Keywords: exercise, myonuclei, p16, p21, SA β-Gal
Abstract
A gradual decline in skeletal muscle mass and function is closely tied to increased mortality and disease risk during organismal aging. Exercise training is the most effective way to enhance muscle health, but the adaptive response to exercise as well as muscle repair potential is blunted in older individuals. Numerous mechanisms contribute to the loss of muscle mass and plasticity as aging progresses. An emerging body of recent evidence implicates an accumulation of senescent (“zombie”) cells in muscle as a contributing factor to the aging phenotype. Senescent cells cannot divide but can release inflammatory factors and create an unfavorable environment for homeostasis and adaptation. On balance, some evidence indicates that cells with senescent characteristics can be beneficial for the muscle adaptive process, specifically at younger ages. Emerging evidence also suggests that multinuclear muscle fibers could become senescent. In this review, we summarize current literature on the prevalence of senescent cells in skeletal muscle and highlight the consequences of senescent cell removal on muscle mass, function, and adaptability. We examine key limitations in the field of senescence specifically in skeletal muscle and identify areas of research that require future investigation.
NEW & NOTEWORTHY There is evidence to suggest that senescent “zombie” cells may or may not accrue in aging skeletal muscle. When muscle is perturbed regardless of age, senescent-like cells do appear, and the benefits of removing them could be age-dependent. More work is needed to determine the magnitude of accumulation and source of senescent cells in muscle. Regardless, pharmacological senolytic treatment of aged muscle is beneficial for adaptation.
INTRODUCTION
The age-mediated decline in health is a multifactorial process defined by a constellation of maladaptive events that lead to tissue dysregulation and loss of function. One of these events, cellular senescence, is now recognized as a major contributing factor to organismal aging. The term “cellular senescence” describes the state of permanent cell-cycle arrest in proliferative cell populations. Various stressors such as oxidative stress, cytotoxic agents, and irradiation can initiate cell cycle arrest via irreparable DNA damage (reviewed in Refs. 1 and 2). With aging, exhaustive replication is a major cause of senescence due to telomere uncapping following a cells’ final division (3, 4). Various nuclear and cytoplasmic markers (5, 6) are used to define senescent cells. Some classic hallmarks that are elevated in senescent cells are senescence-associated β-galactosidase (SA β-Gal) (7), the cell cycle inhibitory proteins p16Ink4a (8) and p21Cip1 (9), and DNA damage and telomere uncapping protein γH2AX (10). More recent markers used to label senescent cells include the nuclear membrane protein Lamin B1 (11) and the secreted factor HMGB1 (12, 13), both of which are low in senescent cell nuclei. Many of these senescent markers are expressed under normal physiological conditions, in multiple pathways, and in conditions unrelated to aging. SA β-Gal is normally expressed in macrophages, osteoclasts, cells undergoing lysosomal activity, and in various inflammatory conditions (14–16). p16Ink4a expression has also been found in nonsenescent cells such as macrophages (14, 17). An increase in expression of the senescent marker p21 may in some cases occur independently from telomere dysfunction and DNA damage associated with senescence; specifically, it is required for myoblast differentiation (18, 19). Since these markers used for senescence are also expressed independently of senescence, multiple markers of senescence should be used to identify senescent cells.
Although mitotically inactive, senescent cells remain metabolically active and secrete an ever-growing list of molecules (i.e., chemokines, inflammatory cytokines, growth factors, proteases, etc.). This list comprises the senescence-associated secretory phenotype (SASP) (20–22). The SASP is generally viewed as deleterious to surrounding healthy cells and may have an effect that is disproportionately large relative to the abundance of senescent cells. Senescent cells and secreted SASP factors contribute to many age-related diseases including metabolic dysfunction (23), neurodegenerative diseases (24), and osteoporosis (25, 26). Removing senescent cells is an attractive approach to slow the progression of various diseases as well as aging (24, 25, 27–31). Specifically, the administration of senescent cell killing compounds, called senolytics, slow the progression of Alzheimer’s disease (24), diabetes (23), and cardiovascular disease (27, 32), while extending lifespan and health span in healthy mice (28, 30). Although not extensively covered in this review, senomorphics are a class of drugs similar to senolytics that modify the SASP by reducing the inflammatory profile without killing the senescent cells (33, 34).
The benefits of senescent cell deletion for whole body health are becoming increasingly apparent, but it is still unclear if senolytics affect all tissue types in the same fashion. It is also not always clear whether tissue-specific benefits of removing senescent cells are mediated by indirect effects throughout the body or direct effects in specific organ systems. We lack the clarity to know if all of the presumed senescent cells that are expressing senescence markers (i.e., p16, p21, SA β-Gal) are truly pathogenic. The removal of senescent cells is generally not deleterious, but there are situations where senescent cells have necessary roles. A study by the Campisi laboratory was among of the first to show a requirement of senescent cells for the appropriate response to wound healing (35). Since then, others have demonstrated a necessity for senescent cells in normal tissue repair (36–38), but it is currently unclear if age plays a role in the effectiveness of senescent cell removal for skeletal muscle repair via senolytics (36, 39).
Skeletal muscle is a unique tissue because it is, by volume, primarily comprised of differentiated multinuclear muscle fibers. The unique syncytial anatomy of a muscle fiber makes the detection of senescence in these cells particularly challenging. Skeletal muscle is also comprised of diverse resident and infiltrating mononuclear cell populations (i.e., macrophages, endothelial cells, fibrogenic cells, etc.). Muscle fibers also have bona fide stem cells, or satellite cells, that are closely associated with the muscle cell membrane and require special attention with respect to discriminating them from muscle fiber nuclei (myonuclei). In addition, muscle fibers can undergo drastic changes in myonuclear proportion and density following a chronic stimulus such as repeated loading (40, 41). At the present time, little is known about the contribution of senescent cells and/or myonuclei to the overall health and adaptability of muscle fibers. This review will discuss the current state of the field regarding the prevalence of senescent cells in skeletal muscle and if muscle health benefits from adjuvant senolytics during exercise and aging.
CELLULAR SENESCENCE IN RESTING MUSCLE
Skeletal muscle is the largest tissue in the body and plays an essential role in short- and long-term physical and metabolic health. A decline in muscle mass is strongly associated with mortality (42, 43), in addition to contributing to numerous metabolic diseases such as insulin resistance and diabetes (44). The accumulation of senescent cells throughout the life span may contribute to muscle mass loss and impaired adaptability to exercise as aging progresses. The prevailing dogma is that all tissues accumulate senescent cells in advanced age, and that removing them is beneficial to muscle health. Recent work by us and others (summarized in Table 1) provide supporting and contrary evidence for these assertions.
Table 1.
Summary of five key studies for or against the accumulation of senescent cells in skeletal muscle with aging
| Group | Study (Reference #) | Model | ↑ SnC in Muscle | Markers | ↑ SnC Genes | Genes/Proteins |
|---|---|---|---|---|---|---|
| Senescent cells accumulate in skeletal muscle with aging | Baker et al. (27) | Progeroid Mice | n/a | n/a | Yes | p16Ink4a, p21Cip1, p19Ink4d |
| Baker et al. (28) | Genetically Modified Mice | n/a | n/a | Yes | p16Ink4a, p21Cip1, p19Ink4d | |
| da Silva et al. (50) | WT Mice | Yes | p21, TAF, HMGB1, LB1, SBB | n/a | n/a | |
| Xiong et al. (26) | Genetically Modified Mice | n/a | n/a | Yes | p16Ink4a | |
| Guzman et al. (49) | Genetically Modified Mice | Yes | SA β-Gal | Yes | p16Ink4a, p53 | |
| Zhang et al. (39) | WT Mice and Humans | Yes | TAF, LB1,HMGB1, p16 | Yes | p16Ink4a, p21Cip1 | |
| Senescent cells do not accumulate in skeletal muscle with aging | Jeyapalan et al. (63) | Primates | No | TAF, 53BP1 | n/a | n/a |
| Dungan et al. (59) | Humans | No | H2AX | n/a | n/a | |
| Yousefzadeh et al. (62) | Progeroid and WT Mice | n/a | n/a | No | p16Ink4a, p21Cip1 | |
| Dungan et al. (36) | WT Mice | No | SA β-Gal | n/a | n/a | |
| Dungan et al. (61) | WT Mice | No | SA β-Gal, p21 | n/a | n/a |
SnC, senescent cell. Data delineates the results from whole muscle analysis of senescent cells or senescent cell gene expression, not the results from experiments performed on isolated SnCs. Markers is in reference to identification via histology.
The Case for an Accumulation of Senescent Cells in Resting Muscle with Advanced Age
A landmark paper by Baker et al. (27) was the first study to indicate that senescent cells accumulate in skeletal muscle with aging. In this study, the authors showed that the removal of senescent cells in the entire body, which included skeletal muscle, of a progeroid model improved muscle fiber size and function. This project was followed up by the same group with another study in naturally aged mice (18 mo) showing that systemic deletion of p16Ink4a-expressing senescent cells extends life span and health span (28). Unlike the first study, there was no improvement in muscle function, nor was there an improvement in gastrocnemius muscle mass (28). This could indicate that the age-mediated senescent cell load is an important determining factor for the beneficial effects of senescent cell removal. The 50% survivability is ∼8 mo in BubR1 mice (45, 46) and ∼27 mo in C57BL/6J wild-type mice (47, 48). The first study used 10-mo-old BubR1 mice (27) and the second used 18-mo-old mice on a C57BL/6J background (28). The BubR1 mice used in the first study are 2 mo older than their 50% survivability, whereas the mice on a C57BL/6J background used in the second study are 9 mo younger and likely have a lower relative senescence burden. More recently, several studies (30, 39, 49), including work that we co-authored (36), report that the removal of senescent cells throughout the body late in life improves muscle mass and function of mice. It is important to note that although this beneficial effect may be due to the direct removal of senescent cells, there could also be beneficial effects due to the removal of senescent muscle fibers and senescent myonuclei, or a systemic reduction in the SASP due to the effect of senolytics on multiple organ systems. Furthermore, senolytics have antioxidant and anti-inflammatory properties that may not directly affect senescent cells, but may mediate the effects of the SASP created by senescent cells. Although the data on whole body deletion of senescent cells collectively point to the possibility that the removal of senescent cells in skeletal muscle can enhance muscle mass and function during aging, many of these studies did not quantify a change in senescent cell abundance. This lack of specificity makes it difficult to determine whether a reduction in the number of senescent cells residing within skeletal muscle is the primary mechanism of action.
Several studies report a greater number of senescent cells in muscle from aged versus young mice, but technical limitations should be considered when interpreting these results. da Silva et al. (50) used five senescence markers to show a greater abundance of senescent cells in muscle from geriatric (32 mo) relative to adult mice (8 mo). These authors did not use a muscle morphology marker to discriminate myonuclei from mononuclear cells. This study confirms the presence of senescent cells in muscle in very advanced age, but the lack of cell type identification makes it difficult to delineate between post-mitotic myonuclei and mononuclear cells. Zhang et al. (39) used three senescence markers to label senescent cells in mouse muscle, showing a greater abundance with age. A muscle morphology marker was also absent in their histochemical analysis of senescent cells. They did, however, perform an RNA-sequencing analysis of skeletal muscle at single cell and nucleus resolution of young and old mice and observed no difference in the abundance of p16Ink4a+ and p21Cip1+ cells or nuclei between ages (39). Alternatively, analysis of isolated myofibers revealed higher p21Cip1 mRNA and protein, suggesting the presence of senescent myonuclei and/or muscle fibers in aged mice. The presence of senescent myonuclei is provocative, especially in light of recent dogma-challenging work, suggesting that nonproliferative myonuclei can synthesize DNA (51). Follow-up work by the same group showed that constitutive overexpression of p21Cip1 leads to muscle atrophy and weakness and induces a transcriptional profile that is consistent with senescence (52). The mechanisms by which myonuclei could be removed from a muscle fiber is unclear and a topic of ongoing debate (40, 41, 53–58). Thus, the action of senolytics on muscle fibers specifically deserves further consideration. Perhaps senolytic-mediated removal of myonuclei could point to a unique process whereby specific myonuclei can be targeted for removal within a syncytium.
The Case against an Accumulation of Senescent Cells in Resting Muscle with Advanced Age
Although there are studies that suggest there is an accumulation of senescent cells in muscle from aged mice and humans, there is also support that senescent cells do not accumulate in muscle with advanced age. In humans, the Peterson laboratory showed no difference in the abundance of senescent cells in muscle from young (18–39) and old (65+) individuals, along with no difference in p16Ink4a and p21Cip1 gene expression in muscle tissue (59). In this study, the appearance of senescent cells in muscle was only apparent in young obese individuals. On balance, only one senescence marker was used to identify senescent cells (γH2AX) in this study (59), which is not the preferred approach (60). Using SA β-Gal alone (36) or SA β-Gal and p21Cip1 (61) to identify senescent cells in muscle from young (4–6 mo) and old (23–24 mo) mice, our data support the human work in that there was no difference in the abundance of senescent cells between young and old groups. In naturally aged and progeroid models, others have also found no elevation in gene expression of p16Ink4a and p21Cip1 in skeletal muscle tissue (62), while separate studies report no change in the number of p16Ink4a+ or SA β-Gal+ cells in muscle of aged animals (63–66). Furthermore, using a p16Ink4a+ reporter mouse, the Muñoz-Cánoves laboratory recently reported no accumulation of senescence cells in muscle by 28 mo (66). Together, data from these studies support the notion that senescent cells may not accumulate in skeletal muscle with aging, at least within the age ranges that were tested.
Although there are conflicting reports between various laboratories on the abundance of senescent cells in aging muscle, there are potential explanations for the discrepancy. Based on work from the Muñoz-Cánoves laboratory (65), the age of “old” animals could play a role. In their study using old (24 mo) and geriatric (>30 mo) mice, they reported no increase in p16Ink4a gene expression in cells isolated from old mice but a substantial increase in p16Ink4a gene expression in muscle from geriatric mice (65). The animal facility where each cohort of animals live may have an effect. There are known differences in life span and health span depending on the geographic location and surrounding conditions (i.e., noise, cage size, temperature) where animals are housed (67). The spatial location of senescent cells could also be important. Defects or loss of the neuromuscular junction (NMJ) is a contributing factor to muscle atrophy and fiber-type shifts that happens during aging (68, 69). An increase in senescent cells and/or myonuclei at the NMJ may facilitate a loss of muscle mass and function while maintaining a relatively low biological footprint. Sex is another biological source of variance, with muscle from aged female mice exhibiting less of an elevation in p16Ink4a expression when compared with males (39). Finally, the fiber-type composition of muscle being evaluated appears to influence the gene expression of p16Ink4a and p21Cip1. The soleus comprises predominately oxidative fibers and shows little to no change in p16Ink4a and p21Cip1 gene expression (39) or improvement in mass and force production upon p16Ink4a+ cell deletion (49). Conversely, the gastrocnemius and tibialis anterior contain mostly glycolytic muscle fibers, have a greater upregulation in p16Ink4a and p21Cip1 with aging (39), and display improvement in muscle mass and function upon the removal of senescent cells (49). This appears to make sense when considering that the glycolytic type 2 fibers are preferentially affected by aging (69, 70).
There are also technical issues that can impact the results of studies that evaluate senescence in muscle. Using two or more senescence markers is the ideal method for identifying senescent cells (60); however, this can be challenging in skeletal muscle due to the relatively low abundance of some of these markers. Baseline p16Ink4a and p21Cip1 expression is exceedingly low in skeletal muscle [nearly undetectable using RT-qPCR (59)], so any increase in their expression could be numerically significant but biologically questionable. Senescence markers can also be tissue or cell type specific. Sudan Black B has been reported as a senescence marker due to its labeling of lipofuscin (71, 72). In skeletal muscle, Sudan Black B also identifies Type 1 myosin heavy chain (59, 73, 74). Nuclear localization of p21Cip1 is a classic senescence marker but is not ideal for skeletal muscle because p21Cip1 is highly upregulated during satellite cell differentiation (18, 19, 75). In the cytoplasm, p21 has anti-apoptotic functions (76) and could be a key mediator of the senescent cell anti-apoptotic pathway (SCAP), a common target of senolytics (77). Given that muscle contains numerous resident and infiltrating cell types (i.e., macrophages, endothelial cells, fibrogenic cells), using a muscle morphology marker (i.e., laminin, dystrophin) is crucial for delineating between mononuclear cell nuclei and muscle fiber nuclei. Studies that neglect to use a morphology marker when identifying senescent cells could in fact be quantifying muscle fiber nuclei instead of senescent cells. It is likely that a senescent muscle fiber, which is extremely large relative to a mononuclear cell, may have a larger impact on muscle mass and function than senescence of another cell type in muscle. Then again, the presence of one myonucleus enriched for senescent markers within a large syncytium may not make the entire muscle fiber senescent and have little relevance; more work is needed in this area.
CELLULAR SENESCENCE DURING MUSCLE ADAPTATION
There is not yet consensus on the role senescent cells play in muscle atrophy with aging. There is, however, a growing body of literature that points to the importance of cellular senescence during the early stages of muscle adaptation throughout the life span, as well as the maladaptive potential if left unresolved long term.
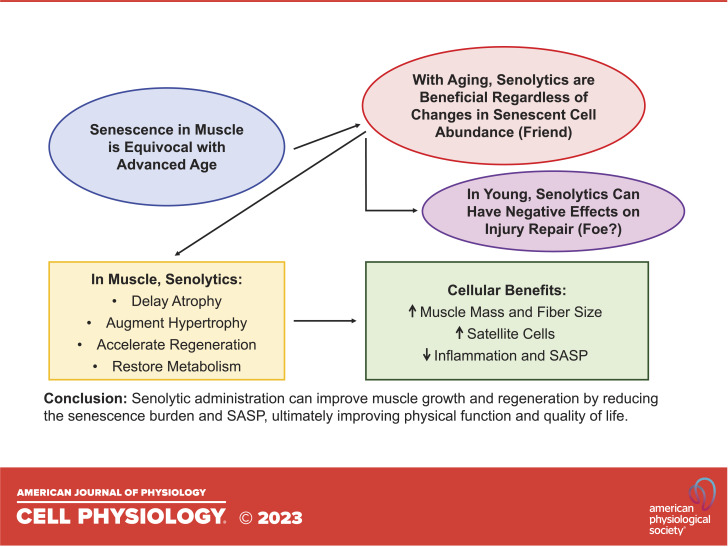
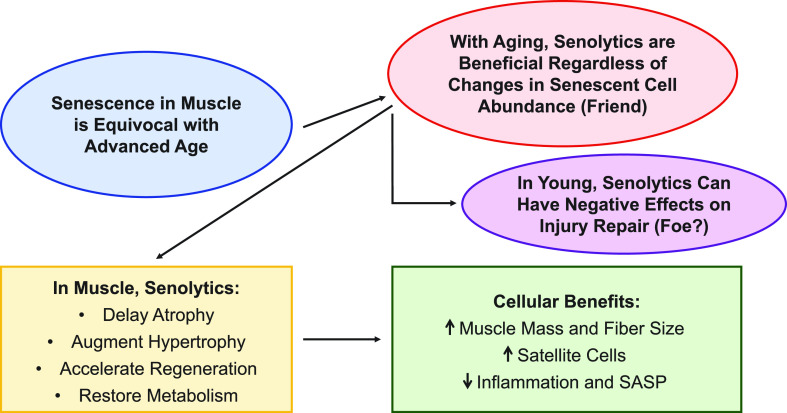
“Senescent” (SA β-Gal+) cells increase dramatically in abundance following acute muscle injury in young and old mice (36, 66, 78, 79). We reported that the influx of SA β-Gal+ cells shortly after injury is predominantly comprised of Cd11b+ macrophage cells (36), which are indispensable for muscle repair and adaptation (80, 81). Another recent report showed that senescent fibro-adipogenic progenitor cells (FAPs) and satellite cells were also abundant after muscle injury (66). Macrophages express high levels of SA β-Gal and p16Ink4a during polarization from a proinflammatory M1 macrophage to a more anti-inflammatory M2 state (14, 17). When this accumulation of “senescent” cells is disrupted in young healthy tissue, there is an impairment of muscle growth and repair (36, 78). The evidence for SA β-Gal+ cells contributing to tissue healing coincides with the requirement of “senescent” cells in other biological processes such as skin wound healing (35) and Yamanaka factor-mediated reprogramming in skeletal muscle (78). In models of exercise training, there is an upregulation of SA β-Gal+ cells that is resolved over time and required for the normal response to exercise and injury (38, 61). The number of SA β-Gal+ cells in muscle is reduced as the injury resolves (36, 78); however, this process is blunted with aging. Fourteen to 28 days following muscle injury or mechanical overload, old mice display a greater abundance of SA β-Gal+ cells than young (36, 61, 66). The accumulation of senescent cells following exercise is not exclusive to aging, as young mice (61) and humans (82) show an increase in the abundance of senescent cells following acute exercise; however, senescent cells persist much longer with aging, leading them to become maladaptive for exercise (61). Deletion of these cells during remodeling in old mice using senolytics ultimately improves muscle mass and muscle fiber size, enhances satellite cell abundance and proliferation, and reduces the overall inflammatory status of the muscle (36, 61, 66). It is important to note two independent laboratories reported that the beneficial effects of senolytics on muscle adaptation appear to be reserved for old mice, as young mice exhibit a blunted response to injury or loading following the removal of senescent cells with the senolytic cocktail, dasatinib and quercetin (D + Q) (36, 61, 78). Alternatively, one recent study (66) reported an accelerated muscle regeneration response in young mice with D + Q. This alternative response could be attributable due to a variety of factors, including the number of days post-injury (28 vs. 7/10 in Moiseeva et al.), the muscle being analyzed (tibialis anterior vs. extensor digitorum longus in Moiseeva et al.), injury model (BaCl2 vs. cardiotoxin in Moiseeva et al.), and/or injury technique used (unilateral injury vs. bilateral injury in Moiseeva et al.). Independent of a senescence burden in aging muscle, senolytics generally appear to be safe and effective at restoring the normal adaptive response of skeletal muscle (Fig. 1).
Figure 1.
Summary of the prevalence of senescent cells in skeletal muscle and the effects of senolytics. SASP, senescence-associated secretory phenotype.
FUTURE DIRECTIONS FOR SENESCENCE IN SKELETAL MUSCLE
Aging is tied to an increased abundance of senescent cells, but there are numerous other conditions and disease states that exhibit a disruption in muscle mass, function, and adaptability that are associated with increased senescent cell burden. Muscle wasting disorders, such as muscular dystrophy, are characterized by a greater abundance of senescent cells (83–85) that are reduced upon administration of senolytics (84, 85). Senolytics improve muscle mass in these models, along with increasing the number of satellite cells (84, 85). Similarly, exercise training reduces the circulating SASP in humans (86) and has been suggested as a nonpharmacological way to reduce the accumulation of senescent cells and promote tissue health (87). Conditions that promote a systemic proinflammatory state, such as obesity, have a greater number of muscle senescent cells (59), and improved insulin sensitivity and glucose uptake coincides with senolytic administration (23). Perhaps conditions characterized by a proinflammatory state and reduced insulin sensitivity (i.e., cancer cachexia) might benefit from senolytic administration. Chemotherapeutic agents such as cisplatin and doxorubicin induce senescence as a way to limit cancer cell growth (88–90); however, these drugs are not isolated to just the tumor and negatively affect all tissues in the body, including muscle. Reducing the senescent cell burden in muscle following chemotherapy might be beneficial for helping these individuals on their path to recovery. There is a growing body of evidence that post-mitotic nuclei can become senescent and display a phenotype and associated SASP similar to conventional senescent cells (91, 92). Considering skeletal muscle fibers are nonproliferative, an accumulation of senescent myonuclei could explain the disparity between a lack of increase, or minimal increase in senescent “cells” with age and the beneficial effects of senolytics in aging muscle. Determining the influence of senescent myonuclei on muscle fibers and overall muscle mass and function could provide clarity on the etiology of muscle mass loss with age, and whether their removal drives improvements in muscle health. Finally, there is a growing need for research on cell-type specific senolytics. To remain viable and avoid death, senescent cells turn on senescent cell anti-apoptotic pathways (SCAPs) that prevent apoptosis (93). Considering that the drivers of senescence (i.e., DNA damage, exhaustive replication, oxidative stress) influence the composition of the SASP, there are likely tissue-specific SCAPs that could be targeted to improving the efficacy of senolytic treatment in the target tissue.
GRANTS
KAM is supported by NIH R00 AG063994.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.M.D. and K.A.M. conceived and designed research; C.M.D. and J.M.W. prepared figures; C.M.D., J.M.W., and K.A.M. drafted manuscript; C.M.D., J.M.W., and K.A.M. edited and revised manuscript; C.M.D., J.M.W., and K.A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Charlotte Peterson, PhD, of the University of Kentucky Center for Muscle Biology for supporting the work we conducted on senescence in muscle during our post-doctoral training.
REFERENCES
- 1. Di Micco R, Krizhanovsky V, Baker D, d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 22: 75–95, 2021. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang W, Hickson LJ, Eirin A, Kirkland JL, Lerman LO. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol 18: 611–627, 2022. doi: 10.1038/s41581-022-00601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiu CP, Harley CB. Replicative senescence and cell immortality: the role of telomeres and telomerase. Proc Soc Exp Biol Med 214: 99–106, 1997. doi: 10.3181/00379727-214-44075. [DOI] [PubMed] [Google Scholar]
- 4. Victorelli S, Passos JF. Telomeres and cell senescence - size matters not. EBioMedicine 21: 14–20, 2017. doi: 10.1016/j.ebiom.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol 28: 436–453, 2018. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 6. Saul D, Kosinsky RL, Atkinson EJ, Doolittle ML, Zhang X, LeBrasseur NK, Pignolo RJ, Robbins PD, Niedernhofer LJ, Ikeno Y, Jurk D, Passos JF, Hickson LJ, Xue A, Monroe DG, Tchkonia T, Kirkland JL, Farr JN, Khosla S. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat Commun 13: 4827, 2022. doi: 10.1038/s41467-022-32552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. and A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367, 1995. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brenner AJ, Stampfer MR, Aldaz CM. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 17: 199–205, 1998. doi: 10.1038/sj.onc.1201919. [DOI] [PubMed] [Google Scholar]
- 9. Chang BD, Swift ME, Shen M, Fang J, Broude EV, Roninson IB. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc Natl Acad Sci USA 99: 389–394, 2002. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198, 2003. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 11. Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23: 2066–2075, 2012. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee JJ, Park IH, Kwak MS, Rhee WJ, Kim SH, Shin JS. HMGB1 orchestrates STING-mediated senescence via TRIM30alpha modulation in cancer cells. Cell Death Discov 7: 28, 2021. doi: 10.1038/s41420-021-00409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 201: 613–629, 2013. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin II, Leonova KI, Consiglio CR, Gollnick SO, Chernova OB, Gudkov AV. p16(Ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY) 9: 1867–1884, 2017. doi: 10.18632/aging.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Mera-Rodriguez JA, Alvarez-Hernan G, Ganan Y, Martin-Partido G, Rodriguez-Leon J, Francisco-Morcillo J. Is senescence-associated beta-galactosidase a reliable in vivo marker of cellular senescence during embryonic development? Front Cell Dev Biol 9: 623175, 2021. doi: 10.3389/fcell.2021.623175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kopp HG, Hooper AT, Shmelkov SV, Rafii S. Beta-galactosidase staining on bone marrow. The osteoclast pitfall. Histol Histopathol 22: 971–976, 2007. doi: 10.14670/HH-22.971. [DOI] [PubMed] [Google Scholar]
- 17. Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin I, Leonova K, Polinsky A, Chernova OB, Gudkov AV. Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY) 8: 1294–1315, 2016. doi: 10.18632/aging.100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev 13: 213–224, 1999. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawke TJ, Meeson AP, Jiang N, Graham S, Hutcheson K, DiMaio JM, Garry DJ. p21 is essential for normal myogenic progenitor cell function in regenerating skeletal muscle. Am J Physiol Cell Physiol 285: C1019–C1027, 2003. doi: 10.1152/ajpcell.00055.2003. [DOI] [PubMed] [Google Scholar]
- 20. Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118, 2010. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Englund DA, Zhang X, Aversa Z, LeBrasseur NK. Skeletal muscle aging, cellular senescence, and senotherapeutics: current knowledge and future directions. Mech Ageing Dev 200: 111595, 2021. doi: 10.1016/j.mad.2021.111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev 34: 1565–1576, 2020. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18: e12950, 2019. doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, Mattson MP. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci 22: 719–728, 2019. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23: 1072–1079, 2017. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiong L, Zhao K, Cao Y, Guo HH, Pan JX, Yang X, Ren X, Mei L, Xiong WC. Linking skeletal muscle aging with osteoporosis by lamin A/C deficiency. PLoS Biol 18: e3000731, 2020. doi: 10.1371/journal.pbio.3000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2011. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530: 184–189, 2016. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Kruger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, Podgorni O, Enikolopov G, Johnson KO, Xu M, Inman C, Palmer AK, Schafer M, Weigl M, Ikeno Y, Burns TC, Passos JF, von Zglinicki T, Kirkland JL, Jurk D. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab 29: 1061–1077.e8, 2019. doi: 10.1016/j.cmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 24: 1246–1256, 2018. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cavalcante MB, Saccon TD, Nunes ADC, Kirkland JL, Tchkonia T, Schneider A, Masternak MM. Dasatinib plus quercetin prevents uterine age-related dysfunction and fibrosis in mice. Aging (Albany NY) 12: 2711–2722, 2020. doi: 10.18632/aging.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 38: e100492, 2019. doi: 10.15252/embj.2018100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ, Robbins PD. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J 290: 1362–1383, 2023. doi: 10.1111/febs.16350. [DOI] [PubMed] [Google Scholar]
- 34. Niedernhofer LJ, Robbins PD. Senotherapeutics for healthy ageing. Nat Rev Drug Discov 17: 377, 2018. doi: 10.1038/nrd.2018.44. [DOI] [PubMed] [Google Scholar]
- 35. Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31: 722–733, 2014. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dungan CM, Murach KA, Zdunek CJ, Tang ZJ, VonLehmden GL, Brightwell CR, Hettinger Z, Englund DA, Liu Z, Fry CS, Filareto A, Franti M, Peterson CA. Deletion of SA beta-Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell 21: e13528, 2022. doi: 10.1111/acel.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reyes NS, Krasilnikov M, Allen NC, Lee JY, Hyams B, Zhou M, Ravishankar S, Cassandras M, Wang C, Khan I, Matatia P, Johmura Y, Molofsky A, Matthay M, Nakanishi M, Sheppard D, Campisi J, Peng T. Sentinel p16(INK4a+) cells in the basement membrane form a reparative niche in the lung. Science 378: 192–201, 2022. doi: 10.1126/science.abf3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saito Y, Chikenji TS, Matsumura T, Nakano M, Fujimiya M. Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat Commun 11: 889, 2020. doi: 10.1038/s41467-020-14734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang X, Habiballa L, Aversa Z, Ng YE, Sakamoto AE, Englund DA, Pearsall VM, White TA, Robinson MM, Rivas DA, Dasari S, Hruby AJ, Lagnado AB, Jachim SK, Granic A, Sayer AA, Jurk D, Lanza IR, Khosla S, Fielding RA, Nair KS, Schafer MJ, Passos JF, LeBrasseur NK. Characterization of cellular senescence in aging skeletal muscle. Nat Aging 2: 601–615, 2022. doi: 10.1038/s43587-022-00250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dungan CM, Murach KA, Frick KK, Jones SR, Crow SE, Englund DA, Vechetti IJ Jr, Figueiredo VC, Levitan BM, Satin J, McCarthy JJ, Peterson CA. Elevated myonuclear density during skeletal muscle hypertrophy in response to training is reversed during detraining. Am J Physiol Cell Physiol 316: C649–C654, 2019. doi: 10.1152/ajpcell.00050.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murach KA, Mobley CB, Zdunek CJ, Frick KK, Jones SR, McCarthy JJ, Peterson CA, Dungan CM. Muscle memory: myonuclear accretion, maintenance, morphology, and miRNA levels with training and detraining in adult mice. J Cachexia Sarcopenia Muscle 11: 1705–1722, 2020. doi: 10.1002/jcsm.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang H, Hai S, Liu Y, Liu Y, Dong B. Skeletal muscle mass as a mortality predictor among nonagenarians and centenarians: a prospective cohort study. Sci Rep 9: 2420, 2019. doi: 10.1038/s41598-019-38893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S, Song Y. Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc 50: 458–467, 2018. doi: 10.1249/MSS.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim G, Lee SE, Jun JE, Lee YB, Ahn J, Bae JC, Jin SM, Hur KY, Jee JH, Lee MK, Kim JH. Increase in relative skeletal muscle mass over time and its inverse association with metabolic syndrome development: a 7-year retrospective cohort study. Cardiovasc Diabetol 17: 23, 2018. doi: 10.1186/s12933-018-0659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wijshake T, Malureanu LA, Baker DJ, Jeganathan KB, van de Sluis B, van Deursen JM. Reduced life- and healthspan in mice carrying a mono-allelic BubR1 MVA mutation. PLoS Genet 8: e1003138, 2012. doi: 10.1371/journal.pgen.1003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet 36: 744–749, 2004. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 47. Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH 3rd, Zhang Y, Becker KG, Perez VI, Richardson A. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One 9: e83988, 2014. [Erratum in PLoS One 9: e92346, 2014]. doi: 10.1371/journal.pone.0083988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395, 2009. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guzman SD, Judge J, Shigdar SM, Paul TA, Davis CS, Macpherson PC, Markworth JF, Van Remmen H, Richardson A, McArdle A, Brooks SV. Removal of p16INK4 expressing cells in late life has moderate beneficial effects on skeletal muscle function in male mice. Front Aging 2: 821904, 2022. doi: 10.3389/fragi.2021.821904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. da Silva PFL, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, Ishaq A, Saretzki G, Nagaraja-Grellscheid S, Nelson G, von Zglinicki T. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18: e12848, 2019. doi: 10.1111/acel.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Borowik AK, Davidyan A, Peelor FF 3rd, Voloviceva E, Doidge SM, Bubak MP, Mobley CB, McCarthy JJ, Dupont-Versteegden EE, Miller BF. Skeletal muscle nuclei in mice are not post-mitotic. Function (Oxf) 4: zqac059, 2023. doi: 10.1093/function/zqac059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Englund DA, Jolliffe A, Aversa Z, Zhang X, Sturmlechner I, Sakamoto AE, Zeidler JD, Warner GM, McNinch C, White TA, Chini EN, Baker DJ, van Deursen JM, LeBrasseur NK. p21 induces a senescence program and skeletal muscle dysfunction. Mol Metab 67: 101652, 2023. doi: 10.1016/j.molmet.2022.101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci USA 107: 15111–15116, 2010. doi: 10.1073/pnas.0913935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hansson KA, Eftestol E, Bruusgaard JC, Juvkam I, Cramer AW, Malthe-Sorenssen A, Millay DP, Gundersen K. Myonuclear content regulates cell size with similar scaling properties in mice and humans. Nat Commun 11: 6288, 2020. doi: 10.1038/s41467-020-20057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Psilander N, Eftestol E, Cumming KT, Juvkam I, Ekblom MM, Sunding K, Wernbom M, Holmberg HC, Ekblom B, Bruusgaard JC, Raastad T, Gundersen K. Effects of training, detraining, and retraining on strength, hypertrophy, and myonuclear number in human skeletal muscle. J Appl Physiol (1985) 126: 1636–1645, 2019. doi: 10.1152/japplphysiol.00917.2018. [DOI] [PubMed] [Google Scholar]
- 56. Egner IM, Bruusgaard JC, Eftestol E, Gundersen K. A cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids. J Physiol 591: 6221–6230, 2013. doi: 10.1113/jphysiol.2013.264457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wen Y, Dungan CM, Mobley CB, Valentino T, von Walden F, Murach KA. Nucleus type-specific DNA methylomics reveals epigenetic “memory” of prior adaptation in skeletal muscle. Function (Oxf) 2: zqab038, 2021. doi: 10.1093/function/zqab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Murach KA, Dungan CM, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. “Muscle memory” not mediated by myonuclear number? Secondary analysis of human detraining data. Sports Med Health Sci 5: 2–9, 2023. doi: 10.1152/japplphysiol.00506.2019. [DOI] [PubMed] [Google Scholar]
- 59. Dungan CM, Peck BD, Walton RG, Huang Z, Bamman MM, Kern PA, Peterson CA. In vivo analysis of gammaH2AX+ cells in skeletal muscle from aged and obese humans. FASEB J 34: 7018–7035, 2020. doi: 10.1096/fj.202000111RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular senescence: defining a path forward. Cell 179: 813–827, 2019. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 61. Dungan CM, Figueiredo VC, Wen Y, VonLehmden GL, Zdunek CJ, Thomas NT, Mobley CB, Murach KA, Brightwell CR, Long DE, Fry CS, Kern PA, McCarthy JJ, Peterson CA. Senolytic treatment rescues blunted muscle hypertrophy in old mice. Geroscience 44: 1925–1940, 2022. doi: 10.1007/s11357-022-00542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, Bank MP, Gurkar AU, McGuckian CA, Calubag MF, Kato JI, Burd CE, Robbins PD, Niedernhofer LJ. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell e13094, 19, 2020. doi: 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 128: 36–44, 2007. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8: 311–323, 2009. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 65. Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, Perdiguero E, Munoz-Canoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506: 316–321, 2014. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 66. Moiseeva V, Cisneros A, Sica V, Deryagin O, Lai Y, Jung S, Andres E, An J, Segales J, Ortet L, Lukesova V, Volpe G, Benguria A, Dopazo A, Benitah SA, Urano Y, Del Sol A, Esteban MA, Ohkawa Y, Serrano AL, Perdiguero E, Munoz-Canoves P. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 613: 169–178, 2023. doi: 10.1038/s41586-022-05535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Akhtar A. The flaws and human harms of animal experimentation. Camb Q Healthc Ethics 24: 407–419, 2015. doi: 10.1017/S0963180115000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One 7: e34640, 2012. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 45: 2191–2199, 2013. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 70. Brunner F, Schmid A, Sheikhzadeh A, Nordin M, Yoon J, Frankel V. Effects of aging on Type II muscle fibers: a systematic review of the literature. J Aging Phys Act 15: 336–348, 2007. doi: 10.1123/japa.15.3.336. [DOI] [PubMed] [Google Scholar]
- 71. Evangelou K, Gorgoulis VG. Sudan Black B, The specific histochemical stain for Lipofuscin: a novel method to detect senescent cells. Methods Mol Biol 1534: 111–119, 2017. doi: 10.1007/978-1-4939-6670-7_10. [DOI] [PubMed] [Google Scholar]
- 72. Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez Marcos PJ, Zoumpourlis V, Trougakos IP, Kletsas D, Bartek J, Serrano M, Gorgoulis VG. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 5: 37–50, 2013. doi: 10.18632/aging.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roberts CJ, Turfrey BA, Bland AP. Lipid deposition in different fiber types of skeletal muscle of periparturient dairy cows. Vet Pathol 20: 23–31, 1983. doi: 10.1177/030098588302000103. [DOI] [PubMed] [Google Scholar]
- 74. Gauthier GF, Padykula HA. Cytological studies of fiber types in skeletal muscle. A comparative study of the mammalian diaphragm. J Cell Biol 28: 333–354, 1966. doi: 10.1083/jcb.28.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267: 1018–1021, 1995. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 76. Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9: 400–414, 2009. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine 21: 21–28, 2017. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Young LV, Wakelin G, Cameron AWR, Springer SA, Ross JP, Wolters G, Murphy JP, Arsenault MG, Ng S, Collao N, De Lisio M, Ljubicic V, Johnston APW. Muscle injury induces a transient senescence-like state that is required for myofiber growth during muscle regeneration. FASEB J 36: e22587, 2022. [Erratum in FASEB J 36: e22641, 2022]. doi: 10.1096/fj.202200289RR. [DOI] [PubMed] [Google Scholar]
- 79. Chiche A, Le Roux I, von Joest M, Sakai H, Aguin SB, Cazin C, Salam R, Fiette L, Alegria O, Flamant P, Tajbakhsh S, Li H. Injury-induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20: 407–414.e4, 2017. doi: 10.1016/j.stem.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 80. Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 17: 165–178, 2017. doi: 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Saclier M, Cuvellier S, Magnan M, Mounier R, Chazaud B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J 280: 4118–4130, 2013. doi: 10.1111/febs.12166. [DOI] [PubMed] [Google Scholar]
- 82. Lee TXY, Wu J, Jean WH, Condello G, Alkhatib A, Hsieh CC, Hsieh YW, Huang CY, Kuo CH. Reduced stem cell aging in exercised human skeletal muscle is enhanced by ginsenoside Rg1. Aging (Albany NY) 13: 16567–16576, 2021. doi: 10.18632/aging.203176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Young LV, Morrison W, Campbell C, Moore EC, Arsenault MG, Dial AG, Ng S, Bellissimo CA, Perry CGR, Ljubicic V, Johnston AP. Loss of dystrophin expression in skeletal muscle is associated with senescence of macrophages and endothelial cells. Am J Physiol Cell Physiol 321: C94–C103, 2021. doi: 10.1152/ajpcell.00397.2020. [DOI] [PubMed] [Google Scholar]
- 84. Sugihara H, Teramoto N, Nakamura K, Shiga T, Shirakawa T, Matsuo M, Ogasawara M, Nishino I, Matsuwaki T, Nishihara M, Yamanouchi K. Cellular senescence-mediated exacerbation of Duchenne muscular dystrophy. Sci Rep 10: 16385, 2020. doi: 10.1038/s41598-020-73315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu L, Yue X, Sun Z, Hambright WS, Feng Q, Cui Y, Huard J, Robbins PD, Wang Z, Mu X. Senolytic elimination of senescent macrophages restores muscle stem cell function in severely dystrophic muscle. Aging (Albany NY)) 14: 7650–7661, 2022. doi: 10.18632/aging.204275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Englund DA, Sakamoto AE, Fritsche CM, Heeren AA, Zhang X, Kotajarvi BR, Lecy DR, Yousefzadeh MJ, Schafer MJ, White TA, Atkinson EJ, LeBrasseur NK. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 20: e13415, 2021. doi: 10.1111/acel.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang X, Englund DA, Aversa Z, Jachim SK, White TA, LeBrasseur NK. Exercise counters the Age-related accumulation of senescent cells. Exerc Sport Sci Rev 50: 213–221, 2022. doi: 10.1249/JES.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Saleh T, Bloukh S, Carpenter VJ, Alwohoush E, Bakeer J, Darwish S, Azab B, Gewirtz DA. Therapy-induced senescence: an “old” friend becomes the enemy. Cancers 12: 822, 2020. doi: 10.3390/cancers12040822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao W, Lin ZX, Zhang ZQ. Cisplatin-induced premature senescence with concomitant reduction of gap junctions in human fibroblasts. Cell Res 14: 60–66, 2004. doi: 10.1038/sj.cr.7290203. [DOI] [PubMed] [Google Scholar]
- 90. Yang MY, Lin PM, Liu YC, Hsiao HH, Yang WC, Hsu JF, Hsu CM, Lin SF. Induction of cellular senescence by doxorubicin is associated with upregulated miR-375 and induction of autophagy in K562 cells. PLoS One 7: e37205, 2012. doi: 10.1371/journal.pone.0037205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. von Zglinicki T, Wan T, Miwa S. Senescence in post-mitotic cells: a driver of aging? Antioxid Redox Signal 34: 308–323, 2021. doi: 10.1089/ars.2020.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sapieha P, Mallette FA. Cellular senescence in postmitotic cells: beyond growth arrest. Trends Cell Biol 28: 595–607, 2018. doi: 10.1016/j.tcb.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 93. Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med 288: 518–536, 2020. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]