Abstract
Background
We performed a nationwide population-based retrospective study to describe the epidemiology of bacterial co-infections in coronavirus disease 2019 (COVID-19)-hospitalized patients in Spain in 2020. We also analyzed the risk factors for co-infection, the etiology and the impact in the outcome.
Methods
Data were obtained from records in the Minimum Basic Data Set (MBDS) of the National Surveillance System for Hospital Data in Spain, provided by the Ministry of Health and annually published with 2 years lag. COVID-19 circulated in two waves in 2020: from its introduction to 31st June and from 1st July to 31st December. The risk of developing a healthcare-associated bacterial co-infection and the risk for in-hospital and intensive care unit (ICU) mortality in co-infected patients was assessed using an adjusted logistic regression model.
Results
The incidence of bacterial co-infection in COVID-19 hospitalized patients was 2.3%. The main risk factors associated with bacterial co-infection were organ failure, obesity and male sex. Co-infection was associated with worse outcomes including higher in-hospital, in-ICU mortality and higher length of stay. Gram-negative bacteria caused most infections. Causative agents were similar between waves, although higher co-infections with Pseudomonas spp. were detected in the first wave and with Haemophilus influenzae and Streptococcus pneumoniae in the second.
Conclusions
Co-infections are not as common as those found in other viral respiratory infections; therefore, antibiotics should be used carefully. Screening for actual co-infection to prescribe antibiotic therapy when required should be performed.
Introduction
SARS-COV2 coronavirus infection responsible for coronavirus disease 2019 (COVID-19), is a major public health problem. Since its emergence in December 2019, it has caused more than 500 million cases and more than 6 million deaths worldwide.1 In Spain, one of the most affected countries in the European Union, it has caused more than 13 million cases and a total of 110 394 deaths.1
At the beginning of the pandemic, little was known about COVID-19 disease, so treatments and decisions were based on experience from previous pandemics caused by other respiratory viruses, such as influenza A(H1N1)pdm09 in 2009. That pandemic was characterized by high bacterial superinfection rates (30–55%)2 in patients infected with the virus that came with worse outcomes including; longer hospital stays, more intensive care unit (ICU) admissions and higher mortality.3 Therefore, antibiotic therapy was prescribed to more than 70% of COVID-19 patients for prevention.4–6 However, lower rates of co-infection (3–15%) have been reported in COVID-19 patients.7 In a study carried out by Garcia-Vidal8 in Spain including 989 patients, bacterial superinfection was only observed in 3% of cases. Another study conducted in England9 reported that only 6% of cases presented bacterial superinfection among 836 patients recruited. The results reported in COVID-19 contrast with those of other viral respiratory infections where co-infections and superinfections are often present.3,10
Although there are previous studies8,11,12 involving bacterial co-infections in COVID-19 patients, we propose a nationwide study that groups all COVID-19 hospitalized patients based on the National Surveillance System for Hospital Data.
The aim of the study was to describe the epidemiology of bacterial co-infections in COVID-19 hospitalized patients, their etiology and risk factors, and their impact in outcomes.
Methods
Study design and data source
A nationwide population-based retrospective study was performed in all COVID-19 hospitalized patients during the first year of the pandemic, 2020. Data were obtained from records in the Minimum Basic Data Set (MBDS) of the National Surveillance System for Hospital Data in Spain, provided by the Ministry of Health, annually published with 2 years lag. The MDBS is a clinical and administrative database, which has an estimated coverage of 99.5% of hospital discharges registered in both public and private hospitals in Spain.13,14 The MDBS includes 20 diagnoses each one with an indicator if the diagnosis was present on admission (POA), and 20 procedures according to the International Classification of Diseases 10th Revision, Clinical Modification (ICD-10-CM).15 The MDBS provides encrypted patient identification, sex, age, dates of hospital admission and discharge, intensive care unit (ICU) admission, length of ICU stay, diagnosis and procedures during hospitalization, as well as outcome at discharge. The MBDS is validated for data quality and methodology by the Spanish Ministry of Health, establishing protocols and periodic audits. The data were treated with full confidentiality according to Spanish legislation. Thus, given the anonymous and mandatory nature of the data, informed consent was not required or necessary. This study was approved by the Ethics Committee of Valladolid East Health Area under the code PI 22-2855.
Study variables
COVID-19 was defined as the presence of ICD-10-CM codes B97.29 and U07.1 as main diagnosis and POA from 1st January to 31st December 2020.16 Sepsis was defined by the codes adapted from MacLaren et al.,17 Esper et al.,18 Dombrovskiy et al.19 and Bateman et al.20 (Supplementary table S1). In addition, severe sepsis was defined as the presence of a source of infection (Supplementary table S2) adapted from Esper et al.18and Wang et al.,21 and organ dysfunction (Supplementary table S3) according to the Angus sepsis abstraction criteria22 adapted by Shen et al.23 and Bateman et al.20 All codes were updated to ICD-10-CM by our group.
Our COVID-19 hospitalized patients were divided in two groups based on the presence or absence of nosocomial bacterial co-infection, named BI group and NBI group, respectively. ICD-10-CM codes for causal agents are described in Supplementary table S4. Regarding this, we studied the impact of co-infections in hospitalized COVID-19 patients on in-hospital and ICU mortality. Also, in 2020 in Spain, two waves of COVID-19 were reported by the Ministry of health:16 the first wave since its introduction in Spain until 30th June 2020, and the second from 1st July 2020, until 31st December 2020. Based on that, we aimed to compare the co-infection profiles and outcomes of both of them.
Statistical analysis
The results were reported as median (interquartile range) for continuous variables and as frequencies and percentages for categorical variables. Categorical data and proportions were analyzed using chi-squared test or Fisher’s exact test, as required. t-Test or Mann–Whitney U test was used to compare continuous variables. The risk of developing a health care-associated bacterial co-infection was analyzed by logistic regression model adjusted by the presence of organ failure and age, sex, tobacco, diabetes, obesity, endocrine and metabolic disorders, respiratory diseases, hypertension, heart disease, liver disease, renal disease and cancer. We also calculated the odds for in-hospital and ICU mortality in patients with COVID-19 diagnosis according to the presence of a health care-associated bacterial co-infection, by using logistic regression models adjusted by presence of bacterial co-infection, and age, sex, tobacco, diabetes, obesity, endocrine and metabolic disorders, respiratory diseases, hypertension, heart disease, liver disease, renal disease and cancer. Statistical analysis was performed using Python 3.9. All tests were two-tailed with P-values < 0.05 considered significant.
Results
Patient characteristics
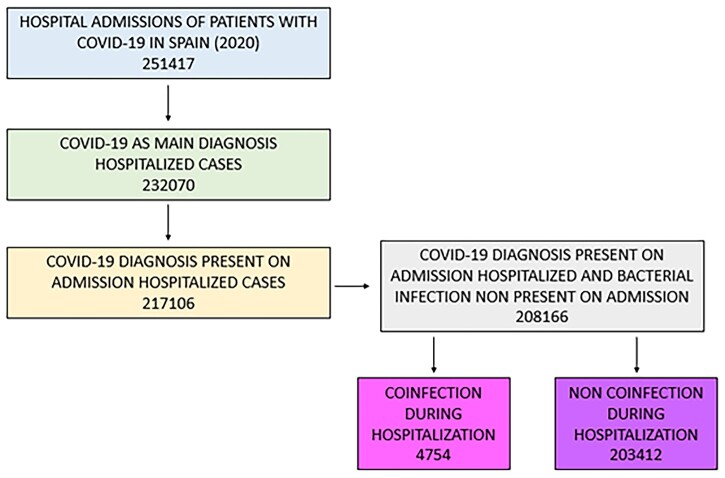
We identified a total of 208 166 patients in Spain from 1st January to 31st December 2020 with a primary diagnosis of COVID-19 and present on admission. Of these, 4754 (2.3%) had an acute bacterial co-infection (figure 1). BI was present more often in males, patients had higher length of stay (LoS), in-hospital mortality, ICU admission, ICU mortality and ICU LoS, as well as a higher need for ventilatory support, compared to NBI patients. BI patients presented with higher obesity, heart disease and respiratory disease rates compared to NBI patients. In addition, more patients of the BI group developed sepsis or septic shock, and more than half presented an acute organ dysfunction, being respiratory, renal and hematologic the most frequent locations. The respiratory tract was the most frequent site of infection in both groups of study (table 1).
Figure 1.
Study flowchart
Table 1.
Patient characteristics in COVID-19 hospitalized patients in Spain during 2020
| Total | NBI | BI | P-value | |
|---|---|---|---|---|
| No. | 208 166 | 203 412 | 4754 | |
| Gender (male) | 118 310 (56.83) | 115 146 (56.61) | 3164 (66.55) | <0.001 |
| Mean age (years) | 66.85 (66.77–66.92) | 66.83 (66.76–66.91) | 67.53 (67.16–67.89) | 0.007 |
| Length of stay (days) | 10.62 (10.57–10.67) | 9.93 (9.89–9.97) | 40.04 (39.23–40.85) | <0.001 |
| In-hospital death | 34 206 (16.43) | 32 616 (16.03) | 1590 (33.45) | <0.001 |
| Charlson Index | 1.33 (1.32–1.34) | 1.33 (1.32–1.34) | 1.49 (1.44–1.54) | <0.001 |
| No comoborbidities | 100 788 (48.42) | 98 787 (48.56) | 2001 (42.09) | <0.001 |
| 1 comoborbidities | 31 421 (15.09) | 30 649 (15.07) | 772 (16.24) | 0.027 |
| 2 comoborbidities | 35 789 (17.19) | 34 874 (17.14) | 915 (19.25) | <0.001 |
| >2 | 40 168 (19.3) | 39 102 (19.22) | 1066 (22.42) | <0.001 |
| ICU and intubation | ||||
| ICU | 18 531 (8.9) | 15 353 (7.55) | 3178 (66.85) | <0.001 |
| ICU death | 5901 (31.84) | 4718 (30.73) | 1183 (37.22) | <0.001 |
| ICU length of stay | 15.44 (15.2–15.68) | 12.3 (12.09–12.51) | 30.6 (29.84–31.37) | <0.001 |
| Mechanical ventilation | 13 487 (6.48) | 10 402 (5.11) | 3085 (64.89) | <0.001 |
| Ventilatory assistance | 11 676 (5.61) | 10 817 (5.32) | 859 (18.07) | <0.001 |
| Morbidities | ||||
| Abuse of tobacco | 6803 (3.27) | 6687 (3.29) | 116 (2.44) | 0.001 |
| Diabetes | 44 310 (21.29) | 43 182 (21.23) | 1128 (23.73) | <0.001 |
| Obesity | 20 017 (9.62) | 19 404 (9.54) | 613 (12.89) | <0.001 |
| Endocrine and metabolic disorders | 77 832 (37.39) | 76 224 (37.47) | 1608 (33.82) | <0.001 |
| Respiratory diseases | 31 088 (14.93) | 30 297 (14.89) | 791 (16.64) | 0.001 |
| Hypertension | 86 312 (41.46) | 84 401 (41.49) | 1911 (40.2) | 0.076 |
| Heart disease | 51 738 (24.85) | 50 477 (24.82) | 1261 (26.53) | 0.007 |
| Peripheral vascular disease | 1273 (0.61) | 1249 (0.61) | 24 (0.5) | 0.390 |
| Liver disease | 10 842 (5.21) | 10 572 (5.2) | 270 (5.68) | 0.148 |
| Renal disease | 14 519 (6.97) | 14 215 (6.99) | 304 (6.39) | 0.119 |
| Cancer | 9783 (4.7) | 9563 (4.7) | 220 (4.63) | 0.840 |
| HIV | 451 (0.22) | 442 (0.22) | 9 (0.19) | 0.801 |
| Sepsis | ||||
| Sepsis | 14 217 (6.83) | 10 883 (5.35) | 3334 (70.13) | <0.001 |
| Sepsis + 1 organ failure | 6776 (3.26) | 4916 (2.42) | 1860 (39.12) | <0.001 |
| Sepsis + 2 organ failure | 3305 (1.59) | 2425 (1.19) | 880 (18.51) | <0.001 |
| Sepsis + >2 organ failure | 1365 (0.66) | 1047 (0.51) | 318 (6.69) | <0.001 |
| Organ failure | ||||
| Number of organ failure | 0.7 (0.7–0.71) | 0.69 (0.68–0.69) | 1.35 (1.32–1.37) | <0.001 |
| No organ failure | 95 588 (45.92) | 95 162 (46.78) | 426 (8.96) | <0.001 |
| 1 organ | 84 651 (40.67) | 81 913 (40.27) | 2738 (57.59) | <0.001 |
| 2 organ | 22 956 (11.03) | 21 767 (10.7) | 1189 (25.01) | <0.001 |
| >2 organ | 4971 (2.39) | 4570 (2.25) | 401 (8.44) | <0.001 |
| Organ failure | ||||
| Cardiovascular | 2939 (1.41) | 2652 (1.3) | 287 (6.04) | <0.001 |
| Hematologic | 11 159 (5.36) | 10 637 (5.23) | 522 (10.98) | <0.001 |
| Hepatic | 7527 (3.62) | 7191 (3.54) | 336 (7.07) | <0.001 |
| Neurologic | 7857 (3.77) | 7562 (3.72) | 295 (6.21) | <0.001 |
| Renal | 22 844 (10.97) | 22 351 (10.99) | 493 (10.37) | 0.186 |
| Respiratory organ | 90 199 (43.33) | 86 105 (42.33) | 4094 (86.12) | <0.001 |
| Metabolic | 3707 (1.78) | 3333 (1.64) | 374 (7.87) | <0.001 |
| Site infection | ||||
| Nervous | 34 (0.02) | 32 (0.02) | 2 (0.04) | 0.406 |
| Circulatory | 250 (0.12) | 226 (0.11) | 24 (0.5) | <0.001 |
| Respiratory | 178 651 (85.82) | 174 088 (85.58) | 4563 (95.98) | <0.001 |
| Digestive | 963 (0.46) | 663 (0.33) | 300 (6.31) | <0.001 |
| Genitourinary | 8109 (3.9) | 6042 (2.97) | 2067 (43.48) | <0.001 |
| Pregnancy | 30 (0.01) | 30 (0.01) | 0 (0.0) | 0.821 |
| Skin soft tissue or bone | 593 (0.28) | 501 (0.25) | 92 (1.94) | <0.001 |
Bacterial infections and antimicrobial resistance
The comparison of BI patients in both waves is described in table 2. First, patients in the first wave had higher LoS in both general and ICU admission, as well as higher need of mechanical ventilation. However, patients in the second wave had higher in-hospital and ICU mortality, and higher need of ventilatory assistance. The profile of bacterial infections was similar in both waves, except for some agents. In the first wave, Gram-negative bacteria caused infections were higher, especially Pseudomonas spp. co-infections (42.08% vs. 38.02%). Meanwhile, in the second wave, Streptococcus pneumoniae (61.64%) and Haemophilus influenzae (3.80%) co-infections were present more often compared to the first wave (44.26 and 1.58%, respectively).
Table 2.
Difference of microorganism specific rate linked to COVID-19 co-infection in both waves, incidence of antimicrobial resistance and the most common antimicrobial resistance in the BI group
| Total | 1st wave | 2nd wave | P-value | |
|---|---|---|---|---|
| No. | 4754 | 1576 | 3178 | |
| Gender (male) | 3164 (66.55) | 1652 (67.37) | 1512 (65.68) | 0.228 |
| Mean age (years) | 67.53 (67.16–67.89) | 66.94 (66.43–67.45) | 68.16 (67.63–68.68) | 0.001 |
| Length of stay (days) | 40.04 (39.23–40.85) | 46.9 (45.59–48.21) | 32.73 (31.91–33.55) | <0.001 |
| In-hospital death | 1590 (33.45) | 714 (29.12) | 876 (38.05) | <0.001 |
| Charlson Index | 1.49 (1.44–1.54) | 1.41 (1.34–1.48) | 1.58 (1.51–1.66) | 0.001 |
| No comoborbidities | 2001 (42.09) | 1105 (45.07) | 896 (38.92) | <0.001 |
| 1 comoborbidities | 772 (16.24) | 399 (16.27) | 373 (16.2) | 0.980 |
| 2 comoborbidities | 915 (19.25) | 440 (17.94) | 475 (20.63) | 0.021 |
| >2 | 1066 (22.42) | 508 (20.72) | 558 (24.24) | 0.004 |
| ICU and intubation | ||||
| ICU | 3178 (66.85) | 1655 (67.5) | 1523 (66.16) | 0.344 |
| ICU death | 1183 (24.88) | 518 (21.13) | 665 (28.89) | <0.001 |
| ICU length of stay | 30.6 (29.84–31.37) | 34.99 (33.77–36.22) | 25.84 (25.02–26.65) | <0.001 |
| Mechanical ventilation | 3085 (64.89) | 1674 (68.27) | 1411 (61.29) | <0.001 |
| Ventilatory assistance | 859 (18.07) | 392 (15.99) | 467 (20.29) | <0.001 |
| Tipo bacteria familia | ||||
| Gram-positive bacteria | 604 (12.71) | 298 (12.15) | 306 (13.29) | 0.256 |
| Staphylococcus spp. | 479 (79.3) | 240 (80.54) | 239 (78.1) | 0.461 |
| S. aureus | 115 (24.01) | 49 (20.42) | 66 (27.62) | 0.065 |
| Other Staphylococci | 382 (79.75) | 199 (82.92) | 183 (76.57) | 0.084 |
| Streptococcus spp. and Enterococcus spp | 134 (22.19) | 61 (20.47) | 73 (23.86) | 0.317 |
| S. pneumoniae | 72 (53.73) | 27 (44.26) | 45 (61.64) | 0.044 |
| Other Streptococci | 65 (48.51) | 35 (57.38) | 30 (41.1) | 0.060 |
| Other Gram-positive bacteria | 1 (0.17) | 1 (0.34) | 0 (0.0) | 0.310 |
| Gram-negative bacteria | 3862 (81.24) | 2025 (82.59) | 1837 (79.8) | 0.015 |
| Haemophilus influenzae | 102 (2.64) | 32 (1.58) | 70 (3.81) | <0.001 |
| Neisseria meningitidis | 2 (0.05) | 1 (0.05) | 1 (0.05) | 0.945 |
| Pseudomonas spp. | 1554 (40.24) | 853 (42.12) | 701 (38.16) | 0.012 |
| Enterobacterales | 180 (4.66) | 92 (4.54) | 88 (4.79) | 0.716 |
| Other Gram-negative bacteria | 537 (13.9) | 294 (14.52) | 243 (13.23) | 0.247 |
| Anaerobic bacteria | 270 (5.68) | 158 (6.44) | 112 (4.87) | 0.022 |
| Other anaerobes | 25 (9.26) | 14 (8.86) | 11 (9.82) | 0.788 |
| Clostridium spp. | 248 (91.85) | 145 (91.77) | 103 (91.96) | 0.955 |
| Other bacterial infections | 327 (6.88) | 138 (5.63) | 189 (8.21) | 0.001 |
| Mycoplasma pneumonia | 16 (4.89) | 6 (4.35) | 10 (5.29) | 0.696 |
| Chlamydia pneumonia | 5 (1.53) | 3 (2.17) | 2 (1.06) | 0.417 |
| Non-classified bacterial infections | 306 (93.58) | 129 (93.48) | 177 (93.65) | 0.950 |
| Antimicrobial drug resistance | 749 (15.76) | 404 (16.48) | 345 (14.99) | 0.171 |
| Resistance to beta-lactam antibiotics | 551 (73.56) | 300 (74.26) | 251 (72.75) | 0.642 |
| Meticilin-resistant S. aureus | 190 (25.37) | 113 (27.97) | 77 (22.32) | 0.076 |
| Resistance to penicilins | 83 (11.08) | 44 (10.89) | 39 (11.3) | 0.857 |
| Resistance to cephalosporins | 45 (6.01) | 25 (6.19) | 20 (5.8) | 0.822 |
| Resistance to other beta-lactam antibiotics | 377 (50.33) | 195 (48.27) | 182 (52.75) | 0.221 |
| Resistance to glucopeptides | 2 (0.27) | 2 (0.5) | 0 (0.0) | 0.191 |
| Resistance to quinolones | 31 (4.14) | 22 (5.45) | 9 (2.61) | 0.052 |
| Resistance to aminoglycoside macrolides sulfonamides or tetracyclines | 26 (3.47) | 14 (3.47) | 12 (3.48) | 0.992 |
| Resistance to other antibiotics | 241 (32.18) | 134 (33.17) | 107 (31.01) | 0.529 |
| Resistance to multiple antibiotics | 150 (20.03) | 74 (18.32) | 76 (22.03) | 0.206 |
No differences in antimicrobial resistance profiles were found in COVID-19 hospitalized patients between waves.
Risk of bacterial co-infection
Supplementary table S5 shows the adjusted risk of bacterial co-infection. The risk of bacterial co-infection risk was not influenced by the seasonality (second vs. first wave, aOR = 0.97, P-values = 0.370).
In-hospital and ICU mortality risks due to bacterial co-infection
Next, we evaluated the risks of mortality associated with bacterial co-infection. Overall, bacterial co-infection was an important risk factor for both, in-hospital and ICU mortality (aOR 3.32 and 1.27, respectively) (Supplementary table S6).
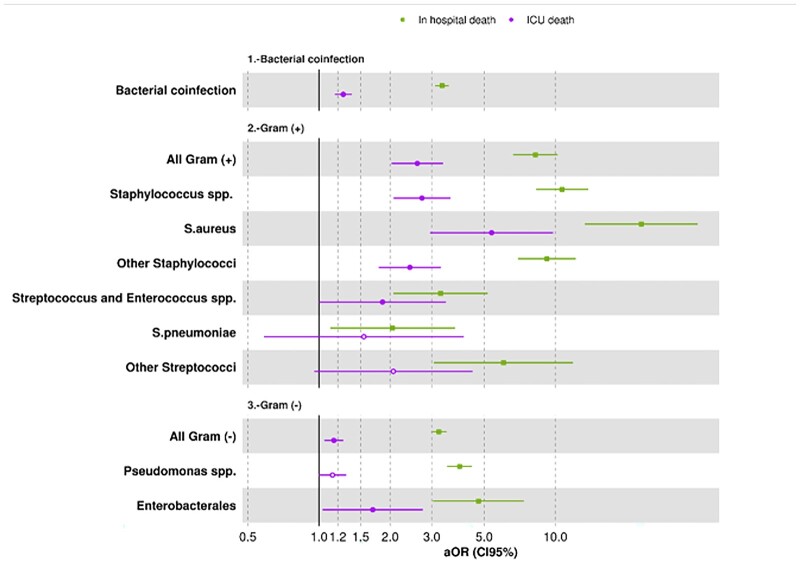
Risks according to bacterial classification was performed to evaluate which bacteria had the highest impact in mortality. Both Gram-positive and Gram-negative bacteria were associated with higher mortality, especially for in-hospital mortality in all cases (figure 2). Additionally, infections due to Gram-positive bacteria had a higher impact than infections caused by Gram-negative bacteria. Among Gram-positive bacteria, higher risks were found for Staphylococcal compared to Streptococcal infections, particularly for Staphylococcus aureus and in-hospital mortality (aOR 23.1). A co-infection with bacteria belonging to Enterobacterales outstands as a higher risk of in-hospital mortality (aOR 4.71) among Gram-negative bacteria.
Figure 2.
In-hospital and ICU mortality risk due to bacterial co-infections in COVID-19 patients
Discussion
In this retrospective study of COVID-19 hospitalized patients in Spain during 2020 with a nosocomial bacterial infection, we found that (i) the incidence of bacterial co-infection was 2,3% and was associated with worse outcomes; (ii) the main factors related with the development of a bacterial co-infection were the presence of an organ failure, obesity and male sex; (iii) Gram-negative bacteria were the most common causal agents of co-infection in both waves. Pseudomonas spp. was higher in the first wave compared to the second wave while S. pneumoniae and H. influenzae were higher in the second.
Many studies have tried to assess the incidence of bacterial co-infections in COVID-19 patients with different outcomes. A meta-analysis performed in 2020 concluded that 7% (95% CI 3–12) of the patients presented bacterial co-infection and raised up to 14% (95% CI 5–26) when it came to the ICU.7 However, another meta-analysis performed in 2021 estimated that the pooled bacterial co-infection prevalence reached up to 20.97%, showing great disparities between studies.24 In our study the prevalence of bacterial co-infection in hospitalized patients with COVID-19 is 2.3% and 17% in the case of ICU admitted patients in our country, which is lower than previously found for general admission, and higher for ICU admission. The biggest pandemic we have records of, occurred in 1918 caused by influenza A(H1N1) where mortality rates were so high due not only to the viral infection but to bacterial co-infections. Most recently, in 2009, another influenza pandemic caused by an emerging influenza A(H1N1) virus of swine origin (A(H1N1)pdm09) occurred and actually replaced the previously A(H1N1) circulating strain. This time mortality was not as high as 1918s thanks to the use of antibiotics and mechanical ventilation among others.25,26 The incidence of bacterial co-infections after viral pneumonia was 12% in hospitalized patients27 and 30% in patients admitted to the ICU.28 In the case of co-infections in COVID-19, the mechanisms that lead to such a lower incidence is not well understood. Several hypotheses have been made, including prophylactic antibiotic therapy at hospital admission and the presence of an immunological factor such as macrophage hyperactivation.8
The most frequent sites of infection were respiratory followed by urinary and digestive, probably due to catheter colonization and antibiotic use. The most often microorganisms found in co-infections were Gram-negative bacteria with higher prevalence of those, particularly Pseudomonas spp. in the first wave, and S. pneumoniae and H. influenzae in the second. The prevalence of one group over the other is a matter of controversy as Gram negatives predominate in some studies29,30 and Gram-positive bacteria in others.31 A study performed in a ICU unit in Spain also showed Pseudomonas spp. and H. influenzae to be among the most often found bacterias causing infection in COVID-19 patients.32 On the other hand, higher mortality risk was associated any bacterial co-infection with a higher risk in case of Gram-positive bacteria, which is probably due to the highest risk association of S. aureus co-infection. This microorganism is known for its virulence and has been previously associated with bacterial co-infection in patients with COVID-19 pneumonia along with S. pneumoniae, H. influenzae and K. pneumonia.33 Among Gram-negative bacteria, the enterobacterales presented the higher mortality risks which includes K. pneumoniae among others, which aligns with the aforementioned review.33
Gram-negative co-infections were higher in the first wave, especially Pseudomonas spp co-infections. One of the risk factors related to Pseudomonas spp. co-infection include mechanical ventilation which could lead to ventilator associated pneumonia.34 In the first wave, the patients required more mechanical ventilation5,35 which would explain the higher incidence of this pathogen. Instead, in the second wave, S. pneumoniae and H. influenzae co-infections were present more often compared to the first wave. S. pneumoniae and H. influenzae usually present seasonality with higher peaks in winter and spring,36,37 which will not explain why higher incidence was found in the second wave. However, it has been shown that circulation patterns of other pathogens have been modified due to the COVID-10 pandemic which could explain our results.38,39
Multiple factors have been associated with the risk of bacterial co-infections,29,40 such as advanced age, male sex and obesity which are consistent with our study. Organ failure is another factor we found significant. Regarding this, invasive techniques would be required in those cases which align with findings that associate higher risk with the use of catheters and invasive mechanical ventilation.41
Previous studies have shown bacterial co-infections upon viral pneumonia present with poorer outcomes.42,43 Additionally, studies have associated lymphocytopenia with COVID-19 severity and ICU admission. This condition is known to encourage the appearance of co-infections more frequently.44 Also, ICU-admitted patients require invasive measures and techniques that increase the susceptibility of nosocomial infections.45 Our results are consistent with previous studies and patients with BI had higher LoS, ICU admission and mortality than NBI patients. The higher LoS need for mechanical ventilation observed in the first wave could be reasonably explained by the lack of knowledge, the severity of the cases and the overwhelmed health system at the first stages of the pandemic. Whereas, in the second wave many admissions were made to follow-up in case of acute respiratory distress syndrome considerably reducing the LoS.46
Our study has limitations since we carried out a retrospective study using data obtained in the Spanish MBDS, with under coding of variables, lack of coding of analytical variables and lack of coding of several admissions of the same patient. The main advantage is the large size of the sample, which gives it high statistical power and allows us to provide a global view of the epidemiological situation of the entire Spanish population.
In conclusion, in this nationwide study, we report bacterial co-infections in 2.3% of COVID-19 hospitalized patients who presented higher in-hospital and ICU LoS and mortality, as well as ICU admission. The main microorganisms causing bacterial infections were Gram-negative bacteria, especially Pseudomonas spp. However, the higher mortality risks were found for Gram-positive bacteria, outstanding S. aureus.
Bacterial co-infections in COVID-19 hospitalized patients are not as common as found in other viral respiratory infections. Due to the low prevalence of bacterial co-infections, antibiotic therapy should be prescribed carefully to avoid complications derived from extensive use of antibiotics in these patients.
Supplementary Material
Contributor Information
R López-Herrero, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Anesthesiology and Critical Care Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain; Department of Surgery, Faculty of Medicine, Universidad de Valladolid, Valladolid, Spain.
L Sánchez-de Prada, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Microbiology and Immunology Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
A Tamayo-Velasco, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Haematology and Hemotherapy Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
M Lorenzo-López, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Anesthesiology and Critical Care Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain; Department of Surgery, Faculty of Medicine, Universidad de Valladolid, Valladolid, Spain.
E Gómez-Pesquera, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Anesthesiology and Critical Care Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain; Department of Surgery, Faculty of Medicine, Universidad de Valladolid, Valladolid, Spain.
B Sánchez-Quirós, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Anesthesiology and Critical Care Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
O de la Varga-Martínez, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Department of Anesthesiology, Hospital Universitario Infanta Leonor, Madrid, Spain.
E Gómez-Sánchez, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Anesthesiology and Critical Care Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain; Department of Surgery, Faculty of Medicine, Universidad de Valladolid, Valladolid, Spain.
S Resino, Unidad de Infección Viral e Inmunidad, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Madrid, Spain.
E Tamayo, BioCritic, Group for Biomedical Research in Critical Care Medicine, Valladolid, Spain; Anesthesiology and Critical Care Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain; Department of Surgery, Faculty of Medicine, Universidad de Valladolid, Valladolid, Spain.
A Álvaro-Meca, Departament of Preventive Medicine and Public Health, Faculty of Health Science, Universidad Rey Juan Carlos, Madrid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III, Madrid, Spain.
Supplementary data
Supplementary data are available at EURPUB online.
Funding
This work was supported by Instituto de Salud Carlos III (COV20/00491, PI18/01238, CIBERINFEC CB21/13/00051), Junta de Castilla y León (VA321P18, GRS 1922/A/19, GRS 2057/A/19), Consejería de Educación de Castilla y León (VA256P20) and Fundación Ramón Areces (CIVP19A5953). L. Sánchez-de Prada received a Río Hortega grant (CM20/00138) from Instituto Carlos III (Co-funded by European Regional Development Fund/European Social Fund ‘A way to make Europe’/’Investing in your future’).
Conflicts of interest: None declared.
Author contributions
All authors contributed to the article and approved the submitted version. A.M. has performed the statistical analysis and has prepared the images with the help of R.L.-H. and L.S. R.L.-H. and L.S. have made the original article under the supervision of A.M. All authors have reviewed the article.
Data availability
All data are available as a part of the article.
Key points.
Coronavirus disease 2019 (COVID-19) infection is a public health problem that has caused more than 6 million cases worldwide.
Bacterial co-infection in patients with COVID-19 increased the length of stay, the admissions to intensive care units and the mortality.
Antibiotherapy should not be prescribed to all patients with COVID-19 infection. It is essential to detect a true co-infection in which case antibiotic therapy should be initiated.
References
- 1.WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available at: https://covid19.who.int/ (14 September 2022, date last accessed).
- 2. Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol 2017;8:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008;198:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020;71:2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clancy CJ, Hong Nguyen M. Coronavirus disease 2019, superinfections, and antimicrobial development: what can we expect? Clin Infect Dis 2020;71:2736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamayo-Velasco Á, Martínez-Paz P, Jesús Peñarrubia-Ponce M, et al. Clinical medicine HGF, IL-1α, and IL-27 are robust biomarkers in early severity stratification of COVID-19 patients. J Clin Med 2021;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 2020; 81:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. ; COVID-19 Researchers Group. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 2021;27:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hughes S, Troise O, Donaldson H, et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 2020;26:1395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA 2013;309:275–82. [DOI] [PubMed] [Google Scholar]
- 11. Falcone M, Tiseo G, Giordano C, et al. ; Pisa COVID-19 Study Group. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother 2021;76:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nebreda-Mayoral T, Miguel-Gómez MA, March-Rosselló GA, et al. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enferm Infecc Microbiol Clin (Engl Ed) 2022;40:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministerio de Sanidad - Portal Estadístico del SNS - Registro de Altas de los Hospitales del Sistema Nacional de Salud. CMBD. Available at: https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbdhome.htm (14 September 2022, date last accessed).
- 14.BOE.es - BOE-A-2015-1235 Real Decreto 69/2015, de 6 de febrero, por el que se regula el Registro de Actividad de Atención Sanitaria Especializada. Available at: https://www.boe.es/buscar/act.php?id=BOE-A-2015-1235 (14 September 2022, date last accessed).
- 15.The Web’s Free 2022 ICD-10-CM/PCS Medical Coding Reference. Available at: https://www.icd10data.com/ (14 September 2022, date last accessed).
- 16.Subdirección General de Información Sanitaria Anuncio de Cambio Y Nueva Normativa Para la Codificación de la Infección Por SARS-CoV-2 (COVID-19) Unidad Técnica de Codificación CIE-10-ES.
- 17. MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med 2008;36:3184–9. [DOI] [PubMed] [Google Scholar]
- 18. Esper AM, Moss M, Lewis CA, et al. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med 2006;34:2576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244–50. [DOI] [PubMed] [Google Scholar]
- 20. Bateman BT, Schmidt U, Berman MF, Bittner EA. Temporal trends in the epidemiology of severe postoperative sepsis after elective surgery: a large, nationwide sample. Anesthesiology 2010;112:917–25. [DOI] [PubMed] [Google Scholar]
- 21. Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007;35:1928–36. [DOI] [PubMed] [Google Scholar]
- 22. Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. [DOI] [PubMed] [Google Scholar]
- 23. Shen HN, Lu CL, Yang HH. Epidemiologic trend of severe sepsis in Taiwan from 1997 through 2006. Chest 2010;138:298–304. [DOI] [PubMed] [Google Scholar]
- 24. Soltani S, Faramarzi S, Zandi M, et al. Bacterial coinfection among coronavirus disease 2019 patient groups: an updated systematic review and meta-analysis. 2021. Available at: 10.1016/j.nmni.2021.100910 (16 January 2023, date last accessed). [DOI] [PMC free article] [PubMed]
- 25. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2021;73:E4208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Short KR, Kedzierska K, van de Sandt CE. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol 2018;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacIntyre CR, Chughtai AA, Barnes M, et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis 2018; 18:637. Available at: https://pubmed.ncbi.nlm.nih.gov/30526505/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rice TW, Rubinson L, Uyeki TM, et al. ; NHLBI ARDS Network. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med 2012;40:1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ripa M, Galli L, Poli A, et al. ; COVID-BioB Study Group. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect 2021;27:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest 2020;50: Available at: https://pubmed.ncbi.nlm.nih.gov/32535894/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barrasa H, Rello J, Tejada S, et al. ; Alava COVID-19 Study Investigators. SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med 2020;39:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Musuuza JS, Watson L, Parmasad V, et al. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One 2021;16:e0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Sig Transduct Target Ther 2022;7:199. 10.1038/s41392-022-01056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iftimie S, Lopez-Azcona AF, Vallverdu I, et al. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus, Spain. PLoS One 2021;16:e0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enfermedad invasora por Haemophilus influenzae antes y después de la campaña de vacunación en la población infantil de la Comunidad Valenciana (1996-2000). Available at: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=s1135-57272002000300004 (21 January 2023, date last accessed). [PubMed]
- 37. Herrera-Lara S, Fernández-Fabrellas E, Cervera-Juan Á, Blanquer-Olivas R. Influyen la estación y el clima en la etiología de la neumonía adquirida en la comunidad? Arch Bronconeumol 2013;49:140–5. [DOI] [PubMed] [Google Scholar]
- 38. Fourgeaud J, Toubiana J, Chappuy H, et al. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur J Clin Microbiol Infect Dis 2021;40:2389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loconsole D, Centrone F, Aprile V, et al. What’s next for flu? Out-of-season circulation of influenza viruses in Southern Italy, August 2022. Viruses 2022; 14:2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 2020;369:m1985. Available at: https://pubmed.ncbi.nlm.nih.gov/32444460/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The references 41 to 46 are available in Supplementary material.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available as a part of the article.
Key points.
Coronavirus disease 2019 (COVID-19) infection is a public health problem that has caused more than 6 million cases worldwide.
Bacterial co-infection in patients with COVID-19 increased the length of stay, the admissions to intensive care units and the mortality.
Antibiotherapy should not be prescribed to all patients with COVID-19 infection. It is essential to detect a true co-infection in which case antibiotic therapy should be initiated.