Abstract
We investigated the role of the two highly conserved cysteine residues, cysteines 67 and 95, of the human immunodeficiency virus type 1 (HIV-1) protease in regulating the activity of that protease during viral maturation. To this end, we generated four HIV-1 molecular clones: the wild type, containing both cysteine residues; a protease mutant in which the cysteine at position 67 was replaced by an alanine (C67A); a C95A protease mutant; and a double mutant (C67A C95A). When immature virions were produced in the presence of an HIV-1 protease inhibitor, KNI-272, and the inhibitor was later removed, limited polyprotein processing was observed for wild-type virion preparations over a 20-h period. Treatment of immature wild-type virions with the reducing agent dithiothreitol considerably improved the rate and extent of Gag processing, suggesting that the protease is, in part, reversibly inactivated by oxidation of the cysteine residues. In support of this, C67A C95A virions processed Gag up to fivefold faster than wild-type virions in the absence of a reducing agent. Furthermore, oxidizing agents, such as H2O2 and diamide, inhibited Gag processing of wild-type virions, and this effect was dependent on the presence of cysteine 95. Electron microscopy revealed that a greater percentage of double-mutant virions than wild-type virions developed a mature-like morphology on removal of the inhibitor. These studies provide evidence that under normal culture conditions the cysteines of the HIV-1 protease are susceptible to oxidation during viral maturation, thus preventing immature virions from undergoing complete processing following their release. This is consistent with the cysteines being involved in the regulation of viral maturation in cells under oxidative stress.
Human immunodeficiency virus type 1 (HIV-1) encodes an aspartyl protease which is responsible for the cleavage of the Gag and Gag-Pol polyproteins during viral maturation and is therefore critical for production of mature viral particles (22). Maturation of HIV-1 begins as the virus particle buds from the cell membrane, with polyprotein processing being complete during or soon after the release of the virion from the cell (14–16, 21). The released mature viral particles contain a condensed core, consisting predominantly of the p24 capsid and p7 nucleocapsid proteins, surrounded by the viral membrane containing the p17 matrix protein. The viral maturation process can be blocked by the use of HIV-1 protease inhibitors, and as a result, the cell releases immature viral particles which are noninfectious (1, 11, 13, 27, 30, 32, 34, 42).
A number of groups, including ours, have studied the ability of isolated immature virions to undergo polyprotein processing following removal of various HIV-1 protease inhibitors from the virus preparations (11, 27, 32, 34, 42). This has been explored, in part, to assess the potential for immature virions to mature and become infectious if protease inhibitor levels were to drop during AIDS therapy. These studies have demonstrated that immature virions can undergo limited polyprotein processing following inhibitor removal, as evidenced by Western blot analysis (11, 27, 32, 34, 42). However, in all cases, the infectivity of the immature viral particles did not increase following removal of protease inhibitors. Our group has also been studying this process to obtain a better understanding of the timing and sequence of events involved in HIV-1 maturation. Previously, we were able to partially restore polyprotein processing following removal of HIV-1 protease inhibitors from immature virions (11). However, even after 48 h, notable levels of unprocessed Gag remained. This was consistent with the observation of only a few partially condensed cores by electron microscopy (EM), with the majority of virions maintaining an immature morphology.
These studies indicated that restoration of protease activity within these virions was not complete. We hypothesized that this might be due in part to inactivation of the protease as a result of oxidation of one or both of the conserved cysteine residues in the enzyme (11). Although neither of the cysteine residues is required for basal enzyme activity, oxidation of either residue can lead to inhibition of protease activity (6). Cysteine 95, a residue located at the dimer interface, is completely conserved in wild-type isolates of HIV-1, and simple oxidation of this residue in vitro leads to inactivation of the protease (19, 20, 31). In addition, we have found that the formation of a disulfide bond between this cysteine and the ubiquitous cellular thiol glutathione (termed glutathionylation) leads to complete but reversible inactivation of the enzyme (6, 7). A decrease in protease activity can also be achieved by chemical oxidation of cysteine 67, another highly conserved residue (6, 20). However, modification of cysteine 67 by glutathione can actually increase protease activity and stabilize the enzyme, suggesting that the chemical nature of the modification is an important determinant of the effect on activity (6). We have proposed that these reversible modifications may, under certain cellular conditions, occur in the viral life cycle and regulate protease activity during virus budding (7). To better understand the role that the two conserved cysteine residues of the HIV-1 protease might play in viral maturation, we generated four HIV-1 molecular clones: wild-type HIV-1, HIV-1 with an alanine substituted for the cysteine at position 67 (C67A), HIV-1 with an alanine substituted for the cysteine at position 95 (C95A), and HIV-1 with alanines substituted for the cysteines at both positions (C67A C95A). Immature virions of each infectious molecular clone were then produced in the presence of KNI-272, a potent HIV-1 protease inhibitor (14), and the ability to restore Gag polyprotein processing was studied under various conditions following removal of the inhibitor.
MATERIALS AND METHODS
Construction of HIV-1 molecular clones.
pSUM9 contains full-length HXB2RIP7 (29) in which the XmaI site has been introduced in pol (nucleotide 2589 of HXB2RIP7). All mutations were made from a subclone of pSUM9 (40) containing the ApaI-XmaI fragment (nucleotides 2006 to 2595 of HXB2RIP7) in the pGEM7f(−) vector (Promega, Madison, Wis.) (pGAX9). The Cys-to-Ala substitution at position 67 and/or 95 of the protease was created by PCR with primers containing the mutations. The PCR was performed with the plasmid pGAX9 as a template and either the forward primer 5′GCTGGACATAAAGCTATAGGTACAGTA3′ and the 5′-phosphorylated reverse primer 5′GATTTCTATGAGTATCTGATCATA3′ (for a Cys-to-Ala substitution at position 67) or the forward primer 5′GGTGCCACTTTAAATTTTCCCATTAGCCCT3′ and the 5′-phosphorylated reverse primer 5′AATCTGAGTCAACAGATTTCTTCC3′ (for a Cys-to-Ala substitution at position 95). The PCR products were subsequently self-ligated following digestion of template pGAX9 with DpnI. The sequences of the PCR-amplified mutated segments were verified after cloning. The cloned ApaI-XmaI fragments containing the substitutions at positions 67 and 95 were designated pGAX-C67A and pGAX-C95A, respectively. Similarly, the double mutant, with Cys-to-Ala substitutions at positions 67 and 95, was created by employing the same procedure with the respective primer pair to yield pGAX-C67/95A. pGAX-C67A, pGAX-C95A, and pGAX-C67/95A were cleaved with ApaI and XmaI, and the respective fragments were inserted between the same sites in plasmid pSUM9 to yield pSUM-C67A, pSUM-C95A, and pSUM-C67/95A, respectively.
Transfection and virus preparation.
To generate infectious HIV-1 molecular clones, COS-7 cells were transfected with the plasmids by using Lipofectamine (Life Technologies, Gaithersburg, Md.) as described previously (45). COS-7 cells were maintained in Dulbecco modified Eagle’s medium (DMEM) (Advanced Biotechnologies Inc., Columbia, Md.) supplemented with 10% heat-inactivated fetal calf serum, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 2 mM glutamine. For transfection, 2 × 105 COS-7 cells were seeded in a six-well plate. At 16 h postinoculation, the cells were incubated for 4 h with the complexes of the plasmid (2 μg) and Lipofectamine (12 μl) in 1 ml of serum-free DMEM, washed once with serum-free DMEM, and resuspended in 2 ml of RPMI 1640 (Life Technologies) supplemented with 10% heat-inactivated fetal calf serum. At 24 h posttransfection, 3 × 105 MT-2 cells were added for propagation of virus. Another 24 h later, the culture medium were harvested, cleared of cells and debris by centrifugation (10 min at 1,000 × g), and used for infection of H9 cells for 2 h. The cells were then washed twice with phosphate-buffered saline and resuspended in complete medium. After 7 days, infection of H9 cells was verified by determining the presence of p24 in the culture medium. These chronically infected cells were maintained for use in generating immature virions as described below. The presence of introduced mutations and the absence of unintended mutations were reconfirmed by DNA sequencing of the protease-encoding region from the proviral DNA isolated from mutant-virus-infected cells. Sequencing was done a second time, using viral supernatants following long-term culture of H9 cells, and the protease mutations were found to be maintained.
Preparation of immature virions and viral polyprotein processing experiments.
H9 cells, chronically infected with each of the viral clones as described above, were incubated at a density of 5 × 105 per ml in complete medium (consisting of RPMI 1640 medium, 2 mM l-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml [all from Gibco Laboratories, Rockville, Md.]) in the presence of the HIV-1 protease inhibitor KNI-272 (5 μM) (14) at 37°C in an atmosphere of 5% CO2. On day 4, the cells were washed by centrifugation (1,000 × g for 10 min) to remove any residual mature virions in the medium and then incubated for 4 to 5 days in the presence of fresh medium containing KNI-272. The cells were then centrifuged as before, and the medium, containing immature virions in the presence of KNI-272, was stored in liquid nitrogen in 1-ml aliquots. For polyprotein processing experiments, 1-ml aliquots of the immature virions were thawed at room temperature and then centrifuged at 100,000 × g for 35 min at 4°C. The supernatants were then removed by aspiration, and the viral pellets were each resuspended in 1 ml of conditioned H9 medium in the absence of protease inhibitor, maintained at 4°C, and centrifuged as before. This washing step was repeated once. This procedure, which is similar to that described previously (11), has been shown to reduce the concentration of KNI-272 in the viral pellets to less than 1 nM. Following removal of the inhibitor, the viral pellets were resuspended in sufficient conditioned medium (4- to 5-day-old medium in which were cultured uninfected H9 cells) to normalize the virus particle counts for the wild-type and mutant virions (the volume required ranged from 75 to 150 μl). We previously demonstrated that measurable but limited polyprotein processing occurs in immature wild-type virions following removal of the protease inhibitor (11). More-recent studies in our laboratory on the processing of wild-type virions demonstrated that the use of conditioned medium improved the rate of processing over that achieved with fresh medium. This increased rate of processing observed with conditioned medium was found to be primarily due to its lower pH (approximately 6.0). This observation is consistent with previous reports showing that a pH of 5.5 to 6.0 is optimal for protease activity in vitro (4, 10, 31, 35). We have confirmed that lowering the pH of fresh medium from 7.2 to 6.0 with sodium acetate buffer leads to improved polyprotein processing of immature virions. For the studies presented here, we used conditioned medium for postrelease processing experiments in an attempt to maximize the rate of polyprotein processing and to simulate the conditions under which mature virions are normally produced.
Polyprotein processing was initiated by incubating aliquots (usually 10 μl per condition) of the immature virions in conditioned medium at 37°C for various periods of time and under a variety of conditions of treatment. To study the effect of dithiothreitol (DTT) or diamide on viral processing, 10× stock solutions of DTT (1 to 1,000 mM), H2O2 (0.05 to 0.5 mM), and diamide (0.1 to 10 mM) were prepared, and following removal of KNI-272, 1-μl volumes of the stocks were added to 9-μl quantities of the viral preparations. After incubation of the preparations for 20 h at 37°C, polyprotein processing was terminated by the addition of 10 μM KNI-272 or saquinavir (Ro318959; Roche Research Center, Welwyn Garden City, Hertfordshire, United Kingdom; a kind gift of Ian Duncan) followed by sodium dodecyl sulfate (SDS) sample buffer (2×) containing 100 mM DTT. These samples were then analyzed for the extent of polyprotein processing by Western blot analysis of the different Gag viral protein products as described below.
Western blotting.
Samples were electrophoresed on a 10% bis-Tris polyacrylamide gel with 2-(N-morphilino)ethanesulfonic acid (MES) running buffer, using the NuPage system (Novex, San Diego, Calif.). Proteins were electroblotted onto nitrocellulose, and p55Gag, Gag intermediates, and the mature p24 capsid protein were detected with a mouse monoclonal anti-p24 antibody (Intracel, Cambridge, Mass.). The identity of p24 in viral preparations was previously confirmed (11) by using a p24 protein standard (Advanced Biotechnologies Inc.). The matrix protein, p17, was also detected on blots, using a mouse monoclonal anti-p17 antibody which has no reactivity to p55Gag (Advanced Biotechnologies Inc.). The band detected with the p17 antibody was found to migrate to a position corresponding to the expected molecular mass of 17 kDa, as determined by comparison to the migration positions of SeeBlue prestained markers (Novex). Blots were incubated with both antibodies for 2 h and then with an anti-mouse secondary antibody conjugated to alkaline phosphatase for 30 min. Bands were detected by using the Western Blue stabilized substrate for alkaline phosphatase (Promega). Densitometry analysis of the Western blots representing time course experiments was performed with the ImageQuant software from Molecular Dynamics (Sunnyvale, Calif.). The time required to decrease the level of p55Gag by 50% following removal of the protease inhibitor from each of the different virus preparations was determined. In some cases, blots were probed with other HIV-1-specific antibodies, including antinucleocapsid antibody (obtained as a kind gift from Larry Arthur, National Cancer Institute, Frederick, Md.) or a monoclonal anti-reverse transcriptase or anti-integrase antibody (Intracel).
Viral particle counts.
Viral particle counts were performed on aliquots of immature virions in order to normalize the amount of virus used in viral processing experiments. Counts were done by EM in a manner similar to that described previously (11). Briefly, viral suspensions were centrifuged at 100,000 × g for 30 min and the pellets were resuspended in phosphate-buffered saline at 1/20 of the original volume. Eighteen microliters of each virus sample was mixed with an equal volume of a latex sphere solution (110-nm-diameter latex spheres, 1.5 × 1010 particles per ml; Structure Probe, Inc., West Chester, Pa.) in a microfuge tube. Aliquots (2.0 μl) of each virus-latex sphere sample, diluted 1:10 in ultrapure water, were placed on separate grids and allowed to dry. The samples were fixed, stained, and then examined in a JEOL model 100CX transmission electron microscope. Four random fields from each sample grid were closely examined, and latex spheres and virus particles were enumerated until 1,000 latex spheres were counted. The concentration of virus particles per milliliter in the original suspension was determined with the following formula: (number of virus particles counted) × (1.5 × 1010 particles/ml)/(number of latex spheres counted) × 20.
Infectivity assay.
The infectivity of viral supernatants was assessed by the syncytium assay of Johnson and Byington (12). However, MT-2 cells were used in place of H9 cells in this assay. Wells that were scored positive for syncytia were subjected to a p24 radioimmunoassay (DuPont, Wilmington, Del.) for confirmation of positivity.
EM.
To evaluate the morphology of viral particles following removal of the protease inhibitor, EM analysis was carried out on viral pellets. Electron micrographs were produced 24 h following removal of the protease inhibitor from wild-type and protease double-mutant virion preparations. As a control, electron micrographs of an identical preparation of virions washed free of inhibitor, but then incubated for 24 h following the readdition of inhibitor (5 μM) to specifically prevent processing due to the activity from the HIV-1 protease, were also produced. For each sample used in EM analysis, a 20-ml preparation of immature virions was centrifuged and the pellet was washed free of protease inhibitor as described above. The pellets were then resuspended in conditioned medium and incubated for 24 h at 37°C. Following incubation, all samples (0.8 ml) were treated with HIV-1 protease inhibitor to prevent any further processing and then centrifuged at 100,000 × g for 30 min. The viral pellets were treated with 100 μl of fresh glutaraldehyde solution (2.5%) for 2 h. Each pellet was then rinsed gently three times with 200 μl of Millonig’s 0.13 M sodium phosphate buffer and then maintained at 4°C. Following fixation, the pellets were minced into small pieces, washed in Millonig’s sodium phosphate buffer, and stored overnight at 4°C. Each sample was postfixed in 1.0% osmium tetroxide and then washed. The samples were then stained with 2.0% aqueous uranyl acetate, dehydrated in a series of graded ethanol solutions, and infiltrated and embedded in Spurr’s plastic resin. The blocks were polymerized overnight at 70°C. An embedded block for each sample was ultrathin sectioned by using a Reicher-Jung Ultracut E ultramicrotome. Sections 60 to 80 nm in thickness were collected from each sample and mounted onto mesh copper grids. The grids from each sectioned block were then poststained with 2.0% aqueous uranyl acetate and Reynold’s lead citrate. The grids were then intensively examined in a Hitachi model HU-12A transmission electron microscope. Representative photomicrographs of each sample were produced.
RESULTS
Characterization of wild-type and mutant molecular clones.
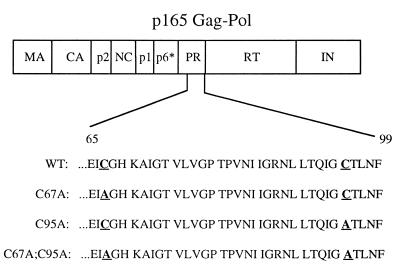
To study the possible role of the conserved cysteine residues of the HIV-1 protease in polyprotein processing, we produced the wild-type clone and three mutant clones, replacing one or both of the cysteines within the protease with alanine. Single-site mutants, with either cysteine 67 or cysteine 95 being replaced by alanine, and a double mutant with both cysteines being replaced by alanine were produced (Fig. 1). It should be noted that enzymatic studies of the HIV-1 protease recombinant forms containing these mutations demonstrated that the activity of each of these proteases is similar to that of the wild-type enzyme when tested under reducing conditions with a standard HIV-1 peptide substrate spanning the p17-p24 junction of Gag (6).
FIG. 1.
Schematic representation of the Gag-Pol polyprotein of HIV-1. The region of the protease (PR) sequence containing the cysteine-to-alanine mutations (in boldface and underlined) is shown for each of the mutant clones generated in this study. Each HIV-1 plasmid construct was introduced into COS-7 cells for the generation of virus used to infect H9 cells. WT, wild type; MA, matrix; CA, capsid; RT, reverse transcriptase; IN, integrase.
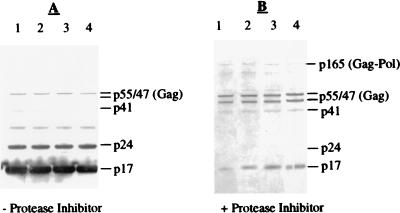
The wild type and all of the mutant virus clones were able to replicate in H9 cells, as evidenced by the presence of p24 in the media of infected cells (the range of p24 values obtained from cultures of the different clones was one- to threefold different from that of the wild type). The virus produced from chronically infected H9 cells in the presence or absence of 5 μM KNI-272 was pelleted, normalized for total viral particle number, and then analyzed by Western blotting with a combination of anti-p17 and anti-p24 monoclonal antibodies as described in Materials and Methods. The Western blot profiles for p17- and p24-reactive bands of the different viral clones were indistinguishable, suggesting that viral maturation in infected cells was not noticeably altered by the introduced mutations (Fig. 2A). The mature viral products corresponding to p17 and p24 were readily detected (Fig. 2A), as were small amounts of unprocessed p55Gag and the partially processed p41Gag. There was also an unidentified band migrating at approximately 34 kDa which might represent dimeric forms of p17, since it was not detected with the anti-p24 antibody alone (data not shown). Thus, as expected from in vitro studies of the forms of the HIV-1 protease with mutations at the cysteine residues, virions encoding these mutant proteases apparently undergo polyprotein processing similar to that of the wild-type virus in H9 cells. In the presence of 5 μM KNI-272, a potent HIV-1 protease inhibitor, p24 was no longer detected. The major antibody-reactive bands observed for all four viral clones corresponded to unprocessed p55Gag, p47, and p41, in addition to higher-molecular-weight bands corresponding to p165Gag-Pol (Fig. 2B). The high-molecular-weight bands were also detected with both anti-reverse transcriptase and anti-integrase antibodies, confirming them as Gag-Pol-derived proteins (data not shown). Gag-Pol and partially processed forms of Gag-Pol detected in the presence of protease inhibitors have been described previously (28). The detection of p47 and small amounts of p17 in the presence of KNI-272 (Fig. 2B) suggested that HIV-1 protease activity in the infected cells was not completely abolished.
FIG. 2.
Western blot analysis of the four viral clones in the absence (A) or presence (B) of protease inhibitor. Viral particles produced in the absence (−) or the presence (+) of the protease inhibitor KNI-272 were obtained from the supernatants (1.0 ml) of infected H9 cells by centrifugation. Gag-related proteins were detected with a combination of anti-p17 antibody and anti-p24 antibody as described in Materials and Methods. Lanes: 1, virus obtained from H9 cells infected with wild-type virus; 2, virus obtained from H9 cells infected with C67A virus; 3, virus obtained from H9 cells infected with C95A virus; 4, virus obtained from H9 cells infected with C67A C95A virus. The positions of Gag and Gag-derived proteins are indicated to the right of each blot. In panel B, the location of Gag-Pol is also indicated.
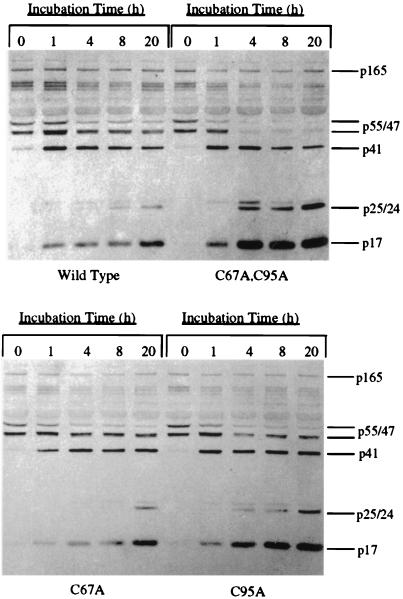
Substitution of alanine for cysteines 67 and 95 of the HIV-1 protease results in enhanced polyprotein processing of immature virions.
Removal of the protease inhibitor KNI-272 from the preparations of immature virions by dilution and centrifugation resulted in the detection of polyprotein processing as early as 1 h postremoval for the wild-type and mutant virions, although to different extents (Fig. 3). Processing for all the virus clones was characterized by a decrease in p55 and p47 and the accumulation of the mature viral proteins p17 and p24. Processing at early time points was most notably evidenced by the appearance of mature viral p17 (viral p17 was more readily detected on blots than was viral p24) (Fig. 3). However, the rate of processing for the wild type was substantially lower than that for the double mutant, suggesting that the presence of the cysteine residues decreases the rate of processing. Densitometry analysis of the remaining p55 at the different time points indicated that the rate of processing for the double mutant was more than five times higher than that measured for the wild type (p55 was decreased by 50% within 0.75 h for the double mutant versus 4 h for the wild type). In addition, viral p24 was recognized only 20 h after the inhibitor was removed from the preparations of wild-type immature virions, whereas the double-mutant virions produced similar levels of p24 within just 4 h after removal of the inhibitor (Fig. 3). Perhaps the most striking difference observed in polyprotein processing between the wild-type and double-mutant virions was the complete and rapid loss of both p55 and p47 in the double mutant after only 4 h of incubation, while the wild type still contained these precursors after 20 h of incubation (Fig. 3, top panel). This difference between wild-type and double-mutant viral polyprotein processing was also observed when blots were probed with an anti-nucleocapsid protein antibody which readily detects the p55 and p47 precursor polyproteins in addition to the mature nucleocapsid protein (data not shown).
FIG. 3.
Western blot analysis of polyprotein processing of immature viral preparations following removal of the protease inhibitor. Each preparation of immature virus, obtained as described in Materials and Methods, was incubated in medium at 37°C for up to 20 h, and aliquots were removed at the times indicated above the lanes. Protease activity was stopped by the addition of 10 μM protease inhibitor and SDS sample buffer. Blots were probed with a combination of anti-p17 and anti-p24 monoclonal antibodies. The positions of Gag-Pol and Gag-derived proteins are indicated to the right of each blot. (Top panel) Wild-type and double-mutant viral preparations; (bottom panel) C67A and C95A viral preparations.
For the single-mutant proteases, the rates of polyprotein processing were similar (Fig. 3, bottom panel). Although mutating either one of the two cysteines seemed to improve the rate of processing, the absence of cysteine 95 appeared to have a greater overall impact throughout our studies. Densitometry analysis of p55Gag at the different time points indicated that the rates of processing for both the C67A and C95A virions were nearly two times higher than that measured for the wild-type (p55 was decreased by 50% within 2.25 and 2 h, respectively, for the single mutants, compared to 4 h for the wild type). Taken together, these results indicate that the presence of either cysteine residue in the protease limits polyprotein processing in immature virions following removal of the protease inhibitor.
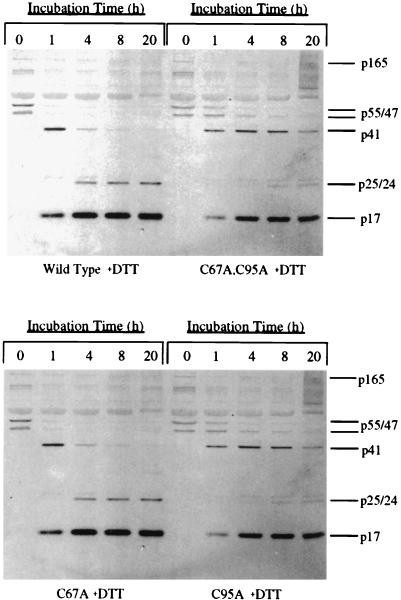
DTT enhances polyprotein processing in virions whose protease contains cysteine residues.
Previous studies have shown that oxidation of cysteine 95 leads to inhibition of HIV-1 protease activity while oxidation of cysteine 67 can either decrease or increase protease activity, depending on the oxidizing agent used (6, 7, 19, 20). Thus, the limited processing in wild-type virions following removal of the protease inhibitor could be due to oxidative inactivation of the cysteine residues in the wild-type protease. To address this possibility, wild-type and mutant immature virions were treated with DTT following removal of the protease inhibitor, and the extent of polyprotein processing over time was then evaluated. Treatment of immature wild-type virions with 50 mM DTT substantially enhanced the rate of polyprotein processing in these virions, but the same treatment somewhat decreased the rate of processing in the immature double-mutant virions (compare Fig. 3, top panel, with Fig. 4, top panel). There was a noticeable decrease in the levels of the Gag polyprotein precursors p55 and p47 within 4 h after the removal of KNI-272 from wild-type immature virions treated with DTT, as well as a corresponding increase in p17 and p24 (Fig. 4, top panel). Densitometry analysis of the remaining p55 at the different time points indicated that the wild-type and double-mutant virions now processed at similar rates (p55 was decreased by 50% within 0.75 h for the wild type versus 1 h for the double mutant). By 20 h, a majority of the p41 in the wild-type virions was also processed to the mature products, p17 and p24, which did not occur in the absence of DTT over the same time period (compare Fig. 4, top panel, with Fig. 3, top panel). By contrast, DTT decreased the rate of processing for the double-mutant virions to a level below that seen for the wild-type virions (Fig. 4, top panel). A dose-response experiment with increasing concentrations of DTT indicated that as little as 100 μM DTT was effective at substantially improving processing for the wild-type virions while causing a slight decrease in processing for the double-mutant virion preparation (data not shown). DTT also improved the processing for the C67A virions while having a slight inhibitory effect on the C95A virions (compare Fig. 4, bottom panel, with Fig. 3, bottom panel). Densitometry analysis of the remaining p55 at the different time points indicated that the C67A and C95A virions now processed at rates similar to the wild type when incubated in the presence of DTT (p55 was decreased by 50% within 0.75 h for both the C67A and C95A virions). These data support the hypothesis that the cysteines in immature virions can become reversibly oxidized and that this oxidation leads to decreases in the rate and extent of polyprotein processing following removal of the inhibitor.
FIG. 4.
Western blot analysis of polyprotein processing of immature viral preparations in the presence of DTT following removal of the protease inhibitor. Each preparation of immature virus, obtained as described in Materials and Methods, was incubated in medium in the presence of 50 mM DTT at 37°C for up to 20 h, and aliquots were removed at the times indicated above the lanes. Protease activity was stopped by the addition of 10 μM protease inhibitor and SDS sample buffer. Blots were probed with a combination of anti-p17 and anti-p24 monoclonal antibodies. The positions of Gag-Pol and Gag-derived proteins are indicated to the right of each blot. (Top panel) Wild-type and double-mutant viral preparations; (bottom panel) C67A and C95A viral preparations.
Effect of oxidizing agents on HIV-1 polyprotein processing.
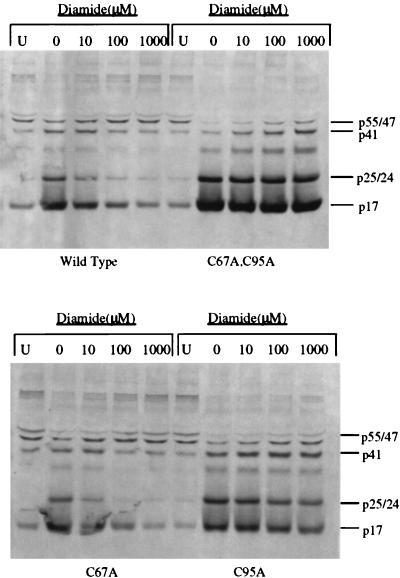
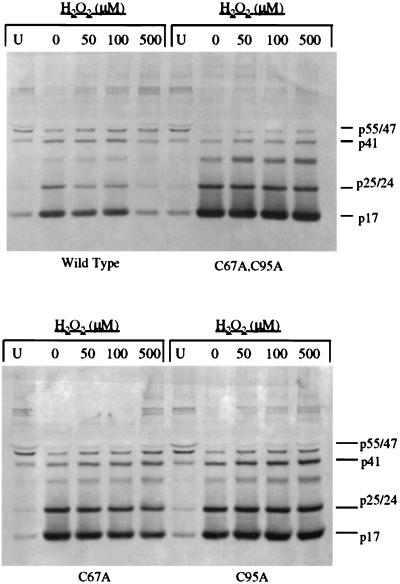
To further study the effect of cysteine oxidation on polyprotein processing, we utilized oxidizing agents, including diamide and hydrogen peroxide. We hypothesized that oxidation of the cysteine residues would inhibit protease activity and thus prevent the limited processing seen in the wild-type virions following removal of the protease inhibitor. Consistent with this hypothesis, treatment of virions with the sulfhydryl-reactive agent diamide (23, 38, 41) at 10, 100, or 1,000 μM followed by incubation at 37°C for 20 h resulted in a dose-dependent inhibition of polyprotein processing in the immature wild-type virion preparations but had little effect on the processing of double-mutant virion preparations (Fig. 5, top panel). Complete inhibition of processing was obtained with 1,000 μM diamide, and this effect was evidenced not only by the absence of Gag processing but also by the continued presence of p165Gag-Pol and Gag-Pol-derived polyproteins. However, concentrations of diamide as high as 1,000 μM had almost no effect on the processing of Gag and Gag-Pol in immature double-mutant virion preparations (Fig. 5, top panel). Diamide treatment also decreased the processing of Gag and Gag-Pol in the C67A virions but had a limited effect on such processing in the C95A virions (Fig. 5, bottom panel). The strong inhibitory effect of diamide on polyprotein processing of the C67A immature virions again demonstrates the impact that oxidation of cysteine 95 alone can have on protease activity. Similarly, treatment of immature virion preparations with hydrogen peroxide at 50, 100, and 500 μM inhibited polyprotein processing in a dose-dependent manner for the wild-type immature virion preparations. However, like diamide, hydrogen peroxide had little effect on the double-mutant virion preparations (Fig. 6, top panel). Also, hydrogen peroxide treatment decreased polyprotein processing in the C67A immature virion preparations but had only a minor effect on such processing in the C95A immature virion preparations (Fig. 6, bottom panel). The results of these experiments correspond well with those of our studies of the in vitro effect of H2O2 on the purified proteases, which indicated that the wild-type protease is particularly more susceptible to hydrogen peroxide-mediated inactivation than any of the mutant proteases (data not shown). Thus, two different oxidizing agents blocked polyprotein processing of immature virions similarly, and this effect was dependent on the presence of cysteines in the protease. These results indicate that oxidation of cysteine 95 plays a more important role than oxidation of cysteine 67 in the inhibition of polyprotein processing.
FIG. 5.
Western blot analysis of polyprotein processing of immature viral preparations in the presence of increasing concentrations of diamide following removal of the protease inhibitor. Each preparation of immature virus, obtained as described in Materials and Methods, was incubated in medium at 37°C for 20 h in the presence of the indicated concentrations of diamide. The first lane (U) for each viral preparation contained the unprocessed virus preparation following removal of the protease inhibitor. Protease activity was stopped by the addition of 10 μM protease inhibitor and SDS sample buffer. Blots were probed with a combination of anti-p17 and anti-p24 monoclonal antibodies. The positions of the viral proteins are indicated to the right of the blots. (Top panel) wild-type and double-mutant viral preparations; (bottom panel) C67A and C95A viral preparations.
FIG. 6.
Western blot analysis of polyprotein processing of immature viral preparations in the presence of increasing concentrations of hydrogen peroxide following removal of the protease inhibitor. Each preparation of immature virus, obtained as described in Materials and Methods, was incubated in the presence of the indicated concentrations of hydrogen peroxide at 37°C for 20 h. The first lane (U) for each viral preparation contained the unprocessed virus preparation following the removal of the protease inhibitor. Protease activity was stopped by the addition of 10 μM protease inhibitor and SDS sample buffer. Blots were probed with a combination of anti-p17 and anti-p24 monoclonal antibodies. The positions of the viral proteins are indicated to the right of the blots. (Top panel) Wild-type and double-mutant viral preparations; (bottom panel) C67A and C95A viral preparations.
Thin-section EM of immature virions following removal of the protease inhibitor.
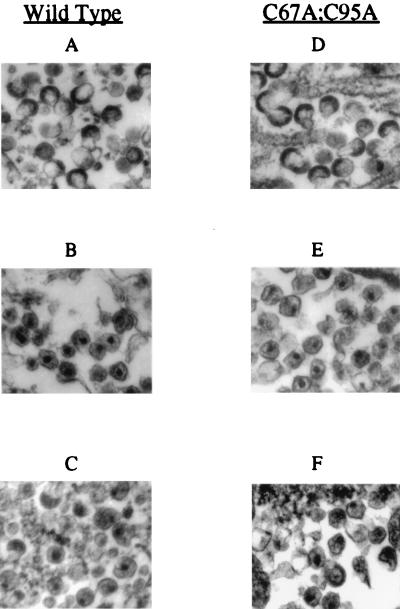
Thin-section EM was performed on mature virions and on immature virions before and after the removal of KNI-272. The membranes of virus particles produced in the presence of KNI-272 were surrounded by electron-dense material which often resided mostly to one side of the membrane (Fig. 7A and D). This is consistent with the previously described immature morphology of virus produced in the presence of protease inhibitors (13, 37). In the absence of inhibitor, the particles contained dense cores, which were sometimes cone shaped, and the membrane appeared relatively translucent (Fig. 7B and E). This appearance is consistent with a mature morphology (8). Following the removal of KNI-272 from wild-type immature virion preparations and incubation at 37°C for 24 h, 56% of the virions appeared to have a mature-virion-like phenotype, with evidence of dense material within the particle and decreased density around the viral membrane (Fig. 7C). By contrast, 85% of the double-mutant virions treated in the same manner had a mature morphology (Fig. 7F). This increase in the number of mature-virion-like particles over that for the wild type is consistent with the greater extent of processing observed for the double mutant by Western blot analysis. Interestingly, the percentages of virions with the mature-virion-like phenotype for the wild type and double mutant (56 and 85%, respectively) correspond closely to the reductions in p55 following incubation for 20 h (55% for the wild type and 87% for the double mutant). This may indicate that a substantial morphological change occurs within the virion following the initial cleavages of p55. The change in viral morphology is consistent with the observed restoration of polyprotein processing as determined by Western blot analysis. The dense cores of these particles often appeared somewhat diffuse and resided closer to the viral membrane than those of the mature-double mutant virion preparations produced in the absence of protease inhibitor. This may indicate that certain steps in polyprotein processing have not been completed in these particles. These results are consistent with a majority of the particles undergoing substantial but incomplete processing rather than a few of the particles being processed to completion.
FIG. 7.
Electron micrographs of virion preparations in the presence and absence of protease inhibitor and following removal of the protease inhibitor and incubation for 20 h. Following incubation of each virus preparation for 20 h at 37°C, the virions were pelleted and prepared for EM analysis as described in Materials and Methods. (A and D) Wild-type and double-mutant (C67A C95A) virions obtained from the media of H9 cells which were treated with 5 μM KNI-272 protease inhibitor. The virions were then incubated for 20 h at 37°C with continued presence of the inhibitor. (B and E) Wild-type and double-mutant (C67A C95A) virions obtained from the media of untreated H9 cells and incubated for 20 h at 37°C in the continued absence of the inhibitor. (C and F) Wild-type and double-mutant (C67A C95A) virions obtained from the media of H9 cells which were treated with 5 μM KNI-272 protease inhibitor; the inhibitor was then removed from the viral preparations by repeated dilution and centrifugation, and the preparations were then incubated for 20 h at 37°C. In panels C and F, note the presence of some mature-virion-like particles as well as particles which appear to be intermediate between mature (B and E) and immature (A and D) virus particles. Magnifications, ×45,000.
Immature virions remain noninfectious following removal of the protease inhibitor.
The infectivity of virions following removal of KNI-272 and incubation for 20 h with or without DTT was tested on MT-2 cells. Both the wild-type and double-mutant immature virions remained noninfectious after incubation in the presence or absence of DTT (data not shown). The lack of infectivity was not due to an insufficient quantity of virus, since the concentration of p24 following incubation exceeded that of the mature viral preparations which retained infectivity. Mature wild-type and double-mutant virions which had undergone an identical treatment, including incubation for 20 h at 37°C, remained infectious. These data suggest that immature virions, even those whose protease cannot be inactivated by oxidation, may not undergo complete maturation following removal of the protease inhibitor. Thus, the maintenance or restoration of protease activity by preventing the oxidation of the cysteine residues in the immature virions may be essential, but not sufficient, to generate infectious particles following removal of the protease inhibitor.
DISCUSSION
Immature viral particles released from HIV-1-infected cells in the presence of protease inhibitors provide a useful model system for delineation of the steps in retroviral polyprotein processing and maturation. The results of this study suggest that the cysteines of the HIV-1 protease are readily susceptible to oxidation and that such oxidation can limit the rate and extent of polyprotein processing of immature viral particles. This conclusion is supported by the results showing that immature viral particles from HIV-1 clones that lack the two conserved cysteine residues of the protease undergo polyprotein processing at a higher rate and to a greater extent than wild-type immature virions following removal of the protease inhibitor KNI-272. Furthermore, the results obtained with HIV-1 mutants containing only cysteine 67 or 95 indicate that oxidation of cysteine 95 plays a more dominant role in limiting the extent of processing observed in immature wild-type virions. This correlates well with in vitro studies demonstrating that modification of cysteine 95 of the HIV-1 protease with a number of different agents leads to inhibition of protease activity (5, 19, 20, 31) while oxidation of cysteine 67 can have variable effects (6, 7). The inhibition of protease activity as a result of cysteine oxidation of the wild-type protease is clearly a reversible phenomenon since it can be substantially reversed by exposure of immature particles to the reducing agent DTT. This appears to be due to an effect on the cysteine residues of the protease since DTT treatment does not increase, but in fact somewhat decreases, the polyprotein processing in virions whose protease lacks the cysteine residues. This decrease in polyprotein processing in the presence of DTT may be due to an alteration in the structure of the Gag protein within virions which alters the accessibility of the virion protease to its substrate. If this is the case, the increased rate of processing in wild-type virions in the presence of DTT may actually be underestimated.
The limited protease activity that is observed in the wild-type and C67A immature virions in the absence of a reducing agent can be blocked by treating the virions with an oxidizing agent, such as diamide or H2O2. This is likely caused by the oxidation of cysteine 95 of the protease by these agents, as has been observed in vitro (unpublished data). While the effects of the oxidizing and reducing agents on viral maturation support a role for the cysteines in regulating polyprotein processing, it should be pointed out that these agents may have other effects on viral particles that could contribute to nonspecific changes in viral maturation. These include oxidation or reduction of other critical cysteine residues in the viral Gag and Gag-Pol proteins as well as nonviral proteins present within the virions. The mechanisms that normally regulate the redox state of the protease within infected cells remain to be determined. The results of the present study indicate that both oxidized and reduced forms of the protease exist in the virions and that these forms are interchangeable.
Although wild-type immature virions incubated in the presence of a reducing agent undergo substantially more processing than do untreated immature virions, they continue to be noninfectious. It remains possible, however, that DTT has secondary effects on viral maturation and viral infectivity which attenuate or nullify the infectivity of particles that otherwise may fully mature and regain infectivity. EM analysis of particles following removal of the protease inhibitor and incubation for 20 h showed that they had developed a mature-virion-like morphology. However, the condensation of the core appeared incomplete, and the core often resided very close to one side of the viral membrane. Thus, the presence of the two reactive cysteines in the HIV-1 protease may be only one of several factors that prevent immature virions from undergoing complete maturation following removal of the protease inhibitor.
The inability of the immature viral preparations to establish infectivity following removal of the protease inhibitor may also indicate that certain factors provided by the infected cell, which are required for regulation of polyprotein processing during the maturation phase, are no longer present within the immature virions. Cellular and viral proteins in addition to those viral proteins contained within the Gag and Gag-Pol precursors (9, 46) have recently been suggested to play a role in altering and/or regulating the activity of the protease. These include the cellular proteins cyclophilin (43) and thioltransferase (7), as well as the viral protein Vif (24). While some of these proteins are found within the virions, they may no longer be present at the concentrations required for complete maturation. For example, Vif, which is known to enhance the infectivity of virions, is found at much higher levels in infected cells than within virions (3). Interestingly, the morphology observed for these virions is quite similar to that recently described for HIV-1 clones with mutations in the basic residues of the nucleocapsid (NC) domain, which were found to have delayed proteolytic processing of the p15NC protein during viral budding and severely impaired infectivity (39). Thus, the timing of events during polyprotein processing and viral maturation may be critical in establishing infectivity.
Cysteines 67 and 95 of the HIV-1 protease are both highly conserved in HIV-1 isolates and are therefore quite likely to be evolutionarily advantageous to the virus. We propose that these cysteine residues play an important role in regulating the rate of polyprotein processing under conditions of oxidative stress, which in turn may be important for optimal viral production and infectivity. A number of studies have focused on the regulation or activation of protease activity during virus maturation since this step appears to be important in determining the resultant infectivity of the released particles. There is evidence that either partial inhibition (17) or premature activation (18) of the HIV-1 protease leads to a reduction in the infectivity of the released viral particles. Interestingly, the cytotoxicity and decreased particle formation arising as a result of protease overexpression can be prevented by the use of low levels of protease inhibitors (25). Together, these studies suggest that HIV-1 establishes a defined level of protease activity for optimal viral replication and the maintenance of cell viability.
Oxidative stress is well known to increase the replication rate of HIV-1 (2, 26, 33, 36, 44), and under these conditions it may be necessary to regulate the activity of the protease through redox mechanisms. This may prevent toxic effects of the protease on infected cells and/or the initiation of apoptosis. In this regard, glutathionylation, which occurs during oxidative stress, was found to reversibly inhibit protease activity (6). The activity could be readily restored by the common cellular enzyme thioltransferase (7). In the presence of a potent HIV-1 protease inhibitor, the majority of the protease is contained within Gag-Pol and precursor forms of Gag-Pol (28). This also occurred in immature virions in the presence of KNI-272 (unpublished data). It is possible, therefore, that oxidative modification of the protease, such as glutathionylation, occurs primarily when the protease exists as part of the Gag-Pol precursor, prior to the association of Gag-Pol with the cell membrane. This could be advantageous to the virus since it would prevent premature activation of the protease within the cytoplasm of cells while still allowing for protease activation during viral budding at a time when the local concentration of Gag-Pol rises substantially. Cellular enzymes, such as thioltransferase, may reverse this modification when the precursors accumulate at the cell membrane, resulting in higher concentrations than that obtained within the cytoplasm of cells. This can serve as a reversible mechanism to optimally regulate HIV-1 protease activity. Ongoing studies are under way in our laboratory to investigate these issues.
ACKNOWLEDGMENTS
Very special thanks to Deborah Goldstein for technical assistance.
This work was supported, in part, by funds from an NIH Intramural AIDS Targeted Antiviral Program grant and NCI project no. ZO1 CM 06737 04M.
REFERENCES
- 1.Bechtold C M, Patick A K, Alam M, Greytok J, Tino J A, Chen P, Gordon E, Ahmad S, Barrish J C, Zahler R, Lin P-F, Colonno R. Antiviral properties of aminodiol inhibitors against human immunodeficiency virus and protease. Antimicrob Agents Chemother. 1995;39:374–379. doi: 10.1128/aac.39.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buttke T M, Sandstrom P A. Redox regulation of programmed cell death in lymphocytes. Free Radic Res. 1995;22:389–397. doi: 10.3109/10715769509147548. [DOI] [PubMed] [Google Scholar]
- 3.Camaur D, Trono D. Characterization of human immunodeficiency virus type 1 Vif particle incorporation. J Virol. 1996;70:6106–6111. doi: 10.1128/jvi.70.9.6106-6111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darke P L, Leu C-T, Davis L J, Heimbach J C, Diehl R E, Hill W S, Dixon A F, Sigal I S. Human immunodeficiency virus protease: bacterial expression and characterization of the purified aspartic protease. J Biol Chem. 1989;264:2307–2312. [PubMed] [Google Scholar]
- 5.Davis D A, Branca A A, Pallenberg A J, Marschner T M, Patt L M, Chatlynne L G, Humphrey R W, Yarchaon R, Levine R L. Inhibition of human immunodeficiency virus-1 protease and human immunodeficiency virus-1 replication by bathocuproine disulphonic acid copper. Arch Biochem Biophys. 1995;322:127–134. doi: 10.1006/abbi.1995.1444. [DOI] [PubMed] [Google Scholar]
- 6.Davis D A, Dorsey K, Wingfield P T, Stahl S J, Kaufman J, Fales H M, Levine R L. Regulation of HIV-1 protease activity through cysteine modification. Biochemistry. 1996;35:2482–2488. doi: 10.1021/bi951525k. [DOI] [PubMed] [Google Scholar]
- 7.Davis D A, Newcomb F M, Starke D W, Ott D E, Mieyal J M, Yarchoan R. Thioltransferase (glutaredoxin) is detected within HIV-1 and can regulate the activity of glutathionylated HIV-1 protease in vitro. J Biol Chem. 1997;272:25935–25940. doi: 10.1074/jbc.272.41.25935. [DOI] [PubMed] [Google Scholar]
- 8.Gelderblom H R, Ozel M, Pauli G. Morphogenesis and morphology of HIV: structure function relations. Arch Virol. 1989;106:1–13. doi: 10.1007/BF01311033. [DOI] [PubMed] [Google Scholar]
- 9.Goobar-Larsson L, Luukkonen B G, Unge T, Schwartz S, Utter G, Strandberg B, Oberg B. Enhancement of HIV-1 proteinase activity by HIV-1 reverse transcriptase. Virology. 1995;206:387–394. doi: 10.1016/s0042-6822(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 10.Grant S K, Deckman I C, Minnich M D, Culp J S, Franklin S, Dreyer G B, Tomaszek T A, Debouck C, Meek T D. Purification and biochemical characterization of recombinant simian immunodeficiency virus protease and comparison to human immunodeficiency virus type 1 protease. Biochemistry. 1991;30:8424–8434. doi: 10.1021/bi00098a021. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey R W, Ohagen A, Davis D A, Fukazawa T, Hayashi H, Höglund S, Mitsuya H, Yarchoan R. Removal of human immunodeficiency virus type 1 (HIV-1) protease inhibitors from preparations of immature HIV-1 virions does not result in an increase in infectivity or the appearance of mature morphology. Antimicrob Agents Chemother. 1997;41:1017–1023. doi: 10.1128/aac.41.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson V A, Byington R E. Quantitative assays for virus infectivity. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 71–76. [Google Scholar]
- 13.Kageyama S, Hoekzema D T, Murakawa Y, Kojima E, Shirasaka T, Kempf D J, Norbeck D W, Erickson J, Mitsuya H. A C2 symmetry-based HIV protease inhibitor, A77003, irreversibly inhibits infectivity of HIV-1 in vitro. AIDS Res Hum Retroviruses. 1994;10:735–743. doi: 10.1089/aid.1994.10.735. [DOI] [PubMed] [Google Scholar]
- 14.Kageyama S, Mimoto T, Murakawa Y, Nomizu M, Ford H, Jr, Shirasaka T, Gulnik S, Erickson J, Takada K, Hayashi H, Broder S, Kiso Y, Mitsuya H. In vitro anti-human immunodeficiency virus (HIV) activities of transition state mimetic HIV protease inhibitors containing allophenylnorstatine. Antimicrob Agents Chemother. 1993;37:810–817. doi: 10.1128/aac.37.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan A H, Manchester M, Swanstrom R. The activity of the protease of human immunodeficiency virus type 1 is initiated at the membrane of infected cells before the release of viral proteins and is required for release to occur with maximum efficiency. J Virol. 1994;68:6782–6786. doi: 10.1128/jvi.68.10.6782-6786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan A H, Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci USA. 1991;88:4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan A H, Zack J A, Knigge M, Paul D A, Kempf D J, Norbeck D W, Swanstrom R. Partial inhibition of the human immunodeficiency virus type 1 protease results in aberrant virus assembly and the formation of noninfectious particles. J Virol. 1993;67:4050–4055. doi: 10.1128/jvi.67.7.4050-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karacostas V, Wolffe E J, Nagashima K, Gonda M A, Moss B. Overexpression of the HIV-1 Gag-Pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology. 1993;193:661–671. doi: 10.1006/viro.1993.1174. [DOI] [PubMed] [Google Scholar]
- 19.Karlstrom A R, Levine R L. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. Proc Natl Acad Sci USA. 1991;88:5552–5556. doi: 10.1073/pnas.88.13.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlstrom A R, Shames B D, Levine R L. Reactivity of cysteine residues in the protease from human immunodeficiency virus: identification of a surface-exposed region which affects enzyme function. Arch Biochem Biophys. 1993;304:163–169. doi: 10.1006/abbi.1993.1334. [DOI] [PubMed] [Google Scholar]
- 21.Katsumoto T, Hattori N, Kurimura T. Maturation of human immunodeficiency virus, strain LAV, in vitro. Intervirology. 1987;27:148–153. doi: 10.1159/000149733. [DOI] [PubMed] [Google Scholar]
- 22.Kohl N E, Emini E A, Schleif W A, Davis L J, Heimbach J C, Dixon R A F, Scolnick E M, Sigal I S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci USA. 1988;85:4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosower N S, Kosower E M, Wertheim B. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969;37:593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- 24.Kotler M, Simm M, Zhao Y S, Sova P, Chao W, Ohnona S-F, Roller R, Krachmarov C, Potash M J, Volsky D J. Human immunodeficiency virus type 1 (HIV-1) protein Vif inhibits the activity of HIV-1 protease in bacteria and in vitro. J Virol. 1997;71:5774–5781. doi: 10.1128/jvi.71.8.5774-5781.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kräusslich H-G. Specific inhibitor of human immunodeficiency virus proteinase prevents the cytotoxic effects of a single-chain proteinase dimer and restores particle formation. J Virol. 1992;66:567–572. doi: 10.1128/jvi.66.1.567-572.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurata S. Sensitization of the HIV-1-LTR upon long term low dose oxidative stress. J Biol Chem. 1996;271:21798–21802. doi: 10.1074/jbc.271.36.21798. [DOI] [PubMed] [Google Scholar]
- 27.Lambert D M, Petteway S R, Jr, McDanal C E, Hart T K, Leary J J, Dreyer G B, Meek T D, Bugelski P J, Bolognesi D P, Metcalf B W, Matthews T J. Human immunodeficiency virus type 1 protease inhibitors irreversibly block infectivity of purified virions from chronically infected cells. Antimicrob Agents Chemother. 1992;36:982–988. doi: 10.1128/aac.36.5.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindhofer H, von der Helm K, Nitschko H. In vivo processing of Pr160Gag-Pol from human immunodeficiency virus type 1 (HIV) in acutely infected, cultured human T-lymphocytes. Virology. 1995;214:624–627. doi: 10.1006/viro.1995.0074. [DOI] [PubMed] [Google Scholar]
- 29.McCune J M, Rabin L B, Feinberg M B, Lieberman M, Kosek J C, Reyes G R, Weissman I L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988;53:55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- 30.McQuade T J, Tomasselli A G, Liu L, Karacostas V, Moss B, Sawyer T. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990;247:454–456. doi: 10.1126/science.2405486. [DOI] [PubMed] [Google Scholar]
- 31.Meek T D, Dayton B D, Metcalf B W, Dreyer G B, Strickler J E, Gorniak J E, Rosenberg M, Moore M L, Magaard V M, Debouck C. Human immunodeficiency virus 1 protease expressed in Escherichia coli behaves as a dimeric aspartic protease. Proc Natl Acad Sci USA. 1989;86:1841–1845. doi: 10.1073/pnas.86.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patick A K, Mo H, Markowitz M, Appelt K, Wu B, Musick L, Kalish V, Kaldor S, Reich S, Ho D, Webber S. Antiviral and resistance studies of AG1343, an orally bioavailable inhibitor of human immunodeficiency virus protease. Antimicrob Agents Chemother. 1996;40:292–297. doi: 10.1128/aac.40.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piette J, Legrand-Poels S. HIV-1 reactivation after an oxidative stress mediated by different reactive oxygen species. Chem Biol Interact. 1994;91:79–89. doi: 10.1016/0009-2797(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 34.Rayner M M, Cordova B C, Meade R P, Aldrich P E, Jadhav P K, Ru Y, Lam P Y S. DMP 323, a nonpeptide cyclic urea inhibitor of human immunodeficiency virus (HIV) protease, specifically and persistently blocks intracellular processing of HIV gag polyprotein. Antimicrob Agents Chemother. 1994;38:1635–1640. doi: 10.1128/aac.38.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richards A D, Phylip L H, Farmerie W G, Scarborough P E, Alvarez A, Dunn B M, Hirel P H, Konvalinka J, Strop P, Pavlickova L, Kostka L, Kay J. Sensitive, soluble chromogenic substrates for HIV-1 proteinase. J Biol Chem. 1990;265:7733–7736. [PubMed] [Google Scholar]
- 36.Sappey C, Legrand-Poels S, Best-Belpomme M, Favier A, Rentier B, Piette J. Stimulation of glutathione peroxidase activity decreases HIV type 1 activation after oxidative stress. AIDS Res Hum Retroviruses. 1994;10:1451–1461. doi: 10.1089/aid.1994.10.1451. [DOI] [PubMed] [Google Scholar]
- 37.Schatzl H, Gelderblom H R, Nitschko H, von der Helm K. Analysis of non-infectious HIV particles produced in presence of HIV proteinase. Arch Virol. 1991;120:71–81. doi: 10.1007/BF01310950. [DOI] [PubMed] [Google Scholar]
- 38.Schuppe I, Moldeus P, Cotgreave I A. Protein-specific s-thiolation in human endothelial cells during oxidative stress. Biochem Pharmacol. 1992;44:1757–1764. doi: 10.1016/0006-2952(92)90069-u. [DOI] [PubMed] [Google Scholar]
- 39.Sheng N, Pettit S C, Tritch R J, Ozturk D H, Rayner M M, Swanstrom R, Erickson-Viitanen S. Determinants of the human immunodeficiency virus type 1 p15NC-RNA interaction that affect enhanced cleavage by the viral protease. J Virol. 1997;71:5723–5732. doi: 10.1128/jvi.71.8.5723-5732.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirasaka T, Kavlick M F, Ueno T, Gao W Y, Kojima E, Alcaide M, Chokekijchai S, Roy B M, Arnold E, Yarchoan R, Mitsuya H. Emergence of human immunodeficiency virus type 1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc Natl Acad Sci USA. 1995;92:2398–2402. doi: 10.1073/pnas.92.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terada T, Oshida T, Nishimura M, Maeda H, Hara T, Hosomi S, Mizoguchi T, Nishihara T. Study on human erythrocyte thioltransferase: comparative characterization with bovine enzyme and its physiological role under oxidative stress. J Biochem (Tokyo) 1992;111:688–692. doi: 10.1093/oxfordjournals.jbchem.a123819. [DOI] [PubMed] [Google Scholar]
- 42.Vacca J P, Dorsey B D, Schleif W A, Levin R B, McDaniel S L, Darke P L, Zugay J, Quintero J C, Blahy O M, Roth E, Sardana V V, Schlabach A J, Graham P I, Condra J H, Gotlib L, Holloway M K, Lin J, Chen I-W, Vastag K, Ostovic D, Anderson P S, Emini E A, Huff J R. L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc Natl Acad Sci USA. 1994;91:4096–4100. doi: 10.1073/pnas.91.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vance J E, LeBlanc D A, Wingfield P, London R E. Conformational selectivity of HIV-1 protease cleavage of X-Pro peptide bonds and its implications. J Biol Chem. 1997;272:15603–15606. doi: 10.1074/jbc.272.25.15603. [DOI] [PubMed] [Google Scholar]
- 44.Westendorp M O, Shatrov V A, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer P H, Droge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-κ B activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yusa K, Kavlick M F, Pope K, Mitsuya H. HIV-1 acquires resistance to two classes of antiviral drugs through homologous recombination. Antivir Res. 1997;36:179–189. doi: 10.1016/s0166-3542(97)00053-3. [DOI] [PubMed] [Google Scholar]
- 46.Zybarth G, Carter C. Domains upstream of the protease (PR) in human immunodeficiency virus type 1 Gag-Pol influence PR autoprocessing. J Virol. 1995;69:3878–3884. doi: 10.1128/jvi.69.6.3878-3884.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]