Abstract
Background & Aims
Bacterial infections are frequent in patients with cirrhosis and increase the risk of death and drop-out from liver transplant (LT) waiting list. In patients with bacterial infections, LT is frequently delayed because of the fear of poor outcomes. We evaluated the impact of pre-LT infections on post-LT complications and survival.
Methods
From 2012 to 2018, consecutive patients transplanted at the Hospital of Padua were identified and classified in two groups: patients surviving an episode of bacterial infection within 3 months before LT (study group) and patients without infections before LT (control group). Post-LT outcomes (complications, new infections, survival) were collected.
Results
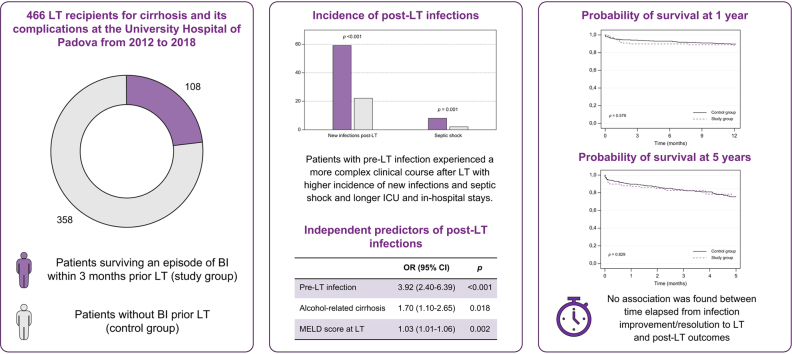
A total of 466 LT recipients were identified (study group n = 108; control group n = 358). After LT, the study group had a higher incidence of new bacterial (57% vs. 20%, p <0.001) and fungal infections (14% vs. 5%, p = 0.001) and of septic shock (8% vs. 2%, p = 0.004) than the control group. Along with the model for end-stage liver disease (MELD) score and alcohol-related cirrhosis, bacterial infection pre-LT was an independent predictor of post-LT infections (odds ratio = 3.92; p <0.001). Nevertheless, no significant difference was found in 1-year (88% vs. 89%, p = 0.579) and 5-year survival rates (76% vs. 75%, p = 0.829) between the study group and control group. Within the study group, no association was found between the time elapsed from infection improvement/resolution to LT and post-LT outcomes.
Conclusions
Patients with pre-LT infections have a higher risk of new bacterial and fungal infections and of septic shock after LT. However, post-LT survival is excellent. Therefore, as soon as the bacterial infection is improving/resolving, transplant should not be delayed, but patients with pre-transplant bacterial infections require active surveillance for infections after LT.
Impact and Implications
Bacterial infections increase mortality and delay transplant in patients with cirrhosis awaiting liver transplantation (LT). Little is known about the impact of adequately treated infections before LT on post-transplant complications and outcomes. The study highlights that pre-LT infections increase the risk of post-LT infections, but post-LT survival rates are excellent despite the risk. These findings suggest that physicians should not delay LT because of concerns about pre-LT infections, but instead should actively monitor these patients for infections after surgery.
Keywords: Liver cirrhosis, Sepsis, Acute-on-chronic liver failure, Liver transplantation, Prioritisation
Graphical abstract
Highlights
-
•
In patients with cirrhosis, the prognostic impact of pre-LT infections is not completely understood.
-
•
Pre-LT infections increase the risk of post-LT infections but do not affect patients’ survival.
-
•
Time from infection resolution to LT does not affect incidence of post-LT complication and survival rates.
-
•
As soon as infections are resolving, it is safe to proceed with LT without any delay.
-
•
Patients with pre-LT infection require active surveillance for infections after LT.
Introduction
Patients with decompensated cirrhosis have a high risk of developing bacterial infections, which can trigger decompensation and organ failures and are associated with a high risk of short-term mortality.1,2 Moreover, in liver transplant (LT) candidates the onset of infections is challenging, because it requires the temporary suspension from the waiting list and it is associated with a high risk of death and/or of permanent de-listing.3,4 The risk of poor outcomes because of the persistence/recurrence of infections after LT is a main barrier in the decision to proceed or not to LT in patients with cirrhosis and infections. In fact, infections increase morbidity and mortality in the early post-transplant period[5], [6], [7] and use of immunosuppression may limit the ability of the host to counteract the pathogens. For these reasons, international guidelines state that active infections should be adequately treated before LT.8 However, the optimal timing of LT in patients surviving an episode of infection as well as their prioritisation on LT waiting list is still to be established.
Moreover, studies on post-LT outcomes of patients surviving an episode of infection showed conflicting results. Some studies showed no difference in survival rates between patients with or without pre-LT infections,9,10 whereas others showed a higher risk of sepsis-related mortality in patients with pre-LT infections, in particular if complicated by septic shock.11,12 The aims of this study were to evaluate: (a) the impact of bacterial infections within 3 months before LT on post-LT outcomes; (b) the impact of time elapsed from infection improvement/resolution to LT on post-LT outcomes.
Patients and methods
Patients
This is a case-control study performed in consecutive patients who underwent LT at the University Hospital of Padova between January 2012 and December 2018. Inclusion criteria were: (1) diagnosis of liver cirrhosis based on histology or on clinical, biochemical, ultrasonographic, and/or endoscopic findings; (2) age >18 years. Exclusion criteria were: (1) indication for LT other than cirrhosis; (2) previous LT; (3) simultaneous liver–kidney transplantation; (4) human immunodeficiency virus infection. Patients surviving an episode of bacterial/fungal infection within 3 months before LT constituted the study group, whereas patients without bacterial infections in the 3 months before LT comprised the control group.
Permission for retrospective data analysis was obtained from the local ethics committee. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Study design
Demographic, clinical, and laboratory data were collected at the time of transplantation for each recipient by reviewing electronic and paper charts. We also collected demographic characteristics of donors and data on cold ischaemia time, time of surgery, surgical technique, and immunosuppressive treatment. For patients in the study group we also collected clinical, laboratory, and microbiological data, and also the treatment administered during the infectious episodes. After LT, we collected data regarding the incidence of complications (new bacterial and fungal infections, septic shock, acute kidney injury [AKI] and need for renal replacement therapy [RRT], vascular and biliary anastomosis complications, primary non-function (PNF), early rejection), the length of intensive care unit (ICU) and in-hospital stay, and 1- and 5-year survival rates.
Definitions
Infections were defined according to well-established criteria.13 Briefly, spontaneous bacterial peritonitis (SBP) was defined as a polymorphonuclear cell (PMNC) count ≥250 cells/μl in ascitic fluid without any intrabdominal, surgically treatable source of infection. Other infections were defined according to the criteria proposed by the Centers for Disease Control and Prevention.14 Sepsis and septic shock were defined according to the Third International Consensus Definitions for Sepsis and Septic Shock.15,16 Infections were classified as community-acquired (CA, onset within 2 days after admission), hospital-acquired (HA, onset >2 days after hospital admission) or healthcare-associated (HCA, onset within 2 days after admission in patients who were hospitalised for at least 2 days in the previous 90 days).17 Bacteria were classified as multi-drug resistant (MDR) if resistant to at least one antibiotic in three or more antimicrobial categories and extensively drug resistant (XDR) if resistant to at least one antibiotic in all but two or fewer antimicrobial categories.18
Infections were considered resolved when normalisation of clinical signs and laboratory parameters of infection were achieved.
For the purpose of the study, the ‘perioperative period’ was considered the period elapsed from LT to discharge and ‘early post-LT complications’ were considered those that occurred during this period. Early rejection was defined as the histologic evidence of rejection in the postoperative period. AKI was defined according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria.19
Local transplant allocation policy
A detailed description of LT policy within our organ procurement agency (North Italian Transplant programme) has been stated elsewhere.20,21 Briefly, potential donors are assigned to a LT unit within a geographic area, and each unit selects a recipient from its own waiting list. Only patients listed for emergency re-LT or acute liver failure get national priority as status 1 A. During the study period, the LT allocation policy in our centre was model for end-stage liver disease (MELD)-based for patients with cirrhosis without hepatocellular carcinoma (HCC), whereas patients with active HCC and a MELD score <20 were prioritised according to the timing of HCC onset and the response to treatment evaluated by the modified Response Evaluation Criteria in Solid Tumours criteria.22
In patients with pre-LT infections, the clearance for LT eligibility was given by infectious disease specialists and transplant hepatologists when the infection showed improvement/resolution and the date of clearance was collected in the chart. In detail, the criteria used for clearance were the following: resolution of signs/symptoms of infection for at least 48 h (absence of fever, cough, dysuria, stranguria, skin signs of cellulitis, septic shock), reduction of biomarkers of infection/inflammation (i.e. white blood cell count, C-reactive protein, procalcitonin), PMNC count <250/μl in patients with SBP, at least one set of negative blood cultures after at least 48 h of antibiotic treatment in patients with bloodstream infection, and source control in secondary bacteraemia (e.g. catheter removal in catheter-related bloodstream infections [CR-BSIs], drainage of abscesses, etc.). Immune suppression was induced with basiliximab and steroids in all patients and maintained with calcineurin inhibitors (CNIs) with or without micophenolate mofetil or everolimus. All patients received perioperative antibiotic prophylaxis.
Statistical analysis
Normally distributed continuous variables were reported as means with SD and compared with Student’s t test. Non-normally distributed continuous variables were reported as median and interquartile range (IQR) and compared with Mann-Whitney’s U test. Categorical variables were reported as frequencies and compared with the Χ2 test or Fisher’s exact test, when appropriate. A univariable analysis was performed to identify predictors of new infections post-LT. Variables found to have a p value <0.05 were included in a multivariable step-wise logistic regression analysis with backward elimination (entry p <0.05; exit p >0.10). Non-normally distributed continuous variables were log-transformed before inclusion in the multivariable model. The odds ratios (ORs) and their 95% CIs were calculated. When scores of liver disease were included in the multivariable analysis their components were excluded to avoid multicollinearity. One- and 5-year survival rates were estimated by Kaplan–Meier’s method and compared with the log-rank test. A multivariable Cox proportional hazards model (adjusted for age, sex, HCC, HCV aetiology, pre-LT infection and MELD score) was used to estimate the risk of post-LT mortality associated with pre-LT infections. The adjusted hazard ratios (aHR) and their 95% CIs were calculated.
All tests were two-tailed and values of p <0.05 were considered significant. The statistical analysis was performed using the SPSS statistical software (SPSS Inc, Chicago, IL), version 28.0.
Results
Study population
During the study period we identified 466 LT recipients who fulfilled the inclusion criteria and without any exclusion criteria. The mean age was 57 ± 9 years. The majority of patients were male (362 patients, 78%) and had alcoholic or viral cirrhosis. At the time of LT, the mean biochemical MELD-Na score was 20 ± 9 and five patients had severe alcoholic hepatitis. Overall, 108 patients survived at least one episode of bacterial/fungal infection within 3 months before LT (study group). The remaining 358 patients had no infections before LT (control group).
The characteristics of infections in the study group are reported in Tables S1 and S2. Sixteen patients had two concomitant infections, therefore a total of 124 infectious episodes have been described. Most of infections were HCA and HA (29% and 53%, respectively). The most common infection was SBP, followed by urinary tract infections (UTIs), pneumonia, and CR-BSIs. Sixty-three patients had positive cultures and two or more pathogens were isolated in 19 patients. Overall, 84 pathogens were isolated (Table S2): 42 (50%) were Gram positive, 35 (42%) were Gram negative and 7 (8%) were fungi. Enterococci and enterobacteriaceae were the most frequently isolated bacteria. Thirty-two (38%) pathogens were MDR; the most common were enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae and methicillin-resistant staphylococci. Five (6%) of the MDR pathogens were XDR: three vancomycin-resistant Enterococcus faecium and two carbapenem-resistant Gram-negative bacteria.
At the time of infection diagnosis, the mean MELD-Na score was 27 ± 7. The majority of patients developed AKI and acute-on-chronic liver failure (ACLF) during the infectious episode (54% and 56%, respectively). Peak ACLF grade was 1, 2, and 3 in 24 (22%), 21 (19%), and 16 (15%) patients, respectively. ACLF resolved before transplantation in 37 of 61 patients (61%). The median time elapsed from infection improvement/resolution to transplantation was 20 (IQR = 9–48) days. Twenty-three patients (21%) underwent transplantation within 7 days from infection improvement/resolution.
Comparison of patients with and without pre-LT infection at the time of LT
Characteristics of patients with and without pre-LT infection at the time of transplantation are reported in Table 1. No significant differences were found between the two groups in terms of age and sex. Prevalence of diabetes and hypertension was not different between the two groups whereas chronic kidney disease (CKD) was significantly more common in the study group (20% vs. 7%; p <0.001). In the study group alcohol-related liver disease was significantly more prevalent than in the control group (59% vs. 29%; p <0.001). At the time of LT, patients with pre-LT bacterial or fungal infection had a worse liver and renal function than those without, as shown by the significantly higher bilirubin, international normalised ratio (INR), creatinine and MELD/MELD-Na scores. Moreover, patients with pre-LT infection had a significantly higher prevalence of ACLF at the time of LT (22% vs. 6%; p <0.001). Patients with pre-LT infection also had significantly lower mean arterial pressure (MAP) than those without. Patients without pre-LT infection had a significantly higher prevalence of HCC (67% vs. 32%; p <0.001). Donor characteristics (age and sex), cold ischaemia time, and duration of surgery were not significantly different between the two groups. Regarding immune suppression after LT, patients with pre-LT infection were more frequently treated with mammalian targer of rapamycin (mTOR) inhibitors (49% vs. 32%; p = 0.003) and a calcineurin inhibitors-sparing regimen (66% vs. 56%; p = 0.049) than patients without pre-LT infection.
Table 1.
Characteristics of patients with and without pre-LT infection at the time of liver transplantation.
| Variable | Controls (n = 358) | Pre-LT infection (n = 108) | p value |
|---|---|---|---|
| Age | 58 (9) | 56 (9) | 0.060 |
| Sex (M) | 283 (79) | 79 (73) | 0.197 |
| Cirrhosis aetiology∗ | |||
| Alcohol-related HCV HBV Autoimmune hepatitis NAFLD Other† |
102 (29) 152 (43) 73 (20) 16 (5) 34 (10) 68 (19) |
53 (49) 35 (32) 18 (17) 4 (4) 16 (15) 17 (16) |
<0.001 0.062 0.392 0.731 0.118 0.443 |
| HCC | 238 (67) | 35 (32) | <0.001 |
| Creatinine (μmol/L) | 79 (65–101) | 104 (75–141) | <0.001 |
| Bilirubin (μmol/L) | 44 (22–101) | 111 (57–261) | <0.001 |
| INR | 1.45 (1.23–1.77) | 1.78 (1.55–2.20) | <0.001 |
| MELD score | 17 (8) | 24 (10) | <0.001 |
| MELD-Na score | 19 (8) | 26 (9) | <0.001 |
| Severe alcoholic hepatitis | 4 (1) | 1 (1) | 1.000 |
| ACLF at LT | 22 (6) | 24 (22) | <0.001 |
| ACLF grade 1 2 3 |
3 (1) 11 (3) 8 (2) |
7 (6) 10 (9) 7 (6) |
<0.001 |
| Sodium (mmol/L) | 137 (135–140) | 135 (131–139) | <0.001 |
| Haemoglobin (g/L) | 11.0 (9.8–13.0) | 9.6 (8.6–10.5) | <0.001 |
| Platelets (×109/L) | 85 (52–119) | 53 (36–70) | <0.001 |
| WBC (×109/L) | 4.51 (3.39–6.38) | 4.65 (3.23–7.24) | 0.584 |
| Albumin (g/L) | 28 (23–33) | 27 (23–31) | 0.267 |
| MAP (mmHg) | 93 (24) | 81 (12) | <0.001 |
| Diabetes | 92 (26) | 31 (29) | 0.617 |
| Hypertension | 109 (30) | 31 (29) | 0.717 |
| CKD | 25 (7) | 22 (20) | <0.001 |
| Donor’s age | 63 (16) | 61 (17) | 0.503 |
| Donor’s sex (M) | 204 (57) | 65 (60) | 0.632 |
| LT technique (piggyback) | 326 (91) | 106 (98) | 0.023 |
| Cold ischaemia time (min) | 480 (420–535) | 480 (436–540) | 0.305 |
| Duration of surgery (min) | 420 (360–480) | 420 (360–480) | 0.629 |
| Calcineurin inhibitors | 351 (98) | 107 (99) | 0.688 |
| Mycophenolic acid | 75 (21) | 28 (26) | 0.275 |
| mTOR inhibitors | 175 (49) | 35 (32) | 0.003 |
| Calcineurin inhibitors sparing regimen | 236 (66) | 60 (56) | 0.049 |
| Single immune suppression | 126 (35) | 49 (45) | 0.069 |
Normally distributed continuous variables are presented as mean ± SD, non-normally distributed continuous variables are expressed as median (IQR) and categorial variables as n (%). Comparisons were made using the Χ2 test or Fisher’s exact test when appropriate, Student’s t test and Mann–Whitney’s U test.
ACLF, acute-on-chronic liver failure; CKD, chronic kidney disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; INR, international normalised ratio; LT, liver transplantation; M, male; MAP, mean arterial pressure; MELD, model for end-stage liver disease; mTOR, mammalian target of rapamycin; NAFLD, non-alcoholic fatty liver disease; WBC, white blood cell.
A total of 115 patients had more than one liver disease aetiology.
This category includes: autoimmune cholestatic liver diseases (primary biliary cholangitis, primary sclerosing cholangitis); hepatitis D virus coinfection; haemochromatosis; Wilson’s disease; alpha1-antitrypsin deficit; cryptogenic cirrhosis.
Incidence of post-operative complications and length of ICU and in-hospital stays
Table 2 shows incidence of post-LT complications and duration of ICU and in-hospital stay in the two groups.
Table 2.
Incidence of early post-LT complications, duration of ICU and in-hospital stay in patients with and without pre-LT infection.
| Variable | Controls (n = 358) | Pre-LT infection (n = 108) | p value |
|---|---|---|---|
| New infection post-LT | 78 (22) | 64 (59) | <0.001 |
| Bacterial infection post-LT | 73 (20) | 61 (57) | <0.001 |
| Fungal infection post-LT | 17 (5) | 15 (14) | 0.001 |
| Septic shock post-LT | 7 (2) | 9 (8) | 0.001 |
| AKI post-LT | 154 (43) | 46 (44) | 0.926 |
| RRT post-LT | 14 (4) | 6 (6) | 0.434 |
| PNF | 8 (2.2) | 1 (0.9) | 0.386 |
| Early rejection | 43 (12) | 9 (8) | 0.374 |
| Vascular anastomosis complications | 34 (10) | 8 (8) | 0.639 |
| Biliary anastomosis complications | 24 (7) | 10 (9) | 0.371 |
| ICU stay | 4 (3–6) | 5 (3–8) | <0.001 |
| In-hospital stay, median | 14 (10–23) | 20 (13–37) | <0.001 |
Non-normally distributed continuous variables are expressed as median (IQR) and categorial variables as n (%). Comparisons were made using the Χ2 test or Fisher’s exact test when appropriate, and Mann–Whitney’s U test.
AKI, acute kidney injury; ICU, intensive care unit; LT, liver transplantation; PNF, primary non-function; RRT, renal replacement therapy.
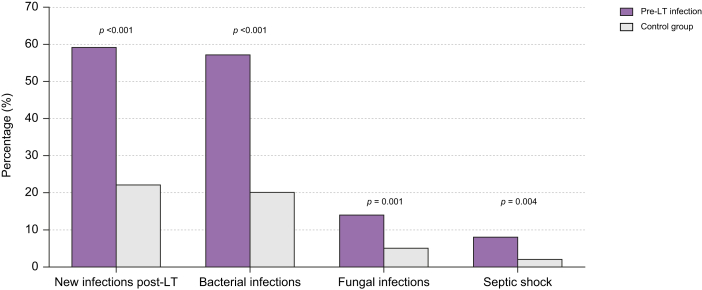
In the perioperative period, the incidence of AKI, vascular or biliary anastomosis complications and early rejection was not significantly different between the two groups. Patients with pre-LT infection had a significantly higher incidence of new infections (59% vs. 22%; p <0.001), both bacterial (57% vs. 20%; p <0.001) and fungal (14% vs. 5%; p = 0.001). The incidence of septic shock was significantly higher in patients with pre-LT infections than in those without (8% vs. 2%; p = 0.004) (Fig. 1).
Fig. 1.
Incidence of post-transplant infectious complications in patients with (study group) and without (control group) infections before liver transplant.
Percentage of patients suffering new infections (59% vs. 22%, p <0.001), bacterial (57% vs. 20%, p <0.001), fungal (14% vs. 5%, p = 0.001), and septic shock (8% vs. 2%, p = 0.004) in the postoperative period in patients with or without pre-LT infection. Comparisons were made using the Χ2 test and Fisher’s exact test. LT, liver transplantation.
Patients with pre-LT infection a had significantly longer ICU stay (5 [3–8] vs. 4 [3–6] days; p <0.001) and in-hospital stay (20 [13–37] vs. 14 [10 – 23] days; p <0.001).
Characteristics and predictors of post-LT infections
In the perioperative period, 142 patients (30.5%) experienced at least one new infection. Characteristics of post-LT infections are reported in Tables S3 and S4. The most frequent sites of infections were intra-abdominal (43.9%), CR-BSI (16.4%), pneumonia (12.9%), and UTI (10.5%). Enterococcus faecium, staphylococci, and Enterobacteriaceae were responsible for almost two-thirds of the infections. Fungal infections were diagnosed in 16% of patients. Rate of infections caused by MDR and XDR pathogens increased after LT (68% and 15%, respectively; Table S5).
Interestingly, 12 of 63 patients with a positive culture in the pre-LT infection (19%) had an infection sustained by the same pathogen in the perioperative period.
In the univariate analysis (Table 3), patients with post-LT infection had higher rates of pre-LT infection (45% vs. 14%, p <0.001), alcohol-related liver disease (45% vs. 28%; p <0.001) and CKD (15% vs. 8%; p = 0.040). They also had a higher prevalence of ACLF at the time of LT (19% vs. 6%; p <0.001), higher MELD-Na scores and white blood cell (WBC) count, and lower haemoglobin level at the time of LT. No significant differences were found between patients with and without post-LT infection in terms of immunosuppressive treatment regimen. Two multivariable logistic regression models were built to avoid multicollinearity (Model 1 adjusted for pre-LT infection, alcohol-related cirrhosis, WBC count and MELD score at LT; Model 2 adjusted for pre-LT infection, alcohol-related cirrhosis, WBC count, and ACLF at LT). In Model 1 pre-LT infection, alcohol-related cirrhosis and MELD score at LT were found to be independent predictors of development of infections in the postoperative period; in Model 2 pre-LT infection, alcohol-related cirrhosis and ACLF at LT were found to be independent predictors of development of infections in the postoperative period (Table 4).
Table 3.
Univariable analysis of factors associated with the development of post-LT infection.
| Variable | No post-LT infection (n = 324) | Post-LT infection (n = 142) | p value |
|---|---|---|---|
| Pre-LT infection | 44 (14) | 64 (45) | <0.001 |
| Age | 58 (9) | 56 (9) | 0.120 |
| Sex (M) | 257 (79) | 105 (74) | 0.199 |
| Cirrhosis aetiology∗ | |||
| Alcohol-related HCV HBV Autoimmune hepatitis NAFLD Other† |
91 (28) 142 (44) 65 (20) 13 (4) 34 (11) 61 (19) |
64 (45) 45 (32) 26 (18) 7 (5) 16 (11) 24 (17) |
<0.001 0.014 0.661 0.653 0.804 0.620 |
| HCC | 210 (65) | 63 (44) | <0.001 |
| Creatinine (μmol/L) | 77.5 (66–102.8) | 95.5 (71–140) | <0.001 |
| Bilirubin (μmol/L) | 45.5 (22–99.2) | 90.4 (29.3–223.4) | <0.001 |
| INR | 1.47 (1.24–1.80) | 1.67 (1.34–2.07) | 0.002 |
| MELD score at LT | 17 (8) | 21 (9) | <0.001 |
| MELD-Na score at LT | 19 (8) | 23 (9) | <0.001 |
| ACLF at LT | 19 (5.9) | 27 (19) | <0.001 |
| ACLF grade 1 2 3 |
5 (1) 7 (2) 7 (2) |
5 (4) 14 (10) 8 (5) |
<0.001 |
| Sodium (mmol/L) | 137 (134–140) | 137 (133–141) | 0.908 |
| Haemoglobin (g/L) | 10.8 (9.6–12.7) | 10.1 (9.1–11.3) | 0.001 |
| Platelets (×109/L) | 69 (45–109) | 57 (38–87) | 0.064 |
| WBC (×109/L) | 4.48 (3.26–6.30) | 4.92 (3.60–6.99) | 0.050 |
| Albumin (g/L) | 27 (23–33) | 27 (23–32) | 0.643 |
| MAP (mmHg) | 89 (12) | 85 (29) | 0.137 |
| Diabetes | 89 (28) | 34 (24) | 0.416 |
| Hypertension | 100 (31) | 40 (28) | 0.546 |
| CKD | 26 (8) | 19 (15) | 0.040 |
| Donor’s age (years) | 63 (16) | 60 (16) | 0.174 |
| Donor’s sex (M) | 191 (59) | 77 (54) | 0.396 |
| LT technique (piggyback) | 304 (94) | 128 (90) | 0.223 |
| Cold ischaemia time (min) | 480 (420–540)) | 480 (420–531) | 0.688 |
| Calcineurin inhibitors sparing regimen | 214 (66) | 82 (58) | 0.109 |
| Single immune suppression | 113 (35) | 62 (44) | 0.089 |
Normally distributed continuous variables are presented as mean ± SD, non-normally distributed continuous variables are expressed as median (IQR) and categorial variables as n (%). Comparisons were made using the Χ2 test or Fisher’s exact test when appropriate, Student’s t test and Mann–Whitney’s U test.
ACLF, acute-on-chronic liver failure; CKD, chronic kidney disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; INR, international normalised ratio; LT, liver transplantation; M, male; MAP, mean arterial pressure; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease; WBC, white blood cell.
A total 115 patients had more than one liver disease aetiology.
This category includes: autoimmune cholestatic liver diseases (primary biliary cholangitis, primary sclerosing cholangitis); hepatitis D virus coinfection; haemochromatosis; Wilson’s disease; alpha1-antitrypsin deficit; cryptogenic cirrhosis.
Table 4.
Independent predictors for development of post-LT infection.
| Variable | OR (95% CI) | p value |
|---|---|---|
| Model 1 | ||
| Pre-LT infection | 3.92 (2.40–6.39) | <0.001 |
| Alcohol-related cirrhosis | 1.70 (1.10–2.65) | 0.018 |
| MELD score at LT |
1.03 (1.01–1.06) |
0.002 |
| Model 2 | ||
| Pre-LT infection | 3.99 (2.48–6.42) | <0.001 |
| Alcohol-related cirrhosis | 1.77 (1.14–2.75) | 0.011 |
| ACLF at LT | 2.68 (1.36–5.27) | 0.004 |
Variables included in Model 1: pre-LT infection, aetiology of cirrhosis, white blood cell count (log-transformed), MELD score at LT.
Variables included in Model 2: pre-LT infection, aetiology of cirrhosis, white blood cell count (log-transformed), ACLF at LT.
Models were developed using logistic regression with stepwise backward elimination. Variables showing a p value <0.05 in the univariable analysis were included in the models.
ACLF, acute-on-chronic liver failure; LT, liver transplantation; MELD, model for end-stage liver disease; OR, odds ratio.
Incidence of post-operative complications in patients with pre-LT infection according to timing of infections improvement/resolution and MDR infections
In the study group, 23 patients underwent LT within 7 days from infection improvement/resolution (defined by the clearance for LT eligibility by the infectious disease specialist). The remaining 85 underwent LT beyond 7 days from infection improvement/resolution. The incidence of early post-LT complications (including infections) were not significantly different between patients receiving LT within 7 days vs. those without (Table S5).
Within the study group, the incidence of early post-LT complications and the length of hospital stay were not significantly different between patients with infections sustained by MDR bacteria and those with either non-MDR bacteria or negative cultures (Table S6).
Survival
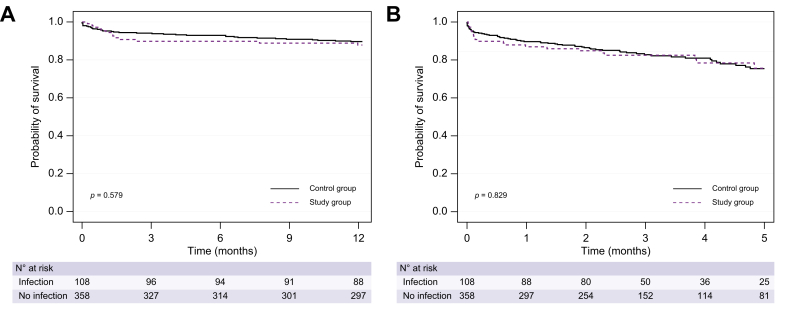
The probability of survival post-LT was not significantly different between patients with and without pre-LT infection (1-year survival: 88% vs. 89%, respectively, p = 0.579; 5-year survival: 76% vs. 75%, respectively, p = 0.829; Fig. 2). Even after adjusting for age, MELD at LT, HCC, and HCV aetiology, pre-LT infection was not associated with post-LT mortality at 5 years (aHR = 1.05; 95% CI 0.62–1.78; p = 0.835). Post-transplant survival was not significantly different between patients with or without ACLF at the time of transplant (1-year survival: 89% vs. 89%, respectively; p = 0.901; 5-year survival: 69% vs. 76%, respectively; p = 0.643).
Fig. 2.
Post-transplant probability of survival according to the presence of infections before liver transplant.
Probability of survival at 1 year (A) and 5 years (B) in patients with (study group) and without (control group) pre-LT infection (88% vs. 89%, p = 0.579 and 76% vs. 75%, p = 0.829, respectively). Comparisons were made using the log-rank test.
Moreover, the probability of post-LT survival was not significantly different in patients transplanted within and beyond 7 days from infection improvement/resolution (1-year survival: 91% vs. 86%, respectively, p = 0.609; 5-year survival 76% vs. 75%, respectively; p = 0.401). Finally, infections caused by MDR bacteria were not associated with worse post-LT survival (1-year survival: 88% vs. 88% in patients with or without MDR, respectively, p = 0.969; 5-year survival: 69% vs. 78% in patients with or without MDR, respectively, p = 0.209).
Discussion
Patients with decompensated cirrhosis are at very high risk of developing bacterial infections. The onset of bacterial infections is a relevant issue in LT candidates, because it requires temporary suspension from the waiting list and it is associated with poor outcomes. Reddy et al.3 reported a very high risk (42%) of permanent de-listing and/or death within 6 months from an episode of bacterial infection, as a result of the development of extrahepatic organ failures. Moreover, because bacterial infections are a leading cause of morbidity and mortality in the early post-transplant period, persistence or recurrence of infections is a main concern in deciding to proceed or not to LT in patients with cirrhosis and infections. Petrowsky et al.12 showed that septic shock within 28 days before LT was an independent predictor of 90-day post-LT mortality in LT candidates with MELD score >40. However, the impact of adequately treated infections before LT on post-transplant complications and outcomes is still controversial. Some experts recommend 2 weeks from infection resolution to transplantation,23 whereas others suggest proceeding to LT earlier, even before infections have been resolved.24 However, there are no data in current literature about a safe time interval between infection treatment and LT.
This single-centre case-control study demonstrated that bacterial infections before transplantation did not affect 1- and 5-year patients' survival, despite patients with pre-LT infections had greater liver disease severity than those without, as indicated by higher MELD and MELD-Na scores. These data show that following adequate antimicrobial treatment, LT should not be delayed in LT candidates with bacterial infections. Our findings are in keeping with results of previous studies with smaller sample size. Sun et al.9 showed that, although higher MELD-score at transplantation and post-transplant infections negatively affected survival, infections within 12 months before transplantation were not associated with higher post-transplant mortality rates. More recently, Bertuzzo et al.10 found that patients with end-stage liver disease and bacterial infections within 1 month before LT had similar short-term (90-day) and long-term (5-year) survival rates than patients without infections, despite higher MELD-scores and higher rates of organ failures at the time of transplantation.
In our series, patients surviving an infectious episode within 3 months before LT had a higher incidence of new infections, both bacterial and fungal, a higher incidence of septic shock, and required longer ICU and in-hospital care. Therefore, although survival is excellent, post-transplant care is more complex in patients undergoing LT after a bacterial infection. Remarkably, pre-LT infection was associated with a fourfold risk of development of infections after LT. These findings suggest that patients with pre-LT infections require an active and intensive surveillance for infections in the postoperative period. Remarkably, the incidence of other postoperative complications such as the occurrence of AKI, rejection, and/or vascular/biliary anastomosis complications was comparable between patients with and without pre-LT infections.
Although our study suggests the outcome of LT is good in patients with infections before LT, the important question is: how long should we wait before we can safely proceed to LT in patients with infections? An important finding of our study is that, in patients with pre-LT infection, time elapsed from infection improvement/resolution to transplantation did not affect patient outcome. Patients who underwent LT within 7 days from infection improvement/resolution had rates of postoperative complications (including new infections and septic shock, length of ICU and in-hospital stays and survival rates) comparable with those of patients who underwent LT beyond 7 days from infection improvement/resolution. This finding has important clinical implications: as soon as bacterial infection is controlled, it is safe to proceed with LT. This is a novel finding, as no previous study assessed a safe time interval from infection improvement/resolution to LT.
In the present study, we found very high rates of MDR pathogens: 38% in the pre-LT and 67.7% in the post-LT period. In the last 25 years the spread of MDR organisms has become a major public health threat worldwide. Antimicrobial resistance is a particularly serious issue in hepatology: infections caused by MDR organisms are associated with increased morbidity and mortality rates, both in the pre-transplant and post-transplant settings.13,17,[25], [26], [27], [28] In our cohort, MDR infections before LT were not associated with poor post-LT outcomes, although it should be highlighted that they had to be adequately treated before proceeding to transplant.
The strengths of our study are the large sample size, the granularity of data collected, and the assessment of timing between infection improvement/resolution and LT. The main limitations of the present study are the retrospective design and the single-centre data, which do not allow generalisation of our results to other patient populations.
In conclusion, this study shows that patients with pre-LT infections have a complex clinical course after LT because of the high risk of new infections and septic shock and long stays in hospital and ICU. In spite of that, post-LT survival is excellent and no association was found between time elapsed from infection improvement/resolution and post-LT outcomes. Therefore, as soon as bacterial infection is resolving, it is safe to proceed with LT, although patients with pre-LT infections require an active surveillance for infections after LT.
Financial support
No specific funding supported this study.
Authors’ contributions
Study concept and design: SP, PA. Data collection: SI, MT, NZ, CG, RG, VC, AB. Statistical analysis: SP, AB. Drafting of the manuscript: SI, SP, PA. Critical revision for important intellectual content: PF, PB, UC, PA.
Data availability statement
The data that support the findings of this study are available from the corresponding author, SP, upon reasonable request.
Conflicts of interest
The authors have nothing to disclose regarding the work under consideration for publication. The following authors disclose conflicts of interests outside the submitted work: SI, MT, NZ, CG, RG, VC, AB. PB, UC, PA received grant/research support from Grifols and CSL Behring; held a patent with Biovie; served as consulting for Sequana Medical. SP received advisory board fees from Mallinckrodt, consulting fees from Plasma Protein Therapeutics Association and Resolution Therapeutics, and speaking fees from Grifols.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100808.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C., et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Moreau R., Jalan R., Gines P., Pavesi M., Angeli P., Cordoba J., et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 3.Reddy K.R., Jacquelin G., O’Leary G., Kamath P.S., Fallon M.B., Biggins S.W., et al. High risk of delisting or death in liver transplant candidate following infections: results from the North American Consortium for the Study of End-Stage Liver Disease. Liver Transpl. 2015;21:881–888. doi: 10.1002/lt.24139. [DOI] [PubMed] [Google Scholar]
- 4.Ferrarese A., Vitale A., Sgarabotto D., Russo F.P., Germani G., Gambato M., et al. Outcome of a first episode of bacterial infection in candidates for liver transplantation. Liver Transpl. 2019;25:1187–1197. doi: 10.1002/lt.25479. [DOI] [PubMed] [Google Scholar]
- 5.Shankar N., Marotta P., Wall W., Albasheer M., Hernandez-Alejandro R., Chandok N. Defining readmission risk factor for liver transplantation recipients. Gastrenterol Hepatol. 2011;7:585–590. [PMC free article] [PubMed] [Google Scholar]
- 6.Yataco M., Cowell A., Waseem D., Keaveny A.P., Taner C.B., Patel T. Predictors and impacts of hospital readmissions following liver transplantation. Ann Hepatol. 2016;15:356–362. doi: 10.5604/16652681.1198805. [DOI] [PubMed] [Google Scholar]
- 7.Pereira A.A., Bhattacharya R., Carithers R., Reyes J., Perkins J. Clinical factors predicting readission after orthotopic liver transplantation. Liver Transpl. 2012;18:1037–1045. doi: 10.1002/lt.23475. [DOI] [PubMed] [Google Scholar]
- 8.Martin P., Di Martini A., Feng S., Brown R., Jr., Fallon M. Evaluation for liver transplantation in adults: 2013 practice guidelines by the American association for the study of liver disease and the American society of transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 9.Sun H.Y., Cacciarelli T.V., Singh N. Impact of pretransplant infections on clinical outcomes of liver transplant recipients. Liver Transpl. 2010;16:222–228. doi: 10.1002/lt.21982. [DOI] [PubMed] [Google Scholar]
- 10.Bertuzzo V.R., Giannella M., Cucchetti A., Pinna A.D., Grossi A., Ravaioli M., et al. Impact of preoperative infection on outcome after liver transplantation. Br J Surg. 2017;104:e172–e181. doi: 10.1002/bjs.10449. [DOI] [PubMed] [Google Scholar]
- 11.Mounzer R., Malik S.M., Nasr J., Madani B., Devera M.E., Ahmad J. Spontaneous bacterial peritonitis before liver transplantation does not affect patient survival. Clin Gastroenterol Hepatol. 2010;8:623–628. doi: 10.1016/j.cgh.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Petrowsky H., Rana A., Kaldas F.M., Sharma A., Hong J.C., Agopian V.G., et al. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg. 2014;259:1186–1194. doi: 10.1097/SLA.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 13.Piano S., Singh V., Caraceni P., Maiwall R., Alessandria C., Fernandez J., et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019;156:1368–1380. doi: 10.1053/j.gastro.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Garner J.S., Jarvis W.R., Emori T.G., Horan T.C., Hughes J.M. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 15.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piano S., Bartoletti M., Tonon M., Baldassarre M., Chies G., Romano A., et al. Assessment of Sepsis-3 criteria and quick SOFA in patients with cirrhosis and bacterial infections. Gut. 2018;67:1892–1899. doi: 10.1136/gutjnl-2017-314324. [DOI] [PubMed] [Google Scholar]
- 17.Fernández J., Acevedo J., Castro M., Garcia O., de Lope C.R., Roca D., et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 18.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 19.Levin A., Stevens P.E., Bilous R.W., Coresh J., De Francisco A.L.M., De Jong P.E., et al. Kidney Disease: improving Global Outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 20.Vitale A., Volk M.L., De Feo T.M., Burra P., Frigo A.C., Ramirez Morales R., et al. A method for establishing allocation equity among patients with and without hepatocellular carcinoma on a common liver transplant waiting list. J Hepatol. 2014;60:290–297. doi: 10.1016/j.jhep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Piano S., Gambino C., Vettore E., Calvino V., Tonon M., Boccagni P., et al. Response to terlipressin and albumin is associated with improved liver transplant outcomes in patients with heptorenal syndrome. Hepatology. 2021;73:1909–1919. doi: 10.1002/hep.31529. [DOI] [PubMed] [Google Scholar]
- 22.Vitale A., D’Amico F., Frigo A.C., Grigoletto F., Brolese A., Zanus G., et al. Response to therapy as a criterion for awarding priority to patients with hepatocellular carcinoma awaiting liver transplantation. Ann Surg Oncol. 2010;17:2290–2302. doi: 10.1245/s10434-010-0993-4. [DOI] [PubMed] [Google Scholar]
- 23.Avery R.K. Recipient screening prior to solid-organ transplantation. Clin Infect Dis. 2002;35:1513–1519. doi: 10.1086/344777. [DOI] [PubMed] [Google Scholar]
- 24.Verna E.C., Pereira M.R. Transplanting patients with active bacterial infection. Clin Liver Dis. 2017;9:81–85. doi: 10.1002/cld.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández J., Piano S., Bartoletti M., Wey E.Q. Management of bacterial and fungal infections in cirrhosis: the MDRO challenge. J Hepatol. 2021;75(Suppl 1):S101–S117. doi: 10.1016/j.jhep.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Merli M., Lucidi C., Di Gregorio V., Falcone M., Giannelli V., Lattanzi B., et al. The spread of multi drug resistant infections is leading to an increase in the empirical antibiotic treatment failure in cirrhosis: a prospective survey. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hand J., Patel G. Multidrug-resistant organisms in liver transplant: mitigating risk and managing infections. Liver Transpl. 2016;22:1143–1153. doi: 10.1002/lt.24486. [DOI] [PubMed] [Google Scholar]
- 28.Santoro-Lopes G., de Gouvêa E.F. Multidrug-resistant bacterial infections after liver transplantation: an ever-growing challenge. World J Gastroenterol. 2014;20:6201–6210. doi: 10.3748/wjg.v20.i20.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, SP, upon reasonable request.