Abstract
Background
Increased disease activity may be a risk factor for sexual dysfunction (SD) in patients with inflammatory bowel disease (IBD). This study investigated associations between objective measures of disease activity and sexual function.
Methods
Adults with IBD undergoing ileocolonoscopy were prospectively recruited. Demographic, sexual function (Female Sexual Function Index and International Index of Erectile Function), disease activity (endoscopic, biomarker, and symptoms), psychological symptoms, and quality-of-life data were collected. Rates of SD and erectile dysfunction (ED) were compared between patients with active and inactive inflammation and symptoms using the Fisher’s exact test. Logistic regression examined associations between SD and ED, and disease characteristics and psychological symptoms.
Results
A total of 159 participants were included, 97 had Crohn’s disease and 85 were women. SD was reported in 36 of 59 and 13 of 59 sexually active women and men, respectively and ED in 22 of 59 sexually active men. Rates of SD and ED were similar between individuals with active and inactive IBD based on endoscopic indices (P > .05) and biomarkers (P > .05). Women with active IBD symptoms experienced significantly higher rates of SD (P < .05), but men did not (P > .05). Multivariable logistic regression identified that symptoms of severe depression (odds ratio, 5.77; 95% confidence interval, 1.59-20.94) were associated with SD in women, and severe anxiety (odds ratio, 15.62; 95% confidence interval, 1.74-140.23) was associated with ED in men.
Conclusions
Objective measures of disease activity are not associated with SD or ED in patients with IBD. Clinicians should consider concomitant psychological symptoms contributing to the sexual health of patients with IBD.
Keywords: inflammatory bowel disease, sexual dysfunction, erectile dysfunction, quality of life
Graphical Abstract
Graphical Abstract.
Key Messages.
What is already known?
Sexual dysfunction is underrecognized in patients with inflammatory bowel disease. It is unclear whether active inflammation is associated with sexual dysfunction, as previous studies examining this relationship used patient-reported symptoms as a surrogate measure of disease activity.
What is new here?
Active inflammation, measured using endoscopy and biomarkers, was not associated with sexual or erectile dysfunction; however, symptoms of severe depression, anxiety, and stress were.
How can this study help patient care?
Sexual functioning is often impaired in patients with inflammatory bowel disease. Clinicians should screen for underlying mental illness when patients are experiencing sexual dysfunction.
Introduction
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), is increasingly recognized as impacting patient’s psychosocial well-being.1,2 An often underrecognized and undertreated element of psychosocial health is sexual dysfunction (SD). SD is more common in patients with IBD than the general population and is a key determinant of quality of life (QoL).3-5 Despite greater emphasis being placed on improving the QoL of patients with IBD, sexual function is rarely addressed by gastroenterologists.6
Knowledge relating to the risk factors for SD in patients with IBD will improve clinicians’ ability to recognize and manage SD. Although there are some well-established risk factors, such as concomitant psychological illness, especially depression; female sex, corticosteroid use; older age; and comorbidities such as diabetes,3,7-9 the effect of IBD activity on sexual function remains controversial, with multiple studies reporting contradictory findings. Importantly, studies examining the relationship between active inflammation and SD have used symptom-based scores, most commonly the Harvey-Bradshaw index (HBI) or Crohn’s Disease Activity Index for CD (CDAI) and Simple Clinical Colitis Activity Index (SCCAI) for UC, to assess disease activity.4,8-13 These scores do not incorporate objective markers of inflammation such as endoscopy or fecal biomarkers, and include variables commonly affected by psychological illness, fatigue, and functional gastrointestinal symptoms, which are also determinants of SD.7,14,15 Therefore, the results from these studies may not reliably assess the association between gastrointestinal inflammation and SD.
This study aimed to determine the factors associated with SD in patients with IBD, with a particular focus on objective measures of intestinal inflammation and disease activity.
Methods
Participants and Data Collection
Patients with IBD undergoing ileocolonoscopy for disease assessment at Christchurch Hospital, a tertiary hospital with specialist IBD care located in Canterbury, New Zealand, were prospectively recruited into the NIDA-IBD (New Indicators of Disease Activity in IBD) study between February 2019 and December 2020.16,17 Eligible study participants had an established diagnosis of CD or UC and were 16 years of age or older. Patients were excluded if they were unable to understand written English.
Recruited study participants completed symptom, sexual function, psychological health, and QoL questionnaires and collected stool samples for biomarker analysis in the week prior to their ileocolonoscopy. Data on demographics, medical and medication history, IBD-related history, and venous blood samples for biomarker analysis were collected during baseline patient interviews conducted by study investigators prior to the participants’ ileocolonoscopy. Questionnaires were completed using the REDCap (Research Electronic Data Capture) software.18 This study was completed in accordance with the World Medical Association’s Declaration of Helsinki and approved by the Health and Disability Ethics Committee of New Zealand (18/NTA/197).
Demographic Data
Sociodemographic data collected included age, sex, and relationship status. Clinical data included body mass index, smoking status, alcohol consumption (≥14 and ≥21 units per week were considered excessive in women and men, respectively),19 comorbid illness, medication use, year of IBD diagnosis, disease type (CD or UC), disease phenotype according to Montreal classification,20 previous intestinal resections, current and past IBD medication use, any previous diagnosis of a psychiatric illness, and hemoglobin concentration.
Variables and Measurement
Disease activity
Objective measures were used to assess disease activity. Endoscopic indices used included the Simple Endoscopic Score for Crohn’s Disease (SES-CD) and Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Active disease was defined as SES-CD ≥321 and UCEIS ≥222 for CD and UC, respectively. Fecal calprotectin (fCal) and C-reactive protein (CRP) concentrations were used as biomarkers of disease activity for all patients. Biomarker thresholds to define active disease were fCal ≥200 µg/g and CRP >3 mg/L.23
Symptom activity
Symptom questionnaires used in this study included the HBI for patients with CD in which scores of ≤4 signified symptom remission and ≥5 indicated active symptoms.24 For patients with UC, the SCCAI was used, with scores of ≤4 indicating symptom remission and ≥5 indicating active symptoms.25,26
Sexual activity and function
Participants were considered as being sexually active if they reported any kind of sexual activity over the past 4 weeks, including intercourse, caressing, foreplay, or masturbation. In women, sexual function was evaluated using the Female Sexual Function Index (FSFI), which assesses 6 domains: desire, arousal, lubrication, orgasmic function, satisfaction, and pain. An FSFI score ≤26.55 defined SD in women.27 In men, sexual function was evaluated using the International Index of Erectile Function (IIEF), which assesses 5 domains: erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. An IIEF score ≤42.9 defined SD and an IIEF erectile function domain score ≤26 defined erectile dysfunction (ED) in men.28,29
Psychological symptoms and QoL
Symptoms of depression and anxiety were assessed using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 (GAD-7) questionnaires, respectively. A PHQ-9 score ≥10 was used to indicate the presence of severe depressive symptoms and a GAD-7 score ≥10 indicated the presence of severe anxiety symptoms.30,31 The Perceived Stress Scale (PSS-10) was used to assess for stress with a PSS-10 score ≥14 indicating the presence of moderate or high stress.32 QoL was assessed using the Inflammatory Bowel Disease Questionnaire-32 (IBDQ-32), which was measured at baseline and at 6 months following recruitment.33 Poor QoL was defined as an IBDQ-32 score <168.34
Statistical analysis
Descriptive statistics were used to assess patient demographics, disease characteristics, and disease and symptom activity. Only patients who had completed sexual function questionnaires were analyzed. Differences in continuous and categorical variables when compared by sex were assessed using the Mann-Whitney U test and Pearson chi-square test, respectively. Differences in the prevalence of SD and ED between sexually active patients with active and inactive symptoms and endoscopically active and inactive disease were assessed using the Fisher’s exact test. Domain subscores of the FSFI and IIEF in sexually active individuals were compared between those with inactive-active symptoms and inactive-active endoscopic disease using the Mann-Whitney U test. Correlations between continuous variables was performed using Spearman’s rank correlation coefficients.
Factors that predicted the presence of SD and ED in sexually active individuals were identified using logistic regression analysis. Variables reaching a significance of P < .05 on univariable analysis were incorporated into a multivariable logistic regression model. Forward and backward stepwise selection were used on the multivariable models to determine significant factors associated with SD and ED (P < .05). These associations are summarized as odds ratio (OR) with 95% confidence interval (CI). All statistical analyses were performed using the SPSS 28 statistical package (IBM Corp, Armonk, NY, USA). Graphs from this data were created using the GraphPad Prism 9 package (GraphPad Software, San Diego, CA, USA).
Results
Baseline Participant Characteristics
Of the 213 eligible patients approached to take part in this study, 46 declined to participate and 8 did not complete sexual function questionnaires, and these patients were excluded as a result. In total, 159 patients were recruited, of which 85 were women and 74 were men. Female participants had higher rates of prior depression (women: 18 of 85 [21.2%] vs men: 5 of 74 [6.8%]; P = .01), anxiety (women: 12 of 85 [14.1%] vs men: 2 of 74 [2.7%]; P = .01), and irritable bowel syndrome (women: 12 of 85 [14.1%] vs men: 1 of 74 [1.4%]; P < .01) diagnoses compared with men (Table 1). Female participants also had higher rates of severe depressive (women: 36 of 85 [42.4%] vs men: 15 of 74 [20.3%]; P < .01) and moderate-severe stress symptoms (women: 50 of 85 [58.8%] vs men: 28 of 74 [37.8%]; P = .04), and higher rates of psychotropic medication use (women: 27 of 85 [31.8%] vs men: 8 of 74 [10.8%]; P < .01) compared with men (Table 1). Endoscopically active IBD was seen in 50 (59%) of 85 women and 40 (54%) of 74 men (P = .63). Median CRP and fCal concentrations were not significantly different between female and male participants (P > .05) (Table 1).
Table 1.
Baseline characteristics of 159 participants with inflammatory bowel disease
| Characteristic | Women (n = 85) | Men (n = 74) |
|---|---|---|
| Crohn’s disease | 54 (64) | 43 (58) |
| Disease characteristics | ||
| Montreal classification for Crohn’s disease | ||
| A1 | 4 (47) | 32 (74) |
| A2 | 42 (49) | 7 (16) |
| A3 | 8 (9) | 4 (9) |
| L1 | 12 (14) | 6 (14) |
| L2 | 11 (13) | 10 (23) |
| L3 | 31 (36) | 27 (63) |
| B1 | 33 (61) | 27 (63) |
| B2 | 15 (28) | 5 (12) |
| B3 | 6 (11) | 11 (25) |
| P | 8 (9) | 7 (9) |
| Montreal classification for ulcerative colitis | ||
| A1 | 0 (0) | 0 (0) |
| A2 | 10 (32) | 6 (19) |
| A3 | 21 (68) | 25 (81) |
| E1 | 4 (13) | 2 (7) |
| E2 | 14 (45) | 14 (45) |
| E3 | 13 (42) | 15 (48) |
| Age, y | 48 (39-62) | 43 (33-54) |
| Time since diagnosis, y | 10 (4-21) | 14 (8-23) |
| Medication use | ||
| Current corticosteroids | 13 (15) | 15 (20) |
| Corticosteroid use in last year | 29 (34) | 24 (32) |
| Immunomodulator | 32 (38) | 31 (42) |
| Biologic | 21 (25) | 14 (19) |
| Psychotropic | 27 (32) | 8 (11) |
| Antihypertensives | 5 (6) | 15 (20) |
| Hormonal contraceptive | 14 (16) | — |
| Previous depression diagnosis | 18 (21) | 5 (7) |
| Previous anxiety diagnosis | 12 (14) | 2 (3) |
| Other major comorbiditya | 23 (27) | 9 (12) |
| Irritable bowel syndrome | 12 (14) | 1 (1) |
| Previous bowel resection | 21 (25) | 16 (22) |
| Active perianal diseaseb | 6 (7) | 4 (5) |
| Excess alcohol intakec | 3 (4) | 3 (4) |
| Opiate use | 8 (9) | 2 (3) |
| Smoking status | ||
| Past smoker | 13 (15) | 12 (16) |
| Current smoker | 7 (8) | 4 (5) |
| Long-term relationshipd | 63 (74) | 52 (70) |
| Sexually active | 59 (69) | 59 (80) |
| Body mass index | 25.9 (22.1-30.1) | 26.5 (23.8-30.0) |
| Hb, g/L | 136 (127-144) | 154 (144-162) |
| Baseline anemia (Hb <130 g/L men, Hb <120 g/L women) | 7 (8) | 4 (5) |
| Active endoscopic disease (SES-CD ≥3, UCEIS ≥2) | 50 (59) | 40 (54) |
| Active symptoms (HBI ≥5, UCEIS ≥5) | 58 (68) | 35 (47) |
| CRP, mg/L | 3.0 (0.0-7.8) | 0.0 (0.0-5.8) |
| Patients with CRP >3 mg/L | 44 (52) | 35 (47) |
| fCal, µg/g | 111.1 (31.9-332.6) | 147.6 (70.1-602.4) |
| Patients with fCal ≥200 µg/g | 30 (35) | 29 (39) |
| Moderate-severe stress symptoms (PSS-10 ≥14) | 50 (59) | 28 (38) |
| Severe depressive symptoms (PHQ-9 ≥10) | 36 (42) | 15 (20) |
| Severe anxiety symptoms (GAD-7 ≥10) | 23 (27) | 12 (16) |
Values are n (%) or median (interquartile range).
Abbreviations: CRP, C-reactive protein; fCal, fecal calprotectin; GAD-7, Generalized Anxiety Disorder-7; Hb, hemoglobin; HBI, Harvey-Bradshaw index; PHQ-9, Patient Health Questionnaire-9; PSS-10, Perceived Stress Scale 10; SCCAI, Simple Clinical Colitis Activity Index; SES-CD, Simple Endoscopic Score for Crohn’s Disease; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
aChronic respiratory, cardiac, gastrointestinal (excluding inflammatory bowel disease and irritable bowel syndrome), gynecological condition, or current/previous malignancy (excluding squamous cell skin cancer or basal cell skin cancer).
bActive fissure, fistula, or abscess (Crohn’s disease only).
c≥14 units/week for women, ≥21 units/week for men.
dSelf-reported relationship for >1 year.
Rates of Sexual Activity, SD, and ED
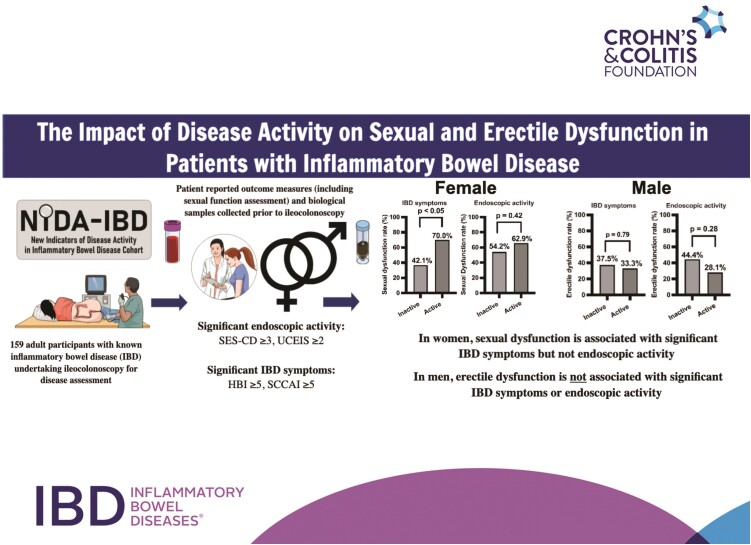
In women, 59 (69.4%) of 85 patients were sexually active, while in men 59 (79.7%) of 74 patients were sexually active. There was no significant difference in the rate of sexual activity between those with endoscopically inactive and active disease for women (24 of 35 [68.6%] and 35 of 50 [70.0%], respectively; P > .05) and men (27 of 34 [79.4%] and 32 of 40 [80.0%], respectively; P > .05).
In individuals who were sexually active, the rate of SD was significantly higher in women compared with men (36 of 59 [61.0%] vs 13 of 59 [22.0%]; P < .001). Sexually active women with SD had a median age of 45 (interquartile range [IQR], 33-55) years and median relationship length of 25 (IQR, 21-31) years; 16 (27.1%) of 59 had a history of depression or anxiety and 16 (27.1%) of 59 had previous abdominal surgery. Sexually active men with SD had a median age of 47 (IQR, 35-62) years and median relationship length of 20 (IQR, 14-26) years; 5 (8.5%) of 59 had a history of depression or anxiety and 3 (5.1%) of 59 had previous abdominal surgery. The rate of ED among sexually active men was 22 (37%) of 59, and all men with SD also had ED. Sexually active men with ED had a median age of 49 (IQR, 39-63) years and median relationship length of 20 (IQR, 9-41) years; 3 (5.1%) of 59 had a history of depression or anxiety and 5 (8.5%) of 59 had previous abdominal surgery. There was no significant difference in the rate of SD between patients with CD (n = 31 [43.1%] of 72) and UC (n = 18 [39.1%] of 46) (P = .70).
Association Between SD and ED With Objective Measures of Disease Activity
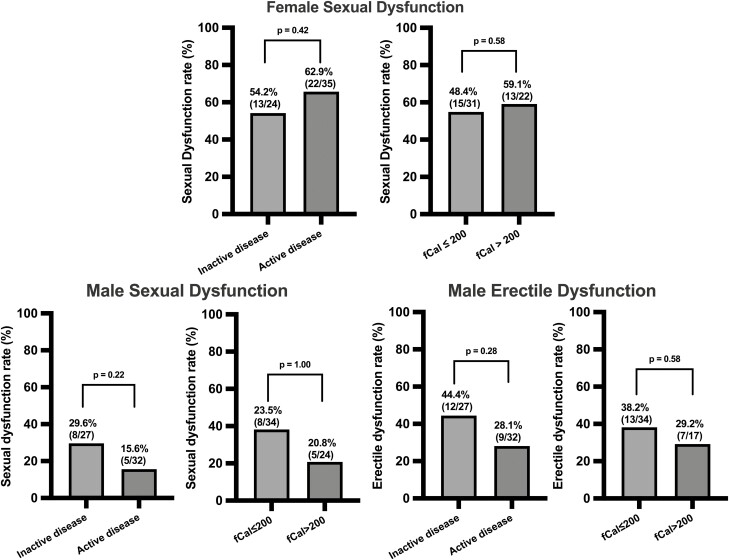
In women who were sexually active, the proportion with SD did not differ between those with endoscopically inactive and active disease (13 of 24 [54.2%] vs 22 of 35 [62.9%]; P = .42), fCal <200 and ≥200 μg/g (15 of 31 [48.4%] vs 13 of 22 [59.1%]; P = .58) (Figure 1), or CRP ≤3 and >3 mg/L (22 of 36 [61.1%] vs 13 of 22 [59.1%]; P = .43). There was also no difference in the median FSFI domain scores between women with endoscopically inactive and active disease (P > .05 for all domains) (Table 2).
Figure 1.
Comparisons of sexual dysfunction and erectile dysfunction rates between patients with inflammatory bowel disease who had inactive and active endoscopic disease, and fecal calprotectin (fCal) ≤200 μg/g and >200 μg/g. Sexual dysfunction defined as Female Sexual Function Index ≤26.55 and International Index of Erectile Function ≤42.9. Erectile dysfunction was defined as a score ≤26 on the erectile dysfunction domain of the International Index of Erectile Function. Endoscopic disease activity was defined as Simple Endoscopic Score for Crohn’s Disease ≥3 and Ulcerative Colitis Endoscopic Index of Severity ≥2.
Table 2.
Comparison of domain scores of sexual function questionnaires between individuals with endoscopically inactive and active disease in sexually active patients
| Domains | Inactive Diseasea | Active Diseaseb | P Value |
|---|---|---|---|
| FSFI (sexually active women) | n = 24 | n = 35 | |
| Desire | 3.0 (2.4-3.6) | 2.4 (1.8-3.6) | .33 |
| Arousal | 4.2 (2.8-5.6) | 4.2 (2.4-4.5) | .18 |
| Lubrication | 5.0 (3.7-5.7) | 4.5 (3.3-5.7) | .65 |
| Orgasm | 5.0 (4.0-6.0) | 4.4 (2.8-5.2) | .12 |
| Global satisfaction | 4.8 (3.6-5.2) | 4.0 (2.4-5.2) | .25 |
| Pain | 5.4 (4.1-6.0) | 4.8 (2.8-6.0) | .28 |
| IIEF (sexually active men) | n = 27 | n = 32 | |
| Intercourse satisfaction | 11.0 (5.0-13.0) | 12.0 (7.5-14.0) | .30 |
| Orgasmic function | 9.0 (6.0-10.0) | 10.0 (7.3-10.0) | .18 |
| Sexual desire | 7.0 (6.0-9.0) | 8.0 (7.0-9.0) | .16 |
| Overall satisfaction | 7.0 (4.0-9.0) | 8.0 (8.0-10.0) | .08 |
Values are median (interquartile range). Comparisons assessed using the Mann-Whitney U test.
Abbreviations: FSFI, Female Sexual Function Index; IIEF, International Index of Erectile Function; SES-CD, Simple Endoscopic Score for Crohn’s Disease; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
aSES-CD <3; UCEIS <2.
bSES-CD ≥3; UCEIS ≥2.
Similarly, in men, there were no significant differences in rates of SD and ED between men with endoscopically inactive and active disease (SD: 8 of 27 [29.6%] vs 5 of 32 [15.6%]; P = .22; ED: 12 of 27 [44.4%] vs 9 of 32 [28.1%]; P = .28), fCal ≤200 and ≥200 μg/g (SD: 8 of 34 [23.5%] vs 5 of 24 [20.8%]; P = 1.00; ED: 13 of 34 [38.2%] vs 7 of 17 [41.1%]; P = .58) (Figure 1), or CRP ≤3 and ≥3 mg/L (SD: 10 of 38 [26.3%] vs 3 of 20 [15%]; P = .51; ED: 15 of 38 [39.5%] vs 6 of 20 [30.0%]; P = .57). There was also no difference in the remaining mean IIEF domain scores between men with endoscopically inactive and active disease (P > .05 for all domains) (Table 2)
Association Between IBD Symptoms and SD and ED
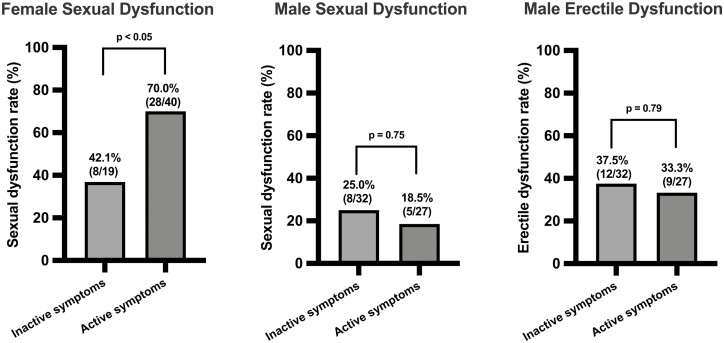
In women, the proportion with SD was higher in those with active compared with inactive IBD symptoms (28 of 40 [70.0%] vs 8 of 19 [42.1%]; P < .05); however, in men, there was no difference in rates of SD or ED between those with inactive and active IBD symptoms (SD: 8 of 32 [25.0%] vs 5 of 27 [18.5%]; P = .75; ED: 12 of 32 [37.5%] vs 9 of 27 [33.3%]; P = .79) (Figure 2).
Figure 2.
Comparisons of sexual dysfunction and erectile dysfunction rates between patients with inflammatory bowel disease who had inactive and active inflammatory bowel disease symptoms. Sexual dysfunction was defined as Female Sexual Function Index ≤26.55 and International Index of Erectile Function ≤42.9. Erectile dysfunction was defined as a score ≤26 on the erectile dysfunction domain of the International Index of Erectile Function. Active inflammatory bowel disease symptoms were defined as Harvey-Bradshaw index ≥5 for Crohn’s disease and Simple Clinical Colitis Activity Index ≥5 for ulcerative colitis.
Association Between Psychological Symptoms and QoL With SD and ED
In sexually active women, symptoms of stress (median PSS-10 score: 18 [IQR, 16-22] vs 13 [IQR, 10-19]; P = .02) and depression (median PHQ-9 score: 11 [IQR, 6-16] vs 5 [IQR, 2-9]; P < .01) were significantly higher in individuals with SD compared with those without (Supplementary Appendix 1). Symptoms of anxiety were not significantly different between sexually active women with and without SD (median GAD-7 score: 5 [IQR, 2-11] vs 5 [IQR, 3-7]; P = .75). Similarly, in sexually active men, symptoms of stress (median PSS-10 score: 18 [IQR, 11-24] vs 11 [IQR, 8-16]; P < .01), depression (median PHQ-9 score: 7 [IQR, 2-13] vs 2 [IQR, 0-5]; P < .01), and anxiety (median GAD-7 score: 7 [IQR, 2-12] vs 2 [IQR, 0-4]; P < .01) were significantly different between those with ED and without ED.
Of the individuals who were sexually active, 27 (45.8%) women and 28 (47.5%) men also completed baseline and 6-month IBDQ-32 questionnaires. SD was correlated with the baseline IBDQ-32 (FSFI: r = 0.30, P = .02, n = 59; IIEF: r = 0.36, P < .01, n = 59) and 6-month IBDQ-32 (FSFI: r = 0.39, P < .05, n = 27; IIEF: r = 0.67, P < .001, n = 28) scores.
Factors Associated With SD and ED
In women, previous abdominal surgery (OR, 5.33; 95% CI, 1.34-21.20), the presence of active IBD symptoms (OR, 3.21; 95% CI, 1.03-9.98), moderate-severe stress (OR, 5.13; 95% CI, 1.50-17.55), and severe depressive symptoms (OR, 4.80; 95% CI, 1.45-15.87) were associated with SD in univariable logistic regression analysis (Table 3). Only the presence of severe depressive symptoms (OR, 5.77; 95% CI, 1.59-20.94) was independently associated with SD on multivariable regression analysis.
Table 3.
Logistic regression analyses assessing factors associated with SD in women and men and ED in men with IBD
| Factor | SD in Women (n = 36)a | SD in Men (n = 13)a | ED in Men (n = 22)b | |||
|---|---|---|---|---|---|---|
| Univariable Analysis (95% CI) | P Value | Univariable Analysis (95% CI) | P Value | Univariable Analysis (95% CI) | P Value | |
| Age | 1.03 (0.99-1.07) | .15 | 1.00 (0.96-1.05) | .88 | 1.02 (0.98-1.07) | .26 |
| Body mass index | 0.99 (0.91-1.08) | .81 | 0.93 (0.79-1.11) | .42 | 0.96 (0.85-1.13) | .77 |
| Long-term relationship (≥1 y) | 1.56 (0.09-26.47) | .76 | >1000 (0.00 -) | 1.00 | >1000 (0.00 -) | 1.00 |
| Crohn’s disease vs ulcerative colitis | 0.77 (0.25-2.37) | .65 | 1.34 (0.38-4.73) | .65 | 2.26 (0.75-6.83) | .15 |
| Disease duration | 1.04 (0.98-1.09) | .19 | 0.89 (0.94-1.06) | .89 | 1.00 (0.95-1.05) | .92 |
| Current corticosteroid use | 1.08 (0.23-5.01) | .93 | 2.00 (0.43-9.42) | .38 | 0.82 (0.18-3.65) | .79 |
| Corticosteroid use in year | 0.78 (0.26-2.35) | .65 | 2.25 (0.60-8.41) | .23 | 1.69 (0.51-5.56) | .39 |
| Immunomodulator use | 0.48 (0.16-1.42) | .18 | 0.81 (0.23-2.87) | .75 | 0.91 (0.31-2.65) | .86 |
| Anti-tumor necrosis factor use | 1.39 (0.41-4.74) | .60 | 1.67 (0.37-7.65) | .51 | 1.15 (0.29-4.62) | .85 |
| Psychotropic medication use | 1.80 (0.54-6.03) | .34 | 3.15 (0.61-16.37) | .17 | 2.75 (0.55-13.67) | .22 |
| Antihypertensive use | — | — | 1.43 (0.32-6.38) | .64 | 2.64 (0.70-10.03) | .15 |
| Opiate use | 0.62 (0.08-4.72) | .64 | — | — | — | — |
| Other major comorbidityc | 1.39 (0.41-4.74) | .60 | 3.64 (0.81-16.33) | .09 | 2.43 (0.58-10.23) | .23 |
| Prior diagnosis of anxiety or depression | 1.80 (0.54-6.03) | .34 | 2.61 (0.39-17.56) | .33 | 2.76 (0.42-18.01) | .29 |
| Irritable bowel syndrome | 1.69 (0.30-9.56) | .55 | — | — | — | — |
| Current smoking | 0.61 (0.11-3.30) | .56 | 4.88 (0.28-86.35) | .28 | 1.85 (0.11-31.12) | .67 |
| Excess alcohol intaked | 1.44 (0.12-17.12) | .77 | — | — | — | — |
| Previous abdominal surgery | 5.33 (1.34-21.20) | .02e | 0.76 (0.18-3.22) | .71 | 0.70 (0.21-2.36) | .56 |
| Hormonal contraceptive use | 0.77 (0.18-3.21) | .72 | — | — | — | — |
| Current or previous perianal disease | 6.56 (0.72-59.85) | .10 | 0.75 (0.07-7.88) | .81 | 0.25 (0.03-2.53) | .24 |
| Baseline Hb | 0.98 (0.93-1.02) | .30 | 0.94 (0.89-0.98) | .01e | 0.95 (0.90-0.99) | .02 |
| Elevated baseline fCal (≥200 μg/g) | 1.19 (0.39-3.59) | .76 | 0.86 (0.24-3.03) | .81 | 0.81 (0.27-2.41) | .70 |
| Active IBD symptoms (HBI ≥5, SCCAI ≥5) | 3.21 (1.03-9.98) | .04e | 0.68 (0.19-2.40) | .55 | 0.98 (0.34-2.83) | .97 |
| Active endoscopic disease (SES-CD ≥3, UCEIS ≥2) | 1.62 (0.56-4.70) | .37 | 0.44 (0.12-1.55) | .20 | 0.57 (0.20-1.65) | .30 |
| Reduced quality of life (IBDQ-32 <169) | 2.29 (0.76-6.88) | .139 | 1.29 (0.36-4.63) | .69 | 1.64 (0.54-4.94) | .38 |
| Moderate or severe stress symptoms (PSS-10 ≥14) | 5.13 (1.50-17.55) | <.01e | 3.70 (0.96-14.29) | .06 | 5.44 (1.58-18.71) | <.01e |
| Severe depressive symptoms (PHQ-9 ≥10) | 4.80 (1.45-15.87) | .01e | 2.96 (0.69-12.72) | .14 | 5.29 (1.20-23.30) | .03e |
| Severe anxiety symptoms (GAD-7 ≥10) | 1.64 (0.44-6.14) | .46 | 4.67 (0.98-22.25) | .05 | 16.80 (1.90-148.64) | .01 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; ED, erectile dysfunction; fCal, fecal calprotectin; FSFI, Female Sexual Function Index; GAD-7, Generalized Anxiety Disorder-7; Hb, hemoglobin; HBI, Harvey-Bradshaw index; IBD, inflammatory bowel disease; IBDQ-32, Inflammatory Bowel Disease Questionnaire-32; IIEF, International Index of Erectile Function; PHQ-9, Patient Health Questionnaire-9; PSS-10, Perceived Stress Scale 10; SCCAI, Simple Clinical Colitis Activity Index; SD, sexual dysfunction; SES-CD, Simple Endoscopic Score for Crohn’s Disease; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
aFSFI ≤26.55 and IIEF ≤42.9.
bED domain score of IIEF ≤26.
cChronic respiratory, cardiac, gastrointestinal (excluding IBD and irritable bowel syndrome), gynecological condition, or current/previous malignancy (excluding squamous cell skin cancer or basal cell skin cancer).
d≥14 units/week (women); ≥21 units/week (men).
eSelected for multivariable analysis.
In men, only baseline hemoglobin was associated with SD (OR, 0.94; 95% CI, 0.89-0.98), while moderate-severe stress (OR, 5.44; 95% CI, 1.58-18.71), severe depressive symptoms (OR, 5.29; 95% CI, 1.20-23.30), and severe anxiety symptoms (OR, 16.8; 95% CI, 1.90-148.64) were associated with ED on univariable logistic regression (Table 3). The presence of baseline anemia (hemoglobin <130 g/L in men) was not associated with SD (OR, 8.00; 95% CI, 0.66-96.46) or ED (OR, 3.79; 95% CI, 0.32-44.53). On multivariable regression analysis, only severe anxiety symptoms were independently associated with ED (OR, 15.62; 95% CI, 1.74-140.23).
Discussion
This prospective observational study assessed patient and disease-related characteristics to identify risk factors for SD and ED in patients with IBD. Previous studies that investigated the association between IBD activity and SD are limited by the use of symptoms as surrogate markers of intestinal inflammation.4,8-13 The present study is the first to directly examine the relationship between sexual function, IBD symptoms, and objectively measured IBD activity.
This study revealed no significant association between active gastrointestinal inflammation, as assessed objectively using ileocolonoscopy and biomarkers (fCal and CRP) and SD in women or SD and ED in men. This is the first study to use objective measures of disease activity to analyze this association and provides a reliable insight into their relationship. This study found that women with active IBD symptoms, as measured by HBI or SCCAI, had a higher rate of SD than women with inactive symptoms, which is consistent with previous studies using these symptom-based scores as a surrogate for disease activity assessment. Multivariable analysis found that psychological illness, namely severe depressive symptoms in women and severe anxiety symptoms in men, were the only risk factors independently associated with SD and ED, respectively. Taken together, these results demonstrate that targeting gastrointestinal inflammation cannot be the sole treatment focus in patients with IBD and SD, with a holistic approach addressing mental well-being and gastrointestinal symptoms being required.
The high prevalence of SD in patients with IBD is again demonstrated in this study, with 61% of women experiencing SD and 22% and 37% of men experiencing SD and ED, respectively.4 Another finding consistent with results from previous studies is the significantly higher rate of SD in female patients compared with male patients.3,4 Interestingly, this observation is also seen in the general population suggesting that factors specific to IBD are unlikely to explain this difference.35 Possible reasons for this difference identified in this study were the significantly higher rates of female patients experiencing severe psychological and IBD symptoms compared with male patients.
Psychological illness, in particular depression, is the most consistent independent risk factor for SD7 and has been exhibited again in this study. This close relationship between SD and mental illness is likely bidirectional with the presence of psychological illness negatively impacting sexual function, and alternatively SD triggering worsening psychological health. This link likely extends beyond just an association with a previous study finding that treating anxious or depressed patients with IBD with antidepressants for 6 months resulted in improved sexual functioning, suggesting a causative role.36 Of interest, in the current study having a prior formal diagnosis of depression or anxiety was not associated with current SD, again suggesting that adequate management of mental illness may improve sexual functioning.
The impact of stress on the QoL of patients with IBD has been widely examined2,16,37,38; however its role in SD and ED is not well defined. This current study used the PSS-1032 to assess for stress because of its previous use in patients with IBD39-41 and to assess the effect of stress on sexual function in the general population.42,43 This study found an association between moderate or high levels of perceived stress and SD and ED in men and women, respectively. This potentially novel finding is of particular importance given the substantial burden of stress experienced by patients with IBD. It also identifies another psychological comorbidity that potentially impacts sexual function, highlighting the complex etiology of SD. Overall, when SD is diagnosed, it is important that patients are screened for comorbid psychological illnesses.
The role of abdominal surgery in SD and ED is unclear, with previous studies providing inconsistent and conflicting results and being of relatively poor quality.7 In this current study, a history of abdominal surgery was associated with SD on univariate analysis but not multivariate analysis, which is most likely explained by higher rates of psychological illness in patients that have undergone IBD-related surgery.44 Importantly, this current study’s sample size was too small to examine the association between different surgeries and sexual function, which is likely important.45-47 It is important that future studies examining the association between surgery and SD also adjust for psychological illness.
Although the findings of the current study suggest SD that is not directly associated with intestinal inflammation, many patients with IBD perceive their disease as negatively impacting their sexual life and blame their IBD for their sexual inactivity, thus making it an important issue when caring for patients with IBD.3,7,11,48-50 Despite this, SD is rarely discussed between patients and clinicians due to a range of patient and clinician factors. Importantly, many patients are unwilling to discuss sexuality with their treating physician, making diagnosis and management particularly challenging.7 On the other hand, IBD physicians rarely discuss sexual function with patients, citing various perceived barriers, such as their discomfort talking about SD or not wanting to make the patient feel uncomfortable, lack of time, not feeling that it is their responsibility to discuss sexual function, and lack of knowledge regarding SD management.6 This study has again demonstrated the high proportion of patients experiencing SD and its negative impact on patients’ QoL. Additionally, the presence of SD can impact the everyday management of patients with IBD; for example, patients with IBD may omit medication because of perceived negative impacts on sexual function.49 It is crucial that efforts are made by multidisciplinary teams caring for patients with IBD to overcome these barriers to improve the QoL of their patients.
Patients are generally more comfortable discussing SD with a clinician of the same gender, highlighting the importance of having female and male members in the IBD team.11 Validated questionnaires to assist clinicians in diagnosing SD may help overcome patient and clinician discomfort. The use of the FSFI and IIEF in patients with IBD has long been criticized due to lack of validation in this group. However, IBD and gender-specific questionnaires have recently been developed and validated.11,12 Although greater emphasis needs to be placed on training IBD physicians in diagnosing and managing SD, its etiology and management is often complex. Additionally, there are also no formal recommendations in current IBD guidelines on how to manage SD in patients with IBD51; this means that an interdisciplinary team approach is required, and improving access to specialists, particularly psychologists and sexual health physicians, should be made a priority.48,52
An intention of this study had been to assess if changes in gastrointestinal inflammation over time, measured by serial fCal measurements, impacted patients’ sexual function. Unfortunately, there was a significant loss of follow-up in this study at 6 months largely due to the impact of the COVID-19 pandemic. As a result of this, no meaningful statistical analyses could be performed on the limited data for fCal and sexual function scores at 6 months of follow-up. These findings would have provided further information about the relationship between gastrointestinal inflammation and SD. Of interest, a recent study in women with newly diagnosed IBD found no improvement in FSFI scores despite improvement in disease activity. Although this study used symptom-based scores to measure disease activity, it supports the current study’s findings and also suggests that reducing intestinal inflammation is unlikely to directly improve sexual function.13
A key strength of this study was the prospective assessment of objective measures of disease activity (endoscopic and biomarkers). Contrary to the current findings, previous studies have demonstrated an association between disease activity and SD.10-12 However, these studies have used IBD symptom-based scores, most commonly the SCCAI for UC and the HBI or the CDAI for CD, to assess disease activity. These scores ask patients to rate their feeling of general well-being, causing criterion contamination when there is comorbid mental illness and possibly SD. Functional gastrointestinal symptoms, which commonly affect both patients with IBD and with psychological illness, can also significantly influence scores.52 Overall, these scores often correlate poorly with true disease activity.15 These issues have been addressed in the present study by using objective measures to assess disease activity.
One of the larger limitations of this study is that it was conducted at a single tertiary center with a predominantly Caucasian population, so the current findings may not be applicable to every patient population. Also, only patients referred for ileocolonoscopy were included. To improve generalizability, all patients with IBD having ileocolonoscopy were invited, including asymptomatic patients having ileocolonoscopy for colorectal cancer surveillance. This study did not include patients with CD having radiological assessments of disease activity, meaning that inflammation proximal to the distal ileum, transmural inflammation, and penetrating disease were likely missed. However, the use of biomarkers, fCal and CRP, to also assess disease activity is likely to have accounted for these patients. It is also possible that the relatively small sample size in this study meant that the identification of certain risk factors for SD was missed. An example of this may be the negative relationship between hemoglobin and SD observed in men but the lack of a significant association between the presence of anemia and SD in these individuals, as reported in other studies.4 Furthermore, although there was no significant association between baseline hemoglobin and SD in women, all sexually active women with anemia had SD. However, the main aim of this study was to assess the association between SD and intestinal inflammation, which was likely achieved with this sample size, particularly given the lack of any significant trends suggesting an association. Of note, the rate of patients declining participation was similar to previous studies researching SD in IBD.3,4,53 Additionally, certain variables in the multivariable analysis, especially IBD symptoms and other psychological illnesses, may not have shown a significant association due to collinearity between these factors, as identified in this study. The exact nature of the IBD symptoms associated with SD in women was unavailable, as this analysis was based on the sum scores of the HBI and SCCAI. This finding would benefit from further investigation of symptom subtypes associated with SD in future studies with a larger cohort of participants. Finally, some recognized risk factors for SD, such as fatigue and negative body image, were not directly assessed in this study.54 Instead, this study assessed some unique potential risk factors, such as stress levels.
Conclusions
Sexual functioning is often impaired in patients with IBD and negatively impacts their quality of life. The current study shows that psychological illness and active IBD symptoms, but not active intestinal inflammation, are associated with SD, emphasizing the need for clinicians to take a holistic approach when caring for patients with IBD and consider underlying mental illness in patients with IBD and SD.
Supplementary Material
Acknowledgments
This study was approved by the New Zealand Health and Disability Ethics Committee (no. 18/NTA/197).
Contributor Information
Thomas C Mules, Department of Gastroenterology, Christchurch Hospital, Canterbury District Health Board, Canterbury, New Zealand.
Akhilesh Swaminathan, Department of Gastroenterology, Christchurch Hospital, Canterbury District Health Board, Canterbury, New Zealand; Department of Medicine, University of Otago, Christchurch, Canterbury, New Zealand.
Esther Hirschfeld, Department of Medicine, University of Otago, Christchurch, Canterbury, New Zealand.
Grace M Borichevsky, Centre for Free Radical Research, University of Otago, Christchurch, Canterbury, New Zealand.
Chris M Frampton, Department of Medicine, University of Otago, Christchurch, Canterbury, New Zealand.
Andrew S Day, Department of Paediatrics, University of Otago, Christchurch, Canterbury, New Zealand.
Richard B Gearry, Department of Gastroenterology, Christchurch Hospital, Canterbury District Health Board, Canterbury, New Zealand; Department of Medicine, University of Otago, Christchurch, Canterbury, New Zealand.
Author Contribution
T.C.M., A.S., G.B., T.S.E., A.S.D., M.B.H., A.J.K., and R.B.G. conceived the study design. A.S., G.B., E.H., and T.C.M acquired the study data and A.S., G.B., and E.H. performed the laboratory analyses. A.S., T.C.M., and C.F. performed the statistical analyses, and A.S.D. and R.B.G assisted in the interpretation of the study results. A.S., T.C.M., and E.H. prepared the initial article draft. All authors contributed the critical revision of this manuscript and approved the final submitted version.
Funding
This work was supported by the New Zealand Society of Gastroenterology Janssen Research Grant and the Royal Australasian College of Physicians Research Entry Grant.
Conflicts of Interest
T.C.M. has received honoraria for educational activities for Janssen and AbbVie (unrelated to this manuscript). A.S. has received honoraria for educational activities for Janssen (unrelated to this manuscript). A.S.D. has served on advisory boards for Janssen, AbbVie, and Nestle (all unrelated to this manuscript). R.B.G. has received research grants, served on advisory boards, and received honoraria for educational activities for Janssen, AbbVie, and Zespri (unrelated to this manuscript).
Data Availability
Data are available upon request to the corresponding author.
References
- 1. Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC.. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021;6(5):359–370. [DOI] [PubMed] [Google Scholar]
- 2. Mules TC, Swaminathan A, Hirschfeld E, et al. The impact of disease activity on psychological symptoms and quality of life in patients with inflammatory bowel disease—results from the Stress, Anxiety and Depression with Disease Activity (SADD) Study. Aliment Pharmacol Ther. 2022;55(2):201–211. [DOI] [PubMed] [Google Scholar]
- 3. Marín L, Mañosa M, Garcia-Planella E, et al. Sexual function and patients’ perceptions in inflammatory bowel disease: a case–control survey. J Gastroenterol. 2013;48(6):713–720. [DOI] [PubMed] [Google Scholar]
- 4. Rivière P, Zallot C, Desobry P, et al. Frequency of and factors associated with sexual dysfunction in patients with inflammatory bowel disease. J Crohns Colitis 2017;11(11):1347–1352. [DOI] [PubMed] [Google Scholar]
- 5. Zhao S, Wang J, Liu Y, et al. Inflammatory bowel diseases were associated with risk of sexual dysfunction in both sexes: a meta-analysis. Inflamm Bowel Dis. 2019;25(4):699–707. [DOI] [PubMed] [Google Scholar]
- 6. Rivière P, Poullenot F, Zerbib F, Laharie D.. Quality of sex life in patients with inflammatory bowel disease: the gastroenterologists’ perspective. Inflamm Bowel Dis. 2017;23(10):E51–E52. [DOI] [PubMed] [Google Scholar]
- 7. Mantzouranis G, Fafliora E, Glanztounis G, Christodoulou DK, Katsanos KH.. Inflammatory bowel disease and sexual function in male and female patients: an update on evidence in the past ten years. J Crohns Colitis. 2015;9(12):1160–1168. [DOI] [PubMed] [Google Scholar]
- 8. Shmidt E, Suárez-Fariñas M, Mallette M, et al. Erectile dysfunction is highly prevalent in men with newly diagnosed inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(8):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Timmer A, Bauer A, Kemptner D, Fürst A, Rogler G.. Determinants of male sexual function in inflammatory bowel disease: a survey-based cross-sectional analysis in 280 men. Inflamm Bowel Dis. 2007;13(10):1236–1243. [DOI] [PubMed] [Google Scholar]
- 10. Bel LGJ, Vollebregt AM, Van der Meulen-de Jong AE, et al. Sexual dysfunctions in men and women with inflammatory bowel disease. J Sex Med. 2015;12(7):1557–1567. [DOI] [PubMed] [Google Scholar]
- 11. de Silva PS, O’Toole A, Marc LG, et al. Development of a sexual dysfunction scale for women with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(11):2350–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Toole A, de Silva PS, Marc LG, et al. Sexual dysfunction in men with inflammatory bowel disease: a new IBD-specific scale. Inflamm Bowel Dis. 2018;24(2):310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shmidt E, Suárez-Fariñas M, Mallette M, et al. A Longitudinal study of sexual function in women with newly diagnosed inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(7):1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Jong MJ, Huibregtse R, Masclee AAM, Jonkers DMAE, Pierik MJ.. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16(5):648–663.e3. [DOI] [PubMed] [Google Scholar]
- 15. Gracie DJ, Williams CJM, Sood R, et al. Poor correlation between clinical disease activity and mucosal inflammation, and the role of psychological comorbidity, in inflammatory bowel disease. Am J Gastroenterol. 2016;111(4):541–551. [DOI] [PubMed] [Google Scholar]
- 16. Swaminathan A, Fan D, Borichevsky GM, et al. The disease severity index for inflammatory bowel disease is associated with psychological symptoms, quality of life and predicts a more complicated disease course. Aliment Pharmacol Ther. [cited 2022;56(4):664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swaminathan A, Borichevsky GM, Edwards TS, et al. Faecal myeloperoxidase as a biomarker of endoscopic activity in inflammatory bowel disease. J Crohns Colitis. Published online July 8, 2022. doi:10.1093/ecco-jcc/jjac098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. International Alliance for Responsible Drinking (IARD). Drinking guidelines: general population. Accessed June 22, 2022. https://iard.org/science-resources/detail/Drinking-Guidelines-General-Population
- 20. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 21. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60(4):505–512. [DOI] [PubMed] [Google Scholar]
- 22. Travis SPL, Schnell D, Krzeski P, et al. Reliability and initial validation of the Ulcerative Colitis Endoscopic Index of Severity. Gastroenterology 2013;145(5):987–995. [DOI] [PubMed] [Google Scholar]
- 23. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160(5):1570–1583. [DOI] [PubMed] [Google Scholar]
- 24. Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14(3):348–354.e17. [DOI] [PubMed] [Google Scholar]
- 25. Bennebroek Evertsz F, Nieuwkerk PT, Stokkers PCF, et al. The patient Simple Clinical Colitis Activity Index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis. 2013;7(11):890–900. [DOI] [PubMed] [Google Scholar]
- 26. Walmsley R, Ayres R, Pounder R, Allan R.. A Simple Clinical Colitis Activity Index. Gut. 1998;43(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiegel M, Meston C, Rosen R.. The Female Sexual Function Index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1–20. [DOI] [PubMed] [Google Scholar]
- 28. Rosen RC, Cappelleri JC, Gendrano N.. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14(4):226–244. [DOI] [PubMed] [Google Scholar]
- 29. Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH.. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54(2):346–351. [DOI] [PubMed] [Google Scholar]
- 30. Spitzer RL, Kroenke K, Williams JBW, Löwe B.. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 31. Kroenke K, Spitzer RL, Williams JB.. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen S, Kamarck T, Mermelstein R.. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 33. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96(3):804–810. [PubMed] [Google Scholar]
- 34. Hlavaty T, Persoons P, Vermeire S, et al. Evaluation of short-term responsiveness and cutoff values of inflammatory bowel disease questionnaire in Crohn’s disease. Inflamm Bowel Dis. 2006;12(3):199–204. [DOI] [PubMed] [Google Scholar]
- 35. Laumann EO, Paik A, Rosen RC.. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537–544. [DOI] [PubMed] [Google Scholar]
- 36. Yanartas O, Kani HT, Bicakci E, et al. The effects of psychiatric treatment on depression, anxiety, quality of life, and sexual dysfunction in patients with inflammatory bowel disease. Neuropsychiatr Dis Treat. 2016;12:673–683. doi: 10.2147/NDT.S106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun Y, Li L, Xie R, Wang B, Jiang K, Cao H.. Stress triggers flare of inflammatory bowel disease in children and adults. Front Pediatr. 2019;7:432. doi: 10.3389/fped.2019.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moradkhani A, Beckman LJ, Tabibian JH.. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7(6):467–473. [DOI] [PubMed] [Google Scholar]
- 39. Targownik LE, Sexton KA, Bernstein MT, et al. The Relationship among perceived stress, symptoms, and inflammation in persons with inflammatory bowel disease. Am J Gastroenterol. 2015;110(7):1001–1012; quiz 1013. [DOI] [PubMed] [Google Scholar]
- 40. Sewitch MJ, Abrahamowicz M, Bitton A, et al. Psychological distress, social support, and disease activity in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96(5):1470–1479. [DOI] [PubMed] [Google Scholar]
- 41. Schoultz M, Beattie M, Gorely T, Leung J.. Assessment of causal link between psychological factors and symptom exacerbation in inflammatory bowel disease: a systematic review utilising Bradford Hill criteria and meta-analysis of prospective cohort studies. Syst Rev. 2020;9(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abedi P, Afrazeh M, Javadifar N, Saki A.. The relation between stress and sexual function and satisfaction in reproductive-age women in Iran: a cross-sectional study. J Sex Marital Ther. 2015;41(4):384–390. [DOI] [PubMed] [Google Scholar]
- 43. Morokoff PJ, Gillilland R.. Stress, sexual functioning, and marital satisfaction. J Sex Res. 1993;30(1):43–53. [Google Scholar]
- 44. Zangenberg MS, El-Hussuna A.. Psychiatric morbidity after surgery for inflammatory bowel disease: a systematic review. World J Gastroenterol. 2017;23(48):8651–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scarberry K, Brady JT, Scarberry K, Stein SL, Steinhagen E.. Surgery for Crohn’s disease affects male sexual function. Ann Colorectal Res.2019;7(1):e85458. doi: 10.5812/acr.85458. [DOI] [Google Scholar]
- 46. Hicks CW, Hodin RA, Savitt L, Bordeianou L.. Does intramesorectal excision for ulcerative colitis impact bowel and sexual function when compared with total mesorectal excision? Am J Surg. 2014;208(4):499–504.e4. [DOI] [PubMed] [Google Scholar]
- 47. Harnoy Y, Desfourneaux V, Bouguen G, et al. Sexuality and fertility outcomes after hand sewn versus stapled ileal pouch anal anastomosis for ulcerative colitis. J Surg Res. 2016;200(1):66–72. [DOI] [PubMed] [Google Scholar]
- 48. Knowles SR, Gass C, Macrae F.. Illness perceptions in IBD influence psychological status, sexual health and satisfaction, body image and relational functioning: a preliminary exploration using structural equation modeling. J Crohns Colitis. 2013;7(9):e344–e350. [DOI] [PubMed] [Google Scholar]
- 49. Muller KR, Prosser R, Bampton P, Mountifield R, Andrews JM.. Female gender and surgery impair relationships, body image, and sexuality in inflammatory bowel disease: patient perceptions. Inflamm Bowel Dis. 2010;16(4):657–663. [DOI] [PubMed] [Google Scholar]
- 50. Walldorf J, Michl P.. Sexual dysfunction in patients with inflammatory bowel disease is not just a matter of quality of life. J Crohns Colitis. 2018;12(4):505–506. [DOI] [PubMed] [Google Scholar]
- 51. Perez de Arce E, Quera R, Ribeiro Barros J, Yukie Sassaki L.. Sexual dysfunction in inflammatory bowel disease: what the specialist should know and ask. Int J Gen Med. 2021;14:2003–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fairbrass KM, Costantino SJ, Gracie DJ, Ford AC.. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(12):1053–1062. [DOI] [PubMed] [Google Scholar]
- 53. Timmer A, Bauer A, Dignass A, Rogler G.. Sexual function in persons with inflammatory bowel disease: a survey with matched controls. Clin Gastroenterol Hepatol. 2007;5(1):87–94. [DOI] [PubMed] [Google Scholar]
- 54. Ghazi LJ, Patil SA, Cross RK.. Sexual dysfunction in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(4):939–947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the corresponding author.