Abstract
The avian digestive tract is an important system for converting ingested food into the nutrients their bodies need for maintenance, growth, and reproduction (meat, table eggs, and fertile eggs). Therefore, preserving digestive system integrity is crucial to bird health and productivity. As an alternative to antibiotics, the world has recently turned to the use of natural products to enhance avian development, intestinal health, and production. Therefore, the primary goal of this review is to explain the various characteristics of the avian digestive tract and how to enhance its performance with natural, safe feed additives such as exogenous enzymes, organic acids, photogenic products, amino acids, prebiotics, probiotics, synbiotics, and herbal extracts. In conclusion, the composition of the gut microbiome can be influenced by a number of circumstances, and this has important consequences for the health and productivity of birds. To better understand the connection between pathogens, the variety of therapies available, and the microbiome of the gut, additional research needs to be carried out.
Key words: avian digestive tract, antibiotic alternatives, bird productivity, feed additives, organic poultry
INTRODUCTION
Organic farming is one of the most rapidly expanding subsectors of the USA agricultural market (USDA-ERS, 2014), and during the last 10 yr, there has also been a rise in organic farming in in Europe (von Borell and Sørensen, 2004; Prache et al., 2022). American consumers have been the primary force behind the rising popularity of organically raised commodities since the 1990s. With an increase from $28.4 billion in 2012, organic food sales in the USA reached $35 billion in 2014 (USDA-ERS, 2014). In recent years, as demand for organic foods has surpassed supply, American retailers have imported organic commodities worth several billion dollars into the domestic market (USDA-ERS, 2014; Borda-Molina et al., 2018; El Jeni et al., 2021; Abd El-Hack et al., 2023).
Organic poultry and eggs are 2 of the most accessible and well-liked animal products on the market. The market for organic poultry products was estimated to be $350 million in 2008 (USDA-ERS, 2014). Despite having 9 million certified broilers, 5.5 million certified layer hens, and 400,000 certified organic turkeys, the industry has been unable to meet increased demand for organic poultry (USDA-ERS, 2014; Gadde et al., 2017; Polovinski-Horvatovi, 2021). This indicates the great potential for the organic poultry business to grow, as predicted by the organic trade association. There is plenty of opportunity for organic chicken farming, but concerns about the safety of organic meat and eggs tainted with foodborne diseases may limit the possibility (USDA-ERS, 2014; Abd El-Hack et al., 2020, 2023).
It is necessary to develop alternative antimicrobial intervention strategies that are also practicable. This is necessary in order to prevent the spread of infections in organic poultry (USDA-ERS, 2014; Abd El-Hack et al., 2023). To ensure the safety of humans, animals, and the environment, the National Organic Program (NOP) prohibits the use of antibiotics, hormones, herbicides, and pesticides in organic agriculture. In addition, customers of organic feed generally consider the products to be safer options for their family and choose to use them because there are fewer or no added preservatives or chemicals in the products. Organic chicken production that abides by the NOP can make use of natural enzymes, antioxidants, and botanicals to combat disease, boost growth, and enhance product quality (Gadde et al., 2017; Shehata et al., 2022; Babot et al., 2023).
Multiple studies have shown that organic and commercial poultry products are equally contaminated with pathogens, proving once again that the present procedures are inadequate (Alagawany et al., 2023). Providing guidance to organic industry customers, such as farmers and processors, on possible antimicrobials to protect their goods from pathogenic microorganisms, is a challenging problem (Clavijo and Flórez, 2018). In accordance with NOP regulations, poultry must have access to an outdoor environment containing pathogens such as Salmonella, Clostridium, and Campylobacter (Baldwin et al., 2018; Clavijo and Flórez, 2018; Rubio, 2018; Abd El-Hack et al., 2021b; Alagawany et al., 2023). When birds are treated for severe diseases with antibiotics, they can no longer be labeled as organic (Li et al., 2023). However, one of the obstacles is the lack of sufficient and reliable scientific evidence on the methods described previously for improving the microbiological quality of organically reared chicken (Clavijo and Flórez, 2018; Abd El-Hack et al., 2020; Li et al., 2023). There are a number of factors that make it challenging to produce organic poultry safely, including slow-growing breeds and a lack of slaughterhouses, both of which can increase the risk of disease transmission (Such et al., 2023).
Antibiotic growth promoters (AGP), much like any other type of feed additive, are believed to be beneficial to both the host's health and the production performance of the animal (Such et al., 2023). AGP has mostly been replaced by a variety of feed additives, including biologically generated nanoparticles, probiotics, prebiotics, synbiotics, herbal extract, essential oils, organic acids, enzymes, and essential amino acids (Goel et al., 2023). Despite the fact that the precise physiological processes of feed additives are unknown, the impacts on health are most visible in the digestive system (Patterson and Burkholder, 2003; Ashayerizadeh et al., 2009; Baffoni et al., 2012; Goel et al., 2023; Such et al., 2023).
Vaccines can prevent a variety of diseases, including Marek's disease, Newcastle disease, infectious bronchitis, Mycoplasma, and Coccidia (Li et al., 2023). Prebiotics are indigestible oligosaccharides that beneficial gut microorganisms can use, whereas probiotics help gut microbe species populate the gut (Ashayerizadeh et al., 2009). Probiotics are beneficial bacteria that can protect chickens against pathogens in their digestive tracts as well as improve overall health and disease prevention in birds (Clavijo and Flórez, 2018). Endogenous enzymes, such as sugars and proteases, promote meal digestion in the gut. Alternatives using phytogenic feed additives show promise as well (de Carvalho et al., 2021). Oleoresins, botanicals, essential oils, and plants are the 4 basic subgroups of this phytogenic class. These herbal ingredients (cinnamon, ginger, pepper, turmeric, and so on) have antibacterial, antioxidant, immune-boosting, and gut-manipulating properties that benefit bird health and performance (Huyghebaert et al., 2011; Lin et al., 2013; Sugiharto, 2016; de Carvalho et al., 2021; Gadde et al., 2017).
This current review focuses on the various organic alternatives to AGPs, the mechanisms of action behind those alternatives, and the consequences those processes have on the poultry industry.
ANATOMY OF THE AVIAN GASTROINTESTINAL TRACT
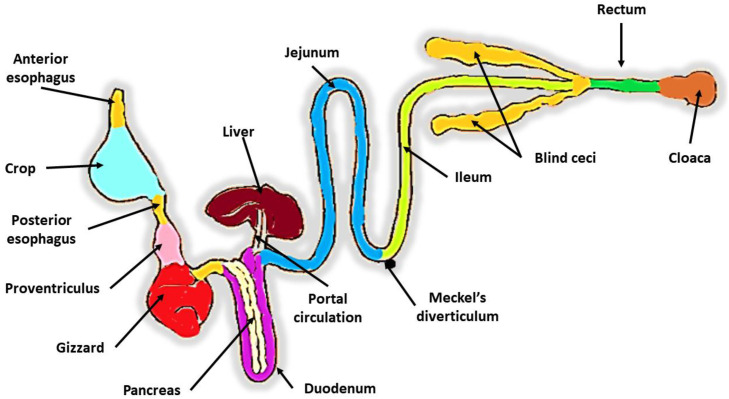
The gastrointestinal tract (GIT) of avian species is the primary factory for converting feed into a product, which can be meat in broiler chickens, eggs in laying hens, or fertilized eggs in breeder flocks (Abd El-Hack et al., 2022a). The avian gut (Figure 1) is anatomically composed of the beak, buccal cavity, anterior esophagus, crop, posterior esophagus, proventriculus, gizzard, duodenum, jejunum, ilium, 2 blind ceci, rectum, and cloaca, as well as accessory organs like as the liver and pancreas (Klasing, 1999). In terms of the anatomy of the avian digestive tract, food is initially stored in the crop, an organ for feed storage where fermentation by lactic acid bacteria takes place; food is then digested and mechanically pulverized in the proventriculus and gizzard, which serve as stomachs (van Leeuwen et al., 2023). The gizzard is the "teeth" of the avian GIT, the location where digestion of feed really takes place, and its low pH makes it a natural microbial barrier (Oakley et al., 2014; Stanley et al., 2014).
Figure 1.
Anatomy of the chicken digestive tract.
Compared to mammals, birds have a shorter digestive tract, which means that their digestion must be extremely efficient (Rodrigues and Choct, 2018). In terms of the physiology of the avian gut, after the bird has swallowed, food moisturization and grounding do not take place in the mouth. This is in contrast to the gut physiology of mammals, in which postcrop fermentation takes place in the proventriculus and gizzard, and digestion takes place with the help of gastric secretions (Grond et al., 2018). The nutrients are then primarily digested and absorbed in the small intestine, with a brief retention time in the duodenum, anatomically forming a loop around the pancreas that receives digestion enzymes (Kohl, 2012). Following that, digestion continues up to the end of the jejunum, which has been identified as the principal site of minimal intestinal absorption. Despite the fact that some nutrient digestion can also take place in the ileum, this final segment of the small intestine is mostly responsible for absorbing nutrients, water, and minerals (Svihus, 2014).
The small intestine, like that of mammals, has a single epithelial cell layer (absorptive, goblet, and enteroendocrine cells) that lines villi and crypts, which is coated by a mucus layer generated by mucins secreted by goblet cells, which serves as the interface between gut and microbiota (Casteleyn et al., 2010). The villi of the small intestine, which are the functional units of the small intestine can be defined as projections of the lamina propria into the gut lumen to increase the porous area, change in size and length as one moves from the proximal to the distal part of the small intestine (Rubin and Langer, 2022). However, since chickens do not have much of a large intestine, the ileum represents the last functional tract to adsorb nutrients (Smith et al., 2022).
The large intestine of birds is also distinguished by the presence of 2 blind-ended bags called ceca at the junction of the ileum and colon. These ceca are typically quite long and well developed, and they are covered in a meshwork of long interdigitating villi that filter, like a sieve, fluids and small particles entering the colon from the ileum (Smith et al., 2022). This villus mesh structure is only found in birds (Rubin and Langer, 2022).
Avian ceca have a key role in microbial carbohydrate degradation and fermentation, microbial vitamins and amino acids synthesis, nitrogen compounds degradation, urea recycling into amino acids, and water absorption and balance (Oakley et al., 2014; Svihus, 2014). Cecum is the most diverse gut section, characterized by the longest feed retention time compared to the upper parts and by the highest short-chain fatty acids (SCFA) concentration that the host absorbs (Svihus, 2014). Cecum represents the main site where complex nutrients such as cellulose and other nonstarch polysaccharides (NSPS) are fermented, with a high fermentation rate of facultative anaerobes and strictly anaerobic bacteria (Józefiak et al., 2011; Borda-Molina et al., 2018).
The large intestine ends with a very short rectum, extending between the ileocecal junction and the cloaca (Borda-Molina et al., 2018). The avian rectum is similar to the small intestine in histological structure but has shorter villi and richer lymphoid follicles (Borda-Molina et al., 2018). Another unusual avian peculiarity is digesta moving in peristaltic and antiperistaltic ways along the GIT (Gofur, 2020). Feed normally moves from the duodenum to the colon due to caudal peristaltic contractions in the intestine; however, poultry use reverse peristalsis (cranial) to increase feed retention duration and digestibility (Gofur, 2020).
There are 3 types of antiperistaltic movements and reflux: i) from the gizzard to the proventriculus due to gizzard contractions to increase feed exposure to proventriculus enzymes; ii) from the jejunum and duodenum back to the stomachs to improve digestion during fasting; and iii) as a continuous physiological process from the cloaca to the ceca (Rodrigues and Choct, 2018).
POSTHATCHING DIGESTIVE TRACT DEVELOPMENT
Small intestine weight grows faster than chick body weight (BW) during incubation and in the late embryonic development, villi begin growing and shaping on d 15, and between d 17 and 20, 3 stages of villus development and maturity can be seen, ranging from elongated pear-shaped villi to shorter ones and nascent villi (Christensen, 2009). The ratio of the small intestine to the BW then rises from 1 to 3.5% between this time and hatching, and minimal enzyme activity is developed (Uni et al., 2003). During the process of incubation, the yolk delivers a significant amount of nutrients to the embryo through circulation afterward, aiding in the growth of the small intestine for up to 48 h after birth (Yegani and Korver, 2008).
During this time, the chick has to shift from getting its nutrients from the yolk which is high in lipids to getting them from the feed which is high in carbohydrates and proteins (Uni et al., 2003). As a result, the balanced growth of the intestine is greater than that of BW at hatching because chicks need to acquire an efficient nutrient uptake capacity quickly following the first intake of yolk nutrients, which is supported by healthy gut maturation (Christensen, 2009).
There is a strict partitioning between the “demand” of the tissues as major users of energy and proteins and the “supply” of the tissues during the first few weeks after hatching (Christensen, 2009). The intestine is the primary nutrient supply organ, and the small intestinal epithelium determines the growth potential of chicks (Hu and Guo, 2008). The sooner the stomach reaches full functional capacity, the sooner the chicks may use the nutrition for effective physiological development, including immunological competence, which is necessary for disease resistance (Lilburn and Loeffler, 2015).
Exogenous feed consumption is associated with rapid development of digestive organs, with the timing and shape of the food, as well as the nutrients accessible to the chick, having a large and crucial impact on gut development (Yegani and Korver, 2008). In fact, fatty acids (especially unsaturated fatty acids) have a faster intestine absorption rate than glucose shortly after hatching (Yegani and Korver, 2008). In a few days, amino acids absorption rate and carbohydrates increase (Cardeal et al., 2015). The ability to obtain feed early in life has been identified as a critical component influencing intestinal function later in life, since studies have shown that delaying feeding delays gut development (Geyra et al., 2001; Yegani and Korver, 2008).
When compared to mammals, chicks' faster gut growth is reflected in an increase in the number of enterocytes during the first few days after hatching (Uni et al., 2003). While hatching, enterocytes are young, similar to mammals postfarrowing, appearing small, round-shaped, and lacking the normally polarized brush-border; they gained polarity in only 24 h, and in the duodenum and jejunum, epithelial surface rises fast through cell hypertrophy (Geyra et al., 2001).
The jejunum, in particular, appears to account for the greatest rise in posthatching absorption, generating a larger absorptive surface with higher and denser villi after 72 h compared to the duodenum, which, in turn, expands denser villi than the ileum (Geyra et al., 2001). In contrast to the previous segments, chicken ileum enterocytes are anatomically mature at hatching, and hypertrophy is limited and delayed (Geyra et al., 2001). In addition to these physical changes, digestive and absorptive capacities increase, with the duodenum showing better functional maturation than the other distal segments (Jamroz et al., 2004). This maturation for digestive activity occurs during enterocyte migration from the crypt to the villus, with an increase in the expression of gut nutrient transporters as well as pancreatic and brush-border enzymes such as disaccharidases, aminopeptidases, and alkaline phosphatase, the latter of which is recognized as an enterocyte maturation marker (Geyra et al., 2001).
The presence of proliferative enterocytes not only at the crypt level but also along the villus has been reported in all segments in chicks, while in the mature bird, like in mammals, cell renewal is guaranteed only by proliferating crypt stem cells that migrate up to villus tip (Geyra et al., 2001). Following that, cell proliferation gradually declines with age, with the jejunum experiencing the slowest reduction, indicating that villus mitosis is more crucial for growth than in the other segments (Jamroz et al., 2004).
In terms of crypt development, undefined crypts after hatching become distinguishable in 24 h, expanding in number and size to provide enterocytes for villus growth and increasing cell renewal rate (Geyra et al., 2001). Villus volume and crypt depth grow, resulting in a full duodenum around d 6 and 7, and then decrease. This rise persists in the jejunum and ileum until d 14 (Jamroz et al., 2004). Along with the development of digestive processes and structures, the modern development of gut-associated lymphoid tissue (GALT) occurs. GALT is a set of lymphoid structures connected to the intestine and representing the immune system at the gut level. Its development in chicks occurs during late embryogenesis (Yegani and Korver, 2008). As a further component of innate host response, the gut mucus layer is formed by goblet cells that release mucins. Goblet cells start their development in the late embryonic and immediate posthatch period, firstly containing only acidic mucins and after hatch also producing neutral mucins (Smirnov et al., 2006).
Eubiosis is defined as an interspecies balance of the microbiota population with maximal feed digestibility and nutrient absorbability. Dybiosis, on the other hand, is a disruption of eubiosis caused by a variety of factors such as poor-quality diets, rancid fats, antinutritional factors (toxins), and pathogens. Affected birds will exhibit signs of diarrhea and increased daily water intake, but there are no specific postmortem lesions (Kohl, 2012).
FEEDING STRATEGIES TO IMPROVE POULTRY GUT HEALTH
Use of Antibiotics and Associated Risks
Antibiotics have been primarily used in animal production for decades. Since their discovery in the 1920s, antibiotics have been pivotal in the progress and growth of animal industries (Gadde et al., 2017; El-Saadony et al., 2022a; Yehia et al., 2022, 2023). Next to those used therapeutically, most antibiotic uses have been practiced for prophylactic aims since the 1950s (Van et al., 2020). The use of in-feed antibiotics at the subtherapeutic level as AGP rose with the intensification of livestock production to maintain gut ecosystem balance, improve average daily weight gain and feed efficiency, as well as to reduce mortality (Gadde et al., 2017; Abd El-Hack et al., 2022b).
AGP's net effect on the poultry industry accounts for about 3 to 5% growth and feed efficiency (Gadde et al., 2017). Animal performance improves as a result of AGP's effect on gut microbiota, which leads to a reduced microbial load, decreased energy consumption, and a more stable microbial population that is less likely to provoke inflammation (Abd El-Hack et al., 2022c; El-Saadony et al., 2022b). This impact appears to boost nutritional energy harvest and assist the animal in reaching its full genetic potential (Gadde et al., 2017). Because microbial utilization is reduced and intestinal permeability increases, with AGP, growth is boosted because more nutrients are readily available (Huyghebaert et al., 2011). Additionally, GI infections are better managed, leading to lower overall maintenance costs (Lin et al., 2013).
A recent study on the effects of medicated or nonmedicated diets on the shift in chicken's ileal microbiota showed a particular influence of AGP on division Firmicutes, with the elimination of Lactobacillus species compared to their high proportion in nonmedicated fed birds (Lin et al., 2013; de Carvalho et al., 2021). It has been hypothesized that one of AGP's indirect effects is a boost to lipid metabolism due to a decrease in Lactobacillus spp. (de Carvalho et al., 2021). Bile salt deconjugation by Lactobacillus species through bile salt hydrolases enzymes leads to a lessen lipid digestion and absorption (Begley et al., 2006). At the same time, a reduction in these bacteria may result in an increase in the amount of energy available for growth (Begley et al., 2006).
The routine and extensive use of antibiotics, mainly as AGP, over the last 50 yr has been linked to the development and increase of drug-resistant bacteria, a real threat and serious problem for animals and public health because the altered microbiota stimulated by antibiotics is transferable to other hosts, with the consequent risk of transfer of antibiotic resistance genes from livestock to human microbiota (Sugiharto, 2016; Gadde et al., 2017).
This increasing problem led to the total ban of AGP in the European Union in 2006 (Regulation EC no. 1831/2003) (Stanley et al., 2014; Ducatelle et al., 2018). The ban on AGP as feed additives in animal feed has led to a rise in gut health problems in poultry, specifically those associated with gut dysbiosis, barrier leakage, and intestinal inflammation (Ranjitkar et al., 2016; Ducatelle et al., 2018). As a result of these issues, researchers began looking for ways to enhance growth performance and animal health while reducing the risk of diseases such necrotic enteritis (NE), gut dysbiosis, and immune system dysregulation (Salem et al., 2021; Abd El-Hack et al., 2022d).
Antibiotic-Free Livestock Production With Natural Feed Additives
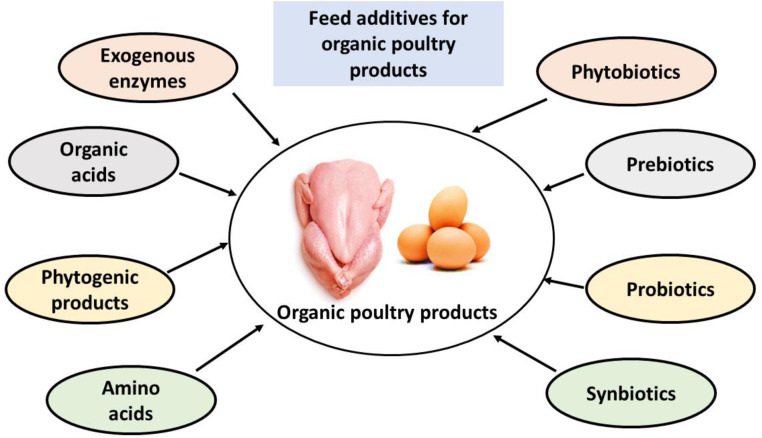
The production of chickens for commercial purposes has developed throughout the years, and the modern poultry industry is dependent on the large-scale production of meat and egg-type hens in order to satisfy the growing demand for meat and eggs on the market (Dittoe et al., 2020; Banday et al., 2023). Recent years have seen an increase in the use of alternative management strategies as a direct result of the growing public interest in locally grown and organically produced foods (Ricke and Rothrock, 2020). As a consequence of this, naturally safe alternative modes of poultry and egg production systems have been developed. These systems include the utilization of herbal extracts, essential oils, organic acids, exogenous enzymes, prebiotics, probiotics, synbiotics, green synthesized nanoparticles, bioactive peptides, polyphenols, and photogenic products in order to improve poultry production and provide products that are free from hazardous organic compounds and are safe for human consumption (El Jeni et al., 2021; Abd El-Hack et al., 2023) (Figure 2).
Figure 2.
The use of natural feed additives in organic poultry production.
Exogenous Enzymes as Feed Additive
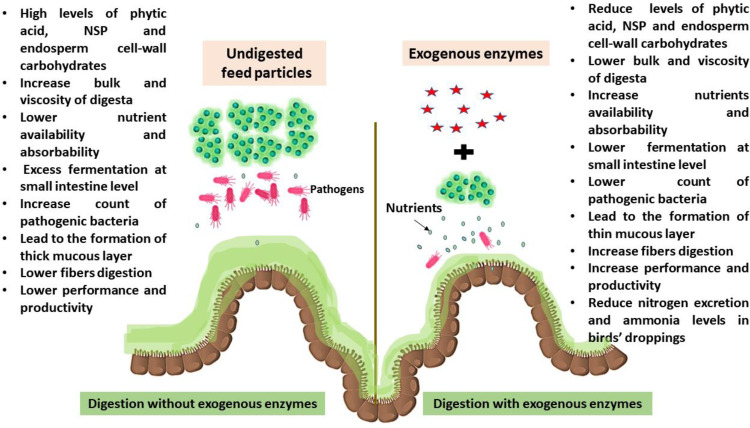
It is a well-known fact that chicken feed efficiency and growth can be boosted by the action of certain exogenous enzymes (Table 1 and Figure 3). Those commonly used enzyme feed additive classes include phytases, carbohydrases (like xylanase, amylase, etc.), and proteases (Llamas-Moya et al., 2019). Most of these exogenous dietary enzymes act on nondigestible and antinutritional factors of animal feeds, such as phytic acid, NSP, and endosperm cell-wall carbohydrates that could hurt poultry production, improving dietary nutrients availability (Gadde et al., 2017). NSP (including pectin, oligosaccharides, and β-glucans) could exert antinutritive effects in poultry, increasing the bulk and viscosity of digesta and mucus layer thickening, hindering the activity of digestive enzymes (Raza et al., 2019).
Table 1.
The effects of exogenous enzymes on poultry production.
| Antibiotic alternatives | Type | Application | Treated birds | Mode of action | Effects | References |
|---|---|---|---|---|---|---|
| Exogenous enzymes | α-Galactosidase enzyme | Applied as 200 g/ton feed | Broiler chickens | Carbohydrase formulations containing α -galactosidase reduced the effect of low-energy-density feed | Improved body weight gain, feed conversion rate, and antibodies titers | Llamas-Moya et al., 2019 |
| β-Glucanase and xylanase | Applied as 2,500 U of xylanase and 250 U of β-glucanase per kg feed | Broiler chickens | β-Glucanase: degraded fiber and β-glucan. Xylanase: digested nonstarch polysaccharides in the fiber, reduced digesta viscosity, and increased nutrients digestibility, release, and digesta passage rates | Improved young bird performance, but ileal or cecal microbiome revealed no significant differences | Munyaka et al., 2016 | |
| Βeta-mannanase | Applied as 200 and 400 mg/kg feed | Broiler chickens | β-Mannan is an antinutritive fiber found in soybean meal and has an antibacterial effect | Improved growth, feed efficiency, morphological status in the gut, blood glucose and anabolic hormone, and homeostasis | Christine et al., 2002; Mehri et al., 2010; Saeed et al., 2019 | |
| Amylase | Feed | Broiler chickens | Increased starch digestibility, and reduced the presence of glucose as a potential substrate for nonbeneficial bacteria in the latter part of the gastrointestinal tract | Increased the weight gain | Gracia et al., 2003; Cowieson et al., 2019 | |
| Protease | Feed | Broiler chickens | Increased protein digestibility through hydrolysis of storage and structural proteins and disrupted interactions of proteins with starch and fiber in the diet. Exogenous proteases are a broader spectrum | Reduced emissions of ammonia in bird droppings and the greatest effects were obtained when protease was used as a tool to allow for lower diet protein content | Oxenboll et al., 2011 | |
| Lipase with emulsifier | Feed | Broiler chickens | Increased the fat digestibility by its hydrolysis and thereby improved its digestibility | Improved the performance of birds given diets with lowered energy | Oliveira et al., 2019 | |
| Xylanase, amylase, and protease | Feed | Broiler chickens | These mixture enzymes are produced using microbial sources. The microbial sources used are selected so that they not only produce the enzyme but also act as probiotics for birds | There was a synergistic effect of enzymes mixture, and they increased birds’ performance and nutrient digestibility | Amerah et al., 2017 | |
| Phytase from bacterial and fungal origins | Feed | Cup broiler chicks | Phosphorous is a key nutritional requirement for poultry to provide bone growth. Most of the phosphorus contained in animal feed of plant origin exists in the storage form of phytate. Poultry cannot digest phosphorus contained within phytate since they lack a phytase enzyme that breaks down this phytate molecule. Therefore, the phytase enzyme in poultry feed is required to release phytate bound phosphorus | The phytase enzyme obtained from bacterial origin revealed better impacts on feed efficiency ratio organ functions, and oxidative status | Awad et al., 2013 | |
| Pectinase and carbohydrases | Feed | Broiler chickens | It degraded indigestible pectin. Reduced digestive viscosity. Degraded pectin α-1,4-linked anhydrogalacuronic acid | No significant effects on body weight gain, feed intake, feed conversion ratio, and abdominal fat content but pectinase increased the digestibility of a corn-soybean meal | Tahir et al., 2006 | |
| Cellulase | Cellulase enzyme to sago palm waste at 0.75 g cellulose/kg sago palm waste | in vitro | Degraded fiber | Improved in vitro dry matter digestibility and in vitro organic matter digestibility | Zulkarnain et al., 2017 | |
| Enzymes as a toxin detoxifier (DETOXIZYME) | Applied a 100 g DETOXIZYME/ton of feed | Boiler chickens | Decreased Clostridium perfringens and E. coli counts and enhanced gut morphometry | Increased birds’ productivity, blood biochemistry and immunity | Saleh et al., 2023 |
Figure 3.
The role of exogenous enzymes in gut health improvement.
In addition, consuming a diet high in NSPs increases the likelihood of an excessive amount of fermentation occurring in the small intestine, as well as an overabundance of bacteria, which leads to decreased activity in the hindgut microbiota and increased competition with the host for nutrients (Elbaz et al., 2023). By increasing the quantity of fermentable material available for cecal microbiota, where fermentation products are rapidly absorbed by an epithelial wall, dietary enzyme supplementation can assist control ileal fermentation and volatile fatty acid production (Huyghebaert et al., 2011). Endo-xylanases, for instance, are responsible for the release of nutrients, which then become accessible to endogenous enzymes. Additionally, endo-xylanases suppress an excessive load of fermentative bacteria in the small intestine by reducing the amount of available nutritive substrate. This is accomplished by increasing the rate at which digesta passes through the body and digestion (Ferket et al., 2005).
The better performance that is attributed to the presence of NSP enzymes is associated with an increase in the digestibility and absorption of nutrients that are not normally digested by host enzymes, the inactivation of antinutritional factors, and an increase in the solubility of nondigested nutrients as well as cecal fermentation (Saleh et al., 2023). In addition, exogenous enzymes have an indirect effect on the composition of the microbiota, which may be influenced by the short-chain oligosaccharides that are released from the NSP. These oligosaccharides have the ability to have prebiotic effects and have varying effects on performance (Gadde et al., 2017; Polovinski-Horvatović, 2021).
According to Borda-Molina et al. (2018), enzymes such as xylanase and beta-glucanases have the ability to boost the growth of lactic acid bacteria. These lactic acid bacteria attach to the gut epithelium and compete with pathogens for binding sites. Furthermore, several enzymes appear to influence the structure of the intestines. It has been observed that the jejunum of broilers fed xylanase had less crypt depth, which was linked to greater chicken growth (Yang et al., 2008), and that the jejunum of chickens fed dietary lysozyme had longer villus hairs, which increased surface area and absorption (Abdel-Latif et al., 2017). It also appears that lysozyme has the ability to alter immunological and oxidant status in the small intestine by raising the mRNA of genes connected to these processes (Abdel-Latif et al., 2017).
According to Sugiharto (2016), endogenous enzymes are frequently combined and utilized in order to increase their efficiency by combining several antinutritive drug targets. This is carried out to enhance the impact of the enzymes. Benefits from feed enzymes may change depending on factors such as the animal's genetic make-up, the feed's composition, and the enzyme's kind and source (Gadde et al., 2017). As a result, incorporating exogenous enzymes into the chicken diet has been shown to improve feed digestibility, the nutritional content of feed ingredients, and have a favorable impact on the environment by reducing the excretion of nitrogen and phosphorus in poultry feces (Alagawany et al., 2018; Llamas-Moya et al., 2019).
Table 1 and Figure 3 summarize the effect of exogenous enzymes as feed additive on poultry performance and production.
Organic Acids as Feed Additive
Organic acids are abundant in nature as both plant and animal tissue components (Bialkowski et al., 2023). They are produced by microbial fermentations of carbohydrates, such as those found in poultry ceca, and are associated with increased development and performance. Organic acids, which can be monocarboxylic acids (e.g., formic, lactic, acetic, propionic, and butyric acid) or carboxylic acids with a hydroxyl group, can be introduced to animal diets as individual acids, salts, or mixes (Huyghebaert et al., 2011; Gadde et al., 2017).
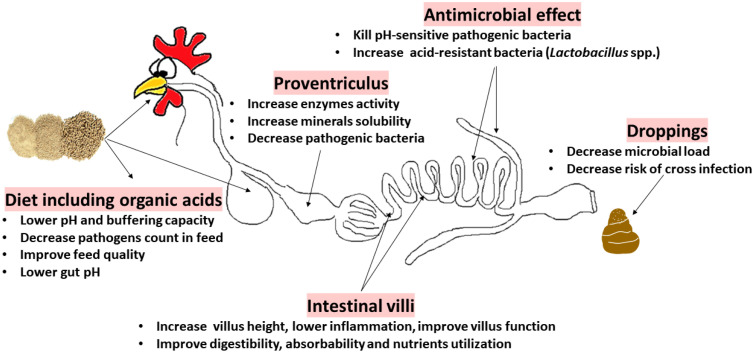
The antimicrobial effect of general in-feed organic acids can be explained by their ability to pass from dissociated to undissociated form (depending on pH) and by their ability to decrease feed pH and the buffering effect at the crop, proventriculus, and gizzard levels (Han et al., 2023). Organic acids are able to pass through bacterial cell membranes and, once inside, they dissociate and reduce pH (Bangar et al., 2022). This results in a bactericidal impact against pH-sensitive bacteria (such as some pathogens), but it has no effect on acid-resistant bacteria (such as Lactobacillus spp.), which instead grow (Huyghebaert et al., 2011; Bangar et al., 2022). The trophic effect has been observed in the duodenum and jejunum of broiler chickens fed butyric or fumaric or lactic acid, where, regardless of the organic acid type used, the dietary supplementation increases villus height, possibly as a result of a reduced bacterial load, less inflammation, and mucosal challenge, with a subsequent increase in villus function. This may be associated with a decrease in the number of potential pathogenic bacterial species (Adil et al., 2010).
In addition, organic acids stimulate digestion by boosting pancreatic secretions (Adil et al., 2010; Ebeid and Al-Homidan, 2022), and they also favor mineral absorption (e.g., SCFA can be used to replenish epithelial cells; Gadde et al., 2017). There is evidence that organic acids are beneficial for gut health, in particular the bacteriostatic effects of fatty acids like butyrate (Rinttilä and Apajalahti, 2013), which have been shown to fortify the mucosal barrier by inducing the release of antimicrobial peptides in mucus and the expression of tight junction proteins (Huyghebaert et al., 2011). However, it is important to keep in mind that absorption and metabolism in the upper intestine diminish the concentration of organic acids in feed as they travel to the hindgut, unless the feed is encapsulated (Adil et al., 2010). In terms of performance, while some favorable impacts have been recorded (Adil et al., 2010), no consistent effects on growth have been observed, possibly due to differences in the types and quantities of organic acids employed and diet ingredients (Gadde et al., 2017; Ebeid and Al-Homidan, 2022).
Table 2 and Figure 4 summarize the effect of organic acid administration as feed additive on poultry performance and production.
Table 2.
Impacts of different organic acids on poultry performance.
| Antibiotic alternatives | Type | Application | Treated birds | Mode of action | Effects | References |
|---|---|---|---|---|---|---|
| Organic acids | Organic acid-based product (Laynexa) |
Feed for 4 or 8 wk | Layer chickens | Improved gut pH | Showed no effect on egg weight and egg quality | Gama et al., 2000 |
| Butyrate (0.4%) | Feed | Broiler chickens | Improved gut health | Maintained body weight gain |
Panda et al., 2009 |
|
| Butyrate (0.2%) |
Feed | Broiler chickens | Improved gut health | Maintained the performance of birds | Leeson et al., 2005; Antongiovanni et al., 2007 | |
| Mixture of 0.06% Galliacid, 0.1% Biacid |
Feed | Broiler chickens | Improved gut health | Improved birds’ performance | Hassan et al., 2010 | |
| Organic acid mixture |
Feed | Layer chickens | Improved gut health | Showed no effect on the egg quality. However, egg weight was increased by 9.08% | Youssef et al., 2013 | |
| Formic and butyric acids | Drinking water | Broiler chickens | Showed broad-spectrum antibacterial and antifungal activity | Showed no impact on birds’ performance. However, it improves the lymphoid organ weight |
Al-Mutairi et al., 2020 | |
| Butyric acid, fumaric acid, and Lactic acid | Feed | Broiler chickens | Reduced pathogen multiplication | Improved birds’ performance | Adil et al., 2010 | |
| Acetic acid |
Feed | Layer chickens | Improved gut health | Improved external egg quality as egg weight, egg length, egg diameter, and eggshell color | Kadim et al., 2008 | |
| Organic |
Feed | Layer chickens | Increased the integrity of the reproductive organs, such as the shell gland in the oviduct | Improved eggshell color | Park et al., 2009 | |
| 1% lactic acid | Feed | Layer chickens | Increased the integrity of the reproductive organs | Improved the albumen and yolk index | Yalcin et al., 2000 | |
| Microencapsulated feed additive including citric acid, sorbic acids, thymol, and vanillin | Feed | Broiler chickens | Lowered inflammation, and improved the intestinal barrier function | Improved chicken feed efficiency | Bialkowski et al., 2023 |
Figure 4.
Impacts of organic acids on birds’ gut health.
Phytogenic as Feed Additive
Phytogenics, also known as phytobiotics or botanicals, are a class of additives containing a wide variety of natural bioactive compounds derived and extracted from various plant sources (principally classified in herbs and spices) that have antimicrobial activity and immune enhancement as significant properties and contain polyphenols and flavonoids as main bioactive components and potent antioxidants (Abdelnour et al., 2020a,b; Abou-Kassem et al., 2021; Abd El-Hack et al., 2022e,f).
The mechanisms of action of these compounds are not completely understood. Their efficiency is debatable, but in general, they act by stimulating cell membrane breakdown in pathogens, stimulating the development, and settling of beneficial bacteria (lactobacilli and bifidobacteria), and acting as immune modulators (such as higher cytokine expression and cell proliferation) (Sugiharto, 2016; Gadde et al., 2017; Abdel-Moneim et al., 2022; Mnisi et al., 2023).
The ability of certain plant extracts to reduce the negative effects of Salmonella enteriditis infection in the cecum of chickens by selectively increasing the number of certain beneficial bacterial species including Faecalibacterium and Lactobacillus has recently been demonstrated (Song et al., 2020; El-Saadony et al., 2023a). In contrast, when plant extracts were supplied without gut infection, there were no impacts documented on microbial diversity (Varmuzova et al., 2015). Another notable effect of phytobiotics in chicken feed is the reduction of oxidative stress, which is accompanied by an improved antioxidant response in tissues, increased pancreatic enzyme production, and promoted proliferation of gut cells (Gadde et al., 2017; Abou-Kassem et al., 2022; Arif et al., 2022). Naturally, given the large range of plant sources, fluctuation in composition due to biological factors, storage, and different types of procedures (Huyghebaert et al., 2011), an extensive range of variables exists (Alagawany et al., 2021a,b).
Processed phytogenic compounds, essential oils, and oleoresins all differ in their effectiveness depending on the type of compound used, the dosage, and the method of preparation, as well as external factors like food, and heredity (Gadde et al., 2017; Alagawany et al., 2022). Essential oils are volatile chemicals extracted from plants, and their influence and efficacy have been the subject of numerous investigations (Abd El-Hack et al., 2022f). It has been shown that they have antioxidant properties and affect the composition of microbiota; for instance, treatment with ginger root essential oil increased the counts of lactic acid bacteria in the jejunum of chickens (Ashour et al., 2020, 2021; El-Tarabily et al., 2021; El-Shall et al., 2022). In addition, essential oil administration increased antioxidant status in broiler chickens exposed to heat stress, as evidenced by a lower concentration of serum malondialdehyde, a primary aldehyde result of lipid peroxidation (Habibi et al., 2014; Sheiha et al., 2020; Reda et al., 2021a,b).
Colony forming units (CFU) counts of Escherichia coli and other enterobacteria in the digesta of ileocolon hens given ginger and garlic essential oils were also reduced (Dieumou et al., 2009; Attia et al., 2022). In contrast to earlier studies that found no enhancement of performance by dietary phytogenic substances in chickens reared until 42 d of age (Dieumou et al., 2009). Habibi et al. (2014) found that chickens fed ginger root powder during the first growing period (at d 22) had a greater BW gain than those fed the powder at any other time.
However, Kamboh et al. (2013) found that supplementing broilers' diets with 2 plant-derived flavonoids during the finisher period of growth (22–42 d) increased weekly performance and had a mitigating effect on chickens subjected to persistent seasonal heat stress by decreasing levels of stress biomarkers in their blood and skeletal muscles (Kamboh et al., 2013).
Table 3 and Figure 5 summarize the effect of phytogenic as feed additives on poultry performance and production.
Table 3.
Impacts of different phytogenic feed additives on poultry performance.
| Antibiotic alternatives | Type | Application | Treated birds | Mode of action | Effects | References |
|---|---|---|---|---|---|---|
| Phytogenic | Quercetin | Dietary supplementation | Layer chickens | Modulated intestinal environment and liver superoxide dismutase content in laying hens | Increased performance | Liu et al., 2014 |
| Hesperetin 0, 0.5, 1, 2, 4 g/kg and Naringenin 0, 0.5, 1, 2, 4 g/kg | Dietary supplementation | Layer chickens | Reduced cholesterol content in eggs | Improved egg production | Ting et al., 2011 | |
| 1.0, 1.5, and 2.0% black cumin | Dietary supplementation | Layer chickens | Reduced egg-yolk cholesterol level | Improved egg quality | Hossain et al., 2016 | |
| Turmeric rhizome extract | Applied as 0, 0.1, 0.2, and 0.3 g/kg feed for 12 wk | Chickens | Turmeric rhizomes extract reduced malondialdehyde concentrations and increased activities of glutathione peroxidase and superoxide dismutase | Elevated the breast muscle weight ratio and reduced the drip loss of both thigh and breast muscles when applied as 0.3 g/kg | Wang et al., 2015 | |
| Resveratrol | Applied as 0.2, 0.4 or 0.6 g resveratrol/kg feed | Heat-stressed chickens | Reduced oxidative stress by elevating serum concentrations of growth hormone and modulated heat shock genes' expression in immune organs | Improved the growth performance | Liu et al., 2014a | |
| Curcumin and thymoquinone | Curcumin (2.5 mg/kg diet) + thymoquinone (2.5 g/kg diet) | Four-wk old mixed-sex turkey poults challenged intranasally with H9N2 avian influenza virus | Showed immunomodulatory activity | Treatment with thymoquinone and curcumin reduced virus shedding | Umar et al., 2016; Reda et al., 2020; Abd El-Hack et al., 2021c | |
| Epigallocatechin gallate | Diet supplemented with 300 and 600 mg Epigallocatechin gallate/kg feed | Heat stressed broiler chickens | Alleviated the oxidative damage caused by heat stress and enhanced the overall antioxidant status | Improved broilers growth performance | Luo et al., 2018 | |
| Terminalia chebula RETZ and Punica granatum L | Oral gavage of the extract dissolved in distilled water at 0.15, 0.3, and 0.6 g/kg for 7 d | Specific pathogen-free chickens challenged with Escherichia coli | Downregulated the activation of NF-κB signaling pathways, reversed the overgene expression of the Toll-like receptor (TLR) 2, 4, and 5, and stopped the generation of proinflammatory cytokines | The extracts decreased the morbidity and inflammation induced by Escherichia coli | Zhang et al., 2014 | |
| Nanocurcumin | Applied as feed with 0.1, 200, 300, 400, and 500 mg/kg of nanocurcumin | Japanese quails | Birds fed nanocurcumin at 0.2 g showed elevated immunoglobulins levels and antioxidant status | Improved feed conversion when applied as a ratio (0.4 g) nanocurcumin-treated bird | Reda et al., 2020 | |
| Resveratrol | Applied as 0.2 or 0.4 g resveratrol/kg feed for 12 wk | Five-wk-old Japanese quails | A 0.4 g/kg diet improved the antioxidant status of both eggs and birds | Improved egg quality | Sahin et al., 2010 |
Figure 5.
Impacts of phytogenics feed additives on poultry performance.
Amino Acids as Feed Additive
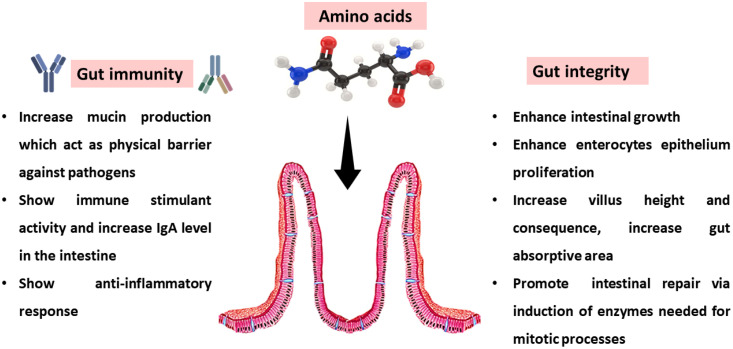
Aside from the primary nutritional function of amino acids and their requirement for protein synthesis for growth optimization, dietary amino acids supplementation has been studied for intestinal protection, immune modulation, microbial maintenance, and better poultry performance (Abdelfatah et al., 2023). Amino acids can regulate metabolic routes, gene expression, hormones, and other essential molecule synthesis (Gottardo et al., 2016; Abdelfatah et al., 2023).
It has been reported that supplying amino acids with higher digestibility is beneficial to restore the gut when absorptive capacity is impaired, and this, in turn, improves feed efficiency and growth, suggesting that an increase in dietary amino acid levels may be helpful in cases of malabsorption during the intestinal challenge (Abdelfatah et al., 2023). Dietary amino acids appear to promote intestinal repair by inducing enzymes necessary for mitotic processes and by stimulating expression of genes for anti-inflammatory response and reparative processes, suggesting that a higher concentration of amino acids in an animal's diet may stimulate a better development of the intestinal mucosa (Bortoluzzi et al., 2018). Threonine (Thr), arginine (Arg), and glutamine (Gln) are well-known amino acids for their roles in mucin synthesis, immunological function, and epithelial proliferation, respectively (Abdelfatah et al., 2023). Lysine and methionine, the other essential amino acids in poultry, are found mostly in intestinal mucins and help maintain the gut barrier. Increased mucus production due to gastrointestinal dysfunction raises the need for Thr (Abdelfatah et al., 2023).
Thr is also important for the synthesis of intestinal IgA and can affect other immunological parameters like the release of proinflammatory cytokines (Bortoluzzi et al., 2018). Arg is needed for the production of polyamines, nitric oxide (a cytotoxic mediator of immune cells), and creatine, all of which have trophic effects on the gut epithelium throughout its formation and repair (Gottardo et al., 2016; Bortoluzzi et al., 2018). The acidic homolog Gln, along with glutamate, is the first energy source for small intestine enterocytes, boosts gut proliferation, and is thought to be crucial in inflammatory circumstances due to its ability to promote mucosal healing (Bortoluzzi et al., 2018).
Dietary Gln supply in chickens under heat stress showed to increase villus height in jejunum and ileum, which may have benefits for digestion due to a larger gut absorptive area, in addition to its role as a nitrogen source for purine and pyrimidine (Wu et al., 2018). Gln also reduced levels of TNF-a and elevated IL-10, which together reduced the inflammatory response in the digestive tract (Wu et al., 2018). Gln also improved intestinal barrier and integrity by limiting increased permeability through the stimulation of RNA expression of tight junction protein-related genes. This contributed to Gln's positive effects on the intestinal barrier and integrity (Wu et al., 2018).
Cell proliferation is a key factor in gut regeneration, and it has been reported that broiler chickens fed high dietary concentrations of amino acids (Thr, Arg, and Gln) have better feed conversion and greater small intestinal recovery and integrity after being subjected to gut infection challenge (Gottardo et al., 2016). Recent research has shown that supplementing the diet with tryptophan (Trp) can mitigate the harmful effects of chronic unpredictable stress on the jejunal mucosal level of broilers. This was seen in terms of intestinal permeability improved by increased tight junction protein abundance, and gut immune regulation improved by increased IL-10 RNA gene expression and IgA level (Yue et al., 2017). In addition, the administration of Trp appeared to lessen the rise in blood corticosterone, and because it is a precursor of the neurotransmitter serotonin which is implicated in intestinal motility and secretion, it stopped the stress-induced change in serotonin signaling (Yue et al., 2017). Taking into account all of these many functional roles that amino acids play; it is important to keep in mind that a correct and adequate level must be specified in order to prevent an excess of amino acids or their loss (Abdelfatah et al., 2023).
Table 4 and Figure 6 summarize the effect of amino acids as feed additives on poultry performance and production.
Table 4.
Impacts of different amino acids on poultry performance.
| Antibiotic alternatives | Type | Application | Treated birds | Mode of action | Effects | References |
|---|---|---|---|---|---|---|
| Amino acids | Betaine with different concentrations of 0, 0.75, 1.5, and 2.25 g/kg | Feed | Japanese quail | Decreased levels of liver enzymes such as alanine aminotransferase, alkaline phosphatase, and aspartate aminotransferase and lowered low-density lipoprotein level | Improved birds’ performance, carcass traits, and blood chemistry | Arif et al., 2021 |
| Total sulfur amino acids | Applied as 1.1 and 0.9 % to starter and finisher diets under a long photoperiod regime | Broiler chickens | Increased the feed consumption and improved feed conversion ratio | Improved broiler performance, carcass traits, and blood parameters | Abou-Kassem et al., 2022 | |
| Betaine | Applied as a dietary supplementation | Poultry | Betaine is an osmolyte and a methyl donor | Improved bird performance | Alagawany et al., 2021 | |
| Threonine | Applied as a threonine dietary supplementation (0, 300, 600, and 900 mg/kg) | Ross broiler chickens | Improved the body weight, weight gain, feed intake, feed conversion ratio, carcass weight, dressing percentage, relative breast weight | Increased birds’ productivity when applied as 900 mg/kg threonine | Khalid and Al-Hayani, 2017 | |
| Glycine | Applied as a 0.62–1.22% from 7 to 20 d of age | Broiler chickens | Increased plasma-free glycine levels | Increased weight gain and feed conversion | Corzo et al., 2004 | |
| Valine | Applied as a 0.52 kg valine/metric ton feed | Broiler chickens | Supported growth and meat yield | Showed the ability to support good production and could potentially offer a useful diet cost-reduction alternative | Corzo et al., 2011 | |
| Valine and isoleucine | Dietary inclusion | Broiler chickens from d 1 to 21 after hatch. | Optimized the effect of leucine supplementation on mRNA expression of mTOR pathway genes in the pectoralis major muscle | Higher valine and isoleucine levels are recommended | Ospina-Rojas et al., 2020 | |
| Phenylalanine, tyrosine, histidine, and leucine | Dietary inclusion | Cobb-broiler chickens | Digestible phenylalanine, tyrosine, histidine, and digestible leucine meets the requirements for the main production parameters of birds during the starter phase |
Improved feed intake, weight gain, and breast muscle yield | Franco et al., 2016 |
Figure 6.
Impact of amino acids on avian gut health.
Probiotics as Feed Additive
Probiotics have long been considered as an alternative to antibiotics in the poultry sector, promoting a "healthy" gut microbiome (Abd El‐Hack et al., 2021a; Salem et al., 2022a). Probiotics are microbial supplements that beneficially affect intestinal balance by maintaining healthy indigenous microbiota and counteracting pathogens (Rubio, 2018; Alagawany et al., 2021c). The further definition states, "probiotics are mono or mixed cultures of live organisms which, when administrated in adequate amounts, confer a health benefit to the host” (FAO/WHO, 2001; Shehata et al., 2022; Babot et al., 2023). Different and variant bacteria (e.g., Lactobacillus, Bifidobacterium, Enterococcus, Bacillus, Streptococcus, and Lactococcus spp.) and yeast (e.g., Candida and Saccharomyces spp.) have been widely tested and studied as probiotics in organic poultry industry (Gadde et al., 2017).
Some of the characteristics of effective probiotics include the following: the ability to colonize the host's gut; the absence of pathogenicity; the suitability of the probiotic for manufacture and delivery; the ability to adhere to the epithelium; the ability to modulate the immune system; the enhancement of gastrointestinal function; and the ability to promote gut health (Clavijo and Flórez, 2018; Abd El‐Hack et al., 2020; Li et al., 2023). Probiotics' positive benefits can be shown indirectly in improved growth performance. An increase in the population of butyric acid-producing bacteria in the cecum content of chickens about changes in metabolic activities (higher abundance of glucosidases) has recently been reported as a result of dietary supplementation with a Lactobacillus acidophilus strain, isolated from GIT of healthy adult chickens on broilers throughout the entire rearing period (De Cesare et al., 2017). Probiotic supplementation enhanced chicken growth performance, BW, and feed conversion rate (FCR), most likely indirectly by increasing gut health (De Cesare et al., 2017).
Directly fed probiotics have been shown to improve growth performance, and this improvement has been linked to an increase in lactic acid bacteria in the bird gut, which may have a secondary effect on performance by making more digestible carbohydrates and enzyme activities available to the bird (Nayebpor et al., 2007). Similarly, Mookiah et al. (2014) observed improved BW and FCR in adult broiler chickens after feeding multistrain Lactobacillus probiotic, as well as an increase in the cecal population of lactobacilli and bifidobacterial and a decrease in E. coli, which could be attributed to the probiotic growth-promoting effect (Mookiah et al., 2014).
Since many factors can influence the efficacy of beneficial bacteria, it is not surprising that studies evaluating the effects of lactic acid bacteria (various Lactobacillus species) as probiotics (Sato et al., 2009) and the probiotic Clostridium butyricum (Zhang et al., 2016) found no differences in growth performance in young chicks. In terms of activating the gut and systemic immune systems, several probiotics have been shown to activate an immunological response, suggesting that they may indirectly contribute to gut health maintenance (Zhang et al., 2016).
For example, dietary administration of Saccharomyces and Bacillus probiotics to chicks from hatching on has been shown to modulate immunity at the jejunum and ileum levels, with an increase in both inflammatory and anti-inflammatory cytokines and an increase in IgA-producing cells (Rajput et al., 2013). In addition, Rajput et al. (2013) also found evidence of a probiotic capacity in the modulation of gut structure, including an increase in tight junction RNA gene expression and an increase in jejunum villus height and goblet cell number, both of which have been hypothesized to play a role in the increased BW seen in chickens fed probiotics (Rajput et al., 2013).
Aside from the conflicting results on the growth-promoting effects of Bacillus spp. in chickens (Gadde et al., 2017), a previous study by Lee et al. (2010) who reported modulation of small intestinal morphology (increased villus height in jejunum and ileum) by direct-fed Bacillus spp. in young chickens. Lee et al. (2010) demonstrated that reducing acute phase proteins and stimulating particular intraepithelial lymphocyte (IEL) subsets modulated immune-related parameters, thereby contributing to gut defense against pathogens in young chicks with incomplete immunological functioning. A substantial alteration in gene expression was also seen in these IELs, particularly for cytokine transcripts such as IL-2 (engaged in cell-mediated immunity regulation), interferon-gamma (IFN-γ) (regulator of acquired immunity), and other immune system mediators (Lee et al., 2010).
Other probiotics and probiotic mixes have also been proven to influence immunity, particularly natural antibody production. Yurong et al. (2005) showed that orally administering a probiotic mix (including Bacillus, Lactobacillus, and Candida species) to posthatched chicks favored an increase in intestinal IgA as well as an increase in Ig-forming cells in cecal tonsils (Yurong et al., 2005). Furthermore, oral inoculation of Lactobacillus, Bifidobacterium, and Streptococcus strains in neonatal chicks might increase serum IgG and IgM and intestinal IgA natural antibody production against several foreign antigens (Haghighi et al., 2006). Brisbin et al. (2011) reported on the different abilities of Lactobacillus species in inducing a systemic immune response to different antigens, where weekly oral administration of different Lactobacillus strains in chicks showed differences in terms of antibodies increasing or decreasing in response to different antigens (Brisbin et al., 2011).
Peyer's patches, which are found in the chicken intestine and are involved in antigen recognition, can be strengthened by probiotics like those of probiotics, even if the bacteria involved are innocuous because of the stimulation of natural nonspecific antibodies and immune cells (Oladokun and Adewole, 2023). Probiotics enter Peyer's patches epithelium and activate lymphocytes B, which transform into plasma cells: these Ig-forming cells diffuse in the lymphoid system and GALT, enter the blood circulation, and exit in the intestinal lamina propria, where they secrete Ig (Yurong et al., 2005).
Attachment (or adhesion) to intestinal epithelial cells is the first step in the infection process (Baldwin et al., 2018). The other critical probiotic capacity is countering and excluding pathogens from epithelial binding sites, which is accomplished through the production of antimicrobial substances, competition for nutrients, inhibition of pathogen adherence, immune response enhancement, and environmental modification to create a hostile environment for harmful bacteria (e.g., pH lowering) (Gadde et al., 2017; Baldwin et al., 2018).
The probiotic bacteria Lactobacillus and Bifidobacterium, for instance, produce hydrogen peroxide, bacteriocins, and organic acids, all of which inhibit the adherence of pathogens (Clavijo and Flórez, 2018; Rubio, 2018). Additionally, it has been shown that the use of Bacillus licheniformis spores or its direct administration as a dietary microbial probiotic in chickens at varying doses can prevent necrotic enteritis (and then in counteracting Clostridium perfringens) in terms of mortality and small intestinal lesions, in addition to improving growth performance (Knap et al., 2010).
Improvements in villus height, crypt depth, cytokine production, and digestive enzyme activity were observed in another study in which Clostridium butyricum was given as a food supplement to chickens that had been challenged with E. coli K88 (Zhang et al., 2016). Lactobacillus and Bifidobacterium, the most often utilized probiotics, have also been linked to improvements in lactose intolerance, blood cholesterol, and bile salt hydrolysis capacity (Begley et al., 2006). This last consequence permits amino acids to be released from bile salt deconjugation and used by bacteria as nitrogen, carbon, and energy sources; also, bile salt hydrolases enzymes function as a bile-detoxifying enzyme for microbial survival (Begley et al., 2006). Increased deconjugated bile salt levels, on the other hand, may not necessarily be appropriate for the host since deconjugated bile salts are less reabsorbed and may harm gut mucosa (Begley et al., 2006).
In any case, it is vital to remember that probiotic efficiency is greatly dependent on changes in bacterial strains employed and their dietary concentration, as well as time-point and method of delivery (Alagawany et al., 2023). Early probiotic administration can aid in microbiota composition balance even later in life, during growth (Clavijo and Flórez, 2018; Rubio, 2018).
The impact of probiotic administration on poultry performance and production is summarized in Table 5.
Table 5.
Impacts of probiotics, prebiotics, and synbiotics on poultry performance.
| Antibiotic alternatives | Type | Application | Treated birds | Mode of action | Effects | References |
|---|---|---|---|---|---|---|
| Probiotics | Bacillus amyloliquefaciens | Applied as 0, 1, 5, 10, and 20 g probiotics/kg feed | Broiler chickens | Improved the immune response of birds | Improved gut health and growth performance | Ahmed et al., 2014 |
| Saccharomyces cerevisiae | Applied as 0.4, 0.8, 1.2, and 1.6% feed | Layer chickens | Usage of 0.4 or 0.8% Saccharomyces cerevisiae increased the productivity and nutrients utilization via the inhibitory effect of yeast against pathogens | Improved growth performance and improved laying performance | Hassanein and Soliman, 2010 | |
| Streptococcus faecium | Dietary supplementation | Poultry | Showed immunomodulating activity | Improved immunity | Alagawany et al., 2018a | |
| Propionibacterium acidipropionici | Dietary supplementation | Broiler chickens | Improved gut microbiome and improved gut integrity | Showed better development of gut mucosa | Martínez et al., 2016 | |
| Bacillus licheniformis | Dietary supplementation | Broiler chickens | Increased growth performance, carcass characteristics, meat quality, muscular antioxidant capacity, and meat mineral contents | Prevented necrotic enteritis and enhanced growth performance | Cheng et al., 2017 | |
| Lactobacillus salivarius | Dietary supplementation | Broiler chickens | Improved gut development and increased beneficial microbial community | Improved performance and enhanced gut histomorphology. | Olnood et al., 2015 | |
| Bacillus subtilis | Dietary supplementation | Layer chickens | Increased egg quality, hatchability, and sperm quality of roosters | Increased laying performance and helped the immune system and gut health | Mazanko et al., 2018 | |
| Lactobacillus plantarum, Enterococcus faecalis, Propionibacterium freudenreichii ssp. | Dietary supplementation | Broiler chickens | Improved digestion, absorption, gut barrier function, and gut structure | Improved immunity, and birds’ productivity | Smolovskaya et al., 2023 | |
| Bacillus amyloliquefaciens H57 | Dietary supplementation | Broiler chickens | Changed the functional capacity of the cecal microbiota, parallel with vitamin and amino acid synthesis pathways | Increased bird productivity | Bajagai et al., 2023 | |
| Bacteroides caecicola, Bacteroides plebeius, Megasphaera stantonii, Megamonas hypermegale, Megamonas funiformis, Phascolarctobacterium faecium, and Sutterella massiliensis | On first day of life, chicks in the experimental group were orally inoculated with 0.1 mL of gut anaerobes | Broiler chickens | Reduced challenged ciprofloxacin-resistant Escherichia coli colonization in birds’ gut | Provided protection during the essential period of the chicken intestinal microflora development | Papouskova et al., 2023 | |
| Prebiotics | Yeast powder | Applied as 1.5–2.0 g/kg feed | Broiler chickens | Increased ileal protein digestibility and pancreatic enzyme activities. | Improved performance and meat yield | Ahiwe et al., 2020 |
| yeast (Saccharomyces cerevisiae) | Feed | Broiler chickens | Modulated gut microflora | Improved performance | Al-Homidan and Fahmy, 2007 | |
| Saccharomyces cerevisiae | Applied as 4.0 g/kg feed | New Zealand White Rabbits | Increased the total thickness of the mucosa, villus heights, crypt depths, and gland depths | Improved gut health | Seyidoglu and Peker, 2015 | |
| Saccharomyces cerevisiae and Kluyveromyces maxianus | Applied as 1:1 feed | Broiler chickens | Increased the number of Lactobacillus and decreased the number of Escherichia coli in the excreta | Increased body weight gain | Sun and Kim, 2019; Abd El-Hack et al., 2021d | |
| Synbiotics | Galactooligosaccharides) and probiotic Bifidobacterium longum | Oral supplementation | Broiler chickens | Increased the colonization of beneficial bacteria and limited the propagation of pathogens | Increased the count of Bifidobacterium and reduced the count of Campylobacter jejuni | Baffoni et al., 2012 |
| Galactooligosaccharides and a Bifidobacterium lactis-based probiotic) | Oral supplementation | Broiler chickens | Increased the colonization of beneficial bacteria and limited the propagation of pathogens | Increased the count of Bifidobacterium | Jung et al., 2008 | |
| fructooligosaccharides with Enterococcus faecium | Oral supplementation | Broiler chickens | Increased the colonization of beneficial bacteria and limited the propagation of pathogens | Reduced total coliform counts while increased lactic acid-producing bacterial count |
Dibaji et al., 2014 |
Prebiotics as Feed Additive
Dietary fiber is a beneficial component of the diet that is made up of insoluble fiber (cellulose and lignin) and soluble fiber (i.e., NSP and polysaccharides) that are not digested by mammalian enzymes (Rafeeq et al., 2023). Over 20 yr ago, expanding on the "probiotic" concept, the term "prebiotic" was coined to describe soluble fiber carbohydrates with a unique property of stimulating beneficial bacterial species (mostly Lactobacillus and Bifidobacterium spp.), thereby indirectly improving host health (Rafeeq et al., 2023). In recent years, the definition of "prebiotic" has shifted from "nondigestible feed ingredients" to "selectively fermented ingredients" that have an impact on the makeup and function of the gut microbiome (Gibson et al., 2017). In 2008, the Food and Agricultural Organization (FAO) updated the definition of prebiotics, defining them as “nonviable food components that confer a health benefit on the host associated with the modulation of the microbiota” so removing the criterion of selective fermentation (Pineiro et al., 2008). Recently, the International Scientific Association for Probiotics and Prebiotics has proposed a new definition of prebiotics, which is “a substrate that is selectively utilized by host microorganisms conferring a health benefit,” extending the possible utilization of prebiotics not only via fermentation but also in other pathways and amplifying prebiotic effects beyond lactobacilli and bifidobacterial species (Gibson et al., 2017).
Plant-based or microbially synthesized prebiotics are defined as feed ingredients that are resistant to gastric acidity and gastrointestinal digestion and absorption, fermentable by specific intestinal bacteria, a nutrient source for commensal bacteria, and selectively stimulate the growth/activity of beneficial bacteria associated with gut health (Rubio, 2018).
Prebiotics can alter the microbiota of poultry guts to a healthier population, boosting resistance to the beneficial bacterial community. Most commercial prebiotics are low molecular weight compounds that are fermented in the lower intestine, creating SCFA and maintaining gut health (Ferket et al., 2005). The major components of the prebiotic category are oligosaccharides, which can be derived from any hexose monosaccharides, including glucose, fructose, galactose, and mannose (Huyghebaert et al., 2011).
Mannan-oligosaccharides (MOS) are widely well-known among the most commercially available prebiotics (Midilli et al., 2008). They are made from the yeast Saccharomyces cerevisiae cell walls. They are known to have a good impact on gut health, due to the yeast-cell antigen stimulating capabilities of the mannan chain (Shashidhara and Devegowda, 2003). Immune system receptors can recognize MOS and boost the host's immune response (Gadde et al., 2017). Midilli et al. (2008), on the other hand, found no differences in serum IgG concentration in broilers fed dietary MOS versus those fed a basal diet, hypothesizing that prebiotic dosages, animal age, and other environmental determinants could influence it (Midilli et al., 2008). After supplementing broilers with MOS during the entire rearing process, Kim et al. (2011) found no alterations in plasma Igs (IgA and IgG).
Similarly, another study found that while chickens given MOS had higher leukocyte counts, they did not produce more antibodies (Taheri et al., 2014). Furthermore, MOS exhibit receptor capabilities for the fimbriae of E. coli and Salmonella spp., which allows them to block the adherence of these pathogens to the epithelium while simultaneously favoring commensal microorganisms (Huyghebaert et al., 2011). Dietary MOS provided to layers has already shown the ability to modify cecal microbiota by stimulating the growth of obligate anaerobes and decreasing enterobacteria load. In addition, when this hen cecal content was orally inoculated in posthatched chicks, the same effects were also observed (Fernandez et al., 2002).
In support of this, dietary MOS supplementation improved gut health status in broiler chickens by decreasing E. coli and C. perfringens and increasing Lactobacillus species and diversity at an ileal level (Kim et al., 2011), as previously observed by Chee et al. (2010), who also reported higher bacterial diversity in the cecum (Chee et al., 2010). Baurhoo et al. (2007) found similar findings, including an increase in bifidobacterial load in ceca of chickens given MOS, as well as a decrease in E. coli load (Baurhoo et al., 2007). Increases in goblet cell number and acidic and sulfate-acidic goblet cell content (Chee et al., 2010), markers of gut maturation and defense, have been attributed to MOS, which has also been shown to positively affect gut health in terms of intestinal morphology (Baurhoo et al., 2007; Chee et al., 2010).
Some Lactobacillus spp. can upregulate genic post-transcripts of mucins (Chee et al., 2010), suggesting that MOS control of intestinal mucin production occurs through upregulation of mucin-related genes (Brennan et al., 2013). According to a further investigation of MOS molecular mechanisms at the jejunal level, MOS altered the expression of various immune-related genes, including those involved in mucosal immunity, which led to an enhanced immune response (Chee et al., 2010).
MOS also increased the activity of mitochondrial pathways involved in energy production and antioxidant response (Xiao et al., 2012). Different results have been provided in terms of growth performance. MOS appears to improve growth performance in broiler chickens in terms of BW and FCR (Chee et al., 2010; Brennan et al., 2013) or BW and feed consumption (Ashayerizadeh et al., 2009), whereas in others, no beneficial effects on performance traits are reported after MOS administration (Midilli et al., 2008; Kim et al., 2011; Taheri et al., 2014). Furthermore, dosages, type, and other external factors have a key impact in prebiotic effectiveness; moreover, MOS influence on growth response is age dependent and performs better on younger than older birds (Chee et al., 2010).
Fructooligosaccharides (FOS) derived from plants have also been investigated as potential prebiotics in poultry, much like MOS. The effects of dietary FOS supplementation at various doses in chickens were studied, and changes in the microbial population in the small intestine and cecum content were observed, with a significant increase in Bifidobacterium and Lactobacillus spp. counts and a decrease in E. coli counts (Xu et al., 2003). FOS also induced a rise in the number of total anaerobes in the cecum. In addition, increased productivity in terms of growth was noted (Xu et al., 2003). Another study also found that FOS had an influence on the bacterial population in the chicken gut, but this time it favored the growth of beneficial bacteria (Kim et al., 2011). Even though no performance boost was noted, the number of E. coli and C. perfringens was reported to have decreased (Kim et al., 2011). Increased digestive enzyme activity (particularly amylase and protease) and enhanced jejunum and ileum morphology due to FOS-induced higher villus height and lower crypt depth, as well as a higher microvillus height, have been linked to alterations in the gut microbiota (Xu et al., 2003).
Inulin, the longer-chained variant of FOS, is a heterogeneous blend of fructose polymers isolated from chicory roots that is well recognized as a prebiotic (Li et al., 2018), being the only one to date to be granted an European Union (EU) health claim for improving gut function (Gibson et al., 2017). Inulin increases the number of Bifidobacterium and Lactobacillus in chickens, enhancing microbial balance and serving as a substrate for SCFA synthesis (Li et al., 2018).
Besides the oligosaccharides already mentioned, galactooligosaccharides (GOS), galactose polymers found in human milk, have also been studied as a prebiotic option in poultry (Wilson and Whelan, 2017). GOS with transgalactosylation activity of lactose is produced commercially by B-galactosidase enzymes; these enzymes are derived from fungi, yeast, or bacteria and are utilized preferentially by bifidobacteria. Studies have shown that these enzymes have positive effects on health and the immune system (Wilson and Whelan, 2017). In rats with severe acute pancreatitis, it has been found that GOS has a protective role and improves gut barrier function in several ways (Zhong et al., 2009). In fact, Zhong et al. (2009) found that GOS-supplemented enteral nutrition significantly increased the number of colonic bifidobacteria, the level of secretory IgA in intestinal mucus, and the level of occludin RNA, all of which are indicative of an improved gut barrier (Zhong et al., 2009).
Although no change in performance parameters was noted, Jung et al. (2008) observed that GOS had an effect on broiler fecal microbiota, with a preferential increase in abundance of Bifidobacterium species, coupled with increases in total anaerobes and lactobacilli. Similar findings were reported by Baffoni et al. (2012), who acknowledged GOS as a good stimulator of Bifidobacterium longum probiotic in vitro and who demonstrated an increase in bifidobacteria-related genes in fecal samples of young broiler chickens in addition to a decrease in the fecal load of Campylobacter spp. (Baffoni et al., 2012).
Based on these findings, Baffoni et al. (2012) reasoned that GOS's lack of lactase would make it a useful ingredient in chicken feed. Therefore, it is probable that all intestinal bacteria employ GOS, and that the increased bifidobacteria in GOS fed chickens has contributed to the modification of expression of Campylobacter spp., implicated in epithelial adherence to prevent its invasion (Baffoni et al., 2012). Nonetheless, it is possible that GOS's prebiotic effects are dose-dependent, host-specific, and modified by several environmental variables, just like those of the aforementioned prebiotics (Baffoni et al., 2012).
The impact of the application of prebiotics as feed additives on poultry performance and production is summarized in Table 5.
Synbiotics as Feed Additive
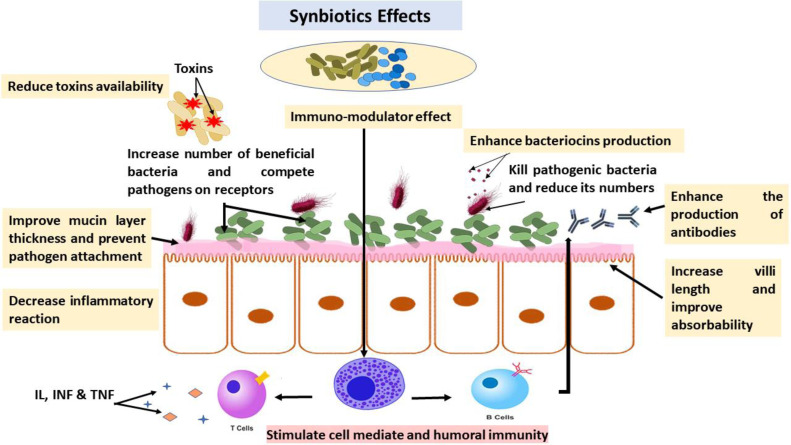
Synbiotics are defined as a mixture of probiotics and prebiotics that have a beneficial effect on the host by increasing the survival and persistence of living microbial dietary supplements in the GIT, by selectively stimulating the growth and by activating the metabolism of one or a limited number of health-promoting bacteria (Patterson and Burkholder, 2003; Goel et al., 2023).
The hypothesis underlying the formulation of this mixture is that the unique substrate (prebiotic) available for fermentation increases the number of beneficial bacteria (probiotic) in the gut and ensures their survival and persistence (Sugiharto, 2016). Some studies found that administering synbiotics formulations to young broiler chickens, which included the prebiotic GOS and the probiotic Bifidobacterium longum, resulted in an increase in the amount of fecal bifidobacteria and a significant reduction in the amount of Campylobacter jejuni in the birds' guts (Baffoni et al., 2012; Such et al., 2023).
Intestinal growth of bifidobacteria was also shown to be improved in broiler chickens by administering a synbiotics (GOS and a Bifidobacterium lactis-based probiotic) (Jung et al., 2008). There were changes in the population of bacteria in the broilers' cecum after they were given a synbiotics containing Enterococcus faecium and FOS for 42 d (during the rearing period; Dibaji et al., 2014). Specifically, there was a decrease in total coliform counts and an increase in lactic acid bacteria, which was positively correlated with the probiotic doses within the symbiotic (Dibaji et al., 2014).
Broilers' growth attributes (BW, FCR, and feed consumption) and carcass trait quality all improved significantly when their meals were supplemented with a symbiotic feed additive (Ashayerizadeh et al., 2009). Symbiotic administration of prebiotic isomalto-oligosaccharides and Lactobacillus multistrain probiotic to adult broiler chickens resulted in enhanced growth performance (BW and FCR) and an increase in cecal volatile and nonvolatile-fatty-acid-concentration compared to probiotic and prebiotic alone (Derakhshan et al., 2023).
However, unlike the sum of probiotic and prebiotic effects, the effects of synbiotics products did not exhibit a 2-fold synergistic impact (Mookiah et al., 2014). On the other hand, not all trials found benefits in growth performance; for example, Jung et al. (2008) found that symbiotic (GOS and Bifidobacterium lactis) inclusion in chicken feed had no effect on chicken growth features.
Table 5 and Figure 7 summarize the effect of synbiotics as feed additives on poultry performance and production.
Figure 7.
Impact of the application of synbiotic on avian gut health.
Herbal Extracts as Feed Additive
In recent years, the use of herbal extracts to boost the conversion rate in birds has become increasingly common because some of these extracts have been shown to have an antimicrobial impact (Swelum et al., 2021; El-Shall et al., 2022). Onion, garlic, clove, cinnamon, oregano, chicory, cumin, sumac, amla, anise, mint, citrus peels, ginger, coriander, parsley, ashwagandha, leek, and aloe vera are among the most studied herbs for use in poultry feed. Research has shown that their extracts are powerful stimulators of the immunological and digestive tracts of birds and have had extremely positive impacts on poultry production (Abo Ghanima et al., 2023; Rafeeq et al., 2023).
According to Adjei-Mensah et al. (2023), garlic is an effective antibacterial agent, and including it in a chicken's diet increases the animal's lifespan and resistance to disease. Withania somnifera has remarkable therapeutic activities due to its unique chemical, pharmacological, biological, and physiological properties, as noted by Salem et al. (2022b). Incorporating W. somnifera, also known as ashwagandha or winter cherry, into the diet of chickens increased feed intake, BW gain, feed efficiency, and FCR, as well as livability, disease resistance, and feed conversion ratio. W. somnifera is widely available all over the world and is considered cardioprotective, antistress, antioxidant, immunostimulant, antiaging, antidiabetic, neuroprotective, antimicrobial, anticancer, and liver protective herb.
The addition of 200 to 400 mg/kg of chamomile oils to broiler feed, enhanced the birds' ultimate weight and decreased the count of pathogenic microorganisms in the birds' stomach, as observed by Jakubcova et al. (2014). Khan et al. (2022) showed that Aloe vera boosts the bird's resistance to microbial infections, improving productivity and immune response. Instant turmeric has been used as a feed additive for a long time due to its beneficial health impact (El-Saadony et al., 2023b). Both El-Sabrout et al. (2023) and Sureshbabu et al. (2023) demonstrated that supplementing the diets of broiler chickens with turmeric improved the feed's digestibility and raised the birds' overall output.
CONCLUSIONS
The health of an avian digestive tract is dependent on the microorganisms that live in the gut, the immune system, and nutrition. Nevertheless, the most significant dangers to gut health come in the form of pathogens, which can include viruses, bacteria, parasites, and fungi, as well as metabolic problems. The current review emphasized the importance of naturally safe and sustainable products, such as exogenous enzymes, organic acids, photogenic products, amino acids, prebiotics, probiotics, synbiotics, and herbal extracts, in sustaining gut health and function.
It is recommended that further research be conducted to clarify the relationship between natural antibiotic alternatives and their nanoformulations and the gut microbiome, immunity, and gut health. Additionally, it is recommended that such research be conducted to explain the appropriate doses, properties, and methods of application of such ingredients that improve the gut health and productivity of birds through large-scale toxicity testing evaluation.
ACKNOWLEDGMENTS
This project was funded by Abu Dhabi Education and Knowledge (Grant # 21S105), UAE to Khaled A. El-Tarabily. The authors also extend their appreciation to the Deanship of Scientific Research at King Khalid University for supporting this work under the grant number (R.G.P.2/121/44).
Author Contributions: All authors were equal contributors in writing this review article. All of them read and approved the final manuscript.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abdelfatah S.H., Salem H.M., Zoelfakar S.A., Mohamed F.F. Effect of adding different levels of glycine amino acid on performance, growth genes expression and immune status of broiler chickens. J. Adv. Vet. Res. 2023;13:76–82. [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Alqhtani A.H., Swelum A.A., El-Saadony M.T., Salem H.M., Babalghith A.O., Taha A.E., Ahmed O., Abdo M., El-Tarabily K.A. Pharmacological, nutritional and antimicrobial uses of Moringa oleifera Lam. leaves in poultry nutrition: an updated knowledge. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Alqhtani A.H., Swelum A.A., Salem H.M., Elbestawy A.R., Noreldin A.E., Babalghith A.O., Khafaga A.F., Hassan M.I., El-Tarabily K.A. The relationship among avian influenza, gut microbiota and chicken immunity: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., El-Tahan A.M., Khafaga A.F., Taha A.E., AbuQamar S.F., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives - a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Gado A.R., Nader M.M., Saad A.M., El-Tahan A.M., Taha A.E., Salem H.M., El-Tarabily K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Ghanima M.M.A., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-Kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y.A., Taha A.E., Mesalam N.M., Abdel-Moneim A.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;7:1668–1677. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E.S., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Salem H.M., Khafaga A.F., Soliman S.M., El-Saadony M.T. Impacts of polyphenols on laying hens' productivity and egg quality: a review. J. Anim. Physiol. Anim. Nutr. 2023;107:928–947. doi: 10.1111/jpn.13758. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif M.A., El-Far A.H., Elbestawy A.R., Ghanem R., Mousa S.A., Abd El-Hamid H.S. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLoS One. 2017;12:1–17. doi: 10.1371/journal.pone.0185153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim A.M.E., Shehata A.M., Selim D.A., El-Saadony M.T., Mesalam N.M., Saleh A.A. Spirulina platensis and biosynthesized selenium nanoparticles improve performance, antioxidant status, humoral immunity and dietary and ileal microbial populations ofheat-stressed broilers. J. Therm. Biol. 2022;104 doi: 10.1016/j.jtherbio.2022.103195. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-Shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambienttemperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abo Ghanima M.M., Aljahdali N., Abuljadayel D.A., Shafi M.E., Qadhi A., Abd El-Hack M.E., Mohamed L.A. Effects of dietary supplementation of amla, chicory and leek extracts on growth performance, immunity and blood biochemical parameters of broilers. Ital. J. Anim. Sci. 2023;22:24–34. [Google Scholar]
- Abou-Kassem D.E., El-Abasy M.M., Al-Harbi M.S., Abol-Ela S., Salem H.M., El-Tahan A.M., El-Saadony M.T., Abd El-Hack M.E., Ashour E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2022;29:1683–1693. doi: 10.1016/j.sjbs.2021.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Adil S., Banday T., Bhat G.A., Mir M.S., Rehman M. Effect of dietary supplementation of organic acids on intestinal histomorphology and serum biochemistry of broiler chicken. Vet. Med. Int. 2010;2010 doi: 10.4061/2010/479485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjei-Mensah B., Koranteng A.A.A., Hamidu J.A., Tona K. Antibacterial activities of garlic (Allium sativum) in broiler and laying hens production. Worlds Poult. Sci. J. 2023;79:155–176. [Google Scholar]
- Ahiwe E.U., Abdallh M.E., Chang'a E.P., Omede A.A., Al-Qahtani M., Gausi H., Graham H., Iji P.A. Influence of dietary supplementation of autolyzed whole yeast and yeast cell wall products on broiler chickens. Asian-Austral. J. Anim. Sci. 2020;33:579–587. doi: 10.5713/ajas.19.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.T., Islam M.M., Mun H.S., Sim H.J., Kim Y.J., Yang C.J. Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult. Sci. 2014;93:1963–1971. doi: 10.3382/ps.2013-03718. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Abd El-Hack M.E., Farag M.R., Sachan S., Karthik K., Dhama K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018;25:10611–10618. doi: 10.1007/s11356-018-1687-x. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Bilal R.M., Elnesr S.S., Elwan H.A., Farag M.R., Dhama K., Naiel M.A. Yeast in layer diets: its effect on production, health, egg composition and economics. Worlds Poult. Sci. J. 2023;79:135–153. [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R. The role of exogenous enzymes in promoting growth and improving nutrient digestibility in poultry. Iran. J. Vet. Res. 2018;19:157–164. [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., El-Naggar K., Taha A.E., Khafaga A.F., Madkour M., Salem H.M., El-Tahan A., El-Saadony M.T., Abd El-Hack M. Betaine and related compounds: chemistry, metabolism, and role in mitigating heat stress in poultry. J. Therm. Biol. 2021;104 doi: 10.1016/j.jtherbio.2021.103168. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., El-Rayes T.K., Madkour M., Loschi A.R., Di Cerbo A., Reda F.M. Evaluation of dried tomato pomace as a non-conventional feed: its effect on growth, nutrients digestibility, digestive enzyme, blood chemistry and intestinal microbiota of growing quails. Food Energy Secur. 2022;11:e373. [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed. Sci. Technol. 2021;276 [Google Scholar]
- Alagawany M., Qattan S.Y., Attia Y.A., El-Saadony M.T., Elnesr S.S., Mahmoud M.A., Reda F.M. Use of chemical nano-selenium as an antibacterial and antifungal agent in quail diets and its effect on growth, carcasses, antioxidant, immunity and caecal microbes. Animals. 2021;11:3027. doi: 10.3390/ani11113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Homidan A., Fahmy M.O. The effect of dried yeast (Saccharomyces cerevisiae) supplementation on growth performance, carcass chemical analysis, immunity, ileum villi heights and bacterial counts of broiler chickens. Egypt. Poult. Sci. J. 2007;27:613–623. [Google Scholar]
- Al-Mutairi H.M.S., Hussein E.O.S., Jar El Nabi A., Swelum A.A., Abd El-Hack M.E., Taha A.E., Al-Mufarrej S.I. Does the consumption of acidified drinking water affect growth performance and lymphoid organs of broilers? Sustainability. 2020;12:3093. [Google Scholar]
- Amerah A.M., Romero L.F., Awati A., Ravindran V. Effect of exogenous xylanase, amylase, and protease as single or combined activities on nutrient digestibility and growth performance of broilers fed corn/soy diets. Poult. Sci. 2017;96:807–816. doi: 10.3382/ps/pew297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antongiovanni M., Buccioni A., Petacchi F., Leeson S., Minieri S., Martini A., Cecchi R. Butyric acid glycerides in the diet of broiler chickens: effects on gut histology and carcass composition. Ital. J. Anim. Sci. 2007;6:19–25. [Google Scholar]
- Arif M., Baty R.S., Althubaiti E.H., Ijaz M.T., Fayyaz M., Shafi M.E., Albaqami N.M., Alagawany M., Abd El-Hack M.E., Taha A.E., Salem H.M., El-Tahan A.M., Elnesr S.S. The impact of betaine supplementation in quail diet on growth performance, blood chemistry, and carcass traits. Saudi J. Biol. Sci. 2021;29:1604–1610. doi: 10.1016/j.sjbs.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif M., Ur Rehman A., Naseer K., Abdel-Hafez S.H., Alminderej F.M., El-Saadony M.T., Alagawany M. Effect of Aloe vera and clove powder supplementation on growth performance, carcass and blood chemistry of Japanese quails. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashayerizadeh A., Dabiri N., Ashayerizadeh O., Mirzadeh K.H., Roshanfekr H., Mamooee M. Effect of dietary antibiotic, probiotic and prebiotic as growth promoterson growth performance, carcass characteristics and hematological indices of broiler chickens. Pak. J. Biol. Sci. 2009;12:52–57. doi: 10.3923/pjbs.2009.52.57. [DOI] [PubMed] [Google Scholar]
- Ashour E.A., El-Hack M.E.A., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Ashour E.A., Farsi R.M., Alaidaroos B.A., Abdel-Moneim A.M.E., El-Saadony M.T., Osman A.O., Abd El-Hack M.E. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcass traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021;20:1357–1372. [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2022;29:1644–1652. doi: 10.1016/j.sjbs.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad G.A., Danial E.N., Kassem S.S., Abdel kader M.M., Hanafi E.M., El-Hawary Z., Hegazy E.M., Helal M.I. A novel phytase enzyme for poultry feed. World Appl. Sci. J. 2013;26:194–199. [Google Scholar]
- Babot J.D., Argañaraz-Martínez E., Apella M.C., Perez Chaia A. Microencapsulation of probiotics with soy protein isolate and alginate for the poultry industry. Food Bioprocess Technol. 2023;16:1478–1487. doi: 10.1007/s11947-023-03007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffoni L., Gaggìa F., Di Gioia D., Santini C., Mogna L., Biavati B. A bifidobacterium-based synbiotic product to reduce the transmission of C. jejuni along the poultry food chain. Int. J. Food Microbiol. 2012;157:156–161. doi: 10.1016/j.ijfoodmicro.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Bajagai Y.S., Yeoh Y.K., Li X., Zhang D., Dennis P.G., Ouwerkerk D., Bryden W.L. Enhanced meat chicken productivity in response to the probiotic Bacillus amyloliquefaciens H57 is associated with the enrichment of microbial amino acid and vitamin biosynthesis pathways. J. Appl. Microbiol. 2023;134:lxad085. doi: 10.1093/jambio/lxad085. [DOI] [PubMed] [Google Scholar]
- Baldwin S., Hughes R.J., Van T.T.H., Moore R.J., Stanley D. At hatch administration of probiotic to chickens can introduce beneficial changes in gut microbiota. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banday M.T., Adil S., Sheikh I.U., Hamadani H., Qadri F.I., Sahfi M.E., Sait H.S., Abd El-Mageed T.A., Salem H.M., Taha A.E., El-Saadony M.T. The use of silkworm pupae (Bombyx mori) meal as an alternative protein source for poultry. Worlds Poult. Sci. J. 2023;79:119–134. [Google Scholar]
- Bangar S.P., Suri S., Trif M., Ozogul F. Organic acids production from lactic acid bacteria: a preservation approach. Food Biosci. 2022;46 [Google Scholar]
- Baurhoo B., Phillip L., Ruiz-Feria C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007;86:1070–1078. doi: 10.1093/ps/86.6.1070. [DOI] [PubMed] [Google Scholar]
- Begley M., Hill C., Gahan C.G.M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialkowski S., Toschi A., Yu L.E., Schlitzkus L., Mann P., Grilli E., Li Y. Effects of microencapsulated blend of organic acids and botanicals on growth performance, intestinal barrier function, inflammatory cytokines, and endocannabinoid system gene expression in broiler chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda-Molina D., Seifert J., Camarinha-Silva A. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput. Struct. Biotechnol. J. 2018;16:131–139. doi: 10.1016/j.csbj.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Rochell S.J., Applegate T.J. Threonine, arginine, and glutamine: influences on intestinal physiology, immunology, and microbiology in broilers. Poult. Sci. 2018;97:937–945. doi: 10.3382/ps/pex394. [DOI] [PubMed] [Google Scholar]
- Brennan K.M., Graugnard D.E., Xiao R., Spry M.L., Pierce J.L., Lumpkins B., Mathis G.F. Comparison of gene expression profiles of the jejunum of broilers supplemented with a yeast cell wall-derived mannan oligosaccharide versus bacitractin methylene disalicylate. Br. Poult. Sci. 2013;54:238–246. doi: 10.1080/00071668.2013.775404. [DOI] [PubMed] [Google Scholar]
- Brisbin J.T., Gong J., Orouji S., Esufali J., Mallick A.I., Parvizi P., Shewen P.E., Sharif S. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccine Immunol. 2011;18:1447–1455. doi: 10.1128/CVI.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardeal P.C., Caldas E.O., Lara L.J.C., Rocha J.S.R., Baiao N.C., Vaz D.P., Da Silva Martins N.R. In ovo feeding and its effects on performance of newly hatched chicks. Worlds Poult. Sci. J. 2015;71:655–662. [Google Scholar]
- Casteleyn C., Doom M., Lambrechts E., Van Den Broeck W., Simoens P., Cornillie P. Locations of gut-associated lymphoid tissue in the 3-month-old chicken: a review. Avian Pathol. 2010;39:143–150. doi: 10.1080/03079451003786105. [DOI] [PubMed] [Google Scholar]
- Chee S.H., Iji P., Choct M., Mikkelsen L.L., Kocher A. Characterization and response of intestinal microflora and mucins to manno-oligosaccharide and antibiotic supplementation in broiler chickens. Br. Poult. Sci. 2010;51:368–380. doi: 10.1080/00071668.2010.503477. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen Y., Li X., Yang W., Wen C., Kang Y., Wang A., Zhou Y. Effects of synbiotic supplementation on growth performance, carcass characteristics, meat quality and muscular antioxidant capacity and mineral contents in broilers: effects of synbiotic supplementation. J. Sci. Food Agric. 2017;97:3699–3705. doi: 10.1002/jsfa.8230. [DOI] [PubMed] [Google Scholar]
- Christensen V.L. Development during the first seven days post-hatching. Avian Biol. Res. 2009;2:27–33. [Google Scholar]
- Christine J.G., Duncan C.J.G., Pasco D.S., Pugh N., Ross S.A. Isolation of a galactomannan that enhances macrophage activation from the edible fungus Morchella esculenta. J. Agric. Food Chem. 2002;50:5683–5685. doi: 10.1021/jf020267c. [DOI] [PubMed] [Google Scholar]
- Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo A., Dozier W.A., Mejia L., Zumwalt C.D., Kidd M.T., Tillman P.B. Nutritional feasibility of l-valine inclusion in commercial broiler diets. J. Appl. Poult. Res. 2011;20:284–290. [Google Scholar]
- Corzo A., Kidd M.T., Burnham D.J., Kerr B.J. Dietary glycine needs of broiler chicks. Poult. Sci. 2004;83:1382–1384. doi: 10.1093/ps/83.8.1382. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Vieira S.L., Stefanello C. Exogenous microbial amylase in the diets of poultry: what do we know? J. Appl. Poult. Res. 2019;28:556–565. [Google Scholar]
- de Carvalho N.M., Oliveira D.L., Saleh M.A.D., Pintado M.E., Madureira A.R. Importance of gastrointestinal in vitro models for the poultry industry and feed formulations. Anim. Feed Sci. Technol. 2021;271 [Google Scholar]
- De Cesare A., Sirri F., Manfreda G., Moniaci P., Giardini A., Zampiga M., Meluzzi A. Effect of dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) on caecum microbioma and productive performance in broiler chickens. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan M., Ghasemian S.O., Gholami-Ahangaran M. The effects of probiotic and phytase on growth performance, biochemical parameters and antioxidant capacity in broiler chickens. Vet. Med. Sci. 2023;9:860–866. doi: 10.1002/vms3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dibaji S.M., Seidavi A., Asadpour L., da Silva F.M. Effect of a synbiotic on the intestinal microflora of chickens. J. Appl. Poult. Res. 2014;23:1–6. [Google Scholar]
- Dieumou F.E., Teguia A., Kuiate J.R., Tamokou J.D., Fonge N.B., Dongmo M.C. Effects of ginger (Zingiber officinale) and garlic (Allium sativum) essential oils on growth performance and gut microbial population of broiler chickens. Livest. Res. Rural Dev. 2009;21:131. [Google Scholar]
- Dittoe D.K., Ricke S.C., Kiess A.S. In: Ricke S.C., editor. Burleigh Dodd Publishing; Cambridge, UK: 2020. Commercial poultry production and gut function − historical perspective; pp. 3–30. [Google Scholar]
- Ducatelle R., Goossens E., De Meyer F., Eeckhaut V., Antonissen G., Haesebrouck F., Van Immerseel F. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet. Res. 2018;49:43. doi: 10.1186/s13567-018-0538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeid T.A., Al-Homidan I.H. Organic acids and their potential role for modulating the gastrointestinal tract, antioxidative status, immune response, and performance in poultry. Worlds Poult. Sci. J. 2022;78:83–101. [Google Scholar]
- Elbaz A.M., El-Sheikh S.E., Abdel‑Maksoud A. Growth performance, nutrient digestibility, antioxidant state, ileal histomorphometry, and cecal ecology of broilers fed on fermented canola meal with and without exogenous enzymes. Trop Anim. Health Prod. 2023;55:46. doi: 10.1007/s11250-023-03476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Jeni R., Dittoe D.K., Olson E.G., Lourenco J., Corcionivoschi N., Ricke S.C., Callaway T.R. Probiotics and potential applications for alternative poultry production systems. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Alqhtani A.H., Swelum A.A., Salem H.M., Elbestawy A.R., Noreldin A.E., Babalghith A.O., Khafaga A.F., Hassan M.I., El-Tarabily K.A. The relationship among avian influenza, gut microbiota and chicken immunity: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Salem H.M., El-Tahan A.M., Abd El-Mageed T.A., Soliman S.M., Khafaga A.F., Swelum A.A., Ahmed A.E., Alshammari F.A., Abd El-Hack M.E. The control of poultry salmonellosis using organic agents: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Yang T., Korma S.A., Sitohy M., El-Mageed T.A., Selim S., Al Jaouni S.K., Salem H.M., Mahmmod Y., Soliman S.M., Mo'men S.A.A., Mosa W.F.A., El-Wafai N.A., Abou-Aly H.E., Sitohy B., Abd El-Hack M.E., El-Tarabily K.A., Saad A.M. Impacts of turmeric and its principal bioactive curcumin on human health: pharmaceutical, medicinal, and food applications: a comprehensive review. Front. Nutr. 2023;9 doi: 10.3389/fnut.2022.1040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehis N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2023;39:2138–2160. [Google Scholar]
- El-Sabrout K., Khalifah A., Mishra B. Application of botanical products as nutraceutical feed additives for improving poultry health and production. Vet. World. 2023;16:369–379. doi: 10.14202/vetworld.2023.369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Heba M.S., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO . Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk With Live Lactic Acid Bacteria. FAO/WHO; Cordoba, Argentina: 2001. pp. 1–34. [Google Scholar]
- Ferket P.R., Santos A.A., Oviedo-Rondon E.O. Dietary factors that affect gut health and pathogen colonization. Sheraton Imperial Hotel, RTP; NC, USA: 2005. pp. 1–22. [Google Scholar]
- Fernandez F., Hinton M., van Gils B. Dietary mannan-oligosaccharides and their effect on chicken caecal microflora in relation to Salmonella Enteritidis colonization. Avian Pathol. 2002;31:49–58. doi: 10.1080/03079450120106000. [DOI] [PubMed] [Google Scholar]
- Franco S.M., Tavernari F.C., Maia R.C., Barros V.R.S.M., Albino L.F.T., Rostagno H.S., Lelis G.R., Calderano A.A., Dilger R.N. Estimation of optimal ratios of digestible phenylalanine + tyrosine, histidine, and leucine to digestible lysine for performance and breast yield in broilers. Poult. Sci. 2016;96:829–837. doi: 10.3382/ps/pew305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gama N.M.S.Q., Olivera M.B.C., Santin E., Berchieri J. Supplementation with organic acids in diets of laying hens. Ciencia Rur. Santa Maria. 2000;30:499–502. [Google Scholar]
- Geyra A., Uni Z., Sklan D. Enterocyte dynamics and mucosal development in the posthatch chick. Poult. Sci. 2001;80:776–782. doi: 10.1093/ps/80.6.776. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., Verbeke K., Reid G. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Goel A., Ncho C.M., Gupta V., Choi Y.H. Embryonic modulation through thermal manipulation and in ovo feeding to develop heat tolerance in chickens. Anim. Nutr. 2023;13:150–159. doi: 10.1016/j.aninu.2023.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofur M.R. Noor Publications; 2020. Pages 192 in Textbook of Avian Anatomy. [Google Scholar]
- Gottardo E.T., Prokoski K., Horn D., Viott A.D., Santos T.C., Fernandes J.I.M. Regeneration of the intestinal mucosa in Eimeria and E. coli challenged broilers supplemented with amino acids. Poult. Sci. 2016;95:1056–1065. doi: 10.3382/ps/pev356. [DOI] [PubMed] [Google Scholar]
- Gracia M.I., Araníbar M.J.R., Lázaro P., Medel G.G. Mateos alpha-amylase supplementation of broiler diets based on corn. Poult. Sci. 2003;82:436–442. doi: 10.1093/ps/82.3.436. [DOI] [PubMed] [Google Scholar]
- Grond K., Sandercock B.K., Jumpponen A., Zeglin L.H. The avian gut microbiota: community, physiology and function in wild birds. J. Avian Biol. 2018;49:e01788. [Google Scholar]
- Habibi R., Sadeghi G., Karimi A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br. Poult. Sci. 2014;55:228–237. doi: 10.1080/00071668.2014.887830. [DOI] [PubMed] [Google Scholar]
- Haghighi H.R., Gong J., Gyles C.L., Hayes M.A., Zhou H., Sanei B., Chambers J.R., Sharif S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006;13:975–980. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Chen B., Dong Y., Miao Z., Su Y., Liu C., Li J. Evaluation of liquid organic acids on the performance, chyme pH, nutrient utilization, and gut microbiota in broilers under high stocking density. Animals. 2023;13:257. doi: 10.3390/ani13020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H.M.A., Mohamed M.A., Youssef A.W., Hassan E.R. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal microflora of broilers. Asian-Austral. J. Anim. Sci. 2010;23:1348–1353. [Google Scholar]
- Hassanein S., Soliman N. Effect of probiotic (Saccharomyces cerevisiae) adding to diets on intestinal microflora and performance of Hy-line layers hens. J. Am. Sci. 2010;6:159–169. [Google Scholar]
- Hossain M.M., Asaduzzaman M., Asad L., Akter M., Rahman A.N.M.I. Use of black cumin in layer diet as cholesterol lowering agents in egg yolk. Int. J. Anim. Res. 2016;1:61–68. [Google Scholar]
- Hu X., Guo Y. Corticosterone administration alters small intestinal morphology and function of broiler chickens. Asian-Australas. J. Anim. Sci. 2008;21:1773–1778. [Google Scholar]
- Huyghebaert G., Ducatelle R., van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Jakubcova Z., Zeman L., Mares P., Mlcek J., Jurikova T., Dostalova L., Mrazkova E., Mrkvicova E., Balla S., Sochor J. Effect of chamomile supplements to feeding doses on antimicrobial parameters in poultry. Potrav. Slovak. J. Food Sci. 2014;8:228–232. [Google Scholar]
- Jamroz D., Wertelecki T., Wiliczkiewicz A., Orda J., Skorupińska J. Dynamics of yolk sac resorption and post-hatching development of the gastrointestinal tract in chickens, ducks and geese. J. Anim. Physiol. Anim. Nutr. 2004;88:239–250. doi: 10.1111/j.1439-0396.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- Józefiak D., Rutkowski A., Martin S.A. Carbohydrate fermentation in the avian ceca: a review. Anim. Feed Sci. Technol. 2011;113:1–15. [Google Scholar]
- Jung S.J., Houde R., Baurhoo B., Zhao X., Lee B.H. Effects of galacto-oligosaccharides and a Bifidobacteria lactis-based probiotic strain on the growth performance and fecal microflora of broiler chickens. Poult. Sci. 2008;87:1694–1699. doi: 10.3382/ps.2007-00489. [DOI] [PubMed] [Google Scholar]
- Kadim I.T., Al-Marzooqi W., Mahgoub O., Al-Jabri A., Al-Waheebi S.K. Effect of acetic acid supplementation on egg quality characteristics of commercial laying hens during hot season. Int. J. Poult. Sci. 2008;7:1015–1021. [Google Scholar]
- Kamboh A.A., Hang S.Q., Bakhetgul M., Zhu W.-Y. Effects of genistein and hesperidin on biomarkers of heat stress in broilers under persistent summer stress. Poult. Sci. 2013;92:2411–2418. doi: 10.3382/ps.2012-02960. [DOI] [PubMed] [Google Scholar]
- Khalid W., Al-Hayani A. Effect of threonine supplementation on broiler chicken productivity traits. Int. J. Poult. Sci. 2017;16:160–168. [Google Scholar]
- Khan R.U., Naz S., De Marzo D., Dimuccio M.M., Bozzo G., V.Tufarelli C.Losacco, Ragni M. Aloe vera: a sustainable green alternative to exclude antibiotics in modern poultry production. Antibiotics. 2022;12:44. doi: 10.3390/antibiotics12010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.B., Seo Y.M., Kim C.H., Paik I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011;90:75–82. doi: 10.3382/ps.2010-00732. [DOI] [PubMed] [Google Scholar]
- Klasing K.C. Avian gastrointestinal anatomy and physiology. Semin. Avian Exot. Pet. Med. 1999;8:42–50. [Google Scholar]
- Knap I., Lund B., Kehlet A.B., Hofacre C., Mathis G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 2010;54:931–935. doi: 10.1637/9106-101509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Kohl K.D. Diversity and function of the avian gut microbiota. J. Comp. Physiol. B. 2012;182:591–602. doi: 10.1007/s00360-012-0645-z. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lee S.H., Lillehoj H.S., Li G.X., Jang S.I., Babu U.S., Park M.S., Kim D.K., Lillehoj E.P., Neumann A.P., Rehberger T.G., Siragusa G.R. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 2010;89:203–216. doi: 10.3382/ps.2009-00418. [DOI] [PubMed] [Google Scholar]
- Leeson S., Namkung H., Antongiovanni M., Lee E.H. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005;84:1418–1422. doi: 10.1093/ps/84.9.1418. [DOI] [PubMed] [Google Scholar]
- Li B., Leblois J., Taminiau B., Schroyen M., Beckers Y., Bindelle J., Everaert N. The effect of inulin and wheat bran on intestinal health and microbiota in the early life of broiler chickens. Poult. Sci. 2018;97:3156–3165. doi: 10.3382/ps/pey195. [DOI] [PubMed] [Google Scholar]
- Li C., Wang S., Chen S., Wang X., Deng X., Liu G., Chang W., Beckers Y., Cai H. Screening and characterization of Pediococcus acidilactici LC-9-1 toward selection as a potential probiotic for poultry with antibacterial and antioxidative properties. Antioxidants. 2023;12:215. doi: 10.3390/antiox12020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilburn M.S., Loeffler S. Early intestinal growth and development in poultry. Poult. Sci. 2015;94:1569–1576. doi: 10.3382/ps/pev104. [DOI] [PubMed] [Google Scholar]
- Lin J., Hunkapiller A.A., Layton A.C., Chang Y-J., Robbins K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog. Dis. 2013;10:331–337. doi: 10.1089/fpd.2012.1348. [DOI] [PubMed] [Google Scholar]
- Liu L.L., He J.H., Xie H.B., Yang Y.S., Li J.C., Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA ex-pression in response to heat stress in black-boned chickens. Poult. Sci. 2014;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Liu H.N., Liu Y., Hu L.L., Suo Y.L., Zhang L., Jin F., Feng X.A., Teng N., Li Y. Effects of dietary supplementation of quercetin on performance, egg quality. Poult. Sci. 2014;93:347–353. doi: 10.3382/ps.2013-03225. [DOI] [PubMed] [Google Scholar]
- Llamas-Moya S., Girdler C.P., Shalash S.M.M., Atta A.M., Gharib H.B., Morsy E.A., Salim H., Awaad M.H.H., Elmenawey M. Effect of a multicarbohydrase containing a-galactosidase enzyme on the performance, carcass yield, and humoral immunity of broilers fed corn–soybean meal-based diets of varying energy density. J. Appl. Poult. Res. 2019;29:142–151. [Google Scholar]
- Luo J., Song J., Liu L., Xue B., Tian G., Yang Y. Effect of epi-gallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018;97:599–606. doi: 10.3382/ps/pex353. [DOI] [PubMed] [Google Scholar]
- Martínez E.A., Babot J.D., Lorenzo-Pisarello M.J., Apella M.C., Chaia A.P. Feed supplementation with avian Propionibacterium acidipropionici contributes to mucosa development in early stages of rearing broiler chickens. Benef. Microbes. 2016;7:687–698. doi: 10.3920/BM2016.0077. [DOI] [PubMed] [Google Scholar]
- Mazanko M.S., Gorlov I.F., Prazdnova E.V., Makarenko M.S., Usatov A.V., Bren A.B., Chistyakov V.A., Tutelyan A.V., Komarova Z.B., Mosolova N.I., Pilipenko D.N., Krotova O.E., Struk A.N., Lin A., Chikindas M.L. Bacillus probiotic supplementations improve laying performance, egg quality, hatching of laying hens, and sperm quality of roosters. Probiotics Antimicrob. Prot. 2018;10:367–373. doi: 10.1007/s12602-017-9369-4. [DOI] [PubMed] [Google Scholar]
- Mehri M., Adibmoradi M., Samie A., Shivazad M. Effects of beta-mannanase on broiler performance, gut morphology, and immune system. Afr. J. Biotechnol. 2010;9:6221–6228. [Google Scholar]
- Midilli M., Alp M., Kocabaǧli N., Muǧlali Ö.H., Turan N., Yilmaz H., Çakir S. Effects of dietary probiotic and prebiotic supplementation on growth performance and serum IgG concentration of broilers. S. Afr. J. Anim. Sci. 2008;38:21–27. [Google Scholar]
- Mnisi C.M., Mlambo V., Gila A., Matabane A.N., Mthiyane D., Kumanda C., Manyeula F., Gajana C.S. Antioxidant and antimicrobial properties of selected phytogenics for sustainable poultry production. Appl. Sci. 2023;13:99. [Google Scholar]
- Mookiah S., Sieo C.C., Ramasamy K., Abdullah N., Ho Y.W. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 2014;94:341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- Munyaka P.M., Nandha N.K., Kiarie E., Nyachoti C.M., Khafipour E. Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poult. Sci. 2016;95:528–540. doi: 10.3382/ps/pev333. [DOI] [PubMed] [Google Scholar]
- Nayebpor M., Farhomand P., Hashemi A. Effects of different levels of direct fed microbial (primalac) on growth performance and humoral immune response in broiler chickens. J. Anim. Vet. Adv. 2007;6:1308–1313. [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Oladokun S., Adewole D. The effect of Bacillus subtilis and its delivery route on hatch and growth performance, blood biochemistry, immune status, gut morphology, and microbiota of broiler chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L.S., Balbino E.M., Silva T.N.S., Ily L., da Rocha T.C., Strada E.S.O., Pinheiro A.M., de Brito J.Á.G. Use of emulsifier and lipase in feeds for broiler chickens. Semin. Ciências Agrárias Londrina. 2019;40:3181–3196. [Google Scholar]
- Olnood C.G., Beski S.S.M., Choct M., Iji P.A. Novel probiotics: their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim. Nutr. 2015;1:184–191. doi: 10.1016/j.aninu.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Pozza P.C., Rodrigueiro R.J.B., Gasparino E., Khatlab A.S., Murakami A.E. High leucine levels affecting valine and isoleucine recommendations in low-protein diets for broiler chickens. Poult. Sci. 2020;99:5946–5959. doi: 10.1016/j.psj.2020.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenboll K.M., Pontoppidan K., Fru-Nji F. Use of a protease in poultry feed offers promising environmental benefits. Int. J. Poult. Sci. 2011;10:842–848. [Google Scholar]
- Panda A.K., Rama Rao S.V., Raju M.V.L.N., Shyam G.S. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian-Austral. J. Anim. Sci. 2009;22:1026–1031. [Google Scholar]
- Papouskova A., Rychlik I., Harustiakova D., Cizek A. A mixture of Bacteroides spp. and other probiotic intestinal anaerobes reduces colonization by pathogenic E. coli strain O78: H4-ST117 in newly hatched chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.W., Rhee A.R., Um J.S., Paik I.K. Effect of dietary available phosphorus and organic acids on the performance and egg quality of laying hens. J. Appl. Poult. Res. 2009;18:598–604. [Google Scholar]
- Patterson J., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Pineiro M., Asp N-G., Reid G., Macfarlane S., Morelli L., Brunser J.O., Tuohy K. FAO technical meeting on prebiotics. J. Clin. Gastroenterol. 2008;42:156–159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- Polovinski-Horvatović M. A mini review of the effects of NSP and exogenous enzymes in broiler diets on digestibility and some intestinal functions. Contemp. Agric. 2021;70:116–122. [Google Scholar]
- Prache S., Lebret B., Baéza E., Martin B., Gautron J., Feidt C., Médale F., Corraze G., Raulet M., Lefèvre F., Verrez-Bagnis V., Sans P. Review: quality and authentication of organic animal products in Europe. Animal. 2022;16 doi: 10.1016/j.animal.2021.100405. [DOI] [PubMed] [Google Scholar]
- Rafeeq M., Bilal R.M., Batool F., Yameen K., Farag M.R., Madkour M., Shaaban S.E., El-Shall A.N., Kuldeep D., Alagawany M. Application of herbs and their derivatives in broiler chickens: a review. Worlds Poult. Sci. J. 2023;79:95–117. [Google Scholar]
- Rajput I.R., Li L.Y., Xin X., Wu B.B., Juan Z.L., Cui Z.W., Yu D.Y. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 2013;92:956–965. doi: 10.3382/ps.2012-02845. [DOI] [PubMed] [Google Scholar]
- Ranjitkar S., Lawley B., Tannock G., Engberg R.M. Bacterial succession in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2016;82:2399–2410. doi: 10.1128/AEM.02549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza, A., S. Bashir, and R. Tabassum. 2019. An update on carbohydrases: growth performance and intestinal health of poultry. Heliyon 5:e01437. [DOI] [PMC free article] [PubMed]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilization, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke S.C., Rothrock M.J. Gastrointestinal microbiomes of broilers and layer hens in alternative production systems. Poult. Sci. 2020;99:660–669. doi: 10.1016/j.psj.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites – implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Rodrigues I., Choct M. The foregut and its manipulation via feeding practices in the chicken. Poult. Sci. 2018;97:3188–3206. doi: 10.3382/ps/pey191. [DOI] [PubMed] [Google Scholar]
- Rubin D.C., Langer J.C. In: In Yamada’s Atlas of Gastroenterology. Wang T.C., Camilleri M., Lok A.S., Sandborn W.J., Wang K.K., Wu G.D., editors. John Wiley & Sons Ltd; Hoboken, NJ: 2022. Small intestine: anatomy and structural anomalies; pp. 22–26. [Google Scholar]
- Rubio L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2018;98:695–706. doi: 10.3382/ps/pey416. [DOI] [PubMed] [Google Scholar]
- Saeed M., Ayaşan T., Alagawany M., El-Hack M.E.A., Abdel-Latif M.A., Patra A.K. The role of ß-mannanase (Hemicell) in improving poultry productivity, health, and environment. Braz. J. Poult. Sci. 2019;21:2019. [Google Scholar]
- Sahin K., Akdemir F., Orhan C., Tuzcu M., Hayirli A., Sahin N. Effects of dietary resveratrol supplementation on egg production and antioxidant status. Poult. Sci. 2010;89:1190–1198. doi: 10.3382/ps.2010-00635. [DOI] [PubMed] [Google Scholar]
- Saleh A.A., Hafez A., Amber K., Abdelhady A.Y., Salem H.M., Fathy M., Kamal M.A., Alagawany M., Alzawqari M.H. Drug-independent control strategy of clostridial infection in broiler chickens using anti-toxin environmentally friendly multienzymes. Sci. Rep. 2023;13:5614. doi: 10.1038/s41598-023-32685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Alqhtani A.H., Swelum A.A., Babalghith A.O., Melebary S.J., Soliman S.M., Khafaga A.F., Selim S., El-Saadony M.T., El-Tarabily K.A., Abd El-Hack M.E. Heat stress in poultry with particular reference to the role of probiotics in its amelioration: an updated review. J. Therm. Biol. 2022;108 doi: 10.1016/j.jtherbio.2022.103302. [DOI] [PubMed] [Google Scholar]
- Salem H.M., El-Saadony M.T., Abd El Mageed T.A., Soliman S.M., Khafaga A.F., Saad A.M., Swelum A.A., AbuQamar S.F., El-Tarabily K.A., Abd El-Hack M.E. Promising prospective effects of Withania somnifera on broiler performance and carcass characteristics: a comprehensive review. Front. Vet. Sci. 2022;9:2022. doi: 10.3389/fvets.2022.918961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Takahashi K., Tohno M., Miura Y., Kamada T., Ikegami S., Kitazawa H. Immunomodulation in gut-associated lymphoid tissue of neonatal chicks by immunobiotic diets. Poult. Sci. 2009;88:2532–2538. doi: 10.3382/ps.2009-00291. [DOI] [PubMed] [Google Scholar]
- Seyidoglu N., Peker S. Effects of different doses of probiotic yeast Saccharomyces cerevisiae on the duodenal mucosa in rabbits. Indian J. Anim. Res. 2015;49:602–606. [Google Scholar]
- Shashidhara R.G., Devegowda G. Effect of dietary mannan oligosaccharide on broiler breeder production traits and immunity. Poult. Sci. 2003;82:1319–1325. doi: 10.1093/ps/82.8.1319. [DOI] [PubMed] [Google Scholar]
- Shehata A.M., Paswan V.K., Attia Y.A., Abougabal M.S., Khamis T., Alqosaibi A.I., Alnamshan M.M., Elmazoudy R., Abaza M.A., Salama E.A.A., El-Saadony M.T., Saad A.M., Abdel-Moneim A.E. In ovo inoculation of Bacillus subtilis and raffinose affects growth performance, cecal microbiota, volatile fatty acid, ileal morphology and gene expression, and sustainability of broiler chickens (Gallus gallus) Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.903847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov A., Tako E., Ferket P.R., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669–673. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- Smith A.L., Powers C., Beal R. In: Pages 303–326 in Avian Immunology. Kaspers B., Schat K.A., Göbel T.W., Vervelde L., editors. Academic Press; London, UK: 2022. The avian enteric immune system in health and disease. [Google Scholar]
- Smolovskaya O., Pleshkov V., Zubova T., Bormina L. Probiotics in industrial poultry farming. Am. J. Anim. Vet. Sci. 2023;18:1–8. [Google Scholar]
- Song F., Liu J., Zhao W., Huang H., Hu D., Chen H., Zhang H., Chen W., Gu Z. Synergistic effect of eugenol and probiotic Lactobacillus plantarum Zs2058 against Salmonella infection in C57bl/6 mice. Nutrients. 2020;12:1611. doi: 10.3390/nu12061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Such N., Mezőlaki Á., Rawash M.A., Tewelde K.G., Pál L., Wágner L., Schermann K., Poór J., Dublecz K. Diet composition and using probiotics or symbiotics can modify the urinary and faecal nitrogen ratio of broiler chicken’s excreta and also the dynamics of in vitro ammonia emission. Animals. 2023;13:332. doi: 10.3390/ani13030332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 2016;15:99–111. [Google Scholar]
- Sun H., Kim I. Dietary supplementation of mixed yeast culture derived from Saccharomyces cerevisiae and Kluyveromyces maxianus: effects on growth performance, nutrient digestibility, meat quality, blood parameters, and gut health in broilers. J. Poult. Sci. 2019;56:140–147. doi: 10.2141/jpsa.0180052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureshbabu A., Smirnova E., Karthikeyan A., Moniruzzaman M., Kalaiselvi S., Nam K., Goff G.L., Min T. The impact of curcumin on livestock and poultry animal's performance and management of insect pests. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1048067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svihus B. Function of the digestive system. J. Appl. Poult. Res. 2014;23:306–314. [Google Scholar]
- Swelum A.A., Elbestawy A.R., El-Saadony M.T., Hussein E.O.S., Alhotan R., Suliman G.M., Taha A.E., Ba-Awadh H., El-Tarabily K.A., Abd El-Hack M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri H.R., Moghadam M.K., Kakebaveh M., Harakinezhad T. Growth performance and immune response of broiler chickens fed diets supplemented with probiotic and (or) prebiotic preparations. J. Livest. Sci. Technol. 2014;2:1–8. [Google Scholar]
- Tahir M., Saleh F., Ohtsuka A., Hayashi K. Pectinase plays an important role in stimulating digestibility of a corn-soybean meal diet in broilers. J. Poult. Sci. 2006;43:323–329. [Google Scholar]
- Ting S., Yeh H.S., Lien T.F. Effects of supplemental levels of hesperetin and naringenin on egg quality, serum traits and antioxidant activity of laying hens. Anim. Feed Sci. Technol. 2011;163:59–66. [Google Scholar]
- Umar S., Shah M.A.A., Munir M.T., Yaqoob M., Fiaz M., Anjum S., Umar W. Synergistic effects of thymoquinone and curcumin on immune response and anti-viral activity against avian influenza virus (H9N2) in turkeys. Poult. Sci. 2016;95:1513–1520. doi: 10.3382/ps/pew069. [DOI] [PubMed] [Google Scholar]
- Uni Z., Tako E., Gal-Garber O., Sklan D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult. Sci. 2003;82:1747–1754. doi: 10.1093/ps/82.11.1747. [DOI] [PubMed] [Google Scholar]
- USDA-ERS. 2014. Organic market review. Accessed Mar. 2015. http://www.ers.usda.gov/topics/natural-resources-environment/organic-agriculture/organicmarket-overview.asp.
- Van T.T.H., Yidana Z., Smooker P.M., Coloe P.J. Antibiotic use in food animals worldwide, with a focus on Africa: pluses and minuses. J. Glob. Antimicrob. Resist. 2020;20:170–177. doi: 10.1016/j.jgar.2019.07.031. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen C.H., Soons M.B., Vandionant L.G., Green A.J., Bakker E.S. Seed dispersal by waterbirds: a mechanistic understanding by simulating avian digestion. Echography. 2023;2023:e06470. [Google Scholar]
- Varmuzova K., Matulova M.E., Gerzova L., Cejkova D., Gardan-Salmon D., Panhéleux M., Robert F., Sisak F., Havlickova H., Rychlik I. Curcuma and Scutellaria plant extracts protect chickens against inflammation and Salmonella Enteritidis infection. Poult. Sci. 2015;94:2049–2058. doi: 10.3382/ps/pev190. [DOI] [PubMed] [Google Scholar]
- von Borell E., Sørensen J.T. Organic livestock production in Europe: aims, rules and trends with special emphasis on animal health and welfare. Livest. Prod. Sci. 2004;90:3–9. [Google Scholar]
- Wang D., Huang H., Zhou L., Li W., Zhou H., Hou G., Liu J., Hu L. Effects of dietary supplementation with turmeric rhizome extract on growth performance, carcass characteristics, antioxidant capability, and meat quality of Wenchang broiler chickens. Ital. J. Anim. Sci. 2015;14:3870. [Google Scholar]
- Wilson B., Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017;32:64–68. doi: 10.1111/jgh.13700. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Liu N., Wu X.H., Wang G.Y., Lin L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018;97:2675–2683. doi: 10.3382/ps/pey123. [DOI] [PubMed] [Google Scholar]
- Xiao R., Power R.F., Mallonee D., Routt K., Spangler L., Pescatore A.J., Cantor A.H., Ao T., Pierce J.L. Effects of yeast cell wall-derived mannan-oligosaccharides on jejunal gene expression in young broiler chickens. Poult. Sci. 2012;91:1660–1669. doi: 10.3382/ps.2011-02035. [DOI] [PubMed] [Google Scholar]
- Xu Z., Zou X., Hu C., Xia M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yalcin S., Yalcin S., Sehu A., Sarifakiogullari K. Pages 600–604 in National Animal Nutrition Congress, Isparta, Turkey. 4–6 September 2000. 2000. Yumurta tavug√u rasyonlarinda laktik asit kullaniminin baziyumurta kalite özelliklerine etkisi. [Google Scholar]
- Yang Y., Iji P.A., Kocher A., Mikkelsen L.L., Choct M. Effects of xylanase on growth and gut development of broiler chickens given a wheat-based diet Asian-australas. J. Anim. Sci. 2008;21:1659–1664. [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia N., AbdelSabour M.A., Erfan A.M., Ali Z.M., Soliman R.A., Samy A., Soliman M., Abd El-Hack M.E., El-Saadony M.T., Ahmed K.A. Selenium nanoparticles enhance the efficacy of homologous vaccine against the highly pathogenic avian influenza H5N1 virus in chickens. Saudi J. Biol. Sci. 2022;29:2095–2111. doi: 10.1016/j.sjbs.2021.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia N., Salem H.M., Mahmmod Y., Said D., Samir M., Mawgod S.A., Sorour H.K., AbdelRahman M.A.A., Selim S., Saad A.M., El-Saadony M.T., El-Meihy R.M., Abd El-Hack M.E., El-Tarabily K.A., Zanaty A.M. Common viral and bacterial avian respiratory infections: an updated review. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef A.W., Hassan H.M.A., Ali H.M., Mohamed M.A. Effect of probiotics, prebiotics and organic acids on layer performance and egg quality. Asian J. Poult. Sci. 2013;7:65–74. [Google Scholar]
- Yue Y., Guo Y., Yang Y. Effects of dietary l-tryptophan supplementation on intestinal response to chronic unpredictable stress in broilers. Amino Acids. 2017;49:1227–1236. doi: 10.1007/s00726-017-2424-3. [DOI] [PubMed] [Google Scholar]
- Yurong Y., Ruiping S., Shimin Z., Yibao J. Effect of probiotics on intestinal mucosal immunity and ultrastructure of cecal tonsils of chickens. Arch. Anim. Nutr. 2005;59:237–246. doi: 10.1080/17450390500216928. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yu D., Sun J., Liu X., Jiang L., Guo H., Ren F. Interaction of plant phenols with food macronutrients: characterization and nutritional–physiological consequences. Nutr. Res. Rev. 2014;27:1–15. doi: 10.1017/S095442241300019X. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang L., Zhan X., Zeng X., Zhou L., Cao G., Chen A., Yang C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016;7:3. doi: 10.1186/s40104-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Cai D., Cai W., Geng S., Chen L., Han T. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin. Nutr. 2009;28:575–580. doi: 10.1016/j.clnu.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Zulkarnain D., Zuprizal W., Supadmo S. Utilization of sago waste with cellulase enzyme fermentation as a local feed for broilers in Southeast Sulawesi. Int. J. Poult. Sci. 2017;16:266–273. [Google Scholar]