Keywords: BFR, cardiac rehabilitation, exercise, muscle metaboreflex, ventricular function
Abstract
Blood flow restriction training (BFRT) employs partial vascular occlusion of exercising muscle and has been shown to increase muscle performance while using reduced workload and training time. Numerous studies have demonstrated that BFRT increases muscle hypertrophy, mitochondrial function, and beneficial vascular adaptations. However, changes in cardiovascular hemodynamics during the exercise protocol remain unknown, as most studies measured blood pressure before the onset and after the cessation of exercise. With reduced perfusion to the exercising muscle during BFRT, the resultant accumulation of metabolites within the ischemic muscle could potentially trigger a large reflex increase in blood pressure, termed the muscle metaboreflex. At low workloads, this pressor response occurs primarily via increases in cardiac output. However, when increases in cardiac output are limited (e.g., heart failure or during severe exercise), the reflex shifts to peripheral vasoconstriction as the primary mechanism to increase blood pressure, potentially increasing the risk of a cardiovascular event. Using our chronically instrumented conscious canine model, we utilized a 60% reduction in femoral blood pressure applied to the hindlimbs during steady-state treadmill exercise (3.2 km/h) to reproduce the ischemic environment observed during BFRT. We observed significant increases in heart rate (+19 ± 3 beats/min), stroke volume (+2.52 ± 1.2 mL), cardiac output (+1.21 ± 0.2 L/min), mean arterial pressure (+18.2 ± 2.4 mmHg), stroke work (+1.93 ± 0.2 L/mmHg), and nonischemic vascular conductance (+3.62 ± 1.7 mL/mmHg), indicating activation of the muscle metaboreflex.
NEW & NOTEWORTHY Blood flow restriction training (BFRT) increases muscle mass, strength, and endurance. There has been minimal consideration of the reflex cardiovascular responses that could be elicited during BFRT sessions. We showed that during low-intensity exercise BFRT may trigger large reflex increases in blood pressure and sympathetic activity due to muscle metaboreflex activation. Thus, we urge caution when employing BFRT, especially in patients in whom exaggerated cardiovascular responses may occur that could cause sudden, adverse cardiovascular events.
INTRODUCTION
Mechanisms to improve the training benefits of exercise have long been sought by individuals recovering from injury, those with reduced mobility and range of motion, as well as individuals looking for innovative ways to maximize the potential benefits of regular exercise without an increase in training duration and/or intensity. One such mechanism that has been shown to improve muscle adaptation (1–13) and potentially provide cardiovascular benefit (2, 14–18) is termed blood flow restriction training (BFRT), also known as the Kaatsu method. This technique involves reducing blood flow to exercising skeletal muscle during exercise via a tourniquet or inflatable exterior cuff. The overarching premise is that through reducing blood flow to the exercising muscle, the threshold to reach fatigue is reduced because of accelerated accumulation of metabolic by-products of exercise. This simple blood flow manipulation technique has been shown to produce significant enhancements in muscle mass, muscle function (1–11, 19–22), and potentially cardiovascular performance after exercise (2, 16, 18, 23–25). Potential molecular mechanisms of muscle and vascular adaptation include activation of mammalian target of rapamycin (mTOR) and MAPK pathways, alterations in mitochondrial synthesis, angiogenesis, as well as alteration in muscle gene regulation (5–8, 11, 12, 23, 24, 26–29). Most BFRT studies lack hemodynamic measurements during the training interval itself and typically only assess cardiovascular function at baseline and after a period of exercise or during a period of recovery from the exercise bout during sustained ischemia at rest (2, 3, 12, 18, 23, 30, 31). Furthermore, a recent meta-analysis assessing cardiovascular hemodynamics before and after BFRT bouts concluded that no significant changes in cardiovascular function occurred immediately after a training event (32). However, no study including those in this meta-analysis has measured cardiovascular hemodynamics during the training bout itself, and thus it is unknown to what degree cardiovascular hemodynamics change during exercise. Evaluation of cardiovascular hemodynamics during BFRT is important because many studies have shown that ischemia of active skeletal muscle can induce a powerful blood pressure-raising reflex triggered by the accumulation of metabolic by-products that activate afferent neurons within the skeletal muscle, termed the muscle metaboreflex (33–67). Muscle metaboreflex activation can elicit profound increases in sympathetic nerve activity causing peripheral vasoconstriction including even constriction of the coronary vasculature, which thereby limits myocardial oxygen consumption and ventricular performance (49, 51, 68–71). These reflex changes in total peripheral resistance in normal healthy subjects induce a positive feedback loop that is likely self-limiting (39, 40, 72) wherein alternative mechanisms of sympathetic control, such as the arterial baroreflex, buffer muscle metaboreflex-induced increases in sympathetic activity (39–41, 51, 73). Conversely, in instances of cardiovascular disease such as heart failure and hypertension, muscle metaboreflex-induced increases in peripheral and coronary vascular resistance are significantly enhanced (49, 67, 69) and the ability of the arterial baroreflex to buffer muscle metaboreflex responses is attenuated (56, 57, 59, 67, 74–86). Combined, this acts to increase the positive feedback amplification of the metaboreflex, as the shift favoring vasoconstriction to increase mean arterial pressure cannot rectify the perfusion deficit caused by attenuated blood flow to ischemic cardiac and skeletal muscle. Thus, cardiac and skeletal muscle afferents are likely stimulated more than the initial response stimulus, and this leads to an even greater ischemia in cardiac and exercising muscle as exercise performance is maintained. In turn, this leads to an enhanced sympathetic response that engenders even greater vasoconstriction of those tissues provoking even greater sympathetic activation. The cycle continues, potentially initiating an uncontrolled positive feedback cycle, causing further and further increases in sympathetic activity that could lead to adverse cardiovascular outcomes including fatal arrythmia (39, 40, 72).
BFRT has been portrayed as beneficial for populations with limited mobility or limited exercise tolerance such as individuals with cardiovascular disease, aging, and incomplete spinal cord injury as well as other mobility-limiting pathophysiological states. However, the levels of muscle ischemia induced during BFRT also likely activate the muscle metaboreflex and thereby could induce profound increases in sympathetic activity in patients with cardiovascular disease. (38, 41, 42, 49, 53, 59, 62, 63, 66, 67, 69, 72, 78, 80, 87, 88). This heightened sympatho-activation could have serious adverse consequences including sudden cardiac death. Though the levels of ischemia in these studies have never been directly related to BFRT, they are of interest, as most of these studies elicited reductions in blood flow that would likely correlate with levels of ischemia observed during BFRT. Furthermore, many of the studies evaluating BFRT have been done in healthy populations, and although the previous studies related to muscle metaboreflex activation have demonstrated negative effects of ischemia on exercising muscle in various cardiovascular pathologies, there is currently no link between levels of BFRT that would induce muscle metaboreflex activation and the subsequent consequences during exercise. Thus, determining a link between BFRT and studies wherein hemodynamics can be measured during exercise with blood flow restriction with and without pathology is of great importance, enabling an assessment of whether the muscle metaboreflex is active during BFRT. Although the muscle metaboreflex has never been measured during BFRT, some studies have evaluated cardiovascular parameters after bouts of BFRT or during the recovery phase of postexercise muscle ischemia, a common method of evaluating the muscle metaboreflex in humans that is limited by the fact that the cardiovascular responses are observed during the recovery from exercise versus actually during the exercise, two settings of markedly different baseline autonomic activity (31, 36, 52, 88, 89). These studies found that the muscle metaboreflex responses before and after BFRT do not change in healthy subjects (30, 31). However, activation of the muscle metaboreflex before or after training is not nearly as concerning as the potential implications of activation during BFRT training, in which much larger pressor responses can occur (34, 35, 37–39, 41, 42, 44, 51–54, 57, 58, 60, 63, 65–67, 89–97). Thus, the goal of this study was to evaluate whether the muscle metaboreflex is active during mild treadmill exercise when a BFRT protocol is utilized.
METHODS
Seven (2 male, 5 female) adult mongrel canines of 18–25 kg were selected based on their willingness to volitionally walk on a motor-driven treadmill at 3.2 km/h, 0% grade for this study. Previously we have not found any significant difference in muscle metaboreflex characteristics regardless of sex (98). All animals in this study underwent a minimum 2-wk acclimation period with laboratory space and personnel before engaging in any part of the protocols or volitional exercise used in this study. All surgical and experimental procedures outlined in this study were approved by and comply with the Wayne State University Institutional Animal Care and Use Committee (IUCAC) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, respectively.
Surgical Instrumentation
All anesthetic, analgesic, and surgical protocols utilized in this study have been documented in previous studies (39–42, 49, 57–60, 67, 72, 88, 89). The specific regimen for this study is outlined as follows: all animals in this study underwent two separate surgical anesthetic events using standard aseptic techniques wherein a minimum 14-day recovery period occurred between procedures and an additional minimum 14-day recovery period occurred before the initiation of any of the volitional exercise experiments utilized in this study. On the day of surgery, ∼30 min before induction of anesthesia, animals were given an intramuscular injection of acepromazine (0.2–0.5 mg/kg) for sedation. Anesthetic induction was achieved through intravenous administration of ketamine (5 mg/kg) and diazepam (0.2–0.3 mg/kg), and animals were anesthetically maintained pre- and intraoperatively with (1–3%) isoflurane gas. Preoperatively, all animals were administered carprofen (4.4 mg/kg iv) as well as buprenorphine (0.03 mg/kg im) and a fentanyl patch (50–125 µg/kg, 72 h, transdermal) for analgesia. The antibiotic cefazolin (30 mg/kg iv) was administered pre- and intraoperatively for acute infection prevention. Postoperatively, animals were administered acepromazine sedation (0.2–0.3 mg/kg iv) and additional analgesic buprenorphine (0.01–0.03 im) as needed, in consultation with on-call veterinarians. All animals were administered prophylactic antibiotic cefalexin (30 mg/kg orally) for the duration of the protocol to prevent surgical site infections.
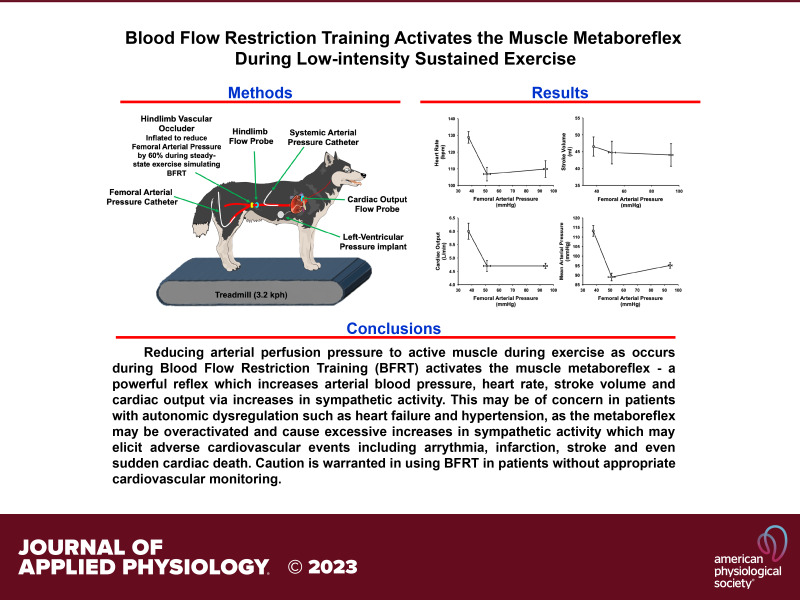
In the first surgical procedure, a left thoracotomy was performed through a vertical incision at the 3rd or 4th intercostal space, in which the pericardium was incised horizontally to expose the apex and ascending aorta. At the apex, a telemetric pressure sensor (Data Sciences International, St. Paul, MN) was placed for measurements of left ventricular pressure. At the ascending aorta, tissue was dissected such that placement of a 20 PAU flow probe (Transonic Systems, Ithaca, NY) to measure cardiac output could be performed. Unrelated to this study, four 0-Flexon steel pacing leads (Ethicon, Summerville, NJ) were placed on the right ventricular free wall epicardium of each animal for induction of heart failure. After placement of the hemodynamic monitoring devices and leads, the pericardium and ribs were reapproximated and the chest was closed in layers. The flow probe cable and Flexon leads were tunneled subcutaneously and were exteriorized between the scapulae. All animals recovered for a minimum of 2 wk before the next surgical procedure.
The second procedure was an abdominal surgery with a retroperitoneal approach to access the terminal aorta, its branches, and the renal artery. The most cranial accessible lumbar artery branch of the terminal aorta was isolated, and a fluid 19-gauge polyvinyl catheter (Tygon, S54-HL; Norton Murdock Industrial Inc., Akron, OH) was placed and advanced above the renal arteries for measurements of systemic arterial pressure. Tissue surrounding the terminal aorta caudal to the catheter was dissected for placement of a 10 PAU flow probe (Transonic Systems) and two hydraulic vascular occluders (Holly Specially Products LLC, Petaluma, CA) for measurement and manipulation of hindlimb blood flow. The left renal artery was isolated, and the tissue surrounding it was dissected for placement of a 4 PSB flow probe (Transonic Systems) for measurements of renal blood flow unrelated to the present study. Finally, the most caudal accessible artery that was caudal to the hydraulic occluders was isolated for placement of a fluid 19-gauge polyvinyl catheter (Tygon, S54-HL; Norton Murdock Industrial Inc) and advanced into the right femoral artery for measurements of hindlimb blood pressure. The retroperitoneal approach was closed in layers, and all cables, catheters, and occluder lines were tunneled and exteriorized at the scapulae near the thoracotomy exit site. All animals recovered for a minimum of 14 days before initiation of any experiments.
Data Acquisition and Experimental Procedures
Before any experiments following the surgical procedures, animals recovered for a minimum of 14 days. Before each experiment, animals were given 10–20 min to acclimatize to the laboratory environment before being guided to the treadmill for connection to hemodynamic monitoring equipment. The fluid catheters were attached to separate pressure transducers (Transpac IV; ICU Medical, San Clemente, CA) for measurements of systemic and hindlimb blood pressure, respectively. Flow probe cables corresponding to the ascending aorta, terminal aorta, and renal artery were connected to their respective flow channels on a TS420 flowmeter (Transonic Systems) for measurements of cardiac output, hindlimb blood flow, and renal blood flow, respectively. The DSI telemetric pressure catheter was turned on and wirelessly connected with the data receiver (Data Sciences International) for measurements of left ventricular pressure. All data were collected continuously during the experiment and later analyzed with LabScribe acquisition and analysis software (iWorx, Dover, NH). For each experiment, measurements began at rest, during which the animals stood still on the treadmill for 3–5 min to achieve steady state. Next the treadmill was started and incrementally increased to a speed of 3.2 km/h with 0% incline over a period of 2–3 min. Animals maintained this speed for the duration of the experiment. Once at speed, animals acclimated for 3–5 min to achieve a steady state, where 1 min of data within this state was taken for analysis. Next while maintaining speed at 3.2 km/h, hindlimb blood flow was incrementally reduced via the hydraulic vascular occluders until a level of 50–30% of the initial hindlimb blood flow value was achieved. At each incremental reduction, animals maintained pace for 3–5 min to achieve steady state. One minute of data was taken during each incremental steady state for analysis including the final reduction, and no exercise bout exceeded 30–35 min. After the final data point for the experiment was taken, the hydraulic vascular occluders were released and normal blood flow was restored, the treadmill was stopped, and all hemodynamic monitoring devices were disconnected.
Data Analysis
Cardiac output, mean arterial pressure, left ventricular pressure, femoral arterial pressure, and hindlimb blood flow were measured and recorded continuously during the experiment. All other variables in this study were derived from waveforms of the above variables or calculated from the above variables. The left ventricular pressure waveform was used to derive heart rate; stroke volume was derived by dividing cardiac output in milliliters by heart rate. Nonischemic vascular conductance, an index of vasoconstriction that corrects for the drop in hindlimb perfusion by assessing the conductance of all vascular beds except the hindlimb, was calculated as (cardiac output − hindlimb blood flow) in milliliters divided by mean arterial pressure. Effective arterial elastance, an index of vascular load and vascular stiffness, was calculated as mean arterial pressure divided by stroke volume. Stroke work, an index of contractility, was calculated as stroke volume in liters divided by mean arterial pressure. Each variable individually was used to generate a linear regression with steady-state points taken from each hindlimb blood flow reduction plotted against the corresponding femoral arterial pressure. To determine the best percentage of hindlimb occlusion for statistical assessment, we generated a linear regression of the hindlimb blood flow values versus the femoral arterial pressure values at each steady state and determined that a 60% reduction in femoral arterial pressure from baseline yielded approximately a 50% reduction in hindlimb blood flow, which is on average the level required for muscle metaboreflex activation. Thus, for our assessments of the BFRT protocol we utilized an occlusion of pressure equal to a 60% reduction in hindlimb blood pressure. In humans, arterial occlusion pressure for cuff inflation is determined as 40–80% of the pressure required to occlude arterial flow. The typical arterial occlusion pressure outlined by Hughes et al. (135) showed that seated participants had on average an arterial occlusion pressure of ∼200 mmHg. Thus a 40% cuff pressure would be 80 mmHg, a 60% occlusion pressure would be 120 mmHg, and 80% would be 160 mmHg, noting that resting blood pressures for this study group were around 120 mmHg systolic and 70 mmHg diastolic, similar to what we see in our canine model. Our mean femoral pressure was on average 94.1 ± 1.4 mmHg; thus we on average reduced blood pressure by 57 mmHg at 60%. Therefore, we feel justified in utilizing our index as a BFRT application as the relative pressure generated to induce a reduction of 57 mmHg at the arteries is similar by our vascular occlusion method to what would likely be observed with an external cuff.
Statistical Analyses
All hemodynamic variables measured and calculated in this study are reported as means ± standard error. For statistical evaluations a Student’s paired t test was used to evaluate the differences between free-flow exercise and the corresponding hemodynamic variable value at 60% reduction in femoral arterial pressure. Statistical significance was determined with an α-level of P < 0.05.
RESULTS
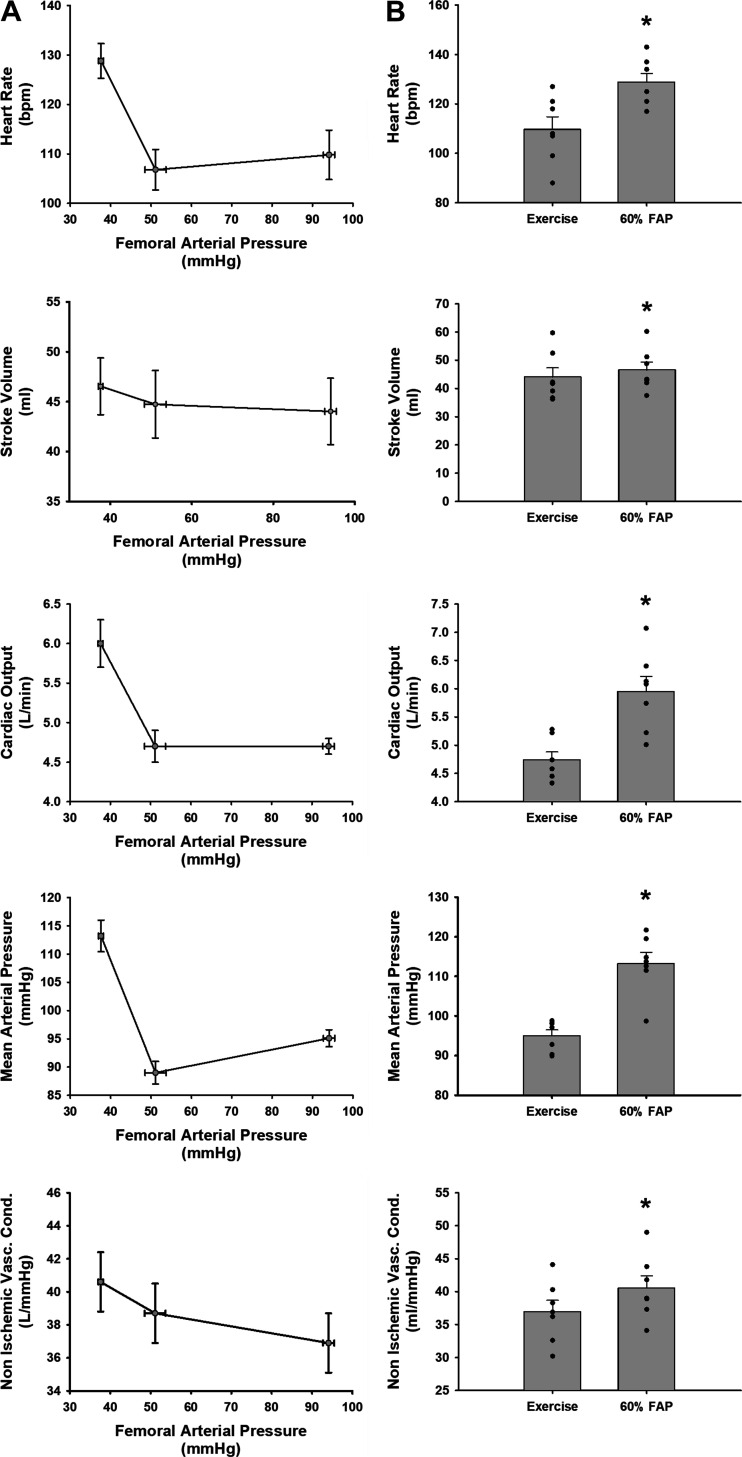
Figure 1A shows the 1-min average steady-state values of heart rate, stroke volume, cardiac output, nonischemic vascular conductance, and mean arterial pressure at free-flow exercise and muscle metaboreflex threshold or the point at which the muscle metaboreflex first becomes active and finally the corresponding value at a 60% reduction on femoral arterial pressure. Figure 1B shows the same data in a graphical format without the threshold value comparing only free-flow conditions to a 60% reduction in femoral arterial pressure. At a 60% reduction in femoral arterial pressure from baseline free-flow conditions we observed significant increases in heart rate, stroke volume, cardiac output, nonischemic vascular conductance, and mean arterial pressure.
Figure 1.
A: linear regressions of steady-state values during exercise and during exercise with 60% reduction in femoral arterial pressure. Graphs should be read right to left: the first point is exercise, the second point is the threshold where the given variable changes because of a reduction in femoral arterial pressure, and the furthest left point is the average peak value for a given variable at a 60% reduction in femoral arterial pressure during exercise. Error bars show SE of the mean in both directions. B: average 1-min steady-state values of heart rate, stroke volume, cardiac output, mean arterial pressure, and nonischemic vascular conductance during free-flow exercise (left bar) and during a 60% reduction in femoral arterial pressure (FAP) during exercise (right bar). Error bars show SE. *Significance vs. the previous setting was determined by a P < 0.05 (N = 7 animals, Student’s paired t test).
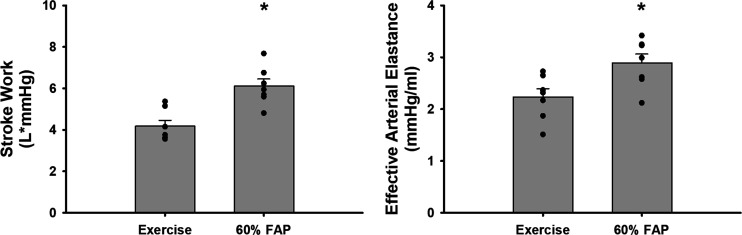
Figure 2 shows the 1-min average steady-state values in stroke work and index of contractility and effective arterial elastance, an index of vascular load and stiffness, at free-flow conditions and at a 60% reduction in femoral arterial pressure. At a 60% reduction in femoral arterial pressure, significant increases in stroke work and effective arterial elastance occurred, likely preserving ventricular vascular coupling.
Figure 2.
Average 1-min steady-state values of stroke work and index of ventricular contractility and effective arterial elastance, an index of vascular compliance, during free-flow exercise (left bar) and during a 60% reduction in femoral arterial pressure (FAP) during exercise (right bar). Error bars show SE. *Significance vs. the previous setting was determined by a P < 0.05 (N = 7 animals, Student’s paired t test).
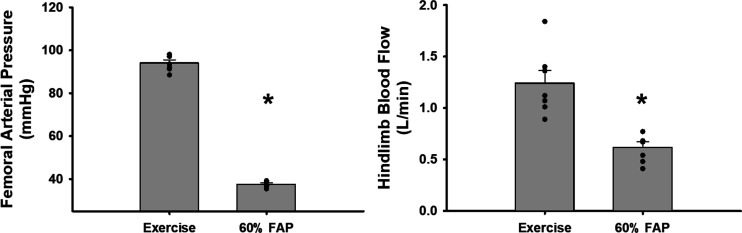
Figure 3 shows the 1-min average responses in hindlimb blood flow at a 60% reduction in femoral arterial pressure as well as the data points corresponding to femoral arterial pressure at free flow and the 60% reduction value. These data illustrate that a 60% reduction in femoral arterial pressure yields approximately a 50% reduction in hindlimb blood flow; thus this level of pressure reduction is comparable to previous studies in which hindlimb blood flow reduction was utilized to assess muscle metaboreflex characteristics.
Figure 3.
Average 1-min steady-state values of femoral arterial pressure (FAP) (left) and terminal aortic blood flow (right) illustrating that a 60% reduction in femoral arterial pressure approximates 50% reduction in hindlimb blood flow during free-flow exercise (left bar) and during a 60% reduction in femoral arterial pressure during exercise (right bar). Error bars show SE. *Significance vs. the previous setting was determined by a P < 0.05 (N = 7 animals, Student’s paired t test).
DISCUSSION
This is the first study to evaluate whether BFRT elicits activation of the muscle metaboreflex during a low-intensity sustained exercise bout. We observed that a 60% reduction in femoral artery pressure activates the muscle metaboreflex as evidenced by significant increases in heart rate, stroke volume, cardiac output, mean arterial pressure, stroke work, and effective arterial elastance. Furthermore, the vasodilation (as indexed by nonischemic vascular conductance) at this level of BFRT could potentially explain studies in which postexercise hypotension was observed (99). We have previously shown that peripheral vasodilation is due to reflex epinephrine release from the adrenal glands, which then activates vascular β2-adrenergic receptors, causing vasodilation (40). Overall, the level of muscle metaboreflex activation observed in this study is on par with previous studies in which the muscle metaboreflex was assessed by 50–60% reductions in hindlimb blood flow. Furthermore, a comparison of femoral pressure reduction and hindlimb blood flow confirms that by reducing femoral arterial pressure by 60% from baseline exercising levels hindlimb blood flow is reduced by ∼50%.
Although this study confirms muscle metaboreflex activation during BFRT, it does not take away the potential benefits that BFRT provides for young healthy subjects. Previous studies have shown that BFRT can induce positive training benefits at reduced workloads and training intervals. For instance, BFRT has been shown to provide the same benefits to muscle endurance, mass, and performance as traditional heavy resistance training, at a much lower workload (3, 9, 10, 20, 100–102). The benefits of BFRT are not limited to effects in muscle. BFRT, similarly to heavy resistance and endurance exercise, has been previously observed to induce its effects through activation of mammalian target of rapamycin (mTOR) (6, 27, 29, 103–106), a pathway associated with cellular growth and energy homeostasis that has been linked to various disease states. Activation of mTOR has been observed to have implications in cardiac hypertrophy (29, 103, 105, 107–109) and insulin signaling (6, 106, 107), both of which when dysregulated can lead to extensive lifelong disease and activation of the mTOR pathway counteracts this. In addition to mTOR activation, BFRT has been shown to improve angiogenesis through activation and upregulation of vascular endothelial growth factors 1 and 2 (VEGF 1 and 2) as well as hypoxia-inducible factor 1 alpha (HIF-1α) and nitric oxide synthase (NOS) (11, 28, 110). These additional benefits alongside the muscular adaptations provide a strong case for the use of BFRT training for healthy subjects with limited training times, those rehabilitating injury, and individuals without any underlying cardiovascular disease. However, many studies have shown that metaboreflex-induced sympathetic activation is exaggerated in heart failure, hypertension, diabetes, and peripheral vascular disease among other cardiovascular pathologies (38, 41, 42, 49, 53, 57, 64, 67, 69, 72, 80, 82, 87, 88, 111–116). In normal subjects, the increase in sympathetic tone with metaboreflex activation is partially buffered by the arterial baroreflex (79, 80, 86, 96, 117–119). However, in many cardiovascular diseases baroreflex function is impaired. This likely contributes to the amplified metaboreflex responses. We have shown that the ischemic muscle itself becomes a target for the increased sympathetic activity, which thereby elicits a positive feedback scenario that further increases sympathetic drive. This elevated sympathetic activity during exercise in heart failure and hypertension even vasoconstricts the coronary circulation (67, 69). This could lead to a serious mismatch between myocardial oxygen supply and oxygen demand that potentially could lead to sudden cardiac death. Since our study shows that BFRT does indeed cause substantial metaboreflex activation even during mild exercise, the use of this technique in patient populations in whom metaboreflex-induced sympathetic activation is known to be accentuated raises concern.
However, one aspect that remains relatively subjective in practice is what is known as arterial occlusion pressure (AOP) or the stimulus that generates the ischemia required to gain the benefits of BFRT. The current recommendation for the AOP required to initiate the benefits of BFRT is between 40% and 80% (1, 4, 9, 100–102, 110, 120, 121). This is, however, also dependent on several factors such as external cuff size, resting blood pressure, and cuff placement, i.e., limb placement. Individuals who use larger cuffs may induce levels of ischemia under the cuff that are detrimental (122). Furthermore, individuals starting at a higher resting blood pressure will inherently require a greater level of AOP to achieve the recommended 40–80% relative to individuals of lower blood pressure who may be more fit. Finally, concerning cuff placement, recommendations shift on the level of AOP relative to either the arm, upper leg, or lower leg, depending on the region being trained, and even then some groups advocate that the greater the ischemic stimulus (60–80% AOP) the greater the benefits (120). Thus, although there are numerous benefits of utilizing BFRT, the mechanisms or guidelines by which those benefits are attained in normal healthy individuals require further assessment. In this study an assessment of multiple AOPs was not evaluated; however, based on previous work observing the trend of muscle metaboreflex activation, increased ischemia progressively increases the extent of muscle metaboreflex activation (34, 35, 37–41, 49, 63, 72). Furthermore, as workload rises toward maximum and the ability to increase cardiac output becomes limited, characteristics of the muscle metaboreflex begin to shift toward more and more peripheral vasoconstriction including constriction of the coronary vasculature and even the active skeletal muscle. (34, 38–40, 44, 49, 51, 57, 62, 69, 72, 87). In disease states such as heart failure, hypertension, and potentially other conditions such as diabetes and metabolic syndrome, in which ventricular function is compromised, this shift in muscle metaboreflex mechanisms happens at even the lowest workloads as a result of enhanced sympathetic activity at rest as well as an inability to adequately buffer muscle metaboreflex-induced sympathetic responses during mild and moderate exercise (56–58, 67, 76, 79, 80, 82, 84, 86, 89, 96, 123–132). Thus, use of BFRT in sympathetically dysregulated populations is potentially unsafe even under supervision, as each patient’s relative sympathetic regulatory state is likely unknown.
Perspectives and Conclusions
Previously calls for concern have arisen regarding whether BFRT is safe in nonhealthy populations such as those with heart failure, hypertension, and peripheral artery disease (30, 122, 133, 134). On the basis of the evidence from this study, we believe those calls for concern to be valid, as we observed significant muscle metaboreflex activation at what is a mild level of blood flow restriction training (60% AOP at 3.2 km/h). If this level of training was performed in patients with heart failure, the muscle metaboreflex would undoubtedly be active, as we have previously shown that in heart failure lower levels of occlusion are required to activate the muscle metaboreflex and at moderate workloads blood flow is already below the threshold level for muscle metaboreflex activation seen in normal subjects (35). Thus, we urge caution in using BFRT even in healthy subjects in whom it appears most, if not all, of the benefits may come without a major cardiovascular event if performed correctly. Our data demonstrate that mimicking BFRT by imposing a 60% reduction in femoral arterial pressure during low-intensity exercise elicits substantial increases in cardiovascular hemodynamics, including mean arterial pressure (∼20 mmHg), heart rate (∼20 beats/min), and cardiac output (∼1.2 L/min) in healthy subjects. However, as it stands now, the guidelines by which a BFRT regimen is practiced are to a degree still under debate, as most previous studies have not assessed changes in cardiovascular hemodynamics during the exercise bout. Thus, further studies in clinical populations are required to investigate the magnitude of cardiovascular responses during BFRT in order to establish the safety of this training technique. Until this is known, caution is warranted for the use of BFRT in patient populations.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-55473, HL-126706, and HL-120822.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M., M.S., and D.S.O’L. conceived and designed research; J.M., M.-H.A., J.K., B.L., A.A., L.M., K.A., M.S., and D.S.O’L. performed experiments; J.M., M.-H.A., J.K., B.L., A.A., L.M., K.A., M.S., and D.S.O’L. analyzed data; J.M., J.K., M.S., and D.S.O’L. interpreted results of experiments; J.M. and D.S.O’L. prepared figures; J.M. and D.S.O’L. drafted manuscript; J.M., J.K., M.S., and D.S.O’L. edited and revised manuscript; J.M., M.-H.A., J.K., B.L., A.A., L.M., K.A., M.S., and D.S.O’L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Audrey Nelson for expert technical assistance and animal care.
REFERENCES
- 1. Barbalho M, Rocha AC, Seus TL, Raiol R, Del Vecchio FB, Coswig VS. Addition of blood flow restriction to passive mobilization reduces the rate of muscle wasting in elderly patients in the intensive care unit: a within-patient randomized trial. Clin Rehabil 33: 233–240, 2019. doi: 10.1177/0269215518801440. [DOI] [PubMed] [Google Scholar]
- 2. Bennett H, Slattery F. Effects of blood flow restriction training on aerobic capacity and performance: a systematic review. J Strength Cond Res 33: 572–583, 2019. doi: 10.1519/JSC.0000000000002963. [DOI] [PubMed] [Google Scholar]
- 3. Cook SB, Brown KA, Deruisseau K, Kanaley JA, Ploutz-Snyder LL. Skeletal muscle adaptations following blood flow-restricted training during 30 days of muscular unloading. J Appl Physiol (1985) 109: 341–349, 2010. doi: 10.1152/japplphysiol.01288.2009. [DOI] [PubMed] [Google Scholar]
- 4. Dankel SJ, Jessee MB, Abe T, Loenneke JP. The effects of blood flow restriction on upper-body musculature located distal and proximal to applied pressure. Sports Med 46: 23–33, 2016. doi: 10.1007/s40279-015-0407-7. [DOI] [PubMed] [Google Scholar]
- 5. Davids CJ, Næss TC, Moen M, Cumming KT, Horwath O, Psilander N, Ekblom B, Coombes JS, Peake J, Raastad T, Roberts LA. Acute cellular and molecular responses and chronic adaptations to low-load blood flow restriction and high-load resistance exercise in trained individuals. J Appl Physiol (1985) 131: 1731–1749, 2021. doi: 10.1152/japplphysiol.00464.2021. [DOI] [PubMed] [Google Scholar]
- 6. Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol (1985) 108: 1199–1209, 2010. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol (1985) 103: 903–910, 2007. [Erratum in J Appl Physiol 104: 1256, 2008]. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 8. Laurentino GC, Ugrinowitsch C, Roschel H, Aoki MS, Soares AG, Neves M Jr, Aihara AY, Fernandes AR, Tricoli V. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc 44: 406–412, 2012. doi: 10.1249/MSS.0b013e318233b4bc. [DOI] [PubMed] [Google Scholar]
- 9. Lowery RP, Joy JM, Loenneke JP, de Souza EO, Machado M, Dudeck JE, Wilson JM. Practical blood flow restriction training increases muscle hypertrophy during a periodized resistance training programme. Clin Physiol Funct Imaging 34: 317–321, 2014. doi: 10.1111/cpf.12099. [DOI] [PubMed] [Google Scholar]
- 10. Miller BC, Tirko AW, Shipe JM, Sumeriski OR, Moran K. The systemic effects of blood flow restriction training: a systematic review. Int J Sports Phys Ther 16: 978–990, 2021. doi: 10.26603/001c.25791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patterson SD, Leggate M, Nimmo MA, Ferguson RA. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol 113: 713–719, 2013. doi: 10.1007/s00421-012-2479-5. [DOI] [PubMed] [Google Scholar]
- 12. Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med 45: 187–200, 2015. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- 13. Vanwye WR, Weatherholt AM, Mikesky AE. Blood flow restriction training: implementation into clinical practice. Int J Exerc Sci 10: 649–654, 2017. [PMC free article] [PubMed] [Google Scholar]
- 14. Barbosa JB, Maia TO, Alves PS, Bezerra SD, Moura EC, Medeiros AI, Fuzari HK, Rocha LG, Marinho PE. Does blood flow restriction training increase the diameter of forearm vessels in chronic kidney disease patients? A randomized clinical trial. J Vasc Access 19: 626–633, 2018. doi: 10.1177/1129729818768179. [DOI] [PubMed] [Google Scholar]
- 15. Cerqueira MS, Costa EC, Santos Oliveira R, Pereira R, Brito Vieira WH. Blood flow restriction training: to adjust or not adjust the cuff pressure over an intervention period? Front Physiol 12: 678407, 2021. doi: 10.3389/fphys.2021.678407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Credeur DP, Jones R, Stanford D, Stoner L, McCoy S, Jessee M. Central cardiovascular hemodynamic response to unilateral handgrip exercise with blood flow restriction. Eur J Appl Physiol 119: 2255–2263, 2019. doi: 10.1007/s00421-019-04209-3. [DOI] [PubMed] [Google Scholar]
- 17. Picón MM, Chulvi IM, Cortell JT, Tortosa J, Alkhadar Y, Sanchís J, Laurentino G. Acute cardiovascular responses after a single bout of blood flow restriction training. Int J Exerc Sci 11: 20–31, 2018. [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao Y, Lin A, Jiao L. Eight weeks of resistance training with blood flow restriction improve cardiac function and vascular endothelial function in healthy young Asian males. Int Health 13: 471–479, 2021. doi: 10.1093/inthealth/ihaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorenz DS, Bailey L, Wilk KE, Mangine RE, Head P, Grindstaff TL, Morrison S. Blood flow restriction training. J Athl Train 56: 937–944, 2021. doi: 10.4085/418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandner CR, Clarkson MJ, Kidgell DJ, Warmington SA. Muscular adaptations to whole body blood flow restriction training and detraining. Front Physiol 10: 1099, 2019. doi: 10.3389/fphys.2019.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Early KS, Rockhill M, Bryan A, Tyo B, Buuck D, McGinty J. Effect of blood flow restriction training on muscular performance, pain and vascular function. Int J Sports Phys Ther 15: 892–900, 2020. doi: 10.26603/ijspt20200892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Minniti MC, Statkevich AP, Kelly RL, Rigsby VP, Exline MM, Rhon DI, Clewley D. The safety of blood flow restriction training as a therapeutic intervention for patients with musculoskeletal disorders: a systematic review. Am J Sports Med 48: 1773–1785, 2020. doi: 10.1177/0363546519882652. [DOI] [PubMed] [Google Scholar]
- 23. Naderi-Boldaji V, Joukar S, Noorafshan A, Raji-Amirhasani A, Naderi-Boldaji S, Bejeshk MA. The effect of blood flow restriction along with low-intensity exercise on cardiac structure and function in aging rat: role of angiogenesis. Life Sci 209: 202–209, 2018. doi: 10.1016/j.lfs.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 24. Horiuchi M, Okita K. Blood flow restricted exercise and vascular function. Int J Vasc Med 2012: 543218, 2012. doi: 10.1155/2012/543218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferreira Junior A, Schamne JC, Altimari LR, Okano AH, Okuno NM. Effect of walk training combined with blood flow restriction on resting heart rate variability and resting blood pressure in middle-aged men. Motriz 25: 2, 2019. doi: 10.1590/s1980-6574201900020005. [DOI] [Google Scholar]
- 26. Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 40: 691–698, 2008. [Erratum in Med Sci Sports Exerc 40: 1191, 2008]. doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gundermann DM, Walker DK, Reidy PT, Borack MS, Dickinson JM, Volpi E, Rasmussen BB. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 306: E1198–E1204, 2014. doi: 10.1152/ajpendo.00600.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc 44: 2077–2083, 2012. doi: 10.1249/MSS.0b013e3182625928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakajima T, Yasuda T, Koide S, Yamasoba T, Obi S, Toyoda S, Sato Y, Inoue T, Kano Y. Repetitive restriction of muscle blood flow enhances mTOR signaling pathways in a rat model. Heart Vessels 31: 1685–1695, 2016. doi: 10.1007/s00380-016-0801-6. [DOI] [PubMed] [Google Scholar]
- 30. Cristina-Oliveira M, Meireles K, Spranger MD, O’Leary DS, Roschel H, Peçanha T. Clinical safety of blood flow-restricted training? A comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. Am J Physiol Heart Circ Physiol 318: H90–H109, 2020. doi: 10.1152/ajpheart.00468.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crisafulli A, de Farias RR, Farinatti P, Lopes KG, Milia R, Sainas G, Pinna V, Palazzolo G, Doneddu A, Magnani S, Mulliri G, Roberto S, Oliveira RB. Blood flow restriction training reduces blood pressure during exercise without affecting metaboreflex activity. Front Physiol 9: 1736, 2018. doi: 10.3389/fphys.2018.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lemos LK, Toledo Teixeira Filho CA, Biral TM, de Souza Cavina AP, Junior EP, Oliveira Damasceno S, Vanderlei FM. Acute effects of resistance exercise with blood flow restriction on cardiovascular response: a meta-analysis. J Comp Eff Res 11: 829–842, 2022. doi: 10.2217/cer-2021-0272. [DOI] [PubMed] [Google Scholar]
- 33. Ansorge EJ, Shah SH, Augustyniak RA, Rossi NF, Collins HL, O'Leary DS. Muscle metaboreflex control of coronary blood flow. Am J Physiol Heart Circ Physiol 283: H526–H532, 2002. doi: 10.1152/ajpheart.00152.2002. [DOI] [PubMed] [Google Scholar]
- 34. Augustyniak RA, Ansorge EJ, O’Leary DS. Muscle metaboreflex control of cardiac output and peripheral vasoconstriction exhibit different latencies. Am J Physiol Heart Circ Physiol 278: H530–H537, 2000. doi: 10.1152/ajpheart.2000.278.2.H530. [DOI] [PubMed] [Google Scholar]
- 35. Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O’Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- 36. Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011. doi: 10.1007/s12576-011-0163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- 38. Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 39. Kaur J, Machado TM, Alvarez A, Krishnan AC, Hanna HW, Altamimi YH, Senador D, Spranger MD, O’Leary DS. Muscle metaboreflex activation during dynamic exercise vasoconstricts ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 309: H2145–H2151, 2015. doi: 10.1152/ajpheart.00679.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O’Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in beta2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mannozzi J, Kaur J, Spranger MD, Al-Hassan MH, Lessanework B, Alvarez A, Chung CS, O’Leary DS. Muscle Metaboreflex-induced increases in effective arterial elastance: effect of heart failure. Am J Physiol Regul Integr Comp Physiol 319: R1–R10, 2020. doi: 10.1152/ajpregu.00040.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mannozzi J, Massoud L, Kaur J, Coutsos M, O’Leary DS. Ventricular contraction and relaxation rates during muscle metaboreflex activation in heart failure: are they coupled? Exp Physiol 106: 401–411, 2021. doi: 10.1113/EP089053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O’Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998. doi: 10.1152/ajpheart.1998.275.1.H220. [DOI] [PubMed] [Google Scholar]
- 44. Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O’Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 45. Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol (1985) 82: 1811–1817, 1997. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- 46. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 47. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol Respir Environ Exerc Physiol 57: 644–650, 1984. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- 48. Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985) 64: 2306–2313, 1988. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- 49. Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O’Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005. doi: 10.1152/ajpheart.00985.2004. [DOI] [PubMed] [Google Scholar]
- 50. Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199: 367–383, 2010. doi: 10.1111/j.1748-1716.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 51. Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O’Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol (1985) 109: 271–278, 2010. doi: 10.1152/japplphysiol.01243.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Crisafulli A, Salis E, Pittau G, Lorrai L, Tocco F, Melis F, Pagliaro P, Concu A. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am J Physiol Heart Circ Physiol 291: H3035–H3042, 2006. doi: 10.1152/ajpheart.00221.2006. [DOI] [PubMed] [Google Scholar]
- 53. Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 54. O’Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. doi: 10.1152/ajpheart.1995.268.3.H980. [DOI] [PubMed] [Google Scholar]
- 55. Ichinose M, Ichinose-Kuwahara T, Kondo N, Nishiyasu T. Increasing blood flow to exercising muscle attenuates systemic cardiovascular responses during dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol 309: R1234–R1242, 2015. doi: 10.1152/ajpregu.00063.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ichinose M, Sala-Mercado JA, O’Leary DS, Hammond RL, Coutsos M, Ichinose T, Pallante M, Iellamo F. Spontaneous baroreflex control of cardiac output during dynamic exercise, muscle metaboreflex activation, and heart failure. Am J Physiol Heart Circ Physiol 294: H1310–H1316, 2008. doi: 10.1152/ajpheart.01187.2007. [DOI] [PubMed] [Google Scholar]
- 57. Mannozzi J, Kim JK, Sala-Mercado JA, Al-Hassan MH, Lessanework B, Alvarez A, Massoud L, Bhatti T, Aoun K, O’Leary DS. Arterial baroreflex inhibits muscle metaboreflex induced increases in effective arterial elastance: implications for ventricular-vascular coupling. Front Physiol 13: 841076, 2022. doi: 10.3389/fphys.2022.841076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaur J, Alvarez A, Hanna HW, Krishnan AC, Senador D, Machado TM, Altamimi YH, Lovelace AT, Dombrowski MD, Spranger MD, O’Leary DS. Interaction between the muscle metaboreflex and the arterial baroreflex in control of arterial pressure and skeletal muscle blood flow. Am J Physiol Heart Circ Physiol 311: H1268–H1276, 2016. doi: 10.1152/ajpheart.00501.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kaur J, Krishnan AC, Senador D, Alvarez A, Hanna HW, O’Leary DS. Altered arterial baroreflex-muscle metaboreflex interaction in heart failure. Am J Physiol Heart Circ Physiol 315: H1383–H1392, 2018. doi: 10.1152/ajpheart.00338.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mannozzi J, Al-Hassan MH, Lessanework B, Alvarez A, Senador D, O’Leary DS. Chronic ablation of TRPV1-sensitive skeletal muscle afferents attenuates the muscle metaboreflex. Am J Physiol Regul Integr Comp Physiol 321: R385–R395, 2021. doi: 10.1152/ajpregu.00129.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol (1985) 74: 1748–1754, 1993. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 62. O’Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol Heart Circ Physiol 287: H2612–H2618, 2004. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 63. O’Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol (1985) 103: 190–194, 2007. doi: 10.1152/japplphysiol.00139.2007. [DOI] [PubMed] [Google Scholar]
- 64. O’Leary DS, Senador D, Augustyniak RA. Muscle metaboreflex-induced central blood volume mobilization in heart failure. Am J Physiol Heart Circ Physiol 316: H1047–H1052, 2019. doi: 10.1152/ajpheart.00805.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Senador D, Kaur J, Alvarez A, Hanna HW, Krishnan AC, Altamimi YH, O’Leary DS. Role of endothelial nitric oxide in control of peripheral vascular conductance during muscle metaboreflex activation. Am J Physiol Regul Integr Comp Physiol 313: R29–R34, 2017. doi: 10.1152/ajpregu.00515.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shoemaker JK, Kunselman AR, Silber DH, Sinoway LI. Maintained exercise pressor response in heart failure. J Appl Physiol (1985) 85: 1793–1799, 1998. doi: 10.1152/jappl.1998.85.5.1793. [DOI] [PubMed] [Google Scholar]
- 67. Spranger MD, Kaur J, Sala-Mercado JA, Krishnan AC, Abu-Hamdah R, Alvarez A, Machado TM, Augustyniak RA, O’Leary DS. Exaggerated coronary vasoconstriction limits muscle metaboreflex-induced increases in ventricular performance in hypertension. Am J Physiol Heart Circ Physiol 312: H68–H79, 2017. doi: 10.1152/ajpheart.00417.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ahmet I, Lakatta EG, Talan MI. Pharmacological stimulation of beta2-adrenergic receptors (beta2AR) enhances therapeutic effectiveness of beta1AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev 10: 289–296, 2005. doi: 10.1007/s10741-005-7543-3. [DOI] [PubMed] [Google Scholar]
- 69. Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O’Leary DS. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol 304: H1029–H1037, 2013. doi: 10.1152/ajpheart.00879.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Feigl EO. Control of myocardial oxygen tension by sympathetic coronary vasoconstriction in the dog. Circ Res 37: 88–95, 1975. doi: 10.1161/01.res.37.1.88. [DOI] [PubMed] [Google Scholar]
- 71. Gwirtz PA, Dodd-O JM, Brandt MA, Jones CE. Augmentation of coronary flow improves myocardial function in exercise. J Cardiovasc Pharmacol 15: 752–758, 1990. doi: 10.1097/00005344-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 72. Kaur J, Senador D, Krishnan AC, Hanna HW, Alvarez A, Machado TM, O’Leary DS. Muscle metaboreflex-induced vasoconstriction in the ischemic active muscle is exaggerated in heart failure. Am J Physiol Heart Circ Physiol 314: H11–H18, 2018. doi: 10.1152/ajpheart.00375.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mannozzi J, Al-Hassan MH, Kaur J, Lessanework B, Alvarez A, Massoud L, Bhatti T, O’Leary DS. Ventricular-vascular uncoupling in heart failure: effects of arterial baroreflex-induced sympathoexcitation at rest and during exercise. Front Physiol 13: 835951, 2022. doi: 10.3389/fphys.2022.835951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Al-Khateeb AA, Limberg JK, Barnes JN, Joyner MJ, Charkoudian N, Curry TB. Acute cyclooxygenase inhibition and baroreflex sensitivity in lean and obese adults. Clin Auton Res 27: 17–23, 2017. doi: 10.1007/s10286-016-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fadel PJ. Reflex control of the circulation during exercise. Scand J Med Sci Sports 25, Suppl 4: 74–82, 2015. doi: 10.1111/sms.12600. [DOI] [PubMed] [Google Scholar]
- 76. Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension 45: 1173–1181, 2005. [Erratum in Hypertension 46: e25, 2005]. doi: 10.1161/01.HYP.0000168056.66981.c2. [DOI] [PubMed] [Google Scholar]
- 77. Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588: 1515–1525, 2010. doi: 10.1113/jphysiol.2009.186387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ichinose M, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, Fano D, O’Leary DS. Dynamic cardiac output regulation at rest, during exercise, and muscle metaboreflex activation: impact of congestive heart failure. Am J Physiol Regul Integr Comp Physiol 303: R757–R768, 2012. doi: 10.1152/ajpregu.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim JK, Augustyniak RA, Sala-Mercado JA, Hammond RL, Ansorge EJ, Rossi NF, O’Leary DS. Heart failure alters the strength and mechanisms of arterial baroreflex pressor responses during dynamic exercise. Am J Physiol Heart Circ Physiol 287: H1682–H1688, 2004. doi: 10.1152/ajpheart.00358.2004. [DOI] [PubMed] [Google Scholar]
- 80. Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O’Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol 289: H2416–H2423, 2005. doi: 10.1152/ajpheart.00654.2005. [DOI] [PubMed] [Google Scholar]
- 81. O’Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- 82. O’Leary DS, Mannozzi J, Augustyniak RA, Ichinose M, Spranger MD. Hypertension depresses arterial baroreflex control of both heart rate and cardiac output during rest, exercise, and metaboreflex activation. Am J Physiol Regul Integr Comp Physiol 323: R720–R727, 2022. doi: 10.1152/ajpregu.00093.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Olivier NB, Stephenson RB. Characterization of baroreflex impairment in conscious dogs with pacing-induced heart failure. Am J Physiol Regul Integr Comp Physiol 265: R1132–R1140, 1993. doi: 10.1152/ajpregu.1993.265.5.R1132. [DOI] [PubMed] [Google Scholar]
- 85. Osterziel KJ, Hänlein D, Willenbrock R, Eichhorn C, Luft F, Dietz R. Baroreflex sensitivity and cardiovascular mortality in patients with mild to moderate heart failure. Br Heart J 73: 517–522, 1995. doi: 10.1136/hrt.73.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sheriff DD, O’Leary DS, Scher AM, Rowell LB. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am J Physiol Heart Circ Physiol 258: H305–H310, 1990. doi: 10.1152/ajpheart.1990.258.2.H305. [DOI] [PubMed] [Google Scholar]
- 87. Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O’Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2007. doi: 10.1152/ajpheart.01240.2006. [DOI] [PubMed] [Google Scholar]
- 88. Spranger MD, Kaur J, Sala-Mercado JA, Machado TM, Krishnan AC, Alvarez A, O’Leary DS. Attenuated muscle metaboreflex-induced pressor response during postexercise muscle ischemia in renovascular hypertension. Am J Physiol Regul Integr Comp Physiol 308: R650–R658, 2015. doi: 10.1152/ajpregu.00464.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Spranger MD, Sala-Mercado JA, Coutsos M, Kaur J, Stayer D, Augustyniak RA, O’Leary DS. Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. Am J Physiol Regul Integr Comp Physiol 304: R657–R663, 2013. doi: 10.1152/ajpregu.00601.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise. Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987. doi: 10.1152/ajpheart.1987.253.5.H1199. [DOI] [PubMed] [Google Scholar]
- 91. Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. doi: 10.1152/ajpheart.1983.245.3.H481. [DOI] [PubMed] [Google Scholar]
- 92. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Campos MO, Mansur DE, Mattos JD, Paiva AC, Videira RL, Macefield VG, da Nóbrega AC, Fernandes IA. Acid-sensing ion channels blockade attenuates pressor and sympathetic responses to skeletal muscle metaboreflex activation in humans. J Appl Physiol (1985) 127: 1491–1501, 2019. doi: 10.1152/japplphysiol.00401.2019. [DOI] [PubMed] [Google Scholar]
- 94. Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJ, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003. doi: 10.1249/01.MSS.0000048639.02548.24. [DOI] [PubMed] [Google Scholar]
- 95. Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306: H132–H141, 2014. doi: 10.1152/ajpheart.00575.2013. [DOI] [PubMed] [Google Scholar]
- 96. Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O’Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005. doi: 10.1152/ajpheart.01040.2004. [DOI] [PubMed] [Google Scholar]
- 97. Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol (1985) 103: 228–233, 2007. doi: 10.1152/japplphysiol.01334.2006. [DOI] [PubMed] [Google Scholar]
- 98. Laprad SL, Augustyniak RA, Hammond RL, O’Leary DS. Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp Physiol 276: R1203–R1208, 1999. doi: 10.1152/ajpregu.1999.276.4.R1203. [DOI] [PubMed] [Google Scholar]
- 99. Neto GR, Sousa MS, Costa PB, Salles BF, Novaes GS, Novaes JS. Hypotensive effects of resistance exercises with blood flow restriction. J Strength Cond Res 29: 1064–1070, 2015. doi: 10.1519/JSC.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 100. Fahs CA, Loenneke JP, Thiebaud RS, Rossow LM, Kim D, Abe T, Beck TW, Feeback DL, Bemben DA, Bemben MG. Muscular adaptations to fatiguing exercise with and without blood flow restriction. Clin Physiol Funct Imaging 35: 167–176, 2015. doi: 10.1111/cpf.12141. [DOI] [PubMed] [Google Scholar]
- 101. Jessee MB, Buckner SL, Mouser JG, Mattocks KT, Dankel SJ, Abe T, Bell ZW, Bentley JP, Loenneke JP. Muscle adaptations to high-load training and very low-load training with and without blood flow restriction. Front Physiol 9: 1448, 2018. doi: 10.3389/fphys.2018.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Loenneke JP, Fahs CA, Rossow LM, Abe T, Bemben MG. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses 78: 151–154, 2012. doi: 10.1016/j.mehy.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 103. Gundermann DM, Fry CS, Dickinson JM, Walker DK, Timmerman KL, Drummond MJ, Volpi E, Rasmussen BB. Reactive hyperemia is not responsible for stimulating muscle protein synthesis following blood flow restriction exercise. J Appl Physiol (1985) 112: 1520–1528, 2012. doi: 10.1152/japplphysiol.01267.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ogasawara R, Jensen TE, Goodman CA, Hornberger TA. Resistance exercise-induced hypertrophy: a potential role for rapamycin-insensitive mTOR. Exerc Sport Sci Rev 47: 188–194, 2019. doi: 10.1249/JES.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Watson K, Baar K. mTOR and the health benefits of exercise. Semin Cell Dev Biol 36: 130–139, 2014. doi: 10.1016/j.semcdb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 106. Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients 9: 1176, 2017. doi: 10.3390/nu9111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Medeiros C, Frederico MJ, da Luz G, Pauli JR, Silva AS, Pinho RA, Velloso LA, Ropelle ER, De Souza CT. Exercise training reduces insulin resistance and upregulates the mTOR/p70S6k pathway in cardiac muscle of diet-induced obesity rats. J Cell Physiol 226: 666–674, 2011. doi: 10.1002/jcp.22387. [DOI] [PubMed] [Google Scholar]
- 108. Pelozin BR, Soci UP, Gomes JL, Oliveira EM, Fernandes T. mTOR signaling-related microRNAs as cardiac hypertrophy modulators in high-volume endurance training. J Appl Physiol (1985) 132: 126–139, 2022. doi: 10.1152/japplphysiol.00881.2020. [DOI] [PubMed] [Google Scholar]
- 109. Schüttler D, Clauss S, Weckbach LT, Brunner S. Molecular mechanisms of cardiac remodeling and regeneration in physical exercise. Cells 8: 1128, 2019. doi: 10.3390/cells8101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ferguson RA, Hunt JE, Lewis MP, Martin NRW, Player DJ, Stangier C, Taylor CW, Turner MC. The acute angiogenic signalling response to low-load resistance exercise with blood flow restriction. Eur J Sport Sci 18: 397–406, 2018. doi: 10.1080/17461391.2017.1422281. [DOI] [PubMed] [Google Scholar]
- 111. De Angelis KL, Oliveira AR, Dall’Ago P, Peixoto LR, Gadonski G, Lacchini S, Fernandes TG, Irigoyen MC. Effects of exercise training on autonomic and myocardial dysfunction in streptozotocin-diabetic rat. Braz J Med Biol Res 33: 635–641, 2000. doi: 10.1590/s0100-879x2000000600004. [DOI] [PubMed] [Google Scholar]
- 112. Kim HK, Hotta N, Ishizawa R, Iwamoto GA, Vongpatanasin W, Mitchell JH, Smith SA, Mizuno M. Exaggerated pressor and sympathetic responses to stimulation of the mesencephalic locomotor region and exercise pressor reflex in type 2 diabetic rats. Am J Physiol Regul Integr Comp Physiol 317: R270–R279, 2019. doi: 10.1152/ajpregu.00061.2019. [DOI] [PubMed] [Google Scholar]
- 113. Grotle AK, Garcia EA, Huo Y, Stone AJ. Temporal changes in the exercise pressor reflex in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 313: H708–H714, 2017. doi: 10.1152/ajpheart.00399.2017. [DOI] [PubMed] [Google Scholar]
- 114. Grotle AK, Stone AJ. Exaggerated exercise pressor reflex in type 2 diabetes: potential role of oxidative stress. Auton Neurosci 222: 102591, 2019. doi: 10.1016/j.autneu.2019.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Harthmann AD, De Angelis K, Costa LP, Senador D, Schaan BD, Krieger EM, Irigoyen MC. Exercise training improves arterial baro- and chemoreflex in control and diabetic rats. Auton Neurosci 133: 115–120, 2007. doi: 10.1016/j.autneu.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 116. Stone AJ, Kaufman MP. The exercise pressor reflex and peripheral artery disease. Auton Neurosci 188: 69–73, 2015. doi: 10.1016/j.autneu.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ahmadian M, Williams AM, Mannozzi J, Konecny F, Hoiland RL, Wainman L, Erskine E, Duffy J, Manouchehri N, So K, Tauh K, Sala-Mercado JA, Shortt K, Fisk S, Kim KT, Streijger F, Foster GE, Kwon BK, O’Leary DS, West CR. A cross-species validation of single-beat metrics of cardiac contractility. J Physiol 600: 4779–4806, 2022. doi: 10.1113/JP283319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sheriff DD. Baroreflex resetting during exercise: mechanisms and meaning. Am J Physiol Heart Circ Physiol 290: H1406–H1407, 2006. doi: 10.1152/ajpheart.01275.2005. [DOI] [PubMed] [Google Scholar]
- 119. Sheriff DD, Augustyniak RA, O’Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 120. Ilett MJ, Rantalainen T, Keske MA, May AK, Warmington SA. The effects of restriction pressures on the acute responses to blood flow restriction exercise. Front Physiol 10: 1018, 2019. doi: 10.3389/fphys.2019.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Laurentino GC, Loenneke JP, Teixeira EL, Nakajima E, Iared W, Tricoli V. The effect of cuff width on muscle adaptations after blood flow restriction training. Med Sci Sports Exerc 48: 920–925, 2016. doi: 10.1249/MSS.0000000000000833. [DOI] [PubMed] [Google Scholar]
- 122. Wernbom M, Schoenfeld BJ, Paulsen G, Bjørnsen T, Cumming KT, Aagaard P, Clark BC, Raastad T. Commentary: Can blood flow restricted exercise cause muscle damage? Commentary on blood flow restriction exercise: considerations of methodology, application, and safety. Front Physiol 11: 243, 2020. doi: 10.3389/fphys.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chang KS, Lund DD. Alterations in the baroreceptor reflex control of heart rate in streptozotocin diabetic rats. J Mol Cell Cardiol 18: 617–624, 1986. doi: 10.1016/s0022-2828(86)80969-5. [DOI] [PubMed] [Google Scholar]
- 124. Choi HM, Stebbins CL, Lee OT, Nho H, Lee JH, Chun JM, Kim KA, Kim JK. Augmentation of the exercise pressor reflex in prehypertension: roles of the muscle metaboreflex and mechanoreflex. Appl Physiol Nutr Metab 38: 209–215, 2013. doi: 10.1139/apnm-2012-0143. [DOI] [PubMed] [Google Scholar]
- 125. Dall’Ago P, Fernandes TG, Machado UF, Belló AA, Irigoyen MC. Baroreflex and chemoreflex dysfunction in streptozotocin-diabetic rats. Braz J Med Biol Res 30: 119–124, 1997. doi: 10.1590/s0100-879x1997000100018. [DOI] [PubMed] [Google Scholar]
- 126. DiCarlo SE, Bishop VS. Central baroreflex resetting as a means of increasing and decreasing sympathetic outflow and arterial pressure. Ann NY Acad Sci 940: 324–337, 2001. doi: 10.1111/j.1749-6632.2001.tb03688.x. [DOI] [PubMed] [Google Scholar]
- 127. Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol 69: 523–531, 1992. doi: 10.1016/0002-9149(92)90998-e. [DOI] [PubMed] [Google Scholar]
- 128. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288: H2271–H2279, 2005. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 129. Ichinose M, Ichinose-Kuwahara T, Watanabe K, Kondo N, Nishiyasu T. The carotid baroreflex modifies the pressor threshold of the muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol 313: H650–H657, 2017. doi: 10.1152/ajpheart.00816.2016. [DOI] [PubMed] [Google Scholar]
- 130. Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during muscle metaboreflex activation in humans. J Physiol 544: 939–948, 2002. doi: 10.1113/jphysiol.2002.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Iellamo F, Sala-Mercado JA, Ichinose M, Hammond RL, Pallante M, Ichinose T, Stephenson LW, O’Leary DS. Spontaneous baroreflex control of heart rate during exercise and muscle metaboreflex activation in heart failure. Am J Physiol Heart Circ Physiol 293: H1929–H1936, 2007. doi: 10.1152/ajpheart.00564.2007. [DOI] [PubMed] [Google Scholar]
- 132. Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 91: 27–36, 2006. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- 133. Spranger MD, Krishnan AC, Levy PD, O’Leary DS, Smith SA. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Heart Circ Physiol 309: H1440–H1452, 2015. doi: 10.1152/ajpheart.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rolnick N, Kimbrell K, Cerqueira MS, Weatherford B, Brandner C. Perceived barriers to blood flow restriction training. Front Rehabil Sci 2: 697082, 2021. doi: 10.3389/fresc.2021.697082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hughes L, Jeffries O, Waldron M, Rosenblatt B, Gissane C, Paton B, Patterson SD. Influence and reliability of lower-limb arterial occlusion pressure at different body positions. PeerJ 6: e4697, 2018. doi: 10.7717/peerj.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.