Abstract
Bovine leukemia virus (BLV) is an oncogenic retrovirus associated with B-cell lymphocytosis, leukemia, and lymphosarcoma in the ovine and bovine species. We have recently reported that in sheep, BLV protects the total population of peripheral blood mononuclear cells (PBMCs) from ex vivo spontaneous apoptosis. This global decrease in the apoptosis rates resulted from both direct and indirect mechanisms which allow extension of cell survival. Although sheep are not natural hosts for BLV, these animals are prone to develop virus-induced leukemia at very high frequencies. Most infected cattle, however, remain clinically healthy. This difference in the susceptibilities to development of leukemia in these two species might be related to alterations of the apoptotic processes. Therefore, we designed this study to unravel the mechanisms of programmed cell death in cattle. We have observed that PBMCs from persistently lymphocytotic BLV-infected cows were more susceptible to spontaneous ex vivo apoptosis than cells from uninfected or aleukemic animals. These higher apoptosis rates were the consequence of an increased proportion of B cells exhibiting lower survival abilities. About one-third of the BLV-expressing cells did not survive the ex vivo culture conditions, demonstrating that viral expression is not strictly associated with cell survival in cattle. Surprisingly, culture supernatants from persistently lymphocytotic cows exhibited efficient antiapoptotic properties on both uninfected bovine and uninfected ovine cells. It thus appears that indirect inhibition of cell death can occur even in the presence of high apoptosis rates. Together, these results demonstrate that the protection against spontaneous apoptosis associated with BLV is different in cattle and in sheep. The higher levels of ex vivo apoptosis occurring in cattle might indicate a decreased susceptibility to development of leukemia in vivo.
Bovine leukemia virus (BLV), a lymphotropic bovine retrovirus, is a member of the Oncovirinae subfamily, which also includes human T-cell leukemia virus types 1 and 2 (HTLV-1 and -2) (13). These viruses are nonacutely lymphotropic retroviruses, inducing lymphoid neoplasia after long latency periods. In most cases, BLV infection remains clinically silent, and infected animals are then referred to as asymptomatic or aleukemic (AL) (2). Only one-third of infected cattle develop persistent lymphocytosis (PL), a polyclonal expansion of B lymphocytes coexpressing CD5, high levels of surface immunoglobulin M (sIgM), and myeloid markers (7, 16). After a latency period of 1 to 8 years, a monoclonal neoplastic transformation of infected B lymphocytes, resulting in fatal leukemia or lymphoma, occurs in fewer than 5% of the infected cows. Since the risk of developing leukemia or lymphoma is greater in animals with PL than in AL cattle, PL is often considered a preneoplastic condition. BLV naturally infects cattle but can also be experimentally transmitted into sheep (14). This ovine host is a particularly convenient experimental model, since infected sheep develop B-cell neoplasia at a higher incidence and after shorter latency periods than cattle.
The molecular mechanisms underlying HTLV- and BLV-induced pathogeneses are still obscure. Nevertheless, it is assumed that the homeostasis of the target cells is perturbed, since infected cells accumulate within the bloodstream. So far, the common dogma has postulated that in virus-induced lymphocytosis, leukemia and tumor formation result mainly from the uncontrolled proliferation of infected lymphocytes. Cellular homeostasis is known to result from a critical balance between proliferation and apoptosis. An increasing number of studies have recently focused on dysregulation of apoptosis as a general component of viral strategies (for reviews, see references 22 and 28). In the case of virus-induced lymphocytosis, artificial prolonged survival of the infected cells caused by the virus is thus likely to contribute to disease progression. In the early stages of the disease, this process would favor viral spread within the host and lead to the expansion of a lymphocyte population. During the late phase, the protection from cell death might also play a key role in virus-induced oncogenesis.
The understanding of the apoptotic processes is strictly dependent on the cellular context and the experimental conditions used. Different protocols have provided conflicting conclusions. For example, while the HTLV-1 Tax protein has been shown to promote cellular death of murine fibroblasts (32), expression of the tax gene in Jurkat T cells could result in either a reduced or an enhanced sensitivity to Fas-mediated apoptosis (5, 6). In contrast, a protective effect was seen when primary human lymphocytes were treated with recombinant soluble Tax protein (6). However, because of the lack of an appropriate experimental model, the biological significance of the mechanisms inhibiting apoptotic cell death during HTLV-1 infection is still elusive.
We and others have previously reported that BLV is able to protect infected sheep B lymphocytes from spontaneous ex vivo programmed cell death (9, 26). In addition, supernatants from BLV-infected ovine peripheral blood mononuclear cells (PBMCs) contain factors that indirectly extend survival of naive cells in culture (9). It thus appears that the presence of the BLV provirus inhibits both directly and indirectly the spontaneous cellular death occurring in ex vivo cultures of lymphocytes. These observations are consistent with a general mechanism based on decreased cell death that would be developed by the virus to disturb the blood cell homeostasis in vivo and would finally lead to leukemia. In sheep, this process might thus be of prime importance for the development of BLV-induced pathogenesis. In order to unravel these processes in the natural host of BLV, we analyzed the precise role of apoptotic dysregulation in the development of lymphocytosis in BLV-infected cattle.
MATERIALS AND METHODS
Animals.
Most of the cows used in this study were adult Holstein cows from the University of Idaho dairy herd. The uninfected (NI) Belgium White Blue, double-muscled cows B1 and B2 were from the herd of the Animal Science Department, Faculty of Agronomy, (Gembloux, Belgium). The NI (no. 6005) and AL (no. 71 and 74) cows were kept at the Veterinary and Agrochemical Centre Research (Uccle, Belgium). Cows were classified as persistently lymphocytotic (PL), AL BLV-infected (AL), or NI based on complete and differential blood counts, phenotypic analysis of PBMCs, and BLV serology. The presence or absence of antibodies against BLV gp51 was assessed by using an agar gel immunodiffusion assay (Leukassay B; Pittman Moore, Mundelein, Ill.). Cows defined as PL were seropositive for BLV and had a lymphocyte count of greater than 8,000 cells/μl which persisted for more than 3 months. AL cows were seropositive for BLV and had a lymphocyte count within the normal range (2,500 to 7,500 cells/μl). NI cows were seronegative for BLV.
All sheep were maintained under controlled conditions at the Veterinary and Agrochemical Research Centre. At regular intervals, the total lymphocyte counts were determined, and sera were analyzed for BLV seropositivity by immunodiffusion and indirect gp51 enzyme-linked immunosorbent assay (19, 20).
PBMC isolation and culture conditions.
Sheep PBMCs were isolated by Percoll gradient centrifugation as previously described (9, 10). For isolation of bovine PBMCs, blood samples were first collected by jugular venipuncture and mixed with heparin. PBMCs were then separated on a Ficoll-Hypaque (Pharmacia; density 1,077 g/ml) density gradient and washed three times with phosphate-buffered saline (PBS). Following isolation, viable cells were counted by trypan blue dye exclusion, and PBMCs were cultured at 2 × 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 5 × 10−5 M β-mercaptoethanol, 2 mM glutamine, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml.
Antibodies.
A mouse monoclonal antibody (MAb) (4′G9, IgG1) against BLV capsid protein p24 was provided by Daniel Portetelle, Faculty of Agronomy, Gembloux, Belgium. Specific MAbs against bovine surface markers were obtained from the Washington State University Monoclonal Antibody Center, Pullman. As a B-cell marker, we used the anti-bovine IgM MAb PIg45A2 (IgG2b).
Flow cytometry.
Flow cytometry analyses were performed on a Becton Dickinson FACScan flow cytometer. Debris was excluded from the analyses by the conventional scatter gating method. Ten thousand events were collected per sample, and data were analyzed with the CELLQUEST software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
DNA ladder assay.
The low-molecular-weight (LMW) DNA was extracted from 2 × 106 PBMCs essentially as previously described (18). Briefly, after 24 h of culture, cells were centrifuged and pellets were resuspended in 40 μl of high buffer (100 mM Tris-HCl, 40 mM EDTA [pH 8.0]). The cells were then lysed by the addition of 400 μl of lysis buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0] with 0.5% Triton X-100) and incubated for 30 min at 37°C. After centrifugation at 14,000 × g for 15 min, the supernatants (containing LMW DNA) were incubated with RNase A (50 μg/ml) at 37°C for 30 min and digested with proteinase K (100 μg/ml) at 50°C for 1 h. After phenol-chloroform extraction and ethanol precipitation, the DNAs were resuspended in TE buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA) and resolved on a 1.5% agarose gel.
In situ detection of apoptosis.
An In Situ Cell Death Detection Kit, Fluorescein (Boehringer Mannheim) was used to measure apoptotic DNA fragmentation in individual cells. The test is based on the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) technique. The TUNEL reaction allows the labeling of DNA strand breaks by incorporation of fluorescein isothiocyanate (FITC)-labeled dUTP on the free 3′-OH DNA ends. This assay was performed directly on cells after culture or after the cells had been previously labeled with MAb PIg45A2 (against sIgM) or MAb 4′G9 (against the p24 viral protein). For detection of apoptosis in the B-cell subset, cultured cells were first labeled with the PIg45A2 MAb for 20 min at room temperature. After two washes, the cells were incubated with phycoerythrin-conjugated goat anti-mouse Ig (isotype specific), washed twice, and processed for the TUNEL reaction. Prior to the terminal deoxynucleotidyltransferase labeling, cells were washed twice in PBS–10% FCS and fixed in 1% paraformaldehyde for 15 min at 4°C. After two washes in PBS–10% FCS, the cells were permeabilized in 70% ethanol at −20°C for at least 30 min. For simultaneous detection of apoptosis in infected and uninfected cells, the cells were first fixed in paraformaldehyde and ethanol as described above. Internal detection of the p24 viral protein was performed on fixed cells by sequential incubation with the 4′G9 MAb and a phycoerythrin-conjugated secondary antibody. After two washes, the TUNEL reaction was performed essentially according to the manufacturer’s instructions.
Semiquantitative PCR analysis.
The PCRs were performed directly on blood samples essentially as previously described (9). Briefly, aliquots of blood were mixed with an equal volume (500 μl) of lysis buffer (0.32 M sucrose, 10 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 1% Triton X-100). After three washes in the same buffer, the samples were resuspended in 500 μl of PCR buffer (50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl [pH 8.3]) and incubated with proteinase K (30 μg/ml) for 1 h at 50°C. The samples were then boiled for 5 min in order to stop the digestion and finally diluted 10 times in PCR buffer. Ten microliters from these dilutions was then amplified by PCR in the presence of 200 μM each deoxynucleotide, 1 U of Taq DNA polymerase (Boehringer Mannheim), and 200 ng of primers. The primers used (PCRTA [5′-CTCTTCGGGATCCATTACCTGA-3′] and PCRTC [5′-CCTGCATGATCTTTCATACAAAT-3′]) encompass the region from position 7999 to 6990 (24) of the BLV tax gene. The samples were overlaid with mineral oil, denatured for 5 min at 95°C, and amplified by 26 cycles of PCR (30 s at 95°C, 30 s at 58°C, and 1 min at 72°C). After a final elongation step of 10 min at 72°C, 20 μl of the amplification product was resolved on a 1% agarose gel, transferred to a Hybond N+ membrane (Amersham), and hybridized with a BLV Tax probe (a 1-kb EcoRI insert from plasmid pSGTax). This construct contains the viral sequences corresponding to the tax gene cDNA isolated from the BLV-infected FLK cell line (30).
RESULTS
PBMCs from cows with PL are prone to high levels of spontaneous ex vivo apoptosis.
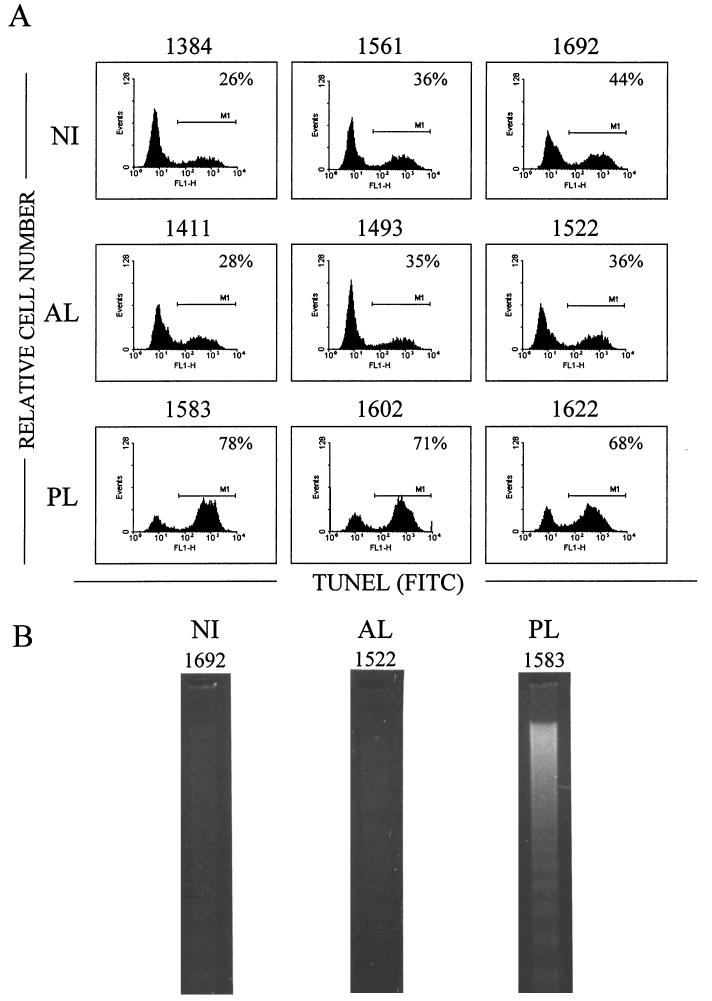
Enzootic bovine leukosis is a complex progressive disease in which infected cows may go through multiple clinical stages. While 30% of infected cows exhibit PL, the majority of the animals remain persistently infected but are clinically healthy and are thus classified as AL. In order to compare the ex vivo survival rates of PBMCs isolated from animals at various stages of the disease, we collected blood from three cows with PL (PL cows 1583, 1602, and 1622) and from three BLV-infected cows without clinical disorders (AL cows 1411, 1493, and 1522). Three NI cows were also used as controls (NI cows 1384, 1561, and 1692). PBMCs were isolated by Ficoll gradient centrifugation and cultured for 24 h, and the occurrence of apoptosis within the cultures was then assessed. The biochemical hallmark of apoptosis is the internucleosomal cleavage of the genomic DNA within the apoptotic cells. This DNA fragmentation can be revealed in situ by the TUNEL procedure, which is based on the enzymatic incorporation of fluorescein-dUTP into the nicks generated in the apoptotic cells. We used this assay to determine cell survival in ex vivo cultures from BLV-infected and NI cows. After the TUNEL reaction, the cell samples were analyzed by flow cytometry in order to evaluate the proportion of cells undergoing apoptosis. As shown in Fig. 1A, about one-third of the cells from NI or AL cows had incorporated the fluorescent marker (26 to 44% and 28 to 36% for NI and AL cows, respectively). In contrast, PBMC cultures from cows with PL showed very high apoptosis rates. Indeed, in independent cultures from three different animals, the proportion of TUNEL-positive cells reached values of 68, 71, and 78%.
FIG. 1.
Detection of apoptosis in PBMC cultures from BLV-infected PL, BLV-infected AL, and NI cows. (A) After 24 h of culture, PBMCs were fixed and the DNA strand breaks were labeled by the TUNEL procedure. Incorporation of FITC-dUTP was assessed by flow cytometry on the FL1 channel over 10,000 events. For each sample, distributions of the cells according to the relative fluorescence on the FL1 channel [TUNEL (FITC) on the x axis] are represented as a histogram. The percentage on each histogram corresponds to the proportion of apoptotic cells (M1). Results from one representative experiment are shown. (B) Visualization of internucleosomal DNA fragmentation by agarose gel electrophoresis. After 24 h of culture, the LMW fraction of the DNA was isolated, electrophoresed through a 1.5% agarose gel, and stained with ethidium bromide. The samples are from PL cow 1583, AL cow 1522, and NI cow 1692.
In order to confirm the occurrence of apoptosis in the cultures, we performed a gel electrophoresis of LMW DNA isolated from PBMCs cultured for 24 h. Indeed, the extensive internucleosomal cleavage of DNA during apoptosis results in LMW DNA fragments that can easily be separated from intact, chromosome-length DNA. After gel electrophoresis, these fragments appear as a typical DNA ladder, whose pattern is considered to be the hallmark of apoptosis. In parallel to the TUNEL assay, we thus cultured PBMCs from the BLV-infected AL cow 1522 and from the PL cow 1583. As a control, NI PBMCs from the BLV-seronegative cow 1692 were also included. For each of the three cows, a typical DNA ladder appeared when LMW DNA was resolved on an agarose gel, demonstrating the occurrence of apoptosis (Fig. 1B). The intensity of the DNA ladder corresponding to the PL cow (1583) revealed massive cell death in comparison with the levels of DNA fragmentation observed in NI and AL samples. This result thus supports the data obtained with the quantitative TUNEL assay and demonstrates that BLV-infected PBMCs from cows with PL are more susceptible to ex vivo apoptotic cell death than those from AL or NI cattle. These observations are unexpected, since we have shown in a previous report that BLV-infected sheep PBMCs are less prone to undergo ex vivo apoptosis than are NI cells (9).
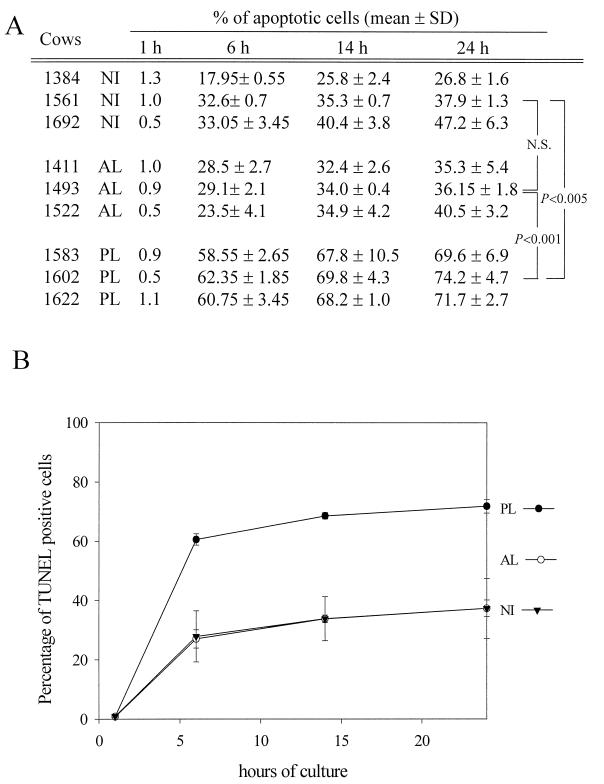
In order to refine these observations, we next performed a kinetic analysis of the PBMC survival during the culture. The PBMCs from the nine cows were cultured for 1, 6, 14, and 24 h, harvested, and analyzed by the TUNEL procedure (Fig. 2A). For each time point, the mean apoptotis rates were calculated according to the stage of the disease (Fig. 2B). After 1 h of culture, the cell mortalities were low (around 1%) and did not differ among the three groups (NI, AL, and PL). This observation indicates that the isolation procedure does not alter the cell survival for the different samples. Within 6 h, the mean apoptosis rates greatly increased for all of the animals. However, a marked difference appeared between PBMCs from cattle with PL and those from NI animals: the mean apoptosis rates reached 60% for PL cows versus 28% for NI cows. On the other hand, there was no difference in the kinetics of apoptosis between NI and AL cows, and both categories had similar apoptosis rates after 6 h of culture (27% for AL cows and 28% for NI cows). At later time points (14 and 24 h), the levels of TUNEL-positive cells exhibited only a slight increase in the different samples. Together, these data demonstrate that PBMCs from persistently infected cows display an increased spontaneous ex vivo apoptosis compared to PBMCs from NI or AL cows.
FIG. 2.
Kinetic analysis of the apoptosis levels in cultures of PBMCs from PL, AL, and NI cows. (A) PBMCs were cultured for 1, 6, 14, and 24 h and processed for detection of DNA strand breaks by the TUNEL procedure. Data are mean values from two independent experiments performed in duplicate. SD, standard deviation. The statistical evaluation of the differences between NI, AL, and PL animals was performed with a Student t test. N.S., not statistically significant. (B) Mean values were calculated from the data presented in panel A, according to the stage of the disease. These values are schematically represented for a kinetic analysis after 1, 6, 14, and 24 h of culture.
PL in cattle is associated with a reduced ex vivo survival of the B-cell population.
Cows with PL exhibit characteristic hematological disorders, with a major perturbation being an increase in circulating B lymphocytes. This kind of anomaly is not found in the AL cows, whose B-cell counts remain within the normal range, accounting for 15 to 30% of the total PBMC population. We thus postulated that these changes in the hematological status of PL versus NI and AL cows could be responsible for the differences in the ex vivo apoptosis rates. Therefore, blood samples were collected by jugular venipuncture of three PL cows (cows 1583, 1602, and 1622), three AL cows (cows 1411, 1493, and 1522) and three NI cows (cows 1384, 1561, and 1692) used as controls. The number of leukocytes and the proportion of lymphocytes were then determined by examination under a light microscope (Table 1). As expected, the highest lymphocyte counts were obtained for the cows with PL, with the numbers of lymphocytes being above 8,000/mm3. On the other hand, neither the AL nor the NI cows showed lymphocytes counts of above 5,000/mm3.
TABLE 1.
Hematological status of NI, AL, and PL cows
| Cow | Status | Leukocytes/ mm3 | Lymphocytes/ mm3 | % of B cells (mean ± SD)a |
|---|---|---|---|---|
| 1384 | NI | 8,000 | 4,960 | 13.25 ± 0.15 |
| 1561 | NI | 7,600 | 4,788 | 29.95 ± 1.05 |
| 1692 | NI | 8,000 | 3,680 | 32.05 ± 1.95 |
| 1411 | AL | 7,100 | 3,479 | 19.90 ± 0.85 |
| 1493 | AL | 6,300 | 3,339 | 26.80 ± 2.30 |
| 1522 | AL | 5,600 | 3,752 | 18.25 ± 1.45 |
| 1583 | PL | 14,100 | 11,562 | 81.80 ± 0.10 |
| 1602 | PL | 9,700 | 8,633 | 69.85 ± 1.25 |
| 1622 | PL | 12,400 | 10,788 | 64.20 ± 1.60 |
Values were determined from two independent experiments and represent the proportion of B cells within the total PBMC population.
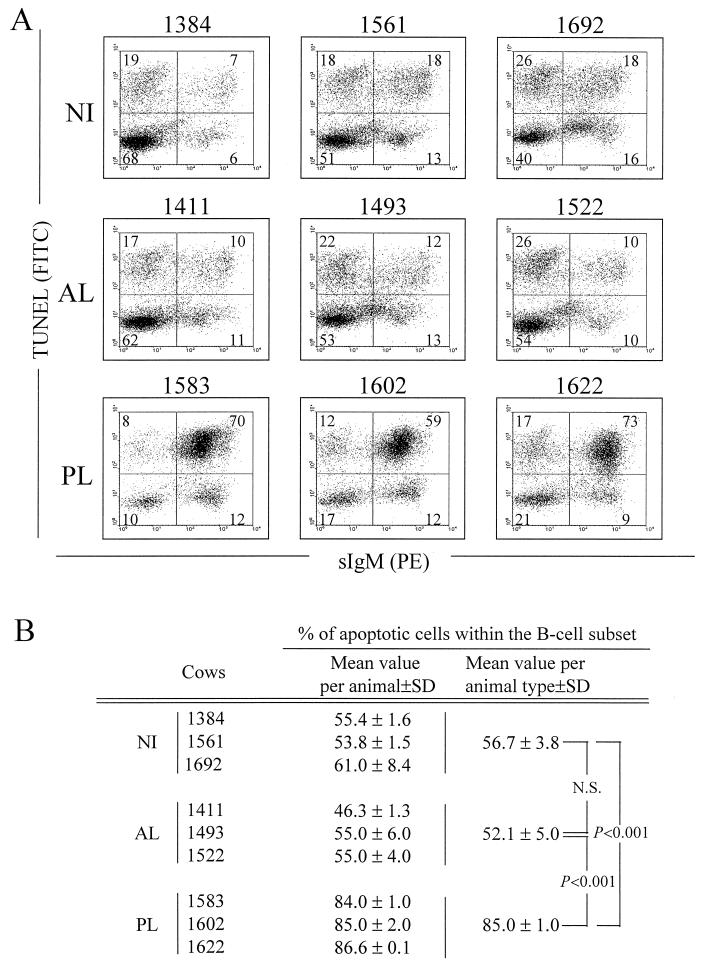
We next assessed the B-lymphocyte survival in ex vivo PBMC cultures from these animals (Fig. 3). After 24 h of culture, the cells were successively labeled with an anti-IgM antibody (PIg45A2) and with a phycoerythrin-conjugated secondary antibody. The cells were then fixed in paraformaldehyde-ethanol and analyzed for programmed cell death by the TUNEL procedure. The proportions of B cells within the PBMC population, estimated by flow cytometry on the basis of the red fluorescence, are summarized in Table 1. As expected, cows with PL exhibited high proportions of IgM+ B cells (64 to 82%), while AL and NI cows have low percentages of B-lymphocytes (13 to 32%). The dual-fluorescence analysis (TUNEL and sIgM) allowed us to analyze the apoptotic process within the B-cell subset (Fig. 3A). In control NI cows 1384, 1561, and 1692, the proportion of B cells undergoing apoptotic DNA cleavage ranged from 53 to 61% (Fig. 3B). Similar apoptosis rates were obtained for AL cows: 46 to 55% of B cells died by apoptosis after 24 h of culture. In contrast, the vast majority of the B lymphocytes isolated from PL cows were positive by the TUNEL assay (84, 85, and 87% for cows 1583, 1602, and 1622, respectively), indicating that massive cell death occurred within this particular lymphocyte subset.
FIG. 3.
Detection of apoptosis in B lymphocytes from BLV-infected PL (no. 1583, 1602, and 1622), BLV-infected AL (no. 1411, 1493, and 1522) and NI (no. 1384, 1561, and 1692) cows. PBMCs were cultured for 24 h and labeled with the PIg45A2 MAb (directed against bovine IgM) and a phycoerythrin (PE)-conjugated secondary antibody. The cells were then fixed and processed by the TUNEL procedure to detect apoptosis, and samples were then analyzed by dual-immunofluorescence flow cytometric analysis. (A) Results from a representative experiment are presented as dot plots. On the basis of control staining, each distribution was divided into four quadrants. The numbers indicate the percentage of PBMCs in each quadrant. (B) Percentages of apoptotic cells within the B-lymphocyte population. The mean values and standard deviations (SD) were calculated with three animals from two independent experiments. The significance of the differences in mean values between groups of animals was established by a Student t test. N.S., not statistically significant.
Together, these data demonstrate that the higher apoptosis rates of PBMCs from PL cows are the consequence of an increased proportion of B cells with a lower ex vivo survival ability.
Most but not all of the BLV-expressing cells exhibit extended ex vivo survival.
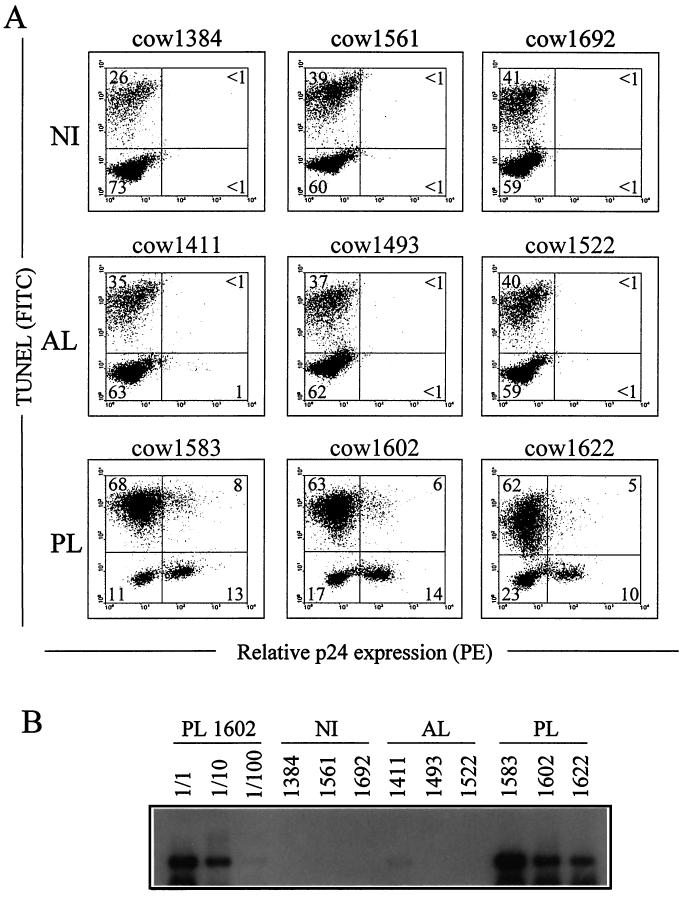
Two recent studies have demonstrated that BLV is capable of increasing the life span of sheep PBMCs in ex vivo cultures (9, 26). Based on the observation that BLV-expressing cells were completely protected from spontaneous programmed cell death, we have assigned a direct role to the virus in the inhibition of the cellular apoptotic programs in sheep (9). To correlate viral expression with apoptosis in cattle, we analyzed the apoptosis levels within the BLV-infected cells. For this purpose, blood samples were collected by venipuncture from three cows with PL (cows 1583, 1602, and 1622). As controls, we also used three NI cows (1384, 1561, and 1692) and three AL cows (1411, 1493, and 1522). PBMCs were isolated, cultured for 24 h, fixed, and incubated with MAb 4′G9, which recognizes the BLV capsid protein p24, and a phycoerythrin-conjugated secondary antibody. Finally, the DNA strand breaks were labeled by the TUNEL reaction, and samples were analyzed by flow cytometry. This procedure allows the detection of apoptotic cells in the p24-positive and p24-negative cell subsets (Fig. 4A). As control, background levels of p24-positive cells (fewer than 1%) were determined in the cultures of PBMCs from NI cows (cows 1384, 1561, and 1692). Similar results were obtained with PBMCs from AL cows 1493 and 1522, but a slightly higher number of p24-expressing cells (about 1%) was detectable in the case of cow 1411. However, the percentage of double-stained cells within sample 1411 was still below the background levels (less than 1%). On the other hand, PBMC cultures from PL cows contained about 15 to 20% of cells expressing the p24 viral protein: 21% (13 + 8), 20% (14 + 6), and 15% (10 + 5) for cows 1583, 1602, and 1622, respectively. Even if the majority appeared to survive the ex vivo culture conditions, about one-third of the p24-producing cells had undergone programmed cell death at 24 h. Indeed, 35% [8/(13 + 8)], 36% [6/(14 + 6)], and 33% [5/(10 + 5)] of the p24-producing cells (for cows 1583, 1602, and 1622, respectively) were strongly positive for DNA fragmentation.
FIG. 4.
In situ detection of apoptosis in BLV-expressing cells. PBMCs from PL cows 1583, 1602, and 1622, AL cows 1411, 1493, and 1522, and NI cows 1384, 1561, and 1692 were cultured for 24 h and fixed in paraformaldehyde-ethanol. Cells were then labeled with an anti-p24 MAb (4′G9) and a phycoerythrin (PE)-coupled secondary antibody. The DNA strand breaks were labeled by the TUNEL procedure. Samples were then analyzed by dual-flow cytometric immunofluorescence analysis. (A) Results from a representative experiment are presented as dot plots. Numbers represent the percentages of positively stained cells in each quadrant. (B) Semiquantitative PCR amplification of viral sequences. The DNAs were prepared from 500-μl aliquots of blood and resuspended in PCR buffer. Specific proviral sequences were amplified by 26 cycles of PCR. The amplification products were resolved on a 1% agarose gel and analyzed by Southern blotting with a BLV probe. Blood samples from NI cows 1384, 1561, and 1692 were used as controls for PCR contamination. The semiquantitative analysis was done by amplification of serial dilutions (1/1, 1/10, and 1/100) from PL cow 1602.
To correlate the number of p24-positive cells with the proviral loads, viral DNA levels within the circulating blood of all of the analyzed animals were determined by semiquantitative PCR. The DNAs were extracted from the samples as previously described (9), and proviral sequences were specifically amplified by PCR. Amplification products were resolved on an agarose gel and analyzed by Southern blotting with a BLV probe (Fig. 4B). As a control for semiquantification, serial dilutions (1/1 to 1/100) of DNA isolated from PL cow 1602 were amplified in parallel (Fig. 4B). As a control for specificity, no hybridization signals were detected with DNAs isolated from NI (no. cows 1384, 1561, and 1692). In contrast, the amplification of viral sequences from the three cows with PL yielded strong hybridization signals (cows 1583, 1602, and 1622). No amplification products were observed under these conditions with samples from AL cows 1493 and 1522, and only a faint signal (about 1/100-fold) was visible for AL cow 1411 (Fig. 4B). These results indicate that the amounts of proviral sequences within the blood samples roughly parallel the numbers of p24-expressing cells as measured by flow cytometry and indicate that the numbers of infected cells differ drastically between AL and PL cows.
Together, these data demonstrate that in PL cows, cells expressing BLV antigens are less prone to undergo spontaneous apoptosis than NI cells. However, expression of the virus alone is not sufficient to ensure full protection against cell death, since one-third of the p24-positive cells have undergone apoptosis after 24 h of ex vivo culture.
Culture supernatants from PL BLV-infected PBMCs protect NI cells from spontaneous apoptosis.
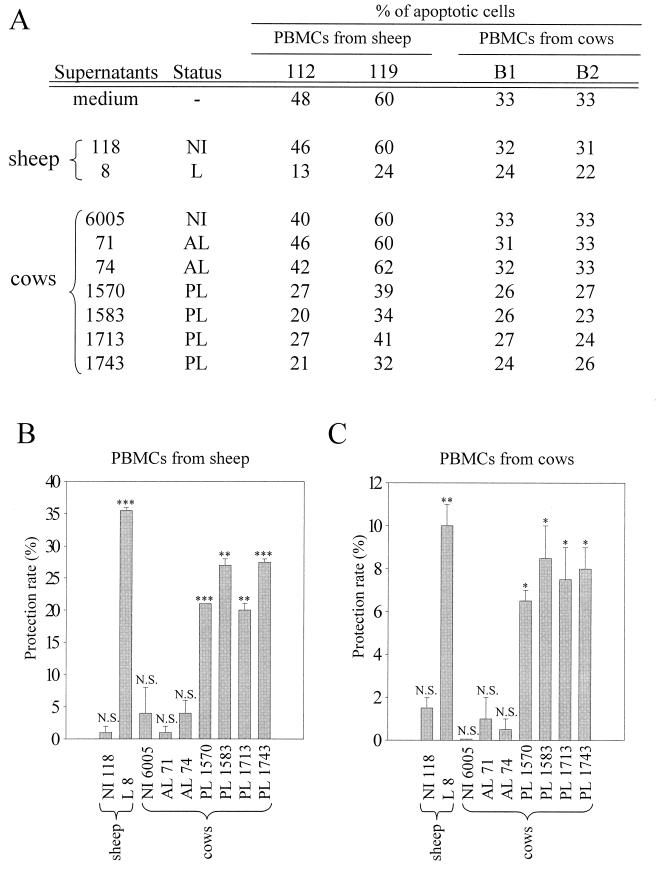
We have recently reported that supernatants from BLV-infected sheep PBMCs can rescue NI ovine cells from undergoing spontaneous programmed cell death (9). This indirect mechanism suggests that a secreted factor, which could diffuse into the culture medium, provides an effective protection to uninfected cells. In this respect, we have proposed that this factor could be responsible, at least in part, for the decreased apoptosis rates observed in cultures of BLV-infected sheep PBMCs. In order to test the antiapoptotic properties of culture supernatants from BLV-infected bovine cells, PBMCs were isolated from four cows with PL (cows 1570, 1583, 1713, and 1743) and two BLV-infected AL animals (cows 71 and 74). These cells were cultured for 48 h, and the corresponding supernatants were recovered, centrifuged, and filtered. As controls, supernatants from an NI cow (cow 6005), an NI sheep (sheep 118), and a sheep infected with a recombinant BLV provirus (sheep 8) (31) were prepared by the same procedure. The supernatants were then diluted (20-fold) and added to the PBMCs isolated from two uninfected cows (cows B1 and B2). After 20 h of culture, the apoptosis rates were determined by the TUNEL procedure (Fig. 5). As a control, the protective effect of the supernatants was first tested on PBMCs from two uninfected sheep (no. 112 and 119). When cultured in medium alone, about half of the cells (48%) from sheep 112 underwent spontaneous cell death (Fig. 5A). A similar apoptosis rate (46%) was obtained when cells were cultured in 20-fold-diluted supernatant from another uninfected sheep (no. 118). On the other hand, supernatant from a BLV-infected sheep (no. 8) led to an effective decrease in the apoptosis rate, since only 13% of the cells from uninfected sheep 112 were positive by the TUNEL reaction. These observations thus support our previous results and demonstrate that media conditioned by infected ovine cells inhibit apoptosis of naive sheep PBMCs (9). Similar conditions were then used to analyze the protective ability of supernatants from bovine cell cultures. Compared to medium alone, no significant decrease in cell death was observed with supernatants from uninfected cow 6005 or BLV-infected AL cows 71 and 74 (apoptosis rates of 40, 46, and 42%, respectively). Surprisingly, when cells from sheep 112 were cultured with supernatants from PL cows, the percentages of apoptotic cells decreased significantly, from 48 to 27, 20, 27, and 21% for cows 1570, 1583, 1713, and 1743, respectively (Fig. 5A). Similar observations were obtained independently with PBMCs from another uninfected sheep (no. 119). In this case, the apoptosis rates decreased from 60% with medium alone to 39, 34, 41, and 32% with supernatants from cows 1570, 1583, 1713, and 1743, respectively. These supernatants thus exert effective antiapoptotic effects on sheep lymphocytes in comparison to nonconditioned medium (protection rates ranging from 20 to 27.5%) (Fig. 5B). We conclude that supernatants which have been conditioned by PBMCs from cows with PL can reduce spontaneous cell death of uninfected sheep PBMCs. These experiments were next performed under similar conditions on NI bovine cells. Supernatants from BLV-infected sheep 8 exhibited an antiapoptotic capability on bovine cells (protection rates of 10%) (Fig. 5C). Interestingly, supernatants from cows with PL showed a weaker, but still effective, cell death-rescuing activity (protection rates of 6.5, 7.5, 8, and 8.5% for cows 1570, 1713, 1743, and 1583, respectively). On the other hand, supernatants from neither an NI cow (no. 6005) nor BLV-infected AL cows were able to decrease spontaneous apoptosis of bovine NI cells (Fig. 5C). Together, these results clearly demonstrate that supernatants from PL cows can rescue uninfected cells from ex vivo programmed cell death. Furthermore, this antiapoptotic effect is not species restricted, since bovine supernatants are also effective on ovine cells (compare Fig. 5B and C).
FIG. 5.
Protection of ovine and bovine uninfected PBMCs by culture supernatants from PBMCs from cows with PL. (A) PBMCs from a BLV-infected sheep (leukemic [L] sheep no. 8), an NI sheep (no. 118), an NI cow (no. 6005), two BLV-infected AL cows (no. 71 and 74), or PL cows (no. 1570, 1583, 1713, and 1743) were cultured for 48 h. The corresponding cell culture supernatants were added to PBMCs from NI sheep 112 and 119 or NI cows B1 and B2 at a 1/20 dilution. After 20 h of culture, the percentages of apoptotic cells were determined by the TUNEL procedure and flow cytometry analysis. As a control, apoptosis rates in culture medium alone were also measured. (B) The protection rate corresponds to the difference between the percentage measured in medium alone and the percentage measured with a conditioned supernatant. Mean values (ranging from 1 to 35.5%) and standard deviations obtained with uninfected PBMCs from sheep 112 and 119 are illustrated in a histogram. (C) The mean values (ranging from 0 to 10%) and standard deviations of protection rates obtained with uninfected PBMCs from cows B1 and B2 are illustrated in a histogram. The significance of the protection rates was analyzed by using a Student t test (N.S., not statistically significant; ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005).
DISCUSSION
In a previous report (9), we demonstrated that the total population of PBMCs isolated from BLV-infected sheep exhibit reduced spontaneous ex vivo apoptosis. In addition, BLV appears to specifically inhibit programmed cell death of the infected ovine B lymphocytes. For the bovine species, we show here that protection against apoptosis is not an absolute characteristic of cells expressing the virus. We have demonstrated that one-third of the cells which express the p24 major capsid antigen are undergoing apoptosis in ex vivo short-term cultures. As a consequence, viral expression does not always correlate, at the PL stage, with a complete protection of all of the infected target cells. The situation thus appears to be quite different in cattle and in sheep, which are the two species prone to develop leukemia after infection by BLV. This differential susceptibility to undergoing apoptosis might be correlated to genetic alterations occurring at late stages (i.e., PL and tumor phases) of the disease in cattle. Indeed, we and others have previously demonstrated that alterations in the p53 gene occur in cattle, but not in sheep, at the transition between PL and the leukemia stage (8, 27). Such neutralization of the p53-dependent proapoptotic pathways through genomic mutations would thus provide a survival advantage to the infected cells. Therefore, these cells would acquire a higher oncogenic potential and propagate to finally yield tumors. In this respect, the expression of the Bax protein, a downstream target of p53 in this pathway, appears to be altered, while the cell progresses towards full transformation. Indeed, an increased ratio of Bcl-2 to Bax expression, which is believed to predetermine the susceptibility to various apoptotic stimuli, has recently been correlated with advanced neoplastic stages of the disease in cattle (23). Mutations within the p53 tumor suppressor gene and subsequent inactivation of apoptosis could thus be essential events required for the infected cell to achieve full malignancy in cattle. Since p53 mutations have never been observed in ovine tumor cells in vivo, the inactivation through genomic mutations of the p53-dependent proapoptotic pathways does not seem to be a prerequisite for tumorigenicity in sheep. In cattle, as long as this rare genomic event did not occur, the p53 guardian would still be able to preclude viral expansion by induction of apoptosis in the infected cells from animals with PL.
Although one-third of the p24-positive cells from cows with PL are undergoing apoptosis ex vivo, the integrity of most of them still appears to be maintained. The most straightforward interpretation of this observation is that the presence of the virus directly protects its host cell from apoptosis. There is, however, a completely opposite model which could explain the same phenomenon. It is indeed possible that BLV preferentially infects a certain cell subtype with intrinsic abilities to survive. In other words, the particular cell which is the potential host for BLV would never die under our experimental conditions, even in the absence of the virus. Therefore, we tried to phenotypically characterize the subpopulation of cells that does not undergo apoptosis. In accordance with previous reports (7, 16), we observed that most, if not all, of the B lymphocytes (sIgM-positive cells) coexpressed both the CD5 and CD11b markers (data not shown). It thus appears that the presence of the CD11b marker on bovine cells does not correlate with a greater ex vivo survival ability. In the sheep model, the global decrease in spontaneous apoptosis of the B lymphocytes has been assigned to a B-1-like (CD11b+/sIgM+) subpopulation which exhibits increased ex vivo survival abilities (3). However, this observation could result from the higher susceptibility to viral infection exhibited by the CD11b+ B-cell subtype. In this case, the presence of the CD11b marker would not correlate with a better survival of the infected cell but would reflect the preference of BLV to replicate in a given subpopulation of B cells (25).
Since a large proportion of the PBMCs from cattle with PL are undergoing apoptosis in ex vivo short-term cultures, the most unexpected observation from this study is that the corresponding supernatants contain an antiapoptotic factor able to protect uninfected cells. Despite being detectable after 48 h, the molecule is unable to efficiently protect cells from cows with PL from undergoing apoptosis in the early hours of culture. The most straightforward interpretation for this observation is that the factor is slowly secreted in the medium by a complex, multiple-step process. In this model, cytokines, like interleukin-2 (IL-2) and IL-10, could be implicated in the regulation of apoptosis during leukemogenesis (12, 17, 21). For example, significant IL-2 functional activity has been observed in concanavalin A-stimulated PBMCs from PL cows (29). Nevertheless, in our hands, a polyclonal anti-recombinant human IL-2 antibody, which was shown to efficiently inhibit bovine IL-2 activity, was unable to abolish the antiapoptotic effect of culture supernatants from unstimulated cells isolated from cattle with PL (data not shown). Alternatively, viral proteins, like the Tax transactivator, could be other factors mediating this antiapoptotic process. Indeed, the Tax protein from the related HTLV-1 is able to inhibit apoptosis of T lymphocytes (1, 6, 15). Although the protective function of Tax in vitro is still a matter of discussion (4, 5, 11, 32), it is becoming clear that HTLV-1 Tax decreases programmed cell death of primary T lymphocytes in vivo. Experiments designed to identify the protective factor(s) in the BLV system are under way.
In addition to the direct protection of its target cell, BLV also modifies the homeostasis of the total B-lymphocyte population. In sheep, this indirect antiapoptotic effect inhibits cell death of the majority (about 80%) of the B lymphocytes (9). This drastic alteration of the B-cell susceptibility to apoptosis is already observed at the asymptomatic stage of the disease when the proviral loads are low (less than 1% of the wild-type levels). In addition, when the sheep are infected by recombinant viruses which exhibit a reduced ability to propagate, a similar protection of the B cells occurs. It thus appears that this indirect protection is independent of the proviral loads, although this process probably requires a certain latency period to be fully efficient (9, 26). The susceptibility to apoptotic cell death of the B lymphocytes in sheep can thus be summarized as follows: NI > AL = L (leukemic). The situation appears to be completely different in cattle (Fig. 3): NI = AL < PL. In contrast to the ovine B cells, the B lymphocytes of leukemic cattle with PL are thus more susceptible to apoptosis under similar experimental conditions. The differential interplay of BLV with the ovine or bovine species might indeed be related to differences in the corresponding induced pathologies. PL in cattle is a stabilized stage at which the numbers of lymphocytes remain high, but relatively constant, over extended periods of time. In contrast, sheep do not strictly develop a real PL. In this species, the proviral loads rise more gradually, indicating that the virus spreads at approximately constant rates (13). Strictly, the lymphocytosis in sheep is thus increasing rather than persistent. The occurrence of massive apoptosis in ex vivo cultures of bovine PBMCs could reflect a difference in pathology observed in vivo. In cattle, the effective control of cellular homeostasis by apoptosis would explain the stabilization of lymphocytosis into a given persistent stage. This virus-host equilibrium has definitely not been achieved in sheep, which are not a natural host for BLV. In this species, BLV is indeed transmissible but is not contagious under natural conditions. Since coevolution could not occur, a lack of symbiosis might explain why virus-induced pathology is more acute. Although these comments are very speculative, they might provide a model to illustrate the interplay between a single pathogen and two different hosts.
ACKNOWLEDGMENTS
The mouse MAb (4′G9, IgG1) against BLV capsid protein p24 was provided by Daniel Portetelle, Faculty of Agronomy, Gembloux, Belgium. Specific MAbs against bovine surface markers were obtained from the Washington State University Monoclonal Antibody Center, Pullman. We warmly thank R. Martin, P. Ridremont, and G. Vandendaele for skillful technical help. We are also grateful to D. S. Hoover for advice and discussion and to E. Wagner for care of animals at the University of Idaho dairy herd.
This work was supported by the Caisse Générale d’Epargne et de Retraite, the Belgian Service of Programmation pour la Politique Scientifique (SSTC P4/30), the Belgian Cancer Association, the Bekales Foundation, the International Union Against Cancer, the Fonds National de la Recherche Scientifique, the U.S. Department of Agriculture NRICGP (G.H.C.), and the National Institutes of Health (G.H.C.). F.D., R.K., and L.W. are, respectively, Chargé de Recherches, Directeur de Recherches, and Maître de Recherches of the Fonds National de la Recherche Scientifique.
REFERENCES
- 1.Arai M, Kannagi M, Matsuoka M, Sato T, Yamamoto N, Fuji M. Expression of FAP-1 (Fas-associated phosphatase) and resistance to Fas-mediated apoptosis in T cell lines derived from human T cell leukemia virus type 1-associated myelopathy/tropical spastic paraparesis patients. AIDS Res Hum Retroviruses. 1998;14:261–267. doi: 10.1089/aid.1998.14.261. [DOI] [PubMed] [Google Scholar]
- 2.Burny A, Cleuter Y, Kettmann R, Mammerickx M, Marbaix G, Portetelle D, Van den Broeke A, Willems L, Thomas R. Bovine leukemia: facts and hypotheses derived from the study of an infectious cancer. Adv Vet Sci Comp Med. 1988;32:149–170. doi: 10.1016/b978-0-12-039232-2.50010-4. [DOI] [PubMed] [Google Scholar]
- 3.Chevallier N, Barthelemy M, Le Rhun D, Lainé V, Levy D, Schwartz-Cornil I. Bovine leukemia virus-induced lymphocytosis and increased cell survival involve the CD11b+ B-lymphocyte subset in sheep. J Virol. 1998;72:4413–4420. doi: 10.1128/jvi.72.5.4413-4420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chlichlia K, Moldenhauer G, Daniel P T, Busslinger M, Gazzolo L, Schirrmacher V, Khazaie K. Immediate effects of reversible HTLV-I tax function: T-cell activation and apoptosis. Oncogene. 1995;10:269–277. [PubMed] [Google Scholar]
- 5.Chlichlia K, Busslinger M, Peter M E, Walczak H, Krammer P H, Schirrmacher V, Khazaie K. ICE-proteases mediate HTLV-I Tax induced apoptotic cell death. Oncogene. 1997;14:2265–2272. doi: 10.1038/sj.onc.1201070. [DOI] [PubMed] [Google Scholar]
- 6.Copeland K F T, Haaksma A G M, Goudsmit J, Krammer P H, Heeney J L. Inhibition of apoptosis in T-cells expressing human T cell leukemia virus type I Tax. AIDS Res Hum Retroviruses. 1994;10:1259–1268. doi: 10.1089/aid.1994.10.1259. [DOI] [PubMed] [Google Scholar]
- 7.Depelchin A, Letesson J J, Lostrie Trussart N, Mammerickx M, Portetelle D, Burny A. Bovine leukemia virus (BLV)-infected cells express a marker similar to the CD5 T cell marker. Immunol Lett. 1989;20:69–76. doi: 10.1016/0165-2478(89)90071-0. [DOI] [PubMed] [Google Scholar]
- 8.Dequiedt F, Kettmann R, Burny A, Willems L. Mutations in the p53 tumor-suppressor gene are frequently associated with bovine leukemia virus-induced leukemogenesis in cattle but not in sheep. Virology. 1995;209:676–683. doi: 10.1006/viro.1995.1303. [DOI] [PubMed] [Google Scholar]
- 9.Dequiedt F, Hanon E, Kerkhofs P, Pastoret P-P, Portetelle D, Burny A, Kettmann R, Willems L. Both wild-type and strongly attenuated bovine leukemia viruses protect peripheral blood mononuclear cells from apoptosis. J Virol. 1997;71:630–639. doi: 10.1128/jvi.71.1.630-639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudler L, Griebel P, Hein W R. Separation of mononuclear cells from blood. In: Lefkovitts I, editor. Immunological methods manual. New York, N.Y: Academic Press; 1997. pp. 2075–2078. [Google Scholar]
- 11.Fujita M, Shiku H. Difference in sensitivity to induction of apoptosis among rat fibroblast cells transformed by HTLV-I tax gene or cellular nuclear oncogenes. Oncogene. 1995;11:15–20. [PubMed] [Google Scholar]
- 12.Keefe R G, Choi Y, Ferrick D A, Stott J L. Bovine cytokine expression during different phases of bovine leukemia virus infection. Vet Immunol Immunopathol. 1997;56:39–51. doi: 10.1016/s0165-2427(96)05727-3. [DOI] [PubMed] [Google Scholar]
- 13.Kettmann R, Burny A, Callebaut I, Droogmans L, Mammerickx M, Willems L, Portetelle D. Bovine leukemia virus. In: Levy J A, editor. The Retroviridae. New York, N.Y: Plenum Press; 1994. pp. 39–81. [Google Scholar]
- 14.Mammerickx M, Palm R, Portetelle D, Burny A. Experimental transmission of leukosis in sheep: latency period of the tumoral disease. Leukemia. 1988;2:103–107. [PubMed] [Google Scholar]
- 15.Marriot S J, Lindholm P F, Reid R L, Brady J N. Soluble HTLV-1 Tax1 protein stimulates proliferation of human peripheral blood lymphocytes. New Biol. 1991;3:678–686. [PubMed] [Google Scholar]
- 16.Matheise J P, Delcommenne M, Mager A, Didembourg C H, Letesson J J. CD5+ B cells from bovine leukemia virus infected cows are activated cycling cells responsive to interleukin-2. Leukemia. 1992;6:304–309. [PubMed] [Google Scholar]
- 17.Meirom R, Moss S, Brenner J, Heller D, Trainin Z. Levels and role of cytokines in bovine leukemia virus (BLV) infection. Leukemia. 1997;11:219–220. [PubMed] [Google Scholar]
- 18.Motyka B, Griebel P J, Reynolds J D. Agents that activate protein kinase C rescue sheep ileal Peyer’s patch B cells from apoptosis. Eur J Immunol. 1993;23:1314–1321. doi: 10.1002/eji.1830230619. [DOI] [PubMed] [Google Scholar]
- 19.Portetelle D, Mammerickx M, Burny A. Use of two monoclonal antibodies in an ELISA test for the detection of antibodies to bovine leukemia virus envelope protein gp51. J Virol Methods. 1989;23:211–222. doi: 10.1016/0166-0934(89)90135-3. [DOI] [PubMed] [Google Scholar]
- 20.Portetelle D, Limbach K, Burny A, Mammerickx M, Desmettre P, Riviere M, Zavada J, Paoletti E. Recombinant vaccinia virus expression of the bovine leukemia virus envelope and protection of immunized sheep against infection. Vaccine. 1991;9:194–200. doi: 10.1016/0264-410x(91)90153-w. [DOI] [PubMed] [Google Scholar]
- 21.Pyeon D, O’Reilly K L, Splitter G A. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J Virol. 1996;70:5706–5710. doi: 10.1128/jvi.70.8.5706-5710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razvi E S, Welsh R M. Apoptosis in viral infections. Adv Virus Res. 1995;45:1–60. doi: 10.1016/s0065-3527(08)60057-3. [DOI] [PubMed] [Google Scholar]
- 23.Reyes R A, Cockerell G L. Increased ratio of bcl-2/bax expression is associated with bovine leukemia virus-induced leukemogenesis in cattle. Virology. 1998;242:184–192. doi: 10.1006/viro.1998.9029. [DOI] [PubMed] [Google Scholar]
- 24.Rice N, Stephens R, Gilden R. Sequence analysis of the bovine leukemia virus genome. In: Burny A, Mammerickx M, editors. Enzootic bovine leukosis and bovine leukemia virus. The Hague, The Netherlands: Nihoff; 1987. pp. 115–144. [Google Scholar]
- 25.Schwartz I, Bensaid A, Polack B, Perrin B, Barthelemy M, Levy D. In vivo leukocyte tropism of bovine leukemia virus in sheep and cattle. J Virol. 1994;68:4589–4596. doi: 10.1128/jvi.68.7.4589-4596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz-Cornil I, Chevallier N, Belloc C, Le Rhun D, Lainé V, Barthelemy M, Levy D. Bovine leukemia virus-induced lymphocytosis in sheep is associated with reduction of spontaneous B cell apoptosis. J Gen Virol. 1997;78:153–162. doi: 10.1099/0022-1317-78-1-153. [DOI] [PubMed] [Google Scholar]
- 27.Tajima S, Zhuang W Z, Kato M V, Okada K, Ikawa Y, Aida Y. Function and conformation of wild-type p53 protein are influenced by mutations in bovine leukemia virus-induced B-cell lymphosarcoma. Virology. 1998;243:235–246. [PubMed] [Google Scholar]
- 28.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trueblood E S, Brown W C, Palmer G H, Davis W C, Stone D M, McElwain T F. B-lymphocyte proliferation during bovine leukemia virus-induced persistent lymphocytosis is enhanced by T-lymphocyte-derived interleukin-2. J Virol. 1998;72:3169–3177. doi: 10.1128/jvi.72.4.3169-3177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willems L, Heremans H, Chen G, Portetelle D, Billiau A, Burny A, Kettmann R. Cooperation between bovine leukemia virus transactivator protein and Ha-ras oncogene product in cellular transformation. EMBO J. 1990;9:1577–1581. doi: 10.1002/j.1460-2075.1990.tb08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willems L, Kettmann R, Dequiedt F, Portetelle D, Voneche V, Cornil I, Kerkhofs P, Burny A, Mammerickx M. In vivo infection of sheep by bovine leukemia virus mutants. J Virol. 1993;67:4078–4085. doi: 10.1128/jvi.67.7.4078-4085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto Y, Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994;68:3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]