Abstract
Background and Objectives
Diabetic polyneuropathy (DPN) is a complication of diabetes characterized by pain or lack of peripheral sensation, but the underlying mechanisms are not yet fully understood. Recent evidence showed increased cutaneous macrophage infiltration in patients with type 2 diabetes and painful DPN, and this study aimed to understand whether the same applies to type 1 diabetes.
Methods
The study included 104 participants: 26 healthy controls and 78 participants with type 1 diabetes (participants without DPN [n = 24], participants with painless DPN [n = 29], and participants with painful DPN [n = 25]). Two immune cells, dermal IBA1+ macrophages and epidermal Langerhans cells (LCs, CD207+), were visualized and quantified using immunohistological labeling and stereological counting methods on skin biopsies from the participants. The IBA1+ macrophage infiltration, LC number density, LC soma cross-sectional area, and LC processes were measured in this study.
Results
Significant difference in IBA1+ macrophage expression was seen between the groups (p = 0.003), with lower expression of IBA1 in participants with DPN. No differences in LC morphologies (LC number density, soma cross-sectional area, and process level) were found between the groups (all p > 0.05). In addition, IBA1+ macrophages, but not LCs, correlated with intraepidermal nerve fiber density, Michigan neuropathy symptom inventory, (questionnaire and total score), severity of neuropathy as assessed by the Toronto clinical neuropathy score, and vibration detection threshold in the whole study cohort.
Discussion
This study showed expressional differences of cutaneous IBA1+ macrophages but not LC in participants with type 1 diabetes–induced DPN compared with those in controls. The study suggests that a reduction in macrophages may play a role in the development and progression of autoimmune-induced diabetic neuropathy.
Introduction
Approximately half of people with diabetes eventually develop diabetic polyneuropathy (DPN), which usually presents in a “stocking and glove distribution”.1 Cutaneous small nerve fibers are unmyelinated or thinly myelinated, making them vulnerable to damage due to hyperglycemia-induced tissue inflammation or oxidative stress.2,3 Symptoms of DPN in patients are heterogeneous, with approximately one-fourth of patients developing neuropathic pain, although the estimate varies between studies.4,5 The underlying mechanisms of DPN are not fully understood, but inflammation is believed to play a significant role.6 Unresolved inflammation has been linked to the development of DPN and neuropathic pain.7,8 Studies have also shown that macrophages play significant roles in the development of neuropathic pain in DPN and in the immune response associated with diabetes.9,10
Ionized calcium-binding adaptor molecule 1 (IBA1) is a polypeptide particularly expressed in the cytoplasm of macrophages or microglia.11,12 The IBA1 gene contributes in encoding the major histocompatibility complex (MHC) III, which is known to initiate inflammatory response in injury and in disease.11,13 Previous animal studies have shown that IBA1+ macrophages contribute to inflammatory responses in the central and peripheral nervous systems at early stages of diabetes.14,15 A previous study by our group examined the expression of IBA1+ macrophages in participants with type 2 diabetes and found an increase in IBA1+ macrophage infiltration in those with painful DPN, suggesting a link between IBA1+ macrophages and neuropathic pain in type 2 diabetes.16 However, the relationship between IBA1+ macrophages and neuropathic pain in type 1 diabetes is still unknown and warrants further investigation because there are several important differences between type 1 and type 2 diabetes.
Recent genetic studies suggest that Langerhans cells (LCs) are macrophages, regardless of their dendritic morphology.17,18 LCs reside in the outermost layer of skin, serving as the first line of defense for the body by detecting pathogens and changes in the environment and pass this information on to lymph nodes.19 Previous studies showed contradictory results in the relationship between LCs and cutaneous nerve fibers, with studies that showed close contact between LCs and nociceptors in epidermis,20,21 others suggested no association between them.22,23 Clinical studies have revealed inconsistent effects of diabetes on LC expression. While some studies have shown a reduction in cutaneous LC density because of hyperglycemia, others have reported an increase in LC density in diabetic cornea and gingiva.23-25 Despite these findings, the relationship between LC expression and diabetes-induced neuropathic pain remains uncertain.
Our study aimed to investigate the relationship between cutaneous macrophages and neuropathic pain in DPN by visualizing and quantifying IBA1+ macrophages and LCs in skin biopsies from participants with type 1 diabetes, including those without DPN, with painless and painful DPN, and healthy controls. The goal was to gain a better understanding of the underlying mechanisms of DPN and neuropathic pain.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was conducted in accordance with the principles outlined in the Helsinki Declaration II and received ethical approval from the Regional Ethics Committee (#1-10-72-103-19) and the Danish Data Protection Agency. Participants provided their informed consent for participation after having been fully informed about the study procedures.
Study Participants and Clinical Diagnosis of DPN and Pain
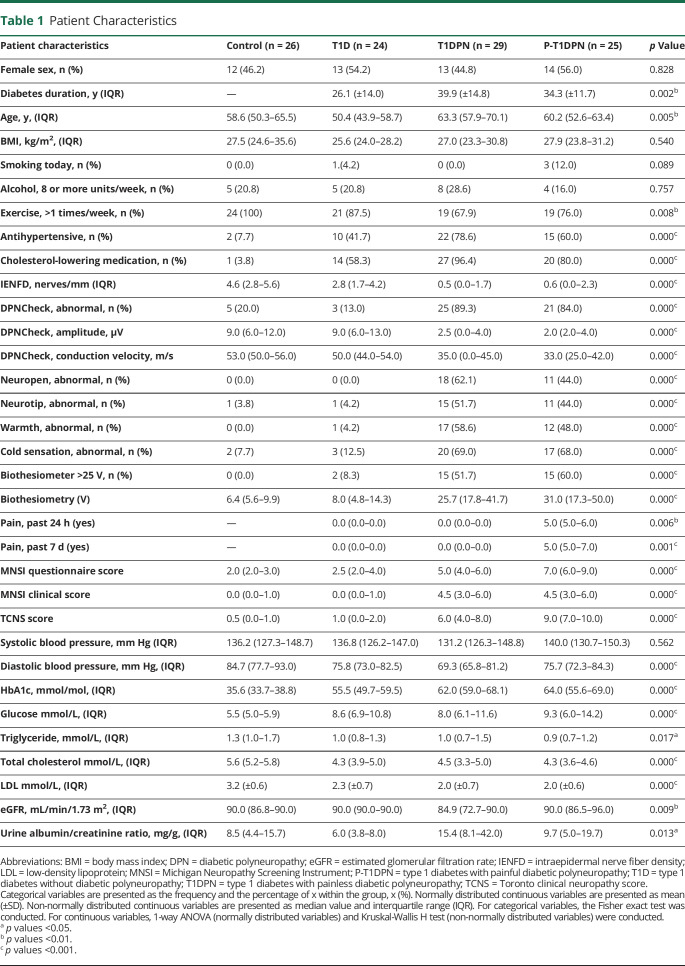
This study was part of a larger cross-sectional study that recruited 216 participants with type 1 diabetes and healthy controls from 3 Steno Diabetes Centers in Denmark from 2019 to 2021. We randomly selected 20 participants from each group (healthy controls, participants with type 1 diabetes but no DPN, and participants with type 1 diabetes and painless or painful DPN) and added additional participants to each group based on sex and age to ensure similarities between the groups. Inclusion criteria for participants with type 1 diabetes were a confirmed diagnosis of type 1 diabetes for at least 5 years and age 30–75 years. Participants were excluded from the study if they were unable to give informed consent, had neuropathy caused by known factors other than diabetes, experienced severe pain caused by factors other than neuropathy, had a history of alcohol or substance abuse, were pregnant, had any skin disorder in the biopsy area, had prior chronic ulcers in the biopsy area, had intermittent claudication, had very weak/nonpalpable foot pulses, had existing diabetic foot ulcers, or had a known allergy to lidocaine. Inclusion criteria for the healthy controls was age 30–75 years, and exclusion criteria were equivalent to those for participants with type 1 diabetes, except that they could not have any known neurologic or metabolic disorders or be experiencing clinical depression or ongoing pain of any cause.
Neuropathy Assessment
All participants were thoroughly examined by using various methods, including ankle and knee reflexes, vibration detection threshold on the hallux using biothesiometer (where values over 25 V are considered abnormal), mechanical detection using Neuropen® (10 g monofilament) under the hallux (where 7 or fewer of 10 stimuli detected is considered decreased sensation), pinprick sensation using Neurotip® on the hallux, and temperature sensation using thermos rollers (Rolltemp II®) of 25°C (cold) and 40°C (warmth) on top of the hallux. In addition, participants were assessed using DPNCheck® that measures the amplitude and velocity of the sural nerve and is considered being a surrogate for a full nerve conduction study (amplitude <4 µV or velocity ≤40 m/s were considered abnormal result), 3 mm punch skin biopsy, and a panel of blood biomarkers, such as HbA1c, triglyceride, and total and LDL cholesterol. The diagnosis of DPN in the participants was performed by using the Toronto Diabetic Neuropathy Expert Group criteria, which include a combination of symptoms and signs of DPN.26 Here, signs of DPN were assessed using the tests described earlier. The diagnosis was confirmed through abnormal IENFD results from skin biopsy and DPNCheck. Participants who reported pain in both feet/legs, reported sensory signs in the feet/legs, had a diagnosis of diabetes, and had abnormal IENFD or DPNCheck results were considered to have neuropathic pain. The level of pain was measured using a numeric rating scale (NRS) and the Neuropathic Pain Symptom Inventory questionnaire (NPSI). DPN severity was assessed using Toronto clinical neuropathy score (TCNS).
Skin Biopsy
Skin samples were obtained using a 3-mm punch biopsy technique (Miltex, York, PA) at the distal leg (10 cm above the lateral malleolus) and processed in accordance with established protocols.27,28 The samples were fixed overnight in Zamboni and 20% sucrose and cryoprotected overnight. They were then snap frozen and stored at −20°C before being cut into 50-µm thick sections for immunohistological staining.
Immunofluorescent Labeling and Imaging
All sections went through antigen retrieval in boiled sodium citrate buffer and incubated in water bath at 80°C for 20 minutes. The cooled sections were washed with washing buffer (0.3% Triton X-100 in 0.05M PBS buffer) and incubated with blocking buffer (1% bovine serum albumin in washing buffer) for 1 hour. The primary antibody rabbit anti-IBA1 (FUJIFILM) with a concentration of 1:1,000 in blocking buffer was applied to skin sections overnight at 4°C, and secondary antibody donkey antirabbit-488 (1:800, Invitrogen) for 90 minutes at room temperature. The sections were rinsed with washing buffer and incubated with DAPI (1:5,000, Sigma) buffer, washed, and finally mounted on slide with 0.5% gelatine and fluorescent mounting medium and coverslipped.
Z-stack confocal images consisting of 15-image stacks with a 21-µm scanning thickness were acquired by a laser scanning spectral confocal microscope (Zeiss LSM 800, Germany) with a 40X oil lens. To reconstruct an image of the entire region of interest (ROI) in 1 skin section, the tile function was used to stitch all single-field images from the same skin section. A minimum of 2 individual skin sections from the same participant were imaged and used for IBA1 quantification.
Immunohistochemistry
Antigen retrieval (Dako) and endogenous peroxidases blocking (3% H2O2) were performed before free-floating staining. After washing in washing buffer (0.3% Triton X-100 in 0.05M Tris-buffered saline), the sections were incubated with 1% bovine serum albumin in washing buffer for an hour, before overnight incubation of primary antibody rabbit anti-CD207 (1:500, Invitrogen). Sections were washed in washing buffer and then incubated with secondary antibody goat antirabbit-HRP (1:400, Dako) at room temperature for 2 hours. After rinsing sections with 0.3% TBST buffer, sections were treated with DAB working solution (0.05% DAK, 0.1% H2O2) for 5 minutes. Sections were rinsed in washing buffer and mounted on slides with gelatine. Skin sections were air dried on slides and rehydrated in distilled water and dehydrated in 70% ethanol (10 minutes), 90% ethanol (10 minutes), 99% ethanol (15 minutes), and xylene (15 minutes) and finally coverslipped.
Quantification
IBA1+ Macrophage Counting
IBA1+ macrophage quantification was analyzed on the original confocal Z-stack images by using the FIJI image-processing package based on ImageJ. 15 Z-stack images were orthogonally projected using Z projection function, and the average intensity of all stacks was shown in a 2-D image for the following measurement. Each image was adjusted by applying a specific intensity threshold to exclude the nonspecific labelling, so the remaining signal showed the positive labeling of IBA1+ macrophages. Then, the dermal IBA1+ macrophages were estimated by measuring the dermal IBA1 area fraction, which was counted by the area that was occupied by the total IBA1+ macrophages (IBA1area), divided by the total region of interest (ROI) area (ROIarea), dermal area within 300 µm below epidermal-dermal border in the orthogonally projected 2-D confocal images. The IBA1 area fraction in each image was measured 3 times, and the average value of the 3 individual double-blind measurements was used for the statistical analysis.
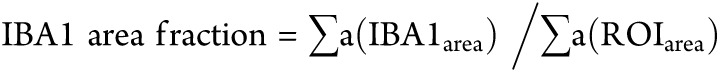 |
Langerhans Cell Counting
Live counting was performed on Olympus BX50 light microscope (Olympus, Japan) with an automatic stage (Prior scientific), encoder (ND281, Germany), and digital camera (Olympus DP79, Japan) connected to a PC with the NewCAST software (ver. 4.4.5.0, Visiopharm A/S, Denmark).
LC number density was estimated by counting the total number of LCs in the epidermis, Q−(LC), divided by the volume of epidermis, Epidermisvolume. The Epidermisvolume was measured by multiplying the area of epidermis (Epidermisarea) and the section thickness (50 µm, disector height). The counting was performed using a 20X magnification lens.
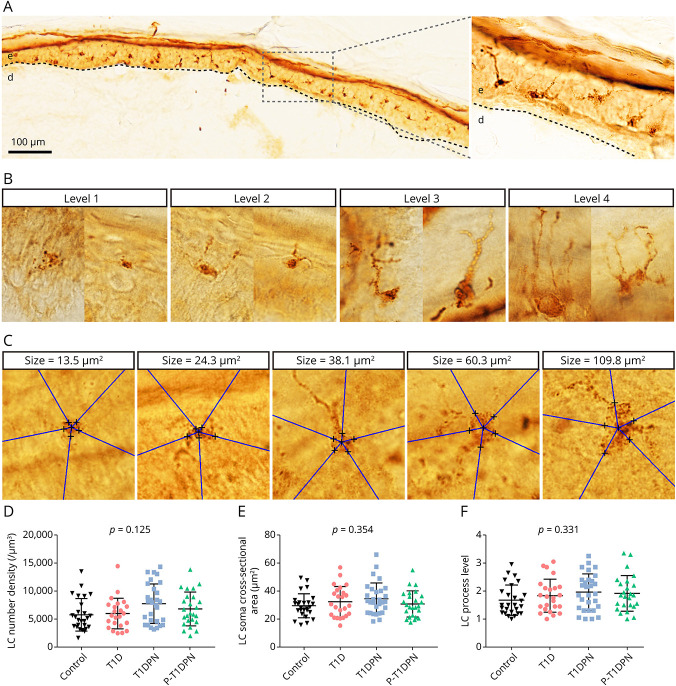
2. LC process grading was estimated by a semiquantitative method with a novel grading system. The LC process levels were graded into 4 levels based on the number and length of the process in the skin sections. Two representative images for each process grading level are shown in Figure 2B. In detail, level 1 is LCs without process; level 2 is LCs with 1–2 processes; level 3 is LCs with 3–4 processes; and level 4 is LCs with more than 4 nicely spread processes. Process levels of 20 LCs were counted from each participant using a ×100 magnification lens.
3. LC soma cross-sectional area was presented by the maximum cross-sectional area of the LC soma, which was unbiasedly estimated using the 2-D Nucleator in the Visiopharm A/S software. For the counting of each soma, we first identified the largest cross-section of the soma in the skin and added 5 random 2-D isotropic test lines. We then marked at the cross between the test lines and the soma border. The length of the test lines within the soma were measured, which automatically generated the estimated cross-sectional area of the soma. The counting method is depicted in Figure 2C and described in previous studies.29 Twenty LC somata were counted from each participant using a ×100 magnification lens.
Figure 2. Quantification of Cutaneous Langerhans Cell Morphologies.
(A) Representative image of Langerhans cells in healthy human epidermis. (B) Representative structures of 4 Langerhans cell process levels. (C) Representative images of Langerhans cell soma cross-sectional area estimation. (D) The quantification of Langerhans cell number density in epidermis. (E) The quantification of Langerhans cell soma cross-sectional area in epidermis. (F) The quantification of Langerhans cell process levels in epidermis. LC = Langerhans cell; T1D = type 1 diabetes without diabetic polyneuropathy; T1DPN = type 1 diabetes with painless diabetic polyneuropathy; P-T1DPN = type 1 diabetes with painful diabetic polyneuropathy.
Statistical Analysis
The distribution of all variables was checked using histograms and quantile-quantile plots. Data were presented as mean (±SD) for normally distributed variables and medians (interquartile range, IQR) for non-normally distributed variables. Comparison between all groups was performed using parametric test (1-way ANOVA) or nonparametric test (Kruskal-Wallis H), depending on the data distribution. p values were presented as raw values and adjusted values for post hoc analysis. Adjustments for sex, age, and HbA1c were performed using a multiple linear regression model. All non-normal variables were transformed to obtain a normal distribution, using either log10 or square root transformations. Correlation analysis was performed using the Pearson correlation (in case some data were not normally distributed, they were transformed to achieve normal distribution). Partial correlations were performed to adjust for confounding variables including age, sex, and HbA1c. Statistical significance (α) was set at a p value ≤0.05.
Data Availability
Deidentified data are available from the corresponding author on request pending signed agreements from Aarhus University.
Results
Participant Characterization
A total of 78 participants (24 participants without DPN, 29 with painless DPN, and 25 with painful DPN) and 26 healthy controls were included in the study. Table 1 summarizes the main clinical characteristics of the participants. There were statistically significant differences in several of the baseline characteristics (Table 1), including age, diabetes duration, HbA1c, total cholesterol, LDL, eGFR, diastolic blood pressure, and urine albumin/creatinine ratio. As for pathophysiologic tests of DPN, there were differences in IENFD and DPNCheck between the groups (all p < 0.001). Among patients with DPN, more than 80% had abnormal DPNCheck results and more than 90% had abnormal IENFD (data not shown), indicating that most patients had both large and small fiber abnormalities. Bedside tests such as mechanical, vibration, and thermal sensation measured by pinprick, biothesiometer, and thermal rollers differed between the groups, as did the MNSI questionnaire (MNSI-Q) and MNSI total score and TCNS (all p < 0.001). Last, the median pain intensity (NRS) was 5 (5;6) and 5 (5;7) in P-T1DPN at 24 hours and 7 days, respectively, before performing the sensory test and skin biopsy acquisition.
Table 1.
Patient Characteristics
Visualization and Quantification of Cutaneous IBA1+ Macrophages
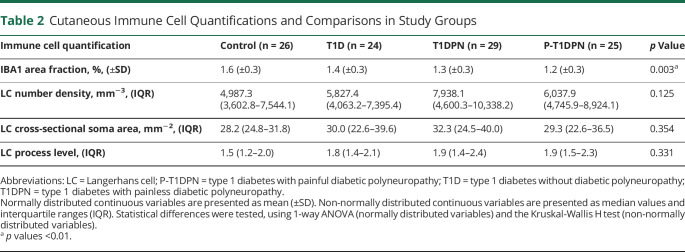
The presence of dermal IBA1+ macrophages was visualized by immunofluorescent labeling. The IBA1 area fraction was measured within 300 µm of the dermal-epidermal border, and results were compared between groups. Representative images of low and high dermal IBA1+ macrophage expression are shown in Figure 1A. The results showed a statistically significant difference in IBA1 area fraction (%) between groups (p = 0.003), with healthy controls having the highest expression (1.6 ± 0.3%), followed by participants without DPN (1.4 ± 0.3%), patients with painless DPN (1.3 ± 0.3%), and last, patients with painful DPN (1.2 ± 0.3%) (Figure 1B, Table 2). Post hoc analysis showed a decrease in IBA1 area fraction in T1DPN and P-T1DPN groups compared with healthy controls before adjusting for confounders (both p < 0.05; eTable 1, links.lww.com/NXI/A881), but no difference was observed between the other 2 groups (all p > 0.05; eTable 1). After adjusting for confounders (age, sex, and HbA1c), a reduction of IBA1 was observed in P-T1DPN compared with healthy controls (p = 0.050; eTable 1).
Figure 1. Immunofluorescent Labeling and Quantification of IBA1+ Macrophages in Human Skin Sections.
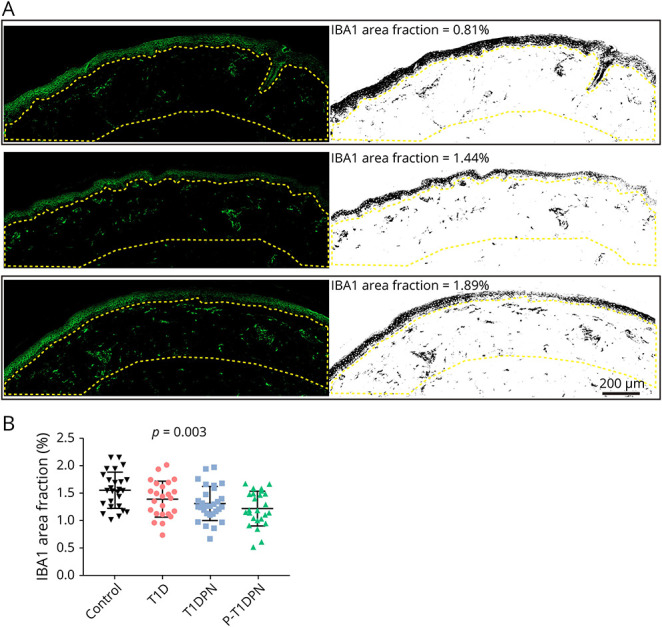
(A) Representative images of different expression levels of IBA1+ macrophages in skin sections. (B) The quantification of IBA1 area fraction in dermis. Enclosed area by yellow dotted line: the region of interest (dermis that was 300 μm below epidermis-dermis border); T1D = type 1 diabetes without diabetic polyneuropathy; T1DPN = type 1 diabetes with painless diabetic polyneuropathy; P-T1DPN = type 1 diabetes with painful diabetic polyneuropathy.
Table 2.
Cutaneous Immune Cell Quantifications and Comparisons in Study Groups
Visualization and Quantification of Cutaneous Langerhans Cells
LCs were visualized in epidermis by immunochemistry labeling of langerin (CD207+). Representative images of different LC morphologies are shown in eFigure 1 (links.lww.com/NXI/A881). The expressional differences of LCs vary not only in number density but also in soma and processes. Therefore, the LC cell number density, soma cross-sectional area, and process level were analyzed separately for better evaluation of LC characteristics (Figure 2). There was no statistically significant difference between all groups when analyzing LC cross-sectional soma area and process level with ANOVA (both p > 0.05; Table 2). However, there was a weak but not statistically significant trend (p = 0.125) toward a difference in LC density between the groups, with higher LC number density seen in participants with diabetes (Table 2).
Post hoc analysis showed no significant difference in the 3 LC quantifications between any 2 groups before adjusting for confounders (all p > 0.05; eTables 2–4, links.lww.com/NXI/A881). After adjusting for confounders (age, sex, and HbA1c), significant differences were observed only in LC number density: (1) between those with T1DPN and healthy controls or those with type 1 diabetes without DPN (both p < 0.05); (2) between those with P-T1DPN and healthy controls (p = 0.019); and (3) no difference was observed between other groups (all p > 0.05) (eTable 2).
Correlations Between Experimental Quantifications
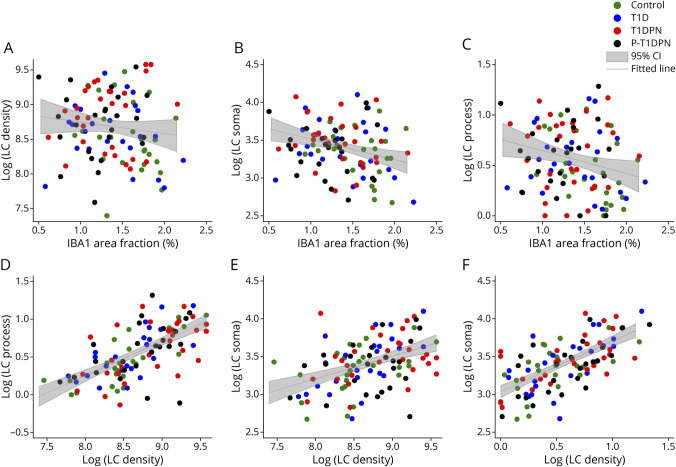
Weak and negative correlations were found between IBA1 area fraction and LC soma cross-sectional area and LC process level (all p < 0.05), but not LC number density (p = 0.237; Figure 3, A–C, eTable 5, links.lww.com/NXI/A881). Furthermore, the 3 LC morphological quantifications, LC number density, LC soma cross-sectional area, and LC process level, were were correlated with each other, meaning that if one LC quantification was high, the two others were high as well, and vice versa. (all p < 0.001; Figure 3, D–F, eTable 5).
Figure 3. Correlations of Immune Cell Quantifications in all Participants.
(A–C) Correlations between IBA1 area fraction and LC number density (A), LC soma cross-sectional area (B), and LC process level (C). (D–E) Correlations between 3 LC quantifications: (D) correlation between LC number density and LC process level (E) correlation between LC number density and LC soma cross-sectional area; and (F) correlation between LC process level and LC soma cross-sectional area. LC_density = LC number density; LC_soma = Langerhans cell soma cross-sectional area; LC_process = Langerhans cell process level.
Correlations Between Experimental Quantifications and Measures of Neuropathy
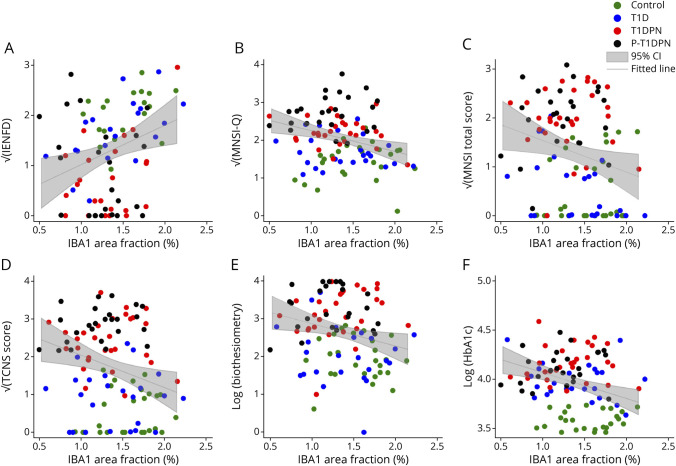
We examined the correlation between LCs and IBA1+ macrophages with measures of DPN. There was a positive correlation between IBA1 area fraction and IENFD (r = 0.29, = 0.007) and weak but significant negative correlations between IBA1 area fraction and MNSI-Q (r = −0.29, p = 0.003), MNSI-total (r = −0.22, p = 0.023), TCNS (r = −0.26, p = 0.009), and biothesiometer threshold (r = −0.23, p = 0.017) in the whole study cohort (Figure 4, A–E, eTable 5, links.lww.com/NXI/A881). IBA1 area fraction also correlated with HbA1c levels (r = −0.32, p = 0.001, Figure 4F), and only the correlation between IBA1 area fraction and IENFD remained statistically significant after adjusting for age, sex, and HbA1c (r = 0.23, p = 0.037, eTable 6). No correlation was found between LC morphology and the abovementioned measures of DPN (eFigure 2, eTables 5–6).
Figure 4. Correlations Between IBA1 Area Fraction and Neuropathy Measurements.
(A) Correlation between IBA1 area fraction and intraepidermal nerve fiber density (IENFD). (B) Correlation between IBA1 area fraction and Michigan Neuropathy Screening Instrument Questionnaire (MNSI-Q). (C) Correlation between IBA1 area fraction and Michigan Neuropathy Screening Instrument total score (MNSI total score). (D) Correlation between IBA1 area fraction and Toronto Clinical Neuropathy score (TCNS score). (E) Correlation between IBA1 area fraction and vibration test (Biothesiometer). (F) Correlation between IBA1 area fraction and HbA1c.
Discussion
In this study, we visualized and quantified 2 cutaneous immune cells, epidermal LCs and dermal IBA1+ macrophages, in carefully phenotyped participants with type 1 diabetes, without and with DPN, the latter with and without pain, and healthy controls without diabetes. We observed significant differences in IBA1+ macrophage infiltration between the groups, with a decrease in IBA1+ macrophage infiltration in patients with DPN. The difference, while not large in absolute values, remained statistically significant after adjusting for confounders (age, sex, and HbA1c). Furthermore, IBA1+ macrophages showed a statistically significant positive correlation with IENFD and 2 of 3 LC measurements (LC somata and processes) but a weak negative correlation with MNSI-Q, MNSI total score, neuropathy severity (TCNS score), and vibration detection. However, after adjusting for confounders, only a weak positive association between IBA1+ macrophages and IENFD remained statistically significant. We found no differences in the density or morphology of LCs between the groups. Taken together, while we found associations between IENFD, macrophages, and LCs, the skin of patients with longstanding type 1 diabetes and DPN is not increasingly innervated by these immune cells.
These findings differ from our recent findings in patients with relatively newly diagnosed (5 to 6 years) screen-detected type 2 diabetes and DPN, where we demonstrated higher IBA1+ macrophage density in patients with painful DPN compared with those without pain and healthy controls and where we found an increasing gradient of IBA1+ macrophages, with healthy controls without diabetes having the lowest density, followed by patients with painless DPN, and last, patients with painful DPN.16 The patient cohorts were in many ways similar, because HbA1c levels, hypertension treatment, pain intensity, and TCNS scores were comparable between the 2 cohorts. There were minor disparities in age, BMI, and smoking status. The differences between these 2 findings could potentially be explained by different types of diabetes and a vast difference in diabetes duration (with a median value up to 40 years in this study compared with up to 6 years in the type 2 diabetes study). Furthermore, while inflammation plays a role in diabetic neuropathy, there are important pathophysiologic differences between type 1 and type 2 diabetes.30 In type 1 diabetes, the immune system attacks and destroys the insulin-producing beta cells in the pancreas, leading to a loss of insulin production.31,32 This chronic lack of insulin can lead to high blood glucose levels, which can damage the nerve fibers, leading to diabetic neuropathy.33 In type 2 diabetes, on the other hand, the cells do not respond properly to insulin, leading to high blood glucose levels.34 However, besides hyperglycemia, other metabolic dysfunctions including hyperlipidemia, hypertension, and obesity are more often seen in type 2 diabetes.35,36 Those multiple metabolic dysfunctions in type 2 diabetes may lead to chronic low-grade inflammation, which is believed to play a role in the development of diabetic neuropathy.37,38 Furthermore, inflammation in the blood vessels can lead to a decrease in blood flow, which further leads to nerve damage.39,40 Taken together, while inflammation may also contribute to the development of diabetic neuropathy in type 1 diabetes, through an increase in the production of cytokines, this does not seem to be reflected by changes in IBA1+ macrophages or LCs in the skin of patients with DPN accompanying type 1 diabetes, at least not in patients with longstanding diabetes.
This study builds on our previous research in patients with type 2 diabetes by including an analysis of the main epidermis-resident immune cell, the Langerhans cells. LCs are found in the epidermis and mucosal membranes and play a crucial role in the immune system as the "health guardians" of peripheral tissue.18,19 However, with previous studies mainly focusing on the density changes of LCs, the relationship between LC morphological changes and pathologic changes is not well understood. Our results revealed that the morphology of LCs, including cell number density, processes, and soma cross-sectional area, varied among the study participants. Correlational analysis showed a positive relationship between the 3 LC morphological characteristics, suggesting degeneration of LCs affects the entire cell and its processes, as represented by number density, soma size, and process density. But no correlation was found between LC morphology and any clinical neuropathy markers in the entire study cohort. In addition, there were no statistically significant differences in LC morphology between the study groups, indicating that there is no clear relationship between LC expression and DPN or neuropathic pain.
Clinical studies have also reported conflicting results in LC expression in diabetes, and this is not the first study reporting a decrease in the skin of patients with diabetes. One study found a significant decrease in epidermal LC density in adult patients with early-diagnosed type 2 diabetes compared with controls, with no correlation between LC density and IENFD or clinical signs.23 A second study assessed LC density using corneal confocal microscopy in type 1 and type 2 diabetes and found that compared with healthy controls without diabetes, patients with early or mild neuropathy were more likely to have increased LCs in the cornea while patients with progressed neuropathy showed a reduction in LC density compared with healthy controls and those with early or mild neuropathy.41 A third study, however, found an increase in epidermal LC density in adult patients with neuropathic pain induced by type 1 (n = 1) or type 2 (n = 12) diabetes compared with healthy controls, but no correlation between LC number density and pain intensity was found.42 It is worth noting that patients in this previous study had a long diabetes duration with a mean value of 14 years,42 but the diabetes duration in this study is twice as long and includes only patients with type 1 diabetes. These contradicting results may be due to differences in study design, study cohorts, and type and duration of diabetes. The complexity of the disease and the different stages of the diabetes may also contribute to the inconsistent findings.
The findings in the literature suggest that LCs may primarily play a role in the early phase of neuropathy and may be supported by preclinical studies. Animal studies have shown both a decrease and an increase in cutaneous LC density in mice with type 1 and type 2 diabetes, respectively. One study reported a decrease in LC density in type 1 diabetes mice at 16 weeks postinduction compared with control mice, while another study reported both a decrease and an increase in LC density in type 2 diabetes mice at different stages.20,43 It is important to note that animal models of type 1 diabetes are typically induced using streptozotocin which are toxic to the beta cells, which does not resemble the human autoimmune condition.44
The potential relationship between lower concentrations of IBA1+ macrophages in the skin and the development and progression of diabetic neuropathy in type 1 diabetes is worth considering. These immune cells are critical in regulating inflammation and oxidative stress in peripheral nerves, and they also play a role in removing cellular debris and waste products that can accumulate and contribute to nerve damage over time.45,46 While the exact mechanisms linking lower concentrations of IBA1+ macrophages to diabetic neuropathy are not yet fully understood and require further research, these data suggest that a reduction in these immune cells could be a contributing factor in the development and progression of autoimmune-induced diabetic neuropathy.
Patients with DPN in this study had relatively mild DPN (with median TCNS of 6 for those without pain and 9 for those with pain) and those with pain had relatively mild to moderate pain (a median of 5 over the last 7 days), resulting in that most patients did not experience severe DPN. Inclusion of more patients with more severe symptoms of DPN may have resulted in more pronounced differences between the groups. In addition, during the random selection of 24–29 patients from each group, while we tried to consider sex and age of the participants, we were unable to completely match the groups of patients. However, the influence these confounders might have had on the results were reduced by adjusting for them. Even so, there were differences between the groups in several patient characteristics that may have influenced the results that we were unable to adjust for. Limitations in the skin biopsy analysis include that we did not measure other parameters related to immune cells, such as the activation status of macrophages or the levels of blood and tissue-resident cytokines. Last, immunohistological and stereological methods can assess only structural changes, and potential functional alterations of the targeted immune cells cannot be addressed by this study. Strengths of the study include carefully phenotyped patients following recommended guidelines and the well-developed immunohistological staining and stereological quantification methods.
Glutamic acid decarboxylase (GAD) enzymes are frequently detected in type 1 diabetes, serving as markers for the autoimmune destruction of pancreatic beta cells.47 Furthermore, they are strongly associated with stiff person syndrome, a condition characterized by painful spasms that affect approximately 30% of patients with type 1 diabetes.47,48 Considering the high prevalence of painful spasms in type 1 diabetes and the findings of this study suggesting a lesser role of the innate immune system compared with that in type 2 diabetes, which is primarily driven by the adaptive immune system, it would be worthwhile for future investigations to explore the potential relationship between macrophages, GAD antibodies, and painful spasms in type 1 diabetes to further understand possible mechanism-based treatment options.49
Moreover, future studies should include a larger cohort of patients to allow both comparison of patients with similar characteristics and comparison of patients with different degree of neuropathy. In addition, future studies should assess immune cells in other etiologies, e.g., chemotherapy-induced neuropathy, and additional biomarkers (including systemic markers such as serum cytokines) to further investigate the role of immune cells in neuropathic pain.
The main finding of this study is that the IBA1+ macrophage expression in peripheral skin biopsies is reduced in this cohort of patients with type 1 diabetes with long-term diabetes diagnosis compared with that in controls and that this reduction is more pronounced in patients with type 1 diabetes with diabetic neuropathy. The reduction in IBA1 area fraction is furthermore correlated with several different measures of neuropathy. These data suggest that reduction in these immune cells may play an important role in the development and progression of autoimmune-induced diabetic neuropathy.
Glossary
- BMI
body mass index
- CDT
cold detection threshold
- Dia BP
diastolic blood pressure
- DPN
diabetic polyneuropathy
- eGFR
estimated glomerular filtration rate
- HDL
high-density lipoprotein
- IBA1
ionized calcium binding adaptor molecule 1
- IENFD
intraepidermal nerve fiber density
- IQR
interquartile range
- LC
Langerhans cell
- LDL
low-density lipoprotein
- MHC
major histocompatibility complex
- MNSI
Michigan Neuropathy Screening Instrument
- MNSI-Q
Michigan Neuropathy Screening Instrument questionnaire
- NRS
numeric rating scale
- P-T1DPN
type 1 diabetes with painful diabetic polyneuropathy
- ROI
region of interest
- Sys BP
systolic blood pressure
- TCNS
Toronto clinical neuropathy score
- T1D
type 1 diabetes without diabetic polyneuropathy
- T1DPN
type 1 diabetes with painless diabetic polyneuropathy
- WDT
warm detection threshold
Appendix. Authors
Study Funding
This research was funded by the Novo Nordisk Foundation (Grant number NNF18OC0052301) and by the Lundbeck Foundation (Grant number R359-2020-2620). Hu X. is funded by a Ph.D. stipend from the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation (Grant number NNF17SA0031406).
Disclosure
P. Karlsson has received personal fees from Grünenthal, Alnylam, and Vertex Pharmaceuticals and has received a research grant from Merck outside the submitted work. Other authors have no conflicts of interest to state. Go to Neurology.org/NN for full disclosures.
References
- 1.Zochodne DW. The challenges of diabetic polyneuropathy: a brief update. Curr Opin Neurol. 2019;32(5):666-675. doi: 10.1097/wco.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 2.Frank T, Nawroth P, Kuner R. Structure-function relationships in peripheral nerve contributions to diabetic peripheral neuropathy. Pain. 2019;160(suppl 1):S29-S36. doi: 10.1097/j.pain.0000000000001530 [DOI] [PubMed] [Google Scholar]
- 3.Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. 2006;29(4):883-887. doi: 10.2337/diacare.29.04.06.dc05-2180 [DOI] [PubMed] [Google Scholar]
- 4.Gylfadottir SS, Weeracharoenkul D, Andersen ST. Painful and non-painful diabetic polyneuropathy: clinical characteristics and diagnostic issues. J Diabetes Investig. 2019;10(5):1148-1157. doi: 10.1111/jdi.13105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gylfadottir SS, Christensen DH, Nicolaisen SK, et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: a cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain. 2020;161(3):574-583. doi: 10.1097/j.pain.0000000000001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diabetes Rep. 2013;13(3):435-444. doi: 10.1007/s11892-013-0375-y [DOI] [PubMed] [Google Scholar]
- 7.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1-12. doi: 10.1016/j.neures.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 8.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871-882. doi: 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- 9.Domoto R, Sekiguchi F, Tsubota M, Kawabata A. Macrophage as a peripheral pain regulator. Cell. 2021;10(8):1881. doi: 10.3390/cells10081881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111-1119. doi: 10.1172/jci25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem biophysical Res Commun. 1996;224(3):855-862. doi: 10.1006/bbrc.1996.1112 [DOI] [PubMed] [Google Scholar]
- 12.Utans U, Arceci RJ, Yamashita Y, Russell ME. Cloning and characterization of allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. J Clin Invest. 1995;95(6):2954-2962. doi: 10.1172/jci118003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao YY, Yan DJ, Chen ZW. Role of AIF-1 in the regulation of inflammatory activation and diverse disease processes. Cell Immunol. 2013;284(1-2):75-83. doi: 10.1016/j.cellimm.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 14.Baum P, Koj S, Klöting N, Blüher M. Treatment-induced neuropathy in diabetes (TIND)-Developing a disease model in type 1 diabetic rats. Int J Mol Sci. 2021;22(4):1571. doi: 10.3390/ijms22041571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu P, Thinschmidt JS, Yan Y, et al. CNS inflammation and bone marrow neuropathy in type 1 diabetes. Am J Pathol. 2013;183(5):1608-1620. doi: 10.1016/j.ajpath.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gylfadottir SS, Itani M, Kristensen AG, et al. Analysis of macrophages and peptidergic fibers in the skin of patients with painful diabetic polyneuropathy. Neurol Neuroimmunol Neuroinflamm. 2021;9(1):e1111. doi: 10.1212/nxi.0000000000001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collin M, Milne P. Langerhans cell origin and regulation. Curr Opin Hematol. 2016;23(1):28-35. doi: 10.1097/moh.0000000000000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doebel T, Voisin B, Nagao K. Langerhans cells - the macrophage in dendritic cell clothing. Trends Immunol. 2017;38(11):817-828. doi: 10.1016/j.it.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 19.Deckers J, Hammad H, Hoste E. Langerhans cells: sensing the environment in health and disease. Front Immunol. 2018;9:93. doi: 10.3389/fimmu.2018.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doss AL, Smith PG. Nerve-Langerhans cell interactions in diabetes and aging. Histol Histopathol. 2012;27(12):1589-1598. doi: 10.14670/hh-27.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doss AL, Smith PG. Langerhans cells regulate cutaneous innervation density and mechanical sensitivity in mouse footpad. Neurosci Lett. 2014;578:55-60. doi: 10.1016/j.neulet.2014.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oaklander AL, Stocks EA, Mouton PR. Number of Langerhans immune cells in painful and non-painful human skin after shingles. Arch Dermatol Res. 2003;294(12):529-535. doi: 10.1007/s00403-002-0362-7 [DOI] [PubMed] [Google Scholar]
- 23.Strom A, Brüggemann J, Ziegler I, et al. Pronounced reduction of cutaneous Langerhans cell density in recently diagnosed type 2 diabetes. Diabetes. 2014;63(3):1148-1153. doi: 10.2337/db13-1444 [DOI] [PubMed] [Google Scholar]
- 24.D'Onofrio L, Kalteniece A, Ferdousi M, et al. Small nerve fiber damage and Langerhans cells in type 1 and type 2 diabetes and LADA measured by corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2021;62(6):5. doi: 10.1167/iovs.62.6.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozsoy N, Gül N, Bostanci H, Ayvali C. Ultrastructural determination of gingival Langerhans cells in alloxan-induced diabetic rats. Cell Biochem Funct. 2005;23(3):181-187. doi: 10.1002/cbf.1138 [DOI] [PubMed] [Google Scholar]
- 26.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285-2293. doi: 10.2337/dc10-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15(3):202-207. doi: 10.1111/j.1529-8027.2010.00271.x [DOI] [PubMed] [Google Scholar]
- 28.Karlsson P, Nyengaard JR, Polydefkis M, Jensen TS. Structural and functional assessment of skin nerve fibres in small-fibre pathology. Eur J Pain. 2015;19(8):1059-1070. doi: 10.1002/ejp.645 [DOI] [PubMed] [Google Scholar]
- 29.Gundersen HJ, Bagger P, Bendtsen TF, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96(10):857-881. doi: 10.1111/j.1699-0463.1988.tb00954.x [DOI] [PubMed] [Google Scholar]
- 30.Sima AA, Kamiya H. Diabetic neuropathy differs in type 1 and type 2 diabetes. Ann N Y Acad Sci. 2006;1084:235-249. doi: 10.1196/annals.1372.004 [DOI] [PubMed] [Google Scholar]
- 31.Lernmark A. Type 1 diabetes. Clin Chem. 1999;45(8 Pt 2):1331-1338. [PubMed] [Google Scholar]
- 32.Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2006;175(2):165-170. doi: 10.1503/cmaj.060244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otto-Buczkowska E, Kazibutowska Z, Sołtyk J, Machnica L. Neuropathy and type 1 diabetes mellitus. Pediatr Endocrinol Diabetes Metab. 2008;14(2):109-116. [PubMed] [Google Scholar]
- 34.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333-1346. doi: 10.1016/s0140-6736(05)61032-x [DOI] [PubMed] [Google Scholar]
- 35.Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397-405. doi: 10.1002/acn3.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS One. 2019;14(2):e0212574. doi: 10.1371/journal.pone.0212574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821-1830. doi: 10.1172/jci19451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796-1808. doi: 10.1172/jci19246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gwathmey KG, Burns TM, Collins MP, Dyck PJ. Vasculitic neuropathies. Lancet Neurol. 2014;13(1):67-82. doi: 10.1016/s1474-4422(13)70236-9 [DOI] [PubMed] [Google Scholar]
- 40.Beachy N, Satkowiak K, Gwathmey KG. Vasculitic neuropathies. Semin Neurol. 2019;39(5):608-619. doi: 10.1055/s-0039-1688990 [DOI] [PubMed] [Google Scholar]
- 41.Tavakoli M, Boulton AJ, Efron N, Malik RA. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye. 2011;34(1):7-11. doi: 10.1016/j.clae.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casanova-Molla J, Morales M, Planas-Rigol E, et al. Epidermal Langerhans cells in small fiber neuropathies. Pain. 2012;153(5):982-989. doi: 10.1016/j.pain.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 43.Dauch JR, Bender DE, Luna-Wong LA, et al. Neurogenic factor-induced Langerhans cell activation in diabetic mice with mechanical allodynia. J Neuroinflammation. 2013;10:64. doi: 10.1186/1742-2094-10-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eleazu CO, Eleazu KC, Chukwuma S, Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013;12(1):60. doi: 10.1186/2251-6581-12-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428-435. doi: 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- 46.Liu G, Ma H, Jiang L, Zhao Y. Allograft inflammatory factor-1 and its immune regulation. Autoimmunity. 2007;40(2):95-102. doi: 10.1080/08916930601083946 [DOI] [PubMed] [Google Scholar]
- 47.Dalakas MC, Li M, Fujii M, Jacobowitz DM. Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies. Neurology. 2001;57(5):780-784. doi: 10.1212/wnl.57.5.780 [DOI] [PubMed] [Google Scholar]
- 48.Dalakas MC. Stiff-person syndrome and GAD antibody-spectrum disorders: GABAergic neuronal excitability, immunopathogenesis and update on antibody therapies. Neurotherapeutics. 2022;19(3):832-847. doi: 10.1007/s13311-022-01188-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalakas MC. Therapies in stiff-person syndrome: advances and future prospects based on disease pathophysiology. Neurol Neuroimmunol Neuroinflamm. 2023;10(3):e200109. doi: 10.1212/nxi.0000000000200109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data are available from the corresponding author on request pending signed agreements from Aarhus University.