Abstract
Eighty percent of antibody secreting cells (ASCs) are found in the intestine, where they produce grams of immunoglobulin (Ig) A daily. immunoglobulin A is actively transcytosed into the lumen, where it plays a critical role in modulating the gut microbiota. Although loss of immune tolerance to bacterial antigens is the likely trigger of the dysregulated immune response that characterizes inflammatory bowel disease (IBD), little effort has been placed on understanding the interface between B cells, IgA, and the microbiota during initiation or progression of disease. This may be in part due to the misleading fact that IgA-deficient humans are mostly asymptomatic, likely due to redundant role of secretory (S) IgM. Intestinal B cell recruitment is critically dependent on integrin α4β7-MAdCAM-1 interactions, yet antibodies that target α4β7 (ie, vedolizumab), MAdCAM-1 (ie, ontamalimab), or both β7 integrins (α4β7 and αE [CD103] β7; etrolizumab) are in clinical use or development as IBD therapeutics. The effect of such interventions on the biology of IgA is largely unknown, yet a single dose of vedolizumab lowers SIgA levels in stool and weakens the oral immunization response to cholera vaccine in healthy volunteers. Thus, it is critical to further understand the role of these integrins for the migration of ASC and other cellular subsets during homeostasis and IBD-associated inflammation and the mode of action of drugs that interfere with this traffic. We have recently identified a subset of mature ASC that employs integrin αEβ7 to dock with intestinal epithelial cells, predominantly in the pericryptal region of the terminal ileum. This role for the integrin had not been appreciated previously, nor the αEβ7-dependent mechanism of IgA transcytosis that it supports. Furthermore, we find that B cells more than T cells are critically dependent on α4β7-MAdCAM-1 interactions; thus MAdCAM-1 blockade and integrin-β7 deficiency counterintuitively hasten colitis in interleukin-10-deficient mice. In both cases, de novo recruitment of IgA ASC to the intestinal lamina propria is compromised, leading to bacterial overgrowth, dysbiosis, and lethal colitis. Thus, despite the safe and effective use of anti-integrin antibodies in patients with IBD, much remains to be learned about their various cell targets.
Keywords: B cells, IgA, IBD, plasma cells, integrins
Introduction
The intestinal environment is a complex ecosystem, wherein trillions of microorganisms (collectively referred to as the gut microbiota) peacefully reside alongside the largest immunological compartment of the human body. Homeostasis is maintained in a precarious state through the acquisition of immune tolerance to microbial and food antigens, while remaining poised to mount a commensurate immune response to pathogenic elements of the microbiota. Encounter of foreign antigens with local immune cells could be deleterious to the host, given the potential for triggering the chronic dysregulated inflammatory response that characterizes inflammatory bowel disease (IBD). In fact, contrary to autoimmunity where the immune system mounts a response to self-antigens, the initial antigenic triggers in IBD likely originate from the microbiota. To minimize such potentially harmful encounters, the gut has acquired a dense and interconnected defensive network, designated as the intestinal barrier. This anatomically and functionally complex structure consists of mechanical, antimicrobial, and immunological components that act in synergy to provide a controlled mutually beneficial interaction between the microbiota and the local immune system, an interspecies relationship known as mutualism/symbiosis. An integral part of this defensive network is immunoglobulin A (IgA), which is locally produced by antibody secreting cells (ASCs). Following secretion, a multistep and highly specialized process is employed, which results in the movement of IgA (transcytosis) from the intestinal lamina propria (LP), through the epithelium into the intestinal lumen, in the form of Ig dimers. The addition of the secretory component, originating from the polymeric immunoglobulin receptor (PIgR) further stabilizes the complex, forming secretory (S) IgA (SIgA), which is anchored to the mucus layer, providing around a 500-micron physical/chemical barrier that maintains most elements of the microbiota separated from the intestinal epithelium. Once inside the lumen, SIgA plays a pivotal role on the mechanisms that shape the composition of the microbiota and preserve homeostasis with the intestinal immune system. Although the role of SIgA in relation to healthy-state conditions has been extensively studied, the effect that derangements in IgA production, secretion, and/or function may have during pathological conditions such as IBD remains largely unexplored. Furthermore, B cells have been often dismissed by some in the field, attributable to the failure of rituximab (an anti-CD20 antibody) as a therapeutic in patients with ulcerative colitis (UC).1 In contrast, rituximab is effective for the treatment of rheumatoid arthritis and other autoimmune diseases.2,3 Despite the peripheral B cell–depleting effect of rituximab, strikingly, IgA(+) plasmablasts and plasma cells demonstrate rituximab-resistance. This observation hints to potential unique characteristics of mucosa resident B cells regarding self-sufficiency, long-lived traits or perhaps absence of CD20(+) on their cell surface.4 Our recent studies in mouse models of IBD lead us to propose that B cell responses may indeed be pivotal for the initiation of the disease process, which eventually becomes self-sustaining and autonomous from its initial bacterial triggers. Here we review the basics of IgA biology and summarize available evidence for its role during homeostasis and IBD.
The Biology of Mucosal IgA
Almost 80% of ASC in mammals are located in the intestine and the majority of these cells produce IgA, by far the most abundant immunoglobulin in mammals.5 The terminology is inconsistent throughout the literature, where plasma cells are often equated to all ASC. More recently, ASC has been used as an inclusive term that includes short-lived, dividing plasmablasts and long-lived terminally differentiated plasma cells (PCs). The latter should be reserved for ASC in their final maturation stages (Table 1). The generation of ASC has been described in detail.6 For simplicity, we refer to various types of B cells (eg, follicular, memory, marginal zone) just as B cells. Some of the surface markers employed to identify these subsets are provided on Table 2. The differentiation of B cells into intestinal IgA+ ASC takes place in the gut-associated lymphoid tissue (GALT), specifically in small intestinal Peyer Patches (PPs), mesenteric lymph nodes (MLNs), cecal patch/appendix, and isolated lymphoid follicles (ILFs)/tertiary lymphoid structures (TLSs)—the latter two found within the intestinal LP.7 These structures carry germinal centers (GCs), where ASC class switch from IgM to IgA, under the control of the enzyme activation-induced cytidine deaminase (AID). The AID-deficient mice lack IgA and IgG, allowing expansion of elements of the microbiota (eg, clostridial species), a situation referred to as bacterial overgrowth. Class switching to IgA has been reported in situ within the LP but remains controversial, as the contribution of ILF/TLS to the process cannot be excluded.8,9 The IgA inductive sites (eg, PP, ILF) lack afferent lymphatics, and thus, transfer of antigenic material is mediated by a population of highly differentiated epithelial cells at the follicle-associated epithelium: microfold (M) cells, goblet cells, as well as CX3CR1+ monocyte-lineage cells that sample antigens directly from the intestinal lumen, acting as antigen transfer modules to CD103+/CD11b- DCs (cDC1).10,11 It should be noted, however, that CD103 expression is not definitive of cDC1, inasmuch as CD103+/CD11b+ intestinal DC that are cDC2 have also been described.12
Table 1.
Features of antibody secreting cells.a
| Antibody Secreting Cells | Plasmablast | Plasma Cells |
|---|---|---|
| Lifespan | + | ++++ |
| Proliferation | ++ | - |
| CD138, CXCR4 | + | +++ |
| CD19, CD20, CD45, MHC class II |
++ | +/- |
| 1. Antibody secreting cells (ASC)- refers to proliferating plasmablasts and nonproliferating plasma cells. | ||
| 2. Plasmablasts- Dividing ASC with migratory potential which may further mature into plasma cells. | ||
| 3. Plasma cells (PC) - Terminally differentiated, non-replicating ASC able to secrete large amounts of antibodies. | ||
a Table modified from Nutt et al in Nature Rev Immunol (2015) 15:160-171.
Table 2.
Selected surface markers for B cell subsets.a
| B Cell Subset | Mouse Positive Markers | Mouse Negative Markers | Human Positive Markers | Human Negative Markers |
|---|---|---|---|---|
| Marginal Zone | CD1d, CD9, CD21high, CD22high CD35high, B220 | CD93, CD23 | CD1c, CD19, CD20 CD21high, CD27var |
|
| Follicular | CD19, CD22, CD23 CD38, B220 |
CD1dlow, CD21/35low, CD93 | CD19, CD20, CD21, CD22, CD23, CD24 | CD10, CD27 CD38low, CD24low |
| Germinal Center | CD19, CD37, CD20, GL7, Siglec2 | CD93, CD38Low | CD10, CD19, CD20, CD23, CD27, CD38, CD269, BCMA | CD24low |
| Memory | B220, CD38var, CD62Lvar, CD80var, CD95low | CD19, CD20, CD40, CD27var, CXCR4,5,7 | CD23low, CD38 | |
| B reg | CD1dhigh, CD5, CD19, CD24 | CD62L, CD93var | CD1dhigh, CD5, CD19, CD21, CD24high | CD27var |
T-dependent and Independent Secretory IgA
Terminology found in the literature over the years subdivides IgA using multiple overlapping terms such as low and high affinity, natural and induced, polyreactive and specific, canonical and noncanonical, and more. Those terms often refer to the same IgA subtypes seen from a distinct perspective. The distinctions have been reviewed in detail by Pabst.13 A commonly used classification is based on whether class switch recombination (CSR) occurs in the absence (T cell-independent [TI]) or presence (T cell-dependent [TD]) of T cells.14 T cell-independent and T cell-dependent IgA responses have been implicated in the control of commensals vs pathogenic elements of the microbiota, respectively.
T cell-dependent IgA induction requires CSR and somatic hypermutation (SHM) after stimulation of the B cell receptor (BCR), costimulatory signals by T-helper cells via CD40:CD40L interaction, and cytokine stimuli (eg, interleukin [IL]-4, IL-21, Transforming Growth Factor β [TGFβ], and IL-10).15 Notably, the production of TGFβ is mediated by the interaction between B cells and DC in the subepithelial dome, the space between the follicle associated epithelium and the B cell follicles in PP. These conditions lead to the expression of activation-induced cytidine deaminase, DNA recombination, and the production of IgA.8 The core step for production of high-affinity IgA is the process of SHM, linked to GC reactions. This process is mediated by the T follicular helper cells in the GCs, which allow the selection of high-affinity B cells and thus the production of antigen-specific IgA ASC.8
By contrast, TI IgA induction depends on extrafollicular interaction between DC in the subepithelial dome and B cells that migrate to this area via CCR6-CXCL20 interactions.16 CSR in the subepithelial dome is mediated through the production of TGFβ, after the interaction of B cells with integrin αvβ8-expressing DC. In addition, several members of the tumor necrosis factor (TNF) superfamily with a CD40L-like structure, including B-cell activating factor (BAFF) and proliferation-inducing ligand (APRIL) appear to facilitate TI class switching.17,18 Toll-like receptor 4 (TLR4) signaling induces the expression of both CCL20 and CCL28 on intestinal epithelial cells and APRIL, as indicated by augmented TI IgA induction in transgenic mice that express activated TLR4, constitutively.19 Hence, TLR signaling triggered by commensal bacteria seems a plausible mechanism of TI IgA class switching.19
Although CSR and SHM have been described in the TI setting,20 the production of high-affinity antigen-specific antibodies was not possible in the absence of T helper cells in a mousess model.21 In addition, Foxp3+ T cells (T regulatory [Treg] cells) are vital for achieving host-microbiome homeostasis.22 Furthermore, decreased IgA ASC in the intestinal mucosa and absence at extraintestinal sites have been described in T cell–depleted mice, underlying the importance of an intact T cell immunity compartment for the development of proper IgA ASC responses.23 It should be noted that in the absence of T cells, there is an almost 80% to 90% depletion of IgA, which indicated a relatively higher contribution of TD to IgA production compared with the T-cell independent counterpart.14 Intriguingly, GC antigen-specific PC populations detected after PC depletion reflected the populations before this intervention, which indicates the ability of mucosal immunity to memorize past triggers and regenerate its cellular composition.24 This also suggests that continuous migration and reentry to GALT GC probably functions as a mean of ongoing specialization and clonal selection of recruited B cells, producing highly specific IgA after repetitive cycles of reentry.24 Additionally, memory IgM B cells can switch to IgA, enriching this cellular pool in the intestinal mucosa, both via TD and TI IgA induction, depending on the requirements of the mucosal microenvironment.25 This observation also supports an interplay between TD and TI IgA responses.25
Structure and Function of Secretory (S) IgA
Immunoglobulin A is present as monomers in circulation, whereas the hallmark of mucosal PCs is their ability to produce polymeric IgA, mainly dimeric (d)IgA.26 More specifically, these IgA dimers are composed of 2 monomers, connected by their Fc, through the joining chain (J chain), a small polypeptide synthesized by long-lived IgA PC. The J-chain provides resistance to proteolysis and also facilitates binding of dIgA to the polymeric immunoglobulin receptor (pIgR).26 The pIgR, located at the basolateral side of intestinal epithelial cells (IECs), is responsible for the transcytosis of dIgA and IgM to the intestinal lumen.27 Upon its transfer to the lumen, proteolytic cleavage of the pIgR-dIgA complex occurs. A portion of PIgR, known as secretory component (SC), and dIgA form what is known as secretory IgA (SIgA).27 The use of a capital “S” distinguishes it from surface (s)IgA, which is spelled witha lowercase “s.” Secretory component is a highly glycosylated peptide that protects SIgA from proteolysis27 and assists in its anchoring to mucus. Moreover, SC appears to play a role in the regulation of the microbiota due to several antimicrobial functions, which include intraluminal sequestration of bacteria, neutralization of bacterial toxins, and binding to pathogenic bacteria.28,29 Free forms of SC are present in mucosa and mucus.30
αEβ7-dependent Transcytosis of IgA
Integrin αEβ7 was first identified as the HML-1 antigen, subsequently recognized as integrin αE or CD10331; it is expressed by malignant B cells and widely used as a marker for hairy cell leukemia, a B cell cancer.32 Integrin αEβ7 is expressed by intraepithelial lymphocytes (IEL)33 and mediates their interactions with IEC via E-cadherin.34 A mucosal DC subset also expresses αEβ734 and is a major producer of retinoic acid (RA),35 critical for induction of a gut-homing phenotype, regulatory T cells (Treg), and IgA class switching.36–38 However, the function of this integrin for this DC subset remains unclear, as CD103-deficient DC are not impaired on their ability to perform known physiologic functions.39 Expression of integrin αEβ7 by intestinal B cells or any role in IgA luminal transport had not been reported so far.40
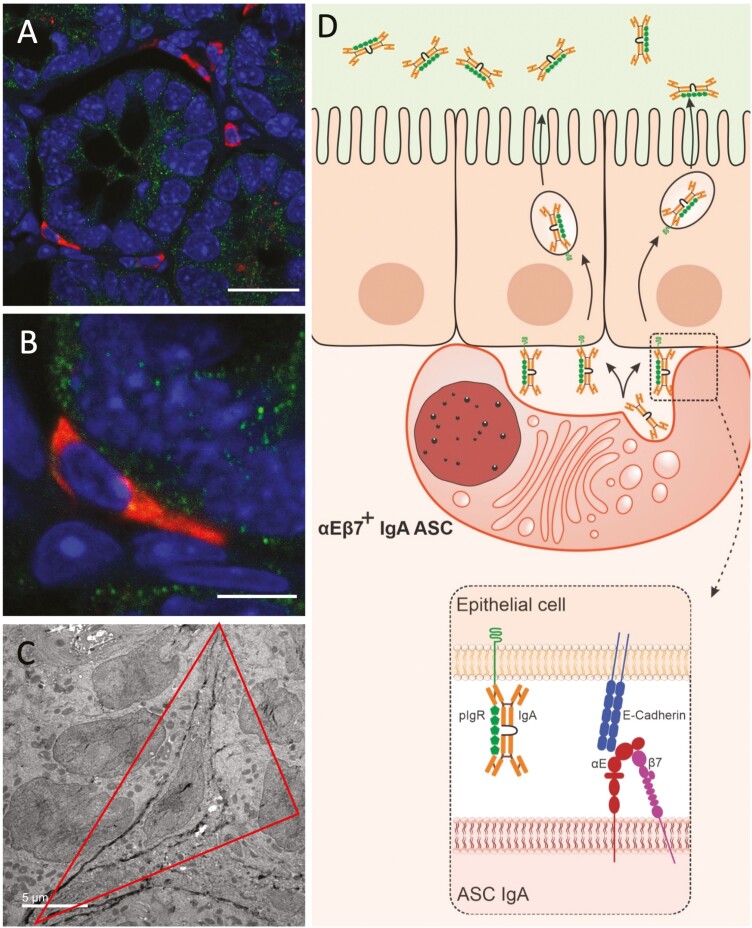
The canonical mechanism for the luminal delivery of IgA was described in 1981 by Per Brandtzaeg and involves the release of dIgA by LP ASC into the extracellular milieu, with subsequent diffusion to reach pIgR on the basolateral side of IECs.41 We have recently reported on a subset of intestinal IgA+ PC which expresses integrin αEβ7 (CD103) and becomes elongated or sickled and abutt with E-cadherin/pIgR-expressing IEC, a property reminiscent of IEL known to express CD103 (Figure 1A-C). Such epithelial-docking PCs were absent in both CD103- or β7-deficient (KO) mice. CD103 KO cells express α4β7, which allows a baseline number of IgA ASC to reach the LP; yet the level of SIgA in the lumen remains low, supporting the functional relevance of CD103-dependent transcytosis for the optimal transport and maintenance of luminal SIgA.42 Transcriptomic analysis of sorted CD103+ IgA PC demonstrated that they bear characteristics of terminal differentiation. They lack expression of markers that are lost in maturation, including CD19, CD20, CD38, and CD79a/CD79b. In addition, they do not express CD40, indicating that these cells no longer present antigen, as it is expected from professional PCs. In contrast, sorted CD103+ IgA PC expressed syndecan-1 (CD138), a classic marker of mature ASC, Tnfrsf17, which encodes for B cell maturation antigen (BCMA), a marker of long-lived PC, and integrin β4. Integrin aEb7-expressing PC predominantly dock with pericryptal epithelium, near the ileal crypt base. This localization allows us to speculate that they play a Paneth-cell like role for the protection of the stem cell niche. Overall, we described a novel subset of intestinal αEβ7-expressing PC, a novel role for integrin αEβ7 for the maintenance of luminal SIgA, and an enhanced mechanism for IgA transcytosis via direct relay of IgA to pIgR by αEβ7+PC.42
Figure 1.
A subset of IgA+ ASCs acquire and elongated/sickled morphology near the crypt base. A, IgA+ ASCs near the crypt base have an elongated (sickle-like) morphology (cross sections). B, Higher magnification of an adherent cell with sickled morphology. C, Direct cell to cell contact between IEC and sickle cell with extensive RER and IgA immunogold particles (representative TEM image). D, Proposed new model of αEβ7-dependent IgA transcytosis in which αEβ7+ IgA+ ASCs dock with IEC to directly relay dIgA to pIgR for transcytosis into the intestinal lumen, from Guzman et al.42
Canonical vs Noncanonical IgA Binding to Antigens
After dIgA is transcytosed to the intestinal lumen, it binds to antigens in canonical and/or noncanonical fashion.43 Canonical binding refers to traditional antibody-antigen interactions through the complementarity determining region (CDR) of Fab portion to the antigen. Immunoglobulin A alternatively binds to antigens, via non-Fab sites (noncanonical binding), with J-chain glycosylated sites and SC being pivotal in this role.43 Apart from the highly mutated, antigen-specific IgA production, poly-reactive IgA (binds to multiple microbial agents) has been described.44 Monoclonal IgA coats multiple microbial species, along with its specific targets.13 Thus, it is reasonable to assume that high-affinity IgA is a result of the TD GC antigen-specific responses, mainly targeted against potentially pathogenic microbial agents, binding canonically to its antigen targets. By contrast, polyreactive IgA is induced by commensals and dietary antigens and binds through nonspecific glycan-mediated interactions.
IgA1 and IgA2
Two IgA isotypes (IgA1 and IgA2) have been recognized in humans. The ratio of their concentrations varies widely, with the highest IgA1 levels found in circulation, whereas equal concentrations of IgA1 and IgA2 are observed within the colonic mucosa.46 The sequences of these 2 isotypes differ mainly in the hinge region between the Fc and the Fab portion of the antibody, with IgA2 having a shorter one, leading to increased proteolytic resistance.26 Furthermore, IgA1 and IgA2 are characterized by different glycosylation profiles, with IgA1 having more sialic acid.45 These differences mirror different effector features, as IgA1 appears to mainly have a regulatory role during homeostasis, whereas IgA2 exerts more pronounced inflammatory functions on neutrophils and macrophages.45 Accordingly, an IgA2 predominant profile in rheumatoid arthritis autoantibodies correlated with high inflammatory burden.45 Interestingly, although 90% of IgA class switching occurs through the direct IgM-to-IgA1 and IgM-to-IgA2 pathways, sequential switching of IgA1 to IgA2 has also been described. This emphasizes the versatility of IgA adaptation to the microbial environment, as IgA2 responses are upregulated in the lipopolysaccharide-rich environment that characterizes the colon.46
Intestinal Trafficking of IgA+ ASC
Naïve B lymphocytes circulate between peripheral blood and secondary lymphoid organs, where they encounter antigens, get activated, and become effector or memory cells. Such induction sites at the intestinal mucosa consist mainly of PP, where the majority of IgA+ ASC are generated. Other inductive sites include the MLN and cecal patch/appendix. During this process, a gut-homing signature is also imprinted on IgA+ ASC via the acquisition of trafficking receptors whose ligands are preferentially expressed by the intestinal microvasculature (ie, CCR9/10, MAdCAM-1). Interestingly, IgA class switch recombination and the acquisition of gut-homing trafficking code on B cells are under the control of the same signals.36,47 Dietary vitamin A is converted to retinoic acid (RA) by retinal dehydrogenase (RALDH) enzymes expressed by IEC and antigen-bearing DC. Retinoic acid induces the expression of α4β7 and CCR9/10 in ASC, rendering them capable of selectively migrating to the intestinal LP. This is in line with several lines of evidence which demonstrate that homing of ASC to a particular peripheral tissue/organ is signified by the respective site where the antigen was initially encountered.
Immunoglobulin A ASCs that are induced in GALT predominantly express integrin α4β7, which facilitates their binding to its ligand, MAdCAM-1. This leads to the selective homing of these cells to the intestinal microvasculature where MAdCAM-1 is expressed.47 By contrast, it is likely that IgA ASC induced at extraintestinal sites for protection of other mucosal surfaces (eg, respiratory, genitourinary epithelium) predominantly express integrin α4β1, which binds to its ligand, vascular cell-adhesion molecule-1 (VCAM-1). Furthermore, intestinally induced IgA ASCs express CCR9 and CCR10 that bind to their ligands CCL25 and CCL28, respectively, with the former ligation being intestinal-specific.47 Homing of IgA ASC to the bone marrow cannot be excluded, as these cells express CXCR4 and remain responsive to CXCL12, which is present in the bone marrow and at the mucosa.47
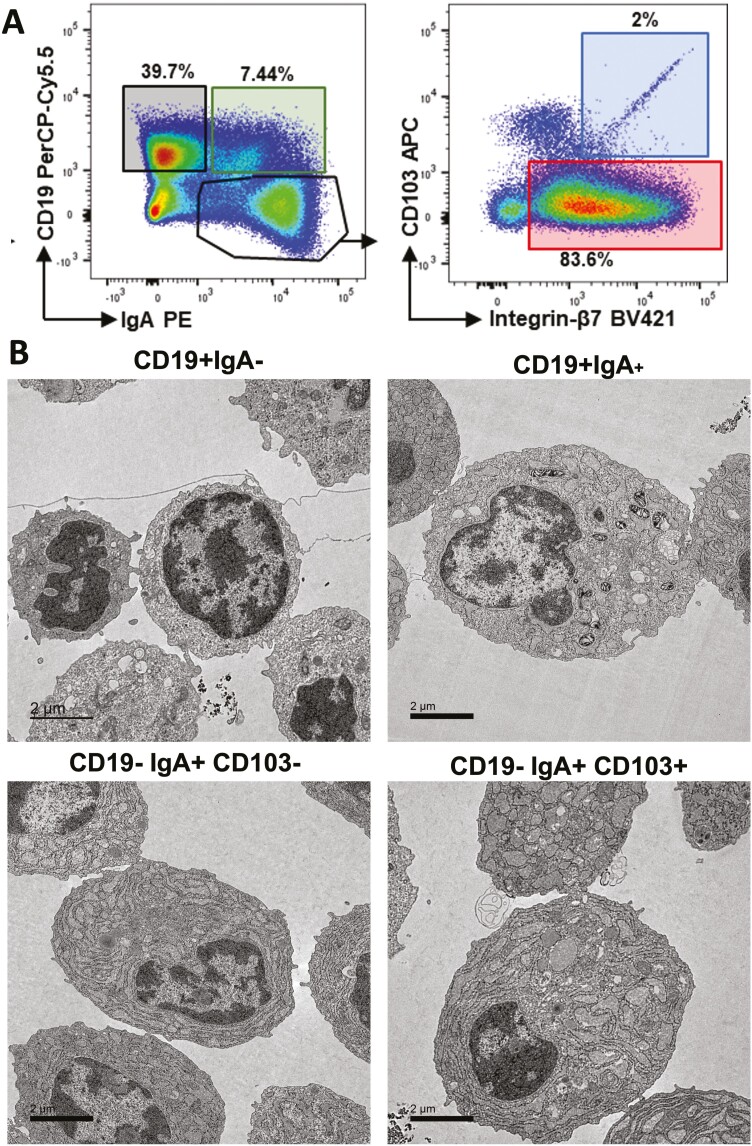
Within the LP, a subset of plasmablasts shed CD19, express surface IgA, and terminally differentiate into long-lived PC with discrete morphology characterized by abundant rough endoplasmic reticulum (Figure 2).48,49 A subset (2%) of the CD19negIgA+ ASC express αEβ7 and have the most abundant rough endoplasmic reticulum (RER). Both CCR9 and β7 KO mice exhibit an ASC deficit in the LP, yet these molecules are not absolutely required, as these mice are able to maintain baseline luminal SIgA levels. Their ability to upregulate luminal SIgA under conditions of chronic inflammation is limited. Regional differences do exist, as indicated by antibody blockade experiments in mice. In particular, migration of IgA+ ASC to the small intestine is inhibited by either CCL25 or CCL28 blockade, whereas only anti-CCL28 but not anti-CCL25 treatment prevents colonic trafficking of this population.50 At the intestinal mucosa, this process is regulated by RA that induces the expression of α4β7 and CCR9/10 in ASC. Dietary vitamin A is converted into RA by retinal dehydrogenase (RALDH) enzymes, which are expressed by IEC and antigen-bearing DC.36 Interestingly, IgA CSR and the acquisition of a gut-homing molecules on B cells are under the control of the same signals.36,47 It was shown that upregulation of CCR10 on developing plasmablasts is dictated by colonic patch DC.51 These chemokine signals act in accord with α4β7:MAdCAM-1 interactions to direct IgA+ ASC to the intestinal microvasculature under homeostatic conditions. Plasmablasts that do not express α4β7 are disadvantaged for intestinal entry and likely employ integrin α4β1 to localize to extraintestinal tissues such as the lung and lacrimal glands. From these data, it is apparent that the expression of α4 integrins on ASC dictate their migration towards the gut (α4β7+ cells) or nonintestinal sites (α4β1+). These expression patterns have recently become of great translational significance because integrins have arisen as prominent treatment targets for chronic immune-mediated diseases (eg, multiple sclerosis [MS], IBD).
Figure 2.
Major subsets of intestinal B cells show distinct morphology. A, Flow cytometric sorting of intestinal LP B cell lineage cells show 3 major subsets which include CD19+ IgAneg, CD19+ IgA+ and CD19neg/IgA positive. Subsequent analysis of the CD19+/IgA+ show that the majority express integrin β7 (likely α4β7) whereas 2% express both αE (CD103) and β7. B, Electron microscopy shows increased rough endoplasmic reticulum and decreased nucleocytoplasmic ratio in cells that express IgA on the cell surface (representative images from Guzman et al.)42
Some additional points are worth mentioning. First, MAdCAM-1 is found at extraintestinal locations, including the lactating mammary gland, the placenta, the inflamed pancreas and liver, and possibly the genitourinary tract.53,54 This expression may allow α4β7+ PC to travel to these locations. Second, it should be noted that although clearly distinct homing signatures have been described under homeostatic conditions, those patterns are blurred during chronic inflammation where multiple homing pathways may contribute to dysregulated inflammatory cell recruitment.54–56 A stepwise reliance on homing mechanisms may also take place from the temporal perspective. The initial orderly cellular influx may be followed by an exaggerated homeostatic pattern, later followed by overlap of inflammatory homing signatures that amplify and perpetuate the disease process.57 Finally, differences between human and murine biology should also be considered when applying mouse data to humans and vice versa.
Differential T and B Cell Dependence on α4β7 and α4β1 for Intestinal Entry
The dependence of B cells on specific α4 integrins for tissue-specific trafficking has recently become of great translational significance since integrins have arisen as prominent treatment targets for chronic immune-mediated diseases, particularly IBD and MS. These include the anti-integrin antibodies (eg, α4 subunit natalizumab; α4β7: vedolizumab; β7: etrolizumab) and the anti-MAdCAM-1 antibody ontamalimab.58–62 Understanding the cellular specificities of such treatments will be of paramount importance for determining their efficacy and safety profiles.
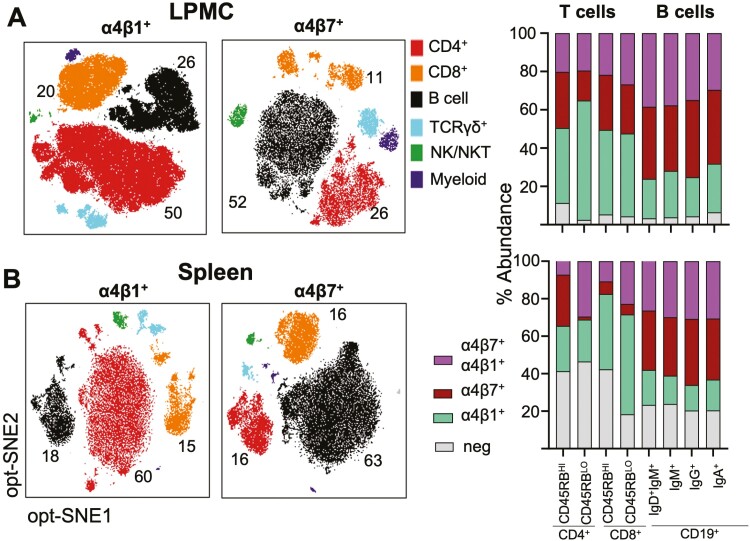
We have recently profiled the cellular expression patterns of α4β7 and α4β1 by cells from distinct lineages in colitis-prone IL-10-/- mice.63 The B cells/ASC in the colonic LP and spleen preferentially expressed α4β7 over α4β1, whereas the reverse is the case for CD4+ lymphocytes (Figure 3). The presence or absence of colitis did not affect those expression profiles, suggesting that B cell/ASC preferentially use α4β7:MAdCAM-1 binding to enter the intestinal LP during both homeostatic and inflammatory conditions, whereas T cells may alternately and efficiently employ α4β1. These results confirm previous reports showing that integrin β7 and MAdCAM-1 deficiencies predominantly affect the B cell lineage, resulting in smaller PP size and less LP B cells and IgA+ ASC.64,65 Interestingly, we observed the same pattern of B cell/α4β7 vs T cell/α4β1 differentiation in patients with UC (unpublished results). In line with the increased expression of α4β7, we observed in our unpublished results that B cells are the primarily targeted population by α4β7:MAdCAM-1 blockade both in mice and in patients with UC.63 Along the same line, Zundler et al recently reported that effector T cells are highly dependent on α4β1 for migration into the inflamed ileum of patients with CD.66
Figure 3.
Preferential α4β1 and α4β7 integrins by T and B cells. A, Cellular distribution of integrins α4β1 and α4β7 among the major leukocyte lineages within the colonic LP (LPMC) and spleen. Cells were pre-gated on live, CD45+ cells, followed by opt-SNE analysis. Major cell subsets are depicted. B, Distribution of integrin expressing subpopulations within the CD4+, CD8+, and B cell lineages. T and B cell populations were divided into the indicated memory subpopulations. From Tyler et al.63
IgA as a Regulator of the Intestinal Microbiota
When SIgA enters the intestinal lumen, it recognizes and interacts with antigens derived from both commensals and pathogens. These interactions allow SIgA to exert a pivotal role in shaping the composition of the gut microbiota during homeostasis with commensals and as an important defense against pathogens. Recent studies have provided evidence for a differential role of SIgA during these 2 settings, inasmuch as substantially diverse responses appear to take place in the healthy state compared with enteric infections, which mainly relate to the specificity of antibody reactivity and the dependence on T cell help. This diversity depends on the ability to SIgA to acquire variable reactivity against bacterial antigens, which can be cross-species, species-specific or strain-specific.67 Cross-species or polyreactivity designates reactivity against structurally disparate antigens such as LPS, CpG, flagellin, DNA, and capsular polysaccharides among others.40,68–73 This is most probably related to natural (non-TD) antibodies and gives polyreactive SIgA the ability to interact with various bacterial taxa. On the other hand, species-specific and strain-specific reactive SIgA also occur, which bind certain bacterial intestinal species (likely by recognizing surface carbohydrate moieties), individual genetic variants or subtypes of a particular species, respectively. Immune exclusion refers to antigens being retained within the lumen, after being bound by IgA, thus precluding access to the epithelium. This “mechanical” blockade is mediated by entrapment within the mucus layer, expulsion via intestinal peristalsis, and/or clearance after antibody agglutination and cross-linkage.74 The luminal specificity of such mechanism is emphasized by the findings of Michetti et al, who used BALB/c mice that bore subcutaneous hybridoma tumors producing monoclonal IgA against Salmonella typhimurium (Sal4) and were capable of secreting monoclonal SIgA into their gastrointestinal tracts. Mice were protected from systemic infection with S. typhimurium when bacteria were administered orally but not after intraperitoneal injection; although Sal4 was detected in the circulation.75 Protection is further enhanced by IgA-mediated neutralization of surface antigens employed by harmful bacteria to breach the epithelial barrier, such as adhesins and pili.76 Immunoglobulin A reactivity against such invasive modules is not confined to bacteria but also include reactivity against proteins that mediate adherence of fungal hyphae adherence and invasion of host cells by Candida albicans.77 Another mechanism is enchained growth, which prevents conjugative plasmid transfer by segregating bacterial plasmid donors and recipient clones.78 This mechanism is relevant for control of low-abundance, fast-growing microorganisms. In addition, SIgA may also modify the expression of bacterial genes that encode for proteins involved in pathogenic mechanisms against the host. As an example, IgA-specific responses against flagellin results in downregulation in bacteria, decreasing motility and pathogenicity.79 It should be noted that molecular modification of intestinal microorganisms by IgA also affects commensals via immune inclusion, which allows residence at mucosal sites.80 Finally, “coating” is used by SIgA to augment antigen sampling by activating resident DC, facilitating entry of coated antigens to PP.81,82 Again, regional differences were noted, as coating was predominantly present at the ileum but not the colon.83 Although these protective mechanisms against pathogenic microorganisms have been clearly demonstrated, the extent to which they participate in homeostatic regulation remains unknown. Nevertheless, it is expected that substantial overlap may take place between IgA reactivity during healthy and pathological states.
Although the epithelial barrier is often referred as being in contact with the microbiota, this is imprecise as there is a 500-micron physical/chemical mucus barrier separating our eukaryotic cells from prokaryotic bacteria. Imunoglobulin A is an essential chemical component, constituting alongside the inner and outer mucus layer and natural antimicrobial peptides, which is the first line of defense against mucosal invasion by intraluminal organisms with pathogenic potential. In fact, constituents of the barrier act in synergy, inasmuch as failure of an individual mechanism may lead to compensatory upregulation of the remaining factors. This was recently shown in mice that were deficient for the natural antimicrobial lectin RegIIIγ.84 Deficiency led to increased bacterial colonization of intestinal epithelium, followed by compensatory increased IgA production.84 Converging lines of evidence support the notion that the homeostatic function of SIgA is mediated via TI polyreactive responses with or without limited somatic hypermutation or affinity maturation.23 Although only 7% of the intraluminal SIgA is cross-species reactive, it has a major impact on the diversification of commensal microbiota. This was shown in studies that demonstrated a genetically defined predisposition of BALB/c mice for higher production of polyreactive SIgA compared with C57BL/6 mice.69 The pathophysiological implications of these differences were further demonstrated by showing that the former strain displayed wider microflora diversity due to higher abundance of polyreactive SIgA, which was also present in germ-free BALB/c mice. Further supporting the concept of naturally occurring polyreactive antibodies is the fact that natural IgA PCs are detected in germ-free mice that are not exposed to external antigenic stimulation and demonstrate specificities that are identical to IgA from specific-pathogen-free (SPF) mice.44,69,85
Recently, IgA-seq methodology, which encompasses bacterial flow cytometric sorting coupled with 16S rRNA sequencing, has allowed delineation of a range of IgA-coated microorganisms and has showed that this process is characterized by taxonomical selectivity.22,86–89 Enriched within the IgA-coated population are members of the phylum Proteobacteria, the Th17-inducing segmented filamentous bacteria (SFB), and members of the Mucispirillum, Prevotella, and Helicobacter species.44,83,90–93 Interestingly, most of those microorganisms have a preferential predilection for localizing in proximity to small intestinal epithelium, which may explain the higher abundance of IgA+ PC in the ileal compared with the colonic LP and of IgA-coated bacteria within the small intestinal lumen. On the other hand, the most abundant commensal phyla, namely Firmicutes and Bacteroidetes, are not bound by IgA antibodies—although exceptions do occur, including the Lactobacilli and Clostridial species, as well as Akkermansia mucinophila.83,90 The importance of SIgA for mucosal homeostasis and microbiota composition is emphasized by dysbiosis in mouse strains that are deficient in luminal IgA (eg, AID, pIgR KO).94
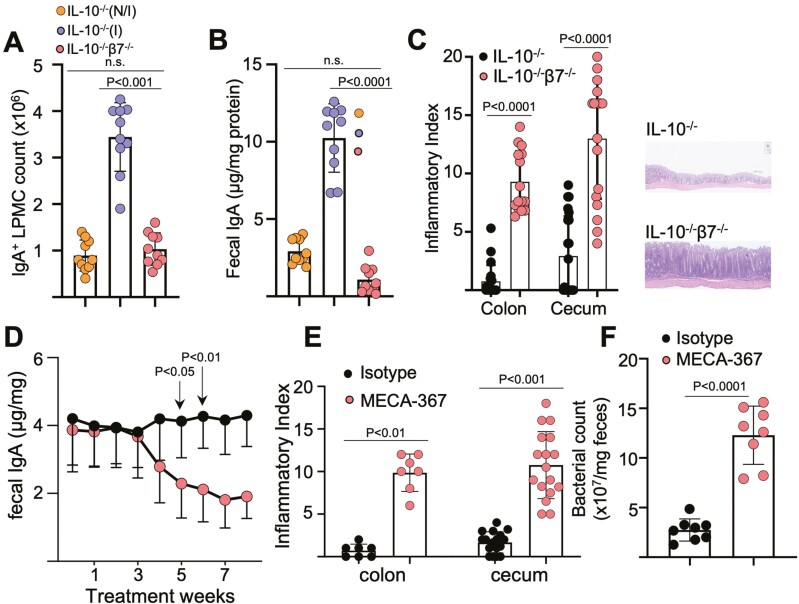
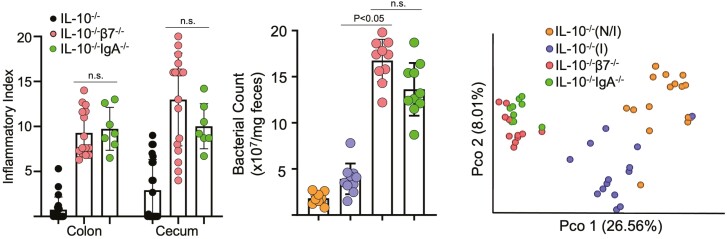
We and others95 have recently reported that IL-10/integrin β7 double KO mice have compromised ASC entry into the LP (Figure 4A), leading to a luminal IgA deficit (Figure 4B) and an accelerated colitic phenotype with early lethality (Figure 4C). Colitis in IL-10 KO is also hastened by MAdCAM-1 blockade, which progressively lowers fecal SIgA (Figure 4D) by blocking intestinal ASC entry. In both the congenital β7 deficit and inducible MAdCAM-1 blockade, we observed bacterial overgrowth (Figure 4F) and an altered microbiota composition, different from that previously reported induced by the development of colitis.59,96
Figure 4.
Integrin β7 deficient IL-10-/- mice and MAdCAM-1 blockade lower fecal IgA and worsens colitis. A, Colonic LP IgA+ ASCs counts of indicated strains at 8 to 12 weeks of age, determined by flow cytometry (non-inflamed [N/I], inflamed [I], as determined by histological scores; total histological scores 0-1 represent N/I mice, scores >1 represent inflamed mice). Freshly isolated LP cells were stained, and events gated on live single cells. B, Fecal IgA of the indicated mouse strains. Expression was normalized to total fecal protein. C, Representative colonic histology and colonic and cecal histological indices. All data are presented as mean ±SD, from n > 9 mice in each data set. Each data point represents an individual mouse. Statistical significance was determined by ANOVA, followed by Tukey multiple comparison test. D, Serial fecal IgA levels of anti-MAdCAM-1-treated IL-10-/- mice. E, colonic and cecal histological indices of treated mice at week 8. F, Fecal bacterial counts from IL-10-/- mice treated with isotype or MECA-367, determined via RT-qPCR for 16S rRNA expression. All data are presented as mean ±SD, from n > 10 mice in each data set. Statistical significance was determined by Student t test (B), or 2-way ANOVA, followed by Sidak multiple comparison test. From Tyler et al.63
In contrast to the polyreactive SIgA dominant homeostatic pathway, responsiveness to microorganisms with pathogenic potential employs IgA responses that are characterized by both high specificity and affinity. These occur in the germinal centers of inductive sites of the GALT and mimic those that occur during systemic immunity to infectious agents or other foreign antigens.23
IgA Defects During Intestinal Inflammation
Plasma cells are amongst the most abundant cells with the inflammatory infiltrate that characterizes IBD. Their presence serves both as an indicator of the chronicity and as a predictor of relapse in UC.97 Moreover, in CD, serum humoral responses against antigens derived from the commensal flora precede clinical inflammation and have prognostic value for assessing disease severity.98,99 In addition, dysbiosis is a core disturbance of the mucosal environment in patients with CD and UC, although whether this is the cause or effect of chronic inflammation remains unanswered.100 Despite those facts, the contribution of B cells, ASC, and IgA to the pathogenesis of IBD has been largely understudied, particularly when compared with T cell responses.
Under healthy-state conditions, a minority (<5%) of the total IgA is employed for bacterial coating. This percentage is, however, substantially increased in patients with IBD. Interestingly, in patients with a disease flare, an increase in IgA-, IgM-, and IgG-coated bacteria has been observed.101 Nevertheless, only IgA-coating persisted during long-term remission, indicating a certain specificity of IgA responses. This was further exemplified in a study by Palm et al93 who colonized germ-free mice with IgA-coated and uncoated bacteria from IBD patients. Upon challenge with dextran sulphate sodium (DSS), recipients of the IgA-coated consortia developed more severe colitis than mice colonized with the IgA-uncoated bacteria, thus suggesting that the IgA-coated fraction bears the most pathogenetic potential. Interestingly, a recent study compared IgA-coated bacteria between patients with CD with or without spondylarthropathy102 and demonstrated selective enrichment with IgA-coated E. coli in in the former, which elicits a Th17 immune response. Therefore, IgA coating may signify the presence of resident bacteria with pathogenetic potential, not only locally but systemically. Additional defective, IgA-related pathways may also exist in subsets of patients with CD, where mutations in the Nod/Card15 gene are prevalent and in certain cases affect retrograde transport of antigen-carrying SIgA in PP. This led to the hypothesis that NOD2 deficiency increases the influx of bound antigens to the intestine and subsequent enhanced inflammatory responses, as it was shown by worse inflammation in NOD2 KO mice that were challenged with S. typhimurium bound with murine IgA.103
In recent years, significant technological advances have taken place in mucosal immunology via the increased incorporation of single-cell immunophenotyping methodologies, which allow investigators to delineate with the utmost detail the changes in the cellular composition in a particular compartment in association to a particular condition (ie, inflammation) or following a specific treatment (ie, biologics). Using this methodology, Uzzan et al characterized the mucosal and peripheral blood B cells in healthy individuals and patients with UC.104 The authors provide evidence for a substantially dysregulated B cell response in UC.104 Changes associated with UC included elevations in the number of naive B cells and IgG+ PC that showed restricted diversity and maturation, clonal expansion of auto-reactive, anti-αvβ6 plasma cells at the inflamed mucosa, and presence of an intestinal CXCL13-bearing peripheral T helper cells reminiscent of TFH-like cells that were associated with pathogenic B cell responses.104 Those changes in local humoral immunity drove similar alterations at the circulating compartment that was enriched in gut-homing plasmablasts.104 Of translational significance was the discovery that the number of those cells were predictive of higher disease activity and a complicated disease course.104
Antibody Responses to Bacterial Antigens in Patients with IBD
Several studies have reported the presence of IgA (and IgG) reactive to E. coli outer-membrane porin C, the Pseudomonas fluorescens–associated sequence I2, anti-Saccharomyces cerevisiae and flagellin in sera from patients with IBD.105,106 Recently, exciting new data have shown that the presence of certain serum biomarkers could predict the eventual development of IBD. Serum samples from the United States Defense Medical Surveillance System were screened for the presence of antimicrobial antibodies and other proteins. Profiles of patients who eventually developed CD or UC were compared with healthy controls, demonstrating that specific biomarkers had a high predictive value up to 5 years prior to diagnosis. The discriminative probability between CD and heathy controls increased when ASCA-IgA positivity was included in the model. These results indicated that IgA reactivity against bacterial antigens preceded the development of clinical IBD and may, therefore, represent an early pathogenetic trigger.107
Mucosal Phenotype in Mice with Luminal IgA Deficiency
Data from animal models provide additional mechanistic information regarding the role of B cell/ASC/IgA during homeostasis and IBD. Mice with differential baseline IgA levels show diverse susceptibility to chemically induced colitis. The CBA/CaJ (CBA) mice with high luminal SIgA and bacterial coating result in lower bacterial load upon challenge with DSS and decreased colitis severity. In contrast, C57BL/6 mice with lower baseline IgA suffer from worse colitis.108
In fact, several studies have shown that murine strains with deficiencies in the production and/or function of IgA uniformly develop intestinal dysbiosis and increased severity of spontaneous or trigger-induced inflammation. Such strains include the pIgR deficient (defective IgA/M transcytosis),109 the inducible costimulator ligand (ICOSL)-deficient (reduced IgA, although control mice may not have been cohoused littermates),110 and the ATF3 (activating transcription factor 3)-deficient mice (compromised development of TFH cells and reduced SIgA production).105,111 Although the precise effects on microbiota differ between those strains, dysbiosis is a common denominator, which emphasizes the pivotal role of SIgA for the preservation of a mucosal homeostasis.
Recently, Nagaishi et al reported their findings on the clinicopathological phenotype, immunological characteristics, and gut-microbiome composition in a mouse line that lacked the constant region of the IgH α chain and was therefore rendered incapable of producing IgA (IgA KO).112 Interestingly, the effects of IgA deficiency were confined to the ileum but not the remainder of the GI segments.112 Such effects consisted of spontaneous ileitis, associated with increased local production of cytokines and LP CD4+ T cells112 and skewed composition of the gut microflora, with increased representation of SFB in ileum.112 The investigators also developed a murine line with a <50 base pair deletion in the cytoplasmic region of the IgA allele; the lack of a phenotype indicated that the cytoplasmic region of IgA is dispensable for protection from ileitis.112 Taken together, these findings point to a critical homeostatic role of IgA, which, however, shows compartmentalization along the GI tract, appearing singularly important in ileum.
We have recently studied the effects of genetic or pharmacological neutralization of the α4β7/MAdCAM-1 trafficking pathway on gut homeostasis.63 We examined IL-10 KO mice that were also rendered deficient in α4β7/MAdCAM-1-pathway by either concomitant β7 deficiency or treatment with anti-MAdCAM-1 neutralizing antibodies (Figures 4, 5). In all cases, we observed a deficit in luminal IgA, which was associated with the development of a dysbiotic microbiota (Figure 4). The latter was indicated by lower α diversity and altered β diversity by principal component analysis.63 We then compared their phenotype with IL-10 KO mice that were also deficient in IgA (IL-10/IgA double [D]KO). Interestingly, we observed that the immunological and microbiome phenotypes of IL-10/IgA DKO mice overlapped with those of IL-10/β7 DKO mice manifested by aggressive colitis and similar changes in microbial counts and community composition (Figure 5). This included decreased representation of Bacteroides and increased Clostridiales in both cases. Similarly to our work, Fagarasan et al also reported prominent expansion of Clostridia in AID-deficient mice which also have compromised SIgA due to their class switch defect.113
Figure 5.
Disease severity and changes in microbiota observed in β7-deficient IL-10-/- mice are recapitulated by IgA deficiency. A, Colon and cecum histological indices of disease severity of indicated genotypes at 8 to 12 weeks of age. B, Fecal bacterial counts from indicated strains determined via RT-qPCR for 16S rRNA expression. C, beta diversity principal coordinate analysis of the indicated mouse groups (mean ±S.D, from n > 10 mice in each data set.) Each data point represents an individual mouse. Statistical significance determined using one-way ANOVA, followed by Sidak multiple comparison test. From Tyler et al.63
Regulatory B Cells
In addition to the dysregulation of IgA secretion and/or function, other abnormalities regarding the B cell lineage also take place during active IBD and may contribute to its pathogenesis. Among those, regulatory B Cells (Bregs) have attracted attention due to their established role in homeostasis and therapeutic potential in patients with CD or UC. The term Bregs was introduced in 2002 in reference to a population of B cells with suppressive function, principally via secretion of IL-10.114 Among other properties, those cells control the mucosal balance of effector and regulatory T cells and exert anti-inflammatory function in murine models of chronic inflammation.115 Apart from IL-10 production they are CD19highCD1dhigh, respond to IL-33, produce TGFβ, and/or express Foxp3.116–118 A cluster of cytokines, mainly of the regulatory type, have been associated with the induction and function of Bregs, including among others IL-10, IL-33, IL-35, IL-21, IL-6, IL-1β, IFNα, BAFF, and APRIL.119 Accordingly in experimental models, Bregs ameliorate the severity of colitis.120 Like for IgA induction, the generation of Bregs also depends on stimulatory signals from the gut microbiota. Recently, Maerz et al reported that the generation and maintenance of mucosal Bregs depend on input driven from specific (but not all) commensals via Toll-like receptors.121 Such stimulation by immunogenic commensals led to the upregulation of IL-10 and rendered Bregs capable of preventing DC activation, direct T-effector polarization from Th1 and Th17 towards the Th2 pathway, and expanding the Treg pool. The clinical significance of such observations was supported by amelioration of DSS colitis in the presence of commensals with the ability to induce Bregs. Recent studies indicate that active CD or UC may be associated with decreased Bregs. In one such study, Bregs, defined as CD24high/CD38high and CD5+ populations (high IL-10 producers), were decreased in active UC, and their percentage correlated with disease activity.122 In a similar manner, it was shown that the population of CD19highCD1dhigh IL-10-producing B cells were decreased in patients with CD.116 Taken together, those studies indicate that a quantitative and/or functional deficit of Bregs may participate in IBD pathogenesis, thus setting the foundation for the therapeutic manipulation of this subset. Nevertheless, the clinical application of such preliminary observations may prove more challenging, given the dichotomous roles of Breg-derived cytokines in the pathogenesis of chronic inflammatory diseases.119
Ongoing work from our laboratory similarly suggests that the attenuation of T cell transfer colitis seen with B cells cotransfer is in part mediated by restoration of luminal SIgA, which restores bacterial IgA coating, reducing bacterial counts and modulating microbiota composition (unpublished results). Lastly, we have recently observed that IL-10-deficient mice have a baseline systemic and luminal IgA deficit, resulting in a blunted IgA response with the development of colitis. We propose that IL-10 has an underappreciated role during class switching that is only partly corrected by TGFβ. Thus, IgA may similarly play a critical role for the pathogenesis of colitis in IL-10 KO mice (unpublished results). Taken together, these data support a critical role of luminal SIgA for the maintenance of a healthy microbiota and the preservation of gut homeostasis.
Conclusions
Luminal SIgA is a critical evolutionary mechanism that allows vertebrates to survive a potentially hostile microbiota, while letting us reap the benefits of our mutual coexistence. It is an integral part of a broader structural and functional network that is the mucosal barrier, which operates under the principle that the best offense is a good defense against constituents of a highly integrated ecological environment comprised of the commensal flora and dietary antigens. The complexity and delicate balance of this “firewall” is demonstrated by the fact that maintenance of adequate SIgA levels is the sum of multiple independent yet interconnected local and systemic processes acquired by mammals over millions of years of coexisting evolution. A simplified version of the required steps and molecules involved within this complex and vital processes include:
B cell progenitor production at bone marrow
Development and organization of inductive sites (PP, MLN, ILF/TLS) required for IgA class switching (LTα,LTβ)
B cell migration into inductive sites (CXCR5)
IgA class switching within inductive sites (AID, IL-10, IL-10R)
Egress from inductive sites to circulation (S1PR1)
IgA ASC recruitment to LP (CCR9, α4β7, MAdCAM-1)
IgA transcytosis (pIgR, αEβ7).
Under homeostatic conditions, disruption of any of these steps may be compensated by expansion of others. As an example, we have observed that decreased LP ASCs recruitment as seen in β7 KO mice leads to overexpression of pIgR.42 Thus, mice with disruption at any of the previously mentioned individual pathways, kept in the relatively clean conditions of a vivarium, reach sexual maturity and reproduce. Indeed, β7-deficient mice show only mild changes in microbiota composition, despite showing marked LP IgA ASC and luminal IgA deficits.123 The phenotype of MAdCAM1-deficient mice is nearly identical, suggesting that these critical molecules are dispensable under homeostatic conditions, where baseline levels of IgA ASC reach the intestinal LP, likely through α4β1/VCAM-1 interactions. Our ongoing studies demonstrate that luminal SIgA levels increase with the development of ileitis or colitis in every IBD model studied, with a relatively intact immune system. Increased luminal SIgA is likely triggered by a hyper-response to bacterial antigens and TLR ligands. In this inflammatory setting, mice that are not able to match the microbial antigenic load from a breached intestinal barrier with a commensurate SIgA response are most susceptible to worse disease and death from colitis. We have tested this hypothesis by adding “second hit” challenges (IL-10 deficit, TNF overproduction, MAdCAM-1 blockade) resulting in uniform worsening of IBD. We predict that disruption of any of the previously mentioned steps that cannot be readily compensated by other mechanisms will similarly lead to worse IBD and or lethality. Interleukin-10 KO mice in B6 background are particularly susceptible because at baseline they have significantly lower levels of IgA than regular B6 counterparts (unpublished results). Thus, a second hit that impairs the necessary IgA response to an antigenic challenge leads to death, as we have shown with both β7- and IgA-deficient IL-10 DKO mice (Figure 4, 5) and after MAdCAM-1 blockade. We anticipate that impairment of any of the steps listed previously might further compromise the ability of IL-10 KO mice to mount the required luminal IgA response and similarly hasten colitis leading to lethality. In practical terms, the only way that we can breed either β7- or IgA-deficient IL-10 DKO mice is by maintaining the IL-10 KO allele as heterozygous in the breeders. Neither DKO strain consistently breeds in the homozygous state, as they develop early and more severe colitis (unpublished results).
An alternative pathophysiologic hypothesis is that during IBD there are exaggerated potentially harmful IgA responses that should be contained by interfering with one or more of these described mechanisms. As is the case with other aspects of mucosal immunity, overlap between healthy and pathological responses may exist, and the effect of a therapeutic intervention may largely depend on the specific clinical context and immunological background. This is particularly relevant as several current therapies may affect the biology of IgA. Currently, α4β7/MAdCAM-1 blockade is a major strategy in IBD. As this trafficking pathway is preferentially utilized by B cell lineage cells, including IgA+ ASC, the prediction from the mouse model data is that patients treated with vedolizumab or ontamalimab might have SIgA deficits. In support of this, a single dose of vedolizumab was shown to lower secretory IgA (SIgA) levels in stool and weaken the immunization response to oral cholera vaccine in healthy volunteers.124 However, it is also possible that millions of years of divergent evolution have rendered us humans less dependent on IgA to maintain homeostasis with our microbiota. Alternatively, B cells may develop less reliance on α4β7 with the sustained blockade of the pathway. Future research should focus on clarifying whether such alterations might be of clinical significance and may affect efficacy and/or safety in subsets of patients with IBD.
The link between mouse and human IBD might not be yet obvious because the B cell/ASC/IgA axis might play its most critical role during the initial triggering response to bacterial antigens leading to a chronic autonomous immune dysregulation. Of note is that the initial immune responses in IBD likely begin years before clinical IBD manifestations appear and patients seek care. We speculate that the eventual confluence of enhanced knowledge of IBD genetics in addition to the microbiota and their interaction with immunity might finally lead to an understanding of the underlying triggers that lead to IBD, opening the door for potentially preventive strategies beyond current therapeutics.
Contributor Information
Giorgos Bamias, GI Unit, 3rd Academic Department of Internal Medicine, National and Kapodistrian University of Athens, Sotiria Hospital, Athens, Greece.
Konstantina Kitsou, GI Unit, 3rd Academic Department of Internal Medicine, National and Kapodistrian University of Athens, Sotiria Hospital, Athens, Greece.
Jesús Rivera-Nieves, Gastroenterology Section, San Diego VA Medical Center, La Jolla Village Drive, San Diego, CA, USA; Division of Gastroenterology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Funding
This work was supported by grants from the National Institutes of Health (DK108670, DK118927, DK131532, DK132242); VA Merit BLRD-I01 BX005951; San Diego Digestive Diseases Research Center (P30 DK120515) and support from Chiba University-UC San Diego Program in Mucosal Immunology, Allergy and Vaccines.
Conflicts of Interest
The authors declare no commercial or financial conflicts of interest.
References
- 1. Leiper K, Martin K, Ellis A, et al. . Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut. 2011;60:1520-1526. [DOI] [PubMed] [Google Scholar]
- 2. Leandro M, Isenberg DA.. Rituximab - the first twenty years. Lupus. 2021;30:371-377. [DOI] [PubMed] [Google Scholar]
- 3. Berghen N, Vulsteke JB, Westhovens R, Lenaerts J, De Langhe E.. Rituximab in systemic autoimmune rheumatic diseases: indications and practical use. Acta Clin Belg. 2018;74:272-279. [DOI] [PubMed] [Google Scholar]
- 4. Mei HE, Frölich D, Giesecke C, et al. . Steady-state generation of mucosal IgA+ plasmablasts is not abrogated by B-cell depletion therapy with rituximab. Blood. 2010;116:5181-5190. [DOI] [PubMed] [Google Scholar]
- 5. Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821-832. [DOI] [PubMed] [Google Scholar]
- 6. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM.. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15:160-171. [DOI] [PubMed] [Google Scholar]
- 7. Mora JR, Andrian UH von.. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1:96-109. [DOI] [PubMed] [Google Scholar]
- 8. Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T.. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639-643. [DOI] [PubMed] [Google Scholar]
- 9. Cerutti A. Location, location, location: B-cell differentiation in the gut lamina propria. Mucosal Immunol. 2007;1:8-10. [DOI] [PubMed] [Google Scholar]
- 10. Rios D, Wood MB, Li J, et al. . Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 2015;9:907-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koscsó B, Kurapati S, Rodrigues RR, et al. . Gut-resident CX3CR1hi macrophages induce tertiary lymphoid structures and IgA response in situ. Sci Immunol. 2020;5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rivera CA, Randrian V, Richer W, et al. Epithelial colonization by gut dendritic cells promotes their functional diversification. Immunity. 2022;55:129-144.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pabst O, Slack E.. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020;13:12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM.. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222-2226. [DOI] [PubMed] [Google Scholar]
- 15. Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci. 2006;1072:52-61. [DOI] [PubMed] [Google Scholar]
- 17. He B, Xu W, Santini PA, et al. . Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812-826. [DOI] [PubMed] [Google Scholar]
- 18. Gommerman JL, Rojas OL, Fritz JH.. Re-thinking the functions of IgA+ plasma cells. Gut Microbes. 2014;5:652-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shang L, Fukata M, Thirunarayanan N, et al. . Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529-538.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheeren FA, Nagasawa M, Weijer K, et al. . T cell–independent development and induction of somatic hypermutation in human IgM+IgD+CD27+ B cells. J Exp Med. 2008;205:2033-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slack E, Hapfelmeier S, Stecher B, et al. . A flexible continuum between adaptive and innate immunity in maintaining host-microbiota mutualism. Science. 2009;325:617-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawamoto S, Maruya M, Kato LM, et al. . Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41:152-165. [DOI] [PubMed] [Google Scholar]
- 23. Bunker JJ, Bendelac A.. IgA responses to microbiota. Immunity. 2018;49:211-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindner C, Wahl B, Föhse L, et al. . Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med. 2012;209:365-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magri G, Comerma L, Pybus M, et al. . Human secretory IgM emerges from plasma cells clonally related to gut memory B cells and targets highly diverse commensals. Immunity. 2017;47:118-134.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schroeder HW, Cavacini L.. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41-S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei H, Wang JY.. Role of polymeric immunoglobulin receptor in IgA and IgM transcytosis. Int J Mol Sci . 2021;22:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dallas SD, Rolfe RD.. Binding of clostridium difficile toxin A to human milk secretory component. J Med Microbiol. 1998;47:879-888. [DOI] [PubMed] [Google Scholar]
- 29. Oliveira IR, AraÃojo AN, Bao SN, et al. . Binding of lactoferrin and free secretory component to enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 2001;203:29-33. [DOI] [PubMed] [Google Scholar]
- 30. Turula H, Wobus CE.. The role of the polymeric immunoglobulin receptor and secretory immunoglobulins during mucosal infection and immunity. Viruses. 2018;10:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwarting R, Dienemann D, Kruschwitz M, Fritsche G, Stein H.. Specificities of monoclonal antibodies B-ly7 and HML-1 are identical [letter; comment]. Blood. 1990;75:320-321. [PubMed] [Google Scholar]
- 32. Visser L, Shaw A, Slupsky J, Vos H, Poppema S.. Monoclonal antibodies reactive with hairy cell leukemia [see comments]. Blood. 1989;74:320-325. [PubMed] [Google Scholar]
- 33. Kilshaw PJ, Murant SJ.. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201-2207. [DOI] [PubMed] [Google Scholar]
- 34. Roberts K, Kilshaw PJ.. The mucosal T cell integrin alpha M290 beta 7 recognizes a ligand on mucosal epithelial cell lines. Eur J Immunol. 1993;23:1630-1635. [DOI] [PubMed] [Google Scholar]
- 35. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y.. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527-538. [DOI] [PubMed] [Google Scholar]
- 36. Mora JR, Iwata M, Eksteen B, et al. . Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157-1160. [DOI] [PubMed] [Google Scholar]
- 37. Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ.. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mora JR, Andrian UH von.. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009;21:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jaensson E, Uronen-Hansson H, Pabst O, et al. . Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schön MP, Arya A, Murphy EA, et al. . Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641-6649. [PubMed] [Google Scholar]
- 41. Brandtzaeg P. Transport models for secretory IgA and secretory IgM. Clin Exp Immunol. 1981;44:221-232. [PMC free article] [PubMed] [Google Scholar]
- 42. Guzman M, Lundborg LR, Yeasmin S, et al. . An integrin αEβ7-dependent mechanism of IgA transcytosis requires direct plasma cell contact with intestinal epithelium. Mucosal Immunol. 2021;14:1347-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Royle L, Roos A, Harvey DJ, et al. . Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140-20153. [DOI] [PubMed] [Google Scholar]
- 44. Bunker JJ, Erickson SA, Flynn TM, et al. . Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017;358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steffen U, Koeleman CA, Sokolova MV, et al. . IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun. 2020;11:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin M, Du L, Brandtzaeg P, et al. . IgA subclass switch recombination in human mucosal and systemic immune compartments. Mucosal Immunol. 2013;7:511-520. [DOI] [PubMed] [Google Scholar]
- 47. Kunkel EJ, Butcher EC.. Plasma-cell homing. Nat Rev Immunol. 2003;3:822-829. [DOI] [PubMed] [Google Scholar]
- 48. Brandtzaeg P, Farstad IN, Johansen F, et al. . The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brynjolfsson SF, Berg LP, Ekerhult TO, et al. . Long-lived plasma cells in mice and men. Front Immunol. 2018;9:2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hieshima K, Kawasaki Y, Hanamoto H, et al. . CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. 2004;173:3668-3675. [DOI] [PubMed] [Google Scholar]
- 51. Masahata K, Umemoto E, Kayama H, et al. . Generation of colonic IgA-secreting cells in the caecal patch. Nat Commun. 2014;5:3704. [DOI] [PubMed] [Google Scholar]
- 52. Fernekorn U, Butcher EC, Behrends J, Hartz S, Kruse A.. Functional involvement of P-selectin and MAdCAM-1 in the recruitment of alpha4beta7-integrin-expressing monocyte-like cells to the pregnant mouse uterus. Eur J Immunol. 2004;34:3423-3433. [DOI] [PubMed] [Google Scholar]
- 53. Salmon H. Mammary gland immunology and neonate protection in pigs. Homing of lymphocytes into the MG. Adv Exp Med Biol. 2000;480:279-286. [DOI] [PubMed] [Google Scholar]
- 54. Ciccia F, Guggino G, Rizzo A, et al. . Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis. 2015;74:1739-1747. [DOI] [PubMed] [Google Scholar]
- 55. Schaible UE, Vestweber D, Butcher EG, et al. . Expression of endothelial cell adhesion molecules in joints and heart during Borrelia burgdorferi infection of mice. Cell Adhes Commun. 2009;2:465-479. [DOI] [PubMed] [Google Scholar]
- 56. Grant AJ, Lalor PF, Hübscher SG, Briskin M, Adams DH.. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology. 2001;33:1065-1072. [DOI] [PubMed] [Google Scholar]
- 57. Bamias G, Cominelli F.. Exploring the early phase of Crohn’s disease. Clin Gastroenterol Hepatol. 2021;19:2469-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [DOI] [PubMed] [Google Scholar]
- 59. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711-721. [DOI] [PubMed] [Google Scholar]
- 60. Vermeire S, O’Byrne S, Keir M, et al. . Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet (London, England). 2014;384:309-318. [DOI] [PubMed] [Google Scholar]
- 61. Vermeire S, Sandborn WJ, Danese S, et al. . Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2017;390:135-144. [DOI] [PubMed] [Google Scholar]
- 62. Sandborn WJ, Lee SD, Tarabar D, et al. . Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut. 2018;67:1824-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tyler CJ, Guzman M, Lundborg LR, et al. . Antibody secreting cells are critically dependent on integrin α4β7/MAdCAM-1 for intestinal recruitment and control of the microbiota during chronic colitis. Mucosal Immunol. 2022;15:109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schippers A, Leuker C, Pabst O, et al. . Mucosal addressin cell-adhesion molecule-1 controls plasma-cell migration and function in the small intestine of mice. Gastroenterology. 2009;137:924-933. [DOI] [PubMed] [Google Scholar]
- 65. Wagner N, Lohler J, Kunkel EJ, et al. . Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366-370. [DOI] [PubMed] [Google Scholar]
- 66. Zundler S, Fischer A, Schillinger D, et al. . The α4β1 homing pathway is essential for ileal homing of Crohn’s disease effector T cells in vivo. Inflamm Bowel Dis. 2017;23:379-391. [DOI] [PubMed] [Google Scholar]
- 67. Abokor AA, McDaniel GH, Golonka RM, et al. . Immunoglobulin A, an Active Liaison for Host-Microbiota Homeostasis. Microorg. 2021;9:2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Benckert J, Schmolka N, Kreschel C, et al. . The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J Clin Invest. 2011;121:1946-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fransen F, Zagato E, Mazzini E, et al. . BALB/c and C57BL/6 mice differ in polyreactive IgA abundance, which impacts the generation of antigen-specific IgA and microbiota diversity. Immunity. 2015;43:527-540. [DOI] [PubMed] [Google Scholar]
- 70. Peterson DA, McNulty NP, Guruge JL, Gordon JI.. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328-339. [DOI] [PubMed] [Google Scholar]
- 71. Peterson CT, Sharma V, Elmén L, Peterson SN.. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol. 2015;179:363-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Quan CP, Berneman A, Pires R, Avrameas S, Bouvet JP.. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun. 1997;65:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shimoda M, Inoue Y, Azuma N, Kanno C.. Natural polyreactive immunoglobulin A antibodies produced in mouse Peyer’s patches. Immunology. 1999;97(1):9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stokes CR, Soothill JF, Turner MW.. Immune exclusion is a function of IgA. Nature. 1975;255:745-746. [DOI] [PubMed] [Google Scholar]
- 75. Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR.. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Williams RC, Gibbons RJ.. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science (80-.). 1972;177:697-699. [DOI] [PubMed] [Google Scholar]
- 77. Ost KS, O’Meara TR, Stephens WZ, et al. . Adaptive immunity induces mutualism between commensal eukaryotes. Nat. 2021;596:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moor K, Diard M, Sellin ME, et al. . High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017;544:498-502. [DOI] [PubMed] [Google Scholar]
- 79. Cullender TC, Chassaing B, Janzon A, et al. . Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14:571-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Donaldson GP, Ladinsky MS, Yu KB, et al. . Gut microbiota utilize immunoglobulin A for mucosal colonization. Science. 2018;360:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kadaoui KA, Corthésy B.. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J Immunol. 2007;179:7751-7757. [DOI] [PubMed] [Google Scholar]
- 82. Rey J, Garin N, Spertini F, Corthésy B.. Targeting of secretory IgA to Peyer’s patch dendritic and T cells after transport by intestinal M cells. J Immunol. 2004;172:3026-3033. [DOI] [PubMed] [Google Scholar]
- 83. Bunker JJ, Flynn TM, Koval JC, et al. . Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity. 2015;43:541-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vaishnava S, Yamamoto M, Severson KM, et al. . The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science (80-.). 2011;334:255-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wijburg OLC, Uren TK, Simpfendorfer K, Johansen F-E, Brandtzaeg P, Strugnell RA.. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dzidic M, Abrahamsson TR, Artacho A, et al. Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J. Allergy Clin. Immunol. 2017;139:1017-1025.e14. [DOI] [PubMed] [Google Scholar]
- 87. Kau AL, Planer JD, Liu J, et al. . Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med. 2015;7(276):ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Koch MA, Reiner GL, Lugo KA, et al. . Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell. 2016;165:827-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kubinak JL, Petersen C, Stephens WZ, et al. . MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe. 2015;17:153-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Planer JD, Peng Y, Kau AL, et al. . Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature. 2016;534:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wilmore JR, Gaudette BT, Gomez Atria D, et al. Commensal microbes induce serum IgA responses that protect against polymicrobial sepsis. Cell Host Microbe. 2018;23:302-311.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jiang HQ, Bos NA, Cebra JJ.. Timing, localization, and persistence of colonization by segmented filamentous bacteria in the neonatal mouse gut depend on immune status of mothers and pups. Infect Immun. 2001;69:3611-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Palm NW, Zoete MD, Cullen TW, et al. . Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Suzuki K, Meek B, Doi Y, et al. . Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101:1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sun H, Kuk W, Rivera-Nieves J, Lopez-Ramirez MA, Eckmann L, Ginsberg MH.. β7 integrin inhibition can increase intestinal inflammation by impairing homing of CD25 hi FoxP3 + regulatory T cells. Cell Mol Gastroenterol Hepatol. 2020;9:369-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Maharshak N, Packey CD, Ellermann M, et al. . Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes. 2013;4:316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kevans D, Kirsch R, Dargavel C, Kabakchiev B, Riddell R, Silverberg MS.. Histological markers of clinical relapse in endoscopically quiescent ulcerative colitis. Inflamm Bowel Dis. 2020;26:1722-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dubinsky MC, Lin YC, Dutridge D, et al. ; Western Regional Pediatric IBD Research Alliance. Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101(2):360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Targan SR, Landers CJ, Yang H, et al. . Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020-2028. [DOI] [PubMed] [Google Scholar]
- 100. Lee M, Chang EB.. Inflammatory bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. 2021;160:524-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Waaij LVD, Kroese FGM, Visser A, et al. . Immunoglobulin coating of faecal bacteria in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16:669-674. [DOI] [PubMed] [Google Scholar]
- 102. Viladomiu M, Kivolowitz C, Abdulhamid A, et al. . IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote T H 17-dependent inflammation. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rochereau N, Roblin X, Michaud E, et al. . NOD2 deficiency increases retrograde transport of secretory IgA complexes in Crohn’s disease. Nat Commun. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Uzzan M, Martin JC, Mesin L, et al. . Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat Med. 2022;28:766-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Landers CJ, Cohavy O, Misra R, et al. . Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689-699. [DOI] [PubMed] [Google Scholar]
- 106. Sitaraman SV, Klapproth JM, Moore DA, et al. . Elevated flagellin-specific immunoglobulins in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2005;288. [DOI] [PubMed] [Google Scholar]
- 107. Torres J, Petralia F, Sato T, et al. . Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology. 2020;159:96-104. [DOI] [PubMed] [Google Scholar]
- 108. Gupta S, Basu S, Bal V, Rath S, George A.. Gut IgA abundance in adult life is a major determinant of resistance to dextran sodium sulfate-colitis and can compensate for the effects of inadequate maternal IgA received by neonates. Immunology. 2019;158:19-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Reikvam DH, Derrien M, Islam R, et al. . Epithelial-microbial crosstalk in polymeric Ig receptor deficient mice. Eur J Immunol. 2012;42:2959-2970. [DOI] [PubMed] [Google Scholar]
- 110. Landuyt AE, Klocke BJ, Duck LW, et al. . ICOS ligand and IL-10 synergize to promote host–microbiota mutualism. Proc Natl Acad Sci USA. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cao Y, Wang X, Yang Q, et al. . Critical role of intestinal microbiota in ATF3-mediated gut immune homeostasis. J Immunol. 2020;205:842-852. [DOI] [PubMed] [Google Scholar]
- 112. Nagaishi T, Watabe T, Kotake K, et al. . Immunoglobulin A-specific deficiency induces spontaneous inflammation specifically in the ileum. Gut. 2022;71:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T.. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424-1427. [DOI] [PubMed] [Google Scholar]
- 114. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM.. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944-950. [DOI] [PubMed] [Google Scholar]
- 115. Wolf SD, Dittel BN, Hardardottir F, Janeway CA.. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Oka A, Ishihara S, Mishima Y, et al. . Role of regulatory B cells in chronic intestinal inflammation: association with pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2014;20:315-328. [DOI] [PubMed] [Google Scholar]
- 117. Sattler S, Ling GS, Xu D, et al. . IL-10-producing regulatory B cells induced by IL-33 (Breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. J Autoimmun. 2014;50(100):107-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Noh J, Choi WS, Noh G, Lee JH.. Presence of Foxp3-expressing CD19(+)CD5(+) B cells in human peripheral blood mononuclear cells: human CD19(+)CD5(+)Foxp3(+) regulatory B Cell (Breg). Immune Netw. 2010;10:247-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mohd Jaya FN, Garcia SG, Borràs FE, et al. . Paradoxical role of Breg-inducing cytokines in autoimmune diseases. J Transl Autoimmun. 2019;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mishima Y, Oka A, Liu B, et al. . Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10–producing regulatory B cells. J Clin Invest. 2019;129:3702-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Maerz JK, Trostel C, Lange A, et al. . Bacterial immunogenicity is critical for the induction of regulatory B cells in suppressing inflammatory immune responses. Front Immunol. 2020;10:3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang X, Zhu Y, Zhang M, Wang H, Jiang Y, Gao P.. Ulcerative colitis is characterized by a decrease in regulatory B cells. J Crohns Colitis. 2016;10:1212-1223. [DOI] [PubMed] [Google Scholar]
- 123. Babbar A, Hitch TCA, Pabst O, et al. . The compromised mucosal immune system of β7 integrin-deficient mice has only minor effects on the fecal microbiota in homeostasis. Front Microbiol. 2019;10:2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wyant T, Leach T, Sankoh S, et al. . Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64:77-83. [DOI] [PubMed] [Google Scholar]