Abstract
Rationale & Objective
The efficacy and safety profile of apixaban remains uncertain in patients receiving peritoneal dialysis (PD) despite increasing use in this population. Accordingly, we assessed the pharmacokinetics of apixaban among patients receiving PD.
Study Design
A pharmacokinetics study in a single center. Patients recruited received 1 week of apixaban at 2.5 mg twice a day to reach steady state. Serial blood samples were then taken before and after the last dose for pharmacokinetics analysis of apixaban.
Setting & Participants
Ten stable PD patients with atrial fibrillation in an outpatient setting.
Analytical Approach/Outcomes
Pharmacokinetic parameters including the area under the concentration-time curve from time 0 to 12 hours after the last dose of apixaban (AUC0-12), peak concentration, trough level, time to peak apixaban concentration, half-life, and drug clearance were analyzed.
Results
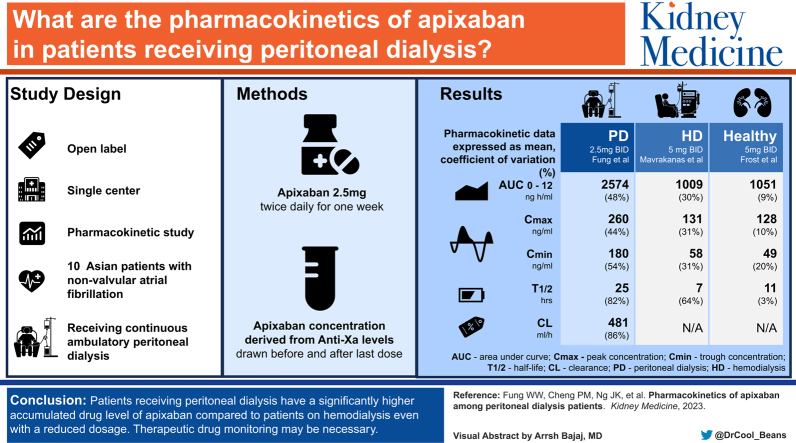
There was a wide variation in the range of apixaban concentration across the 10 patients. The AUC0-12 for the PD group was significantly higher than those reported previously for hemodialysis patients or healthy individuals. Three patients had a supratherapeutic peak concentration whereas 2 patients had a supratherapeutic trough level as compared with the pharmacokinetic parameter in healthy individuals taking equivalent therapeutic dosage.
Limitations
Small sample size with short study duration limits the ability to ascertain the true bleeding risk and to detect any clinical outcomes. Results may be limited to Asian populations only.
Conclusions
A proportion of PD patients had supratherapeutic levels even when the reduced dosage 2.5 mg twice a day was used. Given the large interindividual variation in the drug level, therapeutic drug monitoring should be done if available. Otherwise, one should start the drug at reduced doses with caution and with more frequent clinical monitoring for any signs of bleeding.
Index Words: Apixaban, direct oral anticoagulant, drug monitoring, peritoneal dialysis, pharmacokinetics
Graphical abstract
Plain Language Summary.
The efficacy and safety profile of apixaban remains uncertain in patients receiving peritoneal dialysis (PD) despite increasing use for stroke prevention in these patients. This is the first study to our knowledge analyzing the pharmacokinetics of apixaban in PD patients. Our results suggest that PD patients have a significantly higher accumulated drug level as compared with those reported in hemodialysis patients or healthy individuals, even when a reduced dosage was used. If apixaban is prescribed to PD patients, therapeutic drug monitoring may be necessary, and the patient should be monitored frequently.
Editorial, ●●●
Atrial fibrillation is prevalent in patients with kidney failure.1 Although patients receiving peritoneal dialysis (PD) have a lower incidence of atrial fibrillation as compared with patients receiving hemodialysis, the risk is still substantially higher than in the general population.2,3 The benefit of anticoagulation for stroke prevention is uncertain in the kidney failure population, with limited and often conflicting results for the use of traditional vitamin K antagonists such as warfarin.4,5 There is also an increased risk of bleeding associated with its use in patients with kidney failure.
There is growing interest in advocating the use of direct oral anticoagulants (DOACs) in patients receiving kidney replacement therapy. This is partly attributed to the fact that DOACs are generally safer and more effective than warfarin as determined in the general population.6 DOACs are also more convenient than warfarin as they do not require frequent blood monitoring and can be given safely in fixed doses. In fact, studies have already been performed to assess their safety profile in patients receiving kidney replacement therapy,7,8 and several randomized control trials are being performed.9, 10, 11 However, multiple-dose pharmacokinetics studies involving patients with kidney failure remain scant in the current literature. Furthermore, the currently available studies are limited to hemodialysis populations; there is no data for the PD population. In view of the very limited evidence on the use of DOACs such as apixaban in the PD population, we assessed the pharmacokinetics and the safety profile of apixaban in patients receiving PD.
Methods
This was an open-label pharmacokinetics study in a single center. We recruited 10 stable PD patients with nonvalvular atrial fibrillation and with no significant residual kidney function from our clinic. Patients who had increased risk of bleeding or those with contraindications to anticoagulation such as history of gastrointestinal bleeding, dual antiplatelet therapy, active malignancy, recent trauma, and stroke were excluded from the study. On recruitment at the first visit, baseline clinical information such as past medical history and body weight was reviewed.
The patients then received a total of 14 doses of apixaban at 2.5 mg twice daily for 1 week to ensure they reached steady state. The first dose was taken in the evening on day 1. On day 8, venous blood (3 mL) was collected for pharmacokinetics analysis at various time points and stored in separate citrate collection tubes: immediately before the last dose of apixaban was taken (trough level) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 9, and 12 hours after. No intraperitoneal heparin was used during the study to avoid confounding. Pill counts were performed by study staff at each visit to assess adherence. The medication record was also reviewed during the time the patient was taking apixaban to assess for any presence of cytochrome P450 CYP3A4 inducers or inhibitors. Residual glomerular filtration rate and urinary protein loss was measured from the 24-hour urine collection, and peritoneal dialysate protein loss was calculated from the 24-hour collection of PD fluid, both of which were performed at the end of the study. Patients were also screened for any bleeding complications while they were taking the anticoagulant.
The anti-Xa levels were measured from the blood sample using the ACL TOP 750 LAS coagulation analyzers, and the reagent used for the chromogenic assay was HemosIL Liquid Anti-Xa. Both the analyzers and the reagent used were from Instrumentation Laboratory (Werfen). The apixaban concentration was then derived from the anti-Xa levels using the HemosIL Apixaban Calibrators (Instrumentation Laboratory, Werfen).12 This is a validated method for rapidly quantifying the apixaban level and has been approved for clinical use.13 There is currently no established therapeutic range for apixaban, but the expected peak and trough levels for preventing stroke in atrial fibrillation have been taken from other previous studies to be: median (5th-95th percentile) 123 (69-221) ng/mL and 79 (34-162) ng/mL for 2.5 mg twice a day; and 171 (91-321) ng/mL and 103 (41-230) ng/mL for 5 mg twice a day, respectively.14
The objective was to review several pharmacokinetic parameters after the apixaban level reached steady state. These included the area under the concentration-time curve from time 0 to 12 hours after the last dose of apixaban (AUC0-12), which was calculated from the data using the linear trapezoidal method, the maximum concentration (Cmax), trough level (Cmin), time to peak apixaban concentration (Tmax), half-life (t1/2), and clearance of the drug (Cl). Patients were also screened for any bleeding complications while they were taking the anticoagulant as a secondary objective.
Statistical analysis was performed using SPSS software version 27.0 (SPSS Inc), and the pharmacokinetics analysis was performed using the software PKSolver.15 Descriptive statistics were used to describe the baseline characteristics of patients and the pharmacokinetics parameters of apixaban. Data are presented as mean ± standard deviation or median (interquartile range) as appropriate. Correlations between the pharmacokinetic parameters with various parameters were assessed using Pearson’s correlation coefficient. P values < 0.05 were considered statistically significant. All probabilities were 2-tailed.
The study was performed in accordance with the ethics standards of the institutional research committee at which the studies were conducted and with the Declaration of Helsinki; all patients gave informed consent. The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong (CREC Ref. No. 2020.284).
Results
Ten patients, 5 male and 5 female, were recruited. The mean age was 70.2 ± 6.1 years, and the mean duration of dialysis was 45.2 ± 30.4 months. All the patients except 2 performed 3 PD exchanges per day, which included 1.5% and 2.5% dextrose dialysate. Two patients used 7.5% icodextrin dialysate. Five patients were high-average peritoneal transporters, whereas the other 5 were low-average transporters. All patients had a urine output of <200 mL per 24 hours. All of them were also adherent in taking their anticoagulant, and none of them missed any doses. Their other baseline demographics are shown in Table 1.
Table 1.
Baseline Clinical and Biochemical Data
| No. of Patients | 10 |
|---|---|
| Age (y)a | 70.2 ± 6.1 |
| Sex (M:F) | 5:5 |
| Dialysis vintage (mo)a | 45.2 ± 30.4 |
| Body weight (kg) | 60.5 ± 9.1 |
| Body mass index | 24.5 ± 2.3 |
| Kidney diagnosis | |
| Diabetes | 5 (50%) |
| Hypertensive nephropathy | 2 (20%) |
| Glomerulonephritis | 1 (10%) |
| Obstructive nephropathy | 1 (10%) |
| Polycystic kidney disease | 1 (10%) |
| Comorbidity | |
| Hypertension | 8 (80%) |
| Diabetes | 6 (60%) |
| Ischemic heart disease | 4 (40%) |
| Cirrhosis | 1 (10%) |
| Cancer | 1 (10%) |
| Charlson Comorbidity Index | 8 (6 – 10) |
| CHAD2Vasc scoreb | 5 (3 – 6) |
| Laboratory paramatersa | |
| Hemoglobin (g/dL) | 9.9 ± 1.7 |
| White cell count (×109/L) | 6.7 ± 1.6 |
| Platelets (×109/L) | 244.3 ± 98.6 |
| International Normalized Ratio | 1.2 ± 0.2 |
| Creatinine (μmol/L) | 753.7 ± 192.0 |
| Albumin (g/L) | 23.6 ± 4.9 |
| Residual GFR (mL/min/1.73 m2) | 1.26 ± 1.61 |
| Adequacy Kt/V | 1.92 ± 0.34 |
| Urinary protein loss (g/d) | 0.35 ± 0.44 |
| Peritoneal protein loss (g/d) | 6.52 ± 2.02 |
| CYP3A4 agent | |
| Inducers | 1 (10%) |
| Inhibitors | 2 (20%) |
Abbreviation: GFR, glomerular filtration rate.
Data expressed as mean ± standard deviation.
Data expressed a median (interquartile range).
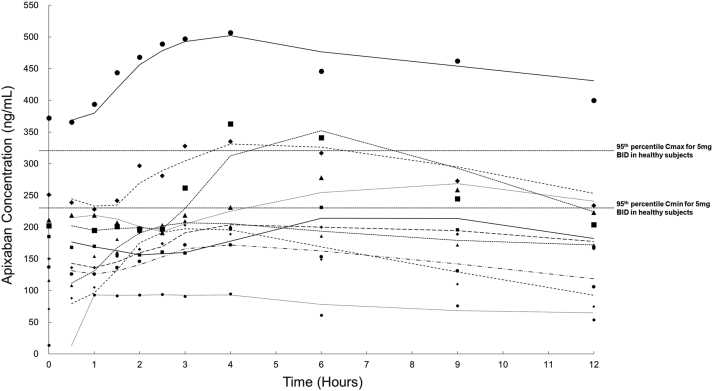
Figure 1 shows the concentration-time curve of apixaban of the patients over the 12 hours after the last dose was taken. There was wide variation in the range of apixaban concentration across the 10 patients. Pharmacokinetics parameters for each of the patients were calculated, and the means of each of the parameters are shown in Table 2. The AUC0-12 for the PD group was significantly higher and was in fact twice as high as that of patients receiving hemodialysis or healthy individuals taking 5 mg apixaban twice a day. Furthermore, 3 of our patients had a supratherapeutic Cmax, and 2 patients had a supratherapeutic Cmin, which were above the 95th percentile of the expected median values in healthy individuals taking 5 mg apixaban twice a day.14,16,17 The number of patients with supratherapeutic Cmax and Cmin was even higher when compared with healthy individuals taking 2.5 mg apixaban twice a day. Among the 3 patients with supratherapeutic Cmax, one was greater than 80 years old whereas 2 had a body weight of <60 kg. During the study period, none of the patients experienced any bleeding events. Only one patient was taking a CYP3A4 inducer (hydrocortisone), whereas 2 patients were taking a CYP3A4 inhibitor (levothyroxine).
Figure 1.
The concentration-time curve from 0 to 12 hours after the last dose of 2.5 mg apixaban among the 10 recruited patients. The trendline is constructed using the “2 period moving average” method. BID, twice a day; Cmax, peak concentration; Cmin, trough concentration.
Table 2.
Pharmacokinetic Parameters of Apixaban 2.5 mg Twice Daily Among 10 PD Patients, Comparing Previous Multiple-Dose Study With HD Patients and Healthy Individuals
| Pharmacokinetic Parametersa | PD: Our study (2.5 mg twice a day) | HD: Mavrakanas et al7 (2.5 mg twice a day) | Healthy individuals: Frost et al21 (5 mg twice a day) |
|---|---|---|---|
| AUC0-12, ng h/mL | 2,574.4 (48.6%) | 1,009.8 (30.7%) | 1,051.9 (9%) |
| AUC0-infinite, ng h/mL | 10,034.4 (84.0%) | N/A | N/A |
| Cmax, ng/mL | 260.2 (44.7%) | 131.5 (31.1%) | 128.5 (10%) |
| Cmin, ng/mL | 180.2 (54.4%) | 58.0 (31.2%) | 49.6 (20%) |
| Tmax, h | 4.0 (28.9%) | 3.6 (48%) | 4.0 |
| t1/2, h | 25.2 (82.3%) | 7.5 (64.3%) | 11.7 (3.3) |
| Cl, ml/h | 481.7 (86.4%) | N/A | N/A |
Abbreviations: AUC, area under curve; Cl, clearance; Cmax, peak concentration; Cmin, trough concentration; HD, hemodialysis; t1/2, half-life; Tmax, time to reach Cmax; N/A, not available; PD, peritoneal dialysis.
Data expressed as mean (coefficient of variation).
Given that apixaban is highly protein bound, we assessed whether its pharmacokinetics could be affected by the degree of protein being lost, either through residual urine or PD effluent. Using the Pearson correlation coefficient, the degree of protein loss from the peritoneal dialysate appeared to have a positive correlation with AUC0-12 (r = 0.654, P = 0.04). There is no significant correlation with the other parameters (AUC0-infinite, Cmax, Cmin, Tmax, t1/2, Cl). Similarly, there was no correlation between any of the pharmacokinetic parameters and the amount of urinary protein lost. We then assessed whether there was any effect of serum albumin on the pharmacokinetics of apixaban, and there seemed to be a negative correlation of Tmax with serum albumin level (r = -0.762, P = 0.01). As for the effect of solute clearance on the pharmacokinetics of apixaban, there was no correlation between any of the pharmacokinetic parameters with transporter status, residual glomerular filtration rate, and total Kt/V.
Discussion
Our study was the first, to our knowledge, to assess the pharmacokinetics of apixaban in the PD population. Our findings showed that a proportion of PD patients had supratherapeutic levels with a significantly higher AUC even when the reduced dosage of 2.5 mg twice a day was used. Our results also showed that there is a wide interindividual variation in apixaban level as shown by the concentration-time curve. Taken together, if apixaban needs to be prescribed to PD patients, therapeutic drug monitoring may be necessary.
The elimination of apixaban involves multiple pathways. This includes metabolic pathways such as O-demethylation, hydroxylation, and sulfation of hydroxylated O-demethyl apixaban via cytochrome P450 CYP3A4, as well as biliary and renal elimination of the unchanged parent compound and direct intestinal excretion.18,19 Apixaban has a total clearance of approximately 3,300 mL/hour and an apparent half-life of about 12 hours after oral administration in healthy individuals.20, 21, 22, 23 However, the pharmacokinetics of apixaban is somewhat less clear in patients with kidney failure.
Two open-label studies have assessed the pharmacokinetics and safety of apixaban in hemodialysis patients. They showed that kidney failure only resulted in a modest increase in apixaban AUC (36% and 44% after a 5-mg or 10-mg single dose of apixaban, respectively) and no increase in Cmax as compared with healthy individuals. Overall, they suggested that dose adjustment of apixaban is not required on the basis of kidney function alone and that it is largely well tolerated.8,24
However, these studies only reflected the pharmacokinetics based on a single dose and may be misleading. Indeed, Mavrakanas et al7 recently showed that, through their multidose study of apixaban to reach steady state, the currently recommended dose of 5 mg twice a day led to supratherapeutic levels in patients receiving hemodialysis and should be avoided. Instead, the reduced dose of 2.5 mg twice a day resulted in drug exposure that was comparable with that of the standard dose in healthy individuals and perhaps should be adopted instead of the recommended 5 mg twice a day. Compared with the hemodialysis population, our study showed that the drug exposure is significantly higher with a larger AUC in the PD population even with this reduced dose of 2.5 mg twice a day. Further detailed analysis of the clearance of apixaban by these dialysis modalities is warranted.
A dose reduction is currently recommended for patients with additional factors: either greater than or equal to 80 years of age or body weight ≤60 kg.25 Our findings suggest that a further dosage reduction may be necessary for these patients. Indeed, among our 3 PD patients with supratherapeutic Cmax, despite a reduced dosage of 2.5 mg twice a day, they all had additional factors. Further pharmacokinetics analysis of an even lower dosage is warranted for this group of patients. Given the large interindividual variation in the drug level, a one-size-fits-all dosage reduction is not advisable.
Given that apixaban is predominantly bound to albumin,26 albumin loss may be an important factor affecting the elimination of apixaban. Our results showed that the higher the peritoneal protein loss, the higher the AUC0-12, whereas the lower the serum albumin level, the longer time it takes to reach peak concentration (Tmax). There are a few possible explanations. Higher peritoneal protein loss may indicate a higher loss of protein-bound middle or high molecular weight substances,27,28 including substrates needed for methylation and sulfation of hydroxylated O-demethyl apixaban.18 This ultimately results in slower degradation of apixaban compound and higher risk of accumulation. Given that the absorption of apixaban appears to occur primarily in the small intestine,29 it is possible that lower albumin level may be an indirect marker of gut edema, causing a delayed gastrointestinal absorption of apixaban and a longer Tmax. However, further study is needed to ascertain these postulations.
Recently, a literature review on the safety and efficacy of apixaban use identified 4 studies that included PD patients.30 However, all but 1 study had a very small sample size of PD patients (1 to 4 patients only), as the PD population was not part of the authors’ primary study design.31, 32, 33, 34 Furthermore, these studies were retrospective and observational in nature and may have inherent selection bias.
The only study with a large sample size was by Siontis et al34 in which 1,377 of the 25,523 patients were receiving PD. They reported that the 5 mg twice a day dosage was associated with lower risks of ischemic stroke/systemic embolism when compared with warfarin (hazard ratio, 0.61; 95% confidence interval, 0.37-0.98; P = 0.04), whereas that of the 2.5 mg twice a day dosage was not statistically significant (hazard ratio, 1.11; 95% confidence interval, 0.82-1.50; P = 0.49). There were also no differences in safety outcomes such as bleeding between both doses of apixaban. However, one should caution that these findings were based on an analysis combining both hemodialysis and PD together (a total of 2,351 patients, of which only 135 cases were patients on PD); crucially, subgroup analyses of patients on PD was not specifically performed by the investigators. Thus, this study may not truly reflect the efficacy and safety profile of apixaban at different doses in PD populations. Indeed, a recent study showed that the relative frequency of hemorrhagic stroke seems to increase as kidney function declines.35
In their study, Amani et al30 stressed the lack of evidence within the PD population and certainly highlights the timeliness and importance of our study assessing the pharmacokinetics of apixaban in PD.
Our study has several limitations. First, we only studied apixaban, and this would not be generalizable to other DOACs. However, it is likely that apixaban would be most suitable as compared with other DOACs such as dabigatran. Apixaban is mostly excreted by the hepatobiliary route with only 25% excreted renally, whereas 85% of dabigatran is renally excreted as active metabolites, and up to 60% of the drug is dialyzable.36,37 Although dabigatran is dialyzable, it is postulated that the periodic nature of hemodialysis may likely result in a fluctuating and unpredictable blood level of dabigatran, which could result in recurring periods of under- and over-anticoagulation.38 The dialysis session may also sometimes be shortened or delayed, which may result in prolonged periods of increased risk for bleeding, especially if the patient continues taking dabigatran in the absence of dialysis.
Second, our sample is small, and a significant difference may simply be due to chance. With no pre-existing study for reference, this is nevertheless the standard sample size for this type of pharmacokinetics study. Furthermore, our results may be limited to Asian populations, who tend to have a much smaller body size, and may not be generalizable to other ethnicities with higher weights. It is possible that the lower body mass index may have influenced the pharmacokinetics result in our cohort, even at a reduced apixaban dosage. Finally, our study was not designed to assess the efficacy and to detect any clinical outcomes. The short duration of the study may also limit the ability to ascertain the true bleeding risk from the reduced dose of apixaban in PD patients.
Nevertheless, our findings suggest that there may be a significant drug exposure to apixaban even using a lower dose for the PD population. Further pharmacokinetics analysis with a larger sample size is needed to confirm the long-term safety profile and to delineate the optimal doses. Until then, the reduced dose of 2.5 mg apixaban twice a day remains recommended for those on dialysis and with low body mass index. However, given the large interindividual variation of drug level, therapeutic drug monitoring should ideally be done if available. In fact, point-of-care testing has been developed in recent years to aid rapid assessment of the coagulation profile, which may enhance therapeutic drug monitoring in clinical practice.39,40 Otherwise, one should start the drug with caution and with more frequent clinical monitoring for any signs of bleeding.
Article Information
Authors’ Full Names and Academic Degrees
Winston Wing-Shing Fung, MBBChir (Cantab), FRCP, Phyllis Mei-Shan Cheng, BN, RN, Jack Kit-Chung Ng, MBChB, MRCP, Gordon Chun-Kau Chan, MBChB, MRCP, Kai Ming Chow, MBChB, FRCP, Philip Kam-Tao Li, MD, FRCP, and Cheuk Chun Szeto, MD, FRCP.
Authors’ Contributions
Research idea and study design: WWSF, CCS; data acquisition: WWSF, PMSC; data analysis/interpretation: WWSF; statistical analysis: WWSF; clinical support: JKCN, GCKC, KMC; supervision or mentorship: PKTL, CCS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This study was supported by the Chinese University of Hong Kong (CUHK) research accounts 6905134 and 7101215. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgments
We would like to express our heartfelt gratitude to Prof Margaret Ng and Dr Natalie Chan of the division of Haematology for their technical support in measuring the anti-Xa level using the chromogenic assay.
Peer Review
Received November 7, 2022. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form February 26, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Königsbrügge O., Posch F., Antlanger M., et al. Prevalence of atrial fibrillation and antithrombotic therapy in hemodialysis patients: cross-sectional results of the Vienna InVestigation of AtriaL Fibrillation and Thromboembolism in Patients on HemoDIalysis (VIVALDI) PLOS ONE. 2017;12(1) doi: 10.1371/journal.pone.0169400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao J.N., Chao T.F., Liu C.J., et al. Incidence and risk factors for new-onset atrial fibrillation among patients with end-stage renal disease undergoing renal replacement therapy. Kidney Int. 2015;87(6):1209–1215. doi: 10.1038/ki.2014.393. [DOI] [PubMed] [Google Scholar]
- 3.Shen C.H., Zheng C.M., Kiu K.T., et al. Increased risk of atrial fibrillation in end-stage renal disease patients on dialysis: a nationwide, population-based study in Taiwan. Medicine (Baltimore) 2016;95(25) doi: 10.1097/MD.0000000000003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C.H., Fan P.C., Lin Y.S., et al. Atrial fibrillation and associated outcomes in patients with peritoneal dialysis and hemodialysis: a 14-year nationwide population-based study. J Nephrol. 2021;34(1):53–62. doi: 10.1007/s40620-020-00713-4. [DOI] [PubMed] [Google Scholar]
- 5.Winkelmayer W.C. Cardiovascular disease: still unresolved: warfarin in ESRD with atrial fibrillation. Nat Rev Nephrol. 2014;10(5):244–245. doi: 10.1038/nrneph.2014.48. [DOI] [PubMed] [Google Scholar]
- 6.Carnicelli A.P., Hong H., Connolly S.J., et al. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145(4):242–255. doi: 10.1161/CIRCULATIONAHA.121.056355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavrakanas T.A., Samer C.F., Nessim S.J., Frisch G., Lipman M.L. Apixaban Pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28(7):2241–2248. doi: 10.1681/ASN.2016090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Tirucherai G., Marbury T.C., et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56(5):628–636. doi: 10.1002/jcph.628. [DOI] [PubMed] [Google Scholar]
- 9.Compare apixaban and vitamin-K antagonists in patients with atrial fibrillation (AF) and end-stage kidney disease (ESKD) (AXADIA). ClinicalTrials.gov identifier: NCT02933697. https://clinicaltrials.gov/ct2/show/NCT02933697 Updated September 22, 2022.
- 10.Oral anticoagulation in haemodialysis patients (AVKDIAL). ClinicalTrials.gov identifier NCT02886962. https://clinicaltrials.gov/ct2/show/NCT02886962 Updated August 23, 2022.
- 11.Trial to evaluate anticoagulation therapy in hemodialysis patients with atrial fibrillation (RENAL-AF). ClinicalTrials.gov identifier: NCT02942407. https://clinicaltrials.gov/ct2/show/results/NCT02942407 Updated December 29, 2020.
- 12.Douxfils J., Chatelain C., Chatelain B., Dogné J.M., Mullier F. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013;110(2):283–294. doi: 10.1160/TH12-12-0898. [DOI] [PubMed] [Google Scholar]
- 13.Cini M., Legnani C., Padrini R., et al. DOAC plasma levels measured by chromogenic anti-Xa assays and HPLC-UV in apixaban- and rivaroxaban-treated patients from the START-Register. Int J Lab Hematol. 2020;42(2):214–222. doi: 10.1111/ijlh.13159. [DOI] [PubMed] [Google Scholar]
- 14.Byon W., Garonzik S., Boyd R.A., Frost C.E. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58(10):1265–1279. doi: 10.1007/s40262-019-00775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Huo M., Zhou J., Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Leil T.A., Feng Y., Zhang L., Paccaly A., Mohan P., Pfister M. Quantification of apixaban’s therapeutic utility in prevention of venous thromboembolism: selection of phase III trial dose. Clin Pharmacol Ther. 2010;88(3):375–382. doi: 10.1038/clpt.2010.106. [DOI] [PubMed] [Google Scholar]
- 17.Pfizer Canada Inc. & Bristol-Myers Squibb Canada. Product Monograph. Eliquis Apixaban Tablets 2.5 mg and 5 mg Anticoagulant. Pages 32–33. https://www.pfizer.ca/files/ELIQUIS_PM_229267_07Oct2019_Marketed_E.pdf
- 18.Raghavan N., Frost C.E., Yu Z., et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37(1):74–81. doi: 10.1124/dmd.108.023143. [DOI] [PubMed] [Google Scholar]
- 19.Wang X., Mondal S., Wang J., et al. Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am J Cardiovasc Drugs. 2014;14(2):147–154. doi: 10.1007/s40256-013-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost C., Wang J., Nepal S., et al. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75(2):476–487. doi: 10.1111/j.1365-2125.2012.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost C., Nepal S., Wang J., et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76(5):776–786. doi: 10.1111/bcp.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frost C., Shenker A., Jhee S., et al. Evaluation of the single-dose pharmacokinetics and pharmacodynamics of apixaban in healthy Japanese and Caucasian subjects. Clin Pharmacol. 2018;10:153–163. doi: 10.2147/CPAA.S169505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frost C., Yu Z., Nepal S., et al. Apixaban, a direct factor Xa inhibitor: single-dose pharmacokinetics and pharmacodynamics of an intravenous formulation. J Clin Pharmacol. 2008;48(9):1132. Abstract 142. [Google Scholar]
- 24.Chang M., Yu Z., Shenker A., et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol. 2016;56(5):637–645. doi: 10.1002/jcph.633. [DOI] [PubMed] [Google Scholar]
- 25.Chan K.E., Giugliano R.P., Patel M.R., et al. Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on dialysis with AF. J Am Coll Cardiol. 2016;67(24):2888–2899. doi: 10.1016/j.jacc.2016.02.082. [DOI] [PubMed] [Google Scholar]
- 26.He K., Luettgen J.M., Zhang D., et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur J Drug Metab Pharmacokinet. 2011;36(3):129–139. doi: 10.1007/s13318-011-0037-x. [DOI] [PubMed] [Google Scholar]
- 27.Guedes A.M. Peritoneal protein loss, leakage or clearance in peritoneal dialysis, where do we stand? Perit Dial Int. 2019;39(3):201–209. doi: 10.3747/pdi.2018.00138. [DOI] [PubMed] [Google Scholar]
- 28.Kagan A., Bar-Khayim Y., Schafer Z., Fainaru M. Kinetics of peritoneal protein loss during CAPD: I. Different characteristics for low and high molecular weight proteins. Kidney Int. 1990;37(3):971–979. doi: 10.1038/ki.1990.73. [DOI] [PubMed] [Google Scholar]
- 29.Byon W., Nepal S., Schuster A.E., Shenker A., Frost C.E. Regional gastrointestinal absorption of apixaban in healthy subjects. J Clin Pharmacol. 2018;58(7):965–971. doi: 10.1002/jcph.1097. [DOI] [PubMed] [Google Scholar]
- 30.Amani A., Fellers C.M., Eyebe A., Hall A., Howard M.L. Safety and efficacy of apixaban use in peritoneal dialysis: a review of the literature. J Pharm Technol. 2021;37(3):147–151. doi: 10.1177/8755122520988728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanton B.E., Barasch N.S., Tellor K.B. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacotherapy. 2017;37(4):412–419. doi: 10.1002/phar.1905. [DOI] [PubMed] [Google Scholar]
- 32.Reed D., Palkimas S., Hockman R., Abraham S., Le T., Maitland H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost. 2018;2(2):291–298. doi: 10.1002/rth2.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herndon K., Guidry T.J., Wassell K., Elliott W. Characterizing the safety profile of apixaban versus warfarin in moderate to severe chronic kidney disease at a Veterans Affairs Hospital. Ann Pharmacother. 2020;54(6):554–560. doi: 10.1177/1060028019897053. [DOI] [PubMed] [Google Scholar]
- 34.Siontis K.C., Zhang X., Eckard A., et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519–1529. doi: 10.1161/CIRCULATIONAHA.118.035418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamberg I., Assouline-Reinmann M., Carrera E., et al. Epidemiology, thrombolytic management, and outcomes of acute stroke among patients with chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2022;37(7):1289–1301. doi: 10.1093/ndt/gfab197. [DOI] [PubMed] [Google Scholar]
- 36.Knauf F., Chaknos C.M., Berns J.S., Perazella M.A. Dabigatran and kidney disease: a bad combination. Clin J Am Soc Nephrol. 2013;8(9):1591–1597. doi: 10.2215/CJN.01260213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khadzhynov D., Wagner F., Formella S., et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost. 2013;109(4):596–605. doi: 10.1160/TH12-08-0573. [DOI] [PubMed] [Google Scholar]
- 38.Chan K.E., Edelman E.R., Wenger J.B., Thadhani R.I., Maddux F.W. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131(11):972–979. doi: 10.1161/CIRCULATIONAHA.114.014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebner M., Birschmann I., Peter A., et al. Point-of-care testing for emergency assessment of coagulation in patients treated with direct oral anticoagulants. Crit Care. 2017;21(1):32. doi: 10.1186/s13054-017-1619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Härtig F., Birschmann I., Peter A., et al. Point-of-care testing for emergency assessment of coagulation in patients treated with direct oral anticoagulants including edoxaban. Neurol Res Pract. 2021;3(1):9. doi: 10.1186/s42466-021-00105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]