Abstract
Human speech and language are among the most complex motor and cognitive abilities. The discovery of a mutation in the transcription factor FOXP2 in KE family members with speech disturbances has been a landmark example of the genetic control of vocal communication in humans. Cellular mechanisms underlying this control have remained unclear. By leveraging FOXP2 mutation/deletion mouse models, we found that the KE family FOXP2R553H mutation directly disables intracellular dynein-dynactin ‘protein motors’ in the striatum by induction of a disruptive high level of dynactin1 that impairs TrkB endosome trafficking, microtubule dynamics, dendritic outgrowth and electrophysiological activity in striatal neurons alongside vocalization deficits. Dynactin1 knockdown in mice carrying FOXP2R553H mutations rescued these cellular abnormalities and improved vocalization. We suggest that FOXP2 controls vocal circuit formation by regulating protein motor homeostasis in striatal neurons, and that its disruption could contribute to the pathophysiology of FOXP2 mutation/deletion-associated speech disorders.
Keywords: basal ganglia, striatum, endosome trafficking, microtubule, vocalization
Kuo et al. identify a mechanism by which the KE family mutation in FOXP2 may give rise to childhood apraxia of speech. Using a mouse model, they show that the mutation disrupts the formation of striatal circuits required for vocalization – ultrasonic in mice and speech in humans – by inhibiting intracellular trafficking.
Introduction
Foxp2 is a forkhead domain transcription factor that is enriched in cortico-basal ganglia and cerebellar circuits. Clinical and genetic studies have linked genetic mutations of FOXP2 to speech and language disorders.1–5 A FOXP2 mutation was first identified in a three-generation British family (the KE family) pedigree, in which childhood apraxia of speech co-segregated with a heterozygous missense mutation that produces an arginine-to-histidine substitution (R553H) at the DNA-binding domain of the encoded protein. In the affected KE family members with this FOXP2R553H mutation, reduction in grey matter was found in movement-related brain regions, including the head of the caudate nucleus, inferior frontal gyrus (Broca's area), precentral gyrus, temporal pole and lobules VIIB and VIIIB of ventral cerebellum.1,6–8 The reduction in the caudate nucleus is of particular interest, as this region is involved in language processing and cognition.9–11 Volumetric analysis indicates ∼20% bilateral reduction in the caudate nuclei of affected members compared with unaffected members, and PET imaging has demonstrated a functional abnormality in the caudate nucleus.6,7 A similar reduction in the size of the caudate nucleus has also been found in a child carrying an intragenic deletion of two nucleotides within the FOXP2 coding region, which leads to a frameshift and consequent loss-of-function; this patient also developed verbal dyspraxia that impaired coordination and sequencing of orofacial movements for speech production.4,12 The aetiology of these pathologic abnormalities in the caudate nucleus remains unknown. Animal model studies have demonstrated, however, that FOXP2 acts as a transcription factor in the regulation of neurite outgrowth and control of neural circuit architecture both during the development of the brain13–22 and in the neuroplasticity occurring during vocal and motor learning.23–29
Genome-wide genetic screening, including expression profiling and chromatin-immunoprecipitation microarray screens in human and mouse brain tissue and cellular models, has identified FOXP2 target genes that are enriched in the regulation of neurite outgrowth30–34 and many putative FOXP2 target genes related to axonal development. It has remained unclear whether FOXP2 is essential for axonal outgrowth and/or navigation in the brain. By contrast, FOXP2 has been shown to be important for the development of dendrites, including dendritic development in the cerebellum, striatum and cerebral cortex.13–17 FOXP2 has also been shown to regulate dendritic spine formation and synaptogenesis in avian and mouse brains.18–22 Notably, dynamic FoxP2 levels have been shown to correlate positively with dendritic arborization and spines of avian Area X neurons and vocal learning in songbirds.19,26,35,36 The capacity to regulate dendritic development and synaptogenesis enables FOXP2 to coordinate circuit assembly in development and synaptic plasticity for adaptation to environmental changes.
A key question posed by these findings is how FOXP2 drives cellular machinery to control dendritic development and synapse formation. A strong clue was given by King,37 who identified p150(Glued) as a candidate target gene of the Drosophila orthologue dFoxp. Dynactin (DCTN)/p150(Glued) protein is a major subunit of dynein, a cytoplasmic protein that functions as a motor protein in the retrograde transport of membranous organelles, including proteasomes, endosomes, lysosomes and Golgi apparatus.38–40 DCTN binds via its largest subunit DCTN1 to the intermediate chain of dynein,41 also binds to microtubules via the glycine-rich cytoskeleton-associated protein domain in its N-terminus, and is critical for stabilizing plus-ends of microtubules in the growth cone of developing neurons. Intracellular endosome trafficking is known to regulate neurite outgrowth and the internalization and trafficking of trophic factors, for example, BDNF-TrkB, enriched in the striatum.42–49 For this function, dynactin protein is a necessary accessory protein in the dynein complex.38
We demonstrate that Dctn1 is a target gene of Foxp2 in neurons of the striatum and that Foxp2-Dctn1 signalling can regulate the development of striatal circuits affecting vocalization through its regulation of TrkB endosome trafficking and microtubule dynamics. We further demonstrate that this regulation can affect vocalization itself. With a mouse model harbouring the KE family mutation, we show that this novel genetic interaction of Foxp2 and Dctn1 is critical by demonstrating that knockdown of Dctn1 rescues deficits in the model. We suggest that dysregulated homeostasis of dynactin protein could contribute to the pathophysiology of FOXP2R553H mutation and FOXP2 deletion-associated speech disorders in humans.
Materials and methods
Details are provided in the Supplementary material.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary material.
Results
FOXP2 represses Dctn1 expression in striatal neurons
We first examined by reverse transcription followed by quantitative PCR (qRT-PCR), the striatal expression of Dctn1 in wild-type (WT) and Foxp2−/− germline knockout (KO) mice at postnatal Day 12 (P12) (Fig. 1A). Dctn1 mRNA was significantly increased in the neonatal striatum of KO mice. To assay DCTN1 protein levels in striatal neurons, we cultivated striatal neurons from the newborn striatum. As cultured neurons did not contain striatal afferents, this allowed us to exclude confounding Foxp2 protein in striatal afferents. Western blotting showed that DCTN1 protein was increased in striatal cultures from the Foxp2 KO mice relative to the WT (Fig. 1B and Supplementary Fig. 1C and E). Consistent with the repression of Dctn1 by Foxp2, overexpression of Foxp2 resulted in a decrease in Dctn1 protein levels in the striatal cultures (Fig. 1C–E). These findings indicated that Dctn1 is a downstream target gene of Foxp2.
Figure 1.
FOXP2 transcriptionally represses Dctn1 in striatal neurons. (A and B) Quantitative RT-PCR and western blotting show that Dctn1 mRNA (A) and protein (B) levels were upregulated in the striatum of Foxp2 KO mice. (C–E) Overexpression of Foxp2 suppresses DCTN1 expression in cultured striatal neurons (C, C′, E) compared with control neurons (D, D′ and E). Three independent experiments were performed. (F) Schematic drawing of the reporter gene construct containing intron 1 region of mouse Dctn1. Putative FOXP DNA-binding sites are indicated by red lines. (G) The ChIP assay shows positive signals with immunoprecipitation of Foxp2 antibody, but not control IgG antibody. PCR primer pair s1-as2 flank the DNA fragment containing the putative Foxp2 motifs at −1891 to −1652 bp (predicted size, 700 bp); s4-as3 flank the fragment containing the putative motifs at −512 to −490 bp (predicted size, 560 bp). (H) Luciferase reporter gene assay in ST14A cells shows that overexpression of WT Foxp2 repressed luciferase activity. By contrast, overexpression of Foxp2R552H mutants failed to suppress luciferase activity. *P < 0.05, **P < 0.01. Student's t-test was used in A, B and E. One-way ANOVA with Tukey's honest significant difference (HSD) post hoc test was used in H. Data are mean ± SEM.
To determine whether Foxp2 directly regulates Dctn1 in striatal neurons, we analysed the genomic locus of the Dctn1 gene. We identified five putative Foxp binding motifs spanning ∼2.5 kb in intron 1 of the Dctn1 gene (Fig. 1F). To test whether FOXP2 was directly bound to the intron 1 region, we performed chromatin immunoprecipitation assays (ChIP) of embryonic Day 16 (E16) striatal cell cultures. Pairs of PCR primers were designed to flank the DNA fragments: PCR primer pair s1-as2 flanked the DNA fragment containing the putative Foxp2 motifs TATTTAT and TATTTGT at −1891 to −1652 bp; s4-as3 flanked the fragment containing the putative motifs TATTTATTTATTTATTTATTTAT at −512 to −490 bp. FOXP2 antibody could immunoprecipitate both DNA fragments containing the putative FOXP2 binding sites (Fig. 1G), suggesting that FOXP2 directly binds to this DNA region in vivo. We subcloned the 2.5 kb DNA region into the pGL3-Luc reporter gene vector, which was then transfected into a striatal progenitor-derived ST14A cell line50 to assay luciferase reporter gene activity. The reporter gene assay indicated that transfection of WT Foxp2 plasmid suppressed luciferase activity by 32.2%. By contrast, transfection of Foxp2R552H mutant plasmid lacking DNA binding ability51 failed to suppress luciferase activity (Fig. 1H). Collectively, these results suggested that FOXP2 transcriptionally represses the Dctn1 gene in striatal neurons.
Deletion of Foxp2 impairs TrkB endosome trafficking in striatal neurons
As KO of Foxp2 increased DCTN1 expression, we postulated that the de-repressed, high levels of DCTN1 might affect dynein-dynactin protein-mediated trafficking of organelles in Foxp2 KO striatal neurons. To test this possibility, we assayed endosome trafficking in striatal neurons of the Foxp2 KO mice, focusing on TrkB endosome trafficking, because BDNF-TrkB signalling is important for the morphological development of medium-sized spiny neurons of the striatum.52,53
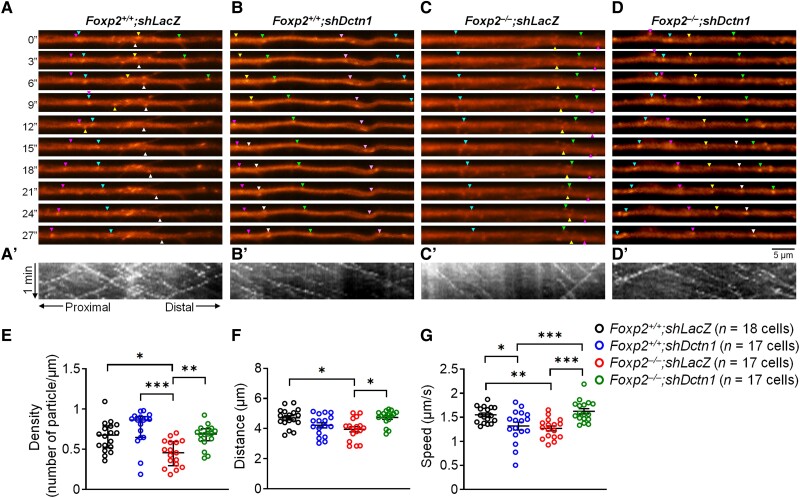
We performed time-lapse video microscopy to assay intracellular TrkB endosome trafficking in cultured striatal neurons. Newborn striatal neurons of Foxp2 WT and KO mice were cultivated for 5 days and then transfected with the pCMV-TrkB-mRFP plasmid.49 Striatal cells were imaged 2 days after transfection. Extensive and intermingled Tau1-positive (+) axons prevented us from tracing axonal outgrowth from single cells (Supplementary Fig. 2A and B). MAP2+ dendrites could, however, be traced from cultured single cells (Supplementary Fig. 2C–F′). We observed a robust phenotype of defective dendritic development in Foxp2 KO striatal neurons (Supplementary Fig. 2G and H), and, accordingly, we focused on the dendrites of striatal neurons to analyse the trafficking of TrkB-mRFP-labelled endosomes. Much evidence suggests that dynein-dynactin motor protein drives cargos retrogradely from plus-ends of microtubules to minus ends. Because the microtubules have mixed polarity of plus-to-minus and minus-to-plus ends in dendrites, the retrogradely transported cargos appear to move bidirectionally, at least in proximal dendrites.54
Many TrkB-mRFP-labelled vesicles moved bidirectionally within dendrites of WT striatal neurons (Supplementary Fig. 2 and Supplementary Video 1). Compared with WT neurons, in Foxp2 KO neurons, the travel distance of TrkB-mRFP-labelled vesicles was markedly decreased as shown in kymographs (Fig. 2A′, C′ and E′ and Supplementary Video 3). The speed of the movement of TrkB-mRFP-labelled vesicles also was reduced in Foxp2 KO neurons compared with WT-derived neurons (Fig. 2A, C and F). These results indicated that TrkB endosome trafficking is impaired in dendrites of Foxp2 KO striatal neurons.
Figure 2.
Dendritic trafficking of TrkB-mRFP-labelled endosomes is impaired in Foxp2 KO striatal neurons. (A–D) TrkB-mRFP-labelled vesicles were transported within proximal dendrites of cultured striatal neurons. The video snapshots show tracing of TrkB-mRFP vesicles every 6 s for 1 min (Supplementary Videos 1–4). The triangles indicate the positions of vesicles in the dendrite, with the same colours of triangles tracking the same vesicles. The motility of TrkB-mRFP vesicles was reduced in Foxp2 KO neurons (C) compared with WT neurons (A). (A′–D′) Kymographs generated from time-lapse video microscopy of trajectories of TrkB-mRFP-labelled vesicles for 180 s. Kymographs of live imaging video show the traces of each vesicle in the 3-min recording. (E and F) The abnormalities in the distance (E) and speed (F) of TrkB-mRFP-labelled vesicles were rescued by shDctn1 knockdown in Foxp2 KO neurons (D). Cultured cells in each group were prepared from at least four litters of P0 neonatal mice. *P < 0.05, **P < 0.01. One-way ANOVA followed by Tukey's HSD post hoc test. Data are mean ± SEM.
Deletion of Foxp2 impairs the microtubule dynamics in striatal neurons
In addition to organelle trafficking, DCTN1 is a plus end-localized microtubule-associated protein that binds to soluble tubulin. DCTN1 regulates the dynamics of microtubule assembly by catalysing nucleation, increasing the polymerization rate and inhibiting what is termed microtubule catastrophe, a drastic shortening of microtubules.55 Delayed recovery of microtubules from depolymerization has been reported in fibroblasts of patients with a DCTN1 G59S heterozygous point mutation.56
We hypothesized that a Foxp2 KO-induced abnormally high level of DCTN1 might also affect microtubule dynamics.55–57 We assayed the dynamics of tubulin polymerization by labelling the plus-end of tubulin with the end-binding protein 3 (EB3), which formed so-called comets close to the growing plus-end of microtubule.58 Newborn striatal cell cultures were transfected with pCMV-EB3-tdTomato plasmids. Time-lapse video microscopy indicated that the overall EB3-tdTomato fluorescence was more diffuse in dendrites of Foxp2 KO striatal neurons than in WT striatal neurons (Fig. 3A and C and Supplementary Videos 5 and 7), suggesting that Foxp2 KO decreased the binding of EB3-tdTomato to tubulin. Quantitative measurement demonstrated a significant decrease in the density of EB3-tdTomato-labelled particles in dendrites of Foxp2 KO striatal neurons (Fig. 3A, C and E). The diffuse EB3-tdTomato fluorescence also blurred the trajectories of EB3-tdTomato-labelled particles in kymographs of the Foxp2 KO group (Fig. 3A′ and C′). The movement of EB3-tdTomato-labelled particles, including their travel distance and speed, was decreased in Foxp2 KO neurons compared with those in WT striatal neurons (Fig. 3A, C, F and G and Supplementary Videos 5 and 7). These results suggested impairment in the dynamics of tubulin polymerization in the dendrites of Foxp2 KO striatal neurons.
Figure 3.
Microtubule dynamics are impaired in dendrites of Foxp2 KO striatal neurons. (A–D) The microtubule dynamics were monitored in proximal dendrites of cultured striatal neurons with time-lapse video microscopy. The video snapshots show tracing of EB3-tdTomato-labelled particles every 3 s (Supplementary Videos 5–8). The triangles indicate the positions of particles in the dendrite, with the same colours of triangles tracking the same particles. The microtubule dynamics were reduced in Foxp2 KO neurons (C) compared with Foxp2 WT neurons (A). The reduction in microtubule dynamics was rescued by knocking down Dctn1 in Foxp2 KO neurons (D). (A′–D′) Kymographs generated from time-lapse video microscopy of trajectories of EB3-tdTomato comet for 1 min. (E–G) The abnormalities in density (E), distance (F) and speed (G) of tdTomato-EB3 particles in Foxp2 KO neurons were rescued by shDctn1 knockdown in Foxp2 KO neurons. Cultured cells in each group were prepared from at least three litters of P0 neonatal mice. *P < 0.05, **P < 0.01, ***P < 0.001. Kruskal–Wallis one-way ANOVA followed by Dunn's pairwise multiple comparisons test is used in E. One-way ANOVA with Tukey's HSD post hoc test is used in F and G. Data are median ± interquartile range in E and mean ± SEM in F and G.
Compromised integrity of dynein-dynactin protein complex in Foxp2 KO striatal neurons
Based on our finding of deficits in TrkB endosome trafficking and microtubule dynamics, we presumed that the dynein-dynactin protein function was impaired in striatal neurons of the Foxp2 KO mice. An important issue to consider thus was how a de-repressed, high level of DCTN1 could lead to a functional deficit in dynein-dynactin motor protein. Dynein binds to the coiled-coil 1 domain of DCTN1 through the dynein intermediate chain (DIC) to form an intracellular transport machine.41 The level of DIC protein was not altered in the Foxp2 KO striatum (Supplementary Fig. 3E). The possibility remained, however, that the dysregulated high level of Dctn1 disrupted the integrity of the assembled protein complex.
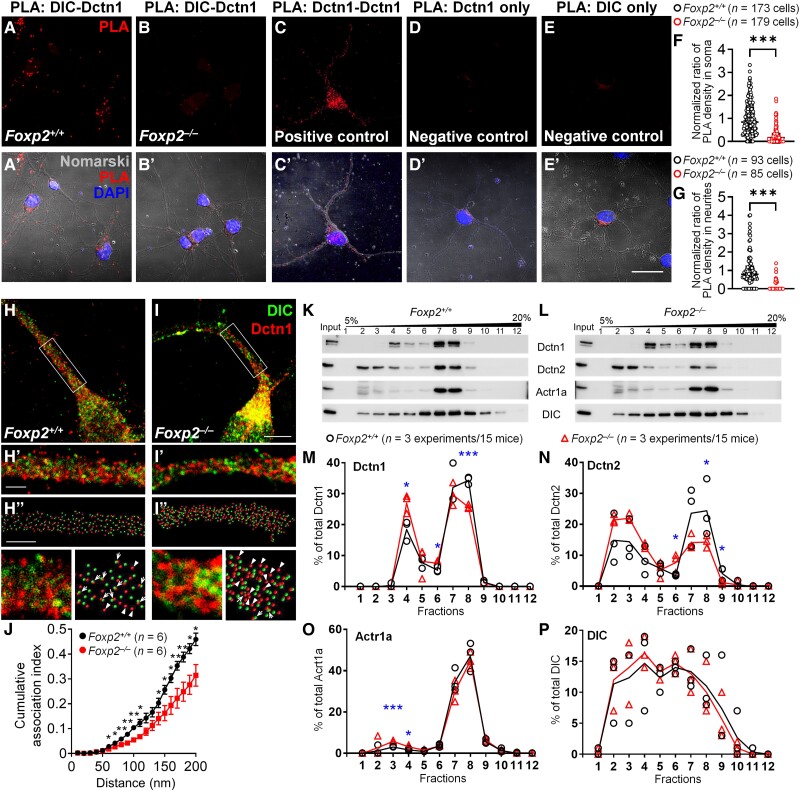
To examine whether physical interaction between DIC and Dctn1 proteins was disrupted in Foxp2 KO neurons, we performed an antibody-based in situ proximity ligation assay (PLA). PLA detects the physical interaction of proteins by generating a fluorescent signal when two proteins of interest are within 40 nm of one another.59 Endogenous DIC and DCTN1 proteins in striatal neurons were labelled with antibody-DNA oligonucleotides. PCR-amplified fluorescent puncta were detected in WT striatal neurons, indicating physical interaction of DIC and DCTN1 proteins (Fig. 4A, A′, F and G). By contrast, PCR-amplified fluorescent puncta were markedly reduced, by 87.7% and 75.7%, respectively, in proximal dendrites and cell bodies of Foxp2 KO striatal neurons (Fig. 4B, B′, F and G). These results indicate that the dysregulated high level of DCTN1 disrupts the physical interaction between DIC and DCTN1 in the protein complex.
Figure 4.
The integrity of the dynein-dynactin protein complex is compromised in Foxp2 KO striatal neurons. (A–E) PLA-generated fluorescent signals using rabbit anti-DIC and mouse anti-DCTN1 antibodies are shown in striatal cultured cells at 7-days in vitro. Signals of fluorescent puncta (red) indicating physical interaction between DIC and DCTN1 proteins are detected in the soma and neurite of WT striatal cells (A). Few fluorescent puncta were found in Foxp2 KO cells (B). (C) Positive control with mouse anti-DCTN1 and rabbit anti-Dctn1 antibodies. (D and E) Negative controls with mouse anti-DCTN1 antibody alone (D) or rabbit anti-DIC antibody alone (E). (A’′–E’′) Striatal cells containing signals of fluorescent puncta (red) are imaged with differential interference contrast (Nomarski) showing the morphology of soma and neurites (white). (F and G) Quantification of PLA fluorescent puncta in Foxp2 WT and KO group. Cultured striatal cells in each group were prepared from at least four litters of P0 newborn mice. (H and I) Photomicrographs of double immunostaining of DCTN1 and DIC in cultured striatal neurons of Foxp2 WT and KO mice shown with maximal intensity projection of STED images on the z-axis. (H′ and I′) High-magnification images of the regions marked with squares in H and I. (H′ and I′) Images of immunoreactive particles in H′ and I′ were processed by the Spots module of Imaris. Scale bars = 5 µm in A and B; 2 µm in H, I, H′ and I′. High magnification images of H′, I′, H′′ and I′′ are shown at the bottom. Arrows and arrowheads indicate DCTN1-immunoreactive particles that are adjacent and non-adjacent to DIC-immunoreactive particles, respectively. (J) The cumulative plot of the association index. The association index is the number of particles with a DIC-Dctn1 distance less than or equal to every interval (10 nm) normalized to the total number of analysed DCTN1 particles. The association index was decreased in Foxp2 KO striatal neurons (n = 6 cells/group from two independent experiments). (K and L) The sucrose gradient sedimentation experiment shows that in Foxp2 WT neurons, peak signals of DCTN1, DCTN2 and ACTR1A proteins were detected in Fractions 7 and 8 (K). In Foxp2 KO neurons, abnormal patterns of dynein-dynactin protein components were found across different fractions compared with that of WT neurons (L). (M–P) Quantitative analysis. *P < 0.05, **P < 0.01 and ***P < 0.001. Mann-Whitney-U test is used in F and G. Two-way ANOVA is used in J. Student's t-test is used in M–P. Data-points with median ± interquartile range are shown in F and G. Data are mean ± SEM in J. Data-points are shown with symbols and the means of each fraction are plotted as connected lines in M–P.
To investigate the structural integrity of the dynein-dynactin complex further, we performed stimulated emission depletion (STED) super-resolution microscopy to resolve the spatial relationship of dynein and dynactin proteins at the nanometer scale. Double immunostaining of DIC and DCTN1 was performed in striatal cultures of newborn Foxp2 WT and KO mice (Fig. 4H and I). Within the dendrites of double DIC and Dctn1 immunostained cells, STED image analysis was performed to measure the shortest distance from the centres of DCTN1-immunoreactive particles to the centres of their neighbouring DIC-immunoreactive particles (Fig. 4H′ and I′). The longer the distance, the farther away DCTN1+ particles would be separated from their neighbouring DIC+ particles in the DCTN1-DIC complex.
We counted the number of DCTN1+ articles with the shortest distance to neighbouring DIC+ particles using incremental bins of 10 nm. This value was normalized to the total number of DCTN1+ particles to derive an association index of Dctn1 and DIC. The cumulative analysis showed that the association index was decreased in the range of 60–110 nm and 140–200 nm in Foxp2 KO striatal cells compared with that in WT striatal cells (Fig. 4J). The STED imaging data thus suggest that Foxp2 deletion compromises the spacing of components of the dynein-dynactin protein complex, which might result in dysfunctional dynein-dynactin complex in Foxp2 KO striatal neurons.
Aberrant composition of the dynein-dynactin protein complex in Foxp2 KO striatal neurons
Dynactin is itself a complex of proteins that mediates the interaction of cytoplasmic dynein and its cargo. The dynactin complex consists of three major component proteins, including DCTN1, DCTN2 and ACTR1A.38 To determine whether the composition of the dynactin protein complex was altered in Foxp2 KO striatal neurons, we performed sucrose gradient sedimentation experiments. The relative levels of DCTN1, DCTN2 and ACTR1A proteins were altered across a broad range of sucrose gradient fractions, from Fraction 3 to Fraction 9, in Foxp2 KO mice compared with those in WT mice. For example, in Fraction 8, the levels of DCTN1 and DCTN2 were, by Student's t-test for WT versus Foxp2 KO, DCTN1: 34.4 ± 0.7 versus 25.7 ± 0.4; DCTN2, 24.5 ± 5.3 versus 14.4 ± 1.2 (Fig. 4K–P). These results indicated that deletion of Foxp2 alters the composition of the dynactin protein complex, which may incapacitate dynactin protein. Foxp2 deletion did not change the total protein levels of DIC and DCTN2, but rather, their relative levels in different fractions (Supplementary Fig. 3E).
We next overexpressed Dctn1 in Neuro2a cells to test whether Dctn1 overexpression altered the composition of the dynein-dynactin complex. It did. As shown in Supplementary Fig. 3, Dctn1 overexpression significantly altered the fractional patterns of DCTN1, DCTN2 and ACTR1A. These findings suggest that dysregulated levels of DCTN1 can perturb the dynein-dynactin protein complex.
Dctn1 knockdown restores endosome trafficking and microtubule dynamics in Foxp2 KO striatal neurons
If the de-repressed high level of DCTN1 were causally involved in the functional deficits in the dynein-dynactin complex, then reducing the Dctn1 level might restore the functionality of the dynein-dynactin protein in Foxp2 KO striatal neurons. We tested this possibility by using short hairpin RNA (shRNA) interference to knock down Dctn1 levels in Foxp2 KO striatal neurons. We validated the shRNA knockdown of Dctn1 (Supplementary Fig. 1). Newborn striatal cell cultures were infected with TRC010-shDctn1-CMVe-hSYNpro-EGFP (shDctn1-EGFP) or with control TRC010-shLacZ-CMVe-hSYNpro-EGFP (shLacZ-EGFP) lentiviruses and then were cultivated for 5 days before being transfected with the pCMV-TrkB-mRFP plasmid. Time-lapse video microscopy demonstrated marked increases in the travel distance and speed of TrkB-mRFP-labelled vesicles in the shDctn1 group of Foxp2 KO striatal neurons compared with the shLacZ control group (Fig. 2C–G and Supplementary Videos 3 and 4).
We then examined the causality of Foxp2 KO-induced upregulation of Dctn1 in producing defective microtubule dynamics. We transfected striatal cell cultures with shDctn1-EGFP or control shLacZ-EGFP lentiviruses, cultivated for 5 days and then transfected with the pCMV-EB3-tdTomato plasmid. The prior shRNA knockdown of Dctn1 rescued the impairments in the density and speed of EB3-labelled particles in Foxp2 KO striatal neurons (Fig. 3C–G and Supplementary Videos 7 and 8).
These findings demonstrate that the Foxp2 KO-induced dysregulation of Dctn1 causally disrupts the functional integrity of dynein-dynactin protein that normally regulates TrkB endosome trafficking and microtubule dynamics.
Dctn1 knockdown rescues defective neurite outgrowth of cultured Foxp2 KO striatal neurons
Previous study has shown that neurite outgrowth is reduced in striatal neurons of Foxp2 mutant mice,30 and genome-wide genetic screening indicates that FOXP2 targets genes that are enriched in signalling pathways promoting neurite outgrowth.30–34 BDNF-TrkB signalling endosomes are involved in neurite outgrowth of hippocampal and cortical neurons,44,49 and BDNF-TrkB signalling regulates neurite outgrowth of striatal neurons.42,43,45–48 Furthermore, microtubule dynamics can regulate neurite outgrowth.60,61
Because reducing the Dctn1 level restored TrkB endsome trafficking and microtubule dynamics in striatal neurons of the Foxp2 KO (Figs 1 and 2), we asked whether the Dctn1 abnormality is causally involved in the defective neurite outgrowth of Foxp2 KO striatal neurons (Fig. 5A, C and E–J). We knocked down Dctn1 in Foxp2 KO striatal neurons. Sholl analysis indicated a marked recovery in neurite lengths and branches of cultured Foxp2 KO striatal neurons in the shRNA Dctn1 group compared with those in the shLacZ control group (Fig. 5C–J). These findings support the conclusion that de-repressed, high levels of DCTN1 in the Foxp2 KO mice impaired neurite outgrowth of Foxp2 KO striatal neurons.
Figure 5.
Dctn1 knockdown rescues impaired neurite outgrowth of Foxp2 KO striatal neurons in vitro. (A and C) Neurite outgrowth was decreased in cultured Foxp2 KO striatal neurons (C) compared with Foxp2 WT striatal neurons (A). (B and D) Dctn1 knockdown significantly increased neurite outgrowth of Foxp2 KO striatal neurons (D). (E) Neurite complexity was analysed by Sholl analysis. The somata of analysed neurons were placed at the centre of concentric circles with the arithmetic progression of radius. (F) The number of neurites that intersected the circles was counted at different radii.92 The blue and ivory colour shedding indicate the quantification at the proximal (0–50 μm) and distal neurites (50–100 μm), respectively. (G–J) The reductions in the neurite lengths (G), the length of longest neurite (H), the number of branch points (I) and the number of terminal points (J) in Foxp2 KO neurons were reversed by Dctn1 knockdown. Two independent experiments from at least two litters for each experiment were performed. *P < 0.05, **P < 0.01, ***P < 0.001. Two-way ANOVA with Tukey's HSD post hoc test was used in F. Kruskal–Wallis one-way ANOVA followed by Dunn's pairwise multiple comparisons test are used in G–J. Data are mean ± SEM in F and median ± interquartile range in G–J.
Dctn1 knockdown restores defective cytoarchitecture of striatonigral neurons of Foxp2 KO mice
The findings so far indicate that FOXP2–DCTN1 signalling regulates neurite outgrowth in striatal cell culture. We next asked whether the cell culture findings applied to the striatum in vivo. Because FOXP2 is expressed preferentially in dopamine D1 receptor (D1R)-expressing striatal projection neurons (SPNs) of the striatonigral direct pathway,30,62 we conditionally knocked out Foxp2 in SPNs of the direct pathway using Drd1a-Cre mice (cKO). We microinjected shDctn1-EGFP and control shLacZ-EGFP lentivirus into the newborn striatum of Drd1a-Cre;Foxp2fl/fl mice63 and harvested the brains at P14. DCTN1 expression was upregulated in striatal neurons of Drd1a-Cre;Foxp2fl/fl;shLacZ conditional cKO mice (Supplementary Fig. 4C) compared with those of control Drd1a-Cre;Foxp2+/+;shLacZ mice (Supplementary Fig. 4A). Neurite lengths and branches were reduced in Drd1a-Cre;Foxp2fl/fl cKO mice compared with those in control Drd1a-Cre;Foxp2+/+ mice (Sholl analysis; Fig. 6A–H). Critically, knocking down Dctn1 in the Drd1a-Cre;Foxp2fl/fl;shDctn1-EGFP group reversed the reductions in neurite lengths and arborization of SPNs relative to those in the control Drd1a-Cre;Foxp2fl/fl;shLacZ-EGFP group (Fig. 6E–H). These results demonstrated that repression of Dctn1 by Foxp2 is important for neurite outgrowth of SPNs in vivo.
Figure 6.
Dctn1 knockdown recovers dendritic arborization of striatal neurons in vivo and partially rescues the USV function of Foxp2 cKO mice. (A) The flow chart of the experiment. (B) Microinjection site of viruses in the neonatal striatum. (C–F) Dendritic arborization was decreased in Drd1a-Cre;Foxp2fl/fl cKO striatal neurons (C) compared with Drd1a-Cre;Foxp2+/+ control mice (A). Dctn1 knockdown rescues dendritic arborization of Foxp2 cKO striatal neurons (D). The numbers of intersections are higher with warm colours. (G) Sholl analysis of dendritic complexity. The heatmap of each neuron represents a 3D volumetric colour rendering of the image stack while each new path segment is traced. (H) The reductions in dendritic lengths were rescued by Dctn1 knockdown in Drd1a-Cre;Foxp2fl/fl cKO neurons. (I–R) Dctn1 knockdown rescued the impairments in the number of events (G), frequency jump (H) and duration (I) of USV features in Drd1a-Cre;Foxp2fl/fl cKO mice. *P < 0.05, **P < 0.01, ***P < 0.001. Two-way ANOVA with Tukey's HSD post hoc test for G. Kruskal–Wallis one-way ANOVA followed by Dunn's pairwise multiple comparisons test was used in H. One-way ANOVA with Tukey's HSD post hoc test for I–R. Data are mean ± SEM in G and I–R, and median ± interquartile range in H. Cx = cortex; Sep = septum.
Dctn1 knockdown partially rescues vocal communication of Foxp2 conditional knockout neonatal mice
Previous studies including our own, have shown specific deficits in the production of ultrasonic vocalization (USV) in Foxp2 mutant mice.14–16,18,64,65 We performed isolation stress-induced USV analysis in neonatal mice to determine whether such defective USVs could be improved by decreasing Dctn1 levels in Foxp2 cKO mice (Fig. 6I–R and Supplementary Fig. 5A). We found that measurements of the events, frequency jump and duration of USVs were all decreased in Drd1a-Cre;Foxp2fl/fl;shLacZ-EGFP cKO mice with conditional deletion of Foxp2, compared with those in Drd1a-Cre;Foxp2+/+;shLacZ control mice (Fig. 6I–L and Supplementary Fig. 5A). By contrast, knocking down Dctn1 rescued the deficits in the events, frequency jump and duration of USV characteristics in Drd1a-Cre;Foxp2fl/fl;shDctn1-EGFP cKO mice compared with those in Drd1a-Cre;Foxp2fl/fl;shLacZ-EGFP control mice (Fig. 6I–L and Supplementary Fig. 5A).
These results demonstrate that Foxp2-Dctn1 genetic interaction is involved in the morphological development of striatonigral circuits, which in turn is critical for the generation of USVs in the mouse.
Homeostasis of DCTN1 is critical to neurite outgrowth
These findings indicated that a de-repressed, high level of DCTN1 is causally linked to impaired TrkB endosome trafficking and microtubule dynamics in Foxp2 KO striatal neurons in which neurite lengths and complexity were reduced. We asked whether the dysregulated high level of DCTN1 is directly responsible for the disruption in TrkB endosome trafficking, microtubule dynamics and neurite outgrowth. We over-expressed Dctn1 in WT striatal neurons by transfecting WT striatal cultures with pcDNA3.1-Dctn1-P2A-EGFP expression vector plasmids. This procedure resulted in impairments of TrkB-mRFP-labelled endosome trafficking and dynamics of EB3-tdTomato-labelled microtubules (Supplementary Figs 6 and 7), along with marked decreases in neurite lengths and branches of the cells (Supplementary Fig. 8).
Paradoxically, depletion of Dctn1 to a low level was also disruptive to neurite outgrowth: Dctn1 deletion by CRISPR-Cas9-mediated genome editing resulted in reductions in neurite lengths and branches of striatal neurons (Supplementary Fig. 9). On this basis, we hypothesized that DCTN1 homeostasis could be essential for the neurite outgrowth of striatal neurons. Changing the homeostasis of DCTN1, either toward a higher or a lower level, could have interfered with organelle transport and microtubule dynamics and hence neurite outgrowth. When we normalized the measured values to levels in the control group (Drd1a-Cre;Foxp2+/+;shLacZ-EGFP), we found that Foxp2 cKO mice (Drd1a-Cre;Foxp2fl/fl;shLacZ-EGFP) had 137% increases in DCTN1 levels, and that neurite length was decreased to 60.8% of the control group. By comparison, knocking down Dctn1 in Foxp2 cKO mice (Drd1a-Cre;Foxp2fl/fl;shDctn1-EGFP) decreased abnormally high levels of DCTN1 to 93.5% of the control group, and restored neurite lengths to levels similar to control levels (Supplementary Fig. 10A). These findings suggest that there is an inverted U-curve relationship between Dctn1 expression and neurite outgrowth, and that this relationship is regulated by FOXP2 (Supplementary Fig. 10A). The implication prompted by these results is that FOXP2 controls neurite outgrowth of striatal neurons by maintaining the homeostasis of DCTN1.
Defective FOXP2–DCTN1 interaction may be involved in the pathophysiology of FOXP2R553H mutation
In an attempt to relate these findings to the human condition produced by FOXP2 missense mutation, we asked whether a genetic-level interaction of FOXP2 with DCTN1 plays a role in the pathogenesis of human FOXP2R553H missense mutation. Previous work has demonstrated that FOXP2R553H mutant protein loses DNA binding ability. Because FOXP2 proteins form homodimers, FOXP2R553H mutant protein can also interfere with transcriptional activity by dimerization with WT FOXP2 protein.51 Given the potential dominant-negative effect of the FOXP2R553H mutation and the high sequence homology between mouse Foxp2 and human FOXP2,66 we infected striatal cell cultures from newborn WT mice with a lentivirus vector containing dual H1 and ubiquitin promoters,67pJHUG-H1-shLacZ-Ubi-FOXP2R553H-p2A-GFP (shLacZ-FOXP2R553H-GFP) or control pJHUG-H1-shLacZ-Ubi-FOXP2WT-p2A-GFP (shLacZ-FOXP2WT-GFP) lentivirus. These lentivirus vectors infected neurons with high efficiency. Time-lapse video microscopy showed that overexpression of the FOXP2R553H mutant protein decreased the travel distance and speed of TrkB-RFP-labelled endosomes in dendrites of WT cultured striatal neurons compared with those in the control shLacZ-FOXP2WT-GFP group (Fig. 7A, B, D, F and G). Paralleling this impaired TrkB-RFP endosome trafficking; neurite lengths and branches were also decreased in the shLacZ-FOXP2R553H-GFP group compared with the control shLacZ-FOXP2WT-GFP group levels (Fig. 7H–K).
Figure 7.
FOXP2R553H mutation impairs intracellular endosome transport and neurite outgrowth of striatal neurons, which is rescued by the knockdown of Dctn1. (A) The flow chart of the experiment in E–H. (B–E) Time-lapse video microscopy showing trafficking of TrkB-RFP endosomes in dendrites of cultured striatal neurons. The kymographs show trajectories of TrkB-RFP vesicles during a 3-min recording. Triangles with the same colours indicate the positions of the same vesicles in the dendrite. The distance and speed of TrkB-RFP vesicles were reduced in FOXP2R553H-expressing neurons (D) compared with control FOXP2WT-expressing neurons (B). The abnormalities of TrkB-RFP-labelled vesicle transport were rescued by knocking down Dctn1 in FOXP2R553H-expressing neurons (E). (B′–E′) Kymographs generated from time-lapse video microscopy of trajectories of TrkB-mRFP vesicles for 180 s. (F and G) Quantitative analysis of TrkB-RFP endosome transport. Cultured cells in each group were prepared from at least four litters of P0 neonatal mice. (H) The flow chart of the experiment in I–N. (I–K) The dendritic complexity (J) and total lengths (K) were reduced in FOXP2R553H-expressing striatal neurons (I′) compared with FOXP2WT-expressing striatal neurons (I) in vivo. The heatmap of each neuron represents a 3D volumetric colour rendering of the image stack while each new path segment is traced. (L–N) The reductions in dendritic arborization (M) and total lengths (N) were rescued in shDctn1;FOXP2R553H-expressing striatal neurons (L′) compared with control shLacZ;FOXP2R553H-expressing striatal neurons (L) in vivo. *,#P < 0.05, **,##P < 0.01, ***,###P < 0.001. One-way ANOVA with Tukey's HSD post hoc test was used in F–G. Two-way ANOVA with Tukey's HSD post hoc test was used in J and M. Significant differences between groups in J and M were quantified by the simple main effect of two-way ANOVA. Student's t-test was used in K and N. Data are mean ± SEM.
As in the Foxp2 KO striatum, in which increased DCTN1 was observed, in mice in which we induced the overexpression of FOXP2R553H in the striatum, we found up-regulation of DCTN1 in striatal neurons (Supplementary Fig. 4F–J). We then decreased the increased level of Dctn1 with shRNA to determine whether this would ameliorate deficits in TrkB-RFP endosome trafficking and neurite outgrowth. The abnormalities of TrkB-RFP-labelled vesicle transport were rescued by this knockdown of DCTN1 in FOXP2R553H-expressing neurons (Fig. 7D–G and Supplementary Videos 9–12). Furthermore, Dctn1 knockdown restored neurite lengths and dendritic arborization of striatal neurons of WT mice infected with pJHUG-H1-shDctn1-Ubi-FOXP2R553H-p2A-GFP (shDctn1-FOXP2R553H-GFP) lentivirus relative to those observed in WT mice infected with the control pJHUG-H1-shLacZ-Ubi-FOXP2R553H-p2A-GFP (shLacZ-FOXP2R553H-GFP) lentivirus in vivo (Fig. 7H and L–N).
Dctn1 knockdown restores morphological and physiological deficits in SPNs of FOXP2R553H mice
We asked whether FOXP2R553H-induced deficits in vocalization could be mitigated by knocking down striatal DCTN1. To study the interaction of human WT FOXP2WT and FOXP2R553H mutant proteins in the mouse brain in vivo, we microinjected pJHUG-H1-shLacZ-Ubi-FOXP2R553H-p2A-mCherry (shLacZ-FOXP2R553H-mCherry) lentivirus into the newborn striatum of Foxp2H/− mice that carried a humanized version of the WT Foxp2 allele (H) and a mouse Foxp2 null allele (−) (Fig. 8A).16 We expressed the FOXP2R553H mutant gene in Foxp2H/− mice to model human KE patients carrying the heterozygous FOXP2R553H mutant allele. Morphological analysis indicated reductions in neurite lengths and branches of mCherry+ cells in the striatum of Foxp2H/−;shLacZ-FOXP2R553H-mCherry mice compared with control values in Foxp2H/−;shLacZ-FOXP2WT-mCherry mice (Fig. 8B, B′, C and D). Remarkably, Dctn1 knockdown restored neurite lengths and arborization of mCherry+ cells in the striatum of Foxp2H/−;shDctn1-FOXP2R553H-mCherry mice compared with control Foxp2H/−;shLacZ-FOXP2R553H-mCherry mice (Fig. 8B, B′, C, D and Supplementary Fig. 10B).
Figure 8.
Dctn1 knockdown restores dendritic arborization of striatal neurons and concomitantly partially rescues vocalization in a mouse model of FOXP2R553H mutation. (A) The flow chart of the experiment. (B–D) Dendritic arborization (C) and total lengths (D) were decreased in striatal neurons of shLacZ;FOXP2R553H-infected Foxp2H/− mice (B′) compared with control shLacZ;FOXP2WT-infected Foxp2H/− mice (B). The dendritic reductions were reversed by Dctn1 knockdown (B′). The heat map of each neuron represents a 3D volumetric colour rendering of the image stack while each new path segment is traced. (E) Whole cell patch-clamp electrophysiological shows that the frequency, but not amplitude, of mEPSCs was reduced in striatal neurons of shLacZ;FOXP2R553H-infected Foxp2H/− mice. Knocking down Dctn1 in FOXP2R553H-expressing neurons reverses the decreased mEPSC frequency. (F–K) The events, frequency jump and duration of USV features were reduced in shLacZ;FOXP2R553H-infected Foxp2H/− mice compared with shLacZ;FOXP2WT-infected Foxp2H/− mice at P8. Decreased USV events in shLacZ;FOXP2R553H-infected Foxp2H/− mice were reversed in shDctn1;FOXP2R553H-infected mice. (L) Schematic drawings summarize the major findings in the present study. *,#,†P < 0.05, **,##,††P < 0.01, ***,###,†††P < 0.001. In C, asterisk indicates the significant difference between Foxp2H/−;shLacZ;FOXP2WT and Foxp2H/−;shLacZ;FOXP2R553H groups; number sign indicates the significant difference between Foxp2H/−;shLacZ;FOXP2R553H and Foxp2H/−;shDctn1;FOXP2R553H groups; dagger indicates the significant difference between Foxp2H/−;shLacZ;FOXP2WT and Foxp2H/−;shDctn1;FOXP2R553H groups. One-way ANOVA with Tukey's HSD post hoc test was used in D, G, H and J. Two-way ANOVA with Tukey's HSD post hoc test were used in C. Significant differences between groups in C were tested by the simple main effect of two-way ANOVA. Kruskal–Wallis one-way ANOVA followed by Dunn's pairwise multiple comparisons test was used in E, F and I. Data are mean ± SEM in C, D, G, H and J, and median ± interquartile range in E, F and I.
We further asked whether the restored neuronal morphology could impact electrophysiological activity. We performed whole-cell patch clamp recording to measure the miniature excitatory postsynaptic currents (mEPSCs) of neonatal striatal neurons. The frequency of mEPSCs was significantly reduced in striatal neurons of Foxp2H/−;shLacZ-FOXP2R553H-EGFP mice compared with that of Foxp2H/−;shLacZ-FOXP2WT-EGFP control mice (Fig. 8E). The amplitude of mEPSCs was, however, not affected. Dctn1 knockdown alleviated the reduction in mEPSC frequency of striatal neurons of Foxp2H/−;shDctn1-FOXP2R553H-EGFP mice compared with that of Foxp2H/−;shLacZ-FOXP2R553H-EGFP controls (Fig. 8E).
Dctn1 knockdown partially rescues vocalization in FOXP2R553H mice
At the behavioural level, Foxp2H/−;shLacZ-FOXP2R553H-mCherry mice exhibited reductions in events, frequency jump and duration of USV characteristics (Fig. 8F–K and Supplementary Fig. 5B). Dctn1 knockdown increased events but not the frequency jump and duration of USVs in the Foxp2H/−;shDctn1-FOXP2R553H-mCherry neonatal mice compared with control Foxp2H/−;shLacZ-FOXP2R553H-mCherry neonatal mice (Fig. 8F–H and Supplementary Fig. 5B).
Taken together, reducing abnormally high levels of DCTN1 not only rescues dendritic morphology and electrophysiological activity of striatal neurons, but also partially alleviates vocalization in a mouse model of FOXP2R553H mutation (Fig. 8L).
Discussion
Increasing evidence suggests that a major function of FOXP2 is to regulate the morphological development of neurons, including their dendrites and spines. Previous studies have uncovered the mechanisms underlying FOXP2-mediated regulation of spine formation and synaptogenesis in both avian and rodent brains.18–22 Here, we complement these studies with the surprise identification of a cellular mechanism by which FOXP2, famed for its potential involvement in human speech, can impact dendritic outgrowth in the striatum. We demonstrate that FOXP2 acts through multiple biological pathways related to intracellular dynein-dynactin molecular motors and TrkB to coordinate the development of dendrites and spines, which as a whole enables striatal circuit assembly for vocal communication.
Our findings lead to three major conclusions. First, FOXP2 acts during the development of the striatum to regulate the homeostasis of intracellular trafficking by directly interacting with the dynactin component of dynein-dynactin protein complexes. Second, accompanying the structural abnormalities induced by loss of FOXP2-driven homeostatic regulation of dynein-dynactin protein motors, functional deficits in vocalization behaviour occur. Third, the FOXP2R553H mutation associated with speech disorders in humans, when expressed in a mouse model, induces dynactin-associated abnormalities strikingly similar to those in Foxp2 knockout mice. These include deficits in TrkB endosome trafficking, neurite extension and dendritic development as well as abnormal vocalizations similar to those exhibited by the Foxp2 deletion mice. These findings suggest a remarkable link between the dynamics of intracellular trafficking and the control of vocalization. They further explicitly point to a potential role for defective FOXP2-DCTN1 genetic interaction in the pathophysiology of FOXP2R553H mutation and FOXP2 deletion-associated speech disorders.
Regulation of DCTN1 by FOXP2 is important for endosome trafficking and microtubule stability
Previous studies have shown that FOXP2 regulates downstream genes involved in neuronal differentiation, neurite outgrowth, migration, synaptogenesis, neurotransmission and synaptic plasticity.30–34,68 Our findings expose a mechanism by which FOXP2 can control critical aspects of development in striatal neurons. FOXP2 acts as a transcriptional repressor of Dctn1, which we found to be important for the morphological development of striatal neurons. As causal evidence, we demonstrated that Foxp2 KO-induced impairments in TrkB endosome trafficking and microtubule dynamics, and defective neurite outgrowth could be rescued by knocking down Dctn1. At the circuit level, knocking down Dctn1 in striatonigral neurons not only restored dendritic lengths and arborization, but also rescued in part the vocalizations of Foxp2 cKO mice. These findings extend the evidence for the importance of Dctn1 in intracellular transport first shown in Drosophila homologue p150(Glued),69,70 in which the p150(Glued) protein participates in dynein-mediated vesicle trafficking at synaptic terminals71 and also enhances microtubule stability through its anti-catastrophe activity at the microtubule plus-end in neuronal cells. Notably, a genetic variant of p150(Glued) abolishes its anti-catastrophic activity and is associated with the Perry syndrome.55 Moreover, genetic mutations of DYNACTIN are associated with other diseases of motor neurons, including amyotrophic lateral sclerosis and frontotemporal dementia.72–75
Foxp2 KO impairs the function of dynein-dynactin motor protein
Our data from proximity ligation assay, STED super-resolution microscopy and sucrose gradient sedimentation assays demonstrated an aberrant assembly of the dynein-dynactin protein complex. Cytoplasmic dynactin is required for intracellular organelle transport.40 Dynactin acts to tether dynein, and the dynactin tethers are released by binding of early endosomes, which in turn permits the endosome-bound dynein to move toward the minus-end of the microtubule.76 Transgenic overexpression of dynamitin (Dctn2), another subunit of the dynactin complex, has been found to induce disassembling of the dynactin complex and impairs vesicle transport in motor neurons of the spinal cord.77 We found here that Foxp2 deletion induced an upregulation of DCTN1 that interfered with the activity of the dynein-dynactin motor proteins used for organelle transport. Therefore, the homeostasis of the components of the dynein-dynactin protein complex is essential for the motor function of the protein complex in mediating intracellular vesicle transport. Indeed, consistent with this hypothesis, Foxp2 overexpression, which decreased the Dctn1 level, also impaired intracellular vesicle transport and neurite outgrowth. Taken together, an optimal level of FOXP2 is important for the regulation of its target genes and thereby their functional actions. This is consistent with the reports that dynamic behaviour-linked regulation of avian FOXP2 is critical to vocal learning of songbirds and that FoxP2 overexpression impairs directed song singing.78,79
FOXP2 mutation may primarily affect the caudate nucleus in FOXP2R553H-associated speech disorder
Foxp2 expression is evolutionarily conserved in motor-related regions in the avian, rodent, non-human primate and human brains.26,66,80–83Foxp2 orthologues are highly expressed in the developing striosome compartment of mouse, rat and monkey brains.18,82–84 In developing non-human primates, FOXP2 expression is higher in the caudate nucleus than in the putamen.83 Evidence from single-nucleus RNA sequencing (snRNA-seq) experiments further suggests that Foxp2 is enriched in striosomes relative to the matrix, though it is expressed in both compartments.85 The striosome compartment takes up a significant part of the head of human caudate nucleus, in which a prominent reduction in volume is observed in KE patients and in a child of a different family carrying a FOXP2 intragenic deletion.4,6,86 If, as snRNA-seq evidence suggests, FOXP2 has at least somewhat elevated levels in human striosomes, then FOXP2 mutation and deletion, by targeting dendritic development, could particularly impact the caudate nucleus as brain imaging studies have reported.4,6,7,87
Limitations and issues for further work
Our study is subject to limitations. We have only focused on the striatum, but other regions of the brain are impacted by Foxp2 mutation or deletion. For example, defective morphological development has been found in dendritic trees of Purkinje neurons in the cerebellum and apical dendrites of layer VI cortical neurons where FOXP2 is highly expressed.13–15,17 Furthermore, we have not fully analysed the input-output circuitry of the Foxp2 KO and mutant mice in which we demonstrated disturbed FOXP2-DCTN1 interaction, although we did document a reduction in dendritic arborization of striatal neurons expressing the FOXP2R553H mutant. We could not, for technical reasons, make direct measurements of the status of axons, and thus lack information on long-distance circuits emanating from striatal projection neurons. We infer that the FOXP2-DCTN1 interaction that we uncovered for striatal neurons has circuit-level effects evident at the behavioural level.
In Drd1a-Cre;Foxp2fl/fl cKO mice, SPNs were infected with constitutively active shDctn1-EGFP and shLacZ-EGFP lentivirus. Although Foxp2 is preferentially expressed in D1R-expressing striatonigral SPNs, Foxp2 is expressed in ∼21–26% of D2R-expressing striatopallidal SPNs.62 It has been reported that ∼26–27% of SPNs co-express D1R and D2R.88 Some FOXP2-positive striatopallidal SPNs co-expressing D1Rs and therefore containing Drd1a-Cre activity might have been included in the dataset that we analysed. The results of our Drd1a-Cre;Foxp2fl/fl cKO analysis thus reflect the effects of Dctn1 knockdown on a majority of GFP-positive striatonigral SPNs, a minority of GFP-positive striatopallidal SPNs, and some GFP-positive SPNs that lack Cre activity in Foxp2 cKO mice.
Another limitation is that we did not investigate potential circuit remodelling associated with FOXP2-mediated neural plasticity, given that FOXP2 is required for vocal learning in songbirds and motor learning in mice.23–29 We focused first here on the developmental function of FOXP2. It would be of great interest to test whether the developmental mechanisms that we have characterized here are shared by neural plasticity in the mature brain. Of note, in the Foxp2H/− heterozygous mouse model, we virally expressed FOXP2WT and FOXP2R553H in striatal cells, but this did not mimic the constitutive expression of FOXP2R553H in all cells of KE patients. This non-uniform experimental situation was nonetheless advantageous, as it permitted the evaluation of the striatum-specific effect of the FOXP2R553H mutant. We realize, however, that the ratio of lentivirus-transduced FOXP2WT to FOXP2R553H protein in striatal cells of Foxp2H/− mice could be different from that driven by the endogenous promoter in physiological conditions. It is also important to point out that the properties of mouse pup isolation calls are not a simple proxy of human speech as has been indicated in prior studies.89,90 The altered pup USVs in Foxp2 mutant mice is not equivalent to speech apraxia in human patients.91
Despite these and other limitations, our findings demonstrate that a key function of FOXP2 in the striatum is to regulate dynein-dynactin molecular motors critical to the development and subsequent function of the striatum and suggest that dysregulation of DCTN1 homeostasis in striatal neurons could underlie pathophysiologic aspects of speech disorders in patients with FOXP2 mutations and deletion.
Supplementary Material
Acknowledgements
We thank Drs W. Enard and S. Pääbo for providing Foxp2 transgenic mice, Drs M. V. Chao, L. Chen and P.-S. Hou for providing reagents, Dr B.-C. Chen for image deconvolution, Drs J.-C. Liao and C.-C. Huang for help with super-resolution microscopy, Dr H.-J. Cheng for reading the manuscript, C.-E. Wu, R.-T. Hsu and C.-W. Huang for assistance in experiments, Dr Y. Kubota for manuscript preparation, Neuroscience Program in Academia Sinica Core Facility for electrophysiological and image analyses, and Academia Sinica RNAi Core Facility for providing shRNA plasmids.
Contributor Information
Hsiao-Ying Kuo, Institute of Neuroscience, National Yang Ming Chiao Tung University, Taipei 112304, Taiwan; Institute of Anatomy and Cell Biology, National Yang Ming Chiao Tung University, Taipei 112304, Taiwan.
Shih-Yun Chen, Institute of Neuroscience, National Yang Ming Chiao Tung University, Taipei 112304, Taiwan.
Rui-Chi Huang, Institute of Neuroscience, National Yang Ming Chiao Tung University, Taipei 112304, Taiwan.
Hiroshi Takahashi, Department of Neurology, National Hospital Organization, Tottori Medical Center, Tottori 689-0203, Japan.
Yen-Hui Lee, Institute of Neuroscience, National Yang Ming Chiao Tung University, Taipei 112304, Taiwan.
Hao-Yu Pang, Institute of Neuroscience, National Yang Ming Chiao Tung University, Taipei 112304, Taiwan.
Cheng-Hsi Wu, Institute of Neuroscience, National Yang Ming Chiao Tung University, Taipei 112304, Taiwan.
Ann M Graybiel, McGovern Institute for Brain Research and Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Fu-Chin Liu, Institute of Neuroscience, National Yang Ming Chiao Tung University, Taipei 112304, Taiwan.
Funding
This work was supported by the Ministry of Science and Technology-Taiwan grants MOST110-2326-B-A49A-504 (to F.-C.L.), MOST111-2326-B-A49-002 (to F.-C.L.), and in part by the following: MOST107-2320-B-010-041-MY3 (F.-C.L.), MOST110-2320-B-A49A-532-MY3 (F.-C.L.), MOST111-2321-B-001-011 (F.-C.L.), MOST107-2321-B-010-010-MY3 (H.-Y.K.), MOST110-2811-B-A49A-031 (S.-Y.C.); Ministry of Education-Taiwan grant (F.-C.L.); National Institute of Mental Health grant R01 MH060379 (A.M.G.); Saks Kavanaugh Foundation (A.M.G.); Kristin R. Pressman and Jessica J. Pourian ‘13 Fund' (A.M.G.); Dr Stephan and Mrs Anne Kott (A.M.G.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Vargha-Khadem F, Watkins KE, Price CJ, et al. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci USA. 1998;95:12695–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. [DOI] [PubMed] [Google Scholar]
- 3. Reuter MS, Riess A, Moog U, et al. FOXP2 Variants in 14 individuals with developmental speech and language disorders broaden the mutational and clinical spectrum. J Med Genet. 2017;54:64–72. [DOI] [PubMed] [Google Scholar]
- 4. Liégeois FJ, Hildebrand MS, Bonthrone A, et al. Early neuroimaging markers of FOXP2 intragenic deletion. Sci Rep. 2016;6:35192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacDermot KD, Bonora E, Sykes N, et al. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005;76:1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watkins KE, Gadian DG, Vargha-Khadem F. Functional and structural brain abnormalities associated with a genetic disorder of speech and language. Am J Hum Genet. 1999;65:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watkins KE, Vargha-Khadem F, Ashburner J, et al. MRI Analysis of an inherited speech and language disorder: Structural brain abnormalities. Brain. 2002;125:465–478. [DOI] [PubMed] [Google Scholar]
- 8. Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 And the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6:131–138. [DOI] [PubMed] [Google Scholar]
- 9. Crinion J, Turner R, Grogan A, et al. Language control in the bilingual brain. Science. 2006;312:1537–1540. [DOI] [PubMed] [Google Scholar]
- 10. Badcock NA, Bishop DV, Hardiman MJ, Barry JG, Watkins KE. Co-localisation of abnormal brain structure and function in specific language impairment. Brain Lang. 2012;120:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. [DOI] [PubMed] [Google Scholar]
- 12. Turner SJ, Hildebrand MS, Block S, et al. Small intragenic deletion in FOXP2 associated with childhood apraxia of speech and dysarthria. Am J Med Genet A. 2013;161a:2321–2326. [DOI] [PubMed] [Google Scholar]
- 13. Druart M, Groszer M, Le Magueresse C. An etiological Foxp2 mutation impairs neuronal gain in layer VI cortico-thalamic cells through increased GABAB/GIRK signaling. J Neurosci. 2020;40:8543–8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujita E, Tanabe Y, Shiota A, et al. Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proc Natl Acad Sci USA. 2008;105:3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shu W, Cho JY, Jiang Y, et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci USA. 2005;102:9643–9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Enard W, Gehre S, Hammerschmidt K, et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137:961–971. [DOI] [PubMed] [Google Scholar]
- 17. Usui N, Co M, Harper M, et al. Sumoylation of FOXP2 regulates motor function and vocal communication through Purkinje cell development. Biol Psychiatry. 2017;81:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen YC, Kuo HY, Bornschein U, et al. Foxp2 controls synaptic wiring of corticostriatal circuits and vocal communication by opposing Mef2c. Nat Neurosci. 2016;19:1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adam I, Mendoza E, Kobalz U, Wohlgemuth S, Scharff C. CNTNAP2 Is a direct FoxP2 target in vitro and in vivo in zebra finches: Complex regulation by age and activity. Genes Brain Behav. 2017;16:635–642. [DOI] [PubMed] [Google Scholar]
- 20. Vernes SC, Newbury DF, Abrahams BS, et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulz SB, Haesler S, Scharff C, Rochefort C. Knockdown of FoxP2 alters spine density in area X of the zebra finch. Genes Brain Behav. 2010;9:732–740. [DOI] [PubMed] [Google Scholar]
- 22. Sia GM, Clem RL, Huganir RL. The human language-associated gene SRPX2 regulates synapse formation and vocalization in mice. Science. 2013;342:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teramitsu I, Poopatanapong A, Torrisi S, White SA. Striatal FoxP2 is actively regulated during songbird sensorimotor learning. PLoS One. 2010;5:e8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Groszer M, Keays DA, Deacon RM, et al. Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr Biol. 2008;18:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haesler S, Rochefort C, Georgi B, et al. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus area X. PLoS Biol. 2007;5:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haesler S, Wada K, Nshdejan A, et al. Foxp2 expression in avian vocal learners and non-learners. J Neurosci. 2004;24:3164–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. French CA, Jin X, Campbell TG, et al. An aetiological Foxp2 mutation causes aberrant striatal activity and alters plasticity during skill learning. Mol Psychiatry. 2012;17:1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fisher SE, Scharff C. FOXP2 As a molecular window into speech and language. Trends Genet. 2009;25:166–177. [DOI] [PubMed] [Google Scholar]
- 29. Schreiweis C, Bornschein U, Burguière E, et al. Humanized Foxp2 accelerates learning by enhancing transitions from declarative to procedural performance. Proc Natl Acad Sci USA. 2014;111:14253–14258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vernes SC, Oliver PL, Spiteri E, et al. Foxp2 regulates gene networks implicated in neurite outgrowth in the developing brain. PLoS Genet. 2011;7:e1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hickey SL, Berto S, Konopka G. Chromatin decondensation by FOXP2 promotes human neuron maturation and expression of neurodevelopmental disease genes. Cell Rep. 2019;27:1699–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spiteri E, Konopka G, Coppola G, et al. Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am J Hum Genet. 2007;81:1144–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oswald F, Kloble P, Ruland A, et al. The FOXP2-driven network in developmental disorders and neurodegeneration. Front Cell Neurosci. 2017;11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vernes SC, Spiteri E, Nicod J, et al. High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am J Hum Genet. 2007;81:1232–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kosubek-Langer J, Scharff C. Dynamic FoxP2 levels in male zebra finches are linked to morphology of adult-born area X medium spiny neurons. Sci Rep. 2020;10:4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teramitsu I, White SA. Foxp2 regulation during undirected singing in adult songbirds. J Neurosci. 2006;26:7390–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. King JW. In silico models of the human language gene FOXP2: Target genes identified from Drosophila promoters. In: Programs and abstracts of the thirty-four annual meeting for society for neuroscience. Society for neuroscience, 2004: program No. 44.25. Neuroscience meeting planner. Online.
- 38. Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. [DOI] [PubMed] [Google Scholar]
- 39. Vallee RB, McKenney RJ, Ori-McKenney KM. Multiple modes of cytoplasmic dynein regulation. Nat Cell Biol. 2012;14:224–230. [DOI] [PubMed] [Google Scholar]
- 40. Moughamian AJ, Holzbaur EL. Dynactin is required for transport initiation from the distal axon. Neuron. 2012;74:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siglin AE, Sun S, Moore JK, et al. Dynein and dynactin leverage their bivalent character to form a high-affinity interaction. PLoS One. 2013;8:e59453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horch HW, Katz LC. BDNF Release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. [DOI] [PubMed] [Google Scholar]
- 44. Lazo OM, Gonzalez A, Ascaño M, et al. BDNF Regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J Neurosci. 2013;33:6112–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. [DOI] [PubMed] [Google Scholar]
- 46. McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. [DOI] [PubMed] [Google Scholar]
- 47. Rauskolb S, Zagrebelsky M, Dreznjak A, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song M, Giza J, Proenca CC, et al. Slitrk5 mediates BDNF-dependent TrkB receptor trafficking and signaling. Dev Cell. 2015;33:690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou B, Cai Q, Xie Y, Sheng ZH. Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell Rep. 2012;2:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ehrlich ME, Conti L, Toselli M, et al. ST14A Cells have properties of a medium-size spiny neuron. Exp Neurol. 2001;167:215–226. [DOI] [PubMed] [Google Scholar]
- 51. Vernes SC, Nicod J, Elahi FM, et al. Functional genetic analysis of mutations implicated in a human speech and language disorder. Hum Mol Genet. 2006;15:3154–3167. [DOI] [PubMed] [Google Scholar]
- 52. Li Y, Yui D, Luikart BW, et al. Conditional ablation of brain-derived neurotrophic factor-TrkB signaling impairs striatal neuron development. Proc Natl Acad Sci USA. 2012;109:15491–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. [DOI] [PubMed] [Google Scholar]
- 54. Kapitein LC, Schlager MA, Kuijpers M, et al. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–299. [DOI] [PubMed] [Google Scholar]
- 55. Lazarus JE, Moughamian AJ, Tokito MK, Holzbaur EL. Dynactin subunit p150(Glued) is a neuron-specific anti-catastrophe factor. PLoS Biol. 2013;11:e1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Levy JR, Sumner CJ, Caviston JP, et al. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J Cell Biol. 2006;172:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Waterman-Storer CM, Karki S, Holzbaur EL. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc Natl Acad Sci USA. 1995;92:1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stepanova T, Slemmer J, Hoogenraad CC, et al. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J Neurosci. 2003;23:2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gullberg M, Gústafsdóttir SM, Schallmeiner E, et al. Cytokine detection by antibody-based proximity ligation. Proc Natl Acad Sci USA. 2004;101:8420–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Athamneh AIM, He Y, Lamoureux P, et al. Neurite elongation is highly correlated with bulk forward translocation of microtubules. Sci Rep. 2017;7:7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Riederer BM, Pellier V, Antonsson B, et al. Regulation of microtubule dynamics by the neuronal growth-associated protein SCG10. Proc Natl Acad Sci USA. 1997;94:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fong L, Kuo HY, Wu HL, Chen SY, Liu FC. Differential and overlapping pattern of foxp1 and foxp2 expression in the striatum of adult mouse brain. Neuroscience. 2018;388:214–223. [DOI] [PubMed] [Google Scholar]
- 63. Chen SY, Kuo HY, Liu FC. Stereotaxic surgery for genetic manipulation in striatal cells of neonatal mouse brains. J Vis Exp. 2018;137:57270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gaub S, Fisher SE, Ehret G. Ultrasonic vocalizations of adult male Foxp2-mutant mice: Behavioral contexts of arousal and emotion. Genes Brain Behav. 2016;15:243–259. [DOI] [PubMed] [Google Scholar]
- 65. Chabout J, Sarkar A, Patel SR, et al. A Foxp2 mutation implicated in human speech deficits alters sequencing of ultrasonic vocalizations in adult male mice. Front Behav Neurosci. 2016;10:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Enard W, Przeworski M, Fisher SE, et al. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869–872. [DOI] [PubMed] [Google Scholar]
- 67. Kim JI, Ganesan S, Luo SX, et al. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015;350:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Konopka G, Bomar JM, Winden K, et al. Human-specific transcriptional regulation of CNS development genes by FOXP2. Nature. 2009;462:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reddy S, Jin P, Trimarchi J, et al. Mutant molecular motors disrupt neural circuits in Drosophila. J Neurobiol. 1997;33:711–723. [DOI] [PubMed] [Google Scholar]
- 70. Martin M, Iyadurai SJ, Gassman A, et al. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell. 1999;10:3717–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lloyd TE, Machamer J, O'Hara K, et al. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 2012;74:344–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Münch C, Sedlmeier R, Meyer T, et al. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology. 2004;63:724–726. [DOI] [PubMed] [Google Scholar]
- 73. Puls I, Jonnakuty C, LaMonte BH, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. [DOI] [PubMed] [Google Scholar]
- 74. Lai C, Lin X, Chandran J, et al. The G59S mutation in p150(glued) causes dysfunction of dynactin in mice. J Neurosci. 2007;27:13982–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Münch C, Rosenbohm A, Sperfeld AD, et al. Heterozygous R1101K mutation of the DCTN1 gene in a family with ALS and FTD. Ann Neurol. 2005;58:777–780. [DOI] [PubMed] [Google Scholar]
- 76. Zhang J, Zhuang L, Lee Y, et al. The microtubule plus-end localization of Aspergillus dynein is important for dynein-early-endosome interaction but not for dynein ATPase activation. J Cell Sci. 2010;123:3596–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. LaMonte BH, Wallace KE, Holloway BA, et al. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. [DOI] [PubMed] [Google Scholar]
- 78. Heston JB, White SA. Behavior-linked FoxP2 regulation enables zebra finch vocal learning. J Neurosci. 2015;35:2885–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Day NF, Hobbs TG, Heston JB, White SA. Beyond critical period learning: Striatal foxp2 affects the active maintenance of learned vocalizations in adulthood. eNeuro. 2019;6:ENEURO.0071-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of foxp2 and foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460:266–279. [DOI] [PubMed] [Google Scholar]
- 81. Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 Expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:2455–2462. [DOI] [PubMed] [Google Scholar]
- 82. Takahashi K, Liu FC, Hirokawa K, Takahashi H. Expression of Foxp2, a gene involved in speech and language, in the developing and adult striatum. J Neurosci Res. 2003;73:61–72. [DOI] [PubMed] [Google Scholar]
- 83. Takahashi K, Liu FC, Oishi T, et al. Expression of FOXP2 in the developing monkey forebrain: Comparison with the expression of the genes FOXP1, PBX3, and MEIS2. J Comp Neurol. 2008;509:180–189. [DOI] [PubMed] [Google Scholar]
- 84. Cirnaru MD, Song S, Tshilenge KT, et al. Unbiased identification of novel transcription factors in striatal compartmentation and striosome maturation. Elife. 2021;10:e65979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Matsushima A, Pineda SS, Crittenden JR, et al. Transcriptional vulnerabilities of striatal neurons in human and rodent models of Huntington's disease. Nat Commun. 2023;14:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Graybiel AM, Ragsdale CW Jr. Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci USA. 1978;75:5723–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liegeois F, Baldeweg T, Connelly A, et al. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003;6:1230–1237. [DOI] [PubMed] [Google Scholar]
- 88. Lester J, Fink S, Aronin N, DiFiglia M. Colocalization of D1 and D2 dopamine receptor mRNAs in striatal neurons. Brain Res. 1993;621:106–110. [DOI] [PubMed] [Google Scholar]
- 89. Konopka G, Roberts TF. Animal models of speech and vocal communication deficits associated with psychiatric disorders. Biol Psychiatry. 2016;79:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hammerschmidt K, Reisinger E, Westekemper K, et al. Mice do not require auditory input for the normal development of their ultrasonic vocalizations. BMC Neurosci. 2012;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gaub S, Groszer M, Fisher SE, Ehret G. The structure of innate vocalizations in Foxp2-deficient mouse pups. Genes Brain Behav. 2010;9:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Langhammer CG, Previtera ML, Sweet ES, et al. Automated sholl analysis of digitized neuronal morphology at multiple scales: Whole cell sholl analysis versus sholl analysis of arbor subregions. Cytometry A. 2010;77:1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary material.