Abstract
Objective
To determine the effect of a multidisciplinary lifestyle program in patients with RA with low–moderate disease activity.
Methods
In the ‘Plants for Joints’ (PFJ) parallel-arm, assessor-blind randomized controlled trial, patients with RA and 28-joint DAS (DAS28) ≥2.6 and ≤5.1 were randomized to the PFJ or control group. The PFJ group followed a 16-week lifestyle program based on a whole-food plant-based diet, physical activity and stress management. The control group received usual care. Medication was kept stable 3 months before and during the trial whenever possible. We hypothesized that PFJ would lower disease activity (DAS28). Secondary outcomes included anthropometric, metabolic and patient-reported measures. An intention-to-treat analysis with a linear mixed model adjusted for baseline values was used to analyse between-group differences.
Results
Of the 83 people randomized, 77 completed the study. Participants were 92% female with mean (s.d.) age of 55 (12) years, BMI of 26 (4) kg/m2 and mean DAS28 of 3.8 (0.7). After 16 weeks the PFJ group had a mean 0.9-point greater improvement of DAS28 vs the control group (95% CI 0.4, 1.3; P < 0.0001). The PFJ intervention led to greater decreases in body weight (difference –3.9 kg), fat mass (–2.8 kg), waist circumference (–3 cm), HbA1c (–1.3 mmol/mol) and low-density lipoprotein (–0.32 mmol/l), whereas patient-reported outcome measures, blood pressure, glucose and other lipids did not change.
Conclusion
The 16-week PFJ multidisciplinary lifestyle program substantially decreased disease activity and improved metabolic status in people with RA with low–moderate disease activity.
Trial Registration
International Clinical Trials Registry Platform; https://www.who.int/clinical-trials-registry-platform; NL7800.
Keywords: RA, diet, physical activity, stress management
Rheumatology key messages.
RA patients with low–moderate disease activity achieved significant improvements in DAS28 and metabolic markers.
This study shows that a lifestyle program based on a whole-food plant-based diet, physical activity and stress management may improve RA outcomes.
Introduction
RA is a chronic systemic inflammatory disease, which manifests primarily as polyarthritis [1]. Modern treatment is centred around drug therapy and aims to induce remission or low disease activity [2, 3]. However, the goal of sustained remission is achieved in only 20% of cases [4], and many patients still suffer from pain, fatigue and loss of function [5–7].
Although the precise aetiology of RA remains unclear, its onset and progression have been linked to various environmental or lifestyle factors, such as smoking, unhealthy diet, obesity, lack of exercise and psychological stress [8–14]. These unhealthy lifestyle factors, but also factors such as microbiome dysbiosis and metabolic syndrome, may drive several other chronic diseases through the shared mechanism of systemic chronic inflammation [15]. Thus, the higher occurrence of conditions such as coronary heart disease and diabetes mellitus in people with RA than in the general population [16] may be partially due to an unhealthy lifestyle.
Reversing and preventing adverse lifestyle factors could potentially reduce the incidence and burden of RA, as well as alleviate its comorbidities [17, 18]. Specifically, beneficial effects have been found in interventions directed at single lifestyle factors, such as dietary interventions with plant-based or Mediterranean diets [19–21], physical exercise programs [22] or stress reduction techniques [23].
A multidisciplinary program including a whole-food plant-based diet, increased physical activity, stress reduction and social support has produced favourable effects that have lasted for up to 5 years in patients with coronary artery disease [24] and low-grade prostate cancer [25]. To date, these nonpharmacologic therapies have not been tested in one integrated intervention in patients with RA. Therefore, we designed a randomized controlled trial (RCT) comparing a multidisciplinary lifestyle program versus usual care, aiming to decrease disease activity in patients with moderately active RA. The ‘Plants for Joints’ (PFJ) intervention, consisting of a whole-food plant-based diet, physical activity, stress-reduction techniques and sleep hygiene, was applied in a group setting for 16 weeks.
Methods
The PFJ project consisted of three trials to investigate the effect of a multidisciplinary lifestyle program in people with RA, a high risk of RA or metabolic syndrome-associated OA. The intervention was executed in mixed groups. The present article covers the RA group. A detailed protocol was published previously [26].
Design
This 16-week observer-blind and open-label RCT with parallel design was conducted between May 2019 and September 2021 at the Reade outpatient clinic for rheumatology and rehabilitation in Amsterdam, The Netherlands. Patient partners were involved in the design of the intervention and selection of patient reported outcome measures and remain engaged for the evaluation, dissemination and implementation of results.
Study visits took place at baseline, and 8 and 16 weeks. The Medical Ethical Committee of the Amsterdam University Medical Centers approved the study protocol (EudraCT number NL66649.048.18). The protocol was prospectively registered (International Clinical Trial Registry Platform number NL7800) and published [26]. Participants gave written informed consent. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline [27].
Recruitment, selection and randomization
Participants aged ≥18 years were referred by healthcare professionals (37%) or enrolled via a dedicated webpage (63%). People included had an RA diagnosis according to the ACR/EULAR 2010 criteria [28] with low to moderate disease activity [2.6 ≤ DAS of 28 joints (DAS28) ≤ 5.1] [29] and were on stable treatment with DMARDs or off DMARD-treatment for at least 3 months. DMARD medication had to remain stable during the study and changes were reported as protocol deviations. People with a low bodyweight (BMI <18.5 kg/m2), following a plant-based diet or unwilling to quit smoking for at least the duration of the RCT,0 and pregnant women were excluded. Participants were randomized in a 1:1 ratio with a variable block randomization in block sizes of 2 and 4.
Intervention
At the start, participants randomized to the PFJ group received individual intakes with a dietitian and a physical therapist. During the program groups of 6–12 participants gathered 10 times for 2–3 h meetings. In the intervention group 17 participants had all meetings live, while the remaining 23 participants received the intervention in hybrid form of two to four live sessions and the rest online due to COVID-19 measures. Peer education and peer support was actively promoted. The PFJ group received theoretical and practical education about a whole-food plant-based diet, physical activity and exercise, and stress management based on previous protocols and guidelines [23, 24, 30–32]. This included a plant-based variation of a diet in line with the 2015 Guidelines on Healthy Nutrition of the Health Council of the Netherlands, personal goals for physical activity in accordance with the 2017 Dutch physical activity guidelines (150 min/week moderately intense physical activity, and 2 days/week muscle and bone-strengthening activities), psychoeducation on the effects of stress on health and stress management, and coaching on sleep.
Intervention participants were facilitated with general information and videos, exercises for at home, fully elaborated weekly menu and daily supplementation with methylcobalamin (1500 µg) and cholecalciferol (50 μg) [33]. The dietary recommendations contained special emphasis on the critical nutrients in a plant-based diet: protein, omega-3 fatty acids, iron, zinc, iodine and calcium [33]. The control group received usual care and was advised not to change their lifestyle habits.
Primary and secondary outcomes
The primary endpoint was the mean change in the DAS28 over time. A research nurse blinded to group allocation assessed the patients. Participants were asked not to discuss their group allocation during the assessments. The DAS28 components ESR, patient’s global assessment, swollen joint count and tender joint count were also included as secondary outcomes.
The validated Dutch-Flemish Patient Reported Outcomes Measurement Information System (PROMIS®) was used to quantify physical function, fatigue, pain interference and depression as elements of health-related quality of life outcomes [34]. Additional secondary outcomes included: waist circumference, fat mass [measured by dual-energy X-ray absorptiometry (DEXA)], blood pressure, low-density lipoproteins (LDLs), high-density lipoproteins (HDLs), triglycerides, fasting glucose, HbA1c, RF, ACPA, ESR and CRP. Additionally, all adverse events and medication changes were recorded.
Adherence
To measure adherence, an adapted version of the Lifestyle Index Adherence Score as developed by Ornish et al. [25] was used, in which adherence is defined by the attendance of meetings, stress-reducing activities, physical activity and diet. In the original version the diet score was defined by total fat and cholesterol intake. Since the PFJ intervention was not based on a low-fat diet, these vectors into were changed into fibre and saturated fatty acids as indicators for a whole-food plant-based diet. Full adherence (100% score) was defined as attendance of all meetings, performing stress-reducing activities 6 days per week for 10 min per day, physical activity 5 days per week for 30 min per day, and mean intake of at least 14 g of fibre per 1000 kilocalories (kcal) and <10% saturated fatty acids of total kcal per day. Adherence was self-assessed with a validated app for participants to keep a 4- to 7-day food diary, and digital questionnaires for physical activity and stress reducing activities. A detailed description of the score was published previously [26].
Sample size calculation
Based on α = 0.05 and power (1 – β) = 0.80, potentially detectable effects ranged from 0.4 to 1.0 point difference in decrease for the DAS28 (estimated from component data of the DAS28 in the case of the trial by Kjeldsen–Kragh) with s.d. ranging from 0.6 to 1.0 (19, 21). We chose a difference of 0.80 as target: this has been suggested as the minimal clinically important improvement in the range of our inclusion criterion of DAS28 (2.6 ≤ DAS28 ≤ 5.1) [35]. With a s.d. of 1.2 for the DAS28, α of 0.05 and β of 0.2, a sample size of 56 was needed, which was rounded to 80 to take possible dropouts into account.
Statistical analysis
Outcomes were measured at baseline, and 8 and 16 weeks. After conclusion of the trial, data were cleaned and verified by two researchers. Baseline values of dropouts and all values of participants included in the full analysis were compared for DAS28, age, BMI and disease duration, using the Mann–Whitney test for independent samples.
Intention-to-treat analyses with a linear mixed model, adjusted for the baseline value of the particular outcome, were performed to calculate the mean difference in change between the groups in continuous outcomes over time. The 95% CIs and P-values for (components of) the DAS28 were calculated.
Furthermore, a logistic generalized estimating equation analysis was used to analyse the average changes in DAS28 over time in terms of whether EULAR Good Response criteria were met (compared with not meeting these criteria or only meeting EULAR Moderate Response criteria) as well as the analysis of whether participants attained minimal disease activity (DAS28 < 2.60).
Based on the Lifestyle Index Adherence Score [25], adherence in the PFJ group at 16 weeks was ranked and differences in DAS28 between quartiles of adherence were analysed over time with a linear mixed model, adjusted for baseline values of the DAS28.
All analyses were performed with R version 4.0.5 (2021–03-31) and P-values <0.05 were considered statistically significant.
Results
Participant characteristics
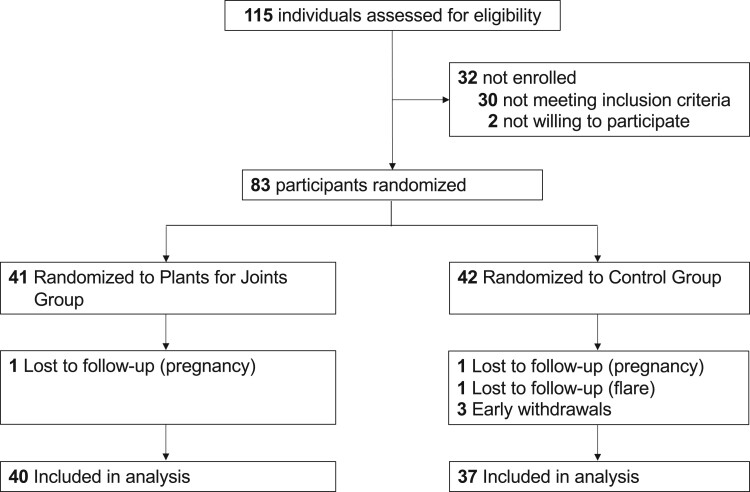
Of the 115 people assessed for eligibility, 83 were randomized (Fig. 1). One person in each group dropped out due to pregnancy, and four people from the control group dropped out (one because of a flare, three were not willing to proceed because they were assigned to the control group). All dropouts occurred shortly after randomization and were lost to follow-up, leaving 77 people for analysis (Fig. 1). Comparison of the dropouts (n = 6) vs people included in the final analysis showed no significant differences regarding DAS28, age, BMI and disease duration at baseline.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram flow of participants in the ‘Plants for Joints’ trial
Study participants had a mean age of 55 years, were mostly female (92%), had a mean BMI of 26 kg/m2, and 74% were seropositive for RF or ACPA. Of all participants, three (4%) used diabetes medication, nine (12%) used antihypertensives and seven (9%) used lipid-lowering medication. Overall, 56 participants (73%) used DMARDs (Table 1). Seventeen participants did not use medication (n = 10 never used medication) for RA at baseline because of an aversion to DMARDs (n = 8), side effects or ineffectiveness of DMARDs (n = 3), a recent diagnosis with the wish to first start this lifestyle program (n = 4) or previously stopped use of DMARDs because of remission or pregnancy (n = 2). Table 1 also shows the history of medication use.
Table 1.
Baseline characteristics ‘Plants for Joints’ RA trial
| Plants for Joints group | Control group | |
|---|---|---|
| Characteristic | (n = 40) | (n = 37) |
| Age, mean (s.d.), years | 56.4 (13.4) | 52.8 (10.3) |
| Female sex, number (%) | 36 (90) | 35 (95) |
| RF positive, number (%) | 20 (50) | 29 (78) |
| ACPA positive, number (%) | 24 (60) | 26 (70) |
| Seropositive, number (%) | 26 (65) | 31 (84) |
| Disease duration, mean (s.d.), years | 10 (9) | 8 (8) |
| BMI, mean (s.d.), kg/m2 | 27.1 (4.6) | 25.1 (3.7) |
| DAS28, mean (s.d.) | 3.90 (0.7) | 3.78 (0.7) |
| Erosive disease, number (%) | 23 (58) | 15 (43) |
| Medication for RA, number (%) | ||
| MTX monotherapy | 11 (28) | 3 (8) |
| MTX combination therapy | 12 (30) | 7 (19) |
| Other csDMARD monotherapy | 3 (8) | 1 (3) |
| Other csDMARD combination therapy | 2 (5) | 2 (5) |
| bDMARD | 6 (15) | 7 (19) |
| tsDMARD | 0 (0) | 3 (8) |
| Glucocorticoids | 2 (5) | 1 (3) |
| No medication | 4 (10) | 13 (35) |
| Previous medication for RA | ||
| csDMARD treatment, median (range), count | 1 (0–4) | 0 (0–4) |
| bDMARD treatment, median (range), count | 0 (0–4) | 0 (0–5) |
| tsDMARD treatment, median (range), count | 0 (0–0) | 0 (0–1) |
| Glucocorticoids, number (%) | 9 (23) | 9 (24) |
| Only csDMARD treatment, number (%) | 10 (25) | 8 (22) |
| 1 bDMARD treatment, number (%) | 6 (15) | 2 (5) |
| 2 or more bDMARD treatments, number (%) | 4 (10) | 4 (11) |
| No prior treatment, number (%) | 5 (13) | 13 (35) |
| Medication for diabetes, number (%) | 2 (5) | 1 (3) |
| Medication for hypertension, number (%) | 6 (15) | 3 (8) |
| Medication for hyperlipidaemia, number (%) | 4 (10) | 3 (8) |
Previous medication for DMARD treatment refers to the median (range) number of DMARDs used before baseline medication and the number (%) of participants who had a certain treatment before baseline. Seropositive: positive for RF or ACPA; BMI: body weight in kilograms divided by the square of the height in metres; DAS28: 28-joint DAS; csDMARD: conventional synthetic DMARD; bDMARD: biological DMARD; tsDMARD: targeted synthetic DMARD.
Primary outcome
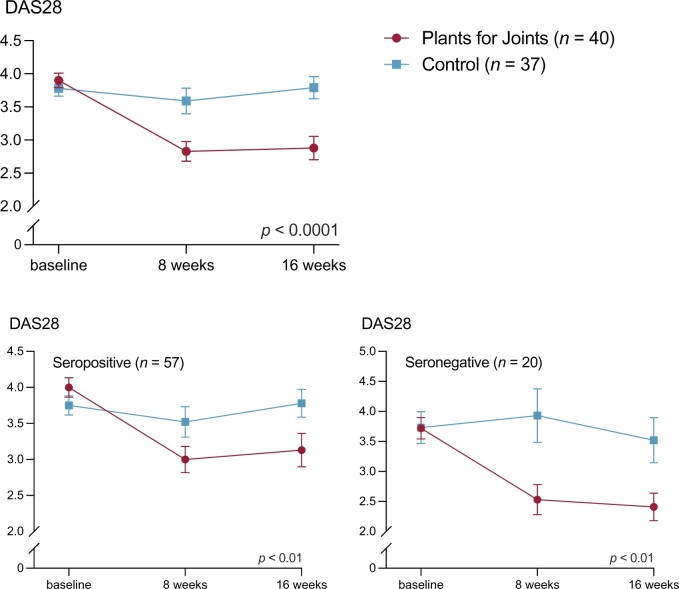
The PFJ group had a greater decrease in DAS28 over time from baseline to 16 weeks than the control group: mean difference was 0.90 (95% CI 0.41, 1.29; P < 0.0001). At 16 weeks, 17 participants from the PFJ group and three participants from the control group met the criteria for EULAR Good Response corresponding to an odds ratio over time of 4.3 (95% CI 1.9, 10.1; P < 0.001). Additionally, in the PFJ group 18 participants had a DAS28 < 2.60 after 16 weeks, compared with three participants in the control group, corresponding to an odds ratio of 4.6 (95% CI 2.0, 10.2; P < 0.001). All changes in DAS28 components (ESR, patient’s global assessment, swollen and tender joint count) differed between groups in favour of the PFJ group, although the difference in ESR was not statistically significant (Table 2 and Supplementary Fig. S1, available at Rheumatology online).
Table 2.
Primary and secondary outcomes of the ‘Plants for Joints’ RA trial
| Plants for Joints group (n = 40) |
Control group (n = 37) |
Difference in change between groups | |||||
|---|---|---|---|---|---|---|---|
| Mean (s.d.) |
Mean (s.d.) |
|
|||||
| Characteristic | Baseline | 8 weeks | 16 weeks | Baseline | 8 weeks | 16 weeks | (95% CI) |
| DAS28 and components | |||||||
| DAS28 | 3.90 (0.7) | 2.83 (1.0) | 2.88 (1.1) | 3.78 (0.7) | 3.59 (1.2) | 3.79 (1.0) | –0.90 (–0.41, –1.29) |
| Seropositive group (n = 57) | 4.00 (0.7) | 3.00 (0.9) | 3.13 (1.2) | 3.75 (0.7) | 3.52 (1.2) | 3.78 (1.1) | –0.72 (–1.21, –0.23) |
| Seronegative group (n = 20) | 3.72 (0.7) | 2.53 (0.9) | 2.41 (0.9) | 3.92 (0.6) | 4.03 (1.2) | 3.85 (0.8) | –1.24 (–1.89, –0.59) |
| ESR, mm/h | 17 (18) | 19 (20) | 17 (14) | 20 (15) | 22 (16) | 24 (16) | –3.0 (–7.2, 1.2) |
| Patient’s global assessment, mm (0–100) | 53 (20) | 32 (22) | 25 (21) | 52 (17) | 47 (23) | 47 (19) | –19 (–26, –12) |
| Swollen joint count of 28 joints | 1.8 (1.9) | 0.7 (1.1) | 1.4 (2.9) | 1.9 (2.7) | 2.4 (3.9) | 2.3 (3.3) | –1.3 (–2.2, –0.3) |
| Tender joint count of 28 joints | 4.6 (3.6) | 1.6 (1.8) | 1.8 (2.8) | 3.8 (3.5) | 3.6 (4.8) | 2.9 (2.9) | –1.7 (–2.7, –0.7) |
| Response and minimal disease activity (number, %) | |||||||
| EULAR good response | 12 (30%) | 17 (43%) | 6 (16%) | 3 (8%) | OR 4.3 (1.9, 10.1) | ||
| Minimal disease activity (DAS < 2.6) | 14 (35%) | 18 (45%) | 7 (19%) | 3 (8%) | OR 4.6 (2.0, 10.2) | ||
| Inflammation | |||||||
| CRP, mg/l | 4.3 (4.1) | 5.6 (7.4) | 3.8 (4.4) | 3.4 (3.9) | 5.5 (10.0) | 4.8 (8.3) | –1.3 (–4.0, 1.4) |
| Anthropometric | |||||||
| BMI, kg/m2 | 27.1 (4.6) | 26.0 (4.7) | 25.9 (5.0) | 25.2 (3.7) | 25.5 (4.3) | 25.3 (3.8) | –1.4 (–1.8, –0.9) |
| Weight, kg | 76.8 (13.2) | 73.7 (13.2) | 73.3 (14.0) | 71.6 (11.9) | 72.6 (13.0) | 72.0 (12.3) | –3.9 (–5.2, –2.6) |
| Fat mass, kg (DEXA) | 30.6 (10.0) | 27.5 (9.9) | 27.7 (8.0) | 28.2 (8.0) | –2.8 (–3.8, –1.7) | ||
| Waist circumference, cm | 93.1 (10.7) | 90.4 (10.9) | 88.6 (11.8) | 88.8 (12.0) | 88.8 (10.7) | 88.7 (11.4) | –3.0 (–5.1, –0.9) |
| Waist circumference (females), cm (n = 72) | 92.4 (10.6) | 89.7 (10.8) | 87.8 (11.7) | 88.1 (11.9) | 88.1 (10.6) | 87.9 (11.3) | –3.0 (–5.3, –0.8) |
| Waist circumference (males), cm (n = 7) | 99.6 (10.7) | 96.9 (10.2) | 95.8 (11.8) | 101.0 (5.7) | 100.5 (3.5) | 101.5 (2.8) | –3.4 (–6.7, 0.0) |
| Metabolic | |||||||
| Fasting blood glucose, mmol/l | 5.1 (0.4) | 5.0 (0.5) | 4.9 (0.5) | 5.5 (1.6) | 5.2 (0.6) | 5.1 (0.5) | –0.1 (–0.3, 0.1) |
| HbA1c, mmol/mol | 35.5 (5.6) | 35.2 (6.0) | 34.7 (5.2) | 37.8 (7.4) | 38.5 (7.2) | 38.5 (7.0) | –1.3 (–2.0, –0.5) |
| Systolic blood pressure, mmHg | 137 (19) | 135 (18) | 132 (16) | 131 (20) | 128 (20) | 130 (18) | 0 (–4, 4) |
| Diastolic blood pressure, mmHg | 87 (11) | 83 (16) | 84 (11) | 86 (13) | 85 (14) | 85 (11) | –3 (–6, 1) |
| LDL, mmol/l | 2.92 (1.0) | 2.58 (0.8) | 2.65 (0.8) | 3.48 (1.1) | 3.22 (0.9) | 3.24 (0.8) | –0.32 (–0.54, –0.10) |
| HDL, mmol/l | 1.63 (0.4) | 1.47 (0.3) | 1.56 (0.4) | 1.69 (0.4) | 1.65 (0.4) | 1.65 (0.4) | –0.07 (–0.15, 0.01) |
| Triglycerides, mmol/l | 1.14 (0.6) | 1.21 (0.6) | 1.05 (0.43) | 1.02 (0.6) | 0.91 (0.3) | 1.03 (0.5) | 0.07 (–0.05, 0.19) |
| Patient-reported outcomes, PROMIS© | |||||||
| Depression | 51 (7) | 49 (8) | 50 (7) | 54 (4) | 54 (6) | 52 (6) | –1 (–4, 1) |
| Fatigue | 53 (12) | 53 (7) | 52 (7) | 58 (6) | 57 (7) | 55 (8) | –2 (–5, 1) |
| Pain interference | 58 (7) | 55 (7) | 55 (7) | 60 (6) | 58 (7) | 56 (6) | –1 (–4, 1) |
| Physical function | 44 (5) | 46 (6) | 46 (8) | 43 (5) | 44 (6) | 44 (5) | 1 (–1, 3) |
All values for the total group (n = 77), except the DAS28 for the seropositive and seronegative group. Seropositive subjects are positive for RF or ACPA, seronegative subjects are negative for RF and ACPA. Patient’s self-reported global assessment of disease activity is based on a visual analogue scale (0–100 mm) with higher values indicating more disease activity. EULAR Good Response = improvement DAS28 > 1.2 and DAS28 ≤ 3.2 (non-responders include responders EULAR Moderate Response). Odds ratio calculation based on logistic generalized estimating equation regression analysis of change between baseline and 16 weeks. DAS28: 28-joint DAS; DEXA: dual-energy X-ray absorptiometry; OR: odds ratio.
The PFJ intervention significantly reduced DAS28 in both seropositive and seronegative RA subgroups in comparison with the control group (Table 2 and Fig. 2).
Figure 2.
DAS with 28 joints (DAS28) at baseline, 8 weeks and at the end of the ‘Plants for Joints’ trial
Protocol deviations
There were several drug treatment intensifications: in the PFJ group two participants started DMARD or prednisolone treatment and two people received glucocorticoid injections. In the control group, seven participants started, switched or increased the dose of DMARDs or prednisolone treatment, while three participants received glucocorticoid injections. Conversely, in the PFJ group 13 people reduced the dose of DMARDs or prednisone, whereas in the control group four participants decreased their dose of DMARDs. Medication changes were divided evenly between the first and second half of the trial period (Supplementary Table S2, available at Rheumatology online).
Secondary outcomes
Loss of weight and fat mass were significantly larger in the PFJ vs the control group with between-group differences of 3.9 and 2.8 kg, respectively. Most of the weight loss within the PFJ group was attributable to a reduction in fat mass (3.1 of the 3.5 kg). Furthermore, compared with the control group, the PFJ group had a significantly larger decrease of waist circumference on average over time (between-group difference 3.0 cm) (Table 2).
The metabolic parameters HbA1c and LDL decreased in the PFJ group vs control group, whereas other metabolic parameters showed minor differences in the changes over time between the PFJ and control group.
All patient-reported outcome measures changed slightly (not statistically significantly) over time in favour of the PFJ group (Table 2).
Program adherence
Mean DAS28 decreased in all adherence quartiles based on the Lifestyle Index Adherence Score of the PFJ group. When compared with the lowest level of adherence (level 1), participants with higher levels of adherence (2–4) had larger reductions of DAS28. When compared with adherence level 1, the average DAS28 decreased 0.3 more on adherence level 2 (P = 0.43), 0.8 more on adherence level 3 (P = 0.05) and 0.6 more on adherence level 4 (P = 0.11).
Energy, carbohydrate and protein intake in both the PFJ and control group did not show relevant changes (Table 3). At baseline the intake of saturated fat was high in both groups (13% of total energy intake; recommendation <10%) while fibre intake was low (13 g/1000 kcal; recommendation ≥14 g/1000 kcal) according to the dietary guidelines. Halfway and at the end of the intervention, the PFJ group reached the healthy intake range of saturated fat (8–9% of total energy intake) and fibre (20–21 g/1000 kcal), while the control group also improved although to a lesser extent (Table 3).
Table 3.
Lifestyle descriptives ‘Plants for Joints’ RA trial
| Plants for Joints group (n = 40) |
Control group (n = 37) |
|||||
|---|---|---|---|---|---|---|
| Mean (s.d.) |
Mean (s.d.) |
|||||
| Characteristic | Baseline | 8 weeks | 16 weeks | Baseline | 8 weeks | 16 weeks |
| Diet | ||||||
| Energy, kcal | 1769 (348) | 1699 (357) | 1752 (340) | 1748 (284) | 1695 (257) | 1699 (196) |
| Fat, g | 72 (21) | 71 (22) | 72 (16) | 75 (21) | 71 (19) | 72 (17) |
| Saturated fat, g | 25 (10) | 15 (6) | 17 (6) | 25 (8) | 24 (7) | 21 (9) |
| Saturated fat, energy% | 13 (4) | 8 (2) | 9 (3) | 13 (4) | 12 (3) | 11 (4) |
| Carbohydrate, g | 190 (47) | 195 (42) | 194 (46) | 178 (42) | 180 (33) | 182 (37) |
| Carbohydrate, energy% | 43 (6) | 47 (10) | 44 (5) | 41 (7) | 43 (7) | 43 (5) |
| Protein, g | 66 (16) | 57 (15) | 58 (13) | 70 (14) | 63 (14) | 64 (17) |
| Protein, g/kg body weight | 0.9 (0.3) | 0.8 (0.3) | 0.8 (0.2) | 1.0 (0.2) | 0.9 (0.2) | 0.9 (0.2) |
| Fibre, g | 24 (6) | 36 (10) | 34 (10) | 23 (5) | 24 (6) | 26 (9) |
| Fibre, g/1000 kcal | 14 (3) | 21 (5) | 20 (5) | 13 (3) | 14 (4) | 15 (4) |
| Physical activity, min/wk | 154 (98) | 211 (117) | 205 (129) | 213 (130) | 194 (140) | 211 (130) |
| Stress-reducing activities, min/week | 24 (26) | 31 (26) | 25 (26) | 35 (28) | 38 (26) | 42 (30) |
All values for the total group (n = 77). Data are self-reported. kcal: kilocalories; energy%: percentage of total energy in kilocalories.
The average self-reported physical activity level was sufficient (recommended >150 min/week) at baseline in both groups and increased further in the PFJ group from 154 to 205 min per week, while staying at baseline level (>200 min per week) in the control group (Table 3). Regarding self-reported average time spent on stress-reducing activities, the PFJ group showed a relatively stable 24–31 min per week, while the control group started at a higher level and increased further to 42 min per week (Table 3).
Adverse events
No serious adverse events occurred during the trial. A total of 22 adverse events were recorded in the PFJ group and 28 in the control group. Most of the events consisted of abdominal symptoms such as stool changes, bloating, cramps and reflux, and were possibly related to the intervention. However, these occurred to a similar extent in the PFJ group (n = 9) and in the control group (n = 11) (Supplementary Table S1, available at Rheumatology online).
Discussion
The 16-week multidisciplinary PFJ lifestyle program, consisting of a whole-food plant-based diet, physical activity and stress management in addition to usual care, decreased disease activity in RA patients with low–moderate disease activity compared with usual care. The result exceeded the minimal clinically important improvement of 0.8 (35) and is comparable to what is generally achieved in drug trials [36]. The improvement was found in both seropositive and seronegative subgroups. In addition, the PFJ intervention caused significant metabolic changes, such as loss of weight and fat mass as well as a decrease of HbA1c and LDL. Depression, fatigue, pain interference and physical function did not change.
The results are in line with previous trials on dietary interventions in RA that showed improvements in disease activity with a plant-based diet for 12 months [19, 20] and with a Mediterranean diet for 3 months [21]. Also, it has been shown that a reduction of fat mass in general as well as visceral fat in particular lowers inflammatory markers [37, 38], which is associated with a smaller risk of metabolic syndrome and other lifestyle-related diseases [15]. A reduced fat mass is therefore beneficial for people with RA, who have a 50% increased risk of cardiovascular disease compared with the general population [39].
In a previous study on the effect of a 13-week intervention based on a hypocaloric, high-protein diet in combination with resistance training, older and obese participants lost a mean 3.4 kg of weight, of which 3.2 kg fat mass [40]. The present study achieved similar reductions of weight and fat mass, although weight loss was not the primary purpose of this intervention and physical training was less intensive. This confirms previous findings on the potential of plant-based diets for weight loss [41, 42].
This study showed ESR and CRP decreased in the PFJ group in comparison with the control group, although these changes were not statistically significant. This is in contrast to previous studies of a plant-based diet in people with RA and may be due to lower baseline values in the PFJ trial (ESR 17 mm/h, CRP 4.3 mg/l) when compared with values in earlier studies (baseline ESR >32 mm/h and CRP >24 mg/l) [19]. On the other hand, improvements in metabolic markers such as HbA1c and LDL were in line with previous studies [24, 41–43].
This study has several strengths, including the low drop-out rate. Most importantly, the study was able to demonstrate substantial effects despite already low disease activity, in a representative group of Dutch RA patients in terms of age and seropositivity [44]. Also, despite protocol deviations, the net effect of all medication changes was a less intensive treatment in the PFJ vs the control group, thus not influencing the results. Furthermore, the study provides evidence of the health benefits of plant-based diets, which strengthens the proposition of a plant-based diet as part of a more sustainable lifestyle [45, 46].
On the other hand, because the study deliberately combined multiple lifestyle factors, the individual contribution of these factors on the results can unfortunately not be defined. Another limitation is that the intervention group received extra attention, therefore we cannot exclude the possibility that this partially explains improvement in subjective measures such as the patient’s global assessment and tender joint count. However, improvement also occurred in objective measures such as swollen joint count, body composition and metabolic markers. Moreover, the adherence to the diet, physical activity and stress management components was not measured by objective means and thus provides room for potential misreporting. The PFJ participants are comparable to the general Dutch population in terms of their level of overweight, the extent to which they adhere to the guideline of physical activity and their intake of saturated fat, whereas the intake of fibre was higher already at baseline than in the general Dutch population (13 vs 9 g per 1000 kcal) [47–49]. The swift increase of fibre intake may have caused some abdominal complaints, which were temporary. Finally, the participants may have been more inclined to try lifestyle changes rather than medication compared with the average RA patient; however, that does not diminish the potential of this treatment.
The present intervention was intensive and short, and long-term effects are still unknown. Therefore, the control group will be offered the intervention after the trial, and we will follow all participants in a 2-year observational extension study including cost effectiveness, body composition, bone mineral density, critical nutrients and a DMARD-tapering protocol for patients in remission [26]. In addition, further research is needed to investigate whether plant-based diets can mimic the anabolic effect of animal-based proteins to ensure preservation of muscle mass during weight loss [50].
In conclusion, the multidisciplinary PFJ program substantially decreased disease activity and improved metabolic status in patients with RA with low–moderate disease activity compared with usual care. The program is readily compatible with drug therapy and could potentially lower the need for medication for both RA as well as for metabolic syndrome–related conditions.
Supplementary Material
Acknowledgements
Study participants of the ‘Plants for Joints’ trial, patient partners of ‘Plants for Joints’, employees of the Reade Biobank (Toni de Jong-de Boer and Corrie Verdoold), registered dietitians Pauline Kortbeek, Anna Kretova, Melissa Dijkshoorn, Michelle Bisschops, Marieke van de Put and Dana Hofland, exercise coaches Sietske de Weers, Tom van Iersel and Jobjan Blonk, physical therapist Boke Dekker and relaxation/sleep coaches Nelleke Doornebal and Marieke Rinkema.
Contributor Information
Wendy Walrabenstein, Reade Center for Rheumatology and Rehabilitation, Amsterdam, The Netherlands; Department of Clinical Immunology and Rheumatology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Rheumatology and Immunology Center, Amsterdam, The Netherlands; Department of Nutrition and Dietetics, Center of Expertise Urban Vitality, Amsterdam University of Applied Sciences, Amsterdam, The Netherlands.
Carlijn A Wagenaar, Reade Center for Rheumatology and Rehabilitation, Amsterdam, The Netherlands; Department of Clinical Immunology and Rheumatology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Rheumatology and Immunology Center, Amsterdam, The Netherlands.
Marike van der Leeden, Reade Center for Rheumatology and Rehabilitation, Amsterdam, The Netherlands; Amsterdam Rheumatology and Immunology Center, Amsterdam, The Netherlands; Department of Rehabilitation Medicine, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands; Amsterdam Movement Sciences Research Institute, Amsterdam, The Netherlands.
Franktien Turkstra, Reade Center for Rheumatology and Rehabilitation, Amsterdam, The Netherlands; Amsterdam Rheumatology and Immunology Center, Amsterdam, The Netherlands.
Jos W R Twisk, Department of Epidemiology and Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
Maarten Boers, Department of Epidemiology and Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
Henriët van Middendorp, Institute of Psychology, Health, Medical, and Neuropsychology Unit, Leiden University, Leiden, The Netherlands.
Peter J M Weijs, Department of Nutrition and Dietetics, Center of Expertise Urban Vitality, Amsterdam University of Applied Sciences, Amsterdam, The Netherlands; Amsterdam Movement Sciences Research Institute, Amsterdam, The Netherlands; Department of Nutrition and Dietetics, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
Dirkjan van Schaardenburg, Reade Center for Rheumatology and Rehabilitation, Amsterdam, The Netherlands; Department of Clinical Immunology and Rheumatology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Rheumatology and Immunology Center, Amsterdam, The Netherlands.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data that support the findings of this study are available from the corresponding author (W.W.) upon reasonable request.
Contribution statement
Study conception and fundraising: W.W. and D.v.S. Study design and statistical analysis: W.W., C.A.W., M.v.d.L., F.T., J.W.R.T., M.B., H.v.M., P.J.M.W. and D.v.S. Patient recruitment: W.W., C.A.W. and F.T. W.W. drafted the manuscript. D.v.S. supervised the overall project. All authors read and approved the final version of the manuscript.
Funding
The RCT is funded by Reade (Amsterdam, The Netherlands), Reade Foundation (Amsterdam, The Netherlands), Stichting Vermeer 14 (private foundation, Amsterdam, The Netherlands) and W.M. de Hoop Stichting (private foundation, Bussum, The Netherlands). The appointment of C.A.W. is funded by The Netherlands Organisation for Health Research and Development (ZonMw) no. 555003210. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 2. Fraenkel L, Bathon JM, England BR et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73:1108–23. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewe RBM, Bijlsma JWJ et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 4. Einarsson JT, Willim M, Ernestam S et al. Prevalence of sustained remission in rheumatoid arthritis: impact of criteria sets and disease duration, a Nationwide Study in Sweden. Rheumatology (Oxford) 2019;58:227–36. [DOI] [PubMed] [Google Scholar]
- 5. Sloot R, Flinterman L, Heins M et al. Reumatische aandoeningen in Nederland - Ervaringen en kengetallen. Utrecht: NIVEL, 2016. [Google Scholar]
- 6. Santos EJF, Duarte C, da Silva JAP, Ferreira RJO. The impact of fatigue in rheumatoid arthritis and the challenges of its assessment. Rheumatology (Oxford) 2019;58:v3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ødegård S, Finset A, Mowinckel P, Kvien TK, Uhlig T. Pain and psychological health status over a 10-year period in patients with recent onset rheumatoid arthritis. Ann Rheum Dis 2007;66:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Giuseppe D, Bottai M, Askling J, Wolk A. Physical activity and risk of rheumatoid arthritis in women: a population-based prospective study. Arthritis Res Ther 2015;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu Y, Sparks JA, Malspeis S et al. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann Rheum Dis 2017;76:1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qin B, Yang M, Fu H et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther 2015;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Hair MJ, Landewé RB, van de Sande MG et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis 2013;72:1654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee YC, Agnew-Blais J, Malspeis S et al. Post-traumatic stress disorder and risk for incident rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song H, Fang F, Tomasson G et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA 2018;319:2388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hahn J, Malspeis S, Choi MY et al. Association of healthy lifestyle behaviors and the risk of developing rheumatoid arthritis among women. Arthritis Care Res (Hoboken) 2022; 10.1002/acr.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furman D, Campisi J, Verdin E et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siebert S, Lyall DM, Mackay DF et al. Characteristics of rheumatoid arthritis and its association with major comorbid conditions: cross-sectional study of 502 649 UK Biobank participants. RMD Open 2016;2:e000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roelsgaard IK, Ikdahl E, Rollefstad S et al. Smoking cessation is associated with lower disease activity and predicts cardiovascular risk reduction in rheumatoid arthritis patients. Rheumatology (Oxford) 2020;59:1997–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sparks JA, Halperin F, Karlson JC, Karlson EW, Bermas BL. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2015;67:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kjeldsen-Kragh J, Haugen M, Borchgrevink CF et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991;338:899–902. [DOI] [PubMed] [Google Scholar]
- 20. Hafström I, Ringertz B, Spangberg A et al. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology (Oxford) 2001;40:1175–9. [DOI] [PubMed] [Google Scholar]
- 21. Skoldstam L, Hagfors L, Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann Rheum Dis 2003;62:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hurkmans E, van der Giesen FJ, Vliet Vlieland TP, Schoones J, Van den Ende EC. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev 2009;2009:CD006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Brouwer SJ, van Middendorp H, Kraaimaat FW et al. Immune responses to stress after stress management training in patients with rheumatoid arthritis. Arthritis Res Ther 2013;15:R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ornish D, Scherwitz LW, Billings JH et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001–7. [DOI] [PubMed] [Google Scholar]
- 25. Ornish D, Lin J, Chan JM et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol 2013;14:1112–20. [DOI] [PubMed] [Google Scholar]
- 26. Walrabenstein W, van der Leeden M, Weijs P et al. The effect of a multidisciplinary lifestyle program for patients with rheumatoid arthritis, an increased risk for rheumatoid arthritis or with metabolic syndrome-associated osteoarthritis: the “Plants for Joints” randomized controlled trial protocol. Trials 2021;22:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schulz KF, Altman DG, Moher D, Group C; CONSORT Group.CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aletaha D, Neogi T, Silman AJ et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 29. Fransen J, van Riel PL. DAS remission cut points. Clin Exp Rheumatol 2006;24:S-29–32. [PubMed] [Google Scholar]
- 30. Barnard N, Gloede L, Cohen J et al. A low-fat vegan diet elicits greater macronutr changes. J Am Diet Assoc 2009;109:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kromhout D, Spaaij CJ, de Goede J, Weggemans RM. The 2015 Dutch food-based dietary guidelines. Eur J Clin Nutr 2016;70:869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weggemans RM, Backx FJG, Borghouts L et al. ; Committee Dutch Physical Activity Guidelines 2017. The 2017 Dutch Physical Activity Guidelines. Int J Behav Nutr Phys Act 2018;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Melina V, Craig W, Levin S. Position of the academy: vegetarian diets. J Acad Nutr Diet 2016;116:1970–80. [DOI] [PubMed] [Google Scholar]
- 34.Terwee CB, Roorda LD, de Vet HCW et al. Dutch-Flemish translation of 17 item banks from the patient-reported outcomes measurement information system (PROMIS). Qual Life Res 2014;23:1733–41. [DOI] [PubMed] [Google Scholar]
- 35. Aletaha D, Funovits J, Ward MM, Smolen JS, Kvien TK. Perception of improvement in patients with rheumatoid arthritis varies with disease activity levels at baseline. Arthritis Rheum 2009;61:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mian AN, Ibrahim F, Scott DL, Galloway J; TITRATE study group. Optimal responses in disease activity scores to treatment in rheumatoid arthritis: is a DAS28 reduction of >1.2 sufficient? Arthritis Res Ther 2016;18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarin HV, Lee JH, Jauhiainen M et al. Substantial fat mass loss reduces low-grade inflammation and induces positive alteration in cardiometabolic factors in normal-weight individuals. Sci Rep 2019;9:3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ranganath VK, La Cava A, Vangala S et al. Improved outcomes in rheumatoid arthritis with obesity after a weight loss intervention: randomized trial. Rheumatology (Oxford) 2022. [DOI] [PubMed] [Google Scholar]
- 39. Hansildaar R, Vedder D, Baniaamam M, Tausche AK et al. Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol 2021;3:e58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verreijen AM, Verlaan S, Engberink MF et al. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr 2015;101:279–86. [DOI] [PubMed] [Google Scholar]
- 41. Barnard ND, Alwarith J, Rembert E et al. A Mediterranean diet and low-fat vegan diet to improve body weight and cardiometabolic risk factors: a randomized, cross-over trial. J Am Nutr Assoc 2022;41:127–139. [DOI] [PubMed] [Google Scholar]
- 42. Hall KD, Guo J, Courville AB et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat Med 2021;27:344–53. [DOI] [PubMed] [Google Scholar]
- 43. Barnard ND, Cohen J, Jenkins DJ et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hooijberg F, Boekel L, Vogelzang EH et al. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol 2020;2:e583–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willett W, Rockström J, Loken B et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019;393:447–92. [DOI] [PubMed] [Google Scholar]
- 46. Hayek MN, Harwatt H, Ripple WJ, Mueller ND. The carbon opportunity cost of animal-sourced food production on land. Nat Sustain 2020;4:21–4. [Google Scholar]
- 47. Rijksinstituut voor Volksgezondheid en Milieu. Overgewicht (overweight). https://www.vzinfo.nl/overgewicht/leeftijd-geslacht (21 June 2022, date last accessed).
- 48. Rijksinstituut voor Volksgezondheid en Milieu. Voedselconsumptiepeiling (National Food Consumption Survey). https://www.rivm.nl/publicaties/diet-of-dutch-results-of-dutch-national-food-consumption-survey-2012-2016 (21 June 2022, date last accessed).
- 49. Rijksinstituut voor Volksgezondheid en Milieu. Feiten en cijfers over sport en bewegen (Facts and numbers on exercise and physical activity). https://www.sportenbewegenincijfers.nl (21 June 2022, date last accessed).
- 50. Berrazaga I, Micard V, Gueugneau M, Walrand S. The role of the anabolic properties of plant- versus animal-based protein sources in supporting muscle mass maintenance: a critical review. Nutrients 2019;11:1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (W.W.) upon reasonable request.