Summary
Background
Accumulating evidence indicates that an early, robust type 1 interferon (IFN) response to SARS-CoV-2 is important in determining COVID-19 outcomes, with an inadequate IFN response associated with disease severity. Our objective was to examine the prophylactic potential of IFN administration to limit viral transmission.
Methods
A cluster randomised open label clinical trial was undertaken to determine the effects of pegylated IFNβ-1a administration on SARS-CoV-2 household transmission between December 3rd, 2020 and June 29th, 2021. Index cases were identified from databases of confirmed SARS-CoV-2 individuals in Santiago, Chile. Households were cluster randomised (stratified by household size and age of index cases) to receive 3 doses of 125 μg subcutaneous pegylated IFNβ-1a (172 households, 607 participants), or standard care (169 households, 565 participants). The statistical team was blinded to treatment assignment until the analysis plan was finalised. Analyses were undertaken to determine effects of treatment on viral shedding and viral transmission. Safety analyses included incidence and severity of adverse events in all treatment eligible participants in the standard care arm, or in the treatment arm with at least one dose administered. Clinicaltrials.gov identifier: NCT04552379.
Findings
5154 index cases were assessed for eligibility, 1372 index cases invited to participate, and 341 index cases and their household contacts (n = 831) enrolled. 1172 participants in 341 households underwent randomisation, with 607 assigned to receive IFNβ-1a and 565 to standard care. Based on intention to treat (ITT) and per protocol (PP) analyses for the primary endpoints, IFNβ-1a treatment did not affect duration of viral shedding in index cases (absolute risk reduction = −0.2%, 95% CI = −8.46% to 8.06%) and transmission of SARS-CoV-2 to household contacts (absolute risk reduction = 3.87%, 95% CI = −3.6% to 11.3%). Treatment with IFNβ-1a resulted in significantly more treatment-related adverse events, but no increase in overall adverse events or serious adverse events.
Interpretation
Based upon the primary analyses, IFNβ-1a treatment did not affect duration of viral shedding or the probability of SARS-CoV-2 transmission to uninfected contacts within a household.
Funding
Biogen PTY Ltd. Supply of interferon as ‘Plegridy (peginterferon beta-1a).’ The study was substantially funded by BHP Holdings Pty Ltd.
Keywords: SARS-CoV-2, COVID-19, Interferon, Ring prophylaxis, Transmission
Research in context.
Evidence before this study
Type I IFNs are critical early mediators of the innate immune response to all virus infections. They achieve viral clearance by directly inhibiting multiple stages of the viral replicative cycle, by protecting uninfected cells from infection and by recruiting and activating immune cell populations to sites of infection. Different clinical studies have identified that early treatment with IFNs-α/β/λ may accelerate SARS-CoV-2 viral clearance and prevent progression to severe disease. Despite effective vaccines that reduce disease severity, and the emergence of Omicron variants that apparently result in less severe disease, these highly transmissible variants continue to fuel the global pandemic. Prophylactic IFN treatment of ‘at risk’ individuals in outbreak or high risk settings may offer a solution to blunt transmission and end the pandemic.
Added value of this study
This randomized cluster trial of 1172 participants in 341 households demonstrated that 3 subcutaneous injections of 125 μg each of pegylated IFNβ-1a had no effect on SARS-CoV-2 viral shedding or transmission from an infected index cases in a household to uninfected household contacts. However, post-hoc sensitivity and exploratory analyses identified a reduction in transmission to household contacts where the index case had a high (>106 copies/mL) viral load. Pegylated IFNβ-1a was safe and well tolerated, with no concerning adverse laboratory events.
Implications of all the available evidence
There is no approved therapy to prevent SARS-CoV-2 transmission. This unique human challenge study demonstrates that prophylactic treatment with IFNβ-1a reduced transmission of SARS-CoV-2 within households. Whilst IFNβ-1a prophylaxis cannot be recommended as a useful intervention given the available evidence, our observations should be considered when designing future clinical trials aimed at preventing the transmission of highly contagious viruses.
Introduction
The SARS-CoV-2 pandemic has claimed over six million lives. Despite the rapid development and deployment of vaccines in many countries, the number of new cases worldwide is approximately 500,000 daily (https://covid19.who.int). With each wave of the pandemic, health systems have been challenged, complicated with emergent mutant strains of the virus. Mutated strains may be more transmissible,1,2 cause more severe disease than the original pandemic strain of SARS-CoV-23 and have the potential to evade available vaccines.4,5 Whilst widespread vaccination has had success limiting the trajectory of the pandemic, the emergence of the Omicron variants demonstrates that even with mutations that appear to cause less severe disease,6 high transmission despite immunization nonetheless results in significant pressures on health services.7 The solution to halting any pandemic is ending community transmission. During the current pandemic, measures such as healthy hygiene, self-isolation when sick, physical distancing and use of face masks have all been effective.8 Moreover, expedited public health responses such as extensive contact tracing, testing for infection and community lockdowns have all been effective in limiting transmission.9 International and local border closures plus strict quarantine measures have reduced community transmission to zero for periods in countries such as Australia and New Zealand.10,11 However, in these countries and elsewhere, as restrictions are relaxed, localized outbreaks have occurred12 that have required rapid, community-wide responses to again supress transmission. Importantly, these community constraints cause unprecedented civil disruption and come at enormous economic13,14 and social costs.15,16
Since the evolution of dominant SARS-CoV-2 virus cannot be easily predicted,17 there remains a need to identify interventions that can be rapidly deployed should highly pathogenic strains emerge despite high levels of community immunization. Furthermore, preparations for the next pandemic must include strategies to limit the potential for infection and transmission on first contact with pathogenic respiratory viruses.
One of the many therapeutic approaches investigated that appeared clinically useful early in the course of the pandemic was treatment with interferons (IFNs), namely IFNs-α/β. Randomised, controlled studies suggest that IFNs-α/β offer clinical benefits in moderate18 and severe disease,19,20 prevent infection in front-line hospital workers,21 and recent data indicate that IFN-λ reduces hospitalization and duration of viral shedding.22, 23, 24 Nonetheless, IFNs are not generally recommended for treatment of proven cases of COVID-1925 and other clinical trials have failed to demonstrate efficacy.26,27
IFNs are sentinel innate immune signalling molecules produced early after first contact with viral pathogens, that mediate their antiviral effects by direct inhibition of viral replication, protection of uninfected cells and also recruitment and activation of immune cells involved in viral clearance.28, 29, 30 Accordingly, we postulated that prophylactic IFN administration might reduce susceptibility to infection of uninfected contacts of individuals with SARS-CoV-2 infection. Such ring prophylaxis could provide non-specific, antiviral protection to curb episodic viral outbreaks,31 help suppress community transmission, even in vaccinated populations, and therefore reduce the risk of emergence of dangerous mutations.4,32 We therefore undertook a cluster, randomised, controlled study of sub-cutaneous pegylated IFNβ-1a (Plegridy. Biogen Inc, Cambridge MA) administration, to determine whether IFNβ-1a given to index cases and household contacts might reduce transmission of SARS-CoV-2.
Methods
The Containing Coronavirus Disease-19 (ConCorD-19) trial was a cluster randomised open label clinical trial of subcutaneous administration of pegylated IFNβ-1a (IFNβ-1a) versus standard care (control),33 completed between December 3rd, 2020 and June 29th, 2021. Each household of an index case (IC) was randomly assigned to either the IFNβ-1a or control arm. The study was approved by the Institutional Review Board of the Pontificia Universidad Católica de Chile and was registered with clinicaltrials.gov (NCT04552379). The published trial protocol is available online.33 All participants provided written informed consent.
Trial population
ICs were identified from databases of those with confirmed SARS-CoV-2 from COVID-19 clinics and emergency room visits in Santiago, Chile. Households were contacted by telephone to determine eligibility prior to enrolment (Supplementary Table S5). Household contacts aged between 18 and 80 years who met inclusion and exclusion criteria were deemed as ‘eligible’ household contacts, with households only enrolled if there was at least one eligible contact.33 Inclusion and exclusion criteria for each participant type (index case, eligible household contact, ineligible household contact) are listed in the full study protocol in the Supplementary Appendices. Household characteristics were captured consistent with recommendations of the World Health Organization for assessing household transmission.34 All participants implemented quarantine measures as mandated by local authorities and maintained a daily symptom diary which was collected and reviewed at each study visit by the study team (see Supplementary Appendices). Index cases were instructed to remain in isolation/quarantine for 11 days from onset of symptoms or, if asymptomatic, 11 days from the sample collection date that resulted in the COVID-19 diagnosis. Household contacts remained in isolation/quarantine for 11 days from date of the sample resulting in the diagnosis of the IC or of a newly diagnosed household member as per recommendations/rulings by local authorities.
Intervention
A mobile health team conducted home visits of all participant households on study days 1 (enrolment), 6, 11, 16, 21 and 29. ICs and eligible HCs in the IFNβ-1a arm received three subcutaneous doses of IFNβ-1a (125 μg/0.5 ml × 0.5 ml) on study days 1, 6 and 11. Ineligible contacts in the IFNβ-1a arm, as well as ICs and all HCs in the control arm, received standard care. All participating households received information regarding hygiene, isolation, social distancing and wearing of face masks as per public health advice at the time of enrolment. The IFNβ-1a injection was given by a trained member of a mobile health team and participants were recommended to take paracetamol (1000 mg, 6 hourly) commencing at the same time as the IFNβ-1a for up to 24 h, in order to mitigate predictable flu-like symptoms.35
Outcomes
The primary outcomes were (i) the proportion of participants in the IC-INF population shedding SARS-CoV-2 at study day 11 in the IFNβ-1a compared to control arm and (ii) the proportion of treatment eligible household contacts shedding SARS-CoV-2 at study day 11 in the IFNβ-1a compared to control arm. Secondary and exploratory outcomes in the trial protocol are listed in Supplementary Methods (Supplementary Table S1). Briefly, secondary outcomes included examination of the effects of IFNβ-1a treatment on SARS-CoV-2 IgG serological conversion, the incidence of adverse events and hospitalizations. Shedding was determined by the presence of SARS-CoV-2 by PCR in saliva collected on days 1, 6, 11, 16, 21, and 29 (see Supplementary Methods). A SARS-CoV-2 PCR Ct value ≥37 was considered negative. Viral load (copies/mL) was estimated from the Ct value using a standard curve of known viral titre (Supplementary Figure S1). Anti-SARS-CoV-2 IgG antibodies to nucleocapsid protein were measured on day 29 (using the Liaison assay according to the manufacturer's instructions [DiaSorin, Saluggia, Italy]).
Biospecimen collections
The full schedule of biospecimen collection is provided in the Supplementary Methods (Supplementary Tables S2 and S3). All consenting, non-eligible HCs also provided biospecimens according to the schedule collection for non-eligible HCs.
Adverse events
These were classified in accordance with Good Clinical Practice,36 as non-serious or serious and as related or unrelated to the trial medication. Because of overlap between symptoms of COVID-19 and potential IFNβ-1a-related adverse events, all symptoms were recorded and categorized, and any symptoms outside of the directed symptom assessment were considered adverse events. An independent data and safety monitoring committee reviewed safety data.
Power calculations
The study was designed at the start of the pandemic and there were few data to guide sample size calculations. Sample size calculations assumed a two-sided alpha level of 0.025 and power >90% to ensure, assuming an extremely conservative correlation between outcome measures of zero, the familywise type 1 error rate remains below 0.05 and power remains above 80%. To estimate the required sample size, we used available transmission data reported at the start of the pandemic, census data to estimate household size in Santiago and an effect size based on a pilot study undertaken in Wuhan.37 Data from Wuhan suggested that the proportion of untreated index cases still shedding virus on study day 11 would be ∼85%. Based on a two tailed Fisher's exact test, a sample size of 278 ICs (310 allowing for a 10% drop-out rate) would have >90% power, at α = 0.025, to detect a difference in the proportion of index cases shedding SARS-CoV-2 at study day 11, if the proportion in the IFNβ-1a arm was 65%. The estimated average household size was 4, based on available census data,38 and we estimated the secondary infection rate (transmission within the household) where there is an untreated IC would be 28%.39,40 A sample of 278 households, providing 834 household contacts, would have >90% power, at alpha 0.025, to detect an odds ratio of 0.5 for a reduction in transmission to a household contact, based on a stratified Cochran-Mantel-Haenszel test (two-tailed with intra-class correlation of 0.15). Based on the uncertainty regarding the effect size in the trial setting, we also planned a Bayesian analysis to estimate the probabilities of transmission for even small effects of therapy.33
Randomisation
Households were randomised as individual clusters using a minimization technique (biased coin, p = 0.7) in order to achieve balance in the total number of people within the household between treatment arms.41 Households were randomized as clusters to receive either IFN β-1a treatment or standard of care at a 1:1 ratio. Households were randomized during the first home visit using minimization software,42 once eligibility was confirmed, participants signed informed consent, and baseline data collection procedures were completed. Participants and study staff were not blinded to randomization of treatment or standard of care, but the statistical team was blinded to treatment assignment until the analysis plan was finalised.
Statistical analyses
We undertook frequentist analyses based upon the primary and secondary outcomes as stated in the study protocol and described in detail in the statistical analysis plan (available in the Supplementary Appendices) which included intent-to-treat and per protocol approaches. Participants were excluded from the populations used in the primary analyses if the study visit was not performed or was performed outside the 1-day window either side of the scheduled visit date as outlined in the Statistical Analysis Plan. Participants who didn't complete the full course of the treatment were excluded from the per protocol populations used in sensitivity analyses. Briefly, the analysis for primary outcome 1 used a generalized linear model (binomial, with a logit link for dichotomous outcomes) and was adjusted for age and sex, reporting an adjusted odds ratio (OR) and its corresponding 95% confidence interval. The analysis for primary outcome 2 used a generalized linear mixed model (binomial with a logit link) adjusted for age and sex and used a random intercept per household with an assumed normal distribution, reporting an adjusted OR and its corresponding 95% confidence interval. Sensitivity analyses were included with additional covariate adjustments and subgroup analyses.
While the frequentist approach allowed us to estimate the effects of IFNβ-1a administration on the risk of an individual becoming infected, the planned Bayesian analysis allowed us to determine whether the ring prophylaxis strategy using IFNβ-1a reduces the probability of transmission within the household of an infected index case. The analysis utilised the household contact population as defined in the Statistical Analysis Plan. Detailed methods are provided in the Supplementary Methods. Briefly, a generalized linear mixed effects model with a binomial logit function was developed to estimate the probability of infection that is influenced by explanatory variables for each contact case using the rstanarm R package.43,44
The analyses of secondary outcomes included duration of SARS-CoV-2 shedding assessed with discrete time-to-survival analysis using a generalized linear model with a complementary log–log link, proportion of participants positive for SARS-CoV-2 at study days 1 and 11 and seroconversion at study day 29 were assessed using Cochran-Mantel-Haenszel tests stratified by baseline immunity, incidence of hospitalization, death, or hospitalization and/or death were assessed using Cochran-Mantel-Haenszel tests stratified by household size, and duration of hospitalization was assessed using Fine-Gray competing risks regression with in-hospital death as a competing risk. Safety analyses comparing incidence of adverse events were performed using Fisher's exact test.
We also undertook post hoc exploratory frequentist analyses based on a modified subgroup analysis that accounted for households where at time of enrolment, it was unknown that all household contacts were SARS-CoV-2 positive or where index cases were no longer shedding virus, and for effects that may only be associated with active treatment. The analysis plan and statistical report for the exploratory analyses are available in the Supplementary Appendices. Briefly, both frequentist analyses fitted generalized linear mixed effects models using lme445 using a random intercept per household with an assumed normal distribution.
Role of funder
The funding bodies for this study had no role in data collection, analysis, or interpretation of data, writing of the manuscript, or decision to submit.
Results
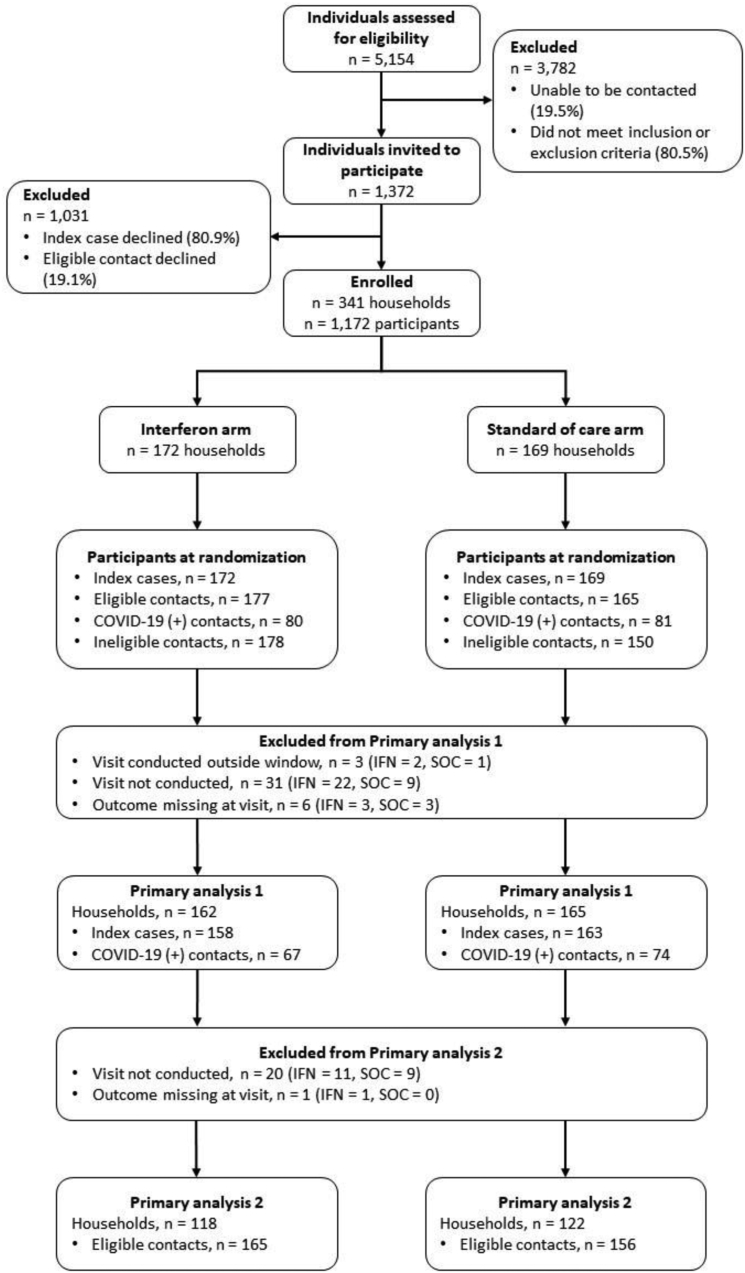
Recruitment, participation, and completion data are shown in Fig. 1. Household characteristics and participant demographics are shown in Tables 1 and 2 respectively. Between December 2020 and May 2021, 5154 index cases were assessed for eligibility, 1372 index cases invited to participate. 341 households were enrolled and randomised, of which 137 (IFNβ-1a arm) and 151 (control arm) completed the study. Of the 1172 individuals randomised (IFNβ-1a arm = 607; standard of care arm = 565), 53 individuals withdrew from the study, of which 15 were ICs: 35 (14 ICs) in the IFNβ-1a arm and 18 (1 IC) in the control arm. The reasons for withdrawal are summarized in the Supplementary Results (Supplementary Table S4). One IC withdrew before randomisation. Eighty-two households where the IC had a negative salivary PCR on Days 1 and 6, or where there were no eligible contacts who tested negative at recruitment, were excluded for the exploratory analyses. (36 households in the treatment arm, 46 households in the standard care arm): 259 households were considered as the ‘at risk’ population.
Fig. 1.
CONSORT diagramdescribing participant screening, enrolment, randomisation, and analysis.
Table 1.
Household demographics at baseline.
| Household demographics |
||
|---|---|---|
| SOC (n = 169) | IFN (n = 172) | |
| Household occupants | ||
| Mean (SD) | 3.74 (1.29) | 3.90 (1.64) |
| Infected household occupants at study start | ||
| Median (IQR) | 1 (1–2) | 1 (1–2) |
| At risk household occupants at study start | ||
| Median (IQR) | 2 (1–3) | 2 (1–3) |
| Household occupants fully vaccinated for COVID-19 | ||
| Median (IQR) | 1 (0–2) | 1 (0–2) |
| Log10viral load of household index case at study start | ||
| Mean (SD) | 5.25 (1.08) | 5.42 (1.11) |
SOC: Standard of care, IFN: IFNβ-1a treatment arm.
Table 2.
Full study population demographics at baseline.
| Full study population |
||
|---|---|---|
| SOC (n = 565) | IFN (n = 607) | |
| Age (years) | ||
| Mean (SD) | 35.0 (19.4) | 33.2 (18.7) |
| Sex | ||
| Female, n (%) | 294 (52.0%) | 323 (53.2%) |
| Household occupants | ||
| Mean (SD) | 3.74 (1.29) | 3.90 (1.64) |
| Fully vaccinated for SARS-CoV-2 | ||
| No, n (%) | 380 (67.3%) | 438 (72.2%) |
| Cancer | ||
| No, n (%) | 559 (98.9%) | 598 (98.5%) |
| Diabetes | ||
| No, n (%) | 542 (95.9%) | 583 (96.0%) |
| Heart disease | ||
| No, n (%) | 533 (94.3%) | 578 (95.2%) |
| Asthma | ||
| No, n (%) | 524 (92.7%) | 581 (95.7%) |
| Chronic lung disease | ||
| No, n (%) | 564 (99.8%) | 606 (99.8%) |
| Chronic kidney disease | ||
| No, n (%) | 564 (99.8%) | 604 (99.5%) |
| Chronic neurological disease | ||
| No, n (%) | 562 (99.5%) | 602 (99.2%) |
| Smoker | ||
| No, n (%) | 502 (88.8%) | 519 (85.5%) |
SOC: Standard of care, IFN: IFNβ-1a treatment arm.
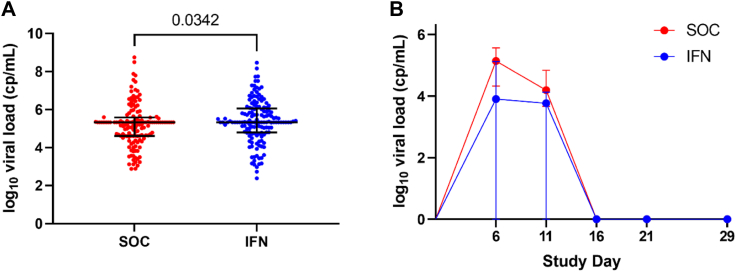
When the study population was assessed for descriptive differences, the baseline (study day 1) viral load of ICs was significantly higher in the IFN treatment arm compared with ICs in the standard care arm (p = 0.034) (Fig. 2A). We then assessed whether IFNβ-1a treatment reduced median viral load when compared to the standard care arm at each study day (1, 6, 11, 16, 21, & 29) including all household contacts (treatment eligible and ineligible). Treatment with IFNβ-1a significantly reduced median viral load at study day 6 in household contacts (p = 0.034; Fig. 2B) but had no effect on median viral load at study days 1, 11, 16, 21, or 29.
Fig. 2.
Descriptive differences in the household contact population. (A) Baseline (study day 1) viral load of index cases in the standard of care (SOC) and IFNβ-1a treatment (IFN) arms in the household contact (HC) (SOC: n = 142. IFN: n = 147) population, with data present as median (circle) with interquartile range (whiskers). (B) Viral load of household contacts who test positive for SARS-CoV-2 in the SOC and IFN treatment arms in the (SOC: n = 66, IFN: n = 64) with data presented as median (circle) and interquartile range at each study visit. Zero values represent data below the limit of quantification, reported as zero per the statistical analysis plan. cp/mL = copies/mL.
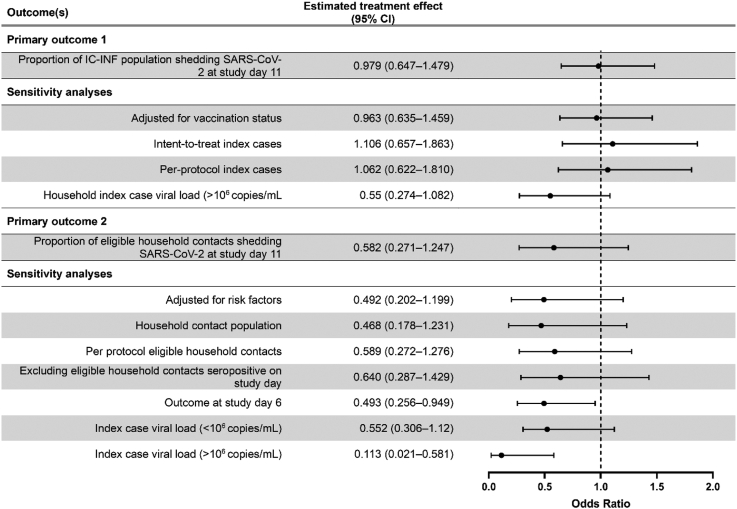
All point estimates and 95% confidence intervals for primary and secondary analyses are available in Table 3. The Statistical Analysis Report with all analyses undertaken as outlined in the Statistical Analysis Plan are included in the Supplementary Appendices. There was no evidence of an effect of IFNβ-1a administration (vs. standard of care) on the probability of viral shedding at study day 11 in the IC-INF population (ICs and treatment eligible HCs infected at study day 1) (Primary outcome 1; OR = 0.979, 95% CI = 0.647–1.479; absolute risk reduction = −0.2%, 95% CI = −8.46% to 8.06%) or in the subsequent sensitivity analyses (Figs. 3 and 4). Similarly, there was no evidence of an effect of IFNβ-1a on the probability of viral shedding in (i.e. transmission to) treatment eligible HCs (EHC-ITT) associated with IFNβ-1a administration (vs. standard of care) at study day 11 (Primary outcome 2: OR = 0.582, 95% CI = 0.271–1.247; absolute risk reduction = 3.87%, 95% CI = −3.6% to 11.3%), however where the day 1 IC viral load was >106 copies/mL, there was evidence that treatment with IFNβ-1a significantly reduced transmission to treatment eligible HCs (OR = 0.121, 95% CI = 0.025–0.524) (Figs. 3 and 4). Additionally, in an unplanned sensitivity analysis with the outcome measure changed to the probability of viral shedding at study day 6, there was a significant reduction in the probability of viral shedding in treatment eligible HCs (EHC-ITT): OR = 0.493, 95% CI = 0.256–0.949.
Table 3.
Primary and secondary outcomes with sensitivity analyses.
| Outcome(s) | IFNβ-1a n (%) | Standard of care n (%) | Estimated treatment effect (95% CI)a |
|---|---|---|---|
| Primary outcome 1 (n = 462) | |||
| Proportion of IC-INF population shedding SARS-CoV-2 at study day 11 | 65 (28.9) | 68 (28.7) | 0.979 (0.647–1.479) ARR = −0.197% (−8.5 to 8.1%) |
| Sensitivity analyses | |||
| Adjusted for vaccination status (n = 462) | – | – | 0.963 (0.635–1.459) |
| Intent-to-treat index cases (n = 321) | – | – | 1.106 (0.657–1.863) |
| Per-protocol index cases (n = 309) | – | – | 1.062 (0.622–1.810) |
| Household index case viral load (>106 copies/mL) as a covariate (n = 309) | – | – | 0.55 (0.274–1.082) |
| Primary outcome 2 (n = 321) | |||
| Proportion of eligible household contacts shedding SARS-CoV-2 at study day 11 | 19 (11.5) | 24 (15.4) | 0.582 (0.271–1.247) ARR = 3.87% (−3.6 to 11.3%) |
| Sensitivity analyses | |||
| Adjusted for risk factors (n = 321) | – | – | 0.492 (0.202–1.199) |
| Household contact population (n = 518) | – | – | 0.468 (0.178–1.231) |
| Per protocol eligible household contacts (n = 311) | – | – | 0.589 (0.272–1.276) |
| Excluding eligible household contacts seropositive on study day 1 (n = 266) | – | – | 0.640 (0.287–1.429) |
| Outcome at study day 6 (n = 329) | 20 (11.8) | 31 (19.5) |
0.493 (0.256–0.949) ARR = 7.7% (−0.1 to 15.6%) |
| Index case viral load (<106 copies/mL) (n = 321) | – | – | 0.552 (0.306–1.12) |
| Index case viral load (>106 copies/mL) (n = 321) | – | – | 0.113 (0.021–0.581) |
| Secondary outcome 1 (n = 502) | |||
| Effect of treatment on duration of viral shedding | – | – | HR = 1.083 (0.964–1.217) |
| Secondary outcome 2 | |||
| Incidence of SARS-CoV-2 shedding in household contacts at study day 11 (n = 518) | 37 (13.6) | 43 (17.6) | 0.680 (0.353–1.309) |
| Seroconversion in household contacts at day 29 (n = 462) | 86 (34.5) | 101 (47.4) | 0.580 (0.336–1.002) |
| Secondary outcome 3 | |||
| Incidence of hospitalisation in IC-ITT (n = 299) | 15 (9.38) | 14 (10.1) | 0.842 (0.385–1.842) |
| Sensitivity analyses | |||
| IC-INF population (n = 437) | 21 (9.29) | 23 (10.9) | 0.789 (0.418–1.489) |
| Per protocol index cases (n = 275) | 4 (2.94) | 14 (10.1) | 0.272 (0.088–0.844) |
| Secondary outcome 3 | |||
| Duration of hospital stay due to COVID-19 (n = 29) | – | – | HR = 0.522 (0.237–1.152) |
| Sensitivity analyses | |||
| IC-INF population (n = 43) | – | – | HR = 0.452 (0.233–0.877) |
| Per protocol index cases (n = 18) | – | – | HR = 2.006 (0.601–6.690) |
| Secondary outcome 4 (n = 844) | |||
| Incidence of adverse events—rate of AE per participant (incidence of any AE) | 2.117 (0.235) | 1.824 (0.280) | p = 0.156 (Fisher's exact test) |
| Incidence of adverse events related to treatment events—rate of related AE per participant (incidence of any related AE) | 0.287 (0.235) | 0.00964 (0.00964) | p < 0.0001 (Fisher's exact test) |
| Incidence of serious adverse events—rate of SAE per participant (incidence of any SAE) | 0.0559 (0.0559) | 0.0627 (0.0578) | p = 1.00 (Fisher's exact test) |
HR: Hazard ratio, ARR: Absolute risk reduction.
Bold text indicates a statistically significant effect.
The estimated treatment effect is an odds ratio unless stated otherwise.
Fig. 3.
Forrest plots describing primary outcomes. The estimated treatment effect is an odds ratio unless stated otherwise. Effects were considered statistically significant if the interval estimates exclude the no-effect value of 1. Data is presented as point estimate (circle) with 95% confidence intervals (whiskers).
Fig. 4.
Absolute risk of positive SARS-CoV-2 test for primary and exploratory outcomes in treatment and standard care arms. SOC (red): Standard of care, IFN (blue): IFNβ-1a treatment. Data is presented as point estimate (circle) with 95% confidence intervals (whiskers).
Analysis of the secondary outcomes revealed no evidence of an effect of IFNβ-1a treatment (vs. standard of care) on duration of viral shedding in the IC-INF population using a discrete time survival analysis or in the subsequent sensitivity analyses. Similarly, there was no evidence of an effect of IFNβ-1a treatment (vs. standard of care) on incidence of saliva PCR positive for SARS-CoV-2 in HCs at day 11 or on seroconversion in HCs at day 29 stratified by seroconversion at baseline.
There were 58 serious adverse events in index cases, 57 hospitalizations due to COVID-19 (25 in the IFNβ-1a treatment arm and 32 in the control arm) and one death in the standard care arm due to COVID-19 in an individual with significant co-morbidities and uncompensated diabetes mellitus. Twenty-six household contacts were hospitalized, 10 in the IFNβ-1a treatment arm and 16 in the control arm. Analysis of safety outcomes (Secondary outcome 3 and 4) found no evidence of an effect of IFNβ-1a treatment on incidence or duration of hospitalization due to COVID-19 in the IC-ITT population (Secondary outcome 3) or on incidence of hospitalization in the IC-INF population. However, in sensitivity analyses for secondary outcome 3, there was evidence of a statistically significant effect of IFNβ-1a treatment (vs. standard of care) on reducing the incidence of hospitalization in the IC-PP population (p = 0.0312), and evidence of a statistically significant effect of IFNβ-1a treatment (vs. standard of care) on increasing the duration of hospitalization in the IC-INF population (HR = 0.452, 95% CI 0.233–0.877). For analysis of safety outcomes of IFNβ-1a treatment (Secondary outcome 4), there was a significant increase in treatment-related adverse events in the IFNβ-1a arm, but no effect on overall adverse events or incidence of serious adverse events.
The Bayesian analysis (see Supplementary Methods) identified a 95% probability of reduction of infection within a household by IFNβ-1a treatment and the credible interval for the reduction in transmission probability was in the order of 0.5% to 15.3% during the active treatment period (study days 1–11). During the active treatment period, there was a significant reduction in the odds of transmission (OR = 0.43, 95% credible interval = 0.21–0.86) with a posterior probability that IFNβ-1a treatment is superior to standard care of 97.5%. The estimated Bayes factor for period 1 was 38.80, indicating strong support for the hypothesis that IFNβ-1a treatment reduces infection rate (∼38 times more likely that observed data agrees with the hypothesis that IFNβ-1a treatment reduces infection than the hypothesis that treatment increases infection rate).46 By contrast, in period 2 (day 12–29), the 95% credible interval for the reduction in transmission probability includes zero and therefore treatment was not effective (Supplementary Table S7). The estimated Bayes factor indicates that the data are only 2 times more likely to be observed under the hypothesis that IFNβ-1a treatment increases infection rate, but the evidence is weak and therefore considered non-significant.46 The effect of IFNβ-1a on transmission was independent of household size (Supplementary Table S8). When the infection reduction is stratified by household size, only households of size 4 in period 1 had a 95% credible interval without zero.
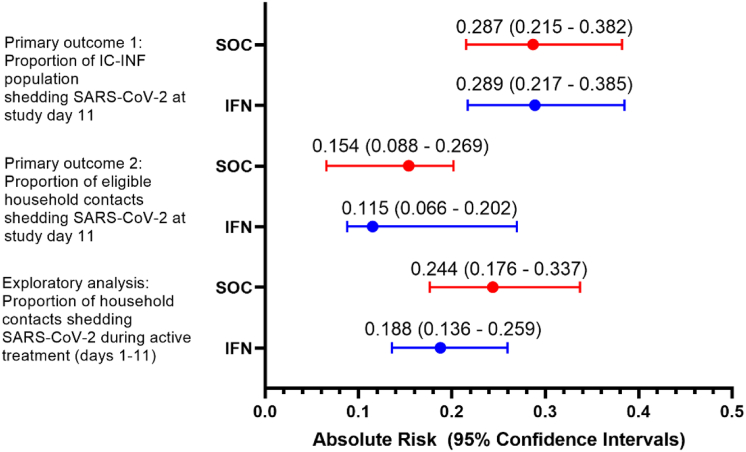
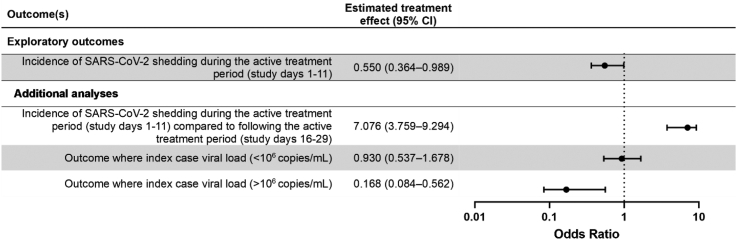
Subsequent exploratory analyses focused on a population of household contacts deemed “at risk”. The at risk population is specifically defined in the Supplementary Appendices, with the significant distinction being that households were excluded from analyses where the index case subsequently tested negative for SARS-CoV-2 on study days 1 and 6, and where no SARS-CoV-2 negative household contacts remain on study day 1, as transmission of SARS-CoV-2 would be biologically implausible. In the at-risk population, 55/293 (absolute risk: 18.7%, 95% CI = 13.6% to 25.9%) household contacts in the IFNβ-1a arm became infected during active treatment (days 1–11) of the study compared to 60/246 (absolute risk: 24.4%, 95% CI = 17.6% to 33.7%) household contacts in the control arm (Fig. 4), indicative of an relative risk reduction in the IFN arm equivalent to 23% (95% CI = −6.4% to 44.4%). When the effect of IFNβ-1a treatment on the probability of a SARS-CoV-2 positive saliva PCR in the at risk population was assessed post-hoc using a binomial generalized linear mixed model with a logit link function (Supplementary Appendices), treatment with IFNβ-1a was associated with a significant reduction in the odds of a SARS-CoV-2 positive saliva PCR for all household contacts compared to standard of care during the treatment period (study days 1–11) (p = 0.033; OR = 0.55, 95% CI = 0.36–0.99; Fig. 5). The treatment effect was not significant in the subsequent period (days 12–29) i.e. after treatment and isolation had ended. Testing positive to SARS-CoV-2 was significant more common in the first period when compared with the second period (OR = 7.08, 95% CI = 3.76–9.29; Fig. 5). To assess whether the protective effect of treatment was more pronounced in households where the index case had a high viral load at the start of the study, we included a covariate dichotomizing index case viral load >106 copies/mL and compared generalized linear mixed effect models with and without an interaction term between this covariate and treatment arm. The treatment by viral load interaction term was significant (p = 0.005), indicating that the treatment effect is significantly different in households with IC with low (<106) vs. high (>106) viral loads. The odds of positive PCR result were significantly higher in households with index cases with high viral load (>106) compared to those with low viral load (p = 0.02; OR = 3.29, 95% CI = 1.14–6.1). In households with index cases with high viral load (>106), treatment with IFNβ-1a significantly reduced the odds of a positive PCR result during the study period (p = 0.0028; OR = 0.17, 95% CI = 0.084–0.56; Fig. 5). Conversely, in households with index cases with low viral load (<106), treatment with IFNβ-1a did not reduce the odds of a positive PCR result during the study period (p = 0.84; OR = 0.93, 95% CI = 0.54–1.68; Fig. 5).
Fig. 5.
Forrest plots describing exploratory outcomes. The estimated treatment effect is an odds ratio unless stated otherwise. Effects were considered statistically significant if the interval estimates exclude the no-effect value of 1. Data is presented as point estimate (circle) with 95% confidence intervals (whiskers).
Discussion
Pegylated IFNβ-1a is an FDA approved therapy for multiple sclerosis for which the pharmacokinetics and safety profile are well-characterized.42 The primary analyses from this prospective, cluster randomised, ring prophylaxis trial demonstrated that IFNβ-1a administration was ineffective as a public health measure to reduce the household transmission of SARS-CoV-2. The chosen formulation allowed us to predict the likely duration of activity of the three-dose regimen to cover the period of peak transmissibility of the virus. Using a mobile medical team to administer doses at home, optimized adherence to therapy and allowed reasons for non-adherence and withdrawal to be accurately documented.
Although the intention to treat and per protocol analyses failed to demonstrate a significant effect on primary outcomes, the sensitivity analyses for primary outcome 2 indicated an effect was observable using day 6 as an outcome measure, and that treatment was only effective in households where the initial viral load of index cases was high. Additionally, these analyses failed to account for index cases not shedding virus at randomization nor an absence of eligible contacts within a household. Using plausible filters to define an at-risk population provided additional insight regarding the biological effects of IFNβ-1a administration on viral transmission. These exploratory analyses indicated that there was a high probability of a small reduction in household transmission. Since point-of-care diagnostics were unavailable at the time of the study, enrichment of the population with high-risk eligible household contacts was not possible. This is perhaps an important point to consider in the design of future ring prophylaxis studies, particularly if a small reduction in viral transmission at the start of a pandemic could translate into large heath and socio-economic benefits.
To our knowledge, only one previous study assessed ring prophylaxis in COVID-19: Labhardt et al. observed that a combination of lopinavir/ritonavir for 5 days as post-exposure prophylaxis was not effective at preventing infection in close contacts of ICs.43 However, a trial of ring vaccination against the Alpha variant demonstrated reduced risk of contracting COVID-19 in areas with high local transmission.47
The overall viral load trajectory we observed was somewhat similar to that observed in a laboratory human challenge experiment using wild-type SARS-CoV-2 virus in healthy volunteers.44 Treatment with IFNβ-1a had no effect on viral load trajectory; therefore, reduced viral shedding by infected individuals is unlikely to explain the protective effect on household transmission of IFNβ-1a treatment that we report. Given that treatment affected the probability of transmission only in the active treatment phase and appears unrelated to viral load, we speculate that the observed effects of IFNβ-1a were direct through protection of the at risk, exposed individual rather than indirectly though effects on the index case. The ConCoRD-19 biorepository will allow further examination of the mechanisms of action of IFNβ-1a on resistance to infection.
This study had several clear limitations due to its setting early in the course of the pandemic that may have impacted the observed outcomes. The trial was undertaken prior to the emergence of the Delta and Omicron strains (Supplementary Figure S3) and prior to widespread vaccination. The effect size observed in this study could be greater for variants with higher transmissibility such as the Omicron strains and derivatives that are now the dominant worldwide. Simulation data suggests antiviral therapy may be more effective against highly transmissible strains,48 and subgroup analysis of a clinical trial of IFN-λ found the treatment effect was only significant against Omicron, not earlier variants.24 Additionally, early intervention is important for effective prophylaxis—this study administered the first dose within 72 h of a positive COVID-19 test or symptom onset. Based upon other recent studies, this appears to likely be within the therapeutic window for antiviral therapies.24,49 Additionally, we identified two sources of potential bias in the study. Bias in primary analyses utilising intent-to-treat analysis cannot be excluded due to missing data at visit 11 for primary analysis 1 (7.9%) and primary analysis 2 (6.1%). However, there is little risk of selection bias in per-protocol analyses due to the minimal amount of non-compliance or missing data for primary outcome 1 (3.7%) and primary outcome 2 (3.1%). We calculated the sample size based upon known household transmission characteristics of the Alpha strain which was the dominant strain of the virus early in the pandemic. Simple hygiene measures and quarantine of affected individuals within households could have contributed to lower rates of transmission than expected. Together with a smaller number of eligible household contacts than anticipated from census data, these factors may have reduced the power of the study for the primary outcomes. Finally, our post-hoc analysis demonstrated that certain assumptions regarding viral shedding by index cases and the likelihood of SARS-CoV-2 negative eligible household contacts at randomisation were incorrect.
In summary, intention to treat and per protocol analyses failed to demonstrate significant effects on primary outcomes. In a sub-population biologically defined as at-risk of infection, IFNβ-1a significantly reduced the probability of household transmission of SARS-CoV-2. These observations suggest that ring prophylaxis with therapies that can interrupt transmission is a strategy worth pursuing and that point of care diagnostics to identify those at highest risk for infection can increase the likelihood of success. These factors should be considered when designing future clinical trials to address transmission of highly contagious viruses.
Contributors
Authors Castro-Rodriguez, Fish, Montgomery, Kollmann, Iturriaga, Karpievitch, and Shannon contributed equally to the conduct of the study and manuscript preparation as first authors. Authors Montgomery, Karpievitch, Shannon, and Chen have directly accessed and verified the underlying data. Authors Tebbutt, Diego García-Huidobro, Perret, Borzutsky and Stick contributed equally to the conduct of the study and manuscript preparation as senior authors.
Data sharing statement
The data that support the findings of this study are available from the corresponding author, SMS, upon reasonable request.
Declaration of interests
Authors Castro-Rodriguez, Kollman, and Stick received funding from BHP Holdings Pty Ltd and Biogen Pty Ltd to conduct this study. Balshaw was paid consulting fees to advise on this study. Castro-Rodriguez received honoraria from AstraZeneca and GlaxoSmithKline to speak at symposia and Eurofarma for participation on advisory board. Kollmann has received research funding from NIH, Medical Research Future Fund, and Bill and Melinda Gates Foundation, honoraria from the Human Immunome Project to attend a conference, and holds two patents unrelated to this study. Montgomery received funding from NHMRC and TSANZ to present findings from this study at conferences. Authors Aniba, Borzutzky, Chen, Fish, Garcia-Huidobro, Gigi-Yunge, Hancock, Hartnell, Ho, Iturriaga, Karpievitch, Othman, Perret, Shannon, Tebbutt, and Urzua have no declared conflicts of interest.
Acknowledgements
Matthew Cooper (Telethon Kids Institute): Advice regarding protocol and establishment of randomization schedule. Alexia Foti (Telethon Kids Institute): Collating, reviewing, and editing documents. Zsuzsanna Hollander (PROOF Centre, Vancouver): Statistical advice and statistical analysis plan review. Nat Eiffler (Telethon Kids Institute): Project management, protocol development, data management. Jessica Meyer (University of Rochester): Assisting collation and formatting of tables and figures. The trial funders had no role in study design, data collection and analyses, data interpretation or writing of the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102082.
Appendix A. Supplementary data
References
- 1.Alizon S., Haim-Boukobza S., Foulongne V., et al. Rapid spread of the SARS-CoV-2 Delta variant in some French regions, June 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.28.2100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra S., Mindermann S., Sharma M., et al. Changing composition of SARS-CoV-2 lineages and rise of Delta variant in England. eClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twohig K., Nyberg T., Zaidi A., et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2021;22:35. doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winger A., Caspari T. The spike of concern-the novel variants of SARS-CoV-2. Viruses. 2021;13 doi: 10.3390/v13061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyngse F.P., Mortensen L.H., Denwood M.J., et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat Commun. 2022;13:5573. doi: 10.1038/s41467-022-33328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyberg T., Ferguson N.M., Nash S.G., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Rutte E., Shattock A., Chitnis N., Kelly S.L., Penny M. Cold Spring Harbor Laboratory; 2021. Assessing impact of Omicron on SARS-CoV-2 dynamics and public health burden. medRXiv. Online server. [Google Scholar]
- 8.Implementation of mitigation strategies for communities with local COVID-19 transmission. CDC; 2021. [Google Scholar]
- 9.Milne G.J., Xie S., Poklepovich D., O'Halloran D., Yap M., Whyatt D. A modelling analysis of the effectiveness of second wave COVID-19 response strategies in Australia. Sci Rep. 2021;11 doi: 10.1038/s41598-021-91418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Team C-NIRS COVID-19 Australia: epidemiology report 38 reporting period ending 28 March 2021. Commun Dis Intell. 2021;45:4–6. doi: 10.33321/cdi.2021.45.19. [DOI] [PubMed] [Google Scholar]
- 11.Houvessou G.M., Souza T.P., Silveira M.F.D. Lockdown-type containment measures for COVID-19 prevention and control: a descriptive ecological study with data from South Africa, Germany, Brazil, Spain, United States, Italy and New Zealand, February–August 2020. Epidemiol Saude. 2021;30 doi: 10.1590/S1679-49742021000100025. [DOI] [PubMed] [Google Scholar]
- 12.Conway S.R., Lazarski C.A., Field N.E., et al. SARS-CoV-2-Specific T cell responses are stronger in children with multisystem inflammatory syndrome compared to children with uncomplicated SARS-CoV-2 infection. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.793197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler D.M., Summers L.H. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324:1495–1496. doi: 10.1001/jama.2020.19759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verschuur J., Koks E.E., Hall J.W. Global economic impacts of COVID-19 lockdown measures stand out in high-frequency shipping data. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chemen S., Gopalla Y.N. Lived experiences of older adults living in the community during the COVID-19 lockdown–the case of Mauritius. J Aging Stud. 2021;57 doi: 10.1016/j.jaging.2021.100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mamun M.A. Suicide and suicidal behaviors in the context of COVID-19 pandemic in Bangladesh: a systematic Review. Psychol Res Behav Manag. 2021;14:695–704. doi: 10.2147/PRBM.S315760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callaway E. Beyond Omicron: what's next for COVID's viral evolution. Nature. 2021;600:204–207. doi: 10.1038/d41586-021-03619-8. [DOI] [PubMed] [Google Scholar]
- 18.Rahmani H., Davoudi-Monfared E., Nourian A., et al. Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial. Int Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alavi Darazam I., Shokouhi S., Pourhoseingholi M.A., et al. Role of interferon therapy in severe COVID-19: the COVIFERON randomized controlled trial. Sci Rep. 2021;11:8059. doi: 10.1038/s41598-021-86859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davoudi-Monfared E., Rahmani H., Khalili H., et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng Z., Wang T., Chen L., et al. The effect of recombinant human interferon alpha nasal drops to prevent COVID-19 pneumonia for medical staff in an epidemic area. Curr Top Med Chem. 2021;21:920–927. doi: 10.2174/1568026621666210429083050. [DOI] [PubMed] [Google Scholar]
- 22.Jagannathan P., Andrews J.R., Bonilla H., et al. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nat Commun. 2021;12:1967. doi: 10.1038/s41467-021-22177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feld J.J., Kandel C., Biondi M.J., et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med. 2021;9:498–510. doi: 10.1016/S2213-2600(20)30566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reis G., Moreira Silva E.A.S., Medeiros Silva D.C., et al. Early treatment with pegylated interferon lambda for Covid-19. N Engl J Med. 2023;388:518–528. doi: 10.1056/NEJMoa2209760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health NIo . Unioted States Goverment; 2021. Covid-19 treatment guidelines. [Google Scholar]
- 26.Ader F., Peiffer-Smadja N., Poissy J., et al. An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clin Microbiol Infect. 2021;27:1826–1837. doi: 10.1016/j.cmi.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Repurposed antiviral drugs for Covid-19 — interim WHO solidarity trial results. N Engl J Med. 2020;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee A.J., Ashkar A.A. The dual nature of type I and type II interferons. Front Immunol. 2018;9:2061. doi: 10.3389/fimmu.2018.02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazewski C., Perez R.E., Fish E.N., Platanias L.C. Type I interferon (IFN)-Regulated activation of canonical and non-canonical signaling pathways. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.606456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B.X., Fish E.N. Global virus outbreaks: interferons as 1st responders. Semin Immunol. 2019;43 doi: 10.1016/j.smim.2019.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization . Worl Health Organization; Geneva: 2020. Informal consultation on the role of therapeutics in COVID-19 prophylaxis and post-exposure prophylaxis. [Google Scholar]
- 32.Gentile I., Maraolo A.E., Piscitelli P., Colao A. COVID-19: time for post-exposure prophylaxis? Int J Environ Res Public Health. 2020;17:3997. doi: 10.3390/ijerph17113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iturriaga C., Eiffler N., Aniba R., et al. A cluster randomized trial of interferon ss-1a for the reduction of transmission of SARS-Cov-2: protocol for the Containing Coronavirus Disease 19 trial (ConCorD-19) BMC Infect Dis. 2021;21:814. doi: 10.1186/s12879-021-06519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization . World Health Organization; Geneva: 2020. Household transmission investigation protocol for 2019-novel coronavirus (COVID-19) infection. Epidemiological protocol. [Google Scholar]
- 35.Reess J., Haas J., Gabriel K., Fuhlrott A., Fiola M. Both paracetamol and ibuprofen are equally effective in managing flu-like symptoms in relapsing-remitting multiple sclerosis patients during interferon beta-1a (AVONEX) therapy. Mult Scler. 2002;8:15–18. doi: 10.1191/1352458502ms771sr. [DOI] [PubMed] [Google Scholar]
- 36.Council NHaMR . National Health and Medical Research Council; Canberra: 2016. Guidance: safety monitoring and reporting in clinical trials involving therapeutic goods. [Google Scholar]
- 37.Zhou Q., Chen V., Shannon C.P., et al. Interferon-alpha2b treatment for COVID-19. Front Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.(Chile) NIoS . National Institute of Statistics (Chile); Santiago, Chile: 2021. Chile population and housing census 2017. [Google Scholar]
- 39.Yi B., Fen G., Cao D., et al. Epidemiological and clinical characteristics of 214 families with COVID-19 in Wuhan, China. Int J Infect Dis. 2021;105:113–119. doi: 10.1016/j.ijid.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curmei M., Ilyas A., Evans O., Steinhardt J. Constructing and adjusting estimates for household transmission of SARS-CoV-2 from prior studies, widespread-testing and contact-tracing data. Int J Epidemiol. 2021;50:1444–1457. doi: 10.1093/ije/dyab108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmeijer J., Anema P.C., van der Tweel I. New algorithm for treatment allocation reduced selection bias and loss of power in small trials. J Clin Epidemiol. 2008;61:119–124. doi: 10.1016/j.jclinepi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Saghaei M., Saghaei S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J Biomed Sci Eng. 2011;4:734–739. [Google Scholar]
- 43.Goodrich B., Gabry J., Ali I., Sam B. 2020. Bayesian applied regression modeling via Stan. R package version 2.21.1. rstanarm. [Google Scholar]
- 44.Brilleman S.L., Crowther M.J., Moreno-Betancur M., Buros Novik J., Wolfe R. StanCon 2018; Pacific Grove C, USA: 2018. Joint longitudinal and time-to-event models via Stan.https://github.com/stan-dev/stancon_talks/ Joint longitudinal and time-to-event models via Stan. [DOI] [PubMed] [Google Scholar]
- 45.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 46.van Doorn J., van den Bergh D., Böhm U., et al. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychon Bull Rev. 2021;28:813–826. doi: 10.3758/s13423-020-01798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Périnet S., Cadieux G., Mercure S.A., Drouin M., Allard R. Analysis of COVID-19 risk following a ring vaccination intervention to address SARS-CoV-2 alpha variant transmission in montreal, Canada. JAMA Netw Open. 2022;5:e2147042. doi: 10.1001/jamanetworkopen.2021.47042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schöning V., Kern C., Chaccour C., Hammann F. Effectiveness of antiviral therapy in highly-transmissible variants of SARS-CoV-2: a modeling and simulation study. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.816429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matrajt L., Brown E.R., Cohen M.S., Dimitrov D., Janes H. Could widespread use of antiviral treatment curb the COVID-19 pandemic? A modeling study. BMC Infect Dis. 2022;22:683. doi: 10.1186/s12879-022-07639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.