Summary
Background
Screening for colorectal cancer (CRC) decreases cancer burden through removal of precancerous lesions and early detection of cancer. The COVID-19 pandemic has disrupted organised CRC screening programs worldwide, with some programs completely suspending screening and others experiencing significant decreases in participation and diagnostic follow-up. This study estimated the global impact of screening disruptions on CRC outcomes, and potential effects of catch-up screening.
Methods
Organised screening programs were identified in 29 countries, and data on participation rates and COVID-related changes to screening in 2020 were extracted where available. Four independent microsimulation models (ASCCA, MISCAN-Colon, OncoSim, and Policy1-Bowel) were used to estimate the long-term impact on CRC cases and deaths, based on decreases to screening participation in 2020. For countries where 2020 participation data were not available, changes to screening were approximated based on excess mortality rates. Catch-up strategies involving additional screening in 2021 were also simulated.
Findings
In countries for which direct data were available, organised CRC screening volumes at a country level decreased by an estimated 1.3–40.5% in 2020. Globally, it is estimated that COVID-related screening decreases led to a deficit of 7.4 million fewer faecal screens performed in 2020. In the absence of any organised catch-up screening, this would lead to an estimated 13,000 additional CRC cases and 7,900 deaths globally from 2020 to 2050; 79% of the additional cases and 85% of additional deaths could have been prevented with catch-up screening, respectively.
Interpretation
COVID-19-related disruptions to screening will cause excess CRC cases and deaths, but appropriately implemented catch-up screening could have reduced the burden by over 80%. Careful management of any disruption is key to improving the resilience of colorectal cancer screening programs.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Cancer Council New South Wales, Health Canada, and Dutch National Institute for Public Health and Environment.
Keywords: Colorectal cancer, Bowel cancer, Cancer screening, COVID-19, COVID, Coronavirus, Public health, Cancer policy, Modelling, Policy evaluation, Epidemiology, Global health
Research in context.
Evidence before this study
Screening asymptomatic individuals with faecal occult blood testing and/or colonoscopy has been found to be effective in reducing colorectal cancer incidence and mortality in long-term cohort follow-up and in trials. Many organised programs provide screening to individuals to help reduce the colorectal cancer burden. Participation is a key determinant in screening program effectiveness, and changes to participation can significantly change long-term cancer incidence and mortality rates. The COVID-19 pandemic has had a significant impact on health systems since 2020 and has affected cancer screening programs worldwide. The short- and long-term implications for cancer burden have not been assessed at a global level.
Added value of this study
This study includes a review of the impact of COVID-19 on organised colorectal cancer screening programs in 2020; where direct data is not available, the extent of disruptions on programs was estimated. These data were used to generate estimates of the long-term impact on colorectal cancer burden using four independent calibrated and validated microsimulation models. This study also assessed the impact of providing catch-up screening for all individuals that missed screening in 2020.
Implications of all the available evidence
COVID-19 significantly disrupted organised colorectal cancer screening globally. Decreases in screening participation in 2020 have potential to result in an increased cancer burden over the decades to come, but this can be largely mitigated through carefully implemented approaches to conducting catch-up screening for those that missed screening.
Introduction
Colorectal cancer (CRC) screening for asymptomatic average-risk individuals typically involves either primary screening with a faecal test followed by a diagnostic colonoscopy for individuals with a positive test result, or primary screening with colonoscopy.1 Organised population-based CRC screening programs have been established in many jurisdictions, particularly in high-income countries.2,3 These programs typically have either national or regional coverage and are organised by government health bodies with oversight of the distribution and processing of faecal tests, usually faecal immunochemical test (FIT), to eligible participants. In some settings, primary colonoscopy screening is also offered alongside faecal testing. Most programs have been established over the last two decades,4 with additional countries initiating pilot programs, notably in South America5,6 and Asia.7
Opportunistic CRC screening, here defined as screening outside of programs administered by government health bodies, also occurs either in lieu of,8,9 or alongside, organised screening.10,11 This is often either ad-hoc screening, frequently by colonoscopy, completed by an individual independently of an organised program, or in many settings screening through an insurer, as is common in the United States12 and Japan13 among other countries.
The COVID-19 pandemic resulted in varying levels of disruption to CRC screening programs between countries. These disruptions have the potential to impact long-term CRC outcomes, and careful management and planning is required to minimise their effect and build more robust programs.14, 15, 16, 17 Both in the wake of the COVID-19 pandemic and in planning for any future disruptions, it is critical to consider the appropriate allocation and continuation of resources, especially where health systems are under strain. Services such as cancer screening can become a lower priority for health systems under pressure and for individuals with competing priorities.18,19
This was exemplified during the COVID-19 pandemic when many countries temporarily paused their organised CRC screening programs; even when screening was available, individuals may have been more reluctant to engage in screening.18 In some settings where programs were not formally paused, decreases in screening volume have been documented. In the Netherlands, for example, primary screening was suspended from March to May 2020,20 while in Australia, the program was not formally suspended but a 55% reduction in organised faecal screening participation rates was observed from March to May 2020 compared to the same period in 2019.21 Reductions were also observed in opportunistic screening rates in settings such as the United States.22
The total reduction in CRC screening rates attributable to COVID-19 may not be clear for some time as waves of COVID-19 infections continue and health systems adjust, and the full extent of the impact on CRC outcomes is unknown.18 Appropriate and timely screening can detect and remove precancerous lesions before they develop into CRC, or detect CRC at earlier stages with better prognosis, leading to a decrease in both CRC cases and deaths. Any shortfall in screening caused by a disruption or delay can diminish these health benefits.23 However, it can be decades before the complex impact of decreases to screening becomes apparent, due to the long sojourn time of precancerous colorectal lesions. Simulation modelling can be useful in providing robust and useful long-term estimates of the impact of screening and disruptions on cancer incidence and mortality rates.24 Modelling outputs can inform policy decision-making when decisive action cannot wait. For example, modelling of hypothetical disruptions to screening informed planning by the Australian Government Department of Health for the National Bowel Cancer Screening Program.16
This study aims to estimate the global impact of screening decreases on CRC outcomes. We harnessed real-world country-level screening data and estimated changes to screening volume in countries for whom direct data were not available. We also estimated the benefits of providing catch-up screening to individuals who missed screening. The focus of this study is on organised screening, as data, such as participation levels and screening frequency is more readily available for these programs unlike opportunistic screening for which data is scarce; additional estimates are provided which may be of relevance to opportunistic screening.
This study was conducted by the International Partnership for Resilience in Cancer Systems (I-PaRCS), formerly the COVID-19 and Cancer Global Modelling Consortium (CCGMC; www.ccgmc.org). I-PaRCS was established in 2020 to support decision-making in cancer control during the COVID-19 pandemic and to support ongoing resilience in cancer systems, and brings together independent modelling groups and other multidisciplinary experts internationally. This study extends the work of prior I-PaRCS studies on COVID-19 and CRC screening conducted at the individual country level.14,15
Methods
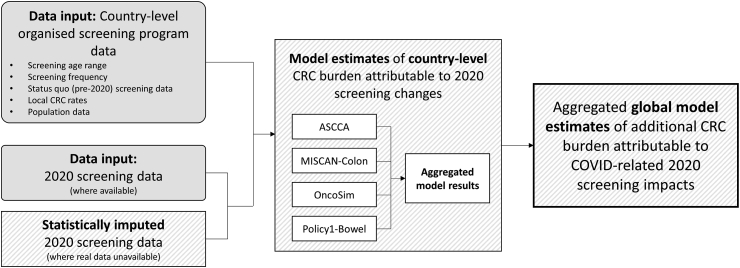
To generate estimates, this work combined a range of data sources and modelling elements (Fig. 1). To summarise:
-
1.
A review of organised screening programs was used to identify program information, pre-2020 screening rates, and, where available, screening rates for 2020.
-
2.
For programs where 2020 screening rates were not available, potential rates were statistically imputed based on local excess mortality rates during 2020.
-
3.
Four independent models were used to estimate the impact of changes to screening participation rates in 2020 on long-term colorectal cancer burden for each organised screening program.
-
4.
Country-level estimates were aggregated to generate global estimates.
Fig. 1.
High-level schematic of the modelling method for generating estimates of the global impact of COVID-related organised screening decreases on long-term CRC burden. For additional details, see Appendix B—Additional modelling methods. CRC: Colorectal cancer.
Further technical details regarding the modelling methods are included in Appendix A—Technical Methods and Appendix B—Additional modelling methods.
Review of organised screening programs
To inform the estimates, a review was completed of existing organised screening programs, including the program design and pre-COVID status-quo screening volume based on the most recent data available prior to 2020. This data was collated by the I-PaRCS team of representatives from many of the countries included in the search, as well as a further review to identify data in countries not represented in the consortium. The most recent data was prioritised in settings where data from 2019 was unavailable; this was used as the “status quo” screening volume for modelling purposes, to estimate screening levels in the absence of any impact of COVID-19. Official data sources, such as governmental reports, were prioritised when there was more than one source; in their absence, we used the most recent and most complete data sources that were identified by the group.
We here use screening volume to refer to the absolute number of people completing screening as part of an organised program, where the decreases in volume potentially reflect both system-level disruptions and individual behaviours. Note that screening volume is related to but distinct from the “screening participation rate” (percentage of people invited who completed screening) or “screening coverage rate” (percentage of people at eligible ages who completed screening). As these measures differ between countries, screening volume was used for modelling purposes, to reduce ambiguity; a change in the absolute number of screening tests completed corresponds to a change in the absolute number of CRC cases and deaths. This avoids possible local variations in the definition of screening participation.
Where 2020 data was available, pre-COVID-19 data were compared to 2020 screening volume during the COVID-19 pandemic to estimate the drop in screening volume. Only screening data identified as of June 2022 was included in the analysis. Based on these countries, a correlation was then estimated between any decreases in screening volume and the WHO-estimated excess all-cause mortality in 2020.25 This correlation was chosen as a proxy for the impact of COVID-19 on health services in general in 2020, and the potential downstream effect on CRC screening. Other indicators of health system disruption, such as COVID cases/deaths, were also evaluated as potential indicators.
The relationship between 2020 excess all-cause mortality and 2020 screening decreases was used to fit a linear regression, which was used to impute estimates of screening decreases for countries where 2020 data was not available. These are here referred to as the imputed COVID-related screening decreases. It must be emphasised that these imputed decreases are not intended to be estimates of the true impact in any specific country, instead providing estimates to be used to generate a global aggregate. Although the correlation is strong enough to suggest a strong estimate in the aggregate, it is not intended to be used to infer realistic local estimates. For this reason, country-level results based on imputed data are included in the appendix only.
Screening programs were stratified into categories based on the recommended age range and frequency of screening (Supplementary Table S1), so that modelling (see below) for countries with similar screening program design could be based on a small set of “template outputs”; this was completed to reduce the computational burden of the modelling by streamlining the analysis where possible. See Appendix A for additional details.
Modelling estimates
To estimate the impact of these decreases to screening volume on short- and long-term health outcomes, four independent microsimulation models of CRC and screening were used to model the relationship between decreases in participation and health outcomes. These models are ASCCA (Adenoma and Serrated pathway to Colorectal CAncer), MISCAN-Colon (MIcrosimulation SCreening ANalysis for colorectal cancer), OncoSim, and Policy1-Bowel. Each of these models have been used at national and international levels to inform health policy, and have been calibrated and validated to ensure they are able to generate meaningful estimates of colorectal cancer burden.26, 27, 28, 29, 30 Key model parameters are included in Supplementary Table S2.
These microsimulation models simulate patients across their lifetime, and estimating their likelihood of developing CRC, including their stage at diagnosis and survival probabilities. The impact of screening, including the removal of precancerous polyps and detection of cancer at earlier stages with higher survival probability, is then modelled. For this project, each of the four models was used to simulate the potential health impacts associated with COVID-related changes to CRC screening for each relevant country based on the participation volumes ascertained in the review and imputation described above. In this way estimates were generated of the downstream impact of screening changes on cancer burden.
Scenarios modelled
Scenarios were simulated based on the possible changes to participation in 2020—see Supplementary Table S3. Scenario A assumed changes were either those observed in each country or the imputed COVID-related screening decreases. Scenarios B, C, and D estimated the impact of 25%, 10%, and 50% decreases in screening volume in 2020 respectively, to represent a likely average drop in screening (Scenario B) as well as low and high possible decreases (Scenarios C and D respectively) as supplementary analyses. These scenarios were simulated even for countries where 2020 screening data was available. Although these scenarios are counterfactual for those countries, by providing a full set of estimates, these outputs can be used to make inferences for future decreases in screening in those countries.
Each scenario was modelled with and without full catch-up screening in 2021 for all individuals who missed screening in 2020, to determine the potential impact of a catch-up screening policies. Full details are included in Appendix A (Technical Methods) and Appendix B (Worked Example). An additional analysis calculating changes in CRC incidence and mortality per screen missed was completed, designed to estimate the relative impact of any disruption to opportunistic screening. A sensitivity analysis exploring the impact of program rollout on outcomes is also included.
Role of the funding source and study approval
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or review of the manuscript; approval of the manuscript, and decision to submit the manuscript for publication. As this was a simulation modelling study with no patient data, no institutional approval or other study approval was required or sought.
Results
Review of organised screening programs
Thirty-one countries and regions were identified which had organised screening programs (Table 1) with sufficient data to inform modelling, with pre-COVID (status quo) participation rates ranging from 13.8% to 74.7%.
Table 1.
Information on the design of national and regional CRC screening programs, and local CRC rates and participation rates.
| Countrya | Age-standardised CRC rates per 100,000 persons |
Screening program |
|||||
|---|---|---|---|---|---|---|---|
| Incidence49 | Mortality49 | Screening age range (years) | Screening interval | Screening categoryb | Status quo screening rate/volume (year)c | Sources | |
| Australia | 33.1 | 8.9 | 50–74 | Biennial | 3 | 43.5% (2019) | 21,50 |
| Belgiumd | 35.3 | 10 | 50–74 | Biennial | 3 | 31.1% (2019) | 51,52 |
| Canada | 31.2 | 9.9 | 50–74 | Biennial | 3 | 42.9% (2017) | 53 |
| Croatia | 36.3 | 19.6 | 50–74 | Biennial | 3 | 15.3% (2014) | 51,54 |
| Czechia | 33.7 | 12.3 | 50−f,g | Biennial | 3 | 30.1% (2019) | 55 |
| Denmark | 40.9 | 11.8 | 50–74 | Biennial | 3 | 60.3% (2019) | 56 |
| France | 30.1 | 10.4 | 50–74 | Biennial | 3 | 30.5% (2019) | 57 |
| Georgia | 15.6 | 8.3 | 50–69 | Biennial | 2 | 74.7% (2015) | 58,59 |
| Germanye | 25.8 | 9.9 | 50−f | Biennialg | 3 | 51.0% (2016) | 60 |
| Hungary | 45.3 | 20.2 | 50–70 | Biennial | 2 | 36.7% (2013) | 51,54 |
| Iceland | 28.5 | 9.5 | 55–75 | Biennial | 2 | 30.0% (2015) | 51,61 |
| Ireland | 34.9 | 12.4 | 60–69 | Biennial | 4 | 112,077h (2018/9) | 62 |
| Israel | 21.9 | 9.0 | 50–74 | Annual | 1 | 1,026,579h (2019) | 63 |
| Italyi | 29.3 | 10.1 | 50–69 | Biennial | 2 | 43.5% (2019) | 64,36 |
| Japan | 38.5 | 11.6 | 40−f | Annual | 1 | 13.8%j (2019) | 65, 66, 67, 68 |
| Lithuania | 27.6 | 11.7 | 50–74 | Biennial | 3 | 17.9% (2018) | 69 |
| Malta | 25.7 | 10.1 | 55–66 | Biennial | 4 | 35.7% (2013) | 51,54 |
| Netherlands | 41.0 | 13.5 | 55–75 | Biennial | 2 | 71.8% (2019) | 70 |
| Portugal, Alentejo and Centro | 39.4 | 13. | 50–69 | Biennial | 2 | 62.8% (2014) | 54 |
| Portugal, Norte | 39.4 | 13. | 50–69 | Biennial | 2 | 29.% (2019) | 71 |
| Singapore | 33.0 | 16.2 | 50−f | Annual | 1 | 27.3% (2016) | 72,73 |
| Slovakia | 43.9 | 21. | 50–74 | Biennial | 3 | 34.0% (2019) | 74 |
| Slovenia | 39.6 | 11.7 | 50–74 | Biennial | 3 | 50.5% (2012) | 60 |
| South Korea | 27.2 | 7.8 | 50–80 | Annual | 1 | 19.1% (2019) | 75 |
| Spaink | 35.8 | 11.5 | 50–69 | Biennial | 2 | 52.9% (2019) | 76,77 |
| Sweden | 27.8 | 10.8 | 60–69 | Biennial | 4 | 68.4% (2016) | 51,78 |
| Switzerland | 22.3 | 7.5 | 50–69 | Biennial | 2 | 16,377h (2014) | 79 |
| Taiwanl | 26.480 | 11.280 | 50–74 | Biennial | 3 | 1,180,000h (2016) | 81 |
| UK–Englandm | 34.1 | 11.4 | 60–74 | Biennial | 4 | 62.4% (2019) | 82 |
| UK—N. Irelandm | 34.1 | 11.4 | 60–74 | Biennial | 4 | 59.4% (2017) | 83 |
| UK–Scotlandm | 34.1 | 11.4 | 50–74 | Biennial | 3 | 63.0% (2019) | 84 |
| UK–Walesm | 34.1 | 11.4 | 58–74 | Biennial | 4 | 58.9% (2019) | 85 |
| Uruguay | 32 | 14.3 | 50–69 | Biennial | 2 | 42.0% (2019) | 86 |
Abbreviations: CRC, Colorectal cancer; UK, United Kingdom.
For Luxembourg, Montenegro, and New Zealand, it was established that there was an organised screening program, but no data was identified on the participation rates in these programs; they were excluded from the analysis.
See Supplementary Table S1. Closest screening category identified to be used in modelling, based on screening interval, start age, and number of lifetime screens.
The year noted is the most recent year prior to 2019 with complete screening data available at the time of review.
Based on data from Flanders, Wallonia, and Brussels regions.
Also offers organized colonoscopy screening; for the purposes of this study, only faecal screening has been included.
No official screening stop age.
Screening is offered annually from ages 50–54.
Where screening is provided as screening volume (number of participants) in official sources rather than participation rate, this has been replicated here.
Calculated from data available by regional programs.
Based on data from municipal screening, as screening participation rates were not available for workplace-based screening. Workplace-based screening is likely to have higher participation rates, but up-to-date data is unavailable.
Calculated from data available for regional programs in Barcelona, Basque Country, Valencia, and Catalonia.
GloboCan data on incidence and mortality was not available for Taiwan.
For England, Northern Ireland, Scotland, and Wales, local screening program data was used, and CRC incidence rates and mortality ASRs were overall United Kingdom rates from GloboCan.
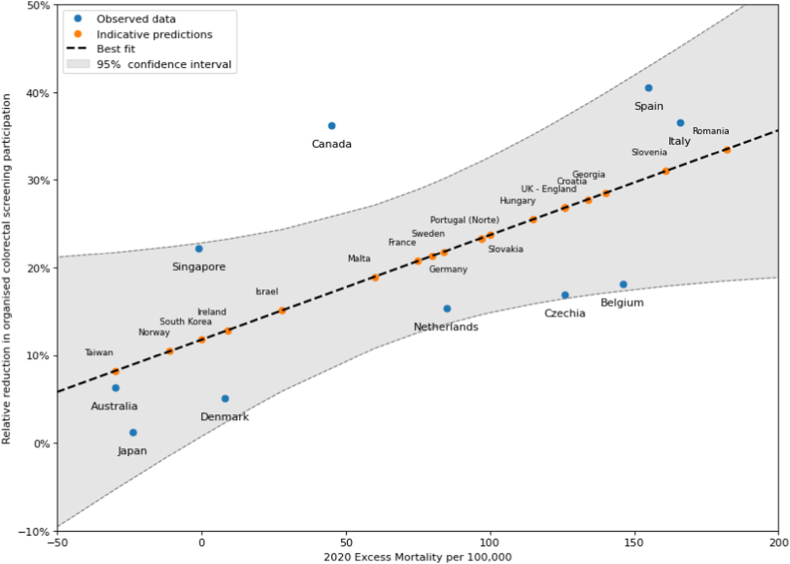
In ten countries, screening data were identified to calculate the relative decrease in screening volume in 2020 compared to pre-2020 rates. These ranged from a 1.27% decrease to a 40.51% decrease (Table 2, Fig. 2).
Table 2.
Scenario A: estimated increase in CRC cases and deaths over 2020–2050 by country, for countries with robust data on the decrease in screening participation in 2020.
| Relative reduction in primary screening in 2020 vs 2019 | Reduction in screening volume in 2020 vs expected | No catch-up (Scenario A.1) |
Full catch-up in 2021 (Scenario A.2) |
Reduction in additional burden attributable to catch-up screening |
Excess all-cause mortality per 100,000, 202,025 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Additional cases, 2020–2050 | Additional deaths, 2020–2050 | Additional cases, 2020–2050 | Additional deaths, 2020–2050 | Cases, 2020–2050 | Deaths, 2020–2050 | ||||
| Australia | 6.33%21 | 111,410 | 221 (109, 297) | 101 (65, 124) | 47 (4, 150) | 15 (−13, 57) | 78.70% | 85.10% | −30 |
| Belgium | 18.08%87,a | 77,262 | 167 (93, 232) | 81 (57, 96) | 36 (3, 115) | 12 (−11, 46) | 78.40% | 85.20% | 146 |
| Canada | 36.22%88,36,b | 1,276,680 | 2,174 (1,002, 3,037) | 1,154 (720, 1,449) | 478 (57, 1,544) | 190 (−135, 694) | 78.00% | 83.50% | 45 |
| Czechia | 16.86%36, 88 | 106,835 | 213 (109, 278) | 132 (89, 160) | 45 (−3, 178) | 20 (−6, 88) | 78.90% | 84.80% | 126 |
| Denmark | 5.06%89,c | 22,991 | 51 (25, 71) | 26 (17, 31) | 11 (1, 35) | 4 (−2, 14) | 78.40% | 84.60% | 8 |
| Italy | 36.58%36, 88 | 878,458 | 1,907 (1,192, 2,475) | 1,102 (837, 1,396) | 365 (−73, 1,507) | 118 (−99, 708) | 80.90% | 89.30% | 166 |
| Japan | 1.27%36, 88 | 79,876 | 114 (67, 138) | 55 (47, 71) | 114 (67, 138) | 55 (47, 71) | −c | −c | −24 |
| Netherlands | 15.36%90 | 250,174 | 553 (243, 813) | 298 (180, 363) | 135 (−7, 542) | 58 (−8, 243) | 75.60% | 80.50% | 85 |
| Singapore | 22.22%36, 88 | 15,131 | 17 (9, 22) | 12 (9, 18) | 17 (9, 22) | 12 (9, 18) | −c | −c | −1 |
| Spain | 40.51%76,77,36,d | 328,217 | 896 (655, 1,197) | 479 (403, 637) | 158 (−41, 676) | 25 (−101, 297) | 82.40% | 94.80% | 155 |
All results are relative to the comparator (status quo participation rates in 2020). Results in brackets are the range of model estimates.
Based on data from Flanders and Brussels regions.
Based on data for Ontario.
Catch-up screening was not simulated for annual programs, as individuals would be invited to screening in 2021 regardless.
Based on data from Barcelona, Basque, Valencia, and Catalonia regions.
Fig. 2.
Relative decreases to organised screening participation in 2020 vs local 2020 excess all-cause mortality rates.25 For areas where 2020 screening data was unavailable, the best fit was used to calculate the imputed screening decrease. These decreases were used to model Scenario A.1 and A.2. The shaded region shows the 95% confidence interval for the imputed values.
Although 2020 screening data were limited at the time of review, the relative decreases were strongly correlated with estimated 2020 excess all-cause mortality rates per 100,000 in each country.25 This was thus used as an indirect measure of the impact of COVID on local health systems, and thus on colorectal cancer screening, in 2020. The Pearson correlation coefficient for this data was R = 0.66.
Based on this correlation, the following linear regression model was fit to describe a linear relationship between the two variables (Fig. 2).
The correlation between other factors and screening decreases in 2020 were calculated, such as COVID-19 cases or deaths. Excess all-cause mortality was found to have the strongest correlation and thus was used for this study. Note that excess CRC mortality in 2020 is unlikely to be a significant contributor to excess short-term (2020) all-cause mortality, both due to the small proportion of deaths in the population and data sources suggesting there have been limited changes to cancer mortality during the COVID-19 pandemic so far.31 The findings of this study demonstrate that the impact on excess CRC mortality is more likely to be a long-term effect. Therefore, excess all-cause mortality was chosen as an appropriate independent indicator of the impact of COVID-19.
Modelled estimates of CRC health outcomes
The screening data identified were used to inform modelled estimates of the impact of screening decreases on CRC incidence and mortality. Across all modelled scenarios, decreases to screening would lead to increases in CRC incidence and mortality rates, with greater decreases in screening volume leading to greater increases in excess CRC incidence and mortality. Globally, it was estimated that the aggregate observed and imputed COVID-related screening decreases (Scenario A) led to a deficit of 7.4 million faecal screens in organised programs in 2020 (Table 3). Among the countries for which 2020 screening data was available, approximately 3.1 million faecal screens in organised programs would be missed (Table 2); 42% of the estimated global total. The remaining 58% of missed screens were estimated to occur in countries without data available on screening in 2020.
Table 3.
Global outcomes for organised screening programs, for observed and imputed COVID-related screening decreases (Table 2 and Supplementary Table S6), and relative 10%, 25%, and 50% screening decreases in 2020.
| Scenario A: Observed and imputed COVID-related screening decrease in 2020 | Scenario B: 25% relative screening decrease in 2020 | Scenario C: 10% relative screening decrease in 2020 | Scenario D: 50% relative screening decrease in 2020 | ||
|---|---|---|---|---|---|
| Screens missed, 2020 | 7,432,858 | 9,829,213 | 3,931,685 | 19,658,427 | |
| No catch-up | Additional cases, 2020–2050 | 13,600 (7,143, 17,733 | 16,848 (8,841, 21,790) | 6,739 (3,536, 8,716) | 33,696 (17,683, 43,581) |
| Additional deaths, 2020–2050 | 7,989 (5,422, 9,857) | 9,639 (6,668, 12,175) | 3,855 (2,667, 4,870) | 19,279 (13,336, 24,351) | |
| Full catch-up | Additional cases, 2020–2050 | 2,883 (566, 8,448) | 5,578 (2,128, 11,739) | 2,231 (851, 4,695) | 11,157 (4,256, 23,478) |
| Additional deaths, 2020–2050 | 1,178 (−482, 4,056) | 2,478 (539, 5,232) | 991 (215, 2,092) | 4,956 (1,078, 10,464) | |
All results are relative to the comparator (status quo participation rates in 2020). Results in brackets are the range of model estimates.
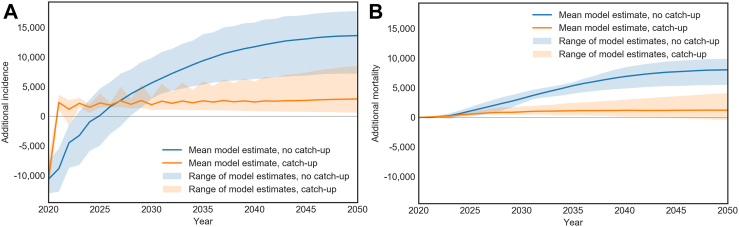
In 2020, there would be an estimated decrease of 10,664 CRC diagnoses, which would be diagnosed either at later screening rounds or symptomatically, potentially at later disease stages. This decrease in diagnoses in 2020 and subsequent increase can be seen in Fig. 3. Over the period 2020–2050, this would lead to 14,000f excess CRC cases and 8,000 excess CRC deaths in the absence of any catch-up screening for individuals who missed screening, compared to status quo screening volume in 2020 (Fig. 3). Between the four models used, the estimated excess cases ranged from 7,100 to 18,000, and excess deaths ranged from 5,400 to 9,900; model ranges are included in the results tables. In scenarios where catch-up screening was modelled, the diagnoses that were missed could be made in 2021 during catch-up screening. Catch-up screening in 2021 for individuals in biennial screening programs who missed screening could prevent up to 79% of the excess cases and up to 85% of the excess deaths from 14,000 to 2,900 and 8,000 to 1,200 respectively. Annual global outcomes are included in Supplementary Table S4.
Fig. 3.
Scenario A: Global cumulative additional CRC cases (panel A) and CRC deaths (panel B), with modelled 2020 observed decreases in screening volume and imputed decreases based on local COVID-19 death rates where local data is not available. Results are shown without (Scenario A.1) and with (Scenario A.2) catch-up in each panel. All results are relative to the comparator (status quo participation rates in 2020). Shaded regions show the range between the estimates generated by the four models.
The health impacts of decreases to screening for countries with 2020 data are shown in Table 2, and results for individual countries with imputed screening decreases are shown in Supplementary Table S5 and Supplementary Fig. S1.
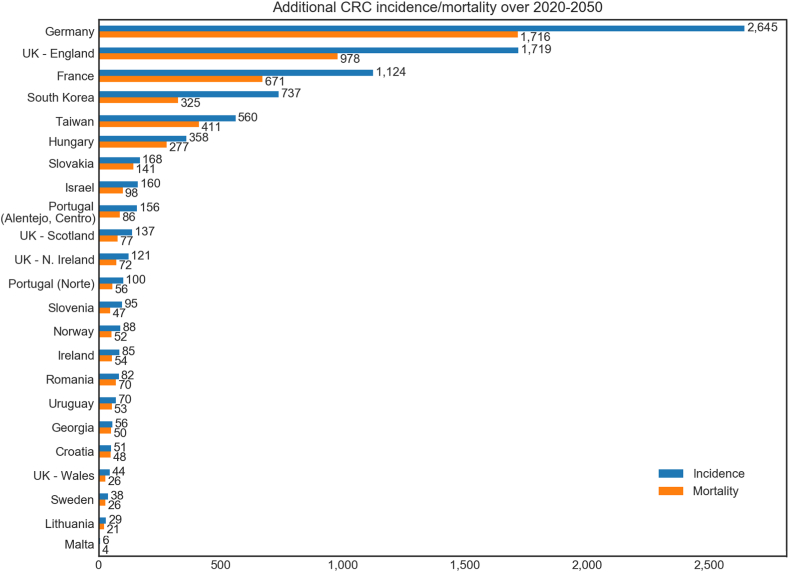
In Scenario B, a 25% relative reduction in screening in 2020 across all organised screening programs was simulated. Per-country results from this analysis are shown in Supplementary Table S6 and Fig. 4, and global results are shown in Table 3 and Supplementary Fig. S2. In this scenario, 11 million screens would be missed in 2020, and there would be an excess 17,000 CRC cases and 9,600 CRC deaths over 2020–2050. With catch-up screening, this would be limited to 5,600 excess cases and 2,500 excess deaths—a 67% and 74% reduction, respectively. Annual global outcomes are included in Supplementary Table S7.
Fig. 4.
Scenario B.1: Additional CRC cases and deaths over 2020–2050 attributable to a hypothetical 25% relative decrease in all countries without robust 2020 screening data available. No catch-up screening was modelled for this scenario. All results are relative to the comparator (status quo participation rates in 2020) and are organised in order of additional cases.
Simulations were also completed with 10% and 50% relative screening decreases in each country (Scenarios C and D)—larger decreases led to proportionally larger impacts on health outcomes (Table 3, Supplementary Table S8, Supplementary Table S9). Per-model global results are also included for comparison purposes (Supplementary Table S10).
Other CRC screening
In all settings with screening, including opportunistic screening, any decrease in screening in 2020 would translate to an increase in cancer burden over 2020–2050, and catch-up screening could reduce the impact on incidence and mortality by 73–88% and 81–94% respectively (Table 4, Supplementary Fig. S3).
Table 4.
Total additional CRC cases and deaths over 2020–2050, per 100,000 screens missed in 2020 (faecal or colonoscopy).
| Screening category | No catch-up |
Full catch-up |
Reduction in additional burden attributable to catch-up screening |
|||
|---|---|---|---|---|---|---|
| Additional CRC cases per 100,000 screens missed, 2020–2050 | Additional CRC deaths per 100,000 screens missed, 2020–2050 | Additional CRC cases per 100,000 screens missed, 2020–2050 | Additional CRC deaths per 100,000 screens missed, 2020–2050 | CRC cases | CRC deaths | |
| SC 1 (annual faecal screening, age 50–74) | 53 (23, 77) | 25 (11, 38) | −a | – | – | – |
| SC 2 (biennial faecal screening, age 50–69) | 101 (43, 180) | 61 (35, 117) | 23 (−5, 86) | 10 (−9, 38) | 77.2% | 83.6% |
| SC 3 (biennial faecal screening, age 50–74) | 92 (35, 145) | 53 (34, 79) | 25 (4, 67) | 10 (−4, 31) | 72.8% | 81.1% |
| SC 4 (biennial faecal screening, age 60–69) | 131 (65, 204) | 85 (59, 127) | 19 (7, 32) | 5 (−9, 9) | 85.5% | 94.1% |
| SC 5 (colonoscopy screening at age 50, 60 and 70) | 1,056 (761, 1,634) | 455 (284, 689) | 124 (−48, 425) | 43 (−15, 137) | 88.3% | 90.5% |
All results are relative to the comparator (status quo participation rates in 2020). Results in brackets are the range of model estimates.
Catch-up screening was not simulated for annual programs, as individuals would be invited to screening in 2021 regardless.
For example, in the United States, it was estimated that 3.8 million screening colonoscopies were missed from March to May 2020 through opportunistic screening,22 which is primarily conducted through individuals’ healthcare insurance providers. These modelling estimates indicate that this chance in opportunistic colonoscopy screening alone could lead to an estimated additional 41,000 CRC cases and 17,000 CRC deaths over 2020–2050 without proper catch-up. There would also be impacts due to disruptions to the use of faecal tests, which were significantly affected in the US; however, quantifying this effect was complicated by recent changes to screening age range recommendations,32 making it difficult to measure the true impact. This is likely to be the case in other high-income countries as well—however, there is not sufficient evidence on the volume or patterns of screening outside of organised programs to make specific estimates in the scope of this project. However, the results regarding the impact of catch-up screening are likely to be generally true across all programs.
Sensitivity analyses
The sensitivity analysis on the impact of program start time showed that excess CRC incidence and mortality rates were highest when the screening program had only been implemented in 2020. Only small differences in outcomes were observed between implementation in 2005, 2010, and 2015, for both faecal and colonoscopy screening (Supplementary Fig. S4, Supplementary Fig. S5). This implies that new screening programs were more likely to be impacted by the pandemic compared to programs that had been established in 2015 or earlier. The impact of differing implementation years is significantly reduced when catch-up screening is included.
Discussion
This is the first modelling study to estimate the impact of decreases to organised CRC screening on a global scale, informed by real-world and estimated screening data on the scale of disruption during the COVID-19 pandemic. Although 2020 screening data was unavailable in many settings, global health impacts were estimated by estimating potential decreases to screening. This study estimated that CRC screening disruptions in 2020 could lead to 14,000 additional CRC cases and 8,000 additional CRC deaths worldwide over the next 30 years. Our results show that catch-up screening can reduce excess cases and deaths by up to 80%.
The current analysis builds on prior studies, which assessed the impact of colorectal screening disruptions for selected individual countries.14,15,33,34 This includes previous studies from the I-PaRCS consortium which modelled the impact of hypothetical pauses to screening, before data on 2020 disruptions was available. The estimates from the current study give, for the first time, an insight into the global impact of the pandemic on cancer burden related to colorectal cancer screening. Although catch-up screening was only simulated in 2021 and later catch-up was not explicitly modelled, these results strongly suggest that countries should attempt to catch-up in those who missed screening as soon as practicable; this is likely to mitigate much of the long-term adverse effects on CRC burden.
A key strength of this study is the comparative analysis harnessing multiple well-established microsimulation models and experienced modelling teams, who completed the analyses independently. The range of estimates across models was aggregated and provides researchers and policymakers with useful data on both the total possible impact and uncertainty around these estimates. As it is infeasible to develop tailored microsimulation models for each country included in this study, multiple models provide a broader and more robust range of estimates, as conditions in each country are likely to resemble the conditions simulated by at least one of the models. This approach to global modelling by simulating a core set of imputed screening programs and extrapolating may be useful in future global epidemiological modelling and builds on similar approaches used previously.35
The scenarios modelled represent a range of decreases to screening volumes in 2020, capturing both observed decreases and hypothetical 10%, 25%, and 50% decreases. The true decrease to screening in any setting is likely to fall in the range captured by these scenarios. Data on catch-up screening conducted in 2021 were not available, but the true rate of catch-up screening in any given country will lie between the “no catch-up” and “full catch-up” scenarios modelled. For example, the observed shortfall in screening in Denmark in 2020 is believed to be attributable to a delay in screening invitations.36 It is therefore likely that all individuals who would have participated in 2020 returned to screening in 2021, meaning health outcomes in Denmark would align more closely with full catch-up (Scenario A.2, Table 2) with a limited impact on CRC outcomes. Screening data for the Flanders region of Belgium showed that formal catch-up screening was provided within 2020, with those that missed screening in March–May or November invited to return in June–July or December respectively.37 Overall, screening participation in Flanders was 48.5% in 2020, compared to 51.5% in 2019—this rapid return to status quo is estimated to lead to a lessened impact on health outcomes, demonstrating the benefit of catch-up screening. Generally, these estimates show the benefit of catch-up screening for individuals that miss a screening round, whether due to COVID-19 or other causes. Although in any given setting it may be difficult to administer catch-up, either due to capacity issues or ongoing disruptions, wherever possible individuals should be returned to screening as soon as possible.
Our global estimates results provide a useful indication of the possible impact of disruptions to organised CRC screening but there are limitations. These fall primarily into two categories: data limitations and modelling limitations. Most notably, screening data from 2020 was not available or was incomplete in many countries. To address this, imputed decreases for countries where data were not available were inferred using a novel method correlating screening with excess all-cause mortality. Though they are not intended to reflect the true local screening impact and are unlikely to be accurate on a country level, these results are useful in generating global estimates of the possible impact on CRC outcomes, and the ranges of estimates including 10%, 25%, and 50% reductions in Scenarios B, C, and D provide a set of realistic outcomes in each country. This range of hypothetical disruptions can also be used to inform planning and policy around future disruptions of various magnitudes. The observed correlation between excess all-cause mortality and a specific health service such as colorectal cancer screening has implications for other similar exercises in terms of understanding the impacts on health services, and suggests that excess all-cause mortality might be a useful general marker of health system resilience.
As in any modelling study, there are limitations to what can be feasibly and accurately modelled. Screening programs can differ in complex and nuanced ways, and we could not directly model each country of interest. Therefore, it was necessary to identify the most important variables to represent each country and simplify the other parameters. Some countries were assigned to a screening category which only approximately matched the local setting; these results may be less accurate. There were also countries where no participation data could be found, either before or during 2020; these countries were excluded from the analysis. These countries had smaller populations and less well-established programs, so are unlikely to have a large volume of screening presently.
Other nuances of CRC screening could not be captured in as much detail as a setting-specific microsimulation model-based analysis. For example, post-polypectomy colonoscopy surveillance recommendations were assumed to be the same as the originally modelled countries, and no COVID-related changes to colonoscopy surveillance could be captured due to a lack of data. Although surveillance recommendations can vary by country, it has previously been noted that differing recommendations would not have a significant impact on population-level health outcomes,38 so this is unlikely to substantially impact our predictions. Similarly, test characteristics of faecal tests were assumed to be the same as the originally modelled country, due to a lack of data around sensitivity and specificity of specific brands and thresholds used in each setting. Additionally, the detailed background rollout of the screening program in each country over time could not be fully captured, as these rollouts can be quite complex, with many countries changing screening technology and/or age range. To address this, the effects of different program initiation years were assessed in a sensitivity analysis and were shown to have minor impact (Appendix C).
Trends in CRC incidence and variations in the specificity and sensitivity of the specific brand of screening tests used in each country were not captured by this study. Overall CRC incidence and mortality rates in each country were accounted for, and other factors were modelled as in the originally modelled countries. Because of this and the other reasons listed above, we did not present estimates of CRC cases and deaths in each individual simulated country, nor the impact of status quo screening; only estimates of the overall changes in CRC cases and deaths attributable to a decrease in screening. We also did not model the impacts of COVID-19 on CRC incidence and survival outside of the direct effects of screening participation–for example, effects on diagnostic rates outside of screening,20,39 the effect of delays to treatment,40 and survival rates in people with cancer who contract COVID-19.41 Future work will address the combined impact of these effects on the long-term CRC burden.
Specific results could not be generated for opportunistic screening, including colonoscopy screening, as longitudinal data on this type of screening and its effectiveness is sparse and cannot be modelled in a generalisable way. Opportunistic screening exists in many countries, and so may also represent a significant increase in CRC burden due to COVID-related disruptions that is not captured in the scope of this study. Instead, we reported rates of additional CRC cases and deaths per 100,000 people who missed screening in 2020. Without reference to specific settings, these rates make a clear case for the importance of catch-up screening, which was estimated to reduce the increased burden in CRC cases and deaths by 72.8–94.1%. Decreases in opportunistic screening are likely to lead to increases to disease burden similar to decreases in organised programs. The qualitative demonstration of the benefits of catch-up screening is clear. Opportunistic screening on a large scale as in the United States experienced massive decreases in screening volumes in 2020; in settings like this, catch-up screening could lead to a dramatic improvement in outcomes. It should also be noted that much of the world, particularly developing countries, lack access to screening, whether organised or opportunistic; although this means the COVID-19 pandemic may not have had an impact on screening in these settings, lack of access screening still contributes significantly to inequities in CRC burden worldwide.
This study, in line with previous studies,14,33,34 highlights the importance of ensuring that individuals return to screening as soon as possible, via organised targeted catch-up screening. By targeting individuals who missed screening during 2020 and/or 2021, screening could return to near-typical effectiveness. Mass-media campaigns aimed at improving screening participation can be very cost-effective,42,43 even at high cost44; similar campaigns could help screening return to pre-COVID levels.
Careful monitoring of this catch-up screening would be needed to manage the increase in follow-up colonoscopy demand.45 Previous estimates suggest that using a higher positivity threshold for faecal testing and/or extending the window for catch-up screening could help manage colonoscopy demand by prioritising individuals at highest risk.42, 43 These results will help manage the diagnostic burden as health systems return to capacity. In many countries program-related colonoscopies make up a small proportion of the overall colonoscopy burden,46 and prioritising patients with positive faecal tests could lead to better outcomes.47 Many countries have already taken measures to address changes to colonoscopy supply and demand.48
Global emergencies such as the COVID-19 pandemic impact all aspects of health services and delivery. For preventative health measures which provide long-term benefits such as CRC screening, the effect may manifest for decades to come, and there is a risk that the lifesaving benefits will not be accessed for many people. The findings of this study show not only the possible extent of this impact globally, but also describes a path forward through appropriate catch-up screening and continued encouragement of screening participation. As these health impacts can take decades to eventuate, it is crucial that this catch-up screening occurs as quickly as possible. By carefully planning and designing screening programs to be resilient to future disruptions, health systems can reduce the impact on population health.
Contributors
Conceptualisation: JW, FvW, ZS, LdJ, JBL, MG, EF, ILV, VMHC, JHEY, KC.
Methodology: JW, FvW, ZS LdJ, JBL, MG, EF, ILV, VMHC, JHEY, KC.
Analysis: JW, FvW, ZS, LdJ, FvdP.
Writing—original draft: JW, FvW.
Writing—review & editing: ZS, LdJ, JBL, MG, RvdP, EF, ILV, VMHC, JHEY, KC.
Supervision: KC.
JW, FvW, ZS, and LdJ accessed and verified the data used in this study. No patient data was used in this study.
Data sharing statement
The data used to generate the findings of this study were collected from publicly available data cited in this manuscript and/or the included supplementary materials. Relevant data used to inform the modelling are included in the supplementary materials, and further details on the modelling are included in previous studies noted in the text. Further details are available from the corresponding author after publication upon reasonable request.
Declaration of interests
Karen Canfell is co-PI of an investigator-initiated trial of cervical screening, “Compass”, run by the Australian Centre for Prevention of Cervical Cancer (ACPCC), which is a government-funded not-for-profit charity. Compass receives infrastructure support from the Australian government and the ACPCC has received equipment and a funding contribution from Roche Molecular Diagnostics, USA. Karen Canfell is co-PI on a major implementation program Elimination of Cervical Cancer in the Western Pacific which has received support from the Minderoo Foundation and the Frazer Family Foundation and equipment donations from Cepheid Inc. Dr. Lew reports grants from National Health and Medical Research Council, during the conduct of the study. Dr. Feletto reports grants from National Health and Medical Research Council, outside the submitted work. Dr Coupé reports grants from Dutch Cancer Foundation, grants from Netherlands Organisation for Health Research and Development, and from Maag Lever Darm Stichting MLDS, outside the submitted work.
Acknowledgements
The authors thank the following contributors.
L. Anderson, A. Cust, E. Dekker, A. Dell’Anna, J.A. Espinas, L. Flander, M. Garcia, I. Idigoras, K. Katanoda, L. Laghi, F. Lamrock, E. McFerran, A. Molina-Barceló, S. Njor, V.K. O'Connor, I. Portillo, C. Senore, E. Symonds, I. Tachecí, G. Taksler, M.A. Tolani, H.A. Valk.
The authors thank the following who contributed data to this study.
J. Gao, J.L. Bulliard, T. Mooney, D. Öhman, D. Novak-Mlakar, A.L. Skrjanec, T. Kofol, T. Sarkeala, C. McKee, T. Owen.
Footnotes
Figures in text cited to two significant figures.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102081.
Appendix A. Supplementary data
References
- 1.International Agency for Research on Cancer . IARC; 2019. Colorectal cancer screening. IARC handbooks of cancer prevention Volume 17. [PubMed] [Google Scholar]
- 2.Ferlizza E., Solmi R., Sgarzi M., Ricciardiello L., Lauriola M. The roadmap of colorectal cancer screening. Cancers. 2021;13(5):1101. doi: 10.3390/cancers13051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreuders E.H., Ruco A., Rabeneck L., et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 4.Chiu H.M., Jen G.H.H., Wang Y.W., et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021;70(12):2321–2329. doi: 10.1136/gutjnl-2020-322545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guimarães D.P., Mantuan L.A., De Oliveira M.A., et al. The performance of colorectal cancer screening in Brazil: the first two years of the implementation program in Barretos Cancer Hospital. Cancer Prev Res. 2021;14(2):241–252. doi: 10.1158/1940-6207.CAPR-20-0179. [DOI] [PubMed] [Google Scholar]
- 6.Reich M., Buki L.P. Colorectal cancer screening in Uruguay: current assessment and roadmap for the future. Psicol Reflexão Crítica. 2021;34:20. doi: 10.1186/s41155-021-00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Li N., Ren J., et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. 2019;68(8):1450–1457. doi: 10.1136/gutjnl-2018-317124. [DOI] [PubMed] [Google Scholar]
- 8.de Moor J.S., Cohen R.A., Shapiro J.A., et al. Colorectal cancer screening in the United States: trends from 2008 to 2015 and variation by health insurance coverage. Prev Med. 2018;112:199–206. doi: 10.1016/j.ypmed.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han A., Maratt J., Kahi C. Colorectal cancer screening decisions in the opportunistic setting. Gastrointest Endosc Clin. 2020;30(3):413–422. doi: 10.1016/j.giec.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Emery J.D., Pirotta M., Macrae F., et al. 'Why don't I need a colonoscopy?': a novel approach to communicating risks and benefits of colorectal cancer screening. Aust J Gen Pract. 2018;47(6):343–349. doi: 10.31128/AJGP-11-17-4386. [DOI] [PubMed] [Google Scholar]
- 11.Ouakrim D.A., Lockett T., Boussioutas A., et al. Screening practices of Australian men and women categorized as “at or slightly above average risk” of colorectal cancer. Cancer Causes Control. 2012;23(11):1853–1864. doi: 10.1007/s10552-012-0067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand S., Liang P.S. A practical overview of the stool DNA test for colorectal cancer screening. Clin Transl Gastroenterol. 2022;13(4) doi: 10.14309/ctg.0000000000000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi H., Machii R., Nakayama T. Analysis of population-based and worksite cancer screening in Japan. Eur J Publ Health. 2020;30(Supplement_5):1348. [Google Scholar]
- 14.de Jonge L., Worthington J., van Wifferen F., et al. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and The Netherlands: a comparative modelling study. Lancet Gastroenterol Hepatol. 2021;6(4):304–314. doi: 10.1016/S2468-1253(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Wifferen F., de Jonge L., Worthington J., et al. Prioritisation of colonoscopy services in colorectal cancer screening programmes to minimise impact of COVID-19 pandemic on predicted cancer burden: a comparative modelling study. J Med Screen. 2021;29 doi: 10.1177/09691413211056777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worthington J., Lew J., Canfell K., Feletto E. Report to the Department of Health; 2020. Modelled analysis of potential hypothetical impacts of COVID-19 related disruptions on the National Bowel Cancer Screening Program. [Google Scholar]
- 17.Smith M.A., Burger E.A., Castanon A., et al. Impact of disruptions and recovery for established cervical screening programs across a range of high-income country program designs, using COVID-19 as an example: a modelled analysis. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basu P., Alhomoud S., Taghavi K., Carvalho A.L., Lucas E., Baussano I. Cancer screening in the coronavirus pandemic era: adjusting to a new situation. JCO Global Oncol. 2021;7(1):416–424. doi: 10.1200/GO.21.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feletto E., Grogan P., Nickson C., Smith M., Canfell K. How has COVID-19 impacted cancer screening? Adaptation of services and the future outlook in Australia. Public Health Res Pract. 2020;30(4) doi: 10.17061/phrp3042026. [DOI] [PubMed] [Google Scholar]
- 20.Toes-Zoutendijk E., Vink G., Nagtegaal I.D., et al. Impact of COVID-19 and suspension of colorectal cancer screening on incidence and stage distribution of colorectal cancers in The Netherlands. Eur J Cancer. 2022;161:38–43. doi: 10.1016/j.ejca.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Australian Institute of Health and Welfare Cancer screening programs: quarterly data. 2021. https://www.aihw.gov.au/reports/cancer-screening/national-cancer-screening-programs-participation [cited 2022 Jul 7]. Available from:
- 22.Chen R.C., Haynes K., Du S., Barron J., Katz A.J. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878–884. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.San Miguel Y., Demb J., Martinez M.E., Gupta S., May F.P. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997–2005.e3. doi: 10.1053/j.gastro.2021.01.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lew J.B., Feletto E., Wade S., et al. Benefits, harms and cost-effectiveness of cancer screening in Australia: an overview of modelling estimates. Public Health Res Pract. 2019;29(2) doi: 10.17061/phrp2921913. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization The true death toll of COVID-19: estimating global excess mortality. 2021. https://wwwwhoint/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality
- 26.Lew J.B., St John D.J., Xu X.M., et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. Lancet Public Health. 2017;2:e331–e340. doi: 10.1016/S2468-2667(17)30105-6. [DOI] [PubMed] [Google Scholar]
- 27.Loeve F., Boer R., van Oortmarssen G.J., van Ballegooijen M., Habbema J.D. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32(1):13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 28.Van Hees F., Zauber A.G., Van Veldhuizen H., et al. The value of models in informing resource allocation in colorectal cancer screening: the case of The Netherlands. Gut. 2015;64(12):1985–1997. doi: 10.1136/gutjnl-2015-309316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greuter M.J., Xu X.M., Lew J.B., et al. Modeling the adenoma and serrated pathway to colorectal CAncer (ASCCA) Risk Anal. 2014;34(5):889–910. doi: 10.1111/risa.12137. [DOI] [PubMed] [Google Scholar]
- 30.Coldman A., Pader J., Gauvreau C., et al. Simulating results from trials of sigmoidoscopy screening using the OncoSim microsimulation model. J Cancer Policy. 2018;15:52–58. [Google Scholar]
- 31.Australian Bureau of Statistics . ABS; 2022. Measuring Australia's excess mortality during the COVID-19 pandemic. [Google Scholar]
- 32.Lee J.K., Lam A.Y., Jensen C.D., et al. Impact of the COVID-19 pandemic on fecal immunochemical testing, colonoscopy services, and colorectal neoplasia detection in a large United States community-based population. Gastroenterology. 2022;163(3):723–731.e6. doi: 10.1053/j.gastro.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yong J.H., Mainprize J.G., Yaffe M.J., et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2021;28(2):100–107. doi: 10.1177/0969141320974711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandrik O., Chilcott J., Thomas C. Modelling the impact of the coronavirus pandemic on bowel cancer screening outcomes in England: a decision analysis to prepare for future screening disruption. Prev Med. 2022;160 doi: 10.1016/j.ypmed.2022.107076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simms K.T., Steinberg J., Caruana M., et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: a modelling study. Lancet Oncol. 2019;20(3):394–407. doi: 10.1016/S1470-2045(18)30836-2. [DOI] [PubMed] [Google Scholar]
- 36.International Cancer Screening Network (ICSN) 2022. Results of the ICSN colorectal cancer screening interest group survey on the impact of COVID-19.https://icsn.global/resources/ Available from: [Google Scholar]
- 37.Jidkova S., Hoeck S., Kellen E., le Cessie S., Goossens M.C. Flemish population-based cancer screening programs: impact of COVID-19 related shutdown on short-term key performance indicators. BMC Cancer. 2022;22(1):1–12. doi: 10.1186/s12885-022-09292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greuter M.J.E., de Klerk C.M., Meijer G.A., Dekker E., Coupe V.M.H. Screening for colorectal cancer with fecal immunochemical testing with and without postpolypectomy surveillance colonoscopy: a cost-effectiveness analysis. Ann Intern Med. 2017;167(8):544–554. doi: 10.7326/M16-2891. [DOI] [PubMed] [Google Scholar]
- 39.Cancer Australia . 2020. National and jurisdictional data on the impact of COVID-19 on medical services and procedures in Australia: breast, colorectal, lung, prostate and skin cancers.https://www.canceraustralia.gov.au/National_and_jurisdictional_data_on_the_impact_of_COVID-19_on_medical_services_and_procedures [cited 2022 Jul 7]. Available from: [Google Scholar]
- 40.Santoro G.A., Grossi U., Murad-Regadas S., et al. DElayed COloRectal cancer care during COVID-19 Pandemic (DECOR-19): global perspective from an international survey. Surgery. 2021;169(4):796–807. doi: 10.1016/j.surg.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenwood E., Swanton C. Consequences of COVID-19 for cancer care—a CRUK perspective. Nat Rev Clin Oncol. 2021;18(1):3–4. doi: 10.1038/s41571-020-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worthington J., Feletto E., Lew J., et al. Evaluating health benefits and cost-effectiveness of a mass-media campaign for improving participation in the national bowel cancer screening program in Australia. Public Health. 2020;179:90–99. doi: 10.1016/j.puhe.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Durkin S., Broun K., Guerin N., Morley B., Wakefield M. Impact of a mass media campaign on participation in the Australian bowel cancer screening program. J Med Screen. 2020;27(1):18–24. doi: 10.1177/0969141319874372. [DOI] [PubMed] [Google Scholar]
- 44.Worthington J., Lew J.B., Feletto E., et al. Improving Australian national bowel cancer screening program outcomes through increased participation and cost-effective investment. PLoS One. 2020;15(2) doi: 10.1371/journal.pone.0227899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kregting L.M., Kaljouw S., de Jonge L., et al. Effects of cancer screening restart strategies after COVID-19 disruption. Br J Cancer. 2021;124(9):1516–1523. doi: 10.1038/s41416-021-01261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worthington J.H.E., Lew J.B., St John J., et al. 2022. Colonoscopies in Australia – how much does the National Bowel Cancer Screening Program contribute to colonoscopy utilisation? Submitted. [DOI] [PubMed] [Google Scholar]
- 47.Miller-Wilson L.-A., Vahdat V., Brooks D., Limburg P.J. Modeling analysis of COVID 19-related delays in colorectal cancer screening on simulated clinical outcomes. J Clin Oncol. 2022;40(16_suppl) [Google Scholar]
- 48.Gastroenterological Society of Australia (GESA) 2020. Guide for triage of endoscopic procedures during the COVID-19 Pandemic.https://www.gesa.org.au/resources/covid-19 [cited 2022 Jul 7]. Available from: [Google Scholar]
- 49.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 50.Australian Institute of Health Welfare . AIHW; Canberra: 2021. National Bowel Cancer Screening Program: monitoring report 2021. Cancer series no.132. Cat. no. CAN 139. [Google Scholar]
- 51.Li N., Lu B., Luo C., et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: a comparison among China, Europe, and northern America. Cancer letters. 2021;522:255–268. doi: 10.1016/j.canlet.2021.09.034. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari A., Tran T.N., Hoeck S., Peeters M., Van Hal G. 2022. Differences and similarities in breast and colorectal cancer screening uptake among municipalities in Flanders. Belgium. [Google Scholar]
- 53.Canadian Partnership Against Cancer . 2021. Equity-focused interventions to increase colorectal cancer screening: program pack.https://www.partnershipagainstcancer.ca/topics/equity-colorectal-cancer-screening/summary/ [Google Scholar]
- 54.International Agency for Research on Cancer Lyon France. Cancer screening in the European union (2017) European Commission; 2017. Report on the implementation of the Council Recommendation on cancer screening. Report No.: 927908934X. [Google Scholar]
- 55.Institute of Health Information and statistics of the Czech republic. National Registry of Reimbursed Health Services; 2021. [Google Scholar]
- 56.Regionernes Kliniske Kvalitetsudviklingsprogram (RKKP) 2021. Dansk tarmkræftscreeningsdatabase årsrapport 2019. [Google Scholar]
- 57.Santé publique France . 2020. Taux de participation au programme de dépistage organisé du cancer colorectal 2018-2019.https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-colon-rectum/articles/taux-de-participation-au-programme-de-depistage-organise-du-cancer-colorectal-2018-2019 [DOI] [PubMed] [Google Scholar]
- 58.Ebell M.H., Thai T.N., Royalty K.J. Cancer screening recommendations: an international comparison of high income countries. Publ Health Rev. 2018;39(1):1–19. doi: 10.1186/s40985-018-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Altobelli E., Lattanzi A., Paduano R., Varassi G., di Orio F. Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med. 2014;62:132–141. doi: 10.1016/j.ypmed.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Cardoso R., Guo F., Heisser T., et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–1013. doi: 10.1016/S1470-2045(21)00199-6. [DOI] [PubMed] [Google Scholar]
- 61.Guðlaugsdóttir S., editor. Implementing colorectal cancer screening program in Iceland. WEO; Barcelona: 2015. [Google Scholar]
- 62.The National Bowel Screening Programme . 2020. BowelScreen programme report 2018– 2019, Round Three. [Google Scholar]
- 63.Paltiel O., Keidar Tirosh A., Paz Stostky O., et al. Adherence to national guidelines for colorectal cancer screening in Israel: comprehensive multi-year assessment based on electronic medical records. J Med Screen. 2021;28(1):25–33. doi: 10.1177/0969141320919152. [DOI] [PubMed] [Google Scholar]
- 64.Observatorio Nazionale Screening Lo Screening Colorettale. 2022. https://www.osservatorionazionalescreening.it/content/lo-screening-colorettale Available from:
- 65.Takahashi N., Nakao M. Social-life factors associated with participation in screening and further assessment of colorectal cancer: a nationwide ecological study in Japanese municipalities. SSM Popul Health. 2021;15 doi: 10.1016/j.ssmph.2021.100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamo K.-I., Fukui K., Ito Y., Nakayama T., Katanoda K. How much can screening reduce colorectal cancer mortality in Japan? Scenario-based estimation by microsimulation. Jpn J Clin Oncol. 2022;52(3):221–226. doi: 10.1093/jjco/hyab195. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi H. Impact of COVID-19 for cancer screening and cancer treatment in Japan. Ann Oncol. 2022;33:S411. [Google Scholar]
- 68.田淵貴大. 日本におけるがん検診受診率格差-医療保険のインパクト. 日医事新報. 2012;4605.
- 69.Dulskas A., Poskus T., Kildusiene I., et al. National colorectal cancer screening program in Lithuania: description of the 5-year performance on population level. Cancers. 2021;13(5):1129. doi: 10.3390/cancers13051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Landelijk Evaluatie team voor Colorectaal kanker bevolkingsonderzoek Landelijke monitoring en evaluatie van het bevolkingsonderzoek naar darmkanker in Nederland. https://www.rivm.nl/sites/default/files/2019-03/monitor-evaluatie-darm-2014-2017.pdf Available from:
- 71.Monteiro H., Tavares F., Reis J., et al. Colorectal screening program in northern Portugal: first findings. Acta Med Port. 2022;35(3):164–169. doi: 10.20344/amp.15904. [DOI] [PubMed] [Google Scholar]
- 72.Chan T.K.C., Tan L.W.L., van Dam R.M., Seow W.J. Cancer screening knowledge and behavior in a multi-ethnic asian population: the Singapore community health study. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.684917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singapore Cancer Society . 2021. Singapore cancer society annual report 2021. [Google Scholar]
- 74.Babela R., Orsagh A., Ricova J., et al. Cost-effectiveness of colorectal cancer screening in Slovakia. Eur J Cancer Prev. 2020;30:415–421. doi: 10.1097/CEJ.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 75.Rim J.H., Youk T., Kang J.G., et al. Fecal occult blood test results of the national colorectal cancer screening program in South Korea (2006–2013) Sci Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-03134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Department of Health Government of Catalonia . 2021. Information system of the colorectal cancer screening programme. [Google Scholar]
- 77.Vives N., Binefa G., Vidal C., et al. Short-term impact of the COVID-19 pandemic on a population-based screening program for colorectal cancer in Catalonia (Spain) Prev Med. 2022;155 doi: 10.1016/j.ypmed.2021.106929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blom J., Löwbeer C., Elfström K.M., et al. Gender-specific cut-offs in colorectal cancer screening with FIT: increased compliance and equal positivity rate. J Med Screen. 2019;26(2):92–97. doi: 10.1177/0969141318804843. [DOI] [PubMed] [Google Scholar]
- 79.Ulyte A., Wei W., Dressel H., et al. Variation of colorectal, breast and prostate cancer screening activity in Switzerland: influence of insurance, policy and guidelines. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeoh K.G. What asia-pacific populations is CRC screening justified? WEO CRC SC meeting. APDW; Taipei: 2015. [Google Scholar]
- 81.Wang Y.W., Chen H.H., Wu M.S., Chiu H.M. Current status and future challenge of population-based organized colorectal cancer screening: lesson from the first decade of Taiwanese program. J Formos Med Assoc. 2018;117(5):358–364. doi: 10.1016/j.jfma.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 82.Public Health England . 2020. Screening KPI data summary factsheets. [Google Scholar]
- 83.HSC Public Health Agency . 2017. Bowel cancer screening factsheet. [Google Scholar]
- 84.Public Health Scotland . 2021. Scottish bowel screening programme statistics. [Google Scholar]
- 85.Sgrinio Coluddion Cymru . 2021. Bowel screening Wales annual statistical report, 2019-20. [Google Scholar]
- 86.Ministerio de Salud Uruguay . 2019. Guia de practica clinica de tamizaje del cancer colo-rectal. [Google Scholar]
- 87.Crott R. The cost-effectiveness of screening for colorectal cancer. Expert Rev Pharmacoecon Outcomes Res. 2001;1(2):157–166. doi: 10.1586/14737167.1.2.157. [DOI] [PubMed] [Google Scholar]
- 88.Walker M.J., Wang J., Mazuryk J., et al. Delivery of cancer care in ontario, Canada, during the first year of the COVID-19 pandemic. JAMA Netw Open. 2022;5(4):e228855–e. doi: 10.1001/jamanetworkopen.2022.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Regionernes Kliniske Kvalitetsudviklingsprogram (RKKP) 2022. Dansk tarmkræftscreeningsdatabase årsrapport 2020. [Google Scholar]
- 90.Baarmoederhalskanker Landelijke Monitoring Bevolkingsonderzoek . 2022. Monitor bevolkingsonderzoek borstkanker. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.