the colon tissues of WT and FTH1 KO mice treated with DSS. Compared with WT mice, the active phosphorylated STAT3 levels were significantly decreased in the colon tissues from the FTH1 KO mice (Figure 3A, 3B). Immunofluorescence staining showed less phosphorylated STAT3 in the colon tissues from the FTH1 KO mice (Figure 3C, 3D)). Interleukin-11, Oncostatin M (Osm), and IL-6 are potent STAT3 activators in intestine,27–29 but they were not significantly changed in our study (Figure S3I-S3K). Interestingly, the expression of 2 STAT3 target genes (S100a830 and Socs331) were significantly reduced in the KO mice (Figure 3E-3F). These data suggest that STAT3 signaling is dampened in colonic myeloid cells from these myeloid-specific FTH1 knockout mice.
Figure 3.
Myeloid cell-specific FTH1 knockout mice show inhibited STAT3 activation during colitis. A, Western blot analysis of p-STAT3 Y705 in colon tissues and (B) quantification. C, Immunofluorescence staining of p-STAT3 in colon tissues and (D) quantification. Quantitative PCR analysis of (E) S100a8, (F) Socs3 expression in colon tissues. *P < 0.05, **P < 0.01, Unpaired Student t test.
Myeloid Cell-specific FTH1 Depletion Suppresses DSS-induced Colitis Through STAT3 Inhibition
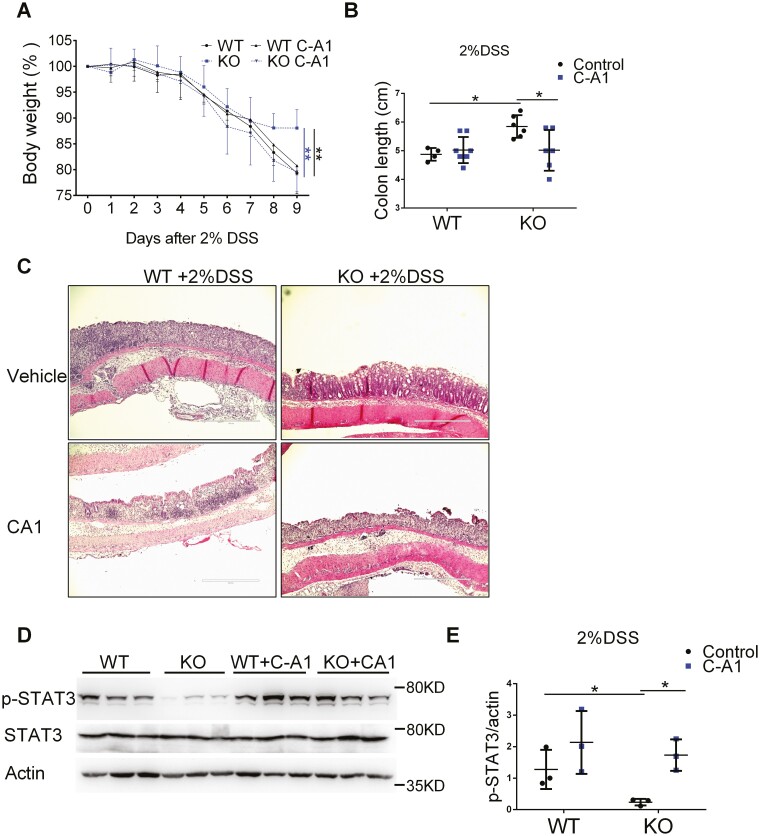
To further confirm that FTH1 knockout suppresses colitis through inhibiting STAT3 activation, we treated FTH1 KO mice with vehicle or JAK2 activator Coumermycin A1 (C-A1). We found that FTH1 KO mice showed less body weight loss (Figure 4A) and longer colon length (Figure 4B) compared with WT mice as expected, and JAK2 activator C-A1 treatment blunted these protective effects. Hematoxylin and eosin staining indicated JAK2 activator did restore inflammation in FTH1 KO mice (Figure 4C). Reactivation of JAK2-STAT3 signaling pathway was confirmed by immunoblot analysis (Figure 4D, 4E). Together, these data indicate that FTH1 KO might suppresses DSS-induced acute colitis via STAT3 inhibition.
Figure 4.
Myeloid cell-specific FTH1 depletion suppresses DSS-induced colitis through STAT3 inhibition. A, Body weight curve, (B) colon length and (C) colon H&E image of WT and KO mice treated daily with vehicle or JAK2 activator C-A1 (i.p. 0.2 mg/kg) since DSS treatment. D, Western blot analysis of p-STAT3 in colon tissues of WT and KO mice treated with vehicle or JAK2 activator C-A1 and (E) quantification. *P < 0.05, **P < 0.01, Unpaired t test or 2-way ANOVA followed by Dunnett’s multiple comparisons test.
Myeloid Cell-specific FTH1 Knockout Mice Show Reduced Colitis-associated Colon Cancer
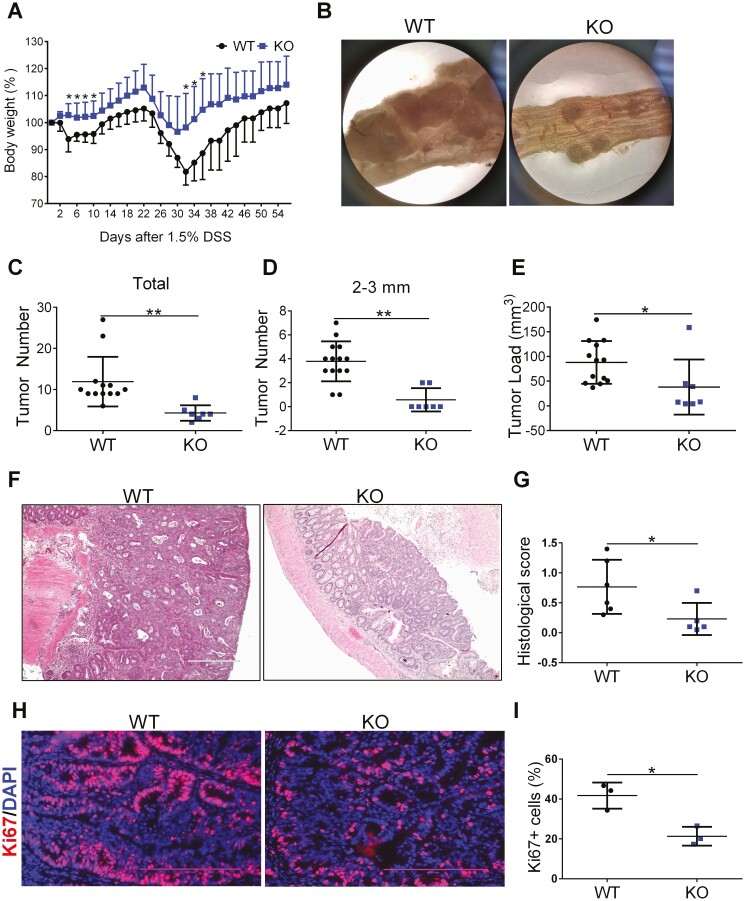
To investigate the role of myeloid cell-specific FTH1 in colitis-associated colon cancer, we used the classic azoxymethane (AOM)/DSS model. We found there was no difference in tumorigenesis between WT and KO mice when fed with a normal chow diet even though there was a trend of higher body weight gain in the KO mice (Figure S4A-4G). Then we further fed these mice with a high-iron diet after AOM injection, followed by 2 cycles of 1.5% DSS. We found that KO mice showed less body weight loss (Figure 5A), which was consistent with our acute colitis results in Figure 2. There were fewer tumors in the KO mice group compared with the WT group under a dissection microscope (Figure 5B). Tumor counting indicated there were fewer colorectal tumors in KO mice (Figure 5C). Although KO mice showed less medium-sized tumors (sizes of 2-3 mm, Figure 5D), there was no difference in the numbers of small tumors (sizes less than 2 mm, Figure S5A) and big tumors (sizes more than 3 mm, Figure S5B) between WT and KO mice. Tumor load was also less in KO mice than WT mice (Figure 5E). Hematoxylin and eosin staining and scoring in colon tumors revealed that KO mice had lower pathological scores than WT mice (Figure 5F, 5G). We analyzed cancer cell proliferation in WT and KO tumors by Ki67 staining and found cell proliferation was inhibited in KO tumors (Figure 5H, 5I). Our data suggest that myeloid cell-specific FTH1 knockout suppresses colitis-associated colon tumorigenesis.
Figure 5.
Myeloid cell-specific FTH1 knockout mice show reduced colitis-associated colon tumor. A, Body weight curve and (B) macroscopy tumor image of WT and KO mice treated with AOM/DSS. Number of total colonic tumors (C) or colonic tumor with its size between 2 mm and 3 mm (D) and total tumor load (E) in WT and KO mice. F, H&E staining (Bar = 400 μm), (G) histological scoring, (H) Ki67 staining (Bar = 200 μm), and (I) quantification of colon tissues of WT and KO mice. *P < 0.05, **P < 0.01, Unpaired Student t test.
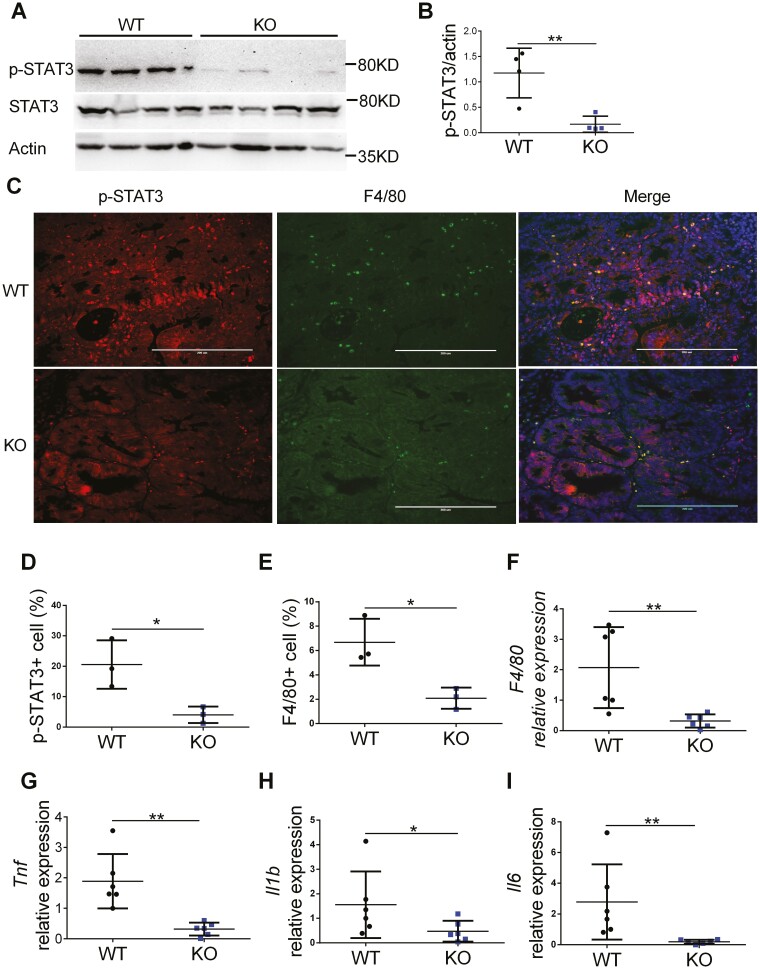
Tumors From Myeloid Cell-specific FTH1 Knockout Mice Have Reduced STAT3 Activation and Inflammation
We further analyzed the potential molecular mechanisms for reduced colitis-associated colon cancer in FTH1 KO mice. We found that STAT3 activation (Figure 6A, 6B) was reduced in the tumor tissues from FTH1 KO. This is consistent with our acute colitis results in Figure 3. Immunofluorescence staining of p-STAT3 indicated there was reduced STAT3 activation within KO tumors (Figure 6C, 6D). The expression of F4/80 protein was also reduced in KO tumors (Figure 6C, 6E) and showed costaining with p-STAT3 protein (Figure 6C). The qPCR analysis further confirmed F4/80 expression was inhibited in KO tumors (Figure 6F). To study if there was reduced inflammation in KO tumors, we analyzed the expression of inflammatory cytokines in WT and KO tumors. We found there was reduced expression of pro-inflammatory cytokines such as TNF (Figure 6G), IL-1b (Figure 6H), and IL-6 (Figure 6I) in KO tumors. Interestingly, the expression of STAT3 target genes Socs3 (Figure S5C) and S100a8 (Figure S5D) were significantly reduced. Together, these data suggest that myeloid FTH1 is critical for maintaining STAT3 activation and the expression of pro-inflammatory cytokines in colitis-associated CRC.
Figure 6.
Tumors from myeloid-specific FTH1 knockout mice show reduced STAT3 activation and inflammation. A, Western analyze of p-STAT3 and (B) quantification of p-STAT3 in tumor tissues of WT and KO mice. C, Immunofluorescence staining of p-STAT3 and F4/80 in WT and KO colon tumors, quantification of p-STAT3(D) and (E) F4/80 positive cells. Quantitative PCR analysis of (F) F4/80, (G) TNF, (H) IL-1b, (I) IL-6 expression in colonic tumor tissues of WT and KO mice. *P < 0.05, **P < 0.01. Unpaired Student t test.
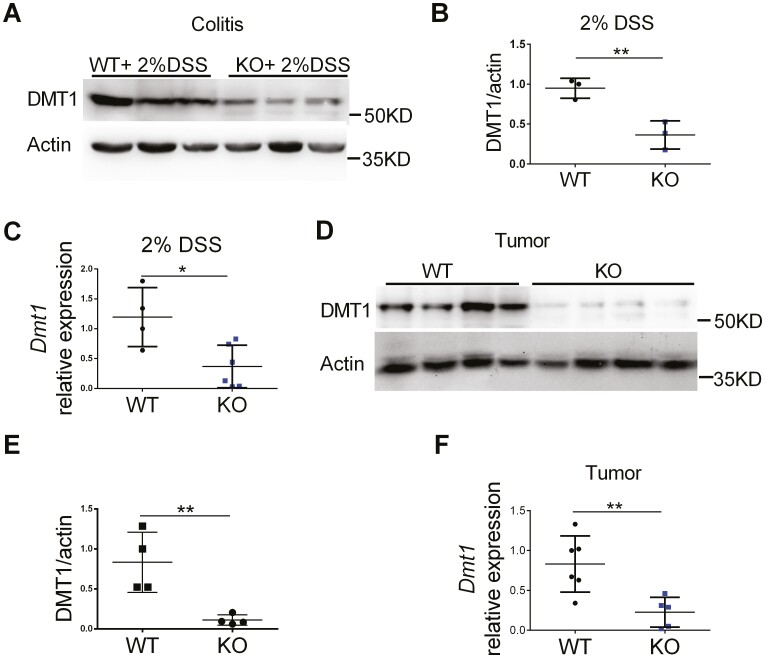
Myeloid Cell-specific FTH1 Knockout Mice Show Repressed DMT1 Expression in Inflamed and Tumor Colon Tissues
Myeloid-specific deletion of the Fth1 gene causes decrease of the Tfrc expression and increase of the Fpn expression in addition to macrophage iron content reduction.21,32 The exact regulatory mechanisms for these changes are not known, but it is reasonable to consider these changes in iron regulators as a compensatory response because FTH1 plays a crucial role in iron metabolism and exerts its cytoprotective action by reducing iron toxicity via its ferroxidase activity.21 We observed reduced spleen iron content, but the expression of Tfrc was only significantly decreased in the colon tumors (Figure S6A, S6B); and Fpn was reduced in the inflamed colon tissues (Figure S6C, S6D) from our FTH1 KO mice. Thus, neither Tfrc nor Fpn can explain the potential reduced cellular iron content, colonic inflammation, or tumors in the KO mice.
Previously, we have shown that DMT1 is important in colon tumor iron uptake, JAK-STAT3 signaling activation, and colon tumorigenesis.4 Thus, we investigated and found that the expression of DMT1 was reduced in the inflamed (Figure 7A-7C) and tumor (Figure 7D-7F) colon tissues from FTH1 KO mice. This may partly contribute to the reduced macrophage iron content, colonic inflammation, and tumors. Together, these data suggest that DMT1/iron-activated STAT3 signaling is dampened in colonic myeloid cells from these myeloid-specific FTH1 knockout mice.
Figure 7.
Myeloid cell-specific FTH1 knockout mice show repressed DMT1 expression in inflamed and tumor colon tissues. A, Quantitative PCR analysis, (B) Western blot analysis, and (C) quantification of DMT1 expression in inflamed colon tissues. D, Quantitative PCR analysis, (E) Western blot analysis, and (F) quantification of DMT1 expression in colorectal tumor tissues. *P < 0.05, **P < 0.01. Unpaired Student t test.
Discussion
Myeloid cell FTH1 deficiency protects mice from various types of inflammation including high fat diet–induced adipose tissue inflammation,21 cecal ligation and puncture-induced sepsis,22 unilateral ureteral obstruction –induced kidney inflammation.20 In this study, we found that myeloid FTH1 deficiency protects mice from DSS-induced acute colitis and colitis-associated colorectal cancer via reducing DMT1/iron-driven STAT3 activation. Our results indicate a pivotal role for myeloid FTH1 and STAT3 in intestinal inflammation and cancer.
In the spleen tissue, we observed that some F4/80+ cells maintained high expression of FTH1. This is consistent with a previous report showing that some spleen myeloid cells do not express lysozyme 2 gene (Lyz2 or LysM).33 In addition, our Perl’s iron staining showed that splenic iron levels were decreased in FTH1 KO mice, suggesting FTH1 is required for maintaining iron retention in the myeloid cells. The splenic iron levels presented here were similar to previous reports.21,32 We observed reduced expression of Dmt1 in the colon tissues from the FTH1 KO mice, suggesting the iron levels are also reduced in the myeloid cells from the colon. Further experiments to isolate these colonic macrophages and measure their iron contents are needed to validate this hypothesis in the future.
It is intriguing that the protective effects of FTH1 KO only occurs under the high iron condition. We believe this to be due to the fact that the disease severity in wild type mice is increased under the high-iron condition. As a result, the difference between WT and KO mice is larger and significant under the high-iron condition.
Ferritin heavy chain is important in iron storage through its ferroxidase activity, which converts ferrous iron into ferric iron and stores it in the ferritin complex.18,19,34 Thus, FTH1 is known as a negative regulator of the iron-dependent cell death, ferroptosis,35 via reducing iron-induced reactive oxygen species production.35,36 In line with this concept, FTH1 deficiency promotes the death of cardiomyocyte and causes heart failure.37 Moreover, FTH1 plays a protective role in endothelial cell and hepatocyte.38 Interestingly, consistent with a previous report,20 we did not observe changes in macrophage and neutrophil numbers after myeloid FTH1 deficiency, suggesting that myeloid cells are more resistant to FTH1 deficiency–induced cell death than other cell types such as cardiomyocyte, which is susceptible to ferroptosis when FTH1 is deficient.
Dietary iron enhances colonic inflammation and IL-6/IL-11-STAT3 signaling, promoting colonic tumor development in mice.39 In neutrophils, STAT3 inhibition reduces the secretion of anti-bacterial proteins S100A8/9 and DSS-induced intestine inflammation.30 Activation of macrophage SOCS3, a downstream target as well as a feedback inhibitor of STAT3 activation, reduces DSS-induced colitis.40 These results are consistent with our observation that the mRNA expression levels of S100a8 and Socs3 were reduced in the colon tissues from FTH1 KO mice, suggesting myeloid STAT3 signaling pathway plays a critical role in intestinal inflammation. However, STAT3 depletion in myeloid cell has also been shown to promote intestine inflammation.41,42 It might be due to difference between partial or total depletion of this pleiotropic gene.43 Also, although we showed that pharmacological reactivation of STAT3 restored colitis in KO mice, it is still not clear whether the reduced p-STAT3 is causative or epiphenomena of reduced DSS damage. A genetic approach to specifically activate or inhibit STAT3 in myeloid cells is needed to corroborate our findings. It is possible STAT3 activation might not be the direct mechanism for FTH1 KO-medicated protection against colitis and colorectal cancer, because STATs in general can bind many JAKs. For example, JAK2 also activates STAT1,44,45 STAT2,46 and STAT5.47 It is possible that these signaling pathways are involved in the protection against colitis in FTH1 KO mice.
Activation of STAT3 in both epithelial cells and myeloid cells is required for colorectal tumor formation.48,49 Mice with intestinal epithelium-specific deletion of STAT3 show reduced colitis-associated colorectal cancer.50,51 Myeloid STAT3 deficiency also leads to T-cell activation in tumor stroma and inhibits colorectal cancer progression.49 Consistently, we found myeloid FTH1 deficiency leads to reduced STAT3 activation and colorectal tumor formation in mice, indicating myeloid FTH1-STAT3 signaling is required for colorectal tumor formation.
In conclusion, myeloid FTH1 deficiency protects mice from colitis and colitis-associated colorectal cancer via reducing DMT1-iron-STAT3 signaling under excess iron condition. Our study suggests that myeloid FTH1 is a new therapeutic target for colitis and colitis-associated colorectal cancer patients with iron-overload conditions.
Supplementary Material
Acknowledgments
We thank Dr. Eliseo Castillo for providing LysM-cre mice, Dr.Sharina P. Desai for help with confocal imaging, and Mark Laguan for proofreading the manuscript.
Glossary
Abbreviations
- AOM
azoxymethane
- CRC
colorectal cancer
- DSS
dextran sodium sulfate
- IP
intraperitoneal
- TAM
tamoxifen;
- DMEM
Dulbecco’s Modified Eagle Medium
- IL
Interleukin
- IFN
Interferon
- M-CSF
Macrophage colony-stimulating factor
- PBS
Phosphate buffered saline
- TNF
Tumor necrosis factor
- FTH1
ferritin heavy chain;
- C-A1
Coumermycin A1
- WT
Fth1 flox/flox
- KO
LysM-cre Fth1flox/flox
Contributor Information
Zhaoli Liu, Department of Biochemistry and Molecular Biology, University of New Mexico, Albuquerque, NM, 87131, USA.
Mariella Arcos, Department of Biochemistry and Molecular Biology, University of New Mexico, Albuquerque, NM, 87131, USA.
David R Martin, Department of Pathology, University of New Mexico, Albuquerque, NM 87131, USA.
Xiang Xue, Department of Biochemistry and Molecular Biology, University of New Mexico, Albuquerque, NM, 87131, USA.
Funding
This research was supported in part by the National Institutes of Health (P20 GM130422) and a Research Scholar Grant from the American Cancer Society (RSG-18-050-01-NEC).
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- [1]. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- [2]. Seril DN, Liao J, West AB, Yang G-Y.. High-iron diet: foe or feat in ulcerative colitis and ulcerative colitis-associated carcinogenesis. J Clin Gastroenterol. 2006;40(5):391-397. doi: 10.1097/00004836-200605000-00006 [DOI] [PubMed] [Google Scholar]
- [3]. Torti SV, Torti FM.. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13(5):342-355. doi: 10.1038/nrc3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Xue X, Ramakrishnan SK, Weisz K, et al. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 2016;24(3):447-461. doi: 10.1016/j.cmet.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Xue X, Taylor M, Anderson E, et al. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 2012;72(9):2285-2293. doi: 10.1158/0008-5472.CAN-11-3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Rivera A, Siracusa MC, Yap GS, Gause WC.. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17(4):356-363. doi: 10.1038/ni.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Haschka D, Hoffmann A, Weiss G.. Iron in immune cell function and host defense. Semin Cell Dev Biol. 2021;115:27-36. doi: 10.1016/j.semcdb.2020.12.005 [DOI] [PubMed] [Google Scholar]
- [8]. Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4(5–6):446-453. doi: 10.1159/000336423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Kim ER, Chang DK.. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20(29):9872-9881. doi: 10.3748/wjg.v20.i29.9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Lukas M. Inflammatory bowel disease as a risk factor for colorectal cancer. Dig Dis. 2010;28(4–5):619-624. doi: 10.1159/000320276 [DOI] [PubMed] [Google Scholar]
- [11]. Puga AM, Samaniego-Vaesken M. de L, Montero-Bravo A, Ruperto M, Partearroyo T, Varela-Moreiras G.. Iron supplementation at the crossroads of nutrition and gut microbiota: the state of the art. Nutrients 2022;14(9):1926. doi: 10.3390/nu14091926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Mei Z, Addo OY, Jefferds ME, Sharma AJ, Flores-Ayala RC, Brittenham GM.. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet. Haematology 2021;8(8):e572-e582. doi: 10.1016/S2352-3026(21)00168-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. DeLoughery TG. Iron deficiency anemia. Med Clin North Am. 2017;101(2):319-332. doi: 10.1016/j.mcna.2016.09.004 [DOI] [PubMed] [Google Scholar]
- [14]. Kaitha S, Bashir M, Ali T.. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6(3):62-72. doi: 10.4291/wjgp.v6.i3.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Wilson MJ, Dekker JWT, Harlaar JJ, Jeekel J, Schipperus M, Zwaginga JJ.. The role of preoperative iron deficiency in colorectal cancer patients: prevalence and treatment. Int J Colorectal Dis. 2017;32(11):1617-1624. doi: 10.1007/s00384-017-2898-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Zimmermann MB, Hurrell RF.. Nutritional iron deficiency. Lancet (London, England). 2007;370(9586):511-520. doi: 10.1016/S0140-6736(07)61235-5 [DOI] [PubMed] [Google Scholar]
- [17]. Verma S, Cherayil BJ.. Iron and inflammation - the gut reaction. Metallomics: Integrated Biometal Sci 2017;9(2):101-111. doi: 10.1039/c6mt00282j [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Harrison PM, Arosio P.. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275(3):161-203. doi: 10.1016/0005-2728(96)00022-9 [DOI] [PubMed] [Google Scholar]
- [19]. Lawson DM, Artymiuk PJ, Yewdall SJ, et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature. 1991;349(6309):541-544. doi: 10.1038/349541a0 [DOI] [PubMed] [Google Scholar]
- [20]. Bolisetty S, Zarjou A, Hull TD, et al. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int. 2015;88(1):95-108. doi: 10.1038/ki.2015.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Ikeda Y, Watanabe H, Shiuchi T, et al. Deletion of H-ferritin in macrophages alleviates obesity and diabetes induced by high-fat diet in mice. Diabetologia. 2020;63(8):1588-1602. doi: 10.1007/s00125-020-05153-0 [DOI] [PubMed] [Google Scholar]
- [22]. Zarjou A, Black LM, McCullough KR, et al. Ferritin light chain confers protection against sepsis-induced inflammation and organ injury. Front Immunol. 2019;10(FEB):1-15. doi: 10.3389/fimmu.2019.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Eshhar Z, Order SE, Katz DH.. Ferritin, a Hodgkin’s disease associated antigen. Proc Natl Acad Sci USA. 1974;71(10):3956-3960. doi: 10.1073/pnas.71.10.3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Muhammad JS, Bajbouj K, Shafarin J, Hamad M.. Estrogen-induced epigenetic silencing of FTH1 and TFRC genes reduces liver cancer cell growth and survival. Epigenetics 2020;15(12):1302-1318. doi: 10.1080/15592294.2020.1770917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Campo E, Palacin A, Benasco C, Condom E, Cardesa A.. Ferritin immunohistochemical localization in normal and neoplastic colonic mucosa. Int J Biol Markers. 1987;2(3):177-183. [DOI] [PubMed] [Google Scholar]
- [26]. Schonberg DL, Miller TE, Wu Q, et al. Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell 2015;28(4):441-455. doi: 10.1016/j.ccell.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Fung KY, Louis C, Metcalfe RD, et al. Emerging roles for IL-11 in inflammatory diseases. Cytokine. 2022;149:15. doi: 10.1016/j.cyto.2021.155750 [DOI] [PubMed] [Google Scholar]
- [28]. West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23(5): 579–589. doi: 10.1038/nm.4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Johnson DE, O’Keefe RA, Grandis JR.. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234-248. doi: 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Loh JT, Lee K, Lee AP, Kay J, Teo H, Lim HL, et al. DOK3 maintains intestinal homeostasis by suppressing JAK2/ STAT3 signaling and S100a8/ 9 production in neutrophils. Cell Death Dis. 2021;12(11):1054. doi: 10.1038/s41419-021-04357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Babon JJ, Nicola NA.. The biology and mechanism of action of suppressor of cytokine signaling 3. Growth Factors (Chur, Switzerland) 2012;30(4):207-219. doi: 10.3109/08977194.2012.687375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Haschka D, Tymoszuk P, Petzer V, et al. Ferritin H deficiency deteriorates cellular iron handling and worsens Salmonella typhimurium infection by triggering hyperinflammation. JCI Insight 2021;6(13):e141760. doi: 10.1172/jci.insight.141760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Shi J, Hua L, Harmer D, Li P, Ren G.. Cre driver mice targeting macrophages. Methods Mol Biol (Clifton, N.J.) 2018;1784:263-275. doi: 10.1007/978-1-4939-7837-3_24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Santambrogio P, Levi S, Cozzi A, Corsi B, Arosio P.. Evidence that the specificity of iron incorporation into homopolymers of human ferritin L- and H-chains is conferred by the nucleation and ferroxidase centres. Biochem J. 1996;314(Pt 1(Pt 1):139-144. doi: 10.1042/bj3140139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Tian Y, Lu J, Hao X, et al. FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson’s disease. Neurotherapeutics 2020;17(4):1796-1812. doi: 10.1007/s13311-020-00929-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Fang Y, Chen X, Tan Q, Zhou H, Xu J, Gu Q.. Inhibiting ferroptosis through Disrupting the NCOA4–FTH1 interaction: a new mechanism of action. ACS Cent Sci. 2021;7(6):980-989. doi: 10.1021/acscentsci.0c01592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Omiya S, Hikoso S, Imanishi Y, et al. Downregulation of ferritin heavy chain increases labile iron pool, oxidative stress and cell death in cardiomyocytes. J Mol Cell Cardiol. 2009;46(1):59-66. doi: 10.1016/j.yjmcc.2008.09.714 [DOI] [PubMed] [Google Scholar]
- [38]. Berberat PO, Katori M, Kaczmarek E, et al. Heavy chain ferritin acts as an anti-apoptotic gene that protects livers from ischemia-reperfusion injury. FASEB J. 2003;17(12):1724-1726. doi: 10.1096/fj.03-0229fje [DOI] [PubMed] [Google Scholar]
- [39]. Chua ACG, Klopcic BRS, Ho DS, et al. Dietary iron enhances colonic inflammation and IL-6/IL-11-Stat3 signaling promoting colonic tumor development in mice. PLoS One. 2013;8(11):e78850. doi: 10.1371/journal.pone.0078850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Zhang X, Wang Y, Yuan J, et al. Macrophage/microglial Ezh2 facilitates autoimmune inflammation through inhibition of Socs3. J Exp Med. 2018;215(5):1365-1382. doi: 10.1084/jem.20171417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Kobayashi M, Kweon M-N, Kuwata H, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111(9):1297-1308. doi: 10.1172/JCI17085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10(1):39-49. doi: 10.1016/S1074-7613(00)80005-9 [DOI] [PubMed] [Google Scholar]
- [43]. Housden BE, Muhar M, Gemberling M, et al. Loss-of-function genetic tools for animal models: cross-species and cross-platform differences. Nat Rev Genet. 2017;18(1):24-40. doi: 10.1038/nrg.2016.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Moon JW, Kong S-K, Kim BS, et al. IFNγ induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci Rep. 20177810;7(1):1. doi: 10.1038/s41598-017-18132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Wang J, Zhang F, Xu H, et al. TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury. Clin Trans Med 2022;12(6):e894. doi: 10.1002/ctm2.894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Xu L, Zhang H, Wang Y, et al. FABP4 activates the JAK2/STAT2 pathway via Rap1a in the homocysteine-induced macrophage inflammatory response in ApoE(-/-) mice atherosclerosis. Lab Invest J Tech Methods Pathol 2022;102(1):25-37. doi: 10.1038/s41374-021-00679-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Guvenir Celik E, Eroglu O.. Combined treatment with ruxolitinib and MK-2206 inhibits the JAK2/STAT5 and PI3K/AKT pathways via apoptosis in MDA-MB-231 breast cancer cell line. Mol Biol Rep. 2022. doi: 10.1007/s11033-022-08034-4 [DOI] [PubMed] [Google Scholar]
- [48]. Han J, Theiss AL.. Stat3: friend or foe in colitis and colitis-associated cancer? Inflamm Bowel Dis. 2014;20(12): 2405–2411. doi: 10.1097/MIB.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Pathria P, Gotthardt D, Prchal-Murphy M, et al. Myeloid STAT3 promotes formation of colitis-associated colorectal cancer in mice. OncoImmunology 2015;4(4):e9985291-e9985299. doi: 10.1080/2162402X.2014.998529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009;15(2):103-113. doi: 10.1016/j.ccr.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Bollrath J, Phesse TJ, von Burstin VA, et al. Gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 2009;15(2):91-102. doi: 10.1016/j.ccr.2009.01.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.